Introduction
Human cervical cancer presents a significant
worldwide health burden, particularly in developing countries
(1), and it accounted for ~500,000
new cases of cervical cancer cases and 250,000 cases of
cancer-related mortality globally in 2012 (2). Human papillomavirus (HPV) infection is
causally associated with the incidence rate of cervical cancer and,
to date, there are two vaccines (Gardasil and Cervarix) available
to prevent the majority of cervical cancer-associated HPV subtypes
(~16 and 18 subtypes accounting for 75% of cervical cancer cases)
(2). Histologically, ~80–85% of
cervical cancer is squamous cell carcinoma and the remaining 15–20%
is adenocarcinoma (3). Clinically,
cervical cancer can be curably treated with surgery at the early
stages of the disease or with radiation therapy and cisplatin-based
chemotherapy at the later stages, or as adjuvant therapy following
surgery (4). The prognosis of
patients with cervical cancer primarily depends on the stage of the
disease. For example, the 5-year survival rate for the earliest
stage of invasive cervical cancer is >90%, whereas the 5-year
survival rate for all other stages is ~70% (1). However, the 5-year survival rate is
only 25–35% for patients with stage III cervical cancer and <15%
for those with stage IV (1).
Therefore, the development of novel therapies or agents is critical
for effectively controlling the later stages of cervical cancer and
improving survival rates.
Osthole (7-methoxy-8-isopentenoxycoumarin) is a
monomer compound that is extracted from Cnidiummonnieri (L.)
Cusson, which has been shown to have anti-proliferative,
anti-inflammatory and anti-leishmanial effects (4,5). This
Traditional Chinese Medicine has been used for years clinically to
treat different disorders, including allergies, inflammation, HIV
infection and diabetes. It has been demonstrated that osthole has
anti-metastatic and anti-proliferative effects in various types of
human cancer. More specifically, osthole enhances tumor cell
apoptosis, arrests cell cycle progression, and inhibits tumor cell
migration (6–13). Furthermore, a previous study showed
that osthole can enhance cisplatin antitumor activity in
rhabdomyosarcoma cells (14) and
another study reported that osthole prevented hepatocellular
carcinoma (11). Together, these
studies indicate the potential role of osthole in the treatment of
human cancer, including cervical cancer.
In the present study, the antitumor activity of
osthole in cervical cancer was investigated in vitro as a
single agent or in combination with irradiation. The underlying
molecular events of osthole treatment in cervical cancer cells were
also investigated. This was expected to provide an initial
assessment of osthole for treating cervical cancer.
Materials and methods
Cell lines and culture
HeLa, SiHa, C-33A and CaSki human cervical cancer
cell lines were obtained from the American Type Culture Collection
(ATCC; Manassas, VA, USA). The HeLa, SiHa and C-33A cells were
cultured in Eagle's minimal essential medium (EMEM) and the CaSki
cells were cultured in Dulbecco's modified Eagle's medium (DMEM),
all of which were supplemented with 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
penicillin (100 U/ml, Gibco; Thermo Fisher Scientific, Inc.) and
streptomycin (100 µg/ml, Gibco; Thermo Fisher Scientific, Inc.),
and maintained in a humidified incubator with 5% CO2 at
37°C.
For radiation treatment, cells were grown and
treated with or without osthole (see below for details) and then
subjected to 6 Gy (the comet assay) or 10 Gy (western blot
analysis) X-ray irradiation at a dose rate of 3.38 Gy/min using
X-320ix (Precision X-Ray, Inc., North Branford, CO, USA) at room
temperature.
Tumor cell viability
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
solution (MTT) assay
The cells were seeded into 96-well plates at a
density of 1×104/well and grown for 24 h and then
treated with different concentrations of osthole (0, 40, 80, 120,
160 or 200 µM; Chengdu Must Bio-Technology Co., Ltd., Sichuan,
China) for 24 or 48 h at 37°C. At the end of each experiment, 5
mg/ml MTT in phosphate-buffered saline (PBS) was added and the
cells were cultured at 37°C for 4 h. The cell culture supernatant
was removed and 150 µl dimethyl sulfoxide (DMSO) was added to
dissolve the formazan crystals for 10 min, following which the
optical density was measured at 490 nm using a spectrophotometer
(PerkinElmer, Inc., Waltham, MA, USA). The experiments were
performed in triplicate and repeated at least three times. Data are
summarized as the percentage of the control.
Tumor cell colony formation assay
The cells were seeded into 6-well plates at a
density of 1,000/well, grown overnight and then treated with
different concentrations of osthole (0, 50, 100 or 200 µM) for 12
days. The culture medium was refreshed every other day. At the end
of the experiments, the cells were stained with 1% crystal violet
solution for 20 min at room temperature. Cell colonies with ≥50
cells were counted using an inverted microscope (Leica Microsystems
GmbH, Wetzlar, Germany). The experiments were performed in
triplicate and repeated at least three times. Data are summarized
as the percentage of the control.
Tumor cell apoptosis assay
The apoptotic rate of cells was measured using the
fluorescence-activated cell sorter (FACS) following staining with
the Annexin-V FITC kit (BD Pharmingen™; BD Biosciences, San Diego,
CA, USA). The cells were grown in 6-well plates and treated with or
without osthole for 24 h, and then collected for staining with the
FITC-labeled Annexin V and PI kit according to the manufacturer's
protocol. The cells were subsequently analyzed using the FACS
Accuri C6 flow cytometer (Genetimes Technology Inc., Shanghai,
China). The experiments were performed in triplicate and repeated
twice. Data are summarized as the percentage of the control.
Acridine orange/ethidium bromide
(AO/EB) fluorescence staining
The cells were seeded onto chamber slides (Corning
Inc., Corning, NY, USA) and treated with 100 µM of osthole for 24
h. Following treatment, the cells were washed with ice-cold PBS to
remove detached cells and then fixed in 95% ethanol for 15 min.
Following brief drying, the chamber slides were stained with 5 µl
AO/EB (50 µg/ml), according to the manufacturer's protocol, and
cell images were captured using a Leica DM 14000B microscope with
digital camera (Leica Microsystems GmbH). The experiments were
performed in triplicate and repeated twice. Data are summarized as
the percentage of the control.
Tumor cell scratch assay
The cells were grown to reach 90–95% confluency in
6-well plates. The cell monolayer was wounded using a sterile
100-µl pipette tip and then washed with cell growth medium to
remove the detached cells. The cells were cultured in serum-free
medium and treated with osthole at different concentrations (0, 20
or 40 µM) for 24 h. Images of the wounded monolayer were captured
at different time points using an inverted microscope (Olympus
Corp., Tokyo, Japan). The experiments were performed in triplicate
and repeated three times. Data are summarized as a percentage of
the control.
Transwell tumor cell migration and
invasion assays
The cells were grown and treated with osthole (0, 20
or 40 µM) for 24 h and then suspended in cell solution, and
2×104 cells in serum-free EMEM were added to the upper
insert of the Transwell chamber (Corning Inc.). The insert membrane
was pre-coated with or without 50 µl Matrigel Matrix (1 mg/ml;
Corning Inc.). EMEM supplemented with 20% FBS was added to the
bottom of the Transwell plates, and the Transwell plates were
incubated at 37°C for 24 h. The tumor cells remaining on the upper
side of the membrane were removed using a cotton swab, and tumor
cells that invaded the reverse side of the membrane were fixed with
95% ethanol and stained with 1% crystal violet solution for 20 min.
Images were captured in five random microscopic fields at ×200
magnifications using an inverted Olympus microscope (Olympus
Corp.). The experiments were performed in triplicate and repeated
twice. Data are summarized as a percentage of the control.
Immunofluorescence staining
The cells were seeded onto coverslips, treated with
osthole (0 or 40 µM) for 24 h and then fixed in 4% paraformaldehyde
for 10 min at room temperature. The cells were then permeabilized
in 0.05% Triton X-100 in PBS for 10 min at room temperature and
subsequently incubated with a monoclonal rabbit anti-E-cadherin
(cat. no. 3195) or vimentin (cat. no. 5741) antibody at a dilution
of 1:100, or a monoclonal rabbit anti-NF-κB p65 (cat. no. 8242) at
a dilution of 1:200 at 4°C overnight, all from Cell Signaling
Technology, Inc. (Beverly, MA, USA). The following day, the cells
were washed with PBS three times and then incubated with a
secondary anti-rabbit IgG (H+L), F(ab')2 fragment (Alexa
Fluor® 555 conjugated; cat. no. 4413; Vector
Laboratories, Inc., Burlingame, CA, USA) at a dilution of 1:500) at
the room temperature for 2 h, and the cell nuclei were
counterstained with 4′,6-diamidino-2-phenylindole (DAPI; Vector
Laboratories, Inc.). Cell images were then captured in five random
microscopic fields using an Olympus fluorescence microscope
(Olympus Corp.) (magnification, ×200). The experiments were
performed in triplicate and repeated three times. Data are
summarized as a percentage of the control.
Tumor cell comet assay
Following the indicated treatment, the cervical
cancer cells were collected, resuspended, and then loaded onto
agarose coated glass slides, which were coated with 150 µl of 0.5%
agarose, at a density of 1.5×103 cells/µl. The slides
were then soaked in a lysis buffer (10 mM Tris-HCl, 2.5 M NaCl, 100
mM EDTA, 1% Triton X-100 and 10% DMSO) for 1 h and washed with
neutralization buffer for 5 min, each for three times. The slides
were then placed into an iced-cold electrophoresis solution (300 mM
NaOH and 1 mM EDTA) and subjected to 25 V at 300 mA electrophoresis
for 25 min. At the end of the experiments, the cells were stained
with an ethidium bromide solution (20 µg/ml), and images were
captured using an Olympus fluorescence microscope (Olympus Corp.).
The numbers of cells with or without comet tails were counted and
averaged. The experiments were performed in triplicate and repeated
three times. Data are summarized as a percentage of the
control.
Western blot analysis
Total cellular protein was extracted using a protein
extraction kit (Thermo Fisher Scientific, Inc.), and protein
concentration was measured using the BCA protein kit (Thermo Fisher
Scientific, Inc.). The denatured protein samples of 30 µg each were
separated on 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis gels and electrophoretically transferred onto
polyvinylidene fluoride membranes (BioTrace; Life Sciences, Port
Washington, NY, USA). For western blot analysis, the membranes were
blocked in 5% skimmed milk solution for 1 h and then incubated with
a specific primary antibody at 4°C overnight. The following day,
the membranes were washed with PBS-Tween-20 three times and then
incubated with an anti-rabbit or mouse secondary antibody at the
room temperature for 2 h. The primary antibodies were rabbit
monoclonal antibodies against Bcl-2 (cat. no. 3498), Bax (cat. no.
14796), cleaved caspase-3 (cat. no. 9664), cleaved caspase-9 (cat.
no. 20750), vimentin (cat. no. 5741), N-cadherin (cat. no. 13116),
E-cadherin (cat. no. 3195), β-catenin (cat. no. 8480), MMP-2 (cat.
no. 40994), MMP-9 (cat. no. 13667), Phospho-ATM (Ser1981; cat. no.
13050), ATM (cat. no. 2873), Phospho-Histone H2A.X (Ser139; cat.
no. 2577), Histone H2A.X (cat. no. 7631), NF-κB p65 (cat. no.
8242), Phospho-IKKα (Ser176)/IKKβ (Ser177) (cat. no. 2078), IKKα
(cat. no. 2682), Phospho-NF-κB p65 (Ser536; cat. no. 3033), NF-κB
p65 (cat. no. 8242) and NF-κB1 p105/p50 (cat. no. 12540; all from
Cell Signaling Technology) and used at a dilution of 1:1,000, while
the secondary antibody was an anti-rabbit IgG SA00001-2
(Proteintech, Wuhan, China) and used at a dilution of 1:5,000. The
protein bands were subsequently visualized using chemiluminescence
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) and x-ray
films.
Statistical analysis
Data are summarized as the mean ± standard deviation
(SD) and were statistically analyzed using GraphPad Prism software
version 5.01 (GraphPad Software, Inc., La Jolla, CA, USA). Analysis
of variance and Student's t-test were used to compare the values of
the test and control samples. P<0.05 was considered to indicate
a statistically significant difference.
Results
Osthole inhibits cervical cancer cell
viability and proliferation
To assess the anti-cervical cancer activity of
osthole, a cell viability MTT assay was performed, and it was found
that osthole reduced the viability of the four cervical cancer cell
lines in a dose-dependent manner (Fig.
1A). In addition, the tumor cell colony formation assay showed
that osthole treatment dose-dependently inhibited growth of
cervical cancer cells (Fig. 1B).
Morphologically, osthole treatment also reduced HeLa and SiHa
cell-to-cell contact, and the osthole-treated tumor cells had more
filopodia and cell layers (Fig.
1C).
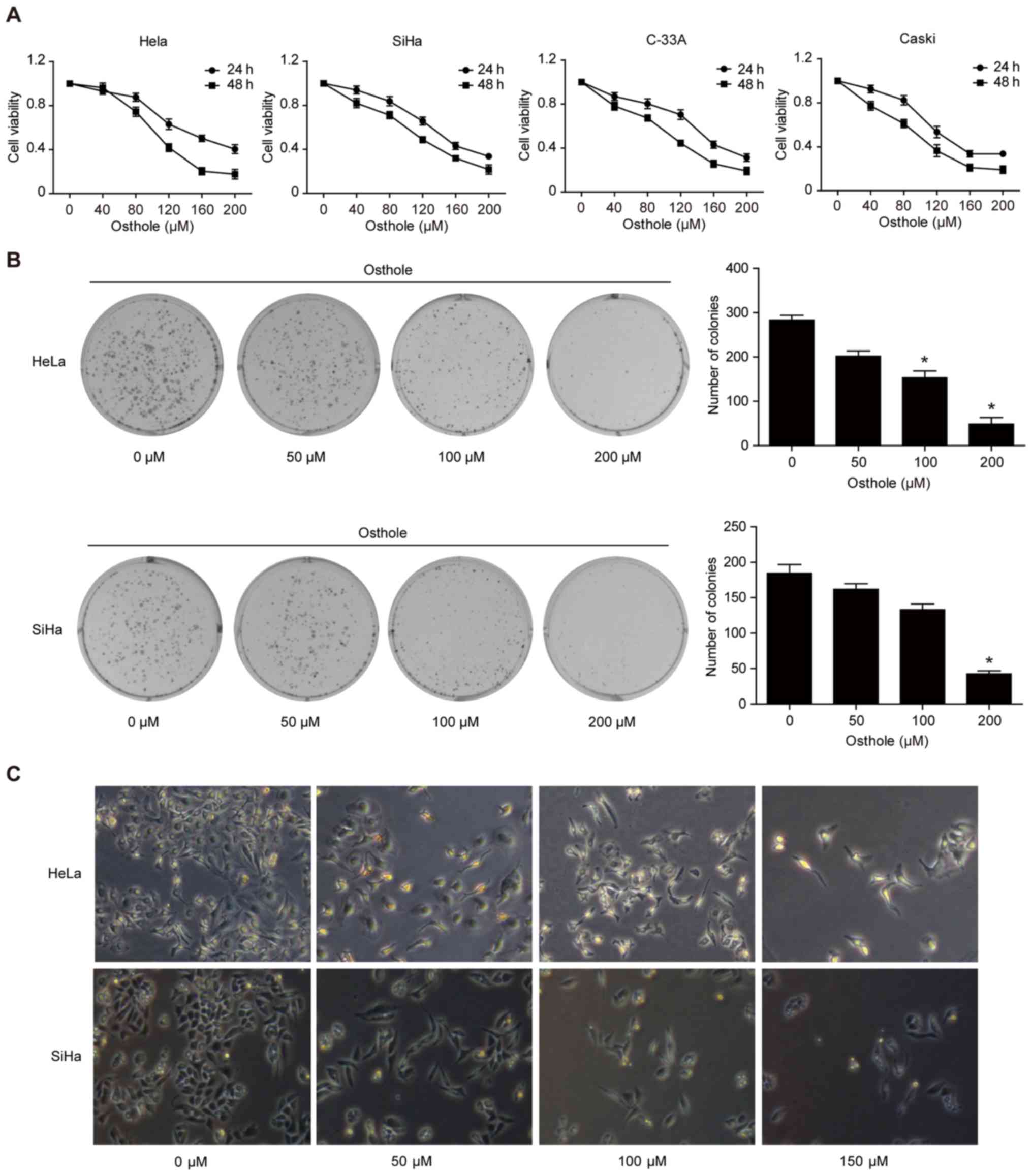 | Figure 1.Osthole inhibits cervical cancer cell
viability and proliferation. (A) MTT assay. HeLa, SiHa, C-33A, and
CaSki human cervical cancer cells were treated with or without
osthole (0, 40, 80, 120, 160, 200 or 240 µM) for 24 or 48 h and
then subjected to the cell viability MTT assay. Osthole suppressed
cervical cancer cell viability in a dose-dependent manner. (B)
Colony formation assay. Cells were grown and treated with 50 µM
osthole for up to 12 days and images were captured. Tumor cells
with ≥50 cells were counted and the data revealed that osthole
inhibited colony formation of HeLa and SiHa cells in a
dose-dependent manner. *P<0.05 compared to the control cells.
(C) Morphology. Tumor cells were grown and treated with or without
osthole (0, 40, 80, 120, 160, 200 or 240 µM) for 24 h and, and
images were captured using an inverted microscope with an attached
digital camera at ×200 magnification. MTT,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide. |
Osthole induces cervical cancer cell
apoptosis
The present study assessed whether the reduction in
tumor cell viability was due to the induction of apoptosis using
Annexin-V and FACS analyses. Osthole treatment significantly
increased the apoptotic rate of the cells, compared with that of
cells in the control group (Fig.
2A). To confirm the osthole-induced tumor cell apoptosis, HeLa
cells were stained with AO/EB. In the control group, the uniform
green staining showed normal morphology of cells, whereas osthole
treatment induced early apoptosis of the tumor cells, which had
condensed chromatin and red apoptotic bodies (Fig. 2B). At the gene level, osthole
treatment increased the B-cell lymphoma 2 (Bcl-2)-associated X
protein (Bax)/Bcl-2 ratio, and the levels of cleaved caspase-3 and
cleaved caspase-9 in a dose-dependent manner, indicating that
osthole activated the caspase-dependent pathway (Fig. 2C).
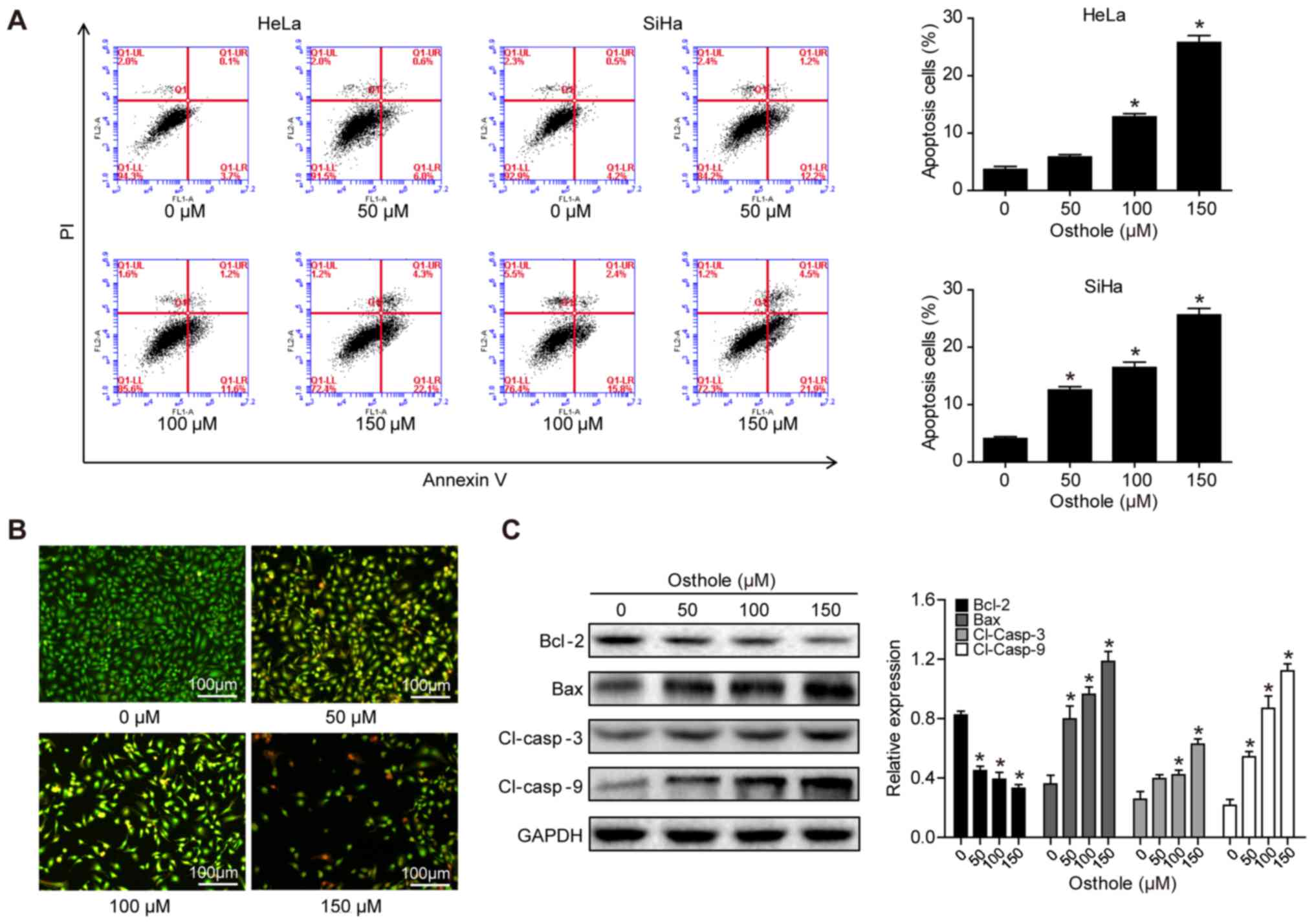 | Figure 2.Osthole induces cervical cancer cell
apoptosis. (A) HeLa and SiHa cells were grown and treated with
osthole (0, 50, 100 or 150 µM) for 24 h and subjected to the
apoptosis assay. (B) Tumor cell AO/EB fluorescence staining. HeLa
cells were grown and treated with osthole (0, 50, 100 or 150 µM)
for 24 h and subjected to staining. (C) Western blot analysis.
Tumor cells were grown and treated with or without osthole (0, 40,
80, 120, 160, 200 or 240 µM) for 24 h and then subjected to western
blot analysis of Bcl-2, Bax, and cleaved caspase-3 and −9 proteins.
*P<0.05 compared to the control group. Bcl-2, B-cell lymphoma 2;
Bax, Bcl-2-associated X protein; Cl, cleaved. |
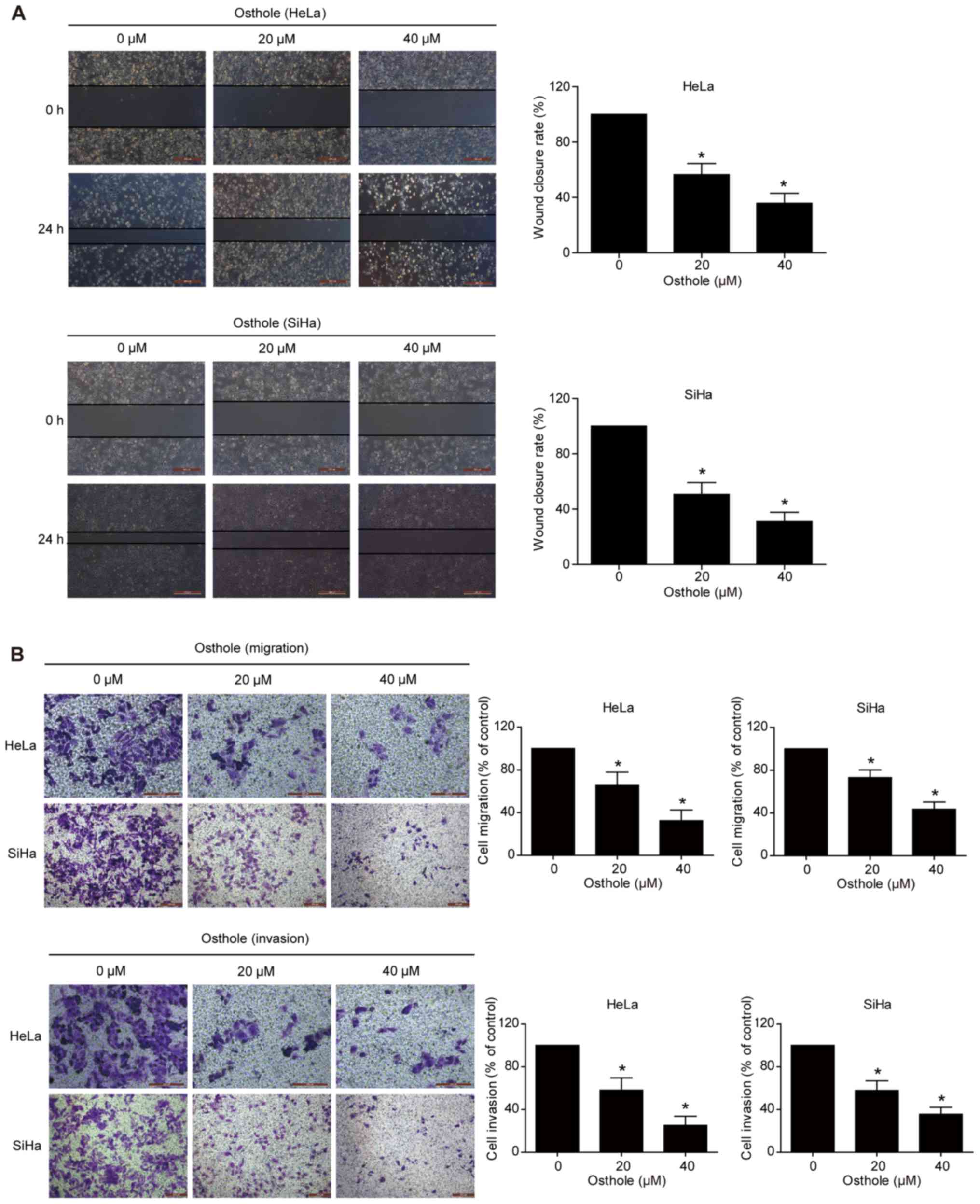
Osthole inhibits cervical cancer cell
migration and invasion
A previous study revealed that osthole suppressed
the invasion capacity of lung cancer cells (13). In the present study, the effect of
osthole on regulating cervical cancer cell migration and invasion
was further examined using Transwell and scratch assays. The data
showed that wound healing was significantly suppressed following
osthole treatment in a dose-dependent manner (Fig. 3A). The Transwell assay data further
supported this finding, as osthole suppressed the tumor cell
migration and invasion capacity of the HeLa and SiHa cells
(Fig. 3B).
Osthole suppresses levels of
epithelial-mesenchymal transition (EMT)-related proteins in
cervical cancer cells
Treatment of the cervical cancer cells with 50 mM
osthole increased the expression of E-cadherin but decreased the
expression of Vimentin in the HeLa cells, as evidenced by the
immunofluorescence analysis (Fig.
4A). This indicated that osthole suppressed cervical cancer
cell EMT. The expression of other EMT biomarkers, including
N-cadherin, β-catenin, MMP-2 and MMP-9, were also analyzed
(Fig. 4B and C), and the results
suggested that osthole suppressed cervical cancer cell EMT. The
osthole-induced suppression of cell adhesion proteins MMP-2 and
MMP-9 in the HeLa and SiHa cells (Fig.
4C) also further support the results of the invasion and
migration assays.
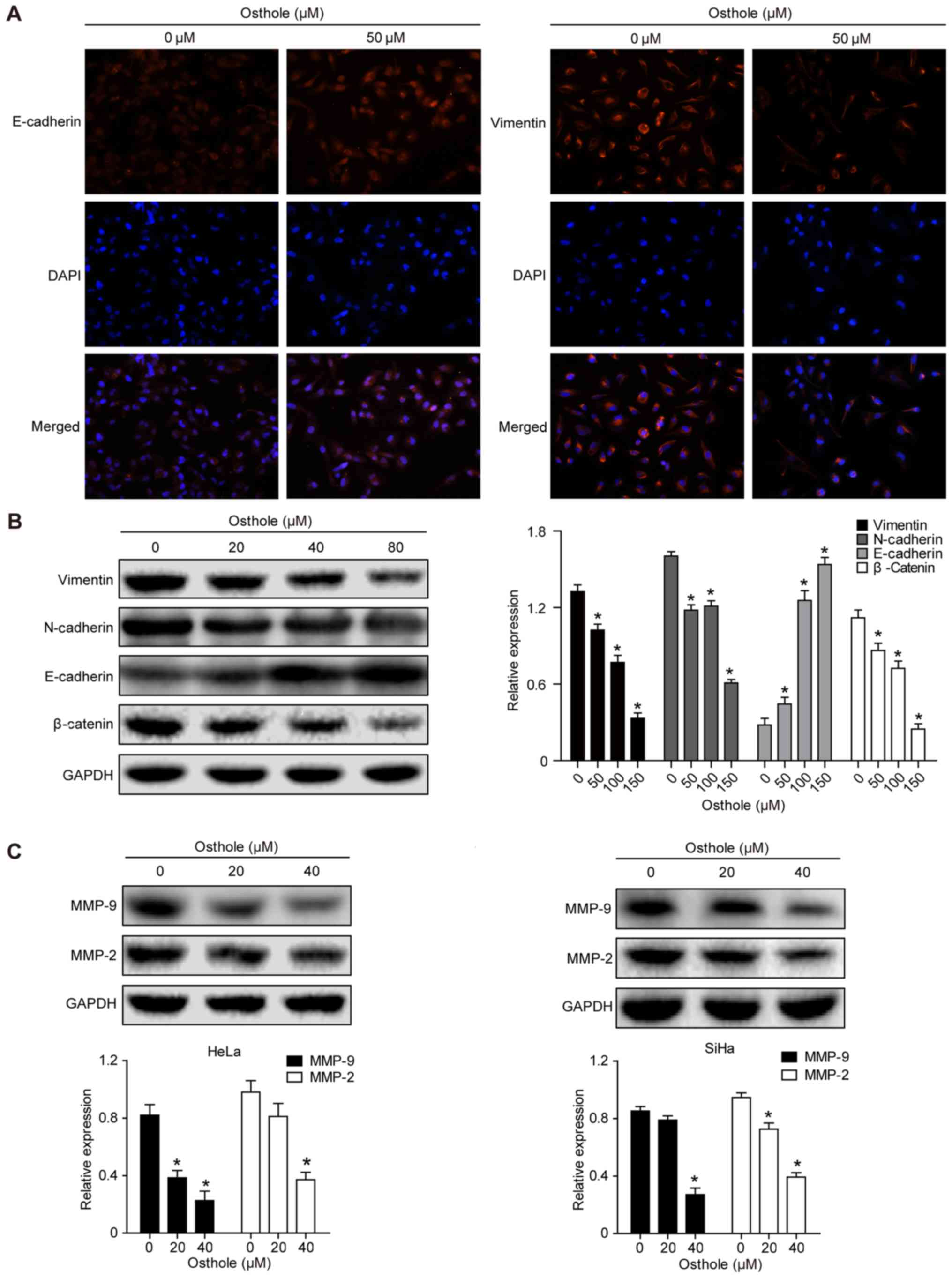 | Figure 4.Osthole suppresses cervical cancer
cell EMT. (A) Immunofluorescence analysis. HeLa cells were grown
and treated with or without 50 µM osthole for 24 h and then
subjected to immunofluorescence analysis of EMT biomarkers
(magnification, ×100). The data showed that the expression of
E-cadherin was increased, whereas that of vimentin was decreased in
HeLa cells. (B) Western blot analysis. Tumor cells were grown and
treated with or without osthole (0, 20, 40 and 80 µM) for 24 h and
then subjected to western blot analysis for detection of EMT
biomarkers vimentin, N-cadherin, E-cadherin and β-catenin proteins.
(C) Western blot analysis. HeLa and SiHa cells were grown and
treated with or without osthole (20 and 40 µM) for 24 h and then
subjected to western blot analysis detection of MMP-2 and MMP-9.
Expression levels of MMP-2 and MMP-9 decreased in a dose-dependent
manner with osthole exposure (*P<0.05, vs. control). EMT,
epithelial-mesenchymal transition; DAPI,
4′,6-diamidino-2-phenylindole; MMP, matrix metalloproteinase. |
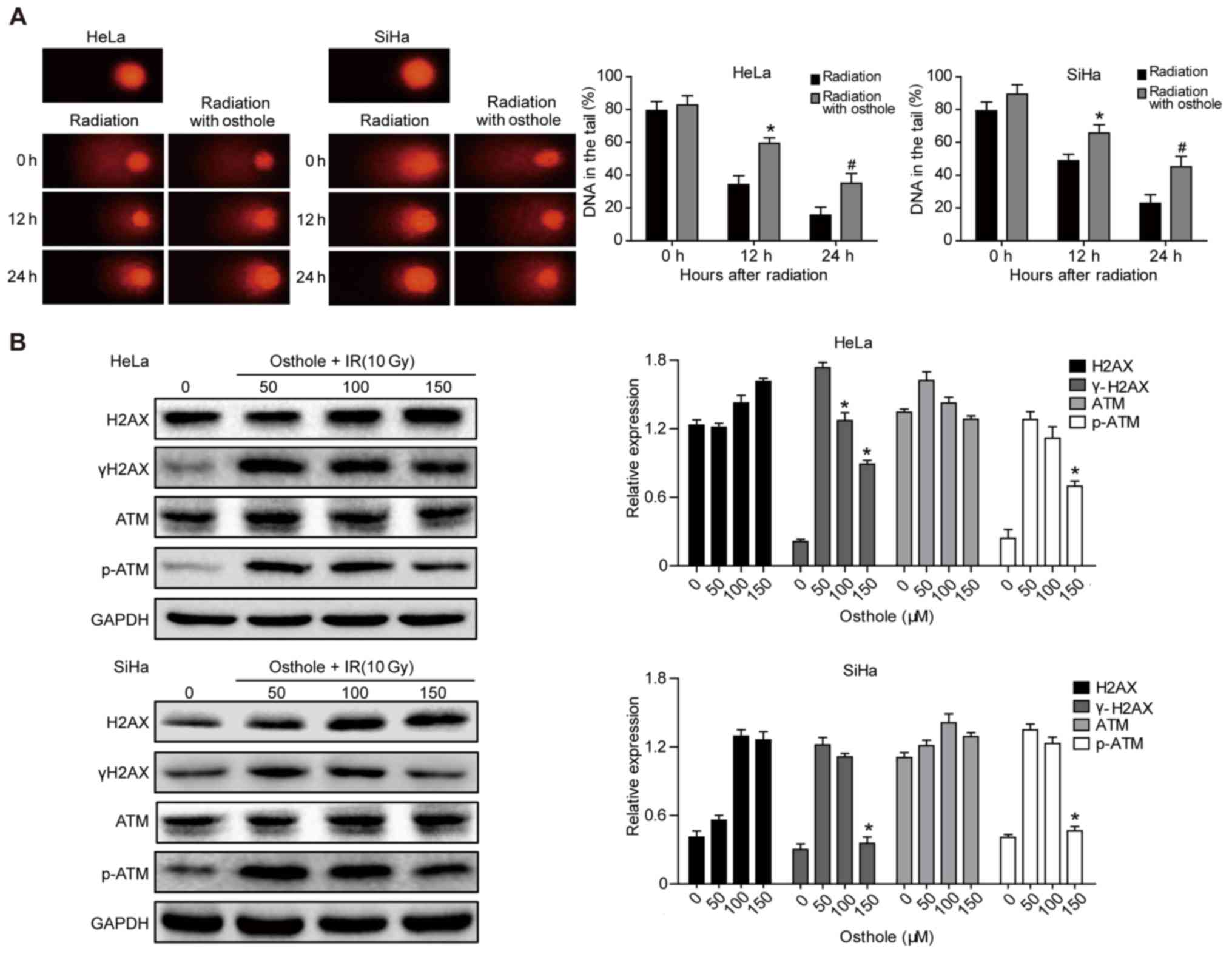
Osthole promotes cervical cancer cell
DNA damage induced by irradiation
Radiotherapy is an important treatment option for
locally advanced cervical cancer. A primary mechanism of
irradiation is to induce tumor cell DNA damage (15). In the present study, a Comet assay
was performed to assess the effects of osthole in combination with
radiation on cervical cancer cells. The pretreatment of HeLa and
SiHa cells with osthole (50 µM) for 24 h and exposure to 6 Gy
radiation markedly increased the irradiation-induced cervical
cancer cell DNA damage, compared with that in the control cells
(Fig. 5A). At the protein level,
this treatment combination significantly inhibited phosphorylation
of the ataxia telangiectasia mutated (ATM) and γH2AX proteins,
whereas no significant changes in the levels of ATM and H2AX were
observed (Fig. 5B). These results
indicated that osthole inhibited the irradiation-induced DNA damage
repair capacity of cervical cancer cells.
Osthole treatment in combination with
irradiation inhibits NF-κB signaling
Previous studies have shown that NF-κB signaling is
involved in the regulation of tumor cell DNA damage (15) and that the osthole-induced
suppression of lung cancer cell invasion capacity is mediated by
suppression of the NF-κB-induced expression of MMP-9 (13). Therefore, it was hypothesized that
osthole-enhanced cervical cancer cell DNA damage and inhibited
tumor cell migration and invasion are also be mediated by
manipulating the NF-κB signaling pathway. The HeLa cells were
treated with osthole (0, 50, 100 or 150 µM) for 24 h and western
blot analysis was performed to detect key proteins of the NF-κB
signaling pathway. It was found that osthole treatment
significantly reduced the phosphorylation of inhibitor of NF-κB
(IκB) kinase (IKK)α and p65 proteins in the cytoplasm, but did not
alter the levels of total IKKα or p65 (Fig. 6A). In addition, osthole treatment
decreased nuclear protein expression of p50 and p65 (Fig. 6B). The subcellular localization of
p65 protein in HeLa cells treated with osthole (0, 50, 100 or 150
µM) was assessed by immunostaining and light microscopy, and the
data were consistent with the results of the western blot analysis
(Fig. 6C). These results suggested
that osthole inhibited irradiation-induced DNA damage repair in
HeLa cells through suppressing NF-κB signaling.
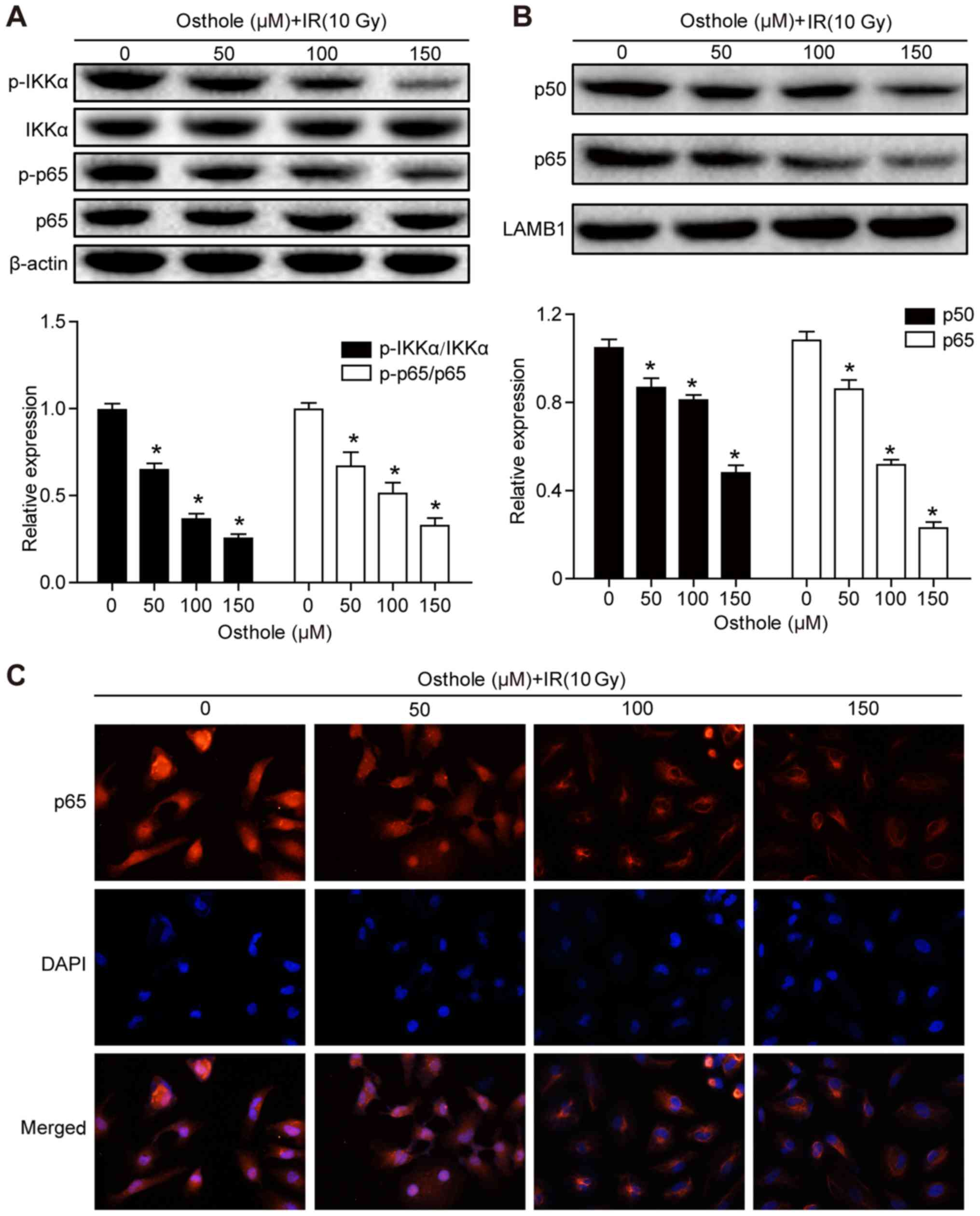 | Figure 6.Osthole promotes NF-κB signaling in
radiation-induced cervical cancer cell DNA damage. HeLa cells were
grown and treated with osthole (50, 100 or 150 µM) for 24 h and
exposed to 10 Gy radiation. Cytoplasmic and nuclear proteins of
HeLa cells were separately extracted for western blot analysis of
key proteins in the NF-κB signaling pathway, including (A) IKKα,
p-IKKα, p65, p-p65 and (B) p50. (C) Subcellular localization of p65
in HeLa cells was examined by analysis with a fluorescence
microscope (magnification, ×50). All results are expressed as the
mean ± standard deviation (*P<0.05, vs. control). NF-κB, nuclear
factor-κB; IKKα, inhibitor of NF-κB kinase α; p-, phosphorylated;
IR, irradiation; DAPI, 4′,6-diamidino-2-phenylindole. |
Discussion
In the present study, the antitumor activity of
osthole in cervical cancer cells was assessed in vitro, and
it was found that osthole treatment dose-dependently reduced tumor
cell viability and proliferation, suppressed tumor cell migration
and invasion, and induced apoptosis. Osthole treatment also
modulated the expression of cervical cancer cell EMT markers,
indicating that osthole inhibited tumor cell EMT. In addition,
osthole treatment sensitized cervical cancer cells to irradiation
and inhibited NF-κB signaling. Therefore, osthole may be further
evaluated as an herbal agent for the adjuvant treatment of cervical
cancer.
Osthole has been reported to induce apoptosis and
have anti-proliferative activity in a variety of human cancer
cells, including ovarian (16),
lung (17), breast cancer (18) and hepatocellular carcinoma (19) cells. Osthole can inhibit the
migration of MCF-7 breast cancer cells and the invasion of
MDA-MB-231 breast cancer cells by suppressing MMP-2 and MMP-9
activity (8). The results of the
present study extend the antitumor activity of osthole to cervical
cancer cells. It is known that the induction of tumor cell
apoptosis is a crucial component of anticancer therapeutic agents.
Osthole treatment significantly increased early and late apoptosis
of cervical cancer cells. The present study demonstrated that the
osthole-induced cervical cancer cell apoptosis was mediated by
modulating the expression of activity of Bcl-2, Bax, caspase-3 and
caspase-9. Caspase-3 and caspase-9 are major factors in the
apoptotic process, whereas Bcl-2, in contrast to Bax, is an
anti-apoptotic protein in the mitochondrial apoptosis pathway
(20). The data obtained in the
present study showed that treatment of cervical cancer cell lines
with osthole increased the protein expression of Bax, cleaved
caspase-3 and caspase-9 and decreased the expression of Bcl-2,
indicating that osthole may be further evaluated as an
anti-cervical cancer agent.
Tumor cell EMT is an important event during normal
cell transformation into malignant cells and cancer metastasis
(21). Morphologically, epithelial
cells transform to mesenchymal cell phenotypes, which is
accompanied by a loss of cell polarization, cell-cell adhesion, and
increased migratory and invasive properties. EMT is essential in
embryonic development, wound healing, cancer development and
metastasis (21). Molecularly, EMT
induces multiple biochemical changes to promote a mesenchymal
phenotype (22). These changes
impart migratory capacity, invasiveness, elevated resistance to
apoptosis, and production of extracellular matrix (23). The changes include the
downregulation of epithelial proteins, including E-cadherin and
β-catenin, and the upregulation of mesenchymal proteins, including
N-cadherin and vimentin (24). In
addition, the expression of different MMPs facilitates cell
mobility, and the increased expression of MMP-2 and MMP-9 is
considered to be important in cancer metastasis (25). A previous study demonstrated that
cervical cancer with an EMT phenotype was associated with an
increased risk of tumor progression, invasion, and metastasis
(26). In the present study, it was
shown that osthole treatment significantly reduced cervical cancer
cell migration and invasion, and at the gene level, osthole
upregulated epithelial markers (E-cadherin and β-catenin) and
downregulated mesenchymal markers (N-cadherin and vimentin) and
MMP-2 and MMP-9, consistent with the hypothesis that osthole
inhibits cervical cancer cell EMT.
Locally advanced stages of cervical cancer are
usually treated with chemotherapy and/or a combination of external
beam radiation therapy and brachytherapy (27). However, 30–40% of patients do not
respond well to these standard treatments, due to tumor cell
resistance to radiotherapy (28).
Therefore, the present study examined the effect of osthole on the
sensitization of cervical cancer cells to radiotherapy. It was
found that osthole co-treatment with radiation enhanced DNA damage
in cervical cancer cells and inhibited DNA damage repair. During
DNA damage repair, the ATM kinase is activated when cells suffer
DNA double-strand breaks, and phosphorylated (p)-ATM regulates cell
cycle checkpoints by phosphorylating p53 at Ser15, and activates
checkpoint kinase 2 (CHK2) by phosphorylating CHK2 at Thr68
(29). Histone H2AX phosphorylation
is among the earliest responders to DNA double-strand breaks
(30), which is activated by the
ATM-induced phosphorylation of γH2AX (31). The data obtained in the present
study support the findings that osthole inhibits p-ATM following
radiation, enhancing the radiosensitivity of cervical cancer cells
in vitro.
Finally, the present study also demonstrated that
osthole attenuated the activation of IKKα and IκBα, and decreased
the translocation of p50/p65 into the cell nucleus, which further
supports data from a previous study showing that osthole inhibited
the NF-κB-mediated expression of MMP-9, lung cancer cell migration
and invasion (13). Activation of
the NF-κB pathway occurs in irradiated tumor cells, and the
activation of NF-κB has been shown to induce cancer cell resistance
to the genotoxic anticancer therapeutic agent, cisplatin (32). However, further investigation is
required to better understand the role of the NF-κB pathway in
cervical cancer.
In conclusion, the present study initially assessed
the anti-cervical cancer activity of osthole in vitro and
confirmed the dose-dependent effects of osthole on reducing
cervical cancer cell proliferation, migration and invasion, and the
induction of apoptosis by suppressing tumor cell EMT and radiation
resistance. The findings also reveal a molecular mechanism by which
osthole suppresses ATM and the NF-κB pathway (Fig. 7). The safety of osthole has been
investigated, and the no-obvious adverse-effect level (NOAEL) of
osthole is considered to be <5 mg/kg in male and female rats. To
validate the safety and efficacy of osthole, randomized, controlled
trials with an adequate sample size are required, according to a
previous study (33). Further
investigations are also required to define the exact mechanism
underlying the effect of osthole in cervical cancer.
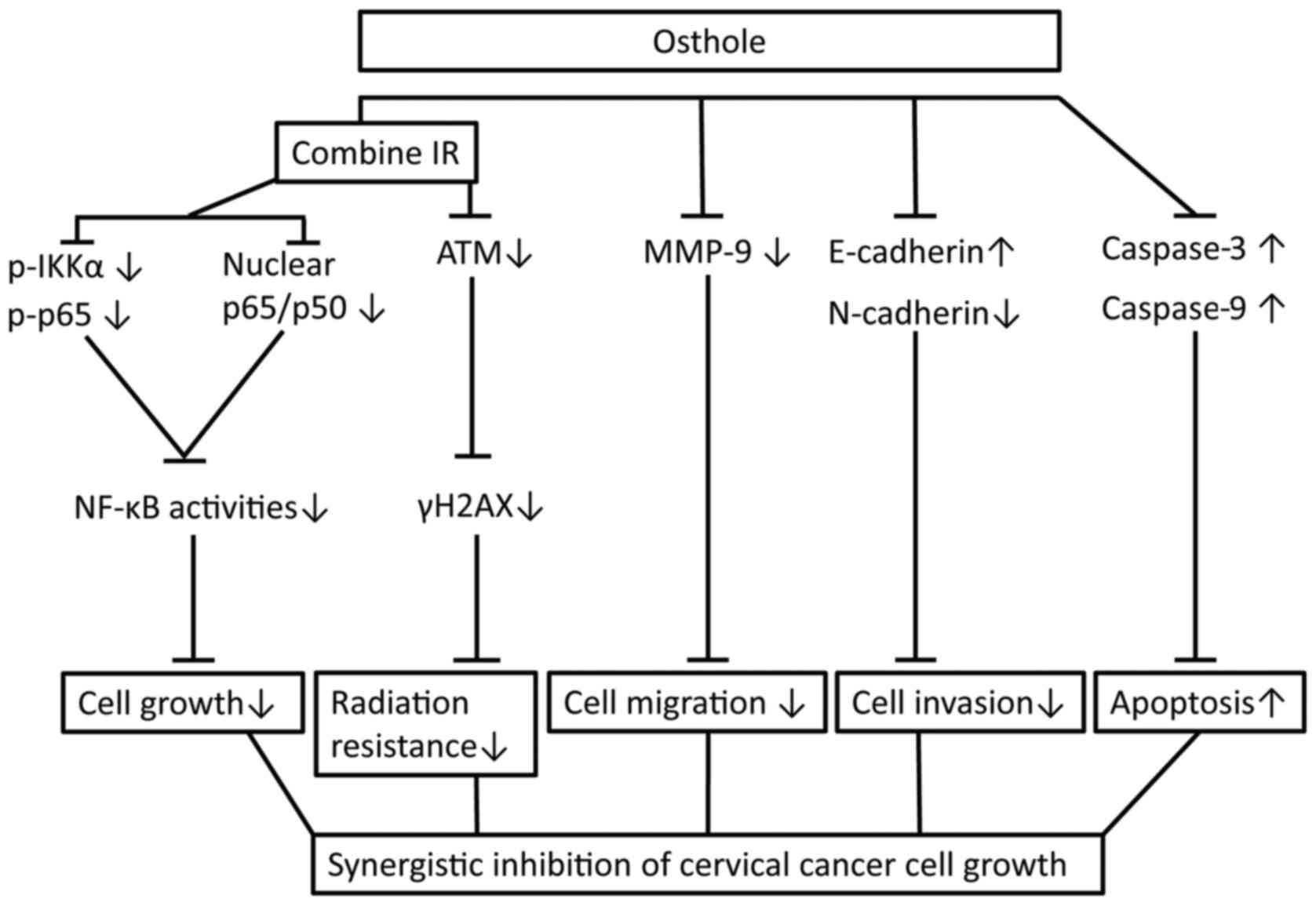 | Figure 7.Osthole sensitization of radiation in
inhibition of cervical cancer cell viability by targeting multiple
signaling pathways. Cervical cancer cells were grown and treated
with osthole in combination with radiation. Combination treatment
suppressed the protein expression of vimentin, N-cadherin, MMP-2
and MMP-9, induced the cleavage of pro-apoptotic caspase, inhibited
the phosphorylation of γH2AX, IKKα and p65 proteins by ATM, and
promoted the translocation of NF-κB from cell nuclei to cytoplasm.
IR, irradiation; NF-κB, nuclear factor-κB; IKKα, inhibitor of NF-κB
kinase α; p-, phosphorylated; ATM, ataxia telangiectasia mutated;
MMP, matrix metalloproteinase. |
Acknowledgements
Not applicable.
Funding
The present study was supported in part by a grant
from the National Natural Science Foundation of China (no.
81473452).
Availability of data and materials
All supporting data and materials are available
within the article.
Authors' contributions
YC and JL performed experiments; ZL and JL and SW
analyzed data; YY, LZ and KZ conceived and designed the
experiments; YC wrote the manuscript. All authors read and approved
the manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PBS
|
phosphate-buffered saline
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
EMT
|
epithelial-mesenchymal transition
|
|
DMSO
|
dimethyl sulfoxide
|
|
HPV
|
human papillomavirus
|
|
FACS
|
fluorescence-activated cell sorter
|
|
DAPI
|
4′,6-diamidino-2-phenylindole
|
References
|
1
|
Ginsburg O, Bray F, Coleman MP, Vanderpuye
V, Eniu A, Kotha SR, Sarker M, Huong TT, Allemani C, Dvaladze A, et
al: The global burden of women's cancers: A grand challenge in
global health. Lancet. 389:847–860. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee MY and Shen MR: Epithelial-mesenchymal
transition in cervical carcinoma. Am J Transl Res. 4:1–13.
2012.PubMed/NCBI
|
|
4
|
Zimecki M, Artym J, Cisowski W, Mazol I,
Włodarczyk M and Gleńsk M: Immunomodulatory and anti-inflammatory
activity of selected osthole derivatives. Z Naturforsch C.
64:361–368. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hao Y and Liu Y: Osthole alleviates
bleomycin-induced pulmonary fibrosis via modulating
angiotensin-converting enzyme 2/angiotensin-(1–7) axis and
decreasing inflammation responses in rats. Biol Pharm Bull.
39:457–465. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang G, Liu J, Ren B, Tang Y, Owusu L, Li
M, Zhang J, Liu L and Li W: Anti-tumor effects of osthole on
ovarian cancer cells in vitro. J Ethnopharmacol. 193:368–376. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ding D, Wei S, Song Y, Li L, Du G, Zhan H
and Cao Y: Osthole exhibits anti-cancer property in rat glioma
cells through inhibiting PI3K/Akt and MAPK signaling pathways. Cell
Physiol Biochem. 32:1751–1760. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang D, Gu T, Wang T, Tang Q and Ma C:
Effects of osthole on migration and invasion in breast cancer
cells. Biosci Biotechnol Biochem. 74:1430–1434. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu C, Sun Z, Guo B, Ye Y, Han X, Qin Y and
Liu S: Osthole inhibits bone metastasis of breast cancer.
Oncotarget. 8:58480–58493. 2017.PubMed/NCBI
|
|
10
|
Chou SY, Hsu CS, Wang KT, Wang MC and Wang
CC: Antitumor effects of Osthol from Cnidium monnieri: An in vitro
and in vivo study. Phytother Res. 21:226–230. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Okamoto T, Kobayashi T and Yoshida S:
Chemical aspects of coumarin compounds for the prevention of
hepatocellular carcinomas. Curr Med Chem Anticancer Agents.
5:47–51. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang LL, Wang MC, Chen LG and Wang CC:
Cytotoxic activity of coumarins from the fruits of Cnidium monnieri
on leukemia cell lines. Planta Med. 69:1091–1095. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kao SJ, Su JL, Chen CK, Yu MC, Bai KJ,
Chang JH, Bien MY, Yang SF and Chien MH: Osthole inhibits the
invasive ability of human lung adenocarcinoma cells via suppression
of NF-κB-mediated matrix metalloproteinase-9 expression. Toxicol
Appl Pharmacol. 261:105–115. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jarząb A, Łuszczki J, Guz M,
Skalicka-Woźniak K, Hałasa M, Smok-Kalwat J, Polberg K and Stepulak
A: Combination of osthole and cisplatin against rhabdomyosarcoma
TE671 cells yielded additive pharmacologic interaction by means of
isobolographic analys. Anticancer Res. 38:205–210. 2018.PubMed/NCBI
|
|
15
|
Small W Jr, Bacon MA, Bajaj A, Chuang LT,
Fisher BJ, Harkenrider MM, Jhingran A, Kitchener HC, Mileshkin LR,
Viswanathan AN, et al: Cervical cancer: A global health crisis.
Cancer. 123:2404–2412. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin VC, Chou CH, Lin YC, Lin JN, Yu CC,
Tang CH, Lin HY and Way TD: Osthole suppresses fatty acid synthase
expression in HER2-overexpressing breast cancer cells through
modulating Akt/mTOR pathway. J Agric Food Chem. 58:4786–4793. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu X, Zhang Y, Qu D, Jiang T and Li S:
Osthole induces G2/M arrest and apoptosis in lung cancer A549 cells
by modulating PI3K/Akt pathway. J Exp Clin Cancer Res. 30:332011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dien PH, Nhan NT, Le Thuy HT and Quang DN:
Main constituents from the seeds of Vietnamese Cnidium monnieri and
cytotoxic activity. Nat Prod Res. 26:2107–2111. 2012.PubMed/NCBI
|
|
19
|
Zhang L, Jiang G, Yao F, He Y, Liang G,
Zhang Y, Hu B, Wu Y, Li Y and Liu H: Growth inhibition and
apoptosis induced by osthole, a natural coumarin, in hepatocellular
carcinoma. PLoS One. 7:e378652012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yao C, Cao X, Fu Z, Tian J, Dong W, Xu J,
An K, Zhai L and Yu J: Boschniakia rossica polysaccharide triggers
laryngeal carcinoma cell apoptosis by regulating expression of
Bcl-2, Caspase-3, and P53. Med Sci Monit. 23:2059–2064. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Samatov TR, Tonevitsky AG and Schumacher
U: Epithelial-mesenchymal transition: Focus on metastatic cascade,
alternative splicing, non-coding RNAs and modulating compounds. Mol
Cancer. 12:1072013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cruz-Solbes AS and Youker K: Epithelial to
Mesenchymal transition (EMT) and endothelial to mesenchymal
transition (EndMT): Role and implications in kidney fibrosis.
Results Probl Cell Differ. 60:345–372. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Verma RP and Hansch C: Matrix
metalloproteinases (MMPs): Chemical-biological functions and
(Q)SARs. Bioorg Med Chem. 15:2223–2268. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee MY, Chou CY, Tang MJ and Shen MR:
Epithelial-mesenchymal transition in cervical cancer: Correlation
with tumor progression, epidermal growth factor receptor
overexpression, and snail up-regulation. Clin Cancer Res.
14:4743–4750. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moreno-Acosta P, Gamboa O, Sanchez de
Gomez M, Cendales R, Diaz GD, Romero A, Balart Serra J, Conrado Z,
Levy A, Chargari C and Magné N: IGF1R gene expression as a
predictive marker of response to ionizing radiation for patients
with locally advanced HPV16-positive cervical cancer. Anticancer
Res. 32:4319–4325. 2012.PubMed/NCBI
|
|
28
|
Yang J, Yue JB, Liu J and Yu JM:
Repopulation of tumor cells during fractionated radiotherapy and
detection methods (Review). Oncol Lett. 7:1755–1760. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Matsuoka S, Rotman G, Ogawa A, Shiloh Y,
Tamai K and Elledge SJ: Ataxia telangiectasia-mutated
phosphorylates Chk2 in vivo and in vitro. Proc Natl Acad Sci USA.
97:10389–10394. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nambiar DK, Rajamani P, Deep G, Jain AK,
Agarwal R and Singh RP: Silibinin preferentially radiosensitizes
prostate cancer by inhibiting DNA repair signaling. Mol Cancer
Ther. 14:2722–2734. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao J, Guo Z, Pei S, Song L, Wang C, Ma
J, Jin L, Ma Y, He R, Zhong J, et al: pATM and γH2AX are effective
radiation biomarkers in assessing the radiosensitivity of 12C6+ in
human tumor cells. Cancer Cell Int. 17:492017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ahmed KM and Li JJ: NF-kappa B-mediated
adaptive resistance to ionizing radiation. Free Radic Biol Med.
44:1–13. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shokoohinia Y, Jafari F, Mohammadi Z,
Bazvandi L, Hosseinzadeh L, Chow N, Bhattacharyya P, Farzaei MH,
Farooqi AA, Nabavi SM, et al: Potential anticancer properties of
osthol: A comprehensive mechanistic review. Nutrients. 10:pii: E36.
2018. View Article : Google Scholar : PubMed/NCBI
|