Introduction
Lung cancer is a malignant tumor of the highest
morbidity and mortality worldwide. It is also a major disease
threatening human life and health. The global cancer burden is
increasingly aggravated, as is suggested in the World Cancer Report
released by the World Health Organization (WHO) International
Agency for Research on Cancer in 2014. Lung cancer was ranked at
the top among new common cancers in 2012 (1). There were approximately 1.8 million
lung cancer cases, accounting for 13% of the total common cancer
cases (1). In addition, lung cancer
was also ranked at the top among the common causes of
cancer-related deaths. There were approximately 1.6 million death
cases, accounting for 19.4% of the total cases. Of these, Chinese
cases accounted for over 1/3 (2).
Primary lung cancer can be divided into small cell
lung cancer (SCLC) and non-small cell lung cancer (NSCLC). NSCLC
accounts for 85% of all lung cancer cases (2). It mainly includes 3 types, namely,
lung adenocarcinoma, lung squamous cell carcinoma and large cell
carcinoma (2). To date, surgical
treatment is the most effective method for NSCLC. In comparison,
SCLC is generally more sensitive to chemotherapy and radiotherapy
(2).
Circulating miRNAs can serve as disease markers.
Such a discovery has aroused extensive interest from numerous
scientists in recent years (1).
Particularly, miRNAs as tumor diagnostic markers have attracted
wide attention (1). A miRNA is an
endogenous non-coding small molecular RNA. It is highly conserved,
with a length of approximately 18–23 bases. Notably, detecting
changes in substances contained in the blood can indicate the
health condition of patients. This is a minimally invasive method
that can alleviate patient suffering (3). Therefore, detecting blood miRNA
content is a good screening method or auxiliary diagnosis (3).
CD4+ T cells can secrete cytokines. Thus,
they can activate monocytes, macrophages and NK cells. In addition,
they can induce immune response in monocytes, macrophages and NK
cells. Therefore, they can exert an antitumor function (4). CD4+ T cells can also
secrete IL-2 as a second signal. Subsequently, it can activate
CD8+ T cells and participate in the immune response
against tumors (4,5). CD8+ T cells are inhibitory
T cells that exert an immunosuppressive function. The body can only
exert normal immune function under the balanced status of the two.
Research has found that lung cancer patients are under
immunosuppressive status (5). This
can be attributed to the joint action of multiple immunosuppressive
factors (4). These inhibitory
factors can suppress the maturation and differentiation of
CD4+ T cells. This can thereby decrease the number of
CD4+ T cells and weaken the immuno-monitoring function
on tumor cells (4).
PD-L1 and PD-L2 are newly discovered B7 family
costimulatory molecule ligands. They share the common receptor PD-1
(6). In addition, they can bind
with receptor PD-1 to inhibit T-cell proliferation and excessive
activation (6). In this way, it
plays a negative regulatory role in cellular immune response
(7). Concurrently, it also exerts a
regulatory role in humoral immunity by affecting cytokine secretion
(7). The PD-L1 and PD-L2/PD-1
pathways play important roles in autoimmune tolerance and the
immune escape mechanism of tumor cells (7). At present, numerous tumors are found
to express PD-L1. PD-L1 expression in tumor cells can weaken the
immunogenicity of tumors. Furthermore, it can influence tumor cells
to produce specific T-cell response (7). Moreover, it can suppress the
production of tumor immune response. PD-L1 expressed in tumor cells
can induce specific CTL apoptosis. Thus, it allows the tumor cells
to develop immune escape (7).
Talebi et al revealed that miR-142 regulates T-cell
differentiation in an animal model of multiple sclerosis (8). The present study aimed to evaluate the
function of miR-142-5p on cancer immunity to induce apoptosis in
human non-small cell lung cancer (NSCLC) and its mechanism.
Materials and methods
Patients and flow cytometry
A total of 20 patients with NSCLC and a total of 20
normal specimens were collected from the Department of Thoracic
Surgery of Shenzhen People's Hospital. The patients were aged from
55 to 65 years. Peripheral blood was collected and rapidly frozen
in liquid nitrogen and stored at −80°C. Ethical approval was
obtained from the Shenzhen People's Hospital.
Serum was collected after centrifugation at 1000 × g
for 10 min at 4°C and used to assess CD4+ T cells.
Immune cell suspensions were prepared and stained with
anti-CD4+CD25hi+Foxp3+ T cell-APC
(anti-mouse antibody; eBioscience; Thermo Fisher Scientific, Inc.)
for 15 min at room temperature. Flow cytometry was performed using
BD AccuriC6 (BD Biosciences, Franklin Lakes, NJ, USA) and data was
analyzed using FlowJo software (FlowJo, LLC, Ashland OR, USA).
Quantitative real-time PCR
(qRT-PCR)
Total RNA from serum and cultured cells samples was
extracted using TRIzol (Invitrogen; Thermo Fisher Scientific,
Inc.). Reverse transcriptase reactions were performed to compound
cDNA using M-MLV reverse transcriptase (Promega Corp., Madison, WI,
USA). miR-142-5p expression was detected using a Bulge-Loop™ miRNA
qRT-PCR Primer Set (Guangzhou Ribobio, Co., Ltd., Guangzhou, China)
with Platinum SYBR-Green qPCR SuperMix-UDG reagents (Invitrogen;
Thermo Fisher Scientific, Inc.) and calculated using the
2−∆∆Ct method. PCR primers of miR-142-5p were as
follows: forward, 5′-AACTCCAGCTGGTCCTTAG-3′ and reverse,
5′-TCTTGAACCCTCATCCTGT-3′; and PCR primers of U6 were: forward,
5′-CTCGCTTCGGCAGCACA-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT. The
qRT-PCR thermocycling conditions were as follows: initial
denaturation at 95°C for 10 min followed by 40 cycles at 95°C for
25 sec, 60°C for 30 sec and 72°C for 30 sec.
Cell culture and reagents
NSCLC cell line A549 was cultured with Dulbecco's
modified Eagle's medium (DMEM; Whittaker BioProducts, Walkersville,
MD, USA) with 10% fetal bovine serum (Invitrogen; Thermo Fisher
Scientific, Inc., Carlsbad, CA, USA), 100 U/ml penicillin, and 100
mg/ml streptomycin in humidified air at 37°C with 5%
CO2. miR-142-5p, anti-miR-142-5p and negative mimics
were transfected into A549 cells using Lipofectamine™ 2000
(Invitrogen, Thermo Fisher Scientific, Inc.). PBMCs were acquired
from the same donor for preparation of non-adherent responder
T-cells (NAC) and monocytes (MN) and incubated in complete
RPMI-1640 (Whittaker BioProducts) supplemented with 5% PHS in 25
cm2 tissue culture flasks (2.5×107
cells/flask) in the presence of MTB H37RvL (1 µg/ml; Invitrogen;
Thermo Fisher Scientific, Inc.) for 5 days. PBMCs
(5×105) were seeded onto the cultured A549 cells by
transfection for 24 h (1:5, A549:PBMCs) in 10 µg/ml of PHA
(Sigma-Aldrich, St. Louis, MO, USA).
MTT assay, LDH activity level and flow
cytometric analysis of apoptosis
Cells were assessed using an MTT assay. MTT solution
(20 µl) was added to the cells after transfection at 24, 48 and 72
h. Following incubation for 4 h, the previous medium was removed
and 150 ml dimethyl sulfoxide (DMSO) was added to the cells for 20
min at 4°C. The optical density (OD) was read at 570 nm using
Bio-Rad Microplate Reader Model 680 (Bio-Rad Laboratories,
Hercules, CA, USA).
To assess the LDH activity level after transfection
at 24 h, the cells were harvested using an LDH level kit (Beyotime
Institute of Biotechnology, Nanjing, China). The OD was read at 450
nm using Bio-Rad Microplate Reader Model 680 (Bio-Rad
Laboratories).
To assess apoptosis using flow cytometry, after
transfection at 24 h, the cells were harvested and stained with
FITC-Annexin V and 7-AAD. The cells were analyzed with BD AccuriC6
(BD Biosciences) and data was analyzed using FlowJo software
(FlowJo, LLC).
Determination of the concentration of
cytokines using ELISA
Cellular supernatant was collected after
centrifugation at 1000 × g for 10 min at 4°C. CCL11, CCL22 and
IFN-γ levels were assessed using ELISA kits. The OD was read at 450
nm using Bio-Rad Microplate Reader Model 680 (Bio-Rad
Laboratories).
Western blotting
Cells were harvested and washed with PBS. Briefly,
total proteins were extracted by disrupting cells in RIPA lysis
buffer and assessed using a BCA assay (both from Beyotime Institute
of Biotechnology). Total protein (50 µg) was separated on 10%
polyacrylamide gels, and transferred to polyvinylidene difluoride
(PVDF) membranes. The membranes were then blocked at room
temperature for 1 h with 5% non-fat milk in TBST and the membranes
were incubated at 4°C overnight with the following antibodies:
PD-L1 (1:500; cat. no. sc-293425), PTEN (1:500; cat. no.
sc-6817-R), PI3K (1:500; cat. no. sc-7175), p-Akt (1:200; cat. no.
sc-7985-R), Akt (1:500; cat. no. sc-8312) and GAPDH (1:5,000; cat.
no. sc-25778; all from Santa Cruz Biotechnology, Inc., Dallas TX,
USA). After being washed with TBST, the membranes were incubated
with goat anti-rabbit peroxidase-conjugated secondary antibodies
(1:5,000; cat. no. sc-2005 or cat. no. sc-2004; Santa Cruz
Biotechnology) at room temperature for 1 h. Protein expression was
detected using an enhanced chemiluminescence kit (Beyotime
Institute of Biotechnology).
Determination of caspase-3/9
activity
Cells were harvested and washed with PBS. Briefly,
total proteins were extracted by disrupting cells in RIPA lysis
buffer and assessed using a BCA assay (both from Beyotime Institute
of Biotechnology). Total protein (10 µg) was used to assess
caspase-3/9 activity levels using Caspase-3/9 activity kits
(Beyotime Institute of Biotechnology). The OD was read at 405 nm
using Bio-Rad Microplate Reader Model 680 (Bio-Rad
Laboratories).
Statistical analyses
All data are represented as the mean ± SD of three
independent experiments. All data were evaluated using Student's
t-test or one-way analysis of variance (ANOVA) and Tukey's post
test. Values were considered significant when P<0.05.
Results
miRNA-142-5p expression and
CD4+ T cells in NSCLC patients
To determine miRNAs in NSCLC patients, we examined
the expression levels of CC chemokines which interact with cell
surface chemokine receptor CCR4, in 136 NSCLC patients by gene chip
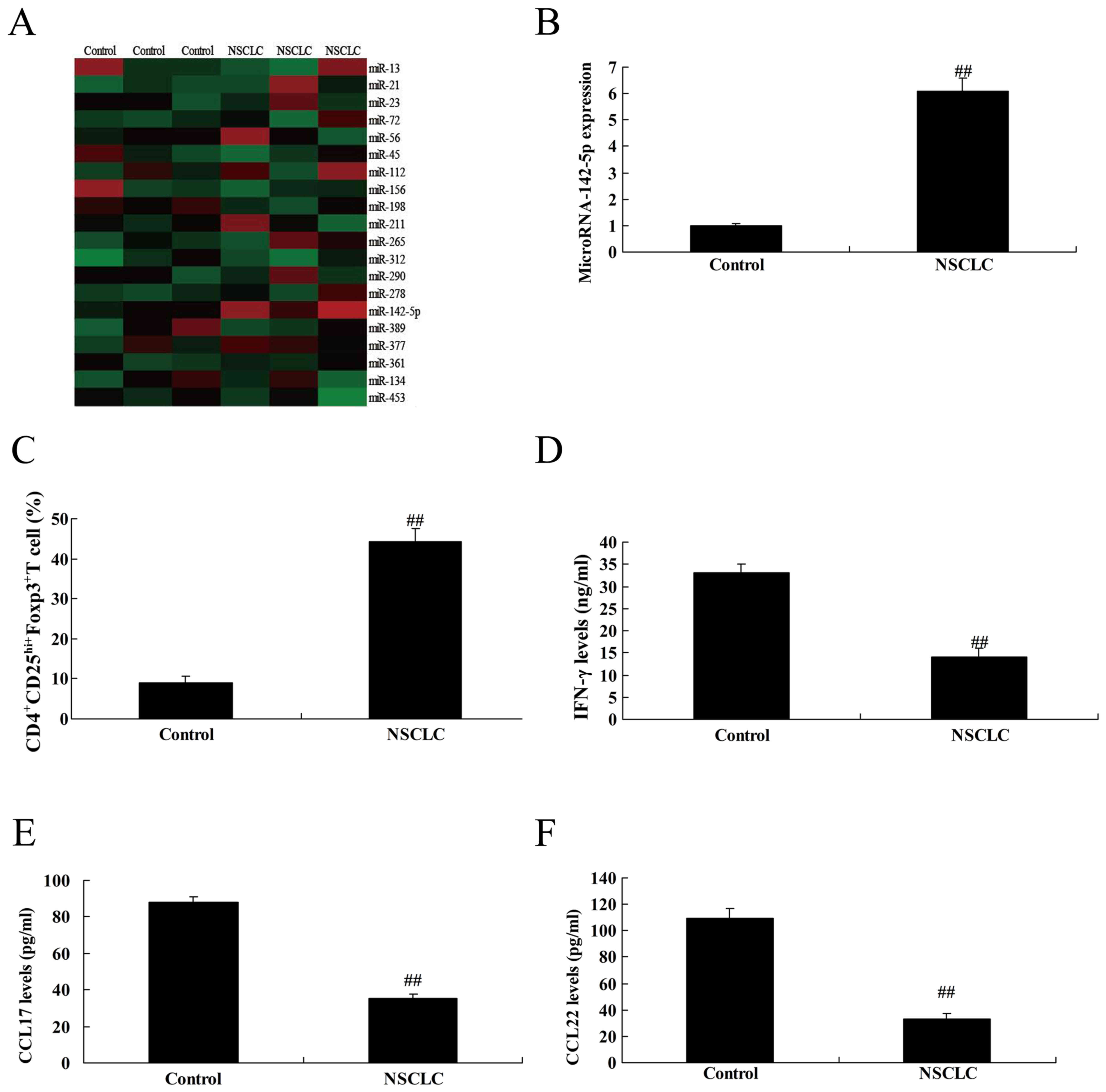
or qPCR analyses. As revealed in Fig.
1A and B, miRNA-142-5p expression was upregulated in NSCLC
patients, compared with the control group. Then, we also found that
the CD4+CD25hi+Foxp3+ T cell
expression level was upregulated in NSCLC patients, compared with
the control group (Fig. 1C). The
levels of IFN-γ, CCL17 and CCL22 were reduced in NSCLC patients,
compared with the control group (Fig.
1D-F).
Overexpression of miR-142-5p
expression inhibits the cancer effects of CD4+ T cells
in NSCLC cell line A549
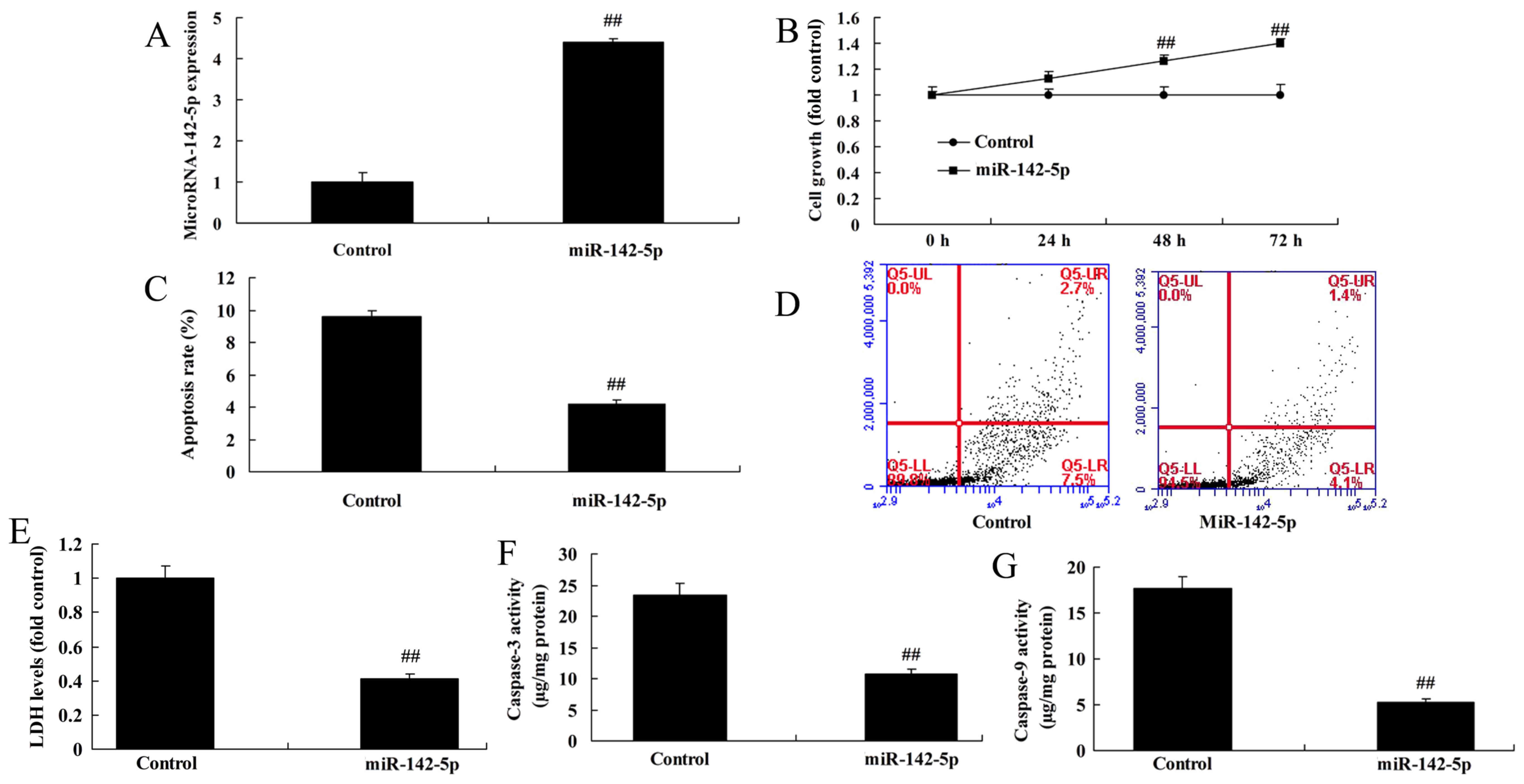
To detect the effect of miR-142-5p on the cancer
effects of CD4+ T cells on NSCLC cell line A549, we
overexpressed miR-142-5p expression using miR-142-5p mimics
(Fig. 2A). Following a significant
increase of miR-142-5p expression in A549 cells, these cells were
co-cultured with CD4+ T cells. Following co-culture for
24, 48 and 72 h, the overexpression of miR-142-5p reduced the
cancer effects of CD4+ T cells. miR-142-5p
overexpression promoted cell growth, and reduced the level of LDH
activity, the apoptosis rate and caspase-3/9 activities in A549
cells compared with the control group (Fig. 2B-G).
Downregulation of miR-142-5p increases
the cancer effects of CD4+ T cells in NSCLC cell line
A549
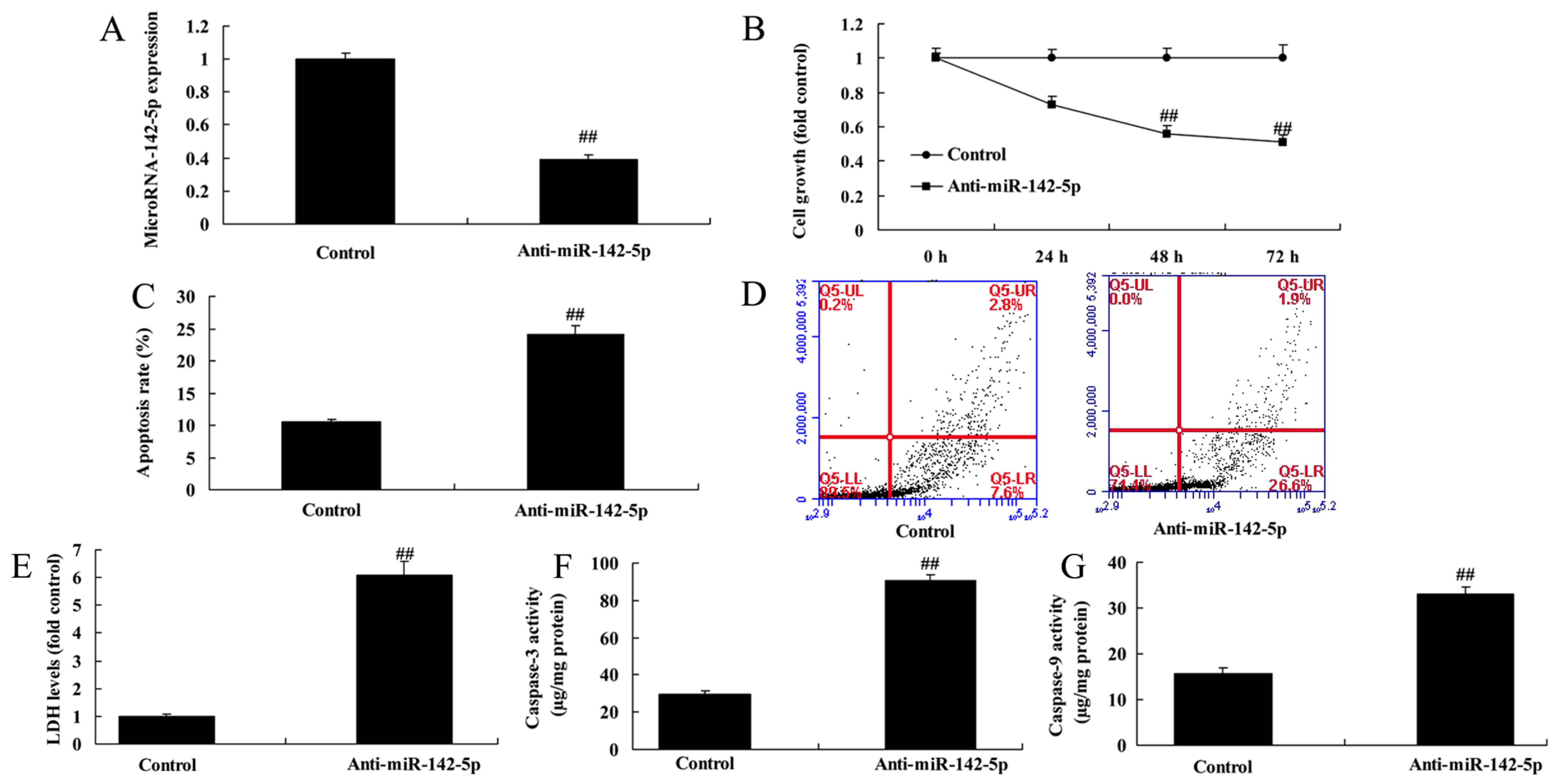
Next, we also used anti-miR-142-5p mimics to
decrease the level of miR-142-5p expression in A549 cells and
compared this level with the control group (Fig. 3A). Following the decrease of
miR-142-5p expression, the cells were co-cultured with
CD4+ T cells. Then, at 24, 48 and 72 h of co-culture, it
was observed that downregulation of miR-142-5p increased the cancer
effects of CD4+ T cells. Downregulation of miR-142-5p
reduced cell growth, and increased the activity level of LDH, the
apoptosis rate and caspase-3/9 activities compared with the control
group (Fig. 3B-G). Therefore, the
anti-effects of miR-142-5p in NSCLC may involve CD4+ T
cells, however, the mechanism warrants further investigation.
The effects of miR-142-5p on
CD4+ T cells in NSCLC cell line A549 via the PTEN
pathway
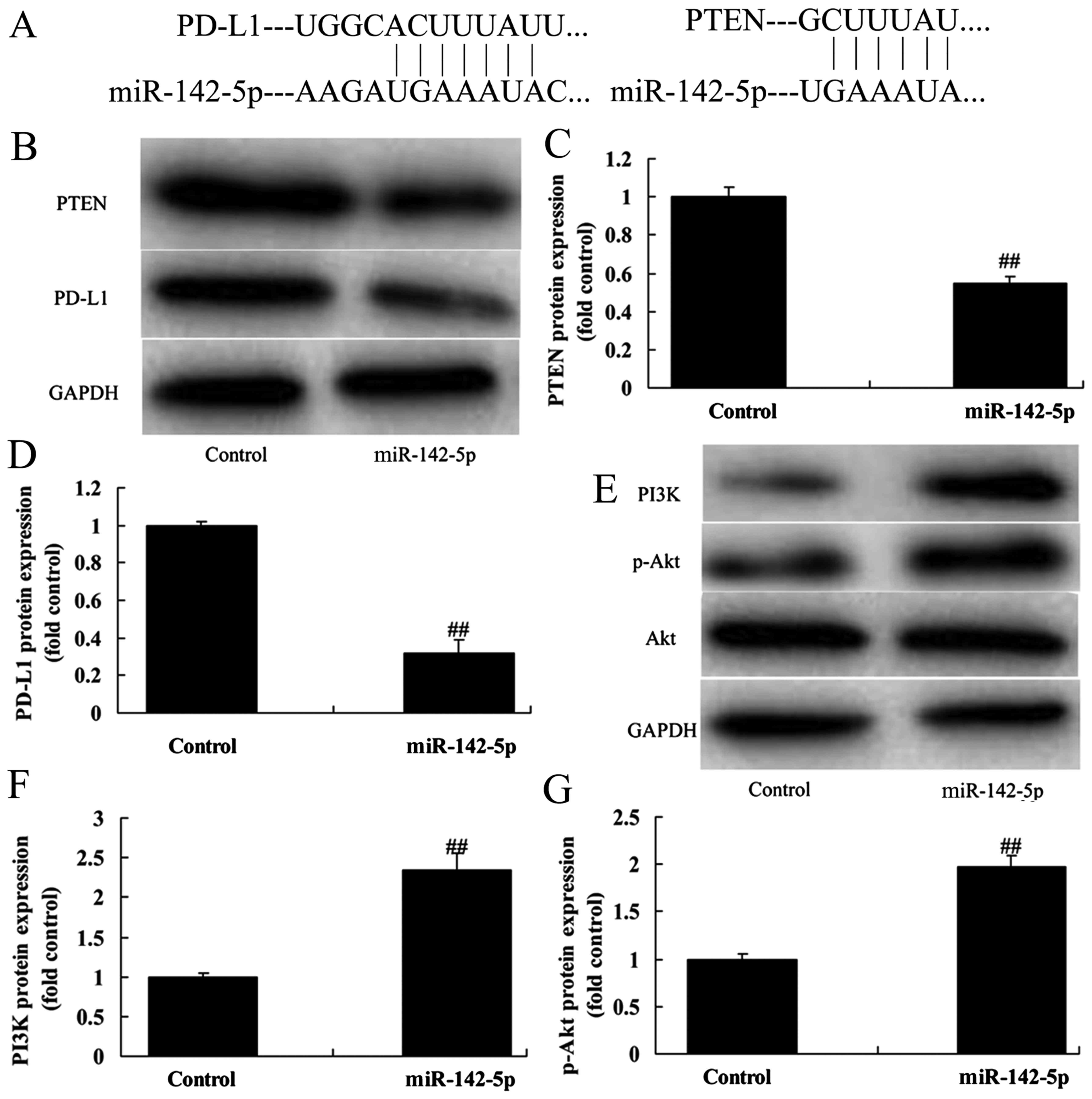
We investigated the effects of miR-142-5p on the
PTEN pathway, to validate the bioinformatics analysis. As revealed
in Fig. 4A, PD-L1 and PTEN are the
potential target genes of miR-142-5p. Then, in the co-culture
model, overexpression of miR-142-5p suppressed PTEN and PD-L1
protein expression in A549 cells compared with the control group
(Fig. 4B-D). Furthermore, we also
found that overexpression of miR-142-5p induced PI3K and p-Akt
protein expression in the co-culture model, compared with the
control group (Fig. 4E-G). In the
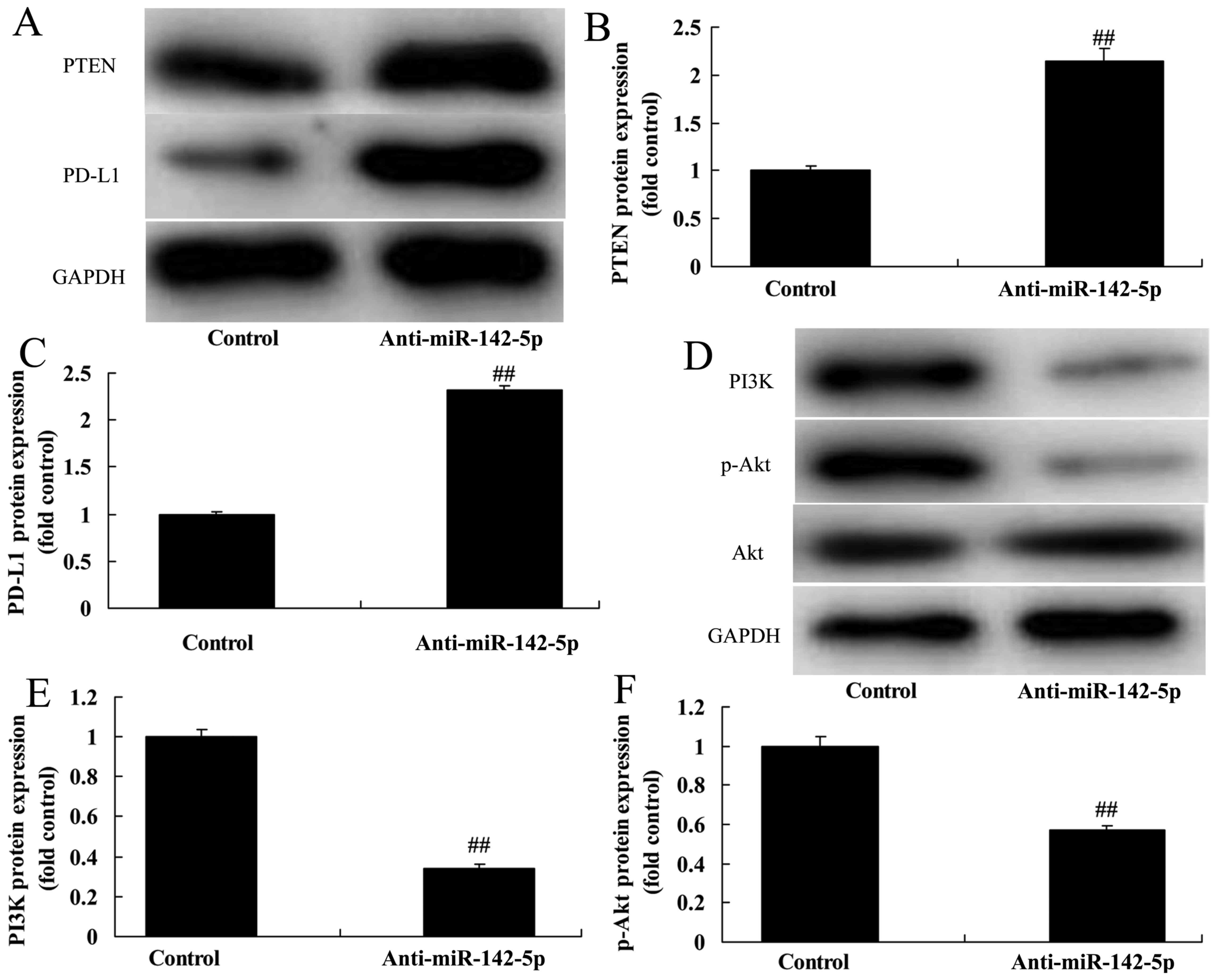
co-culture model, downregulation of miR-142-5p induced PTEN and
PD-L1 protein expression in A549 cells compared with the control
group (Fig. 5A-C). Moreover,
downregulation of miR-142-5p suppressed PI3K and p-Akt protein
expression in the co-culture model compared with the control group
(Fig. 5D-F). Collectively,
miR-142-5p promoted antitumor immunity in NSCLC by blocking the
PD-L1/PD-1 pathway via the PTEN pathway.
Effects of miR-142-5p on the
concentration of CCL17, CCL22 and IFN-γ levels
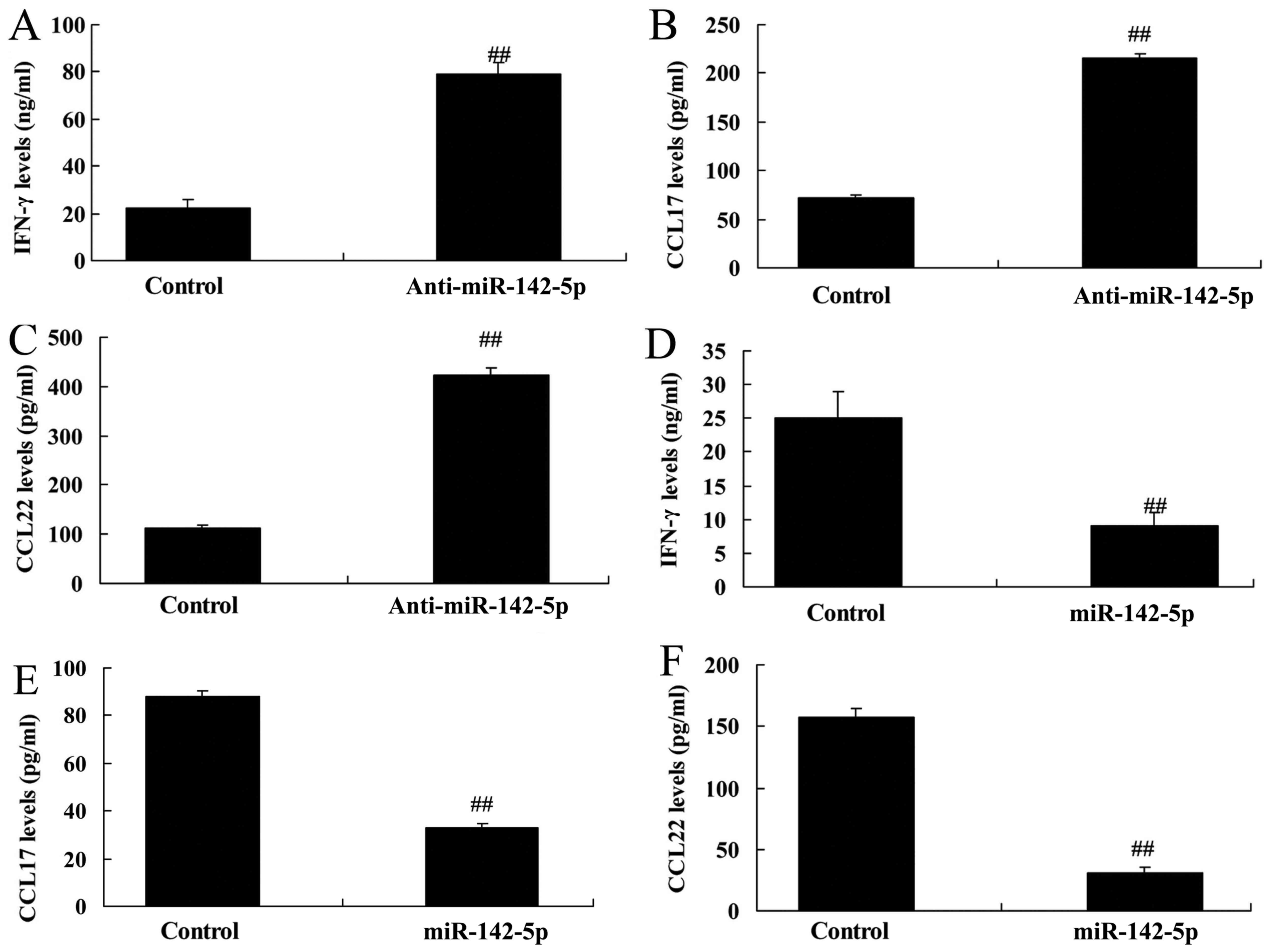
Next, we analyzed CCL17, CCL22 and IFN-γ levels in
the medium of the co-culture model using ELISA kits. Downregulation
of miR-142-5p increased CCL17, CCL22 and IFN-γ levels in the medium
of the co-culture model compared with the control group (Fig. 6A-C). Overexpression of miR-142-5p
reduced CCL17, CCL22 and IFN-γ levels in the medium of the
co-culture model compared with the control group (Fig. 6D-F). These results revealed that
miR-142-5p regulates the PD-L1/PD-1 pathway to secrete CCL17, CCL22
and IFN-γ into the tumor microenvironment for antitumor immunity in
NSCLC cells.
Inhibition of PTEN also reduces the
cancer effects of CD4+ T cells in NSCLC cell line A549
following miR-142-5p downregulation
Considering the potential role of the PTEN/PI3K/Akt
signaling pathway in miR-142-5p-promoted antitumor immunity in
NSCLC cells, we wanted to further elucidate the involvement of PTEN
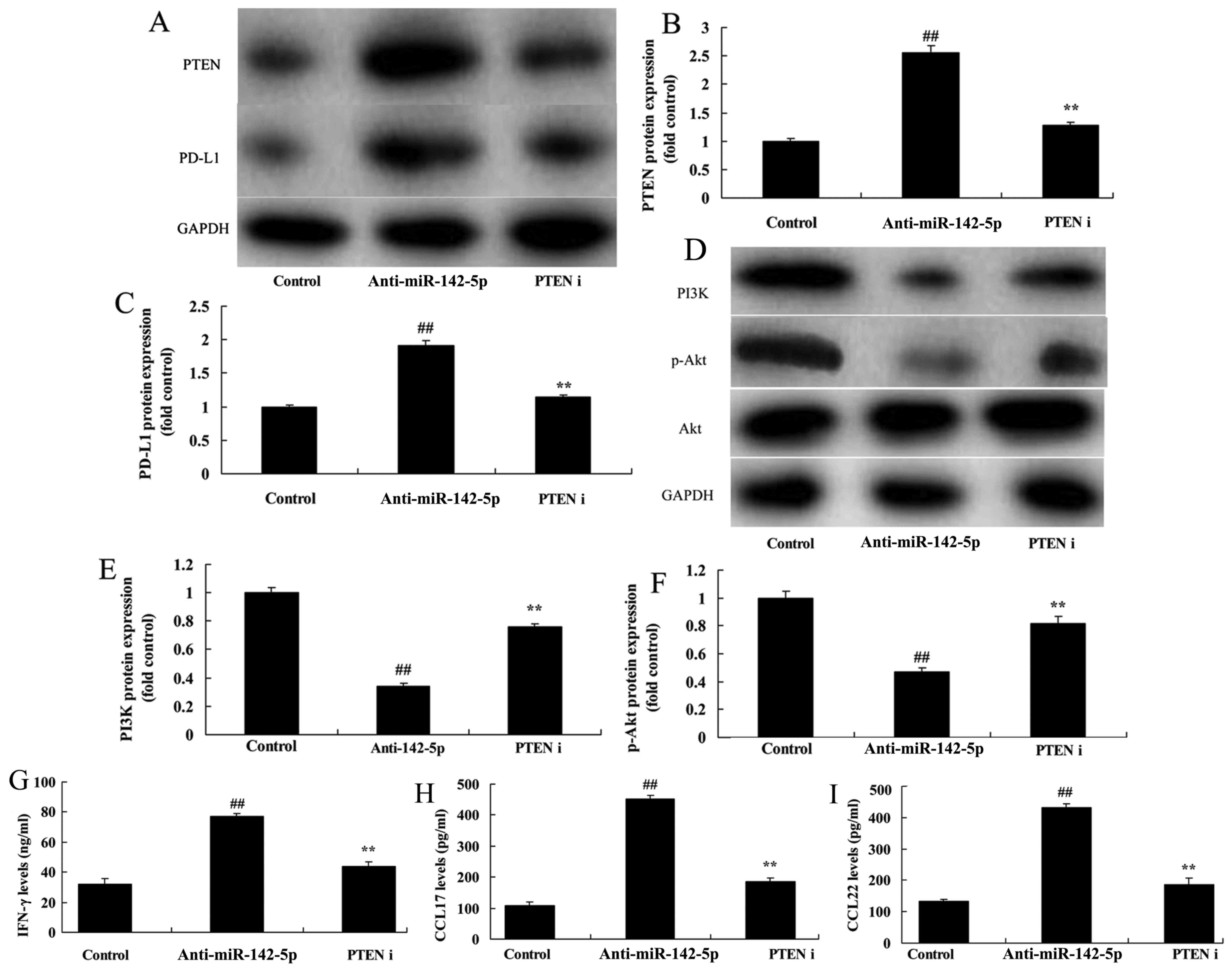
in the stability regulation of PD-L1. The PTEN inhibitor (VO-Ohpic
trihydrate) suppressed PTEN and PD-L1 protein expression in A549
cells in the co-culture model compared with the miR-142-5p
downregulation group (Fig. 7A-C).
In addition, the inhibition of PTEN induced PI3K and p-Akt protein
expression in the co-culture model compared with the miR-142-5p
downregulation group (Fig. 7D-F).
Moreover, the inhibition of PTEN inhibited the levels of CCL17,
CCL22 and IFN-γ in the medium of the co-culture model compared with
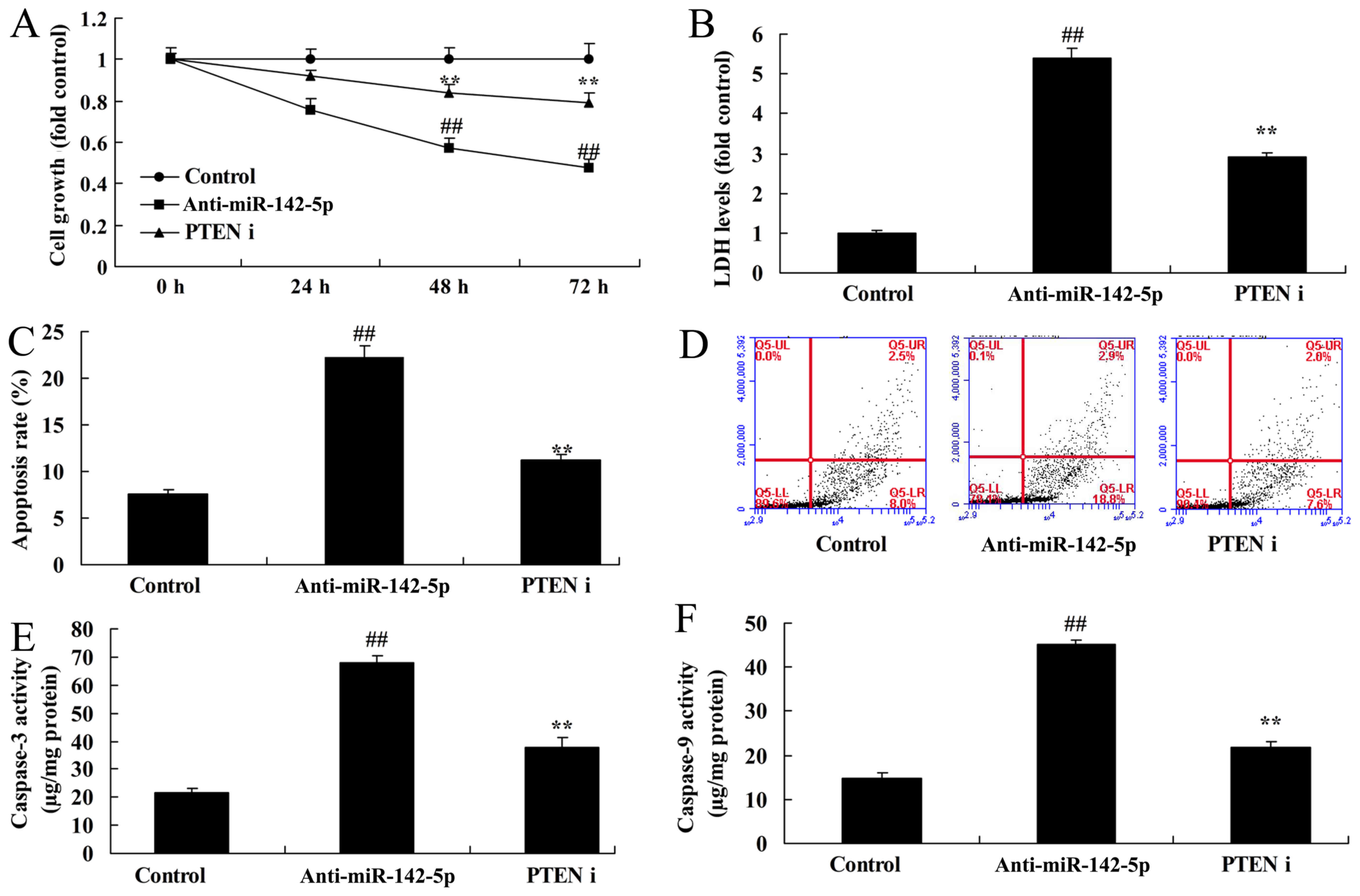
the miR-142-5p downregulation group (Fig. 7G-I). Finally, inhibition of PTEN
also reduced the cancer effects of CD4+ T cells in A549
cells following miR-142-5p downregulation compared with the
miR-142-5p downregulation group (Fig.
8).
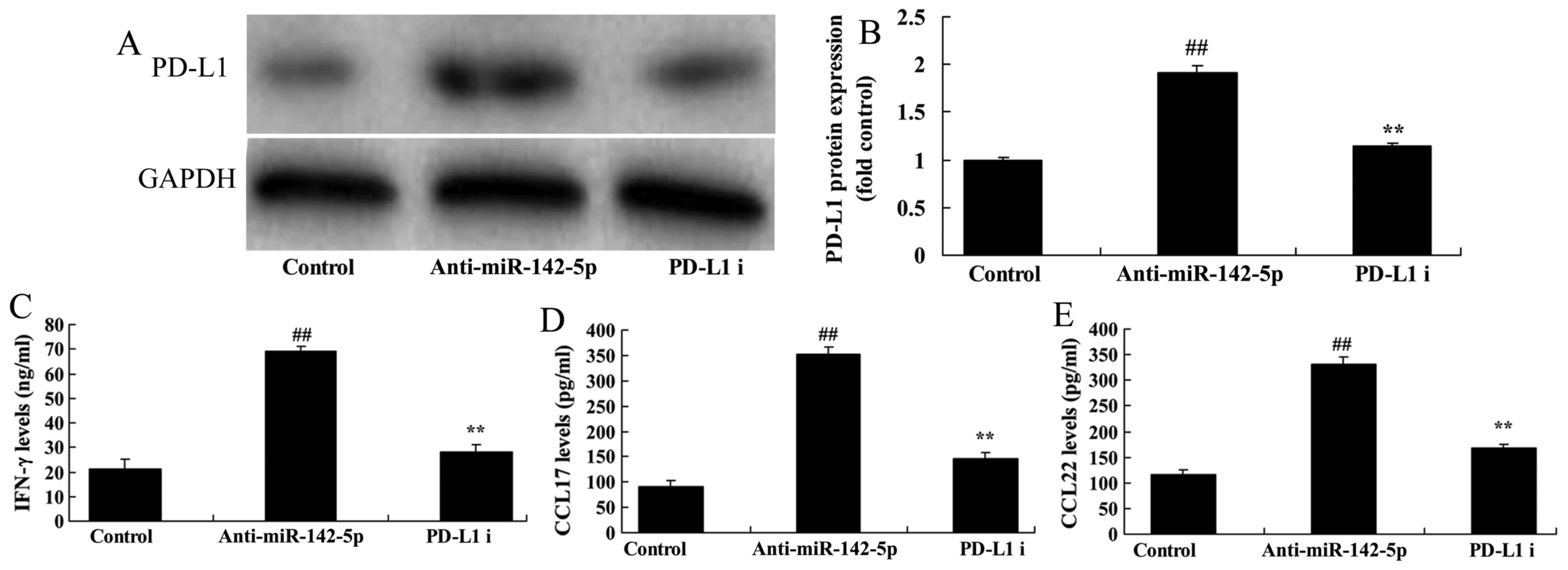
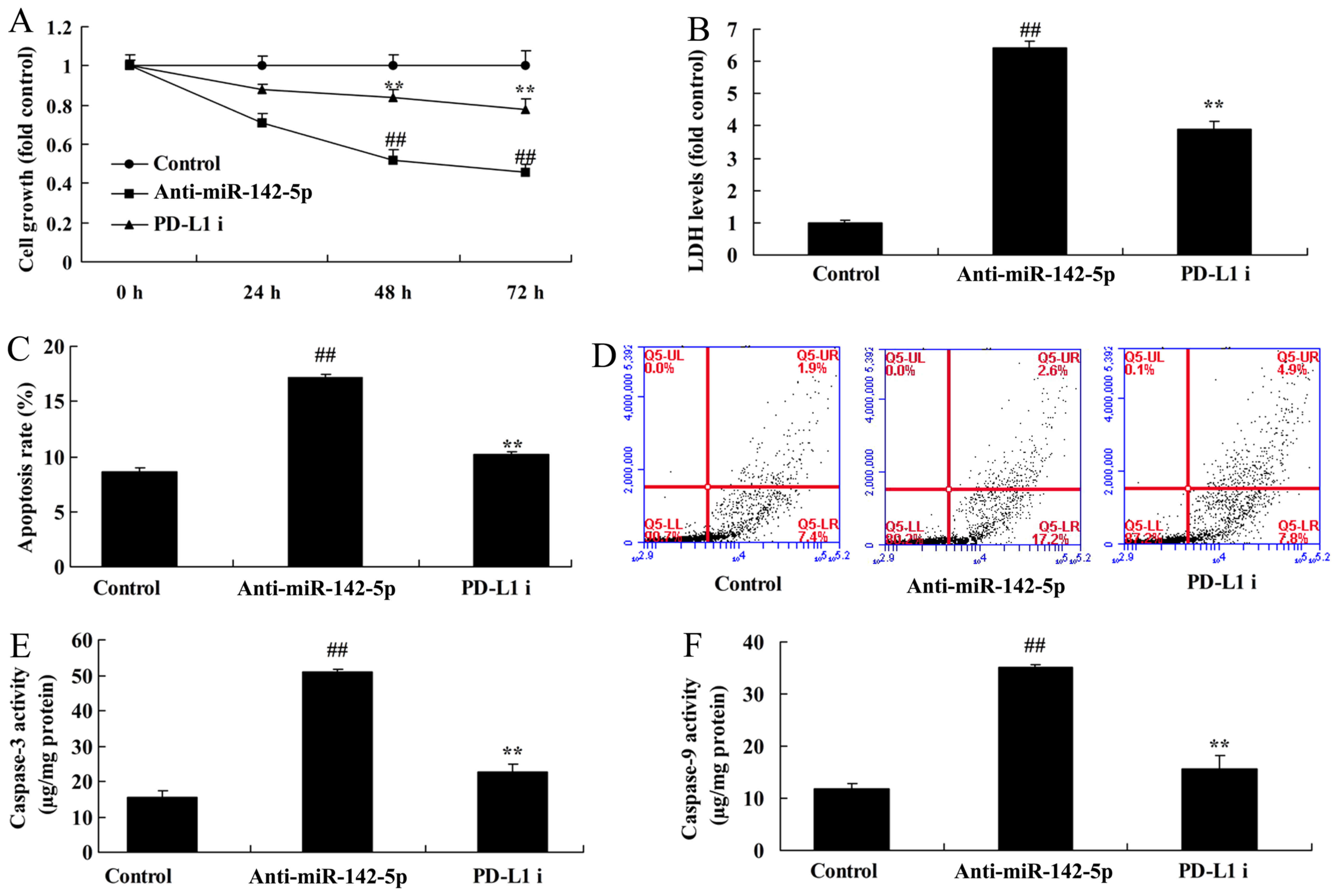
Suppression of PD-L1 reduces the
cancer effects of CD4+ T cells in NSCLC cell line A549
following miR-142-5p downregulation
Investigation of the function of PD-L1 in the cancer
effects of CD4+ T cells in NSCLC cell line A549
following miR-142-5p downregulation revealed that PD-L1 inhibitor
(PD1-PDL1 inhibitor 1) suppressed the PD-L1 protein expression in
the co-culture model of A549 cells as well as the levels of CCL17,
CCL22 and IFN-γ in the medium of the co-culture model compared with
the miR-142-5p downregulation group (Fig. 9). The suppression of PD-L1 reduced
the cancer effects of CD4+ T cells on cell growth, the
LDH activity levels, the apoptosis rate and caspase-3/9 activities
in A549 cells in the co-culture model, compared with miR-142-5p
downregulation group (Fig.
10).
Discussion
Lung cancer is one of the high-risk factors of
malignancy-induced deaths at present (9). No detection means with strong
specificity are presently available due to the lack of obvious
early clinical features and symptoms (10). As a result, most lung cancer
patients are at the advanced stage at the time of diagnosis. This
has severely affected the therapeutic effect and prognosis of
patients. Cellular immunity is the major mechanism against tumors.
Moreover, T cells are important effector cells in antitumor
immunity. It can complete immune regulatory function through
lymphocyte subsets with various functions (10). Therefore, we hypothesized that
miRNA-142-5p expression was upregulated in NSCLC patients. Talebi
et al revealed that miR-142 regulates T-cell differentiation
in an animal model of multiple sclerosis (8). Hou et al revealed that the
levels of
CD4+CD25+FOXP3+/CD4+ T
cells and FOXP3 mRNA were significantly higher in lung cancer
patients than in healthy controls (11), which is in keeping with Fig. 1. Kotsakis et al revealed that
particular CD4+ Treg subtypes are elevated in NSCLC
patients (12), which is in keeping
with Fig. 1.
Tumor genesis, development, infiltration and
metastasis are the collective action of multiple factors (9). However disordered autoimmune
monitoring and immune tolerance are the basic factors of tumor
formation (10). In recent years,
the discovery of CD4+CD25+ regulatory T cells
(Tregs) has added to the understanding of immune tolerance and
immune regulation. CD4+CD25+ Treg cells are
derived from the thymus. It is an important component of the immune
system which helps to maintain immune homeostasis (10). It is characterized by immune
nullipotence and immune suppression. It can directly contact
effector cells (9). Thus, it can
inhibit the activation and proliferation of potential autoimmune T
cells in the body (9). Notably, it
plays an important role in regulating tumor immunity and
autoimmunity (10). Therefore, we
hypothesized that miRNA-142-5p expression was upregulated in NSCLC
patients. Talebi et al revealed that miR-142 regulates
T-cell differentiation in an animal model of multiple sclerosis
(8).
CCL17 can be secreted by dendritic cells and
endothelial cells. CCL22 is mainly derived from macrophages and
dendritic cells differentiated from monocytes. CCL17 shares the
common receptor molecule CCR4 with CCL22 (13,14).
The chemokine receptor plays a key role in guiding cell migration
in tissue along with the chemokine gradient. CCR4 is expressed in T
cells, NK cells, monocytes and the eosinophil surface (14,15).
Among them, CCR4+ T cells are the important effector
cells of CCL17 and CCL22. Both CCL17 and CCL22 act on
CCR4+ T cells. However, they play distinct roles in
tumor immunity (14). CCL17 may
play a role against tumor cell immunity (15). However, CCL22 may promote the
formation of tumor immunity tolerance of Treg cells. IFN-γ is the
potent activator of monocytes/macrophages. It can extensively
enhance the expression of the MHC-6-type antigen by all types of
cells (16). Therefore, it can
amplify the recognition stage of immune response. IFN-γ can
directly stimulate T- and B-cell differentiation as well as CTL
maturation. In addition, it can stimulate B cells to secrete
antibodies (6). IFN-γ can suppress
lymphocytes under certain circumstances, especially Th2 cells
(6). We found that the
downregulation of miR-142-5p increased CCL17, CCL22 and IFN-γ
levels in the medium of the co-culture model. Karu et al
revealed that miR-142-5p regulates tumor cell PD-L1 expression and
enhances antitumor immunity via increase of IFN-γ and TNF-α
(17).
Malignant tumor patients are mostly associated with
immunological dysfunction. T lymphocytes are the most important
components in cellular immunity (10). They play an immune regulatory role
through T lymphocyte subsets with various functions (10). Peripheral T cell subsets can also be
divided into CD4+ helper/inducer T cells and
CD8+ inhibitory/cytotoxic T cells (9). CD4+ cells can assist B
cells in secreting antibodies and regulating the immune responses
of other T cells (18).
CD8+ cells mostly manifest cytotoxicity. They are the
major cytotoxic effector cells (9).
In this study, we found that overexpression of miR-142-5p
expression inhibited the cancer effects of CD4+ T cells
in NSCLC cell line A549. Ding et al indicated that the
inhibition of miR-142-3p/5p causes CD4+ T-cell
activation in systemic lupus erythematosus (19).
PD-L1 can transfer immune inhibitory signals to T
cells after binding with receptor PD-1. Thus, it can inhibit T-cell
immunity (20). Moreover, it plays
a negative regulatory role in the immune response. The PD-1/PD-L1
interaction can suppress proliferation and activation of
CD4+ and CD8+ T cells. In addition, it can
downregulate the expression and secretion of IL-2 and IFN-γ.
Furthermore, it induces cell cycle arrest at the G0/G1 stage
(20). The outcome of their
interaction depends on T-cell antigen receptor, which accounts for
the major biological effect of the PD-1/PD-L1 pathway (21). In addition, this pathway exhibits
more evident inhibitory effects on CD8+ T cells than on
CD4+ T cells (21). Our
study revealed that the effects of miR-142-5p on CD4+ T
cells in NSCLC cell line A549 were achieved via the PTEN pathway.
Jia et al revealed that miR-142-5p enhances antitumor
immunity via tumor cell PD-L1 expression (22).
The PD-L1 signaling pathway can regulate T-cell
activation. One of its mechanisms of action is the direct
inhibition of T cells (23). It
manifests as T-cell proliferation inhibition, cytokine secretion
suppression and cytotoxic reaction (24). PD-L1 protein can induce massive
transformation of CD4+ T cells into Treg cells. This
molecular mechanism includes blocking PI3K to initiate new signals
(25). In addition, it is
accompanied with the upregulation of PTEN expression (25). PTEN is the first tumor suppressor
gene discovered with dual specific phosphatase activity of lipid
phosphatase and protein phosphatase. Aberrant PTEN changes can be
observed in most tumors (25). Loss
of PTEN function can activate the PI3k/Akt signaling pathway, thus
participating in cancerogenesis (25,26).
In addition, in the present study we revealed that the suppression
of the PD-L1 or PTEN inhibitor reduced the cancer effects of
CD4+ T cells in NSCLC cell line A549 following
miR-142-5p downregulation. Bai et al revealed that
miR-142-5p induced cancer stem cell-like properties via inhibition
of PTEN in cutaneous squamous cell carcinoma (19). These results are consistent with our
study which revealed that miR-142-5p/PTEN regulates CD4+
T cells in NSCLC cells.
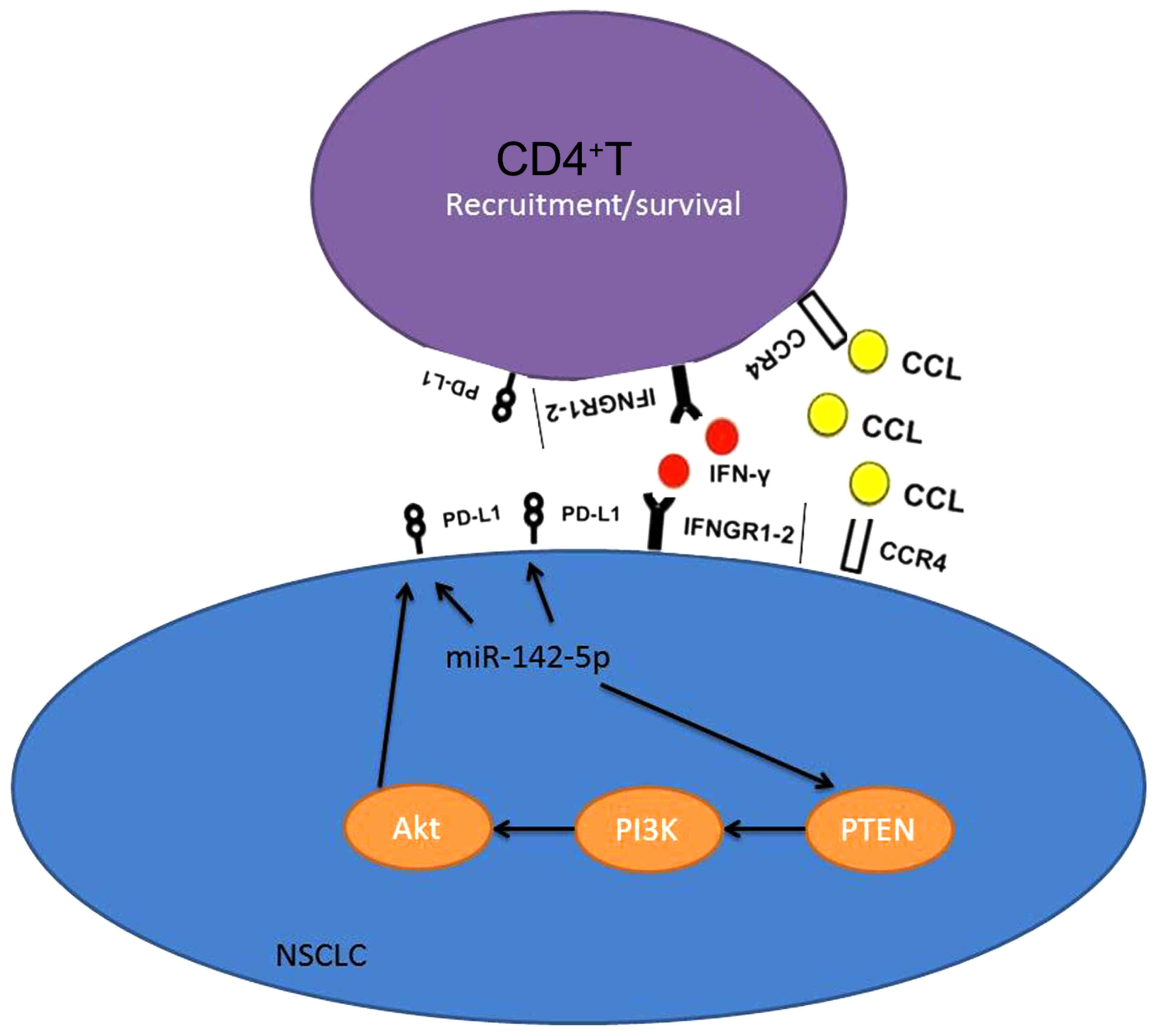
Collectively, we revealed that miR-142-5p expression
was upregulated in NSCLC patients. In this study, we found that
miR-142-5p regulated CD4+ T cells in human non-small
cell lung cancer through PD-L1 expression via the PTEN pathway
(Fig. 11). Our findings revealed
that miR-142-5p may be a novel therapeutic target for NSCLC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81302028).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
JW designed the experiment; XL, BP and GD performed
the experiment; JW and XL analyzed the data; JW wrote the
manuscript. All authors read and approved the manuscript and agree
to be accountable for all aspects of the research in ensuring that
the accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Ethical approval was obtained from the Shenzhen
People's Hospital.
Consent for publication
Not applicable.
Competing interests
The authors state that they have no competing
interests.
References
|
1
|
Song M, Chen D, Lu B, Wang C, Zhang J,
Huang L, Wang X, Timmons CL, Hu J, Liu B, et al: PTEN loss
increases PD-L1 protein expression and affects the correlation
between PD-L1 expression and clinical parameters in colorectal
cancer. PLoS One. 8:e658212013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Atefi M, Avramis E, Lassen A, Wong DJ,
Robert L, Foulad D, Cerniglia M, Titz B, Chodon T, Graeber TG, et
al: Effects of MAPK and PI3K pathways on PD-L1 expression in
melanoma. Clin Cancer Res. 20:3446–3457. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao L, Li C, Liu F, Zhao Y, Liu J, Hua Y,
Liu J, Huang J and Ge C: A blockade of PD-L1 produced antitumor and
antimetastatic effects in an orthotopic mouse pancreatic cancer
model via the PI3K/Akt/mTOR signaling pathway. Onco Targets Ther.
10:2115–2126. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fujimoto N, Kubo T, Inatomi H, Bui HT,
Shiota M, Sho T and Matsumoto T: Polymorphisms of the androgen
transporting gene SLCO2B1 may influence the castration resistance
of prostate cancer and the racial differences in response to
androgen deprivation. Prostate Cancer Prostatic Dis. 16:336–340.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sharifi N, Hamada A, Sissung T, Danesi R,
Venzon D, Baum C, Gulley JL, Price DK, Dahut WL and Figg WD: A
polymorphism in a transporter of testosterone is a determinant of
androgen independence in prostate cancer. BJU Int. 102:617–621.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cai W, Jiang H, Yu Y, Xu Y, Zuo W, Wang S
and Su Z: miR-367 regulation of DOC-2/DAB2 interactive protein
promotes proliferation, migration and invasion of osteosarcoma
cells. Biomed Pharmacother. 95:120–128. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Talebi F, Ghorbani S, Chan WF, Boghozian
R, Masoumi F, Ghasemi S, Vojgani M, Power C and Noorbakhsh F:
MicroRNA-142 regulates inflammation and T cell differentiation in
an animal model of multiple sclerosis. J Neuroinflammation.
14:552017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou G, Sprengers D, Boor PPC, Doukas M,
Schutz H, Mancham S, Pedroza-Gonzalez A, Polak WG, de Jonge J,
Gaspersz M, et al: Antibodies against immune checkpoint molecules
restore functions of tumor-infiltrating T cells in hepatocellular
carcinomas. Gastroenterology. 153:1107–1119.e10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yi H, Guo C, Yu X, Gao P, Qian J, Zuo D,
Manjili MH, Fisher PB, Subjeck JR and Wang XY: Targeting the
immunoregulator SRA/CD204 potentiates specific dendritic cell
vaccine-induced T-cell response and antitumor immunity. Cancer Res.
71:6611–6620. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hou PF, Zhu LJ, Chen XY and Qiu ZQ:
Age-related changes in
CD4+CD25+Foxp3+ regulatory T cells
and their relationship with lung cancer. PLoS One. 12:e01730482017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kotsakis A, Koinis F, Katsarou A,
Gioulbasani M, Aggouraki D, Kentepozidis N, Georgoulias V and
Vetsika EK: Prognostic value of circulating regulatory T cell
subsets in untreated non-small cell lung cancer patients. Sci Rep.
6:392472016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee TM, Lin SZ and Chang NC:
Antiarrhythmic effect of lithium in rats after myocardial
infarction by activation of Nrf2/HO-1 signaling. Free Radic Biol
Med. 77:71–81. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu H, Carvalhais LC, Kazan K and Schenk
PM: Development of marker genes for jasmonic acid signaling in
shoots and roots of wheat. Plant Signal Behav. 11:e11766542016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao H, Chai W, Gao W, Xu L, Zhang H and
Yang Y: Hyperoxygenated solution: Effects on acute hypobaric
hypoxia-induced oxidative damage in rabbits. High Alt Med Biol.
10:283–291. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao C, Zhang G, Sun X, Zhang H, Kuai J,
Zhao H, Yao L, Yu D, Yang Y, Xu L, et al: The effects of
intravenous hyperoxygenated solution infusion on systemic
oxygenation and intrapulmonary shunt during one-lung ventilation in
pigs. J Surg Res. 159:653–659. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Karu I, Loit R, Zilmer K, Kairane C,
Paapstel A, Zilmer M and Starkopf J: Pre-treatment with hyperoxia
before coronary artery bypass grafting - effects on myocardial
injury and inflammatory response. Acta Anaesthesiol Scand.
51:1305–1313. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dumitriu IE, Dunbar DR, Howie SE, Sethi T
and Gregory CD: Human dendritic cells produce TGF-beta 1 under the
influence of lung carcinoma cells and prime the differentiation of
CD4+CD25+Foxp3+ regulatory T
cells. J Immunol. 182:2795–2807. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bai X, Zhou Y, Chen P, Yang M and Xu J:
MicroRNA-142-5p induces cancer stem cell-like properties of
cutaneous squamous cell carcinoma via inhibiting PTEN. J Cell
Biochem. 119:2179–2188. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gong X, Li X, Jiang T, Xie H, Zhu Z, Zhou
F and Zhou C: Combined radiotherapy and anti-PD-L1 antibody
synergistically enhances antitumor effect in non-small cell lung
cancer. J Thorac Oncol. 12:1085–1097. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee SY, Jung DK, Choi JE, Jin CC, Hong MJ,
Do SK, Kang HG, Lee WK, Seok Y, Lee EB, et al: Functional
polymorphisms in PD-L1 gene are associated with the prognosis of
patients with early stage non-small cell lung cancer. Gene.
599:28–35. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jia L, Xi Q, Wang H, Zhang Z, Liu H, Cheng
Y, Guo X, Zhang J, Zhang Q, Zhang L, et al: miR-142-5p regulates
tumor cell PD-L1 expression and enhances anti-tumor immunity.
Biochem Biophys Res Commun. 488:425–431. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Y, Liu Z, Tian M, Hu X, Wang L, Ji J
and Liao A: The altered PD-1/PD-L1 pathway delivers the ‘one-two
punch’ effects to promote the Treg/Th17 imbalance in pre-eclampsia.
Cell Mol Immunol. Sep 11–2017.(Epub ahead of print). View Article : Google Scholar :
|
|
24
|
Li QS, Meng FY, Zhao YH, Jin CL, Tian J
and Yi XJ: Inhibition of microRNA-214-5p promotes cell survival and
extracellular matrix formation by targeting collagen type IV alpha
1 in osteoblastic MC3T3-E1 cells. Bone Joint Res. 6:464–471. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pressler H, Sissung TM, Venzon D, Price DK
and Figg WD: Expression of OATP family members in hormone-related
cancers: Potential markers of progression. PLoS One. 6:e203722011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu L, Gao H, Wang H, Zhang Y, Xu W, Lin
S, Wang H, Wu Q and Guo J: Catalpol promotes cellular apoptosis in
human HCT116 colorectal cancer cells via microRNA-200 and the
downregulation of PI3K-Akt signaling pathway. Oncol Lett.
14:3741–3747. 2017. View Article : Google Scholar : PubMed/NCBI
|