Introduction
Activating mutations in epidermal growth factor
receptor (EGFR) were reported to be potential targets for
the treatment of non-small cell lung cancer (NSCLC) (1,2).
EGFR mutation frequency was reported to vary by population
type; for example, in North America and Western Europe,
approximately 5–10% of adenocarcinoma patients contain mutations,
whereas approximately 60–70% of non-smokers in East Asia have
EGFR mutations (3,4). EGFR tyrosine kinase inhibitors
(EGFR-TKIs) including gefitinib, erlotinib, and afatinib have
demonstrated marked radiographic and clinical improvement in
patients with EGFR mutations and are recommended for the
treatment of EGFR-mutant NSCLC (5,6). A
longer progression-free survival (PFS) was reported in NSCLC
patients with such mutations who were treated with an EGFR-TKI as a
first-line therapy compared with those receiving platinum-based
chemotherapy (7–11). The expression of PD-L1,
BCL2L11 (BIM), p53 upregulated modular of apoptosis
(PUMA), human epidermal growth factor receptor 2
(HER2), vascular endothelial growth factor A (VEGFA),
EGFR and mesenchymal-epithelial transition (MET) were
reported to be prognostic factors for patients with EGFR
mutations receiving EGFR-TKI therapy (12–18).
Programmed death 1 (PD-1) is a co-inhibitory
receptor expressed on activated T and B cells and is involved in
tumor immune escape (19–21). The PD-1 ligand, termed programmed
death-ligand 1 (PD-L1), has been reported to be overexpressed in
many cancers (22). Recent clinical
trials have shown promising efficacy for PD-L1 and PD-1 antibody
blockade in NSCLC (23–25). A recent study reported that PD-L1
was expressed in 19.6–65.3% of NSCLC patients (26–30)
and that EGFR mutation status was associated with PD-L1
expression as assessed by immunohistochemistry (IHC) (31, 32). Chen
et al (33) reported three
pathways of EGFR activation: i) EGF simulation; ii)
EGFR-19 del; and iii) EGFR-L858R mutation, which
induced PD-L1 expression. Therefore, constitutive oncogenic pathway
activation may upregulate PD-L1 expression. Azuma et al
(32) reported that high PD-L1
expression was associated with the presence of EGFR
mutations in surgically resected NSCLC indicating it may be an
independent negative prognostic factor.
Several studies have reported an association between
PD-L1 and apoptotic activity and angiogenesis in addition to
other prognostic factors of EGFR-TKI (34,35).
For this reason, HER2, EGFR and MET genes were
selected as prognostic markers of EGFR-TKI, VEGFA was
selected as an angiogenic marker, and BIM and PUMA
were selected as apoptotic markers. This study investigated the
association between PD-L1 mRNA expression and other
prognostic factors for EGFR-TKI therapy, including BIM, PUMA,
HER2, VEGFA, EGFR and MET in lung tissue from patients
with EGFR-mutant NSCLC.
Patients and methods
Clinical samples
Samples from 33 patients with recurrent
postoperative EGFR-mutant lung adenocarcinoma (exon 19
deletion in 16, L858R in 15, G719C in 2 patients) treated with
gefitinib between January 2008 and January 2016 were obtained. The
inclusion criteria were: i) patients with advanced and
postoperative recurrent NSCLC; ii) patients with an EGFR
mutation (Del 19, L858R mutation, and minor mutation); iii)
patients treated with gefitinib; iv) patients aged <80 years;
and v) either male or female patients. The exclusion criteria were:
i) patients with complications or a history of serious lung
disorder; ii) pregnant women, women who may possibly be pregnant,
women who hope to be pregnant, lactating women; and iii) men who
declined contraception.
mRNA expression of PD-L1, BIM, PUMA, HER2, VEGFA,
EGFR and MET were investigated by the real-time PCR
analysis of 33 formalin-fixed paraffin-embedded (FFPE) slides of
intratumoral and paratumoral lung tissue surgical samples.
mRNA extraction from intratumoral and
paratumoral tissues
Total RNA including miRNA was extracted from FFPE
sections of intratumoral and paratumoral lung tissues using a
miRNeasy FFPE kit (Qiagen KK, Tokyo, Japan) according to the
manufacturer's protocol. Paratumoral tissues were defined as normal
lung cells including inflammatory cells, and/or mesenchymal cells
in the same section with a 1–2 cm distance from the tumor edge.
Detection of PD-L1, BIM, PUMA, HER2,
VEGFA, EGFR and MET
Total RNA was stored at −80°C until use. cDNA was
synthesized using PrimeScript RT MasterMix (Perfect Real-Time;
Takara Bio, Inc., Otsu, Japan). Quantitative real-time PCR was
performed using a Thermal Cycler Dice Real-Time System TP800
(Takara Bio, Inc.), using SYBR Premix Ex Taq II (Tli RNaseH Plus;
Takara Bio, Inc.). Each PCR reaction used Perfect Real Time primers
(Takara Bio, Inc.) as follows: PD-L1 forward,
5′-CGTCTCCTCCAAATGTGTATCA-3′ and reverse,
5′-TGGTAATTCTGGGAGCCATC-3′; BIM-EL forward,
5′-GAGCCACAAGGTAATCCTGAA-3′ and reverse,
5′-ATACCCACTGGAGGATCGAG-3′; BIM-L forward,
5′-GACAGAGCCACAAGACAGGA-3′ and reverse,
5′-GGAAGCCATTGCACTGAGATA-3′; BIM-S forward,
5′-AGACAGAGCCACAAGCTTCC-3′ and reverse,
5′-TGCATAGTAAGCGTTAAACTCG-3′; PUMA forward,
5′-GACGACCTCAACGCACAGTA-3′ and reverse,
5′-GAGATTGTACAGGACCCTCCA-3′; HER2 forward,
5′-GGAAACCTGGAACTCACCTACCTG-3′ and reverse,
5′-AGTGGGACCTGCCTCACTTG-3′; VEGFA forward,
5′-TCACAGGTACAGGGATGAGGACAC-3′ and reverse,
5′-CAAAGCACAGCAATGTCCTGAAG-3′; EGFR forward,
5′-GTGGCGGGACATAGTCAGCA-3′ and reverse, 5′-CCCATTGGGACAGCTTGGA-3′;
MET forward, 5′-TCCCATCAACAGGACTACACACTT-3′ and reverse,
5′-GCTGCAGGTATAGGCAGTGACAA-3; and GAPDH forward,
5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse,
5′-TGGTGAAGACGCCAGTGGA-3′.
Quantification of PD-L1, BIM, PUMA,
HER2, VEGFA, EGFR and MET expression
The targets were obtained from the same mRNA
preparations. The relative expression of PD-L1, BIM, PUMA, HER2,
VEGFR, EGFR and MET in mRNA isolated from tissue
sections of intratumoral and paratumoral lung tissues, normalized
to the reference gene (GAPDH), were calculated using the
KCL22 or H2228 cell line for calibration (35–37).
PD-L1 negative was defined as no detection of PD-L1 mRNA in this
study.
Validation between PD-L1 mRNA levels
and IHC
We confirmed the validity between PD-L1 mRNA levels
and PD-L1 expression by IHC. PD-L1 IHC was performed using an
automated IHC assay (Dako; Agilent Technologies, Inc., Santa Clara,
CA, USA) with rabbit anti-human PD-L1 antibody (clone 28-8, cat.
no. ab205921; Epitomics; Abcam, Burlingame, CA, USA). Tumor PD-L1
protein expression was confirmed when staining of the tumor-cell
membrane (at any intensity) was observed at a prespecified
expression in a section that included at least 100 tumor cells that
could be evaluated.
Clinical outcomes
We retrospectively analyzed the clinical
characteristics, response rate, and disease control rate for
gefitinib in patients with and without PD-L1. We then
estimated the PFS and overall survival (OS). OS was defined as the
interval from the date of diagnosis until death from any cause. The
PFS of patients treated with gefitinib was assessed from the date
of induction of gefitinib therapy until the first sign of disease
progression, as determined by computed tomographic or magnetic
resonance imaging, according to the Response Evaluation Criteria in
Solid Tumors (RECIST) criteria.
Statistical analysis
Statistical analyses were performed using SPSS
software for Windows, version 12.0 (SPSS Inc., Tokyo, Japan).
Differences in the relative expression of PD-L1, BIM, PUMA,
HER2, VEGFA, EGFR and MET between patients with and
without PD-L1 expression were compared with the Wilcoxon
rank-sum test. Survival curves were plotted using the Kaplan-Meier
method, and the log-rank test was used for statistical analysis. A
P-value <0.05 indicated a statistically significant
difference.
We used univariate analysis and multivariate Cox
regression analysis to identify factors associated with a shorter
PFS and OS. The investigated prognostic factors were age, sex (male
vs. female), performance status (PS; 2 vs. 1 vs. 0), brain
metastasis (yes vs. no), bone metastasis (yes vs. no), pulmonary
metastasis (yes vs. no), pleura metastasis (yes vs. no), liver
metastasis (yes vs. no), lymph node metastasis (yes vs. no),
EGFR mutation [major mutations (L858R and exon 19 deletion)
vs. minor mutations (other mutations)], smoking history
[pack(s)-year], and intratumoral PD-L1 expression (yes vs.
no).
This single-center study was conducted at Toho
University Omori Medical Center (Tokyo, Japan) and was approved by
its Human Genome/Gene Analysis Research Ethics Committee
(authorization no. 27128).
Results
PD-L1 mRNA expression in EGFR-positive
NSCLC
We analyzed PD-L1 mRNA expression in 33
patients with EGFR mutation-positive NSCLC patients who were
treated with gefitinib. The patient characteristics are presented
in Table I. Intratumoral
PD-L1 mRNA expression was noted in 11 out of 33 patients
(33.3%), and paratumoral expression was noted in 14 out of 33
patients (42.4%) (Table II). Six
patients had both intratumoral and paratumoral PD-L1
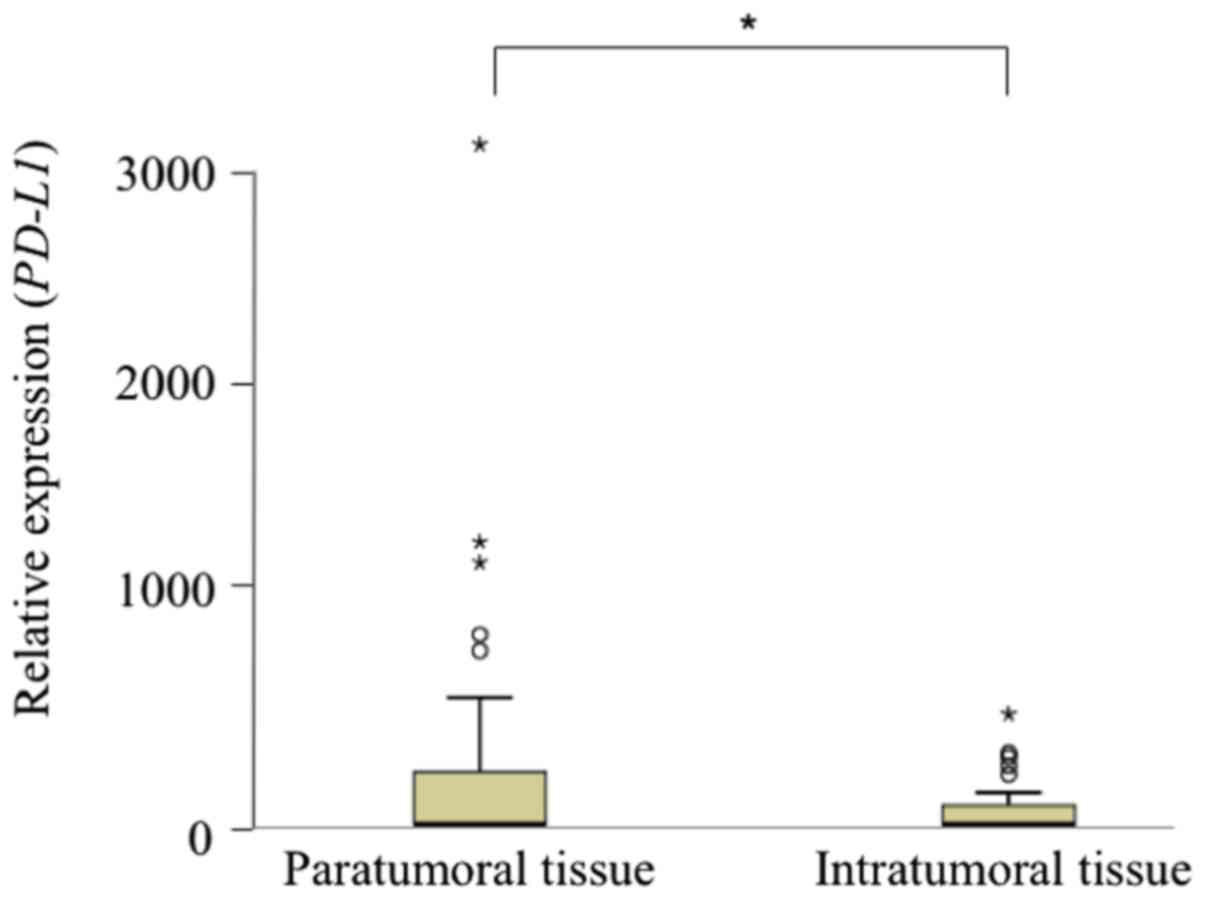
expression. There was no significant difference in the relative
expression of PD-L1 mRNA between intratumoral and
paratumoral tissues (P=0.056) (Fig.
1).
 | Table I.Characteristics of patients
(n=33). |
Table I.
Characteristics of patients
(n=33).
| Parameters | Values |
|---|
| Age (years)
range | 25–82 |
|
Mean | 64.7 |
| Sex |
|
|
Male | 26 |
|
Female | 7 |
| ECOG Performance
status |
|
| 0 | 21 |
| 1 | 10 |
| 2 | 2 |
| Histological
pattern |
|
| Ad | 33 |
| Clinical stage |
|
|
Rec | 33 |
| EGFR
mutation at primary site |
|
|
19del | 16 |
|
L858R | 15 |
|
G719C | 2 |
| Line of gefitinib
therapy |
|
|
First | 16 |
|
Second | 16 |
|
Third | 1 |
 | Table II.PD-L1 mRNA expression
(n=33). |
Table II.
PD-L1 mRNA expression
(n=33).
| PD-L1
expression | N | % |
|---|
| Intratumoral | 11a | 33.3 |
| Paratumoral | 14a | 42.4 |
| Absent | 14a | 43.4 |
Validation between PD-L1 mRNA levels
and IHC results
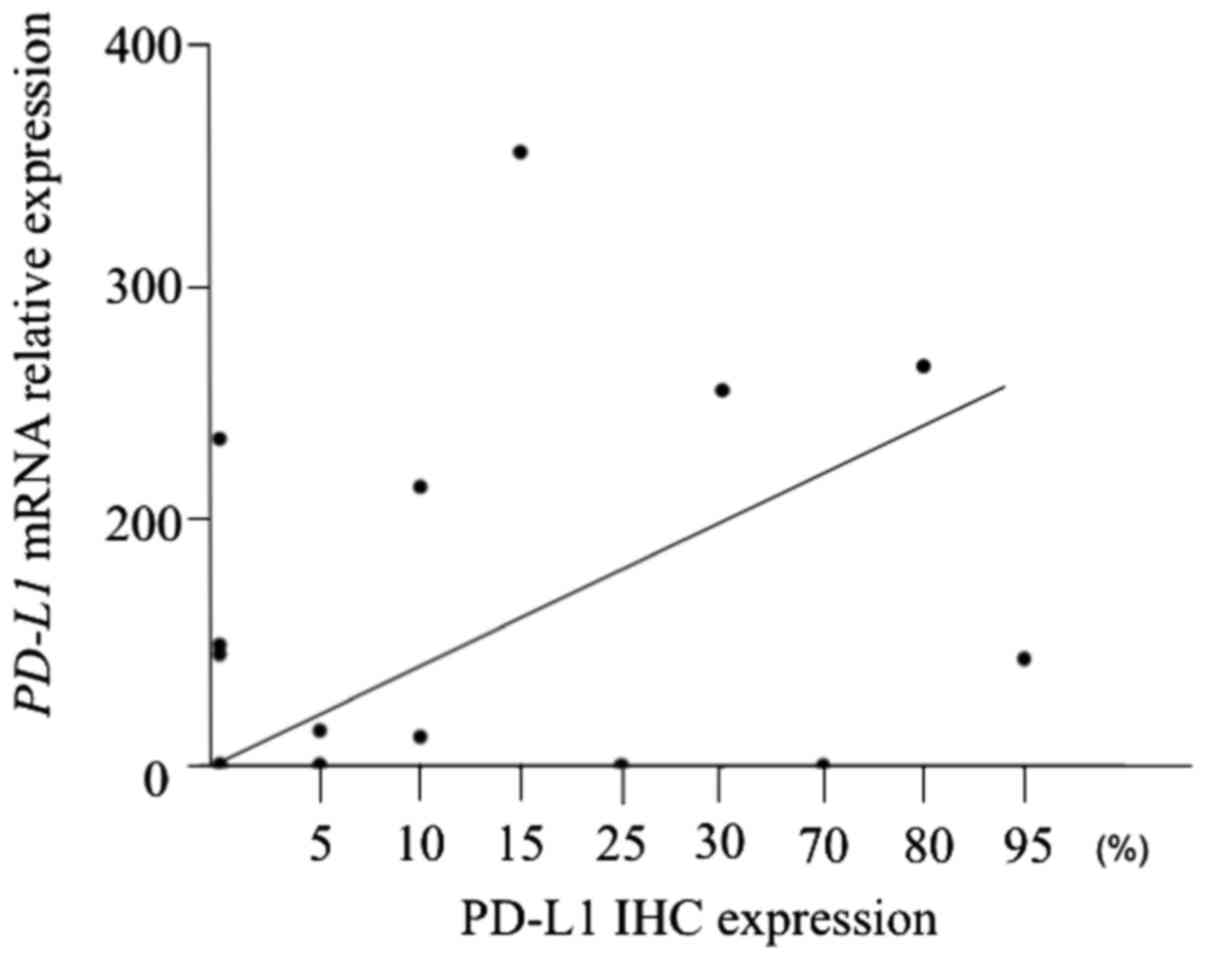
We confirmed the validity between the PD-L1 mRNA
levels and PD-L1 expression by IHC. There was a significant
correlation between PD-L1 mRNA levels and PD-L1 IHC expression
(r=0.44, P=0.015) (Fig. 2).
Association of PD-L1 expression with
BIM, PUMA, HER2, VEGFR, EGFR and MET expression
Patients with intratumoral PD-L1 mRNA
expression had significantly higher BIM expression and
significantly lower VEGFA expression compared with those
without PD-L1 expression (P=0.049 and P=0.009, respectively)
(Table III). The expression of
PUMA, HER2, EGFR, and MET was not associated with
PD-L1 mRNA expression status. Paratumoral PD-L1 mRNA
expression was not associated with the expression of BIM, PUMA,
HER2, VEGFA, or EGFR (Table III).
 | Table III.Associations of PD-L1
expression with BIM, PUMA, HER2, VEGFA, EGFR, and MET
expression (n=33). |
Table III.
Associations of PD-L1
expression with BIM, PUMA, HER2, VEGFA, EGFR, and MET
expression (n=33).
|
| Intratumoral
PD-L1 expression (mean ± SD) |
| Paratumoral
PD-L1 expression (mean ± SD) |
|
|---|
|
|
|
|
|
|
|---|
|
| Positive
(n=11) | Negative
(n=22) | P-value | Positive
(n=14) | Negative
(n=19) | P-value |
|---|
| BIM | 57.4±87.7 | 14.5±34.3 | 0.049 | 6.1±8.5 | 4.4±9.1 | 0.85 |
| PUMA | 15.9±13.7 | 17.3±47.5 | 0.93 | 29.8±69.2 | 42.2±127 | 0.45 |
| HER2 | 1071±874 | 1354±941 | 0.41 | 1020±819 | 1648±1577 | 0.17 |
| VEGFA | 549±294 | 1440±1032 | 0.009 | 1328±1050 | 1474±1108 | 0.70 |
| EGFR | 34.1±26.2 | 38.7±45.6 | 0.76 | 29.3±27.6 | 26.1±37.0 | 0.78 |
| MET | 33.9±36.6 | 22.7±18.9 | 0.25 | 9.8±14.8 | 14.3±15.1 | 0.41 |
Correlations of PD-L1 mRNA expression
with BIM and VEGFA expression
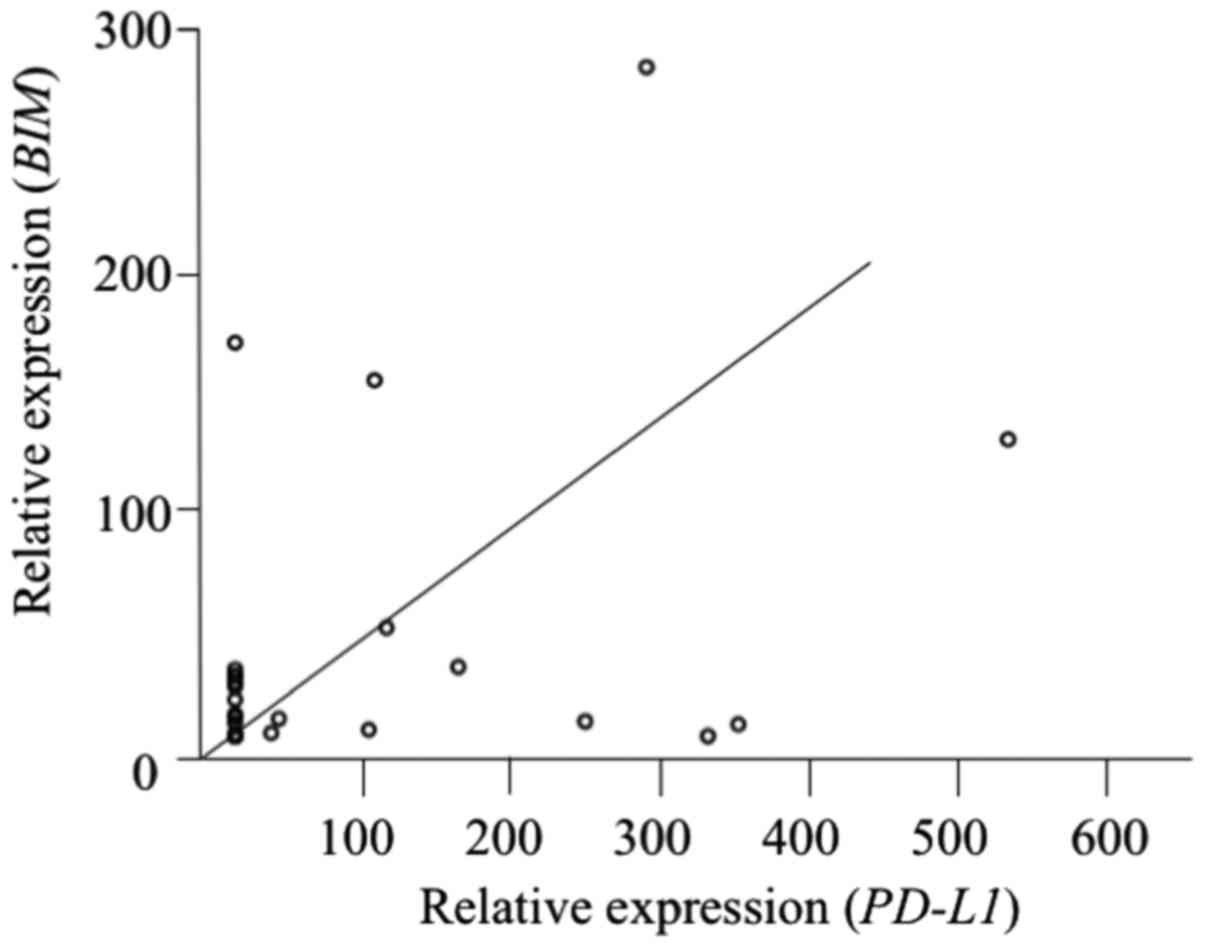
We assessed the correlations of intratumoral
PD-L1 mRNA expression with BIM and VEGFA mRNA
expression. PD-L1 mRNA expression was positively correlated
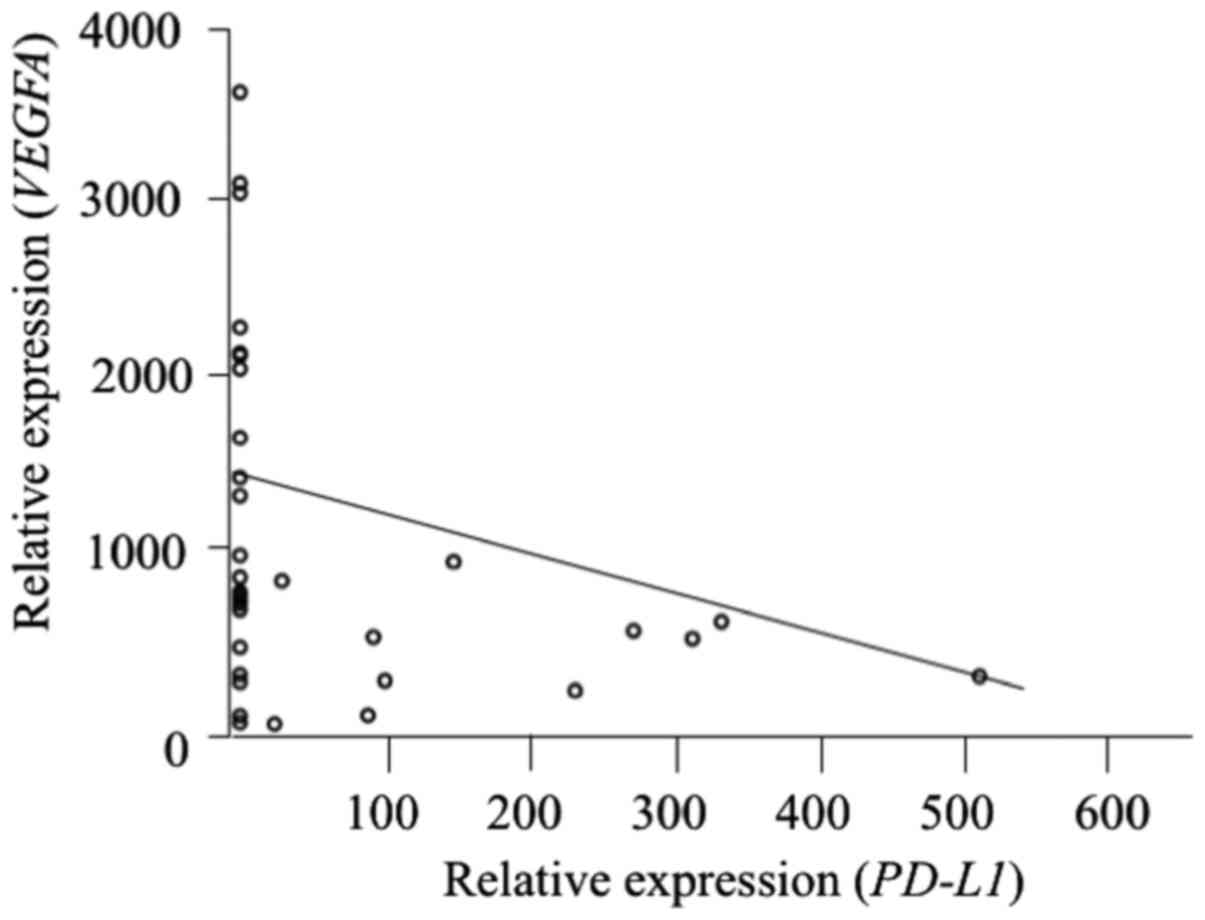
with BIM expression (r=0.41, P=0.017) (Fig. 3) and inversely correlated with
VEGFA expression (r= −0.33, P=0.043) (Fig. 4). However, PD-L1 mRNA
expression was not correlated with the expression of HER2, EGFR,
MET, and PUMA expression (Fig. 5).
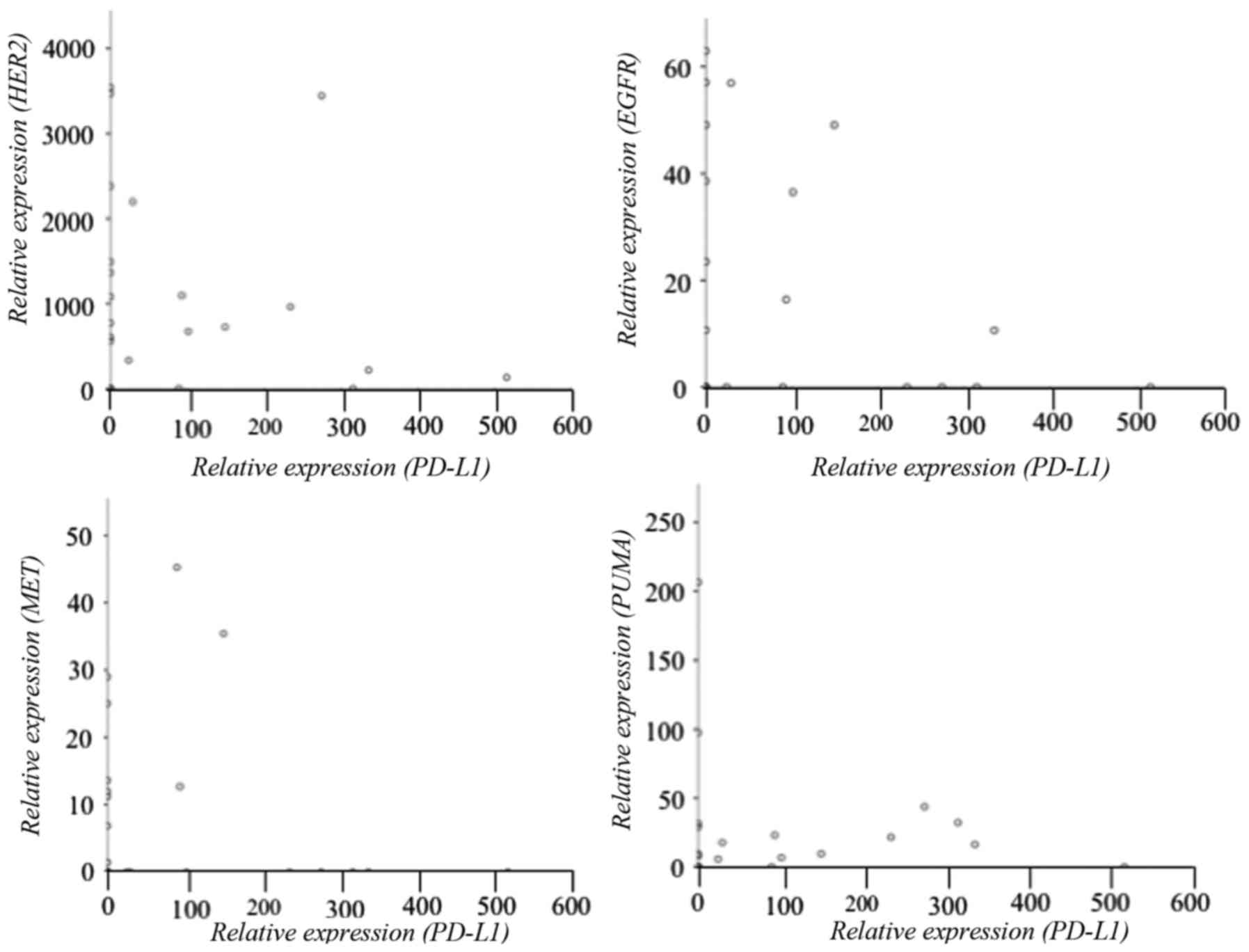 | Figure 5.PD-L1 mRNA expression is not
correlated with HER2 (r=−0.11, P=0.56), EGFR
(r=−0.67, P=0.22), MET (r= −0.44, P=0.81), and PUMA
(r=0.06, P=0.93) expression. PD-L1, programmed death-ligand
1; HER2, human epidermal growth factor receptor 2;
EGFR, epidermal growth factor receptor; MET,
mesenchymal-epithelial transition; PUMA, p53 upregulated
modular of apoptosis. |
Clinical response and survival
There were no significant differences in the
response rate or disease control rate between patients with (n=11)
or without (n=22) intratumoral PD-L1 expression (Table IV). Patients with intratumoral
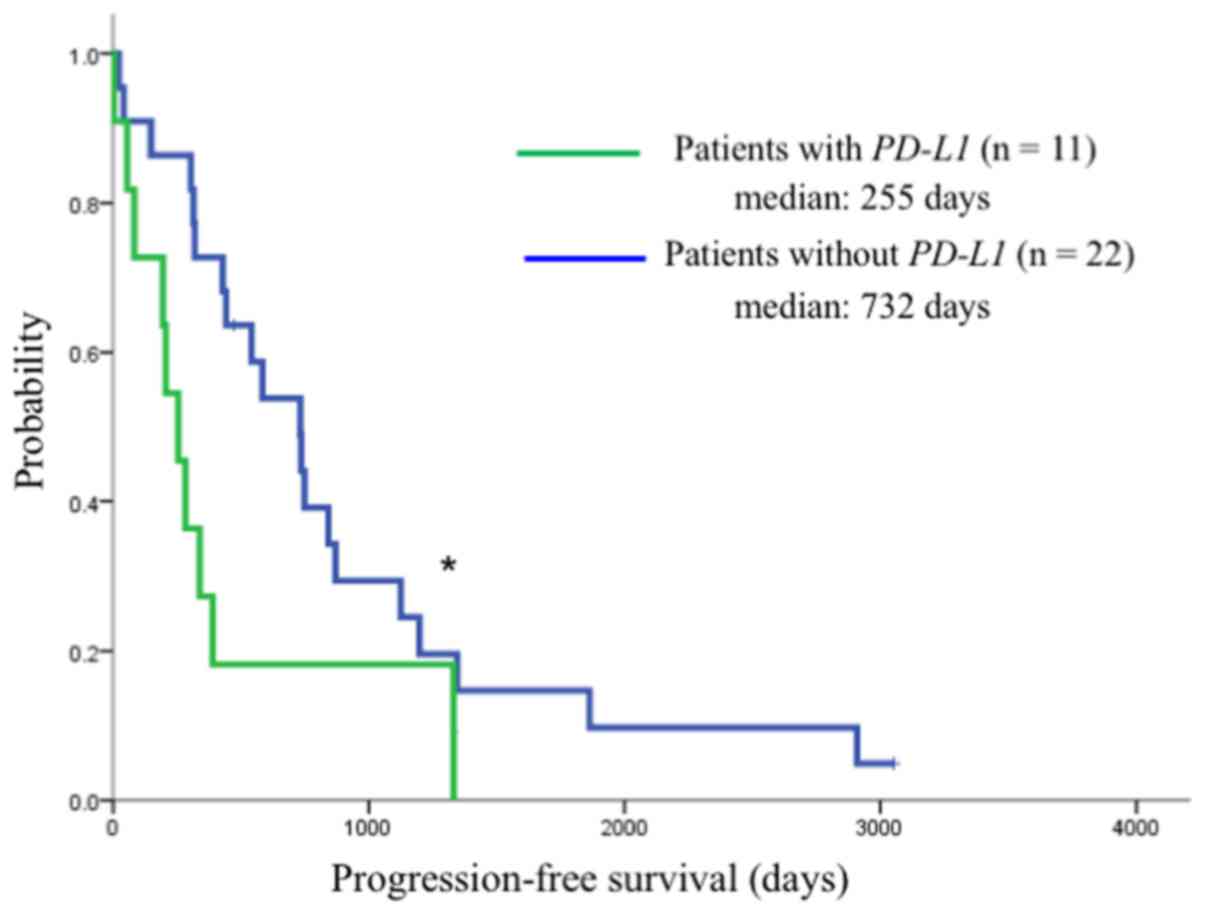
PD-L1 mRNA expression had a significantly shorter median PFS
after gefitinib therapy compared with those without PD-L1
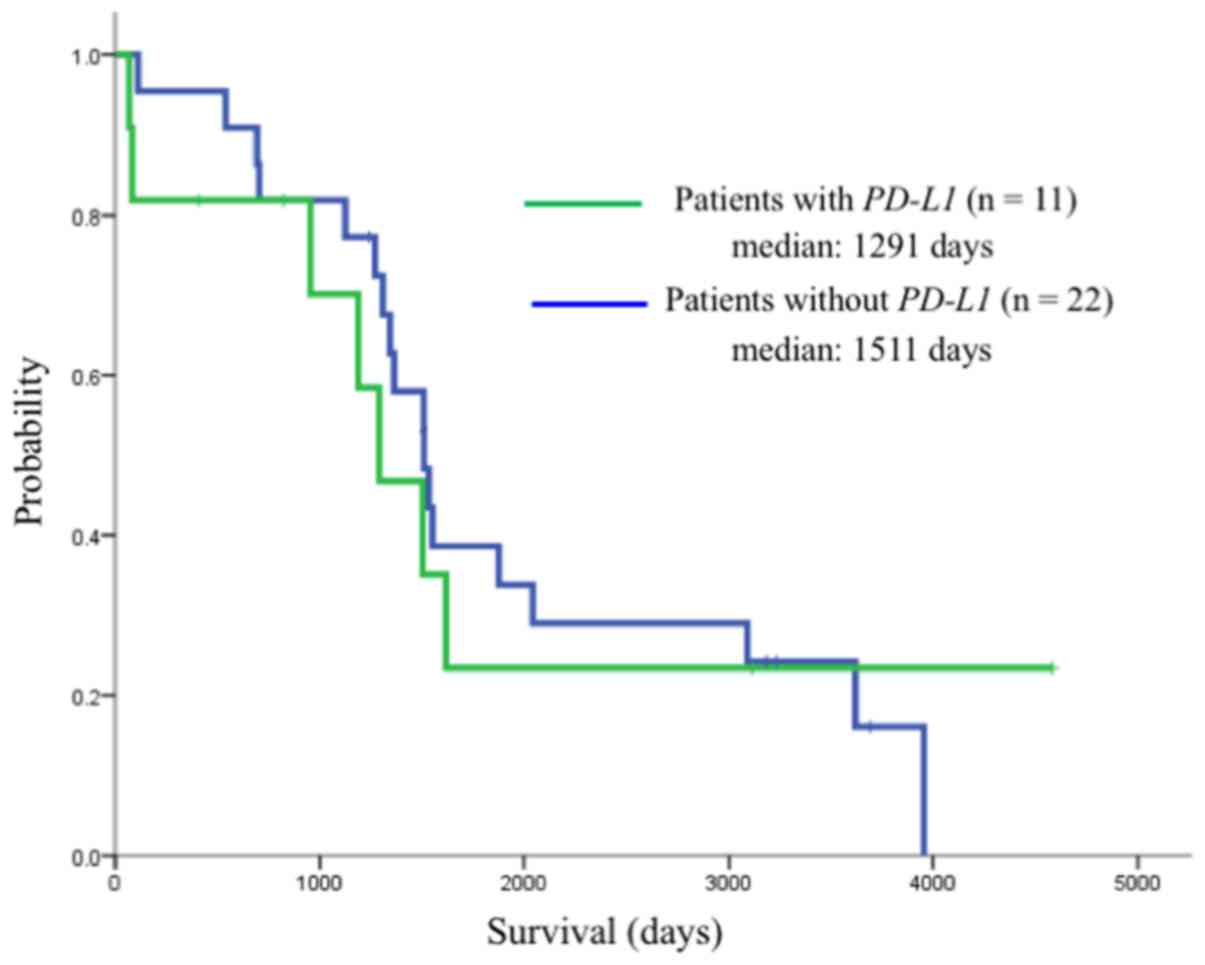
expression (255 vs. 732 days, respectively; P=0.032) (Fig. 6). However, the median OS did not
significantly differ between these groups (1,291 vs. 1,511 days,
P=0.24) (Fig. 7).
 | Table IV.Clinical response after EGFR-TKI
therapy (n=33). |
Table IV.
Clinical response after EGFR-TKI
therapy (n=33).
| Clinical response
(%) | Patients with
intratumoral PD-L1 expression (n=11) | Patients without
intratumoral PD-L1 expression (n=22) | P-value |
|---|
| Response rate | 45.5 | 59.1 | 0.45 |
| Disease control
rate | 91.0 | 95.5 | 0.60 |
Multivariate Cox regression analysis revealed that
intratumoral PD-L1 expression was the most important
independent indicator of a shorter PFS (hazard ratio, 2.953; 95%
confidence interval, 1.270–6.868; P=0.012) (Table V). However, intratumoral
PD-L1 expression was not an indicator of a shorter OS.
 | Table V.Indicators of shorter PFS after
gefitinib treatment. |
Table V.
Indicators of shorter PFS after
gefitinib treatment.
| Parameters | HR | 95% CI | P-value |
|---|
| Univariate Cox
Regression Analysis |
| Pleura
metastasis (yes vs. no) | 2.06 | 0.773–5.484 | 0.15 |
| Bone
metastasis (yes vs. no) | 3.86 | 1.506–9.887 | 0.005 |
|
Intratumoral PD-L1
expression | 2.29 | 1.054–4.953 | 0.036 |
| Multivariate Cox
Regression Analysis |
| Pleura
metastasis (yes vs. no) | 3.47 | 1.193–10.107 | 0.02 |
| Bone
metastasis (yes vs. no) | 5.03 | 1.830–13.803 | 0.002 |
|
Intratumoral PD-L1
expression | 2.95 | 1.270–6.868 | 0.012 |
Discussion
We investigated the association between PD-L1
mRNA expression and prognostic factors associated with EGFR-TKI
therapy, including BIM, PUMA, HER2, VEGFA, EGFR and
MET in the lung tissues of patients with EGFR-mutant
NSCLC. PD-L1 mRNA expression in EGFR-mutant lung
adenocarcinoma was associated with BIM and VEGFA mRNA
expression and with a shorter PFS after gefitinib therapy. To the
best of our knowledge, this is the first study of the association
of BIM and VEGFA mRNA expression in human NSCLC
clinical samples.
EGFR activation induced PD-L1 expression, indicating
that this constitutive oncogenic pathway activation may upregulate
PD-L1 (33). IHC analysis revealed
that PD-L1 was positive in 53.6–58.8% of tumor specimens in
patients with EGFR-mutant NSCLC (38–40).
In the present study, intratumoral PD-L1 mRNA expression was
noted in 11 out of 33 patients (33.3%). This lower ratio may be
explained by the degradation of mRNA in the specimens used in this
study. Future studies should examine the correlation between
PD-L1 mRNA expression and PD-L1 protein expression
deternined by IHC.
BIM is a proapoptotic protein of the B-cell
CLL/lymphoma 2 (Bcl-2) family of proteins and is a key modulator of
apoptosis induced by EGFR-TKI (41). It has been reported that BIM
upregulation is related to the expression of PD-L1 by
tumor-reactive CD8+ T cells in patients with malignant
melanoma (42). In the present
study, patients with detectable PD-L1 mRNA expression had
significantly higher BIM expression (P=0.049), and
PD-L1 mRNA expression was positively correlated with
BIM expression. Recently, Dronca et al (34) reported that BIM, regulated by PD-1
and PD-L1, was crucial for T-cell activation and apoptosis,
especially in effector CD8+ T cells from melanoma
patients, and that T-cell BIM levels reflected the patient response
to anti-PD-1 cancer therapy. Future studies should examine the
association between PD-L1 and BIM.
Although VEGF pathway activation is most commonly
associated with increased angiogenesis, recent studies reported
that increased angiogenesis promoted an immunosuppressive tumor
microenvironment (43–45). Other studies suggested that VEGF
inhibition increased the number of tumor-infiltrating lymphocytes
(46). Joseph et al reported
that PD-L1 expression assessed by IHC was inversely correlated with
the expression of VEGFA, VEGFR1, and VEGFR2 in clear cell renal
carcinoma (35). In the present
study, VEGFA expression was significantly lower in patients
with intratumoral PD-L1 mRNA expression compared with
patients lacking PD-L1 mRNA expression (P=0.009). In
addition, the relative PD-L1 mRNA expression was inversely
correlated with VEGFA expression (r= −0.33, P=0.043). These
findings indicated that tumors with increased VEGF expression have
decreased immune infiltration and therefore, there is less adaptive
pressure to express PD-L1.
High IHC staining of PD-L1 was associated with a
poor prognosis in several human malignancies, indicating that high
intratumoral PD-L1 expression may drive tumor recurrence by
preventing antitumor immunity (47,48).
PD-L1-positive patients treated with EGFR-TKI had a faster disease
progression compared with PD-L1-negative patients (49,50).
Data from the present study revealed that as PD-L1 expression
increased, VEGFA expression decreased leading to the suppression of
angiogenesis, tumor growth and metastasis, which consequently
shortened the PFS of EGFR-TKI. In EGFR mutation-positive NSCLC, BIM
reflects the expression of PD-L1 because it is a downstream signal
of PD-L1; therefore, future clinical applications are expected.
PD-L1 expression has been reported to change after EGFR-TKI
treatment (51). Han et al
(51) reported that intratumoral
PD-L1 IHC expression was markedly increased in 38.9% of patients
after gefitinib treatment. Since samples were obtained and used
before treatment in the present study, there appears to be no
correlation between the expression of PD-L1 and EGFR,
MET, and HER2. Our future study will investigate the
relationship between PD-L1 expression and EGFR, MET and
HER2 after EGFR-TKI resistance. Furthermore, it was suggested
that PD-L1 expression was also related to BIM-mediated
apoptosis and VEGFA-mediated angiogenesis in EGFR-mutated
lung cancer.
This study had some limitations. First, it was a
retrospective single-center study with a small sample size. We
revealed differences in clinical outcome according to PD-L1
expression; however, the number of patients enrolled was too small
to consider the association of PD-L1 expression and PFS.
Furthermore, the OS was not different between patients with and
without PD-L1 expression. Thus, a large-scale multicenter study is
required to confirm the validity of our results. Second, there is a
possibility of the influence of mRNA deterioration in the specimens
used in this study. In addition, since we used a cell line as a
control and samples to produce the standard curve, we cannot
assess/confirm that the clinical samples were of sufficient high
quality to produce meaningful results in the present study.
In conclusion, PD-L1 mRNA expression in
EGFR-mutant lung adenocarcinoma was associated with
BIM and VEGFA mRNA expression and with a shorter PFS
after gefitinib therapy. The present results should help treatment
planning for patients with EGFR-mutant NSCLC.
Acknowledgements
We thank Yoshihisa Otsuka of SRL Inc. (Tokyo,
Japan), Yusuke Hashizawa and Akimitsu Iwama of Astra Zeneca Inc.
(Tokyo Japan) and Yusuke Hozumi of Ono Pharmaceutical Co., Ltd.
(Tokyo Japan). We are also grateful to David Kipler and N.A.I. Inc.
for the review of the language of this study.
Funding
The present study was supported by JSPS KAKENHI
(grant nos. JP15K09195 and JP2642140) and by a Research Promotion
Grant from Toho University Graduate School of Medicine (no. 16-3,
to KI).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Author's contributions
KI, AK, TMi and SH conceived and designed the study.
KK, HK, TY and YN performed the experiments. KI and AK wrote the
paper. TMa, HO, GS, KS, SS, YT, NT, AI and SH reviewed and edited
the manuscript. All authors read and approved the manuscript and
agree to be accountable for all aspects of the research in ensuring
that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
This single-center study was conducted at Toho
University Omori Medical Center (Tokyo, Japan) and was approved by
its Human Genome/Gene Analysis Research Ethics Committee
(authorization no. 27128).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PD-L1
|
programmed death-ligand 1
|
|
PD-1
|
programmed death 1
|
|
BIM
|
BCL2-like 11
|
|
NSCLC
|
non-small cell lung cancer
|
|
EGFR
|
epidermal growth factor receptor
|
|
EGFR-TKI
|
epidermal growth factor receptor
tyrosine kinase inhibitor
|
|
PUMA
|
p53 upregulated modular of
apoptosis
|
|
HER2
|
human epidermal growth factor
receptor 2
|
|
VEGFA
|
vascular endothelial growth factor
A
|
|
MET
|
mesenchymal-epithelial transition
|
|
FFPE
|
formalin-fixed paraffin-embedded
|
|
PFS
|
progression-free survival
|
|
OS
|
overall survival
|
|
PCR
|
polymerase chain reaction
|
|
CTC
|
National Cancer Institute Common
Terminology Criteria
|
References
|
1
|
Paez JG, Jänne PA, Lee JC, Tracy S,
Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et
al: EGFR mutations in lung cancer: Correlation with clinical
response to gefitinib therapy. Science. 304:1497–1500. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lynch TJ, Bell DW, Sordella R,
Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat
SM, Supko JG, Haluska FG, et al: Activating mutations in the
epidermal growth factor receptor underlying responsiveness of
non-small-cell lung cancer to gefitinib. N Engl J Med.
350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tan DS, Mok TS and Rebbeck TR: Cancer
genomics: Diversity and disparity across ethnicity and geography. J
Clin Oncol. 34:91–101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tan DS, Yom SS, Tsao MS, Pass HI, Kelly K,
Peled N, Yung RC, Wistuba II, Yatabe Y, Unger M, et al: The
International Association for the Study of Lung Cancer consensus
statement on optimizing management of EGFR mutation-positive
non-small cell lung cancer: Status in 2016. J Thorac Oncol.
11:946–963. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Masters GA, Temin S, Azzoli CG, Giaccone
G, Baker S Jr, Brahmer JR, Ellis PM, Gajra A, Rackear N, Schiller
JH, et al: Systemic Therapy for Stage IV Non-Small-Cell Lung
Cancer: American Society of Clinical Oncology Clinical Practice
Guideline Update. J Clin Oncol. 33:3488–3515. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kuan FC, Kuo LT, Chen MC, Yang CT, Shi CS,
Teng D and Lee KD: Overall survival benefits of first-line EGFR
tyrosine kinase inhibitors in EGFR-mutated non-small-cell lung
cancers: A systematic review and meta-analysis. Br J Cancer.
113:1519–1528. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mok TS, Wu YL, Thongprasert S, Yang CH,
Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et
al: Gefitinib or carboplatin-paclitaxel in pulmonary
adenocarcinoma. N Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maemondo M, Inoue A, Kobayashi K, Sugawara
S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I,
et al: North-East Japan Study Group: Gefitinib or chemotherapy for
non-small-cell lung cancer with mutated EGFR. N Engl J Med.
362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou C, Wu YL, Chen G, Feng J, Liu XQ,
Wang C, Zhang S, Wang J, Zhou S, Ren S, et al: Erlotinib versus
chemotherapy as first-line treatment for patients with advanced
EGFR mutation-positive non-small-cell lung cancer (OPTIMAL,
CTONG-0802): A multicentre, open-label, randomised, phase 3 study.
Lancet Oncol. 12:735–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang JC, Wu YL, Schuler M, Sebastian M,
Popat S, Yamamoto N, Zhou C, Hu CP, O'Byrne K, Feng J, et al:
Afatinib versus cisplatin-based chemotherapy for EGFR
mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6):
Analysis of overall survival data from two randomised, phase 3
trials. Lancet Oncol. 16:141–151. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mitsudomi T, Morita S, Yatabe Y, Negoro S,
Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, et
al: West Japan Oncology Group: Gefitinib versus cisplatin plus
docetaxel in patients with non-small-cell lung cancer harbouring
mutations of the epidermal growth factor receptor (WJTOG3405): An
open label, randomised phase 3 trial. Lancet Oncol. 11:121–128.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bean GR, Ganesan YT, Dong Y, Takeda S, Liu
H, Chan PM, Huang Y, Chodosh LA, Zambetti GP, Hsieh JJ, et al: PUMA
and BIM are required for oncogene inactivation-induced apoptosis.
Sci Signal. 6:ra202013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Isobe K, Kakimoto A, Mikami T, Kaburaki K,
Kobayashi H, Yoshizawa T, Makino T, Otsuka H, Sano GO, Sugino K, et
al: Association of BIM deletion polymorphism and BIM-γ RNA
expression in NSCLC with EGFR mutation. Cancer Genomics Proteomics.
13:475–482. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Costa C, Molina MA, Drozdowskyj A,
Giménez-Capitán A, Bertran-Alamillo J, Karachaliou N, Gervais R,
Massuti B, Wei J, Moran T, et al: The impact of EGFR T790M
mutations and BIM mRNA expression on outcome in patients with
EGFR-mutant NSCLC treated with erlotinib or chemotherapy in the
randomized phase III EURTAC trial. Clin Cancer Res. 20:2001–2010.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Garofalo M, Romano G, Di Leva G, Nuovo G,
Jeon YJ, Ngankeu A, Sun J, Lovat F, Alder H, Condorelli G, et al:
EGFR and MET receptor tyrosine kinase-altered microRNA expression
induces tumorigenesis and gefitinib resistance in lung cancers. Nat
Med. 18:74–82. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Naumov GN, Nilsson MB, Cascone T, Briggs
A, Straume O, Akslen LA, Lifshits E, Byers LA, Xu L, Wu HK, et al:
Combined vascular endothelial growth factor receptor and epidermal
growth factor receptor (EGFR) blockade inhibits tumor growth in
xenograft models of EGFR inhibitor resistance. Clin Cancer Res.
15:3484–3494. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hirsch FR, Varella-Garcia M and Cappuzzo
F: Predictive value of EGFR and HER2 overexpression in advanced
non-small-cell lung cancer. Oncogene. 28 Suppl 1:S32–S37. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rho JK, Choi YJ, Lee JK, Ryoo BY, Na II,
Yang SH, Lee SS, Kim CH, Yoo YD and Lee JC: The role of MET
activation in determining the sensitivity to epidermal growth
factor receptor tyrosine kinase inhibitors. Mol Cancer Res.
7:1736–1743. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Agata Y, Kawasaki A, Nishimura H, Ishida
Y, Tsubata T, Yagita H and Honjo T: Expression of the PD-1 antigen
on the surface of stimulated mouse T and B lymphocytes. Int
Immunol. 8:765–772. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nishimura H and Honjo T: PD-1: An
inhibitory immunoreceptor involved in peripheral tolerance. Trends
Immunol. 22:265–268. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Watanabe N, Gavrieli M, Sedy JR, Yang J,
Fallarino F, Loftin SK, Hurchla MA, Zimmerman N, Sim J, Zang X, et
al: BTLA is a lymphocyte inhibitory receptor with similarities to
CTLA-4 and PD-1. Nat Immunol. 4:670–679. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Keir ME, Butte MJ, Freeman GJ and Sharpe
AH: PD-1 and its ligands in tolerance and immunity. Annu Rev
Immunol. 26:677–704. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brahmer J, Reckamp KL, Baas P, Crinò L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus docetaxel in advanced
squamous-cell non-small-cell lung cancer. N Engl J Med.
373:123–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Herbst RS, Baas P, Kim DW, Felip E,
Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al:
Pembrolizumab versus docetaxel for previously treated,
PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010):
A randomised controlled trial. Lancet. 387:1540–1550. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Afreen S and Dermime S: The
immunoinhibitory B7-H1 molecule as a potential target in cancer:
Killing many birds with one stone. Hematol Oncol Stem Cell Ther.
7:1–17. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boland JM, Kwon ED, Harrington SM,
Wampfler JA, Tang H, Yang P and Aubry MC: Tumor B7-H1 and B7-H3
expression in squamous cell carcinoma of the lung. Clin Lung
Cancer. 14:157–163. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Konishi J, Yamazaki K, Azuma M, Kinoshita
I, Dosaka-Akita H and Nishimura M: B7-H1 expression on non-small
cell lung cancer cells and its relationship with tumor-infiltrating
lymphocytes and their PD-1 expression. Clin Cancer Res.
10:5094–5100. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen YY, Wang LB, Zhu HL, Li XY, Zhu YP,
Yin YL, Lü FZ, Wang ZL and Qu JM: Relationship between programmed
death-ligand 1 and clinicopathological characteristics in non-small
cell lung cancer patients. Chin Med Sci J. 28:147–151. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mu CY, Huang JA, Chen Y, Chen C and Zhang
XG: High expression of PD-L1 in lung cancer may contribute to poor
prognosis and tumor cells immune escape through suppressing tumor
infiltrating dendritic cells maturation. Med Oncol. 28:682–688.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Akbay EA, Koyama S, Carretero J, Altabef
A, Tchaicha JH, Christensen CL, Mikse OR, Cherniack AD, Beauchamp
EM, Pugh TJ, et al: Activation of the PD-1 pathway contributes to
immune escape in EGFR-driven lung tumors. Cancer Discov.
3:1355–1363. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Azuma K, Ota K, Kawahara A, Hattori S,
Iwama E, Harada T, Matsumoto K, Takayama K, Takamori S, Kage M, et
al: Association of PD-L1 overexpression with activating EGFR
mutations in surgically resected nonsmall-cell lung cancer. Ann
Oncol. 25:1935–1940. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen N, Fang W, Zhan J, Hong S, Tang Y,
Kang S, Zhang Y, He X, Zhou T, Qin T, et al: Upregulation of PD-L1
by EGFR activation mediates the immune escape in EGFR-driven NSCLC:
Implication for optional immune targeted therapy for NSCLC patients
with EGFR mutation. J Thorac Oncol. 10:910–923. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dronca RS, Liu X, Harrington SM, Chen L,
Cao S, Kottschade LA, McWilliams RR, Block MS, Nevala WK, Thompson
MA, et al: T cell Bim levels reflect responses to anti-PD-1 cancer
therapy. JCI Insight. 1:12016. View Article : Google Scholar
|
|
35
|
Joseph RW, Parasramka M, Eckel-Passow JE,
Serie D, Wu K, Jiang L, Kalari K, Thompson RH, Ho Huu T, Castle EP,
et al: Inverse association between programmed death ligand 1 and
genes in the VEGF pathway in primary clear cell renal cell
carcinoma. Cancer Immunol Res. 1:378–385. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vandesompele J, De Preter K, Pattyn F,
Poppe B, Van Roy N, De Paepe A and Speleman F: Accurate
normalization of real-time quantitative RT-PCR data by geometric
averaging of multiple internal control genes. Genome Biol.
3:RESEARCH00342002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wong ML and Medrano JF: Real-time PCR for
mRNA quantitation. Biotechniques. 39:75–85. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
D'Incecco A, Andreozzi M, Ludovini V,
Rossi E, Capodanno A, Landi L, Tibaldi C, Minuti G, Salvini J,
Coppi E, et al: PD-1 and PD-L1 expression in molecularly selected
non-small-cell lung cancer patients. Br J Cancer. 112:95–102. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tang Y, Fang W, Zhang Y, Hong S, Kang S,
Yan Y, Chen N, Zhan J, He X, Qin T, et al: The association between
PD-L1 and EGFR status and the prognostic value of PD-L1 in advanced
non-small cell lung cancer patients treated with EGFR-TKIs.
Oncotarget. 6:14209–14219. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lin C, Chen X, Li M, Liu J, Qi X, Yang W,
Zhang H, Cai Z, Dai Y and Ouyang X: Programmed death-ligand 1
expression predicts tyrosine kinase inhibitor response and better
prognosis in a cohort of patients with epidermal growth factor
receptor mutation-positive lung adenocarcinoma. Clin Lung Cancer.
16:e25–e35. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gong Y, Somwar R, Politi K, Balak M,
Chmielecki J, Jiang X and Pao W: Induction of BIM is essential for
apoptosis triggered by EGFR kinase inhibitors in mutant
EGFR-dependent lung adenocarcinomas. PLoS Med. 4:e2942007.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gibbons RM, Liu X, Pulko V, Harrington SM,
Krco CJ, Kwon ED and Dong H: B7-H1 limits the entry of effector
CD8(+) T cells to the memory pool by upregulating Bim.
Oncoimmunology. 1:1061–1073. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Huang Y, Goel S, Duda DG, Fukumura D and
Jain RK: Vascular normalization as an emerging strategy to enhance
cancer immunotherapy. Cancer Res. 73:2943–2948. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wei J, Wu A, Kong LY, Wang Y, Fuller G,
Fokt I, Melillo G, Priebe W and Heimberger AB: Hypoxia potentiates
glioma-mediated immunosuppression. PLoS One. 6:e161952011.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ott PA, Hodi FS and Buchbinder EI:
Inhibition of immune checkpoints and vascular endothelial growth
factor as combination therapy for metastatic melanoma: An overview
of rationale, preclinical evidence, and initial clinical data.
Front Oncol. 5:2022015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Huang Y, Yuan J, Righi E, Kamoun WS,
Ancukiewicz M, Nezivar J, Santosuosso M, Martin JD, Martin MR,
Vianello F, et al: Vascular normalizing doses of antiangiogenic
treatment reprogram the immunosuppressive tumor microenvironment
and enhance immunotherapy. Proc Natl Acad Sci USA. 109:17561–17566.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zou W and Chen L: Inhibitory B7-family
molecules in the tumour microenvironment. Nat Rev Immunol.
8:467–477. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chen J, Jiang CC, Jin L and Zhang XD:
Regulation of PD-L1: A novel role of pro-survival signalling in
cancer. Ann Oncol. 27:409–416. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Okita R, Maeda A, Shimizu K, Nojima Y,
Saisho S and Nakata M: PD-L1 overexpression is partially regulated
by EGFR/HER2 signaling and associated with poor prognosis in
patients with non-small-cell lung cancer. Cancer Immunol
Immunother. 66:865–876. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Shimoji M, Shimizu S, Sato K, Suda K,
Kobayashi Y, Tomizawa K, Takemoto T and Mitsudomi T: Clinical and
pathologic features of lung cancer expressing programmed cell death
ligand 1 (PD-L1). Lung Cancer. 98:69–75. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Han JJ, Kim DW, Koh J, Keam B, Kim TM,
Jeon YK, Lee SH, Chung DH and Heo DS: Change in PD-L1 expression
after acquiring resistance to gefitinib in EGFR-mutant
non-small-cell lung cancer. Clin Lung Cancer. 17:263–270.e262.
2016. View Article : Google Scholar : PubMed/NCBI
|