Introduction
Nasopharyngeal carcinoma (NPC) is a common head and
neck tumor with mortality rates of 15–50 per 100,000 people in
Southeast Asia, especially in southern China (1). Since >90% of the NPC cases are
poorly distinguished squamous cell carcinomas, they display
radiosensitivity, and thus radical radiotherapy is the first choice
of primary treatment (2). However,
radiotherapy still has many obstacles to overcome, like
radioresistance (3). To overcome
this critical issue and correctly predict the required radiation
levels for individualized treatment, the role of many
radiosensitive biomarkers has been analyzed, with the hope that
some of them will help to predict clinical radiosensitivity, and
improve the therapeutic efficacy along with revealing the molecular
mechanism of NPC radioresistance. We determined that
glucose-regulated protein 78 (GRP78) was a radioresistant protein
of NPC in our previous study (4).
GRP78, a multifunctional protein folding chaperone
and co-receptor is highly expressed on the cell surface, and holds
promise for cancer-specific targeting. Its role has specifically
been studied in colon and breast cancer (5,6). It
has also been revealed to play an analeptic role in invasive
procedures and possibly an anti-invasive target for treating
hepatocellular carcinoma (7). GRP78
expression is induced in multiple cancers as a result of
endoplasmic reticulum stress induced either by acidosis, glucose
deprivation or severe hypoxia. Chemotherapeutic drugs and radiation
have also been revealed to induce GRP78 protein expression, which
in turn confers resistance in the tumor cells against these agents
(8). In addition, the changes of
GRP78 expression have also been revealed to play key functions in
the development and progression of malignant tumors (9). GRP78 has been revealed to be a
therapeutic target of NPC (10).
Our previous efforts in identifying the proteins
responsible for NPC radioresistance indicated GRP78 as a potential
player for predicting NPC response to radiotherapy (4). However, the mechanism of its
functional/expression regulation in NPC following radiotherapy
remains unclear.
MicroRNAs (miRNAs) are small non-coding RNAs (20–24
nucleotides in length) which post-transcriptionally modulate gene
expression through negative regulation of the stability or
translational efficiency of their target mRNAs (11,12).
These effects are obtained by miRNA binding to the 3′-untranslated
region (3′UTR) of their target mRNAs, which can reduce the
expression of the associated protein.
Thus, in the present study, we investigated the
mechanism of GRP78 regulation by first performing bioinformatics
analysis to identify putative miRNA targeting GRP78. Our initial
analysis led to the identification of miR-495 as the miRNA binding
to GRP78 mRNA.
In the present study, we examined the impact of
miR-495-mediated targeting of cell surface associated GRP78, and
its effects on cell survival and growth when combined with X-ray
treatment. In the present study, miR-495 mimics suppressed cell
proliferation and induced cell death. GRP78 was targeted by miR-495
and miR-495 mimics also enhanced the efficacy of radiotherapy.
Subsequently, we analyzed the mechanism of miR-495-mediated
regulation of GRP78 expression and its role in regulating the
radiosensitivity of NPC cells.
Materials and methods
Patients and tissue specimens
A total of 92 NPC patients (52 males and 40 females)
were included in the present study with a median age of 48 years
(range, 12–85 years) and 30 patients (18 males and 12 females) with
chronic rhinitis were enrolled as controls (median age 44 years;
range, 20–73 years). The information of all NPC patients that
underwent curative-intent radiotherapy treatment between August
2012 and July 2014 was reviewed and it was observed that the
overall radiation dose of 60–70 Gy was administered to each patient
using a modified type of linear accelerator (Xiangya Hospital of
Central South University, Changsha, China). Based on this therapy,
39 patients were diagnosed as radioresistant, while 53 were
radiosensitive. The patients were characterized as radioresistant,
if they exhibited persisting disease (incomplete regression of
tumors) after 6 weeks of completing radiotherapy, or those with
recurrent disease at the nasopharynx and/or neck nodes after 2
months post-radiotherapy (13).
Similarly, the patients were characterized as radiosensitive, if
they did not display any local residual lesions after 6 weeks or
any recurrence of symptoms after 2 months of completing
radiotherapy (13). NPC tissue
biopsies (pre-therapy) were obtained after informed consent was
received from all participants and were used for
immunohistochemical (IHC) staining. All the patient specimens were
separately reviewed by two experienced pathologists and the
diagnoses were confirmed by histopathological examination. The
diagnoses were based on the 1978 WHO classification (14). This study was certified by the
Ethics Committee of Xiangya School of Medicine (Central South
University, Changsha, China).
Cell culture
NPC cell line 5–8F was purchased from the Cell Bank
of Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China). 5-8F was seeded in a 6-cm plate
(1×105 cells). The radioresistant NPC cell line 5-8F-IR
was derived from NPC cell line 5-8F. 5-8F-IR was established by our
laboratory (Xiangya Hospital of Central South University). These
cells were cultured in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), supplemented with 10% fetal
bovine serum (FBS; Hangzhou Sijiqing Biological Engineering
Materials, Co., Ltd., Hangzhou, China) and incubated at 37°C in a
5% CO2 atmosphere. After 24 h of culture, the radiation
of the sublethal 10 Gy dose was delivered at room temperature at
300 cGy/min with a linear accelerator (2100EX; Varian Medical
Systems, Inc., Palo Alto, CA, USA). Following treatment, the
radioresistant cells that had survived produced the generation of
the subclones 15 days later.
Other reagents
The IHC kit (S-P) and 3,3′-diaminobenzidine (DAB)
developing solution were purchased from Fuzhou Maixin Biotech Co.,
Ltd., (Fuzhou, China). Mouse anti-human monoclonal antibodies to
GRP78 (1:500; cat. no. sc-376768), E-cadherin (1:500; cat. no.
sc-71007), N-cadherin (1:500; cat. no. sc-53488), vimentin (1:500;
cat. no. c-80975) and β-actin (1:1,000; cat. no. sc-58673) were all
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Lipofectamine 2000 was purchased from Invitrogen (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and the Transwell assay kit was
procured from Corning Inc. (Corning, NY, USA).
Bioinformatics analysis
The prediction analysis of miRNAs binding to GRP78
mRNA was performed using miRWalk online program (http://zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk/predictedmirnagene.html).
The predicted miRNAs with the highest binding scores were further
identified using miRanda software (http://www.microrna.org/microrna/home.do). This
software computes thermodynamic stability and sequence conservation
scores.
Immunohistochemistry staining
IHC staining of NPC tissue specimens was performed
using the previously described S-P IHC method (4). The cytoplasm staining intensity was
scored by two pathologists as follows: No color, negative; pale
yellow, weakly positive; brown, positive; and tan, strongly
positive. Overall the percentage of tissue samples with positive
expression was calculated by the following formula: (Total number
of samples with weakly positive + positive + strongly positive
staining/total number of samples evaluated) × 100.
RT-qPCR analysis
Total RNA was extracted from fresh NPC and chronic
rhinitis tissue samples by homogenization in TRIzol reagent (Gibco;
Thermo Fisher Scientific, Inc.). PCR amplification of miR-495 was
carried out using a two-step method. The following primers were
designed based on the precursor sequence of miR-495: Forward
primer, 5′-UGGUACCUGAAAAGAAGUUG-3′ and reverse,
5′-GCACUUCUUUUUCGGUAUCA-3′. For U6 gene amplification, the
following primers were used: Forward primer,
5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse,
5′-CGCTTCACGAATTTGCGTGTCAT-3′. All these primers were synthesized
by Guangzhou RiboBio Co., Ltd. (Guangzhou, China), and the
amplification reactions were carried out using a fluorescence
RT-qPCR instrument, based on the manufacturer's instructions. The
following RT-qPCR reaction protocol was used: 2.5 µl of dNTPs (2.5
mM each); 2.5 µl of 10× PCR buffer; 1.5 µl of MgCl2 (MW
95.21) solution; 1 unit of Taq polymerase; SYBR-Green I,
final concentration 0.25×; 1 µl of forward primer and reverse
primer (10 µM stock); 1 µl cDNA; and water to a total volume of 25
µl. The U6 gene amplification reaction was as follows: 95°C for 5
min; 35 cycles (95°C for 10 sec; 59°C for 15 sec; 72°C for 20 sec;
and 82°C for 5 sec). The miRNA reaction was performed as follows:
95°C for 15 min; 40 cycles (94°C for 15 sec; 55°C for 30 sec; and
70°C for 30 sec).
Western blotting
To extract the total cellular protein, tissues or
cells were incubated with pre-cooled RIPA lysis buffer (Thermo
Fisher Scientific, Inc.), vortexed and then placed on ice for 30
min. After centrifugation, the supernatants were removed and the
protein concentrations were estimated using the Bradford method.
The proteins were denatured by incubation for 5 min at 100°C and
then the loading buffer was added. Subsequenlty, 20 µg of the
denatured proteins per lane were separated by 12% gel
electrophoresis and transferred to polyvinylidene difluoride (PVDF)
membranes. The membranes were blocked by incubating them in a
blocking buffer containing 5% non-fat milk powder for 1–2 h and
then washed and incubated with the appropriate primary antibody
overnight at 4°C. The primary antibodies were diluted as follows:
GRP78 (1:500), E-cadherin (1:500), N-cadherin (1:500), vimentin
(1:500) and β-actin (1:1,000). For detection enhanced
chemiluminescence (ECL; Santa Cruz Biotechnology, Inc.) was used.
The protein bands were analyzed using Band leader 3.0.
Construction of GRP78 3UTR
plasmids
The bioinformatics software predicted the binding
between miR-495 and GRP78 mRNA. RT-PCR was used to amplify a
sequence encompassing these 501 base pairs. The primers for
amplification were designed as follows: GRP78_WT_forward,
5′-ACTGCTGTTTTCAGATGGAGGT-3′ and reverse,
5′-CTAGGAGCCAGCTCAGATGC-3′; GRP78_mut_forward,
5′-TGCGGAGATCTATCTATCATGGC-3′ and reverse,
5′-GGTGTCAGGCGATTCTGGTC-3′. The amplified fragments were cloned
into the pmiR-RB-REPORT™ dual luciferase reporter vector (Guangzhou
RiboBio Co., Ltd., Guangzhou, China). The hRluc vector was used to
report fluorescence, and the 3′UTR of GRP78 was cloned downstream
of the hRluc gene. The directly targeted region was determined by
cloning the 3′UTR seed region and the mutated seed region into the
pmiR-RB-REPORT™ luciferase reporter vectors (Guangzhou RiboBio Co.,
Ltd.).
Luciferase reporter assay
The plasmids containing the 3′UTR of GRP78, miR-495
mimics and NC sequences were transfected into 5-8F cells, using
Lipofectamine 2000 reagent. The cells were incubated for 48 h after
transfection, and the activity of firefly luciferase (hRluc) and
the internal control (hluc) were detected using Dual-Glo Luciferase
Assay system (Promega Corp., Madison, WI, USA).
Clonogenic survival assay
Radioresistance was determined by colony survival
assay after irradiation. Briefly, the cells were plated in 6-well
plates and exposed to a series of radiation doses (2–10 Gy), and
were then cultured for 12 days. Subsequently, the surviving
colonies (defined as a colony with >50 cells) were counted and
the survival fraction was calculated as the number of colonies
divided by the number of cells seeded and multiplied by the plating
efficiency. Plating efficiency was calculated as colonies/10 cells.
Three separate experiments were performed.
Cell growth analysis
Cells were plated in 24-well culture plates
(2.5×104/well), and incubated for 24 h. Subsequently,
they were irradiated with radiation of 6 Gy, and cell growth was
monitored by counting the number of cells at various time
intervals. Three independent experiments were carried out in
triplicate.
Statistical analysis
Data were analyzed using SPSS software version 17.0
(SPSS, Inc., Chicago, IL, USA), and the results were expressed as
the mean ± standard deviation (SD). Comparisons were performed
using the Student t-test or χ2 test. Spearman's and
Pearson's analysis was performed for correlation analysis. A
P-value <0.05 was considered to indicate a statistically
significant difference.
Results
Analysis of GRP78 protein expression
in NPC and chronic rhinitis tissues
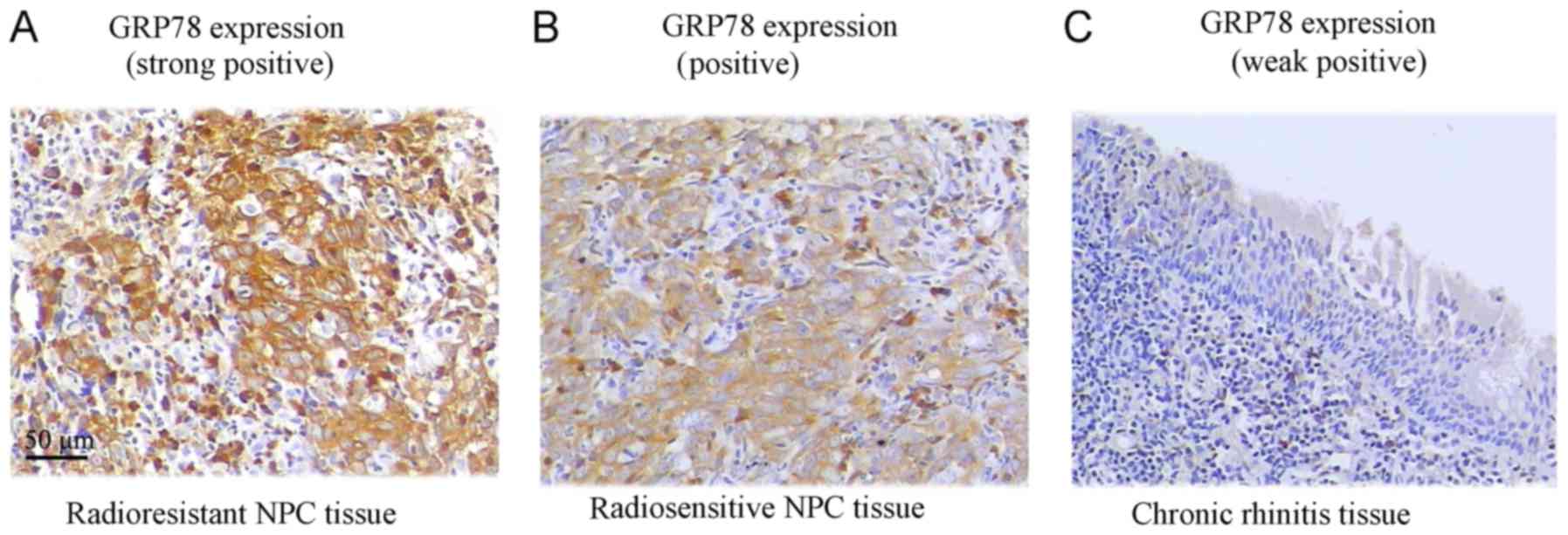
GRP78 protein expression was assessed in tissue
specimens by IHC. The ratio of positive GRP78 expression was
significantly higher in NPC tissue samples (70.7%), where 65
samples out of 92, exhibited positive expression (P<0.05).
However, in chronic rhinitis control tissue samples only 13.3% of
the samples (4/30) exhibited positive expression. From further
analysis of NPC tissue samples based on radiosensitivity
information, we observed that 87.2% (34/39) of the radioresistant
NPC patient samples significantly exhibited positive GRP78
staining, in comparison to 58.5% of the radiosensitive samples
(31/53) (Fig. 1 and Table I).
 | Table I.Expression of GRP78 in NPC and chronic
rhinitis tissues. |
Table I.
Expression of GRP78 in NPC and chronic
rhinitis tissues.
|
|
| Scores of
immunohistochemical staining |
|
|
|---|
|
|
|
|
|
|
|---|
|
| N | Weak
positive/negative (0–2) | Positive (3–4) | Strong positive
(5–6) | χ2 | P-value |
|---|
| Chronic
rhinitis | 30 | 26 | 3 | 1 | 30.621 | 0.000a |
| NPC | 92 | 27 | 30 | 35 |
|
|
| Radioresistant | 39 | 5 | 14 | 20 | 9.644 | 0.008b |
| Radiosensitive | 53 | 22 | 16 | 15 |
|
|
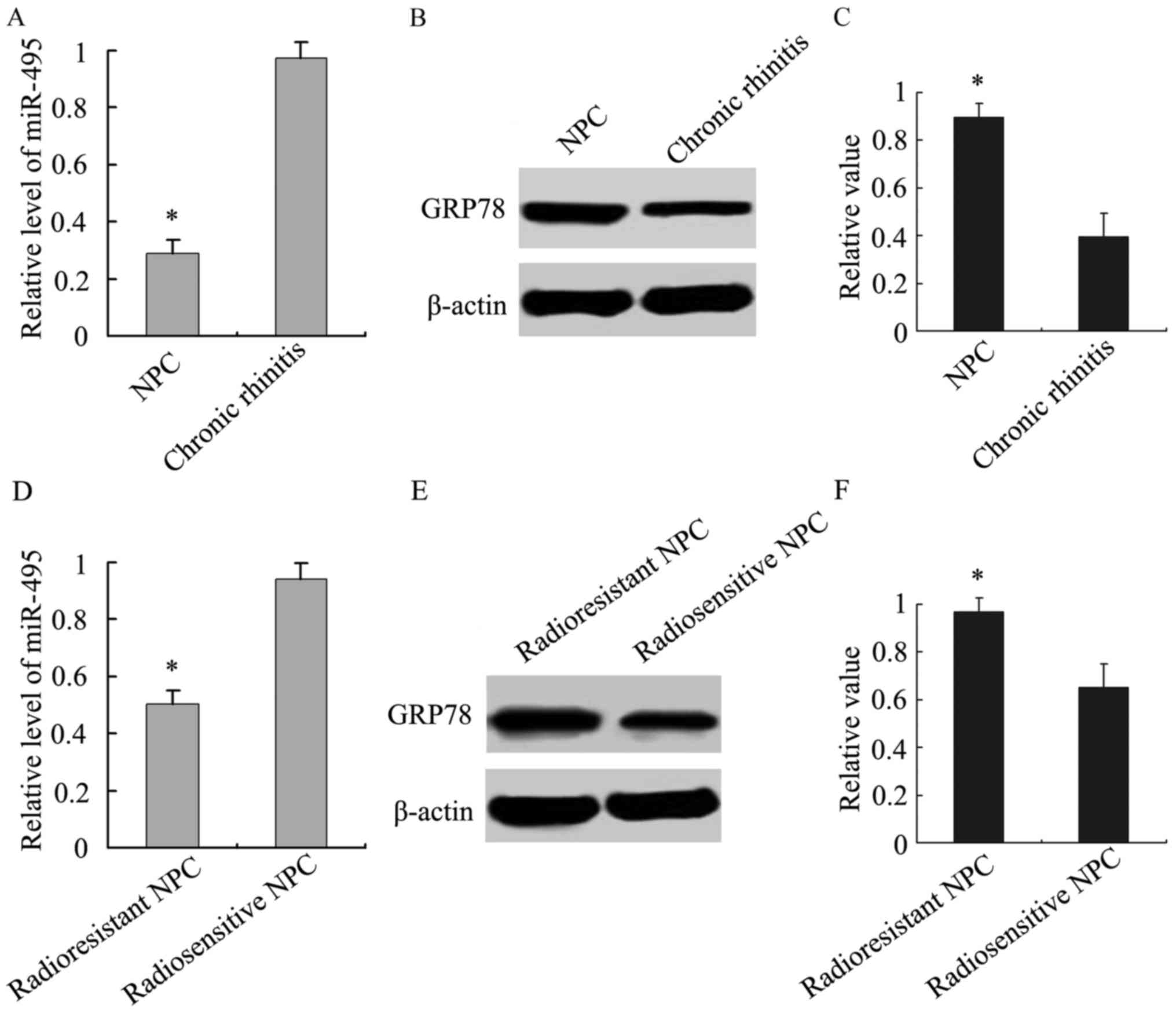
Analysis of the relationship between miR-495 and
GRP78 expression was then performed. We analyzed the expression of
miR-495 in NPC and chronic rhinitis tissue samples, and our results
demonstrated that its expression was lower in NPC tissue samples
than chronic rhinitis tissues (Fig.
2A). Similarly, we also observed that miR-495 expression was
lower in radioresistant tissue samples than radiosensitive NPC
tissue samples (Fig. 2D).
Accordingly, GRP78 was highly expressed in NPC tissue samples,
especially in radioresistant NPC (Fig.
2B, C, E and F). An inverse association between miR-495 and
GRP78 expression was revealed using Spearman's and Pearson's
correlation analysis.
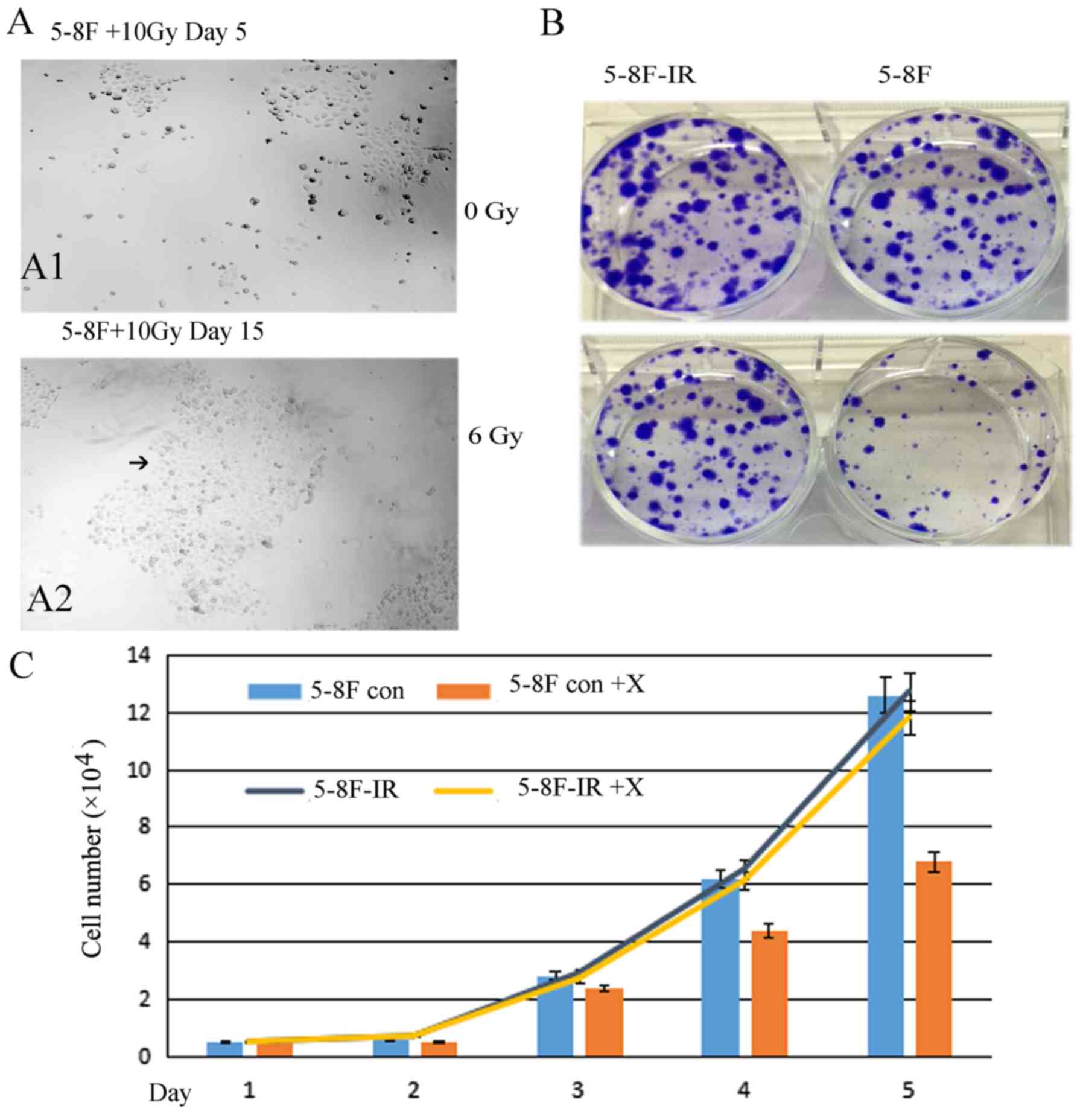
5-8F-IR with radioresistance from the
irradiated 5-8F cells
From the 5-8F cells that were irradiated with 10 Gy
X-ray only 5% of the cells survived. Fifteen days later, the
5%-surviving cells formed subclones. A large subclone was selected
and named 5-8F-IR. The subclone 5-8F-IR was more resistant than the
parent 5-8F (Fig. 3). The 5-8F-IR
subclone was cultured as a radioresistant sample.
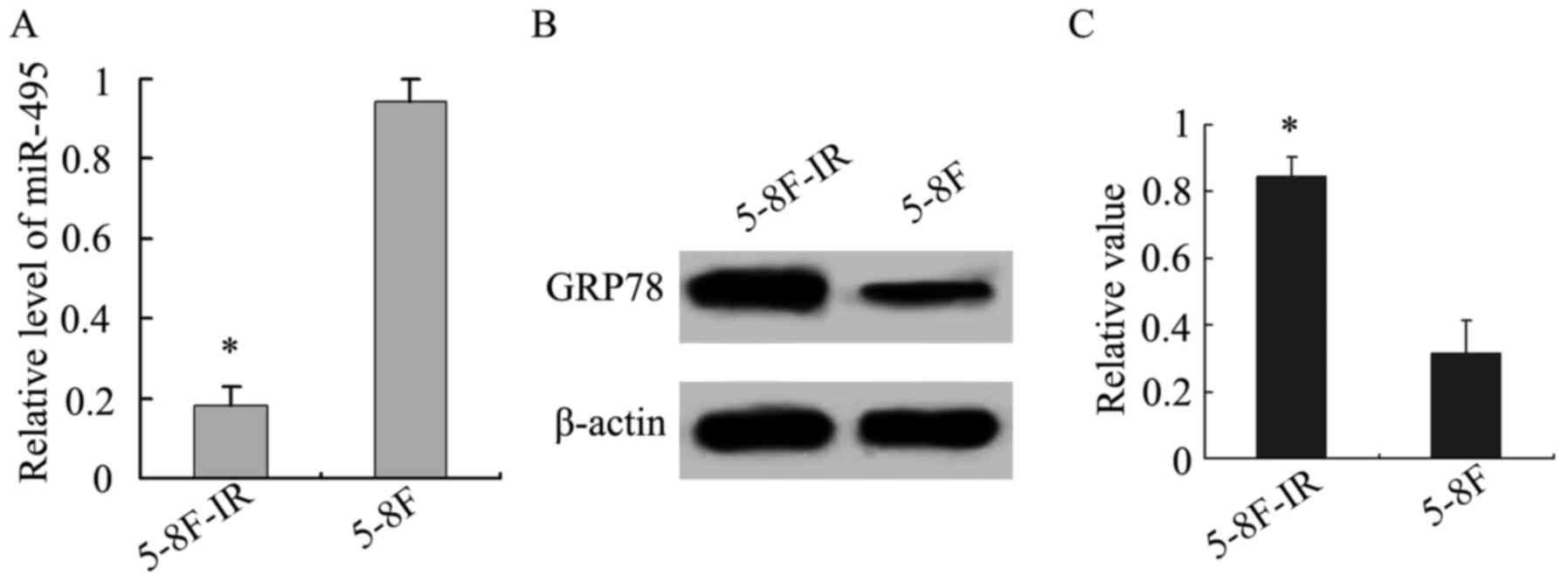
In addition, we analyzed miR-495 and GRP78
expression in the 5-8F-IR and 5-8F NPC cells. The radioresistant
5-8F-IR cells exhibited lower expression of miR-495, in comparison
to the parent 5-8F cells, while the opposite was obtained for GRP78
(P<0.05; Fig. 4), and this
further confirmed the existence of an inverse relationship between
the expression of miR-495 and GRP78.
Prediction of the interactions between
miRNAs and GRP78 mRNA
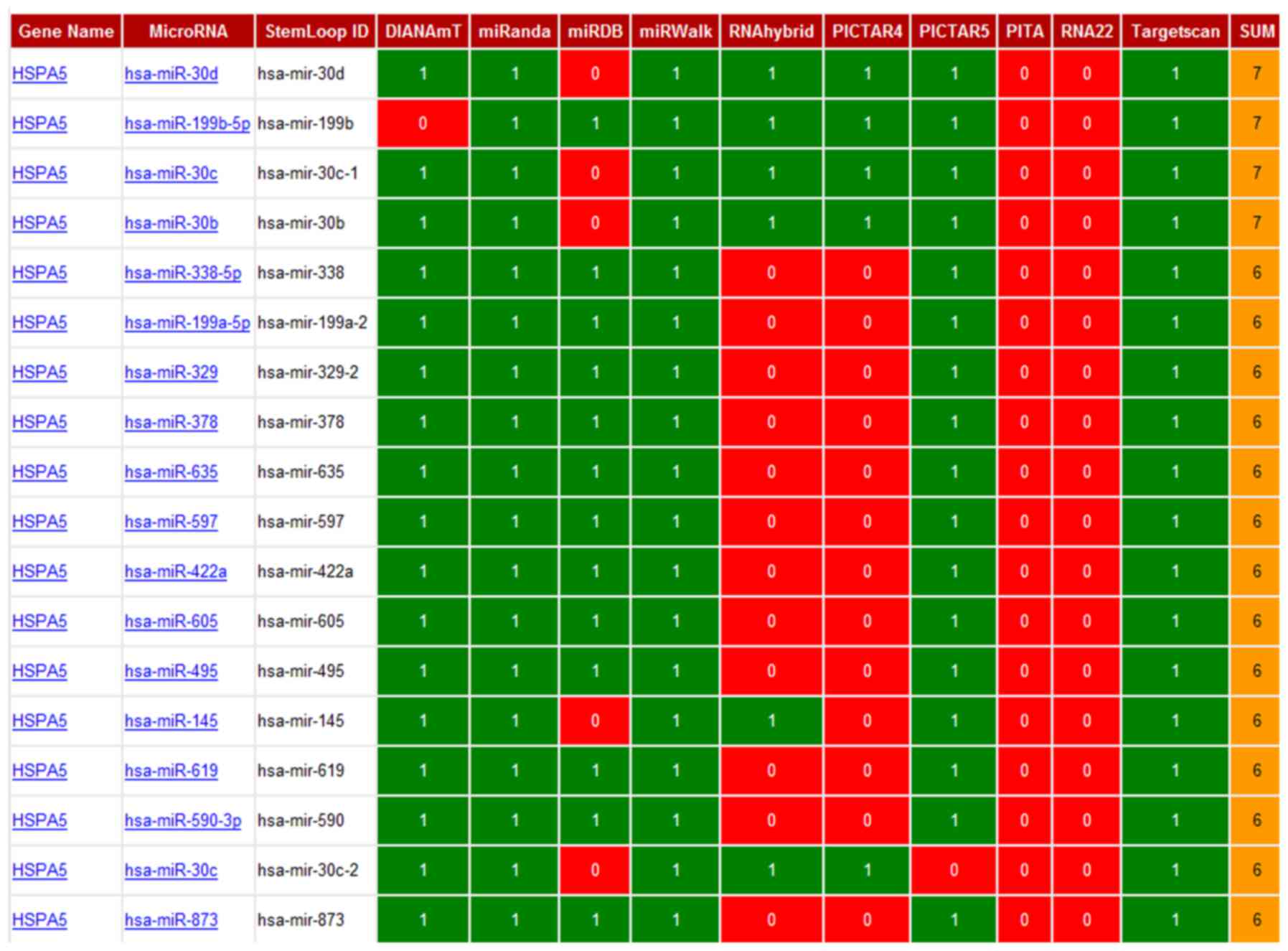
Using the miRWalk program, which has the combined
power of ten different programs, we predicted 18 different miRNAs
that appeared to interact with HSPA5 (Gene alias: GRP78) mRNA
(Fig. 5). Among these 18 predicted
miRNAs, we further narrowed our search based on the highest binding
scores for GRP78 mRNA, using miRanda software, which incorporates
thermodynamic and sequence conservation scores. This analysis led
to the identification of four miRNAs based on the following
selection criteria (mirSVR ≤-0.1, phastCons 0.5–0.8) (Table II). Among these miRNAs, miR-495 was
predicted to bind GRP78 mRNA with the highest stability and
specificity.
 | Table II.mirSVR and PhastCons scores of 18
predicted miRNAs. |
Table II.
mirSVR and PhastCons scores of 18
predicted miRNAs.
|
| mirSVR score | Ranking | PhastCons
score |
|---|
| hsa-miR-495 | −1.2492 | 1 | 0.7212 |
|
hsa-miR-199b-5p | −1.1437 | 2 | 0.7212 |
|
hsa-miR-199a-5p | −1.1437 | 2 | 0.7212 |
| hsa-miR-597 | −1.1257 | 4 | 0.7212 |
| hsa-miR-338-5p | −1.0612 | 5 | 0.6955 |
| hsa-miR-873 | −0.8443 | 6 | 0.5165 |
| hsa-miR-590-3p | −0.8084 | 7 | 0.5061 |
| hsa-miR-605 | −0.7911 | 8 | 0.4909 |
| hsa-miR-635 | −0.6991 | 9 | 0.5104 |
| hsa-miR-619 | −0.6745 | 10 | 0.5165 |
| hsa-miR-378 | −0.3412 | 11 | 0.5165 |
| hsa-miR-422a | −0.3412 | 11 | 0.5165 |
| hsa-miR-329 | −0.1813 | 13 | 0.6127 |
| hsa-miR-30e | −0.1665 | 14 | 0.7104 |
| hsa-miR-30b | −0.1649 | 15 | 0.7104 |
| hsa-miR-30c | −0.1649 | 15 | 0.7104 |
| hsa-miR-30d | −0.1634 | 17 | 0.7104 |
| hsa-miR-145 | −0.0698 | 18 | 0.6840 |
Analysis of the mechanism of
miR-495-mediated regulation of GRP78 expression
The inverse association between miR-495 and GRP78
expression in both NPC patient tissue samples and cell lines, led
us to investigate whether miR-495 could influence GRP78 expression.
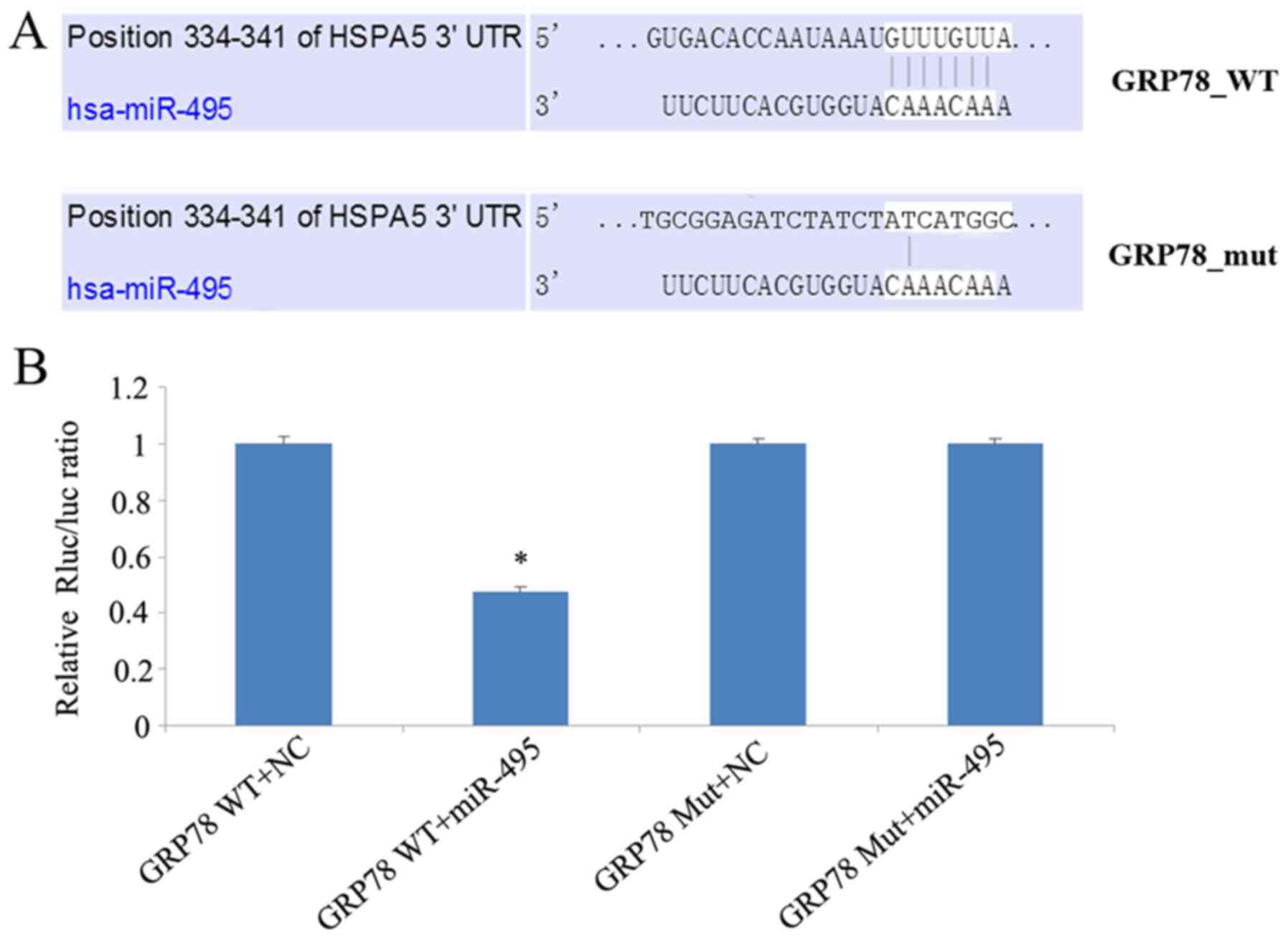
We investigated whether miR-495 directly targeted the 3′UTR of
GRP78, using a dual luciferase assay. In this regard, 5-8F cells
were co-transfected with either miR-495 mimics or corresponding
negative control, along with a dual luciferase plasmid of wild-type
(WT) or mutant (Mut) 3′UTR GRP78. This luciferase assay revealed
that miR-495 mimics significantly decreased the luciferase activity
of WT GRP78-transfected cells, in comparison to cells transfected
with its 3′UTR mutant (Fig. 6).
Overall our data revealed that miR-495 mimics could not target
mutant GRP78, and could only reduce the expression of wild-type
GRP78, thereby demonstrating that miR-495 targets the 3′UTR of
GRP78.
Analysis of the relationship of
miR-495 and GRP78 expression with EMT-related proteins
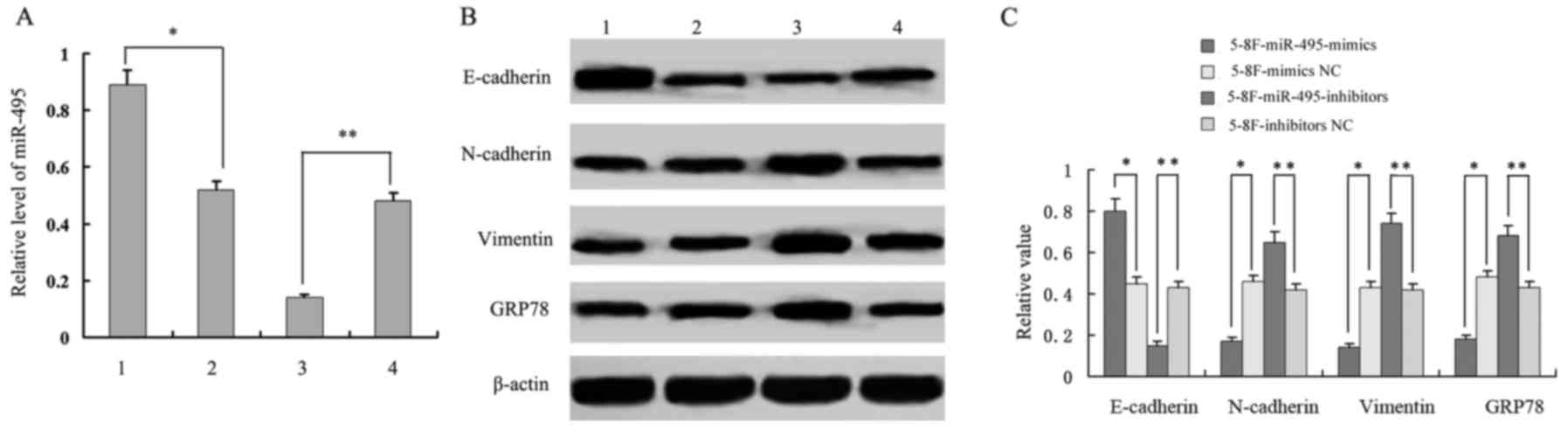
In addition to downregulation of GRP78 expression by
miR-495 mimics in 5-8F cells, we also observed the reduced
expression of vimentin and N-cadherin expression, and the increased
expression of E-cadherin. This may indicate a EMT phenotype
(reduced EMT). In parallel, when miR-495 expression was inhibited
in 5-8F cells by treating them with miR-495 inhibitors, we observed
an increased expression of GRP78, vimentin and N-cadherin, and a
decreased expression of E-cadherin (P<0.05, Fig. 7). This may increase the EMT
phenotype. These results indicated that miR-495 not only mediated
the expression of GRP78, but was also involved in the expression of
EMT-related proteins E-cadherin, N-cadherin and vimentin. Following
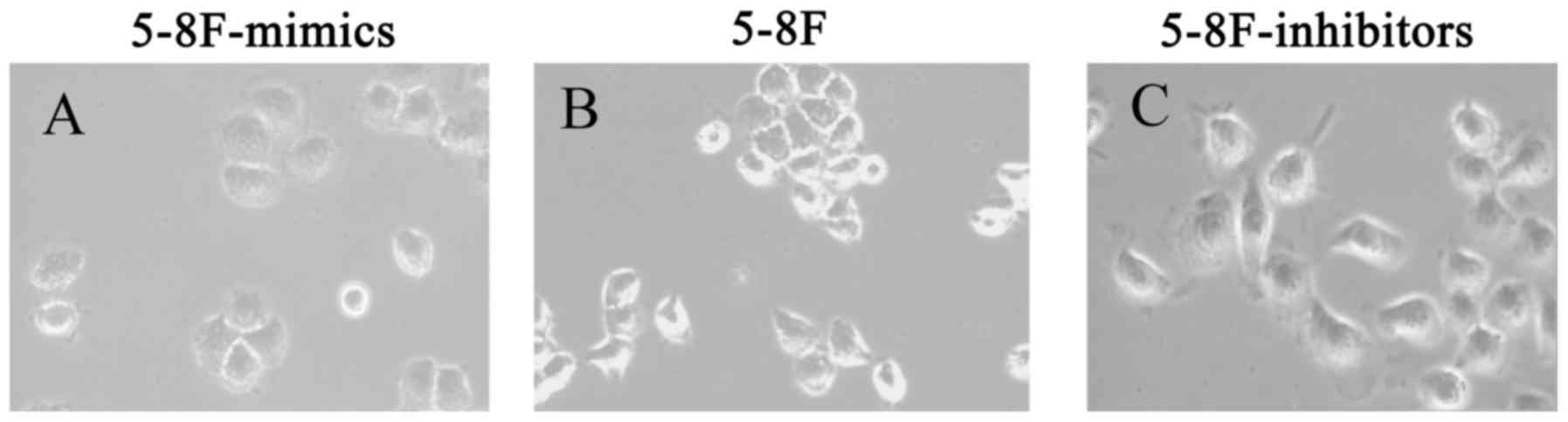
miR-495 treatment, 5-8F cells underwent morphological changes. The
5-8F cells became rounder and the pseudopodiums became smaller with
reduced EMT phenotype. Therefore, it was revealed that miR-495
influenced the EMT process (Fig.
8).
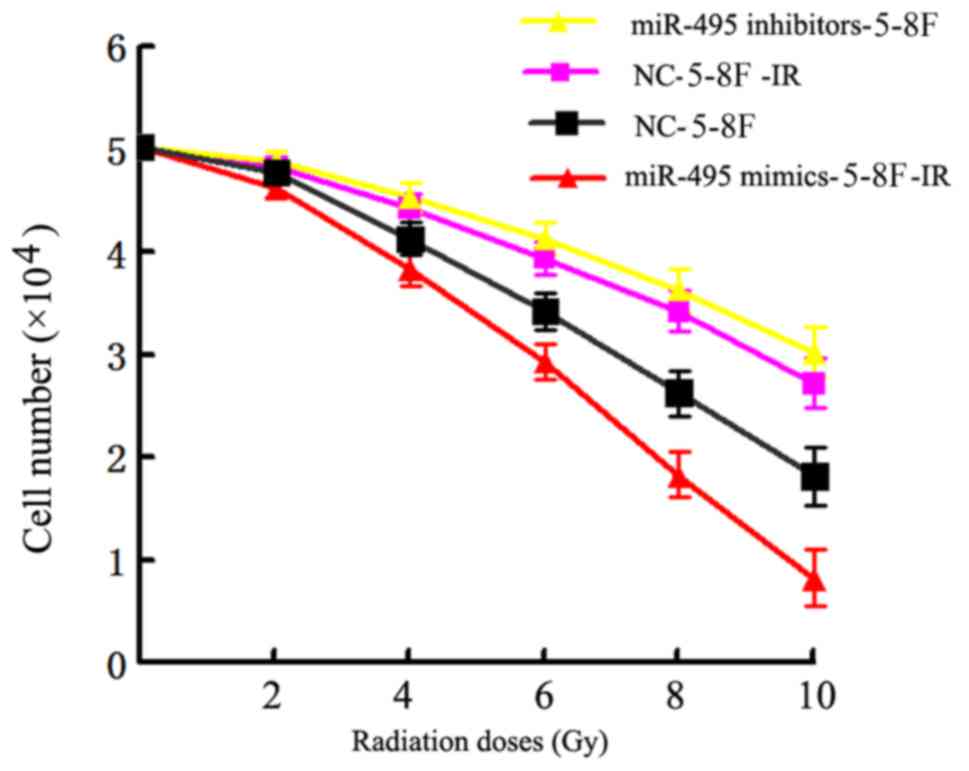
miR-495 upregulation enhances the
efficacy of radiation therapy in NPC cells
Finally, we assessed whether regulation of miR-495
expression could influence the sensitivity of NPC cells against
radiation therapy. The miR-495 mimics, inhibitors and additional
negative control sequences were transfected into radioresistant
5-8F cells (5-8F-IR), which were later irradiated with a range of
radiation doses (2–10 Gy) for 24 h. The analysis of cell viability
by MTT assay revealed that the 5-8F-IR cells transfected with
miR-495 mimics had significantly reduced viability in comparison to
the negative control sequence-transfected cells, as observed in
Table III. In contrast, the
5-8F-IR cells transfected with miR-495 inhibitors exhibited
significantly increased viability compared to the NC-transfected
cells (P<0.05; Fig. 9 and
Table III).
 | Table III.Cell viability of transfected NPC
cell lines. |
Table III.
Cell viability of transfected NPC
cell lines.
|
| Survival fraction
(%) |
|---|
|
|
|
|---|
| Radiation doses
(Gy) | miR-495
mimics-5-8F-IR | NC-5-8F-IR | miR-495
inhibitors-5-8F | NC-5-8F |
|---|
| 2 | 92±2.0 | 96±2.2 | 97±2.0 | 95±1.8 |
| 4 | 76±3.0 | 88±3.0 | 90±2.8 | 82±2.8 |
| 6 | 58±2.8 | 78±3.1 | 82±3.2 | 68±3.0 |
| 8 | 36±2.6 | 68±2.4 | 72±2.6 | 52±3.1 |
| 10 | 16±2.8 | 54±2.5 | 60±3.1 | 36±3.0 |
| aRPF |
RPF1=1.04; P<0.05 |
RPF2=1.02; P<0.05 |
Discussion
The glucose-regulated protein GRP78, an endoplasmic
reticulum stress-induced molecular chaperone, plays an important
role in endoplasmic reticulum (ER) stress and in maintaining
cellular homeostasis as a vital stress response survival protein
(15,16). Its expression is induced in cancer
cells not only as a result of ER stress, due to glucose
deprivation, severe hypoxia and acidosis, but also because of
chemotherapeutic drugs and radiation therapy. It has been revealed
to confer tumor resistance to chemotherapeutic agents and radiation
therapy (17,18). The GRP78 expression and distribution
changes have been observed in multiple instances, including
thrombotic disease and cancer (19,20).
It had been demonstrated that GRP78 is highly expressed in many
tumors, thereby suggesting that it may play a vital role in tumor
biology (21). In our previous
study, we reported that GRP78 may act as radioresistant factor in
NPC (4). In the present study based
on IHC analysis, we established that NPC patient tissue samples
exhibited significantly higher positive GRP78 staining, in
comparison to chronic rhinitis patient samples. Specifically, a
higher percentage of GRP78 positive staining was mostly observed in
radioresistant NPC patient samples than radiosensitive NPC patient
samples. These observations revealed that GRP78 may play a vital
role in the progression of NPC and may act as a radioresistant
agent in NPC.
To further shed light on the regulation of GRP78
expression in NPC, we performed bioinformatics analysis to identify
miRNAs targeting GRP78, using miRWalk software. Typically, the
interaction between miRNAs and mRNAs can occur through
complementary binding of the base pairs. However, the interaction
is not usually fully complementary, and several software programs
have been developed to accurately predict miRNA-mRNA binding and to
find specific target genes of miRNAs (22). Programs use algorithms to predict
interaction, and the prediction results are not completely
consistent between different programs. Thus, many researchers use a
combination of predictive programs for definitive analysis. Among
these miRWalk is an effective online software tool for predicting
miRNA-mRNA binding, and is based on ten separate programs that help
to predict results with more accuracy and credibility (23). In the present study, using this
software, we predicted 18 miRNAs that could interact with GRP78
mRNA. Among them, the interaction of miR-495 was predicted to have
the highest stability and specificity, indicating its preferential
binding to GRP78 mRNA.
Notably, the parallel analysis of miR-495 expression
in NPC and chronic rhinitis tissue samples revealed an inverse
association with GRP78 expression. In addition, miR-495 expression
was observed to be lower in radioresistant NPC tissues, than in
radiosensitive NPC tissue samples. Similarly, radioresistant
5-8F-IR cells also displayed lower expression of miR-495 than
radiosensitive parental 5-8F cells. In contrast, GRP78 was higher
in radioresistant 5-8F-IR cells, while low in radiosensitive
parental 5-8F cells. These observations led us to hypothesize that
GRP78 may be a target of miR-495, and may play a role in NPC
radioresistance.
Ahmadi et al (24) found that miR-495 and miR-199-5p
could bind to the 3′-UTR of GRP78 in non-small cell lung cancer
(NSCLC) and miR-495 may play a causative role in tumorigenesis of
lung cancer. Their results revealed upregulation of GRP78 and a
concomitant downregulation of miR-495 and miR-199a-5p in NSCLC,
which was consistant with our study on NPC. Based on the findings
of Ahmadi et al and our primary study of GRP78 which
revealed its association with NPC radioresistance, this study
emphasized and elucidated the mechanism of mediation of NPC
radioresistance by miR-495 targeting GRP78 in more depth. To
further explore our hypothesis, we directly explored the
relationship between miR-495 and GRP78 by undertaking a dual
luciferase assay, where miR-495 mimics were transfected into 5-8F
cells along with either wild-type GRP78 or 3′UTR mutant, both
cloned into luciferase vectors. Our experiments revealed that
miR-495 mimics were not capable of targeting mutant GRP78, while
they targeted wild-type GRP78 and led to decreased GRP78
expression. Therefore, our study concluded that miR-495 targeted
the 3′UTR of GRP78.
Recently some studies have suggested a link between
EMT and radioresistance. A study by Jong et al (25) indicated that EMT and low expression
of EMT-inhibiting miRNAs (especially miR203A), were observed in
pretreatment samples, and caused intrinsic radioresistance in head
and neck squamous cell carcinoma (HNSCC). Thus EMT, a complex and
dynamic process and one of the early events in remodeling of the
cytoskeleton, is characterized by increased expression of
mesenchymal markers, such as N-cadherin and vimentin, and decreased
expression of epithelial markers, such as E-cadherin (26,27).
In addition, multiple studies have shown that EMT plays a vital
role in the occurrence, development, invasion and metastasis of
tumors (28,29). Thus, in the present study, we
observed that upregulation of miR-495 expression in 5-8F cells
after miR-495-mimic transfection, resulted in downregulation of
GRP78, vimentin and N-cadherin expression and upregulation of
E-cadherin expression. Conversely, downregulation of miR-495 in
5-8F cells by its inhibitor reversed the expression trends of GRP78
and EMT proteins. These data demonstrated that miR-495 not only
targets GRP78 but may also be involved in the regulation of EMT
markers.
Finally, by assessing the effects of miR-495 mimics
on post-exposure with different doses of radiation it was observed
that the viability of 5-8F-IR cells was significantly decreased. In
contrast, miR-495 inhibitors significantly enhanced 5-8F cell
viability. These experiments indicated that miR-495 may regulate
radiation sensitivity of NPC cells.
In conclusion, the present study revealed that the
GRP78 protein was highly expressed in NPC than in chronic rhinitis
patient tissue samples. In addition, the enhanced expression of
GRP78 was associated with radioresistant NPC samples.
Bioinformatics analysis indicated that miR-495 interacted strongly
with GRP78 and notably followed an inverse expression trend.
Additional studies suggested that miR-495 not only reduced GRP78
expression by targeting its 3′UTR, but may also be involved in the
expression of EMT-related proteins. Elucidation of the influence of
miR-495-targeting GRP78 on EMT and a knockdown assay of GRP78,
which are limitations of the present study, need to be performed in
a future study. Finally, we also observed that miR-495 mimics
sensitized NPC cells against various doses of radiation. However,
further studies are required to ascertain the significance and
mechanism of miR-495-mediated regulation of EMT, and the subsequent
contributions to radiation sensitivity in NPC cells.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (81372905), the Hunan
Provincial Natural Science Foundation of China (2015JJ4075) and the
Hunan Provincial Health and Family Planning Commission, China (no.
C2017044).
Availability of data and materials
The data sets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
XF and QH planned the study. XF, WL, SW and QH
performed the experiments and data analysis. QH wrote the study
with input from XF, WL and SW. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
NPC tissue biopsies (pre-therapy) were obtained
after receiving informed consent from all patients and were used
for immunohistochemical (IHC) staining. This study was certified by
the Ethics Committee of Xiangya School of Medicine (Central South
University, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EMT
|
epithelial-mesenchymal transition
|
|
UTR
|
untranslated region
|
|
miRNA
|
microRNA
|
|
NPC
|
nasopharyngeal carcinoma
|
|
GRP78
|
glucose-regulated protein 78
|
|
RT-qPCR
|
real-time fluorescence quantitative
polymerase chain reaction
|
References
|
1
|
Chen W and Hu GH: Biomarkers for enhancing
the radiosensitivity of nasopharyngeal carcinoma. Cancer Biol Me.
12:23–32. 2015.
|
|
2
|
Yi HM, Yi H, Zhu JF, Xiao T, Lu SS, Guan
YJ and Xiao ZQ: A five-variable signature predicts radioresistance
and prognosis in nasopharyngeal carcinoma patients receiving
radical radiotherapy. Tumour Biol. 37:2941–2949. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu YH, Xia WX, Shi JL, Ma WJ, Li Y, Ye YF,
Liang H, Ke LR, Lv X, Yang J, et al: A model to predict the risk of
lethal nasopharyngeal necrosis after re-irradiation with
intensity-modulated radiotherapy in nasopharyngeal carcinoma
patients. Chin J Cancer. 35:592016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Feng XP, Yi H, Li MY, Li XH, Yi B, Zhang
PF, Li C, Peng F, Tang CE, Li JL, et al: Identification of
biomarkers for predicting nasopharyngeal carcinoma response to
radiotherapy by proteomics. Cancer Res. 70:3450–3462. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tian S, Chang W, Du H, Bai J, Sun Z, Zhang
Q, Wang H, Zhu G, Tao K and Long Y: The interplay between GRP78
expression and Akt activation in human colon cancer cells under
celecoxib treatment. Anticancer Drugs. 26:964–973. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cook KL, Soto-Pantoja DR, Clarke PA, Cruz
MI, Zwart A, Wärri A, Hilakivi-Clarke L, Roberts DD and Clarke R:
Endoplasmic reticulum stress protein GRP78 modulates lipid
metabolism to control drug sensitivity and antitumor immunity in
breast cancer. Cancer Res. 76:5657–5670. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao S, Li H, Wang Q, Su C, Wang G, Song
H, Zhao L, Luan Z and Su R: The role of c-Src in the invasion and
metastasis of hepatocellular carcinoma cells induced by association
of cell surface GRP78 with activated α2M. BMC Cancer. 15:3892015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cook KL and Clarke R: Role of GRP78 in
promoting therapeutic-resistant breast cancer. Future Med Chem.
7:1529–1534. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu T, Guo Z, Fan H, Song J, Liu Y, Gao Z
and Wang Q: Cancer-associated fibroblasts promote non-small cell
lung cancer cell invasion by upregulation of glucose-regulated
protein 78 (GRP78) expression in an integrated bionic microfluidic
device. Oncotarget. 7:25593–25603. 2016.PubMed/NCBI
|
|
10
|
Li C, Zhang B, Lv W, Lai C, Chen Z, Wang
R, Long X and Feng X: Triptolide inhibits cell growth and GRP78
protein expression but induces cell apoptosis in original and
radioresistant NPC cells. Oncotarget. 7:49588–49596.
2016.PubMed/NCBI
|
|
11
|
He L and Hannon GJ: MicroRNAs: Small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
To EW, Chan KC, Leung SF, Chan LY, To KF,
Chan AT, Johnson PJ and Lo YM: Rapid clearance of plasma
Epstein-Barr virus DNA after surgical treatment of nasopharyngeal
carcinoma. Clin Cancer Res. 9:3254–3259. 2003.PubMed/NCBI
|
|
14
|
Shanmugaratnam K and Sobin LH: The World
Health Organization histological classification of tumours of the
upper respiratory tract and ear. A commentary on the second
edition. Cancer. 71:2689–2697. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Flodby P, Li C, Liu Y, Wang H, Marconett
CN, Laird-Offringa IA, Minoo P, Lee AS and Zhou B: The 78-kD
Glucose-regulated protein regulates endoplasmic reticulum
homeostasis and distal epithelial cell survival during lung
development. Am J Respir Cell Mol Biol. 55:135–149. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kureel J, John AA, Raghuvanshi A, Awasthi
P, Goel A and Singh D: Identification of GRP78 as a molecular
target of medicarpin in osteoblast cells by proteomics. Mol Cell
Biochem. 418:71–80. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kuang XY, Jiang HS, Li K, Zheng YZ, Liu
YR, Qiao F, Li S, Hu X and Shao ZM: The phosphorylation-specific
association of STMN1 with GRP78 promotes breast cancer metastasis.
Cancer Lett. 377:87–96. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gifford JB, Huang W, Zeleniak AE, Hindoyan
A, Wu H, Donahue TR and Hill R: Expression of GRP78, master
regulator of the unfolded protein response, increases
chemoresistance in pancreatic ductal adenocarcinoma. Mol Cancer
Ther. 15:1043–1052. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang B, Han H, Fu S, Yang P, Gu Z, Zhou Q
and Cao Z: Dehydroeffusol inhibits gastric cancer cell growth and
tumorigenicity by selectively inducing tumor-suppressive
endoplasmic reticulum stress and a moderate apoptosis. Biochem
Pharmacol. 104:8–18. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kaira K, Toyoda M, Shimizu A, Imai H,
Sakakura K, Nikkuni O, Suzuki M, Iijima M, Asao T and Chikamatsu K:
Decreasing expression of glucose-regulated protein GRP78/BiP as a
significant prognostic predictor in patients with advanced
laryngeal squamous cell carcinoma. Head Neck. 38:1539–1544. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nami B, Ghasemi-Dizgah A and Vaseghi A:
Overexpression of molecular chaperons GRP78 and GRP94 in
CD44hi/CD24lo breast cancer stem cells.
Bioimpacts. 6:105–110. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dweep H, Sticht C, Pandey P and Gretz N:
miRWalk-database: Prediction of possible miRNA binding sites by
‘walking’ the genes of three genomes. J Biomed Inform. 44:839–847.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dweep H, Gretz N and Sticht C: miRWalk
database for miRNA-target interactions. Methods Mol Biol.
1182:289–305. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ahmadi A, Khansarinejad B, Hosseinkhani S,
Ghanei M and Mowla SJ: miR-199a-5p and miR-495 target GRP78 within
UPR pathway of lung cancer. Gene. 620:15–22. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
de Jong MC, Ten Hoeve JJ, Grénman R,
Wessels LF, Kerkhoven R, Te Riele H, van den Brekel MW, Verheij M
and Begg AC: Pretreatment microRNA expression impacting on
Epithelial-to-mesenchymal transition predicts intrinsic
radiosensitivity in head and neck cancer cell lines and patients.
Clin Cancer Res. 21:5630–5638. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shenoy AK, Jin Y, Luo H, Tang M, Pampo C,
Shao R, Siemann DW, Wu L, Heldermon CD, Law BK, et al:
Epithelial-to-mesenchymal transition confers pericyte properties on
cancer cells. J Clin Invest. 126:4174–4186. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nishitani S, Noma K, Ohara T, Tomono Y,
Watanabe S, Tazawa H, Shirakawa Y and Fujiwara T: Iron
depletion-induced downregulation of N-cadherin expression inhibits
invasive malignant phenotypes in human esophageal cancer. Int J
Oncol. 49:1351–1359. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang Z, Bu X, Chen H, Wang Q and Sha W:
Bmi-1 promotes the invasion and migration of colon cancer stem
cells through the downregulation of E-cadherin. Int J Mol Med.
38:1199–1207. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Petrova YI, Schecterson L and Gumbiner BM:
Roles for E-cadherin cell surface regulation in cancer. Mol Biol
Cell. 27:3233–3244. 2016. View Article : Google Scholar : PubMed/NCBI
|