Introduction
Primary liver cancer is a common aggressive
malignant disease, and the third leading cause of cancer-related
deaths worldwide. An estimated 40,710 new cases of disease and
28,920 cancer-associated deaths were reported in 2017 in the USA
(1). More than 90% of primary liver
cancers are hepatoma, which is mainly caused by infection with
hepatitis B or C. The incidence of liver cancer has been increasing
in Asia and ~50% of reported mortalities are Chinese individuals
(2). As early detection is
difficult, ~70% of all hepatoma patients cannot be treated by
surgical resection at the time of diagnosis, which results in a
poor prognosis and a median overall survival time of a few months
(3). The molecular pathogenesis of
liver cancer has been extensively investigated in recent years;
however, no effective biomarker for the early diagnosis and
treatment of hepatoma has been established.
Emerging evidence has demonstrated that abnormal
homologous recombination repair (HRR) is closely related to the
development and progression of human cancers (4). The function of proteins that
participate in homologous recombination (HR) is primarily to
maintain genomic stability and suppress tumor development by
repairing DNA double strand breaks (DSBs) and damaged replication
forks (5,6). RAD51 is a central member of the HRR
signaling pathway, which is expressed in a number of cancer cell
lines and is associated with cell sensitivity to DNA-damaging
agents (7,8). RAD51-associated protein 1 (RAD51AP1)
mRNA expression is increased in liver cancer tissues compared with
corresponding normal liver tissues (9). Obama et al revealed that
RAD51AP1 expression was increased in intrahepatic
cholangiocarcinoma, and the downregulation of RAD51AP1 by shRNA
could effectively suppress the proliferation of cholangiocarcinoma
cells (10). Recent findings have
revealed that RAD52 may be a potential therapeutic target for BRCA1
and BRCA2-deficient familial breast and ovarian cancer (11–13).
It has also been demonstrated that disease-free survival is
correlated with RAD50 expression in tissues from patients with
non-small cell lung cancer (NSCLC); RAD50 knockdown increases cell
sensitivity to radiation whereas RAD50 upregulation induces
radioresistence in NSCLC cells (14). These previous studies indicated that
many HR-associated proteins are involved in the development of
cancers, including liver cancer; however, there are still many
HRR-associated proteins that regulate the growth and function of
cancer cells and need to be further elucidated.
Recently, our group investigated RAD54B, which is a
vital motor protein of HR. RAD54B belongs to the SNF2/SWI2
superfamily and plays an important role in the DNA repair system.
It has been revealed that distant metastasis was significantly
increased in colorectal cancer patients with high RAD54B expression
compared with the low expression group, which may be associated
with the degradation of p53 protein in clinical samples (15). In addition, the high expression of
Rad54B may act as an independent prognostic factor for lung
adenocarcinoma (16). To the best
of our knowledge, few studies have reported the effect of RAD54B on
the development of cancer and the mechanism by which it functions.
Furthermore, there have been no previous reports regarding the
expression and biological function of RAD54B in liver cancer.
In the present study, we investigated the expression
of RAD54B in liver cancer and analyzed its relationship with liver
cancer patient prognosis. Furthermore, we explored the effect of
RAD54B silencing on hepatoma cell proliferation, colony formation,
cell cycle distribution and apoptosis. This study aimed to identify
a potential new biomarker or treatment target that could be used
for the prognosis of liver cancer patients.
Materials and methods
Gene expression profiles
RAD54B mRNA expression data from 50 liver cancer
tissues and 50 matched adjacent tissue samples were obtained from
the TCGA data portal (https://tcga-data.nci.nih.gov/tcga/).
Human tissue samples, cell culture,
reagents and antibodies
The human tissue protocol utilized in this study was
approved by the Ethics Committee of Bengbu Medical College. A total
of 83 samples were obtained from patients with liver cancer who
underwent surgery at the First Affiliated Hospital of Bengbu
Medical College (Bengbu, China) between January 2012 and November
2015. Preoperative informed consent was obtained from each patient
registered in the study, in accordance with the institutional
guidelines. Harvested specimens were subjected to
immunohistochemistry (IHC). Human LO2, BEL-7404, BEL-7402, HepG2
and SMMC-7721 cell lines were purchased from the Cell Bank of the
Shanghai Institute of Cell Biology, Chinese Academy of Sciences
(Shanghai, China). The cells were cultured at 37°C in 5%
CO2 in Dulbecco's modified Eagle's medium (DMEM;
Corning, Inc., Corning, NY, USA) containing 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). MTT
[3-(4,5-dimethythiazol- 2-yl)-2,5-diphenyl tetrazolium bromide] was
purchased from Genview Scientific, Inc. (El Monte, CA, USA). An
Annexin V Apoptosis Detection kit was obtained from BD Biosciences
(San Diego, CA, USA). Rabbit polyclonal anti-RAD54B antibodies
(cat. no. ab83311) and mouse anti-β-actin monoclonal antibodies
(cat. no. ab8226) were obtained from Abcam (Cambridge, MA,
USA).
Immunohistochemistry (IHC)
Formalin-fixed and paraffin-embedded liver cancer
tissue samples were cut into 4-µm-thick slices. After
deparaffinization, 3% H2O2 was used for 15
min to block the endogenous peroxidase and reduce non-specific
staining. Antigen retrieval was then performed with sodium citrate
buffer (pH 6.0). The slices were incubated with primary antibodies
(rabbit anti-RAD54B polyclonal antibodies, dilution 1:50; Abcam)
overnight at 4°C. After washing, the slices were incubated for 1 h
at 37°C with the goat anti-rabbit IgG second antibodies (dilution
1:1,000; cat. no. ab6720; Abcam). The sections were treated using
the SABC kit (Wuhan Boster Biological Technology, Ltd., Wuhan,
China) according to the manufacturer's instructions and stained
using a 3,3′-diaminobenzidine tetrahydrochloride (DAB) substrate
kit. Normal rabbit serum was used in place of the primary antibody
as the negative control. Representative images were visualized
using an Olympus inverted microscope (Olympus Corp., Tokyo, Japan).
Staining was evaluated and scored by two independent researchers.
The percentage ratio of positively stained cells vs. all cells was
calculated in 10 randomly selected microscopic fields at
magnification ×400. Staining localized to the nucleus was graded on
a 0–3 intensity scale (0, negative; 1, weakly positive; 2,
moderately positive; 3, strongly positive). Positive staining was
defined as >5% of the tumor cells being stained by the
antibodies.
Real-time quantitative reverse
transcription-PCR
Total RNA from the liver cancer cells was extracted
using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.), which
was then treated with an RNeasy Mini kit and RNase-free DNase Set
(Promega Corp., Madison, WI, USA) according to the manufacturer's
protocols. The primers used in the PCR reactions were as follows:
RAD54B forward, 5′-GCCAAACACTGATGATTTGTGG-3′ and reverse,
5′-CCTGAGAAGAATGCGAGATAGC-3′; GAPDH forward,
5′-TGACTTCAACAGCGACACCCA-3′ and reverse,
5′-CACCCTGTTGCTGTAGCCAAA-3′. GAPDH was used as the internal
control. The fold amplification for gene expression was determined
using the 2−∆∆Cq method (17).
Western blot analysis
Harvested hepatoma cells were washed with
phosphate-buffered saline (PBS) and lysed using a RIPA lysis buffer
(Wuhan Boster Biological Technology, Ltd.). The concentrations of
protein were detected using a Bio-Rad protein assay kit (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Equal amounts of total
protein were separated by 10% sodium dodecyl sulfate-polyacrylamide
gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene
difluoride (PVDF) membranes. The membranes were blocked with 5%
skimmed milk-TBST and incubated with primary antibodies against
RAD54B (1:500; Abcam) at 4°C overnight. The membranes were washed
three times using TBST before being incubated with HRP-conjugated
secondary antibodies (dilution 1:2000; cat. no. ab6721; Abcam) at
room temperature for 1 h. The membranes were then washed three
times with TBST and proteins were visualized using an
electrochemiluminescence (ECL) assay. Images were taken by
fusion-capture software (Fusion FX7; Vilber Lourmat, Marne
Le-Vallée, France).
Lentivirus infection assay
The targeting sequences of RAD54B
(AGATTGTTGATGGCTTTAA) and a nonsense sequence
(TTCTCCGAACGTGTTCACGT) were designed and constructed. RAD54B-shRNA
and control-shRNA were inserted into linearized GV115 plasmid
vectors (Shanghai GeneChem, Co., Ltd., Shanghai, China), which
carried the green fluorescent protein (GFP) gene and
U6-MCS-CMV-puror. The RAD54B and control shRNA vectors and two
packaging auxiliary plasmids (pHelper1.0 and pHelper2.0) were used
for lentivirus production. The plasmid vectors were transformed
Escherichia coli competent cells. The positive colonies were
selected and identified by PCR and sequencing. Next, the effective
plasmid vectors were extracted and packed into lentivirus particles
using 293T cells. BEL-7404 and SMMC-7721 cells were transfected
with the RAD54B-shRNA-lentivirus (shRAD54B group) or negative
control lentivirus (shCtrl group) at the recommended multiplicity
of infection (MOI) when the cells reached 80% confluence in the
6-well plates. Fluorescence expression was observed under a
fluorescence microscope (Olympus Corp.) 3 days after lentiviral
infection.
MTT assay
BEL-7404 and SMMC-7721 cells were seeded at equal
densities in 96-well culture plates and incubated overnight at 37°C
in 5% CO2. The cells were incubated with a medium
containing 5 mg/ml MTT for 4 h, following which the medium solution
was removed and 100 µl dimethyl sulfoxide (DMSO) was added to the
plates to dissolve the precipitated formazan. The absorbance at 450
nm was detected using an ELISA microplate reader (Tecan
infinite).
Cell proliferation assay
After infection with shRAD54B or shCtrl, the
BEL-7404 and SMMC-7721 cells were seeded at a density of 1,000
cells/well in 96-well plates, and then cultured at 37°C in 5%
CO2. The Celigo Imaging Cytometry System (Nexcelom
Bioscience, Lawrence, MA, USA) was used to observe the fluorescence
intensity and calculate the corresponding cell count.
Colony formation assay
After lentiviral infection, the BEL-7404 and
SMMC-7721 cells were seeded at a density of 1,000 cells/well in
6-well plates with three duplicates of each and cultured under
standard conditions. After 2 weeks, the cells were washed with PBS,
stained with Giemsa for 30 min, and washed three times with
deionized distilled water to remove any background interference.
The number of colonies was counted and images were obtained using a
digital camera under light microscopy. The assay was performed
independently a minimum of three times.
Cell cycle distribution analysis
For the cell cycle assay, BEL-7404 and SMMC-7721
cells were seeded into 6-well plates and transfected with the
shRAD54B or shCtrl vectors. After the cells were washed with PBS
and digested by trypsin, the corresponding dyes and solution were
added from the cell cycle kit and incubated according to the
manufacturer's instructions. The cell cycle assay was performed
using a flow cytometer (EMD Millipore, Billerica, MA, USA) and
FlowJo software (FlowJo, LLC, Ashland, OR, USA) was used for
analysis.
Apoptosis analysis
The apoptosis rate of BEL-7404 and SMMC-7721 cells
infected with shRAD54B or shCtrl was evaluated by Annexin-V PE and
7-AAD staining, using an Apoptosis Detection kit (BD Biosciences,
San Diego, CA, USA) according to manufacturer's instructions.
Quantification and analysis of the apoptotic cells was performed by
flow cytometry (EMD Millipore) and FlowJo software (FlowJo, LLC),
respectively.
Statistical analysis
The Student's t-test was performed to estimate the
statistical significance of the data. All statistical analysis was
performed using SPSS 19.0 software (IBM Corp., Armonk, NY, USA).
Data were presented as the mean ± standard deviation (SD).
Kalpan-Meier survival analysis was adopted for overall survival
rate analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
RAD54B expression is elevated in liver
cancer tissues and cells
To explore the expression of RAD54B in liver cancer
tissues, we collected results from a TCGA dataset. It was found
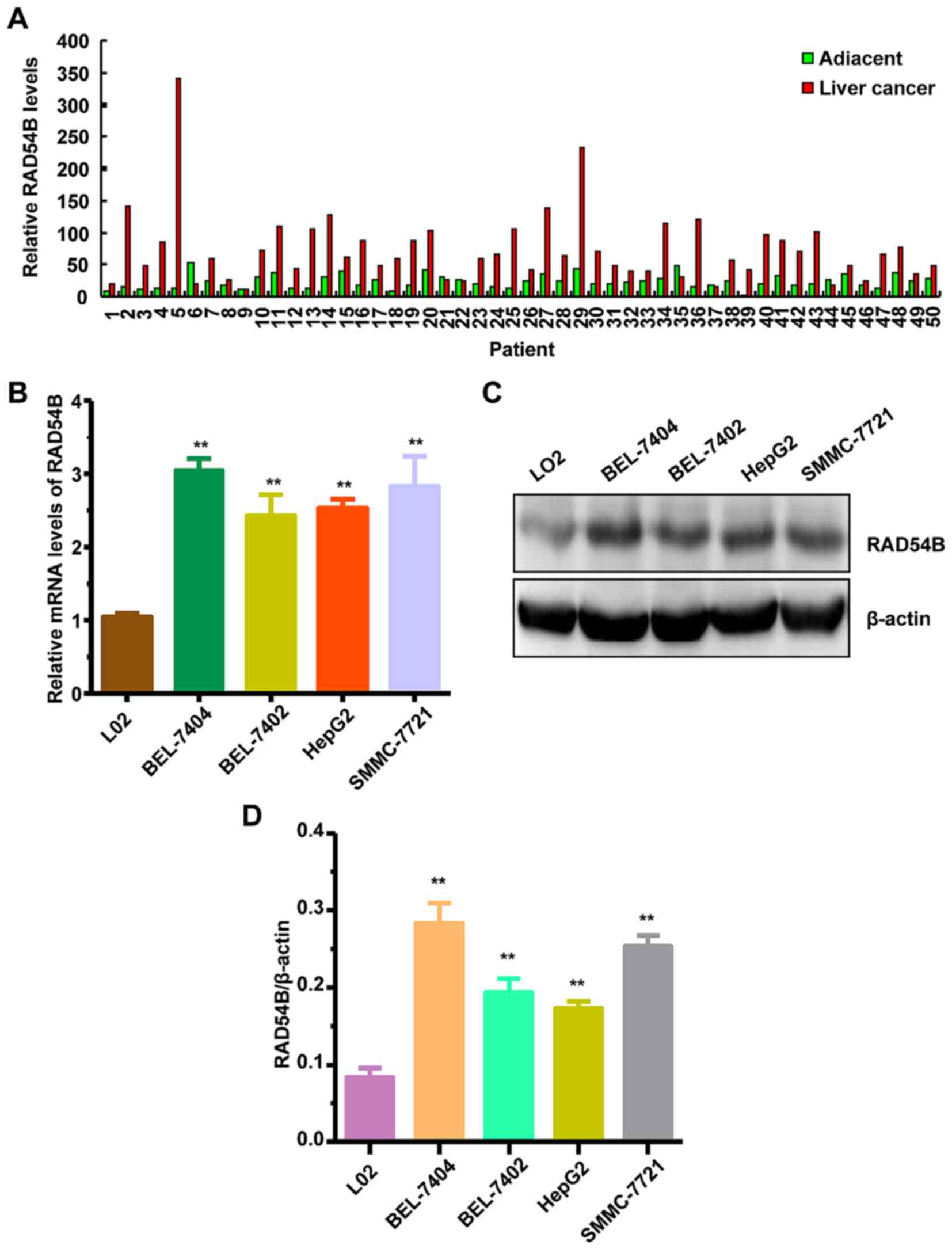
that RAD54B mRNA expression was increased by 2–3-fold in liver
cancer tissues compared with paired adjacent tissues (Fig. 1A). Furthermore, we assessed RAD54B
mRNA and protein expression in the liver cancer cell lines
BEL-7404, BEL-7402, HepG2 and SMMC-7721, and the normal hepatic
cell line LO2 using RT-qPCR and western blotting, respectively. The
results revealed that RAD54B mRNA and protein expression were both
significantly increased in all four liver cancer cell lines
compared with the normal LO2 cell line (Fig. 1B-D). These results revealed that
RAD54B mRNA and protein expression was elevated in liver cancer
tissues and cell lines.
Downregulation of RAD54B inhibits the
proliferation of BEL-7404 and SMMC-7721 cells
To further investigate the effect of RAD54B on the
proliferation of hepatoma cells, we inhibited the expression of
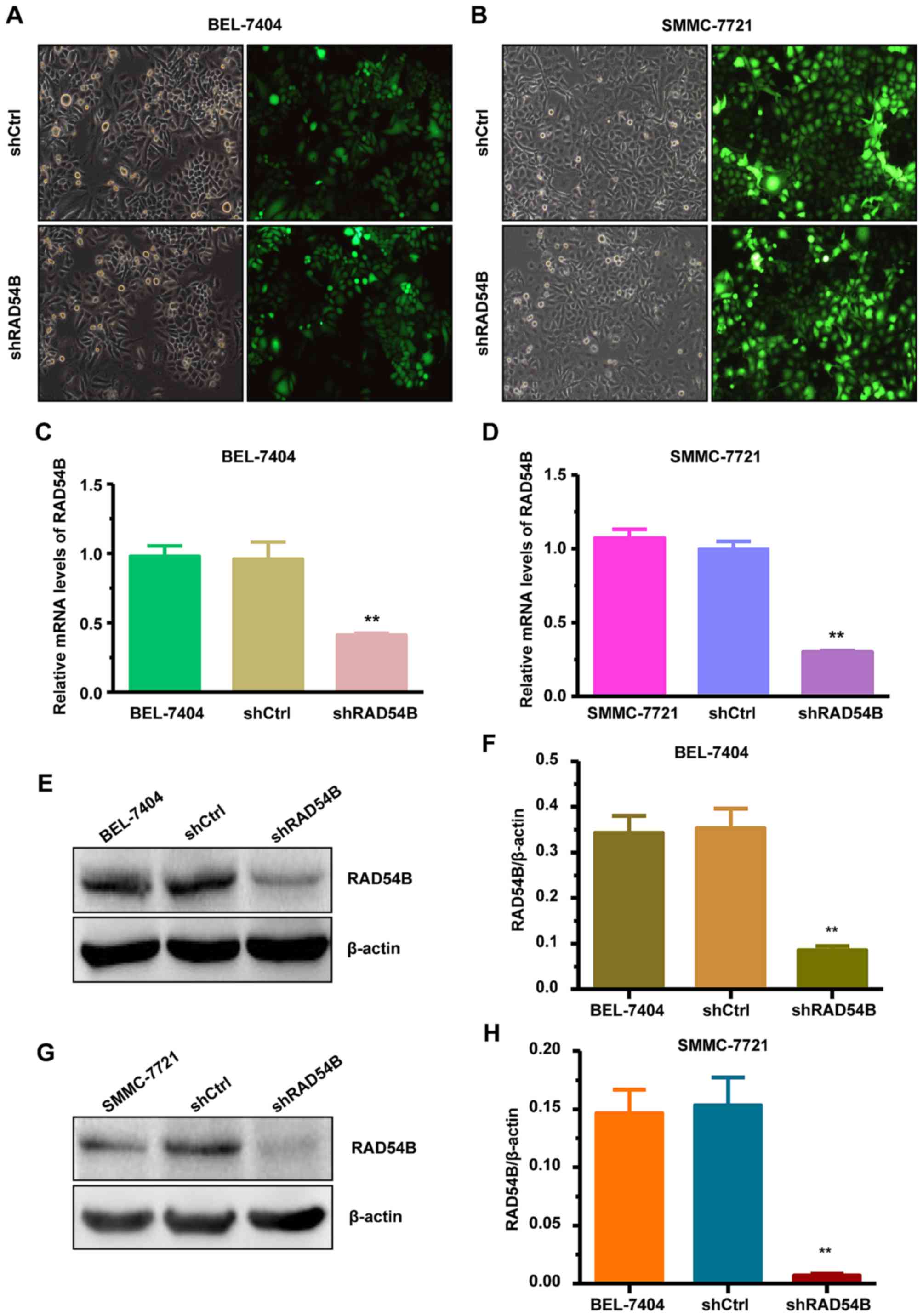
RAD54B in the BEL-7404 and SMMC-7721 cell lines. The cells were
infected with lentivirus-shRAD54B (shRAD54B) and the changes to
cell proliferation were observed. Lentivirus-shCtrl (shCtrl)
infected cells were used as the control. We observed that >80%
of the cells were infected by shRAD54B or the shCtrl, which was
confirmed by fluorescence microscopy (Fig. 2A and B). The mRNA and protein
expression of RAD54B were obviously suppressed in cells infected by
shRAD54B compared with those cells infected with shCtrl, as
determined by RT-qPCR and western blot analysis (Fig. 2C-H).
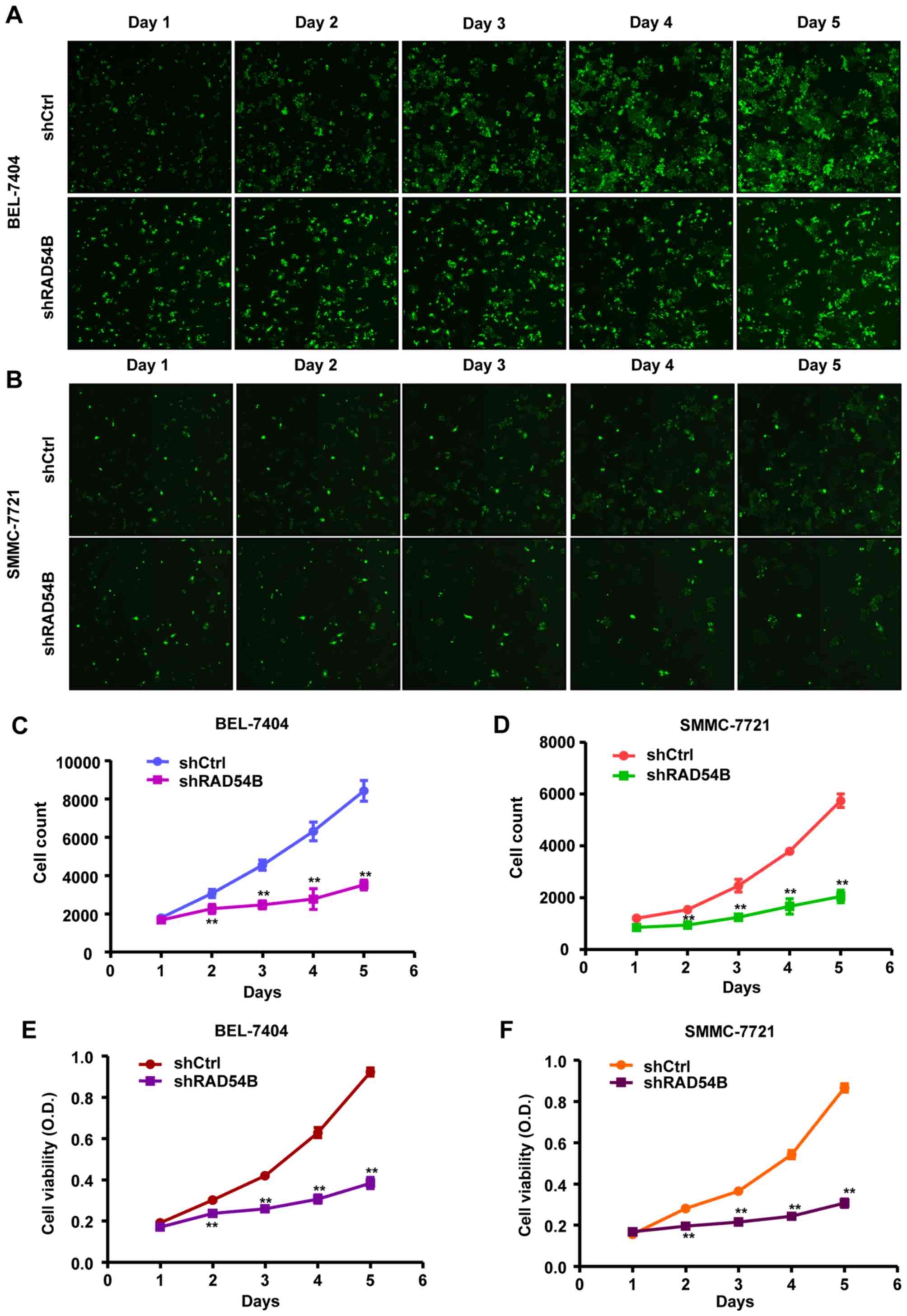
In addition, Celigo calculations and an MTT assay
were used to determine the effect of RAD54B inhibition on the
proliferation of infected cells. The results of the Celigo
calculation revealed that the fluorescence intensities of the
shRAD54B groups were reduced compared with the shCtrl groups from
days 2 to 5 in both the BEL-7404 and SMMC-7721 cell lines (Fig. 3A and B). In addition, cell counts of
the shRAD54B groups were significantly lower than the shCtrl groups
from days 2 to 5 (Fig. 3C and D).
Similarly, the MTT assay revealed that cell growth in the shRAD54B
groups was significantly suppressed compared with the shCtrl groups
from days 2 to 5 (Fig. 3E and F).
These results revealed that the downregulation of RAD54B inhibited
cell proliferation in the BEL-7404 and SMMC-7721 cell lines.
RAD54B downregulation significantly
inhibits colony formation in BEL-7404 and SMMC-7721 cells
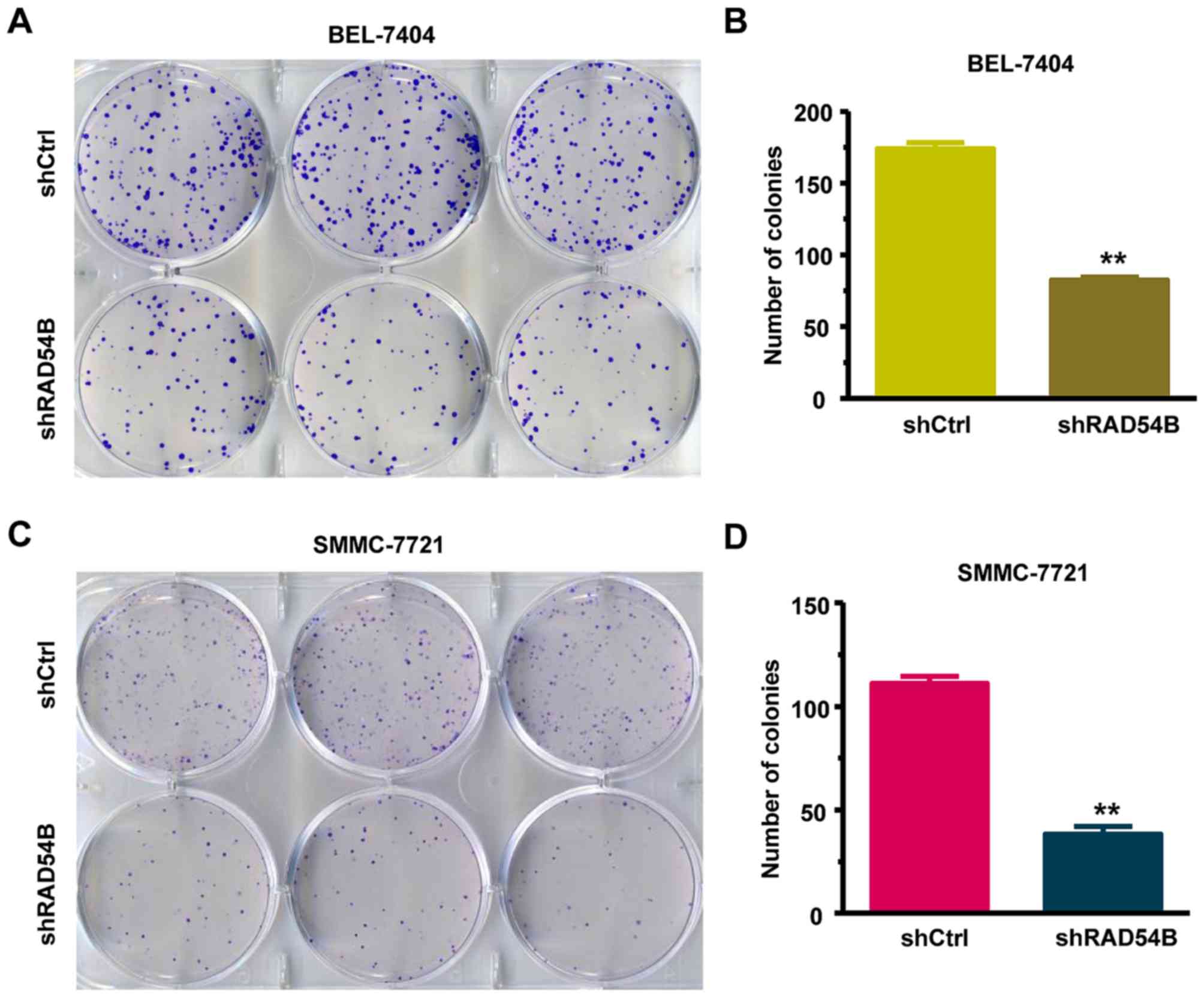
We also analyzed the effect of shRAD54B on colony
formation in the BEL-7404 and SMMC-7721 cell lines using a colony
formation assay. As observed in Fig.
4A-D, there was a clear decrease in the number of colonies in
the shRAD54B groups compared with the shCtrl groups. The results
indicated that RAD54B downregulation significantly inhibited the
colony-forming abilities of BEL-7404 and SMMC-7721 cells.
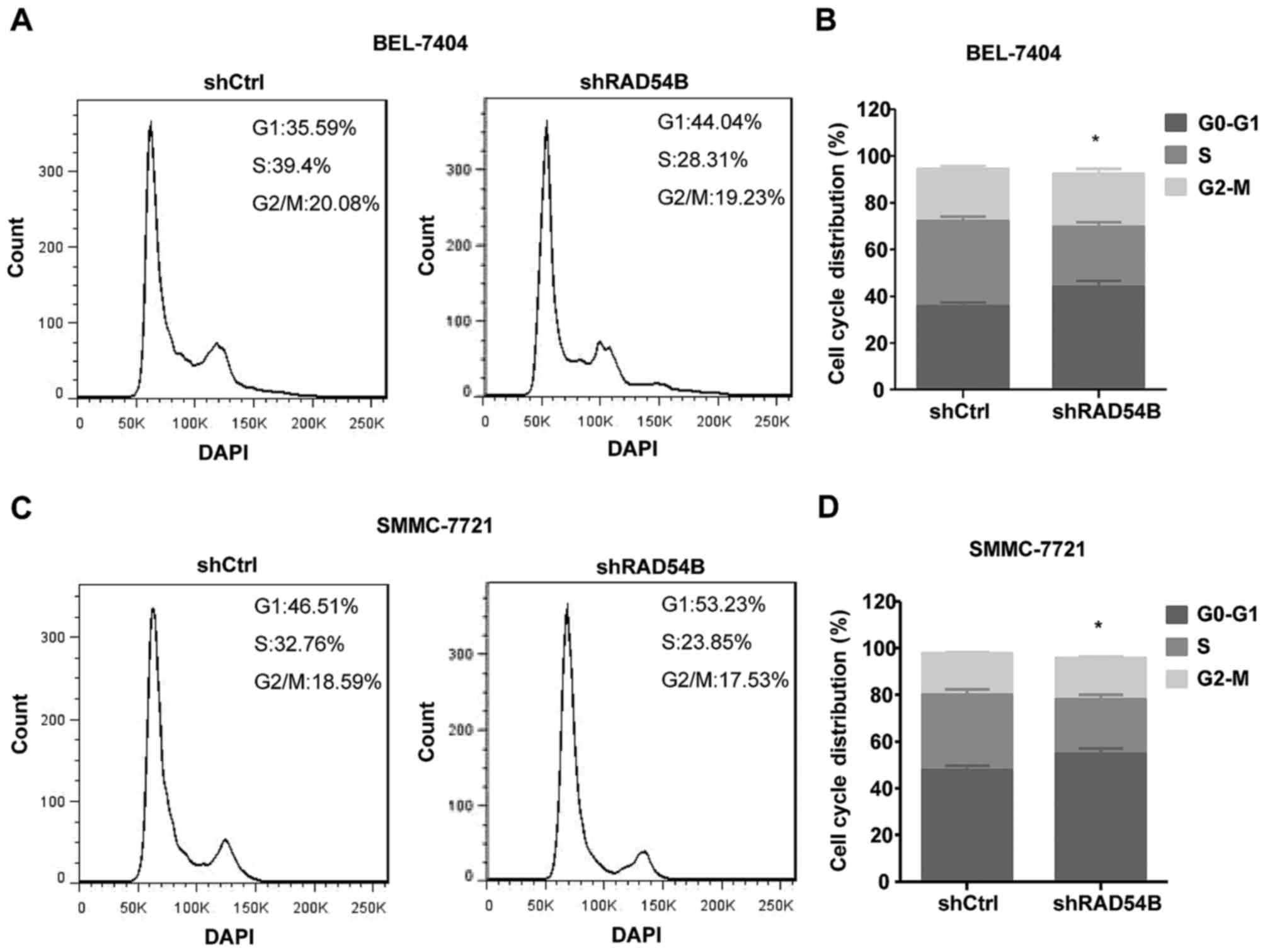
Inhibition of RAD54B induces cell
cycle arrest in the G1/S phase
Flow cytometry was used to detect the cell cycle
distribution and thereby determine the effects of RAD54B on liver
cancer cells, including the BEL-7404 and SMMC-7721 cell lines. As
shown in Fig. 5A-D, the percentage
of cells in the G1 phase was significantly increased and the
population of cells in the S phase was significantly decreased in
the shRAD54B group compared with the control group, which indicated
that RAD54B downregulation induced cell cycle arrest in the G1/S
phase.
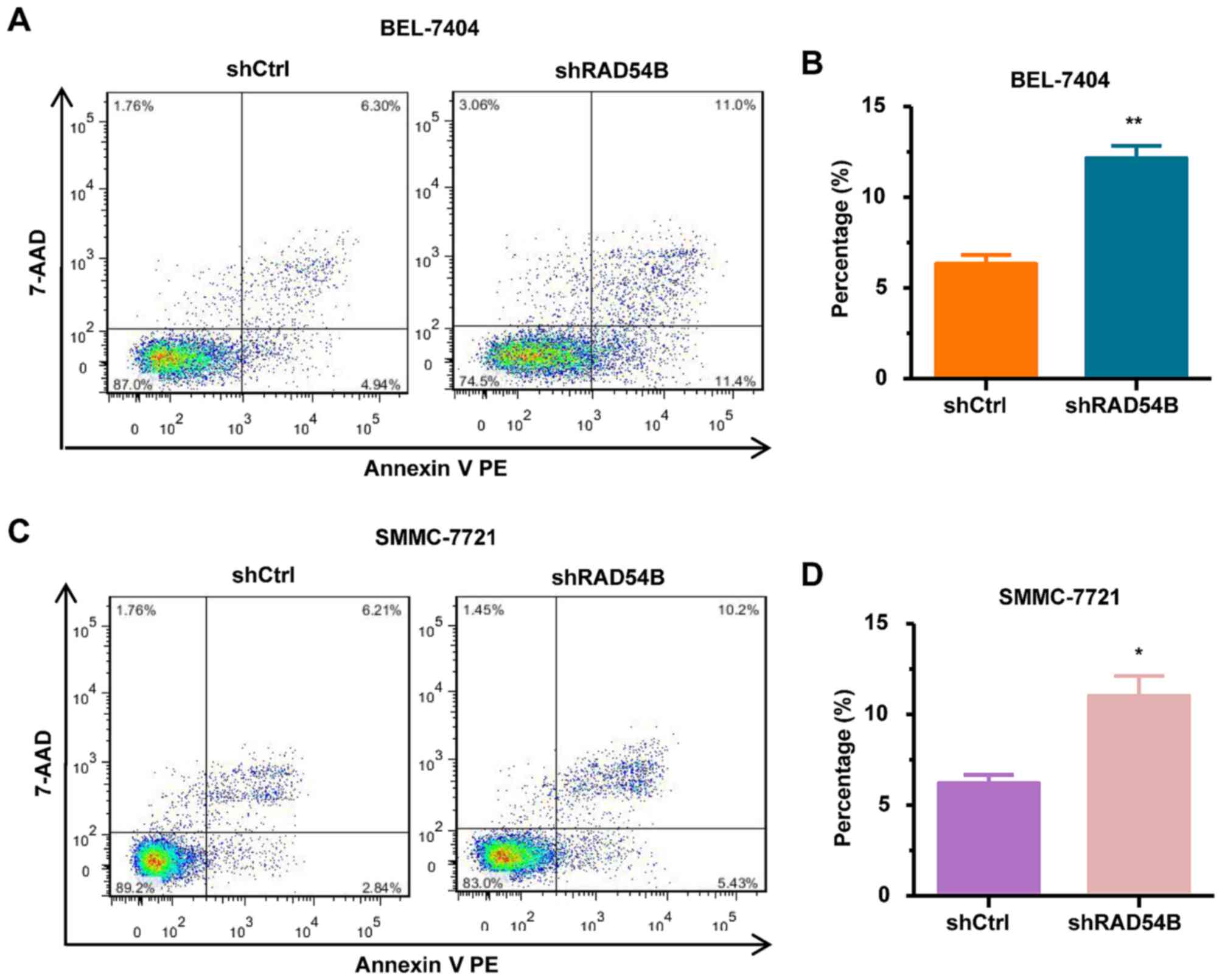
RAD54B downregulation increases the
apoptosis of BEL-7404 and SMMC-7721 cell lines
To further elucidate whether RAD54B affects
apoptosis in hepatoma cells, we assessed the levels of apoptosis in
shRAD54B and shCtrl cells. As shown in Fig. 6A-D, the number of apoptotic
shRAD54B-infected cells was significantly increased compared with
the shCtrl-infected cells in both BEL-7404 and SMMC-7721 cell
lines. These results demonstrated that RAD54B downregulation
induced apoptosis in BEL-7404 and SMMC-7721 cells.
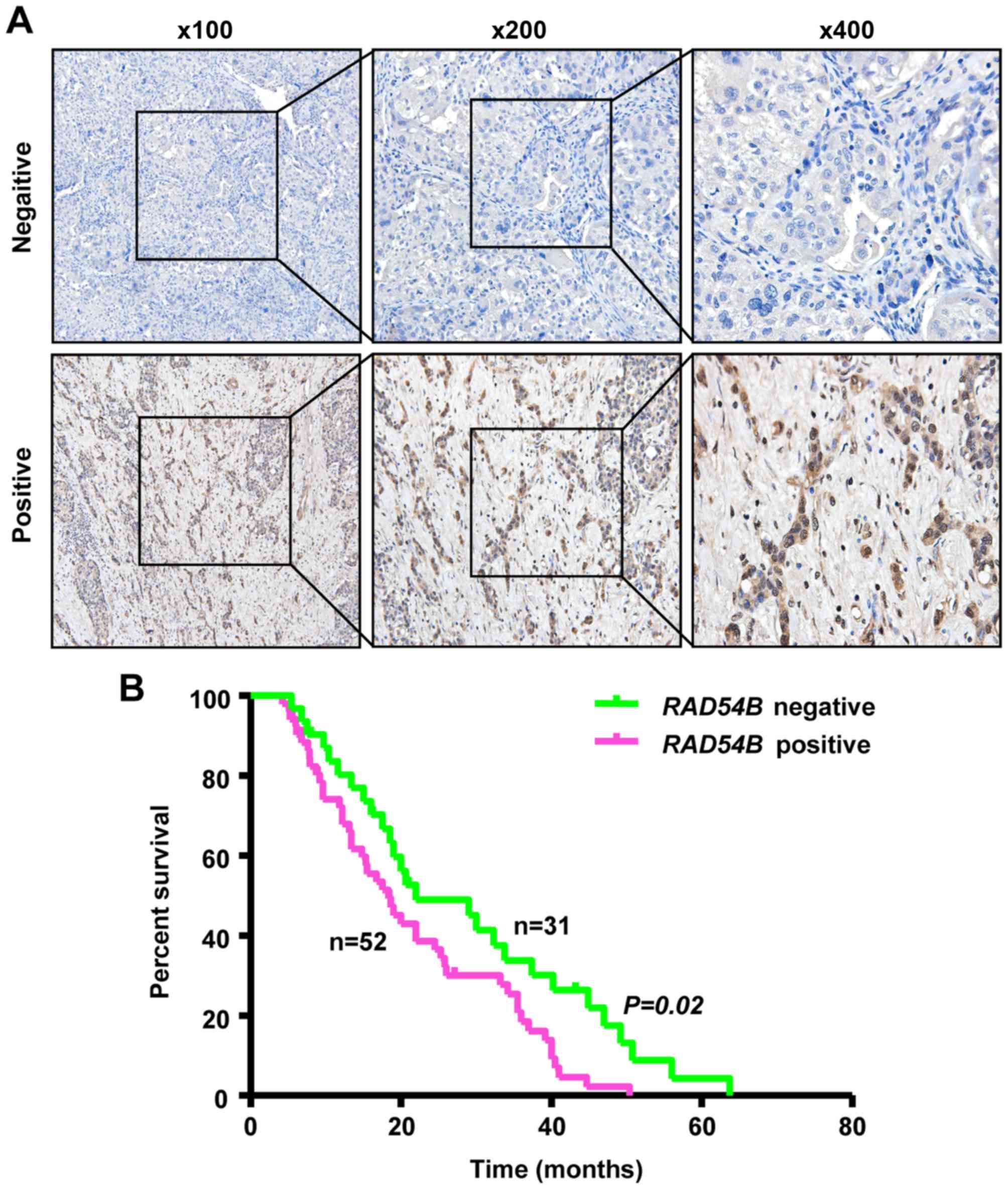
RAD54B expression is associated with a
poor prognosis in liver cancer patients
IHC staining was performed to determine the
relationship between RAD54B expression and the prognosis of liver
cancer patients. RAD54B protein expression was investigated in 83
samples of liver cancer tissue. The overall survival (OS) data was
obtained for all 83 patients. The results revealed that 52/83
samples of liver cancer were positive for nuclear staining and
RAD54B protein expression, while the other 31 samples were negative
(Fig. 7A). Using Kaplan-Meier
survival analysis we found that the median OS time was 18.3 months
for patients with positive expression, whereas the OS was 22.0
months for patients with RAD54B negative expression. Liver cancer
patients with positive RAD54B expression were associated with a
reduced OS (P=0.02, Fig. 7B).
Collectively, these results indicated that the expression of RAD54B
could be a prognostic marker for liver cancer.
Discussion
Homologous recombination (HR) is an important
pathway in DSB repair; however, a number of genes and proteins
associated with HR have been reported to serve a critical role in
the development of cancer. Human RAD54B is an important DNA repair
and recombination protein, which belongs to the DEAD-like helicase
superfamily and plays an important role in the DNA damage response
(DDR) by binding to double-stranded DNA and displaying ATPase
activity (18–20). A number of DNA re-sequencing studies
have revealed that somatic alterations in human RAD54B, including
gene amplifications, mutations, homozygous deletions and
single-nucleotide polymorphisms, are observed in distinct types of
human cancers (21–26). The influence of RAD54B on a few
types of cancer has been previously reported, however, the effect
of RAD54B on hepatoma cells and its mechanism of action remain
unclear. Therefore, our study focused on the role of RAD54B during
the development and progression of liver cancer, and clarified that
RAD54B expression was increased in liver cancer tissues, which may
be associated with the poor prognosis observed in liver cancer
patients. Moreover, we confirmed that RAD54B downregulation
inhibited proliferation and colony formation in liver cancer cells,
while inducing apoptosis. These findings indicate that RAD54B could
be a potential prognostic factor for hepatoma patients.
As aforementioned, RAD54B has been previously
revealed to participate in the development of some cancers,
although this has not yet been demonstrated in liver cancer. Nagai
et al found that RAD54B mRNA expression was elevated in the
majority of colorectal cancer (CRC) tissues compared to the
corresponding normal mucosa. Furthermore, CRC patients with high
RAD54B expression had significantly reduced recurrence-free
survival compared with patients with low RAD54B expression. Based
on these results, RAD54B expression is considered to be an
independent prognostic factor for distant recurrence in stage I–III
CRC patients (15). In addition, it
has been observed that the expression of RAD54B protein was
increased in patients with lung adenocarcinoma (16). Thus, we speculated that RAD54B
expression could be related to the prognosis of liver cancer
patients.
A number of studies have shown that other HR
proteins associated with the normal repair process are closely
related to the progression of liver cancer. Recently, HR proteins
have been viewed as potential treatment targets for different
cancers, including liver cancer. A recent study reported that
gefitinib and harmine inhibited the proliferation of hepatoma cells
and suppressed HR repair mediated by RAD51, which forms a protein
complex with RAD54B (27). Another
study performed genotype-phenotype correlation analysis of 44 human
liver tissue samples, and found that RAD52 single nucleotide
polymorphisms (SNPs) contributed to the susceptibility of
developing hepatoma (28).
Similarly, we initially found that RAD54B overexpression resulted
in a poor patient prognosis in liver cancer, and that RAD54B
downregulation inhibited the proliferation of hepatoma cells.
However, the molecular mechanisms of this process still need to be
studied further.
In conclusion, our results indicated that RAD54B
inhibition suppresses proliferation and colony formation, inducing
cell cycle arrest in the G1/S phase and apoptosis in liver cancer
cells. Moreover, we found that RAD54B was highly expressed in
hepatoma tissues, which may contribute to poor prognosis in liver
cancer patients. Collectively, these results revealed that RAD54B
could be a potential target for the treatment of liver cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by funding from the
National Natural Sciences Fund (no. 81572315) and the Anhui
Provincial Natural Science Foundation (no. 1708085QH216).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
RW and LiW participated in the design of the study,
the data interpretation and manuscript drafting. YaL, YC, LeW and
JL performed the experiments. QW, YG and YuL participated in the
clinical sample collection. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The human tissue protocol utilized in this study was
approved by the Ethics Committee of Bengbu Medical College (Bengbu,
China). Preoperative informed consent was obtained from each
patient registered in the study, in accordance with the
institutional guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zuo TT, Zheng RS, Zhang SW, Zeng HM and
Chen WQ: Incidence and mortality of liver cancer in China in 2011.
Chin J Cancer. 34:508–513. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Trovato FM, Tognarelli JM, Crossey MM,
Catalano D, Taylor-Robinson SD and Trovato GM: Challenges of liver
cancer: Future emerging tools in imaging and urinary biomarkers.
World J Hepatol. 7:2664–2675. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
O'Kane GM, Connor AA and Gallinger S:
Characterization, detection, and treatment approaches for
homologous recombination deficiency in cancer. Trends Mol Med.
23:1121–1137. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Talens F, Jalving M, Gietema JA and Van
Vugt MA: Therapeutic targeting and patient selection for cancers
with homologous recombination defects. Expert Opin Drug Discov.
12:565–581. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kolinjivadi AM, Sannino V, de Antoni A,
Técher H, Baldi G and Costanzo V: Moonlighting at replication forks
- a new life for homologous recombination proteins BRCA1, BRCA2 and
RAD51. FEBS Lett. 591:1083–1100. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Klein HL: The consequences of Rad51
overexpression for normal and tumor cells. DNA Repair (Amst).
7:686–693. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tennstedt P, Fresow R, Simon R, Marx A,
Terracciano L, Petersen C, Sauter G, Dikomey E and Borgmann K:
RAD51 overexpression is a negative prognostic marker for colorectal
adenocarcinoma. Int J Cancer. 132:2118–2126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Song H, Xia SL, Liao C, Li YL, Wang YF, Li
TP and Zhao MJ: Genes encoding Pir51, Beclin 1, RbAp48 and aldolase
b are up or down-regulated in human primary hepatocellular
carcinoma. World J Gastroenterol. 10:509–513. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Obama K, Satoh S, Hamamoto R, Sakai Y,
Nakamura Y and Furukawa Y: Enhanced expression of RAD51 associating
protein-1 is involved in the growth of intrahepatic
cholangiocarcinoma cells. Clin Cancer Res. 14:1333–1339. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chandramouly G, McDevitt S, Sullivan K,
Kent T, Luz A, Glickman JF, Andrake M, Skorski T and Pomerantz RT:
Small-molecule disruption of RAD52 rings as a mechanism for
precision medicine in BRCA-deficient cancers. Chem Biol.
22:1491–1504. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang F, Goyal N, Sullivan K, Hanamshet K,
Patel M, Mazina OM, Wang CX, An WF, Spoonamore J, Metkar S, et al:
Targeting BRCA1- and BRCA2-deficient cells with RAD52 small
molecule inhibitors. Nucleic Acids Res. 44:4189–4199. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cramer-Morales K, Nieborowska-Skorska M,
Scheibner K, Padget M, Irvine DA, Sliwinski T, Haas K, Lee J, Geng
H, Roy D, et al: Personalized synthetic lethality induced by
targeting RAD52 in leukemias identified by gene mutation and
expression profile. Blood. 122:1293–1304. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Y, Gudikote J, Giri U, Yan J, Deng W,
Ye R, Jiang W, Li N, Hobbs BP, Wang J, et al: RAD50 expression is
associated with poor clinical outcomes after radiotherapy for
resected non-small cell lung cancer. Clin Cancer Res. 24:341–350.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nagai Y, Yamamoto Y, Yasuhara T, Hata K,
Nishikawa T, Tanaka T, Tanaka J, Kiyomatsu T, Kawai K, Nozawa H, et
al: High RAD54B expression: An independent predictor of
postoperative distant recurrence in colorectal cancer patients.
Oncotarget. 6:21064–21073. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hwang JC, Sung WW, Tu HP, Hsieh KC, Yeh
CM, Chen CJ, Tai HC, Hsu CT, Shieh GS, Chang JG, et al: The
overexpression of FEN1 and RAD54B may act as independent prognostic
factors of lung adenocarcinoma. PLoS One. 10:e01394352015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Smith CL and Peterson CL: A conserved
Swi2/Snf2 ATPase motif couples ATP hydrolysis to chromatin
remodeling. Mol Cell Biol. 25:5880–5892. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miyagawa K, Tsuruga T, Kinomura A, Usui K,
Katsura M, Tashiro S, Mishima H and Tanaka K: A role for RAD54B in
homologous recombination in human cells. EMBO J. 21:175–180. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sarai N, Kagawa W, Fujikawa N, Saito K,
Hikiba J, Tanaka K, Miyagawa K, Kurumizaka H and Yokoyama S:
Biochemical analysis of the N-terminal domain of human RAD54B.
Nucleic Acids Res. 36:5441–5450. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bass AJ, Thorsson V, Shmulevich I,
Reynolds SM, Miller M, Bernard B, Hinoue T, Laird PW, Curtis C,
Shen H, et al: Cancer Genome Atlas Research Network: Comprehensive
molecular characterization of gastric adenocarcinoma. Nature.
513:202–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ciriello G, Gatza ML, Beck AH, Wilkerson
MD, Rhie SK, Pastore A, Zhang H, McLellan M, Yau C, Kandoth C, et
al: TCGA Research Network: Comprehensive molecular portraits of
invasive lobular breast cancer. Cell. 163:506–519. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Imielinski M, Berger AH, Hammerman PS,
Hernandez B, Pugh TJ, Hodis E, Cho J, Suh J, Capelletti M,
Sivachenko A, et al: Mapping the hallmarks of lung adenocarcinoma
with massively parallel sequencing. Cell. 150:1107–1120. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hodis E, Watson IR, Kryukov GV, Arold ST,
Imielinski M, Theurillat JP, Nickerson E, Auclair D, Li L, Place C,
et al: A landscape of driver mutations in melanoma. Cell.
150:251–263. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dulak AM, Stojanov P, Peng S, Lawrence MS,
Fox C, Stewart C, Bandla S, Imamura Y, Schumacher SE, Shefler E, et
al: Exome and whole-genome sequencing of esophageal adenocarcinoma
identifies recurrent driver events and mutational complexity. Nat
Genet. 45:478–486. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hiramoto T, Nakanishi T, Sumiyoshi T,
Fukuda T, Matsuura S, Tauchi H, Komatsu K, Shibasaki Y, Inui H,
Watatani M, et al: Mutations of a novel human RAD54 homologue,
RAD54B, in primary cancer. Oncogene. 18:3422–3426. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shao J, Xu Z, Peng X, Chen M, Zhu Y, Xu L,
Zhu H, Yang B, Luo P and He Q: Gefitinib synergizes with irinotecan
to suppress hepatocellular carcinoma via antagonizing
Rad51-mediated DNA-repair. PLoS One. 11:e01469682016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Z, Guo Y, Zhou L, Ge Y, Wei L, Li L,
Zhou C, Wei J, Yuan Q, Li J, et al: Association of a functional
RAD52 genetic variant locating in a miRNA binding site with risk of
HBV-related hepatocellular carcinoma. Mol Carcinog. 54:853–858.
2015. View
Article : Google Scholar : PubMed/NCBI
|