Introduction
Colorectal cancer (CRC) is one of the most common
cancer types worldwide with the highest incidence rate in western
countries (1). The prognosis of CRC
patients is poor, due to frequent metastasis and recurrence.
Therefore, it is important to further study the pathogenesis of CRC
and explore novel effective treatments. Risk factors, including
smoking, alcohol consumption, environmental factors, diet and
obesity lead to oxidative stress in the colorectal environment,
causing an overproduction of reactive oxygen species (ROS)
(2). ROS are in certain ways
detrimental to various cellular macromolecules, interfering with
cellular function (3). DNA damage
may occur, causing chromosomal instability and aneuploidy (4). This resulting oxidative damage is the
first step involved in mutagenesis and carcinogenesis (5,6).
Furthermore, excessive and uncontrollable production of ROS for an
extended period of time leads to persistent inflammation (7). It is widely known that inflammation in
the colonic environment is a vital risk factor for CRC. Patients
with inflammatory bowel disease, including ulcerative colitis or
Crohn's disease, have a 6-fold increased risk of developing
colitis-associated cancer compared with the general population
(8). Overall, the association
between oxidative stress, inflammation and CRC has been broadly
evidenced.
ATP binding cassette (ABC) subfamily G member 2
(ABCG2), also known as breast cancer resistant protein, is an ABC
transporter and is abundantly expressed in the apical membrane of
the normal colonic epithelium (9),
playing an important role in maintaining normal physiological
function by protecting normal cells via efflux of a variety of
carcinogens, drugs or toxic substances (10,11).
ABCG2 has been demonstrated to be associated with chemoresistance
in CRC (12). However, the
association between ABCG2 and the onset or development of CRC
remains to be fully elucidated and the expression pattern of ABCG2
in CRC also remains controversial. Gupta et al (13) reported that ABCG2 mRNA expression
was present in normal colorectal tissues and was 6-fold decreased
in cancer tissues. Furthermore, Liu et al (14) indicated that the expression rate of
ABCG2 in carcinomatous tissues was higher compared with that in
tissues from the non-carcinomatous margin. It may therefore be
suggested that ABCG2 was associated with colorectal carcinogenesis,
while the mechanism and its influence on patient prognosis remains
elusive.
A previous study by our group indicated that ABCG2
has a protective role against oxidative stress by decreasing the
level of cellular heme and inhibiting ROS signaling-mediated
nuclear factor (NF)-κB activation, leading to a decreased
expression of inflammatory genes (15). Due to the fact that oxidative stress
and inflammation have an effect on the development of CRC, and
ABCG2 has a protective role during oxidative stress and is
aberrantly expressed in cancer tissues, it may be hypothesized that
the upregulation of ABCG2 in CRC tissues is an adaptive response to
relieve oxidative stress and protect colonic epithelial cells
against ROS-induced damage and inflammation by inhibiting the NF-κB
signaling pathway.
In the present study, immunohistochemical analysis
of CRC specimens was performed and the association of ABCG2 with
the clinicopathological factors of patients was analyzed to
evaluate the significance of ABCG2. The role of ABCG2 in the
mechanism of colorectal carcinogenesis was also explored by
examining the association between ABCG2, ROS generation,
inflammatory gene expression and the probable signaling
pathways.
Materials and methods
Patients
Eighty-three CRC, 24 colorectal adenoma patients and
24 normal colorectal tissues from the same adenoma patients with
complete clinical information, who were treated at Nanjing Drum
Tower Hospital (Nanjing, China) between January 2007 and August
2013 were collected. The experimental study was approved by the
Ethics Committee of Nanjing Drum Tower Hospital, and the informed
consent forms were obtained when the patients were accepted for the
study by the hospital.
ROS assay
For the ROS assay, negative control and
ABCG2-knockdown HT-29 cells were treated with 0, 1 and 2 mM
H2O2 for 4 h, following which cells were
incubated with 5 µM 2′,7′-dichlorofluoresceindiacetate (DCFDA;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 25 min. To
remove excess DCFDA, the cells were washed twice with PBS. Labeled
cells were then trypsinized and re-suspended in PBS. The mean
fluorescence intensity was analyzed by flow cytometry to determine
the intracellular ROS concentration.
To detect ROS levels in tissues, fresh normal human
colorectal mucosal tissues, colorectal adenoma or tumor tissues
from patients were collected and lysed, and the supernatant was
collected to detect ROS levels using a ROS detection kit (cat. no.
E004; Nanjing Jiancheng Bioengineering Institute, Nanjing, China)
according to the manufacturer's protocol.
Detection of oxidative
stress-associated markers and inflammatory factors
Glutathione (GSH) detection. Equal numbers of
negative control and ABCG2-knockdown HT-29 cells were seeded in
6-well plates and cultured overnight. Cells were treated with 0 or
1 mM H2O2 for 4 h and then collected to
detect GSH levels in the cell lysate using a Glutathione
Colorimetric Assay kit (cat. no. K261-100; BioVision, Milpitas, CA,
USA) according to the manufacturer's protocol.
IL-8 detection in cell culture
medium
Equal numbers of negative control and
ABCG2-knockdown HT-29 cells were seeded in 6-well plates and grown
overnight. Cells were treated with 0, 1 or 2 mM
H2O2 for 4 h and the culture medium was
collected to detect IL-8 using a Human IL-8 Coated ELISA kit (cat.
no. BMS204-3; Invitrogen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) according to the manufacturer's protocols.
Serum superoxide dismutase (SOD),
malondialdehyder (MDA) and GSH detection
Venous blood (5 ml) was drawn from the antecubital
vein of patients using a sterile disposable syringe and transferred
to an EDTA-containing vial. The blood was centrifuged for 10 min at
4°C at 1,500 × g and plasma was separated. Serum SOD, MDA and GSH
levels were detected using a SOD Activity Colorimetric sssay kit
(cat. no. K335-100; BioVision), a Lipid Peroxidation (MDA)
Colorimetric/fluorometric assay kit (cat. no. K739-100; Biovison)
and a Glutathione Colorimetric Assay kit (cat. no. K261-100;
BioVision), respectively, according to the manufacturer's
protocols.
Tumor necrosis factor (TNF)-α
detection in tissues
Fresh human normal colorectal mucosal tissues,
colorectal adenoma or tumor tissues from patients were collected
and lysed, and the supernatant was collected to detect TNF-α levels
using a Human TNF-α Platinum ELISA kit (cat. no. BMS223/4;
Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol.
Immunohistochemistry
For histological analysis, normal human colorectal
mucosal tissues, colorectal adenoma or tumor tissues from patients
were fixed in 10% buffered formalin (Sigma-Aldrich) and embedded in
paraffin. Paraffin sections were then processed for either
hematoxylin and eosin (H&E) staining or immunohistochemistry.
Antibodies used for immunostaining are listed in Table I. The proportion of stained areas
was evaluated as follows: 0, <5%; 1, ≥5% and <25%; 2, ≥25%
and <50%; 3, ≥ 50% and <75%; 4, ≥75%. The intensity of
staining was scored as follows: 0, yellow; 1, brown; 2, dark brown
and 3 for the absence of staining. The final scores were obtained
by multiplying the area and intensity scores, producing a range of
0–12. For ABCG2, samples with scores of at least 2 were considered
positive. For NF-κB, samples scoring 4 points and above were
considered positive. The stained slides were independently
evaluated by two experienced pathologists.
 | Table I.Antibodies used in the present
study. |
Table I.
Antibodies used in the present
study.
| Antibody | Assay | Supplier/catalogue
no. |
|---|
|
Anti-BCRP/ABCG2 | WB, IHC | Abcam; ab24115 |
| Anti-NF-κB p65 | IHC | Abcam; ab16502 |
| Phospho-IκBα
(Ser32) (14D4) rabbit mAb | WB | Cell Signaling
Technology; #2859 |
| β-actin | WB | Bioworld;
AP0060 |
| HRP-linked
anti-mouse IgG | WB | Cell Signaling
Technology; #7076 |
| HRP-linked
anti-rabbit IgG | WB | Cell Signaling
Technology; #7074 |
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated using TRIzol reagent (Takara
Bio Inc., Otsu, Japan) according to the manufacturer's
instructions. RT reactions were performed with 1 µg total RNA using
the PrimeScript™ RT Master Mix (Takara Bio Inc.). qPCR was
performed in a total reaction volume of 20 µl in 96-well reaction
plates using SYBR® Advantage® qPCR Premix
(Takara Bio, Inc.) according to the manufacturer's protocols. The
sequences of the primers used are listed in Table II. qPCR was performed at 95°C for
30 sec, followed by 40 cycles at 95°C for 5 sec and 60°C for 30 sec
using LightCycler® 96 system (Roche Diagnostics, Basel,
Switzerland). Reactions were run in triplicate in three independent
experiments. The 2−∆∆Cq method (16) was used to determine the relative
levels of mRNA expression in experimental samples and controls.
Specific primers were listed in Table
II. β-actin was amplified as an internal control.
 | Table II.Sequences of the primers used. |
Table II.
Sequences of the primers used.
| Primer | Sequence |
|---|
| ABCG2 | F:
5′-GTGTTTATGATGGTCTGTTGGTCA-3′ |
|
| R:
5′-TGCTGCAAAGCCGTAAATCC-3′ |
| IL-8 | F:
5′-AGCTGGCCGTGGCTCTCT-3′ |
|
| R:
5′-TTTAGCACTCCTTGGCAAAACTG-3′ |
| MCP-1 | F:
5′-GACCATTGTGGCCAAGGAGAT-3′ |
|
| R:
5′-TGCTTGTCCAGGTGGTCCAT-3′ |
| GRO-β | F:
5′-ACTCAAGAATGGGCAGAAAGCTT-3′ |
|
| R:
5′-TCAGCATCTTTTCGATGATTTTCTTA-3′ |
| β-actin | F:
5′-AGCGAGCATCCCCCAAAGTT-3′ |
|
| R:
5′-GGGCACGAAGGCTCATCATT-3′ |
Western blot analysis
Cell and tissue lysates were obtained as previously
described (17). Protein
concentrations were determined using the bicinchoninic acid assay
(Beyotime Institute of Biotechnology, Haimen, China). Protein
lysates (30–60 µg/lane) were separated by 6–12% SDS-PAGE and
transferred to polyvinylidene difluoride membranes (EMD Millipore,
Billerica, MA, USA). Tris-buffered saline containing Tween-20 with
5% non-fat milk or bovine serum albumin (BioFroxx GmbH, Einhausen,
Germany) was used to block non-specific binding for 2 h at room
temperature. The membranes were then incubated with primary
antibodies (1:50 dilution) according to the manufacturer's
protocols overnight at 4°C, followed by appropriate horseradish
peroxidase-conjugated secondary antibodies for 1 h at room
temperature (1:5,000 dilution). Signals generated by enhanced
chemiluminescence (EMD Millipore) were recorded with a CCD camera
(Tanon, Shanghai). Primary and secondary antibodies are listed in
Table I.
Cell culture
The human CRC cell lines HCT-8, HCT-116, HT-29,
Colo-205, SW480, SW620, Caco-2 and SW116 were purchased from the
Cell Bank of the Chinese Academy of Sciences (Shanghai, China), and
LS-180 (ATCC CL-187TM) (18) was
purchased from ATCC. All cell lines were cultured in RPMI-1640
medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with
10% fetal bovine serum (FBS; Biological Industries, Cromwell, CT,
USA) in a humidified atmosphere with 5% CO2 at 37°C. The HT-29 cell
line with high ABCG2 expression was selected for further study.
Lentiviral transfection
The ABCG2 small hairpin (sh)RNA lentiviral vector
was constructed by GeneChem Co. Ltd (Shanghai, China) from GV298
vector (U6-MCS-Ubiquitin-Cherry-IRES-puromycin). The targeting
sequence of ABCG2 shRNA was 5′-CTCGATATTCCATCTTCAA-3′. An empty
vector was used as a negative control. To generate the HT-29 cell
line with stable ABCG2 knockdown, cells were seeded in a 6-well
plate at a density of 1×105 cells/well and following 1
day of culture, 1×106 units of lentivirus were added to
the cell culture medium supplemented with 5 µg/ml Polybrene to
initiate transfection. Following 12 h of culture, the medium was
replaced with fresh normal cell culture medium. At 3 days
post-transfection, 5 µg/ml puromycin was added to the cell culture
medium to generate the stable ABCG2-knockdown HT-29 cell line.
Statistical analysis
Data were analyzed using GraphPad Prism v6.0
(GraphPad Inc., La Jolla, CA, USA). Unless otherwise indicated, all
experiments were performed in triplicate, and the results were
expressed as the mean and standard deviation where appropriate.
Differences among detected marker values (oxidative stress markers,
inflammatory factors and antioxidants) in in vitro studies
were evaluated using analysis of variance (ANOVA) or Student's
t-test. Multiple comparison between the groups was performed using
Student-Newman-Keuls (SNK) method. The differences in ABCG2 and
NF-κB expression by immunohistochemistry among patients were
analyzed by a Chi-squared test for categorical variables. The
differences in ABCG2 expression among patients with diffferent
clinicopathological characteristics were analyzed by a chi-squared
test for categorical variables. Correlations between ABCG2 and
NF-κB expression were analyzed by the Spearman's Rank-Order method.
Log-rank tests were performed on Kaplan-Meier survival curves to
determine any significant associations between ABCG2 expression and
patient outcomes. P<0.05 was considered to indicate
statistically significant differences.
Results
Oxidative stress and inflammation in
CRC tissues
Previous studies have indicated a close association
between oxidative stress, inflammation and CRC. To further identify
this association, 83 CRC, 24 colorectal adenomas and 24 normal
colorectal mucosa tissues from the same adenoma patients with
complete clinical information, treated at Nanjing Drum Tower
Hospital (Nanjing, China) between January 2007 and August 2013 were
collected. The serum and tissue homogenate supernatants were
obtained to detect oxidative stress-associated markers and the
expression of inflammatory genes. The results indicated that the
ROS levels in the serum and homogenate supernatants were increased
from normal tissue to adenoma to cancer (P<0.05, P<0.01;
Fig. 1A). To determine the redox
state of the cells, the antioxidants SOD and GSH and the oxidative
damage product MDA were detected in the tissues and serum of the
patients. In the serum, the levels of SOD in the CRC group were
lower than those in the normal groups (P<0.01; Fig. 1B, left) and GSH was significantly
decreased in CRC samples compared with that in normal samples
(P<0.01; Fig. 1C). In the
homogenate supernatants, the results regarding SOD were similar to
those obtained in serum (Fig. 1B,
right), while GSH was not detected. In terms of oxidative damage
products, the serum levels of MDA in the CRC patients were higher
than those in the normal group (P<0.05; Fig. 1D, left), while no difference was
observed in fresh tissues from the different groups (Fig. 1D, right). These results indicated
that oxidative stress prevailed in CRC and that it may play a role
in the onset and development of tumors.
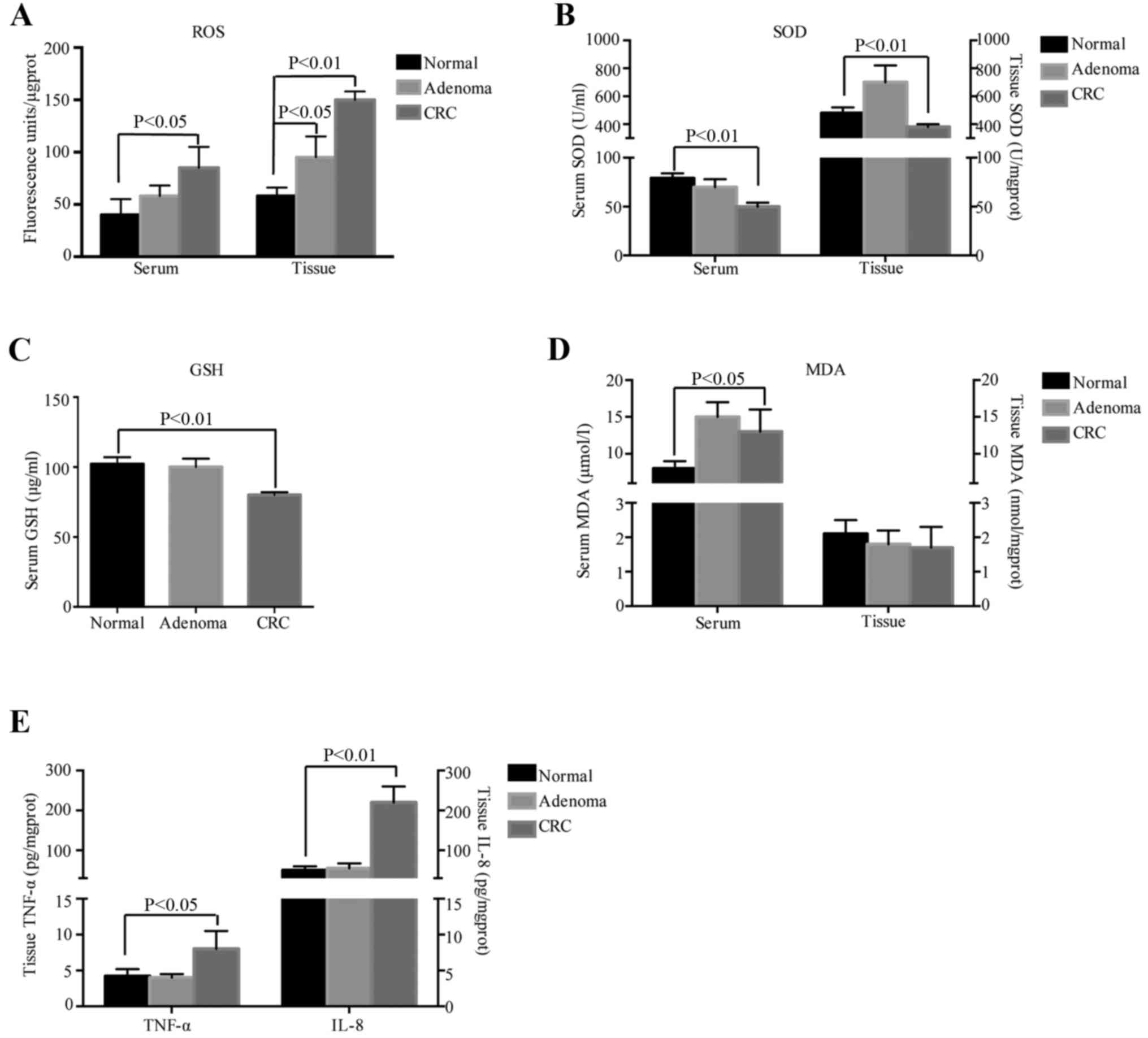 | Figure 1.Oxidative stress and inflammation in
CRC tissues. (A) Levels of ROS, (B) SOD, (C) GSH, (D) MDA, (E)
TNF-α and IL-8. CRC, colorectal cancer; MDA, malondialdehyde; TNF,
tumor necrosis factor; IL, interleukin; ROS, reactive oxygen
species; SOD, superoxide dismutase; GSH, glutathione |
Excessive and uncontrollable production of ROS for a
long period of time may result in persistent inflammation, which is
an important factor in carcinogenesis. In the present study,
inflammatory factors were also detected in tissue homogenates from
the different groups by ELISA to determine whether any inflammatory
reaction was present. The levels of TNF-α in cancer tissues
significantly surpassed those in normal tissues (P<0.05) and
IL-8 exhibited a similar trend to that of TNF-α (P<0.01;
Fig. 1E). These results indicated
that inflammatory factors are activated in CRC.
ABCG2 expression increases during
human CRC progression and metastasis
ABCG2 has been reported to be aberrantly expressed
in CRC and to play a role in regulating oxidative stress, which may
be associated with tumor development (14). To further examine this, ABCG2
expression was assessed in fresh tissues from the different groups
in the present study. The mRNA levels of ABCG2 were detected by
RT-qPCR and the protein levels were assessed by western blotting
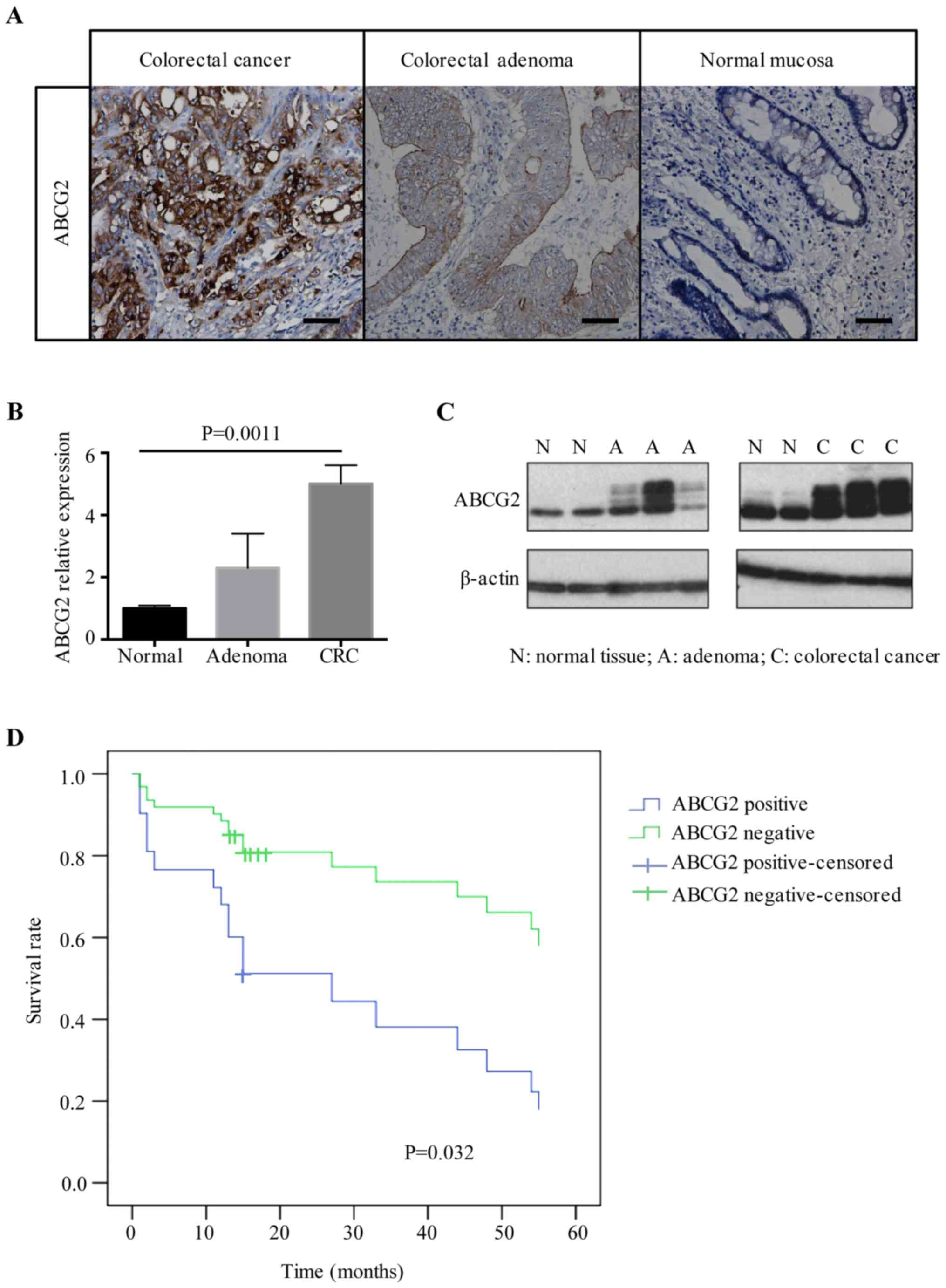
and immunohistochemistry. Immunohistochemical staining indicated
that ABCG2 was mainly localized in the membrane and/or cytoplasm of
colonic epithelial cells. Staining for ABCG2 was identified in
86.7% (72/83) of CRC tissues, and 54.2% (13/24) of colon adenoma
tissues, while 66% (16/24) of normal colon mucosa tissues were
negative (Fig. 2A). A significant
difference in ABCG2 expression was observed among malignant,
pre-malignant and normal tissues (P<0.001; Table III). In fresh tissues, RT-qPCR and
western blotting results indicated that the expression of ABCG2 in
CRC and adenoma tissues at the mRNA (P=0.0011) and protein level
was higher than that in normal tissues (Fig. 2B and C).
 | Table III.ABCG2 expression by
immunohistochemistry in different groups. |
Table III.
ABCG2 expression by
immunohistochemistry in different groups.
| Tissues | Total (n) | Positive (n) | Negative (n) | χ2
value | P-value |
|---|
| CRC | 83 | 72 | 11 | 29.832 | <0.001 |
| Adenoma | 24 | 13 | 11 |
|
|
| Normal | 24 | 8 | 16 |
|
|
Subsequently, the association between ABCG2
expression in tumor tissues and clinicopathological parameters was
analyzed. No correlation was identified between the expression of
ABCG2 and age, sex, tumor size, depth of infiltration and tumor
grade. However, ABCG2 expression levels in tumor tissues were
significantly associated with tumor differentiation and lymph node
metastasis. Of the 83 CRC samples, 21 had low differentiation
according to the histology, among which 85.7% (18/21) had high
expression of ABCG2, which was significantly higher than that in
CRC samples with moderate or high differentiation [50.0% (31/62),
P=0.005]. Among the 35 cancer samples with lymph node metastasis,
88.6% (31/35) were positive for ABCG2, and in the 48 samples
without metastasis, the rate of positivity for ABCG2 was
significantly lower [66.7% (32/48), P=0.005; Table IV). To investigate the prognostic
value of ABCG2 expression, 49 cancer patients whose follow-up data
were available were enrolled for analysis. Patients were classified
into ABCG2-positive and ABCG2-negative groups according to the
assessment criteria listed in Materials and methods, and the 5-year
survival rates were 28.6 and 71.4%, respectively (P=0.032; Fig. 2D). Notably, these results indicated
that overexpression of ABCG2 which may be the feedback of
over-oxidative reaction, was associated with a worse prognosis of
CRC, though we suppose ABCG2 could play a protective role in
cancer.
 | Table IV.Relationship of ABCG2 expression and
clinicopathological characteristics in colorectal cancer. |
Table IV.
Relationship of ABCG2 expression and
clinicopathological characteristics in colorectal cancer.
|
|
| ABCG2 staining |
|
|---|
|
|
|
|
|
|---|
| Variable | No. of cases | Negative | Positive | P-value |
|---|
| Sex |
|
Male | 40 | 6 | 34 | 0.751 |
|
Female | 43 | 5 | 38 |
|
| Age (years) |
|
≤60 | 36 | 6 | 30 | 0.52 |
|
>60 | 47 | 5 | 42 |
|
| Tumor location |
|
Colon | 45 | 6 | 39 | 0.981 |
|
Rectum | 38 | 5 | 33 |
|
|
Differentiation |
|
MD-HD | 62 | 31 | 31 | 0.005 |
| LD | 21 | 3 | 18 |
|
| Tumor size
(cm) |
| ≤4 | 42 | 6 | 36 | 0.779 |
|
>4 | 41 | 5 | 36 |
|
| Depth of
invasion |
| Not
serous | 25 | 2 | 23 | 0.491 |
| Serous
and above | 58 | 9 | 49 |
|
| Lymphatic
invasion |
| No | 48 | 16 | 32 | 0.005 |
|
Yes | 35 | 4 | 31 |
|
| Distant
metastasis |
| No | 71 | 8 | 63 | 0.194 |
|
Yes | 12 | 3 | 9 |
|
| TNM stage |
|
I/II | 43 | 5 | 38 | 0.751 |
|
III/IV | 40 | 6 | 34 |
|
Downregulation of ABCG2 suppresses the
antioxidant capacity of HT-29 cells
In order to explore the potential mechanism of ABCG2
in regulating the degree of malignancy of CRC, ABCG2 expression was
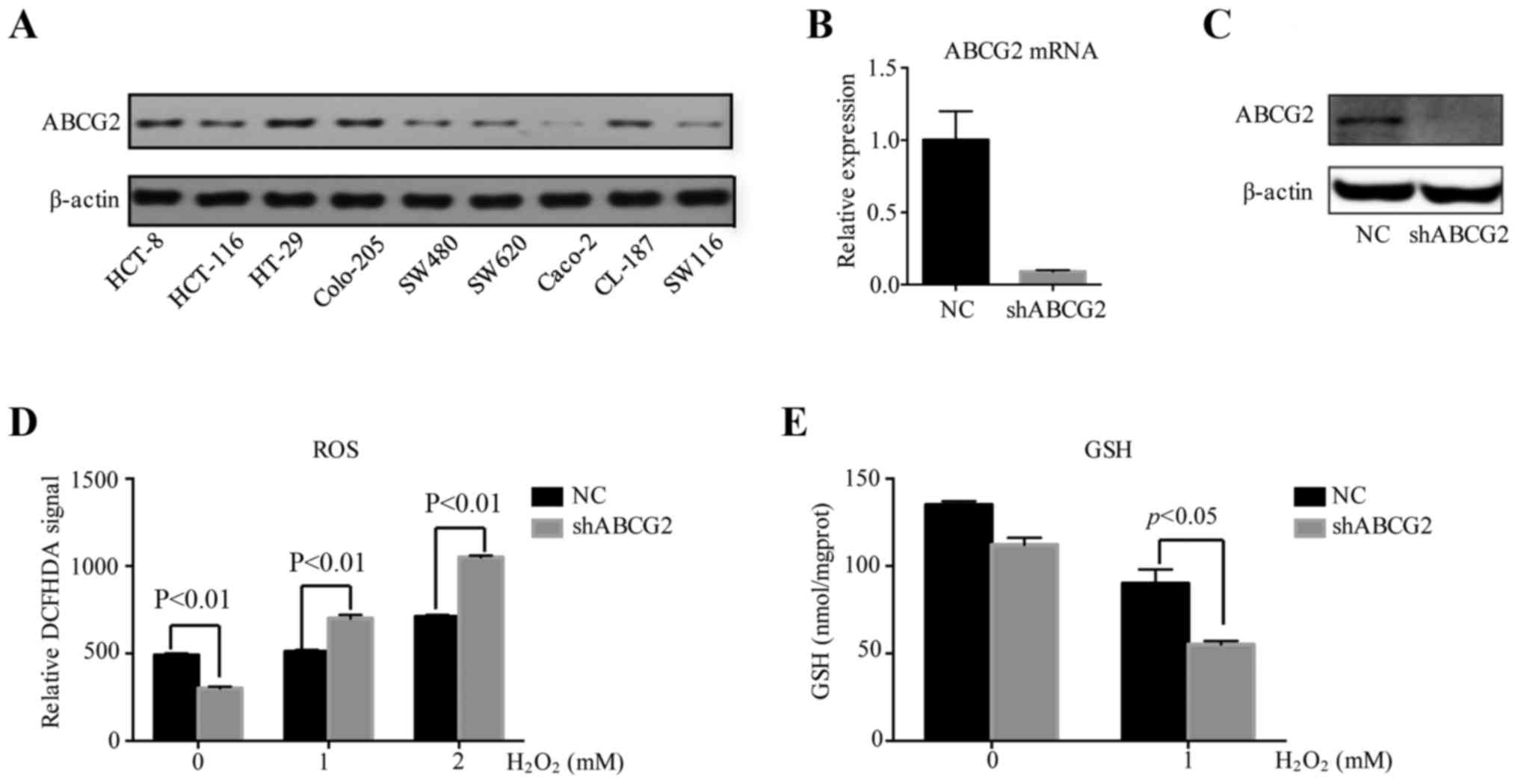
examined in 9 widely used human CRC cell lines (HCT-8, HCT-116,
HT-29, Colo-205, SW480, SW620, Caco-2, CL-187 and SW116). The HT-29
cell line with the highest expression levels of ABCG2 was selected
for in vitro study (Fig.
3A). shRNA-mediated knockdown resulted in decreased ABCG2
expression at the mRNA and protein levels (Fig. 3B and C). The transfection efficiency
of the lentivirus was almost 100% and the gene silencing efficiency
reached 90% compared with the negative control cells transfected
with blank vector.
To assess the antioxidative capacity of the cells,
H2O2 was added to the culture media of HT-29
cells to emulate an oxidative stress environment. ROS activation
reflecting redox intensity was assessed via a DCFDA fluorescence
assay. At baseline, shABCG2 HT-29 cells produced less ROS than
negative control cells. When H2O2 at
different concentrations (1 or 2 mM) was added to the culture media
of the cells for 4 h, ROS levels in shABCG2 HT-29 cells were
significantly higher than in the negative control cells (Fig. 3D). To further study whether ABCG2
influenced the redox balance by increasing antioxidative products,
the levels of GSH were determined in HT-29 cells treated with
hydrogen peroxide. At baseline, there was no difference in the
levels of GSH between control cells and ABCG2-knockdown HT-29
cells. However, when cells were treated with
H2O2 (1 mM) for 4 h, the levels of GSH in the
shABCG2 HT-29 cells decreased by 40% compared with those in the
negative control cells (Fig. 3E).
These results indicated that under oxidative stress, ABCG2 had an
important role in redox homeostasis by increasing the expression of
antioxidative products.
Downregulation of ABCG2 increases the
inflammatory reaction in HT-29 cells
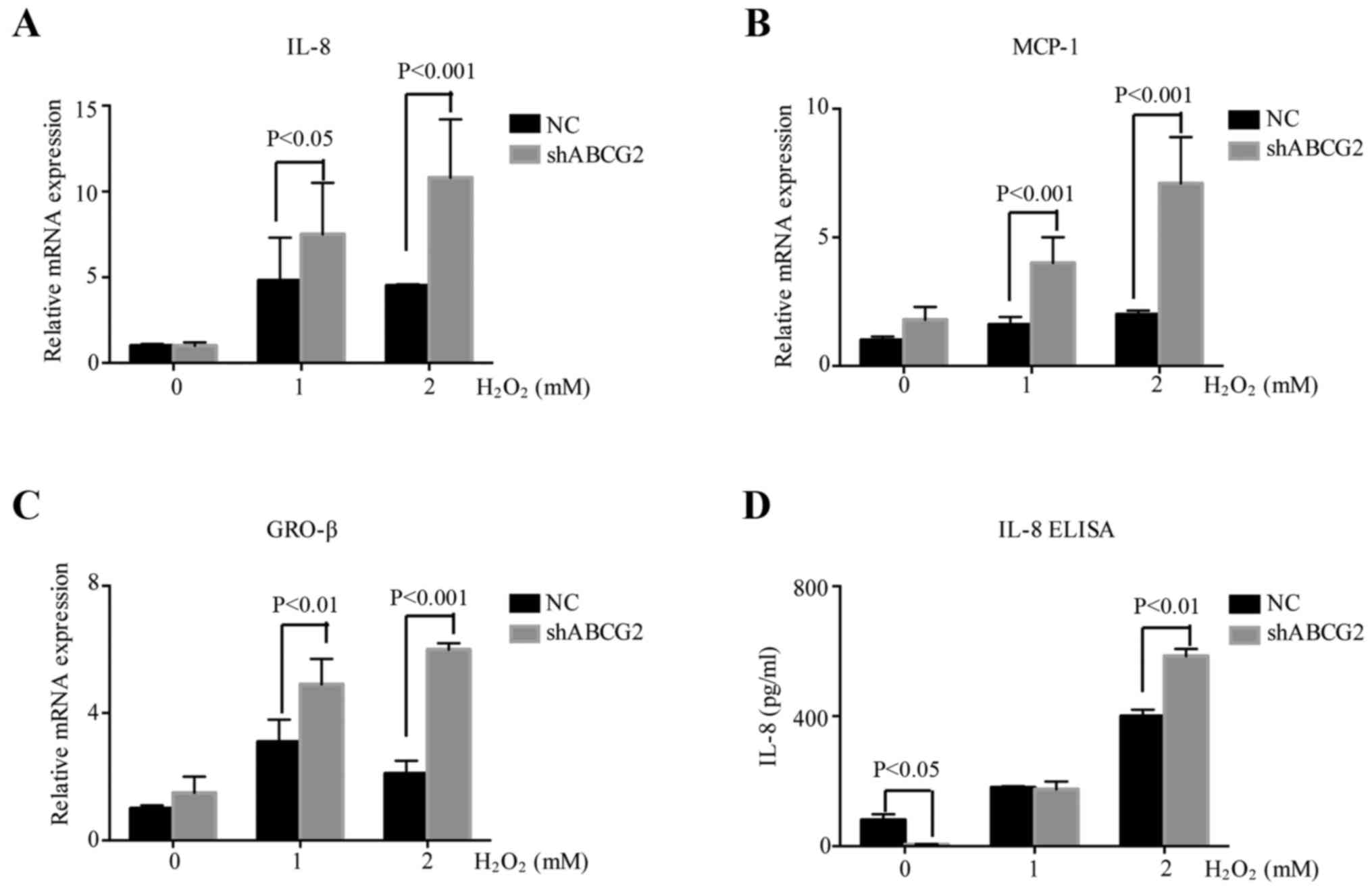
ROS is known to activate the expression of
inflammatory genes responsive to oxidative stress. To assess
whether ROS induced a cellular inflammatory response in CRC, the
expression of IL-8, monocyte chemoattractant protein (MCP-1) and
growth-related oncogene (GRO-β) was detected in HT-29 cells treated
with various concentrations of H2O2 (1 or 2
mM) for 4 h. Furthermore, to explore the association between ABCG2
and the inflammatory reaction, RNA samples were collected from
shABCG2 HT-29 cells and control HT-29 cells treated with
H2O2 (1 or 2 mM) for 4 h. RT-qPCR analysis
indicated that IL-8, MCP-1 and GRO-β were induced by ROS, and a
further increase was observed in shABCG2 HT-29 cells compared with
that in the negative control cells (Fig. 4A-C). ELISA also confirmed that
shABCG2 HT-29 cells produced more IL-8 than the control cells
(Fig. 4D). These results indicated
that oxidative stress promoted inflammation, which was more intense
when ABCG2 expression was decreased.
Effects of downregulation of ABCG2 on
the NF-κB signaling pathway
The NF-κB transcription factor is present in the
cytosol in a complex with the inhibitory IκB protein, remaining
inactive. Activation occurs via phosphorylation of IκB at Ser32 and
Ser36, followed by proteasome-mediated degradation, which results
in the release and nuclear translocation of active NF-κB (19).
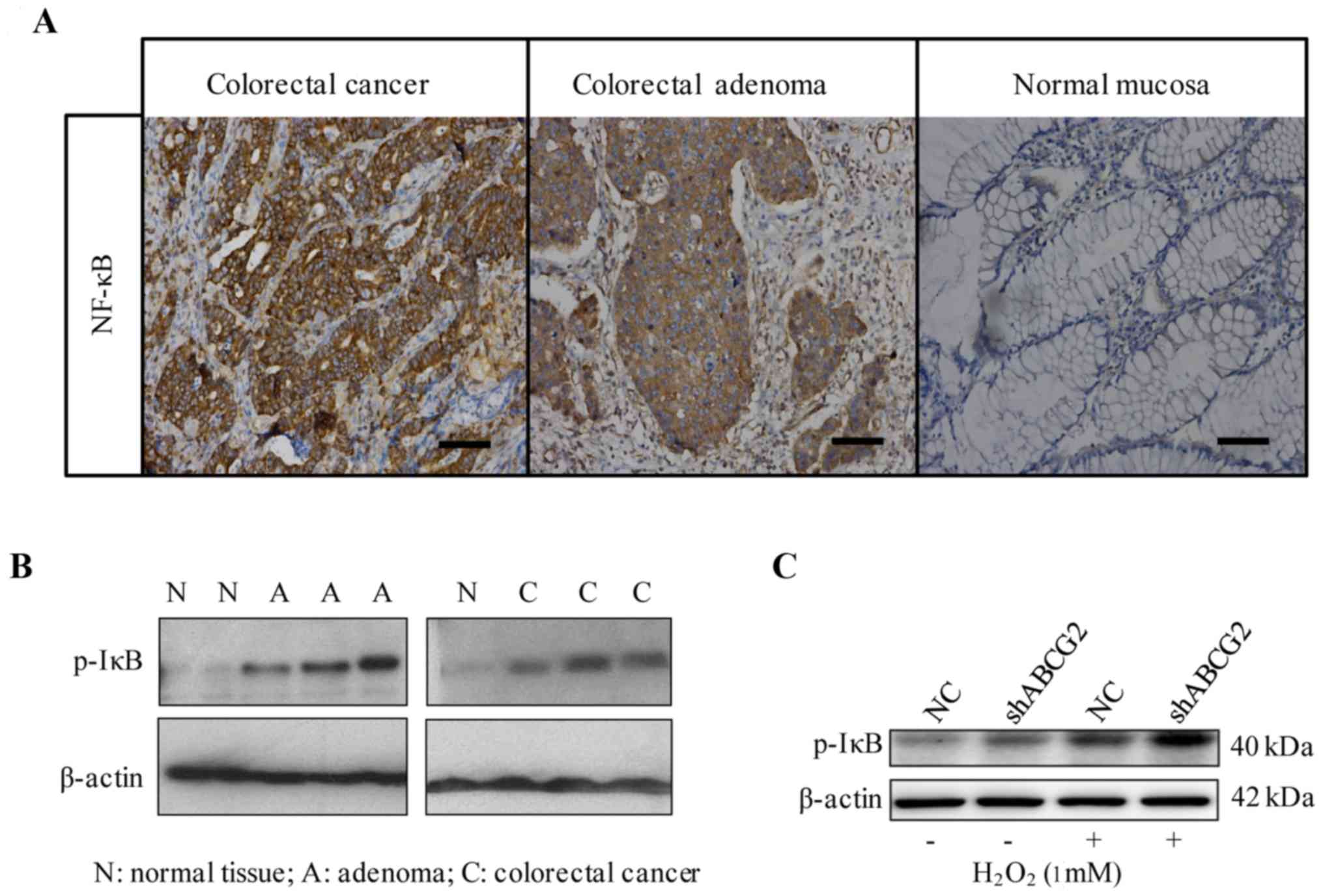
To explore the association between ABCG2 and NF-κB,
the expression of NF-κB was detected in human CRC, colon adenoma
and normal colorectal tissues by immunohistochemistry. NF-κB was
located mainly in the cytoplasm and/or nucleus. In CRC and colon
adenoma, 84.3% (70/83) and 62.5% (15/24) of paraffin-embedded
samples were positive for NF-κB, respectively, while in normal
tissues, only 20.8% were positive (Fig.
5A). Similar to the results of ABCG2 immunostaining obtained in
the clinical samples, a significant difference in NF-κB expression
was identified among malignant, pre-malignant and normal tissues
(P<0.001; Table V). Furthermore,
western blot analysis demonstrated that phosphorylated IκB was
higher in CRC and adenoma than in normal tissues (Fig. 5B). In addition, Spearman's rank
correlation analysis revealed a positive correlation between ABCG2
and NF-κB in CRC tissues (r=0.302, P=0.026).
 | Table V.NF-κB expression by
immunohistochemistry in different tissues. |
Table V.
NF-κB expression by
immunohistochemistry in different tissues.
| Tissues | Total (n) | Positive (n) | Negative (n) | χ2
value | P-value |
|---|
| CRC | 83 | 70 | 13 | 35.442 | <0.001 |
| Adenoma | 24 | 15 | 9 |
|
|
| Normal | 24 | 5 | 19 |
|
|
To further confirm the association between ABCG2 and
NF-κB, the level of phosphorylated IκB (Ser32) was examined in
H2O2-treated shABCG2 cells. Exposure to
H2O2 (1 mM) for 4 h significantly stimulated
the phosphorylation of IκB in HT-29 cells compared with vehicle
treatment. Furthermore, compared with the negative control cells,
phosphorylation of IκB was significantly increased in shABCG2 HT-29
cells treated with H2O2 (at 1 mM) for 4 h
(Fig. 5C).
Discussion
The association between oxidative stress and CRC has
been intensively studied in the last decade. Oxidative stress,
accompanied with overproduction of ROS, is detrimental to various
cellular macromolecules, interfering with cellular function
(3). Damage may result in
chromosomal instability (4), which
is the first step involved in mutagenesis and carcinogenesis
(5). CRC originates from the
epithelial cells, whose self-renewal and differentiation largely
depend on the redox environment in the gut mucosa. These cells
divide rapidly and have a high metabolic rate, as a potential
factor responsible for the increased oxidation of DNA (20). It was revealed that human colorectal
tumor (adenoma and carcinoma) cells had increased levels of ROS and
lipid peroxidation product MDA, and decreased levels of the
antioxidants SOD and GSH (6). MDA
is one of the best known breakdown products of lipid peroxides,
acting as signal transducers to modulate several cell functions,
including gene expression and cell proliferation (21). Evidence has indicated that MDA could
be a marker of oxidative stress which may promote the onset and
development of cancer, including CRC (22). Regarding antioxidants, GSH and SOD
are essential to protect cells from excessive ROS and to detoxify
harmful agents or metabolites (11). The present study revealed increased
levels of ROS and MDA, as well as decreased levels of GSH and SOD
in the tumor tissues or serum of CRC patients, which indicated that
oxidative stress did exist and was associated with CRC.
ABCG2 is abundantly expressed in the apical membrane
of the normal colonic epithelium (9), protecting normal cells by effluxing a
variety of carcinogens, drugs or toxic substances (11). Aberrant ABCG2 transport function is
linked to disease development, including carcinogenesis. The
present clinicopathological study indicated that ABCG2 expression
was higher in CRC than in non-carcinoma tissues. Furthermore, among
CRC patients, high expression of ABCG2 was associated with lower
histological differentiation, lymph node metastasis and even
shorter 5-year survival. These results indicated that ABCG2
overexpression was linked to poorer prognosis of CRC, which was
consistent with the studies by Yuan et al (23) and Liu et al (14). However, Gupta et al (13) reported that ABCG2 mRNA levels in CRC
tissues exhibited a 6-fold decrease, and they suggested that
downregulation of ABCG2 may have a role in the initial stage of
cancer by allowing for the accumulation of genotoxins and
overproduction of nitric oxide. Several studies have indicated that
ABCG2 was induced by hypoxic stress to regulate toxic levels of
cellular heme and porphyrin, so as to protect the cell from toxic
substrate accumulation (24–26).
Thus, it was presumed that ABCG2 expression may vary between
different stages of carcinogenesis. In the early stage, ABCG2 may
be downregulated to sensitize cells to carcinogens. However, in
more advanced stages of CRC, overexpression of ABCG2, as the
feedback of ROS or other oxidant overproduction, exerts a
protective effect on cancer cells. The possible mechanism in which
ABCG2 has dual roles at different cancer stages may explain the
positive association between ABCG2 and worse prognosis of patients,
though we suppose that ABCG2 may play an antioxidative and
protective role in CRC according to our present results. Thus, in a
complicated tumour environment with oxidative stress, ABCG2 may
have an important antioxidative role, but not be sufficient to
control the development of cancer.
The results of the present study indicated that
ABCG2 inhibited ROS generation. Since ABCG2 is an efflux pump
located in the cellular membrane, one possible mechanism is that
ABCG2 prevents exogenous ROS inducers from entering the cells,
thereby leading to a reduction in cellular ROS production (10). A previous study indicated that in
the brains of patients with Alzheimer's disease, overexpression of
ABCG2 protected cells from ROS-mediated toxicity and inflammatory
response (15). Furthermore, ABCG2
was reported to be a novel GSH transporter (27,28).
The present in vitro study revealed a decreased level of
intracellular GSH and higher levels of oxidative damage markers in
shABCG2 cells, which indicated an antioxidative role of ABCG2 in
CRC.
The inflammatory reaction is one of the ROS-induced
cellular responses. Excessive and uncontrollable production of ROS
for a longer period of time may result in persistent inflammation
(7). It has been indicated that
chronic inflammation is an important factor in carcinogenesis
(29). In accordance with the
results of other studies (15,30),
the present study indicated that ROS stimulated the expression of
inflammatory genes in cells, including IL-8, MCP-1 and GRO-β. One
important aspect in assessing the role of ABCG2 is to determine
whether ABCG2 attenuates the ROS-induced inflammatory response.
Notably, knockdown of ABCG2 significantly increased the
ROS-stimulated expression of IL-8 and GRO-β at the mRNA protein
levels. Furthermore, when ABCG2 was knocked down, the
ROS-stimulated expression of IL-8, MCP-1 and GRO-β at the mRNA
and/or protein level was significantly increased compared with that
in control cells. Therefore, it was revealed that ABCG2 antagonized
oxidative stress, which has an anti-inflammatory effect.
The expression of NF-κB target genes typically
promotes cellular survival, having a critical role in cancer
development. NF-κB signaling controls the ability of pre-neoplastic
as well as malignant cells to evade apoptosis-based tumor
surveillance mechanisms (31). As
observed in the present study, NF-κB was overexpressed in CRC
tissues, indicating its vital role in carcinogenesis. Furthermore,
NF-κB is linked with diverse groups of extracellular signal
inducers, including oxidants and inflammatory cytokines. The
transcription of NF-κB-dependent genes may also influence the level
of ROS in cells (32). Numerous
studies have demonstrated that the NF-κB signaling pathway is
activated by oxidative stress, which in turn leads to the
upregulation of the expression of inflammatory genes, including
IL-8 and GRO-β, as NF-κB binding sites are present in the promoter
regions of these genes. NF-κB is of vital importance in regulating
the expression of inflammatory genes. A previous study by our group
also provided evidence that ABCG2 inhibited the NF-κB signaling
pathway in cell models and in mouse brains, exerting a protective
role against the ROS-induced inflammatory response (11). In the present study, NF-κB was
activated in an oxidative stress environment in CRC cells,
particularly when ABCG2 was downregulated. It was therefore
indicated that NF-κB signaling participated in ABCG2-associated
processes in CRC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Youth
National Natural Science Foundation of China (grant nos. 81201908
and 81602147), the Youth Natural Science Foundation of Jiangsu
Province (grant no. BK20160110), the Outstanding Youth Project of
Nanjing City (grant no. JQX16026) and the Nanjing Medical Science
and Technique Development Foundation (grant nos. QRX17117 and
QRX17037).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SN, YH and MS carried out the in vitro cell
experiments, manuscript preparation and statistical analysis; XQ
and HL collected the clinical specimens, wrote the IHC analysis
section and revised the manuscript; CP and BK contributed to the
conception and design of the study, made the interpretation of data
for the study and provided critical review. XZ and SS conceived,
designed, supervised, analyzed and interpreted the data and
provided critical review. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the study are appropriately investigated and resolved.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Nanjing Drum Tower Hospital, and the informed consent
forms were obtained when the patients were accepted for the study
by the hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stone WL, Krishnan K, Campbell SE and
Palau VE: The role of antioxidants and pro-oxidants in colon
cancer. World J Gastrointest Oncol. 6:55–66. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sreevalsan S and Safe S: Reactive oxygen
species and colorectal cancer. Curr Colorectal Cancer Rep.
9:350–357. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saud SM, Li W, Morris NL, Matter MS,
Colburn NH, Kim YS and Young MR: Resveratrol prevents tumorigenesis
in mouse model of Kras activated sporadic colorectal cancer by
suppressing oncogenic Kras expression. Carcinogenesis.
35:2778–2786. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bond CE, Liu C, Kawamata F, McKeone DM,
Fernando W, Jamieson S, Pearson SA, Kane A, Woods SL, Lannagan TRM,
et al: Oncogenic BRAF mutation induces DNA methylation changes in a
murine model for human serrated colorectal neoplasia. Epigenetics.
13:40–48. 2018.PubMed/NCBI
|
|
6
|
Myers JN, Schäffer MW, Korolkova OY,
Williams AD, Gangula PR and M'Koma AE: Implications of the colonic
deposition of free hemoglobin-α chain: A previously unknown tissue
by-product in inflammatory bowel disease. Inflamm Bowel Dis.
20:1530–1547. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Federico A, Morgillo F, Tuccillo C,
Ciardiello F and Loguercio C: Chronic inflammation and oxidative
stress in human carcinogenesis. Int J Cancer. 121:2381–2386. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Barrett CW, Ning W, Chen X, Smith JJ,
Washington MK, Hill KE, Coburn LA, Peek RM, Chaturvedi R, Wilson
KT, et al: Tumor suppressor function of the plasma glutathione
peroxidase gpx3 in colitis-associated carcinoma. Cancer Res.
73:1245–1255. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maliepaard M, Scheffer GL, Faneyte IF, van
Gastelen MA, Pijnenborg AC, Schinkel AH, van De Vijver MJ, Scheper
RJ and Schellens JH: Subcellular localization and distribution of
the breast cancer resistance protein transporter in normal human
tissues. Cancer Res. 61:3458–3464. 2001.PubMed/NCBI
|
|
10
|
Pavek P, Merino G, Wagenaar E, Bolscher E,
Novotna M, Jonker JW and Schinkel AH: Human breast cancer
resistance protein: Interactions with steroid drugs, hormones, the
dietary carcinogen 2-amino-1-methyl-6-phenylimidazo(4,5-b)pyridine,
and transport of cimetidine. J Pharmacol Exp Ther. 312:144–152.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shen S and Zhang W: ABC transporters and
drug efflux at the blood-brain barrier. Rev Neurosci. 21:29–53.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Westover D and Li F: New trends for
overcoming ABCG2/BCRP-mediated resistance to cancer therapies. J
Exp Clin Cancer Res. 34:1592015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gupta N, Martin PM, Miyauchi S, Ananth S,
Herdman AV, Martindale RG, Podolsky R and Ganapathy V:
Down-regulation of BCRP/ABCG2 in colorectal and cervical cancer.
Biochem Biophys Res Commun. 343:571–577. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu HG, Pan YF, You J, Wang OC, Huang KT
and Zhang XH: Expression of ABCG2 and its significance in
colorectal cancer. Asian Pac J Cancer Prev. 11:845–848.
2010.PubMed/NCBI
|
|
15
|
Shen S, Callaghan D, Juzwik C, Xiong H,
Huang P and Zhang W: ABCG2 reduces ROS-mediated toxicity and
inflammation: A potential role in Alzheimer's disease. J Neurochem.
114:1590–1604. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ, Wills QF, Tipping AJ, Datta K,
Mittal R, Goldson AJ, Sexton DW and Holmes CC: Methods for qPCR
gene expression profiling applied to 1440 lymphoblastoid single
cells. Methods. 59:71–79. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Duan G, Tang Q, Yan H, Xie L, Wang Y,
Zheng XE, Zhuge Y, Shen S, Zhang B, Zhang X, et al: A strategy to
delay the development of cisplatin resistance by maintaining a
certain amount of cisplatin-sensitive cells. Sci Rep. 7:4322017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li W, Chen H, Yu M and Fang J: Targeted
delivery of doxorubicin using a colorectal cancer-specific ssDNA
aptamer. Anat Rec (Hoboken). 297:2280–2288. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Karin M: NF-kappaB and cancer: Mechanisms
and targets. Mol Carcinog. 45:355–361. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park MY, Kim MY, Seo YR, Kim JS and Sung
MK: High-fat diet accelerates intestinal tumorigenesis through
disrupting intestinal cell membrane integrity. J Cancer Prev.
21:95–103. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Perše M: Oxidative stress in the
pathogenesis of colorectal cancer: Cause or consequence? BioMed Res
Int. 2013:7257102013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Obtułowicz T, Winczura A, Speina E,
Swoboda M, Janik J, Janowska B, Cieśla JM, Kowalczyk P, Jawien A
and Gackowski D: Aberrant repair of etheno-DNA adducts in
leukocytes and colon tissue of colon cancer patients. Free Radic
Biol Med. 49:1064–1071. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yuan Y, Yang Z, Miao X, Li D, Liu Z and
Zou Q: The clinical significance of FRAT1 and ABCG2 expression in
pancreatic ductal adenocarcinoma. Tumour Biol. 36:9961–9968. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Krishnamurthy P, Xie T and Schuetz JD: The
role of transporters in cellular heme and porphyrin homeostasis.
Pharmacol Ther. 114:345–358. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ogino T, Kobuchi H, Munetomo K, Fujita H,
Yamamoto M, Utsumi T, Inoue K, Shuin T, Sasaki J, Inoue M, et al:
Serum-dependent export of protoporphyrin IX by ATP-binding cassette
transporter G2 in T24 cells. Mol Cell Biochem. 358:297–307. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gnana-Prakasam JP, Reddy SK,
Veeranan-Karmegam R, Smith SB, Martin PM and Ganapathy V: Polarized
distribution of heme transporters in retinal pigment epithelium and
their regulation in the iron-overload disease hemochromatosis.
Invest Ophthalmol Vis Sci. 52:9279–9286. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brechbuhl HM, Gould N, Kachadourian R,
Riekhof WR, Voelker DR and Day BJ: Glutathione transport is a
unique function of the ATP-binding cassette protein ABCG2. J Biol
Chem. 285:16582–16587. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Krzyżanowski D, Bartosz G and Grzelak A:
Collateral sensitivity: ABCG2-overexpressing cells are more
vulnerable to oxidative stress. Free Radic Biol Med. 76:47–52.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Reuter S, Gupta SC, Chaturvedi MM and
Aggarwal BB: Oxidative stress, inflammation, and cancer: How are
they linked? Free Radic Biol Med. 49:1603–1616. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Satapati S, Kucejova B, Duarte JA,
Fletcher JA, Reynolds L, Sunny NE, He T, Nair LA, Livingston KA, Fu
X, et al: Mitochondrial metabolism mediates oxidative stress and
inflammation in fatty liver. J Clin Invest. 125:4447–4462. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Karin M: Nuclear factor-kappaB in cancer
development and progression. Nature. 441:431–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Morgan MJ and Liu ZG: Crosstalk of
reactive oxygen species and NF-κB signaling. Cell Res. 21:103–115.
2011. View Article : Google Scholar : PubMed/NCBI
|