Introduction
Colorectal cancer (CRC) is the third most common
type of cancer, comprising 9.7% of all cancer cases, and the fourth
most common cause of mortalities due to cancer globally (8.5% of
all cancer mortalities), accounting for ~1.4 million new cases and
697,000 mortalities per year (1).
The incidence of CRC increases with age; the median age at the time
of diagnosis for CRC is 68 for men and 72 for women (2). The 5-year survival rate for CRC ranges
from 50% in developing countries to 65% in developed countries
(3). The treatment of CRC depends
on the location of the tumor and the disease stage at diagnosis.
For early-stage CRC, surgery alone can eliminate cancer (4). For late-stage CRC, chemotherapy is
used alone or in combination with radiotherapy to treat metastases
(5). The metastasis of CRC is a
critical indicator of survival rate. The 5-year survival rate is
>90% if CRC is diagnosed at an early stage (6). If the tumor has spread to the adjacent
lymph nodes, the 5-year survival rate is <70%. Nevertheless, if
the tumor has metastasized to distant organs, the 5-year survival
rate is 11.7% (2). The clinical
outcome for patients with CRC is far from satisfactory,
particularly for patients with advanced cancer (stage III or IV).
Therefore, the identification of molecular markers for tumor
progression and metastasis, which may also be targets for
therapeutic intervention, is urgently required for personalized and
accurate treatment and diagnosis.
Lysyl oxidase-like 2 (LOXL2) is a member of the
lysyl oxidase family of proteins, which includes lysine oxidase
(LOX) and four lysyl oxidase-like proteins (LOXLl, LOXL2, LOXL3 and
LOXL4) (7). The LOX family is
crucial for the formation of connective tissues. The proteins in
the LOX family of are extracellular copper-dependent amine oxidases
that catalyze the first step in the formation of collagen-elastin
crosslinks. Numerous studies have implicated LOXL2 in fibrotic
diseases (8,9). A number of studies have also
demonstrated an association between LOXL2 and cancer progression.
LOXL2 is upregulated in many types of cancer and is associated with
a poor outcome (10). LOXL2
expression is also positively associated with cancer metastasis due
to its role in the formation of covalent collagen-elastin
crosslinks in the extracellular matrix (ECM) (11–14).
In a previous study by the authors, the differences
in the mRNA expression profile for 8 CRC tumor samples compared
with paired normal mucosa were determined using microarray analysis
(15). LOXL2 expression was
upregulated in the CRC tissue samples compared with the paired
normal tissues. In the present study, the intracellular expression
of LOXL2 protein in CRC tissues compared with corresponding normal
tissues was examined, and the potential associations with the
disease clinicopathological features and prognosis were analyzed.
The mechanism of action for the effect of LOXL2 in CRC cell lines
was also evaluated.
Materials and methods
Collection of CRC specimens
A total of 228 human CRC and matched normal samples
were collected by biopsy from the Department of Colorectal Surgery,
Xinhua Hospital, Shanghai Jiaotong University School of Medicine
(Shanghai, China). Informed consent was obtained for the collection
of all specimens, and the present study was approved by the
institutional review board. These experiments were conducted in
accordance with the ethical standards of the Helsinki Declaration
of 1964 and its subsequent amendments or comparable ethical
standards. A total of 40 CRC samples were analyzed by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR).
Tissue microarrays were prepared from 228 CRC specimens and
subjected to immunohistochemical analysis.
Cell culture
A total of 6 human CRC cell lines, including SW480,
SW620, HT29, HCT116, DLD1 and LOVO cells, and 293T cells for
luciferase reporter assays, were cultured in Dulbecco's modified
Eagle's medium (Corning Life Sciences, Tewksbury, MA, USA) with 10%
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA). The cells were cultured in a 5% CO2 humidified
atmosphere at 37°C and passaged every 3–4 days.
RNA isolation and RT-qPCR
Total RNA was extracted with RNAiso (Takara
Biotechnology Co., Ltd., Dalian, China), and the
PrimeScript® RT-PCR kit (Takara Biotechnology Co., Ltd.)
was used to generate cDNA. qPCR was performed using an ABI 7500
cycler (Thermo Fisher Scientific, Inc.) and SYBR® Premix
Ex Taq™ (Takara Biotechnology Co., Ltd.). ACTB served as an
internal control. The primers used are as follows: LOXL2-F,
5′-CCTGTCTTCGGGCTGATG-3′ and LOXL2-R, 5′-CACTGCGGATCCCTGAAAC-3′;
ACTB-F, 5′-GTTGTCGACGACGAGCG-3′ and ACTB-R,
5′-GCACAGAGCCTCGCCTT-3′. The Thermal Cycler Dice system (Takara
Biotechnology Co., Ltd.) was used for qPCR with the following
conditions: 95°C for 5 min, 50 cycles of 95°C for 5 sec and 60°C
for 10 sec. Each value was normalized to the levels of ACTB. The
PCR reaction was performed in triplicate, and the relative gene
expression was analyzed by the 2−∆∆Cq method (16).
Immunohistochemistry
The paraffin sections were deparaffinized,
rehydrated and treated according to the standard protocol.
Following incubation with a monoclonal anti-human LOXL2 antibody
(dilution, 1:1,000; catalog no., ab179810, Abcam, Cambridge, MA,
UK) or phosphate-buffered saline (PBS; negative control) overnight,
the sections were washed three times with PBS and incubated with a
horseradish peroxidase-labeled secondary antibody (dilution,
1:1,000; catalog no., GK500710; Gene Company Ltd., Shanghai, China)
for 30 min at room temperature. The sections were stained with
3′,3-diaminobenzidine solution after washing in PBS. The sections
were then counterstained with 0.1% hematoxylin and sealed with
coverslips. LOXL2 staining was graded according to staining
intensity and staining rate. The criteria for staining intensity
are as follows: 0, no staining; 1, mild staining; 2, moderate
staining or 3, strongly positive. The criteria for staining rate
are as follows: 1, 0–25% positive cells; 2, 26–50%; 3, 51–75% or 4,
>75%, as previously described (17). For each section, a semi-quantitative
score was calculated by multiplying these two values (from 0 to
12). A total of 2 histopathologists blinded to the clinical data
were assigned to review and score the slides.
Immunoblotting
Immunoblotting was performed as previously described
(18). The proteins were separated
by 10% SDS-PAGE polyacrylamide gel electrophoresis and transferred
to a nitrocellulose membrane. The membranes were blocked with 5%
skimmed milk in PBS buffer for 1 h at room temperature then
incubated overnight at 4°C with the primary antibodies, including
anti-LOXL2 (dilution, 1:500; catalog no., ab179810, Abcam);
anti-vimentin (dilution, 1:1,000; catalog no., ab92547, Abcam);
anti-CCNE1 (dilution, 1:1000; catalog no., sc-377100, Santa Cruz
Biotechnology, Inc., Dallas, TX, USA); anti-CDK2 (cyclin dependent
kinase 2; dilution, 1:1,000; catalog no., 2546, Cell Signaling
Technology, Inc., Danvers, MA, USA); anti-cleaved poly(ADP-ribose)
polymerase (PARP; dilution, 1:5,000; catalog no., ab32064, Abcam);
and anti-ACTB (dilution, 1:20,000; catalog no., A3854,
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). After washing with
TBST for 3 times (10 min for each wash), the membranes were
incubated with a horseradish peroxidase-labeled secondary antibody
(HRP-labeled goat anti-rabbit IgG (H+L), catalog no., A0208 or
HRP-labeled goat anti-mouse IgG (H+L), catalog no., A0216,
dilution, 1:1,000; Beyotime Institute of Biotechnology, Haimen,
China) for 1 h at room temperature and then visualized with a
chemiluminescence reagent (EMD Millipore, Billerica, MA, USA).
RNA interference
For the shRNA-mediated knockdown of LOXL2 induced by
doxycycline (DOX), oligonucleotides encoding LOXL2 targeting shRNAs
and their complement were synthesized, including LOXL2-sh1,
5′-CCGGACAATACCAAAGTGTACAACTCGAGTTGTACACTTTGGTATTGTTTTTTG-3′ and
LOXL2-sh2,
5′-CCGGCGATTACTCCAACAACATCATCTCGAGATGATGTTGTTGGAGTAATCGTTTTTG-3′.
The sequence published by Sigma-Aldrich (Merck KGaA) was referred
to. After transfection for 8 h in 293T cells (American Type Culture
Collection, Manassas, VA, USA), the cell culture medium was changed
to normal DMEM high glucose medium containing fetal bovine serum
and penicillin-streptomycin. Viral particles were harvested after
48 h of transfection. HCT116 and DLD1 were incubated with viral
particles for 8 h. The viral titer was determined by positive count
of green fluorescent protein. The cells that were not infected
would be killed by puromycin. The 4 pmol oligonucleotide sense and
antisense pairs were inserted into the lentiviral expression
system, pLKO-TET-ON vector (20 ng; Addgene, Inc., Cambridge, MA,
USA) (19). The cells stably
expressing shRNA induced by DOX were cultured in
puromycin-containing (1 µg/ml) medium. The cells cultured without
DOX were used for control. The incubation of the cells for 48 h at
37°C with 500 ng/ml DOX induced the knockdown of LOXL2.
Cell proliferation assay
Cell growth was measured using a Cell Counting Kit-8
(CCK-8 kit; Dojindo Molecular Technologies, Inc., Kumamoto, Japan),
which detects the dehydrogenase activity of the viable cells.
Briefly, the cells (2×103/well) were seeded in 96-well
plates of 5 repeats and incubated for 24 h at 37°C. The cells
cultured without DOX were used for control. The incubation of the
cells for 48 h at 37°C with 500 ng/ml DOX induced the knockdown of
LOXL2. CCK-8 solution (10 µl) was added to each well and incubated
at 37°C for a further 1 h in an incubator. The absorbance at 450 nm
was measured using a microplate reader. In addition, a colony
formation assay was performed. A total of 1×103 cells
were seeded in 6-well plates and incubated at 37°C for 2 weeks.
After incubation, the cells were fixed with 100% methanol and
stained with 0.1% crystal violet for 10 min at room temperature.
The cells were observed under an inverted microscope, and images
were captured in a vertical field of view. The experiment was
performed in triplicate.
Cell cycle analysis
The cells were treated with 70% ice-cold ethanol
overnight. The cells were treated with propidium iodide (PI; 20
µg/ml, catalog no., 550825; BD Biosciences, San Jose, CA, USA) at
4°C in the dark for 30 min. A flow cytometer (Beckman Coulter,
Inc., Brea, CA, USA) was used for analyzing the DNA content for
cell cycle analysis. The software CytExpert (version no., 1.2.11.0,
Beckman Coulter, Inc., Suzhou, Jiangsu, China) was used for
anlaysis. The experiment was performed in triplicate.
Detection of apoptosis
The detection of the rate of apoptosis was performed
using an FITC Annexin V Apoptosis Detection kit (BD Pharmingen; BD
Biosciences). The cells were trypsinized, washed in PBS and
resuspended in 1X binding buffer at a density of 106
cells/ml. A total of 100 µl of the suspension was transferred to a
5 ml culture tube. FITC-Annexin V (5 µl) and PI (5 µl) were added
to the tube. The samples were vortexed for 15 min at 25°C in the
dark. After incubation, 400 µl 1X binding buffer was added to each
tube. The rate of apoptosis was determined by CytExpert (version
no., 1.2.11.0, Beckman Coulter, Inc., Suzhou, Jiangsu, China) using
flow cytometry (Beckman Coulter, Inc.) within 1 h.
Wound-healing assay, Transwell and
luciferase assays
For the wound-healing assays, the cells
(106) were cultured in complete medium [Dulbecco's
modified Eagle's medium (Corning Life Sciences) with 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.)] and seeded in
6-well plates. After 8 h, the medium was changed to a low-serum
medium (Dulbecco's modified Eagle's medium with 1% fetal bovine
serum). A wound was produced in the cell monolayer, and the cells
were cultured for an additional 72 h at 37°C. Images were acquired
immediately after wound scratching and at 72 h. For Transwell
assay, the cells (105) were seeded in a growth
factor-deficient upper chamber. Complete medium was added to the
lower chamber. Cell migration was analyzed after 48 h as previously
described (20). Luciferase assays
were performed as previously described (21). Briefly, 200 ng vimentin-Luc reporter
plasmid was co-transfected with pGL3 basic plasmid (200 ng) or
pGL3-LOXL2 plasmid (200 ng) into 293T cells along with
Renilla luciferase plasmid (20 ng; Promega, Madison, WI,
USA).
Xenograft tumor formation and lung
metastasis mouse model
A total of 10 male nude mice (4–6 weeks old; weight,
~20 g) were obtained from SLAC Lab Animal (Shanghai SLAC Laboratory
Animal Co., Ltd., Shanghai, China). The animal care and use
committees of Xinhua Hospital approved all mouse procedures. To
establish xenograft tumors, shLOXL2 TET-ON DLD1 cells
(106) were subcutaneously injected into both axillary
fat pads. The mice were randomized into two groups (5 mice/group)
and received either normal water (control group) or water
containing 1 mg/ml DOX for 4 weeks. Water was renewed every other
day. The growth of the tumors was measured 4 weeks later by
sacrificing the mice and determining the weight of the tumors. To
establish a lung metastasis model, DLD1 cells (106) were
injected into the tail veins of 12 mice (n=6 per group), which then
received the same treatments as described above. After 8 weeks, the
mice were sacrificed, and both lungs were resected and imaged. The
lung tissue sections were stained with hematoxylin and eosin for 5
min at room temperature, and lung metastases were observed under a
microscope by a pathologist who was blinded to the treatment status
of the mice. The animal experiments were approved by the Xinhua
hospital review board.
Statistical analysis
Statistical analysis was performed using SPSS
software (version 18; SPSS, Inc., Chicago, IL, USA). Student's
t-test was used to compare continuous variables. Pearson's
χ2 test was used to assess the association between the
expression of LOXL2 and clinicopathological parameters. Survival
curves were drawn using the Kaplan-Meier method and compared using
the log-rank test. Univariate and multivariate analyses were
performed using the Cox proportional hazards regression model. A
two-tailed P-value of 0.05 was considered statistically
significant.
Results
Upregulation of LOXL2 in CRC cells and
its prognostic value for patients with CRC
LOXL2 was identified as an upregulated gene in CRC
by a mRNA microarray in a previous study by the authors
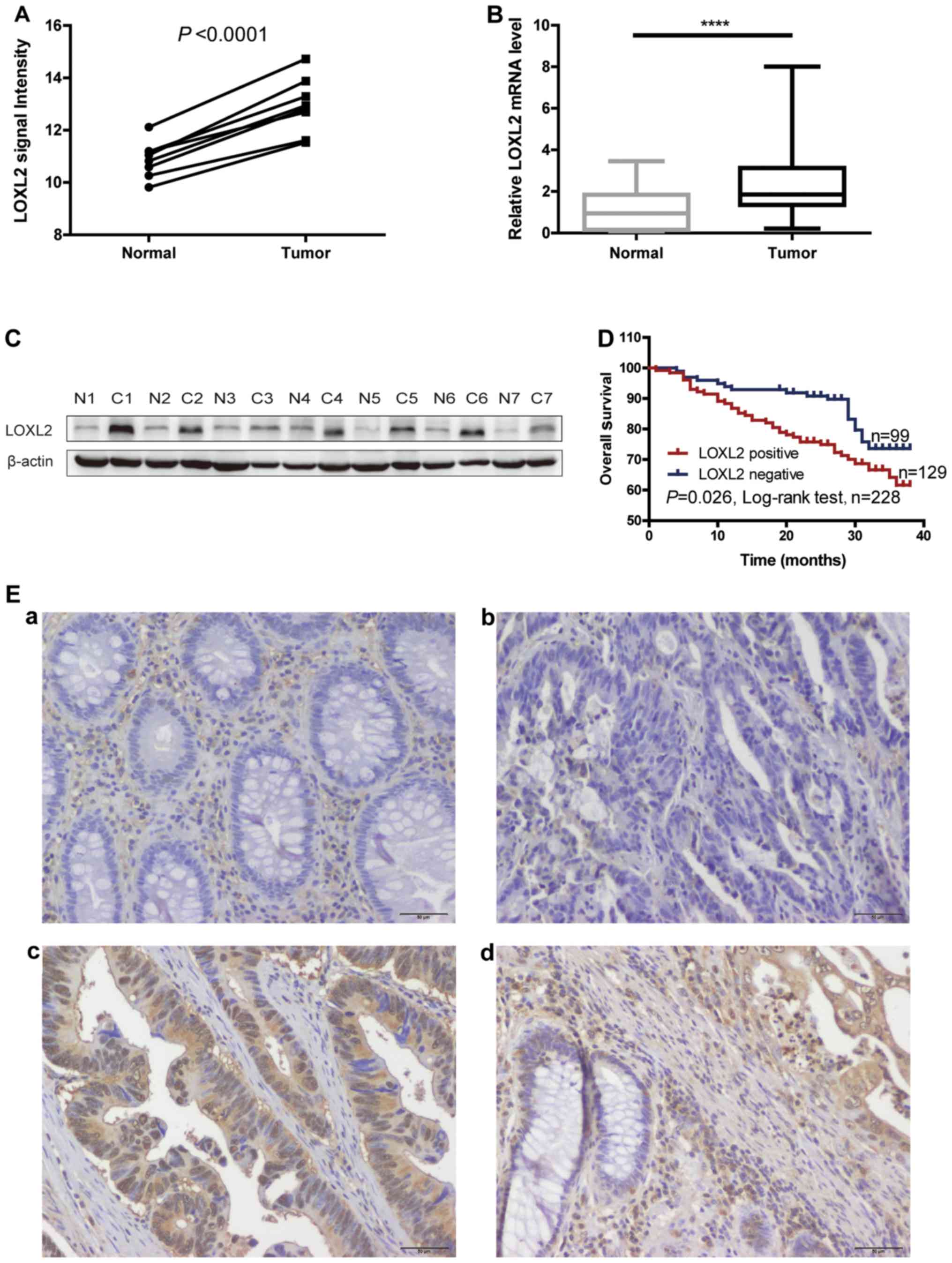
(P<0.0001; Fig. 1A) (15). Therefore, in the present study, the
microarray results were validated. RT-qPCR was performed to detect
the level of LOXL2 mRNA expression in 40 paired CRC and normal
adjacent tissues. The level of LOXL2 mRNA expression was
significantly elevated in CRC tissues compared with normal tissues
(P<0.0001; Fig. 1B). Western
blot analysis was performed on 7 paired CRC and normal adjacent
tissues, and observed that the level of LOXL2 protein was also
elevated in CRC tissues compared with normal colorectal tissues
(Fig. 1C).
Immunohistochemistry was next performed in 228 CRC
tissues and adjacent normal colon tissues. Representative images
are shown in Fig. 1E. Low LOXL2
expression was detected in the epithelial cells of normal tissues
(Fig. 1Ea), while a low to high
LOXL2 expression was identified in the epithelial cells of tumor
tissues (Fig. 1Eb and c). LOXL2
expression was observed not only in the ECM but also in the CRC
cells. Notably, in a considerable number of LOXL2-positive cells,
LOXL2 was localized to the cell nucleus (Fig. 1Ec). The expression pattern of LOXL2
was different between normal and CRC tissues (Fig. 1Ed). The positive expression ratio
for LOXL2 in epithelial cells was 56.6% (129/228) in CRC tissues
and 7.89% (18/228) in normal tissues.
The association between LOXL2 protein expression and
pathological features, including sex, age, tumor size, T, N and M
classifications, tumor-node metastasis (TNM) stage and tumor
differentiation, were analyzed in patients with CRC (Table I). The analysis revealed that a high
LOXL2 protein expression was associated with TNM stage (P=0.001), N
classification (P=0.006) and M classification (P=0.032), but there
was no significant association with age, sex, tumor diameter, T
classification or differentiation.
 | Table I.Association of LOXL2 epithelial/tumor
cell staining with the pathological and clinical features of
patients with colorectal cancer. |
Table I.
Association of LOXL2 epithelial/tumor
cell staining with the pathological and clinical features of
patients with colorectal cancer.
|
| LOXL2
epithelial/tumor cell staining |
|
|---|
|
|
|
|
|---|
| Variables | All cases
(n=228) | Absent, n (%)
(n=100) | Positive, n (%)
(n=128) | P-values |
|---|
| Age,
yearsc |
|
|
| 0.930a |
|
≤63 | 119 | 52 (43.7) | 67 (56.3) |
|
|
>63 | 109 | 47 (43.1) | 62 (56.9) |
|
| Sex |
|
|
| 0.978a |
|
Male | 108 | 47 (43.5) | 61 (56.5) |
|
|
Female | 120 | 52 (43.3) | 68 (56.7) |
|
| Tumor size
(cm) |
|
|
| 0.230a |
| ≤5
cm | 98 | 47 (48.0) | 51 (52.0) |
|
| >5
cm | 130 | 52 (40.0) | 78 (60.0) |
|
| TNM stage |
|
|
|
<0.001b |
| I | 42 | 30 (71.4) | 12 (28.6) |
|
| II | 69 | 29 (42.0) | 40 (58.0) |
|
|
III | 75 | 28 (37.3) | 47 (62.7) |
|
| IV | 42 | 12 (28.6) | 30 (71.4) |
|
| T stage |
|
|
| 0.188b |
| I | 4 | 2
(50.0) | 2 (50.0) |
|
| II | 53 | 28 (52.8) | 25 (47.2) |
|
|
III | 66 | 22 (33.3) | 44 (66.7) |
|
| IV | 105 | 47 (44.8) | 58 (55.2) |
|
| Lymphatic
metastasis |
|
|
| 0.006b |
|
N0 | 117 | 61 (52.1) | 56 (47.9) |
|
|
N1 | 65 | 18 (27.7) | 47 (72.3) |
|
|
N2 | 46 | 20 (43.5) | 26 (56.5) |
|
| Distal
metastasis |
|
|
| 0.032a |
|
M0 | 186 | 87 (46.8) | 99 (53.2) |
|
|
M1 | 42 | 12 (28.6) | 30 (71.4) |
|
|
Differentiation |
|
|
| 0.623b |
|
Well | 31 | 15 (48.4) | 16 (51.6) |
|
|
Moderate | 190 | 82 (43.2) | 108 (56.8) |
|
|
Poor | 7 | 2
(28.6) | 5
(71.4) |
|
Next, the Kaplan-Meier method was used to
investigate the significance of tumor LOXL2 expression in survival
prognosis. As indicated in Fig. 1D,
log-rank tests revealed that LOXL2 expression was significantly
associated with shortened patient survival time (P=0.026). In
addition, univariate and multivariate Cox regression hazard
analyses indicated that LOXL2 expression was an independent
prognostic indicator for CRC (Table
II). These data indicated that LOXL2 might be an appropriate
marker for predicting the prognosis of patients with CRC.
 | Table II.Univariate and multivariable OS
analyses for LOXL2 expression in CRC cells in patients with
CRC. |
Table II.
Univariate and multivariable OS
analyses for LOXL2 expression in CRC cells in patients with
CRC.
|
| OS |
|
|---|
|
|
|
|
|---|
| Parameters | HR (95% CI) | P-value | n
(events) |
|---|
| Univariate |
|
Negative LOXL2 staining | 1 |
| 99
(19) |
|
Positive LOXL2 staining | 1.832
(1.063–3.159) | 0.029a | 129 (41) |
| Multivariable |
|
Positive LOXL2 staining | 1.830
(1.032–3.244) | 0.039a |
|
|
Sex | 0.663
(0.383–1.149) | 0.143 |
|
|
Age | 1.626
(0.963–2.747) | 0.069 |
|
| TNM
stage | 0.494
(0.246–0.991) | 0.047a |
|
| T
stage | 1.417
(0.945–2.125) | 0.092 |
|
| N
stage | 1.729
(1.080–2.769) | 0.023a |
|
| M
stage | 10.822
(3.419–34.249) |
<0.0001a |
|
|
Grade | 1.021
(0.496–2.104) | 0.954 |
|
LOXL2 knockdown impairs the
proliferation of CRC cells in vitro
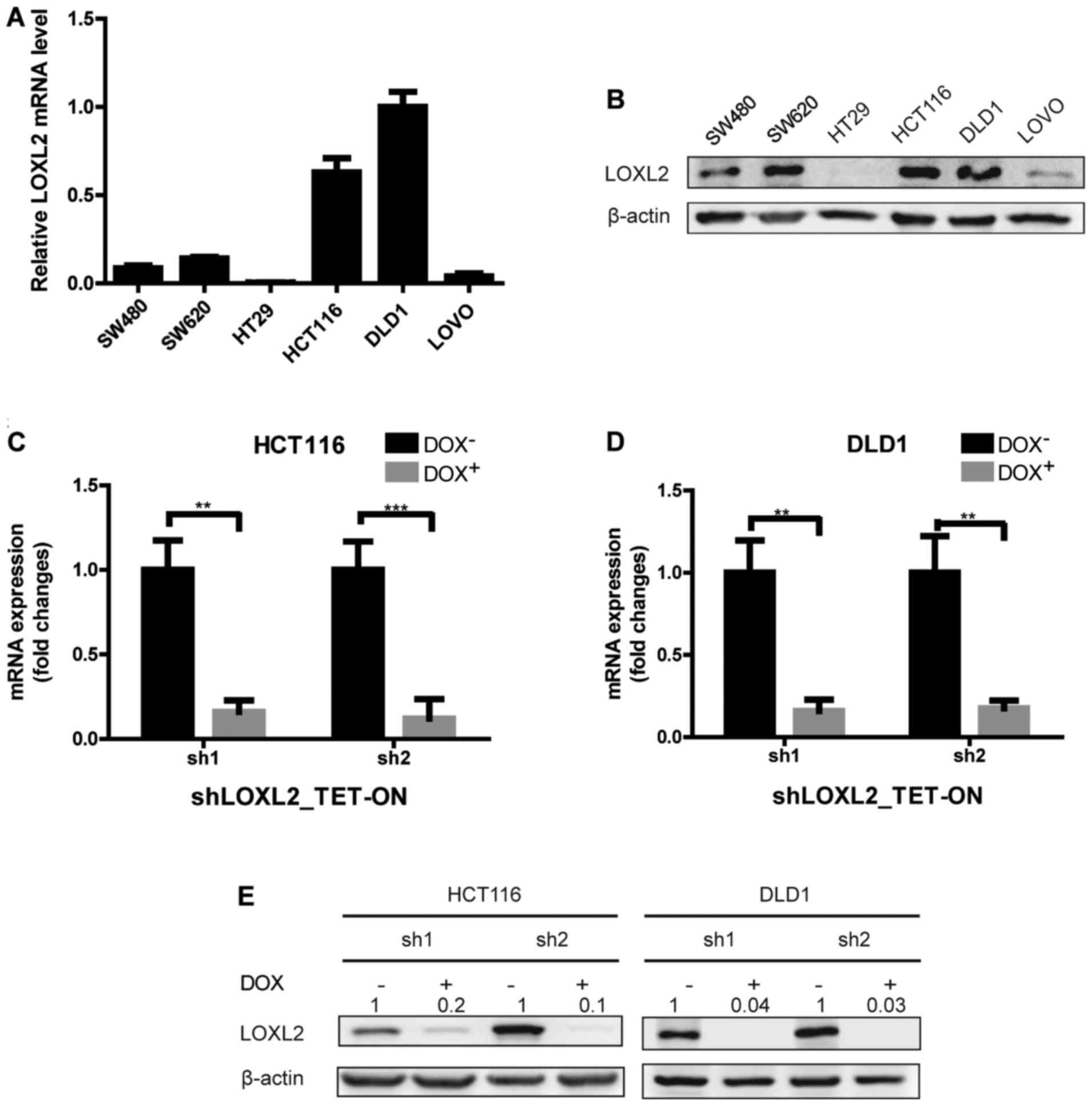
To further determine the expression of LOXL2 in CRC
epithelial cells, LOXL2 mRNA and protein levels were examined in 6
CRC cell lines (Fig. 2A and B).
Among the 6 cell lines, HCT116 and DLD1 cells expressed relatively
higher levels of LOXL2 mRNA and protein, and were therefore
selected for further analysis. To examine the function of LOXL2,
LOXL2 knockdown cells were generated by infecting HCT116 and DLD1
with two TET-ON pLKO lentiviruses that encode LOXL2-targeting
shRNAs (shRNA1 and shRNA2). Stable lines were selected, and the
LOXL2 knockdown efficiency after 48 h of DOX treatment was
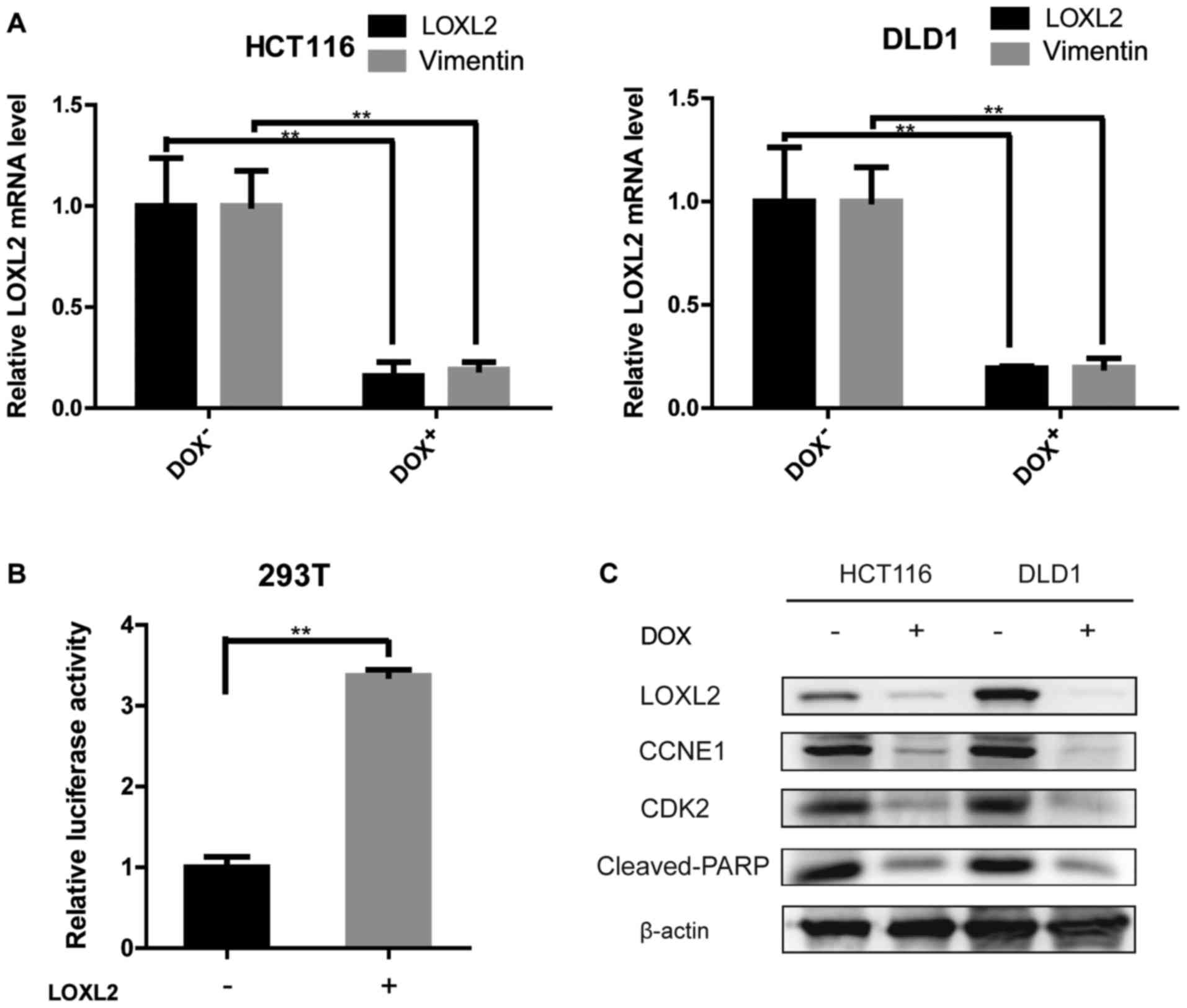
confirmed by qPCR and immunoblotting (Fig. 2C-E).
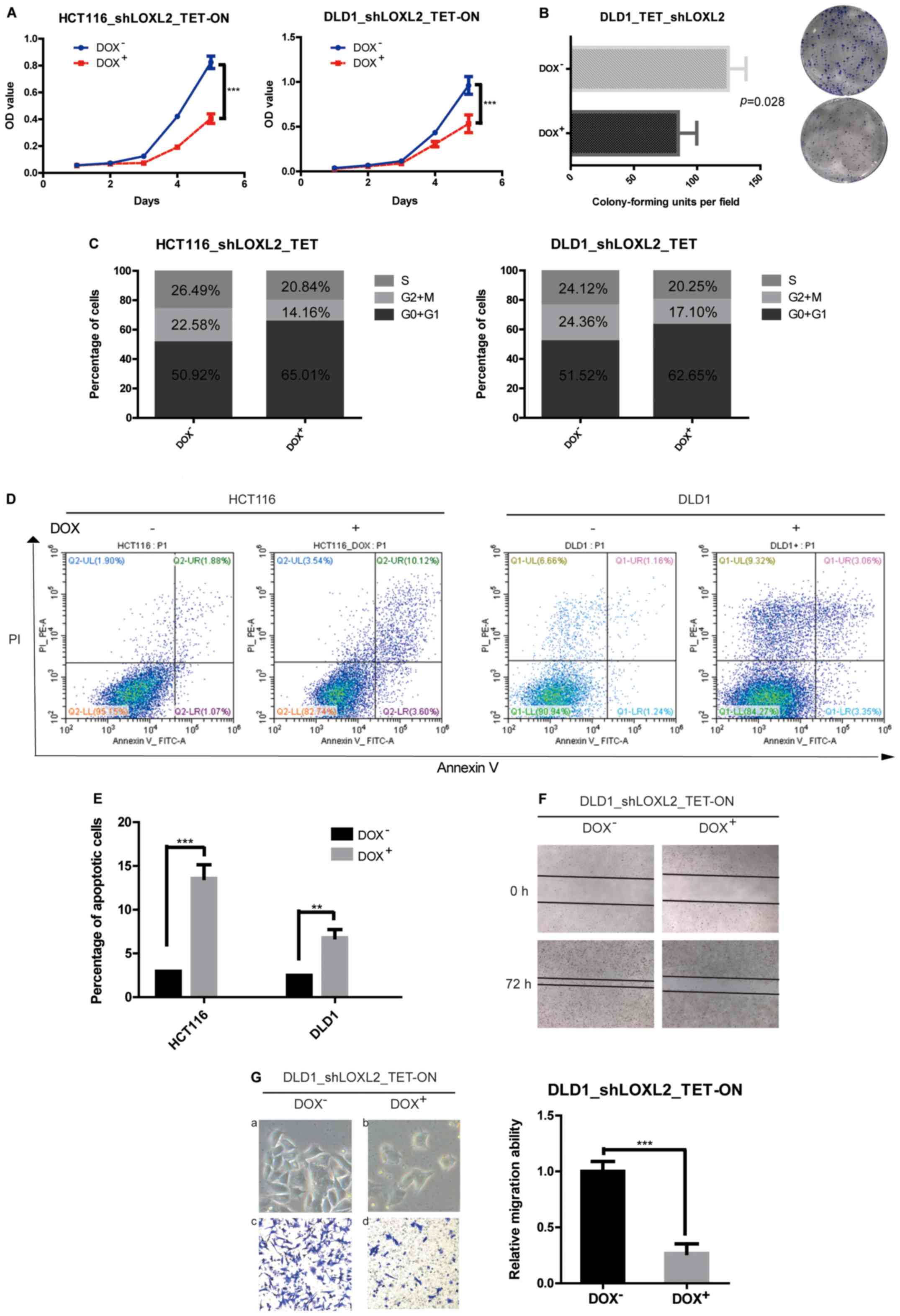
The effects of LOXL2 knockdown (shRNA2) in the
stable cell lines on cell proliferation were evaluated using CCK8
assays. It was indicated that the knockdown of LOXL2 in HCT116 and
DLD1 cells significantly decreased the growth of CRC cells in
vitro (HCT116, P<0.0001; DLD1, P=0.0002) (Fig. 3A). A similar pattern was observed in
the colony-forming assays, in which the knockdown of LOXL2
significantly decreased the clonogenicity of DLD1 cells compared
with the cells without DOX (85.00±8.505 vs. 124.7± 8.110,
respectively; P=0.0279) (Fig.
3B).
LOXL2 knockdown induces cell cycle
arrest and apoptosis in CRC cells
To investigate the mechanism for the
anti-proliferative effect of the knockdown of LOXL2 in CRC cells,
the cell cycle distribution was analyzed using flow cytometry. The
silencing of LOXL2 in HCT116 and DLD1 cells resulted in an increase
in cells in the G0/G1 phase with a decrease
in the proportion of S-phase cells compared with the control cells
(Fig. 3C). The data showed that the
depletion of LOXL2 decreased cell proliferation by cell cycle
arrest. The effects of LOXL2 silencing on apoptotic cell death were
also examined. Flow cytometry analysis showed that while only 3.0%
of HCT116 and 2.5% of DLD1 cells in the absence of DOX were Annexin
V-positive, ~13.6% of HCT116 and 6.8% of DLD1 cells with silenced
LOXL2 exhibited Annexin V-positive staining (Fig. 3D and E). These results suggested
that LOXL2 might be involved in the inhibition of apoptosis.
LOXL2 knockdown impairs the migration
of CRC cells and induces mesenchymal-epithelial transition in
vitro
As the overexpression of LOXL2 in the clinical data
analysis was associated with distant metastasis, wound-healing and
Transwell assays were performed to assess the effect of LOXL2
knockdown on the migratory ability of CRC cells. Wound closure
rates and cell migration were decreased after LOXL2 knockdown
compared with the control group (Fig.
3F and G). Furthermore, LOXL2 knockdown also induced
morphological changes in CRC cells, including a loss of cell
dispersion, and the formation of intercellular junctions and tight
clusters (Fig. 3Ga and b),
suggesting a mesenchymal-epithelial transition.
LOXL2 knockdown impairs the growth of
tumor xenografts in vivo
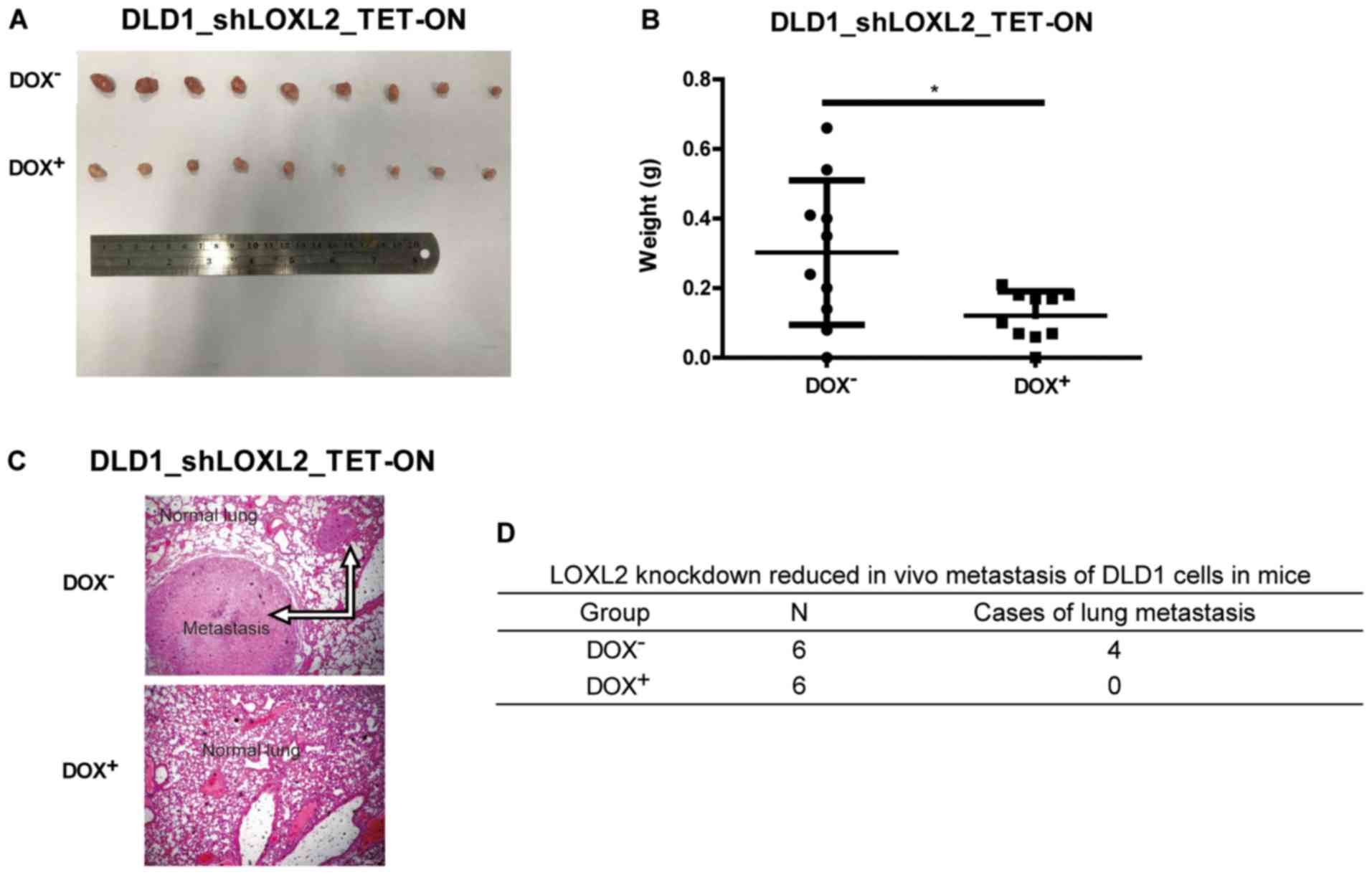
The effect of LOXL2 expression on CRC cells was
examined in vivo. Immunodeficient nude mice were
subcutaneously injected with DLD1 cells harboring DOX-inducible
LOXL2 shRNA and randomized into two groups. In one group, DOX was
administered in the drinking water to induce shRNA expression and
LOXL2 knockdown, while normal water was administered in the control
group. The growth of the tumor xenografts was monitored over the
following 4 weeks. As shown in Fig.
4A, LOXL2 depletion resulted in a marked reduction in tumor
size compared with the controls. The tumors from the
LOXL2-knockdown group were significantly smaller after 4 weeks
compared with tumors from the control group (P=0.0177; Fig. 4B). This finding demonstrated that
LOXL2 silencing was able to reduce the growth of CRC cell in
vivo.
The role of LOXL2 in tumor metastasis was further
investigated by injecting DLD1 cells harboring DOX-inducible LOXL2
shRNA into the tail vein of nude mice. The mice were treated every
other day with normal water (control) or water with DOX (treatment
group; 1 mg/ml) for 8 weeks. Compared with the control mice, the
mice receiving DOX developed significantly fewer lung metastases
(Fig. 4C and D). This finding
demonstrated that silencing LOXL2 reduced CRC metastasis in
vivo.
LOXL2 may regulate the expression of
vimentin, cyclin E1 and cleaved-PARP-1
The knockdown of LOXL2 induced morphological changes
in CRC cells, which are indicative of mesenchymal-epithelial
transition (Fig. 3Ga and b). These
results prompted us to examine the effect of LOXL2 knockdown on
epithelial-mesenchymal transition (EMT) transcriptional regulators
and markers. In the LOXL2 knocked down-HCT116 and DLD1 cells,
vimentin expression, as determined by qPCR, was decreased compared
with the control cells (Fig. 5A).
The protein levels of vimentin in DLD1 and HCT116 cell lines were
repeatedly analyzed. Unfortunately, vimentin expression was
extremely low in western blots in both cell lines. Previous
published studies also confirmed the results of the present study
(22). To evaluate the mechanism by
which LOXL2 affects the expression of vimentin, luciferase assays
were performed using a luciferase reporter driven by the vimentin
promoter (Fig. 5B). The
transfection of 293T cells with pGL3-LOXL2 was able to
significantly increase luciferase activity that was driven by the
vimentin promoter, implying that LOXL2 may induce vimentin
expression. The levels of the cell cycle regulator, CDK2, and its
partner cyclin E were examined. The activity of CDK2 is maximal
during S and G2 phases; CDK2 is activated by interaction
with cyclin E during the early stages of DNA synthesis to permit
G1-S transition (23).
Decreased levels of CDK2 and cyclin E were demonstrated in
LOXL2-knocked down cells compared with the control cells (Fig. 5C). PARP degradation was also
detected by western blotting to confirm the ability of LOXL2 to
inhibit apoptosis (Fig. 5C). These
data may explain why a depletion of LOXL2 reduced cell
proliferation and induced apoptosis.
Discussion
The LOX family contributes to fibrotic matrix
crosslinking and stabilization by catalyzing the covalent
interchain crosslink of collagen in the ECM (24). The role of LOXL2 has been
intensively studied in fibrotic diseases. Ikenaga et al
(8) revealed that the selective
targeting of LOXL2 suppressed the progression of hepatic fibrosis.
LOXL2 levels are elevated in the heart tissues and serum of
patients with heart failure (HF). Additionally, heart dysfunction
and a high level of HF biomarkers are associated with high LOXL2
levels. In mice, LOXL2 activation is crucial for myocardial
fibrosis and the development of HF. LOXL2 crosslinks with collagen
fibers to activate fibroblasts, and activated fibroblasts secrete
further collagen as well as LOXL2. Through this positive feedback
loop, LOXL2 triggers cardiac interstitial fibrosis and HF (9).
LOXL2 has also been shown to serve an important role
in cancer, mostly through its functions in the ECM. LOXL2 is
critical for metastatic niche and tumor ECM formation in
hepatocellular carcinoma (13).
LOXL2 is overexpressed in cancer-associated fibroblasts (CAFs) in
colon cancer (12). Targeting LOXL2
with an allosteric antibody was effective in reducing the number of
CAFs in xenograft models of cancer (25). LOXL2 expression is associated with
metastasis and poor survival in patients with breast cancer
(26), and promotes cancer invasion
by downregulating the extracellular protein tissue inhibitor of
matrix metalloproteinase-9 and −1 (27). Furthermore, LOXL2 has been verified
to be associated with the metastasis of colorectal cancer cells
in vitro through EMT (28).
In the present study, LOXL2 expression was markedly
higher in CRC tissues compared with the adjacent noncancerous
tissues at both the mRNA and protein level. These results are
consistent with previous microarray data by the authors (15). Several studies have shown that LOXL2
functions as a prognostic marker in various types of cancer,
including colon cancer (12).
Whereas other reports have focused on its role in the ECM, the
nuclear localization of LOXL2 in CRC cells was revealed in the
present study. It was demonstrated with the retrospective analysis
of 228 CRC tissues and normal adjacent colon tissues and clinical
data that high intracellular expression levels of LOXL2 were
associated with a poor prognosis. In addition, elevated LOXL2
expression was associated with more advanced clinical and
pathological features, including TNM staging and distant
metastasis. Univariate and multivariate Cox regression hazard
analyses showed that LOXL2 might be a valuable independent
biomarker for the prognosis of patients with CRC. These findings
prompted further study of the molecular mechanisms of LOXL2 in CRC.
It was identified that the depletion of LOXL2 by DOX-induced
expression of shRNA inhibited the proliferation of CRC cells via
cell cycle arrest and apoptosis. Furthermore, the depletion of
LOXL2 also impaired the migratory activity of CRC cells in
vitro and in vivo.
EMT has been demonstrated as a key event in cancer
metastasis, and plays a key role in driving cancer cells to invade
the adjacent normal tissues (29).
Two mechanisms have previously been demonstrated for the function
of LOXL2 in EMT. Firstly, LOXL2 interacts with Snail1, which
results in the repression of CDH1 to induce EMT (30). Secondly, LOXL2 has been implicated
in the activation of FAK kinase in a Snail1-independent pathway,
which downregulates genes that are associated with epidermal
differentiation and cell polarity to promote the mesenchymal
phenotype (14). The luciferase
assay results in the present study indicated that LOXL2 expression
was also able to significantly increase the promoter activity of
vimentin, suggesting that LOXL2 may exert its effects by inducing
vimentin expression.
In conclusion, it was observed that a high level of
LOXL2 mRNA and protein expression in CRC patients was associated
with impaired overall survival. The knockdown of LOXL2 in
vitro and in vivo induced cell cycle arrest and
apoptosis. However, a phase II study previously indicated that the
outcomes in patients with metastatic CRC and KRAS mutation were not
improved following the addition of simtuzumab (a monoclonal
antibody to LOXL2) (31). The
results of the present study provide novel insights into the
biology of CRC cells and suggest that LOXL2 may be a potential
target for tumor therapy.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National High
Technology Research and Development Program of China (863 Program;
grant no. SQ2014SFOZD00314) and the National Natural Science
Foundation of China (grant nos. 81372636, 81572378 and
81302089).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XC conducted most of the molecular and animal
experiments, and was a major contributor in writing the manuscript.
GW analyzed the patient data. WS made substantial contributions to
conception and design. ZH and HH performed substantial parts of the
molecular experiments. LC made substantial contributions to
conception and design, and was a major contributor in revising
manuscript critically for important intellectual content. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Approval for experiments was obtained from the
institutional ethics committee. All applicable international,
national, and/or institutional guidelines for the care and use of
animals were followed.
Consent for publication
The patient, or their parent, guardian or next of
kin provided written informed consent for the publication of any
associated data and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
International Agency for Research on
Cancer: Population Fact Sheets: World. http://gco.iarc.fr/today/fact-sheets-populations?population=900&sex=0July
25–2017.
|
|
2
|
Siegel R, DeSantis C, Virgo K, Stein K,
Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, et al:
Cancer treatment and survivorship statistics, 2012. CA Cancer J
Clin. 62:220–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sankaranarayanan R, Swaminathan R, Brenner
H, Chen K, Chia KS, Chen JG, Law SC, Ahn YO, Xiang YB, Yeole BB, et
al: Cancer survival in Africa, Asia, and Central America: A
population-based study. Lancet Oncol. 11:165–173. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Heald RJ and Ryall RD: Recurrence and
survival after total mesorectal excision for rectal cancer. Lancet.
1:1479–1482. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
van Gijn W, Marijnen CAM, Nagtegaal ID,
Kranenbarg EM, Putter H, Wiggers T, Rutten HJ, Påhlman L, Glimelius
B and van de Velde CJ; Dutch Colorectal Cancer Group: Preoperative
radiotherapy combined with total mesorectal excision for resectable
rectal cancer: 12-year follow-up of the multicentre, randomised
controlled TME trial. Lancet Oncol. 12:575–582. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Polónia A, Coimbra M, Reis S, Tavares A,
Raimundo A, Santos LL, Jeronimo C and Henrique RM: Expression of
histone modifying enzymes and histone post-translational marks in
colorectal cancer. Virchows Arch. 463:1252013.
|
|
7
|
Barker HE, Cox TR and Erler JT: The
rationale for targeting the LOX family in cancer. Nat Rev Cancer.
12:540–552. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ikenaga N, Peng ZW, Vaid KA, Liu SB,
Yoshida S, Sverdlov DY, Mikels-Vigdal A, Smith V, Schuppan D and
Popov YV: Selective targeting of lysyl oxidase-like 2 (LOXL2)
suppresses hepatic fibrosis progression and accelerates its
reversal. Gut. 66:1697–1708. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang J, Savvatis K, Kang JS, Fan P, Zhong
H, Schwartz K, Barry V, Mikels-Vigdal A, Karpinski S, Kornyeyev D,
et al: Targeting LOXL2 for cardiac interstitial fibrosis and heart
failure treatment. Nat Commun. 7:137102016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu L and Zhu Y: The function and
mechanisms of action of LOXL2 in cancer (Review). Int J Mol Med.
36:1200–1204. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park JS, Lee JH, Lee YS, Kim JK, Dong SM
and Yoon DS: Emerging role of LOXL2 in the promotion of pancreas
cancer metastasis. Oncotarget. 7:42539–42552. 2016.PubMed/NCBI
|
|
12
|
Torres S, Garcia-Palmero I, Herrera M,
Bartolomé RA, Peña C, Fernandez-Aceñero MJ, Padilla G,
Peláez-García A, Lopez-Lucendo M, Rodriguez-Merlo R, et al: LOXL2
is highly expressed in cancer-associated fibroblasts and associates
to poor colon cancer survival. Clin Cancer Res. 21:4892–4902. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wong CC, Tse AP, Huang YP, Zhu YT, Chiu
DK, Lai RK, Au SL, Kai AK, Lee JM, Wei LL, et al: Lysyl
oxidase-like 2 is critical to tumor microenvironment and metastatic
niche formation in hepatocellular carcinoma. Hepatology.
60:1645–1658. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moreno-Bueno G, Salvador F, Martín A,
Floristán A, Cuevas EP, Santos V, Montes A, Morales S, Castilla MA,
Rojo-Sebastián A, et al: Lysyl oxidase-like 2 (LOXL2), a new
regulator of cell polarity required for metastatic dissemination of
basal-like breast carcinomas. EMBO Mol Med. 3:528–544. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fu J, Tang W, Du P, Wang G, Chen W, Li J,
Zhu Y, Gao J and Cui L: Identifying microRNA-mRNA regulatory
network in colorectal cancer by a combination of expression profile
and bioinformatics analysis. BMC Syst Biol. 6:682012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cui X, Liu B, Zheng S, Dong K and Dong R:
Genome-wide analysis of DNA methylation in hepatoblastoma tissues.
Oncol Lett. 12:1529–1534. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang G, Shen W, Liu CY, Liu Y, Wu T, Cui
X, Yu T, Zhu Y, Song J, Du P, et al: Phosphorylase kinase β affects
colorectal cancer cell growth and represents a novel prognostic
biomarker. J Cancer Res Clin Oncol. 143:971–980. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cui X, Yang Y, Jia D, Jing Y, Zhang S,
Zheng S, Cui L, Dong R and Dong K: Downregulation of bone
morphogenetic protein receptor 2 promotes the development of
neuroblastoma. Biochem Biophys Res Commun. 483:609–616. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wiederschain D, Wee S, Chen L, Loo A, Yang
G, Huang A, Chen Y, Caponigro G, Yao YM, Lengauer C, et al:
Single-vector inducible lentiviral RNAi system for oncology target
validation. Cell Cycle. 8:498–504. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu CY, Yu T, Huang Y, Cui L and Hong W:
ETS (E26 transformation-specific) up-regulation of the
transcriptional co-activator TAZ promotes cell migration and
metastasis in prostate cancer. J Biol Chem. 292:9420–9430. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu Y, Wang G, Yang Y, Mei Z, Liang Z, Cui
A, Wu T, Liu CY and Cui L: Increased TEAD4 expression and nuclear
localization in colorectal cancer promote epithelial-mesenchymal
transition and metastasis in a YAP-independent manner. Oncogene.
35:2789–2800. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Findlay VJ, Wang C, Nogueira LM, Hurst K,
Quirk D, Ethier SP, Staveley O'Carroll KF, Watson DK and Camp ER:
SNAI2 modulates colorectal cancer 5-fluorouracil sensitivity
through miR145 repression. Mol Cancer Ther. 13:2713–2726. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Terret ME, Lefebvre C, Djiane A, Rassinier
P, Moreau J, Maro B and Verlhac MH: DOC1R: A MAP kinase substrate
that control microtubule organization of metaphase II mouse
oocytes. Development. 130:5169–5177. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu SB, Ikenaga N, Peng ZW, Sverdlov DY,
Greenstein A, Smith V, Schuppan D and Popov Y: Lysyl oxidase
activity contributes to collagen stabilization during liver
fibrosis progression and limits spontaneous fibrosis reversal in
mice. FASEB J. 30:1599–1609. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barry-Hamilton V, Spangler R, Marshall D,
McCauley S, Rodriguez HM, Oyasu M, Mikels A, Vaysberg M, Ghermazien
H, Wai C, et al: Allosteric inhibition of lysyl oxidase-like-2
impedes the development of a pathologic microenvironment. Nat Med.
16:1009–1017. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barker HE, Chang J, Cox TR, Lang G, Bird
D, Nicolau M, Evans HR, Gartland A and Erler JT: LOXL2-mediated
matrix remodeling in metastasis and mammary gland involution.
Cancer Res. 71:1561–1572. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kouhkan F, Motovali-Bashi M and Hojati Z:
The influence of interstitial collagenase-1 genotype polymorphism
on colorectal cancer risk in Iranian population. Cancer Invest.
26:836–842. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park PG, Jo SJ, Kim MJ, Kim HJ, Lee JH,
Park CK, Kim H, Lee KY, Kim H, Park JH, et al: Role of LOXL2 in the
epithelial-mesenchymal transition and colorectal cancer metastasis.
Oncotarget. 8:80325–80335. 2017.PubMed/NCBI
|
|
29
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Peinado H, Del Carmen Iglesias-de la Cruz
M, Olmeda D, Csiszar K, Fong KS, Vega S, Nieto MA, Cano A and
Portillo F: A molecular role for lysyl oxidase-like 2 enzyme in
snail regulation and tumor progression. EMBO J. 24:3446–3458. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hecht JR, Benson AB III, Vyushkov D, Yang
Y, Bendell J and Verma U: A phase II, randomized, double-blind,
placebo-controlled study of simtuzumab in combination with FOLFIRI
for the second-line treatment of metastatic KRAS mutant colorectal
adenocarcinoma. Oncologist. 22:243–e23. 2017. View Article : Google Scholar : PubMed/NCBI
|