Introduction
Retinoblastoma (RB), the most prevalent primary
intraocular malignancy, is initiated from immature cells in the
retina (1). RB typically arises in
infancy and childhood and accounts for ~2–4% of all childhood
malignancies (2). An estimated
9,000 novel RB cases occur each year globally (3). Untreated RB usually rapidly progresses
to blindness and is fatal because RB can spread into the brain via
the optic nerve (4). To date,
surgical operations, such as enucleation, combined with
chemotherapy and radiotherapy are the predominant therapeutic
methods for treating patients with RB (5,6).
Despite considerable development in diagnosis and therapy, the
long-term survival of patients with RB remains unfavourable
(7). Deregulations of oncogenes or
tumour suppressors are implicated in RB occurrence and development;
however, the pathogenesis of RB remains poorly understood (8,9).
Hence, identifying the molecular mechanisms by which RB occurs,
determining factors that affect RB development and developing novel
promising therapeutic strategies to improve the prognosis of
patients with this fatal disease are urgently needed.
MicroRNAs (miRNAs), a large group of single-stranded
non-coding small RNA molecules, have emerged as important gene
regulators by binding to the 3′-untranslated regions (UTRs) of
their target genes in a base-pairing manner, thereby promoting the
formation of a miRNA-mRNA-induced silencing complex and resulting
in the degradation and/or translational suppression of the target
mRNAs (10). In the human genome,
>1,000 mature miRNAs have been identified, and these miRNAs are
estimated to regulate ~30% of human protein-coding genes (11). miRNAs may act as tumour suppressors
or oncogenes, in which aberrantly downregulated miRNAs play
tumour-suppressive roles, whereas highly expressed miRNAs serve
oncogenic roles in carcinogenesis and cancer progression (12). Emerging studies have indicated that
numerous miRNAs are dysregulated in RB, such as miR-29a (13), miR-320 (14), miR-613 (15) and miR-143 (16). Aberrantly expressed miRNAs are
closely related with RB formation and progression by regulating
various pathological behaviours, such as cellular proliferation,
apoptosis, invasion, metastasis and angiogenesis (17–19).
Thus, in-depth investigation on miRNAs in RB may contribute to the
identification of therapeutic targets for patients with this
aggressive malignancy.
miR-758 has been well studied in cervical cancer
(20) and hepatocellular carcinoma
(21). However, the expression
pattern of miR-758 in RB and its roles in the development of RB, as
well as the mechanisms by which miR-758 exerts its functions, are
still unknown. Therefore, this study aimed to detect miR-758
expression in RB tissues and cell lines and determine its effects
and underlying mechanisms on RB progression. The results of this
study may provide novel insights into the pathogenesis of RB and
may provide an effective therapeutic target for the treatment of
patients with RB.
Materials and methods
Tissue specimens
A total of 26 RB tissues were obtained from patients
(17 males and 9 females; age range, 15–53 years) with RB who
received surgical resection at Liaocheng People's Hospital between
September 2014 and February 2017. Normal retinal tissues were
collected from 10 patients with ruptured globes. All tissues were
quickly snap-frozen in liquid nitrogen and stored at −80°C until
RNA isolation. None of these subjects had been treated with
radiotherapy or chemotherapy prior to recruitment in this study.
This research was approved by the Ethics Committee of Liaocheng
People's Hospital. Written informed consent was also provided by
all subjects.
Cell lines
The three human RB cell lines Y79, WERI-RB1 and
SO-RB50 and the normal retinal pigmented epithelial cell line
ARPE-19 were acquired from the American Type Culture Collection
(ATTC; Manassas, VA, USA) and cultured in Dulbecco's modified
Eagle's medium (DMEM) supplemented with 10% fetal bovine serum
(FBS) and 1% penicillin/streptomycin mixture (all from Gibco;
Thermo Fisher Scientific, Inc., Grand Island, NY, USA). All cells
were grown at 37°C under normoxic conditions of 95% air and 5%
CO2.
Transfection experiments
The oligonucleotides of the miR-758 mimics and their
negative control (miR-NC) were chemically produced by Guangzhou
RiboBio Co., Ltd. (Guangzhou, China). For PAX6 overexpression,
pCMV-PAX6 plasmid and empty PAX6 plasmid were synthesised by
Shanghai GenePharma Co., Ltd. (Shanghai, China). For the
transfection experiments, cells were inoculated into 6-well plates
at a density of 5×105 cells/well one day prior to
transfection and maintained in antibiotic-free DMEM containing 10%
FBS. miRNA mimics or plasmid were transfected into cells using
Lipofectamine® 2000 (Invitrogen Life Technologies;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) following the
manufacturer's instructions.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
TRIzol® reagent (Invitrogen Life
Technologies; Thermo Fisher Scientific, Inc.) was applied to
isolate total RNA from tissue samples or cells. The quality and
concentration of total RNA was determined using a NanoDrop 2000
spectrophotometer (Thermo Fisher Scientific, Inc.). For the
quantification of miR-758 expression, total RNA was converted into
cDNA using a TaqMan® MicroRNA Reverse Transcription kit
(Applied Biosystems; Thermo Fisher Scientific, Inc.). Subsequently,
miR-758 expression was detected by performing qPCR using a TaqMan
MicroRNA Assay kit on an ABI 7300 Plus thermal cycler (both from
Applied Biosystems; Thermo Fisher Scientific, Inc.). U6 snRNA
served as an internal control for miR-758 expression. The standard
SYBR-Green RT-PCR kit (Takara Biotechnology Co., Ltd., Dalian,
China) was used to quantify the mRNA level of PAX6, with β-actin
employed as an internal control. Relative expression was analysed
using the 2−∆∆Cq method (22).
Cell Counting Kit-8 (CCK-8) and colony
formation assays
After transfection for 24 h, cells were harvested
and seeded in 96-well plates with a density of 3,000 cells/well.
Following various incubation times (0–72 h), a CCK-8 assay was
conducted to detect cell proliferative ability. Briefly, 10 µl of
CCK-8 solution (Dojindo Molecular Technologies, Inc., Kumamoto,
Japan) was added into each well and further incubated at 37°C for 2
h. Finally, cell proliferation was determined by detecting the
absorbance at a wavelength of 450 nm on a multifunction microplate
reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Transfected cells were harvested at 24 h
post-transfection and prepared into a cell suspension. A total of
1,000 transfected cells were transferred into 6-well plates and
incubated for 2 weeks at 37°C under 95% air and 5% CO2.
On day 15, the cells were fixed in 4% paraformaldehyde, stained
with methyl violet (Nanjing KeyGen Biotech Co., Ltd., Nanjing,
China) and washed with phosphate-buffered saline (PBS). The number
of colonies (>50 cells/colony) was counted using an inverted
microscope (Olympus IX53; Olympus Corp., Tokyo, Japan).
Detection of cell apoptosis by flow
cytometric analysis
An Annexin V fluorescein isothiocyanate (FITC)
Apoptosis Detection kit (BioLegend, San Diego, CA, USA) was
employed to evaluate cell apoptosis. Briefly, transfected cells
were collected at 48 h post-transfection, washed with cold PBS and
suspended in 100 µl of binding buffer. Subsequently, the cells were
further incubated with 500 µl of Annexin V-FITC and 5 µl of
propidium iodide (PI) in the dark at room temperature for 15 min.
Finally, cell apoptosis was detected by flow cytometry (FACScan; BD
Biosciences, Franklin Lakes, NJ, USA) within 1 h and analysed with
CellQuest software version 3.3 (BD Biosciences).
Migration and invasion assays
Transwell chambers with 8-µm pore size (Corning
Inc., Corning, NY, USA) coated with or without Matrigel (BD
Biosciences) were used for the detection of cellular migration and
invasion, respectively.
At 48 h post-transfection, a total of
1×105 transfected cells were suspended in FBS-free DMEM
and placed into the top chambers. The bottom chambers were filled
with 5 µl of DMEM containing 10% FBS. After 24 h of incubation at
37°C with 95% air and 5% CO2, non-migrated and
non-invaded cells were removed from the surface of the membrane
with a cotton swab. The migrated and invaded cells were fixed with
100% methanol, stained with 0.5% crystal violet, washed with PBS
and photographed using an inverted microscope. Values for migration
and invasion were determined by counting five randomly selected
fields per membrane.
Bioinformatics analysis
TargetScan online software (www.targetscan.org) and microRNA.org
(www.microrna.org/microrna/) were used
to predict the potential targets of miR-758.
Luciferase reporter assay
PAX6 was predicted as a major target gene of
miR-758, and this association was further confirmed by luciferase
reporter assay. To synthesise luciferase reporter plasmids, we
amplified the 3′-UTR fragment of PAX6 containing wild-type (Wt)
putative or mutant-type binding sites for miR-758 (GenePharma Co.,
Ltd.), inserted it into the pmirGLO luciferase reporter vector
(Promega Corp., Madison, WI, USA) and labelled it as
pmirGLO-Wt-PAX6-3′-UTR or pmirGLO-Mut-PAX6-3′-UTR, respectively.
For the luciferase assay, the cells were seeded into 24-well plates
and co-transfected with miR-758 mimics or miR-NC and
pmirGLO-Wt-PAX6-3′-UTR or pmirGLO-Mut-PAX6-3′-UTR using
Lipofectamine® 2000, in accordance with the
manufacturer's protocol. Luciferase activities were detected at 48
h after transfection using a Dual-Luciferase Reporter Assay System
(Promega Corp.). Renilla luciferase activity was determined
for normalisation.
Western blot analysis
The total protein of cells or tissues was extracted
using ice-cold radioimmunoprecipitation assay buffer (Beyotime
Institute of Biotechnology, Shanghai, China). The concentration of
total protein was quantified with a BCA Protein Assay kit (Beyotime
Institute of Biotechnology). Equal amounts of protein were
separated by 10% SDS-PAGE and then transferred to polyvinylidene
difluoride membranes (PVDF; EMD Millipore, Billerica, MA, USA).
Subsequently, the membranes were blocked with 5% skimmed dry milk
in Tris-buffered saline-Tween (TBST) at room temperature for 2 h
and incubated with primary antibodies at 4°C overnight. After
washing with TBST thrice, the membranes were incubated with goat
anti-mouse horseradish peroxidase-conjugated secondary antibodies
(1:5,000 dilution; cat. no. sc-2005; Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA) or goat anti-rabbit horseradish
peroxidase-conjugated secondary antibodies (1:5,000 dilution; cat.
no. sc-2004; Santa Cruz Biotechnology, Inc.) at room temperature
for 1 h. The membranes were washed thrice with TBST and subjected
to protein signal detection using an enhanced chemiluminescence
reagent (Bio-Rad Laboratories). The primary antibodies used in the
present study included mouse anti-human monoclonal PAX6 (1:500
dilution; cat. no. ab197768), rabbit anti-human monoclonal p-pi3k
(1:500 dilution; cat. no. ab182651) and mouse anti-human monoclonal
pi3k (1:500 dilution; cat. no. ab86714; all from Abcam, Cambridge,
UK), mouse anti-human monoclonal p-Akt (1:1,000 dilution; cat. no.
sc-81433) and mouse anti-human monoclonal Akt (1:1,000 dilution;
cat. no. sc-56878; both from Santa Cruz Biotechnology, Inc.), and
rabbit anti-human monoclonal GAPDH antibody (1:500 dilution; cat.
no. ab181603; Abcam). GAPDH was employed as the internal
reference.
Statistical analysis
The data were expressed as the median ± standard
deviation (SD) and analysed with SPSS 17.0 (SPSS, Inc., Chicago,
IL, USA). Differences between groups were compared with Student's
t-test or one-way ANOVA, followed by the SNK multiple comparison
test. The possible correlation between miR-758 and PAX6 mRNA in RB
tissues was evaluated using Spearman's correlation analysis.
P<0.05 indicated a statistically significant difference.
Results
miR-758 is downregulated in RB tissues
and cell lines
To illustrate the potential roles of miR-758 in RB,
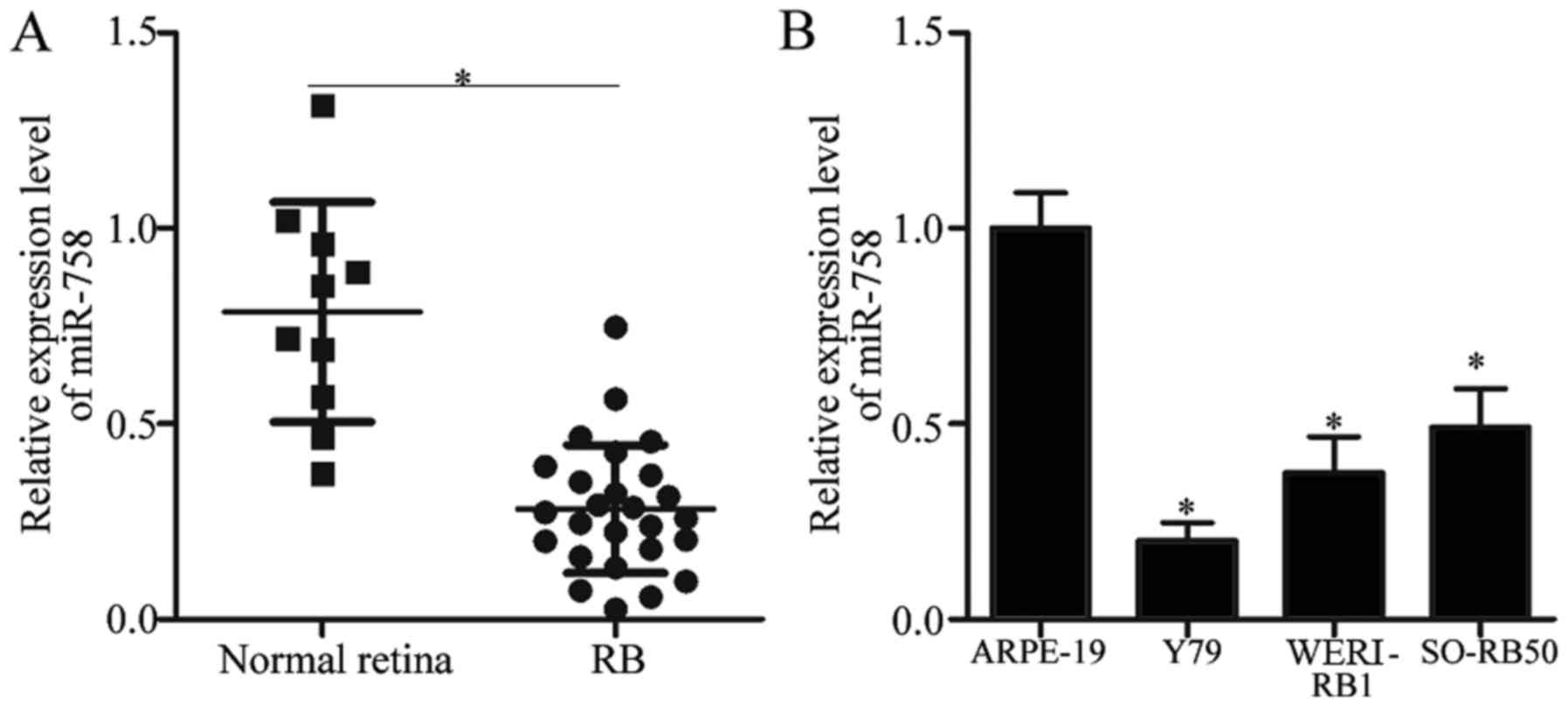
we first detected miR-758 expression in 26 RB tissues and 10 normal
retinal tissues. The RT-qPCR analysis data revealed that miR-758
was significantly downregulated in RB tissues compared with that in
normal retinal tissues (Fig. 1A,
P<0.05). In addition, miR-758 expression level was determined in
the three RB cell lines (Y79, WERI-RB1 and SO-RB50) and in the
normal retinal pigmented epithelium cell line ARPE-19. Compared
with that in ARPE-19, the expression levels of miR-758 were
markedly reduced in all three RB cell lines (Fig. 1B, P<0.05). These results
indicated that the dysregulation of miR-758 may be involved in the
progression of RB.
MiR-758 overexpression inhibits
proliferation and promotes apoptosis of RB cells
To explore the role of miR-758 in the progression of
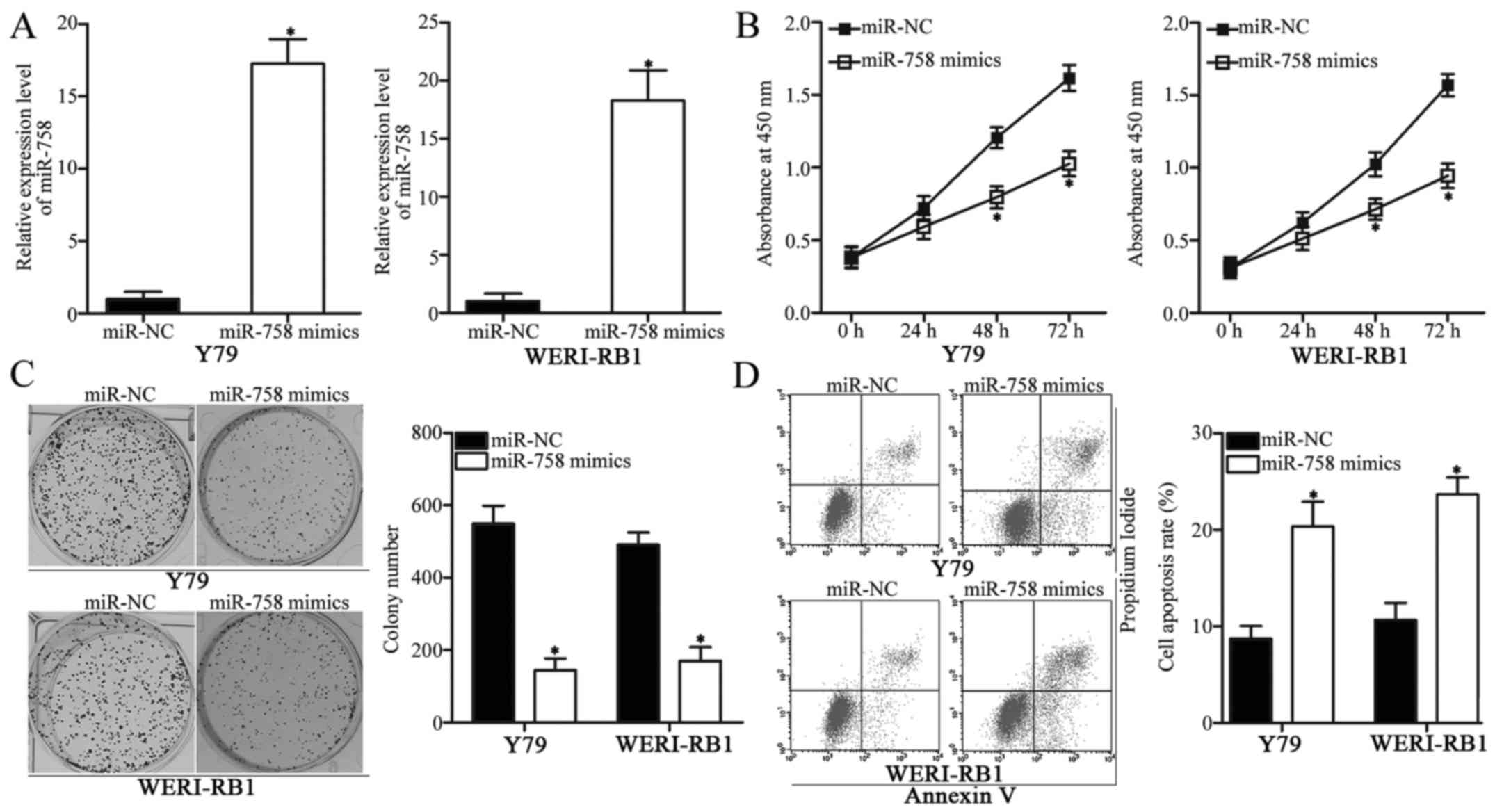
RB, we transfected miR-758 mimics into Y79 and WERI-RB1 cells to
upregulate the endogenous miR-758 expression level. Following
transfection, miR-758 was markedly overexpressed in Y79 and
WERI-RB1 cells transfected with miR-758 mimics compared with that
in cells transfected with miR-NC (Fig.
2A, P<0.05). The effect of miR-758 overexpression on
cellular proliferation was investigated using a CCK-8 assay. As
displayed in Fig. 2B, the
proliferation of Y79 and WERI-RB1 cells transfected with miR-758
mimics was delayed compared with that in cells transfected with
miR-NC (P<0.05).
Colony formation assay was performed to further
confirm the inhibition of RB cell proliferation caused by miR-758
overexpression. The results revealed that the colony numbers of Y79
and WERI-RB1 cells transfected with miR-758 mimics were
significantly decreased relative to those in the miR-NC groups
(Fig. 2C, P<0.05). Furthermore,
flow cytometric assay revealed that the enforced expression of
miR-758 increased the percentage of cell apoptosis in Y79 and
WERI-RB1 cells (Fig. 2D,
P<0.05). These findings revealed that miR-758 may be important
in regulating the proliferation and apoptosis of RB cells.
Overexpression of the expression of
miR-758 inhibits cell migration and invasion in RB cells
Migration and invasion assays were employed to
determine the role of miR-758 on cell metastasis in RB cells. As
shown in Fig. 3A, overexpression of
miR-758 suppressed the migratory capacities of Y79 and WERI-RB1
cells compared with those in the miR-NC groups (P<0.05).
Similarly, the number of invaded cells was lower in the miR-758
mimics-transfected Y79 and WERI-RB1 cells than those in cells
transfected with miR-NC (Fig. 3B,
P<0.05). These results indicated that miR-758 may have
suppressive roles in RB metastasis.
PAX6 is direct target of miR-758 in
RB
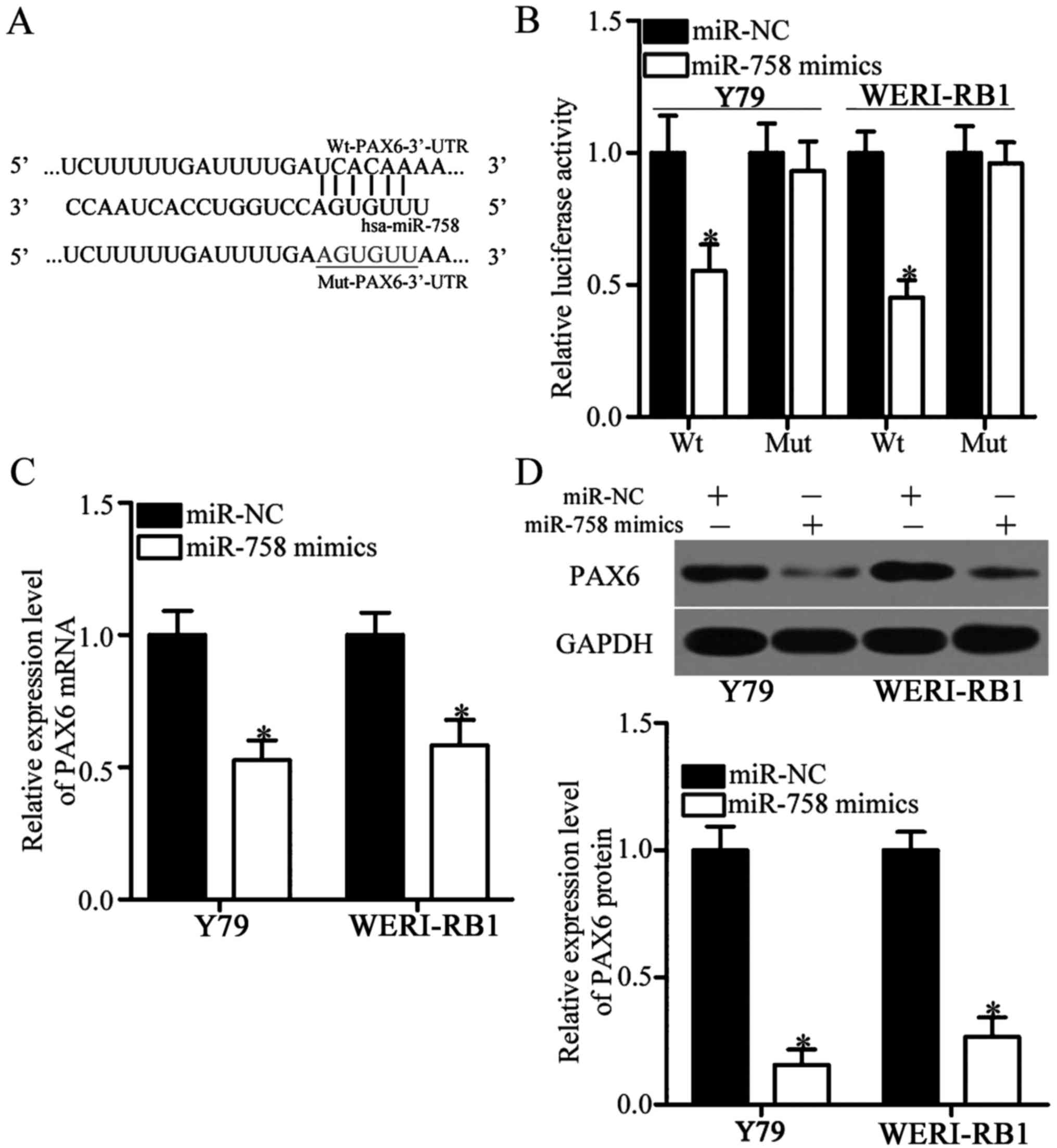
To gain insights into the mechanisms underlying the
action of miR-758 in RB, we conducted bioinformatics analysis to
predict the potential targets of miR-758. PAX6, a major highly
conserved putative target of miR-758, plays oncogenic roles in RB
(23–25) and was therefore selected for further
confirmation (Fig. 4A). To clarify
whether miR-758 can directly interact with the 3′-UTR of PAX6, we
performed luciferase reporter assays in Y79 and WERI-RB1 cells
transfected with miR-758 mimics or miR-NC in the presence of
pmirGLO-Wt-PAX6-3′-UTR or pmirGLO-Mut-PAX6-3′-UTR. Luciferase
activities were considerably reduced in Y79 and WERI-RB1 cells
co-transfected with miR-758 mimics and the luciferase reporter
plasmid carrying the wild-type miR-758 binding sequences
(P<0.05). However, miR-758 upregulation did not affect the
luciferase activities of the plasmid carrying the mutant PAX6
3′-UTR (Fig. 4B). To further
determine the regulatory roles of miR-758 on endogenous PAX6
expression, we utilised RT-qPCR and western blot analysis to assess
the expression levels of PAX6 in Y79 and WERI-RB1 cells after
transfection with miR-758 mimics or miR-NC. The ectopic expression
of miR-758 decreased the mRNA (Fig.
4C, P<0.05) and protein (Fig.
4D, P<0.05) expression of PAX6 in both Y79 and WERI-RB1
cells. In summary, these data demonstrated that PAX6 is a direct
target of miR-758 in RB.
Inverse correlation between PAX6 and
miR-758 expression in RB tissues
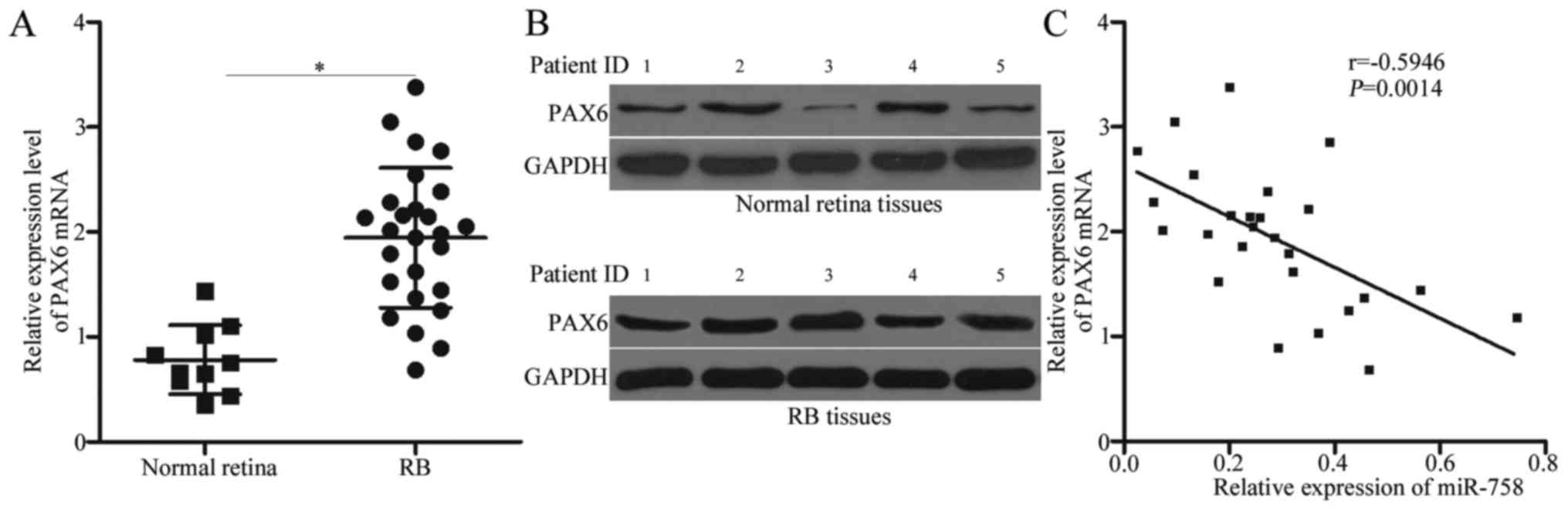
To examine the possible clinical relevance of PAX6
in RB, we detected PAX6 mRNA and protein expression in RB and
normal retinal tissues. As indicated in Fig. 5A and B, the mRNA and protein levels
of PAX6 were significantly higher in the RB tissues than those in
the normal retinal tissues (P<0.05). We analysed the correlation
between miR-758 and PAX6 mRNA in RB tissues using Spearman's
correlation analysis. An inverse correlation was validated between
miR-758 and PAX6 mRNA level in RB tissues (Fig. 5C; r=−0.5946, P=0.0014). These data
further indicated that PAX6 is a direct target of miR-758 in
RB.
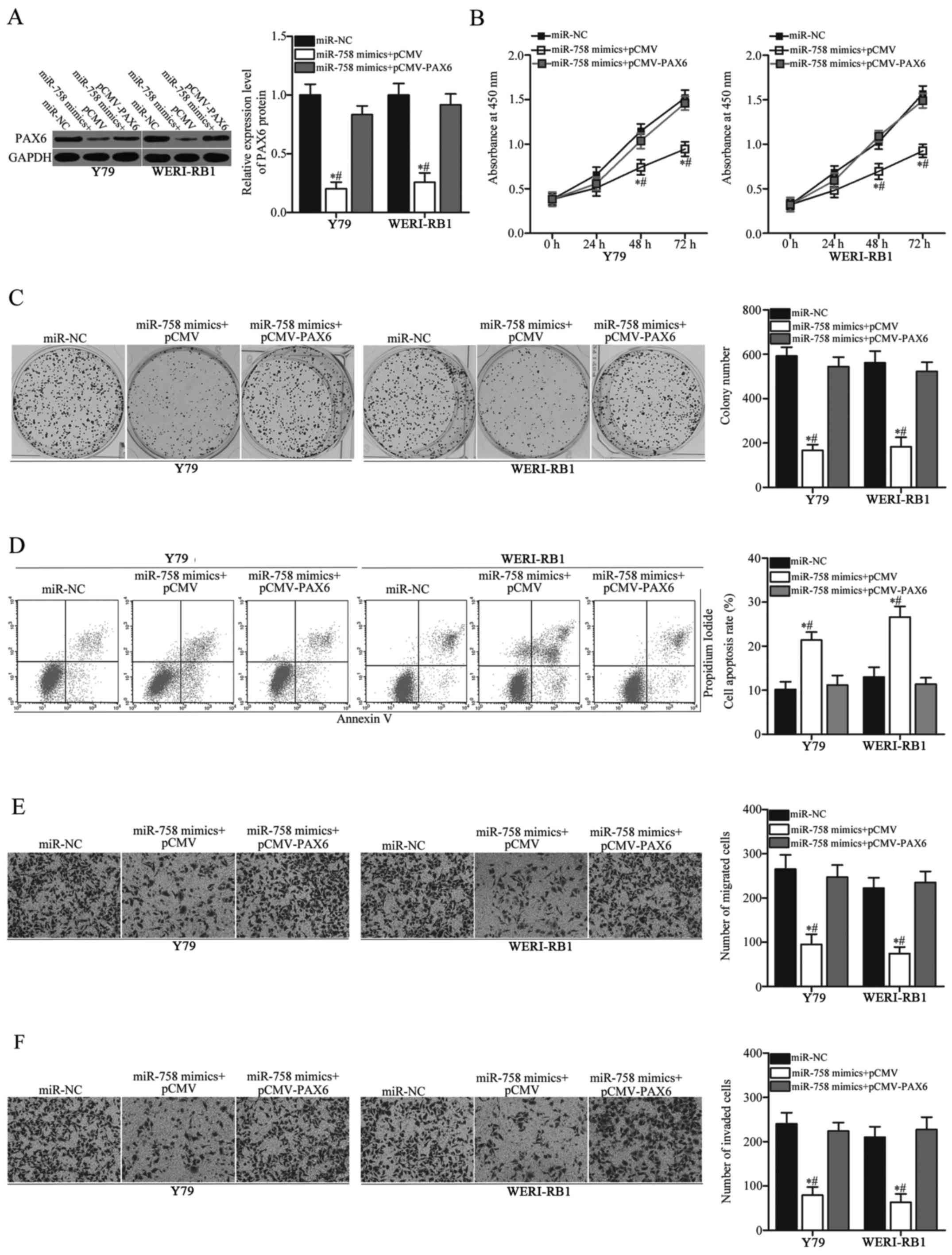
PAX6 overexpression abrogates the
impact of miR-758 overexpression on RB cells
After determining that PAX6 is a direct target of
miR-758, we further performed a series of rescue experiments to
investigate whether miR-758 mediates its tumour-suppressive roles
in RB cells by inhibiting PAX6. PAX6 overexpression plasmid
pCMV-PAX6 or empty pCMV plasmid, together with miR-758 mimics, were
transfected into Y79 and WERI-RB1 cells. After transfection,
western blot analysis confirmed that the decreased PAX6 protein
induced by miR-758 overexpression was restored in Y79 and WERI-RB1
cells after co-transfection with pCMV-PAX6 (Fig. 6A, P<0.05). Functional assays
revealed that restored PAX6 expression significantly abrogated the
effects of miR-758 overexpression on cell proliferation (Fig. 6B, P<0.05), colony formation
(Fig. 6C, P<0.05), apoptosis
(Fig. 6D, P<0.05), migration
(Fig. 6E, P<0.05) and invasion
(Fig. 6F, P<0.05). These results
revealed that miR-758 may exert tumour-suppressive effects in RB
cells, at least partly, by downregulating PAX6 expression.
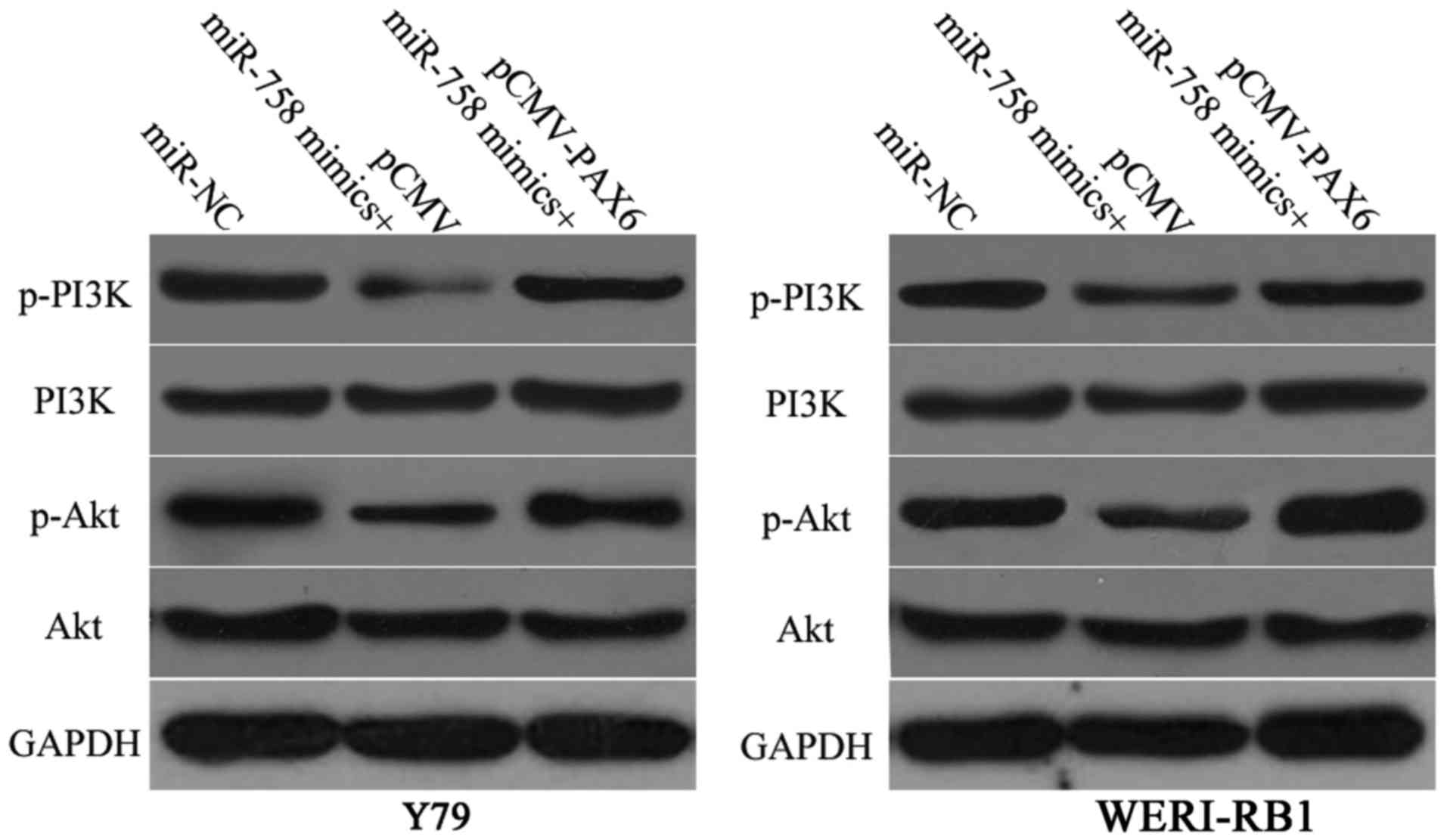
miR-758 inactivates the PI3K/Akt
pathway in RB cells
PAX6 participates in the regulation of the PI3K/Akt
pathway (26,27). Therefore, we investigated whether
miR-758 could regulate the PI3K/Akt pathway in RB cells via PAX6
modulation. Y79 and WERI-RB1 cells were transfected with miR-758
mimics in the presence of pCMV-PAX6 or pCMV. Following transfection
for 72 h, the ectopic expression of miR-758 significantly reduced
p-PI3K and p-Akt protein levels in Y79 and WERI-RB1 cells relative
to those in the miR-NC groups. Furthermore, the downregulation of
p-PI3K and p-Akt protein levels caused by miR-758 overexpression
was restored in Y79 and WERI-RB1 cells after co-transfection with
pCMV-PAX6 (Fig. 7). These results
confirmed that miR-758 may act as a tumour suppressor in RB by
negatively regulating the PAX6/PI3K/Akt pathway.
Discussion
In recent decades, accumulated studies have
highlighted that the dysregulation of miRNAs in RB is a leading
cause for tumourigenesis and tumour development (28–30).
Therefore, the elucidation of the expression, functional roles and
underlying mechanisms of miRNAs in RB would help develop promising
therapeutic methods to improve the prognosis of RB patients.
miR-758 is downregulated in cervical cancer, and this
downregulation is correlated with infiltration and invasion of
cervical cancer (20). Jiang et
al (21) reported that miR-758
was downregulated in hepatocellular carcinoma tissues and cell
lines. Enforced expression of miR-758 decreased cell growth and
metastasis in hepatocellular carcinoma. However, the expression
pattern and detailed roles of miR-758 in RB remain unknown. Thus,
the present study aimed to detect miR-758 expression in RB and
determine the biological roles and underlying mechanisms of miR-758
in RB cells and tissues.
The key finding in this study is that miR-758 was
downregulated in RB tissues and cell lines compared with that in
normal retinal tissues and a normal retinal pigmented epithelium
cell line, respectively. Overexpression of miR-758 inhibited
cellular proliferation, migration and invasion and promoted
apoptosis in RB cells in vitro. In addition, PAX6 was
confirmed as a direct target of miR-758 in RB. PAX6 was
overexpressed in RB tissues and negatively correlated with the
expression level of miR-758. Overexpression of PAX6 rescued the
tumor-suppressive roles of miR-758 overexpression in RB cells.
Notably, ectopic expression of miR-758 inhibited the activation of
the PI3K/Akt pathway in RB cells by inhibiting PAX6. To the best of
our knowledge, our present study is the first to demonstrate that
miR-758 exerts tumour-suppressive effects on the malignant
phenotypes of RB cells, at least in part, by directly targeting
PAX6 and inactivating the PI3K/Akt pathway. These findings also
suggest that the miR-758/PAX6 axis may be developed as a potential
therapeutic target for patients with this aggressive disease.
Recognising the targets of miR-758 in RB is
essential to understand the mechanisms on the regulatory roles of
miR-758 in RB occurrence and development. It is also important for
the identification of novel therapeutic targets for patients with
RB. Several targets of miR-758 have been identified, including MDM2
(21), mTOR (21), CD36 (31) and ABCA1 (32). In the present study, PAX6 was
demonstrated to be a novel target of miR-758 in RB. PAX6, a highly
conserved transcription factor, is a member of the PAX gene family
(33). It plays important roles in
the development of the eyes, central nervous system and pancreas
(34,35). Emerging studies have demonstrated
that PAX6 is implicated in the carcinogenesis and progression of
multiple human cancers. For example, PAX6 is overexpressed in
breast cancer tissues and cell lines. Breast cancer patients with
an increased expression level of PAX6 exhibited poorer prognosis
than those with a decreased PAX6 level (36). PAX6 knockdown was revealed to
inhibit cell viability, proliferation, colony formation and
metastasis in vitro and decrease tumour growth in
vivo (37,38). Hence, PAX6 may be a promising
biomarker for diagnosis and prognosis and an effective therapeutic
target in tumour patients.
PAX6 is closely related with RB onset and
development. It is highly expressed in RB tissues and cell lines.
Its suppression was revealed to significantly reduce cell
proliferation, migration, invasion, cell cycle arrest and cell
apoptosis in RB cells (23–25). PAX6 is directly targeted by several
miRNAs in human cancers. For example, miR-433 (25) and miR-365b-3p (39) directly target PAX6 to inhibit the
malignant progression of RB. Furthermore, miR-223 (27), miR-7 (40), miR-335 (33) and miR-448 (41) directly bind to PAX6 and therefore
inhibit the progression of glioblastoma, non-small cell lung,
breast and gastric cancer, respectively. These findings indicated
that miRNA-based therapy targeting PAX6 may represent a novel
therapeutic method for the treatment of tumour patients.
In conclusion, the present study provides new
evidence that miR-758 may play tumour-suppressive roles in RB by
directly targeting PAX6 and regulating the PI3K/Akt pathway. Our
findings regarding miR-758 are encouraging and suggest that this
miRNA can be explored as a potential therapeutic target for
patients with RB in the future. However, we did not analyze the
correlation between miR-758 expression and the prognosis of
patients with RB. This is a limitation of the present study and we
will explore this in our future experiments.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL and XY designed this research, and performed all
functional experiments. Both authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
This research was approved by the Ethics Committee
of the Liaocheng People's Hospital. Written informed consent was
also provided by all subjects.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dimaras H, Kimani K, Dimba EA, Gronsdahl
P, White A, Chan HS and Gallie BL: Retinoblastoma. Lancet.
379:1436–1446. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Broaddus E, Topham A and Singh AD:
Incidence of retinoblastoma in the USA: 1975–2004. Br J Ophthalmol.
93:21–23. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kivelä T: The epidemiological challenge of
the most frequent eye cancer: Retinoblastoma, an issue of birth and
death. Br J Ophthalmol. 93:1129–1131. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dong C, Liu S, Lv Y, Zhang C, Gao H, Tan L
and Wang H: Long non-coding RNA HOTAIR regulates proliferation and
invasion via activating Notch signalling pathway in retinoblastoma.
J Biosci. 41:677–687. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shields CL, Say EA, Pointdujour-Lim R, Cao
C, Jabbour PM and Shields JA: Rescue intra-arterial chemotherapy
following retinoblastoma recurrence after initial intra-arterial
chemotherapy. J Fr Ophtalmol. 38:542–549. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chantada GL, Qaddoumi I, Canturk S, Khetan
V, Ma Z, Kimani K, Yeniad B, Sultan I, Sitorus RS, Tacyildiz N and
Abramson DH: Strategies to manage retinoblastoma in developing
countries. Pediatr Blood Cancer. 56:341–348. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abramson DH, Shields CL, Munier FL and
Chantada GL: Treatment of retinoblastoma in 2015: Agreement and
disagreement. JAMA Ophthalmol. 133:1341–1347. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Beta M, Venkatesan N, Vasudevan M,
Vetrivel U, Khetan V and Krishnakumar S: Identification and
insilico analysis of retinoblastoma serum microrna profile and gene
targets towards prediction of novel serum biomarkers. Bioinform
Biol Insights. 7:21–34. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li J, Zhang Y, Wang X and Zhao R:
microRNA-497 overexpression decreases proliferation, migration and
invasion of human retinoblastoma cells via targeting vascular
endothelial growth factor A. Oncol Lett. 13:5021–5027. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shenouda SK and Alahari SK: MicroRNA
function in cancer: Oncogene or a tumor suppressor? Cancer
Metastasis Rev. 28:369–378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu S, Zhang X, Hu C, Wang Y and Xu C:
miR-29a inhibits human retinoblastoma progression by targeting
STAT3. Oncol Rep. 39:739–746. 2018.PubMed/NCBI
|
|
14
|
Liang Y, Chen X and Liang Z: MicroRNA-320
regulates autophagy in retinoblastoma by targeting hypoxia
inducible factor-1α. Exp Ther Med. 14:2367–2372. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Y, Zhu X, Zhu X, Wu Y, Liu Y, Yao B
and Huang Z: MiR-613 suppresses retinoblastoma cell proliferation,
invasion, and tumor formation by targeting E2F5. Tumour Biol.
39:10104283176916742017.PubMed/NCBI
|
|
16
|
Wang LL, Hu HF and Feng YQ: Suppressive
effect of microRNA-143 in retinoblastoma. Int J Ophthalmol.
9:1584–1590. 2016.PubMed/NCBI
|
|
17
|
Xu X, Ge S, Jia R, Zhou Y, Song X, Zhang H
and Fan X: Hypoxia-induced miR-181b enhances angiogenesis of
retinoblastoma cells by targeting PDCD10 and GATA6. Oncol Rep.
33:2789–2796. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu S, Hu C, Wang Y, Shi G, Li Y and Wu H:
miR-124 inhibits proliferation and invasion of human retinoblastoma
cells by targeting STAT3. Oncol Rep. 36:2398–2404. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang L, Wei N, Wang L, Wang X and Liu QH:
miR-498 promotes cell proliferation and inhibits cell apoptosis in
retinoblastoma by directly targeting CCPG1. Childs Nerv Syst.
34:417–422. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Meng X, Zhao Y, Wang J, Gao Z, Geng Q and
Liu X: Regulatory roles of miRNA-758 and matrix extracellular
phosphoglycoprotein in cervical cancer. Exp Ther Med. 14:2789–2794.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang D, Cho W, Li Z, Xu X, Qu Y, Jiang Z,
Guo L and Xu G: MiR-758-3p suppresses proliferation, migration and
invasion of hepatocellular carcinoma cells via targeting MDM2 and
mTOR. Biomed Pharmacother. 96:535–544. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li L, Li B, Zhang H, Bai S, Wang Y, Zhao B
and Jonas JB: Lentiviral vector-mediated PAX6 overexpression
promotes growth and inhibits apoptosis of human retinoblastoma
cells. Invest Ophthalmol Vis Sci. 52:8393–8400. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bai SW, Li B, Zhang H, Jonas JB, Zhao BW,
Shen L and Wang YC: Pax6 regulates proliferation and apoptosis of
human retinoblastoma cells. Invest Ophthalmol Vis Sci.
52:4560–4570. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li X, Yang L, Shuai T, Piao T and Wang R:
MiR-433 inhibits retinoblastoma malignancy by suppressing Notch1
and PAX6 expression. Biomed Pharmacother. 82:247–255. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Y, Li Y, Liu Y, Xie P, Li F and Li G:
PAX6, a novel target of microRNA-7, promotes cellular proliferation
and invasion in human colorectal cancer cells. Dig Dis Sci.
59:598–606. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang BS, Luo QZ, Han Y, Huang D, Tang QP
and Wu LX: MiR-223/PAX6 axis regulates glioblastoma stem cell
proliferation and the chemo resistance to TMZ via regulating
PI3K/Akt pathway. J Cell Biochem. 118:3452–3461. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Z, Yao YJ, Zheng F, Guan Z, Zhang L,
Dong N and Qin WJ: Mir-138-5p acts as a tumor suppressor by
targeting pyruvate dehydrogenase kinase 1 in human retinoblastoma.
Eur Rev Med Pharmacol Sci. 21:5624–5629. 2017.PubMed/NCBI
|
|
29
|
Che X, Qian Y and Li D: Suppression of
disheveled-axin domain containing 1 (DIXDC1) by MicroRNA-186
inhibits the proliferation and invasion of retinoblastoma cells. J
Mol Neurosci. 64:252–261. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li J, Xu ZW, Wang KH, Wang N, Li DQ and
Wang S: Networks of MicroRNAs and genes in retinoblastomas. Asian
Pac J Cancer Prev. 14:6631–6636. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li BR, Xia LQ, Liu J, Liao LL, Zhang Y,
Deng M, Zhong HJ, Feng TT, He PP and Ouyang XP: miR-758-5p
regulates cholesterol uptake via targeting the CD36 3′UTR. Biochem
Biophys Res Commun. 494:384–389. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ramirez CM, Dávalos A, Goedeke L, Salerno
AG, Warrier N, Cirera-Salinas D, Suárez Y and Fernández-Hernando C:
MicroRNA-758 regulates cholesterol efflux through
posttranscriptional repression of ATP-binding cassette transporter
A1. Arterioscler Thromb Vasc Biol. 31:2707–2714. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Meng Y, Zou Q, Liu T, Cai X, Huang Y and
Pan J: microRNA-335 inhibits proliferation, cell-cycle progression,
colony formation, and invasion via targeting PAX6 in breast cancer
cells. Mol Med Rep. 11:379–385. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Georgala PA, Carr CB and Price DJ: The
role of Pax6 in forebrain development. Dev Neurobiol. 71:690–709.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hanson IM: PAX6 and congenital eye
malformations. Pediatr Res. 54:791–796. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xia X, Yin W, Zhang X, Yu X, Wang C, Xu S,
Feng W and Yang H: PAX6 overexpression is associated with the poor
prognosis of invasive ductal breast cancer. Oncol Lett.
10:1501–1506. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zong X, Yang H, Yu Y, Zou D, Ling Z, He X
and Meng X: Possible role of Pax-6 in promoting breast cancer cell
proliferation and tumorigenesis. BMB Rep. 44:595–600. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zou Q, Yi W, Huang J, Fu F, Chen G and
Zhong D: MicroRNA-375 targets PAX6 and inhibits the viability,
migration and invasion of human breast cancer MCF-7 cells. Exp Ther
Med. 14:1198–1204. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang J, Wang X, Wu G, Hou D and Hu Q:
MiR-365b-3p, down-regulated in retinoblastoma, regulates cell cycle
progression and apoptosis of human retinoblastoma cells by
targeting PAX6. FEBS Lett. 587:1779–1786. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Luo J, Li H and Zhang C: MicroRNA-7
inhibits the malignant phenotypes of nonsmall cell lung cancer in
vitro by targeting Pax6. Mol Med Rep. 12:5443–5448. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhao Y, Lu G, Ke X, Lu X, Wang X, Li H,
Ren M and He S: miR-488 acts as a tumor suppressor gene in gastric
cancer. Tumour Biol. 37:8691–8698. 2016. View Article : Google Scholar : PubMed/NCBI
|