Introduction
Glioma is the most common and the most lethal brain
tumor in the adult central nervous system, with characteristics of
being highly heterogeneous, having a strong invasive ability and
radiotherapy and chemotherapy resistance (1). Due to the fact that its pathogenesis
is not completely clear, there is still a lack of effective
treatments for glioma. Using radiotherapy combined with
temozolomide, we treated newly diagnosed glioblastoma, which
doubled the 2-year survival rate of patients up to 27% (2). However, the patient overall prognosis
remains poor. In addition to the traditional diagnostic criteria
for histopathology, molecular diagnostics play a vital part in the
auxiliary typing of glioma, incorporating the information about the
entire deletions of 1p/19q, the shifts in the EGFR signaling
pathway, and the gene mutations of MGMT promoter methylation and
isocitrate dehydrogenases 1 (IDH1) (3,4). A
large and growing body of literature has investigated new molecular
biomarkers in glioma that satisfied the needs of precise treatment
for glioma (5,6). Emerging evidence demonstrates that the
activation of amino acid biosynthetic pathways has an important
role in the progression of cancer (7,8).
Enolase-phosphatase 1 (ENOPH1, also called mtnC) is
a new enzyme of research interest, which is not only involved in
the synthesis of polyamine, but is also required for methionine
remediation synthesis (9).
Recently, Barth et al (10)
found that ENOPH1 was extensively expressed in the brain and that
ENOPH1 protein levels in the brain tissue of C57BL/6J mice could be
enhanced by stress responses (10).
Polyamines are present in all eukaryotic cells, their synthesis is
closely related to cell growth or tumor cell proliferation and play
a key role in regulating tumor cell differentiation (11,12).
Polyamine accumulation has a significant effect on glioma growth
(13). Since ENOPH1 participates
indirectly via S-adenosyl methionine (SAM) in the synthesis of
polyamine (14–17), we can logically conclude that ENOPH1
has a role in glioma. However currently, the mechanisms controlling
the expression and function of ENOPH1 in glioma are not well
understood.
Thus, in the present study, we used in vitro
cultured human glioma cell lines and in vivo human glioma
tissues to explore the role of ENOPH1 in glioma progression. Apart
from evaluating the expression pattern of ENOPH1 gene and protein,
we also examined the effect of targeted silencing of the ENOPH1
gene in glioma cell proliferation and migration. Our data revealed
that ENOPH1 was upregulated in human glioma cell lines, and glioma
samples and knockdown of ENOPH1 decreased glioma cell proliferation
and migration along with translocation of its downstream protein
aci-reductone dioxygenase 1 (ADI1) and downregulation of membrane
type 1-matrix metalloproteinase (MT1-MMP).
Materials and methods
Clinical tissue sample collection
Tumor samples as well as patient
clinical-pathological data were acquired from Wuhan General
Hospital of PLA (Wuhan, China) between January 2013 and December
2014. All the experiments using the human samples were authorized
by the Ethics Committee of Southern Medical University. All
patients willingly consented to participate in the study by signing
an informed consent form. In terms of the World Health Organization
(WHO) classification (18), a total
of 86 gliomas were classified as 12 pilocytic astrocytomas (WHO I),
15 diffuse astrocytomas (WHO II), 18 anaplasia astrocytomas (WHO
III) and 41 primary glioblastomas (WHO IV). None of the patients
had been treated with chemotherapy or radiotherapy before surgery.
Resected tumor tissues were snap-frozen in liquid nitrogen and
maintained at −80°C and liquid nitrogen before use, and the other
tissues were formalin-fixed and paraffin-embedded for conventional
histopathology and immunohistochemistry (IHC). Clinical parameters,
including age, sex and pathological grading of all patients are
presented in Table I. The period of
follow-up was from 0.2 to 68.4 months.
 | Table I.Clinicopathological characteristics
of glioma patients. |
Table I.
Clinicopathological characteristics
of glioma patients.
|
|
| ENOPH1
expression |
|---|
|
|
|
|
|---|
| Clinical
characteristics | Patient number | High, n (%) | Low, n (%) |
|---|
| Age years |
|
<45 | 30 | 13 (43) | 17 (57) |
|
≥45 | 56 | 25 (47) | 31 (53) |
| Sex |
|
Male | 45 | 20 (45) | 25 (55) |
|
Female | 41 | 21 (51) | 20 (49) |
| WHO grade |
| I | 12 | 3
(25) | 9 (75) |
| II | 15 | 4
(27) | 11 (73) |
|
III | 18 | 14 (78) | 4
(22) |
| IV | 41 | 34 (83) | 7
(17) |
IHC
Immunohistochemical staining was performed as
previously mentioned (19). The
tissues sections were formalin-fixed and paraffin-embedded. The
sections were deparaffinized in xylene and rehydrated by graded
alcohol to water, and by incubating the slides in 3%
H2O2 in water at room temperature for 30 min
endogenous peroxidase activity was eliminated. Subsequently, after
being incubated in 1% BSA for 30 min, the sections were wiped off,
and then a dilution of anti-ENOPH1 (1:250; cat. no. sc-365155;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) was applied to the
slides and incubated at room temperature overnight. Subsequently,
the slides were incubated at room temperature with a secondary
antibody (1:1,000; cat. no. 323-005-021; Jackson ImmunoResearch
Labs, West Grove, PA, USA) for 2 h, following the manufacturer's
instructions. At the same concentration as for the primary antibody
(ENOPH1; 1:250; cat. no. sc-365155; Santa Cruz Biotechnology), the
negative control slides were processed in parallel with a
non-specific immunoglobulin IgG (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany). Finally, the stained sections were examined
under a light microscope (Leica Microsystems, Wetzlar,
Germany).
Cell culture and transfection
The human glioma cell lines U87 and U251 were
obtained from the Type Culture Collection of the Chinese Academy of
Sciences (Shanghai, China). The U87 cell line is glioblastoma of
unknown origin. The glioma cells were grown in Dulbecco's modified
Eagle's medium (DMEM) supplemented with 10% fetal bovine serum
(FBS) (both from Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) at 37°C in humidified 5% CO2/95% air. To obtain
cells in the exponential growth phase, cell cultures for the assays
were initiated at a density of 5×105 cells/ml. We used a
haemocytometer (Adam MC; Digital Bio, Seoul, Korea) to count the
number of cells. Following the manufacturer's instructions, at
60–70% confluence on dishes of different sizes (Corning Glass
Works, Corning, NY, USA), glioma cells were transfected with 100 nM
ENOPH1 siRNA (si-ENOPH1; cat. no. sc-88932) or scrambled control
siRNA (si-control; cat. no. sc-37007) with the use of siRNA
transfection reagent (cat. no. sc-29528; all from Santa Cruz
Biotechnology, Inc.). The cells were not used in the following
experiments until 48 h post-transfection, and the specific
silencing effect was verified by western blot analysis. The cell
lines have been authenticated by STR profiling.
RNA extraction and quantitative
real-time PCR
Total RNA was obtained from glioma cells using
TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) following the
manufacturer's instructions. After being reversely transcribed
using TaqMan® Reverse Transcription kit and the Vii7
Real-Time PCR System, the products were amplified in a 10-µl final
reaction volume using SYBR® Green PCR Master Mix (all
from Applied Biosystems; Thermo Fisher Scientific, Inc.). This was
performed under the following conditions: 30 sec at 95°C, followed
by a total of 40 cycles of two temperature cycles (15 sec at 95°C
and 1 min at 60°C). The primer sequences used were as follows:
human ENOPH1 forward, 5′-AGAAGACTTACTACAGCCTCA-3′ and reverse,
5′-AACTACACTTTGCCCTCA-3′; GAPDH served as the endogenous control,
and the primers were as follows: forward,
5′-CAATGTGTCCGTCGTGGATCT-3′ and reverse,
5′-GTCCTCAGTGTAGCCCAAGATG-3′. Using the SDS Enterprise Database
v2.0.6 software (Applied Biosystems; Thermo Fisher Scientific,
Inc.), the Cq value was calculated using the comparative
2−ΔΔCq method (20).
Cell viability and proliferation
assays
Using a 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl
tetrazolium bromide (MTT) assay, the viability and proliferation of
glioma cells were determined. After being seeded in 96-well plates
(2×103 cells/well), the U251 cells were transfected with
siRNAs. The cells were cultured for an appropriate time period and
then 0.5 mg/ml MTT (MedChem Express, Monmouth Junction, NJ,
USA).
USA) was added to each well and
cultured for 4 h at 37°C
By measuring the absorption at a wavelength of 490
nm, the number of viable growing cells was calculated after the
cells were lysed for 10 min at 37°C in 200 µl dimethyl sulfoxide
(Sigma-Aldrich; Merck KGaA). This allowed for the establishment of
cell growth curves in terms of the optical density (OD) value. The
proliferation rate was calculated according to the following
formula: survival rate = (OD test/OD negative control) ×100%. In
addition to being repeated at least three times, all the
experiments were performed in triplicate.
Cell migration scratch assay
Scratch assays were conducted to determine the
glioma cell migration ability. Before being transfected with the
control siRNA or the ENOPH1 siRNA (50 nM) 24 h later, the cells
were seeded in 12-well plates (2×105 cells/well). Next,
the cells were cultured for a corresponding time period, treated
with colchicine (10 µg/ml) for 1 h, and then a 10-µl sterile
micropipette tip was used to create an artificial wound.
Subsequently, the displaced cells and debris were removed by
rinsing the plates with PBS before replacing the culture media.
Using ImageJ software (Java 1.8.0_112; NIH, Bethesda, MD, USA) the
distance covered by the cells at the leading edge of the wound at
each time-point was calculated, and the scratched area was imaged
under a fluorescence microscope at 0 and 24 h. The results were
reported by percentage of migrated cells.
Cell cycle analysis
DNA content analysis was determined by propidium
iodide (PI) staining. In brief, after the cells in each group were
washed with PBS twice and centrifuged at 1,000 rpm for 5 min to
adjust their density to 1×106 cells/well, cold 70% ethyl
alcohol was added, and the cells were maintained at 4°C for
overnight fixation. In addition, before being incubated with 400 µl
PI staining buffer (50 µg/ml; NanJing KeyGen Biotech Co., Ltd.,
Nanjing, China) at 4°C in the dark for 30 min, the fixed cells were
washed with PBS, and then, 100 µl RNase was added to the sample for
incubation for 30 min at 37°C. Using a flow cytometer (Beckman
Coulter, Brea, CA, USA), the cell cycle distribution was
determined, and the percentage of cells in the G1, S and G2/M
phases with the FlowJo software (FlowJo V10; Tree Star, Inc.,
Ashland, OR, USA) was determined. The experiment was performed at
least three times.
Western blot analysis
Glioma tissues and glioma cell lines were
solubilized in RIPA lysis buffer (Thermo Fisher Scientific, Inc.).
With the help of a Pierce™ BCA Protein Assay kit (Thermo Fisher
Scientific, Inc.) the cell lysates were centrifuged at 12,000 rpm
and the protein concentrations were estimated. The cell lysates
were separated by electrophoresis on 10–12% sodium dodecyl sulfate
(SDS)-PAGE and 25 µg of protein was loaded per lane, before being
transferred to polyvinylidene difluoride (PVDF) membranes. The
membranes were then blocked in TBS-T containing 5% non-fat milk for
1 h at room temprature, and then incubated with primary antibodies
overnight. The membranes were washed and incubated with horseradish
peroxidase (HRP)-conjugated secondary antibodies (1:1,000; cat. no.
323-005-021; Jackson Immuno Research Labs, West Grove, PA, USA) for
1 h at room temperature and developed with the use of a
chemiluminescence kit (Thermo Fisher Scientific, Inc.). The primary
antibodies were ENOPH1 (1:1,000; cat. no. 11763-1-AP), ADI1
(1:2,000; cat. no. ab154689), MT1-MMP (1:1,000; cat. no. ab51074),
p21 (1:2,000; cat. no. ab109520), p27 (1:4,000; cat. no. ab32034),
cyclin B (1:20,000; cat. no. ab32053), cyclin D (1:30,000; cat. no.
ab134175) and β-actin (1:1,000; cat. no. sc-47778). The anti-ENOPH1
antibody was obtained from ProteinTech Group, Inc. (cat. no.
11763-1-AP; Chicago, IL, USA). In addition, the other primary
antibodies were acquired from Abcam (Cambridge, MA, USA). β-actin
was used as an internal standard for overall protein levels.
Immunocytochemistry
The U87 and U251 glioma cells, which were cultured
on type I collagen-coated coverslips, were fixed with 4%
paraformaldehyde and permeabilized with 0.1% Triton X-100 during
fixation. After applying a blocking solution (3% BSA, 0.1% Tween-20
and 5% goat serum in PBS) non-specific binding was blocked, and
then the cells were cultured with anti-ENOPH1 (1:200) primary
antibodies at 4°C overnight. Subsequently, FITC anti-mouse
secondary antibodies (1:200; Invitrogen; Thermo Fisher Scientific,
Inc.) were added and incubated at room temperature for 1 h. After
applying the anti-fade sealing solution (Maixin Biotech. Co., Ltd.,
Fuzhou, China), the coverslips were mounted on glass slides,
immunostaining was imaged with the use of a DMI6000B fluorescence
microscope (Leica Microsystems GmbH, Wetzlar, Germany). The average
fluorescence intensity of ENOPH1 in the cells in the image was
determined as previously described (21).
Statistical analysis
With the help of SPSS version 13.0 (SPSS, Chicago,
IL, USA), statistical analyses were performed. All the data are
presented as the mean values ± standard deviation (SD). Student's
t-test or one-way ANOVA followed by Tukey's post-hoc test was
applied to determine significant differences between groups.
Overall survival curves were plotted by the Kaplan-Meier method and
were compared by log-rank tests. P<0.05 was considered to
indicate a statistically significant difference.
Results
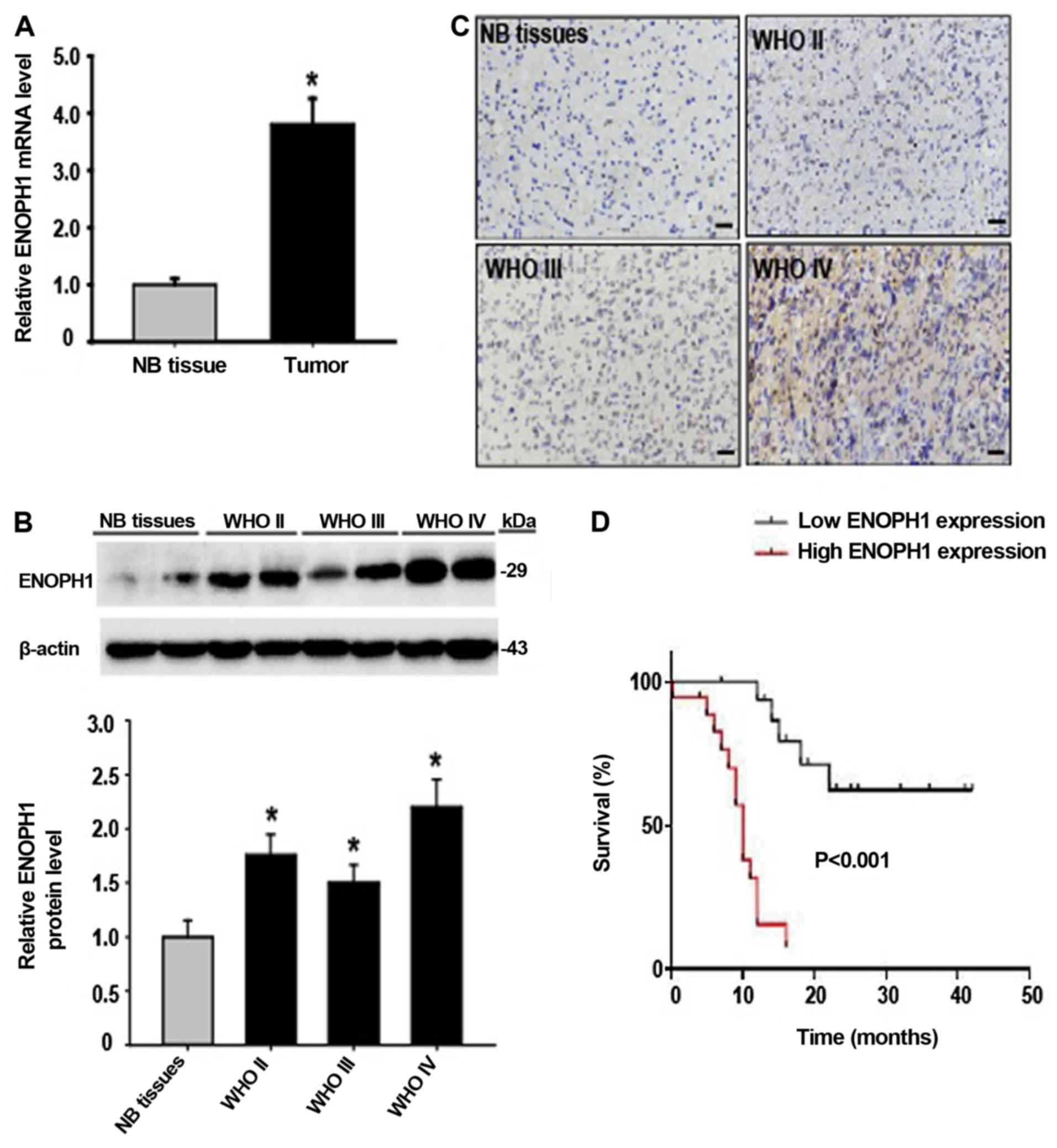
ENOPH1 is upregulated in human glioma
tissues
ENOPH1 has been demonstrated to be expressed in a
wide range of cells in the brain, including neurons and
microvascular endothelial cells, and is involved in stress response
and cell apoptosis (10,21). To identify whether ENOPH1
participates in the progression of glioma, we first studied the
ENOPH1 expression changes in human glioma tissue. By real-time
RT-PCR, we observed that ENOPH1 mRNA expression in glioma tissue
was significantly increased (~4-fold) compared to normal brain
tissue (Fig. 1A). In accordance
with its mRNA change, ENOPH1 protein levels were markedly increased
as well in human glioma tissue, especially in the WHO grade IV
samples (Fig. 1B). IHC analysis
revealed that the expression of ENOPH1 was mainly localized in the
nucleus in the WHO grade II and III glioma samples, in contrast to
normal brain samples, while in the WHO grade IV glioma samples,
ENOPH1 was mainly localized in the cytoplasm of the cells (Fig. 1C). Subsequenlty, we investigated the
association between ENOPH1 expression and overall survival using
Kaplan-Meier survival curve analysis. We observed that a high level
of ENOPH1 expression was associated with a shorter overall survival
(Fig. 1D). Thus, these results
indicated that ENOPH1 was upregulated in glioma patients, and its
expression was significantly associated with clinicopathological
grade and survival status. For the remainder of the present study
we selected extensively used in vitro human glioma malignant
cell lines (i.e., U87 and U251), in order to investigate the role
of ENOPH1 in glioma pathophysiology and to demonstrate the
underlying mechanisms involved.
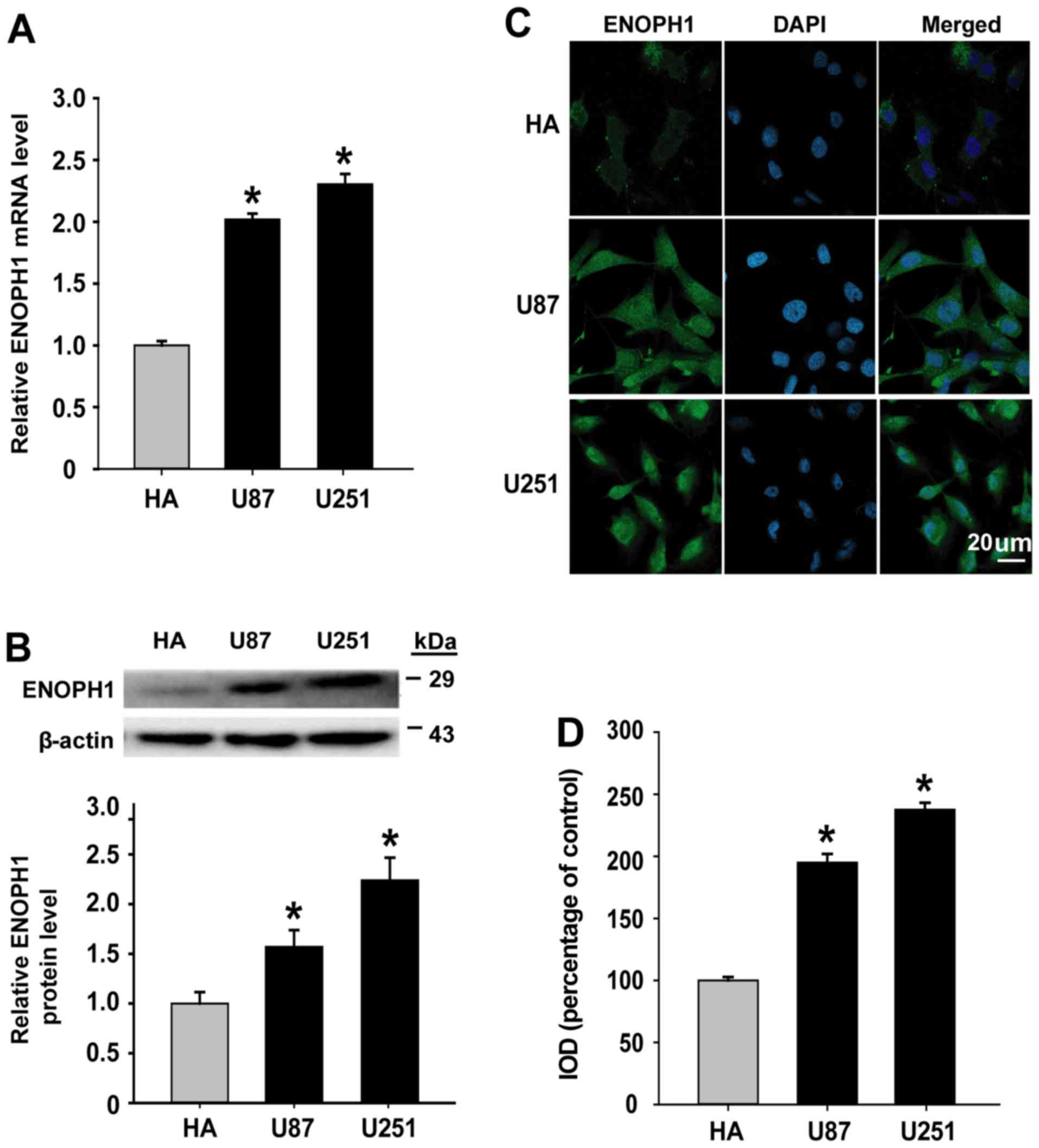
ENOPH1 expression is increased in
glioma cell lines
Taking into account the findings that ENOPH1
expression was increased in human glioma tissues, we determined the
level of ENOPH1 in vitro. We performed qPCR analysis as well
as western blotting to determine the expression of ENOPH1 in glioma
cell lines (U87 and U251). ENOPH1 mRNA expression was significantly
increased in U87 (2.02-fold) and U251 (2.30-fold) glioma cells
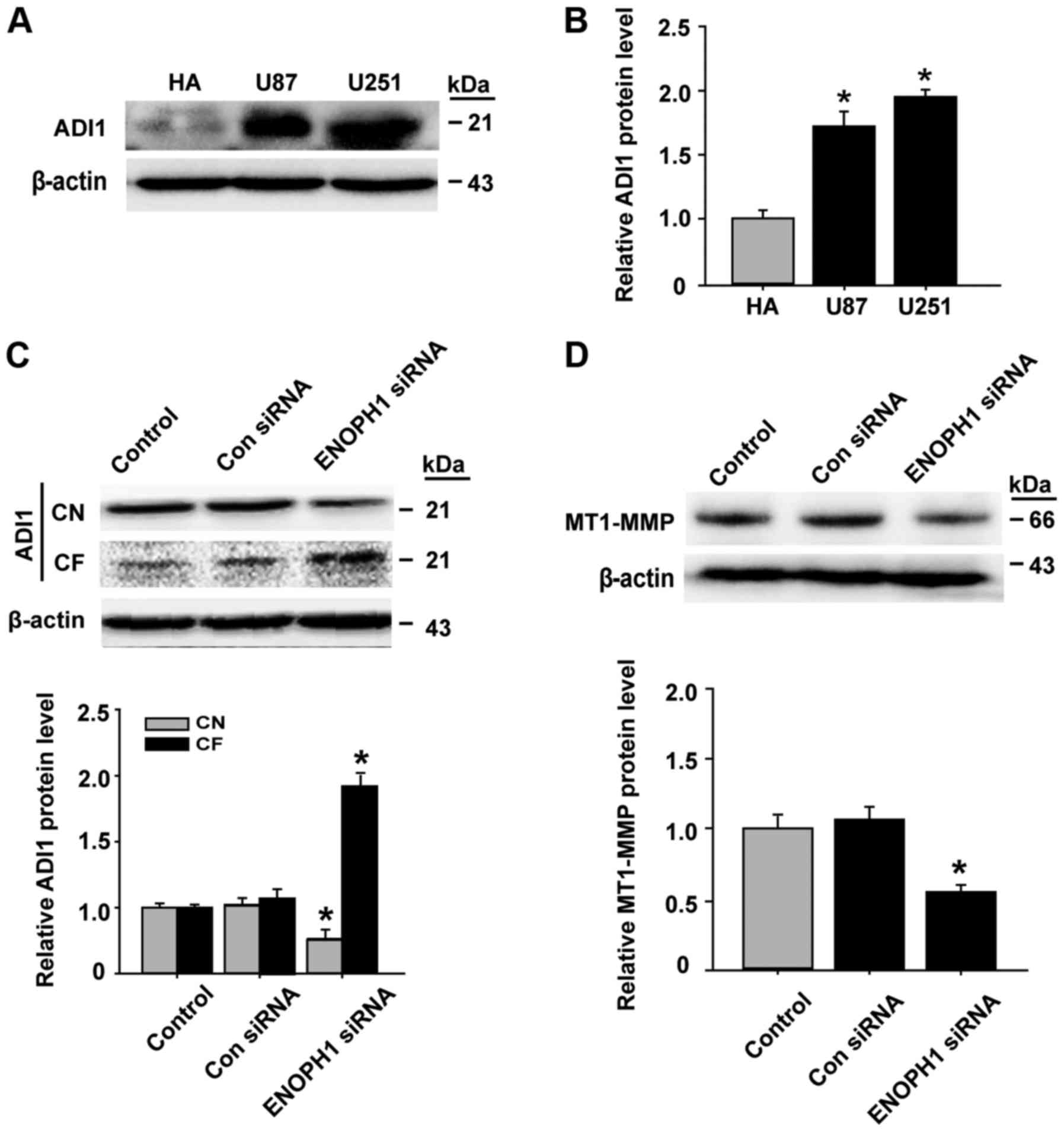
compared with human astrocyte cells, as demonstrated in Fig. 2A. Similar results were also observed
at the protein level (Fig. 2B). To
reveal the intracellular localization of ENOPH1 in glioma cell
lines, we performed immunocytochemical staining to further verify
the immunoblot findings. It was demonstrated by immunostaining that
ENOPH1 protein was found in the cytosol and nucleus (Fig. 2C) and the fluorescence intensity of
ENOPH1 was significantly increased in U87 (~1.95-fold increase) and
U251 (~2.38-fold increase) glioma cells compared with human
astrocyte cells (Fig. 2D). Notably
the expression of ENOPH1 in glioma cells was upregulated.
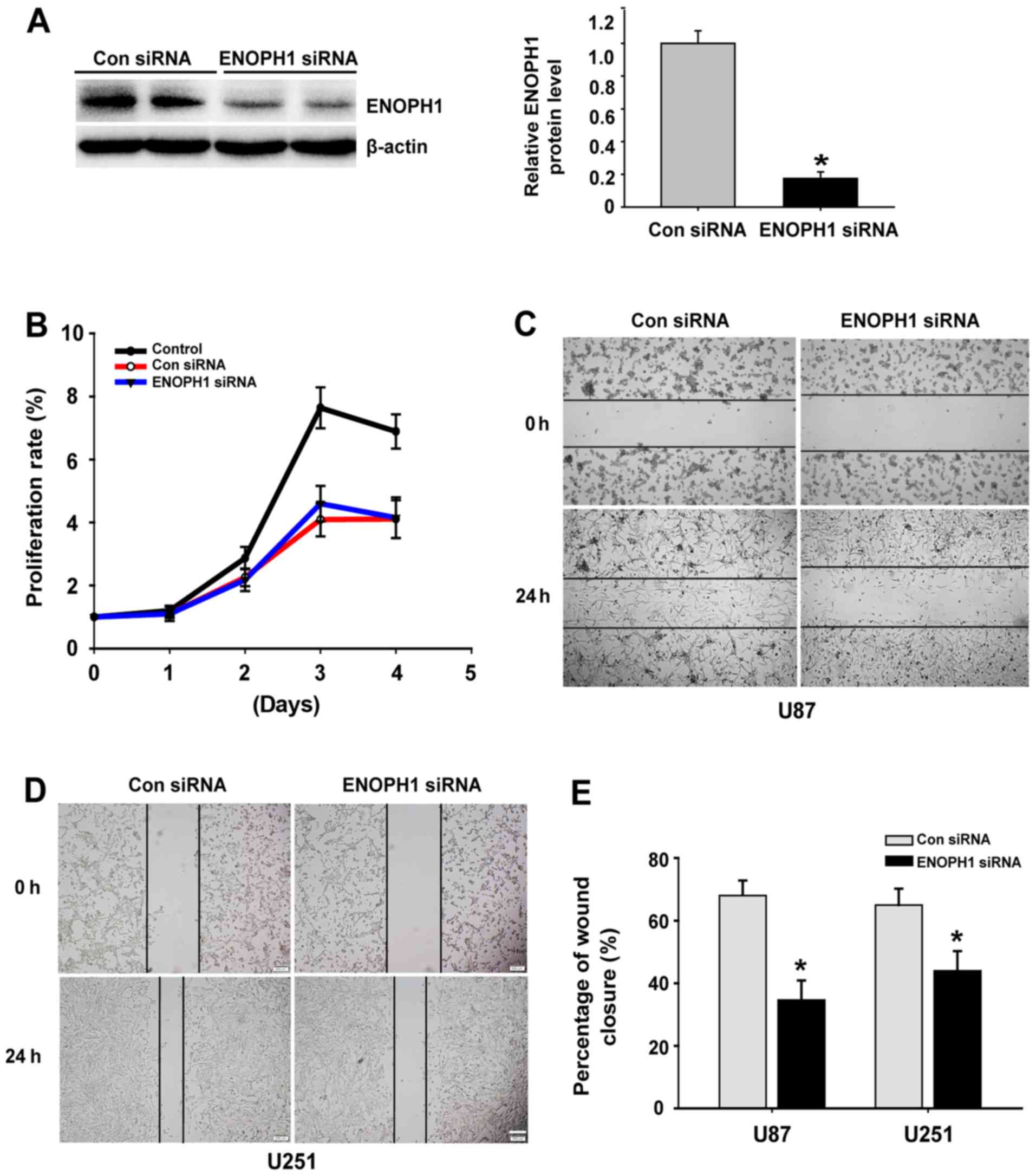
Knockdown of ENOPH1 inhibits glioma
cell proliferation and migration
To identify the cellular mechanisms responsible for
tumor proliferation and migration, MTT and scratch assays were
performed, respectively. First, by RNA disturbance (RNAi) in
high-grade glioma cells we inhibited ENOPH1 gene expression, using
both the U87 and U251 cells. The evaluation of the silencing
effects of siRNA was also confirmed by western blotting (Fig. 3A). Subsequently, using MTT assay we
detected the proliferation of U251 cells following transfection
with ENOPH1 siRNA. A powerful growth ability was demonstrated by
U251 cells, which was attenuated significantly by knockdown of
ENOPH1 in a time-dependent manner, particularly at 72 and 96 h
(P<0.05) post-transfection of ENOPH1 siRNA (Fig. 3B). Subsequently, using a microscope,
the degree of wound healing was evaluated by scratch analysis every
24 h. Representative images of U87 and U251 cells acquired at 48 h
are displayed (Fig. 3C and D); the
former (U87) was inhibited by 36% and the latter (U251) by 44%
(Fig. 3E). Our data indicated that
downregulation of ENOPH1 inhibited glioma cell proliferation and
migration.
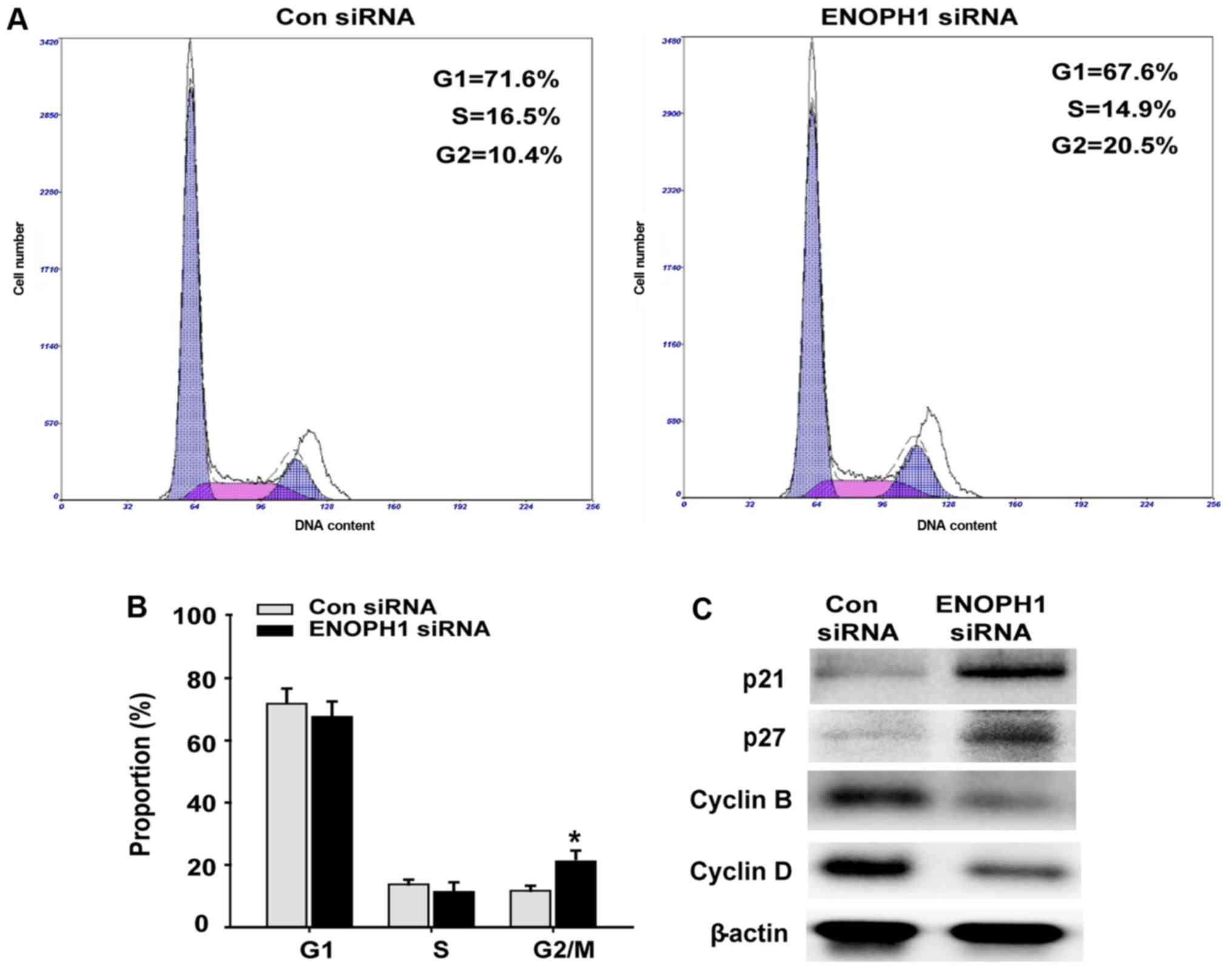
Knockdown of ENOPH1 results in a block
of the G2/M transition
By PI staining and flow cytometry, the possible
effects of ENOPH1 knockdown on cell cycle progression were
evaluated. An increase in cells at the G2/M phase was found as a
result of depletion of ENOPH1 in U251 cells (Fig. 4A and B). The assessment of the
effects of ENOPH1 siRNA transfection on the protein levels of the
cell-cycle key regulators p21, p27, cyclin B and cyclin D was
performed using western blotting. It was demonstrated in Fig. 4C that the expression of p21 and p27
was upregulated, while that of cyclin B and cyclin D was
downregulated in the ENOPH1 siRNA group, when compared with the
astrocyte group. These results demonstrated that knocked down
ENOPH1 expression blocks the cell cycle G2/M transition.
Downregulation of ENOPH1 inhibits
MT1-MMP expression in glioma cells
Previous studies have revealed that the downstream
molecule ADI1 of ENOPH1 can tie up and knock down the activity of
membrane-type matrix metalloproteinase (MT1-MMP) (22,23),
and it is known that, MT1-MMP contributes to human malignant glioma
aggressiveness (24,25). Thus, we hypothesized that the effect
of ENOPH1 on glioma progression may be mediated by the ADI1/MT1-MMP
pathway. To investigate this possibility, we examined the effect of
ENOPH1 silencing on its downstream protein ADI1 and MT1-MMP
expression. We first used western blotting to investigate the ADI1
protein level in human malignant cell lines. We observed that the
increase of ADI1 protein levels was significant in U87 and U251
glioma cells compared with human astrocyte cells (Fig. 5A and B). Subsequently, we determined
the effect of ENOPH1 knockdown on ADI1 intracellular distribution.
The data demonstrated that ENOPH1 downregulation reduced ADI1
protein levels in CN (cytosolic nuclei), while its protein level in
the CF (cytosolic fraction) was increased in U251 glioma cells
(Fig. 5C). In addition, the
expression of MT1-MMP protein was significantly decreased in the
ENOPH1-siRNA transfection group compared with the control group
(Fig. 5D). Collectively, these data
indicated that ENOPH1 may indirectly promote MT1-MMP expression
through the inhibition of ADI1 translocation from the nucleus to
the cytosol in human malignant glioma cells.
Discussion
ENOPH1, as the methionine salvage pathway enzyme,
has been revealed to play a role in stress reactivity (10) and ischemic blood-brain barrier (BBB)
dysfunction (21). However, its
possible effects on glioma progression and potential mechanisms
have not been fully elucidated. The present study provided the
first evidence that ENOPH1 contributed to glioma proliferation and
migration in vitro. The major findings included the
following: i) ENOPH1 was markedly upregulated in human glioma
tissues and malignant glioma cells (U87 and U251 cell lines),
respectively; ii) knockdown of ENOPH1 with siRNA significantly
attenuated glioma cell proliferation; iii) knockdown of ENOPH1
attenuated glioma cell migration; and iv) knockdown of ENOPH1
induced ADI1 to translocate from the nucleus to the cytosol and may
promote the separation of ADI1 from MT1-MMP in the cytoplasm, and
in glioma cells, downregulating MT1-MMP expression.
In glioma, polyamine synthesis is increased and can
spread to the surrounding tissues. Key enzyme-ODC activity
increases in a positive correlation with the degree of tumor
malignancy and, can be used as a reliable indicator of glioma
malignancy (26,27). Other evidence revealed that ENOPH1
via S-adenosyl methionine (SAM) plays an indirect role in the
synthesis of polyamine (14–17).
At present, the biological functions of ENOPH1 are still mainly not
known. It has been revealed in a few recent studies that there is
wide expression of ENOPH1 in the brain and it is related to
neurodevelopmental disorders, anxiety behavior and BBB function
integrity damage (10,21,28).
The present study was the first investigation of the role of ENOPH1
in the pathophysiology of gliomas. Our data revealed that ENOPH1
upregulation existed not only in the glioma cell lines but also in
human glioma tissue, especially in glioblastomas. Notably, ENOPH1
was mainly located in the nucleus in normal brain tissue, WHO II
and WHO III grade tissue samples, but in WHO IV grade tissue,
ENOPH1 was translocated to the cytoplasm, which was consistent with
the findings in U87 and U251 malignant glioma cells. According to
these results, we hypothesized that the occurrence of gliomas and
the transition of gliomas from low- to high-grade may be
facilitated by the translocation of ENOPH1 from the nucleus to the
cytoplasm. In previous studies, there was no similar data to
support this translocation, and we believe a possible reason for
this is that polyamine accumulation promoted ENOPH1 activation and
that ENOPH1 is a tumor microenvironment stress factor. Further
experiments are warranted to analyze this phenomenon and
mechanism.
This is the first study of the role of ENOPH1 in
cell proliferation and migration in order to determine the
molecular mechanism of the effect of ENOPH1 on the progression of
glioma. We revealed a reduction of cellular proliferation rate and
the degree of wound healing produced by knockdown of ENOPH1 with
siRNA, which obviously implicated ENOPH1 in glioma cell growth and
migration. In addition, in the assay of cell cycle progression, we
observed downregulation of cyclin B and cyclin D following knockout
of ENOPH1 which promoted a G2/M phase arrest, whereas there was
upregulation of p21 and p27, further supporting a promoting role of
ENOPH1 in glioma development.
ADI1 (also known as MTCBP1), a methionine salvage
pathway enzyme, is a downstream molecule of ENOPH1 and has been
indicated to be connected with cell apoptosis, oxidative stress,
microbial infection and reproductive development (29–32).
In addition, as an oxidoreductase, ADI1 can collect molecular
oxygen donor to generate ROS (30).
A recent study illustrated a new role for ADI1 in regulating
MT1-MMP-mediated autophagy, which is important in glioblastoma
cells (33). The high expression of
MT1-MMP in malignant glioma tissue and its close relationship to
the progression and invasion of glioma are well-known (34–36).
These research data led us to hypothesize that the effect of ENOPH1
on glioma progression may be mediated by the ADI1/MT1-MMP pathway.
Our in vitro study revealed that knockdown of ENOPH1
promoted ADI1 translocation from the nucleus to the cytoplasm and
the expression of MT1-MMP was decreased. According to these
evidence, we hypothesized that ENOPH1 may promote ADI1 migration
into the nucleus and separate MT1-MMP from ADI1 in the cytoplasm,
attenuating the inhibitory effect of ADI1 on MT1-MMP, and
indirectly enhancing MMP-2 activity, further promoting glioma cell
proliferation and migration. Future studies are warranted to
perform animal experiments in vivo to detect the activity of
MT1-MMP and the interaction of ENOPH1 with ADI1, as well as the
interaction between ADI1 and MT1-MMP in glioma cell lines.
In conclusion, our results collectively provided
preliminary evidence of the role of ENOPH1 in glioma progression,
in which increased cellular growth and migration can be caused by
ENOPH1 upregulation in glioma cells. In addition, Knockdown of
ENOPH1 facilitated its downstream molecule ADI1, to translocate
from the nucleus to the cytoplasm, and indirectly decreased the
expression of MT1-MMP in glioma cells. Investigation of the
mechanisms underlying this change need to be identified in future
studies. In addition, the expression of ENOPH1 can be used as an
unfavorable progression indicator for glioma patients.
Acknowledgements
Not applicable.
Funding
The present study was funded by grants from the
National Natural Science Foundation of China (no. 81760227), the
Natural Science Foundation of Inner Mongolia Autonomous Region of
China (no. 2016MS0802), the Natural Science Foundation of Guangdong
Province (no. 2017A030313568) and the Science Research Fund Project
of Baotou Medical College (no. BYJJ-DF201601).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LS, YZ and GX conceived and planned the experiments.
LS, JH and KY conducted the experiments. SL and CL collected the
clinical samples. LS, YZ and KY analyzed the data. LS and KY
contributed to the reagent material and analysis tools. YZ and GX
wrote the paper. All authors read and approved the manuscript and
agree to be accountable for all aspects of the research in ensuring
that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
All experiments using human samples were approved by
the Ethics Committee of Southern Medical University. All patients
willingly consented to participate in the study by signing an
informed consent form.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MGMT
|
O−6-methylguanine-DNA
methyltransferase
|
|
EGFR
|
epidermal growth factor receptor
|
|
EDTA
|
eathylene diamine tetraacetic acid
|
|
PBS
|
phosphate buffered saline
|
|
BSA
|
bovine albumin
|
|
DAPI
|
4,6-diamidino-2-phenylindole
|
|
FITC
|
fluorescein isothiocyanate
|
|
SDS
|
sodium dodecyl sulfate
|
|
RIPA
|
radio-immunoprecipitation assay
|
References
|
1
|
Eckel-Passow JE, Lachance DH, Molinaro AM,
Walsh KM, Decker PA, Sicotte H, Pekmezci M, Rice T, Kosel ML,
Smirnov IV, et al: Glioma Groups Based on 1p/19q, IDH, and TERT
Promoter Mutations in Tumors. N Engl J Med. 372:2499–2508. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Omuro A and DeAngelis LM: Glioblastoma and
other malignant gliomas: A clinical review. JAMA. 310:1842–1850.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pesenti C, Paganini L, Fontana L, Veniani
E, Runza L, Ferrero S, Bosari S, Menghi M, Marfia G, Caroli M, et
al: Mass spectrometry-based assay for the molecular diagnosis of
glioma: Concomitant detection of chromosome 1p/19q codeletion, and
IDH1, IDH2, and TERT mutation status. Oncotarget. 8:57134–57148.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Karsy M, Guan J, Cohen AL, Jensen RL and
Colman H: New molecular considerations for glioma: IDH, ATRX, BRAF,
TERT, H3 K27M. Curr Neurol Neurosci Rep. 17:192017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Holland EC: Progenitor cells and glioma
formation. Curr Opin Neurol. 14:683–688. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tang W, Wang X, Chen Y, Zhang J, Chen Y
and Lin Z: CXCL12 and CXCR4 as predictive biomarkers of glioma
recurrence pattern after total resection. Pathol Biol (Paris).
63:190–198. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun L, Song L, Wan Q, Wu G, Li X, Wang Y,
Wang J, Liu Z, Zhong X, He X, et al: cMyc-mediated activation of
serine biosynthesis pathway is critical for cancer progression
under nutrient deprivation conditions. Cell Res. 25:429–444. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Adams S, Teo C, McDonald KL, Zinger A,
Bustamante S, Lim CK, Sundaram G, Braidy N, Brew BJ and Guillemin
GJ: Involvement of the kynurenine pathway in human glioma
pathophysiology. PLoS One. 9:e1129452014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sauter M, Moffatt B, Saechao MC, Hell R
and Wirtz M: Methionine salvage and S-adenosylmethionine: Essential
links between sulfur, ethylene and polyamine biosynthesis. Biochem
J. 451:145–154. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Barth A, Bilkei-Gorzo A, Drews E, Otte DM,
Diaz-Lacava A, Varadarajulu J, Turck CW, Wienker TF and Zimmer A:
Analysis of quantitative trait loci in mice suggests a role of
Enoph1 in stress reactivity. J Neurochem. 128:807–817. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Morita K, Lee MS, Her S and Nishibori N:
Polyamines cause elevation of steroid 5α-reductase mRNA levels by
suppressing mRNA degradation in C6 glioma cells. Cell Biol Int.
38:1132–1137. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Elworthy P and Hitchcock E: Red blood cell
polyamines as a diagnostic indicator of glioma presence and
recurrence. J Neurooncol. 7:31–38. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Redgate ES, Boggs S, Grudziak A and
Deutsch M: Polyamines in brain tumor therapy. J Neurooncol.
25:167–179. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takano K, Ogura M, Nakamura Y and Yoneda
Y: Neuronal and glial responses to polyamines in the ischemic
brain. Curr Neurovasc Res. 2:213–223. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li J, Doyle KM and Tatlisumak T:
Polyamines in the brain: Distribution, biological interactions, and
their potential therapeutic role in brain ischaemia. Curr Med Chem.
14:1807–1813. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Duan B, Wang YZ, Yang T, Chu XP, Yu Y,
Huang Y, Cao H, Hansen J, Simon RP, Zhu MX, et al: Extracellular
spermine exacerbates ischemic neuronal injury through sensitization
of ASIC1a channels to extracellular acidosis. J Neurosci.
31:2101–2112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim GH, Komotar RJ, McCullough-Hicks ME,
Otten ML, Starke RM, Kellner CP, Garrett MC, Merkow MB, Rynkowski
M, Dash KA, et al: The role of polyamine metabolism in neuronal
injury following cerebral ischemia. Can J Neurol Sci. 36:14–19.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Y, Zeng Y, Wang M, Tian C, Ma X,
Chen H, Fang Q, Jia L, Du J and Li H: Cardiac-specific
overexpression of E3 ligase Nrdp1 increases ischemia and
reperfusion-induced cardiac injury. Basic Res Cardiol. 106:371–383.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Y, Wang T, Yang K, Xu J, Ren L, Li W
and Liu W: Cerebral Microvascular Endothelial Cell Apoptosis after
ischemia: Role of enolase-phosphatase 1 activation and
aci-reductone dioxygenase 1 translocation. Front Mol Neurosci.
9:792016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Uekita T, Gotoh I, Kinoshita T, Itoh Y,
Sato H, Shiomi T, Okada Y and Seiki M: Membrane-type 1 matrix
metalloproteinase cytoplasmic tail-binding protein-1 is a new
member of the Cupin superfamily. A possible multifunctional protein
acting as an invasion suppressor down-regulated in tumors. J Biol
Chem. 279:12734–12743. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chang ML, Huang YH, Cheng JC and Yeh CT:
Interaction between hepatic membrane type 1 matrix
metalloproteinase and acireductone dioxygenase 1 regulates
hepatitis C virus infection. J Viral Hepat. 23:256–266. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen S, Han M, Chen W, He Y, Huang B, Zhao
P, Huang Q, Gao L, Qu X and Li X: KIF1B promotes glioma migration
and invasion via cell surface localization of MT1-MMP. Oncol Rep.
35:971–977. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mou L, Kang Y, Zhou Y, Zeng Q, Song H and
Wang R: Neurokinin-1 receptor directly mediates glioma cell
migration by up-regulation of matrix metalloproteinase-2 (MMP-2)
and membrane type 1-matrix metalloproteinase (MT1-MMP). J Biol
Chem. 288:306–318. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ernestus RI, Röhn G, Schröder R, Els T,
Klekner A, Paschen W and Klug N: Polyamine metabolism in brain
tumours: Diagnostic relevance of quantitative biochemistry. J
Neurol Neurosurg Psychiatry. 71:88–92. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hoelzinger DB, Nakada M, Demuth T,
Rosensteel T, Reavie LB and Berens ME: Autotaxin: A secreted
autocrine/paracrine factor that promotes glioma invasion. J
Neurooncol. 86:297–309. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Komlósi K, Duga B, Hadzsiev K, Czakó M,
Kosztolányi G, Fogarasi A and Melegh B: Phenotypic variability in a
Hungarian patient with the 4q21 microdeletion syndrome. Mol
Cytogenet. 8:162015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hirano W, Gotoh I, Uekita T and Seiki M:
Membrane-type 1 matrix metalloproteinase cytoplasmic tail binding
protein-1 (MTCBP-1) acts as an eukaryotic aci-reductone dioxygenase
(ARD) in the methionine salvage pathway. Genes Cells. 10:565–574.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Oram SW, Ai J, Pagani GM, Hitchens MR,
Stern JA, Eggener S, Pins M, Xiao W, Cai X, Haleem R, et al:
Expression and function of the human androgen-responsive gene ADI1
in prostate cancer. Neoplasia. 9:643–651. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cheng JC, Yeh YJ, Pai LM, Chang ML and Yeh
CT: 293 cells over-expressing human ADI1 and CD81 are permissive
for serum-derived hepatitis C virus infection. J Med Virol.
81:1560–1568. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chou HY, Lin YH, Shiu GL, Tang HY, Cheng
ML, Shiao MS and Pai LM: ADI1, a methionine salvage pathway enzyme,
is required for Drosophila fecundity. J Biomed Sci.
21:642014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pratt J, Iddir M, Bourgault S and Annabi
B: Evidence of MTCBP-1 interaction with the cytoplasmic domain of
MT1-MMP: Implications in the autophagy cell index of high-grade
glioblastoma. Mol Carcinog. 55:148–160. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Markovic DS, Vinnakota K, Chirasani S,
Synowitz M, Raguet H, Stock K, Sliwa M, Lehmann S, Kälin R, van
Rooijen N, et al: Gliomas induce and exploit microglial MT1-MMP
expression for tumor expansion. Proc Natl Acad Sci USA.
106:12530–12535. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Markovic DS, Vinnakota K, van Rooijen N,
Kiwit J, Synowitz M, Glass R and Kettenmann H: Minocycline reduces
glioma expansion and invasion by attenuating microglial MT1-MMP
expression. Brain Behav Immun. 25:624–628. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huang M, Liu T, Ma P, Mitteer RA Jr, Zhang
Z, Kim HJ, Yeo E, Zhang D, Cai P, Li C, et al: c-Met-mediated
endothelial plasticity drives aberrant vascularization and
chemoresistance in glioblastoma. J Clin Invest. 126:1801–1814.
2016. View Article : Google Scholar : PubMed/NCBI
|