Introduction
Colorectal cancer (CRC) is the second leading cause
of cancer-related deaths among all ages in the US, with nearly
130,000 new cases and ~50,000 deaths in 2015 (1). The primary treatment modality for CRC
is surgery. Radiotherapy (RT) may be used in combination with
chemotherapy postoperatively to reduce the frequency of local
recurrence (2). Adjuvant therapies
have also been extensively studied due to the high incidence of
postoperative recurrences. Concurrent chemoradiotherapy (CCRT)
plays an important role in controlling the progression of CRC and
in the palliation of CRC-related symptoms (3). Large portions of the small intestine,
colorectum and urinary bladder are also included in the radiation
field during RT with pelvic lymphatic drainage, thus limiting the
delivery dose of RT. Therefore, radioprotectors such as amifostine
and misoprostol have been used to improve therapeutic efficacy
(4). Alternatively,
radiosensitizers may be potential candidates for increasing the
efficacy of RT for CRC.
The root of Stephania tetrandra has been used
in traditional Chinese medicine for several decades to treat
patients with arthritis, rheumatic disorders, silicosis, edema,
inflammatory diseases and hypertension (5,6).
Tetrandrine (TET), a bisbenzylisoquinoline alkaloid isolated from
the dried root of Hang-Fang-Chi (Stephania tetrandra S.
Moore), possesses a number of medicinal properties, including
proliferation, angiogenesis, migration and invasion. The
cytotoxicity of TET may be via the induction of apoptosis and
autophagy, the reversal of multidrug resistance and the enhancement
of radiation sensitization (7–9). The
antitumor effects of TET were demonstrated in several studies,
including leukemia, lung carcinoma, hepatoblastoma, neuroblastoma
and colorectal carcinomas (10–14).
Furthermore, TET was revealed to enhance the radiosensitivity of
human glioblastoma U138MG cells in vitro, suggesting its
potential as an adjunct to RT (15). However, its potential in
chemoradiotherapy of CRC in vivo remains to be
elucidated.
Characteristics of apoptosis include loss of
cellular contact with the matrix, cytoplasmic contraction,
chromatin condensation, plasma membrane blebbing and DNA
fragmentation (16). Both caspase-8
and −3, which are involved in the death receptor pathways, are
considered to play important roles in TET-induced apoptosis
(17,18). IR can also act on the cellular
membrane to generate ceramides via hydrolysis of sphingomyelin,
resulting in apoptosis (19). TET
has been reported to enhance the radiosensitivity of human
esophageal carcinoma cells by arresting cells at G2/M, which are
the most radiosensitive phases of the cell cycle (20). Here, we used a BALB/c
CT26/tk-luc colorectal adenocarcinoma cell line and a
tumor-bearing animal model to investigate the cytotoxic effects and
therapeutic efficacy of TET alone and combined with IR in
vitro and in vivo. In addition to digital caliper
measurements for evaluating tumor growth, multiple modalities of
molecular imaging, including bioluminescent imaging (BLI) and gamma
scintigraphy, were used to monitor the inhibition of tumor
growth
Materials and methods
Cell culture and transfection of HSV-1
thymidine kinase and luciferase genes
Standard cloning techniques were used to establish
the pC1-tk-IRES-luc vector with dual reporter genes in the
present study, as previously described (21). In brief, murine CT26 colorectal
adenocarcinoma cells were cultured in RPMI-1640 medium (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal bovine serum (FBS; HyClone Laboratories; GE Healthcare
Life Sciences, Logan, UT, USA). A plasmid vector carrying HSV-1
thymidine kinase (tk) and luciferase (luc) genes was
transfected into parental CT26 cells using Lipofectamine™ 2000
transfection reagent (Invitrogen; Thermo Fisher Scientific, Inc.),
and the cells were renamed CT26/tk-luc cells, as previously
described (22). The
CT26/tk-luc stable clones were cultured under the same
condition as the parental cells. G418 (600 µg/ml) was added to the
medium to maintain the stable expression of tk-luc
genes.
TET preparation
For the in vitro study, TET (cat. no. 365629;
Sigma-Aldrich; Merck, St. Louis, MO, USA) was dissolved in dimethyl
sulfoxide (DMSO) to 10 mM, sterilized by filtration through a
0.22-µm filter as a stock solution, then stored at −20°C. For the
working solution, the stock solution was diluted to desired
concentrations with serum-free medium immediately before each
experiment. The final concentration of DMSO was <0.5%. For the
in vivo study, TET was dissolved in a drop of 1 N HCl and
then adjusted to pH 7.0 with 1 N NaOH, then further diluted with
0.9% NaCl solution to desired concentrations. Finally, it was
sterilized by filtration through a 0.22-µm filter and stored at
4°C.
Irradiation
For the in vitro study, cultured monolayer
cells were irradiated with a Co-60 AECL Eldorado-78 irradiator at a
dose rate of 32 cGy/min at room temperature, 80 cm
source-to-surface distance (SSD) and a field size of 30×30 cm.
Before and immediately after irradiation, cells were maintained on
ice to arrest the cell cycle. For the tumor-bearing animal model, 6
mice per group were placed in an acrylic restraint and irradiated
with 18 Gy (field size of 30×5 cm) administered in 6 fractions, one
fraction a day, three times a week.
Clonogenic survival assay
CT26/tk-luc cells were seeded in T-25 flasks
(1×106 cells/flask) overnight, then treated with 0, 4,
8, 16, 24 and 32 µM of TET for 3 h, different doses of radiation,
or 20 µM TET for 3 h followed by irradiation. The plating
efficiency (PE) was defined as the number of colonies divided by
the number of cells plated in the control group. The surviving
fraction (SF) under various treatments was the number of counted
colonies divided by the number of colonies plated and corrected for
PE. The mean lethal dose (D0) was calculated from the
radiation survival curve using D10 = 2.3×D0.
The experiments were repeated more than three times.
Sub-G1 population assayed by flow
cytometric analysis
CT26/tk-luc cells were cultured in 60
mm-diameter dishes (1×106 cells/dish) overnight. When
the cell growth reached ~80% confluence, the cells were treated
with different concentrations of TET for 3 h, or TET for 3 h
followed by 4 Gy irradiation. Cells were washed twice with
phosphate-buffered saline (PBS), then cultured in fresh medium
containing 10% FBS for a further 24 h prior to analysis with a
FACScan flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA).
Cells in the sub-G1 phase (i.e. apoptotic cells) could be
distinguished from cells with the normal diploid DNA peak
(G0/G1 phase) on the fluorescence profiles of
propidium iodide-stained cells. The percentage of cells in the
sub-G1 phase was estimated with CellQuest software (BD
Biosciences). CT26/tk-luc cells treated with 50 µM
camptothecin (CAM; cat. no. 7689-03-4; Sigma-Aldrich; Merck KGaA)
for 24 h were used as the positive control.
DNA fragmentation assay
CT26/tk-luc cells were cultured in 60
mm-diameter dishes (1×106 cells/dish) and incubated at
37°C overnight, then treated with 0, 10, 20 and 50 µM TET for 3 h.
The combination group was irradiated with 4 Gy immediately after
TET treatment. Cells were harvested and centrifuged at 80 × g for 5
min and supernatants were aspirated. DNA fragmentation was
performed using Suicide Track™ DNA Ladder Isolation kit [cat. no.
AM41; Calbiochem; EMD Millipore (Billerica, MA, USA)]. Total DNA
was extracted using the kit, then dissolved in 50 µl resuspension
buffer. A volume of 21 µl DNA sample was transferred to a clean
centrifuge tube and 4 µl of 6× gel loading buffer was added. Then,
it was loaded onto a 1.5% agarose gel and stained with ethidium
bromide for electrophoresis in 0.5 M Tris/EDTA buffer.
Total protein isolation
CT26/tk-luc cells were harvested at 24 h
after TET alone and combined with 4 Gy irradiation, then washed
twice with PBS and centrifuged at 80 × g for 3 min. The cell pellet
was resuspended in 100 µl protein lysis buffer (for
1.5×106−2×107). Suspended cells were
transferred to an Eppendorf tube on ice, then centrifuged at 10,956
× g for 10 min at 4°C. The supernatant was transferred to a new
tube on ice and the pellet was discarded. The total protein
concentration was determined using bovine serum albumin (BSA) as
the standard by measuring the absorbance at 595 nm. Samples were
stored at −20°C for western blot analysis and caspase-3 activity
assay.
Western blotting
Lysis buffer (50 mM Tris-HCl, pH 8.0, 120 mM NaCl,
0.5% NP-40 and 1 mM phenylmethanesulfonyl fluoride) was used for
the extraction of total proteins from cells of each group at 4°C.
The proteins (40 µg/lane) were separated via 10% SDS-PAGE and
transferred onto a polyvinylidene difluoride (PVDF) membrane (EMD
Millipore, Billerica, MA, USA). The membrane was blocked with 5%
non-fat milk in TBST buffer solution (10 mM Tris-base, 150 mM NaCl,
0.1% Tween-20) for 1 h at room temperature, followed by incubation
with the appropriate primary antibodies against caspase-3 (1:300;
cat. no. IMG-144A; Imgenex, Novus Biologicals, LLC, Littleton, CO,
USA), β-actin (1:3,000; cat. no. MAB1501; Chemicon International,
Inc., Billerica, MA, USA) overnight at 4°C. The membranes was
further incubated with anti-mouse IgG-horseradish peroxidase (HRP)
for 1 h at room temperature (1:5,000; cat. no. AP124P; Chemicon
International, Inc.), then detected using ECL Western Blot
Chemiluminescence Reagent Plus (cat. nos. NEL104 and NEL105;
PerkinElmer Life Science, Inc., Waltham, MA, USA). Membranes were
dried by dabbing with tissue, then exposed to FUJI Medical X-ray
film (cat. no. 50407; Fujifilm, Tokyo, Japan) and quantified with
Scion Image software (version 4.0.3.2; Scion Corp., Frederick, MD,
USA).
Caspase-3 activity analysis
Caspase-3 plays an important role in triggering the
apoptotic process, and its activity has been suggested to be an
index of apoptosis (23). To
determine whether caspase activation was involved in TET-induced
cell death, CT26/tk-luc cells were exposed to TET for 3 h
with or without radiation, and incubated for a further 3, 9 and 21
h at 37°C. The protein expression of caspase-3 was assayed by
western blotting. Total protein (50 µg) was added to the buffer to
a final volume of 500 µl. Ac-DEVD-AFC (20 µM), the substrate for
caspase-3, was added to the sample and incubated at 37°C for 30
min. After cleavage by activated caspase-3, the substrate released
a yellow-green fluorescent compound, AFC, which could be detected
with a spectrophotometer (Hitachi F-4500; Hitachi, Ltd., Tokyo,
Japan), with excitation and emission at 380 and 508 nm,
respectively. Cells treated with 50 µM CAM for 24 h were used as
the positive control. The results were expressed as the percentage
change in activity compared with the untreated control. The
experiments were performed three times independently.
Tumor-bearing animal model
Six-week-old male BALB/c mice (number of mice, 85;
body weight, 20±2 g) were housed in the following conditions:
22±1°C, 55–60% relative humidity, 12-h light/dark cycle control and
food and water with ad libitum. The mice were anesthetized
with an i.p. injection of 45 mg/kg ketamine and 15 mg/kg
xylazine, and 2×105 CT26/tk-luc cells in 0.2 ml
serum-free medium were subcutaneously injected into the right hind
legs. Perpendicular tumor diameters were measured using a digital
caliper and the tumor volume was calculated using the formula:
0.523 × (length × width × thickness) (24) Treatment was initiated when the tumor
volume reached 100 mm3. Mice were randomly separated
into six groups (6 mice for the control group, 7 mice per group for
the rest groups): Normal saline, 5 mg/kg TET for 12 consecutive
days, 10 mg/kg TET (three times a week for 2 weeks), 20 mg/kg TET
(three times in 2 weeks), radiation alone (3 Gy/fraction, three
fractions per week for 2 weeks) and concurrent 10 mg/kg TET and 3
Gy radiation. Normal saline and TET were i.p. injected.
Tumor growth inhibition was measured by a digital caliper,
non-invasive BLI and gamma scintigraphy. All animal experiments
were repeated twice.
Tumor growth inhibition assay
Body weight and tumor volume were assayed twice per
week throughout the experimental period. The time required to reach
8-fold of the initial tumor volume (i.e. 100 mm3) was
used as the biological endpoint. Tumor growth delay (TGD) was
calculated as follows: TGD = TGTtreated-
TGTcontrol, where TGT represents tumor growth time
(25). The enhancement ratio (ER)
was calculated as follows: ER = (TGDTET+IR -
TGDIR)/TGDTET, where TGDTET+IR
stands for the tumor growth delay of the combination group;
TGDIR stands for the tumor growth delay of the radiation
alone group and TGDTET represents the tumor growth delay
of the TET alone group.
In vivo survival curve
Survival curves were determined by the Kaplan-Meier
method. Mice were considered expired when the tumor volume reached
2,500 mm3 post-treatment in accordance with the
Institutional Animal Care and Use Committee regulations (26). IACUC approval no. for the animal
experiments in the present study was 950501 issued by the National
Yang-Ming University.
Bioluminescent imaging (BLI)
CT26/tk-luc murine CRC cells
(2×105 cells) were inoculated into the flank of BALB/c
mice to monitor tumor growth and the therapeutic efficacy of TET
with or without radiation, assayed by direct measurement with
calipers and BLI using the Xenogen IVIS50 Imaging system (Xenogen
Corp., Alameda, CA, USA). Images and measurements of bioluminescent
signals were acquired and analyzed using Living Image software
(Xenogen Corp.). Mice were i.p. injected with D-luciferin 150 mg/kg
in PBS and anesthetized using 1–3% isoflurane 15 min prior to
imaging. Mice were placed on a warmed platform inside the camera
box and received continuous exposure to 1–2% isoflurane to sustain
sedation during imaging. The image acquisition time ranged from 30
sec to 5 min depending on the bioluminescence of the tumors.
Regions of interest (ROIs) from displayed images were drawn around
the tumor and quantified as photons/second (ph/s) using the Living
Image software (version 3.1; Information Technologies LLC, St.
Louis, MO, USA). The serial bioluminescent signals were quantified
and displayed over time.
Gamma scintigraphy
Planar imaging was performed on BALB/c mice bearing
CT26/tk-luc tumors derived from 2×105 cells as
aforementioned. After mice were injected with 100 µCi
[131I] FIAU via the tail vein, static imaging was
obtained from anaesthetized animals at 24 h post-injection with
e.Cam Multiangle Cardiac camera (Siemens AG, Munich,
Germany) equipped with a pinhole collimator. ROIs were selected
over the tumor area and the reference organs.
Statistical analysis
All data are presented as the mean ± standard error
(SE). Student's t-test was used for comparison between two groups.
The differences among multiple groups was analyzed using ANOVA and
the Tukey-Kramer method for the post hoc test. A difference between
the means was considered significant at P<0.05.
Results
Cytotoxicity of TET and radiation in
CT26/tk-luc cells
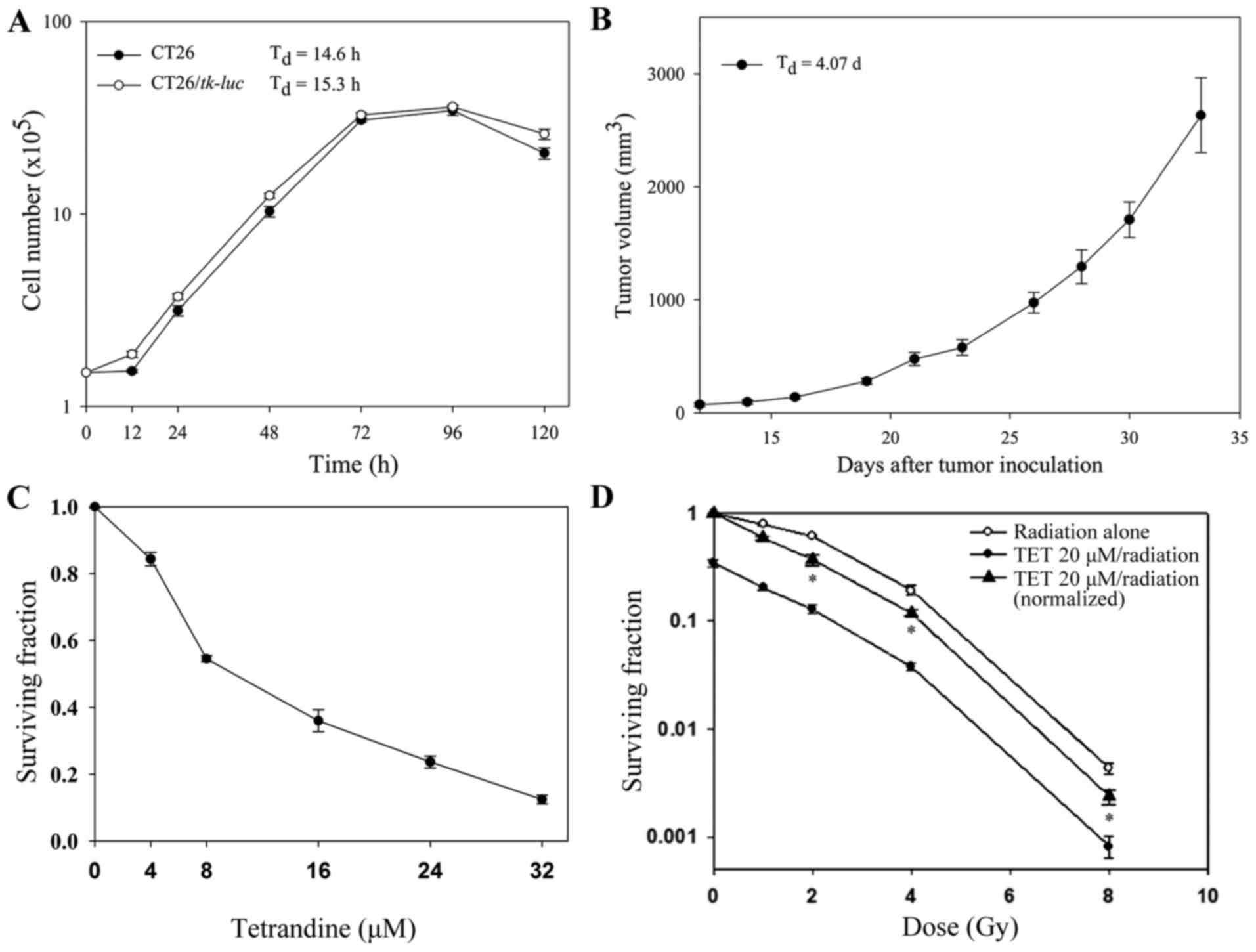
The cell growth curves of CT26 and CT26/tk-luc in
vitro are shown in Fig. 1A. The
cell doubling time of CT26 and CT26/tk-luc were 14.6 and
15.3 h, respectively. Fig. 1B shows
the tumor growth curve of C26/tk-luc in vivo. The tumor
doubling time of CT26/tk-luc-bearing mice was 4.07 days. To
evaluate the cytotoxicities of TET and radiation, surviving
fractions of CT26/tk-luc cells were determined by colony
formation assay. Cells were treated with various concentrations of
TET for 3 h. The growth inhibition by TET was found to occur in a
dose-dependent manner. The IC50 (50% inhibition
concentration) was ~10 µM, as shown in Fig. 1C. The radiation response of
CT26/tk-luc cells was examined following single doses of
Co-60 gamma irradiation. The radiation survival curve is shown in
Fig. 1D. The D0 was ~
2.0 Gy, as calculated from the formula D10=2.3
D0 using D10=4.7 Gy. In addition,
CT26/tk-luc cells were treated with 20 µM TET for 3 h, and
then combined with different doses of radiation. The survival curve
for the combination of TET and radiation shifted the radiation
survival curve downward without changing the curve shape,
suggesting that the combined effect of TET and radiation on
CT26/tk-luc cells was additive.
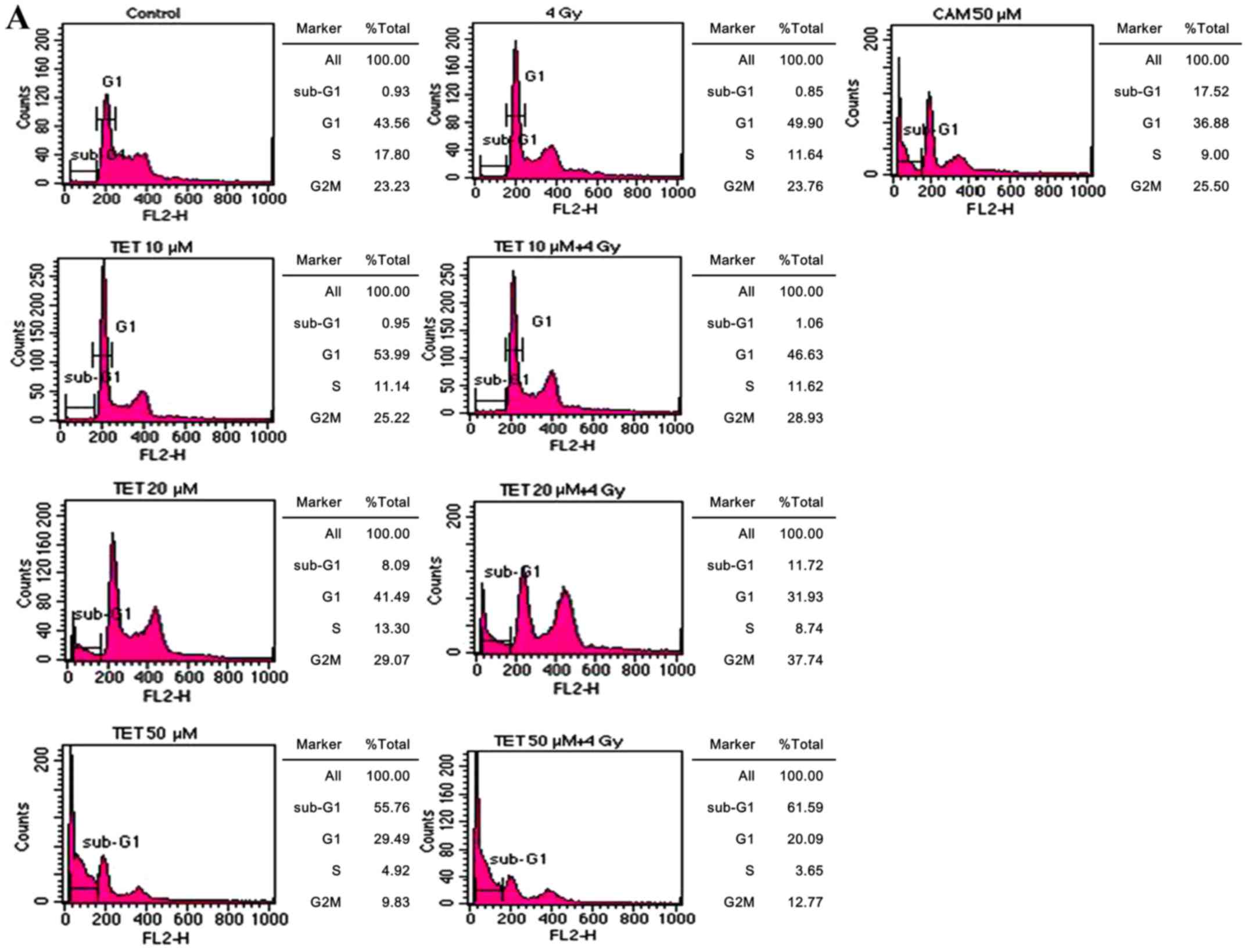
Analysis of the sub-G1 fraction by
flow cytometry and apoptosis by DNA fragmentation assay
Apoptosis induced by TET with or without radiation
in CT26/tk-luc cells was also determined via the sub-G1 cell
population by flow cytometry. Fig.
2A reveals the DNA histograms of CT26/tk-luc cells
post-treatment. The sub-G1 populations of CT26/tk-luc cells
were 0.93, 0.95, 8.09 and 55.76% for 0, 10, 20 and 50 µM,
respectively, following TET treatment for 3 h. The sub-G1
populations of CT26/tk-luc cells were 0.85, 1.06, 11.72 and
61.59% for 0, 10, 20 and 50 µM TET treatment for 3 h combined with
4 Gy, respectively. Notably, significant G1 phase arrest was found
in all TET-treated groups. CAM (50 µM) was used as the positive
control. The mean sub-G1 populations in CT26/tk-luc cells
induced by TET with or without radiation determined by flow
cytometric assay are displayed in Fig.
2B. The decrease in cell viability due to apoptosis rather than
necrosis was detected by DNA ladder assay. To evaluate the nature
of TET-induced apoptosis, CT26/tk-luc cells were treated
with the indicated TET concentrations or treated with TET in
combination with radiation. Internucleosomal DNA fragmentation was
observed, as revealed in Fig. 2C.
The results indicated that TET alone or in combination with
radiation induced apoptosis in CT26/tk-luc cells.
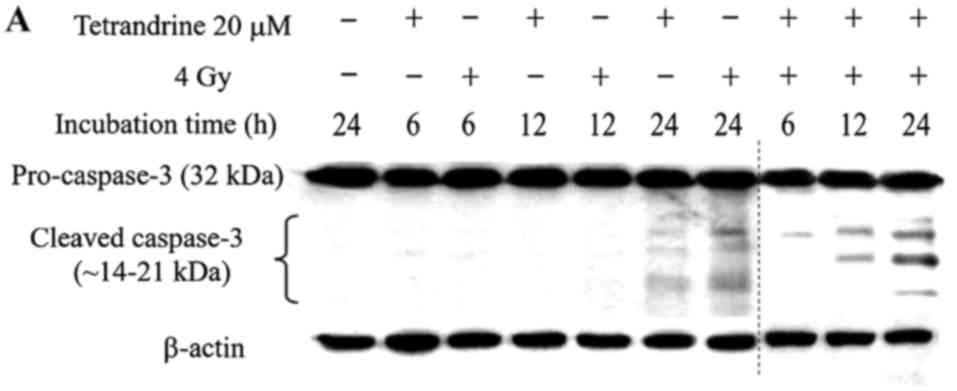
Western blotting for caspase-3
activity assay
Activation of caspase-3 can be demonstrated from the
cleavage of pro-caspase-3 into cleaved caspase-3. Cleaved caspase-3
was detected in CT26/tk-luc cells after 20 mM TET treatment
for 6, 12 or 24 h with or without 4 Gy irradiation, respectively,
as shown in Fig. 3A. In addition,
CT26/tk-luc cells were treated with various concentrations
of TET for 24 h with or without 4 Gy. The expression levels of
cleaved caspase-3 quantified by Scion imaging software were higher
when concurrently treated with TET and radiation, as shown in
Fig. 3B. These results
unambiguously indicated that activation of caspase-3 contributed to
TET-induced apoptosis in murine colorectal CT26/tk-luc
cancer cells.
TET combined with radiation enhances
the regression of CT26/tk-luc tumors in vivo
Antitumor activity of TET combined with radiation
was assessed in a CT26/tk-luc tumor-bearing Balb/c mouse
model. The experimental protocol is presented in Fig. 4A. The monitoring of
CT26/tk-luc tumor volumes was initiated on day 12 post-tumor
cell inoculation, when bulges were observed. Treatment was
administered when the tumor volume reached ~100 mm3. The
tumor growth inhibition caused by TET with or without irradiation
is shown in Fig. 4B. No significant
differences were observed among the groups treated with TET alone
in any fractionation protocol. However, radiation alone and
combination therapy exhibited a significant decrease in tumor
volume as compared with either TET alone or the control group
(P<0.01). To determine whether TET could enhance tumor
growth inhibition in irradiated tumors, mice were i.p.
injected with 10 mg/kg TET 24 h before 3 Gy irradiation for a total
of six administrations. The time required to reach 800
mm3 tumor volume for the control, 5, 10 and 20 mg/kg of
TET, radiation alone (3 Gyx6) and combination therapy (TET 10
mg/kg+3 Gy) groups was 8.0±1.8, 10.7±1.6, 10.7±1.0, 11.3±1.8,
18.4±2.1 and 23.1±1.3 days, respectively. These results are
presented in Table I. The TGD times
were 2.7±2.4, 2.7±2.1, 3.3±2.5, 10.4±2.8 and 15.1±2.2 for 5, 10 and
20 mg/kg of TET, radiation alone and combination therapy,
respectively. The calculated ER for the combination group compared
with the radiation alone group based on the TGD time was 1.45,
indicating that TET combined with IR for the treatment of
CT26/tk-luc cancer in vivo was synergistic.
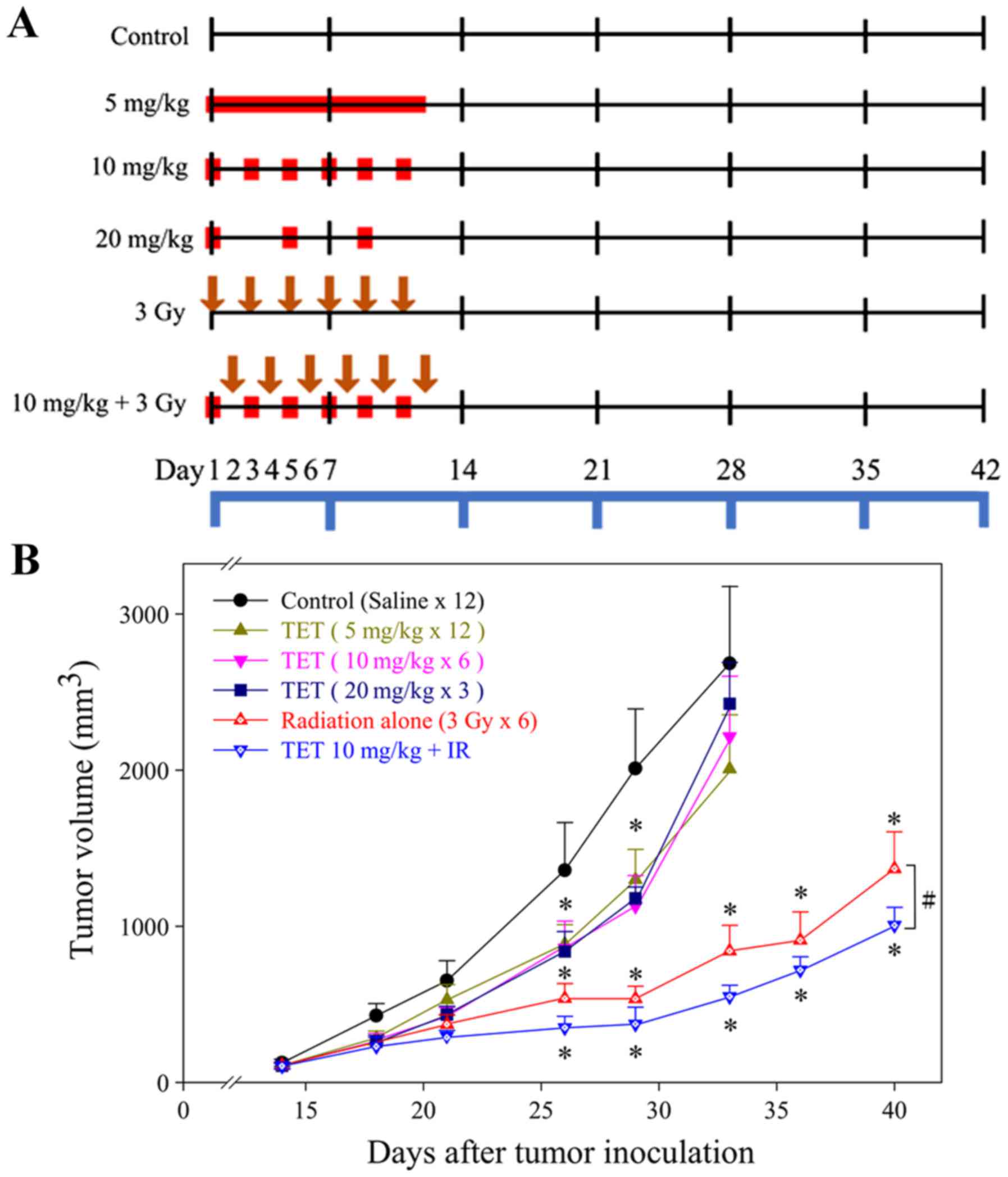 | Figure 4.Tumor growth curves in
CT26/tk-luc tumor-bearing mice. (A) Experimental design for
the evaluation of therapeutic efficacy of tetrandrine (TET).
Treatment was initiated when the tumor volume reached 100
mm3. Mice were randomly assigned to six groups: Control,
20, 10 and 5 mg/kg TET, radiation alone and combination. TET was
administered every 4 days for 2 weeks in the 20 mg/kg group, every
2 days for 2 weeks in the 10 mg/kg group and for 12 consecutive
days in the 5 mg/kg group by intraperitoneal injection (i.p.), i.e.
3, 6 and 12 fractions for a total of 60 mg/kg. For the radiation
alone group, 3 Gy was administered every other day in 6 fractions,
and for the combination group, 10 mg/kg TET or 3 Gy were
alternately administered every day for 2 weeks. The red symbols
indicate the time points for the administration of TET, while the
downward brown arrows indicate irradiation. (B) Tumor growth curves
of CT26/tk-luc tumor-bearing mice following different
treatments. The time required to reach an 8-fold increase from the
initial tumor volume was used as the endpoint for determining the
tumor growth delay assays. n=7 for all mouse groups, except n=6 in
the control group. *P<0.05 vs. the control, and
#P<0.05, the radiation alone group vs. the
combination group. |
 | Table I.Therapeutic efficacy of TET with or
without RT on CT26/tk-luc tumor growth. |
Table I.
Therapeutic efficacy of TET with or
without RT on CT26/tk-luc tumor growth.
| Groups | No. of mice | Tumor growth
timea (days ± SE) | Tumor growth delay
timeb (days ± SE) |
|---|
| Control | 6 | 8.0±1.8 | 0.0±0.0 |
| TET 5 mg/kg ×
12 | 7 | 10.7±1.6 | 2.7±2.4 |
| TET 10 mg/kg ×
6 | 7 | 10.7±1.0 | 2.7±2.1 |
| TET 20 mg/kg ×
3 | 7 | 11.3±1.8 | 3.3±2.5 |
| RT (3 Gy × 6) | 7 | 18.4±2.1 | 10.4±2.8 |
| TET 10 mg/kg +
RT | 7 | 23.1±1.3 | 15.1±2.2 |
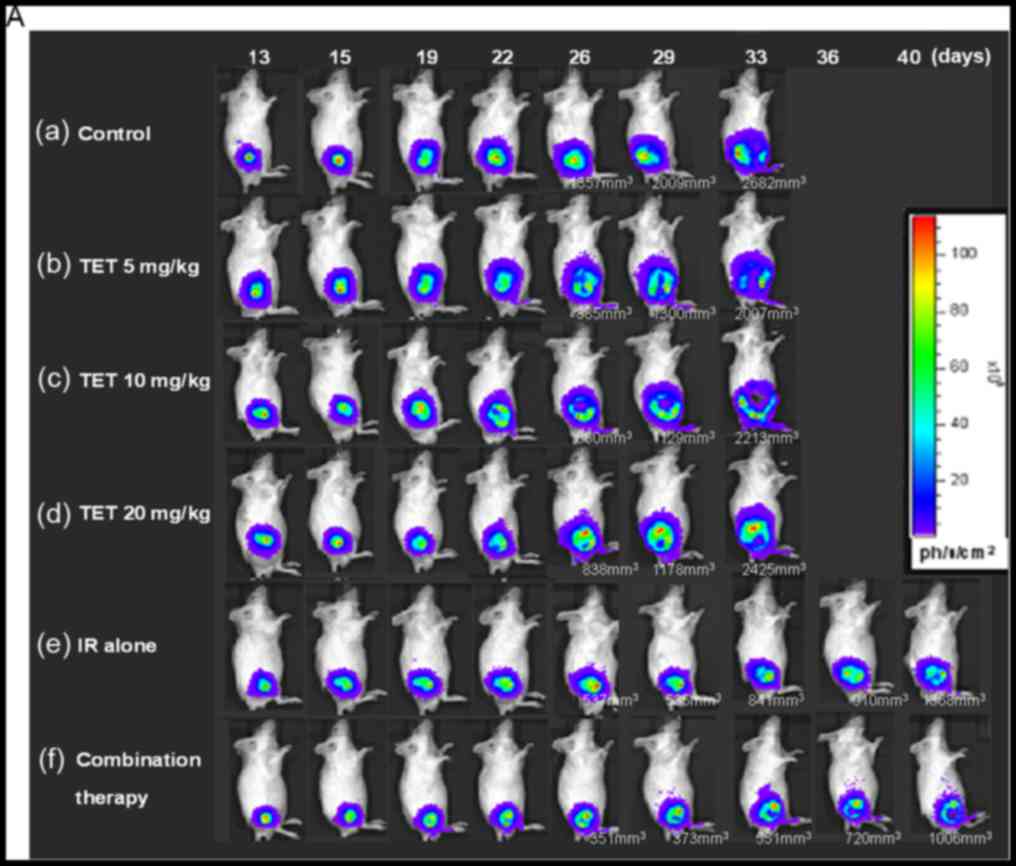
Evaluation of therapeutic efficacy
with BLI and gamma scintigraphy
CT26/tk-luc cells (2×105) were
inoculated into the right flank of BALB/c mice to monitor
subcutaneous tumor growth and the therapeutic efficacy of TET with
or without radiation by non-invasive molecular imaging modalities:
BLI using the Xenogen IVIS50 system and gamma scintigraphy.
Fig. 5A shows the longitudinal
monitoring of the tumor growth by BLI. In addition, the mice were
also monitored by gamma scintigraphy at 24 h after
131I-FIAU intravenous administrations. The
representative images of the CT26/tk-luc tumor-bearing
animals are shown in Fig. 5B. ROIs
of the tumor exhibited higher radioactivity compared with normal
soft tissue. The radioactive counts over the tumor area were
normalized against the tumor size (pixels). The ROI was also
created over a non-tumor area (muscle) to determine the
radioactivity of the background. Notably, the tumor/muscle (T/M)
ratios were decreased as the therapeutic time increased. The
tumor/muscle (T/M) ratios were 90.52, 74.51, 69.37, 66.25, 64.86
and 41.95 for the control, 5, 10 or 20 mg/kg TET, radiation alone
and combination therapy, respectively.
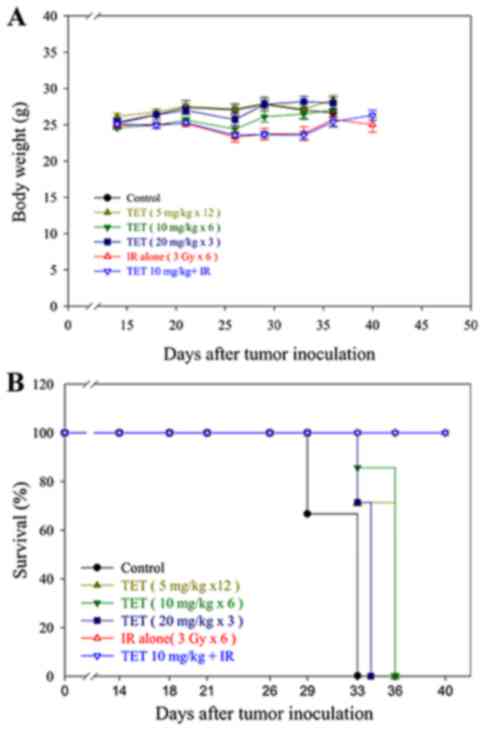
Body weight and survival curves in
vivo
The general toxicity of TET with or without
radiation was monitored via body weight changes during the
experimental period, as shown in Fig.
6A. No significant body weight changes among the six groups
were found. Additionally, no other adverse effects, such as skin
changes or hair loss, were observed in the mice post-radiation or
with combination therapy. The survival curves in vivo were
calculated using the Kaplan-Meier method, as presented in Fig. 6B. Mice were considered expired when
the tumor volume reached 2,500 mm3.
Discussion
The antiproliferation effect of TET has been
revealed in several different cell lines, and this inhibitory
effect is reportedly exerted through apoptotic pathways (27–30).
Ionizing radiation may cause DNA damage and induce apoptosis
through the intrinsic pathway. Ionizing radiation may also cause
rapid sphigomyelin hydrolysis to ceramide and initiate the
extrinsic apoptotic pathway. In the present study, the
IC50 of TET on CT26/tk-luc cells was 10 µM
(Fig. 1C). The survival curves of
radiation alone and radiation combined with 20 µM TET were similar
and exhibited the same slope (Fig.
1D). This is indicative of an additive effect of combined
therapeutic drug and ionizing radiation. This finding is different
from that reported by Sun et al, who found a synergistic
effect using a human CNE nasopharyngeal carcinoma cell line
(30). This difference in
radiosensitivity may be due to the following reasons: Firstly, the
inherent radiosensitivity among various cancer cell lines is
different; secondly, the TET dose used in combination with
radiation was 20 µM in the present study vs. 2 µM in the study by
Sun et al; thirdly, the sequence of the two treatments may
affect the outcome. In this study, CT26/tk-luc cells were
treated with 20 µM TET for 3 h followed by various doses of
radiation, while CNE cells were treated with 2 mM TET
post-irradiation in the study of Sun et al (30). The apoptosis was induced by TET in a
dose-dependent manner, as demonstrated by the sub-G1 fraction and
DNA fragmentation assays (Fig.
2A-C). In addition, cleaved caspase-3 in CT26/tk-luc
cells treated with TET with or without radiation was also
identified (Fig. 3A and B). The
non-invasive BLI modality used for tumor growth monitoring obtains
high-throughput and real-time images of therapeutic efficacy in
animals (24). In the present
study, the CT26/tk-luc cell line carrying HSV-1 thymidine
kinase and firefly luciferase dual reporter genes was suitable for
BLI and nuclear imaging, such as gamma scintigraphy (Fig. 5A and B).
Animals bearing CT26/tk-luc tumor xenografts
treated with 5, 10 or 20 mg/kg TET exhibited similar inhibition
rates of tumor growth, since the total dosage was the same. The
control of tumor growth was significantly improved in the radiation
alone and combination groups compared with the control and TET
alone groups. Notably, TET pretreatment followed by radiation
exhibited a synergistic effect on tumor control, rather than an
additive effect as determined in the in vitro study
(Fig. 1D and Table I). The therapeutic ER for the
combination group in vivo was 1.45. The enhancement in
radiosensitization effect of TET in vivo may be partly due
to the biochemical metabolism of TET into active ingredients.
Alternatively, the enhanced effect may involve modulation of the
tumor microenvironment, such as downregulation of the ERK/NF-kB or
Akt/NF-kB/MMP-9 signaling pathways, which can be induced by
ionizing radiation (31–33). Activation of these signaling
pathways often results in an increase in effector proteins that are
involved in tumor angiogenesis, anti-apoptosis, proliferation and
invasion (31). However, this
phenomenon requires further investigation. In addition, no general
toxic effects were found in all experimental groups. These results
demonstrated that TET is a radiosensitizer with a synergistic
effect in vivo, and may be used in combination with ionizing
radiation for cancer treatment in the clinic.
In conclusion, the cytotoxic effect of TET on
CT26/tk-luc cells occured via the induction of apoptosis,
and the combined effect of TET with ionizing radiation was additive
in vitro. However, the combined effect of TET with ionizing
radiation was synergistic in a CT26/tk-luc tumor-bearing
animal model, and may have potential applications as a treatment
adjuvant for cancer chemoradiotherapy.
Acknowledgements
The authors thank both the National Science Council
(Taipei, Taiwan) and the Taipei City Hospital (Taipei, Taiwan) for
the financial support.
Funding
The present study was supported by grants NSC94-
2314-B-010-003 from the National Science Council (Taipei, Taiwan),
and 95003-62-121 from the Taipei City Hospital Research Program
(Taipei, Taiwan).
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
Authors' contributions
WCL, WHW, YJC and JJH contributed to the conception
and design of the study. WCL, WHW, YHL and SYC performed the
experiments and wrote the first draft of the manuscript. JDL, YJC
and JJH organized the research, contributed to the revision of the
manuscript and gave final approval of the version to be published.
All authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The animal use protocol listed below (PDF file:
950501_IACUC_Jeng-Jong Hwang.pdf) has been reviewed and approved by
the Institutional Animal Care and Use Committee (IACUC).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sebag-Montefiore D, Stephens RJ, Steele R,
Monson J, Grieve R, Khanna S, Quirke P, Couture J, de Metz C, Myint
AS, et al: Preoperative radiotherapy versus selective postoperative
chemoradiotherapy in patients with rectal cancer (MRC CR07 and
NCIC-CTG C016): A multicentre, randomised trial. Lancet.
373:811–820. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin JK, Lee LK, Chen WS, Lin TC, Jiang JK,
Yang SH, Wang HS, Chang SC, Lan YT, Lin CC, et al: Concurrent
chemoradiotherapy followed by metastasectomy converts to survival
benefitin stage IV rectum cancer. J Gastrointest Surg.
16:1888–1896. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Abbasakoor F, Vaizey CJ and Boulos PB:
Improving the morbidity of anorectal injury from pelvic
radiotherapy. Colorectal Dis. 8:2–10. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen YJ: Potential role of tetrandrine in
cancer therapy. Acta Pharmacol Sin. 23:1102–1106. 2002.PubMed/NCBI
|
|
6
|
Lai JH: Immunomodulatory effects and
mechanisms of plant alkaloid tetrandrine in autoimmune diseases.
Acta Pharmacol Sin. 23:1093–1101. 2002.PubMed/NCBI
|
|
7
|
Wang H, Liu T, Li L, Wang Q, Yu C, Liu X
and Li W: Tetrandrine is a potent autophagy agonist via activated
intracellular reactive oxygen species. Cell Biosci. 5:42015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu T, Liu X and Li W: Tetrandrine, a
Chinese plant-derived alkaloid, is a potential candidate for cancer
chemotherapy. Oncotarget. 7:40800–40815. 2016.PubMed/NCBI
|
|
9
|
Wong VK, Zeng W, Chen J, Yao XJ, Leung EL,
Wang QQ, Chiu P, Ko BCB and Law BYK: Tetrandrine, an activator of
autophagy, induces autophagic cell death via PKC-α inhibitionand
mTOR-dependent mechnisms. Front Pharmacol. 8:3512017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lai YL, Chen YJ, Wu TY, Wang SY, Chang KH,
Chung CH and Chen ML: Induction of apoptosis in human leukemic U937
cells by tetrandrine. Anticancer Drugs. 9:77–81. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee JH, Kang GH, Kim KC, Kim KM, Park DI,
Choi BT, Kang HS, Lee YT and Choi YH: Tetrandrine-induced cell
cycle arrest and apoptosis in A549 human lung carcinoma cells. Int
J Oncol. 21:1239–1244. 2002.PubMed/NCBI
|
|
12
|
Yoo SM, Oh SH, Lee SJ, Lee BW, Ko WG, Moon
CK and Lee BH: Inhibition of proliferation and induction of
apoptosis by tetrandrine in HepG2 cells. J Ethnopharmacol.
81:225–229. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen Y, Chen JC and Tseng SH: Effects of
tetrandrine plus radiation on neuroblastoma cells. Anticancer Res.
29:3163–3171. 2009.PubMed/NCBI
|
|
14
|
Wu K, Zhou M, Wu QX, Yuan SX, Wang DX, Jin
JL, Huang J, Yang JQ, Sun WJ, Wan LH and He BC: The role of IGFBP-5
in mediating the anti-proliferation effect of tetrandrine in human
colon cancer cells. Int J Oncol. 46:1205–1213. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chang KH, Chen ML, Chen HC, Huang YW, Wu
TY and Chen YJ: Enhancement of radiosensitivity in human
glioblastoma U138MG cells by tetrandrine. Neoplasma. 46:196–200.
1999.PubMed/NCBI
|
|
16
|
Gupta S: Molecular steps of death receptor
and mitochondrial pathways of apoptosis. Life Sci. 69:2957–2964.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Salvesen GS: Caspase 8: Igniting the death
machine. Structure. 7:R225–R229. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stennicke HR, Jürgensmeier JM, Shin H,
Deveraux Q, Wolf BB, Yang X, Zhou Q, Ellerby HM, Ellerby LM,
Bredesen D, et al: Pro-caspase-3 is a major physiologic target of
caspase-8. J Biol Chem. 273:27084–27090. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Haimovitz-Friedman A, Kan CC, Ehleiter D,
Persaud RS, McLoughlin M, Fuks Z and Kolesnick RN: Ionizing
radiation acts on cellular membranes to generate ceramide and
initiate apoptosis. J Exp Med. 180:525–535. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu J, Liu F, Sun M, Sun Z and Sun S:
Enhancement of radiosensitivity and the potential mechanism on
human esophageal carcinoma cells by tetrandrine. Cancer Biother
Radiopharm. 26:437–442. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chang YF, Lin YY, Wang HE, Liu RS, Pang F
and Hwang JJ: Monitoring of tumor growth and metastasis potential
in MDA-MB-435s/tk-luc human breast cancer xenografts. Nucl Instrum
Meth A. 571:155–159. 2007. View Article : Google Scholar
|
|
22
|
Chen CC, Hwang JJ, Ting G, Tseng YL, Wang
SJ and Peng JW: Monitoring and quantitative assessment of tumor
burden using in vivo bioluminescence imaging. Nucl Instrum Meth A.
571:437–441. 2007. View Article : Google Scholar
|
|
23
|
Jänicke RU, Sprengart ML, Wati MR and
Porter AG: Caspase-3 is required for DNA fragmentation and
morphological changes associated with apoptosis. J Biol Chem.
273:9357–9360. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Burgos JS, Rosol M, Moats RA, Khankaldyyan
V, Kohn DB, Nelson MD Jr and Laug WE: Time course of bioluminescent
signal in orthotopic and heterotopic brain tumors in nude mice.
Biotechniques. 34:1184–1188. 2003.PubMed/NCBI
|
|
25
|
Cividalli A, Ceciarelli F, Livdi E,
Altavista P, Cruciani G, Marchetti P and Danesi DT:
Radiosensitization by oxaliplatin in a mouse adenocarcinoma:
Influence of treatment schedule. Int J Radiat Oncol Biol Phys.
52:1092–1098. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Heckelsmiller K, Rall K, Beck S, Schlamp
A, Seiderer J, Jahrsdörfer B, Krug A, Rothenfusser S, Endres S and
Hartmann G: Peritumoral CpG DNA elicits a coordinated response of
CD8 T cells and innate effectors to cure established tumors in a
murine colon carcinoma model. J Immunol. 169:3892–3899. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jang BC, Lim KJ, Paik JH, Cho JW, Baek WK,
Suh MH, Park JB, Kwon TK, Park JW, Kim SP, et al:
Tetrandrine-induced apoptosis is mediated by activation of caspases
and PKC-δ in U937 cells. Biochem Pharmacol. 67:1819–1829. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kuo PL and Lin CC: Tetrandrine-induced
cell cycle arrest and apoptosis in Hep G2 cells. Life Sci.
73:2432522003. View Article : Google Scholar
|
|
29
|
Ng LT, Chiang LC, Lin YT and Lin CC:
Antiproliferative and apoptotic effects of tetrandrine on different
human hepatoma cell lines. Am J Chin Med. 34:125–135. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun X, Xu R, Deng Y, Cheng H, Ma J, Ji J
and Zhou Y: Effects of Tetrandrine on apoptosis and
radiosensitivity of nasopharyngeal carcinoma cell line CNE. Acta
Biochim Biophys Sin. 39:869–878. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hsu FT, Chang B, Chen JC, Chiang IT, Liu
YC, Kwang WK and Hwang JJ: Synergistic effect of sorafenib and
radiation on human oral carcinoma in vivo. Sci Rep. 5:153912015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu CJ, Wang YH, Lin CJ, Chen HH and Chen
YJ: Tetrandrine down-regulaties ERK/NF-κB signaling and inhibits
activtion of mesangial cells. Toxicol in vitro. 25:1834–1840. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen S, Liu W, Wang K, Fan Y, Chen J, Ma
J, Wang X, He D, Zeng J and Li L: Tetrandrine inhibits migration
and invasion of human renal cell carcinoma by regulating
Akt/NF-κB/MMP-9 signaling. PLoS One. 12:e01737252017. View Article : Google Scholar : PubMed/NCBI
|