Introduction
Thyroid cancer (TC) is the most common endocrine
tumor and its incidence has continually increased over recent
decades (1–5). Thyroid nodules are found in 50–60% of
the population in adulthood, while only 5% of thyroid nodules are
malignant (6). A previous study
found that preoperative image examination and operation process
missed 33% of lymph node metastases in TC patients (7). As a result, the main challenge in the
management of thyroid nodules is to rule out malignancy from
massive thyroid nodules and predict lymph node metastasis.
The diagnosis of TC is typically obtained through
ultrasound (US) examination and fine-needle aspiration (FNA) biopsy
(8). However, current US has an
accuracy of only 72.6% (9). Between
20 and 30% of FNA biopsy samples may draw incorrect or
indeterminate conclusions based on simple cytology (10,11).
The current predictive value of US and FNA cannot fully meet the
requirements of accurate diagnosis of TC. Accurate identification
of the pathological subtype and lymph node metastasis status of TC
preoperatively is important in developing individualized
therapeutic strategies.
Genetic examination, as a newly-developed technique
for cancer diagnosis, has been demonstrated to have advantages in
the early diagnosis of cancer and in accelerating the examination
process (12,13). As the most common gene mutation
found in TC, BRAF plays an important role in TC diagnosis and risk
stratification (12). With the
introduction of next-generation sequencing (NGS), high throughput
sequencing is able to detect target mutations using only a small
amount of DNA. The detection of mutations in TC is useful for
providing a specific diagnosis in cytologically indeterminate cases
(12,14).
In this study, we focused on the application of NGS
for a panel of mutations in TC and evaluated its predictive
efficacy in classifying benign and malignant nodules and their
metastasis status, compared with conventional US examination and
histological diagnosis.
Materials and methods
Thyroid samples
A total of 98 formalin-fixed, paraffin-embedded
(FFPE) tissue specimens from surgically removed thyroid samples
were collected at the Department of Pathology of the First
Affiliated Hospital of Sun Yat-sen University between 2011 and
2016. Subsequent analyses were performed on 16 thyroid benign
nodules and 82 TC specimens. Based on histological results, all
tumors were classified by two independent pathologists. All
patients provided informed consent before enrollment in the study,
which was approved by the Ethics Committee of the First Affiliated
Hospital of Sun Yat-sen University.
Tissue DNA extraction
DNA was extracted using QIAamp DNA FFPE tissue kit
(Qiagen, Inc., Valencia, CA, USA) according to the manufacturer's
instructions. DNA concentration was measured using a Qubit dsDNA
assay.
NGS library preparation
DNA shearing was performed using Covaris M220,
followed by end repair, phosphorylation and adaptor ligation.
Fragments of 200–400 bp in size were selected by beads (Agencourt
AMPure XP kit; Beckman Coulter, Inc., Brea, CA, USA), followed by
hybridization with capture probe baits, hybrid selection with
magnetic beads and PCR amplification. A bioanalyzer
high-sensitivity DNA assay was performed to assess the quality and
size of the fragments. Indexed samples were sequenced on a NextSeq
500 sequencer (Illumina, Inc., San Diego, CA, USA) with pair-end
reads.
Capture-based targeted DNA
sequencing
Genetic profiles of all tissue samples were assessed
by capture-based targeted deep sequencing, using a lymphoma panel
consisting of critical exons and introns of 31 genes (Burning Rock
Biotech, Ltd., Guangzhou, China), covering 209 kb of human genomic
regions. DNA quality and size were assessed by high sensitivity DNA
assay using a bioanalyzer. All indexed samples were sequenced on a
NextSeq 500 (Illumina, Inc.) with pair-end reads. When the NGS
analysis was performed in thyroid-derived tumor samples, the white
blood cells (WBCs) from the same patient were used as their own
inner controls to ensure that the detected mutations were
definitely somatic.
Sequence data analysis
After a successful sequencing reaction, the raw
sequence data were mapped to the human genome (hg19) using BWA
Aligner 0.7.10. Local alignment optimization, variant calling and
annotation were performed using GATK 3.2, MuTect and VarScan.
Variants were filtered using the VarScan fpfilter pipeline. At
least 5 supporting reads were required for INDELs, while 8
supporting reads were required for SNVs to be defined. According to
the ExAC, 1000 Genomes, dbSNP and ESP6500SI–V2 databases, variants
with population frequency >0.1% were grouped as SNPs and
excluded from further analysis. Remaining variants were annotated
with ANNOVAR and SnpEff v3.6. DNA translocation analysis was
performed using both TopHat2 and Factera 1.4.3.
Thyroid Imaging Reporting and Data
System (TI-RADS) category
According to TI-RADS (15), thyroid nodules classified as
category 4 with at least one suspicious US feature are believed to
have a high probability of malignancy. The following US features
showed a significant association with malignancy: solid component,
hypoechogenicity, marked hypoechogenicity, microlobulated or
irregular margin, microcalcification and taller-than-wide shape.
Thyroid nodules without the above features were classified as
category <4 (low risk).
Statistical analysis
Calculations of specificity, sensitivity, positive
predictive value (PPV), negative predictive value (NPV) and
receiver operator characteristic (ROC) were conducted using IBM
SPSS 16.0 for Windows (SPSS, Inc., Chicago, IL, USA). Correlations
were studied using Spearman's rank correlation coefficients.
Significance level was set as α=0.05.
Results
Thyroline NGS panel design
To design a NGS sequencing panel for thyroid nodule
patients, we conducted a review-based search in PubMed to collect
TC-related genetic information. These gene mutations and fusions
were found in particular subtypes of TC (Fig. 1). Papillary thyroid cancer (PTC) and
anaplastic thyroid cancer (ATC) had the most related gene
mutations. The literature reported that BRAF, RAS, TERT, ETV6,
EIF1AX, GNAS, PIK3CA, TP53 and NTRK1 mutations, as well as RET and
ALK fusions, were found in PTC. BRAF, TERT, ALK fusion, GNAS, AKT1,
PIK3CA, TP53 and PTEN were found in ATC. Fewer gene mutations were
found in medullary thyroid cancer (MTC) and follicular thyroid
cancer (FTC). RAS, TERT, TSHR, GNAS, PENT and TP53 were found in
FTC, while only RET and RAS mutations were found in MTC. The
workflow of the NGS mutation analysis is shown in Fig. 1.
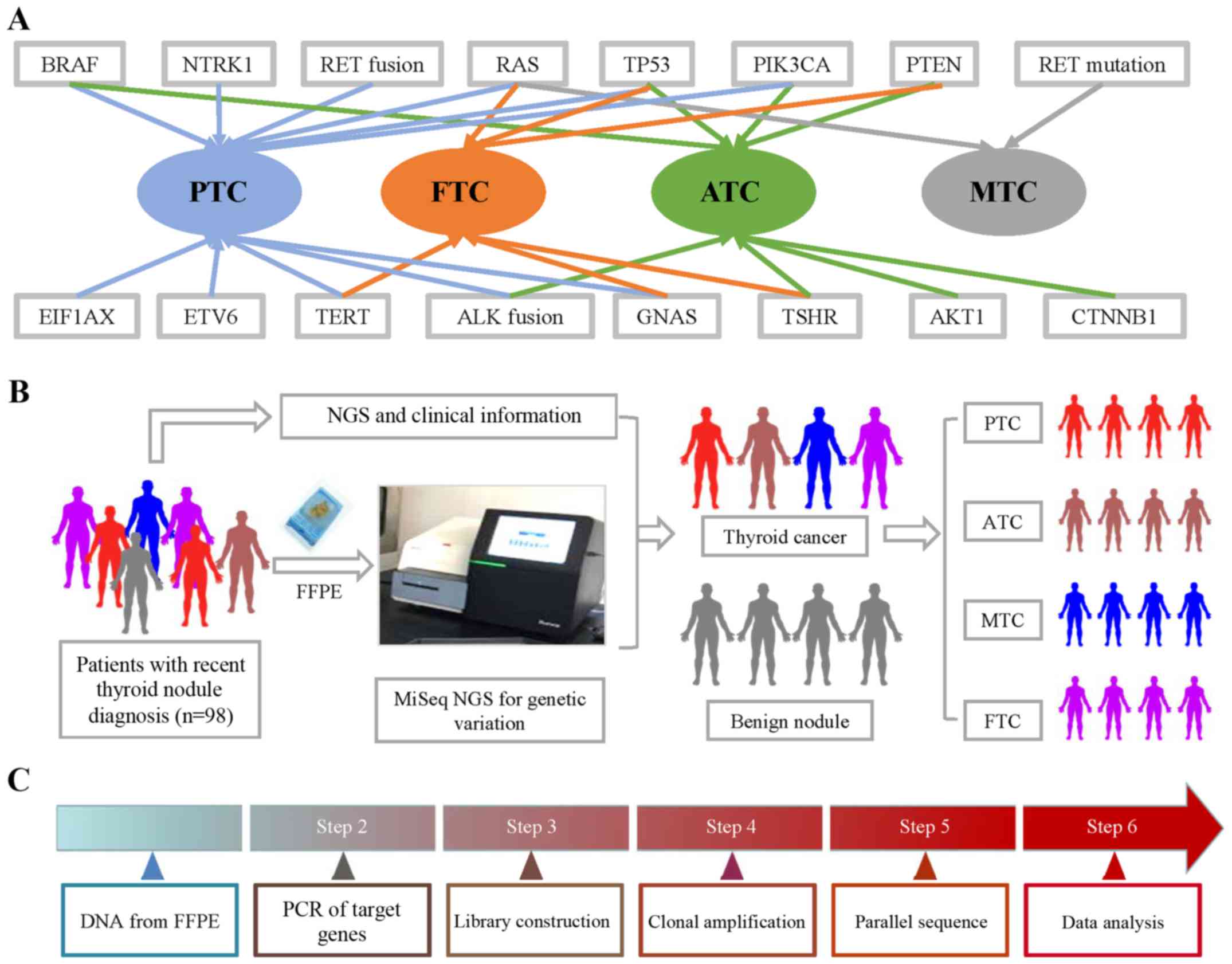 | Figure 1.Gene mutations and fusions in
subtypes of TC and workflow of NGS. (A) BRAF, RAS, TERT, ETV6,
EIF1AX, GNAS, PIK3CA, TP53 and NTRK1 mutations, as well as RET and
ALK fusions, were found in PTC. BRAF, TERT, ALK fusion, GNAS, AKT1,
PIK3CA, TP53 and PTEN were found in ATC. RAS, TERT, TSHR, GNAS,
PENT and TP53 were found in FTC, while only RET and RAS mutations
were found in MTC. (B) FFPE samples were obtained from 98 thyroid
nodule patients, which was followed by CTC enumeration on
NanoVelcro Chips. After collecting clinical information, we
analyzed the correlation between pathological information and NGS
results. (C) DNA from FFPE tissue was amplified for enrichment of
target regions in a multiplex PCR reaction. Then, the library was
prepared by ligating the PCR amplicons into platform-specific
adapters and adding bar codes for specimen multiplexing. Finally,
the library was enriched by clonal amplification (emPCR) and
sequenced by massively parallel sequencing on the Ion Torrent PGM.
The data analysis and variant calling were performed using
bioinformatic pipelines followed by a custom SeqReporter algorithm
for filtering and annotation of genetic variants. TC, thyroid
cancer; NGS, next-generation sequencing; PTC, papillary thyroid
cancer; ATC, anaplastic thyroid cancer; FTC, follicular thyroid
cancer; MTC, medullary thyroid cancer; FFPE, formalin-fixed,
paraffin-embedded. |
Demographic and nodule
characteristics
A total of 98 patients with thyroid nodules who had
undergone US and pathological examination were included (Table I). DNA samples were taken from FFPE
tissue and amplified for sequencing. Based on the histological
diagnosis, the demographic data of these patients were separately
presented in two groups: benign (16 patients) and malignant (82
patients). Age and sex ratio (M/F) in the two groups were
48.00±6.07 years and 6/10, and 45.95±3.20 years and 25/57,
respectively. Characteristics of the nodules, including number,
size and various US features (echogenicity, blood flow,
microcalcification, aspect ratio and boundary) were collected.
Fifteen patients in the benign group and 63 patients in the
malignant group were assigned to TI-RADS category 4. The 82 TC
patients included 52 PTC, 13 FTC, 10 MTC and 7 ATC cases. Of these,
27 patients were also pathologically diagnosed with lymph node
metastasis.
 | Table I.Demographic and nodule
characteristics. |
Table I.
Demographic and nodule
characteristics.
|
| Histological
diagnosis |
|---|
|
|
|
|---|
|
| Benign group
(n=16) | Malignant group
(n=82) |
|---|
| Patient
information |
|
|
| Age
(years) | 48.00±6.07 | 45.95±3.20 |
| Sex
ratio (M/F) | 6/10 | 25/57 |
| Nodule
characteristic |
|
Unique/multinodular | 16/0 | 73/9 |
| Maximun
size |
| (mm;
mean ± SD) |
7.3±6.1 |
13.7±11.6 |
| Echo genicity |
|
Hypoechoic | 8 | 39 |
|
Isoechoic | 3 | 12 |
|
Hyperechoic | 2 | 10 |
|
Unknown | 3 | 21 |
| Blood flow |
|
Rich | 9 | 35 |
|
Normal | 1 | 0 |
|
Low | 4 | 22 |
|
Unknown | 2 | 25 |
|
Microcalcification |
|
Positive | 9 | 38 |
|
Negative | 5 | 15 |
|
Unknown | 2 | 29 |
| Aspect ratio |
|
<1 | 11 | 46 |
|
>1 | 4 | 5 |
|
Unknown | 1 | 24 |
| Boundary |
|
Clear | 7 | 33 |
|
Unclear | 6 | 18 |
|
Unknown | 3 | 31 |
| TI-RADS category
4 |
15/16 | 63/82 |
| Pathological
subtype |
|
PTC | – | 52 |
|
ATC | – | 13 |
|
MTC | – | 10 |
|
FTC | – | 7 |
| Lymph node
metastasis |
|
Positive | – | 27 |
|
Negative | – | 55 |
Gene spectrum between benign and
malignant thyroid nodules
The gene spectrum of the 98 patients is presented in
Fig. 2. In general, 86 mutations of
all types of target genes were detected in 69 patients (70.4% of 98
patients). Thyroline reported 66 cases (67.3%) in high risk,
slightly lower than the number of cases with gene mutation. A total
of 16 patients (16.3%) simultaneously carried two gene mutations
and no one had >3 different gene mutations.
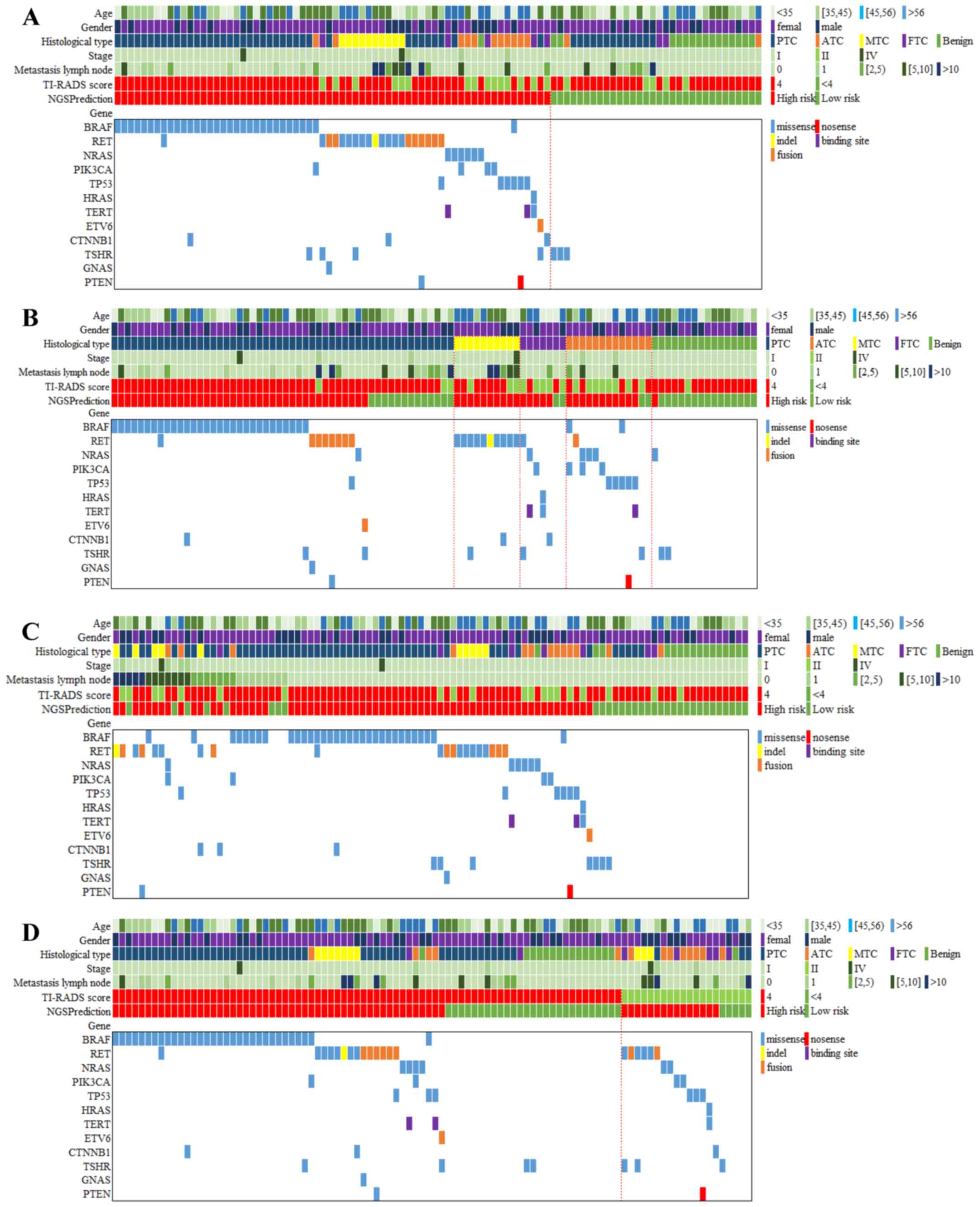 | Figure 2.Gene spectrum grouped by benign and
malignant thyroid nodules, pathological subtypes, metastatic lymph
nodes and TI-RADS. (A) The gene spectrum of the 98 patients
contained age, sex, histological diagnosis of cancer type, tumor
stage, metastasis lymph node number, TI-RADS category, NGS
prediction result and detailed genetic information. (B) The gene
spectra of the four TC subtypes varied. For PTC, BRAF mutations
were predominant, while RET mutations were detected among all the
MTC. A wide range of mutations were positive but none of them
showed dominance in ATC and FTC. (C) A total of 27 patients had 1
or more metastasis lymph nodes, 19 of whom carried 23 mutations,
including BRAF (n=8), RET (n=8), NRAS (n=1), PIK3CA (n=2), TP53
(n=1), CTNNB1 (n=2) and PTEN (n=1). (D) Among the 78 US-predicted
high-risk patients, 63 cases were diagnosed with TC by histological
examination. Out of the 20 US-predicted low-risk patients, only 1
case was diagnosed with benign thyroid nodule by histological
examination. TI-RADS, Thyroid Imaging Reporting and Data System;
NGS, next-generation sequencing; TC, thyroid cancer; PTC, papillary
thyroid cancer; MTC, medullary thyroid cancer; ATC, anaplastic
thyroid cancer; FTC, follicular thyroid cancer; US, ultrasound. |
The majority of mutations were missense (73 cases,
84.8%), while other mutation types included nonsense (1 case,
1.0%), indel (1 case, 1.0%), binding site (2 cases, 2.0%) and
fusion (9 cases, 9.2%). In addition to those cases confirmed to be
malignant, 16 patients were diagnosed with benign thyroid nodules
through pathological examination. Of these, 3 patients (18.75%)
were positive for gene mutations, including NRAS (n=1) and TSHR
(n=2). None of the 16 patients carried ≥2 mutations. Missense
mutations were the only mutation type for both NRAS and TSHR.
Gene spectrum in different
pathological subtypes of TC
The gene spectra of the four TC subtypes varied. For
PTC, BRAF mutations were predominant, while RET mutations were
detected among all the MTC cases. A wide range of mutations were
positive but none of them showed dominance in ATC or FTC (Fig. 2). Among the 52 PTC patients, 39
(75%) patients were identified with mutations, an indication for
high risk in NGS prediction. Specifically, BRAF mutations occurred
in 30 patients (57.69%), followed by RET (n=8), TSHR (n=2), NRAS
(n=1), TP53 (n=1), ETV6 (n=1), CTNNB1 (n=1), GNAS (n=1) and PTEN
(n=1). Seven patients (13.46%) had multiple mutations, including
BRAF/CTNNB1, BRAF/RET, BRAF/TSHR, RET/GNAS, RET/PTEN, RET/TP53 and
ETV6/TSHR (Fig. 2). Missense
mutations were the most common type of mutation (38 of 46
mutations), detected in all BRAF, NRAS, TP53, CTNNB1, TSHR, GNAS
and PTEN genes, while fusion was only observed in RET and ETV6 (8
of 46 mutations). Ten patients were confirmed to be MTC by
histological test and all of them were positive for gene mutations,
including only RET (n=10), CTNNB1 (n=1) and TSHR (n=1). Other
mutations were negative among MTC patients. Two patients (20%)
exhibited multiple mutations, RET/CTNNB1 (n=1) and RET/TSHR (n=1).
Missense mutations still accounted for the most mutations (11 of 12
overall mutations) and just 1 case was detected to be an indel
mutation. FTC was diagnosed in 7 patients, among whom 5 (71.4%)
were detected with gene mutations. A wide range of mutations were
positive, including RET (n=1), NRAS (n=1), PIK3CA (n=1), HRAS
(n=1), TERT (n=2), CTNNB1 (n=1) and TSHR (n=1). Three patients
(23.1%) had multiple mutations, NRAS/TERT, RET/TSHR and HRAS/TERT.
Missense mutation was also the most common (7 of 8 overall
mutations) mutation pattern, and only 1 TERT mutation was reported
to occur in the DNA-binding site. A total of 13 patients were
diagnosed with ATC, 12 of whom (92.31%) had gene mutations in BRAF
(n=2), RET (n=1), NRAS (n=3), PIK3CA (n=3), TP53 (n=5), TERT (n=1),
TSHR (n=1) and PTEN (n=1). Five patients had multiple mutations,
such as BRAF/PIK3CA, NRAS/PIK3CA, BRAF/TP53, TP53/PTEN and
TP53/TERT. The greatest diversity in mutation patterns occurred in
ATC patients. Missense mutations were again predominant (14 of 17
overall mutations). There was also 1 case of RET fusion mutation, 1
case of TERT binding site mutation, and 1 PTEN nonsense
mutation.
Greater prediction value for NGS
compared to US
The overall predictive abilities for thyroid nodule
malignancy by NGS and US are presented in Table II. Among the NGS high-risk
patients, 65 were confirmed to be malignant, with only 1 benign
sample. All patients with multiple mutations were malignant and
reported with high risk. In the low-risk patients, 15 were benign
and 17 were malignant. Thus, the sensitivity and specificity of NGS
prediction were 79.27 and 93.75%, respectively. The PPV and NPV for
NGS were 98.48 and 46.88%, respectively. Among the high-risk
patients identified by US examination, 63 were confirmed to be
malignant and 15 were benign. In the low-risk patients, 1 was
benign and 19 were malignant. Thus, the sensitivity and specificity
of US examination were 76.83 and 6.25%, respectively. The PPV and
NPV were 80.77 and 5.00%, respectively.
 | Table II.Comparison of the prediction value of
NGS and US for clinical TC diagnosis. |
Table II.
Comparison of the prediction value of
NGS and US for clinical TC diagnosis.
| A, NGS |
|---|
|
|---|
| Pathological
diagnosis | High risk | Low risk | Sensitivity(%) | Specificity(%) |
|---|
| Malignant | 65 | 17 | 79.27 |
|
| Benign | 1 | 15 |
| 93.75 |
| PPV | 98.48% |
|
|
|
| NPV |
| 46.88% |
|
|
|
| Histological
type | Count |
Malignant | Benign | Sensitivity
(%) |
|
| PTC | 53 | 39 | 13 |
75.00 |
| ATC | 13 | 11 | 2 |
84.62 |
| MTC | 10 | 10 | 0 | 100.00 |
| FTC | 7 | 5 | 2 |
71.43 |
|
| B, US |
|
| Pathological
diagnosis | 4 | <4 | Sensitivity
(%) | Specificity
(%) |
|
| Malignant | 63 | 19 | 76.83 |
|
| Benign | 15 | 1 |
| 6.25 |
| PPV | 80.77% |
|
|
|
| NPV |
| 5.00% |
|
|
|
| Histological
type | Count | 4 | <4 | Sensitivity
(%) |
|
| PTC | 52 | 49 | 3 | 94.23 |
| ATC | 13 | 5 | 8 | 38.46 |
| MTC | 10 | 7 | 3 | 70.00 |
| FTC | 7 | 2 | 5 | 28.57 |
In terms of different pathological subtypes, the
sensitivity of NGS was consistently >70%, particularly in MTC,
when it was up to 100%. US had the highest sensitivity in PTC, up
to 94.23%. The panel reported all the cases (100%) to be at high
risk, while only 7 cases (70%) were defined as high risk based on
US. However, the sensitivity in ATC and FTC was only 38.46 and
28.57%, respectively. A total of 11 ATC patients (84.62%) were
reported as having high risk through NGS test, slightly lower than
the number of patients with mutations. However, US only found 5 ATC
cases to be at high risk. The panel reported 5 in 7 FTC cases
(71.43%) to be high risk, and only 2 cases (28.57%) were defined as
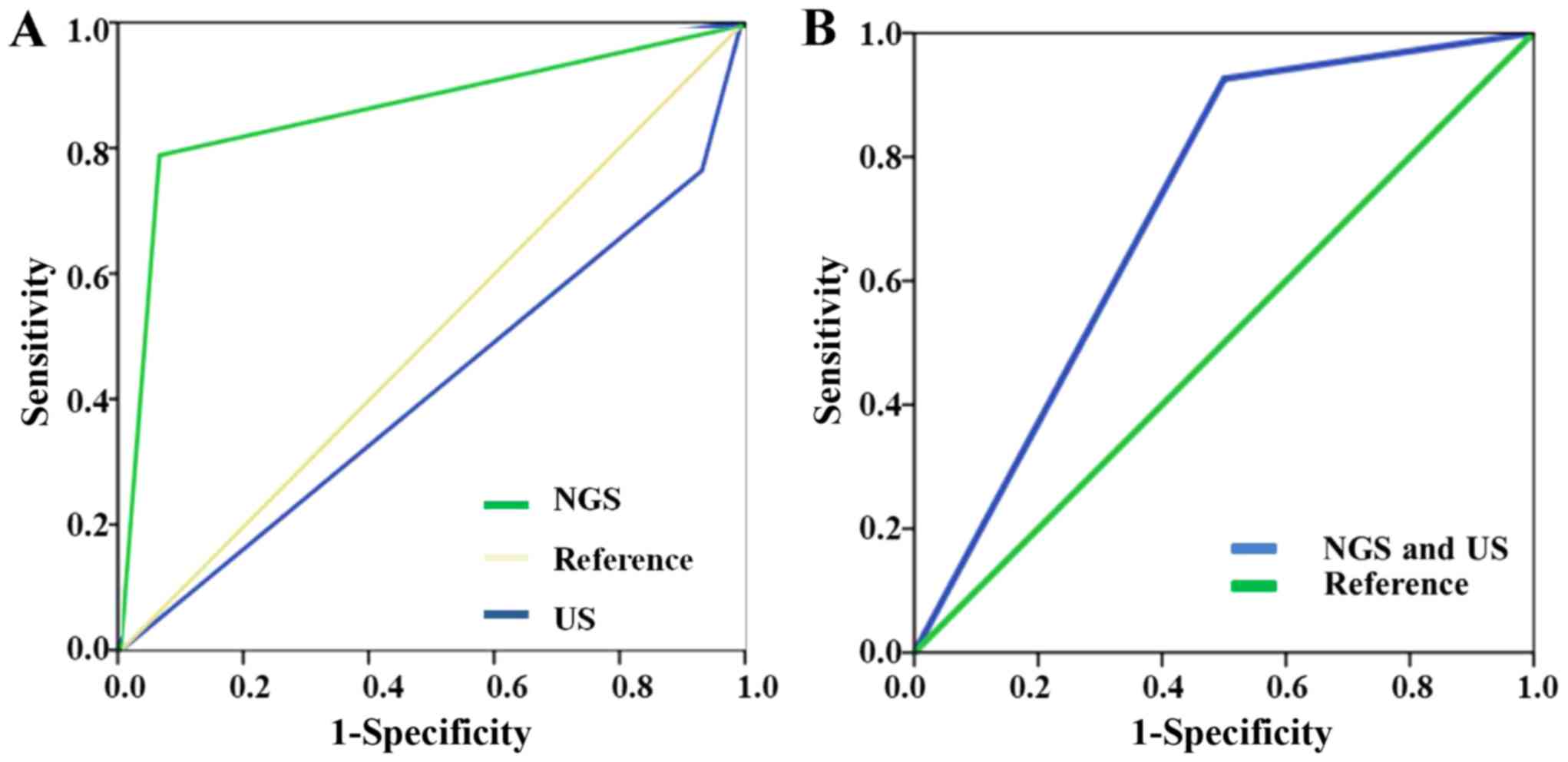
high risk through US. The ROCs of NGS and US are presented in
Fig. 3. The area under curve (AUC)
of NGS test was 0.865, while the AUC of US test was 0.415. The
prediction value of NGS was higher than that of US. In situations
where NGS and US drew the same conclusion, the sensitivity of
combined NGS and US was 92.6% and the specificity was 50%. The AUC
of combined NGS and US was 0.712.
Differential profile among lymph node
metastasis degrees of thyroid nodules
By examining the lymph nodes of all patients with
thyroid nodules, 27 patients had ≥1 metastasis lymph nodes,
including PTC (n=18), MTC (n=5), FTC (n=1) and ATC (n=3) patients.
Specifically, 5 (18.52%) patients had >10 metastasis lymph
nodes, 7 (25.93%) had 5–10 nodes, 7 (25.93%) had 2–5 nodes and 8
(29.63%) had 1 node. Patients with >1 metastatic lymph node
tended to show a greater diversity in cancer subtype, while all
patients with only 1 metastasis lymph node were diagnosed with PTC.
A total of 8 patients (29.63%) with metastasis lymph nodes were
detected with no gene mutation by NGS and reported to be at low
risk as well. The remaining patients had a variety of mutations
that differed in both gene and mutation pattern. Nineteen patients
carried 23 mutations, including BRAF (n=8), RET (n=8), NRAS (n=1),
PIK3CA (n=2), TP53 (n=1), CTNNB1 (n=2) and PTEN (n=1). However, no
correlation was found between the variant allele frequency of BRAF,
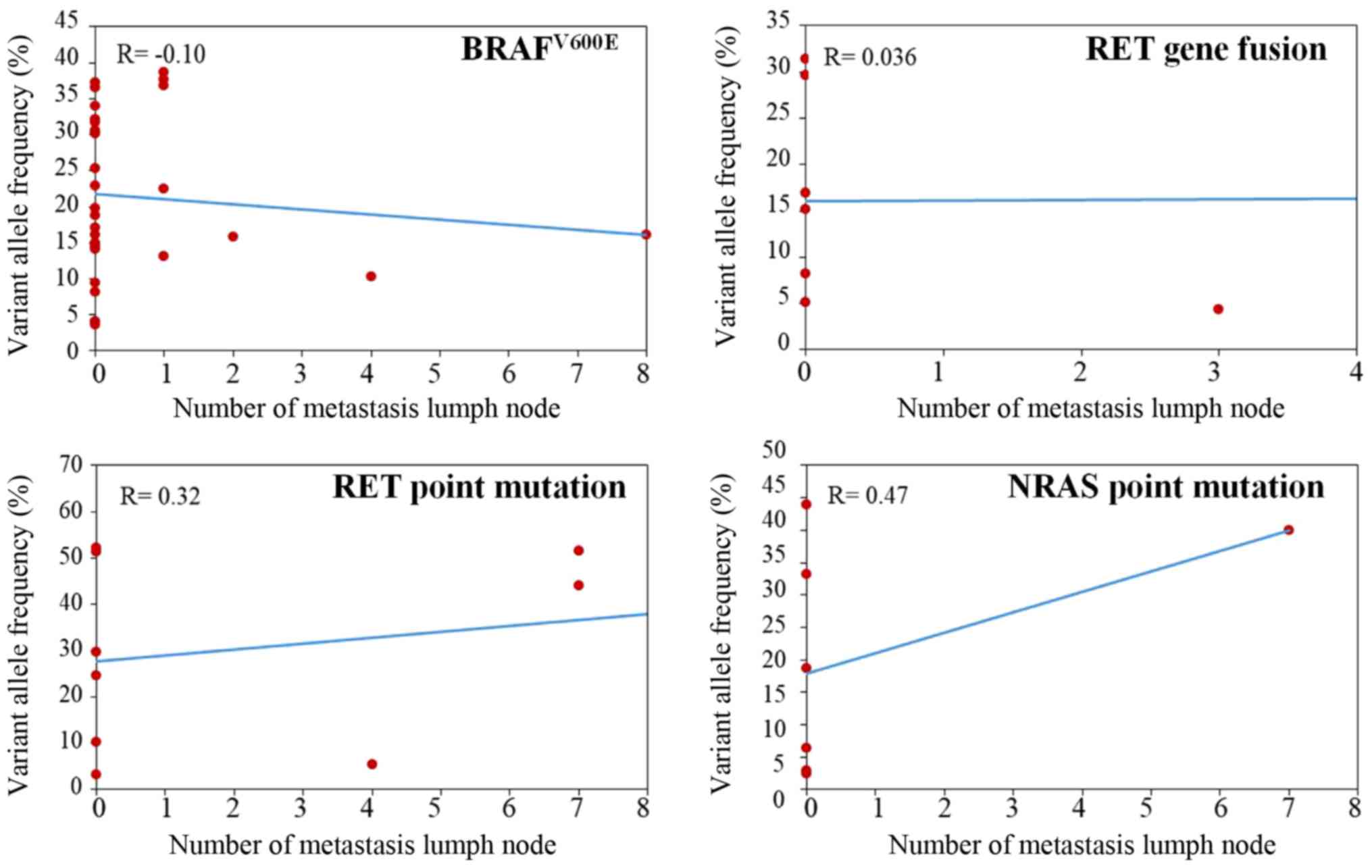
NRAS, RET mutation, RET fusion and number of metastatic lymph nodes
(Fig. 4; P>0.05).
Prediction difference for the
high-risk thyroid nodules between NGS and US
There was inconsistency in high-risk identification
between NGS and US (Fig. 2). Among
the 78 US-predicted high-risk patients, 51 (65.38%) were reported
as high risk by NGS. Among 20 US-predicted low-risk patients, only
5 (25%) were reported as low risk by NGS. We picked out two
patients (case 1 and 2) diagnosed with benign thyroid nodule by
ultrasonic diagnosis but assessed as malignant by pathological
results (Fig. 5). US showed no
typical features such as hypoechogenicity, unclear margins, rich
blood flow, microcalcification or taller-than-wide shape. However,
both cases were PTC with BRAF mutation. Simultaneously, we also
selected two other patients (case 3 and 4) with consistent results
among NGS, US and pathological diagnosis. For these cases, US
demonstrated typically malignant features, including
microcalcification and hypoechogenicity (Fig. 5). Consistently, NGS also detected
TC-related driver mutation, such as NRAS and TERT in case 3, and
TP53 and TERT in case 4.
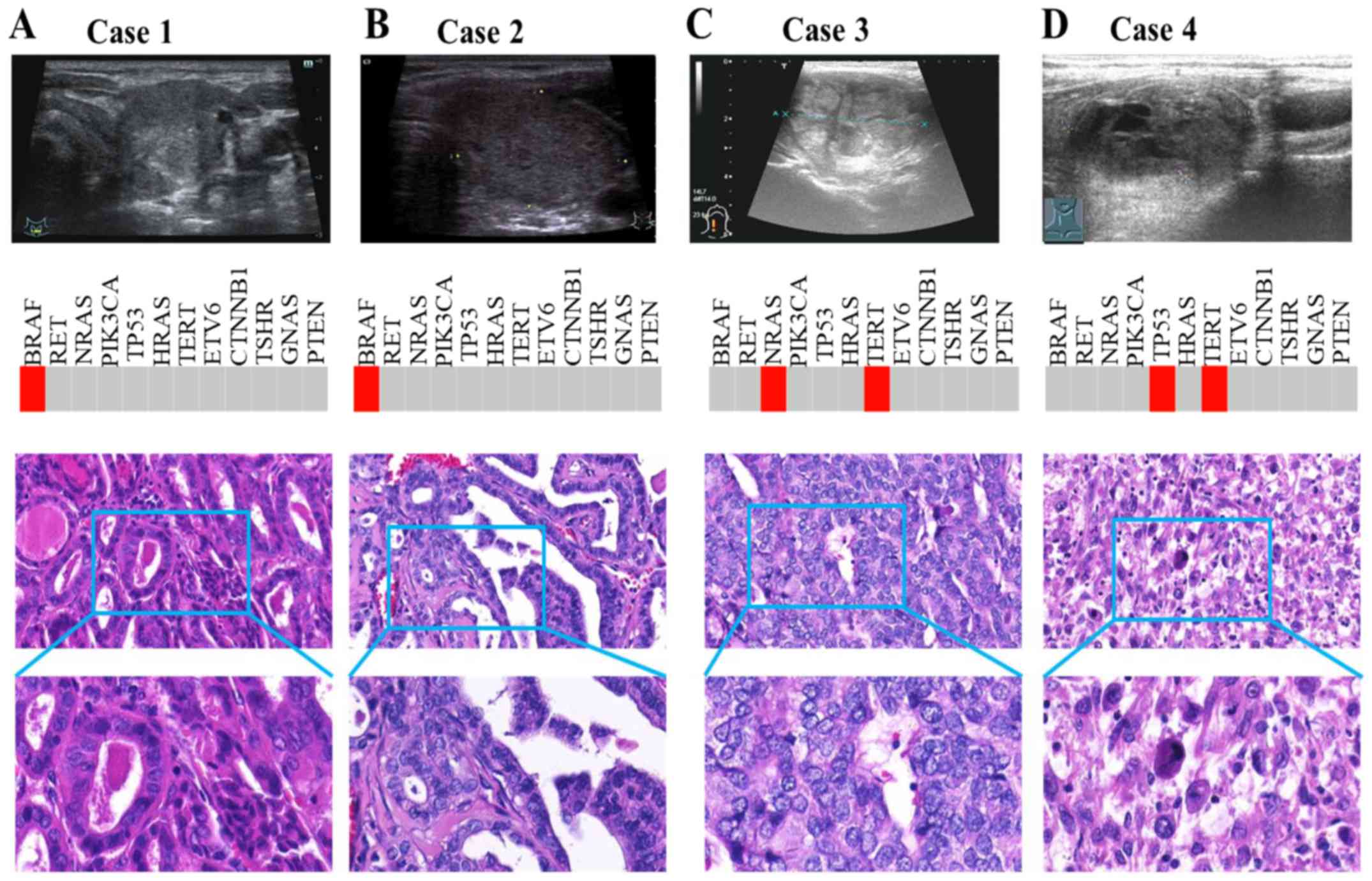 | Figure 5.Prediction difference between NGS and
US for high-risk thyroid nodules. (A and B) Case 1 and 2 were
diagnosed via US with benign thyroid nodule, but pathological
results revealed malignancy. US showed no typical features.
However, both cases were PTC with BRAF mutation. (C and D) Case 3
and 4 had consistent results among NGS, US and pathological
diagnosis. US demonstrated typically malignant features, including
microcalcification and hypoechogenicity. Consistently, NGS also
detected TC-related driver mutations, such as NRAS and TERT in case
3, and TP53 and TERT in case 4. NGS, next-generation sequencing;
US, ultrasound; PTC, papillary thyroid cancer; TC, thyroid
cancer. |
Discussion
A complete understanding of the molecular mechanisms
of tumor formation is essential for providing accurate diagnoses
and personalized treatments. In the past, single gene assays have
been commonly used for finding molecular alterations in TC. TC
harbors characteristic genetic alterations, including point
mutations for proto-oncogenes (BRAF and RAS) and chromosomal
rearrangements (RET), which vary with histological subtypes
(12,16). Presently, NGS technology can
simultaneously analyze hundreds of genes of interest using targeted
sequencing panels, indicating that NGS-based molecular tests for
oncology research and clinical practice are rapidly evolving
(17). NGS allows for simultaneous
high-throughput sequencing analysis of variable genetic alterations
and provides a comprehensive understanding of tumor biology, which
can improve diagnostic accuracy and is useful for providing
personalized treatments for TC patients.
Published literature increasingly indicates that
gene mutations can be specific biomarkers for clinical TC diagnosis
(13,16,18).
As the most common gene mutation of TC, BRAF mutations were found
in ~45–60% of PTC cases (12,19).
RAS mutations (HRAS, KRAS and NRAS) were found in 40–53% of FTC
cases (20,21). RET was commonly found to be mutated
in both familial and sporadic MTC (22). In our study, analysis of patients
with malignant nodules showed great heterogeneity in gene and
mutation patterns between different TCs. BRAF mutations were
detected in 57.69% of PTC, while RAS mutations were only found in
28.57% (2/7) of FTC cases. A previous study including 35 FTCs found
that 16 patients (45.7%) harbored RAS mutations (23). Compared with this, the low positive
rate of RAS in the current study may be attributed to the recruited
sample size (7 FTC patients). Interestingly, in the FTC subgroup,
two patients had either NRAS/TERT or HRAS/TERT multiple mutation
patterns. TERT promoter mutations are more prevalent in advanced
TCs, particularly those harboring BRAF and RAS mutations (24,25).
In addition, RET mutations were detected in all MTC patients.
Specifically, RET mutations may suggest a strong genotype-phenotype
correlation with MTC (26),
indicating that it may be a reliable biomarker for MTC
diagnosis.
In addition to well-known BRAF, RAS and RET
mutations, NGS technology facilitated detection of new somatic
alterations in TC, the significance of which has not yet been
explored in detail. We found many gene mutations in particular
tumor subtypes that are seldom reported, such as CTNNB1 and TSHR in
PTC; NRAS and TSHR in ATC; CTNNB1 in MTC; and PIK3CA and CTNNB1 in
FTC. Interestingly, TSHR was traditionally reported in FTC
(27), but found in all subtypes by
our gene spectrum. Thus, we hypothesize that TSHR may be a common
driver gene for TC. Due to sample size limitations, our gene
spectrum results may vary from previous studies (27–29),
and some mutations in our panel were negative among TC
patients.
US is the preferred non-invasive diagnostic method
recommended by current American Thyroid Association guidelines
(8). Nonetheless, the diagnosis
value of TI-RADS is controversial. A prospective study including
>2,000 patients reported that the diagnostic accuracy of US of
≥1 malignant features was only 72.6% (9). In our study, we focused on the
application of NGS for predicting biological characteristics of
thyroid nodules, including a novel comparison of the predictive
efficacy between NGS and US. Both NGS and US demonstrated a high
sensitivity in diagnosis of TC, but both showed inconsistency in
high-risk identification. TC patients with BRAF were all classified
as high risk by US. However, for those without BRAF mutations,
prediction accuracy dropped markedly. BRAF mutations were mainly
detected in PTC (12,19), and the US features varied between
subtypes (30,31). The clinical application of US-based
TI-RADS may be confined to PTC, which may lead to missed and
delayed diagnosis of other subtypes. The results suggest that NGS
technology in differentiation of benign and malignancy possess more
advantages in terms of sensitivity, specificity, PPV and NPV
compared to US. The sensitivity and specificity of combined NGS and
US were superior to US alone. Therefore, NGS in combination with US
may have better diagnostic and/or prognostic value for TC
patients.
Another purpose of the panel was to investigate its
ability to reflect lymph node metastasis status. Previous studies
found that 19.4–84% of TC patients have lymph node metastases
(32,33), 33% of which are missed by
preoperative US examination (7,34).
Patients with lymph node metastasis often need more aggressive
therapy, including radiative iodine treatment and more frequent
follow-up (8). Based on
histological examination in the current study, 27 patients had ≥1
metastatic lymph nodes. Mutations in genes including BRAF, RET,
NRAS, PIK3CA, TP53, CTNNB1 and PTEN were found in 19 patients.
These mutations may be a key driving factor for lymph node
metastasis. For deeper insight into the relationship between the
abundance of mutant allele and metastasis status, we selected BRAF,
RET and NRAS mutations as well as RET fusion for correlation
analysis. However, we did not find a significant correlation
between the variant allele frequency of aforementioned gene
mutations and the number of metastatic lymph nodes. This finding
may have been due to the small sample size. Therefore, the impact
of genetics on lymph node metastasis has yet to be determined.
The association of BRAF mutation with more
aggressive clinicopathological features and poorer outcomes has
been under debate for some time. Certain studies have demonstrated
a role of BRAF in tumor aggressiveness in PTC (35,36),
but others have failed to do so (37,38). A
new genetic alteration, the point mutation in the TERT promoter,
was recently described in TC and has been shown to be associated
with increased aggressiveness and poorer clinical prognosis
(39,40). Notably, Xing et al found that
PTC patients with coexisting BRAF and TERT mutations had the worst
clinicopathological outcomes (41).
TERT mutations were found in 12.9% (21/163) of Portuguese PTC
patients (42). However, we did not
find TERT mutations in PTC patients, or coexisting BRAF and TERT
mutations. TERT mutation was found in 4.4% (20/455) and 4.1%
(27/653) PTC patients in another two Chinese studies, respectively
(43,44). Additionally, the prevalence (14.3%,
1/7) of TERT promoter mutation in ATC patients from our study was
significantly <46.3% (25/54) and 38.7% (41/106) from Liu et
al and Shi et al respectively (39,45).
TERT promoter mutations may play an important role in distant
metastases of TC (42). However, no
ATC patients in our cohort presented distant metastases. Thus, the
reason why the mutation rate of TERT promoters was low in our
detection platform may be attributed to the small sample size of
ATC. On the other hand, it may be related with the heterogeneity of
ATC biological behavior.
In conclusion, NGS exhibits advantages in
discriminating benign and malignant thyroid nodules compared with
traditional US-based TI-RADS, simultaneously providing insight into
the pathological subtypes of TC. The novel use of NGS-based
Thyroline in conjunction with US may pave the way for increasingly
accurate and timely diagnoses of TC compared to conventional
methods. However, the current findings may assist the application
of NGS in FNA samples. A limitation of our analysis was that it was
performed in FFPE tissues, not in FNA samples. In the future, the
combined evaluation of target-medicine-related gene status included
in the Thyroline panel may be used to assist precision treatment
for TC patients, leading to improved clinical outcomes.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (81272932, 30900650, 81372501,
81572260), the Natural Science Foundation of Guangdong Province
(S2012040007756, S201281572260010008378, S2013010015327,
2013B021800126, 2015A020214010, 2016A020215055 and
2013B021800259).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZK, YL, YZ and HX conceived and designed the study.
ZK, LC and CS performed the histological examination. JLiang was a
contributor to the US examination. SP and BL were contributors in
the data statistics. ZK and YL wrote the report. JLi, MK, LS, SY,
JC and WL reviewed and edited the manuscript and were also involved
in the conception of the study. All authors read and approved the
final manuscript. All authors agreed to be accountable for all
aspects of the work in ensuring that questions related to the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The study had got the ethics approval of the Ethics
Committee of the First Affiliated Hospital of Sun Yat-sen
University and the reference number was 2017224.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
TC
|
thyroid cancer
|
|
US
|
ultrasound
|
|
FNA
|
fine-needle aspiration
|
|
NGS
|
next-generation sequencing
|
|
FFPE
|
formalin-fixed, paraffin-embedded
|
|
TI-RADS
|
Thyroid Imaging Reporting and Data
System
|
|
PPV
|
positive predictive value
|
|
NPV
|
negative predictive value
|
|
PTC
|
papillary thyroid cancer
|
|
ATC
|
anaplastic thyroid cancer
|
|
MTC
|
medullary thyroid cancer
|
|
FTC
|
follicular thyroid cancer
|
|
ROC
|
receiver operator characteristic
|
|
AUC
|
area under curve
|
References
|
1
|
Kilfoy BA, Zheng T, Holford TR, Han X,
Ward MH, Sjodin A, Zhang Y, Bai Y, Zhu C, Guo GL, et al:
International patterns and trends in thyroid cancer incidence,
1973–2002. Cancer Causes Control. 20:525–531. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rego-Iraeta A, Perez-Mendez LF, Mantinan B
and Garcia-Mayor RV: Time trends for thyroid cancer in northwestern
Spain: Τrue rise in the incidence of micro and larger forms of
papillary thyroid carcinoma. Thyroid. 19:333–340. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dal Maso L, Lise M, Zambon P, Falcini F,
Crocetti E, Serraino D, Cirilli C, Zanetti R, Vercelli M, Ferretti
S, et al: AIRTUM Working Group Incidence of thyroid cancer in
Italy, 1991–2005: Time trends and age-period-cohort effects. Ann
Oncol. 22:957–963. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Haggar FA, Preen DB, Pereira G, Holman CD
and Einarsdottir K: Cancer incidence and mortality trends in
Australian adolescents and young adults, 1982–2007. BMC Cancer.
12:1512012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ellison LF and Wilkins K: Canadian trends
in cancer prevalence. Health Rep. 23:7–16. 2012.PubMed/NCBI
|
|
6
|
Gharib H, Papini E, Garber JR, Duick DS,
Harrell RM, Hegedüs L, Paschke R, Valcavi R and Vitti P;
AACE/ACE/AME Task Force on Thyroid Nodules: American Association of
Clinical Endocrinologists, American College of Endocrinology, and
Associazione Medici Endocrinologi medical guidelines for clinical
practice for the diagnosis and management of thyroid nodules - 2016
Update. Endocr Pract. 22:622–639. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moo TA, McGill J, Allendorf J, Lee J,
Fahey T III and Zarnegar R: Impact of prophylactic central neck
lymph node dissection on early recurrence in papillary thyroid
carcinoma. World J Surg. 34:1187–1191. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Haugen BR, Alexander EK, Bible KC, Doherty
GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM,
Schlumberger M, et al: 2015 American Thyroid Association management
guidelines for adult patients with thyroid nodules and
differentiated thyroid cancer: The American Thyroid Association
Guidelines Task Force on thyroid nodules and differentiated thyroid
cancer. Thyroid. 26:1–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim SJ, Moon WK and Cho N: Sonographic
criteria for fine-needle aspiration cytology in a Korean female
population undergoing thyroid ultrasound screening. Acta Radiol.
51:475–481. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nikiforov YE, Carty SE, Chiosea SI, Coyne
C, Duvvuri U, Ferris RL, Gooding WE, Hodak SP, LeBeau SO, Ohori NP,
et al: Highly accurate diagnosis of cancer in thyroid nodules with
follicular neoplasm/suspicious for a follicular neoplasm cytology
by ThyroSeq v2 next-generation sequencing assay. Cancer.
120:3627–3634. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Suh YJ, Son EJ, Moon HJ, Kim EK, Han KH
and Kwak JY: Utility of thyroglobulin measurements in fine-needle
aspirates of space occupying lesions in the thyroid bed after
thyroid cancer operations. Thyroid. 23:280–288. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nikiforova MN, Wald AI, Roy S, Durso MB
and Nikiforov YE: Targeted next-generation sequencing panel
(ThyroSeq) for detection of mutations in thyroid cancer. J Clin
Endocrinol Metab. 98:E1852–E1860. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hsiao SJ and Nikiforov YE: Molecular
approaches to thyroid cancer diagnosis. Endocr Relat Cancer.
21:T301–T313. 2014.PubMed/NCBI
|
|
14
|
Nikiforov YE, Ohori NP, Hodak SP, Carty
SE, LeBeau SO, Ferris RL, Yip L, Seethala RR, Tublin ME, Stang MT,
et al: Impact of mutational testing on the diagnosis and management
of patients with cytologically indeterminate thyroid nodules: A
prospective analysis of 1056 FNA samples. J Clin Endocrinol Metab.
96:3390–3397. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kwak JY, Han KH, Yoon JH, Moon HJ, Son EJ,
Park SH, Jung HK, Choi JS, Kim BM and Kim EK: Thyroid imaging
reporting and data system for US features of nodules: A step in
establishing better stratification of cancer risk. Radiology.
260:892–899. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cha YJ and Koo JS: Next-generation
sequencing in thyroid cancer. J Transl Med. 14:3222016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
LeBlanc VG and Marra MA: Next-generation
sequencing approaches in cancer: Where have they brought us and
where will they take us? Cancers (Basel). 7:1925–1958. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nikiforov YE and Nikiforova MN: Molecular
genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol.
7:569–580. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chiosea S, Nikiforova M, Zuo H, Ogilvie J,
Gandhi M, Seethala RR, Ohori NP and Nikiforov Y: A novel complex
BRAF mutation detected in a solid variant of papillary thyroid
carcinoma. Endocr Pathol. 20:122–126. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lemoine NR, Mayall ES, Wyllie FS, Williams
ED, Goyns M, Stringer B and Wynford-Thomas D: High frequency of ras
oncogene activation in all stages of human thyroid tumorigenesis.
Oncogene. 4:159–164. 1989.PubMed/NCBI
|
|
21
|
Motoi N, Sakamoto A, Yamochi T, Horiuchi
H, Motoi T and Machinami R: Role of ras mutation in the progression
of thyroid carcinoma of follicular epithelial origin. Pathol Res
Pract. 196:1–7. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kloos RT, Eng C, Evans DB, Francis GL,
Gagel RF, Gharib H, Moley JF, Pacini F, Ringel MD, Schlumberger M,
et al: American Thyroid Association Guidelines Task Force Medullary
thyroid cancer: Management guidelines of the American Thyroid
Association. Thyroid. 19:565–612. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jeong SH, Hong HS, Kwak JJ and Lee EH:
Analysis of RAS mutation and PAX8/PPARγ rearrangements in
follicular-derived thyroid neoplasms in a Korean population:
Frequency and ultrasound findings. J Endocrinol Invest. 38:849–857.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Song YS, Lim JA, Choi H, Won JK, Moon JH,
Cho SW, Lee KE, Park YJ, Yi KH, Park DJ, et al: Prognostic effects
of TERT promoter mutations are enhanced by coexistence with BRAF or
RAS mutations and strengthen the risk prediction by the ATA or TNM
staging system in differentiated thyroid cancer patients. Cancer.
122:1370–1379. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Landa I, Ganly I, Chan TA, Mitsutake N,
Matsuse M, Ibrahimpasic T, Ghossein RA and Fagin JA: Frequent
somatic TERT promoter mutations in thyroid cancer: Higher
prevalence in advanced forms of the disease. J Clin Endocrinol
Metab. 98:E1562–E1566. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Frank-Raue K, Rondot S and Raue F:
Molecular genetics and phenomics of RET mutations: Impact on
prognosis of MTC. Mol Cell Endocrinol. 322:2–7. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lado-Abeal J, Celestino R, Bravo SB,
Garcia-Rendueles ME, de la Calzada J, Castro I, Castro P, Espadinha
C, Palos F, Soares P, et al: Identification of a paired box gene
8-peroxisome proliferator-activated receptor γ (PAX8-PPARγ)
rearrangement mosaicism in a patient with an autonomous functioning
follicular thyroid carcinoma bearing an activating mutation in the
TSH receptor. Endocr Relat Cancer. 17:599–610. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim SH, Kang JG, Kim CS, Ihm SH, Choi MG,
Yoo HJ and Lee SJ: 17-Allylamino-17-demethoxygeldanamycin and
herbimycin A induce cell death by modulating β-catenin and PI3K/AKT
signaling in FRO anaplastic thyroid carcinoma cells. Anticancer
Res. 35:5453–5460. 2015.PubMed/NCBI
|
|
29
|
García-Rostán G, Costa AM, Pereira-Castro
I, Salvatore G, Hernandez R, Hermsem MJ, Herrero A, Fusco A,
Cameselle- Teijeiro J and Santoro M: Mutation of the PIK3CA gene in
anaplastic thyroid cancer. Cancer Res. 65:10199–10207. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jeh SK, Jung SL, Kim BS and Lee YS:
Evaluating the degree of conformity of papillary carcinoma and
follicular carcinoma to the reported ultrasonographic findings of
malignant thyroid tumor. Korean J Radiol. 8:192–197. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Machens A, Holzhausen HJ and Dralle H: The
prognostic value of primary tumor size in papillary and follicular
thyroid carcinoma. Cancer. 103:2269–2273. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee YC, Shin SY, Kwon KH and Eun YG:
Incidence and clinical characteristics of prelaryngeal lymph node
metastasis in papillary thyroid cancer. Eur Arch Otorhinolaryngol.
270:2547–2550. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Noguchi M, Yamada H, Ohta N, Ishida T,
Tajiri K, Fujii H and Miyazaki I: Regional lymph node metastases in
well-differentiated thyroid carcinoma. Int Surg. 72:100–103.
1987.PubMed/NCBI
|
|
34
|
Rotstein L: The role of lymphadenectomy in
the management of papillary carcinoma of the thyroid. J Surg Oncol.
99:186–188. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xing M: BRAF mutation in papillary thyroid
cancer: Pathogenic role, molecular bases, and clinical
implications. Endocr Rev. 28:742–762. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim TH, Park YJ, Lim JA, Ahn HY, Lee EK,
Lee YJ, Kim KW, Hahn SK, Youn YK, Kim KH, et al: The association of
the BRAF(V600E) mutation with prognostic factors and poor clinical
outcome in papillary thyroid cancer: A meta-analysis. Cancer.
118:1764–1773. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gouveia C, Can NT, Bostrom A, Grenert JP,
van Zante A and Orloff LA: Lack of association of BRAF mutation
with negative prognostic indicators in papillary thyroid carcinoma:
The University of California, San Francisco, experience. JAMA
Otolaryngol Head Neck Surg. 139:1164–1170. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee KC, Li C, Schneider EB, Wang Y,
Somervell H, Krafft M, Umbricht CB and Zeiger MA: Is BRAF mutation
associated with lymph node metastasis in patients with papillary
thyroid cancer? Surgery. 152:977–983. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu X, Bishop J, Shan Y, Pai S, Liu D,
Murugan AK, Sun H, El-Naggar AK and Xing M: Highly prevalent TERT
promoter mutations in aggressive thyroid cancers. Endocr Relat
Cancer. 20:603–610. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Melo M, da Rocha AG, Vinagre J, Batista R,
Peixoto J, Tavares C, Celestino R, Almeida A, Salgado C, Eloy C, et
al: TERT promoter mutations are a major indicator of poor outcome
in differentiated thyroid carcinomas. J Clin Endocrinol Metab.
99:E754–E765. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xing M, Liu R, Liu X, Murugan AK, Zhu G,
Zeiger MA, Pai S and Bishop J: BRAF V600E and TERT promoter
mutations cooperatively identify the most aggressive papillary
thyroid cancer with highest recurrence. J Clin Oncol. 32:2718–2726.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Melo M, Gaspar da Rocha A, Batista R,
Vinagre J, Martins MJ, Costa G, Ribeiro C, Carrilho F, Leite V,
Lobo C, et al: TERT, BRAF, and NRAS in primary thyroid cancer and
metastatic disease. J Clin Endocrinol Metab. 102:1898–1907. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sun J, Zhang J, Lu J, Gao J, Ren X, Teng
L, Duan H, Lin Y, Li X, Zhang B, et al: BRAF V600E and TERT
promoter mutations in papillary thyroid carcinoma in Chinese
patients. PLoS One. 11:e01533192016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jin L, Chen E, Dong S, Cai Y, Zhang X,
Zhou Y, Zeng R, Yang F, Pan C, Liu Y, et al: BRAF and TERT promoter
mutations in the aggressiveness of papillary thyroid carcinoma: A
study of 653 patients. Oncotarget. 7:18346–18355. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shi X, Liu R, Qu S, Zhu G, Bishop J, Liu
X, Sun H, Shan Z, Wang E, Luo Y, et al: Association of TERT
promoter mutation 1,295,228 C>T with BRAF V600E mutation, older
patient age, and distant metastasis in anaplastic thyroid cancer. J
Clin Endocrinol Metab. 100:E632–E637. 2015. View Article : Google Scholar : PubMed/NCBI
|