Introduction
Small cell lung cancer (SCLC), a poorly
differentiated and highly aggressive tumor, constitutes
approximately 15% of all lung cancers (1). SCLC patients often present with
metastasis at diagnosis, ruling out surgery as a treatment option
(2). There is currently no approved
targeted drugs for SCLC, and no effective method for early
diagnosis is available; meanwhile, most patients treated with
conventional therapies, including chemotherapy and radiotherapy,
show recurrence after a short period of time, which results in poor
patient prognosis (3). To improve
the survival of patients with SCLC, developing new and efficacious
candidate agents is urgently required.
Emerging evidence suggests that cancer stem cells
(CSCs), a subpopulation of tumor cells, have the properties of
self-renewal, heterogeneous progeny, drug-resistance, and
carcinogenesis in vitro and in vivo (4,5).
Multiple SCLC characteristics, such as aggressiveness and high
metastatic potential, suggest that this cancer could be enriched in
CSCs (6). Furthermore, drug
resistance may be attributable to the occurrence of a CSC
subpopulation in SCLC (6).
Therefore, a therapeutic strategy targeting CSCs may help cure
malignant tumors, including SCLC (7).
FOXO3a is considered an evolutionarily conserved
transcription factor involved in various cellular processes,
including cell cycle arrest, DNA repair and tumor suppression
(8). In cancer progression, FOXO3a
inhibition stimulates cell transformation and angiogenesis
(9). Conversely, FOXO3a
overexpression suppresses cancer cell growth, induces apoptosis,
and reduces tumor size by regulating downstream effectors (10). These findings suggest a tumor
suppressor role for FOXO3a, which could constitute a potential
target for cancer treatment. Our previous findings demonstrated
that casticin, a natural polymethoxyflavone isolated from A.
annua, V. trifolia, and V. agnus-castus that has been
widely used in traditional Chinese medicine as an anti-inflammatory
drug for thousands of years, not only induces growth suppression,
apoptosis and cell cycle arrest in hepatocellular carcinoma
(11) and breast cancer cells
(12), but also promotes apoptosis
in ovarian cancer SKOV3 cells (13)
through FOXO3a activation. However, whether casticin inhibits in
vitro carcinogenesis and CSC characteristics in the SCLC H446
cell line, and activates FoxO3a remains unclear.
Yung et al (9) demonstrated that AMPK activation
inhibited cervical cancer cell growth through AKT/FOXO3a/FOXM1
signaling. Meanwhile, Shrestha et al (14) reported that the AMPK/FoxO3A axis
plays a critical role in the antiproliferative effects of
adiponectin in cancer cells. Furthermore, Sato et al
proposed that metformin efficaciously eliminates glioma stem
cell-like cells by activating FOXO3 via AMPK (8). Moreover, Zhao et al provided
evidence that GL-V9, a new synthetic flavonoid derivative, improved
the state of animals with DSS-induced colitis from oxidative stress
by upregulating Trx-1 via activation of the AMPK/FOXO3a pathway
(15). However, whether the
anticancer effects of casticin involve AMPK/FoxO3a signaling
remains undefined.
The present study showed that casticin inhibited
in vitro carcinogenesis and CSC characteristics in LCSCs
derived from the H446 cell line, as demonstrated by sphere- and
colony-formation assays, as well as western blot analysis.
Mechanistically, the effects of casticin were partly associated
with activation of AMPK/FoxO3a signaling. These findings suggest
that casticin may be used as a novel candidate agent for SCLC
treatment targeting lung cancer stem-like cells.
Materials and methods
Cell culture and reagents
The human small cell lung cancer NCI-H446, H209 and
H69 cells were obtained from the Cell Bank of the Chinese Academy
of Sciences (Shanghai, China), and maintained in Dulbecco's
modified Eagle's medium (DMEM, Life Technologies, Grand Island, NY,
USA) supplemented with 10% fetal bovine serum (FBS) (Invitrogen,
Shanghai, China), 100 U/ml penicillin and 100 U/ml streptomycin, in
a humidified atmosphere with 5% CO2 at 37°C. Casticin
(purity ≥98%) was purchased from Chengdu Biopurify Phytochemicals
Ltd. (Chengdu, China), as yellow crystals (molecular weight, 374.3
Da). It was dissolved in dimethyl-sulfoxide (DMSO) to prepare a 10
mmol/l stock solution, diluted in cell culture medium immediately
before use. The following reagents were purchased from Hunan
Clonetimes Biotech Co., Ltd. (Changsha, China): Antibodies against
AMPKα (cat. no. 2532), p-AMPK (cat. no. 8324), ACC (cat. no. 9957),
FoxO3a (cat. no. 2497), p-FoxO3a (cat. no. 9465), uPAR (cat. no.
9692) and CD133 (cat. no. 3570S) (Cell Signaling Technology, Inc.,
Danvers, MA. USA); antibodies targeting p-ACC (cat. no. sc-271965;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and human β-actin
(cat. no. A4700; Sigma Chemicals; Merck KGaA, Darmstadt, Germany)
were employed as well. Other reagents included Invitrogen™
Lipofectamine 2000 (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) and the growth supplements B-27 and N-2 (Invitrogen; Thermo
Fisher Scientific, Inc.).
Sphere culture and self-renewal
assay
To obtain spheres, the cells were cultured in stem
cell-conditioned medium (DMEM/F12 medium supplemented with 0.02×
B27, 20 ng/ml EGF, 20 ng/ml bFGF, 0.4% BSA, 4 µg/ml insulin, 100
U/ml penicillin and 100 µg/ml streptomycin (Invitrogen; Thermo
Fisher Scientific, Inc.) in ultra-low attachment 6-well plates
(Corning Inc., Corning, NY, USA). When spheres reached ≥20 cells,
the suspension cultures were passaged every six days. Spheres were
counted in 10 different high power fields using an inverted
microscope (Nikon TS100; Nikon, Tokyo, Japan).
For future generation of spheres in vitro,
sphere cells were collected by gentle centrifugation at 200 × g for
10 min, dissociated into single-cell suspensions, and cultured to
allow sphere regeneration.
To determine the sphere-formation rate, the
dissociated cells or second-generation spheres treated with
casticin (final concentrations of 1.0, 3.0 and 10.0 µmol/l,
respectively) were seeded at a density of 1,000 cells/ml in 6-well
plates to generate new spheres. The total number of spheres was
recorded after 6 days of culture. Sphere formation rate was
calculated by dividing the total number of spheres formed by that
of live cells seeded multiplied by 100.
Clonogenic assay on soft agar
Each well of a 6-well culture plate was coated with
2 ml bottom agar-medium mixture (DMEM, 10% FBS, and 0.6% agar).
After solidification, 2 ml top agar-medium mixture (DMEM, 10% FBS,
and 0.3% Noble agar; BD Difco™; BD Biosciences, Franklin Lakes, NJ,
USA) containing 1,000 cells treated as described above were added.
After 14 days, the colonies formed (≥20 cells) were counted under
an inverted fluorescent microscope (Olympus CK40; Olympus Corp.,
Tokyo, Japan), with the representative views imaged. Colony
formation rate was calculated by dividing the total number of
colonies formed by that of live cells seeded multiplied by 100.
Silencing by siRNA
Dissociated second-generation spheres were plated at
2.5×105 cells/well in 6-well plates. After 24 h, the
siRNA-negative control (si-NC; Santa Cruz Biotechnology, Inc.) and
AMPK- or FoxO3a-specific siRNAs (siAMPK or siFoxO3a; Shanghai
GenePharma Co., Ltd., Shanghai, China) were transfected into cells,
respectively, using Invitrogen™ Lipofectamine 2000 reagent (Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. Separate siRNAs were used for FoxO3A
(5′-GACAAUAGCAACAAGUAUA-3′) and AMPK (5′-GAGGAGCUAUUUGAUUA-3′)
(16). On-TARGET-plus control
siRNAs (Thermo Fisher Scientific, Inc.) were used as control
sequences.
Western blot analysis
Western blot analysis was carried out as described
by Liu et al (17). Primary
antibodies raised against AMPKα, p-AMPK, FoxO3a, p-FoxO3a, uPAR,
ALDH1, Bmi1, SOX2 and β-actin were used. Cells were lysed in lysis
buffer for 20 min at 4°C. Protein amounts were determined with the
Bio-Rad assay system (Bio-Rad Laboratories, Hercules, CA, USA).
Equal amounts (50 µg) of total protein were fractionated by
SDS-PAGE and transferred onto polyvinylidene fluoride membranes
(PVDF) (Millipore, Billerica, MA, USA). Signals were detected using
an ECL Advance Western blot analysis system (Amersham Pharmacia
Biotech Inc., Piscataway, NJ, USA). Experiments were carried out in
triplicate (ImageJ v.1.84; National Institutes of Health, Bethesda,
MD, USA).
Immunohistochemical (IHC)
analysis
Four micron-thick tissue sections were immunostained
with uPAR-specific antibody. Immunostaining was performed using a
Ventana Discovery Ultra (Ventana Medical Systems, Tucson, AZ, USA).
Antigen retrieval was performed using CC1 for 40 min at 95°C. IHC
staining was followed by hematoxylin counterstaining. Slides were
rinsed, dehydrated though alcohol and xylene and coverslipped.
In vivo tumorigenesis assessment
Twenty-four pathogen-free BALB/c-nu female mice
(13–15 g) aged 4 weeks were purchased from the Animal Institute of
the Chinese Academy of Medical Science (CAMS). All animal studies
were performed in accordance with the standard protocols, and
approved by the Ethics Committee of Hunan Normal University and the
Committee of Experimental Animal Feeding and Management. Mice were
randomly divided into 3 groups (4 mice/group), and maintained under
standard conditions. Varying amounts of H446 cells (103,
104 and 105, respectively) were
subcutaneously injected into 4-week-old female nude mice in the
left flank; in parallel, second generation sphere-derived cells
(LCSLCs, 102, 103 and 104,
respectively) were subcutaneously injected into the right flank.
Tumor growth was monitored visually every week, and the maximum
tumor volume allowed was consistent with the IACUC guidelines
(diameter, 1.5 cm; area, 1.8 cm2; volume 1.8
cm3). Tumor volumes were calculated in accordance with
the formula: V (transplanted tumor volume, mm3) = L
(longest diameter, mm) × W (minimum diameter, mm)2 ×
0.5. After 8 weeks of tumor growth, the mice were euthanized using
cervical vertebra luxation. The obtained tumor tissues were fixed
in formalin and embedded in paraffin. Hematoxylin and eosin
(H&E) staining and immunohistochemical analysis were performed
to assess tumor histology and tumor markers in the mouse
xenografts.
Statistical analysis
SPSS 20.0 software (IBM Corp., Armonk, NY, USA) was
used for analysis. Data are expressed as the mean ± standard
deviation (SD); n=number of measurements. Multiple groups
were compared by one-way analysis of variance (ANOVA); comparisons
of group means were performed by the LSD method for normally
distributed variables. Two-tailed t-test was also used as
appropriate. P<0.05 was considered statistically
significant.
Results
Spheres from the H446 cell line show
cancer stem-like cell characteristics
To evaluate the sphere-forming capabilities of the
SCLC H446, H209 and H69 cell lines, sphere-formation rates of the
three cell lines were assessed. The results showed that the
sphere-forming capability of H446 cells was higher than that of
both H209 and H69 cell lines (Fig.
1A). To determine the capability of H446, H209 and H69 cells
for self-renewal initiation, these cells were submitted to several
serial passages. As shown in Fig.
1B, sphere-formation rate was highest for second-generation
spheres at the third sphere-formation process in the H446 cell line
compared with other cell lines or spheres of another generation.
Therefore, second-generation spheres of H446 cells were considered
to be lung cancer stem-like cells (LCSLCs), and used for subsequent
experiments.
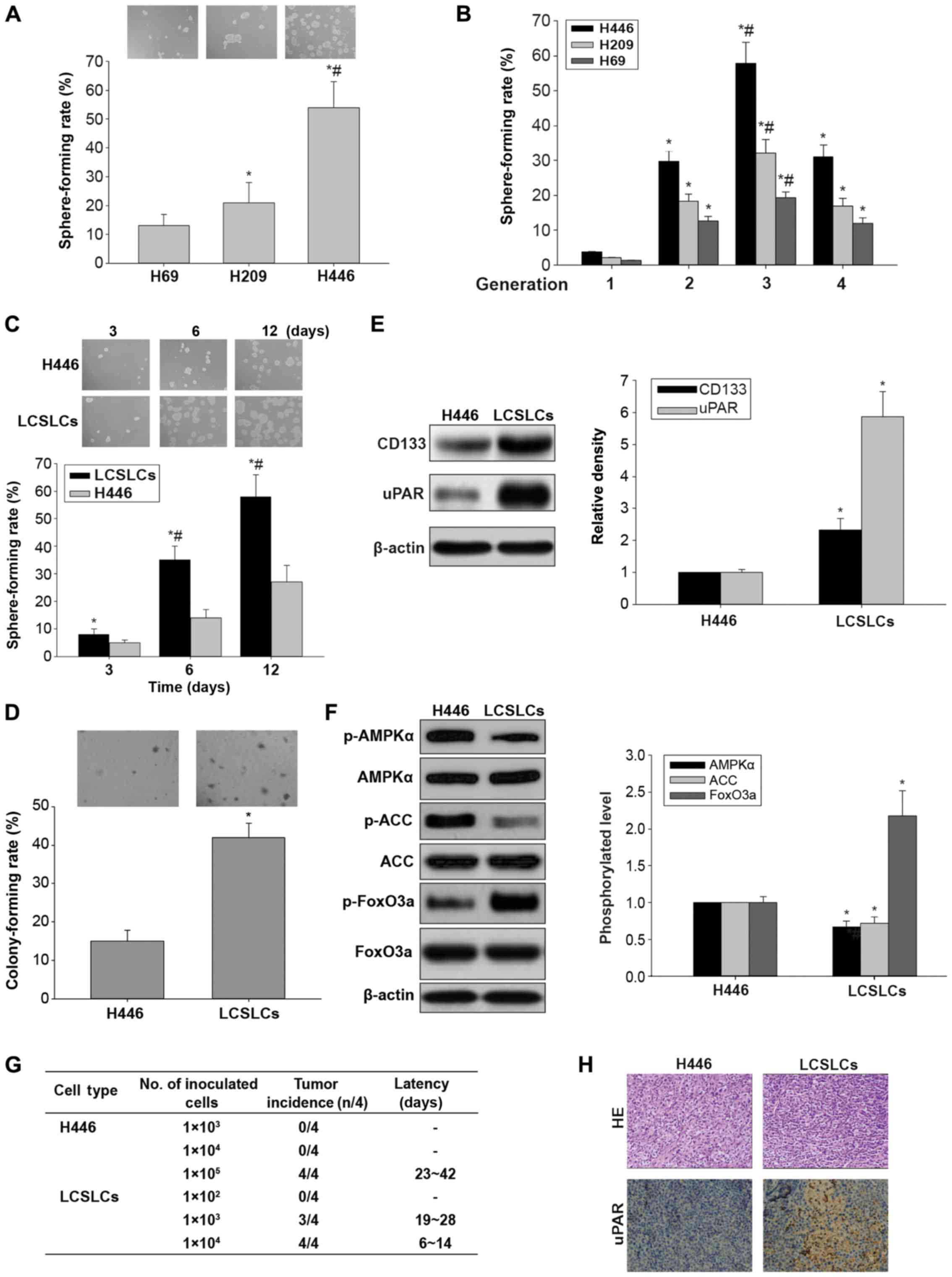 | Figure 1.In vitro and in vivo
carcinogenesis and AMPK/FoxO3a signaling activation in LCSLCs and
H446 cells. (A) Representative micrographs of spheres obtained
under a phase contrast microscope (magnification, ×10, upper panel)
and sphere-forming rates in H69, H209 and H446 cells (lower panel).
*P<0.05 vs. H69 cells. #P<0.05 vs. H209 cells. (B)
Sphere forming rates of H69, H209, and H446 in different
generations. *P<0.05 vs. first generation. #P<0.05
vs. second or fourth generation. (C) Representative micrographs of
spheres obtained under a phase contrast microscope (magnification,
×10, upper panel) and sphere-forming rates in LCSLCs and H446 cells
(lower panel) at 3, 6 and 12 days, respectively. *P<0.05 vs.
H446 cells at the same time point. #P<0.05 vs. LCSLCs
at 3 days. (D) Representative micrographs of colonies obtained
under a phase contrast microscope (magnification, ×10, upper panel)
and colony-forming rates in LCSLCs and H446 cells (lower panel).
(E) Representative western blot bands (left panel), and
quantitative analysis of CD133 and uPAR protein expression levels
in LCSLCs and H446 cells (right panel). (F) Representative western
blot bands (left panel), and phosphorylation levels of AMPKα, ACC
and FoxO3a in LCSLCs and H446 cells (right panel). *P<0.05 vs.
H446 cells. (G) Ability of LCSLCs and H446 cells to form tumors in
BALB/c-nu mice. (H) Hematoxylin and eosin (H&E) staining and
uPAR immunohistochemistry of tumor xenografts derived from LCSLCs
and H446 cells. LCSLCs, lung cancer stem-like cells. |
We next compared the in vitro oncogenic
capabilities of LCSLCs and H446 cells by sphere- and colony
formation assays. The results showed that the size and population
of tumor spheres from LCSLCs were larger than the values obtained
for H446 cells (Fig. 1C). In
addition, the colony formation ability was significantly enhanced
in LCSLCs compared with H446 cells (Fig. 1D). These data suggested that LCSLCs
had the capacity of self-renewal and oncogenic capabilities in
vitro, and were highly enriched in second-generation spheres of
H446 cells.
To further confirm the stem-like properties of
LCSLCs, the protein expression levels of SCLC CSC-related markers
were assessed in LCSLCs and H446 cells. Western blot analysis
demonstrated that the protein expression levels of uPAR and CD133
were higher in the LCSLCs than these levels in the H446 cells
(Fig. 1E).
To explore the role of AMPK/FoxO3a signaling in the
in vitro carcinogenesis of H446-derived LCSLCs, the
phosphorylated protein levels of AMPK, ACC and FoxO3a were
determined. As shown in Fig. 1F,
the phosphorylation levels of AMPK and ACC were lower, while FoxO3a
protein phosphorylation was increased, in LCSLCs compared with H446
cells. These results suggest that inactivation of AMPK and FoxO3a
were associated with carcinogenesis maintenance in vitro and
the stem-like properties of H446-derived LCSLCs.
In addition, the abilities of LCSLCs and H446 cells
to form tumors in BALB/c-nu mice were assessed. As many as
1×105 H446 cells were required to initiate stable tumor
formation for 23–42 days after injection, while, as few as
1×103 LCSLCs were sufficient to generate visible tumors
only 19–28 days post-injection (Fig.
1G). Furthermore, H&E staining revealed similar
histological characteristics in tumor xenografts derived from
LCSLCs and H446 cells (Fig. 1H).
Furthermore, uPAR protein amounts were higher in transplanted
tumors of LCSLCs than those of H446 cells, as indicated by
immunohistochemistry (Fig. 1H).
These results provide sufficient evidence that H446
second-generation spheres possess LCSLC features such as increased
in vitro carcinogenesis and in vivo tumorigenic
potential.
Casticin inhibits in vitro
carcinogenesis and activates AMPK/FoxO3a signaling in H446-derived
LCSLCs
Similar to our previous findings (17), this study demonstrated that the
indicated concentrations (1.0, 3.0 and 10.0 µmol/l) of casticin
suppressed self-renewal capability by reducing the sphere-formation
rate in H446-derived LCSLCs (Fig.
2A), in a dose-dependent manner. In addition, colony formation
assay on soft agar showed that casticin significantly inhibited the
colony forming ability of H446-derived LCSLCs (Fig. 2B), in a dose-dependent manner. To
further confirm the inhibitory effects of casticin on CSC
characteristics, we next assessed the expression levels of SCLC CSC
bio-markers, including uPAR and CD133, in H446-derived LCSLCs
treated with casticin at the indicated concentrations. The results
showed that the protein levels of uPAR and CD133 were
dose-dependently reduced by casticin (Fig. 2C). Taken together, these findings
demonstrated that casticin could inhibit in vitro
carcinogenesis and CSC characteristics in H446-derived LCSCs.
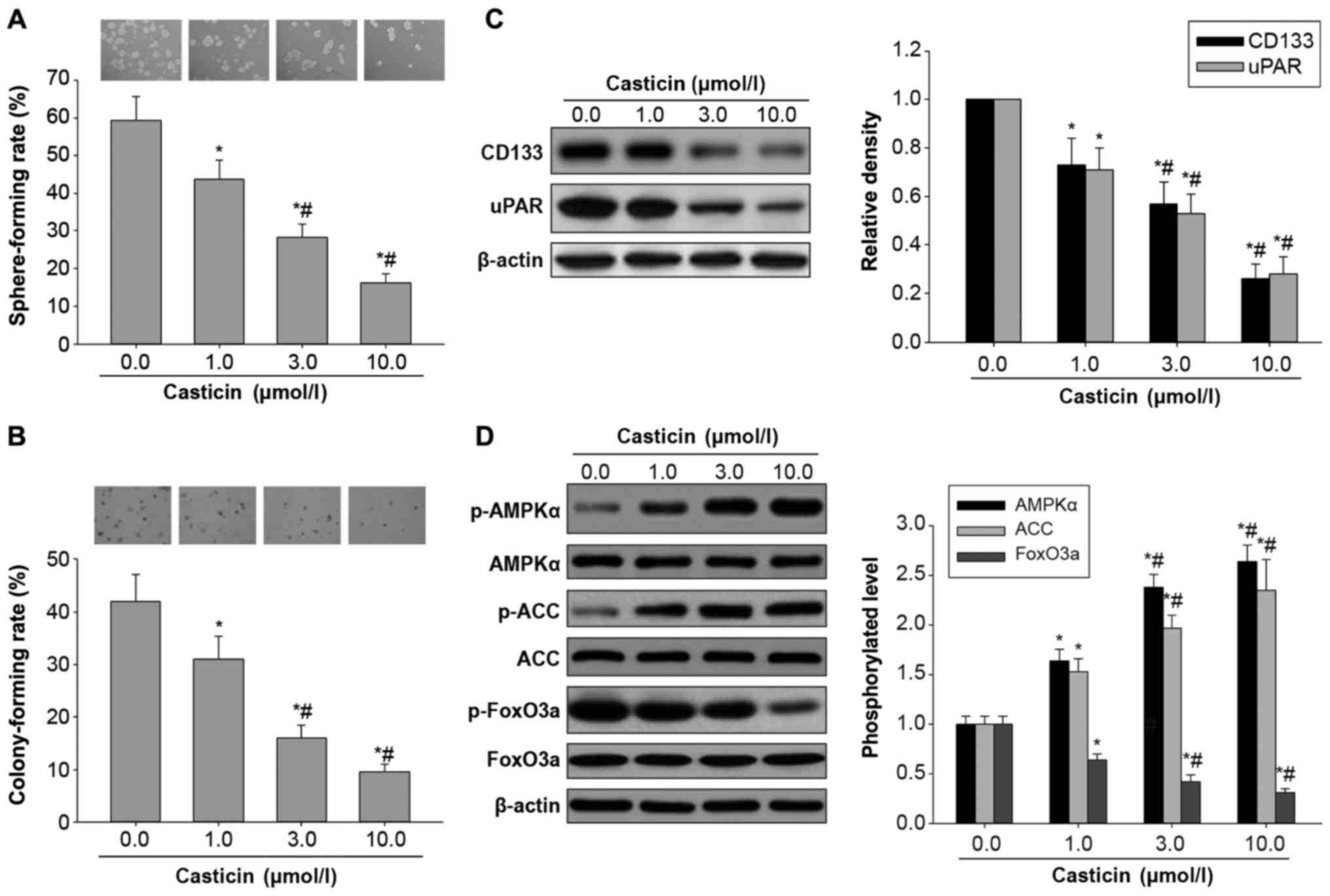 | Figure 2.Effects of casticin on in
vitro carcinogenesis and AMPK/FoxO3a signaling activation in
H446-derived LCSLCs. (A) Representative micrographs of spheres
obtained under a phase contrast microscope (magnification, ×10,
upper panel) and sphere-forming rates in H446-derived LCSLCs
treated with casticin (0.0, 1.0, 3.0 and 10.0 µmol/l) (lower
panel). (B) Representative micrographs of colonies obtained under a
phase contrast microscope (magnification, ×10, upper panel) and
colony-forming rates in H446-derived LCSLCs treated with casticin
(0.0, 1.0, 3.0 and 10.0 µmol/l) (lower panel). (C) Representative
western blot bands (left panel) and quantitative analysis of CD133
and uPAR protein expression levels in H446-derived LCSLCs treated
with casticin (0.0, 1.0, 3.0 and 10.0 µmol/l) (right panel). (D)
Representative western blot bands (left) and phosphorylation levels
of AMPKα, ACC and FoxO3a in H446-derived LCSLCs treated with
casticin (0.0, 1.0, 3.0 and 10.0 µmol/l) (right panel). *P<0.05
vs. untreated group. #P<0.05 vs. LCSLCs treated with
1.0 µmol/l casticin. LCSLCs, lung cancer stem-like cells. |
To test the hypothesis that casticin inhibits in
vitro carcinogenesis and CSC characteristics in H446-derived
LCSCs through activation of AMPK/FoxO3a signaling, the effects of
casticin on the phosphorylated protein levels of AMPK, ACC and
FoxO3a were evaluated by western blot analysis. As shown in
Fig. 2D, treatment with casticin
resulted in significantly elevated phosphorylation levels of AMPK
and ACC, and reduced FoxO3a phosphorylation, in a
concentration-dependent manner. Collectively, these findings
indicated that casticin inhibited in vitro carcinogenesis
and CSC characteristics of H446-derived LCSCs, likely involving the
activation of AMPK/FoxO3a signaling.
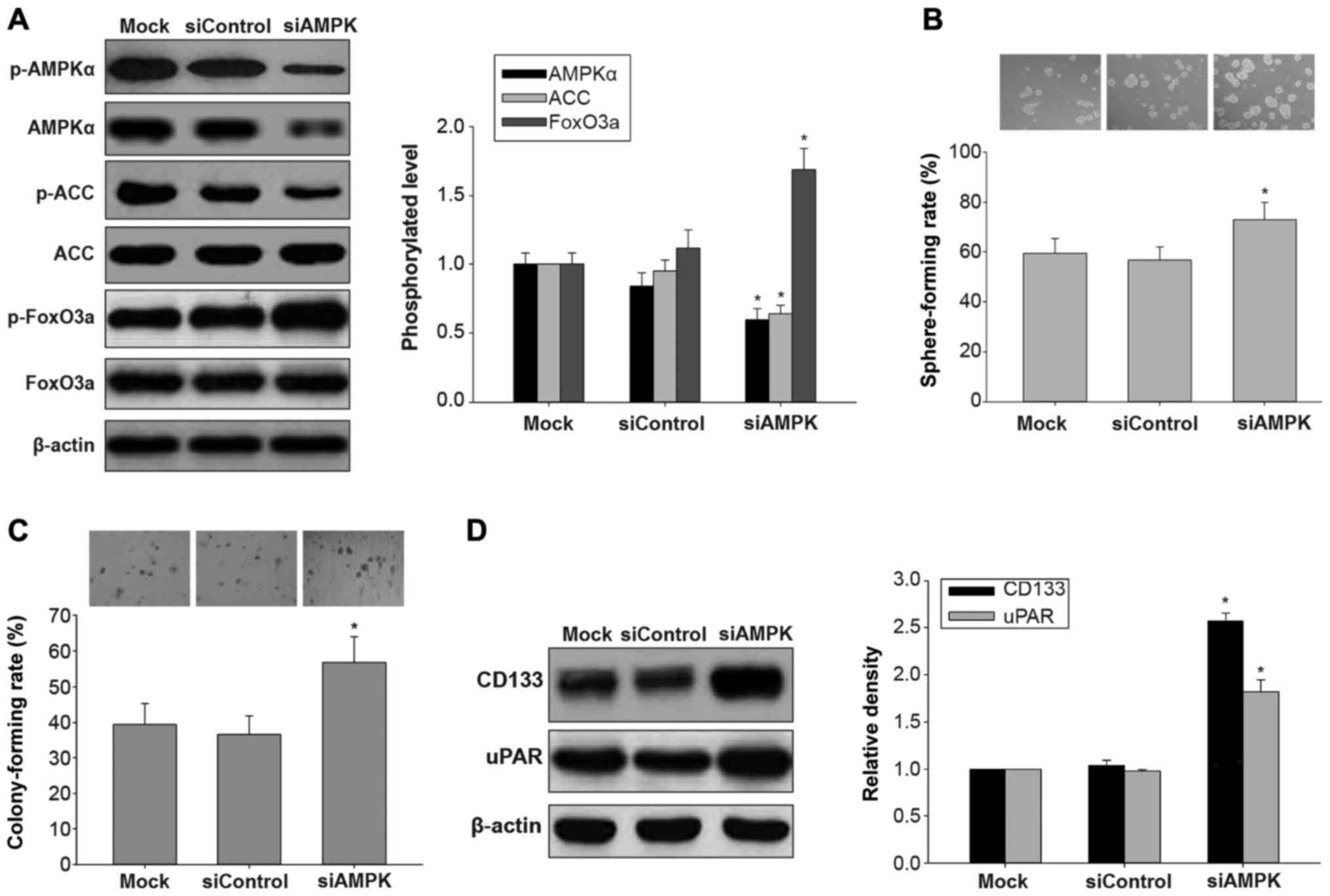
Effects of AMPK silencing on in vitro
carcinogenesis and AMPK/FoxO3a signaling in H446-derived
LCSLCs
AMPK is a central cellular energetic biosensor that
regulates a broad array of cellular metabolic routes activated by
nutrient deprivation, mitochondrial dysfunction, oxidative stress
and cytokines (18). Previous
studies have demonstrated that casticin induces apoptosis through
reactive oxygen species-mediated mitochondrial signaling pathways
in cervical (19) and lung
(20) cancers. Therefore, we
hypothesized that the inhibitory effects of casticin on
H446-derived LCSLCs may also involve AMPK activation. To test this,
we firstly knocked down AMPK with siRNA in H446-derived LCSLCs, and
evaluated the phosphorylated protein levels of AMPK, ACC and
FoxO3a. As shown in Fig. 3A,
transfection with AMPK siRNA resulted in reduced AMPK protein
expression, decreased ACC phosphorylation, and increased
phosphorylation levels of FoxO3a, compared with the untreated or
control siRNA-treated H446-derived LCSLCs. Moreover, AMPK knockdown
enhanced sphere and colony formation abilities, compared with the
untreated or control siRNA-treated H446-derived LCSLCs (Fig. 3B and C). Accordingly, the protein
expression levels of uPAR and CD133 were increased in the
H446-derived LCSLCs transfected with AMPK siRNA compared with the
untreated or control siRNA-treated counterparts (Fig. 3D). These results suggested that AMPK
knockdown blocked AMPK/FoxO3a signaling, and enhanced in
vitro carcinogenesis and CSC characteristics in H446-derived
LCSLCs.
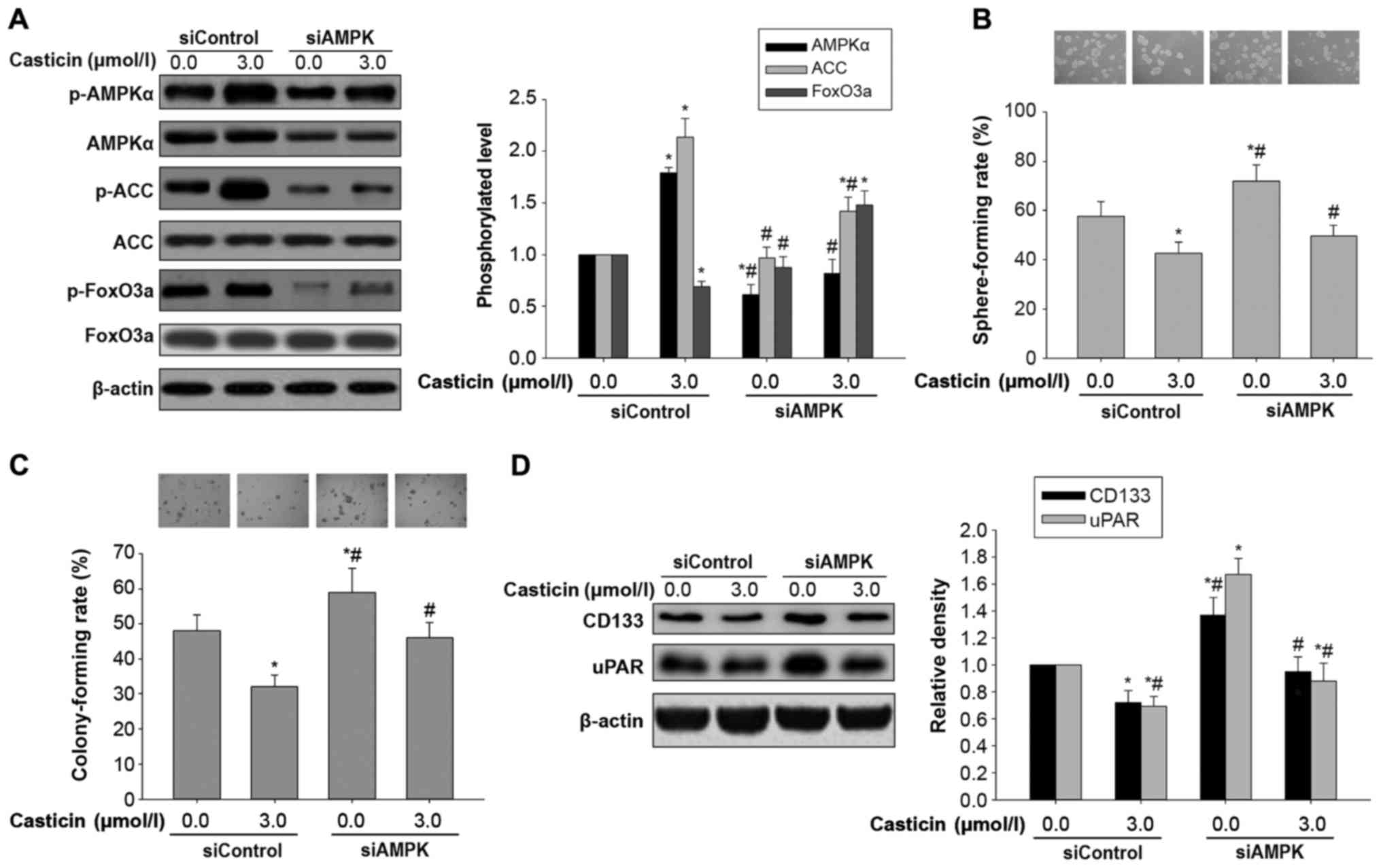
Effects of casticin on in vitro
carcinogenesis and AMPK/FoxO3a signaling activation in H446-derived
LCSLCs transfected with AMPK siRNA
To assess whether the inhibitory effects of casticin
on oncogenicity in H446-derived LCSLCs is affected by AMPK
regulation, casticin (0 or 3.0 µmol/l) was administered to
H446-derived LCSLCs transfected with AMPK- and control siRNAs,
respectively. Compared with the control siRNA group, AMPK silencing
not only reduced AMPK levels and ACC phosphorylation, but also
antagonized elevated phosphorylation levels of AMPK and ACC in
response to casticin administration (3.0 µmol/l) in H446-derived
LCSLCs (Fig. 4A). In addition, AMPK
knockdown also elevated the phosphorylation levels of FoxO3a, while
attenuating the reduction of FoxO3a phosphorylation after treatment
with casticin (3.0 µmol/l) in H446-derived LCSLCs (Fig. 4A). Furthermore, AMPK knockdown not
only enhanced self-renewal and colony formation capabilities, but
also counteracted the inhibitory effects of casticin on
carcinogenesis in H446-derived LCSLCs in vitro (Fig. 4B and C). Meanwhile, transfection
with AMPK siRNA resulted in increased uPAR and CD133 protein
levels, reversing the inhibitory effects of casticin on uPAR and
CD133 expression in H446-derived LCSLCs (Fig. 4D). These results suggested that AMPK
activation may be upstream of FoxO3a activation in response to
casticin stimulation in H446-derived LCSLCs.
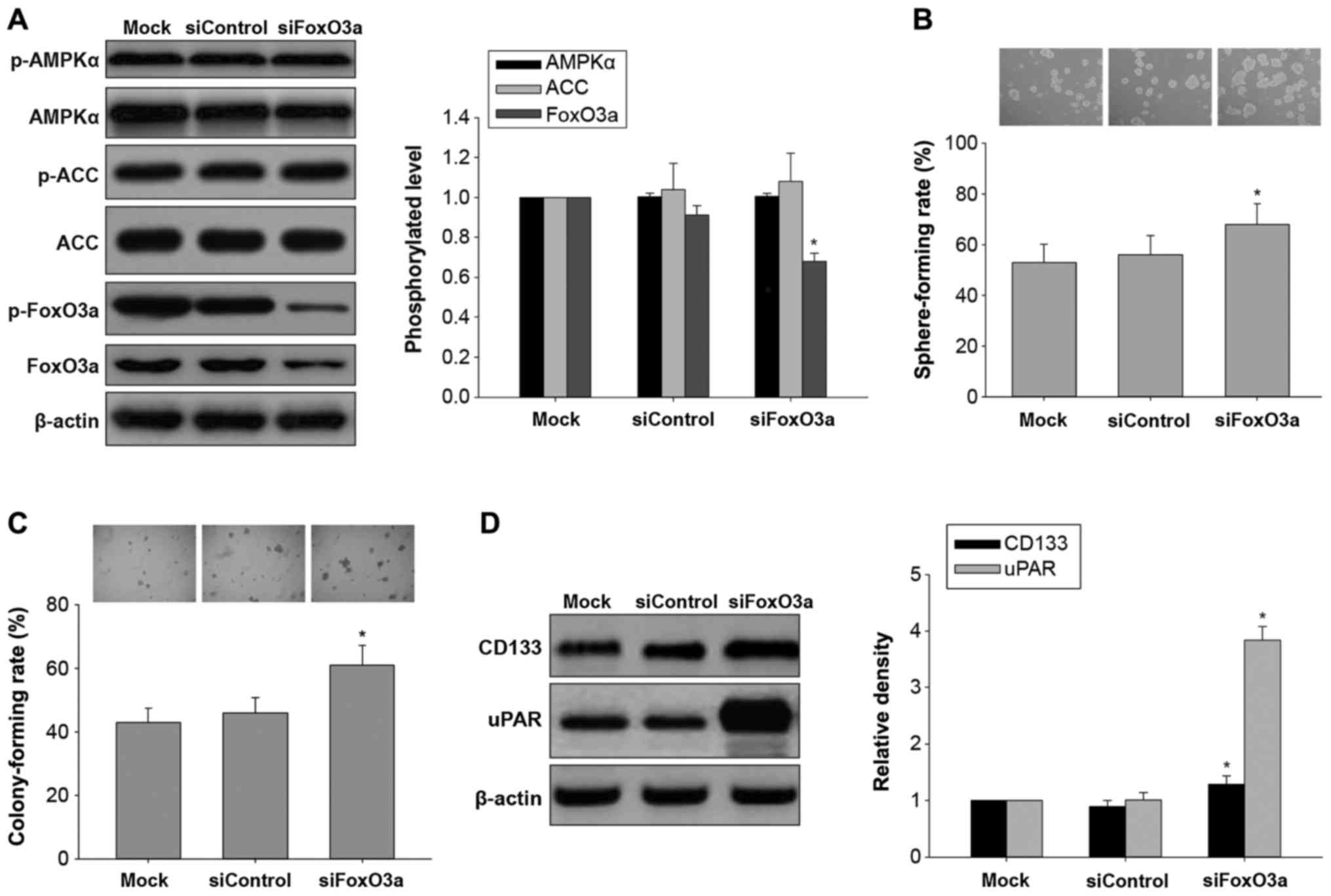
Effects of FoxO3a silencing on in
vitro carcinogenesis and AMPK/FoxO3a signaling activation in
H446-derived LCSLCs
FoxO3a, a downstream effector of AMPK, is associated
with a variety of cell processes, including cell cycle progression,
apoptosis, stress, detoxification, DNA repair, glucose metabolism
and differentiation (21). To
assess whether the in vitro effects of casticin on
carcinogenesis and CSC characteristics in H446-derived LCSLCs
involve AMPK/FoxO3a signaling, FoxO3a was silenced. As shown in
Fig. 5A, FoxO3a knockdown mainly
resulted in decreased FoxO3a protein levels, with no effects on
AMPK and ACC levels or phosphorylation. Furthermore, FoxO3a
knockdown increased sphere and colony formation rates (Fig. 5B and C) as well as uPAR and CD133
protein levels (Fig. 5D) in
H446-derived LCSLCs. These results indicated that activation of
FoxO3a could inhibit in vitro carcinogenesis and CSC
characteristics in H446-derived LCSLCs, and FoxO3a may be a
downstream target of AMPK.
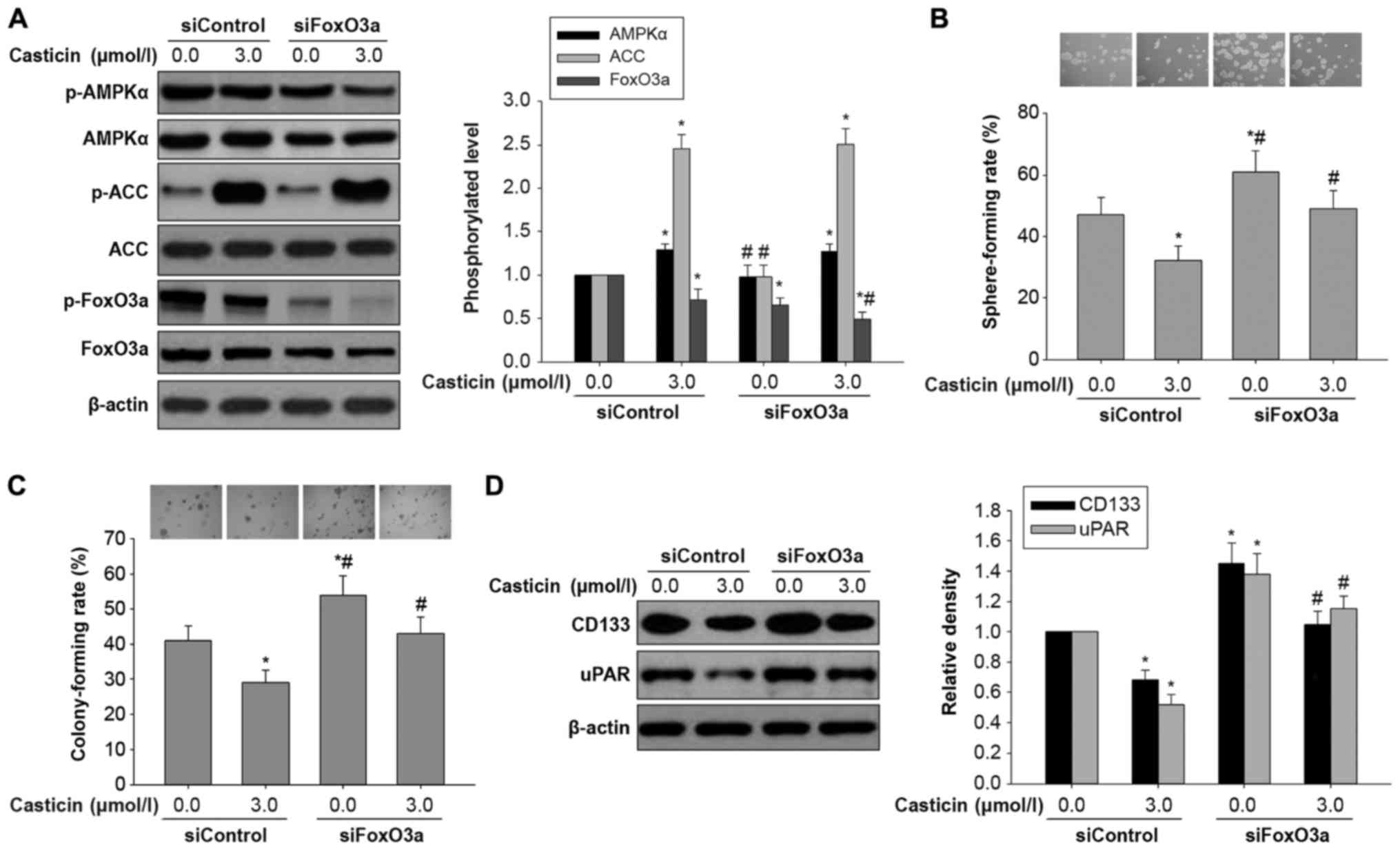
Effects of casticin on in vitro
carcinogenesis and AMPK/FoxO3a signaling activation in H446-derived
LCSLCs transfected with FoxO3a siRNA
To further assess whether the effects of casticin on
in vitro carcinogenesis and CSC characteristics are mediated
by AMPK/FoxO3a signaling, casticin (0 or 3.0 µmol/l) was
administered to H446-derived LCSLCs transfected with FoxO3a or
control siRNA. As expected, FoxO3a knockdown synergistically
reduced FoxO3a protein phosphorylation, with slight effects on
elevated AMPK and ACC phosphorylation levels associated with
casticin (3.0 µmol/l) in H446-derived LCSLCs (Fig. 6A). As shown in Fig. 6B and C, FoxO3a silencing neutralized
the inhibitory effects of casticin (3.0 µmol/l) on sphere and
colony formation abilities in H446-derived LCSLCs. Consistently,
FoxO3a knockdown weakened the decreased expression levels of uPAR
and CD133 observed in H446-derived LCSLCs in response to treatment
with casticin (3.0 µmol/l) (Fig.
6D). These findings clearly indicated that the inhibitory
effects of casticin on in vitro carcinogenesis and CSC
properties were mediated by AMPK/FoxO3a signaling in H446-derived
LCSLCs.
Discussion
The present study confirmed that casticin reduced
the in vitro carcinogenesis and cancer stem cell (CSC)
characteristics, and the molecular mechanism involved activation of
AMPK/FoxO3 signaling in H446-derived lung cancer stem-like cells
(LCSLCs). CSCs are a small population of tumor cells with the
properties of self-renewal, multi-differentiation potential, and
high aggressiveness and tumorigenesis in vivo (4–6). This
study firstly examined second-generation spheres from the H446 cell
line, namely, H446-derived LCSLCs, using sphere- and
colony-formation assays, western blot analysis and xenografts in
nude mice. The H446-derived LCSLCs were characterized by stronger
sphere- and colony-forming capacities and overexpression of SCLC
CSC biomarkers, including uPAR and CD133, compared with H446 cells.
In addition, xenograft assays in nude mice provided important
evidence that H446-derived LCSLCs had higher capability of
tumorigenesis in vivo compared with H466 cells. Therefore,
second-generation spheres from the H446 cell line could be
considered as LCSLCs.
He et al (22) found that casticin inhibited
epithelial-mesenchymal transition (EMT) in liver cancer stem cells
from the SMMC-7721 cell line by downregulating Twist. He et
al (23) demonstrated casticin
also suppressed self-renewal ability in liver cancer stem cells
from the MHCC97 cell line. Our previous study showed that casticin
suppressed the self-renewal and invasion abilities of LCSLCs from
NSCLC A549 cells through p-Akt downregulation (17). The present study demonstrated that
casticin efficaciously reduced sphere and colony formation
abilities, and downregulated SCLC CSC biomarkers such as uPAR and
CD133, in H446-derived LCSLCs. These results clearly indicated that
casticin reduced in vitro carcinogenesis and CSC
characteristics in SCLC LCSLCs. However, the molecular mechanism by
which casticin inhibits SCLC LCSLCs remained poorly understood.
Given that casticin suppresses cell growth in
ovarian cancer (SKOV3) cells through FOXO3a activation (13), we aimed to ascertain whether
casticin inhibits in vitro carcinogenesis and CSC
characteristics in SCLC LCSLCs by activating FOXO3a, a
transcription factor inactivated by AMPK (14–16,24).
We first found that casticin significantly increased the
phosphorylation levels of AMPK and ACC, in a
concentration-dependent manner. These findings indicated that
casticin increased AMPK activity. More importantly, AMPK knockdown
not only reduced AMPK expression and ACC phosphorylation levels,
but also antagonized the elevated phosphorylation levels of AMPK
and ACC in response to casticin administration in H446-derived
LCSLCs. Therefore, these findings suggest that AMPK activation may
be required for response to casticin stimulation in H446-derived
LCSLCs.
It was reported that AMPK acts as an upstream
regulator of Foxo3a (8–10,14–16,24,25).
This study provided several lines of evidence that casticin
activates Foxo3a in an AMPK-dependent manner. Indeed, AMPK
knockdown resulted in elevated phosphorylation levels of FoxO3a,
and attenuated the decreased FoxO3a phosphorylation associated with
casticin administration in H446-derived LCSLCs. In addition, FoxO3a
silencing mainly led to decreased FoxO3a protein levels, with no
effects on AMPK and ACC expression and phosphorylation associated
with casticin administration. Mechanistically, casticin had no
effect on AMPK protein expression, but suppressed AMPK-mediated
FoxO3a phosphorylation. Functionally, FoxO3a knockdown mimicked the
effects AMPK knockdown on sphere- and colony-forming capabilities,
as well as on protein expression levels of the CSC markers uPAR and
CD133, in H446-derived LCSLCs. These findings suggest that the
inhibitory effects of casticin on in vitro carcinogenesis
and CSC properties may be mediated by activation of AMPK/FoxO3a
signaling in H446-derived LCSLCs.
Overexpression of uPAR is strongly correlated with
the malignant cancer phenotype and poor prognosis (26,27).
In breast cancer, elevated uPAR expression is an independent
prognostic marker of reduced relapse-free survival (27). Consistently, high uPAR levels in
primary tumors indicate aggressive gastric cancer (28). LeBeau et al and others
demonstrated that a stem cell-like cell population may be enriched
in spheres expressing the uPAR cell surface marker in H446 cells
(27,29). Furthermore, inhibition of uPAR
results in prominently increased p27Kip1 expression,
which correlates with decreased p-PI3K and p-AKT levels, as well as
increased FOXO3a protein amounts (30,31).
The current findings suggest that the inhibitory effects of
casticin on in vitro carcinogenesis and CSC properties
involve uPAR downregulation. However, the interaction of FoxO3a
with uPAR warrants further investigation.
In summary, this study firstly provided evidence
that AMPK plays a role in regulating in vitro carcinogenesis
and CSC properties by activating FoxO3a in H446-derived LCSLCs. In
addition, the above findings indicated that the inhibitory effects
of casticin on in vitro carcinogenesis and CSC properties in
H446-derived LCSLCs, including sphere and colony formation
abilities, and increased levels of CSC biomarkers (e.g. uPAR and
CD133), are associated with activation of AMPK/FoxO3a signaling.
Overall, this study raises the novel and intriguing idea that
interventions to modulate AMPK, a known cellular metabolic sensor
by casticin, could contribute to preventing and treating human SCLC
by targeting LCSLCs.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Project of
NSFC (nos. 30760248 and 81172375), the Project of Scientific
Research of Hunan Province, granted by the Health and Family
Planning Commission of Hunan Province (no. B2013-098).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
Professor JC conceived and designed the study. QG,
XC and XY performed the experiments. QG and XC wrote the
manuscript. Professor JC and WZ reviewed and edited the manuscript.
Professor WZ was also involved in the conception of the study. All
authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
All animal studies were performed in accordance with
the standard protocols, and approved by the Ethics Committee of
Hunan Normal University and the Committee of Experimental Animal
Feeding and Management.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of
interest.
References
|
1
|
DeSantis CE, Lin CC, Mariotto AB, Siegel
RL, Stein KD, Kramer JL, Alteri R, Robbins AS and Jemal A: Cancer
treatment and survivorship statistics, 2014. CA Cancer J Clin.
64:252–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zakaria N, Satar NA, Abu Halim NH, Ngalim
SH, Yusoff NM, Lin J and Yahaya BH: Targeting lung cancer stem
cells: Research and clinical impacts. Front Oncol. 7:802017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang Z, Zhou Y, Qian H, Shao G, Lu X,
Chen Q, Sun X, Chen D, Yin R, Zhu H, et al: Stemness and inducing
differentiation of small cell lung cancer NCI-H446 cells. Cell
Death Dis. 4:e6332013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rahman M, Deleyrolle L, Vedam-Mai V, Azari
H, Abd-El-Barr M and Reynolds BA: The cancer stem cell hypothesis:
Failures and pitfalls. Neurosurgery. 68:531–545. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tysnes BB and Bjerkvig R: Cancer
initiation and progression: Involvement of stem cells and the
microenvironment. Biochim Biophys Acta. 1775:283–297.
2007.PubMed/NCBI
|
|
6
|
Qiu X, Wang Z, Li Y, Miao Y, Ren Y and
Luan Y: Characterization of sphere-forming cells with stem-like
properties from the small cell lung cancer cell line H446. Cancer
Lett. 323:161–170. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xiao J, Mu JS, Liu TR and Xu HN: Dig the
root of cancer: Targeting cancer stem cells therapy. J Med Discov.
2:1–6. 2017.
|
|
8
|
Sato A, Sunayama J, Okada M, Watanabe E,
Seino S, Shibuya K, Suzuki K, Narita Y, Shibui S, Kayama T, et al:
Glioma-initiating cell elimination by metformin activation of FOXO3
via AMPK. Stem Cells Transl Med. 1:811–824. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yung MM, Chan DW, Liu VW, Yao KM and Ngan
HY: Activation of AMPK inhibits cervical cancer cell growth through
AKT/FOXO3a/FOXM1 signaling cascade. BMC Cancer. 13:3272013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Queiroz EA, Puukila S, Eichler R, Sampaio
SC, Forsyth HL, Lees SJ, Barbosa AM, Dekker RF, Fortes ZB and
Khaper N: Metformin induces apoptosis and cell cycle arrest
mediated by oxidative stress, AMPK and FOXO3a in MCF-7 breast
cancer cells. PLoS One. 9:e982072014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He L, Yang X, Cao X, Liu F, Quan M and Cao
J: Casticin induces growth suppression and cell cycle arrest
through activation of FOXO3a in hepatocellular carcinoma. Oncol
Rep. 29:103–108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu LP, Cao XC, Liu F, Quan MF, Sheng XF
and Ren KQ: Casticin induces breast cancer cell apoptosis by
inhibiting the expression of forkhead box protein M1. Oncol Lett.
7:1711–1717. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang L, Cao XC, Cao JG, Liu F, Quan MF,
Sheng XF and Ren KQ: Casticin induces ovarian cancer cell apoptosis
by repressing FoxM1 through the activation of FOXO3a. Oncol Lett.
5:1605–1610. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shrestha A, Nepal S, Kim MJ, Chang JH, Kim
SH, Jeong GS, Jeong CH, Park GH, Jung S, Lim J, et al: Critical
role of AMPK/FoxO3A axis in globular adiponectin-induced cell cycle
arrest and apoptosis in cancer cells. J Cell Physiol. 231:357–369.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao Y, Sun Y, Ding Y, Wang X, Zhou Y, Li
W, Huang S, Li Z, Kong L, Guo Q, et al: GL-V9, a new synthetic
flavonoid derivative, ameliorates DSS-induced colitis against
oxidative stress by up-regulating Trx-1 expression via activation
of AMPK/FOXO3a pathway. Oncotarget. 6:26291–26307. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zheng F, Wu J, Zhao S, Luo Q, Tang Q, Yang
L, Li L, Wu W and Hann SS: Baicalein increases the expression and
reciprocal interplay of RUNX3 and FOXO3a through crosstalk of
AMPKalpha and MEK/ERK1/2 signaling pathways in human non-small cell
lung cancer cells. J Exp Clin Cancer Res. 34:412015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu F, Cao X, Liu Z, Guo H, Ren K, Quan M,
Zhou Y, Xiang H and Cao J: Casticin suppresses self-renewal and
invasion of lung cancer stem-like cells from A549 cells through
down-regulation of pAkt. Acta Biochim Biophys Sin. 46:15–21. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bonini MG and Gantner BN: The multifaceted
activities of AMPK in tumor progression-why the ‘one size fits all’
definition does not fit at all? IUBMB Life. 65:889–896. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen D, Cao J, Tian L, Liu F and Sheng X:
Induction of apoptosis by casticin in cervical cancer cells through
reactive oxygen species-mediated mitochondrial signaling pathways.
Oncol Rep. 26:1287–1294. 2011.PubMed/NCBI
|
|
20
|
Zhou Y, Peng Y, Mao QQ, Li X, Chen MW, Su
J, Tian L, Mao NQ, Long LZ, Quan MF, et al: Casticin induces
caspase-mediated apoptosis via activation of mitochondrial pathway
and upregulation of DR5 in human lung cancer cells. Asian Pac J
Trop Med. 6:372–378. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Myatt SS and Lam EW: The emerging roles of
forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 7:847–859.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
He M, Cao XC, He GC, Sheng XF, Ai XH and
Wu YH: Casticin inhibits epithelial-mesenchymal transition of liver
cancer stem cells of the SMMC-7721 cell line through downregulating
Twist. Oncol Lett. 7:1625–1631. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He G, Cao X, He M, Sheng X, Wu Y and Ai X:
Casticin inhibits self-renewal of liver cancer stem cells from the
MHCC97 cell line. Oncol Lett. 7:2023–2028. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chou CC, Lee KH, Lai IL, Wang D, Mo X,
Kulp SK, Shapiro CL and Chen CS: AMPK reverses the mesenchymal
phenotype of cancer cells by targeting the Akt-MDM2-Foxo3a
signaling axis. Cancer Res. 74:4783–4795. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guo Q, Liu Z, Jiang L, Liu M, Ma J, Yang
C, Han L, Nan K and Liang X: Metformin inhibits growth of human
non-small cell lung cancer cells via liver kinase B-1-independent
activation of adenosine monophosphate-activated protein kinase. Mol
Med Rep. 13:2590–2596. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gutova M, Najbauer J, Gevorgyan A, Metz
MZ, Weng Y, Shih CC and Aboody KS: Identification of uPAR-positive
chemoresistant cells in small cell lung cancer. PLoS One.
2:e2432007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
LeBeau AM, Duriseti S, Murphy ST, Pepin F,
Hann B, Gray JW, VanBrocklin HF and Craik CS: Targeting uPAR with
antagonistic recombinant human antibodies in aggressive breast
cancer. Cancer Res. 73:2070–2081. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma YY and Tao HQ: Role of urokinase
plasminogen activator receptor in gastric cancer: A potential
therapeutic target. Cancer Biother Radiopharm. 27:285–290. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Codony-Servat J, Verlicchi A and Rosell R:
Cancer stem cells in small cell lung cancer. Transl Lung Cancer
Res. 5:16–25. 2016.PubMed/NCBI
|
|
30
|
Chiacchiera F, Matrone A, Ferrari E,
Ingravallo G, Lo Sasso G, Murzilli S, Petruzzelli M, Salvatore L,
Moschetta A and Simone C: p38alpha blockade inhibits colorectal
cancer growth in vivo by inducing a switch from HIF1alpha- to
FoxO-dependent transcription. Cell Death Differ. 16:1203–1214.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gopinath S, Malla RR, Gondi CS, Alapati K,
Fassett D, Klopfenstein JD, Dinh DH, Gujrati M and Rao JS:
Co-depletion of cathepsin B and uPAR induces G0/G1 arrest in glioma
via FOXO3a mediated p27 upregulation. PLoS One. 5:e116682010.
View Article : Google Scholar : PubMed/NCBI
|