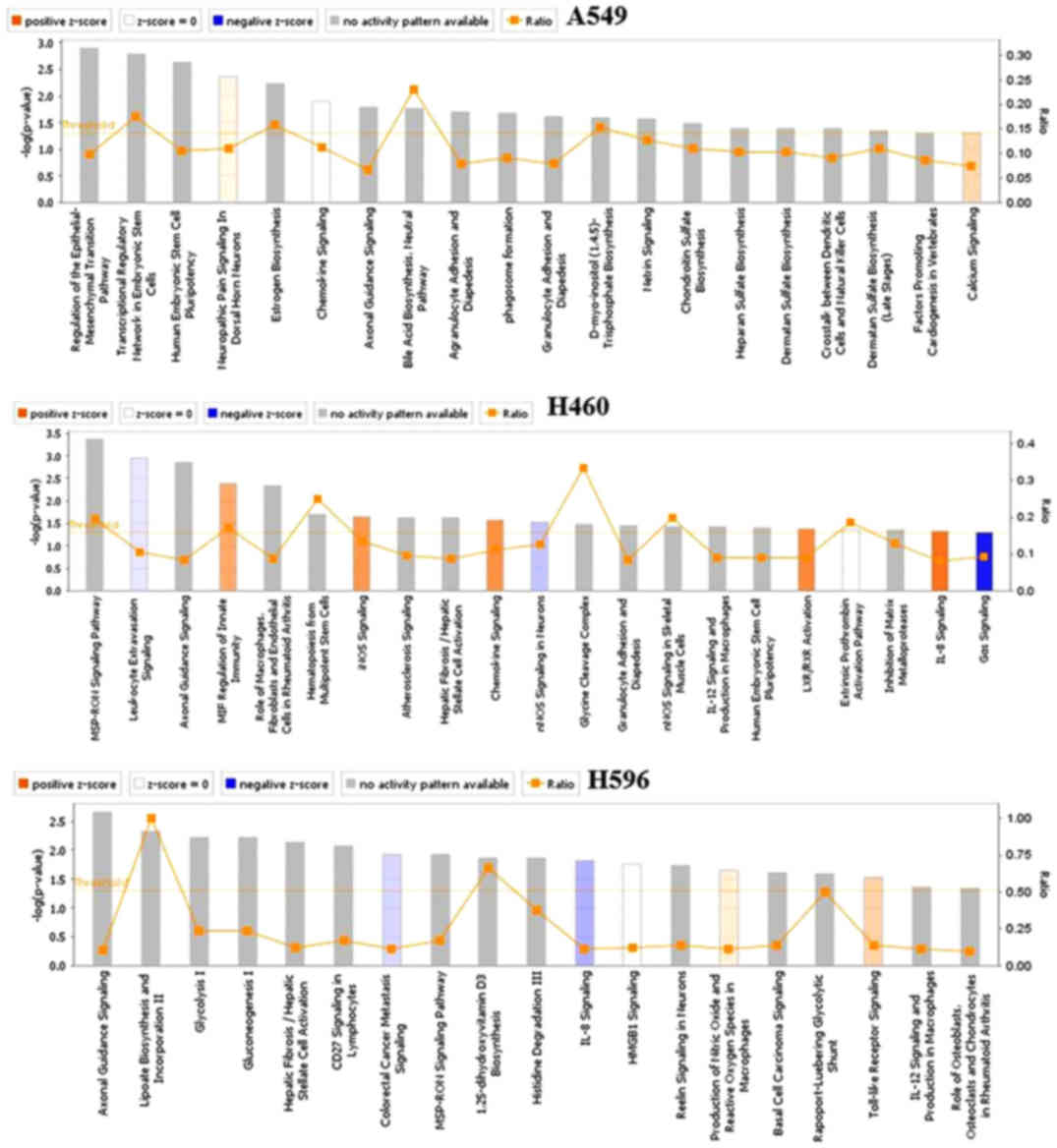
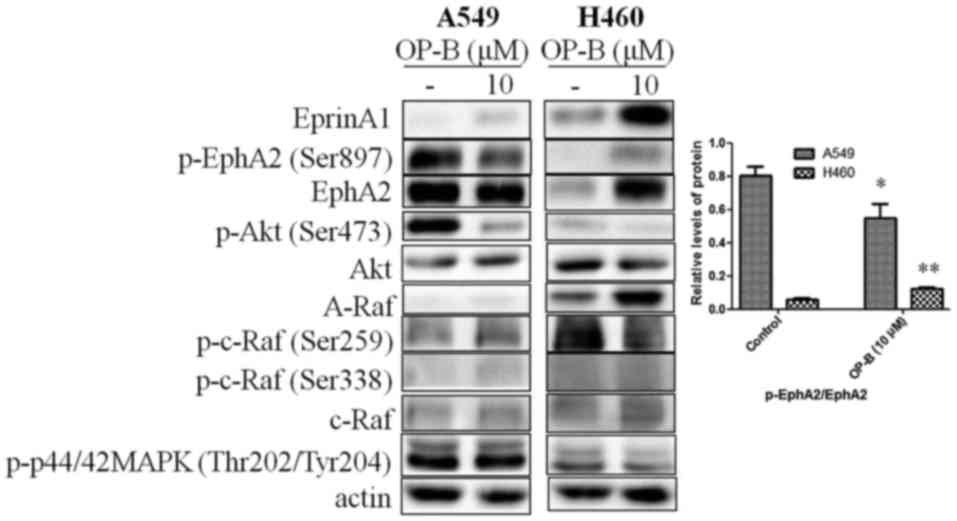
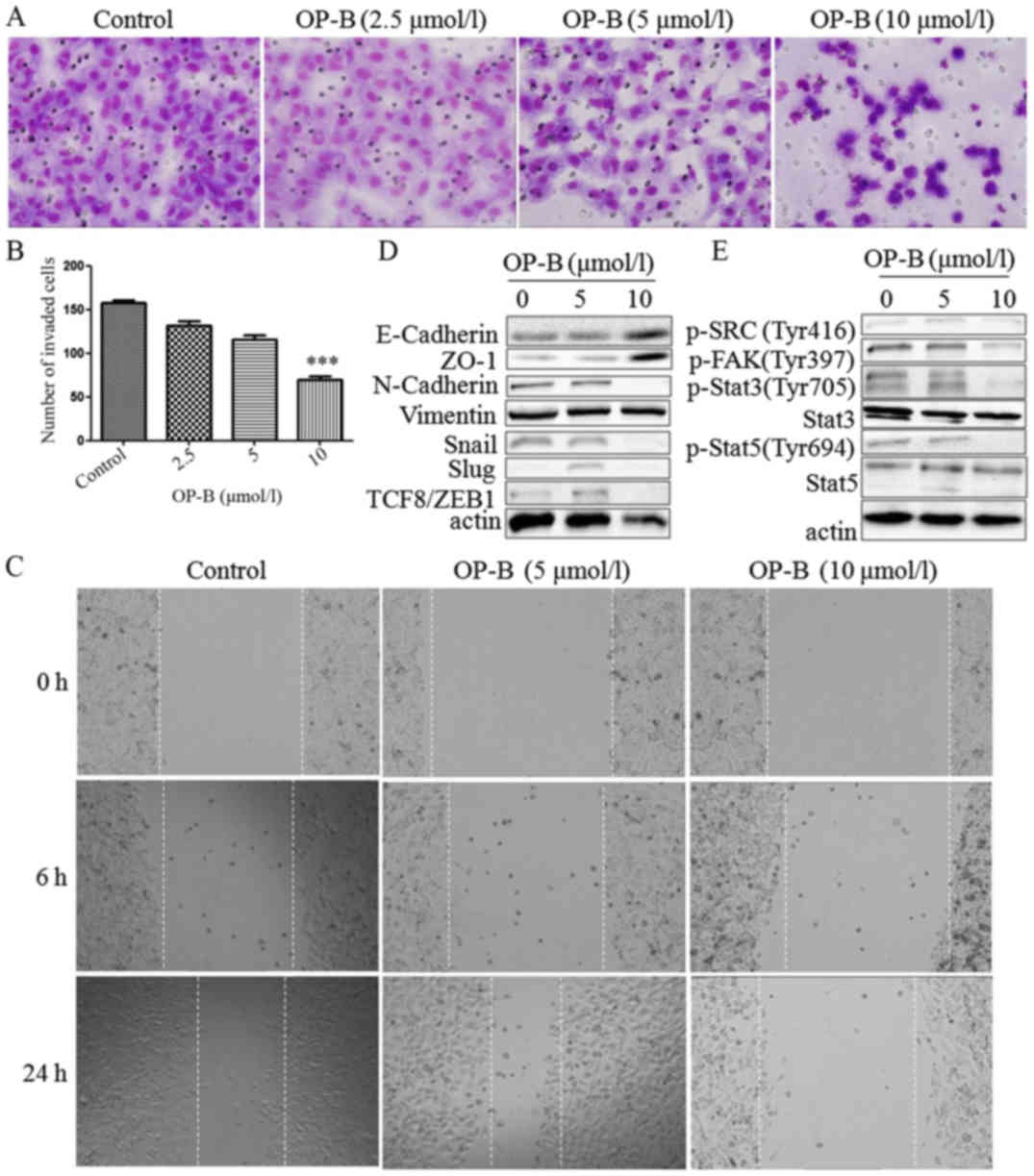
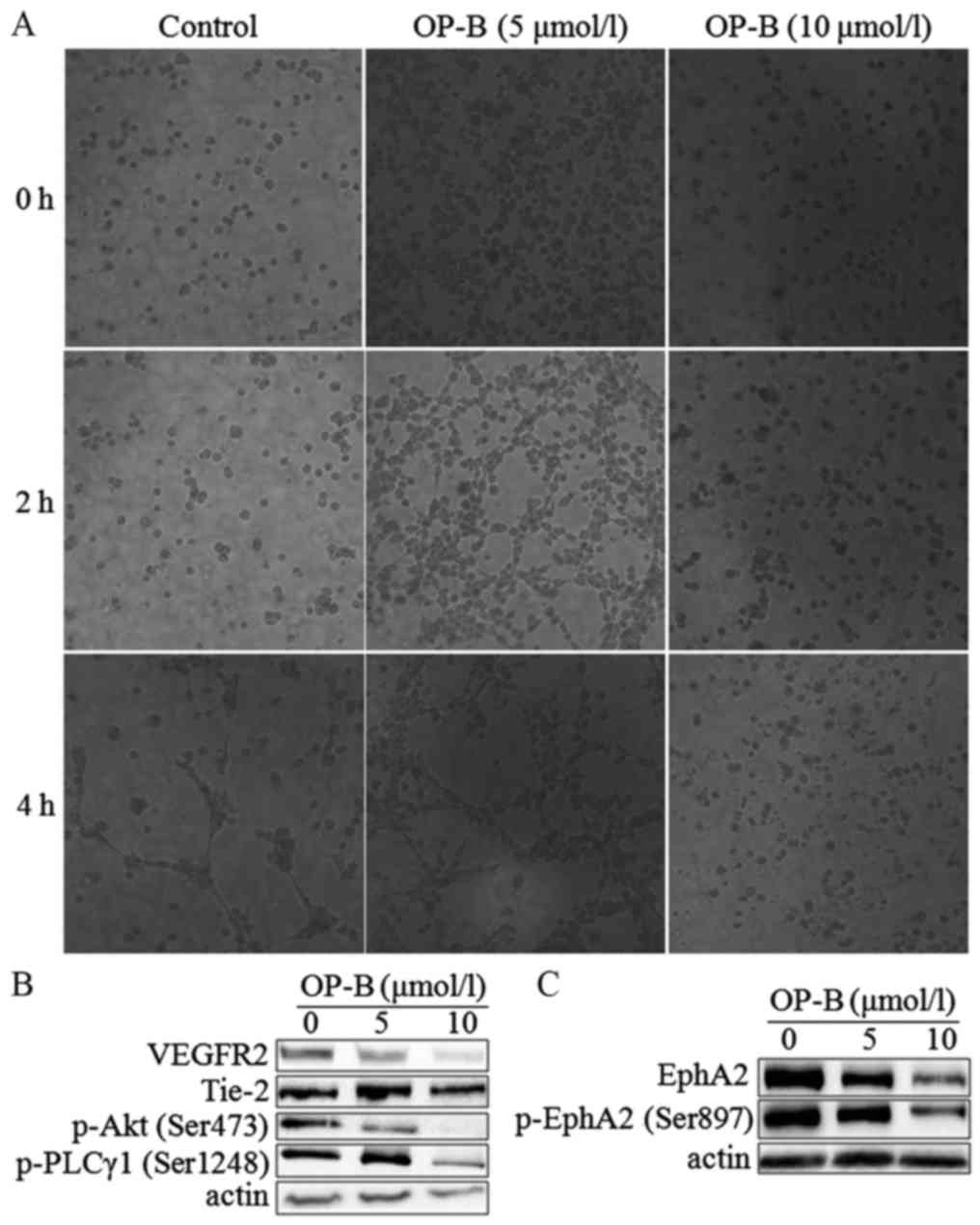
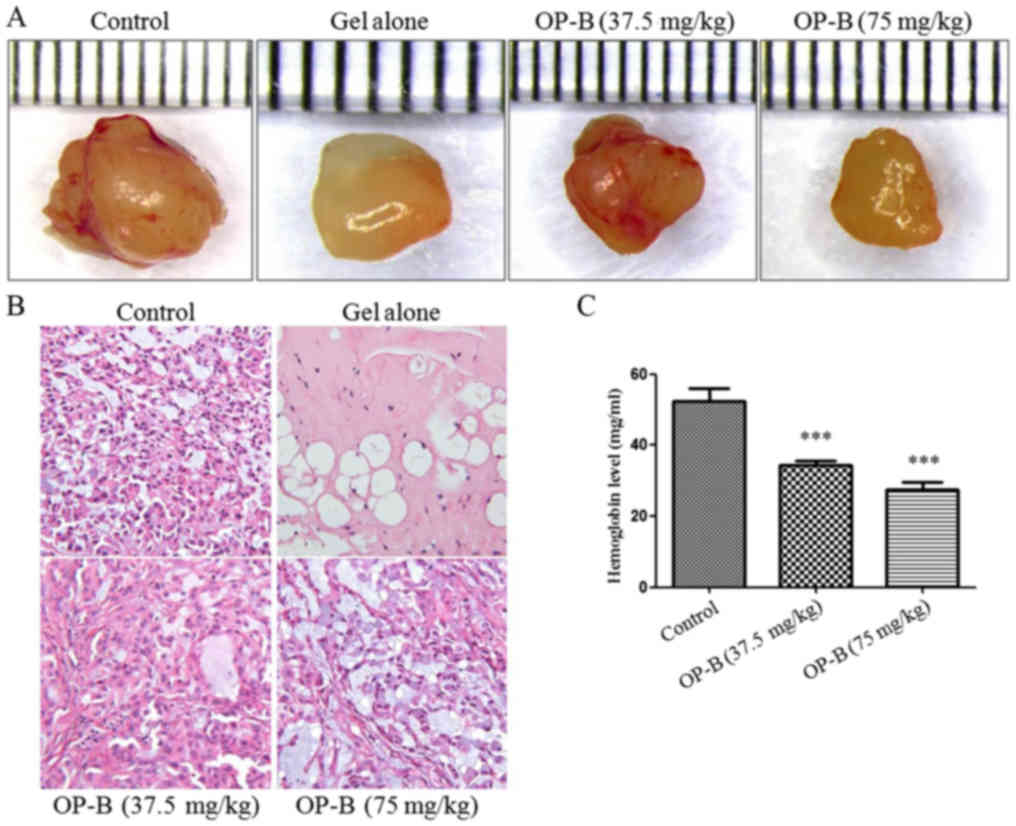
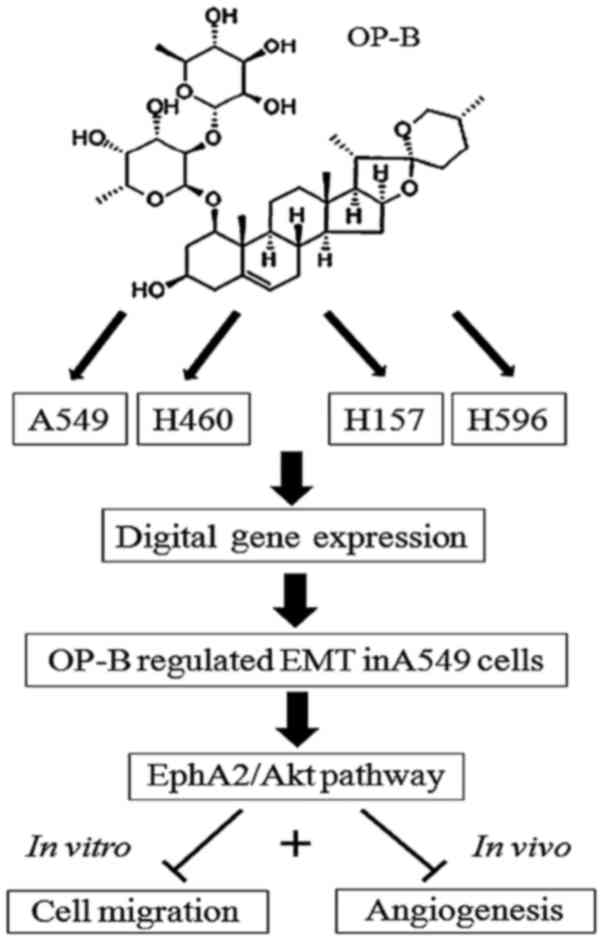
|
1
|
Collins LG, Haines C, Perkel R and Enck
RE: Lung cancer: Diagnosis and management. Am Fam Physician.
75:56–63. 2007.PubMed/NCBI
|
|
2
|
Riihimaki M, Hemminki A, Fallah M, Thomsen
H, Sundquist K, Sundquist J and Hemminki K: Metastatic sites and
survival in lung cancer. Lung Cancer. 86:78–84. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
De Craene B and Berx G: Regulatory
networks defining EMT during cancer initiation and progression. Nat
Rev Cancer. 13:97–110. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang G, Liang Y, Zheng T, Song R, Wang J,
Shi H, Sun B, Xie C, Li Y, Han J, et al: FCN2 inhibits
epithelial-mesenchymal transition-induced metastasis of
hepatocellular carcinoma via TGF-β/Smad signaling. Cancer Lett.
378:80–86. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tandon M, Vemula SV and Mittal SK:
Emerging strategies for EphA2 receptor targeting for cancer
therapeutics. Expert Opin Ther Targets. 15:31–51. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang J, Xiao D, Li G, Ma J, Chen P, Yuan
W, Hou F, Ge J, Zhong M, Tang Y, et al: EphA2 promotes
epithelial-mesenchymal transition through the Wnt/β-catenin pathway
in gastric cancer cells. Oncogene. 33:2737–2747. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Udayakumar D, Zhang G, Ji Z, Njauw CN,
Mroz P and Tsao H: EphA2 is a critical oncogene in melanoma.
Oncogene. 30:4921–4929. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dunne PD, Dasgupta S, Blayney JK, McArt
DG, Redmond KL, Weir JA, Bradley CA, Sasazuki T, Shirasawa S, Wang
T, et al: EphA2 expression is a key driver of migration and
invasion and a poor prognostic marker in colorectal cancer. Clin
Cancer Res. 22:230–242. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang W, Lin P, Sun B, Zhang S, Cai W, Han
C, Li L, Lu H and Zhao X: Epithelial-mesenchymal transition
regulated by EphA2 contributes to vasculogenic mimicry formation of
head and neck squamous cell carcinoma. Biomed Res Int.
2014:8039142014.PubMed/NCBI
|
|
10
|
Song W, Ma Y, Wang J, Brantley-Sieders D
and Chen J: JNK signaling mediates EPHA2-dependent tumor cell
proliferation, motility, and cancer stem cell-like properties in
non-small cell lung cancer. Cancer Res. 74:2444–2454. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Parri M, Buricchi F, Giannoni E, Grimaldi
G, Mello T, Raugei G, Ramponi G and Chiarugi P: EphrinA1 activates
a Src/focal adhesion kinase-mediated motility response leading to
rho-dependent actino/myosin contractility. J Biol Chem.
282:19619–19628. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang S and Yu D: Targeting Src family
kinases in anti-cancer therapies: Turning promise into triumph.
Trends Pharmacol Sci. 33:122–128. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen M, Du Y, Qui M, Wang M, Chen K, Huang
Z, Jiang M, Xiong F, Chen J, Zhou J, et al: Ophiopogonin B-induced
autophagy in non-small cell lung cancer cells via inhibition of the
PI3K/Akt signaling pathway. Oncol Rep. 29:430–436. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen M, Guo Y, Zhao R, Wang X, Jiang M, Fu
H and Zhang X: Ophiopogonin B induces apoptosis, mitotic
catastrophe and autophagy in A549 cells. Int J Oncol. 49:316–324.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Naudin C, Sirvent A, Leroy C, Larive R,
Simon V, Pannequin J, Bourgaux JF, Pierre J, Robert B, Hollande F
and Roche S: SLAP displays tumour suppressor functions in
colorectal cancer via destabilization of the SRC substrate EPHA2.
Nat Commun. 5:31592014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wykosky J, Palma E, Gibo DM, Ringler S,
Turner CP and Debinski W: Soluble monomeric EphrinA1 is released
from tumor cells and is a functional ligand for the EphA2 receptor.
Oncogene. 27:7260–7273. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang NY, Fernandez C, Richter M, Xiao Z,
Valencia F, Tice DA and Pasquale EB: Crosstalk of the EphA2
receptor with a serine/threonine phosphatase suppresses the
Akt-mTORC1 pathway in cancer cells. Cell Signal. 23:201–212. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miao H, Li DQ, Mukherjee A, Guo H, Petty
A, Cutter J, Basilion JP, Sedor J, Wu J, Danielpour D, et al: EphA2
mediates ligand-dependent inhibition and ligand-independent
promotion of cell migration and invasion via a reciprocal
regulatory loop with Akt. Cancer Cell. 16:9–20. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Garg M: Epithelial-mesenchymal
transition-activating transcription factors-multifunctional
regulators in cancer. World J Stem Cells. 5:188–195. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alizadeh AM, Shiri S and Farsinejad S:
Metastasis review: From bench to bedside. Tumour Biol.
35:8483–8523. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bolos V, Peinado H, Pérez-Moreno MA, Fraga
MF, Esteller M and Cano A: The transcription factor Slug represses
E-cadherin expression and induces epithelial to mesenchymal
transitions: A comparison with Snail and E47 repressors. J Cell
Sci. 116:499–511. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Turner FE, Broad S, Khanim FL, Jeanes A,
Talma S, Hughes S, Tselepis C and Hotchin NA: Slug regulates
integrin expression and cell proliferation in human epidermal
keratinocytes. J Biol Chem. 281:21321–21331. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Playford MP and Schaller MD: The interplay
between Src and integrins in normal and tumor biology. Oncogene.
23:7928–7946. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Barrett CL, DeBoever C, Jepsen K, Saenz
CC, Carson DA and Frazer KA: Systematic transcriptome analysis
reveals tumor-specific isoforms for ovarian cancer diagnosis and
therapy. Proc Natl Acad Sci USA. 112:E3050–E3057. 2015. View Article : Google Scholar : PubMed/NCBI
|