Introduction
Multiple myeloma (MM) is an incurable disease of
hematological malignancies, characterized by the abnormal
proliferation of plasma cells and overexpression of monoclonal
immunoglobulin, which accounts for ~10% of hematopoietic neoplasias
with increasing incidence worldwide. MM can be divided into
hyperdiploid multiple myeloma (HD-MM) and non-HD-MM subtypes by
fluorescence in situ hybridization (1). The majority of cases of MM are from
the original premalignant state, known as monoclonal gammopathy of
undetermined significance, to smoldering MM, truly overt and
symptomatic MM, and finally extramedullary MM/plasma cell leukemia
(2,3). Despite agents, including proteasome
inhibitors and immunomodulatory drugs, and rapidly developing stem
cell transplantation technology, which have significantly improved
the efficacy of treatment and prognosis of patients with MM,
treatment for relapse remains limited.
Nuclear protein-1 (NUPR1), also known as p8 and
candidate of metastasis 1, was first described in pancreatic acinar
cells of rats when evaluating the molecular changes in the injured
pancreas (4). The NUPR1 gene is
located on human chromosome 16p11.2. The length of its cDNA is 719
bp, and its open reading frame is 249 bp, encoding a protein with a
molecular weight of 8,872.7 Da (5).
Studies have found that Nupr1 has a wide regulatory role in cell
proliferation, migration and apoptosis, and is involved in the
development of various types of tumor, including pancreatic cancer,
liver cancer, bladder cancer and breast cancer (6–9). It
has been shown that NUPR1 also regulates cell sensitivity to drugs
in hepatocarcinoma cells (7). It
has been reported that there is a specific NUPR1 expression profile
in HD-MM, which may become a novel drug target (10). However, the characterization of its
effects on MM cells remains to be fully elucidated.
In the present study, the effect of NUPR1 in MM
cells was examined; it was found that NUPR1 was upregulated in MM
cell lines (U266 and RPMI8226), and NUPR1 was then silenced using
lentiviral short hairpin (sh)RNA in the U266 and RPMI8226 cells.
The functional effect of NUPR1 was examined by proliferation,
apoptosis and cell cycle assays. In order to examine the possible
mechanism underlying these effects, the expression of genes known
to be associated with proliferation and apoptosis were examined by
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) and western blot analyses.
Materials and methods
Cell culture and sample
collection
The U266 and RPMI8226 human MM cell lines were
donated by Professor Jian Hou at The Second Military Medical
University (Shanghai, China). The U266 and RPMI8226 cells were
cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), supplemented with 10% heat-inactivated
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) at
37°C in a humidified incubator with 5% CO2. Bone marrow
specimens were obtained from four patients with MM (two females and
two males aged 77, 63, 75 and 61 years), and four healthy
individuals (one female and three males aged 61, 65, 53 and 55
years) admitted to The First Affiliated Hospital of Chongqing
Medical University (Chongqing, China). These specimens were
collected between April 2016 and October 2016. The diagnosis was
established according to standard morphological and
immunophenotypic criteria and revised international staging system
(11). All specimens were obtained
in accordance with the Research Ethics Board of the First
Affiliated Hospital of Chongqing Medical University.
Lentiviral vector construction and
cell infection
The lentiviral shRNAs were designed and synthesized
by Shanghai GeneChem Co., Ltd. (Shanghai, China). The RNA
interference (RNAi) target sequence for NUPR1 was
5′-CCAAGCTGCAGAATTCAGA-3′ whereas the control non-silencing RNA
sequence was 5′-TTCTCCGAACGTGTCACGT-3′. The lentivirus was produced
in 293T cells (American Type Culture Collection, Manassas, VA, USA)
as discussed previously (12). The
U266 and RPMI8226 cells in the exponential phase were plated in
24-well plates (5×104 cells/well), infected with
NUPR1-shRNA lentivirus or NC-shRNA lentivirus in the presence of 1
µg/ml polybrene at a set multiplicity of infection of 100. After 5
days, the transfection efficiency was estimated by flow
cytometry.
RNA extraction and RT-qPCR
analysis
Total RNA was extracted from cells with TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. The RT reaction was performed using
the Prime Script™ RT reagent kit according to the manufacturer's
protocol (Takara Biotechnology Co., Ltd., Dalian, China). cDNA was
reverse transcripted from 1,000 ng total RNA RT-qPCR analysis was
performed using a CFX96 Real-Time PCR system (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) and a SYBR Premix Ex Taq™ II PCR kit
(Takara Biotechnology Co., Ltd.). The primer pairs were as follows:
NUPR1, forward 5′-AGGACTTATTCCCGCTGACTGA-3′ and reverse
5′-TGCCGTGCGTGTCTATTTATTG-3′; B-cell lymphoma 2 (Bcl-2), forward
5′-AACATCGCCCTGTGGATGAC-3′ and reverse 5′-GACTTCACTTGTGGCCCAGAT-3′;
proliferating cell nuclear antigen (PCNA), forward
5′-CTCGTCCCACGTCTCTTTGG-3′ and reverse 5′-CGCGTTATCTTCGGCCCTTA-3′;
phosphatase and tensin homolog (PTEN), forward
5′-ACACGACGGGAAGACAAGTT-3′ and reverse 5′-CTGGTCCTGGTATGAAGAATG-3′;
β-actin, used as an internal control, forward
5′-CCACGAAACTACCTTCAACTCC-3′ and reverse
5′-GTGATCTCCTTCTGCATCCTGT-3′. The PCR reactions consisted of 30 sec
at 95°C, followed by 40 cycles at 95°C for 5 sec and annealing at
60°C for 30 sec. Relative gene expression was calculated using the
2−ΔΔCq method (13).
Western blot analysis
Total protein was extracted from the MM cells, and
protein quantification was performed using the BCA method (Beyotime
Institute of Biotechnology, Haimen, China). Subsequently, equal
quantities of protein (40 µg) were separated by SDS-PAGE (12%),
transferred onto PVDF membranes, and then blocked with 5% non-fat
milk. The membranes and corresponding specific antibodies were
incubated at 4°C overnight. The following antibodies were used as
primary antibodies: Rabbit anti-human NUPR1 antibody (1:1,000; cat.
no. MBS420484; Novus Biologicals LLC, Littleton, CO, USA); PCNA
(1:1,000; cat. no. ab18197; Abcam, Cambridge, UK) and PTEN
(1:1,000; cat. no. ab31392; Abcam); cleaved caspase-3 (1:1,000;
cat. no. Asp175; Cell Signaling Technology, Inc., Danvers, MA,
USA,), cleaved caspase-8 (1:1,000; cat. no. Asp391; Cell Signaling
Technology), cleaved caspase-9 (1:1,000; cat. no. Asp315; Cell
Signaling Technology); Bcl-2 (1:800; cat. no. 12789–1-AP;
ProteinTech Group, Inc., Wuhan, China); GAPDH (1:5,000; cat. no.
10494–1-AP; ProteinTech Group). These membranes were incubated with
secondary antibodies (1:3,000; cat. no. 7074; Cell Signaling
Technology) for 2 h at 37°C, following washing with TBS-Tween-20
the following day. Excess antibody was removed with TBS-Tween-20
prior to incubation in ECL. The western blot assays were repeated
three times. The band intensity was analyzed using Quantity One
software (version 4.5.0; Bio-Rad Laboratories) according to the
manufacturer's instructions.
Cell Counting Kit-8 (CCK-8) assay for
cell proliferation
The proliferation of the U266 and RPMI8226 cells was
evaluated following lentivirus infection for 5 days. The cells were
resuspended and plated in 96-well plates at 2,000 cells per well.
CCK-8 (10 µl; Dojindo Molecular Technologies, Inc., Kumamoto,
Japan) reagent was added to each well, and the plates were
incubated at 37°C for 2 h. Cell growth was estimated by measuring
the absorbance at 450 nm with a microplate reader (M88; Thermo
Fisher Scientific, Inc.) at the end of incubation.
Cell cycle analysis by
fluorescence-activated cell sorting (FACS)
The cells were collected and centrifuged at 1,500 ×
g at 22°C for 5 min. The cells were then washed with PBS, and fixed
with cold 70% ethanol at 4°C overnight. The cells were then stained
with 50 µg/ml propidium iodide (PI) for 30 min at room temperature.
Finally, each group of cells was determined using a FACScan cell
sorter (BD Influx; BD Biosciences, Franklin Lakes, NJ, USA).
Assessment of apoptosis by flow
cytometry
The cells were collected and stained with annexin
V-APC to differentiate intact cells from apoptotic cells. All cells
were washed with ice-cold PBS twice and incubated for 30 min in a
binding buffer (1 mg/ml annexin V-APC), respectively. FACS analysis
for annexin V-APC staining was performed by flow cytometry using
CellQuest software version 4.02 (BD Biosciences).
Statistical analysis
The differences between groups were analyzed using
Student's t-test or one-way analysis of variance using SPSS 22
software (IBM Corp., Armonk, NY, USA). Data are expressed as the
mean ± standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
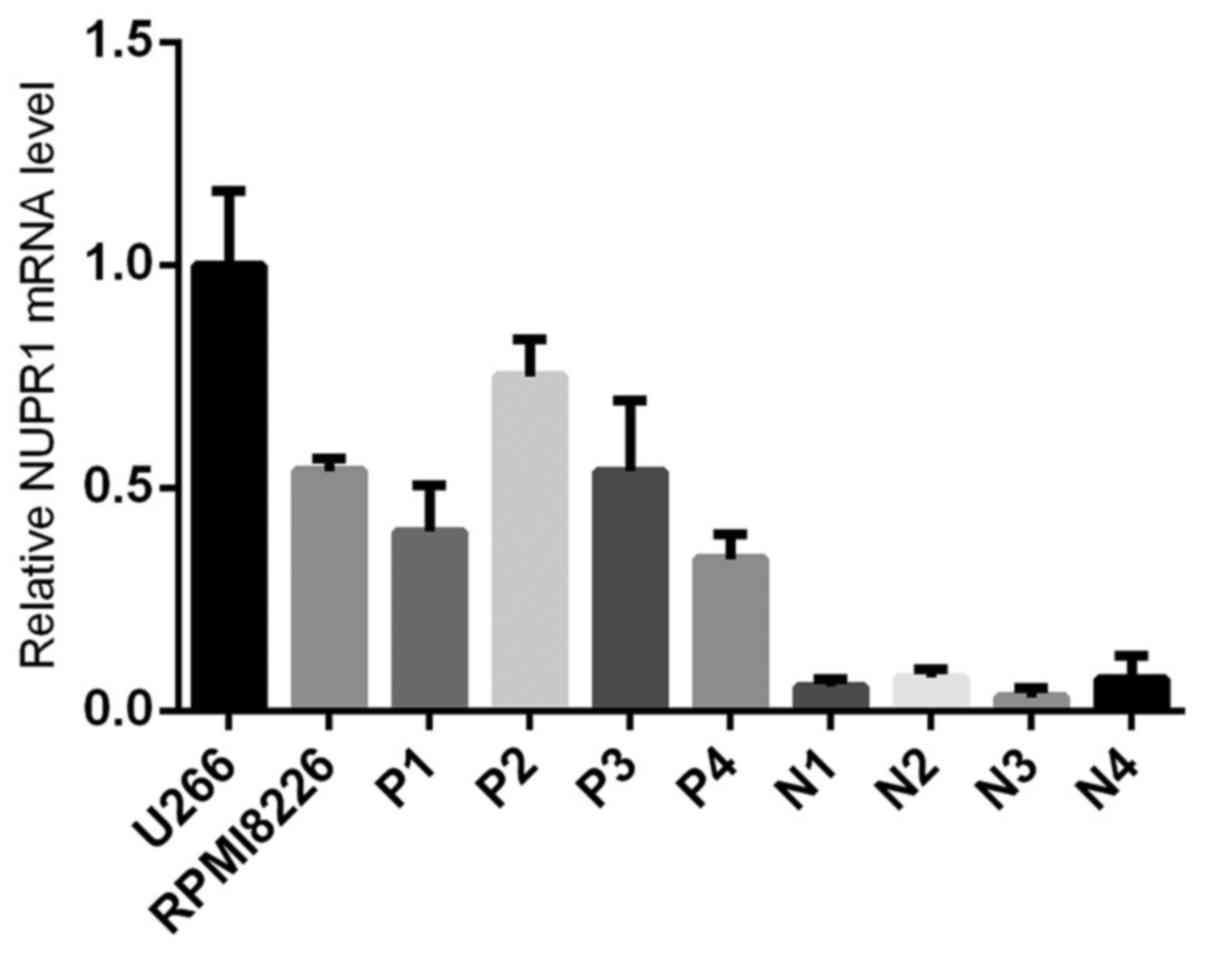
Expression pattern of NUPR1 in human
MM
The present study determined the mRNA expression of
NUPR1 in U266 and RPMI8226 MM cell lines, and the specimens of
patients with MM, with cells from normal human bone marrow as a
control. As shown in Fig. 1, the MM
cell lines and MM patient specimens showed higher expression levels
of NUPR1, compared with those in the normal bone marrow cells
(P<0.05). Therefore, a specific lentiviral vector was designed
to knock down the NUPR1 gene and examine the role of the gene in
the U266 and RPMI8226 MM cell lines.
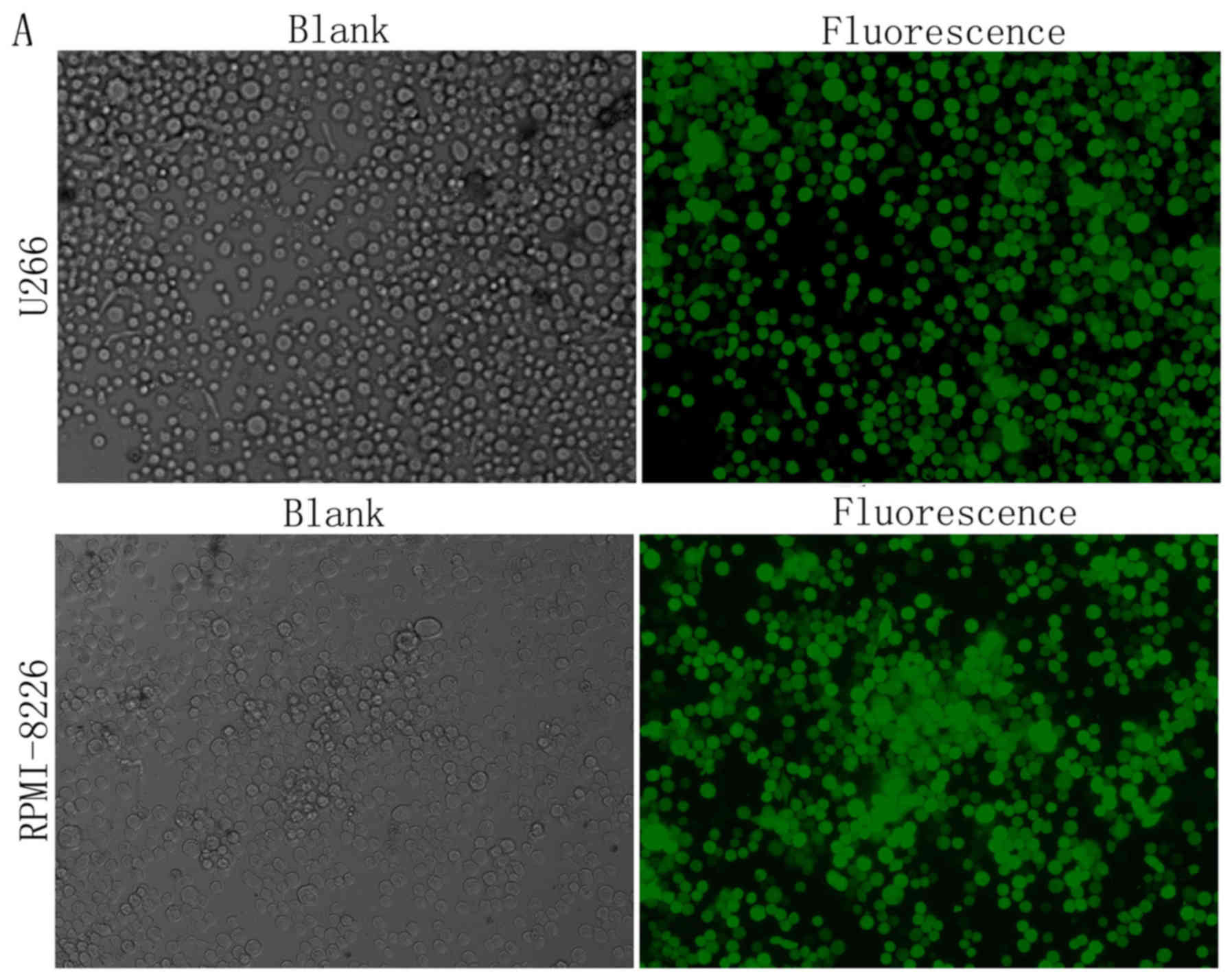
Downregulation of the expression of
NUPR1 in U266 and RPMI8226 cell lines following lentivirus
transfection
To better understand the significance of the
upregulation of NUPR1 in the MM cell lines, the lentivirus
expressing shRNA against NUPR1 was used for gene silencing in the
U266 and RPMI8226 cells. The transfection efficiency of the cells
was detected using a fluorescence microscope (Fig. 2A) and flow cytometry (Fig. 2B), the results of which showed that
the transfection efficiency was >80%. The expression mRNA and
protein levels of NUPR1 were determined by RT-qPCR and western blot
analyses, respectively, following infection of the cells with the
lentiviral vector (shCtrl or shNUPR1). The shNUPR1-transfected
cells showed a significant reduction in mRNA and protein levels of
NUPR1 when compared with the cells infected with the control
lentivirus (Fig. 2C).
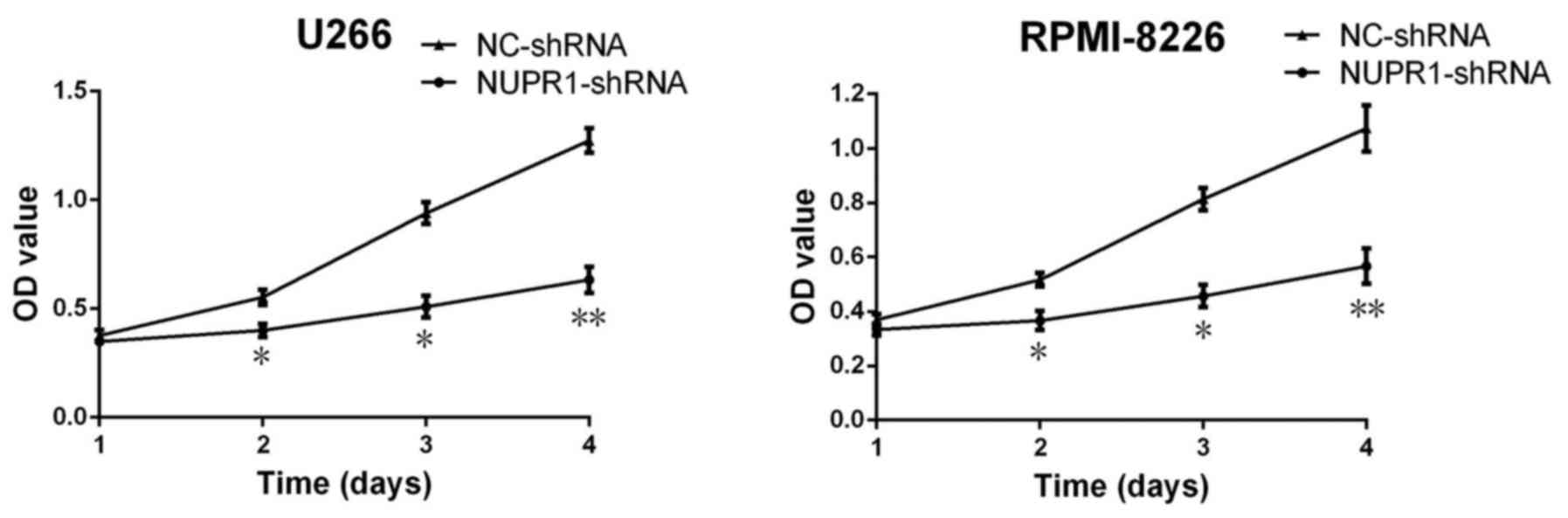
Downregulation of NUPR1 suppresses
cell growth in U266 and RPMI8226 cells
To examine the potential function of NUPR1 in the
growth of U266 and RPMI8226 cells, CCK-8 assays were used to
analyze cell proliferation. Following a 3-day period, the
proliferation of cells in the NUPR1-shRNA group was significantly
suppressed compared with those in the negative control group
(Fig. 3).
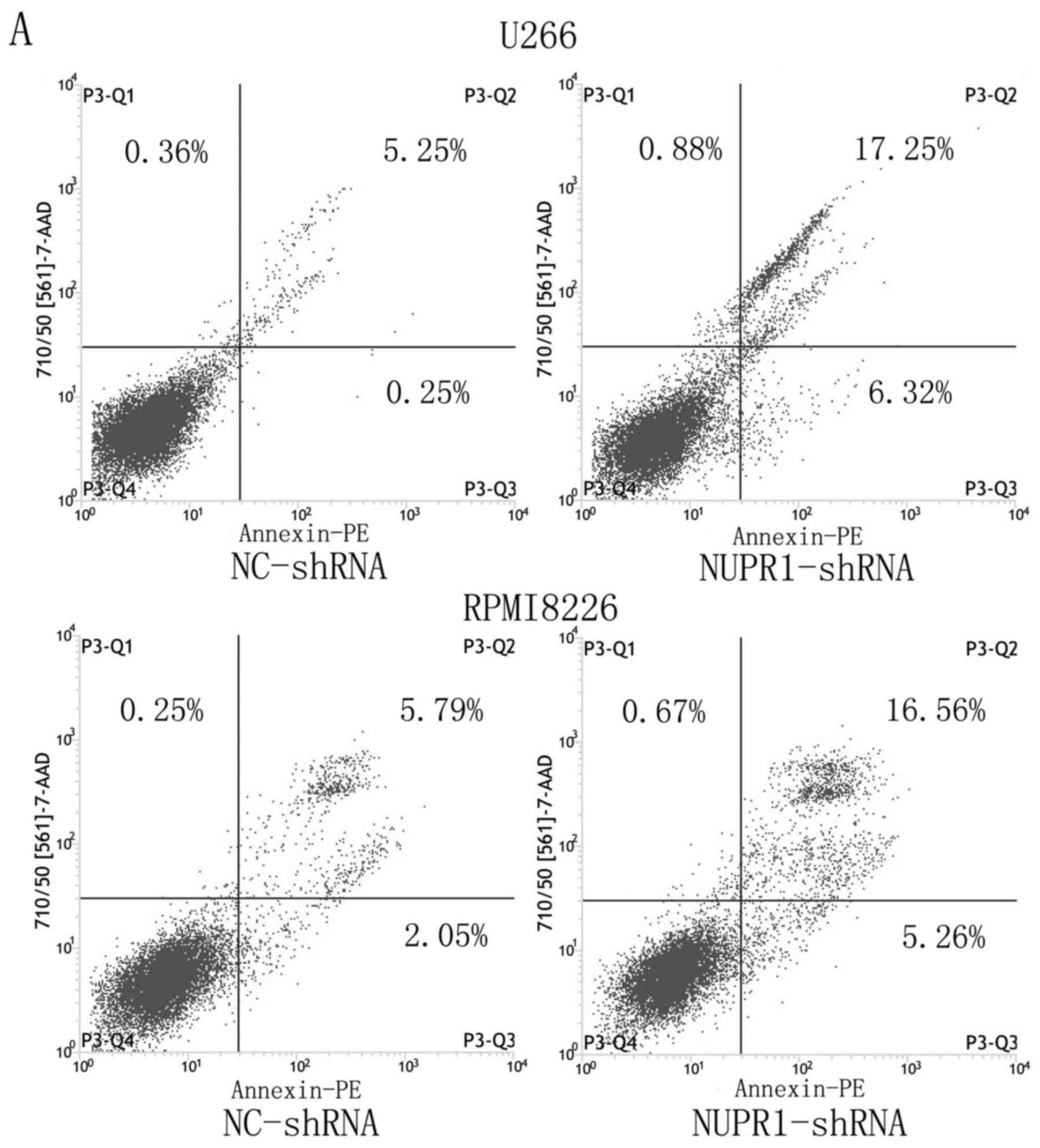
Analysis of cell cycle and
apoptosis
Flow cytometry was performed to analyze apoptosis,
as shown in Fig. 4A and B, the
average apoptotic rates of the NUPR1-shRNA group and negative group
U226 cells were 29.61±11.90 and 6.18±1.13%, and those of the
RPMI8226 cells were 23.98±9.29 and 8.11±2.75%, respectively
(P<0.05). This result revealed that knockdown of the expression
of NUPR1 significantly promoted apoptosis of the U226 and RPMI8226
cells. FACS was used to examine the effect of NUPR1 knockdown on
the cell cycle. As shown in Fig. 4C and
D, compared with the negative group, the U266 and RPMI8226
cells in the NUPR1-shRNA group were arrested at the
G0/G1 phase (P<0.01).
Knockdown of NUPR1 affects the
expression of apoptotic and proliferation proteins in U266 and
RPMI8226 cells
To investigate the molecular mechanism by which
NUPR1 affects apoptosis and proliferation, the expression of
relevant factors, including Bcl-2, cleaved caspase-3, −8 and −9,
PTEN and PCNA, were examined in U266 and RPMI8226 cells with NUPR1
knockdown. The RT-qPCR and western blot analyses showed that the
mRNA and protein levels of Bcl-2 and PCNA were significantly
decreased in the NUPR1 shRNA-infected cells, whereas the expression
level of PTEN was notably higher compared with that in the negative
group. Western blot analysis revealed that the protein levels of
cleaved caspase-3, −8 and −9 were higher in the U266 and RPMI8226
cells infected with the NUPR1-shRNA lentivirus, compared with those
in the negative control group (Fig.
5A-C).
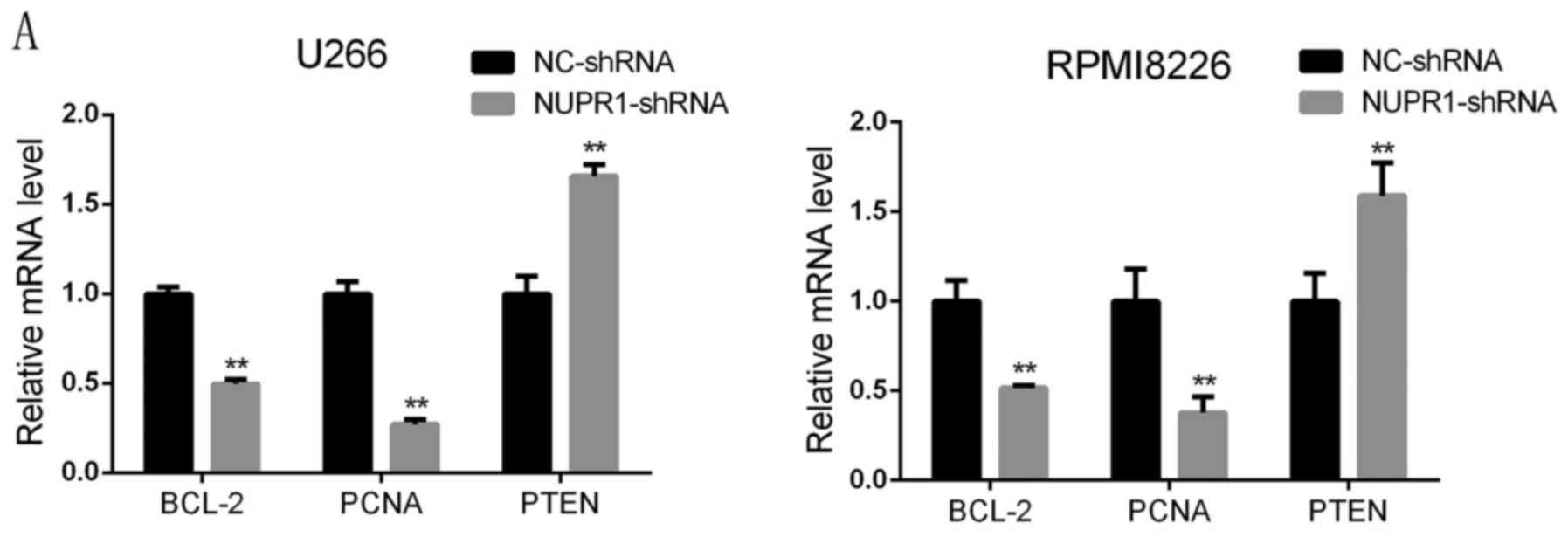 | Figure 5.NUPR1 knockdown inhibits the
expression of BCL-2 and PCNA, increases the expression of PTEN, and
activates caspase-3, −8 and −9. (A) Reverse
transcription-quantitative polymerase chain reaction analysis
revealed that the mRNA expression levels of BCL-2 and PCNA
decreased and the expression of PTEN increased in the NUPR1-shRNA
group, compared with those in the NC group of U266 and RPMI18226
cells (**P<0.01). (B and C) Protein expression levels of PCNA,
PTEN, BCL-2, and cleaved caspase-3, −9 and −8 were determined using
a western blot assay. NUPR1, nuclear protein-1; PCNA, proliferating
cell nuclear antigen; PTEN; phosphatase and tensin homolog; BCL-2,
B-cell lymphoma 2; shRNA, short hairpin RNA; NC, negative
control. |
Discussion
NUPR1 was originally identified from the rat
pancreas during the acute phase of pancreatitis (5). As a stress induced protein, it appears
to be involved in a variety of stress-related functions and can
produce different effects based on the physiological scenario. It
is capable of promoting tumor growth and aggressiveness (14,15),
however, the same molecule appears to have tumor suppressive
activity in prostate cancer (16),
which depends on cancer cell type. This may be due to the different
microenvironment affecting the activity of NUPR1. Previous studies
have shown that NUPR1 affects the biological functions of tumor
cells by regulating apoptosis and proliferation (17–19).
NUPR1 modulates the apoptosis and proliferation of glioblastoma
cells via caspase activation and the extracellular signal-regulated
kinase signaling pathway (20). As
an anti-apoptotic molecule, NUPR1 exerts an anti-apoptotic effect
in breast cancer specimens and pancreatic cancer (8,21,22).
However, whether and how NUPR1 affects MM cell
viability remains to be fully elucidated, and few studies have been
performed to determine the effects of NUPR1 on the behavior of MM
cells in MM. In the present study, RT-qPCR was used to detect NUPR1
and it was found that the levels of NUPR1 in MM cell lines and
specimens of patients with MM were significantly higher, compared
with those in normal bone marrow cells. These results indicated
that NUPR1 may be important in the progression of MM. The effects
of NUPR1 on U266 and RPMI8226 MM cell line proliferation and
apoptosis were also examined by infecting cells with an
NUPR1-specific RNAi-expressing lentivirus. Apoptosis occurs in
various physiological and pathological situations, and is involved
in maintaining tissue homeostasis in multicellular organisms.
Apoptosis may be initiated through different entry sites, including
death receptors or mitochondria, which results in the activation of
effector caspases (23,24). Resistance to apoptosis is one of the
hallmarks of human cancer, and promotes the development and
progression of cancer (25). In
addition, evasion from apoptosis represents one of the leading
causes of failure of antileukemic therapy as numerous anticancer
treatments act by triggering apoptosis in cancer cells (26). The present study indicated that the
NUPR1-shRNA-infected cells exhibited reduced cell proliferation and
increased apoptosis, and FACS analysis demonstrated that the
NUPR1-shRNA-infected U266 and RPMI8226 cells were arrested at the
G0/G1 phase, compared with the negative
control group cells.
Furthermore, the present study found that the
knockdown of NUPR1 reduced the expression of PCNA and increased the
expression of PTEN in U266 and RPMI8226 cells. PCNA is closely
associated with cell DNA synthesis and repair, and promotes cell
proliferation by increasing the synthesis of DNA polymerase
(27). Following the knocking down
of NUPR1, the gene and protein levels of PCNA were significantly
downregulated, indicating that downregulating NUPR1 inhibited the
proliferation activity of cells, possibly by affecting DNA
synthesis and repair in MM cells. PTEN has been identified as a
tumor suppressor, and mutation of this gene may lead to the
development of several types of cancer. It has been reported that
PTEN can regulate proliferation and apoptosis in different types of
cancer (28–31). Studies have show that via the
phosphoinositide 3-kinase (PI3K)/AKT signaling pathway, PTEN can
regulate cell proliferation, and caspase-9 also regulated by Akt
(32,33). As the activation of phosphorylated
Akt can lead to the decrease of Bcl-2 and the increase of cleaved
caspase-3 (34), the present study
examined the expression of Bcl-2, and cleaved caspase-3, −8 and −9,
which are general apoptosis-related proteins. It was found that the
knockdown of NUPR1 inhibited the expression of Bcl-2 and promoted
the expression of cleaved caspase-3, −8 and −9, showing that
inhibiting the expression of NUPR1 may directly or indirectly lead
to the downregulation of Bcl-2 and activation of caspase-3, −8 and
−9 via the PTEN/PI3K/AKT signaling pathway, which is responsible
for the inhibition of U266 and RPMI8226 cell growth in
vitro. However, for specific signaling pathways of NUPR1 in MM
cells and its in vivo mechanisms, further investigations are
required in subsequent experiments. Only a preliminary judgment can
be made on the expression level of NUPR1 in patients with MM. In
subsequent experiments, an increase in the number of specimens is
required to analyze the expression of NUPR1, and its association
with patient outcomes and prognosis.
In conclusion, the present study showed that
lentivirus-mediated NUPR1-shRNA (RNAi targeting NUPR1)
significantly inhibited the proliferation of U266 and RPMI8226 MM
cell lines, and induced apoptosis in vitro. NUPR1 may be
associated with the induction of apoptosis by activating caspases
and increasing the expression of PTEN in U266 and RPMI8226 cells.
Further investigation of the functional role of NUPR1 may lead to
an improved understanding of the molecular mechanism of MM. Drugs
suppressing the effects of NUPR1 and inducing apoptosis may be a
valid strategy for the treatment of MM.
Acknowledgements
The authors would like to thank Professor Jian Hou
of The Second Military Medical University for providing the MM cell
lines, and the Laboratory of Hematology of The First Affiliated
Hospital of Chongqing Medical University for providing specimens.
The authors would also like to thank Lixue Chen and Xiaojuan Deng
(Experimental Research Center of the First Affiliated Hospital of
Chongqing Medical University) for their technical assistance.
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
CZ and JC conceived and designed the study. CZ, XL,
AL and BY performed the experiments. CZ, XP and XH wrote the
manuscript. CZ, XP, XH and JC reviewed and edited the manuscript.
All authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
All specimens were obtained in accordance with the
Research Ethics Board of the First Affiliated Hospital of Chongqing
Medical University. Informed consent was provided.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mohamed AN, Bentley G, Bonnett ML, Zonder
J and Al-Katib A: Chromosome aberrations in a series of 120
multiple myeloma cases with abnormal karyotypes. Am J Hematol.
82:1080–1087. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dupéré-Richer D and Licht JD: Epigenetic
regulatory mutations and epigenetic therapy for multiple myeloma.
Curr Opin Hematol. 24:336–344. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Manier S, Salem KZ, Park J, Landau DA,
Getz G and Ghobrial IM: Genomic complexity of multiple myeloma and
its clinical implications. Nat Rev Clin Oncol. 14:100–113. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mallo GV, Fiedler F, Calvo EL, Ortiz EM,
Vasseur S, Keim V, Morisset J and Lovanna JL: Cloning and
expression of the rat p8 cDNA, a new gene activated in pancreas
during the acute phase of pancreatitis, pancreatic development, and
regeneration, and which promotes cellular growth. J Biol Chem.
272:32360–32369. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cano CE, Hamidi T, Sandi MJ and Iovanna
JL: Nupr1: The Swiss-knife of cancer. J Cell Physiol.
226:1439–1443. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sandi MJ, Hamidi T, Malicet C, Cano C,
Loncle C, Pierres A, Dagorn JC and Lovanna JL: p8 expression
controls pancreatic cancer cell migration, invasion, adhesion, and
tumorigenesis. J Cell Physiol. 226:3442–3451. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Emma MR, Iovanna JL, Bachvarov D, Puleio
R, Loria GR, Auqello G, Candido S, Libra M, Gulino A, Cancila V, et
al: NUPR1, a new target in liver cancer: Implication in controlling
cell growth, migration, invasion and sorafenib resistance. Cell
Death Dis. 7:e22692016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Veerla S, Panagopoulos I, Jin Y, Lindgren
D and Höglund M: Promoter analysis of epigenetically controlled
genes in bladder cancer. Genes Chromosomes Cancer. 47:368–378.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ito Y, Yoshida H, Motoo Y, Lovanna JL,
Nakamura Y, Kakudo K, Uruno T, Takamura Y, Miya A, Noquchi S, et
al: Expression of p8 protein in breast carcinoma; an inverse
relationship with apoptosis. Anticancer Res. 25:833–837.
2005.PubMed/NCBI
|
|
10
|
Di Martino MT, Guzzi PH, Caracciolo D,
Aqnelli L, Neri A, Walker BA, Morqan GJ, Cannataro M, Tassone P and
Taqliaferri P: Integrated analysis of microRNAs, transcription
factors and target genes expression discloses a specific molecular
architecture of hyperdiploid multiple myeloma. Oncotarget.
6:19132–19147. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Palumbo A, Avet-Loiseau H, Oliva S,
Lokhorst HM, Goldschmidt H, Rosinol L, Richardson P, Caltagirone S,
Lahuerta JJ, Facon T, et al: Revised international staging system
for multiple myeloma: A report from international myeloma working
group. J Clin Oncol. 33:2863–2869. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Scherr M and Eder M: Gene transfer into
hematopoietic stem cells using lentiviral vectors. Curr Gene Ther.
2:45–55. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo X, Wang W, Hu J, Feng K, Pan Y, Zhang
L and Feng L: Lentivirus-mediated RNAi knockdown of NUPR1 inhibits
human nonsmall cell lung cancer growth in vitro and in vivo. Anat
Rec. 295:2114–2121. 2012. View
Article : Google Scholar
|
|
15
|
Pedrola N, Devis L, Llauradó M, Campoy I,
Martinez-Garcia E, Garcia M, Muinelo-Romay L, Alonso-Alconada L,
Abal M, Alameda F, et al: Nidogen 1 and nuclear protein 1: Novel
targets of ETV5 transcription factor involved in endometrial cancer
invasion. Clin Exp Metastasis. 32:467–478. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang WG, Davies G, Martin TA, Kynaston H,
Mason MD and Fodstad O: Com-1/p8 acts as a putative tumour
suppressor in prostate cancer. Int J Mol Med. 18:981–986.
2006.PubMed/NCBI
|
|
17
|
Kong DK, Georgescu SP, Cano C, Aronovitz
MJ, Lovanna JL, Patten RD, Kyriakis JM and Goruppi S: Deficiency of
the transcriptional regulator p8 results in increased autophagy and
apoptosis, and causes impaired heart function. Mol Biol Cell.
21:1335–1349. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Goruppi S and Iovanna JL: Stress-inducible
protein p8 is involved in several physiological and pathological
processes. J Biol Chem. 285:1577–1581. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li J, Ren S, Liu Y, Lian Z, Dong B, Yao Y
and Xu Y: Knockdown of NUPR1 inhibits the proliferation of
glioblastoma cells via ERK1/2, p38 MAPK and caspase-3. J
Neurooncol. 132:15–26. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lorente M, Carracedo A, Torres S, Natali
F, Eqia A, Hernández-Tiedra S, Salazar M, Blázquez C, Guzman M and
Velasco G: Amphiregulin is a factor for resistance of glioma cells
to cannabinoid-induced apoptosis. Glia. 57:1374–1385. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Su SB, Motoo Y, Iovanna JL, Berthézène P,
Xie MJ, Mouri H, Ohtsubo K, Matsubara F and Sawabu N:
Overexpression of p8 is inversely correlated with apoptosis in
pancreatic cancer. Clin Cancer Res. 7:1320–1324. 2001.PubMed/NCBI
|
|
22
|
Malicet C, Giroux V, Vasseur S, Dagorn JC,
Neira JL and Iovanna JL: Regulation of apoptosis by the
p8/prothymosin alpha complex. Proc Natl Acad Sci USA.
103:2671–2676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fulda S and Debatin KM: Extrinsic versus
intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene.
25:4798–4811. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Reed JC and Pellecchia M: Apoptosis-based
therapies for hematologic malignancies. Blood. 106:408–418. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mahler M, Miyachi K, Peebles C and
Fritzler MJ: The clinical significance of autoantibodies to the
proliferating cell nuclear antigen (PCNA). Autoimmun Rev.
11:771–775. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gregorian C, Nakashima J, Dry SM,
Nghiemphu PL, Smith KB, Ao Y, Dang J, Lawson G, Mellinghoff IK,
Mischel PS, et al: PTEN dosage is essential for neurofibroma
development and malignant transformation. Proc Natl Acad Sci USA.
106:19479–19484. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Song L, Liu S, Zhang L, Yao H, Gao F, Xu D
and Li Q: MiR-21 modulates radiosensitivity of cervical cancer
through inhibiting autophagy via the PTEN/Akt/HIF-1alpha feedback
loop and the Akt-mTOR signaling pathway. Tumour Biol.
37:12161–12168. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang DD, Yang SJ, Chen X, Shen HY, Luo LJ,
Zhang XH, Zhong SL, Zhao JH and Tang JH: miR-222 induces Adriamycin
resistance in breast cancer through PTEN/Akt/p27kip1
pathway. Tumour Biol. 37:15315–15324. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhu DY, Li XN, Qi Y, Liu DL, Yang Y, Zhao
J, Zhang CY, Wu K and Zhao S: MiR-454 promotes the progression of
human non-small cell lung cancer and directly targets PTEN. Biomed
Pharmacother. 81:79–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xiaozhou L, Xing Z, Haidong X, Zhiwei H,
Xin S and Sujia W: SLC34A2 regulates the proliferation, migration,
and invasion of human osteosarcoma cells through PTEN/PI3K/AKT
signaling. DNA Cell Biol. 36:775–780. 2017.PubMed/NCBI
|
|
33
|
Chappell WH, Steelman LS, Long JM, Kempf
RC, Abrams SL, Franklin RA, Bäsecke J, Stivala F, Donia M, Fagone
P, et al: Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors:
Rationale and importance to inhibiting these pathways in human
health. Oncotarget. 2:135–164. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang F, Shi L, Liang T, Ji L, Zhang G,
Shen Y, Zhu F and Xu L: Anti-tumor effect of evodiamine by inducing
Akt-mediated apoptosis in hepatocellular carcinoma. Biochem Biophys
Res Commun. 485:54–61. 2017. View Article : Google Scholar : PubMed/NCBI
|