Introduction
Lung cancer generally has a poor prognosis and it
remains the leading cause of cancer-related deaths worldwide
(1). Non-small cell lung cancer
(NSCLC) represents approximately 85% of all newly diagnosed lung
cancer cases (2). Currently,
chemotherapy with cytotoxic agents is used for the treatment of
lung cancer patients (3).
Significant advances have also been made in the availability of
targeted molecules for the inhibition of critical pathways in
NSCLC, such as the targeting of epidermal growth factor receptor
(EGFR) by afatinib (4). However,
the 5-year survival rate for lung cancer still remains very
low.
Gemcitabine (GEM) is a type of deoxycytidine
analogue that has exhibited strong antitumor activity (5) and has been widely approved for the
treatment of advanced lung cancer, pancreatic cancer and ovarian
cancer (6). However, some studies
have demonstrated that GEM resistance often limits its efficacy
(7). Thus, new treatment strategies
for NSCLC are needed. Considering the advances that have been made
in the development of targeted therapies, a better understanding of
lung cancer cell biology is needed to facilitate further advances
(8,9).
Notch is a protein that plays an important role in
embryogenesis and organogenesis by regulating cell proliferation
and differentiation (10). This
transmembrane heterodimeric receptor has four distinct forms
(Notch1-4) in both rodents and humans. In particular, Notch-3 is a
receptor of the Notch signaling pathway and it plays an important
role in regulating self-renewal, differentiation and apoptosis in
cancer cells. Consequently, it represents a promising target for
the development of novel therapies for the treatment of aggressive
cancers such as NSCLC (11).
Clinical studies have revealed that overexpression of Notch-3 is
common in NSCLC and it correlates with a shorter progression-free
period and shorter overall disease-free survival (12). Clinical studies have also revealed
that a high level of Notch-3 expression is a poor prognostic factor
for NSCLC (13). Additionally,
Notch-3 overexpression has been reported to be related to the
proliferative and apoptotic capacity of cancer cells (14). Overall survival is significantly
lower for patients with Notch-3-positive tumors compared with those
with Notch-3-negative tumors (15)
and high levels of Notch-3 expression have been associated with
drug resistance in NSCLC and ovarian cancer (16,17).
Thus, Notch-3 may be a potential target for cancer therapies,
either alone or in combination with GEM.
Based on our current understanding of the structure,
function and regulation of the Notch signaling pathway, there are
several steps which have been identified as potential targets for
inhibiting this pathway, as well as Notch-3 activity. Accumulating
evidence has revealed that activation of Notch proteins largely
depends on γ-secretase activity (18). Thus, γ-secretase is a promising
target for Notch-3 inhibition (19).
N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine
t-butyl ester (DAPT) is a γ-secretase that is often referred
to as a ‘Notch inhibitor’ in oncology. Moreover, DAPT is widely
considered to act as an inhibitor in terms of biological activity
and to mediate cytotoxic activities in various types of cancer
cells (20). In clinical trials,
DAPT has had a variety of indications (21). To investigate whether DAPT is an
effective treatment for GEM-resistant tumors, DAPT inhibition of
Notch-3 activity was tested. DAPT was found to effectively
downregulate protein levels of NICD3 and to potentially target
Notch-3 in gene therapy experiments conducted in vitro
(22). Therefore, in the present
study, a combined treatment involving DAPT and GEM was examined for
its potential to effectively mediate antitumor activity in NSCLC
cells.
Materials and methods
Cell lines
The NSCLC cell lines, H1299 and A549, were purchased
[American Type Culture Collection (ATCC), Manassas, VA, USA] and
cultured as recommended. Dulbecco's modified Eagle's medium (Gibco™
DMEM; Thermo Fisher Scientific, Inc., Shanghai, China) was
supplemented with 10% fetal bovine serum (FBS; Sijiqing, Hangzhou,
China) and a 5-ml penicillin/streptomycin solution (100X; Beyotime
Institute of Biotechnology, Shanghai, China) for each 500 ml of
medium. The cells were cultured at 37°C in a humidified incubator
containing 5% CO2.
Treatment
DAPT was purchased from Abcam (Shanghai, China)
(120633) and GEM was a gift from Jiangsu Hansen Pharmaceutical Co.
Ltd. (Jiangshu, China). The cells in this study received: i) no
treatment (NC group); ii) 1 µl dimethyl sulfoxide (DMSO,
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany); iii) 20 µM DAPT
(DAPT); iv) GEM (0.05 and 0.1 µM for A549 and H1299 cells,
respectively); v) 1 µl DMSO + GEM (0.05 and 0.1 µM for A549 and
H1299 cells, respectively); vi) 20 µM DAPT + GEM (0.05 and 0.1 µM
for A549 and H1299 cells, respectively).
Western blot analysis
Cells were plated at 1×105 cells per well
in 6-well plates and treated as described above. The cells were
subsequently harvested with lysis buffer (RIPA, Beyotime Institute
of Biotechnology) and an equal volume of 1X SDS buffer (Beyotime
Institute of Biotechnology) was added to each protein sample. After
the samples were placed in boiling water for 10 min, they were
subsequently separated by 10% SDS-polyacrylamide gel
electrophoresis (PAGE) and transferred to 0.45-µm or 0.22-µm
nitrocellulose membranes. The membranes were blocked in Tris-HCl
buffered saline Tween (TBST) containing 0.5% dry milk and then were
incubated with antibodies recognizing Notch-3 and NICD3 (1:5,000;
cat. no. ab23426; Abcam, Cambridge, UK), and anti-Bcl-2 and
anti-Bax (1:1,000; cat. nos. YM3041 and YT0459, respectively;
ImmunoWay Biotechnology Co., Plano, TX, USA), overnight. The
membranes were then incubated with appropriate goat anti-mouse IgG
secondary antibodies (1:10,000; cat. no. ZB-2305; Beijing Zhongshan
Jinqiao Biotechnology Co., Ltd., Beijing, China) for 1 h. Bound
antibodies were visualized with enhanced chemiluminescence reagents
and imaged on film (Kodak X-ray film). Actin was detected with a
mouse anti-actin monoclonal antibody (1:5,000; cat. no. ZM-0001;
Beijing Zhongshan Jinqiao Biotechnology, Co., Ltd., Beijing, China)
as an internal control for protein quantification. Each experiment
was repeated 3 times and similar results were obtained by the
ImageJ bundled with Java 1.8.0_101 (imagej.nih.gov/ij/download/).
Cultivating higher expression of
Notch-3
H1299 and A549 cells were plated at 1×106
cells per well in 6-well plates. Twenty-four hours later, GEM was
added to the culture medium. After 4 days, both sets of cells were
harvested, subjected to protein extraction, and expression of
Notch-3 was detected by western blot analysis. In parallel, an
additional set of cells from each cell line were cultured with GEM
for two months. These cells were also harvested, subjected to
protein extraction, and expression of Notch-3 was detected by
western blot analysis. Each experiment was repeated 3 times and
expression of Notch-3 was compared with and without GEM treatment.
The cells induced with GEM for two months were used in subsequent
experiments.
Cell viability assay
Tumor cells were plated in 96-well plates with 3,000
cells per well and were allowed to attach overnight. To detect the
sensitivity of GEM, H1299 and A549 cells were incubated with
various concentrations of GEM. Different concentrations of DAPT
were subsequently added to the two cell lines for 48 h, and then
24, 48 and 72 h later, 10%
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
was added to each well. After 4 h, cell viability for each well was
measured based on optical density measurements obtained at a
wavelength of 490 nm. Each cell viability assay was performed six
times.
H1299 and A549 cells overexpressing Notch-3 were
plated in 96-well plates (3,000 cells/well) and were allowed to
attach overnight. The cells were then treated with DMSO or 20 µM
DAPT for 24 h, followed by treatment with a specific concentration
of GEM (0.05 or 0.1 µM for A549 and H1299 cells, respectively). Two
days later, cell viability was evaluated with the addition of MTT
as described above.
Apoptosis assay
H1299 and A549 cells overexpressing Notch-3 were
plated in 6-well plates (1×105 cells/well) and were
allowed to attach overnight. The cells were then incubated with 1
µl DMSO or 20 µM DAPT for 24 h, then they were treated with GEM at
varying concentrations (e.g., H1299 cells received 10 µM GEM and
A549 cells received 1 µM GEM). Two days later, the cells were
trypsinized, collected, centrifuged, and washed with pre-cooled
phosphate-buffered saline (PBS). To each sample
(105-106 cells each), 5 µl Annexin V-FITC
(BestBio, Shanghai, China) and 10 µl propidium iodide (PI) were
added. The samples were mixed and then incubated at room
temperature in the dark. After 15 min, fluorescence was determined
using a flow cytometer (Beckman, USA) within 1 h. The percentages
of cells staining for apoptosis were calculated with WinMDI 2.9
(http://facs.scripps.edu) and compared. All of
these experiments were performed in triplicate.
Cell colony assay
H1299 and A549 cells overexpressing Notch-3 were
seeded in 96-well plates (3,000 cells/well) and were allowed to
attach overnight. The next day, DMSO or 20 µM DAPT was added to
each well. Twenty-four hours later, the cells were treated with GEM
at varying concentrations. Two days later, the number of colonies
that formed were counted. After an additional 15 days, the colonies
were washed with PBS, fixed with formaldehyde for 20 min, stained
with hematoxylin for 30 min, and counted. Clusters of cells
containing >50 cells were scored by fluorescence electron
microscope (Shanghai Changfang Optical Instrument, Co., Ltd.,
Shanghai, China) as colonies.
Cell count assay
H1299 and A549 cells were cultured in 24-well plates
(104 cells/well). According to the treatment groups, all
of the wells were treated with DMSO or 20 µM DAPT on the second day
after plating. Twenty-four hours later, GEM was added. Cells were
subsequently counted each day for 7 days. Counting was performed by
adding 100 µl of pancreatic enzyme (0.25% Gibco; Thermo Fisher
Scientific, Inc.) to each well. After the cells were detached and
dissociated from one another, 100 µl medium was added to terminate
digestion. All of the samples were subsequently counted with cell
counter. Each group was counted 3 times.
Statistical analysis
Data are presented as the mean ± standard deviation
(SD). Comparisons between the treatment groups were performed by
applying a t-test. Multiple factors were analyzed by using the
Mann-Whitney U-test. Values with a P-value <0.05 were considered
statistically significant (*P<0.05, **P<0.01 vs. control; as
indicated in the figures).
Results
Induction of Notch-3 overexpression in
tumor cells
GEM resistance currently represents a major
challenge to cancer treatments. A majority of the research that has
been conducted regarding this resistance has been at a nonclinical
research stage. However, valuable experimental experience has been
gained in the construction of drug-resistant cell models as a
result of these efforts. Most in vitro studies of NSCLC have
focused on A549 cells, a human lung adenocarcinoma cell line. Here,
two commonly used NSCLC cell lines (H1299 and A549) were compared,
with a focus on Notch-3 protein expression in response to different
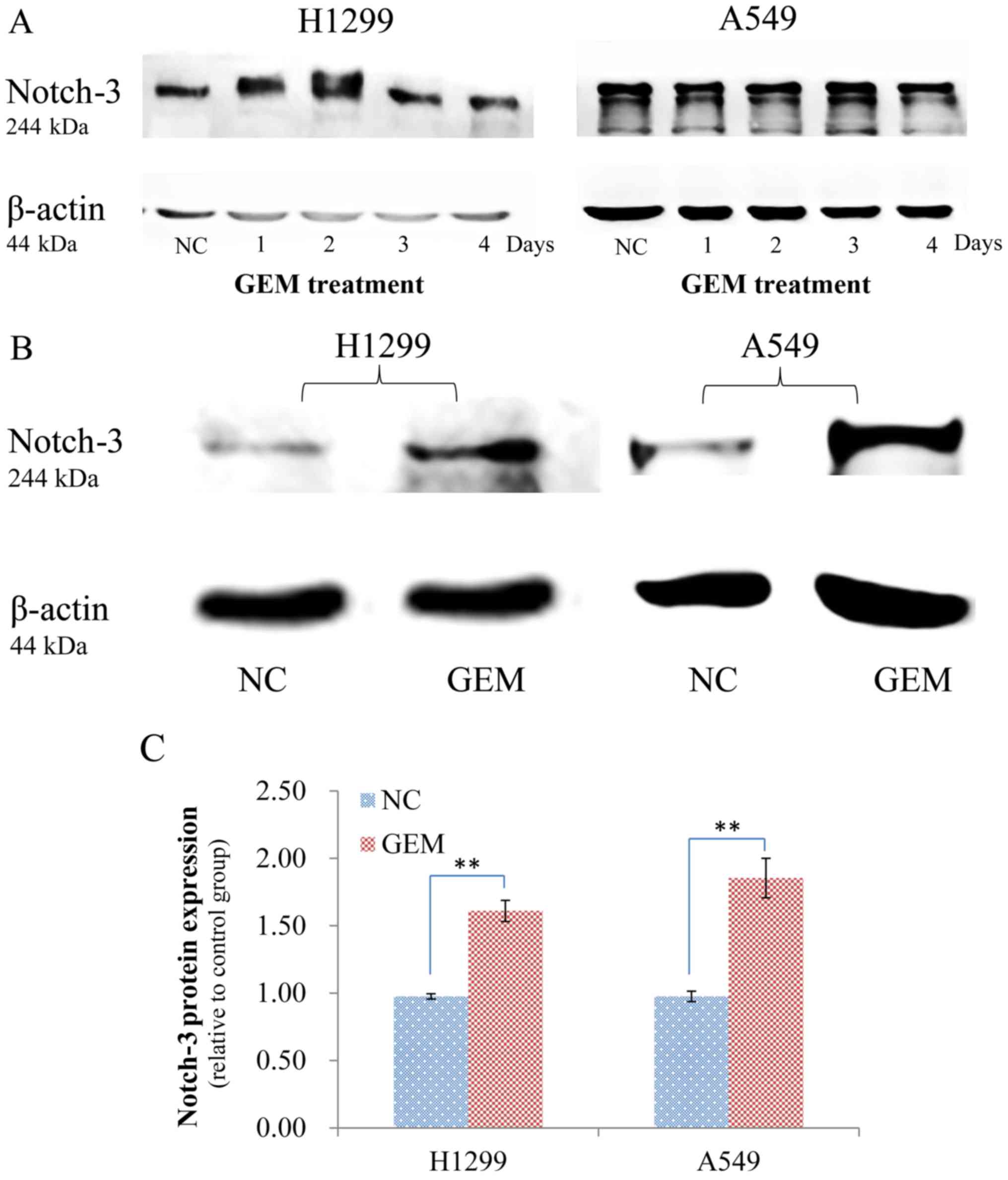
durations of GEM induction. In the western blot assays performed,
no significant differences in protein expression were observed
between the no treatment group and induction with GEM for 1, 2, 3
and 4 days (Fig. 1A). However,
significant differences in Notch-3 expression were detected in both
cell lines after they were induced with GEM for 2 months (Fig. 1B and C). Therefore, H1299 and A549
cells that were subjected to the latter induction method were
analyzed in subsequent experiments.
DAPT efficiently downregulates NICD3
expression
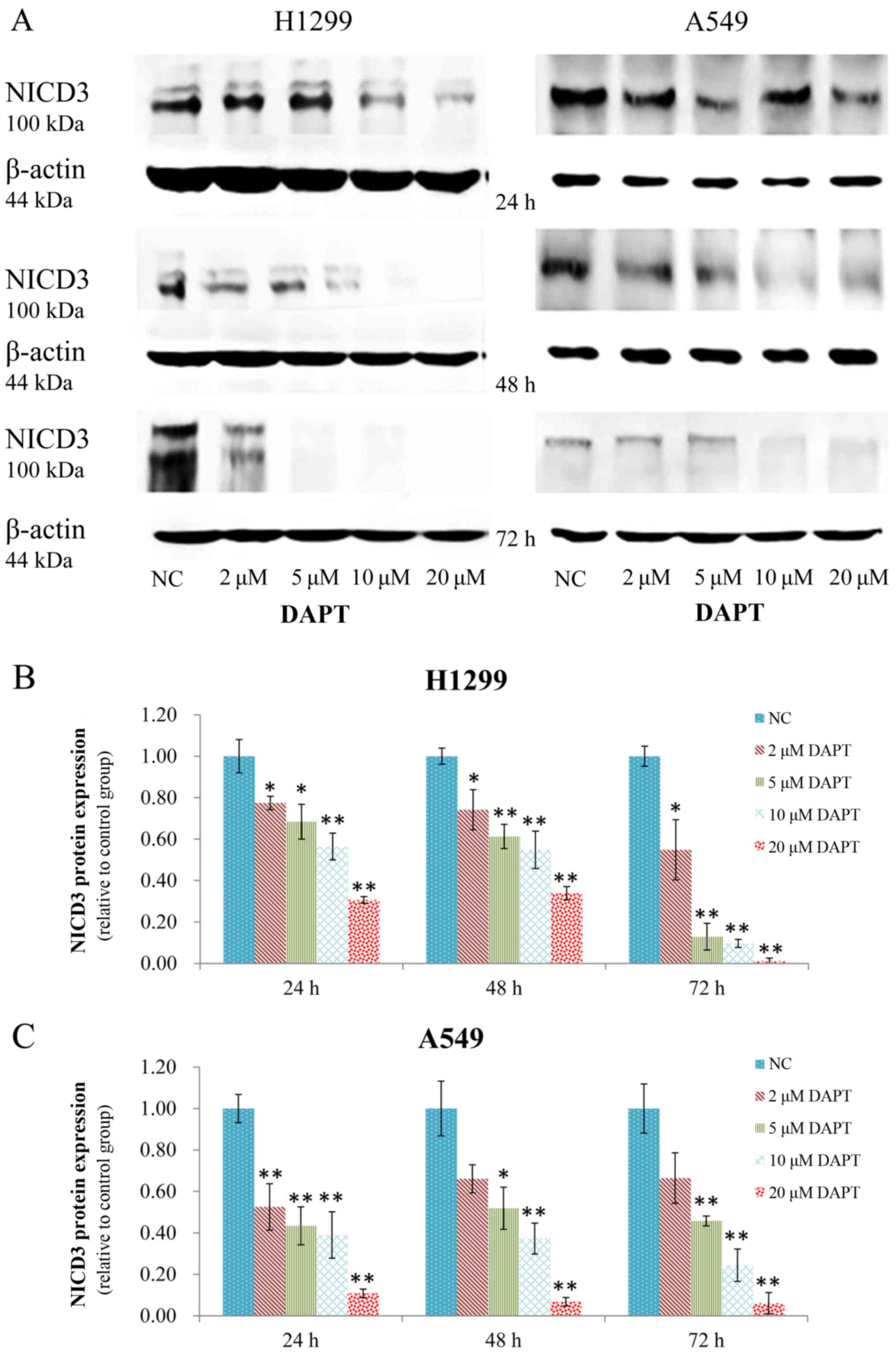
H1299 and A549 cells overexpressing Notch-3 were
incubated with various concentrations of DAPT (2, 5, 10, and 20
µM). As a result, they exhibited a significant dose-dependent
decrease in NICD3 protein expression. Moreover, marked decreases in
expression were most notably detected at 24, 48 and 72 h after
treatment with 10 µM DAPT or 20 µM DAPT. These results suggest that
DAPT effectively reduced expression of NICD3 (Fig. 2).
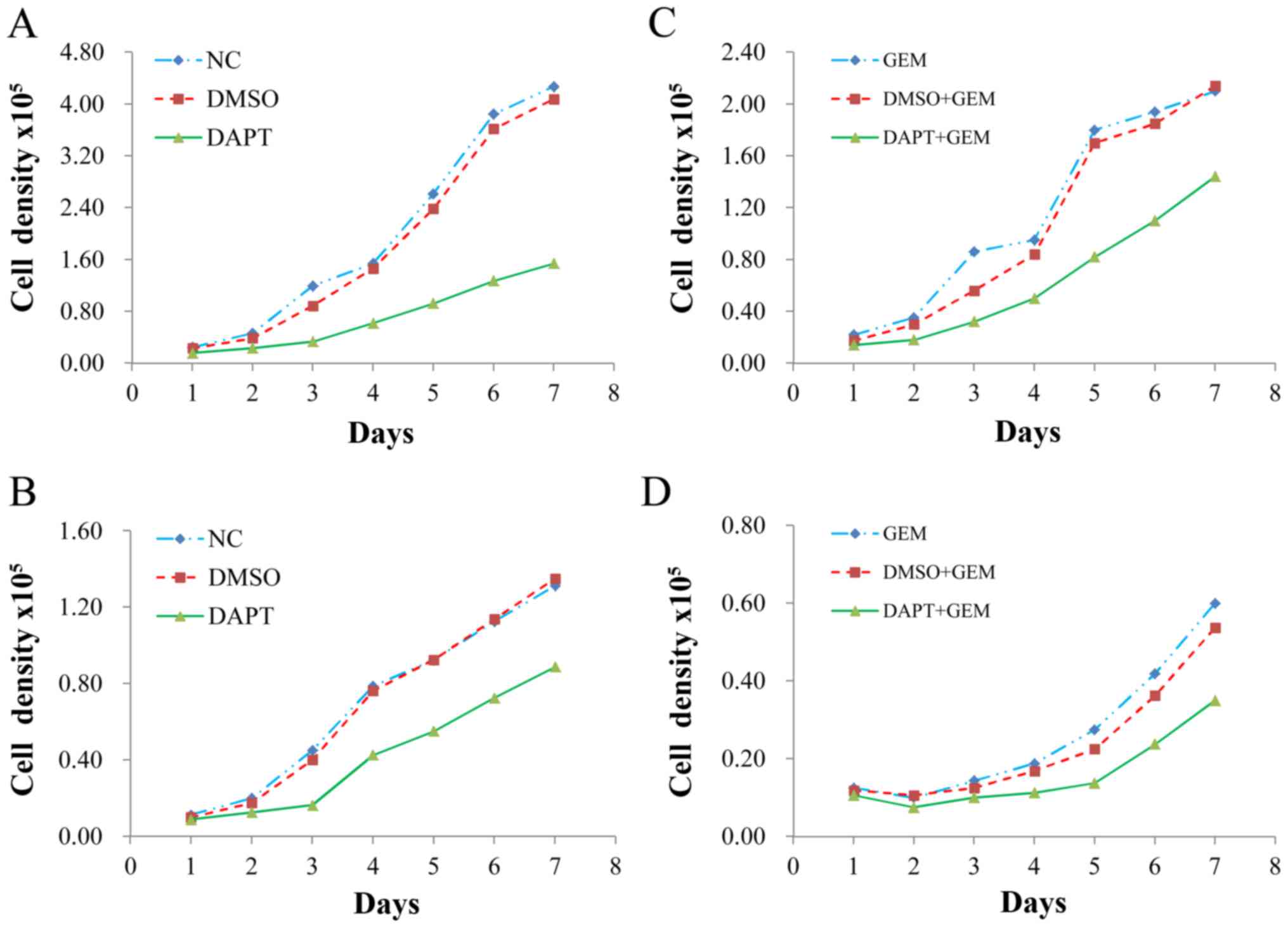
Treatment with GEM and DAPT affects
cell growth
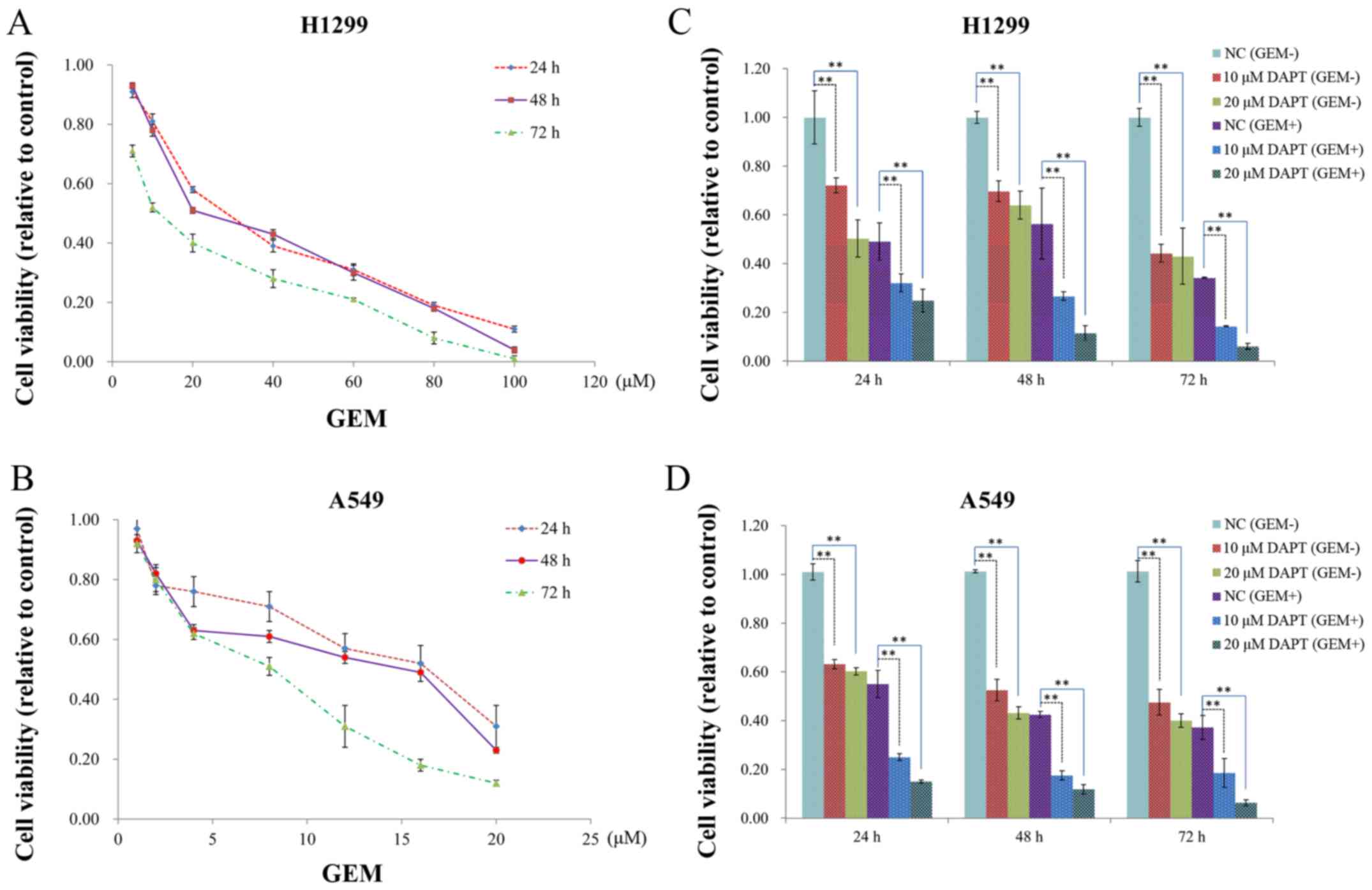
When A549 and H1299 cells were incubated with GEM,
an inhibitory effect on cell growth was observed 24, 48 and 72 h
after the start of GEM treatment (Fig.
3A and B). Both time-dependent decreases and decreases in the
half maximal inhibitory concentration (IC50) values for
GEM in each cell line were observed at the three time points
assayed. In cell viability assays that were conducted with both
cell lines in the presence of DAPT and DAPT + GEM, the inhibitory
effect of GEM was further enhanced compared to the control groups
at the 48 h time-point (Fig. 3C and
D). It is generally believed that drug inhibition of tumor
cells is related to apoptosis rates and colony numbers. Therefore,
we subsequently investigated the apoptosis rate and colony growth
of these 2 cell lines.
DAPT enhances GEM sensitivity to
increase apoptosis and colony numbers
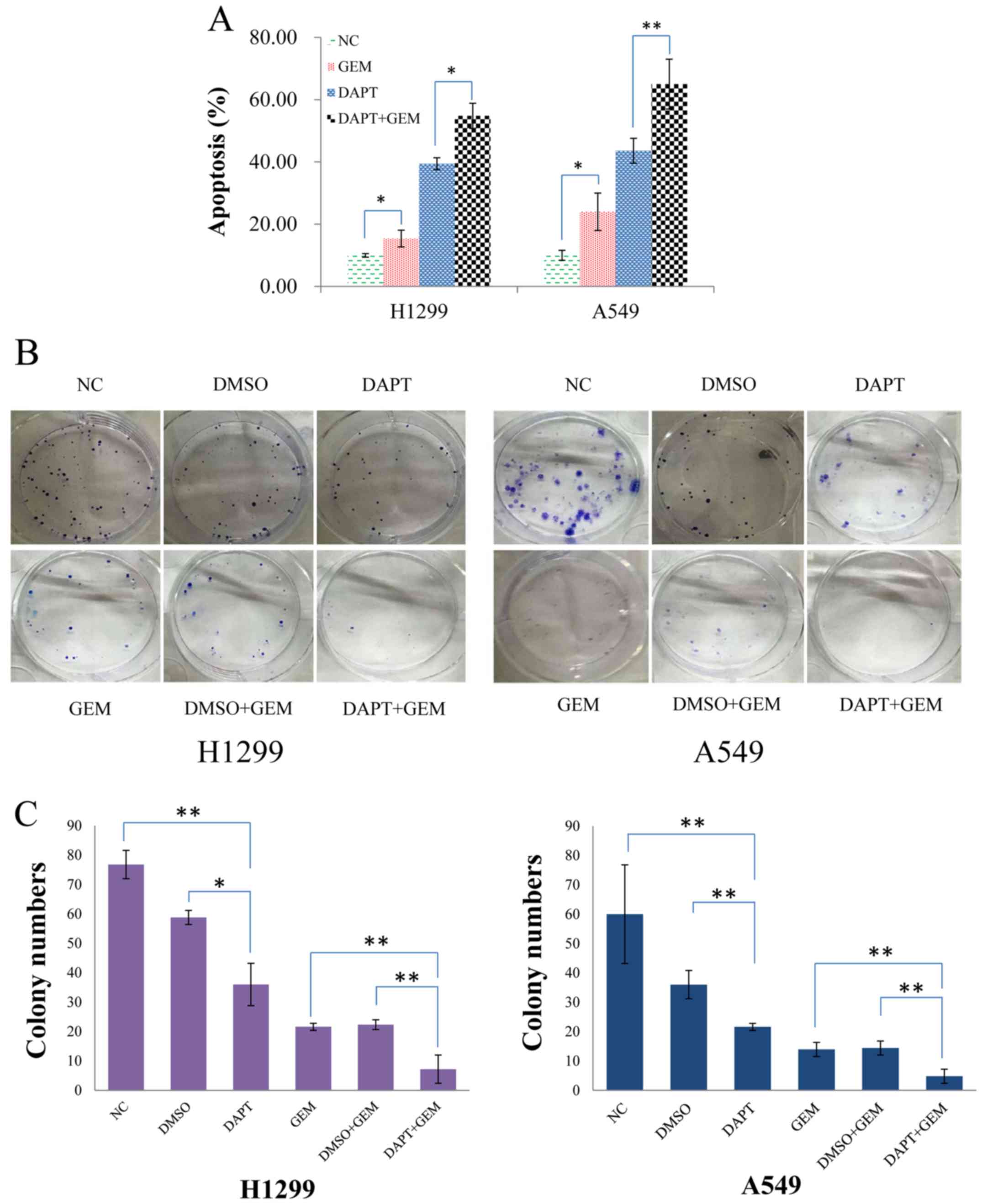
The percentage of cells undergoing apoptosis was
significantly increased in both the H1299 and A549 cells that were
treated with DAPT compared with no treatment (Fig. 4A). Moreover, when these two cells
lines were treated with DAPT + GEM vs. GEM alone, a marked increase
in the percentage of apoptotic cells was also observed (Fig. 4A). Correspondingly, the number of
colonies formed following treatment of the H1299 and A549 cells
with DAPT + GEM was markedly lower than the number of colonies
formed following the treatment of H1299 and A549 cells with GEM
alone (Fig. 4B and C). These
results suggest that DAPT is able to enhance the efficacy of GEM
and this positive impact is consistent with our hypothesis that
DAPT enhances the drug sensitivity of NSCLC cell lines, thereby
delaying or blocking GEM resistance.
Inhibition of DAPT specifically
increases lung cancer cell sensitivity to GEM
Cell proliferation was assayed for H1299 and A549
cells that were treated with and without DAPT and GEM individually
and in combination. An obvious effect of DAPT on cell proliferation
was observed in both sets of cells (Fig. 5A and B), while treatment with DAPT +
GEM significantly enhanced the effect of GEM on cell proliferation
(Fig. 5C and D). These results
revealed that DAPT positively promoted the pharmacological effects
of GEM.
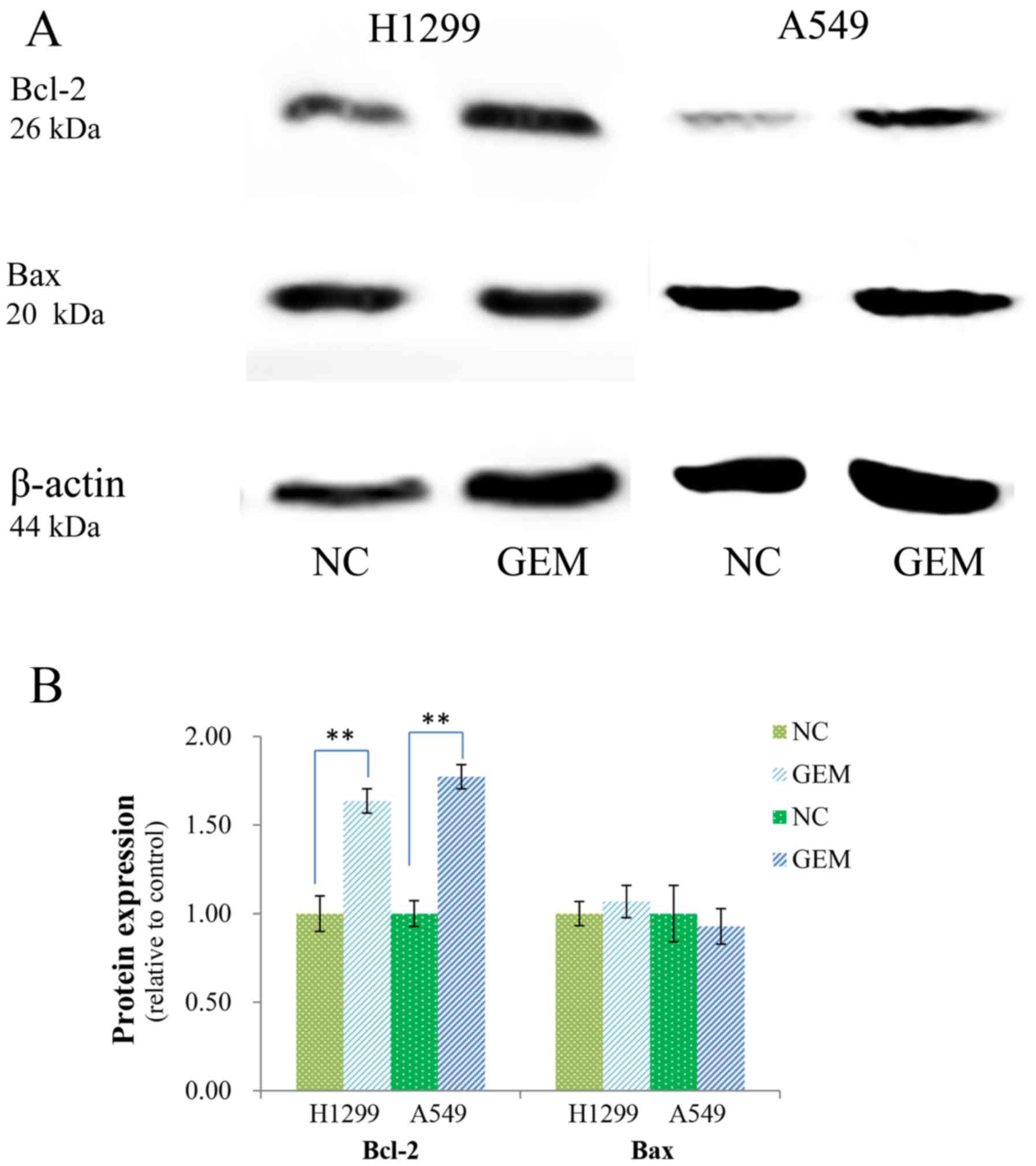
Role of apoptosis-related proteins in
DAPT-enhanced GEM sensitivity
It has been shown that Bcl-2 family proteins can be
regulated by direct interactions with Bax, Bak, Bcl-2, and Bcl-xL
to allow mitochondrial outer membrane permeabilization (MOMP) and
apoptosis to occur. Expression levels of Bcl-2 and Bax have also
been found to be related to GEM resistance. Following the treatment
of H1299 and A549 cells with GEM, Bcl-2 was found to be
upregulated, while expression of Bax exhibited no obvious changes
(Fig. 6A and B). However, when
these two cell lines were incubated with various concentrations of
DAPT (2, 5, 10 and 20 µM) for 48 h, expression of Bcl-2 decreased
in both cell lines, while expression of Bax increased (Fig. 6C-E).
Discussion
To date, there are many methods available that
provide inhibition of Notch signaling pathway activity. Various
approaches include the targeting of Notch ligands, Notch receptors,
ADAM-mediated cleavage of Notch, γ-secretase-mediated cleavage of
Notch, and specific targeting of Notch-3 (19). Targeting of Notch-3 by silencing RNA
(siRNA) has been reported and it is an important method. Moreover,
treatment with DAPT can decrease cleavage of Notch-3. Direct
comparisons of Notch-3-targeted siRNAs and DAPT treatments have
shown that both approaches can achieve a similar silencing effect,
although other aspects of the Notch signaling pathway appear to be
affected as well (23).
In the present study, the γ-secretase inhibitor,
DAPT, was selected to target Notch-3. Our results demonstrated that
DAPT treatment is able to effectively inhibit NICD3 expression at
the protein level and can significantly inhibit cell proliferation,
with higher concentrations associated with stronger inhibitory
effects. These results provide evidence that DAPT is able to
effectively inhibit Notch-3 secretion, and Notch-3 is a highly
important target for cancer treatment as demonstrated in previous
studies. Many articles have also reported that effective inhibition
of Notch-3 activity by DAPT results in a decrease in cell
proliferation (24). In
vivo, Tammam et al demonstrated that tumor growth was
inhibited in a mouse model that received systemic administration of
DAPT (22). In the present study,
DAPT inhibited the proliferation of cells overexpressing Notch-3,
it significantly decreased the percentage of cells undergoing
apoptosis, and the numbers of colonies formed were decreased. Thus,
Notch-3 appears to be important for cell viability by promoting
cell proliferation and inhibiting cell apoptosis. Specific
mechanisms involving the influence of Notch-3 on tumor biology have
been widely studied. Notch-3 has been found to regulate many
signaling pathways related to tumor development, as well as other
important proteins in cells, and to promote tumorigenesis or
inhibit tumor progression (25).
Moreover, in our recent clinical study, high levels of Notch-3
expression were detected in immunohistochemistry assays and this
was identified as a poor prognostic factor for NSCLC patients
regardless of treatment (26).
In the clinic, chemotherapeutic drugs and
molecular-targeted drugs (e.g. inhibitors of EGFR) are used to
treat NSCLC. However, only a few chemotherapeutic drugs are
available for long-term treatment of NSCLC due to the potential for
chemoresistance (27). Thus, a
combination of molecular-targeted drugs and chemotherapy drugs may
be important for the treatment of cancer. In several clinical
studies, chemoresistance of NSCLC was found to be related to the
overexpression of certain proteins, including EGFR, Notch and RIPK1
(28–31). It has been recognized that Notch-3
expression is related to the efficacy of GEM. Moreover, a role for
Notch-3 in tumor development has been established; indeed, high
levels of Notch-3 expression have been associated with resistance
to chemotherapy drugs in various types of cancers (23,32,33).
GEM is used in the clinic due to its structural similiarities with
deoxycytidine, a molecule which affects cells and promotes cell
apoptosis. In some clinical studies of patients with
cholangiocarcinoma, overexpression of Notch-3 was found to
potentially correlate with resistance to GEM-based chemotherapy and
poor survival (34). Consistent
with these data, preclinical studies have shown that Notch-3 is
related to GEM sensitivity in other tumors (30,35).
However, the mechanism responsible for GEM's ability to enhance
Notch-3 expression in specific cells remains unclear. Thus, the
role of Notch-3, as well as Notch1, in predicting GEM efficacy is
an active area of research. Meanwhile, it is apparent that Notch
family proteins affect the effectiveness of GEM for NSCLC and
enhancement of GEM-mediated resistance by Notch-3 overexpression
has been observed in other types of cancer, including pancreatic
cancer.
Previously, Aoki et al reported that poor
survival was associated with Notch-3 expression in cases of
extrahepatic cholangiocarcinoma (34). The results of the present study
confirm that cells with long-term exposure to GEM have enhanced
expression of Notch-3 and increased resistance to GEM. There are
multiple genes that are involved in mediating induced resistance
that results from continuous exposure to GEM. In the present study,
H1299 and A549 cells were exposed to GEM to induce overexpression
of Notch-3 and to avoid the controversy mentioned above. Our
results further confirmed that Notch-3 is a major determinant in
mediating the cytotoxic effect of GEM in NSCLC cells. Further
research is still needed to explain how GEM is able to enhance
expression of Notch-3 and suppress secretion of Notch-3 to improve
sensitivity to GEM chemotherapy. The results of previous studies
indicate that the process of apoptosis may be involved (36). The results of the present study are
consistent with this hypothesis, since GEM treatment was found to
significantly improve Notch-3 expression, and Bcl-2 expression was
also increased. Bcl-2 family proteins are major regulators of
apoptosis that primarily act in mitochondria where the
mitochondrial apoptotic pathway involves a caspase-9-dependent
caspase signaling cascade (37).
One component of this cascade, caspase-3, is usually activated by
caspase-9 and this leads to the cleavage and inactivation of key
cellular proteins such as PARP and DNA fragmentation factor
(38). Some members of the Bcl-2
family modulate the activation of caspases (39). For example, Bcl-2 inhibits the
release of cytochrome c by mitochondria, thereby preventing
cell death (40,41). Therefore, in the present study, we
focused on Bcl-2 in a preliminary investigation of a possible role
for apoptosis in GEM resistance. Based on the results obtained,
further in-depth studies of the mechanistic details involved will
be pursued.
Thus, further evidence is provided that the drug
resistance and sensitivity of GEM in relation to Notch-3 involve
apoptosis. Moreover, support for the inhibition of Notch-3 by DAPT
for the treatment of patients with NSCLC was demonstrated in the
present study, especially for patients presenting with resistance
to GEM. Furthermore, we demonstrated that DAPT could be used alone
or in combination with GEM.
In summary, Notch-3 is not only important for
regulating cell viability, but it is also an important target for
GEM-mediated antitumor effects. Therefore, Notch-3 may be a good
candidate for use in the development of new treatment strategies
for cancer patients who can undergo GEM-based chemotherapy and
overexpress Notch-3. Furthermore, based on the results obtained, a
prospective study and clinical treatment are under consideration,
with the latter including a combined treatment region of DAPT and
GEM for tumor patients with GEM-resistant and
Notch-3-overexpressing tumors.
Acknowledgements
Not applicable.
Funding
The present study was funded by grants from the
Scientific and Technological Programs of the Science and Technology
Department of Anhui Province (no. 1501041144) and the Natural
Science Foundation of Anhui Province (no. KJ2015A164).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author upon.
Authors' contributions
YBZ designed the research. BD and JG performed the
research and wrote the manuscript. All authors analyzed the data
and were involved in writing the manuscript. All authors read and
approved the manuscript and agree to be accountable for all aspects
of the research in ensuring that the accuracy or integrity of any
part of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The present study does not contain any studies
involving human participants or animals that were performed by any
of the authors.
Consent for publication
Not applicable.
Competing interests
The authors state that they have no competing
interests to report.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ridge CA, McErlean AM and Ginsberg MS:
Epidemiology of lung cancer. Semin Intervent Radiol. 30:93–98.
2015. View Article : Google Scholar
|
|
3
|
D'Addario G and Felip E: ESMO Guidelines
Working Group: Non-small-cell lung cancer: ESMO clinical
recommendations for diagnosis, treatment and follow-up. Ann Oncol.
20 Suppl 4:S68–S70. 2009. View Article : Google Scholar
|
|
4
|
Neal JW, Dahlberg SE, Wakelee HA, Aisner
SC, Bowden M, Huang Y, Carbone DP, Gerstner GJ, Lerner RE, Rubin
JL, et al: Erlotinib, cabozantinib, or erlotinib plus cabozantinib
as second-line or third-line treatment of patients with EGFR
wild-type advanced non-small-cell lung cancer (ECOG-ACRIN 1512): A
randomised, controlled, open-label, multicentre, phase 2 trial.
Lancet Oncol. 17:1661–1671. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reck M, Heigener DF, Mok T, Soria JC and
Rabe KF: Management of non-small-cell lung cancer: Recent
developments. Lancet. 382:709–719. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wong A, Soo RA, Yong WP and Innocenti F:
Clinical pharmacology and pharmacogenetics of gemcitabine. Drug
Metab Rev. 41:77–88. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dyawanapelly S, Kumar A and Chourasia MK:
Lessons learned from gemcitabine: Impact of therapeutic carrier
systems and gemcitabine's drug conjugates on cancer therapy. Crit
Rev Ther Drug Carrier Syst. 34:63–96. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nakano Y, Tanno S, Koizumi K, Nishikawa T,
Nakamura K, Minoguchi M, Izawa T, Mizukami Y, Okumura T and Kohgo
Y: Gemcitabine chemoresistance and molecular markers associated
with gemcitabine transport and metabolism in human pancreatic
cancer cells. Br J Cancer. 96:457–463. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vandana M and Sahoo SK: Synergistic
activity of combination therapy with PEGylated pemetrexed and
gemcitabine for an effective cancer treatment. Eur J Pharm
Biopharma. 94:83–93. 2015. View Article : Google Scholar
|
|
10
|
D'Amato G, Luxán G and de la Pompa JL:
Notch signalling in ventricular chamber development and
cardiomyopathy. FEBS J. 283:4223–4237. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takebe N, Nguyen D and Yang SX: Targeting
notch signaling pathway in cancer: Clinical development advances
and challenges. Pharmacol Ther. 141:140–149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dang TP, Gazdar AF, Virmani AK, Sepetavec
T, Hande KR, Minna JD, Roberts JR and Carbone DP: Chromosome 19
translocation, overexpression of Notch-3, and human lung cancer. J
Natl Cancer Inst. 92:1355–1357. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ye YZ, Zhang ZH, Fan XY, Xu XL, Chen ML,
Chang BW and Zhang YB: Notch-3 overexpression associates with poor
prognosis in human non-small-cell lung cancer. Med Oncol.
30:5952013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin L, Mernaugh R, Yi F, Blum D, Carbone
DP and Dang TP: Targeting specific regions of the Notch-3
ligand-binding domain induces apoptosis and inhibits tumor growth
in lung cancer. Cancer Res. 70:632–638. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi C, Qian J, Ma M, Zhang Y and Han B:
Notch 3 protein, not its gene polymorphism, is associated with the
chemotherapy response and prognosis of advanced NSCLC patients.
Cell Physiol Biochem. 34:743–752. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rahman MT, Nakayama K, Rahman M, Katagiri
H, Katagiri A, Ishibashi T, Ishikawa M, Iida K, Nakayama S, Otsuki
Y and Miyazaki K: Notch-3 overexpression as potential therapeutic
target in advanced stage chemoresistant ovarian cancer. Am J Clin
Pathol. 138:535–544. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Groeneweg JW, DiGloria CM, Yuan J,
Richardson WS, Growdon WB, Sathyanarayanan S, Foster R and Rueda
BR: Inhibition of notch signaling in combination with Paclitaxel
reduces platinum-resistant ovarian tumor growth. Front Oncol.
4:1712014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Groth C and Fortini ME: Therapeutic
approaches to modulating notch signaling: Current challenges and
future prospects. Semin Cell Dev Biol. 23:465–472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Konishi J, Kawaguchi KS, Vo H, Haruki N,
Gonzalez A, Carbone DP and Dang TP: Gamma-secretase inhibitor
prevents Notch-3 activation and reduces proliferation in human lung
cancers. Cancer Res. 67:8051–8057. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Patrad E, Niapour A, Farassati F and Amani
M: Combination treatment of all-trans retinoic acid (ATRA) and
γ-secretase inhibitor (DAPT) cause growth inhibition and apoptosis
induction in the human gastric cancer cell line. Cytotechnology.
70:865–877. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang J, Miao Y, Xiao S, Zhang Z and Hu Z:
DAPT in the control of human hair follicle stem cell proliferation
and differentiation. Postepy Dermatol Alergol. 31:201–206. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tammam J, Ware C, Efferson C, O'Neil J,
Rao S, Qu X, Gorenstein J, Angagaw M, Kim H, Kenific C, et al:
Down-regulation of the Notch pathway mediated by a gamma-secretase
inhibitor induces anti-tumour effects in mouse models of T-cell
leukaemia. Br J Pharmacol. 158:1183–1195. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kang H, Jeong JY, Song JY, Kim TH, Kim G,
Huh JH, Kwon AY, Jung SG and An HJ: Notch-3-specific inhibition
using sirna knockdown or gsi sensitizes paclitaxel-resistant
ovarian cancer cells. Mol Carcinog. 55:1196–1209. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tolcher AW, Messersmith WA, Mikulski SM,
Papadopoulos KP, Kwak EL, Gibbon DG, Patnaik A, Falchook GS, Dasari
A, Shapiro GI, et al: Phase I study of RO4929097, a gamma secretase
inhibitor of Notch signaling, in patients with refractory
metastatic or locally advanced solid tumors. J Clin Oncol.
30:2348–2353. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Roy M, Pear WS and Aster JC: The
multifaceted role of Notch in cancer. Curr Opin Genet Dev.
17:52–59. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yuan X, Wu H, Xu H, Xiong H, Chu Q, Yu S,
Wu GS and Wu K: Notch signaling: An emerging therapeutic target for
cancer treatment. Cancer Lett. 369:20–27. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cheng B, Cai XJ, Chen LY, Wang Z, Weng L
and Li QL: Predictors and impact of cytotoxic second-line
chemotherapy for stage IIIa-IV nonsmall lung cancer patients in
China: A retrospective institution analysis of 132 patients. J
Cancer Res There. 1 Suppl 1:C84–C88. 2015.
|
|
28
|
Lin JJ and Shaw AT: Resisting resistance:
Targeted therapies in lung cancer. Trends Cancer. 2:350–364. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Eto K, Kawakami H, Kuwatani M, Kudo T, Abe
Y, Kawahata S, Takasawa A, Fukuoka M, Matsuno Y, Asaka M, et al:
Human equilibrative nucleoside transporter 1 and Notch-3 can
predict gemcitabine effects in patients with unresectable
pancreatic cancer. Br J Cancer. 108:1488–1494. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tokunaga Y, Liu D, Nakano J, Zhang X, Nii
K, Go T, Huang CL and Yokomise H: Potent effect of adenoviral
vector expressing short hairpin RNA targeting ribonucleotide
reductase large subunit M1 on cell viability and chemotherapeutic
sensitivity to gemcitabine in non-small cell lung cancer cells. Eur
J Cancer. 51:2480–2489. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li B, Yao Z, Wan Y and Lin D:
Overexpression of OCT4 is associated with gefitinib resistance in
non-small cell lung cancer. Oncotarget. 7:77342–77347.
2016.PubMed/NCBI
|
|
32
|
Park JT, Chen X, Trope CG, Davidson B,
Shih IeM and Wang TL: Notch-3 overexpression is related to the
recurrence of ovarian cancer and confers resistance to carboplatin.
Am J Pathol. 177:1087–1094. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
McAuliffe SM, Morgan SL, Wyant GA, Tran
LT, Muto KW, Chen YS, Chin KT, Partridge JC, Poole BB, Cheng KH, et
al: Targeting Notch, a key pathway for ovarian cancer stem cells,
sensitizes tumors to platinum therapy. Proc Natl Acad Sci USA.
109:E2939–E2948. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Aoki S, Mizuma M, Takahashi Y, Haji Y,
Okada R, Abe T, Karasawa H, Tamai K, Okada T, Morikawa T, et al:
Aberrant activation of Notch signaling in extrahepatic
cholangiocarcinoma: Clinicopathological features and therapeutic
potential for cancer stem cell-like properties. BMC Cancer.
16:8542016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Du X, Zhao YP, Zhang TP, Zhou L, Chen G,
Wang TX, You L and Shu H: Arch Med Res. Alteration of the intrinsic
apoptosis pathway is involved in Notch-induced chemoresistance to
gemcitabine in pancreatic cancer. Arch Med Res. 45:15–20. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yao J and Qian C: Inhibition of Notch-3
enhances sensitivity to gemcitabine in pancreatic cancer through an
inactivation of PI3K/Akt-dependent pathway. Med oncol.
27:1017–1022. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gross A: BCL-2 family proteins as
regulators of mitochondria metabolism. Biochim Biophys Acta.
1857:1243–1246. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jia SS, Xi GP, Zhang M, Chen YB, Lei B,
Dong XS and Yang YM: Induction of apoptosis by D-limonene is
mediated by inactivation of Akt in LS174T human colon cancer cells.
Oncol Rep. 29:349–354. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang HB, Lu P, Guo QY, Zhang ZH and Meng
XY: Baicalein induces apoptosis in esophageal squamous cell
carcinoma cells through modulation of the PI3K/Akt pathway. Oncol
Lett. 5:722–728. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang X, Wang S, Mu Y and Zheng Y:
Schisandrin B inhibits cell proliferation and induces apoptosis in
human cholangiocarcinoma cells. Oncol Rep. 36:1799–1806. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ferreira Cda S, Maganhin CC, Rdos Simões
S, Girão MJ, Baracat EC and Soares JM Jr: Melatonin: Cell death
modulator. Rev Assoc Med Bras. 56:715–718. 2010.(In Portuguese).
View Article : Google Scholar : PubMed/NCBI
|