Introduction
Amino acids are essential for different cellular
functions including synthesis of protein, nitric oxide, urea,
creatine and polyamines (1). Thus,
amino acid depletion can potentially serve as an effective
treatment for cancers.
Arginine is a semi-essential amino acid in some
tumours, but non-essential in normal cells. Therefore,
arginine-degrading enzymes (arginase and arginine deiminase) have
recently been studied in the treatment of different types of cancer
that lack the ability to re-synthesize arginine (2,3).
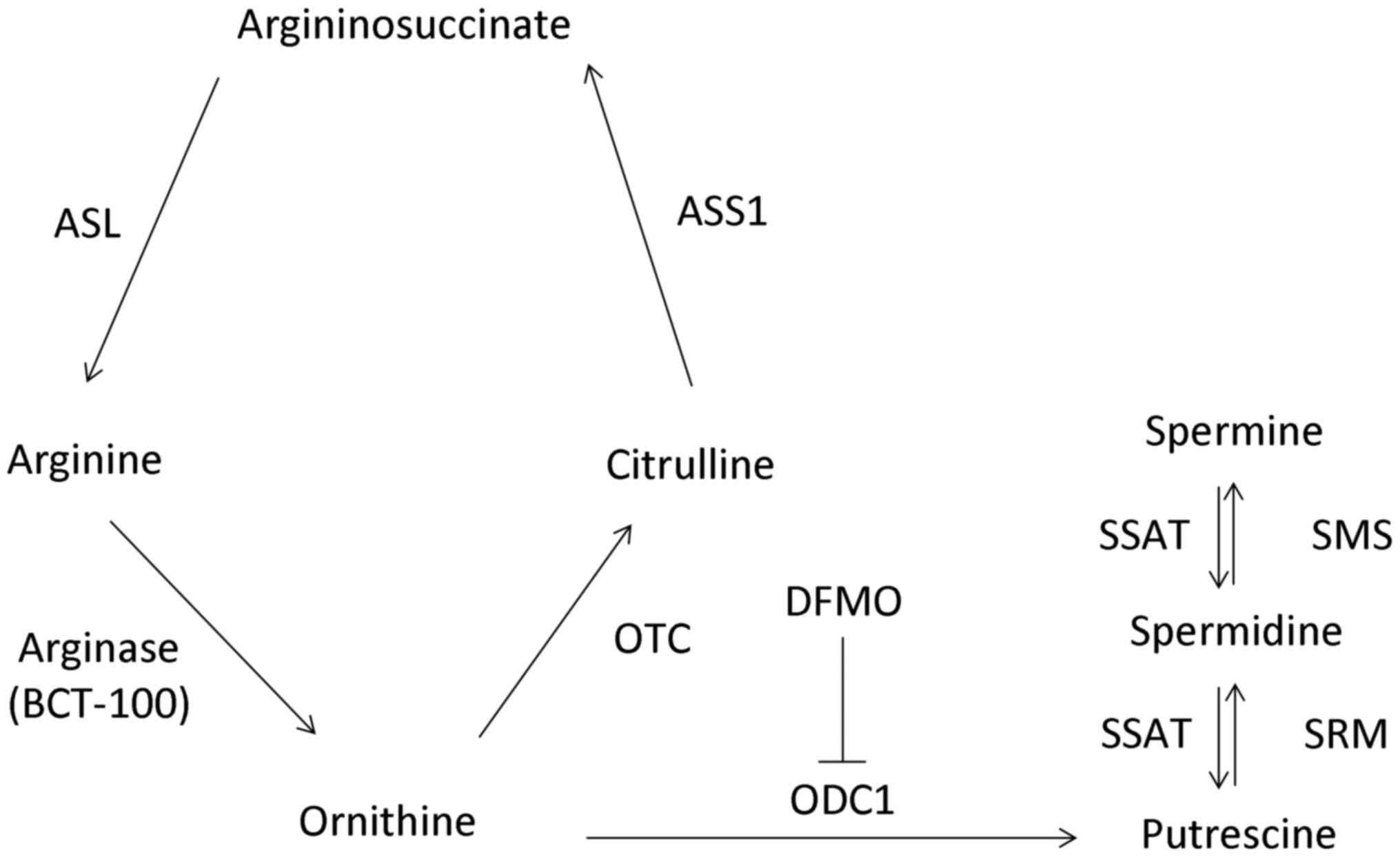
Arginine can be converted to ornithine, citrulline
and argininosuccinate by arginase, ornithine transcarbamylase (OTC)
and argininosuccinate synthetase (ASS1), respectively. Polyamines
(putrescine, spermidine and spermine) are aliphatic cations with
pleiotropic functions and are found in various cell types. The
biosynthesis of polyamines involves ornithine decarboxylase 1
(ODC1) and S-adenosylmethionine decarboxylase, as well as
spermidine synthase (SRM) and spermine synthase (SMS). Nonetheless
spermidine/spermine N1-acetyltransferase (SSAT), FAD-dependent
polyamine oxidase and spermine oxidase are responsible for the
degradation of polyamines (Fig. 1).
Polyamines are essential to cell proliferation that can also
promote tumour growth (4). High
expression of ASS1 and OTC, which are the key enzymes in the urea
cycle that are responsible for replenishing arginine, is
biologically predictive of refractoriness to arginase
treatment.
BCT-100 (Bio-Cancer Treatment International Ltd.,
Hong Kong) is a pegylated (PEG) formulation of arginase (US FDA IND
granted in March 2012) that was developed as an anticancer agent.
An anti-proliferative effect of BCT-100 has been demonstrated in
acute myeloid leukaemia (5),
hepatocellular carcinoma (HCC) (2,6) and
mesothelioma (7), with an
IC50 value of 0.1–1.25, 100–250 and 13–23 mU/ml,
respectively. Cell cycle arrest and apoptosis are induced by
BCT-100 in human melanoma (8), HCC
(9) and mesothelioma (7). BCT-100 has recently demonstrated
clinical activity in a phase I/II clinical trial in HCC (10). The present study aimed to
investigate the role of BCT-100 as an anticancer treatment for lung
adenocarcinoma.
Materials and methods
Cell lines and reagents
A panel of seven lung adenocarcinoma cell lines
[epidermal growth factor receptor (EGFR) wild-type and K-ras
mutated (H23 and H358) or EGFR mutated (HCC827, H1650, H1975,
HCC2935 and HCC4006)] was obtained from The American Type Culture
Collection (ATCC, Manassas, VA, USA). The passage of cells used for
various experiments was authenticated by ATCC in December 2016 by
comparing the ATCC reference database profile. Cells were cultured
in Gibco® RPMI-1640 medium (Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum
(FBS; Gibco®; Thermo Fisher Scientific, Inc.) in a
humidified atmosphere of 5% CO2 at 37°C.
Pegylated arginase (BCT-100) and
α-difluoromethylornithine (DFMO)
Pegylated arginase (BCT-100, PEG-BCT-100 or
rhArg1peg5000) was manufactured and donated by Bio-Cancer Treatment
International. DFMO was purchased from Shijiazhuang Aopharm Import
& Export Trading Co., Ltd. (Hebei, China).
Cell viability assay
The
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
cell viability assay was performed as previously described
(11). Briefly, 5,000 cells were
plated in each well and treated for 72 h with BCT-100. Culture
medium alone was served as the control.
Protein expression by western blot
analysis and spermidine level by dot blot
Specific primary antibodies [mouse monoclonal
anti-human β-actin (1:1,000; cat. no. A1978) (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany), anti-ASS1 (1:1,000; cat. no. SC-99178),
anti-ODC1 (1:1,000; cat. no. SC-33539), anti-OTC (1:1,000; c cat.
no. SC-102051), anti-spermidine synthase (SRM) (1:1,000; cat. no.
SC-374524), anti-spermine synthase (SMS) (1:1,000; cat. no.
SC-99159), anti-cleaved PARP (1:1,000; cat. no. SC-9542) (Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA), anti-PEG (1:1,000;
cat. no. 31-1008-00) (RevMAb Biosciences, San Francisco, CA, USA),
anti-survivin (1:1,000; cat. no. 2808), (Cell Signaling Technology,
Inc., Danvers, MA, USA)] and corresponding horseradish peroxidase
(HRP)-conjugated secondary antibody (anti-mouse IgG, 1:1,000; cat.
no. 7076 or anti-rabbit IgG, 1:1,000; cat. no. 7074) (from Cell
Signaling Technology, Inc.) were purchased. Western blot analysis
was performed as previously reported (12). Cells (5×106) were
collected and lysed for 1 h on ice with RIPA buffer (20 mM Tris-HCl
(pH 7.5), 150 mM NaCl, 1% sodium deoxycholate, 1 mM EGTA, 2.5 mM
sodium pyrophosphate, 1 mM Na2EDTA, 1% NP-40, 1 mM
Na3VO4, 1 mM β-glycerophosphate and 1 µg/ml
leupeptin) including a protease inhibitor cocktail. Tissue samples
collected from xenograft models were lysed for 1 h on ice with
T-PER® Tissue Protein Extraction Reagent (cat. no.
78510) (Thermo Fisher Scientific, Inc., Waltham, MA, USA) including
a protease inhibitor cocktail. For dot blotting, samples were
directly spotted onto a nitrocellulose membrane and allowed to dry
for 1 h at room temperature. The membranes were blocked, incubated
with anti-spermidine antibody (1:1,000; cat. no. NB100-1847) (Novus
Biologicals, LCC, Littleton, CO, USA) and corresponding secondary
antibody (anti-rabbit IgG, 1:1,000; cat. no. 7074; Cell Signaling
Technology, Inc.), similar to western blotting. An enhanced
chemiluminescence (ECL) kit (GE Healthcare, Buckinghamshire, UK)
was used to detect protein expression. β-actin served as a
housekeeping protein.
Tumour xenograft growth in vivo
H358, HCC827, H1650, H1975 and HCC4006 ×enografts
were established by subcutaneous injection of the corresponding
(107) cells in phosphate-buffered saline (PBS) into the
upper back of 12 (6 mice ×2 groups) or 32 (8 mice ×4 groups) nude
mice (female; age, 4- to 6-weeks; weight, 10–14 g, BALB/cAnN-nu;
Charles River Laboratories, Wilmington, MA, USA). The mice were
kept in 12-h/light/dark cycle with temperature (20–25°C) and
humidity (60–70%) control and ad libitum diet was provided.
Treatment started when the tumor size reached ~50 mm3.
To study the effect of BCT-100, mice were randomized to one of two
groups, after tumour growth was established (n=6). PBS (control) or
BCT-100 (20 mg/kg twice a week, intraperitoneally) was
administered. To study the combined effect of DFMO and BCT-100,
mice were randomized into four groups after tumour growth was
established (n=8). PBS (control), 2% DFMO (in drinking water),
BCT-100 (20 mg/kg twice a week, intraperitoneally) or DFMO/BCT-100
was given accordingly. Tumour dimension (using standard calipers)
and body weight of mice were assessed twice a week and tumour
volume was calculated as follows: Volume = length × width ×
width)/2 (11). For humane reasons,
mice were sacrificed (by administration of 100 µl pentobarbital
sodium solution, intraperitoneally) when tumour size reached 600
mm3. Tumour xenografts were harvested. The study
protocol was approved by the institutional Animal Ethics Committee
of The University of Hong Kong (approval ref. no. CULATR 3781-15)
and standard humane endpoints for animal research were applied in
compliance with the instructions of the U.S. Public Health Service
(policy on humane care and use of laboratory animals).
Serum arginine concentration
L-arginine ELISA kit was purchased from
Immundiagnostik (Bensheim, Hessen, Germany) and the assay was
performed according to the manufacturer's protocol. In brief,
control, standards and samples were derivatized and incubated with
L-arginine antibody overnight. After washing with washing buffer,
peroxidase conjugate was added. The reaction was stopped following
incubation with tetramethybenzidine substrate (13). Absorbance (450 nm) was determined
with a reference (620 nm) using a FLUOstar Optima microplate reader
(Bmg Labtec GmbH, Ortenberg, Germany).
Putrescine concentration assessement
by high performance liquid chromatography (HPLC)
The concentration of putrescine in different tumour
lysates was analyzed according to a previously reported methodology
(14). Putrescine dihydrochloride
and o-phthaldialdehyde (OPA) reagent solution (Sigma-Aldrich; Merck
KGaA) and HPLC grade methanol (Tedia Company, Fairfield, OH, USA)
were purchased. The standards and samples were centrifuged at
13,400 × g for 10 min. Supernatant (30 µl) was mixed with 5%
perchloric acid (30 µl) to precipitate proteins. The mixtures were
then centrifuged (13,400 × g, 10 min). The supernatant of acidic
extract (50 µl) was neutralized with borate buffer (100 µl, 0.1 M,
pH 9.0) and OPA reagent (60 µl) was then added. The derivatized
mixtures were centrifuged (13,400 × g, 10 min) and the supernatant
(20 µl) was injected into the HPLC system. Nucleosil ODS column
(250×4.6 mm, internal diameter 5 mm) (Macherey-Nagel GmbH &
Co., Düren, Germany) was connected to Agilent 1260 Infinity
(Agilent Technologies, Santa Clara, CA, USA) and eluted with buffer
A (water) and buffer B (methanol) at a flow rate of 1 ml/min.
Following injection of standards or samples, the column was eluted
with 70% buffer B for 1 min and an isocratic gradient from 70%
buffer B to 90% solvent B for 13 min. The column was washed with
100% buffer B for 5 min and re-equilibrated with 100% buffer A for
5 min. Signals were detected with an excitation wavelength of 360
nm and emission wavelength of 510 nm.
Terminal deoxynucleotidyl
transferase-dUTP nick end labeling (TUNEL) assay
TUNEL assay was performed using Click-iT®
Plus TUNEL assay (Invitrogen; Thermo Fisher Scientific, Inc.).
De-paraffinization, fixation and permeabilization of
formalin-fixed, paraffin-embedded tumour xenograft sections were
performed first. Sections were incubated with terminal
deoxynucleotidyl transferase (TdT) reaction buffer, and then
incubated with TdT buffer containing EdUTP, TdT and TdT enzyme.
TUNEL reaction cocktail (Alexa Fluor® picoyl azide,
copper protectant, TUNEL reaction buffer additive and TUNEL
reaction buffer) was added to each section. The slides were mounted
with Prolong® Gold antifade reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) containing 4′,6-diamidino-2-phenylindole
(DAPI). Images were captured using a Nikon Ni-U fluorescence
microscope (Nikon, Tokyo, Japan) equipped with a camera/detector
Diagnostic Instrument RT3 slider (Meyer Instruments, Houston, TX,
USA). Images were captured at ×400 magnification using CFI Plan
Fluor DLL 40X objective (Nikon). Images were captured using
NIS-Elements Basic Research software (SPOT™ Software 5.0)
(Laboratory Imaging Ltd., Prague, Czech Republic).
Statistical analysis
Experiments were repeated at least three times and
data were analysed (mean ± standard error of the mean). Student's
two-tailed t-test was used for comparison in pairs. The differences
between groups (>2 groups) were analyzed with one way analysis
of variance (ANOVA) and Tukey's multiple comparison test using
GraphPad Prism software version 5.01 (GraphPad Software, Inc., La
Jolla, CA, USA). Comparisons without significance are indicated as
‘NS’. A P-value <0.05 is considered to indicate statistically
significant difference (*P<0.05, **P<0.01, ***P<0.001 as
indicated in the figures). Kaplan-Meier analysis was performed for
tumour xenograft models in different treatment arms, with the
humane endpoint or death of mice as the outcome measure. The
difference in median survival between arms was analysed using
log-rank test by Prism.
Results
In vitro activity of BCT-100 in lung
adenocarcinoma cell lines
Treatment with BCT-100 induced a dose-dependent
anti-proliferative effect in all lung adenocarcinoma cell lines.
The IC50 value of H23, H358, HCC827, H1650, H1975,
HCC2935 and HCC4006 cells was 13.1±1.2, 12.8±1.5, 19.1±7.7,
25.9±5.0, 12.3±0.5, 620±65 and 15.9±5.1 mU/ml respectively
following a 72-h treatment.
Basal expression of ASS1 and OTC in
vitro
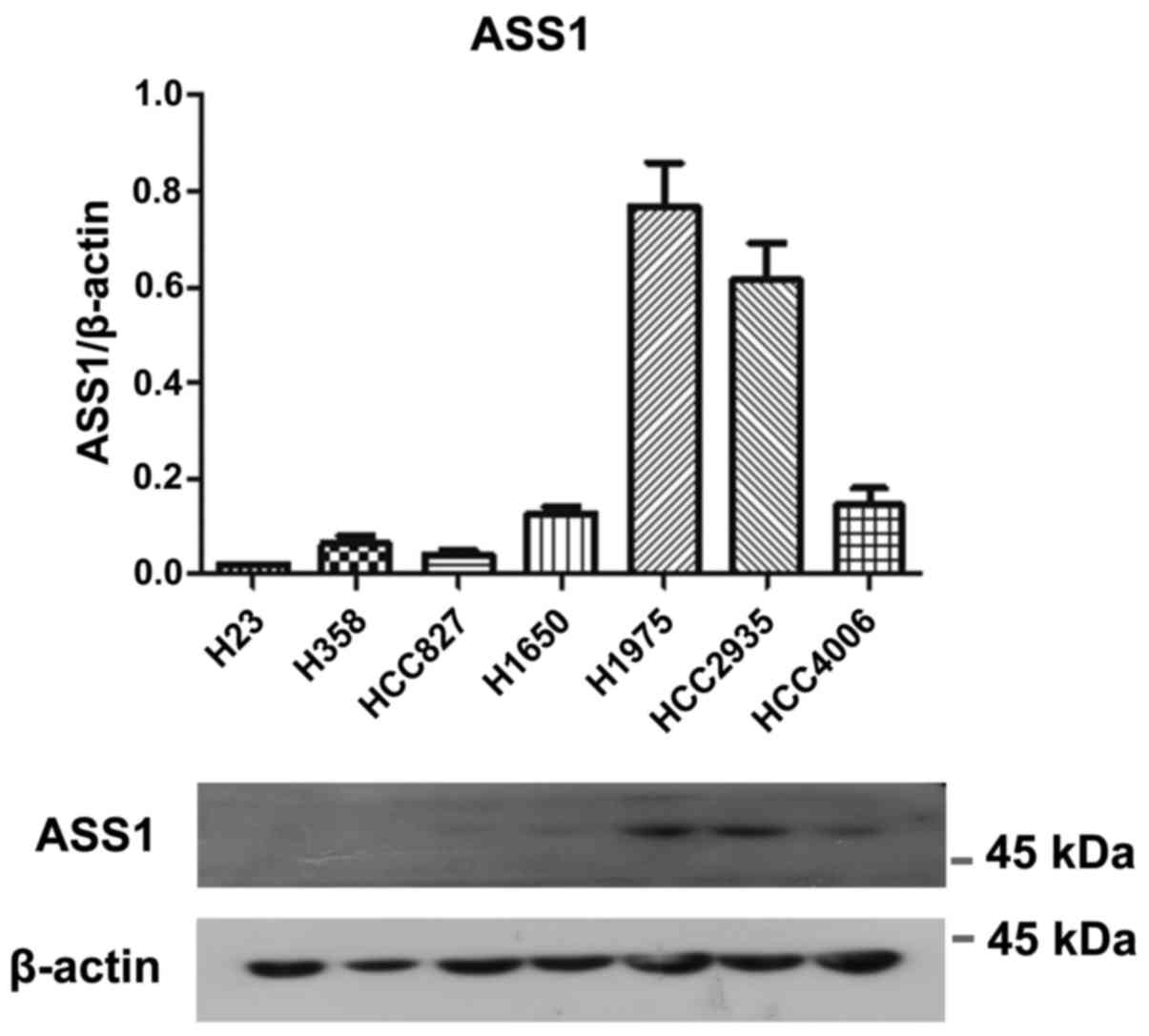
The protein expression of ASS1 was relatively higher
in H1975 and HCC2935 cells, lower in H1650 and HCC4006 cells and
almost undetectable in H23, H358 and HCC827 cells (Fig. 2). All cell lines were OTC negative
(data not shown).
Effects of BCT-100 on tumour xenograft
growth
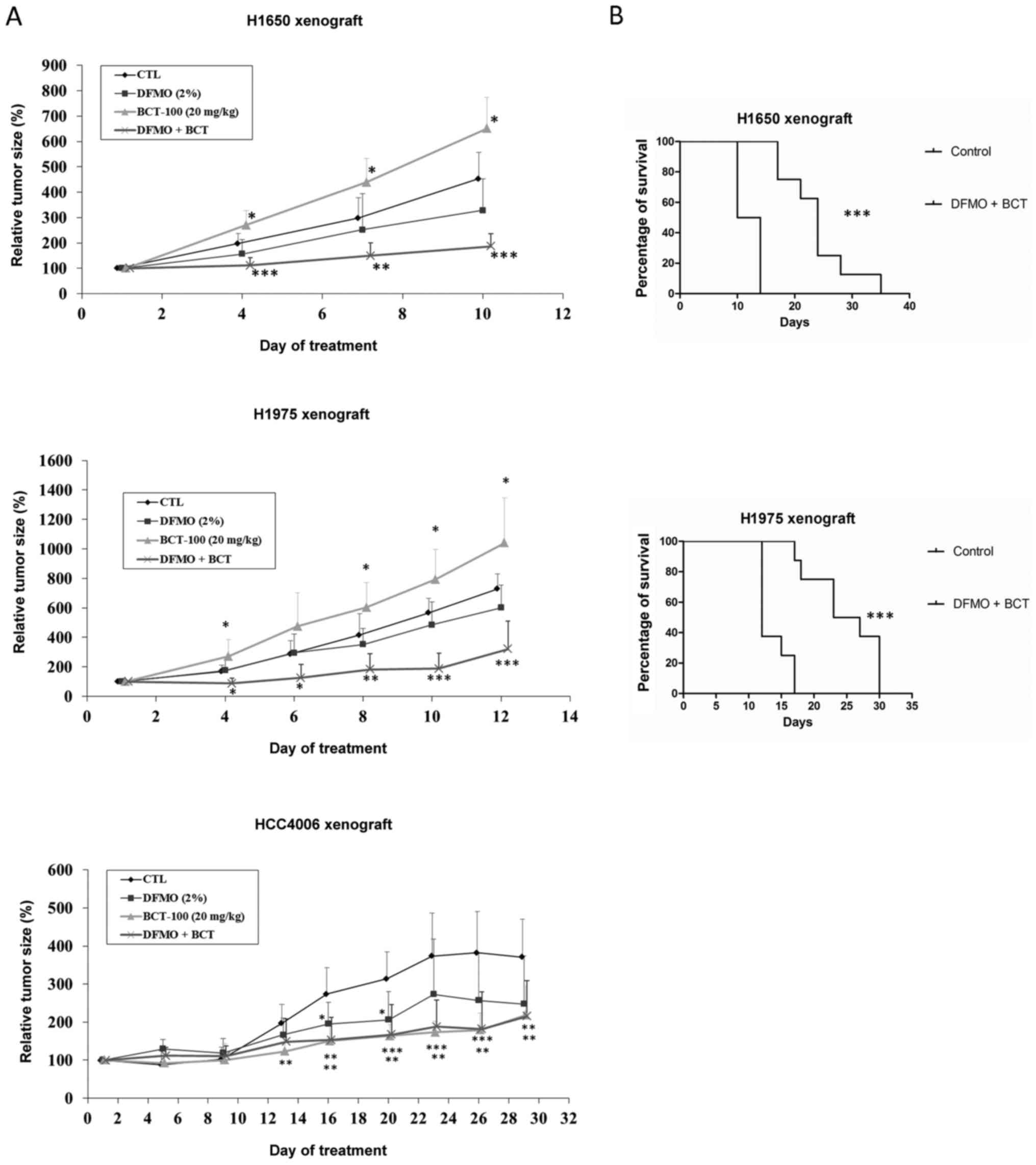
BCT-100 (20 mg/kg) promoted tumour growth in H358,
H827, H1650 and H1975 ×enograft models, but suppressed growth in
the HCC4006 ×enograft (Fig. 3A).
H358 and HCC827 ×enografts were cystic tumours, H1650, H1975 and
HCC4006 were solid.
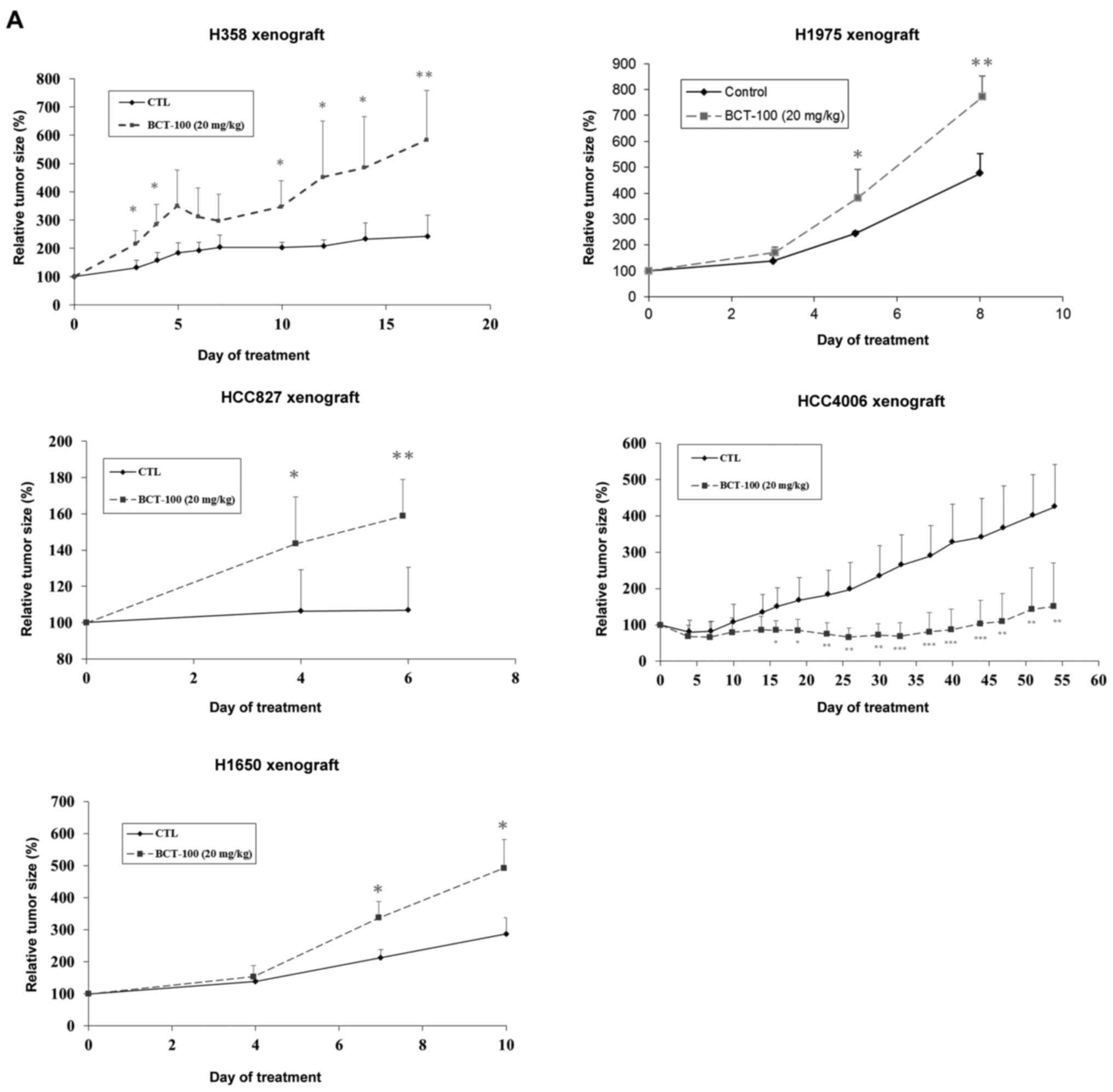 | Figure 3.Alteration of tumour size as well as
ASS1, OTC, ODC1, SRM and SMS expression in xenograft models
following treatment with BCT-100. (A) BCT-100 (dotted lines)
stimulated tumour growth in H358, HCC827, H1650 and H1975 ×enograft
models but inhibited growth in HCC4006 ×enograft models.
*P<0.05, **P<0.01, ***P<0.001. (B) ASS expression was
downregulated in the BCT-100 arm in the H1975 ×enograft model. OTC
was found and unaltered in HCC827 ×enograft. Upregulation of ODC1
was noted in the BCT-100 arm in both H1650 and H1975 ×enograft
models. Expression of SRM was unchanged in H358 and HCC827
×enografts but upregulated in H1650, H1975 and HCC4006 ×enografts.
SMS was unchanged in HCC827, H1650, H1975 and HCC4006 ×enografts.
*P<0.05, **P<0.01. |
Protein expression of ASS1, OTC, ODC1,
SRM and SMS after BCT-100 treatment
Alteration in protein expression of key molecules of
arginine metabolism was determined between control and BCT-100
treatment arms. ASS1 was downregulated by BCT-100 in the H1975
×enograft but unaltered in other xenografts. OTC was unchanged in
the HCC827 ×enograft and undetectable in others. ODC1 was unaltered
in H358 ×enograft but upregulated by BCT-100 in H1650 and H1975
×enografts. ODC1 was undetectable in both control and BCT-100
treatment arms in HCC4006 ×enograft (data not shown). SRM was
unchanged in H358 and HCC827 ×enografts but upregulated in H1650,
H1975 and HCC4006 ×enografts. SMS was unaltered in HCC827, H1650,
H1975 and HCC4006 ×enografts (Fig.
3B).
Effects of combined DFMO and BCT-100
on xenograft models
Relative tumour size increased with BCT-100
treatment in H1650 and H1975 ×enografts and decreased with BCT-100
in HCC4006 ×enograft as described above. There was no significant
difference in tumour size between DFMO and control arms in any
xenograft model. Combination treatment with DFMO/BCT-100
significantly suppressed tumour growth in H1650 and H1975 ×enograft
models, in contrast to enhanced tumour growth evident with BCT-100
alone. The effect of DFMO/BCT-100 and BCT-100 remained similar in
the HCC4006 ×enograft model (Fig.
4A).
Increased median survival with
DFMO/BCT-100 in xenograft models
The median survival was increased from 12 days in
the control arm to 24 and 25 days in the DFMO/BCT-100 treatment arm
(P<0.01) in H1650 and H1975 ×enograft models respectively
(Fig. 4B).
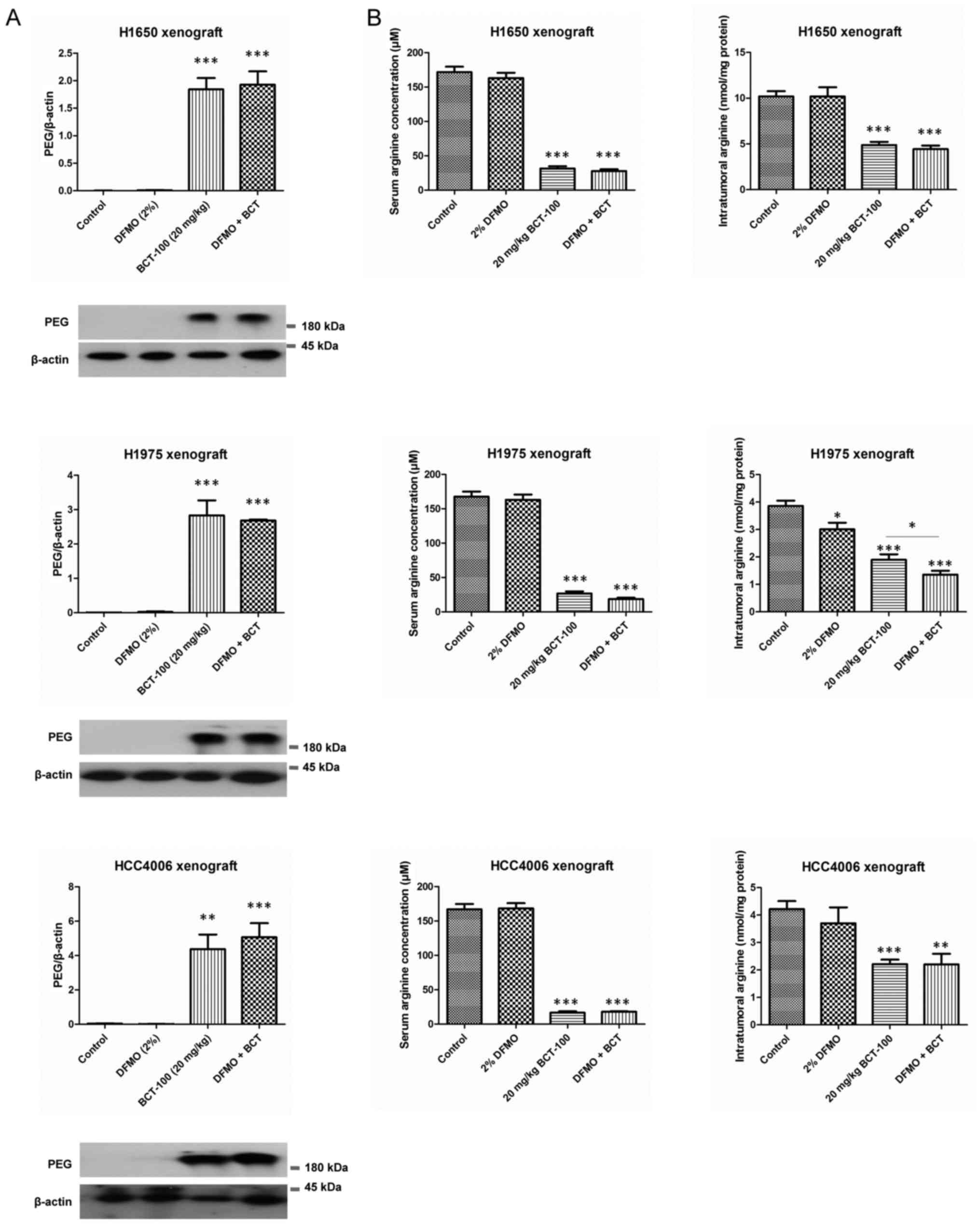
Intratumoral penetration of
BCT-100
As BCT-100 was tagged with PEG, immunoreactivity to
anti-PEG antibody was used to quantify the amount of BCT-100 within
tumour xenografts. BCT-100 was significantly accumulated within
tumours in BCT-100 and DFMO/BCT-100 treatment arms in all three
xenograft models (Fig. 5A).
Arginine depletion by DFMO and/or
BCT-100 in vivo
Serum arginine concentration and intratumoral
arginine content in both BCT-100 and DFMO/BCT-100 treatment arms
were significantly decreased in all xenograft models (Fig. 5B). The serum arginine was equally
depleted in BCT-100 alone or DFMO/BCT-100 combination across
different xenografts. Except for the H1975 ×enograft, the
DFMO/BCT-100 treatment arm and BCT-100 arm decreased intratumoral
arginine to comparable levels (Fig.
5B).
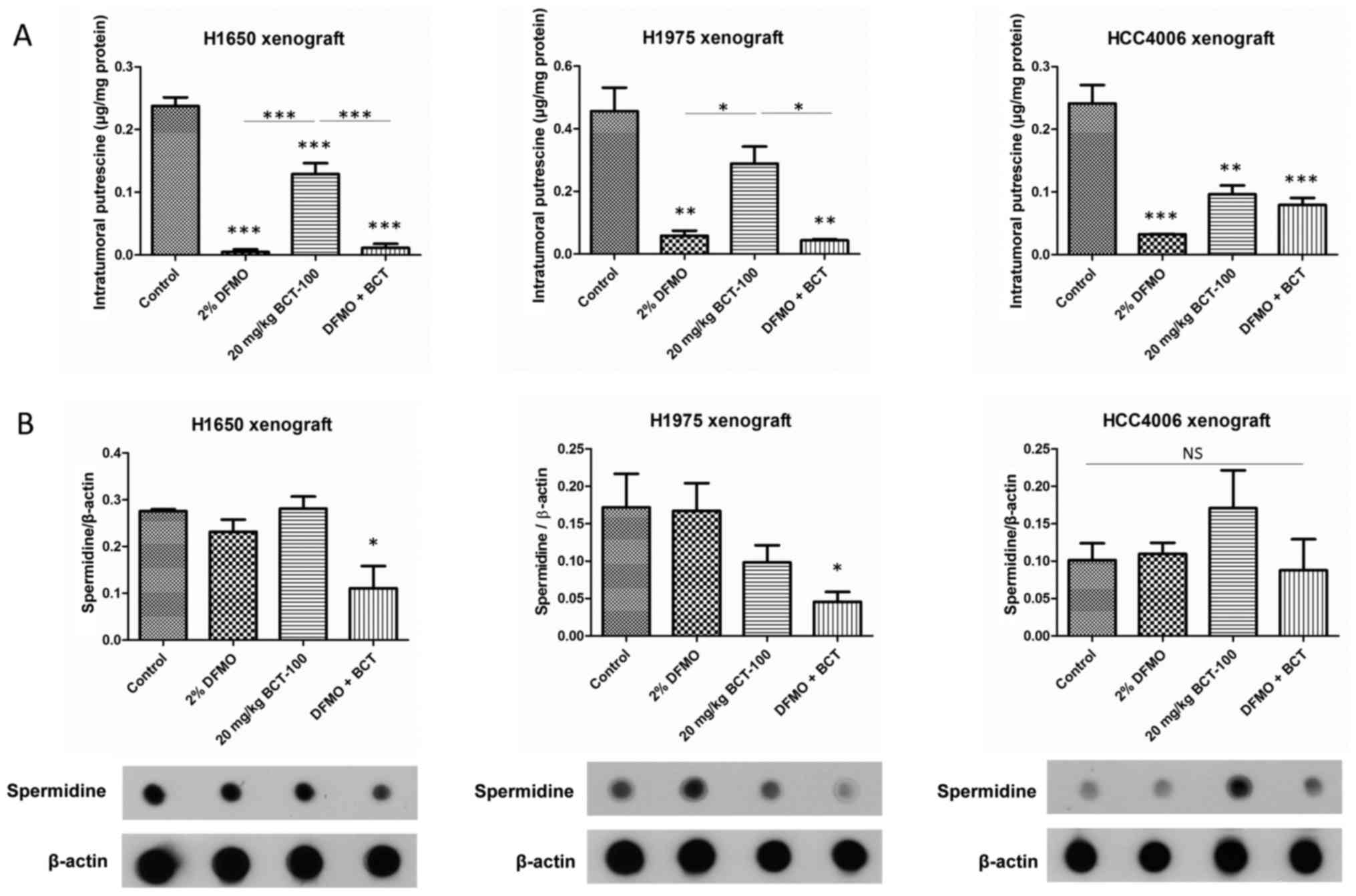
Putrescine and spermidine level in
DFMO and/or BCT-100 treatment arms in vivo
Putrescine was decreased in DFMO, BCT-100 and
DFMO/BCT-100 groups in all xenograft models except for H1975
×enograft in the BCT-100 arm (Fig.
6A). Decrease in putrescine level in BCT-100 arms was possibly
due to putrescine consumption with enhanced tumour growth.
Conversely, DFMO declined putrescine level in DFMO ± BCT-100 arms
resulting from inhibition of ODC1, i.e. decreased putrescine
production.
Spermidine level was significantly reduced in
DFMO/BCT-100 arm only in H1650 and H1975 ×enografts, but remained
unaltered in HCC4006 ×enograft (Fig.
6B). Spermidine level may be a more important marker than
putrescine as an indicator of efficacy in DFMO/BCT-100
combination.
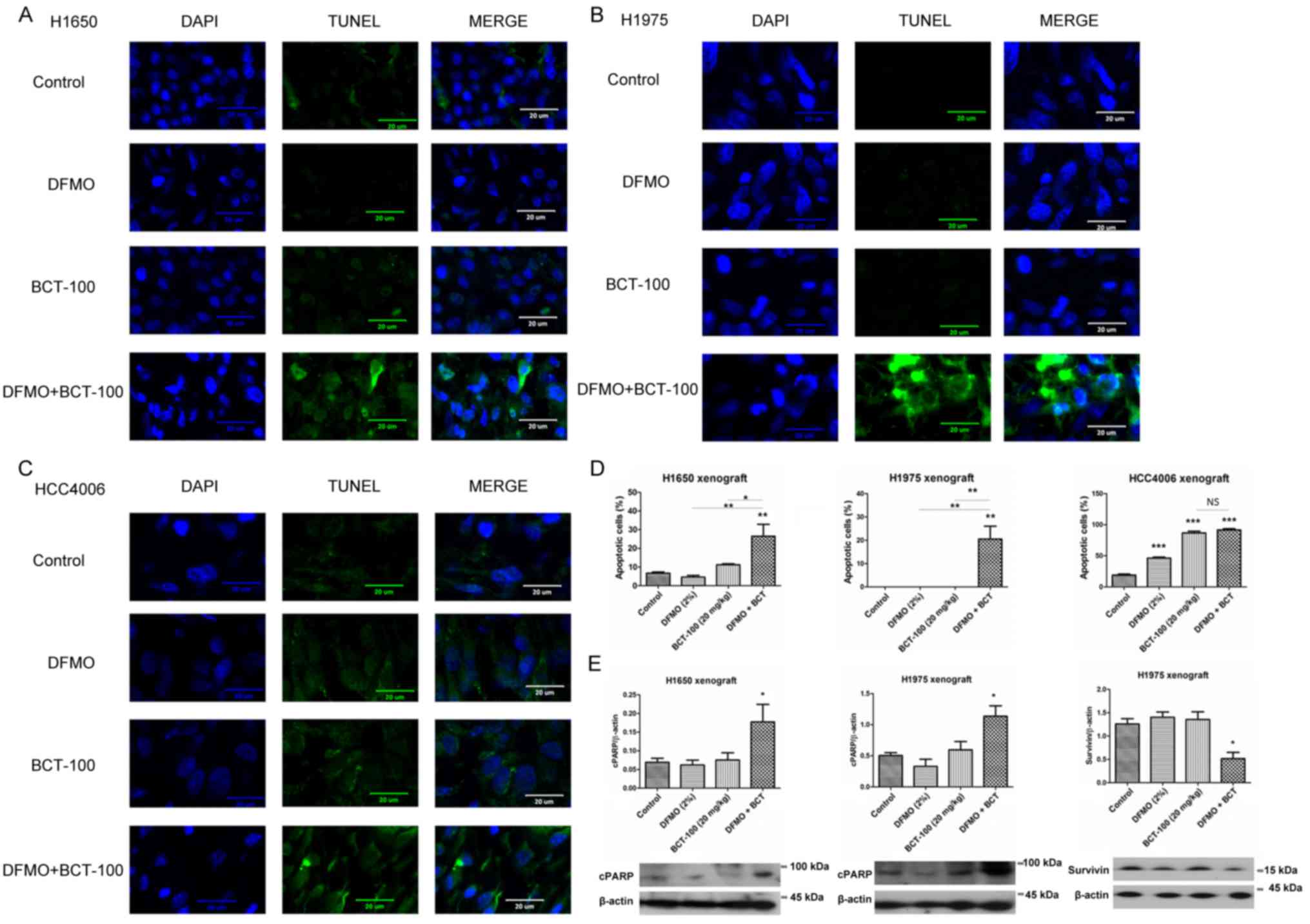
Apoptosis induced by BCT-100 in
xenograft models
TUNEL-positive DNA strand breaks and nuclei were
stained green and blue, respectively. Apoptotic cells (TUNEL and
DAPI-positive) were indicated with double positive staining
(Fig. 7). In the H1650 ×enograft,
the apoptotic signal remained low in the control and DFMO groups,
slightly increased in the BCT-100 arm and was further elevated in
the DFMO/BCT-100 combination group (Fig. 7A). In the H1975 ×enograft, the level
of apoptosis was maintained at a low level in control, DFMO and
BCT-100 groups, but significantly enhanced in the DFMO/BCT-100
combination arm (Fig. 7B). In the
HCC4006 ×enograft, apoptotic signal was noted in DFMO, BCT-100 and
DFMO/BCT-100 arms but not in the control group (Fig. 7C). The percentage of cells that
underwent apoptosis is displayed in Fig. 7D. Furthermore, the upregulation of
cleaved PARP (cPARP) was observed in the DFMO/BCT-100 treatment arm
in both H1650 and H1975 ×enograft models (Fig. 7E), but not in the HCC4006 ×enograft
(data not shown). Downregulation of survivin, serving as an
anti-apoptotic factor, was also observed in DFMO/BCT-100 treatment
group in H1975 ×enograft (Fig.
7E).
Discussion
BCT-100 demonstrated anti-proliferative effects
in vitro, however, demonstrated a paradoxical tumour
promoting effect in most of our in vivo xenograft models.
ODC1 was upregulated by BCT-100 treatment in H1650 and H1975
×enograft models. We postulated that ornithine produced from
arginine by BCT-100 was converted to polyamines by upregulated
ODC1, thus leading to stimulation of tumour growth. The combination
of an ODC1 inhibitor (DFMO) with BCT-100 restored the tumour
suppressive effects of BCT-100 in the H1650 and H1975 ×enograft
models with an increased median survival, mediated by decreasing
serum and intratumoral arginine levels as well as intratumural
putrescine and spermidine levels. Furthermore, apoptosis was
enhanced by DFMO/BCT-100 in H1650 and H1975 ×enografts, in keeping
with the observed antitumoral effects. Nonetheless there was no
beneficial or detrimental effect in the HCC4006 ×enograft model
(whereby ODC1 was not induced by BCT-100) when DFMO and BCT-100
were combined.
Based on the updated GLOBOCAN project of the World
Health Organization in 2012, lung cancer is the top cancer killer
(http://globocan.iarc.fr/). The incidence and
mortality rates of lung cancer were 16.7 and 23.2%, respectively.
Lung cancer can be classified as non-small cell lung carcinoma or
small cell lung carcinoma. Eighty-five percent of lung cancer cases
are NSCLC and adenocarcinoma is the major subtype. Systemic
chemotherapy remains the cornerstone treatment for NSCLC with only
a modest survival benefit. Although targeted therapies (e.g.
against EGFR) have been developed for lung cancer patients with
different types of mutations, development of acquired drug
resistance around one year following targeted therapy is
practically unavoidable (15). As
such, novel treatment for NSCLC is highly desired.
Amino acid depletion is a potentially promising
approach of anticancer therapy, akin to the use of L-asparaginase
in the treatment of acute leukaemia. Arginine is an essential amino
acid for cancer cells that are incapable of replenishing their
arginine store, but not normal cells. Arginine deiminase (ADI) and
arginase have been used to deplete arginine in in vivo and
clinical studies. ADI induces an anticancer effect on
ASS1-deficient cancers including those of the head and neck,
lymphoma, pancreas, breast and small cell lung carcinoma (16) by cell cycle arrest and apoptosis.
Arginase (BCT-100) also displays anticancer activity in melanoma
(8) and human hepatoma (2) via apoptosis, as well as cell cycle
arrest in melanoma (8), HCC
(9) and acute myeloid leukaemia
(17).
BCT-100 reduced cell viability in a panel of lung
adenocarcinoma cell lines but promoted tumour growth in most lung
adenocarcinoma xenograft models. Concurrently, ODC1 was upregulated
when treated with BCT-100. For tumour xenografts (H1650 and H1975)
that did not express OTC, ornithine accumulated upon exposure to
BCT-100. Of note, ODC1 is an inducible enzyme. An increased
ornithine level (substrate of ODC1) has been shown to induce ODC1
expression (18). Conversely, ODC1
can be upregulated by translational and transcriptional mechanisms
via PI3-kinase/Akt/mTOR and Ras/Raf/MEK/Erk/c-myc pathways
respectively (19). In addition,
ODC1 can be induced during hypoxia which increased polyamines
production and promoted cell survival in different cell lines.
Notably, apoptosis was induced when depleting polyamines during
hypoxic stress (20). BCT-100
depleted arginine to ornithine and may have been responsible for
upregulation of ODC1 expression in H1650 and H1975 solid tumour
xenografts. Notably, ODC1 expression was not induced with in
vitro exposure to BCT-100 (data not shown), which was
postulated to be due to the limited amount of arginine in in
vitro culture medium, but relatively abundant source of
arginine from food in vivo. The excessive arginine in
vivo was then converted to ornithine by BCT-100 which induced
ODC1 expression.
Ornithine was then converted to polyamines by ODC1,
SRM and SMS. Polyamines are known to promote cell proliferation and
tumour growth (21). In addition,
SRM is also an inducible enzyme by its own substrate (putrescine)
(22). BCT-100 not only upregulated
ODC1, but also SRM, and this may speed up the conversion of
putrescine to spermidine. Increased polyamines production may
counteract or even overwhelm the tumour suppression effect of
arginase treatment. Nonetheless, conversion of polyamines from
ornithine could be inhibited by ODC1 inhibitors (e.g.
α-difluoromethylornithine or DFMO). In order to prevent the
catastrophic effect due to increased polyamines synthesis, we
proposed that a combination of ODC1 inhibitor with BCT-100 could
restore the therapeutic function of BCT-100 in lung
adenocarcinoma.
DFMO is a specific ODC1 inhibitor that has been used
in a phase III clinical trial for chemoprevention of sporadic
colorectal adenomas (23). It has
been shown to significantly decrease putrescine and spermidine
level, but not spermine level, in both human colon cancer (24) and xenograft mouse models of skin
squamous cell carcinoma (25).
Nonetheless there was no direct tumour-suppressive effect of DFMO
in our lung adenocarcinoma xenograft models. In the present study,
the putrescine level was decreased in the BCT-100 treatment arms,
yet DFMO inhibited ODC1 that also suppressed putrescine production.
In our study, BCT-100 did not increase the spermidine level that
was probably used up for protein synthesis and cell proliferation
(26). As previously reported in
the literature, activation of ODC1 often decreases the level of
polyamines (26) and is consistent
with our findings.
Depletion of arginine (BCT-100 treatment) and
polyamine (DFMO treatment) suppressed tumour growth in solid tumour
xenograft models with inducible ODC1. At the same time, the
tumour-suppressive effect of BCT-100 was not hampered by DFMO
treatment in a solid tumour xenograft model without ODC1 induction.
It is of note that high basal ODC1 expression has been found in
liver and colon cancers (27),
MYCN-amplified neuroblastoma (28)
and leukaemia (29). Whether
induction of ODC1 expression by BCT-100 occurs in cancers other
than lung adenocarcinoma need to be elucidated. Theoretically,
upregulation of polyamines by high basal or inducible ODC1
expression can be potentially aborted by DFMO. Our findings
indicated that a combination of DFMO with BCT-100 can abrogate a
compensatory mechanism (via production of polyamines) that hinders
the therapeutic effects of BCT-100 in lung adenocarcinoma.
BCT-100 has been tested in different cancer models,
including hepatocellular carcinoma, acute myeloid leukemia, acute
lymphoblastic leukemia, glioblastoma, melanoma, prostate and
pancreatic cancer and mesothelioma. BCT-100 induced apoptosis
and/or cell cycle arrest in most cases (30), while promoted tumour growth
paradoxically in the present study. A combination of DFMO and
BCT-100 re-directed cancer cells to apoptosis. However, cell cycle
arrest was not observed as there was no alteration in the
expression of different cyclins and cyclin-dependent kinases (data
not shown).
A recent early phase clinical trial of BCT-100
treatment (1,600 U/kg/week) in HCC was completed in our
institution. A significant increase in progression-free survival
and overall survival was observed in patients with adequate
arginine depletion for >2 months. The adverse effects associated
with BCT-100 treatment were mild and included loss of appetite,
pain, vomiting, constipation, insomnia, fatigue and nausea
(10). Further clinical exploration
of BCT-100 in the treatment of HCC and acute leukaemia are
ongoing.
In a phase III chemoprevention trial for sporadic
colorectal adenomas, DFMO 500 mg daily in combination with sulindac
150 mg daily for 3 years was associated with few reported adverse
events. There was no significant difference (P>0.05) between
placebo and treatment groups in grade 3 or above adverse events,
frequency of overnight hospitalizations, cardiovascular events,
deaths, hearing problems and gastrointestinal events (23).
In conclusion, an ODC1-inhibitor (e.g. DFMO) should
be used in conjunction with pegylated arginase (BCT-100) in the
treatment of lung adenocarcinoma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
SKL, KPU, YYL, SX, PNMC and JCMH substantially
contributed to the conception or design of the work, to the
acquisition, analysis and interpretation of data. SKL and JCMH
drafted the work or revised it critically for important
intellectual content. SKL and JCMH approved the final version to be
published. All authors agree to be accountable for all aspects of
the work in ensuring that questions related to the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
The study protocol was approved by the institutional
Animal Ethics Committee of The University of Hong Kong (approval
ref. no. CULATR 3781-15) and standard humane endpoints for animal
research were applied in compliance with the instructions of U.S.
Public Health Service (policy on humane care and use of laboratory
animals).
Patient consent for publication
Not applicable.
Competing interests
YYL and SX report no potential conflict of interest.
PNC is the Chief Executive Officer of Bio-Cancer Treatment
International Limited and holds stocks or shares in Bio-Cancer
Treatment International Limited. KPU was a Scientific Officer at
Bio-Cancer Treatment International Limited. SKL, KPU, PNMC and JCH
hold patents relating to the content of the manuscript.
Glossary
Abbreviations
Abbreviations:
|
ODC1
|
ornithine decarboxylase 1
|
|
DFMO
|
α-difluoromethylornithine
|
|
OTC
|
ornithine transcarbamylase
|
|
ASS1
|
argininosuccinate synthetase
|
|
SRM
|
spermidine synthase
|
|
SMS
|
spermine synthase
|
|
SSAT
|
spermidine/spermine
N1-acetyltransferase
|
|
HCC
|
hepatocellular carcinoma
|
|
EGFR
|
epidermal growth factor receptor
|
|
ATCC
|
American Type Culture Collection
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
HRP
|
horseradish peroxidase
|
|
ECL
|
enhanced chemiluminescence
|
|
PBS
|
phosphate-buffered saline
|
|
OPA
|
o-phthaldialdehyde
|
|
TUNEL
|
terminal deoxynucleotidyl
transferase-dUTP nick end labeling
|
|
TdT
|
terminal deoxynucleotidyl
transferase
|
|
DAPI
|
4′,6-diamidino-2-phenylindole
|
References
|
1
|
Delage B, Fennell DA, Nicholson L, McNeish
I, Lemoine NR, Crook T and Szlosarek PW: Arginine deprivation and
argininosuccinate synthetase expression in the treatment of cancer.
Int J Cancer. 126:2762–2772. 2010.PubMed/NCBI
|
|
2
|
Chow AK, Ng L, Li Sing H, Cheng CW, Lam
CS, Yau TC, Cheng PN, Fan ST, Poon RT and Pang RW: Anti-tumor
efficacy of a recombinant human arginase in human hepatocellular
carcinoma. Curr Cancer Drug Targets. 12:1233–1243. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Feun L and Savaraj N: Pegylated arginine
deiminase: A novel anticancer enzyme agent. Exp Opin Invest Drugs.
15:815–822. 2006. View Article : Google Scholar
|
|
4
|
Thomas T and Thomas TJ: Polyamines in cell
growth and cell death: Molecular mechanisms and therapeutic
applications. Cell Mol Life Sci. 58:244–258. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tanios R, Bekdash A, Kassab E, Stone E,
Georgiou G, Frankel AE and Abi-Habib RJ: Human recombinant arginase
I(Co)-PEG5000 [HuArgI(Co)-PEG5000]-induced arginine depletion is
selectively cytotoxic to human acute myeloid leukemia cells. Leuk
Res. 37:1565–1571. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cheng PN, Lam TL, Lam WM, Tsui SM, Cheng
AW, Lo WH and Leung YC: Pegylated recombinant human arginase
(rhArg-peg5,000mw) inhibits the in vitro and in vivo
proliferation of human hepatocellular carcinoma through arginine
depletion. Cancer Res. 67:309–317. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lam SK, Li YY, Xu S, Leung LL; U KP, ;
Zheng YF, Cheng PN and Ho JC: Growth suppressive effect of
pegylated arginase in malignant pleural mesothelioma xenografts.
Respir Res. 18:802017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lam TL, Wong GK, Chow HY, Chong HC, Chow
TL, Kwok SY, Cheng PN, Wheatley DN, Lo WH and Leung YC: Recombinant
human arginase inhibits the in vitro and in vivo proliferation of
human melanoma by inducing cell cycle arrest and apoptosis. Pigment
Cell Melanoma Res. 24:366–376. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lam TL, Wong GK, Chong HC, Cheng PN, Choi
SC, Chow TL, Kwok SY, Poon RT, Wheatley DN, Lo WH, et al:
Recombinant human arginase inhibits proliferation of human
hepatocellular carcinoma by inducing cell cycle arrest. Cancer
Lett. 277:91–100. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yau T, Cheng PN, Chan P, Chen L, Yuen J,
Pang R, Fan ST, Wheatley DN and Poon RT: Preliminary efficacy,
safety, pharmacokinetics, pharmacodynamics and quality of life
study of pegylated recombinant human arginase 1 in patients with
advanced hepatocellular carcinoma. Invest New Drugs. 33:496–504.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lam SK, Li YY, Zheng CY and Ho JC:
Downregulation of thymidylate synthase and E2F1 by arsenic trioxide
in mesothelioma. Int J Oncol. 46:113–122. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li YY, Lam SK, Mak JC, Zheng CY and Ho JC:
Erlotinib-induced autophagy in epidermal growth factor receptor
mutated non-small cell lung cancer. Lung Cancer. 81:354–361. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brenner T, Fleming TH, Spranz D, Schemmer
P, Bruckner T, Uhle F, Martin EO, Weigand MA and Hofer S: Reactive
metabolites and AGE-RAGE-mediated inflammation in patients
following liver transplantation. Mediators Inflamm.
2013:5014302013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nicoletti R, Venza I, Ceci G, Visalli M,
Teti D and Reibaldi A: Vitreous polyamines spermidine, putrescine,
and spermine in human proliferative disorders of the retina. Br J
Ophthalmol. 87:1038–1042. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ho JC, Tam TC and Lam SK: Salvage therapy
beyond targeted therapy in lung adenocarcinoma. Semin Respir Crit
Care Med. 34:837–844. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qiu F, Huang J and Sui M: Targeting
arginine metabolism pathway to treat arginine-dependent cancers.
Cancer Lett. 364:1–7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mussai F, Egan S, Higginbotham-Jones J,
Perry T, Beggs A, Odintsova E, Loke J, Pratt G; U KP, ; Lo A, et
al: Arginine dependence of acute myeloid leukemia blast
proliferation: A novel therapeutic target. Blood. 125:2386–2396.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Majumdar R, Shao L, Minocha R, Long S and
Minocha SC: Ornithine: The overlooked molecule in the regulation of
polyamine metabolism. Plant Cell Physiol. 54:990–1004. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shantz LM and Levin VA: Regulation of
ornithine decarboxylase during oncogenic transformation: Mechanisms
and therapeutic potential. Amino Acids. 33:213–223. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Svensson KJ, Welch JE, Kucharzewska P,
Bengtson P, Bjurberg M, Påhlman S, Dam Ten GB, Persson L and
Belting M: Hypoxia-mediated induction of the polyamine system
provides opportunities for tumor growth inhibition by combined
targeting of vascular endothelial growth factor and ornithine
decarboxylase. Cancer Res. 68:9291–9301. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miller-Fleming L, Olin-Sandoval V,
Campbell K and Ralser M: Remaining mysteries of molecular biology:
The role of polyamines in the cell. J Mol Biol. 427:3389–3406.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Russell DH and Snyder SH: Amine synthesis
in regenerating rat liver: Extremely rapid turnover of ornithine
decarboxylase. Mol Pharmacol. 5:253–262. 1969.PubMed/NCBI
|
|
23
|
Meyskens FL Jr, McLaren CE, Pelot D,
Fujikawa-Brooks S, Carpenter PM, Hawk E, Kelloff G, Lawson MJ,
Kidao J, McCracken J, et al: Difluoromethylornithine plus sulindac
for the prevention of sporadic colorectal adenomas: A randomized
placebo-controlled, double-blind trial. Cancer Prev Res (Phila).
1:32–38. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mackenzie GG, Ouyang N, Xie G, Vrankova K,
Huang L, Sun Y, Komninou D, Kopelovich L and Rigas B:
Phospho-sulindac (OXT-328) combined with difluoromethylornithine
prevents colon cancer in mice. Cancer Prev Res. 4:1052–1060. 2011.
View Article : Google Scholar
|
|
25
|
Chen Y, Hu J, Boorman D, Klein-Szanto A
and O'Brien TG: Therapy of murine squamous cell carcinomas with
2-difluoromethylornithine. J Carcinog. 3:102004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schipper RG, Penning LC and Verhofstad AA:
Involvement of polyamines in apoptosis. Facts and controversies:
Effectors or protectors? Semin Cancer Biol. 10:55–68. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tomasi ML, Ryoo M, Skay A, Tomasi I,
Giordano P, Mato JM and Lu SC: Polyamine and methionine
adenosyltransferase 2A crosstalk in human colon and liver cancer.
Exp Cell Res. 319:1902–1911. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rounbehler RJ, Li W, Hall MA, Yang C,
Fallahi M and Cleveland JL: Targeting ornithine decarboxylase
impairs development of MYCN-amplified neuroblastoma. Cancer
Res. 69:547–553. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang MF, Liao YF, Hung YC, Lin CL, Hour
TC, Lue KH, Hung HC and Liu GY: Hydroxydibenzoylmethane induces
apoptosis through repressing ornithine decarboxylase in human
promyelocytic leukemia HL-60 cells. Exp Mol Med. 43:189–196. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fung MKL and Chan GC: Drug-induced amino
acid deprivation as strategy for cancer therapy. J Hematol Oncol.
10:1442017. View Article : Google Scholar : PubMed/NCBI
|