Introduction
Hypopharyngeal squamous cell carcinoma (HPSCC) is a
common type of head and neck cancer that is associated with a high
invasiveness and a poor prognosis. Recently, surgical resection
along with chemoradiation therapy have been indicated as a good
modality for HPSCC treatment; however, the majority of patients
with HPSCC have an unfavorable prognosis due to diagnosis at an
advanced stage with the presence of distant metastasis and
therefore, the opportunity for radical treatment is lost. It has
been reported that the overall survival rate of patients with HPSCC
is only 15–45% (1,2). The etiological factors of HPSCC are
unclear. A series of recent studies have described that smoking
(active and passive), alcohol consumption and exposure to the human
papilloma virus may be risk factors for the development of HPSCC
(3–5).
The pathophysiological mechanisms of HPSCC are not
yet well understood. Previous studies have demonstrated that
ectopically expressed genes, microRNAs (miRNAs or miRs) and long
non-coding RNAs were involved in the initiation, development and
prognosis of HPSCC. Tumor necrosis factor receptor 1 (TNFR1) is a
membranous receptor that anchors on the surface of the cell
membrane, which belongs to the TNF receptor superfamily (6,7). The
upregulation of TNFR1 has been shown to be associated with clinical
staging, T stage, cervical lymph node metastasis and histological
grade in HPSCC (8). The
immunoglobulin heavy chain binding protein (BiP)/glucose-regulated
protein 78 (GRP78) plays an important role in the endoplasmic
reticulum stress and is known to be highly expressed in various
human neoplasms. A decreased expression of GRP78/BiP has been
reported to predict a poor overall survival and progression-free
survival of patients with advanced HPSCC (9). The downregulation of miR-140-5p has
also been shown to be associated with tumor stage and lymph node
metastasis, and the restoration of miR-140-5p inhibits cell
migration and invasion in HPSCC by targeting ADAM metallopeptidase
(ADAM10), which is involved in the Notch1 signaling pathway
(10). The expression of AB209630,
a long non-coding RNA, has been shown to be markedly lower in HPSCC
cell lines and tumor tissues compared with the normal cells and
tissues, and functions as a tumor suppressor in HPSCC. A high
expression of AB209630 significantly has been shown to inhibit the
growth, metastasis and invasion of HSPCC in vitro, and a
decreased expression of AB209630 predicts a poorer prognosis
(11). In addition, abnormal
methylation is associated with tumorigenesis in HSPCC. The
hypermethylation of G protein-coupled receptor kinase 6 (GRK6) has
been observed in HPSCC tissues compared to the matched adjacent
normal tissues. GRK6 is also associated with tumor invasion and TNM
stage in HPSCC (12).
HPSCC is a common malignancy with a poor prognosis.
To the best of our knowledge, the expression profiling of mRNAs and
miRNAs via RNA-sequencing has not yet been documented in HPSCC. In
the present study, we used high-throughput RNA-sequencing to
examine gene and miRNA expression and to investigate the functional
significance of aberrantly expressed genes and miRNAs in HSPCC,
with the aim of providing the groundwork for the elucidation of
HPSCC tumorigenesis and potential biomarkers for the early
diagnosis of HPSCC.
Materials and methods
Patients and samples
A total of 3 patients diagnosed with HPSCC were
enrolled in this study from the First People's Hospital of Jining
from August, 2015 to January, 2016. The mean age of the 3 patients
with HPSCC was 60.7 years (range, 56–64 years). All 3 patients with
HPSCC patients with squamous cell carcinoma were male. All patients
had lymph node metastasis tumor loci. Primary HPSCC tissues and
adjacent normal tissues of patients were obtained through surgical
resection. The TNM stage and tumor grade of the patients are
presented in Table I. The study was
approved by the Ethics Committee of the First People's Hospital of
Jining, and informed written consent was obtained from all
patients. Primary tumors and adjacent normal tissues of patients
with HPSCC were obtained based on the Declaration of Helsinki.
 | Table I.Basic characteristics of patients
with HPSCC. |
Table I.
Basic characteristics of patients
with HPSCC.
| Patient ID | Age (years) | Sex | Cigarettes per
day | Years of
smoking | Alcohol consumption
each day (g) | Years of alcohol
consumption | TNM stage | Histological
feature | Tumor grade |
|---|
| 1 | 62 | Male | 20 | 40 | 100 | 30 | T2N1M0 | SCC | Medium |
| 2 | 56 | Male | 20 | 30 | 250 | 30 | T2N1M0 | SCC | Medium |
| 3 | 64 | Male | 20 | 30 | No details | No details | T3N1M0 | SCC | Medium |
Isolation of RNA
A total of 6 specimens from the 3 patients were used
for RNA isolation using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) according to the manufacturer's
instructions. RNA quality and quantity were assessed using the
NanoDrop 2000 spectrophotometer (NanoDrop Technologies; Thermo
Fisher Scientific) and Agilent 2100 bioanalyzer (Agilent
Technologies GmbH, Waldron, Germany). The average RNA quantity for
the specimens was 73.42 µg (0.84–174.98 µg) with RNA integrity
number (RIN) values between 1.0 and 7.6.
Library preparation and RNA
sequencing
The Illumina TruSeq RNA Sample Prep kit (Illumina,
Inc., San Diego, CA, USA) was used for the library preparation
according to the manual. PolyT oligo-conjugated magnetic beads were
used to enrich the RNA with polyA+ tail, followed by mRNA
fragmentation, first strand cDNA synthesis, second strand cDNA
synthesis and end repair. Subsequenlty, the product was ligated to
Illumina TruSeq adaptors. Following PCR amplification, enriched
cDNA libraries were sequenced using the Illumina HiSeq 2500
sequencing platform (Illumina).
Data preprocessing
The raw data from high-throughput RNA-sequencing was
translated into raw FASTQ sequence data by base calling. Raw
RNA-sequencing data were filtered and trimmed using FASTx-Tool
SeqPrep (https://github.com/jstjohn/SeqPrep) and Sickle
(https://github.com/najoshi/sickle).
Clean and trimmed FASTQ reads were aligned to the human hg19 genome
by using TopHat (version 1.3.1; Center for Computational Biology,
Johns Hopkins University, Baltimore, MD, USA) (13). The aligned read files were then
processed using Cufflinks version 1.2.1 software package (Trapnell
Lab, Seattle, WA, USA) which assessed the abundance of genes
(14). Fragments per kilobase of
transcript per million mapped reads (FPKM) was used to determine
the transcription abundance of each gene.
Differentially expressed genes (DEGs)
and miRNAs
The DEGs between HPSCC tumor and adjacent normal
tissues were carried out using paired t-tests and limma package in
R (15). The false discovery rate
(FDR) was performed; multiple testing corrections of raw P-values
were performed using the Benjamini and Hochberg method (16,17).
The genes with an FDR <0.05 and abs (log2 fold
change) >1 were screened as DEGs. A two-way hierarchical
clustering analysis was conducted to assess the similarity of gene
expression patterns between samples, and the results were displayed
in a heatmap using the ‘pheatmap’ package (18). In addition, DEGseq (http://bioconductor.org/packages/DEGseq/) was applied
to quantify the expression of miRNAs. miRNAs with P-value <0.001
and abs (log2FC) >2 were screened as differentially
expressed miRNAs.
Analysis of the miRNA target
genes
Identifying the target genes of miRNAs is a key step
towards studying the function of miRNAs in specific tissues and
cells. In the present study, 6 miRNA-target prediction tools
(DIANAmT, miRanda, miRDB, miRWalk, PICTAR5 and TargetScan) were
applied to predict the target genes of differentially expressed
miRNAs. The miRNA-targets that were predicted by >4 algorithms
or verified by experiment in the miRWalk database were identified.
All miRNA-target pairs were obtained, which were not only predicted
by algorithms (or verified by experiment), but also negatively
associated in the expression level. The miRNA-target regulatory
network was then constructed, which was visualized using Cytoscape
(19).
Functional annotation of DEGs
The Gene Ontology (GO) function was used to
elucidate the biological function of DEGs in biological process,
molecular function and cellular component using the GeneCodis 3
online software (http://genecodis.cnb.csic.es/analysis) (20). P<0.01 was set as the cut-off for
selecting significantly enriched functional GO terms. KOBAS version
2.0 software (http://kobas.cbi.pku.edu.cn) was used to enrich the
signaling pathways of DEGs (21).
P<0.05 was set as the threshold for determining significantly
enriched pathways.
Protein-protein interaction network
(PPI)
In order to elucidate the protein interactions
between upregulated and downregulated DEGs in HPSCC, the BioGRID
database was used to screen pairs of interacting proteins (22). The PPI network was visualized using
Cytoscape (http://cytoscape.org/) (23). In the network, nodes represent
proteins and edges represent interaction between two proteins.
Validation of the expression of
selected DEGs in The Cancer Genome Atlas (TCGA)
TCGA dataset was used to validate the expression of
selected DEGs in HPSCC patients with a large sample size by using
the online software SurvExpress (http://bioinformatica.mty.
itesm.mx:8080/Biomatec/SurvivaX.jsp).
Results
RNA sequencing of HPSCC specimens
Total RNA was extracted from HPSCC tissues and
adjacent normal controls from 3 patients. A total of 5 out of 6
samples, including 1C, 1N, 3C, 3N and 2C passed the assessment for
RNA quality and quantity. Sample 2N did not pass the assessment, as
the RIN value was not applicable (Table II). A total of 5 samples were used
for library construction and RNA-sequencing. For each sample,
RNA-sequencing reads were generated and yielded 34–44 million
sequencing reads per sample (Table
III). All sequencing reads were aligned to the human hg19
genome by using TopHat and FPKM and the relative expression of
genes was determined using the Cufflinks tool.
 | Table II.RNA quantity and quality measurements
of 6 samples. |
Table II.
RNA quantity and quality measurements
of 6 samples.
| Sample ID | RNA volume
(µl) | RNA concentration
(ng/µl) | RNA amount
(µg) | RIN |
|---|
| 1C | 38 | 2,723 | 103.47 | 4 |
| 2C | 65 | 2,692 | 174.98 | 7.6 |
| 3C | 27 | 3,288 | 88.78 | 7.5 |
| 1N | 14 | 334 | 4.68 | 5.7 |
| 2N | 12 | 70 | 0.84 | N/A |
| 3N | 38 | 1,784 | 67.79 | 4.7 |
 | Table III.RNA-sequencing of the specimens. |
Table III.
RNA-sequencing of the specimens.
| Sample_ID | Total reads | Total bases | Q20% | Q30% | Average
coverage |
|---|
| 1C |
4.4×107 |
5.6×109 | 96.66 | 93.47 |
99.1863479139598 |
| 2C |
3.5×107 |
4.3×109 | 96.61 | 93.33 |
99.4073847620807 |
| 3C |
3.4×107 |
4.2×109 | 96.68 | 93.41 |
99.2552832336352 |
| 1N |
4.3×107 |
5.4×109 | 96.27 | 92.79 |
99.348671268904 |
| 3N |
3.5×107 |
4.4×109 | 96.58 | 93.32 |
99.2810150213976 |
DEGs and differentially expressed
miRNAs between primary HPSCC and adjacent normal controls
A total of 160 DEGs, including 16 upregulated and
144 downregulated genes, were identified in the HPSCC tumors
compared to the adjacent normal controls using the screening
criteria FDR<0.05 and abs (log2FC) >1 (data not
shown). In addition, a total of 79 differentially expressed miRNAs
(48 upregulated and 31 downregulated miRNAs) were identified in the
HPSCC tumors compared to adjacent normal controls using the
screening criteria P<0.001 and abs (log2FC) >2
(data not shown).
As shown in Table
IV, zinc activated ion channel (ZACN), chorionic
gonadotropin β subunit 3 (CGB3) and zinc finger protein 385C
(ZNF385C) were the top 3 upregulated genes. Ribosomal
protein L26 (RPL26), ribosomal protein S6 (RPS6) and
S-phase kinase associated protein 1 (SKP1) were the top 3
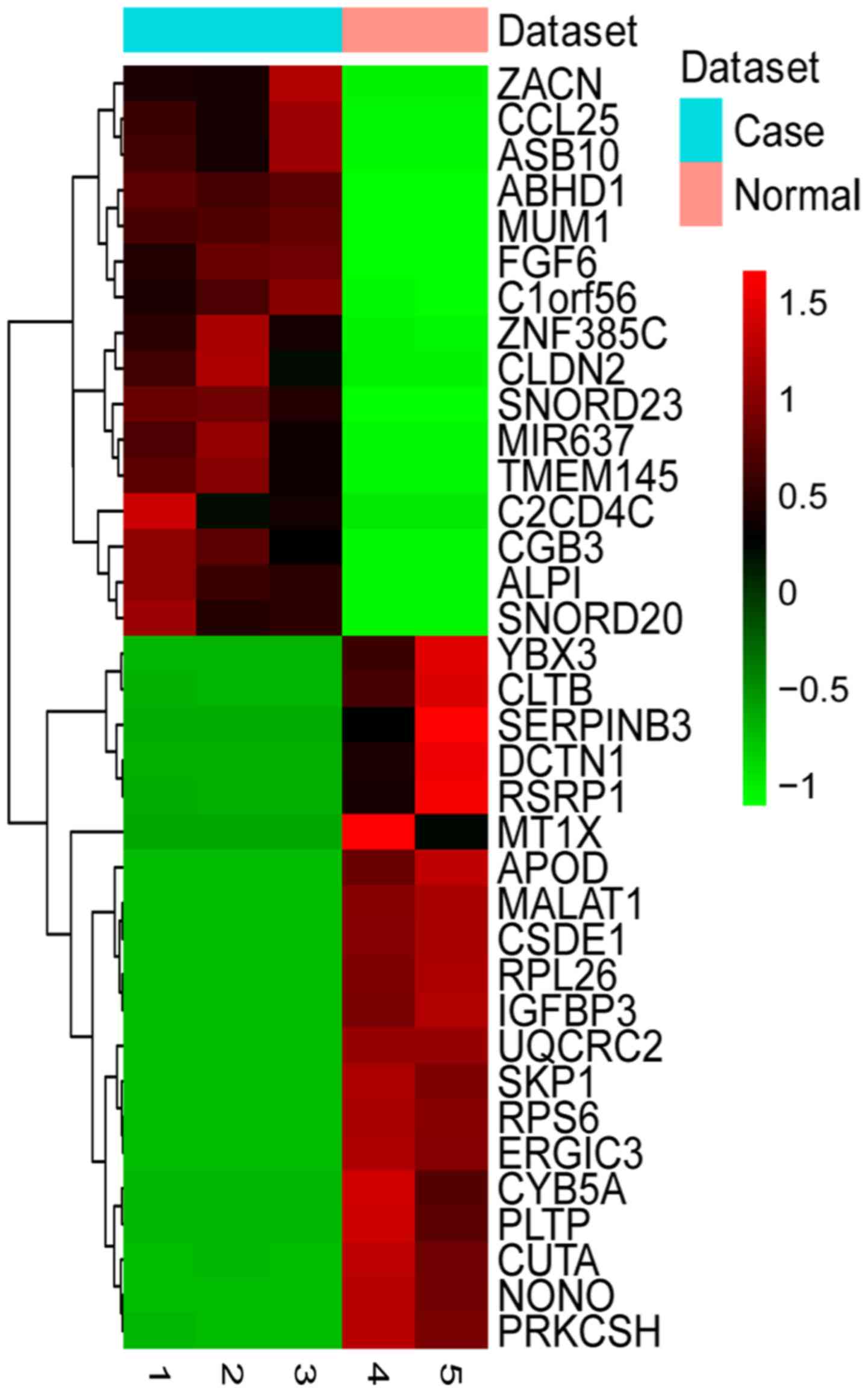
downregulated genes in HPSCC. The expression pattern of the top 20
upregulated and downregulated DEGs was examined by two-way
hierarchical clustering analysis (Fig.
1). Compared to the normal control, metastasis-associated lung
adenocarcinoma transcript 1 (MALAT1) was a significantly
downregulated long non-coding RNA in HPSCC based on our
RNA-sequencing data (FDR=0.014705). The top 20 differentially
expressed miRNAs are shown in Table
V. As shown in Table V,
hsa-let-7i-5p, hsa-miR-136-5p and hsa-miR-143-5p were the top 3
upregulated miRNAs in HPSCC, and hsa-miR-15a-5p, hsa-miR-2278 and
hsa-miR-125a-5p were the top 3 downregulated miRNAs in HPSCC.
 | Table IV.DEGs in HPSCC. |
Table IV.
DEGs in HPSCC.
| Gene ID | Gene symbol | Log2FC | P-value | FDR |
|---|
| Upregulated
genes |
|
353174 | ZACN | 6.288149 | 8.86E-05 | 0.038292 |
|
1082 | CGB3 | 5.765826 | 4.12E-05 | 0.034011 |
|
201181 | ZNF385C | 5.310858 | 0.000127 | 0.039757 |
|
126567 | C2CD4C | 5.256596 | 0.000222 | 0.049962 |
|
84696 | ABHD1 | 5.10952 | 4.07E-05 | 0.034011 |
|
6370 | CCL25 | 5.035685 | 5.76E-05 | 0.035804 |
|
693222 | MIR637 | 4.830281 | 6.51E-05 | 0.035863 |
|
692091 | SNORD23 | 4.628719 | 3.74E-05 | 0.033502 |
|
9075 | CLDN2 | 4.580931 | 0.000195 | 0.047531 |
|
6082 | SNORD20 | 4.49704 | 8.54E-05 | 0.038072 |
|
284339 | TMEM145 | 4.395233 | 8.36E-05 | 0.038072 |
|
2251 | FGF6 | 3.962287 | 6.55E-05 | 0.035863 |
|
136371 | ASB10 | 3.961177 | 0.000138 | 0.040535 |
|
248 | ALPI | 3.959662 | 7.79E-05 | 0.038041 |
|
54964 | C1orf56 | 3.849454 | 0.000166 | 0.044327 |
|
84939 | MUM1 | 3.145926 | 9.19E-05 | 0.038292 |
| Downregulated
genes |
|
6154 | RPL26 | −10.6837 | 1.06E-06 | 0.014705 |
|
6194 | RPS6 | −9.89489 | 1.14E-06 | 0.014705 |
|
6500 | SKP1 | −7.78013 | 3.26E-06 | 0.030707 |
|
8531 | YBX3 | −7.37473 | 2.26E-05 | 0.033502 |
|
347 | APOD | −7.36529 | 6.56E-06 | 0.033502 |
|
51614 | ERGIC3 | −7.28305 | 0.000122 | 0.03936 |
|
4501 | MT1X | −6.78769 | 0.000215 | 0.049189 |
|
3486 | IGFBP3 | −6.56019 | 6.70E-06 | 0.033502 |
|
7812 | CSDE1 | −6.49789 | 5.73E-06 | 0.033502 |
|
4841 | NONO | −6.45686 | 8.40E-06 | 0.033502 |
|
6317 |
SERPINB3 | −6.45555 | 0.000213 | 0.049086 |
|
5360 | PLTP | −6.29414 | 2.00E-05 | 0.033502 |
|
1528 | CYB5A | −6.28125 | 2.21E-05 | 0.033502 |
|
51596 | CUTA | −6.24975 | 0.000199 | 0.048247 |
|
57035 | RSRP1 | −6.20271 | 0.000205 | 0.048326 |
|
7385 | UQCRC2 | −6.01731 | 8.71E-06 | 0.033502 |
|
1639 | DCTN1 | −5.79807 | 0.000143 | 0.040725 |
|
1212 | CLTB | −5.70891 | 0.000204 | 0.048326 |
|
5589 | PRKCSH | −5.58244 | 8.30E-05 | 0.038072 |
|
6194 | RPS6 | −9.89489 | 1.14E-06 | 0.014705 |
|
340277 | FAM221A | −3.26588 | 1.30635 | −17.5303 |
 | Table V.Top 20 differentially expressed
miRNAs in HPSCC. |
Table V.
Top 20 differentially expressed
miRNAs in HPSCC.
| miRNA symbol | Log2FC | P-value |
|---|
| Upregulated
miRNAs |
|
hsa-let-7i-5p | 2.122547495 | 0 |
|
hsa-miR-136-5p | 2.444466751 | 0 |
|
hsa-miR-143-5p | 2.976688335 | 0 |
|
hsa-miR-370-3p | 3.344940583 | 0 |
|
hsa-miR-140-3p | 3.395556571 | 0 |
|
hsa-let-7g-5p | 9.933859336 | 8.71E-85 |
|
hsa-miR-106b-3p | 9.933859336 | 8.71E-85 |
|
hsa-miR-127-3p | 9.933859336 | 8.71E-85 |
|
hsa-miR-148b-3p | 9.933859336 | 8.71E-85 |
|
hsa-miR-187-3p | 9.933859336 | 8.71E-85 |
| Downregulated
miRNAs |
|
hsa-miR-15a-5p | −12.79407766 | 0 |
|
hsa-miR-2278 | −12.05711064 | 1.56E-243 |
|
hsa-miR-125a-5p | −10.472141 | 3.27E-107 |
|
hsa-miR-1269a | −10.472141 | 3.27E-107 |
|
hsa-miR-135b-5p | −10.472141 | 3.27E-107 |
|
hsa-miR-18a-3p | −10.472141 | 3.27E-107 |
|
hsa-miR-19b-3p | −10.472141 | 3.27E-107 |
|
hsa-miR-200b-3p | −10.472141 | 3.27E-107 |
|
hsa-miR-203b-3p | −10.472141 | 3.27E-107 |
|
hsa-miR-20a-5p | −10.472141 | 3.27E-107 |
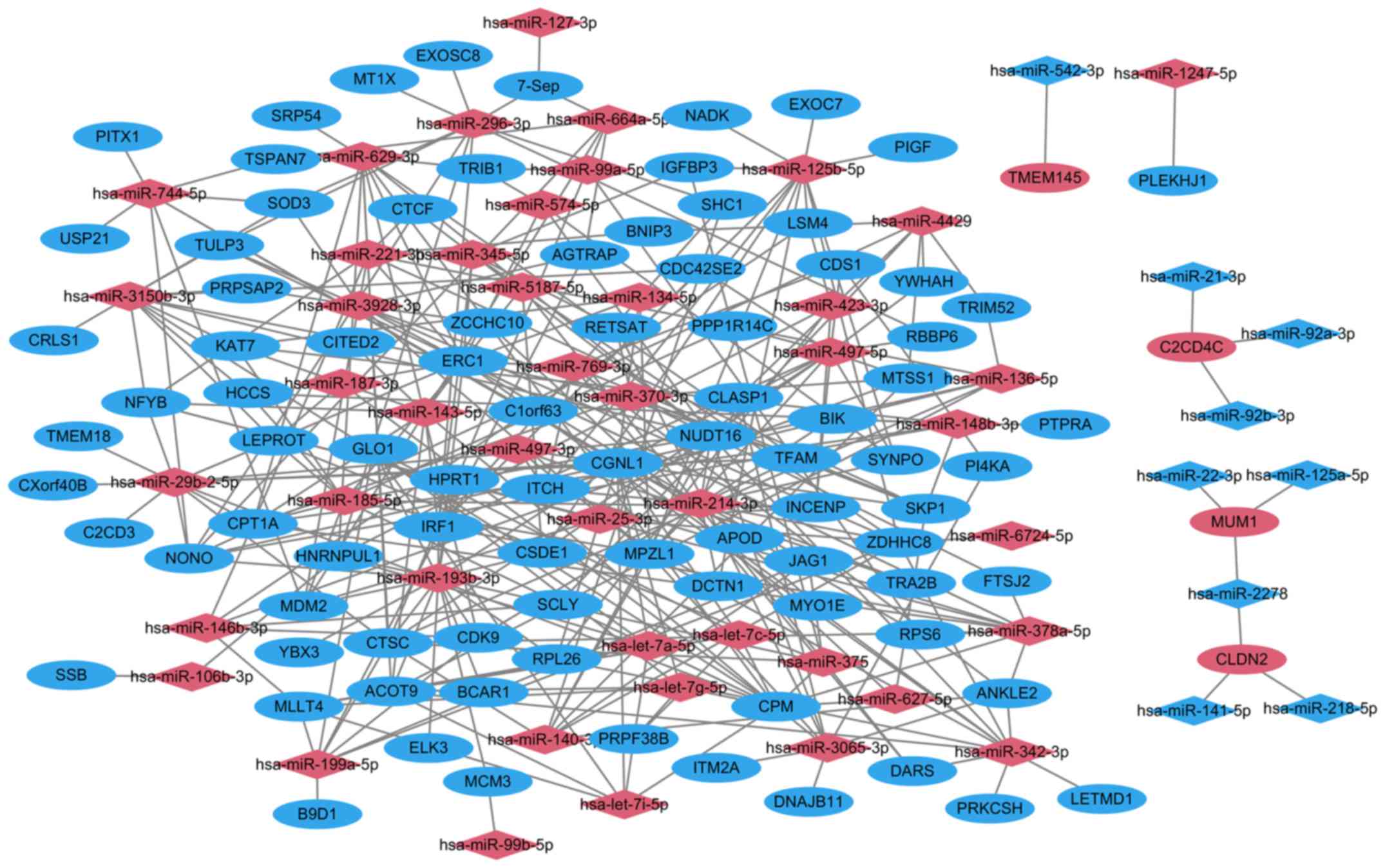
miRNA target gene interactions
Following target correlation analysis, a total of
339 miRNA-target pairs were obtained in HPSCC. A total of 228
upregulated miRNA-target pairs and 9 downregulated miRNA-target
pairs were predicted using 6 prediction tools. A total of 101
upregulated miRNAs-target pairs and 1 downregulated miRNAs-target
pair were validated in the miRWalk dataset. The top 10 miRNAs that
targeted the majority of genes are listed in Table VI. The miRNA-target network between
DEGs and differentially expressed miRNAs is presented in Fig. 2.
 | Table VI.Top 10 miRNAs that targeted the
majority of genes. |
Table VI.
Top 10 miRNAs that targeted the
majority of genes.
| miRNA |
Up/downregulated | Count of
targets | Target genes |
|---|
|
hsa-miR-193b-3p | Upregulated | 18 | CDK9, HNRNPUL1,
ELK3, ACOT9, GLO1, HPRT1, IRF1, MCM3, NONO, SCLY, RPL26, YBX3,
CTSC, ERC1, MDM2, CSDE1,CGNL1, BCAR1 |
|
hsa-miR-214-3p | Upregulated | 17 | ERC1, CDK9, SYNPO,
NUDT16, DCTN1, CLASP1, ZDHHC8, APOD, INCENP, MYO1E, BIK, TRA2B,
TFAM, ITCH, CGNL1, MPZL1, JAG1 |
|
hsa-miR-497-5p | Upregulated | 14 | RBBP6, YWHAH, CDS1,
CTSC, SYNPO, NUDT16, INCENP, IRF1, CSDE1, PPP1R14C, CGNL1, MPZL1,
MTSS1, CDC42SE2 |
|
hsa-miR-29b-2-5p | Upregulated | 13 | TMEM18, NUDT16,
CPM, CPT1A, ERC1, C2CD3, NFYB, NONO, CXorf40B, LEPROT, TFAM, CGNL1,
CTSC |
|
hsa-miR-125b-5p | Upregulated | 12 | EXOC7, LSM4, PIGF,
SYNPO, DCTN1, ZDHHC8, IGFBP3, IRF1, NADK, CSDE1, ITCH, MPZL1 |
|
hsa-miR-3150b-3p | Upregulated | 12 | KAT7, NUDT16, CPM,
ERC1, HCCS, NFYB, CRLS1, LEPROT, PRPSAP2, CDC42SE2, SOD3,
TULP3 |
|
hsa-miR-185-5p | Upregulated | 11 | KAT7, INCENP, MDM2,
NUDT16, CPM, CPT1A, ERC1, HCCS, LEPROT, PPP1R14C, MPZL1 |
|
hsa-miR-342-3p | Upregulated | 11 | DARS, ERC1, ACOT9,
LETMD1, PRKCSH RPL26, NUDT16, ZDHHC8, CGNL1, MPZL1 |
|
hsa-miR-378a-5p | Upregulated | 11 | IRF1, RPS6, CTSC,
CPM, CPT1A, ERC1, ANKLE2, FTSJ2, NFYB, TULP3, ITCH |
|
hsa-miR-629-3p | Upregulated | 11 | HNRNPUL1, ACOT9,
TRIB1, CDK9, CTSC, NUDT16, CPM, CPT1A, ERC1, SRP54, TFAM |
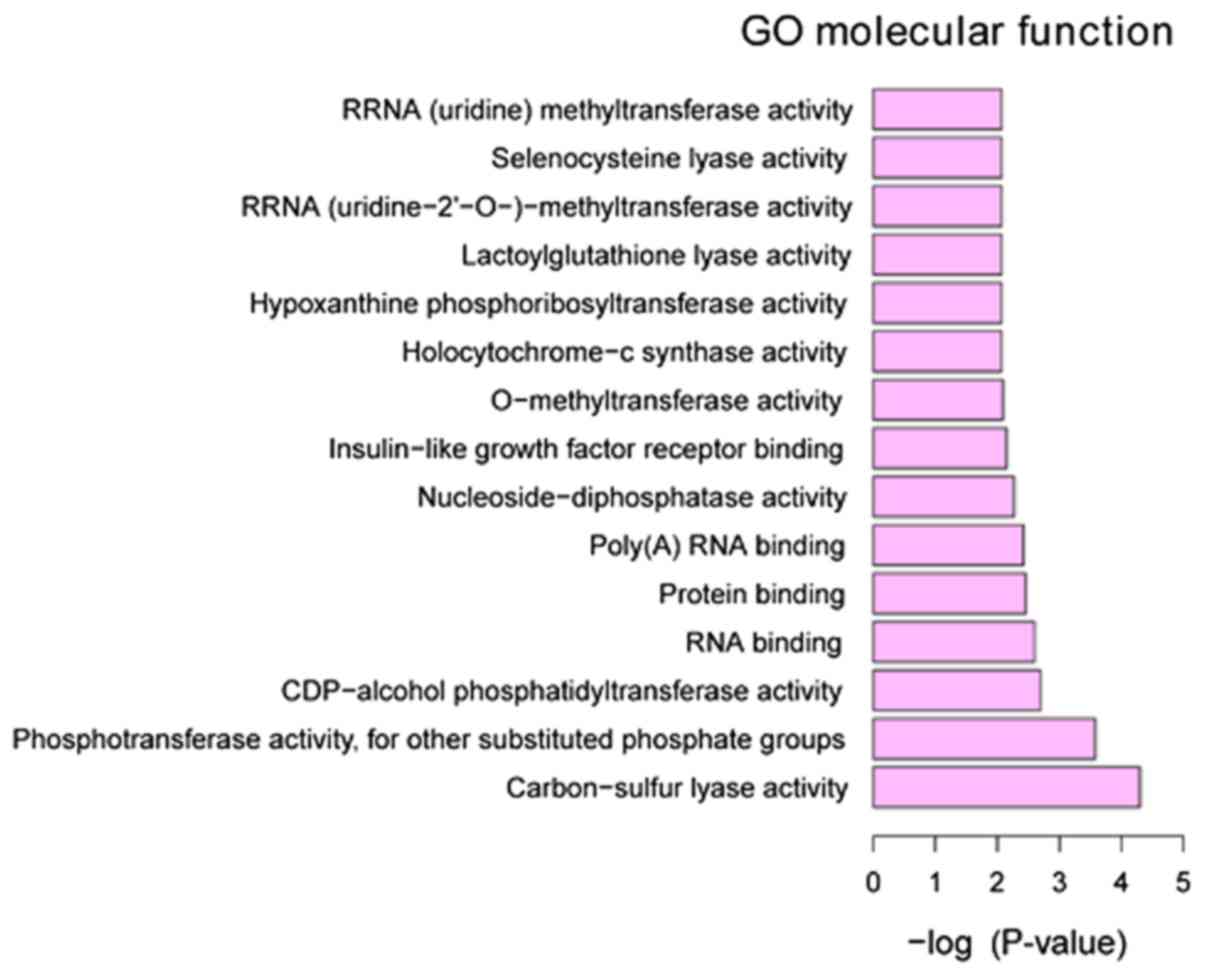
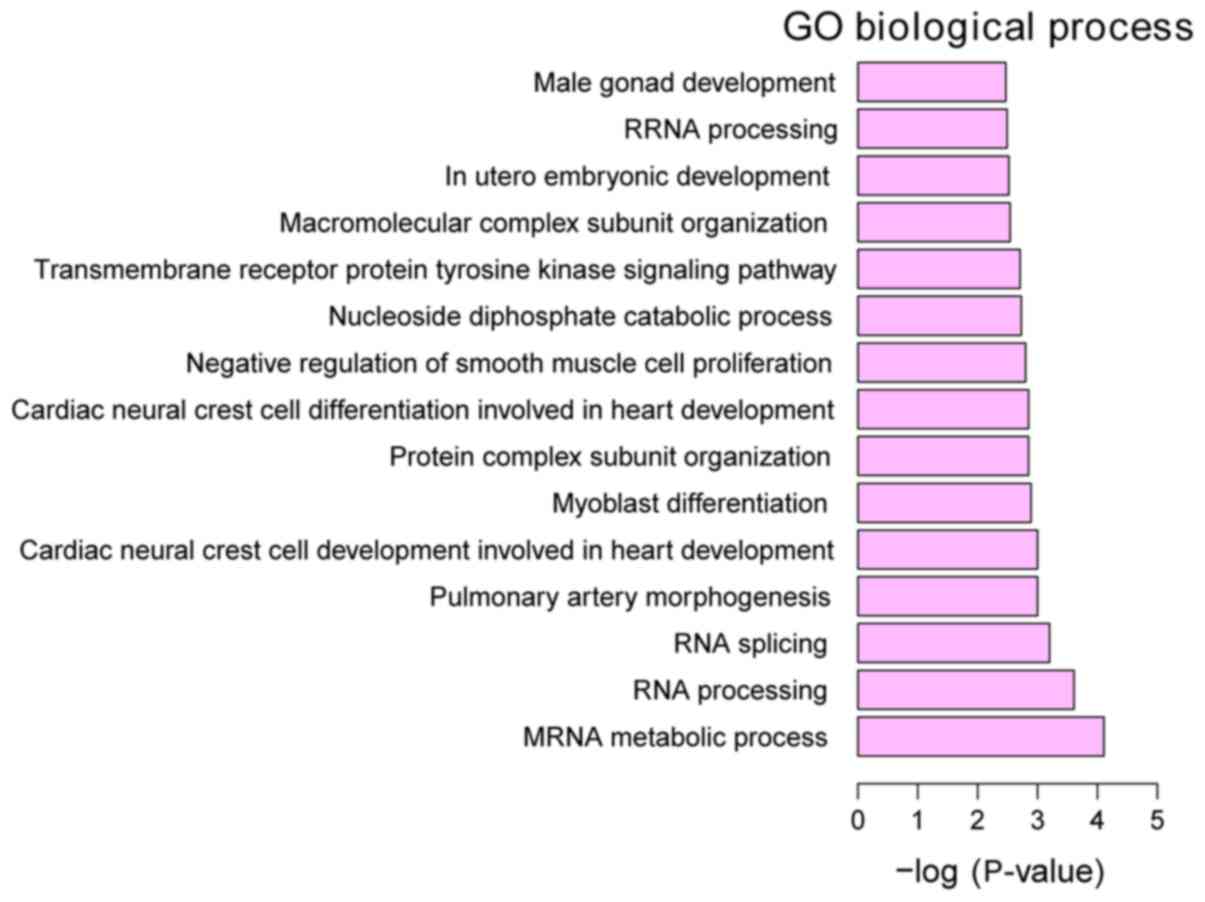
Functional annotation of DEGs
GO annotation of the 160 identified DEGs in HPSCC
was performed to elucidate the biological roles of DEGs using the
GeneCodis 3 online software. P<0.05 was used to determine
significantly enriched GO terms. The most enriched ‘molecular
functions’ of the DEGs were carbon-sulfur lyase activity (GO,
0016846), phosphotransferase activity, for other substituted
phosphate groups (GO, 0016780) and CDP-alcohol
phosphatidyltransferase activity (GO, 0017169). The most enriched
‘cellular components’ were intracellular organelle part (GO,
0044446), organelle part (GO, 0044422) and cytoplasm (GO, 0005737).
The most enriched ‘biological processes’ were mRNA metabolic
process (GO, 0016071), RNA processing (GO, 0006396) and RNA
splicing (GO, 0008380) (Figs.
3–5).
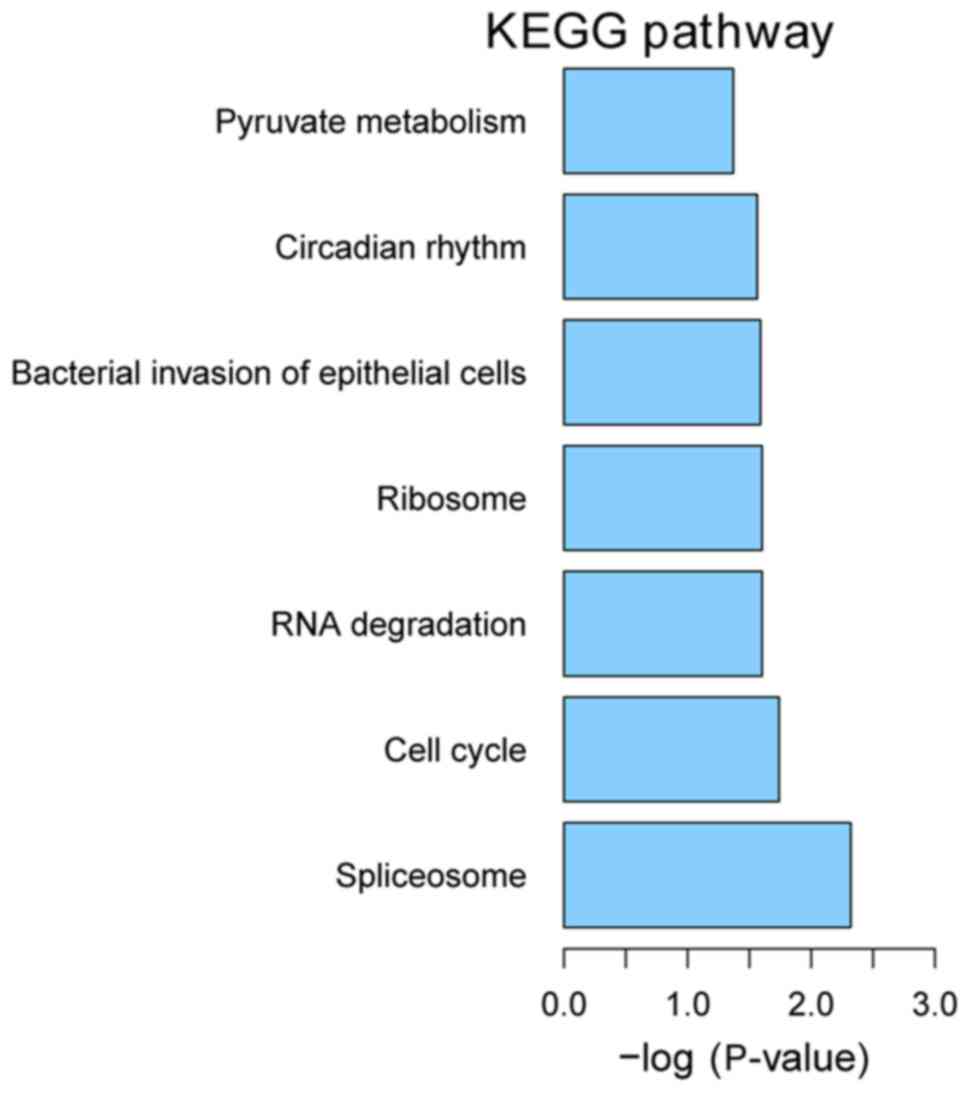
KEGG pathway enrichment
We performed KEGG pathway enrichment analysis for
DEGs using KOBAS version 2.0. P<0.05 was used as the criteria
for pathway enrichment. The most enriched pathways were spliceosome
(hsa03040), cell cycle (hsa04110) and RNA degradation (hsa03018)
(Fig. 6).
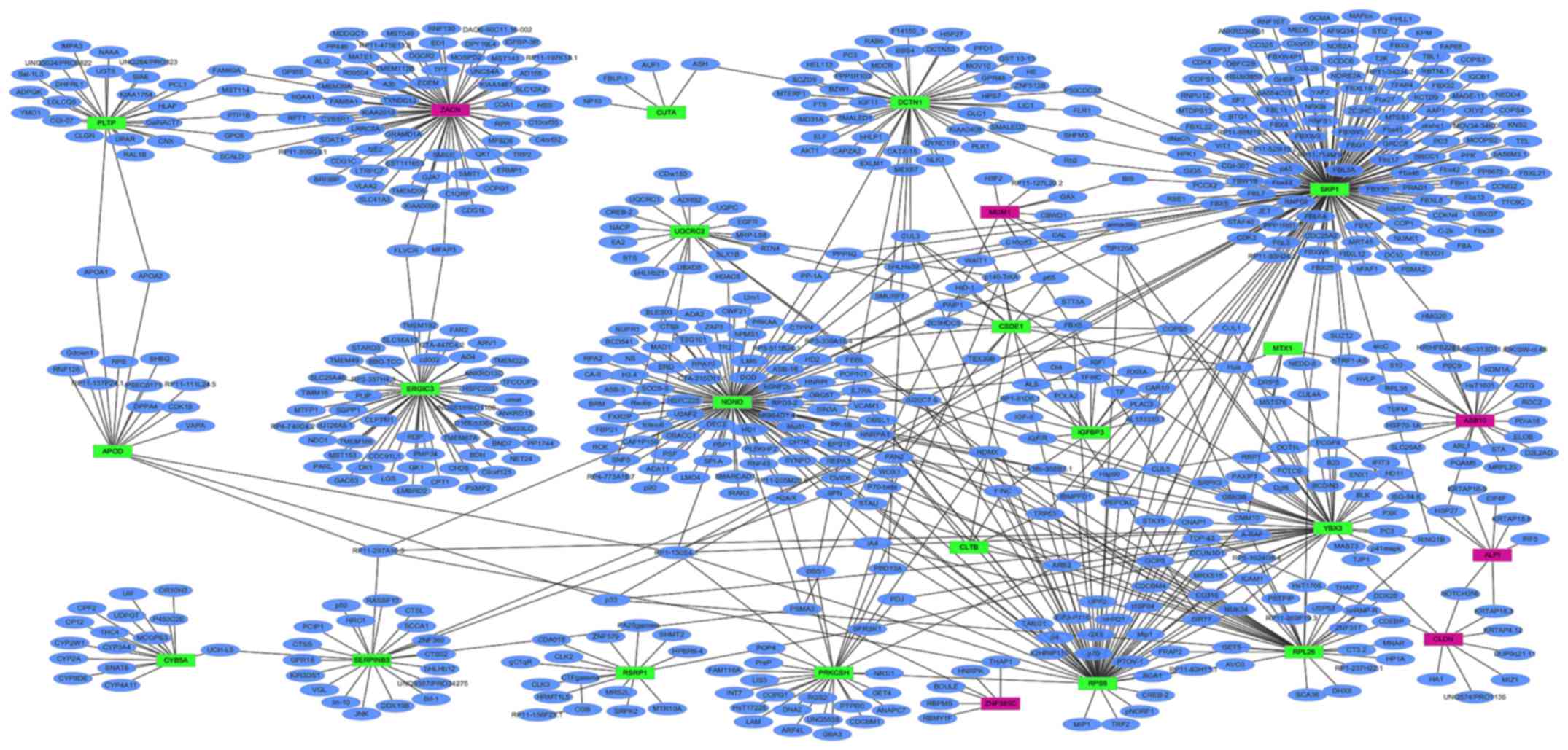
PPI network of DEGs in primary HPSCC
compared to adjacent normal controls
PPI networks of the top 20 upregulated and
downregulated DEGs in HPSCC compared to adjacent normal controls
were determined using Cytoscape, which consisted of 673 nodes and
812 edges (data not shown). The proteins that had high connectivity
with other proteins were hub proteins. In the PPI network, SKP1,
NONO and ZACN were the hub proteins, which interacted with 166, 101
and 70 proteins, respectively (Fig.
7).
Expression validation of selected DEGs
in TCGA
A total of 5 DEGs [SKP1, ZACN, NONO, cold
shock domain containing E1 (CSDE1), chorionic gonadotropin β
subunit 3 (CGB3) and MALAT1] were selected to perform
the tumor risk evaluation in HPSCC by using the head and neck
squamous cell carcinoma datasets (283 samples) in TCGA. CGB3,
SKP1 and ZACN were upregulated while MALAT1, NONO
and CSDE1 were downregulated in the HPSCC high-risk group
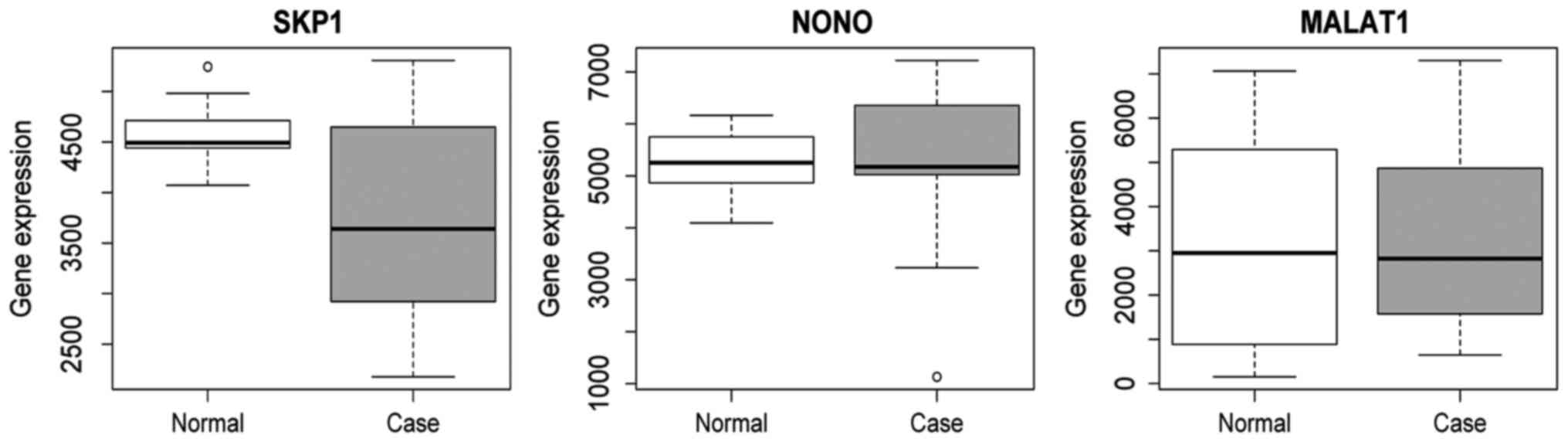
(Fig. 8). Additionally, we also
performed validation of SKP1, NONO and MALAT1 using
the HPSCC dataset in TCGA. The result showed that the expression of
SKP1, NONO and MALAT1 was decreased in HPSCC tissues
compared to the normal control, which was consistent with the
RNA-sequencing analysis (Fig.
9).
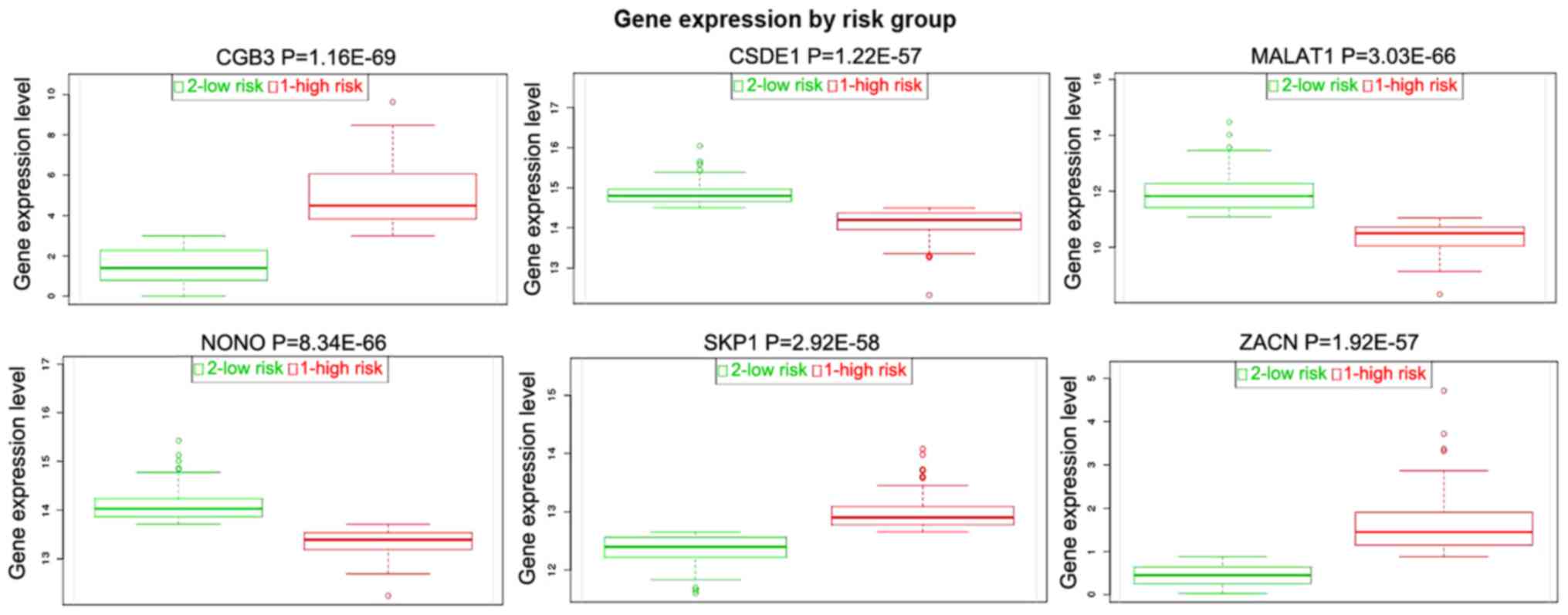 | Figure 8.Tumor risk evaluations of SKP1,
ZACN, NONO, CSDE1, CGB3 and MALAT1 in the TCGA dataset.
CGB3, chorionic gonadotropin beta subunit 3; CSDE1, cold shock
domain containing E1; MALAT1, metastasis associated in lung
adenocarcinoma transcript 1; NONO, non-POU domain containing
octamer binding; SKP1, S-phase kinase associated protein 1; TCGA,
The Cancer Genome Atlas; ZACN, zinc activated ion channel. |
Discussion
At present, there is a lack of early detection
biomarkers in clinical practice for HPSCC. To the best our
knowledge, this is the first study to report the expression pattern
of mRNAs and miRNAs in HPSCC via high-throughput RNA
sequencing.
SKP1 was significantly downregulated in HPSCC
compared to adjacent normal controls (Table IV). SKP1 had the highest
connectivity with 193 proteins (Fig.
7). According to KEGG signaling pathway enrichment, SKP1
was significantly enriched in cell cycle and circadian rhythm. In
homo sapiens, SKP1 encodes S-phase kinase-associated protein
1, which is a scaffold protein of the ubiquitin E3 ligase
Skp1/Cullin1/Rbx1/F-box protein complex (SCF complex). The SCF
complex catalyzes the ubiquitination of proteins, which are
involved in cell-cycle progression, signal transduction and
transcription (24). SKP1
was overexpressed in 56.3% of the non-small cell lung cancer
(NSCLC) specimens and elevated SKP1 was associated with a
poor prognosis (25). The other
component of the SCF complex, cullin, is highly expressed in NSCLC
tissues and significantly associated with histological
differentiation and clinical stage (26). Our finding indicated that
SKP1 may play vital roles in the development of HSPCC, which
indicates that it may serve as a diagnostic and prognostic
biomarker for HSPCC.
Apart from SKP1, ZACN (also termed L2, ZAC) was also
a hub protein according to the HSPCC-specific PPI network.
ZACN is located at chromosome 17, and it encodes
zinc-activated ion channel, which belongs to the cysteine-loop
superfamily of ligand-gated ion channels. ZACN mRNA is
expressed in various organs and tissues, including the brain,
pancreas, liver, lung, heart, kidney and skeletal muscle (27). The overexpression of ZACN was
implicated in transient neonatal diabetes mellitus, which is a rare
inherited diabetic syndrome apparent in neonate and again during
early adulthood. In a transgenic mouse model, the expression of
human ZACN and hydatidiform mole associated and imprinted
(HYMAI) impaired the development of the endocrine pancreas
and the function of β cells (28).
In the present study, ZACN was the most significantly
upregulated gene in the HPSCC dataset compared to the adjacent
normal control. The overexpression of ZACN in HPSCC was
first reported in this study. The biological roles of ZACN
overexpression in HPSCC warrant further investigation in in
vitro and in vivo studies.
NONO encodes an RNA-binding protein and
functions to regulate transcription and RNA splicing in the
nucleus. NONO has been implicated in the progression of
various tumor types, including breast and colorectal cancer and
melanoma. NONO was also reported to regulate lipid
metabolism by binding to SREBP-1A in breast cancer (29).
Gastric adenocarcinoma associated, positive CD44
regulator, long intergenic non-coding RNA (GAPLINC) promotes
cell invasion by targeting snail family transcriptional repressor 2
(SNAI2) via binding with NONO in colorectal cancer
(30). As a hub protein of the
HSPCC-specific PPI network, NONO was significantly
downregulated in HPSCC in this study. In addition, NONO was
targeted by hsa-miR-29b-2-5p (Table
VI). It has been reported that miR-29b-2-5p was potentially
associated with survival in high-grade serous ovarian carcinoma
(31). Furthermore, miR-29b-2-5p
has been shown to be downregulated in gallbladder cancer (32). It was suggested that NONO may
be involved in HPSCC under the regulation of hsa-miR-29b-2-5p.
CGB3 is a member of the glycoprotein hormone
β chain family and encodes the β 3 subunit of chorionic
gonadotropin (33). Previous
studies have indicated that the overexpression of CGB3 plays
a role in carcinogenesis (34,35).
The synthesis of human chorionic gonadotropin β subunit has been
detected in 30–50% of various malignant tumors (36,37).
In addition, upregulated CGB3 has been found to be closely
associated with the metastatic cancer phenotype, resistance to
therapy, as well as with a poor prognosis (37,38).
To date, to the best of our knowledge, no study has reported the
association between CGB3 and HSPCC. In the present study, an
increased CGB3 expression was detected in patients with
HSPCC. Hence, it can be concluded that CGB3 may be involved
in the pathogenesis of HSPCC; however, its precise function
warrants further investigation.
MALAT1 was the first long non-coding RNA
prognostic biomarker identified for early-stage NSCLC (39). The deregulation of MALAT1 has
been found in various types of cancer, including lung, breast,
pancreas, colon, prostate and liver cancer (40). Previous studies have demonstrated
that MALAT1 plays a role in cell proliferation, invasion and
tumorigenesis (41–43). In addition, the prognostic value of
MALAT1 for cancer metastasis of cancer has been reported
(44). The expression of
MALAT1 was significantly downregulated in HSPCC in this
study, which suggested that MALAT1 may be a key regulator of
tumorigenesis in HSPCC. In addition, long-term studies are required
in order to identify whether MALAT1 is a predictor for
metastasis of HSPCC.
CSDE1 (also known as UNR) encodes the
RNA-binding protein, which is found to be upregulated in melanoma
tumors, and promotes invasion and metastasis (45). The upregulation of CSDE1 has
also been identified in breast cancer (46), which suggests that CSDE1 may
be involved in tumorigenesis. In the present study, the expression
of CSDE1 was downregulated in HSPCC, which may be due to the
difference in the type of cancer tissues used. Additionally, this
study demonstrated that CSDE1 is under the regulation of
hsa-miR-497-5p and hsa-miR-125b-5p (Table VI). The expression of
hsa-miR-497-5p has been reported to be significantly decreased in
triple-negative breast cancer (47)
and to be negatively associated with survival in oropharyngeal
squamous cell carcinoma (48).
hsa-miR-125b-5p is involved in regulating the proliferation,
migration and invasion of tumor cells. The overexpression of
hsa-miR-125b-5p has been shown to inhibit cell proliferation,
migration and invasion in esophageal squamous cell carcinoma
(49). The underlying molecular
mechanism of CSDE1 under the regulation of hsa-miR-497-5p
and hsa-miR-125b-5p in HPSCC need to be elaborated in future
studies.
In differentially expressed miRNAs, we observed that
hsa-let-7i-5p, hsa-miR-136-5p and hsa-miR-143-5p were the top 3
upregulated miRNAs in HPSCC. hsa-miR-15a-5p, hsa-miR-2278 and
hsa-miR-125a-5p were the top 3 downregulated miRNAs in HPSCC.
hsa-let-7i-5p has been shown to be upregulated in triple-negative
breast cancer cells, colon cancer cells and non-muscle invasive
bladder cancer cells (50–52). The upregulation of hsa-let-7i-5p is
associated with an advanced stage, a high grade and therefore, with
the progression of clear cell renal cell carcinoma (53). hsa-miR-136-5p has been shown to be
significantly upregulated in osteosarcoma and colorectal
adenocarcinoma (54,55). hsa-miR-143-5p acts as a strong tumor
suppressive factor. It has been confirmed that hsa-miR-143-5p
inhibits the proliferation, invasion and migration of cervical
cancer cells (56). It has also
been shown that hsa-miR-15a-5p acted as a tumor suppressor by
silencing the expression of growth promoting oncogenes (57–59).
In chronic myeloid leukemia, hsa-miR-15a-5p has been shown to
suppress cell growth and metastasis (60). In addition, hsa-miR-15a-5p has been
shown to be associated with patient survival in lung adenocarcinoma
(61). hsa-miR-2278 has been shown
to be associated with survival in both rectal and colon cancer
(62). hsa-miR-125a-5p is known to
act as a tumor suppressor and is downregulated in various malignant
tumors, such as laryngeal carcinoma and verrucous carcinoma of the
head and neck (63,64). In multiple myeloma cells, the
inhibition of hsa-miR-125a-5p has been shown to reduce cell growth,
increased cell apoptosis and attenuated cell migration (65). On the whole, these differentially
expressed miRNAs may play an important role in the development of
HPSCC. In the present study, spliceosome, cell cycle and RNA
degradation were significantly enriched signal pathways in HPSCC.
Deregulated genes regulated the progression and development of
cancer via the cell cycle in various cancer types, including lung
adenocarcinoma, colorectal and head and neck cancer (44–46),
which indicated that the cell cycle pathway was implicated in the
tumorigenesis of HPSCC.
In conclusion, in the present study, we identified
160 DEGs and 79 differentially expressed miRNAs in HPSCC tissues
compared to adjacent normal tissues. The top 20 upregulated and
downregulated genes in HPSCC were used to construct PPI networks,
where SKP1, NONO and ZACN were the hub proteins. DEGs were
significantly enriched in the spliceosome, cell cycle and RNA
degradation. The present study was the first to investigate the
gene and mRNA expression profiles in HPSCC. Our findings may
provide the groundwork for the identification of early diagnosis
biomarkers for HPSCC and the mechanisms that underlie its
pathogenesis, as well as make a contribution for future drug
design. However, there are limitations to this study. Firstly, the
sample size in the RNA-sequencing was small. Therefore, large
numbers of HPSCC tumor samples are needed for further research.
Secondly, although deregulated genes, miRNAs and pathways in HPSCC
were identified, the biological functions of these genes, miRNAs
and pathways were not investigated in this study. In vitro
and in vivo experiments are essential for the elucidation of
the biological roles of DEGs and differentially expressed miRNAs in
HPSCC in future investigations.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FW, YF and YL collected the data. FL, WL and XX
analyzed and interpreted the data. HL and PG were major
contributors in writing the manuscript. HL organized all the data
and finally revised the manuscript. PG designed the project. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the First People's Hospital of Jining, and informed
written consent was obtained from all patients. Primary tumors and
adjacent normal tissues of HPSCC patients were obtained based on
the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ADAM10
|
ADAM metallopeptidase
|
|
CGB3
|
chorionic gonadotropin beta subunit
3
|
|
CSDE1
|
cold shock domain containing E1
|
|
DEGs
|
differentially expressed genes
|
|
FDR
|
false discovery rate
|
|
FPKM
|
fragments per kilobase of exon per
million fragments mapped
|
|
GRK6
|
G protein-coupled receptor kinase
6
|
|
GAPLINC
|
gastric adenocarcinoma associated,
positive CD44 regulator, long intergenic non-coding RNA
|
|
GO
|
Gene Ontology
|
|
GRP78
|
glucose-regulated protein 78
|
|
HYMAI
|
hydatidiform mole associated and
imprinted
|
|
HPSCC
|
hypopharyngeal squamous cell
carcinoma
|
|
MALAT1
|
metastasis associated lung
adenocarcinoma transcript 1
|
|
NONO
|
non-POU domain containing octamer
binding
|
|
NSCLC
|
non-small cell lung cancers
|
|
PPI
|
protein-protein interaction
|
|
RPL26
|
ribosomal protein L26
|
|
RPS6
|
ribosomal protein S6
|
|
RIN
|
RNA integrity number
|
|
SNAI2
|
snail family transcriptional repressor
2
|
|
SKP1
|
S-phase kinase associated protein
1
|
|
TCGA
|
The Cancer Genome Atlas
|
|
TNFR1
|
tumor necrosis factor receptor 1
|
|
ZACN
|
zinc activated ion channel
|
|
ZNF385C
|
zinc finger protein 385C
|
References
|
1
|
Chaturvedi AK, Engels EA, Pfeiffer RM,
Hernandez BY, Xiao W, Kim E, Jiang B, Goodman MT, Sibug-Saber M,
Cozen W, et al: Human papillomavirus and rising oropharyngeal
cancer incidence in the United States. J Clin Oncol. 29:4294–4301.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van Monsjou HS, Balm AJ, van den Brekel MM
and Wreesmann VB: Oropharyngeal squamous cell carcinoma: A unique
disease on the rise? Oral Oncol. 46:780–785. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hashibe M, Brennan P, Chuang SC, Boccia S,
Castellsague X, Chen C, Curado MP, Dal Maso L, Daudt AW, Fabianova
E, et al: Interaction between tobacco and alcohol use and the risk
of head and neck cancer: Pooled analysis in the International Head
and Neck Cancer Epidemiology Consortium. Cancer Epidemiol
Biomarkers Prev. 18:541–550. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chaturvedi AK, Engels EA, Anderson WF and
Gillison ML: Incidence trends for human papillomavirus-related and
-unrelated oral squamous cell carcinomas in the United States. J
Clin Oncol. 26:612–619. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gillison ML, Alemany L, Snijders PJ,
Chaturvedi A, Steinberg BM, Schwartz S and Castellsagué X: Human
papillomavirus and diseases of the upper airway: Head and neck
cancer and respiratory papillomatosis. Vaccine. 30 (Suppl
5):F34–F54. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tartaglia LA and Goeddel DV: Two TNF
receptors. Immunol Today. 13:151–153. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ashkenazi A and Dixit VM: Death receptors:
Signaling and modulation. Science. 281:1305–1308. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma X, Li X, Lu X, Jia L, Li H and Song Q:
Interaction between TNFR1 and TNFR2 dominates the clinicopathologic
features of human hypopharyneal carcinoma. Tumour Biol.
36:9421–9429. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kaira K, Toyoda M, Shimizu A, Imai H,
Sakakura K, Nikkuni O, Suzuki M, Iijima M, Asao T and Chikamatsu K:
Prognostic significance of GRP78/BiP expression in patients with
stage III/IV hypopharyngeal squamous cell carcinoma. Neoplasma.
63:477–483. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jing P, Sa N, Liu X, Liu X and Xu W:
MicroR-140-5p suppresses tumor cell migration and invasion by
targeting ADAM10-mediated Notch1 signaling pathway in
hypopharyngeal squamous cell carcinoma. Exp Mol Pathol.
100:132–138. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou J, Li M, Yu W, Li W, Wang J, Xiang X,
Li G, Pan X and Lei D: AB209630, a long non-coding RNA decreased
expression in hypopharyngeal squamous cell carcinoma, influences
proliferation, invasion, metastasis, and survival. Oncotarget.
7:14628–14638. 2016.PubMed/NCBI
|
|
12
|
Qiu X, Chen J, Zhang Z, You Y and Wang Z:
Aberrant GRK6 promoter methylation is associated with poor
prognosis in hypopharyngeal squamous cell carcinoma. Oncol Rep.
35:1027–1033. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Trapnell C, Pachter L and Salzberg SL:
TopHat: Discovering splice junctions with RNA-Seq. Bioinformatics.
25:1105–1111. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ghosh S and Chan CK: Analysis of RNA-Seq
data using tophat and cufflinks. Methods Mol Biol. 1374:339–361.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW,
Shi W and Smyth GK: Limma powers differential expression analyses
for RNA-sequencing and microarray studies. Nucleic Acids Res.
43:e472015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Reiner-Benaim A: FDR control by the BH
procedure for two-sided correlated tests with implications to gene
expression data analysis. Biom J. 49:107–126. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Benjamini Y and Hochberg Y: Controlling
the false discovery rate-a practical and powerful approach to
multiple testing. J Royal Stat Soc. 57:289–300. 1995.
|
|
18
|
Gołębiowski M, Sosnowska A, Puzyn T, Boguś
MI, Wieloch W, Włóka E and Stepnowski P: Application of two-way
hierarchical cluster analysis for the identification of
similarities between the individual lipid fractions of Lucilia
sericata. Chem Biodivers. 11:733–748. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Smoot ME, Ono K, Ruscheinski J, Wang PL
and Ideker T: Cytoscape 2.8: New features for data integration and
network visualization. Bioinformatics. 27:431–432. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ochs C, Perl Y, Halper M, Geller J and
Lomax J: Quality assurance of the gene ontology using abstraction
networks. J Bioinform Comput Biol. 14:16420012016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kanehisa M, Sato Y, Kawashima M, Furumichi
M and Tanabe M: KEGG as a reference resource for gene and protein
annotation. Nucleic Acids Res. 44:D457–D462. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chatr-Aryamontri A, Breitkreutz BJ,
Oughtred R, Boucher L, Heinicke S, Chen D, Stark C, Breitkreutz A,
Kolas N, O'Donnell L, et al: The BioGRID interaction database: 2015
update. Nucleic Acids Res. 43:D470–D478. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Keaton MA: Morgan DO: The cell cycle:
Principles of control (Primers in Biology). Cell Division.
2:272007. View Article : Google Scholar :
|
|
25
|
Liu YQ, Wang XL, Cheng X, Lu YZ, Wang GZ,
Li XC, Zhang J, Wen ZS, Huang ZL, Gao QL, et al: Skp1 in lung
cancer: Clinical significance and therapeutic efficacy of its small
molecule inhibitors. Oncotarget. 6:34953–34967. 2015.PubMed/NCBI
|
|
26
|
Xu M, Yang X, Zhao J, Zhang J, Zhang S,
Huang H, Liu Y and Liu J: High expression of Cullin1 indicates poor
prognosis for NSCLC patients. Pathol Res Pract. 210:397–401. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Houtani T, Munemoto Y, Kase M, Sakuma S,
Tsutsumi T and Sugimoto T: Cloning and expression of ligand-gated
ion-channel receptor L2 in central nervous system. Biochem Biophys
Res Commun. 335:277–285. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma D, Shield JP, Dean W, Leclerc I, Knauf
C, Burcelin R Ré, Rutter GA and Kelsey G: Impaired glucose
homeostasis in transgenic mice expressing the human transient
neonatal diabetes mellitus locus, TNDM. J Clin Invest. 114:339–348.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu Z, Zhao X, Zhao L, Yang H, Liu L, Li
J, Wu J, Yang F, Huang G and Liu J: p54nrb/NONO
regulates lipid metabolism and breast cancer growth through
SREBP-1A. Oncogene. 35:1399–1410. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang P, Chen T, Xu Z, Zhu H, Wang J and He
Z: Long noncoding RNA GAPLINC promotes invasion in colorectal
cancer by targeting SNAI2 through binding with PSF and NONO.
Oncotarget. 7:42183–42194. 2016.PubMed/NCBI
|
|
31
|
Vilming Elgaaen B, Olstad OK, Haug KB,
Brusletto B, Sandvik L, Staff AC, Gautvik KM and Davidson B: Global
miRNA expression analysis of serous and clear cell ovarian
carcinomas identifies differentially expressed miRNAs including
miR-200c-3p as a prognostic marker. BMC cancer. 14:802014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chandra V, Kim JJ, Mittal B and Rai R:
MicroRNA aberrations: An emerging field for gallbladder cancer
management. World J Gastroenterol. 22:1787–1799. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kubiczak M, Walkowiak GP, Nowak-Markwitz E
and Jankowska A: Human chorionic gonadotropin beta subunit genes
CGB1 and CGB2 are transcriptionally active in ovarian
cancer. Int J Mol Sci. 14:12650–12660. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hotakainen K, Lintula S, Jarvinen R, Paju
A, Stenman J, Rintala E and Stenman UH: Overexpression of human
chorionic gonadotropin beta genes 3, 5 and 8 in tumor tissue and
urinary cells of bladder cancer patients. Tumour Biol. 28:52–56.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hotakainen K, Lintula S, Ljungberg B,
Finne P, Paju A, Stenman UH and Stenman J: Expression of human
chorionic gonadotropin beta-subunit type I genes predicts adverse
outcome in renal cell carcinoma. J Mol Diagn. 8:598–603. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Iles RK: Ectopic hCGbeta expression by
epithelial cancer: Malignant behaviour, metastasis and inhibition
of tumor cell apoptosis. Mol Cell Endocrinol. 260-262:264–270.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Iles RK, Delves PJ and Butler SA: Does hCG
or hCGβ play a role in cancer cell biology? Mol Cell Endocrinol.
329:62–70. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hotakainen K, Ljungberg B, Haglund C,
Nordling S, Paju A and Stenman UH: Expression of the free
beta-subunit of human chorionic gonadotropin in renal cell
carcinoma: Prognostic study on tissue and serum. Int J Cancer.
104:631–635. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wu Y, Lu W, Xu J, Shi Y, Zhang H and Xia
D: Prognostic value of long non-coding RNA MALAT1 in cancer
patients. Tumour Biol. 37:897–903. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lin R, Maeda S, Liu C, Karin M and
Edgington TS: A large noncoding RNA is a marker for murine
hepatocellular carcinomas and a spectrum of human carcinomas.
Oncogene. 26:851–858. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang MH, Hu ZY, Xu C, Xie LY, Wang XY,
Chen SY and Li ZG: MALAT1 promotes colorectal cancer cell
proliferation/migration/invasion via PRKA kinase anchor protein 9.
Biochim Biophys Acta. 1852:166–174. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wu XS, Wang XA, Wu WG, Hu YP, Li ML, Ding
Q, Weng H, Shu YJ, Liu TY, Jiang L, et al: MALAT1 promotes the
proliferation and metastasis of gallbladder cancer cells by
activating the ERK/MAPK pathway. Cancer Biol Ther. 15:806–814.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ying L, Chen Q, Wang Y, Zhou Z, Huang Y
and Qiu F: Upregulated MALAT-1 contributes to bladder cancer cell
migration by inducing epithelial-to-mesenchymal transition. Mol
Biosyst. 8:2289–2294. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tano K and Akimitsu N: Long non-coding
RNAs in cancer progression. Front Genet. 3:2192012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wurth L, Papasaikas P, Olmeda D, Bley N,
Calvo GT, Guerrero S, Cerezo-Wallis D, Martinez-Useros J,
García-Fernández M, Hüttelmaier S, et al: UNR/CSDE1 drives a
post-transcriptional program to promote melanoma invasion and
metastasis. Cancer Cell. 30:694–707. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fang H, Yue X, Li X and Taylor JS:
Identification and characterization of high affinity antisense PNAs
for the human unr (upstream of N-ras) mRNA which is uniquely
overexpressed in MCF-7 breast cancer cells. Nucleic Acids Res.
33:6700–6711. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chang YY, Kuo WH, Hung JH, Lee CY, Lee YH,
Chang YC, Lin WC, Shen CY, Huang CS, Hsieh FJ, et al: Deregulated
microRNAs in triple-negative breast cancer revealed by deep
sequencing. Mol Cancer. 14:362015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wong N, Khwaja SS, Baker CM, Gay HA,
Thorstad WL, Daly MD, Lewis JS Jr and Wang X: Prognostic microRNA
signatures derived from The Cancer Genome Atlas for head and neck
squamous cell carcinomas. Cancer Med. 5:1619–1628. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mei LL, Wang WJ, Qiu YT, Xie XF, Bai J and
Shi ZZ: miR-125b-5p functions as a tumor suppressor gene partially
by regulating HMGA2 in esophageal squamous cell carcinoma. PLoS
One. 12:e01856362017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Qattan A, Intabli H, Alkhayal W, Eltabache
C, Tweigieri T and Amer SB: Robust expression of tumor suppressor
miRNA's let-7 and miR-195 detected in plasma of Saudi female breast
cancer patients. BMC Cancer. 17:7992017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Choo KB, Soon YL, Nguyen PN, Hiew MS and
Huang CJ: MicroRNA-5p and −3p co-expression and cross-targeting in
colon cancer cells. J Biomed Sci. 21:952014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Armstrong DA, Green BB, Seigne JD, Schned
AR and Marsit CJ: MicroRNA molecular profiling from matched tumor
and bio-fluids in bladder cancer. Mol Cancer. 14:1942015.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Gowrishankar B, Ibragimova I, Zhou Y,
Slifker MJ, Devarajan K, Al-Saleem T, Uzzo RG and Cairns P:
MicroRNA expression signatures of stage, grade, and progression in
clear cell RCC. Cancer Biol Ther. 15:329–341. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lauvrak SU, Munthe E, Kresse SH, Stratford
EW, Namløs HM, Meza-Zepeda LA and Myklebost O: Functional
characterisation of osteosarcoma cell lines and identification of
mRNAs and miRNAs associated with aggressive cancer phenotypes. Br J
Cancer. 109:2228–2236. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zheng G, Du L, Yang X, Zhang X, Wang L,
Yang Y, Li J and Wang C: Serum microRNA panel as biomarkers for
early diagnosis of colorectal adenocarcinoma. Br J Cancer.
111:1985–1992. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Jin X, Chen X, Hu Y, Ying F, Zou R, Lin F,
Shi Z, Zhu X, Yan X, Li S and Zhu H: LncRNA-TCONS_00026907 is
involved in the progression and prognosis of cervical cancer
through inhibiting miR-143-5p. Cancer Med. 6:1409–1423. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Calin GA, Cimmino A, Fabbri M, Ferracin M,
Wojcik SE, Shimizu M, Taccioli C, Zanesi N, Garzon R, Aqeilan RI,
et al: MiR-15a and miR-16-1 cluster functions in human leukemia.
Proc Natl Acad Sci USA. 105:5166–5171. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Pekarsky Y and Croce CM: Role of miR-15/16
in CLL. Cell Death Differ. 22:6–11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Huang E, Liu R and Chu Y: miRNA-15a/16: As
tumor suppressors and more. Future Oncol. 11:2351–2363. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chen D, Wu D, Shao K, Ye B, Huang J and
Gao Y: MiR-15a-5p negatively regulates cell survival and metastasis
by targeting CXCL10 in chronic myeloid leukemia. Am J Transl Res.
9:4308–4316. 2017.PubMed/NCBI
|
|
61
|
Yanaihara N, Caplen N, Bowman E, Seike M,
Kumamoto K, Yi M, Stephens RM, Okamoto A, Yokota J, Tanaka T, et
al: Unique microRNA molecular profiles in lung cancer diagnosis and
prognosis. Cancer Cell. 9:189–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Slattery ML, Pellatt AJ, Lee FY, Herrick
JS, Samowitz WS, Stevens JR, Wolff RK and Mullany LE: Infrequently
expressed miRNAs influence survival after diagnosis with colorectal
cancer. Oncotarget. 8:83845–83859. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yao XD, Li P and Wang JS: MicroRNA
differential expression spectrum and microRNA-125a-5p inhibition of
laryngeal cancer cell proliferation. Exp Ther Med. 14:1699–1705.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Odar K, Boštjančič E, Gale N, Glavač D and
Zidar N: Differential expression of microRNAs miR-21, miR-31,
miR-203, miR-125a-5p and miR-125b and proteins PTEN and
p63 in verrucous carcinoma of the head and neck. Histopathology.
61:257–265. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Leotta M, Biamonte L, Raimondi L,
Ronchetti D, Di Martino MT, Botta C, Leone E, Pitari MR, Neri A,
Giordano A, et al: A p53-dependent tumor suppressor network is
induced by selective miR-125a-5p inhibition in multiple myeloma
cells. J Cell Physiol. 229:2106–2116. 2014. View Article : Google Scholar : PubMed/NCBI
|