Introduction
The increasing rate of cancer morbidity has become a
serious public health issue worldwide (1). It has been reported that there were
14.1 million newly diagnosed cancer cases, and 8.2 million cases of
cancer-associated mortality in 2012 (2). Lung cancer is among the most common
malignant tumors and the leading cause of cancer-associated cases
of mortality worldwide (3). In lung
cancer, non-small cell lung cancer (NSCLC) accounts for ~80% of all
clinical cases, with a poor prognosis and a 5-year survival rate as
low as 15% (4). Treatment of lung
cancer usually includes a combination of surgery, radiation therapy
and chemotherapy. Although considerable advances have been made in
lung cancer research, there is still an urgent need to improve
understanding of the molecular pathogenesis of lung cancer,
particularly to identify novel therapeutic targets (5).
MicroRNAs (miRNAs) are a class of highly conserved
non-coding RNAs of ~20–22 nucleotides in length (6). The regulatory roles of miRNAs in gene
expression are associated with their pairing to the 3′untranslated
region (3′UTR) of their target mRNAs (7–9).
Following processing by the consecutive actions of Drosha and Dicer
ribonucleases, miRNAs are subsequently loaded onto RNA-induced
silencing complexes to interact with their target mRNAs and induce
post-transcriptional silencing (2,9,10).
Numerous studies have indicated that miRNAs perform their functions
by regulating various biological processes, including cell
proliferation, differentiation, angiogenesis and apoptosis
(2,11–13).
MiRNAs also serve as oncogenes or tumor suppressor genes. Let-7a,
as a tumor-suppressive miRNA, has been identified to be deregulated
in multiple types of malignant cells. For instance, overexpression
of let-7a was demonstrated to suppress the proliferation, migration
and invasion of gastric cancer cells by downregulating the
expression of pyruvate kinase muscle isozyme M2 (11,14).
As a dysplastic disease, cancer formation is largely
attributed to the deregulation of cell proliferation, which is
strictly controlled by checkpoints of the cell cycle.
G1/S and G2/M are the classic cell cycle
checkpoints (11,15). As a member of the cyclin family,
cyclin D1 is an important regulator of cell proliferation. Cyclin
D1 reaches a peak level at the G1 stage, indicating that
it is involved in the checkpoint of G1/S. Therefore,
overexpression of cyclin D1 may lead to transition through the
G1/S checkpoint and promotion of cell proliferation,
which may eventually lead to the formation of cancer (16).
Although it has been observed that certain miRNAs
are unconventionally expressed in lung cancer and associated with
poor outcomes, the role of let-7a in lung cancer has not yet been
fully elucidated (17). The present
study aimed to investigate the effects of let-7a on cell
proliferation, apoptosis, migration and invasion in A549 and H1299
cells.
Materials and methods
Lung adenocarcinoma tissues
The present study was conducted between August 1,
2014 and July 31, 2015 at the Inpatient Department of Medical
Oncology, Yantai Shan Hospital, The Teaching Hospital of Binzhou
Medical University (Yantai, China). The experiments were performed
according to the relevant guidelines of the Code of Ethics of the
World Medical Association for experiments involving humans and the
Medical Ethics Committee of Binzhou Medical University (18).
A total of 20 patients (10 males and 10 females),
who were pathologically diagnosed with lung adenocarcinoma for the
first time and had not yet received chemotherapy, were included in
the present study. Fresh lung adenocarcinoma and control tissues
were obtained from the patients who underwent surgery. Written
informed consent was obtained from all patients prior to the
collection of lung tissue samples. The control and lung
adenocarcinoma tissues were collected from the same patients, and
control samples were normal adjacent control tissues.
Quantitative polymerase chain reaction
(qPCR)
Lung adenocarcinoma cells were incubated with
let-7a-mimics or let-7a-inhibitor and harvested 48 h after miRNA
treatment. Small RNA was isolated using RNAiso for Small RNA
reagent (Takara Biotechnology Co., Ltd., Dalian, China). qPCR was
performed as previously described (14). The primers used to amplify let-7a
(Shanghai GenePharm Co., Ltd., Shanghai, China) were as follows:
Forward, 5′-ACACTCCAGCTGGGTGAGGTAGTAGGTTGT-3′ and reverse,
5′-AACATGTACAGTCCATGGATG-3′. Human 5S rRNA served as the positive
control. The primers used to amplify 5S rRNA (GenePharm Co., Ltd.)
were as follows: Forward, 5′-GCCATACCACCCTGAACG-3′ and reverse,
5′-AACATGTACAGTCCATGGATG-3′. Total RNA was isolated using TRIzol
reagent (Takara Biotechnology Co., Ltd.). The primers used for
amplifying cyclin D1 (GenePharm Co., Ltd.) were as follows:
Forward, 5′-CTGGCCATGAACTACCTGGA-3′ and reverse,
5′-GTCACACTTGATCACTCTGG-3′. qPCR was performed on an RG3000 system
(Qiagen GmbH, Hilden, Germany) under the following reaction
conditions: Initial denaturation at 95°C for 30 sec, followed by 40
cycles at 95°C for 5 sec, annealing at 60°C for 20 sec and
extension at 72°C for 30 sec. GAPDH cDNA served as the positive
control (18). The primers used for
amplifying GAPDH (Shanghai GenePharm, Co., Ltd.) were as follows:
Forward, 5′-GTCTTCACCACCATGGAGAAGG-3′ and reverse,
5′-GCCTGCTTCACCACCTTCTTGA-3′.
Construction of cyclin D1-3′UTR GFP
plasmid
The sequence of cyclin D1-3′ UTR was obtained from
GenBank. The primers were designed using Primer Premier 5.0
software (Premier Biosoft International, Palo Alto, CA, USA) and
then synthesized by Shanghai GenePharma Co., Ltd. The primers used
to amplify cyclin D1-3′ UTR were as follows: forward,
5′-TGCTCTAGATGAATTCTTATCCCCTGCCC-3′ and reverse,
5′-CGCGGATCCAAGAGAAGAGGGACACAGCC-3′. The amplification template was
human genomic DNA. Then, cyclin D1-3′ UTR was inserted into the
pcDNA3.1-GFP-neo (+) expression vector.
Cell culture and transfection
Lung adenocarcinoma cell lines (A549 and H1299) were
obtained from the Shanghai Institute of Cell Biology (Shanghai,
China). HBE 135E6E7 cells were obtained from the American Type
Culture Collection (human bronchus epithelial; ATCC®
CRL-2741; Manassas, VA, USA). The cells were cultured in RPMI-1640
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.) at 37°C with 5% CO2. Cells
(2×105) were seeded into 6-well plates. Let-7a mimics,
let-7a inhibitors, negative controls and si-cyclin D1 were
synthesized by Shanghai GenePharma Co., Ltd. The target sequence of
si-cyclin D1 was as follows: Sense, 5′-CAAACAGAUCAUCCGCAA-3′ and
antisense, 5′-UUUGCGGAUGAUCUGUU-3′. Transfection was performed in
triplicate at ~60% confluence using Lipofectamine™ 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol (19).
MTT assay
Cell proliferation assays were conducted using a
modified colorimetric MTT assay as previously described (20). All procedures were repeated three
times. Cell colony formation rate was assessed using a plate colony
formation assay. The plate was gently washed and stained with
crystal violet. Then, the number and size of colonies was
analyzed.
Apoptosis assays
An apoptosis assay was performed 48 h after the
oligonucleotides were transfected into lung adenocarcinoma cells.
The assay was performed using Annexin V-FITC/PI (BD Pharmingen; BD
Biosciences, Franklin Lakes, NJ, USA) according to the
manufacturer's protocol (19).
Cell cycle analysis by flow
cytometry
A cell cycle assay was performed 48 h after
transfection. The cells were collected using a Cell Cycle Detection
kit (Nanjing KeyGen Biotech, Co., Ltd., Nanjing, China) according
to the manufacturer's protocols and counted by flow cytometry
(21).
Western blot analysis
Western blot analysis was performed as previously
described (22). The antibodies
used were as follows: Rabbit antibodies against GAPDH (1:800; cat.
no. AP0063), Rb (1:800; cat. no. BS1311), p-Rb (1:800; cat. no.
BS4164), Bcl-2 (1:800; cat. no. BS1511), Bax (1:800; cat. no.
BS2538; all from Bioworld Technology, Inc., St. Louis Park, MN,
USA) and caspase-9 (1:1,000; cat. no. 9502), caspase-8 (1:1,000;
cat. no. 9748) and caspase-3 (1:1,000; cat. no. 9662; all from Cell
Signaling Technology, Inc., Danvers, MA, USA). GAPDH was used as an
internal reference.
Cell migration and invasion
assays
An invasion assay was performed using a modified
two-chamber plate with a pore size of 8.0 µm (23) as previously described (14). The Transwell migration assay was
conducted according to the same protocol as the invasion assay,
with the exception that the cell suspension was added into the
upper chamber directly, without Matrigel.
Statistical analysis
SPSS 22.0 software (IBM Corp., Armonk, NY, USA) was
used to analyze the significance of all results. One-way analysis
of variance (ANOVA) was used to analyze the differences among three
or more groups. A post-hoc test of ANOVA was conducted by
performing a Tukey's test. Correlations were calculated with
Spearman's rank correlation coefficiant. Group means were compared
using an unpaired, two-sided, Student's t-test. Wilcoxen
signed-rank test was used to compare the expression of let-7a and
cyclin D1 in para-carcinoma and carcinoma tissues. Array data of
cyclin D1 were downloaded from data link(s): https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE13213.
Overall survival was determined using Kaplan-Meier survival
analysis by a log-rank test. P<0.05 was considered to indicate a
statistically significant difference (11,23).
All experiments were performed in triplicate, and all data are
expressed as the mean ± standard deviation.
Results
Let-7a is downregulated in human lung
adenocarcinoma tissues
Although let-7a has previously been investigated in
other types of cancer (24), the
molecular mechanism of let-7a in lung adenocarcinoma remains
unclear. In the present study, we first collected the
clinicopathological features of patients with lung adenocarcinoma
(Table I). Lung cancer
tumor-node-metastasis (TNM) staging was based on the 8th edition of
the Union for International Cancer Control (UICC) (25). The number of nuclear fission images
or proliferation index, necrosis range, invasion status and other
parameters were used to determine the lung cancer grade according
to the tumor structure and cell atypia in H&E stained sections
(26–29). Then, let-7a expression was analyzed
in lung adenocarcinoma tissues to investigate the roles of let-7a
in lung cancer. The current results indicated that let-7a levels
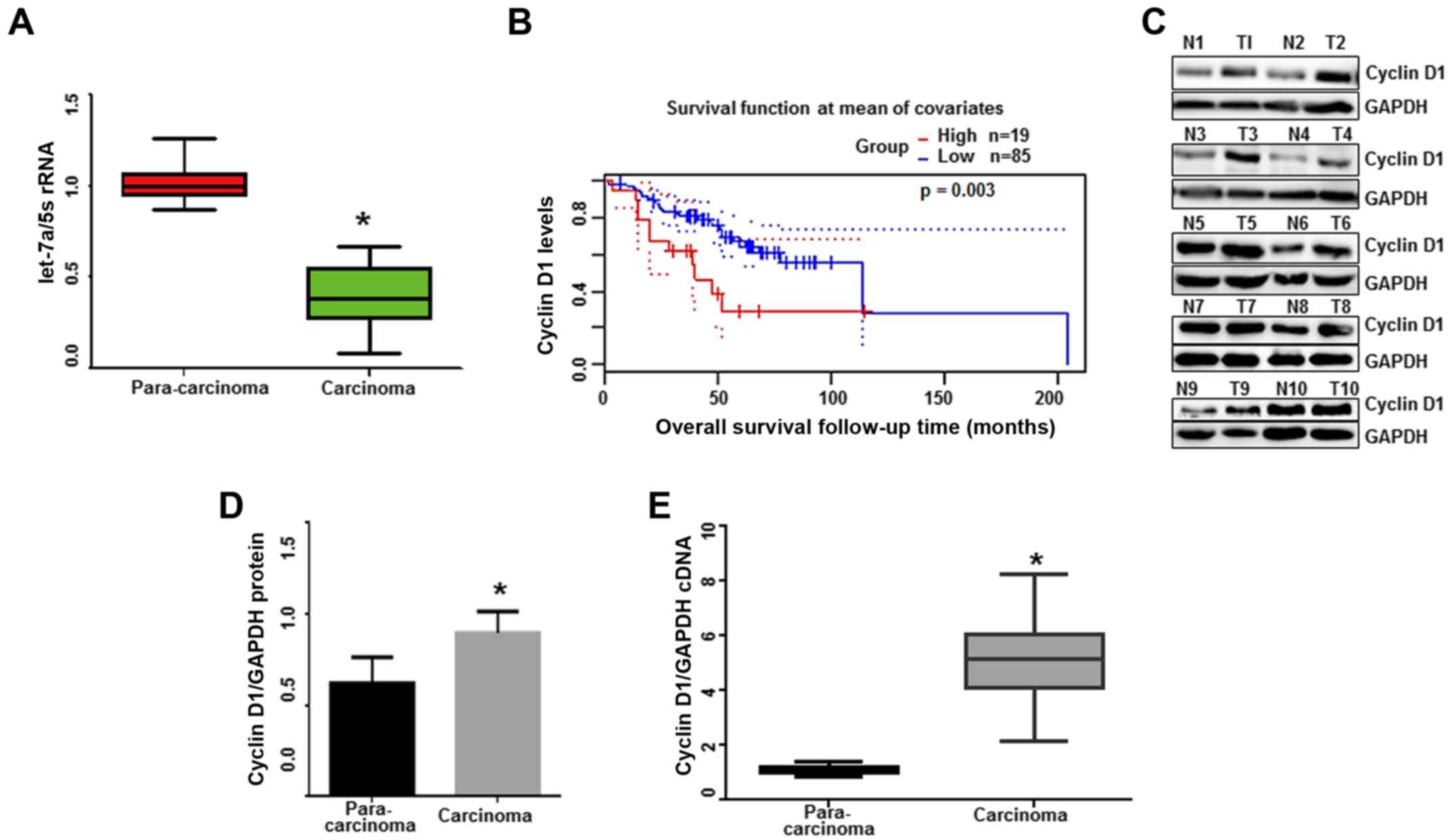
were significantly lower in lung adenocarcinoma tissues (n=20)
compared with para-carcinoma tissues (2 cm from the para-carcinoma
tissues, n=20, Fig. 1A;
P<0.001). The current results indicated that let-7a is involved
in the progression of lung adenocarcinoma.
 | Table I.The clinicopathological features of
the patients with lung cancer. |
Table I.
The clinicopathological features of
the patients with lung cancer.
| Sample no. | Sex | Age (years) | Tumor stage | TNM stage | Tumor grade |
|---|
| 1 | Female | 61 | IIA | T2aN1M0 | G1 |
| 2 | Female | 36 | IA | T1aN0M0 | G1 |
| 3 | Female | 67 | IA | T1aN0M0 | G1 |
| 4 | Female | 64 | IB | T2aN0M0 | G1 |
| 5 | Female | 48 | IA | T1aN0M0 | G1 |
| 6 | Female | 61 | IA | T1aN0M0 | G1 |
| 7 | Female | 71 | IA | T1aN0M0 | G1 |
| 8 | Female | 42 | IB | T2aN0M0 | G1 |
| 9 | Female | 61 | IA | T1aN0M0 | G1 |
| 10 | Female | 44 | IA | T1aN0M0 | G1 |
| 11 | Male | 56 | IA | T1aN0M0 | G1 |
| 12 | Male | 69 | IA | T1aN0M0 | G1 |
| 13 | Male | 59 | IA | T1aN0M0 | G1 |
| 14 | Male | 71 | IA | T1aN0M0 | G1 |
| 15 | Male | 68 | IA | T1aN0M0 | G1 |
| 16 | Male | 61 | IB | T2aN0M0 | G1 |
| 17 | Male | 53 | IA | T1aN0M0 | G1 |
| 18 | Male | 64 | IIA | T1bN0M0 | G1 |
| 19 | Male | 64 | IA | T1aN0M0 | G1 |
| 20 | Male | 75 | IA | T1aN0M0 | G1 |
Kaplan-Meier survival analysis was performed to
determine the significance of cycin D1 expression in affecting the
outcome of lung cancer patients from the GSE13213 dataset. The poor
survival time of patients was not significantly correlated with age
(P=0.360) or sex (P=0.278). Notably, patients with high cyclin D1
levels exhibited short survival time (P=0.003; Fig. 1B) through a log-rank test analysis,
suggesting that higher cyclin D1 levels increase the risk of lung
adenocarcinoma. Then, the expression of cyclin D1 was evaluated in
lung adenocarcinoma tissues. The results indicated that the
expression of cyclin D1 was considerably higher in lung
adenocarcinoma tissues (n=10) compared with para-carcinoma tissues
(2 cm from the para-carcinoma tissues, n=10, Fig. 1C). Subsequently, we assessed the
protein and mRNA expression levels of cyclin D1 in para-carcinoma
and carcinoma tissue samples, and our results revealed that cyclin
D1 levels were significantly higher in lung adenocarcinoma tissues
(n=20) than those in para-carcinoma tissues (Fig. 1D and E; P<0.0001). These results
indicated that lower levels of let-7a and higher levels of cyclin
D1 may be associated with the development of lung
adenocarcinoma.
Let-7a is downregulated in lung
adenocarcinoma cell lines
Let-7a levels were detected in lung adenocarcinoma
cell lines (A549 and H1299) and control human bronchus epithelial
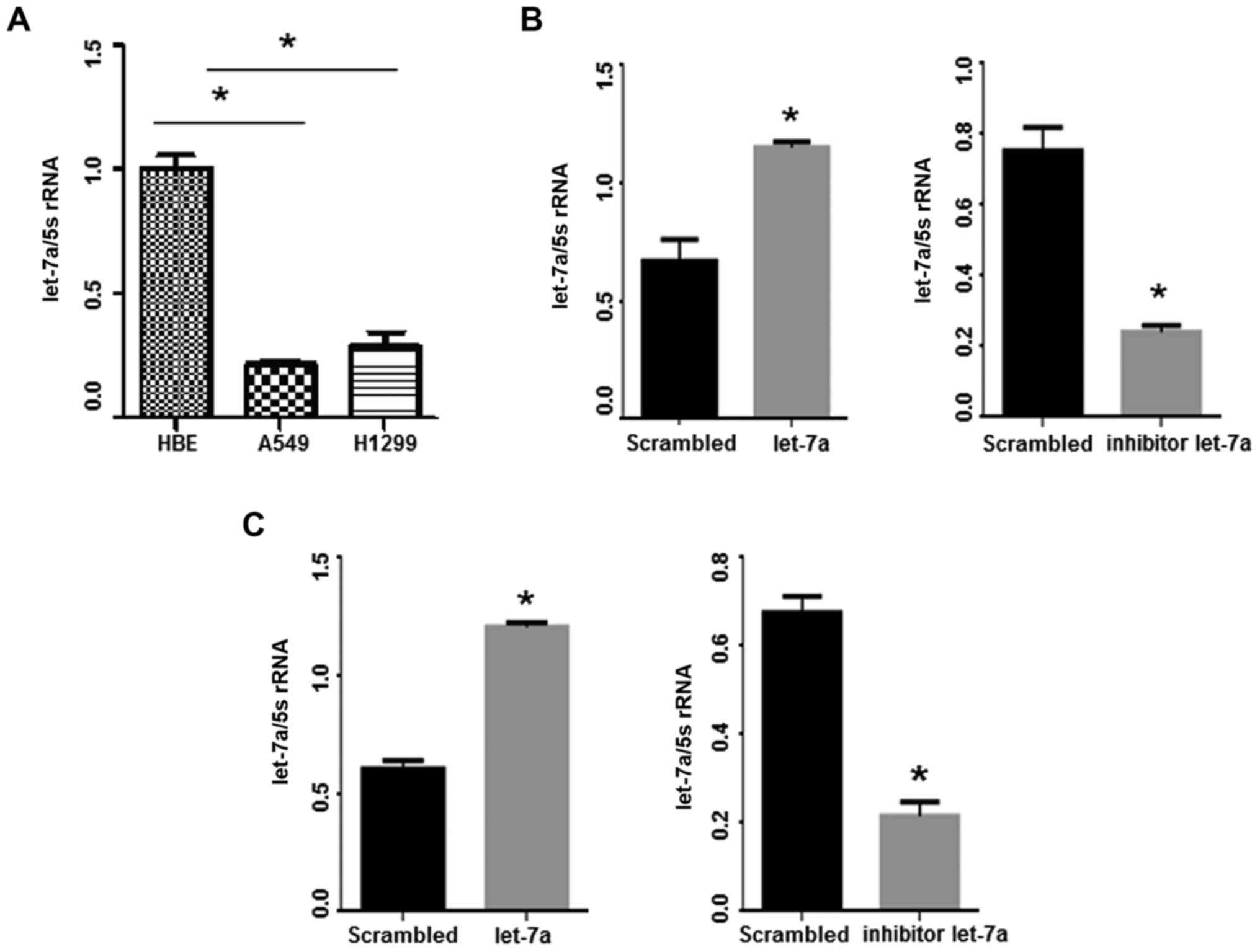
(HBE) cell line by qPCR. Notably, let-7a levels were reduced in the
two lung adenocarcinoma cell lines compared with the HBE cells
(Fig. 2A; P<0.001). Let-7a-mimic
oligo treatment led to significant upregulation of let-7a compared
with the control group (P<0.05). Conversely, the expression of
let-7a was significantly reduced in lung adenocarcinoma cell lines
following let-7a-inhibitor transfection (P<0.05; Fig. 2B and C).
Let-7a directly targets cyclin D1
To elucidate the molecular mechanisms of let-7a in
the suppression of the growth of lung adenocarcinoma cells, the
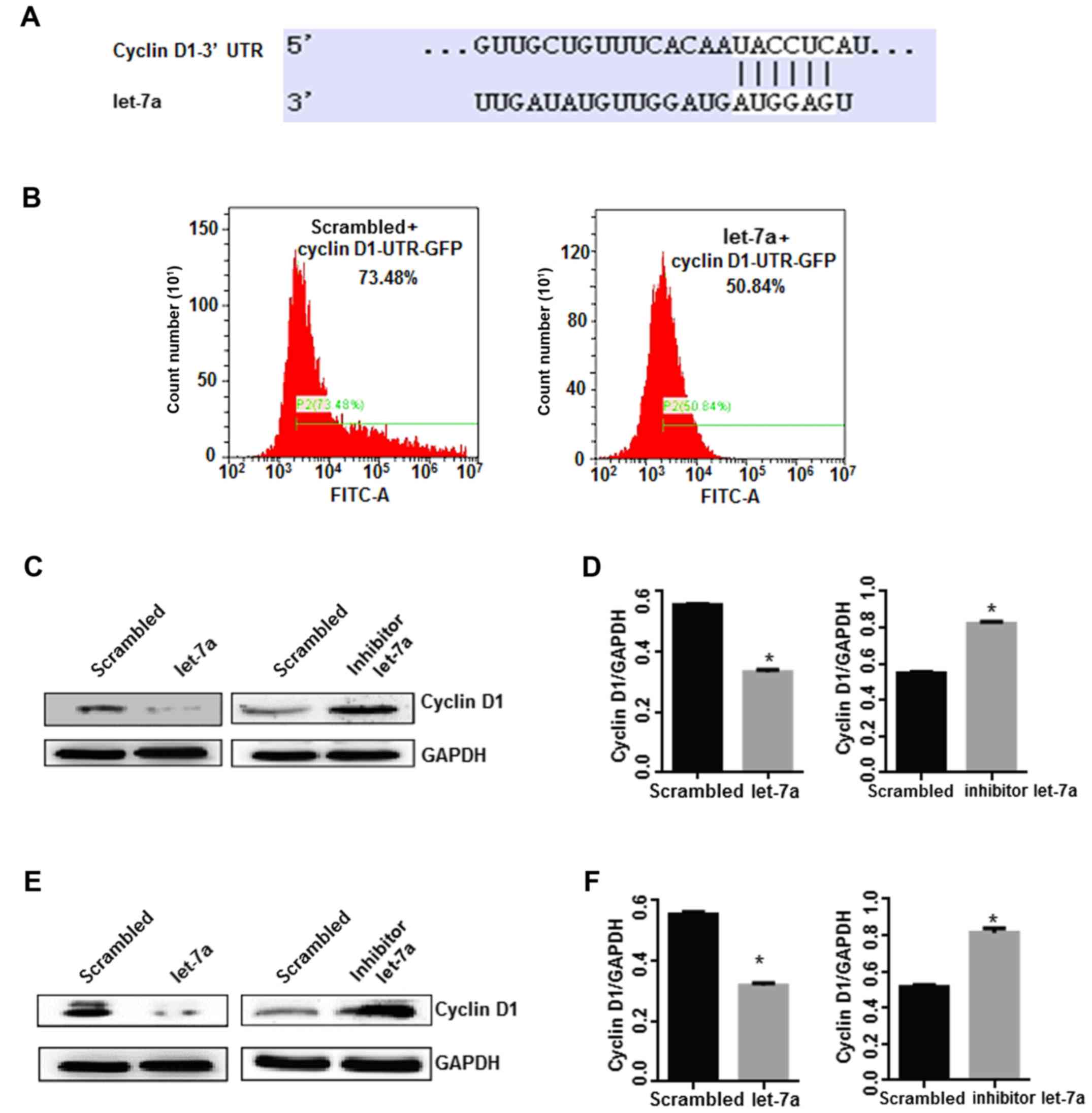
putative target gene of let-7a was identified. It was indicated
that cyclin D1 was a target gene of let-7a with high probability
using TargetScan software online (Fig.
3A; http://www.targetscan.org/vert_71/). Then, a cyclin
D1-3′ UTR GFP plasmid was constructed to transfect cells with
let-7a. The results indicated that GFP fluorescence intensity and
positive rate decreased significantly in let-7a-treated cells
compared with the control treatment (P<0.05; Fig. 3B), indicating that cyclin D1-3′ UTR
was directly targeted by let-7a. Next, the effects of let-7a on
cyclin D1 expression in lung cancer cell lines were investigated by
western blot analysis. Notably, the expression of cyclin D1 was
significantly reduced in lung adenocarcinoma cell lines following
overexpression of let-7a (P<0.05). Conversely, the let-7a
inhibitor resulted in the significant upregulation of cyclin D1
compared with the control group (P<0.05; Fig. 3C-F). Cyclin D1 is a positive
regulator of cell cycle progression (11), and these results indicated that
let-7a suppresses lung cancer cell growth by regulating the pathway
of cyclin D1.
Let-7a inhibits cellular proliferation
and colony formation
To further assess the potential inhibitory roles of
let-7a in lung adenocarcinoma, the effects of let-7a on cell
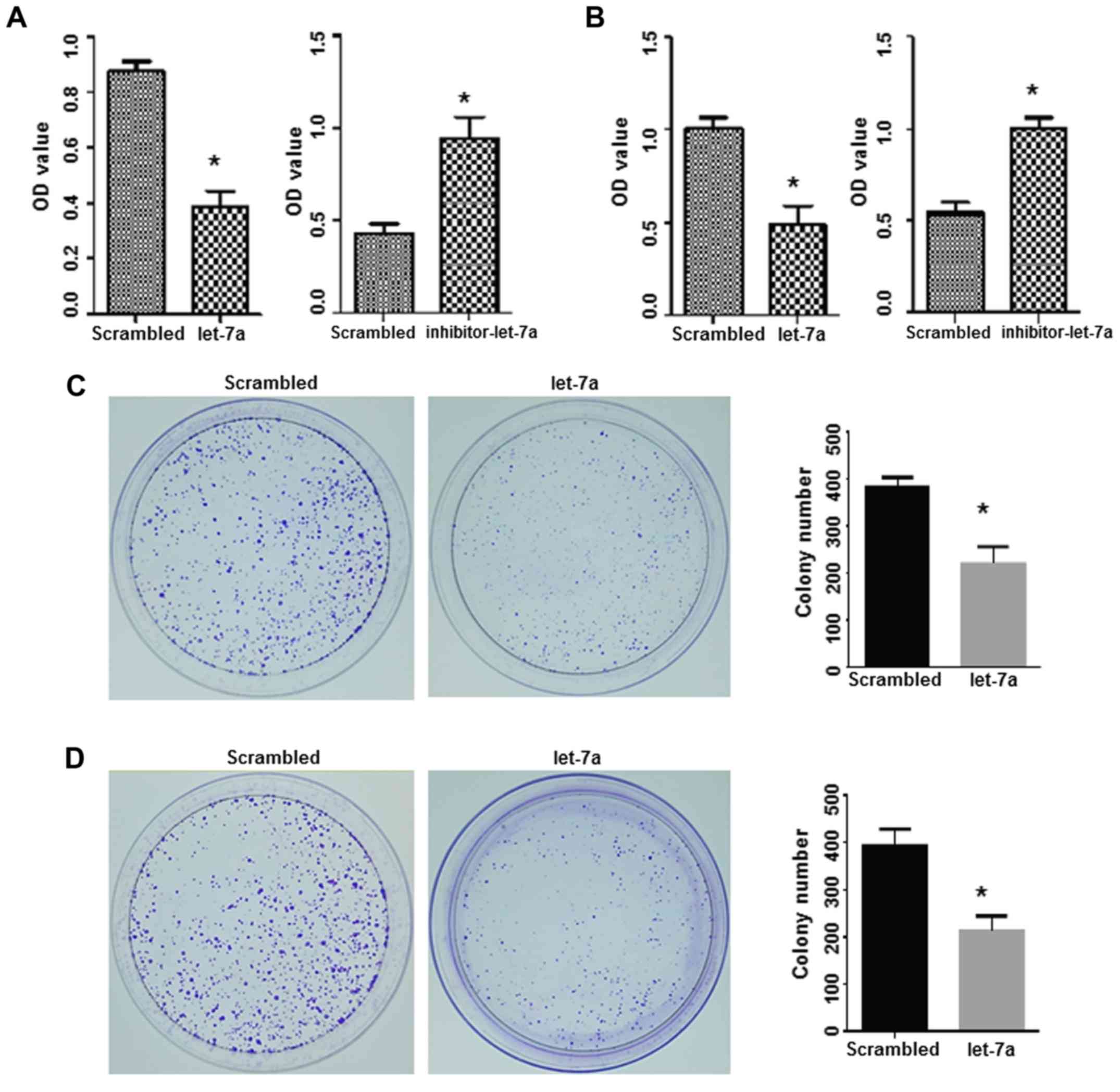
proliferation and colony formation were studied. An MTT assay
indicated that the proliferation of lung adenocarcinoma cells in
the let-7a-treated groups was significantly decreased compared with
the scrambled oligo-treated cells (P<0.05). In contrast, the
let-7a-inhibitor caused a significant increase in cell
proliferation compared with the scrambled oligo treatment
(P<0.05; Fig. 4A and B).
A colony formation assay was performed to evaluate
the long-term effect of let-7a on cell proliferation. Compared with
the scrambled control treatment, a smaller size and a decreased
number of clones were observed in the let-7a-treated cells,
suggesting that the upregulation of let-7a significantly suppressed
the colony formation ability of A549 and H1299 cells (Fig. 4C and D).
Let-7a regulates cell apoptosis and
apoptosis-associated proteins
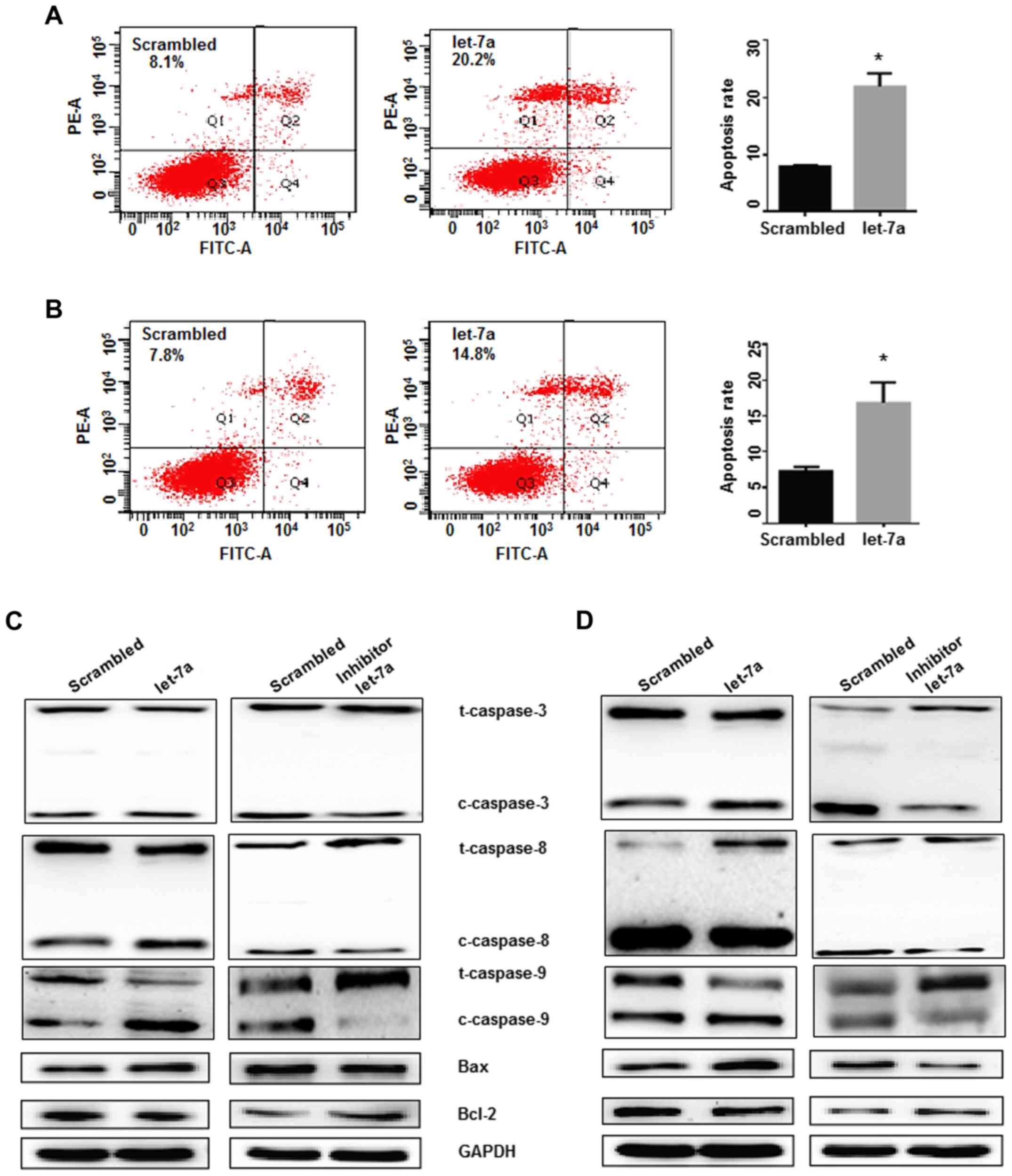
Cell apoptosis was detected following transfection
of lung adenocarcinoma cells with let-7a mimics and controls. Cell
apoptosis was significantly increased in let-7a-treated A549 and
H1299 cells, indicating that let-7a overexpression induced lung
adenocarcinoma cell apoptosis (P<0.05; Fig. 5A and B). To further characterize the
possible mechanisms of let-7a in inducing cell apoptosis, the
apoptotic genes, Bcl-2 (as an anti-apoptotic protein) and Bax (as a
pro-apoptotic protein), were studied. It was determined that let-7a
treatment significantly downregulated Bcl-2 expression and
upregulated the expression of Bax and cleaved caspase-3, −8 and −9,
compared with the control treatment in A549 and H1299 cells. In
contrast, the opposite trends for these proteins were observed in
cells transfected with the let-7a-inhibitor (Fig. 5C and D). These findings demonstrated
that the Bcl-2/Bax pathways and the activation of caspase-3, −8 and
−9 were constitutively downregulated by let-7a overexpression, and
that the Bcl-2 family proteins are involved in the apoptosis
induced by let-7a.
Let-7a functions as an inhibitor of
cell cycle progression
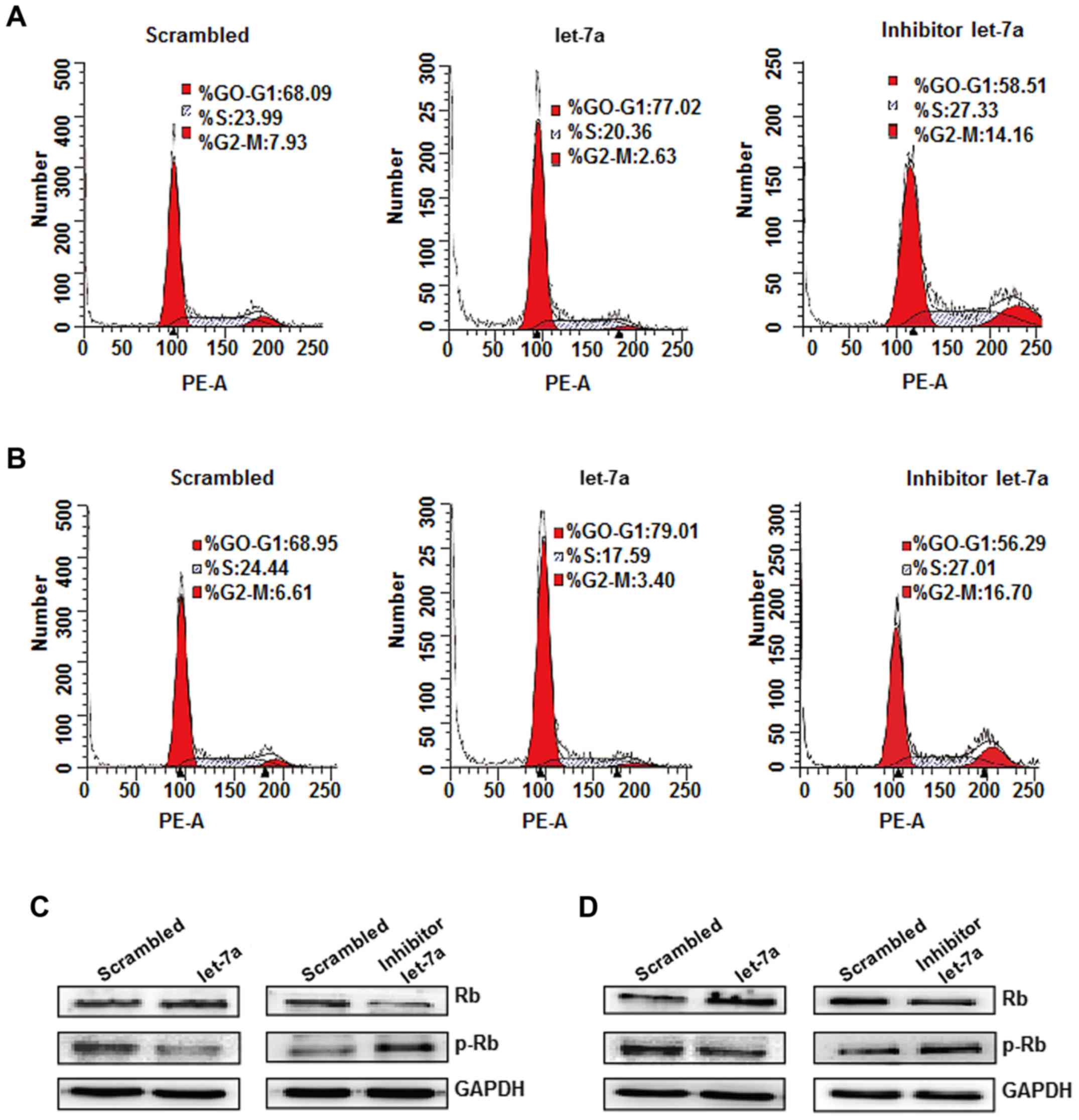
The effects of let-7a on cell cycle regulation were
evaluated by flow cytometric analysis (30). The results indicated that the
distribution of the cell cycle in A549 and H1299 cells was notably
affected by let-7a overexpression compared with the scrambled
group. The cell cycle profile indicated that 77.02% of A549 cells
and 79.01% of H1299 cells were arrested at the
G0/G1 phase at 48 h after let-7a treatment,
compared with 68.09% of NC-transduced A549 cells and 68.95% of
NC-transduced H1299 cells. However, the let-7a inhibitor-transduced
cells exhibited a smaller percentage of arrest at the
G0/G1 phase and increased cells inhibited in
the G2/M phase compared with let-7a treatment (Fig. 6A and B). These experiments
demonstrated that let-7a exerts an inhibitory effect on cell cycle
progression. To investigate the potential molecular mechanism of
let-7a in cell cycle arrest, the expression of cell cycle
molecules, namely cyclin D1, Rb and p-Rb, was detected. Let-7a
treatment significantly decreased the expression of cyclin D1 and
p-Rb, but increased the Rb protein expression compared with the
controls. The opposite trends were observed in let-7a
inhibitor-transduced cells (Fig. 6C and
D). Therefore, let-7a induces G0/G1 arrest in A549 and H1299
cells, which may be mediated by downregulation of cyclin D1 and
upregulation of Rb.
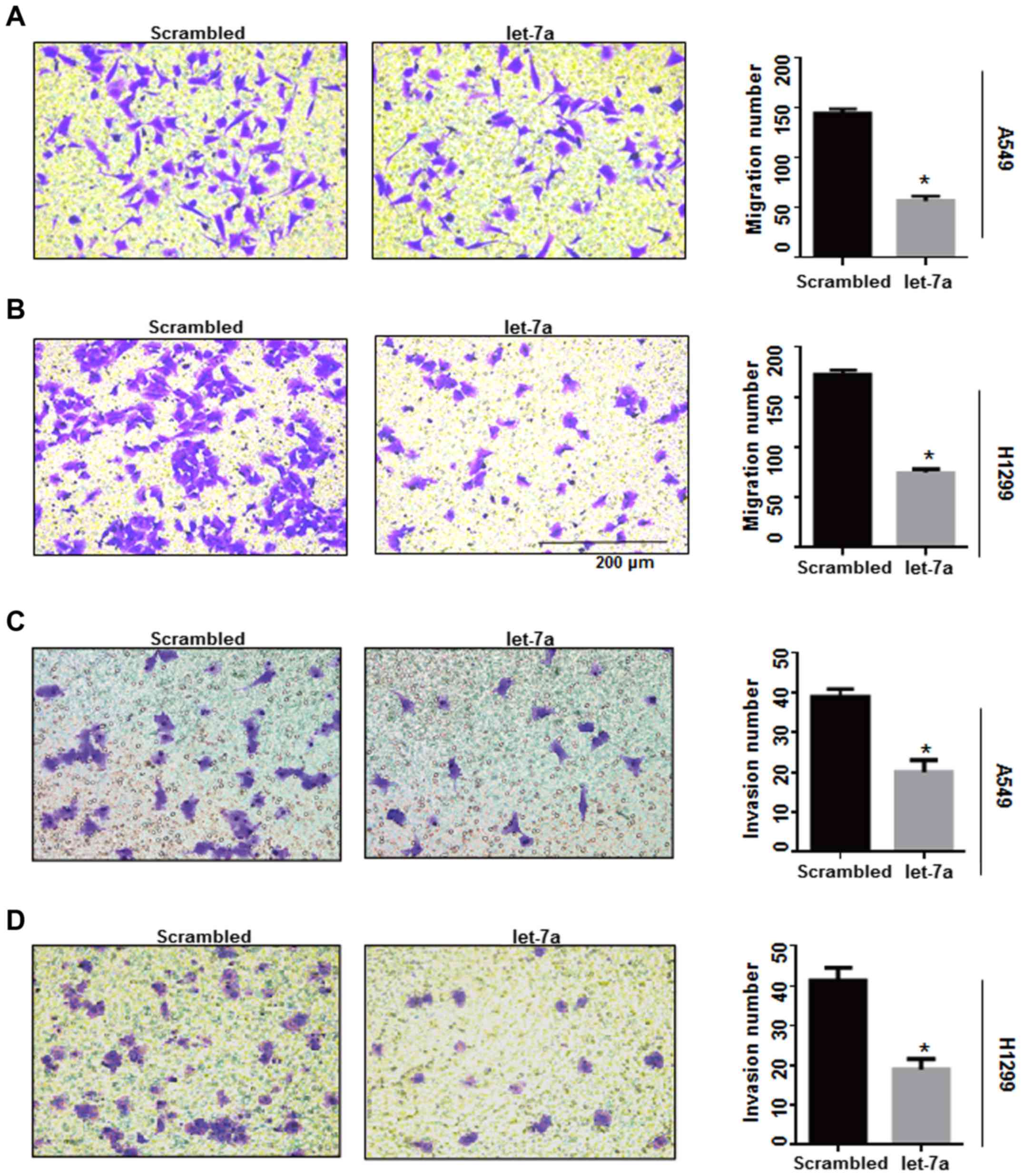
Let-7a inhibits cell migration and
invasion
Cyclin D1-associated factors may affect the
migration and invasion potential of breast cancer cells (31). In the present study, Transwell
experiments were performed to detect whether let-7a inhibits lung
cancer cell migration and invasion by influencing cyclin
D1-associated factors. The number of migrated cells in
let-7a-treated A549 and H1299 cells was significantly reduced
compared with the controls (P<0.0001; Fig. 7A and B), while the let-7a inhibitor
increased the number of migrated cells. There was no significant
increase in the rate of migration in A549 and H1299 cells
co-transfected with let-7a-inhibitor and si-cyclin D1 (P<0.001;
Fig. 7E and F). Furthermore, an
invasion assay demonstrated that invasive ability was significantly
decreased in let-7a-transfected A549 and H1299 cells compared with
the control groups (P<0.0001; Fig.
7C and D), and the effects of let-7a on invasion could be
reversed by knockdown of cyclin D1 using siRNA (P<0.001;
Fig. 7G and H). These results
indicated that let-7a inhibits cell migration and invasion by
targeting cyclin D1.
Discussion
As small non-coding RNAs, miRNAs are able to
influence numerous biological processes through
post-transcriptional regulation of gene expression (18). Over the past two decades, numerous
studies have indicated miRNA changes are associated with cancer
biology, including cancer formation, development and metastasis
(18,32). Let-7 miRNAs are underexpressed in
the blood of patients with NSCLC (33) and expressed at lower levels in lung
cancer (34). Similarly, the
current results indicated that the expression of let-7a in lung
adenocarcinoma tissues is reduced, suggesting that loss of let-7a
may be a key factor in lung cancer development.
Gain-of-function and loss-of-function experiments
were performed to observe the effects of let-7a on the biological
characteristics of A549 and H1299 cells. It was determined that
let-7a treatment inhibits proliferation and induces apoptosis of
lung adenocarcinoma cells, via a decrease of cyclin D1. It was
previously demonstrated that cyclin D1/cyclin-dependent kinase 4
may interact with filamin A and impact the migration and invasion
potential of breast cancer cells (31). In the present study, it was
determined that migration and invasion abilities were reduced
following let-7a upregulation in A549 and H1299 cells.
Aberrant expression of cyclin D1 is observed
frequently in a variety of tumor types, and cyclin D1 is regarded
as a prognostic marker in multiple cancer types (35,36).
Recently, researchers have aimed to explore the roles of miRNAs in
regulating cyclin D1. Lower expression of miR-138 increases cyclin
D1 expression, which serves a key function in the pathogenesis of
OLP mucosal disease (37).
MiRNA-520a-3p induces breast cancer cell apoptosis by directly
targeting cyclin D1 (38). In the
present study, it was revealed that let-7a, as a novel miRNA,
directly suppresses cyclin D1-associated signals, which effectively
inhibits cell growth and induces cell apoptosis. As downstream
factors of cyclin D1, Bcl-2 was decreased, whereas Bax was
increased, in let-7a-treated A549 and H1299 cells. These phenomena
may be associated with the inhibitory effect of let-7a on lung
cancer cell proliferation. The regulation of Bcl-2 promotes the
mitochondria to release cytochrome c, which further induces
cell apoptosis through caspase signals (39). The current results demonstrated that
let-7a could induce lung adenocarcinoma cell apoptosis by reducing
Bcl-2 and upregulating cleaved caspase-3, −8 and −9.
Although it was revealed in the present study that
let-7a inhibits the malignant behavior of lung cancer cells by
targeting cyclin D1, the mechanisms of let-7a remain to be
elucidated. The regulatory networks between miRNAs and mRNAs
indicate that their interactions are complex. Therefore, it is
necessary to assess the roles of other miRNAs in the regulation of
cyclin D1 in lung adenocarcinoma. Further investigations are
warranted in order to extend these findings, and in vivo
studies are required to provide more convincing data about the
roles of cyclin D1 and let-7a in lung adenocarcinoma (31).
In summary, the present study demonstrates novel
roles of let-7a in lung cancer. Let-7a significantly inhibits cell
proliferation and enhances apoptosis of lung adenocarcinoma cells
by directly regulating cyclin D1 signals. The current findings
suggest that let-7a may be a novel therapeutic target in patients
with lung cancer.
Acknowledgements
We thank Lixia Zhang (Binzhou Medical University)
for their help of electron microscopy and flow cytometry
analysis.
Funding
The present study was supported by the National
Natural Science Foundation of China (nos. 31371321, 81772281 and
81502536), the Shandong Science and Technology Committee (nos.
2017GSF221011, ZR2016CL09 and ZR2015HL060), the Health and Family
Planning Commission of Shandong Province (no. 2014WS0186,
2015WS0499), and the Shandong Province Taishan Scholar Project (no.
ts201712067).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
SYX and CZ conceived and designed the study. WZ,
JXH, RMH, QZ, JQG, YJL, NX, LYL and PYW performed the experiments.
WZ, JXH and SYX wrote the paper. CZ and SYX reviewed and edited the
manuscript. All authors read and approved the manuscript and agree
to be accountable for all aspects of the research in ensuring that
the accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The experiments were performed according to the
relevant guidelines of the Code of Ethics of the World Medical
Association for experiments involving humans and the Medical Ethics
Committee of Binzhou Medical University. Written informed consent
was obtained from all patients prior to the collection of lung
tissue samples.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
miRNAs
|
microRNAs
|
|
NSCLC
|
non-small cell lung cancer
|
|
3′ UTR
|
3′ untranslated region
|
|
qRT-PCR
|
real-time polymerase chain
reaction
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
FLNa
|
filamin A
|
References
|
1
|
Geyik E, Igci YZ, Pala E, Suner A, Borazan
E, Bozgeyik I, Bayraktar E, Bayraktar R, Ergun S, Cakmak EA, et al:
Investigation of the association between ATP2B4 and
ATP5B genes with colorectal cancer. Gene. 540:178–182. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ozcan O, Kara M, Yumrutas O, Bozgeyik E,
Bozgeyik I and Celik OI: MTUS1 and its targeting miRNAs in
colorectal carcinoma: Significant associations. Tumour Biol.
37:6637–6645. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ye L, Wang H and Liu B: miR-211 promotes
non-small cell lung cancer proliferation by targeting SRCIN1.
Tumour Biol. 37:1151–1157. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Castro D, Moreira M, Gouveia AM, Pozza DH
and De Mello RA: MicroRNAs in lung cancer. Oncotarget.
8:81679–81685. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pan C, Wang D, Zhang Y and Yu W:
MicroRNA-1284 inhibits cell viability and induces apoptosis of
ovarian cancer cell line OVCAR3. Oncol Res. 24:429–435. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kumar MS, Erkeland SJ, Pester RE, Chen CY,
Ebert MS, Sharp PA and Jacks T: Suppression of non-small cell lung
tumor development by the let-7 microRNA family. Proc Natl
Acad Sci USA. 105:3903–3908. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu WL, Chang JM, Chong IW, Hung YL, Chen
YH, Huang WT, Kuo HF, Hsieh CC and Liu PL: Curcumin inhibits
LIN-28A through the activation of miRNA-98 in the lung cancer cell
line A549. Molecules. 22:E9292017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhong Z, Dong Z, Yang L, Chen X and Gong
Z: Inhibition of proliferation of human lung cancer cells by green
tea catechins is mediated by upregulation of let-7. Exp The Med.
4:267–272. 2012. View Article : Google Scholar
|
|
9
|
Pan L, Gong Z, Zhong Z, Dong Z, Liu Q, Le
Y and Guo J: Lin-28 reactivation is required for let-7 repression
and proliferation in human small cell lung cancer cells. Mol Cell
Biochem. 355:257–263. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Braga TV, Evangelista FC, Gomes LC, Araujo
S, Carvalho MD and Sabino AP: Evaluation of MiR-15a and MiR-16-1 as
prognostic biomarkers in chronic lymphocytic leukemia. Biomed
Pharmacother. 92:864–869. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ru Y, Chen XJ, Zhao ZW, Zhang PF, Feng SH,
Gao Q, Gao SG and Feng XS: CyclinD1 and p57kip2 as
biomarkers in differentiation, metastasis and prognosis of gastric
cardia adenocarcinoma. Oncotarget. 8:73860–73870. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou X, Sun F, Luo S, Zhao W, Yang T,
Zhang G, Gao M, Lu R, Shu Y, Mu W, et al: let-7a is an
antihypertrophic regulator in the heart via targeting calmodulin.
Int J Biol Sci. 13:22–31. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He M and Xue Y: MicroRNA-148a suppresses
proliferation and invasion potential of non-small cell lung
carcinomas via regulation of STAT3. Onco Targets Ther.
10:1353–1361. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tang R, Yang C, Ma X, Wang Y, Luo D, Huang
C, Xu Z, Liu P and Yang L: MiR-let-7a inhibits cell proliferation,
migration, and invasion by down-regulating PKM2 in gastric cancer.
Oncotarget. 7:5972–5984. 2016.PubMed/NCBI
|
|
15
|
Shirali S, Aghaei M, Shabani M, Fathi M,
Sohrabi M and Moeinifard M: Adenosine induces cell cycle arrest and
apoptosis via cyclinD1/Cdk4 and Bcl-2/Bax pathways in human ovarian
cancer cell line OVCAR-3. Tumour Biol. 34:1085–1095. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li ZCJ, Yu Q, Wu X, Pan A and Li L:
Evaluation of CCND1 amplification and CyclinD1 expression: Diffuse
and strong staining of CyclinD1 could have same predictive roles as
CCND1 amplification in ER positive breast cancers. Am J Transl Res.
8:142–153. 2016.PubMed/NCBI
|
|
17
|
Wang YY, Ren T, Cai YY and He XY: MicroRNA
let-7a inhibits the proliferation and invasion of nonsmall cell
lung cancer cell line 95D by regulating K-Ras and
HMGA2 gene expression. Cancer Biother Radiopharm.
28:131–137. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen Z, Wang D, Gu C, Liu X, Pei W, Li J,
Cao Y, Jiao Y, Tong J and Nie J: Down-regulation of let-7 microRNA
increased K-ras expression in lung damage induced by radon. Environ
Toxicol Pharmacol. 40:541–548. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang YX, Yan YF, Liu YM, Li YJ, Zhang HH,
Pang M, Hu JX, Zhao W, Xie N, Zhou L, et al: Smad3-related miRNAs
regulated oncogenic TRIB2 promoter activity to effectively suppress
lung adenocarcinoma growth. Cell Death Dis. 7:e25282016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Y, Cheng N and Luo J: Downregulation
of lncRNA ANRIL represses tumorigenicity and enhances
cisplatin-induced cytotoxicity via regulating microRNA let-7a in
nasopharyngeal carcinoma. J Biochem Mol Toxicol. 31:72017.
View Article : Google Scholar
|
|
21
|
Wang PY, Sun YX, Zhang S, Pang M, Zhang
HH, Gao SY, Zhang C, Lv CJ and Xie SY: Let-7c inhibits A549 cell
proliferation through oncogenic TRIB2 related factors. FEBS Lett.
587:2675–2681. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cheng G, Wang X, Li Y and He L:
let-7a-transfected mesenchymal stem cells ameliorate
monocrotaline-induced pulmonary hypertension by suppressing
pulmonary artery smooth muscle cell growth through STAT3-BMPR2
signaling. Stem cell Res Ther. 8:342017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fan YC, Zhu YS, Mei PJ, Sun SG, Zhang H,
Chen HF, Chen C and Miao FA: Cullin1 regulates proliferation,
migration and invasion of glioma cells. Med Oncol. 31:2272014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang G, Wang J, Zhao H, Wang J and Tony To
S: The role of Myc and let-7a in glioblastoma, glucose metabolism
and response to therapy. Arch Biochem Biophys. 580:84–92. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Detterbeck FC, Boffa DJ, Kim AW and Tanoue
LT: The 8th edition lung cancer stage classification. Chest.
2016.doi:10.1016/j.chest.2016.10.010.
|
|
26
|
Nakashima Y, Yao T, Hirahashi M, Aishima
S, Kakeji Y, Maehara Y and Tsuneyoshi M: Nuclear atypia grading
score is a useful prognostic factor in papillary gastric
adenocarcinoma. Histopathology. 59:841–849. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kadota K, Suzuki K, Kachala SS, Zabor EC,
Sima CS, Moreira AL, Yoshizawa A, Riely GJ, Rusch VW, Adusumilli
PS, et al: A grading system combining architectural features and
mitotic count predicts recurrence in stage I lung adenocarcinoma.
Mod Pathol. 25:1117–1127. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Espinosa AM, Alfaro A, Roman-Basaure E,
Guardado-Estrada M, Palma Í, Serralde C, Medina I, Juárez E,
Bermúdez M, Márquez E, et al: Mitosis is a source of potential
markers for screening and survival and therapeutic targets in
cervical cancer. PLoS One. 8:e559752013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Giordano TJ: The argument for mitotic
rate-based grading for the prognostication of adrenocortical
carcinoma. Am J Surg Pathol. 35:471–473. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li X, Hao Z, Fan R, Zou X, Jin H, Pan Y,
He L, Du R, Gao L, Liu D, et al: CIAPIN1 inhibits gastric cancer
cell proliferation and cell cycle progression by downregulating
CyclinD1 and upregulating P27. Cancer Biol Ther. 6:1539–1545. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhong Z, Yeow WS, Zou C, Wassell R, Wang
C, Pestell RG, Quong JN and Quong AA: CyclinD1/cyclin-dependent
kinase 4 interacts with filamin A and affects the migration and
invasion potential of breast cancer cells. Cancer Res.
70:2105–2114. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cho KJ, Song J, Oh Y and Lee JE:
MicroRNA-let-7a regulates the function of microglia in
inflammation. Mol Cell Neurosci. 68:167–176. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jeong HC, Kim EK, Lee JH, Lee JM, Yoo HN
and Kim JK: Aberrant expression of let-7a miRNA in the blood of
non-small cell lung cancer patients. Mol Med Rep. 4:383–387.
2011.PubMed/NCBI
|
|
34
|
Zhao BW, Zhou LF, Liu YL, Wan SM and Gao
ZX: Evolution of Fish Let-7 MicroRNAs and their expression
correlated to growth development in Blunt Snout Bream. Int J Mol
Sci. 18:E6462017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hunter T and Pines J: Cyclins and cancer.
II: Cyclin D and CDK inhibitors come of age. Cell. 79:573–582.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Seiler R, Thalmann GN, Rotzer D, Perren A
and Fleischmann A: CCND1/CyclinD1 status in metastasizing bladder
cancer: A prognosticator and predictor of chemotherapeutic
response. Mod Pathol. 27:87–95. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ghallab NA, Kasem RF, El-Ghani SF and
Shaker OG: Gene expression of miRNA-138 and cyclin D1 in oral
lichen planus. Clin Oral Investig. 21:2481–2491. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li J, Wei J, Mei Z, Yin Y, Li Y, Lu M and
Jin S: Suppressing role of miR-520a-3p in breast cancer through
CCND1 and CD44. Am J Transl Res. 9:146–154. 2017.PubMed/NCBI
|
|
39
|
Gómez-Crisóstomo NP, López-Marure R,
Zapata E, Zazueta C and Martínez-Abundis E: Bax induces cytochrome
c release by multiple mechanisms in mitochondria from MCF7
cells. J Bioenerg Biomembr. 45:441–448. 2013. View Article : Google Scholar : PubMed/NCBI
|