Introduction
Hepatocellular carcinoma (HCC) is one of the most
common malignant tumors worldwide, with relatively high incidence
and mortality rates, particularly in Africa and Southeast Asia
(1). Surgical resection remains the
first choice for the treatment of early-stage HCC. However, despite
a variety of comprehensive treatment strategies for patients with
advanced liver cancer, the prognosis remains poor due to
intrahepatic and distant metastasis. Metastasis and recurrence of
HCC is a multistep, complex process involving numerous regulatory
factors (2). The identification of
novel biological regulating factors of HCC recurrence and
metastasis is crucial for the targeted treatment of HCC
patients.
Octamer-binding transcription factor 4 (Oct4) is a
member of the POU transcription factor family that plays a key role
in the development of almost all organisms (3). A number of studies have suggested that
Oct4 may maintain the characteristics of cancer stem cells
(4) and mediate the development of
chemoresistance in tumor cells (5,6). The
aberrant expression of Oct4 is implicated in the initiation and
development of several malignant tumors, such as esophageal,
gastric and breast, non-small-cell lung and prostate cancer and
oral squamous cell carcinoma (7–13).
However, little is known on the mechanisms underlying the
involvement of Oct4 in the malignant progression of HCC.
Survivin is a new member of the apoptosis suppressor
protein family. Survivin is only expressed in tumors and embryonic
tissues, and is closely associated with differentiation,
proliferation, cell cycle regulation, neovascularization,
resistance to radiotherapy and chemotherapy, and infiltration and
metastasis of tumor cells (14–19).
In view of the abovementioned biological characteristics of
survivin, it is likely to become a broad-spectrum cancer marker for
diagnosis and clinical treatment.
The Janus kinase/signal transducers and activators
of transcription (JAK/STAT) pathway represents a fast signal
transduction pathway from the cytoplasm to the nucleus. Recent
studies have demonstrated that the activation of the JAK/STAT
signaling pathway, particularly of STAT3, has a major effect on
cell growth, proliferation, transformation, resistance to
chemotherapy-induced apoptosis and chemosensitivity in several
tumors (20–24).
Oct4 has been found to exert important
anti-apoptotic effects on embryonic stem cells under survival
stress, and it has been suggested that these effects may be
regulated by the survivin/STAT3 signaling pathway (25). Based on previous and the present
findings, we hypothesized that Oct4 may be associated with
malignant progression and poor prognosis in HCC through the
survivin/STAT3 signaling pathway. By performing a series of in
vitro and ex vivo experiments presented in this study,
we demonstrated that Oct4 is involved in the malignant progression
of HCC cells and poor prognosis of HCC patients, and its effects
are likely mediated through the survivin/STAT3 signaling
pathway.
Materials and methods
Patients and samples
Tissues from 53 patients with primary HCC treated at
the Wujiang No. 1 People's Hospital (Jiangsu, China) between June
1, 2009 and December 31, 2011 were included in this study. These
patients included 39 men and 14 women with a median age of 50 years
(range, 36–67 years). The HCC tissues were obtained from patients
who received hepatectomy and the diagnosis was pathologically
confirmed. Long-term follow-up was conducted and the median time
was 39.0 months (range, 5–60.0 months). The study protocol was
approved by the Ethics Committee of Wujiang No. 1 People's Hospital
and all patients signed a written informed consent form.
Cell lines and cell culture
The human normal liver cell line LO2 and the HCC
cell lines SMMC-7721, Huh-7, MHCC97H, PLC/PRF/5 and Hep3B were
purchased from the Cell Bank of Type Culture Collection of the
Chinese Academy of Sciences (Shanghai, China). All cells were
routinely maintained as a monolayer in high-glucose Dulbecco's
modified Eagle's medium (DMEM) supplemented with 10% fetal bovine
serum (FBS), 100 mg/ml streptomycin and 100 IU/ml penicillin G.
Immunohistochemical staining
All patient tissues were obtained from the specimens
resected during surgery. Paraffin-embedded consecutive sections
(4-µm) were examined for Oct4, survivin and phosphorylated STAT3
(pSTAT3) expression. The primary antibodies were as follows: mouse
anti-human Oct4 (dilution 1:100; cat. no. sc-5279; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), rabbit anti-human
survivin (71G4B7) (dilution 1:200; cat. no. 2808) and p-STAT3
(Tyr705; dilution 1:100; cat. no. 9145; both from Cell Signaling
Technology, Inc., Danvers, MA, USA). The MaxVision™
HRP-polymer anti-mouse/rabbit IHC kit (Kit-5010; Fuzhou Maixin
Biotechnology Development, Co., Ltd., Fuzhou, China) was used. The
evaluation criteria included the number and proportion of positive
cells. Five random high-power fields were examined and the
evaluation of staining reaction was performed with the
immunoreactive score (26). A score
of >4 was considered to indicate high expression.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tissue samples and cell
lines using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). The RNA concentration was assessed using a UV
spectrophotometer (Ultrospec 2,100 pro; Amersham; GE Healthcare,
Chicago, IL, USA). RNA (0.8 µg) was reversely transcribed into cDNA
using the PrimeScript RT reagent kit (Takara Bio, Inc., Otsu,
Japan) according to the manufacturer's instructions. The qPCR was
performed using SYBR® Premix Ex Taq (Takara Bio, Inc.)
on the Applied Biosystems 7500 Real-time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.), with GAPDH used as an
internal control. The primers used were as follows: Oct4 forward,
5′-AGGGCTTCTCCTTCTGGGTCT-3′ and reverse,
5′-TGAGAAAGGAGACCCAGCAG-3′; GAPDH forward,
5′-TGACTTCAACAGCGACACCCA-3′ and reverse,
5′-CACCCTGTTGCTGTAGCCAAA-3′. The pre-amplification PCR was
performed at one cycle 95°C for 5 min, 30 cycles at 95°C for 30
sec, 55°C for 30 sec and then 72°C for 30 sec. The product was
stored at 4°C until needed. All reactions were performed in
triplicate and the relative expression of Oct4 mRNA was calculated
using the 2−ΔΔCq method (24).
Construction and transfection of shRNA
vectors
The Oct4-shRNA and the control vector were purchased
from Santa Cruz Biotechnology, Inc. (sc-36123-SH). HCC cells were
transfected with shRNA vectors at a concentration of 4
µg/105 cells using the Lipofectamine 2000 transfection
reagent (Invitrogen Corp., Shanghai, China). Following routine
culture for 48 h after transfection, the cells were harvested for
further experiments.
Cell viability assay
The proliferation of Hep3B cells with different
pretreatment was detected using the MTT assay. Hep3B cells were
cultured in 96-well plates at a density of 5,000 cells/well and
transfected with shRNA vector as aforementioned. MTT (5 mg/ml) was
added into each well after transfection for 48 h and continuously
incubated for 4 h. Finally, 150 µl dimethyl sulfoxide (DMSO) was
added into each well and the absorbance was measured at 490 nm
using a microplate reader.
Soft agar colony formation assay
A soft agar colony formation assay was used to
evaluate cell growth capacity. HCC cells were collected and counted
after transfection, and 1×104 cells were mixed with agar
solution (0.6%) in high-glucose DMEM containing 20% fetal bovine
serum and layered on top of an agar layer (1.2%) in 6-well plates.
Cells were continuously incubated for 2–3 weeks in a 5%
CO2 humidified incubator at 37°C until colonies were
formed. High-glucose DMEM supplemented with 10% FBS was added to
the culture every 3–4 days. Each colony was defined as a spheroid
containing >50 cells.
Cell mobility assays
The Transwell chamber assay (pore size 8 µm; EMD
Millipore, Billerica, MA, USA) was used to evaluate the mobility of
HCC cells according to the manufacturer's instructions. The cell
migration assay was performed with an 8-µm Transwell chamber and
the cell invasion assay was performed with a Transwell chamber
pre-coated with Matrigel (1:6; BD Biosciences, Franklin, Lakes, NJ,
USA). HCC cells were collected and counted after transfection for
48 h, and 5×104 cells were seeded into the upper
chamber, whereas DMEM with 20% FBS was placed into the lower
chamber. After incubation for 24 h, HCC cells that migrated through
the membranes were fixed using 90% ethanol and stained with crystal
violet solution (0.1%). Five random fields were selected and the
cells were counted under a microscope.
Western blotting
HCC cells were collected and counted after
transfection for 48 h. Approximately 1×106 cells were
lysed using RIPA lysis buffer (cat. no. P0013C; Beyotime Institute
of Biotechnology, Jiangsu, China). The enhanced BCA protein assay
kit (cat. no. P0010S; Beyotime Institute of Biotechnology) was used
to quantify protein concentration. The primary antibodies were as
follows: anti-survivin (71G4B7) (dilution 1:1,000; cat. no. 2808;
Cell Signaling Technology), Oct4 (dilution 1:500; cat. no. sc-5279;
Santa Cruz Biotechnology), STAT3 (124H6) (dilution 1:1,000; cat.
no. 9139) and pSTAT3 (Tyr705) (1:1,000; cat. no. 9145; both from
Cell Signaling Technology). The results were visualized using an
enhanced chemiluminescence reagent (PerkinElmer, Inc., Shanghai,
China). The resultant film images were analyzed by Quantity One
analysis software (Bio-Rad Laboratories, Hercules, CA, USA).
Immunofluorescence staining
HCC cells transfected with Oct4-shRNA were grown on
coverslips in 6-well dishes. After transfection for 48 h, the HCC
cells were rinsed with phosphate-buffered saline (PBS) twice, then
fixed with 15% formaldehyde and permeabilized with 1% Triton X-100.
Next, the cells were blocked by incubation with non-immune animal
serum at room temperature for 1 h, followed by incubation with one
of the primary antibodies (dilution 1:200) at 4°C overnight. The
following day, the cells were washed and incubated in the dark for
1 h with Alexa Fluor Cy3-conjungated goat anti-mouse (cat. no.
A0521), Alexa Fluor 488-conjungated goat anti-rat (cat. no. A0428)
and Alexa Fluor 647-conjungated goat anti-rabbit IgG secondary
antibodies (dilution 1:400; cat. no. A0468) (Beyotime Institute of
Biotechnology) at room temperature, and then co-stained with DAPI
(1 µg/ml; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Images of
the immunostained cells were obtained using a fluorescence
microscope (magnification, ×200).
Statistical analysis
All presented data are expressed as the median ±
standard deviation and were compared with the Student's t-test or
one-way analysis of variance (ANOVA) using SPSS software, version
19.0 for Windows (SPSS, Inc., Chicago, IL, USA). Multigroup
comparisons of the means were carried out by ANOVA test with post
hoc contrasts by Student-Newman-Keuls test. The association between
the Oct4 expression level and the clinicopathological factors was
analyzed using Pearson's χ2 test. Survival time analysis
was performed using the Kaplan-Meier survival method. P<0.05 in
the univariate analyses were further tested using the multivariate
Cox proportional hazards model. P<0.05 was considered to
indicate statistically significant differences.
Results
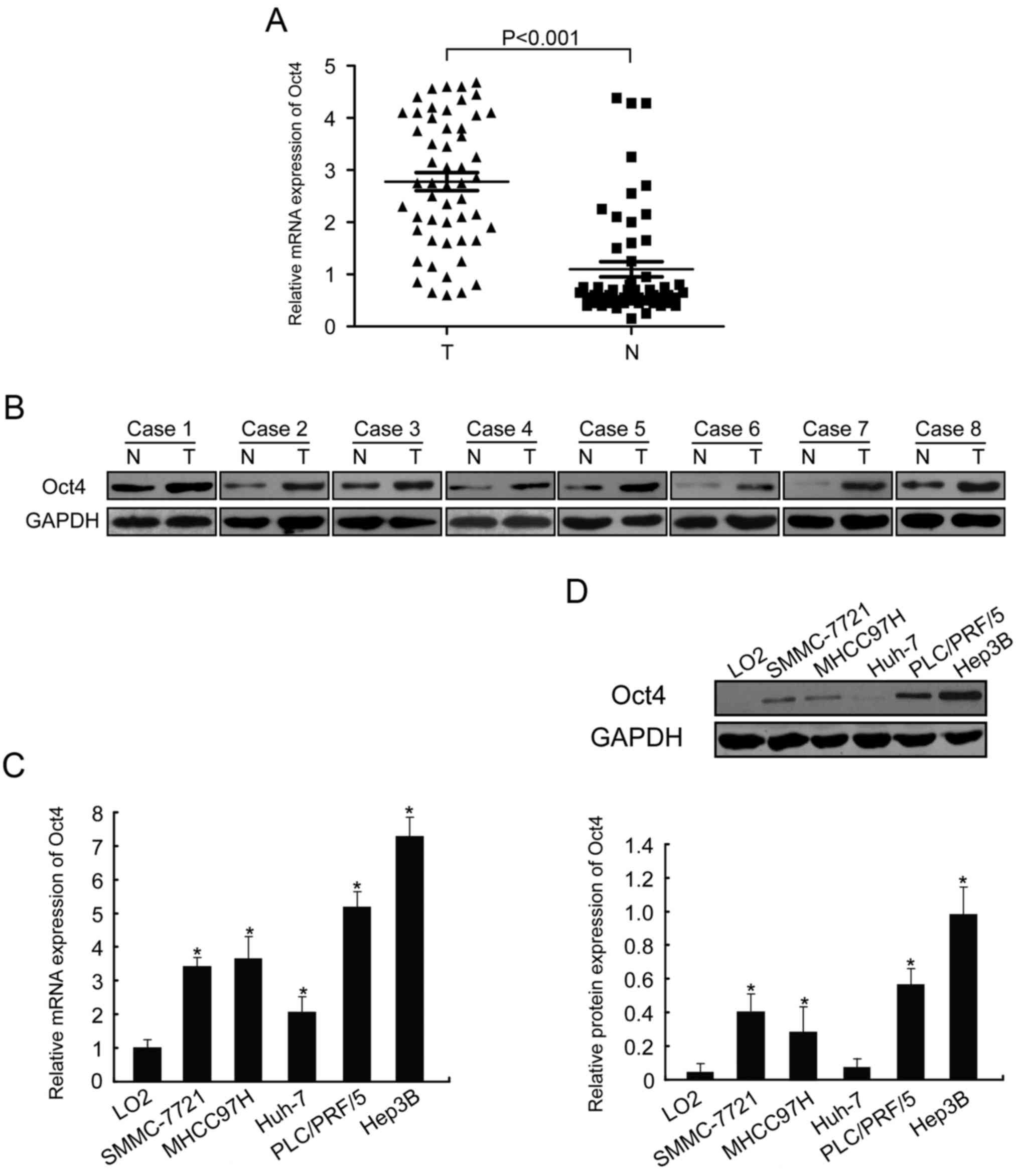
Oct4 is overexpressed in HCC tissues
and cell lines
In the present study, Oct4 mRNA expression was
determined in HCC tissues and adjacent liver tissues using RT-qPCR.
As shown in Fig. 1A, the relative
expression levels of Oct4 were higher in HCC tissues compared with
those in adjacent normal liver tissues (n=53, P<0.001).
Furthermore, the expression levels of the Oct4 protein were higher
in tumors compared with those in the corresponding non-malignant
liver tissues (Fig. 1B, n=8).
In addition, Oct4 expression was assessed in HCC
cell lines (SMMC-7721, Huh-7, MHCC97H, PLC/PRF/5 and Hep3B) and in
the normal liver cell line LO2. Similar to the expression pattern
in HCC tissues, the expression of Oct4 at the mRNA and protein
levels was upregulated in HCC cell lines compared with normal liver
cells (Fig. 1C and D; P<0.05).
We also found that Oct4 was expressed at different levels in the
HCC cell lines. These data revealed that the aberrant expression of
Oct4 may affect HCC initiation and progression. Hep3B cells, which
exhibited the highest expression of Oct4, were selected for
subsequent experiments.
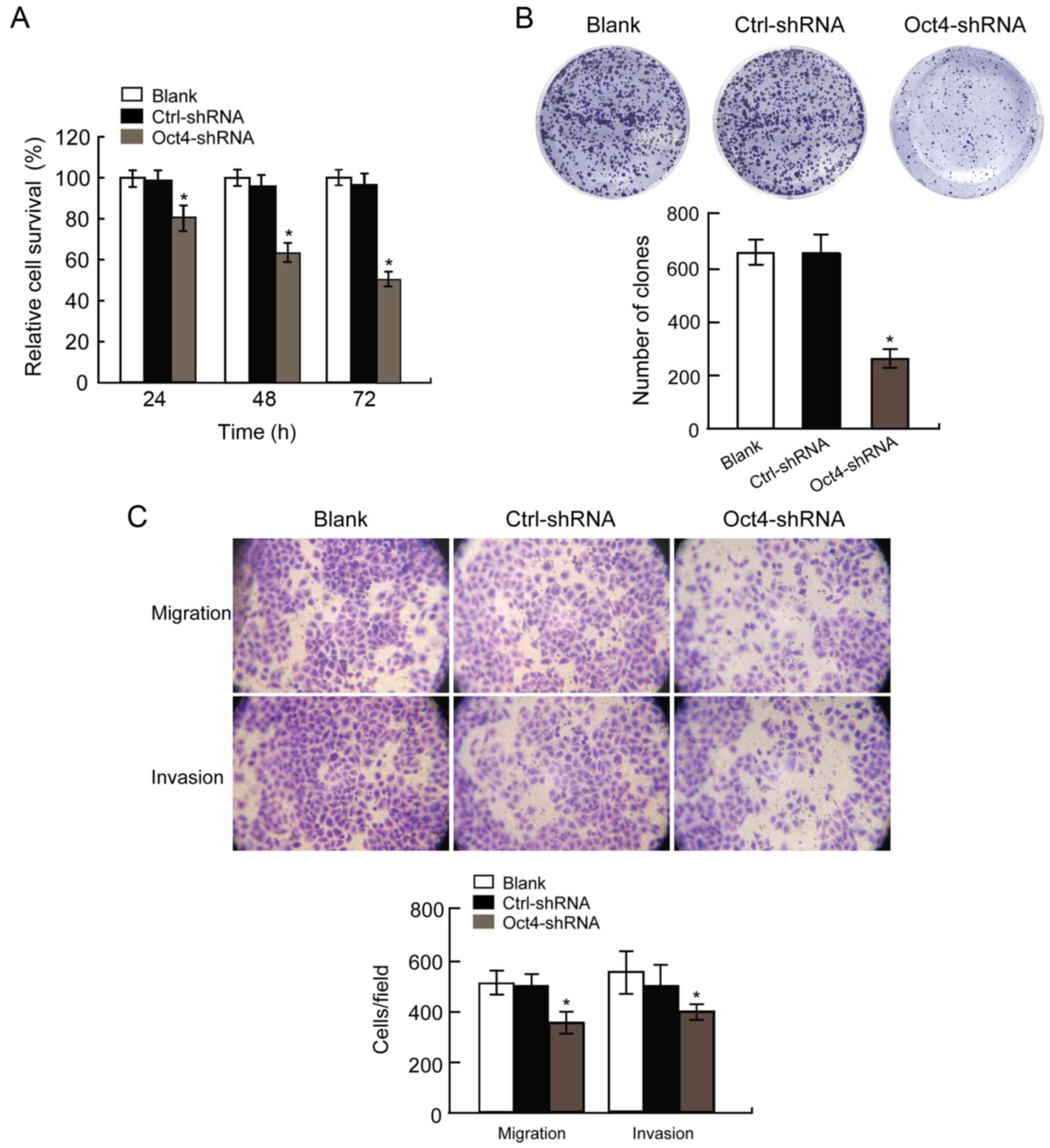
The loss of Oct4 inhibits HCC cell
viability and mobility in vitro
We evaluated three time-points (24, 48 and 72 h)
following transfection of Hep3B cells with Oct4-shRNA. The results
revealed that Hep3B cells transfected with Oct4-shRNA exhibited
significantly decreased viability compared with those transfected
with Ctrl-shRNA (Fig. 2A;
P<0.05). Furthermore, we also evaluated the rate of colony
formation of Hep3B cells at 2 weeks after transfection. Our results
revealed that Hep3B cells transfected with Oct4-shRNA formed fewer
colonies compared with those transfected with Ctrl-shRNA (Fig. 2B, P<0.05).
To assess the effect of Oct4-shRNA on cell motility,
Hep3B cells underwent migration and invasion assays following
transfection. The results confirmed that the number of migratory
and invasive cells in the Oct4-shRNA transfected Hep3B cell group
was markedly decreased compared with that in the Ctrl-shRNA group
(Fig. 2C, P<0.05). These results
revealed that the downregulation of Oct4 contributed to the
inhibition of migration and invasion of Hep3B cells in
vitro. Therefore, Oct4 may play a key role in the regulation of
Hep3B cell motility, including invasion and metastasis.
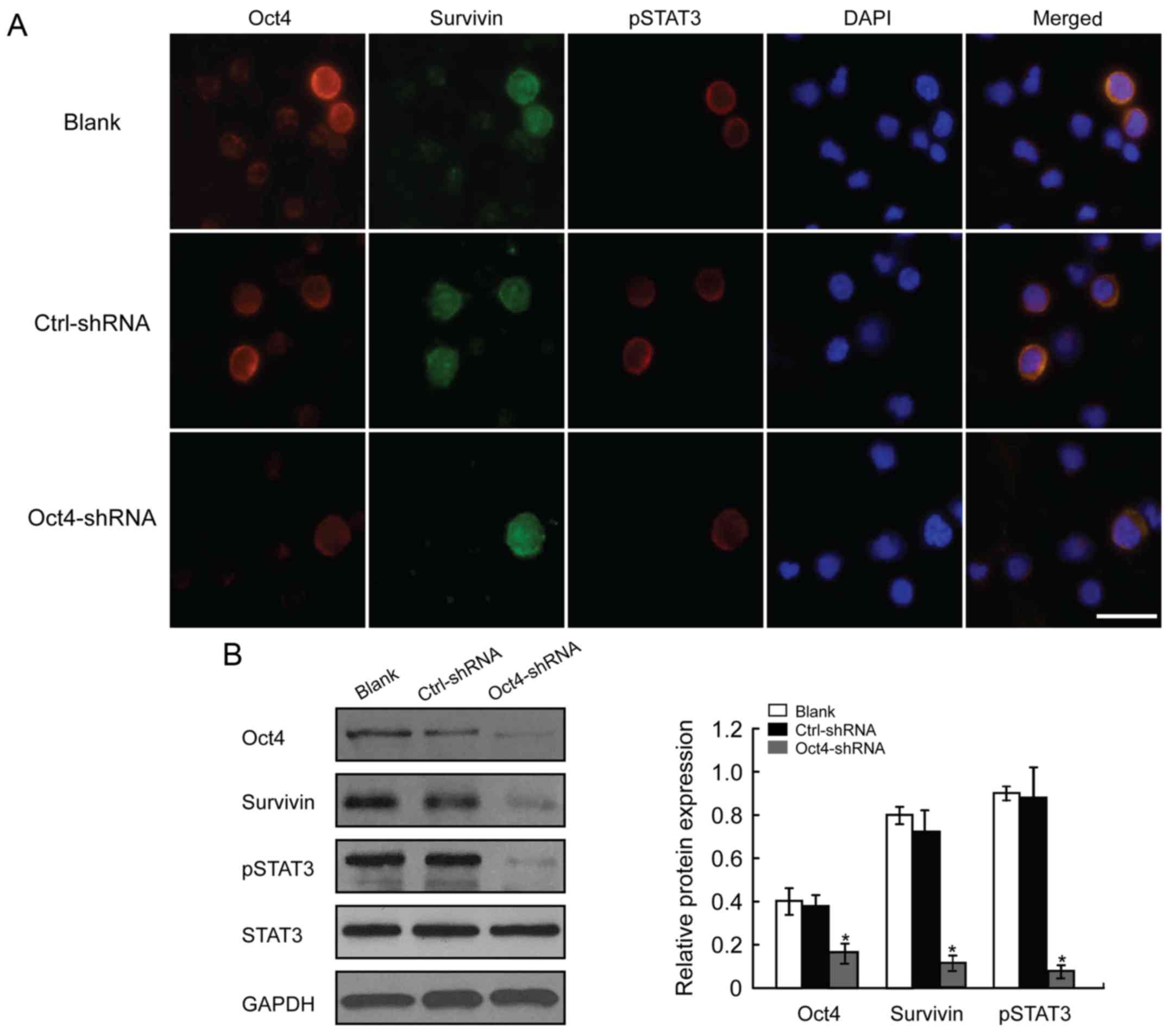
Depletion of Oct4 affects the
survivin/STAT3 signaling pathway
In this study, western blotting and cell
immunofluorescence assays were used to investigate Oct4-related
signaling pathway proteins. The results of the immunofluorescence
assay revealed that Oct4-depleted HCC cells exhibited notably
decreased survivin and p-STAT3 expression levels at the same time
(Fig. 3). Western blot analysis
confirmed these results. The protein levels of survivin and p-STAT3
were decreased following downregulation of Oct4 (Fig. 3), suggesting that Oct4 depletion
adversely affects the activation of the survivin/STAT3 signaling
pathway, which may play an important role in the malignant
progression of HCC cells.
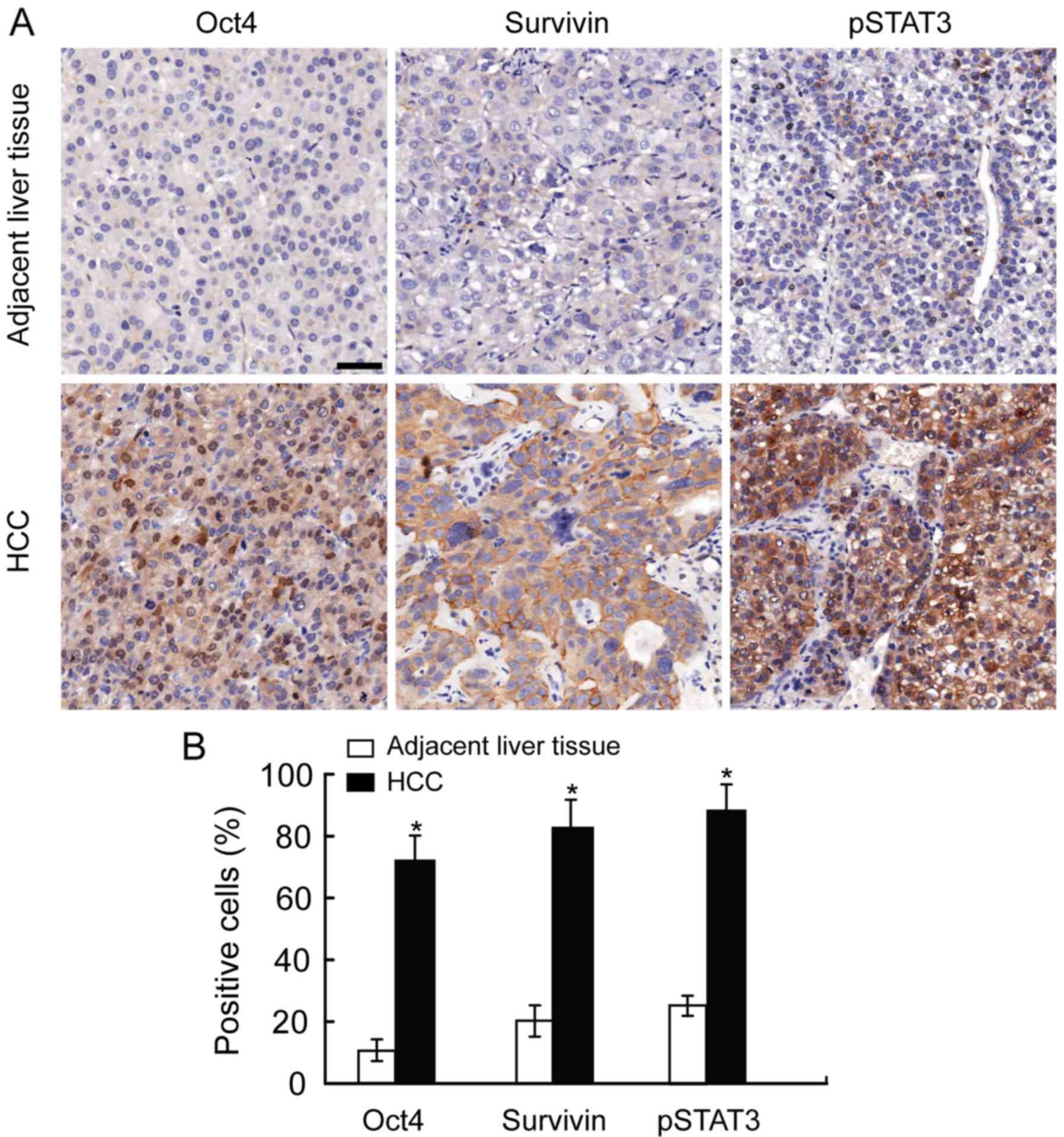
Associations of Oct4 expression in HCC
with clinicopathological factors
Oct4 is mainly expressed in the nuclei and
survivin/STAT3 are mainly distributed in the cytoplasm of tumor
cells (Fig. 4). A score of >4
was considered to indicate high expression. The 53 patients were
divided into two groups, namely the Oct4-high and Oct4-low groups.
The clinicopathological variables in these two groups are compared
in Table I. Our results revealed
that the Oct4-high subtype was significantly correlated with tumor
size (P=0.031), TNM stage (P=0.029) and vascular invasion
(P=0.010). The associations are detailed in Table II and Oct4 expression was found to
be positively correlated with survivin (r=0.299, P=0.031) and
pSTAT3 expression (r=0.349, P=0.012).
 | Table I.Associations of Oct expression with
clinicopathological features in 53 HCC patients. |
Table I.
Associations of Oct expression with
clinicopathological features in 53 HCC patients.
| Variables | Total (n=53) | Oct4-high (n=36) | Oct4-low (n=17) | P-value |
|---|
| Age (≤50/>50
years) | 16/37 | 11/25 | 5/12 | 0.933 |
| Sex
(male/female) | 39/14 | 28/8 | 11/6 | 0.314 |
| Hepatitis
(HBV/HCV/none) | 31/9/13 | 21/6/9 | 10/3/4 | 0.991 |
| Serum AFP
(≤400/>400 ng/ml) | 25/28 | 15/21 | 10/7 | 0.243 |
| Liver cirrhosis
(yes/no) | 43/10 | 30/6 | 13/4 | 0.551 |
| BCLC stage
(0-A/B/C) | 38/9/6 | 24/7/5 | 14/2/1 | 0.484 |
| Tumor size
(≤5.0/>5.0 cm) | 26/27 | 14/22 | 12/5 | 0.031 |
| Tumor number
(single/multiple) | 37/16 | 23/13 | 14/3 | 0.172 |
| TNM
stagea (I/II/III) | 18/23/12 | 8/19/9 | 10/4/3 | 0.029 |
| Tumor encapsulation
(yes/no) | 28/25 | 16/20 | 12/5 | 0.075 |
| Vascular
invasionb
(yes/no) | 33/20 | 26/10 | 6/11 | 0.010 |
 | Table II.Association of Oct4 with
survivin/pSTAT3 expression in 53 HCC patients. |
Table II.
Association of Oct4 with
survivin/pSTAT3 expression in 53 HCC patients.
|
| Oct4 |
|
|
|---|
|
|
|
|
|
|---|
|
| High | Low | r | P-value |
|---|
| Survivin |
|
|
|
|
|
High | 26 | 7 | 0.299 | 0.031 |
|
Low | 10 | 10 |
|
|
| pSTAT3 |
|
|
|
|
|
High | 24 | 5 | 0.349 | 0.012 |
|
Low | 12 | 12 |
|
|
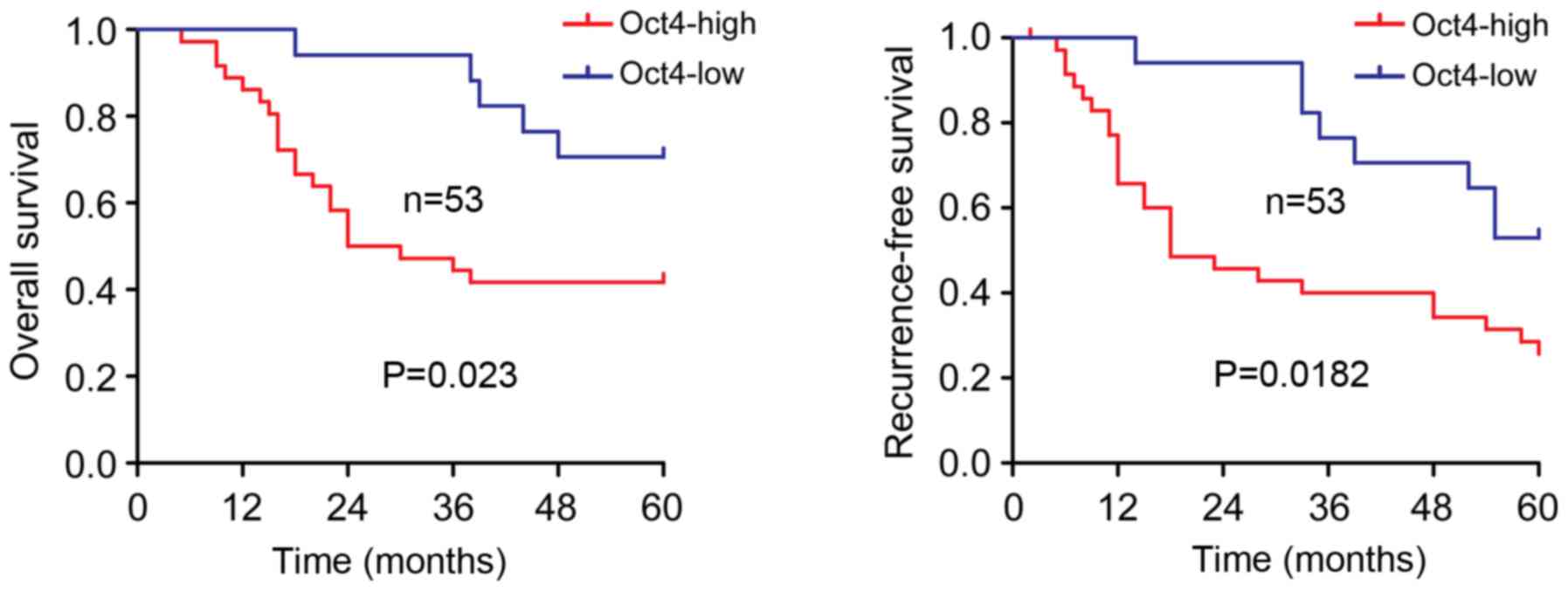
Oct4-high HCC subtype has a poor
prognosis
As shown in Fig. 5,
HCC patients with Oct4-high tumors had a significantly shorter
overall survival time (P=0.023, Fig.
5) and recurrence-free survival time (P=0.0182, Fig. 5) compared with those with Oct4-low
tumors. Through univariate analysis, 5 clinicopathological
variables were selected to be further investigated by multivariate
analysis (Table III). The data
revealed that Oct4 overexpression was one of the potential
independent prognostic factors for overall and recurrence-free
survival, although not as significant as were advanced TNM stage
and vascular invasion (Table
III).
 | Table III.Univariate and multivariate analyses
of the factors associated with survival and recurrence in 53 HCC
patients. |
Table III.
Univariate and multivariate analyses
of the factors associated with survival and recurrence in 53 HCC
patients.
|
| OS | RFS |
|---|
|
|
|
|
|---|
|
| Univariate | Multivariate | Univariate | Multivariate |
|---|
|
|
|
|
|
|
|---|
| Factors | P-value | HR (95% CI) | P-value | P-value | HR (95% CI) | P-value |
|---|
| Age |
|
|
|
|
|
|
| (≤50
vs. >50 years) | 0.564 |
| NA | 0.961 |
| NA |
| Sex |
|
|
|
|
|
|
| (male
vs. female) | 0.699 |
| NA | 0.963 |
| NA |
| Hepatitis
history |
|
|
|
|
|
|
| (Yes
vs. no) | 0.173 |
| NA | 0.278 |
| NA |
| Serum AFP |
|
|
|
|
|
|
| ≤400
vs. >400 ng/ml | 0.076 |
| NA | 0.197 |
| NA |
| Liver
cirrhosis |
|
|
|
|
|
|
| (Yes
vs. no) | 0.189 |
| NA | 0.223 |
| NA |
| BCLC stage |
|
|
|
|
|
|
| (0-A
vs. B-C) | 0.019 | 1.027 | 0.953 | 0.001 | 1.423 | 0.378 |
|
|
| (0.427–2.469) |
|
| (0.645–3.177) |
|
| Tumor size |
|
|
|
|
|
|
| (≤5.0
vs. >5.0 cm) | 0.086 |
| NA | 0.129 |
| NA |
| Tumor number |
|
|
|
|
|
|
| (single
vs. multiple) | 0.063 |
| NA | 0.074 |
| NA |
| TNM stage |
|
|
|
|
|
|
| (I vs.
II–III) | <0.001 | 4.665 | 0.041 | <0.001 | 3.018 | 0.036 |
|
|
| (1.068–20.376) |
|
| (1.075–8.472) |
|
| Tumor
encapsulation |
|
|
|
|
|
|
| (yes
vs. no) | 0.001 | 0.554 | 0.205 | 0.003 | 0.774 | 0.522 |
|
|
| (0.222–1.381) |
|
| (0.353–1.697) |
|
| Vascular
invasion |
|
|
|
|
|
|
| (yes
vs. no) | <0.001 | 3.756 | 0.044 | <0.001 | 2.609 | 0.049 |
|
|
| (1.037–13.606) |
|
| (1.005–6.772) |
|
| OCT4 |
|
|
|
|
|
|
| (high
vs. low) | 0.023 | 1.890 | 0.218 | 0.018 | 1.503 | 0.329 |
|
|
| (0.687–5.201) |
|
| (0.663–3.407) |
|
Discussion
Despite the significant improvements in early
diagnosis and treatment strategies for hepatocellular carcinoma
(HCC) in recent years, postoperative recurrence and metastasis
occur frequently, and the survival time and prognosis of HCC
patients remain unsatisfactory (27). Therefore, novel treatment modalities
and sensitive prognostic markers that can decrease the mortality
rate of HCC are required. HCC is a complex and heterogeneous tumor
with multiple genetic aberrations. Recent research revealed that
accumulation of genetic and epigenetic changes leads to the clonal
selection of cancer cells harboring malignant behavior potential.
Aberrant expression of cancer-related genes is one of the hallmarks
of cancer cells and plays a key role in hepatocarcinogenesis
(28).
Oct4 is a transcription factor that has been
reported to play a vital role in pluripotency maintenance of
embryonic cells and tumor cells. Accumulating studies have
suggested that Oct4 is indispensable for the development of drug
resistance in prostate and liver cancer (5,6).
According to these studies and our previous study, we hypothesized
that Oct4 may be implicated in hepatocarcinogenesis and malignant
progression of HCC cells. First, we analyzed Oct4 mRNA and protein
expression in HCC tissues and cell lines. Our results revealed that
Oct4 expression at the mRNA and protein level was significantly
higher in HCC tissues compared with that in the corresponding
non-malignant liver tissues (Fig. 1A
and B). The results from the HCC cell lines were consistent
with those from the clinical samples (Fig. 1C and D).
Furthermore, Oct4-shRNA was used in our study to
investigate its effects on the biological behavior of HCC cells.
The results indicated that downregulation of Oct4 expression in HCC
inhibited cell viability and mobility in vitro (Fig. 2). Latest research has demonstrated
that Oct4 may be detected in a number of solid tumors, including
HCC, and has a major effect on patient prognosis (7–13). The
biological function of Oct4 may regulate several signaling
pathways, such as the Wnt/β-catenin, TGF-β/Smad, JAK/STAT and
survivin/STAT3 signaling pathways (25,29,30).
Upon examination of the expression of signaling pathway-related
proteins in clinical samples, we observed that the protein
expression of survivin and phosphorylated STAT3 was increased,
which was associated with Oct4 overexpression (Fig. 4). Western blotting results and
cellular immunofluorescence analysis revealed that downregulating
Oct4 expression decreased the expression of survivin and the
phosphorylation of STAT3 in HCC cells, which were consistent with
the results from clinical samples (Fig.
3). Our data revealed that silencing Oct4 exerted a stronger
inhibitory effect on HCC cell viability and mobility in
vitro. Furthermore, we observed that the involvement of Oct4 in
the malignant progression of HCC cells may be through the
survivin/STAT3 pathway.
Next, to determine the clinical significance of Oct4
in HCC patients, we analyzed the association of the expression of
Oct4 with surgical outcome and clinicopathological characteristics.
Our data revealed that patients with high Oct4 expression had a
worse prognosis compared with those with low Oct4 expression. When
comparing the two groups, patients with Oct4-high HCC exhibited
shorter overall and recurrence-free survival time (Fig. 5). In addition, increased expression
of Oct4 in HCC patients was found to be significantly associated
with vascular invasion (P=0.010), TNM stage (P=0.029) and tumor
size (P=0.031). Multivariate and univariate analyses revealed that
the overexpression of Oct4 was found to be associated with patient
prognosis, although not as significantly as advanced TNM stage and
vascular invasion (Table III). In
subsequent studies, we aim to expand the sample size to confirm
this result.
The present study suggested that aberrant expression
of Oct4 was crucial for the viability, migration and invasion of
HCC cells, possibly through the survivin/STAT3 signaling pathway.
The clinical findings revealed that overexpression of Oct4 was
associated with poor patient prognosis, and Oct4 was considered as
an independent factor for predicting overall survival time. These
results revealed that Oct4 may be promising as a novel therapeutic
target for HCC. However, the molecular mechanisms underlying the
function of Oct4 in the survivin/STAT3 signaling pathway remain to
be fully understood. Further studies are required to elucidate the
genetic characteristics of Oct4 and its potential as a target in
anti-HCC therapy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
GW, QG and GS conceived and designed the
experiments; GW and HZ performed the experiments; ZG analyzed the
data; GW and GS wrote the study. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committee of Wujiang No. 1 People's Hospital and all patients
signed a written informed consent form.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Su CQ: Survivin in survival of
hepatocellular carcinoma. Cancer Lett. 379:184–190. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cherepanova OA, Gomez D, Shankman LS,
Swiatlowska P, Williams J, Sarmento OF, Alencar GF, Hess DL, Bevard
MH, Greene ES, et al: Activation of the pluripotency factor OCT4 in
smooth muscle cells is atheroprotective. Nat Med. 22:657–665. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu T, Liu S, Breiter DR, Wang F, Tang Y
and Sun S: Octamer 4 small interfering RNA results in cancer stem
cell-like cell apoptosis. Cancer Res. 68:6533–6540. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang XQ, Ongkeko WM, Chen L, Yang ZF, Lu
P, Chen KK, Lopez JP, Poon RT and Fan ST: Octamer 4 (Oct4) mediates
chemotherapeutic drug resistance in liver cancer cells through a
potential Oct4-AKT-ATP-binding cassette G2 pathway. Hepatology.
52:528–539. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Linn DE, Yang X, Sun F, Xie Y, Chen H,
Jiang R, Chen H, Chumsri S, Burger AM and Qiu Y: A role for OCT4 in
tumor initiation of drug-resistant prostate cancer cells. Genes
Cancer. 1:908–916. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ezeh UI, Turek PJ, Reijo RA and Clark AT:
Human embryonic stem cell genes OCT4, NANOG, STELLAR, and GDF3 are
expressed in both seminoma and breast carcinoma. Cancer.
104:2255–2265. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gu G, Yuan J, Wills M and Kasper S:
Prostate cancer cells with stem cell characteristics reconstitute
the original human tumor in vivo. Cancer Res. 67:4807–4815. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen YC, Hsu HS, Chen YW, Tsai TH, How CK,
Wang CY, Hung SC, Chang YL, Tsai ML, Lee YY, et al: Oct-4
expression maintained cancer stem-like properties in lung
cancer-derived CD133-positive cells. PLoS One. 3:e26372008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Atlasi Y, Mowla SJ, Ziaee SA and Bahrami
AR: OCT-4, an embryonic stem cell marker, is highly expressed in
bladder cancer. Int J Cancer. 120:1598–1602. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chiou SH, Yu CC, Huang CY, Lin SC, Liu CJ,
Tsai TH, Chou SH, Chien CS, Ku HH and Lo JF: Positive correlations
of Oct-4 and Nanog in oral cancer stem-like cells and high-grade
oral squamous cell carcinoma. Clin Cancer Res. 14:4085–4095. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen Z, Xu WR, Qian H, Zhu W, Bu XF, Wang
S, Yan YM, Mao F, Gu HB, Cao HL, et al: Oct4, a novel marker for
human gastric cancer. J Surg Oncol. 99:414–419. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou X, Huang GR and Hu P: Over-expression
of Oct4 in human esophageal squamous cell carcinoma. Mol Cells.
32:39–45. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang R, Jin S, Rao W, Song F, Yin Q, Wang
Y, Wang L, Xi Y, Zhang X, Wang M, et al: OVA12, a novel tumor
antigen, promotes cancer cell growth and inhibits
5-fluorouracil-induced apoptosis. Cancer Lett. 357:141–151. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Athanasoula KCh, Gogas H, Polonifi K,
Vaiopoulos AG, Polyzos A and Mantzourani M: Survivin beyond
physiology: Orchestration of multistep carcinogenesis and
therapeutic potentials. Cancer Lett. 347:175–182. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Salzano G, Riehle R, Navarro G, Perche F,
De Rosa G and Torchilin VP: Polymeric micelles containing
reversibly phospholipid-modified anti-survivin siRNA: A promising
strategy to overcome drug resistance in cancer. Cancer Lett.
343:224–231. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang J, Li Z, Lin Z, Zhao B, Wang Y, Peng
R, Wang M, Lu C, Shi G and Shen Y: 17-DMCHAG, a new geldanamycin
derivative, inhibits prostate cancer cells through Hsp90 inhibition
and survivin downregulation. Cancer Lett. 362:83–96. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kanda N, Seno H, Konda Y, Marusawa H,
Kanai M, Nakajima T, Kawashima T, Nanakin A, Sawabu T, Uenoyama Y,
et al: STAT3 is constitutively activated and supports cell survival
in association with survivin expression in gastric cancer cells.
Oncogene. 23:4921–4929. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gritsko T, Williams A, Turkson J, Kaneko
S, Bowman T, Huang M, Nam S, Eweis I, Diaz N, Sullivan D, et al:
Persistent activation of stat3 signaling induces survivin gene
expression and confers resistance to apoptosis in human breast
cancer cells. Clin Cancer Res. 12:11–19. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Glienke W, Hausmann E and Bergmann L:
Downregulation of STAT3 signaling induces apoptosis but also
promotes anti-apoptotic gene expression in human pancreatic cancer
cell lines. Tumour Biol. 32:493–500. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao C, Li H, Lin HJ, Yang S, Lin J and
Liang G: Feedback activation of STAT3 as a cancer drug-resistance
mechanism. Trends Pharmacol Sci. 37:47–61. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zeng R, Tang Y, Zhou H, Liu Y, Huang J, Li
L, Liu W, Feng Y, Zhou Y, Chen T, et al: STAT3 mediates multidrug
resistance of Burkitt lymphoma cells by promoting antioxidant
feedback. Biochem Biophys Res Commun. 488:182–188. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang S, Chen M, Shen Y, Shen W, Guo H,
Gao Q and Zou X: Inhibition of activated Stat3 reverses drug
resistance to chemotherapeutic agents in gastric cancer cells.
Cancer Lett. 315:198–205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nagaraj NS, Washington MK and Merchant NB:
Combined blockade of Src kinase and epidermal growth factor
receptor with gemcitabine overcomes STAT3-mediated resistance of
inhibition of pancreatic tumor growth. Clin Cancer Res. 17:483–493.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guo Y, Mantel C, Hromas RA and Broxmeyer
HE: Oct-4 is critical for survival/antiapoptosis of murine
embryonic stem cells subjected to stress: effects associated with
Stat3/survivin. Stem Cells. 26:30–34. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Friedrichs K, Gluba S, Eidtmann H and
Jonat W: Overexpression of p53 and prognosis in breast cancer.
Cancer. 72:3641–3647. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bruix J, Gores GJ and Mazzaferro V:
Hepatocellular carcinoma: Clinical frontiers and perspectives. Gut.
63:844–855. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Umeda S, Kanda M and Kodera Y: Emerging
evidence of molecular biomarkers in hepatocellular carcinoma.
Histol Histopathol. 33:343–355. 2018.PubMed/NCBI
|
|
29
|
Yuan F, Zhou W, Zou C, Zhang Z, Hu H, Dai
Z and Zhang Y: Expression of Oct4 in HCC and modulation to
wnt/β-catenin and TGF-β signal pathways. Mol Cell Biochem.
343:155–162. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee TI, Jenner RG, Boyer LA, Guenther MG,
Levine SS, Kumar RM, Chevalier B, Johnstone SE, Cole MF, Isono K,
et al: Control of developmental regulators by Polycomb in human
embryonic stem cells. Cell. 125:301–313. 2006. View Article : Google Scholar : PubMed/NCBI
|