Introduction
Colorectal cancer (CRC) is the third most commonly
diagnosed cancer in men and the second in women; it is a malignant
digestive tumor, with ~1,360,600 new cases diagnosed and 693,900
deaths from CRC occurring in 2012 (1). The incidence rate of CRC is higher in
men than in women in most parts of the world (1), and CRC is currently the fourth leading
cause of cancer-related death worldwide (2). The incidence of CRC varies among
countries, and the mortality rates of CRC are decreasing in many
countries worldwide because of CRC screening, reduced prevalence of
risk factors, and improved treatments (1). CRC incidence and mortality rates in
China showed an upward trend between 2000 and 2011 (3). CRC is the fifth most commonly
diagnosed cancer and the fifth leading cause of cancer-related
death in China (3). The
age-standardized 5-year relative survival from CRC in China, which
is determined from the cancer registries, is estimated at 47.2%
(4). CRC can be divided into three
types according to histological classification, and most colon
cancers are colon adenocarcinoma (COAD). The major subtypes of COAD
are non-mucinous adenocarcinoma, mucinous or colloid
adenocarcinoma, and signet ring cell carcinoma.
MicroRNAs (miRNAs) are small, single-stranded RNAs
of 21–23 nucleotides (nt) in length that play important roles in
the post-transcriptional control of gene expression (5). An increasing number of studies show
that miRNAs play crucial roles in cancer. Abnormal miRNA levels in
CRC have been reported in many studies, and these miRNAs may have
potential applications as biomarkers in the diagnosis and prognosis
of CRC (6,7). Therefore, using whole genome
technology to screen for potential prognostic miRNA biomarkers of
CRC is necessary and effective. Advances in genome-wide
high-throughput technology led to the development of a project in
the United States named The Cancer Genome Atlas (TCGA), which
attempted to map out the genome variations of human cancers by
applying genomic analysis techniques (8,9). In
addition, multiple genome-wide datasets of cancers are open access,
including the COAD miRNA-sequencing (miRNA-seq) dataset. The aim of
the present study was to identify potential prognostic miRNA
biomarkers for patients with COAD using the miRNA-seq dataset from
TCGA. An miRNA expression-based prognostic signature was generated,
and the potential role of the corresponding miRNA target genes in
the overall survival (OS) of patients with COAD was
investigated.
Materials and methods
Data source and pre-processing
The miRNA-seq, RNA-sequencing (RNA-seq) dataset, and
corresponding clinical information were download from TCGA
(https://portal.gdc.cancer.gov/, accessed
February 11, 2018) (10). The raw
data of miRNA-seq and RNA-seq were normalized by the DESeq
package in the R platform, and miRNAs showing mean expression
values >1 were included in the subsequent analysis (11). Since all datasets of COAD included
in the present study were downloaded from TCGA, additional approval
by an Ethics Committee was not needed.
Screening of prognosis-related
miRNAs
Survival analyses were performed in patients with
normalized expression of miRNAs and OS profiles. Patients were
divided into low- and high-expression groups according to the
median gene expression levels. The prognostic value of each miRNA
was assessed by multivariate Cox proportional hazards regression
analysis using a survival package in the R platform, and the
low expression group was set as a reference group. An adjusted
P-value cutoff of 0.05 was considered statistically significant and
identified as prognosis-related miRNAs.
Construction of an miRNA
expression-based prognostic signature
A prognosis risk score was established based on a
linear combination of gene expression level multiplied by a
regression coefficient (β)-identified as the weight derived from
multivariate Cox proportional hazards regression analysis, in which
the prognostic miRNAs were fitted in the multivariate Cox
regression model with OS as a dependent variable. The risk score
formula was as follows (12–15):
Risk score = expression of miRNA1 × β1
miRNA1 + expression of miRNA2 × β2
miRNA2 + …expression of miRNAn ×
βn miRNAn. Patients were divided into high-
and low-risk groups according to the risk score median values. To
evaluate the predictive accuracy of this miRNA expression-based
prognostic signature for CRC outcome, a time-dependent receiver
operating characteristic (ROC) curve was constructed using the
survivalROC package in the R platform (16).
Comprehensive survival analysis of the
miRNA expression-based prognostic signature
A stratified and joint effect survival analysis was
performed to investigate the association between the risk score and
the clinical characteristics of CRC in respect to the miRNA
expression-based prognostic signature. A nomogram was constructed
to assess the individualized prognosis prediction model based on
the clinical characteristics and risk score.
Target gene prediction and enrichment
analysis
The TargetScan (http://www.targetscan.org/, accessed February 28,
2018) (17,18), miRDB (http://www.mirdb.org/, accessed February 28, 2018)
(19,20), and miRTarBase (http://mirtarbase.mbc.nctu.edu.tw/, accessed
February 28, 2018) (21,22) algorithms were used to predict the
target genes of these prognostic miRNAs. The overlapping target
genes in these three databases were identified as miRNA-target
genes and used for further enrichment analysis. The miRNA-target
gene interaction networks were constructed using Cytoscape v3.4.0.
The functional enrichment of these miRNA-target genes was performed
using the Database for Annotation, Visualization and Integrated
Discovery v6.8 (DAVID v6.8; https://david.ncifcrf.gov/home.jsp, accessed February
28, 2018) (23,24) and visualized with the ggplot2
package.
Statistical analysis
Clinical features associated with OS were analyzed
using the log-rank test, and those with a P<0.05 were entered
into the multivariate Cox proportional hazards regression model for
adjustment. A value of P<0.05 was considered statistically
significant. All statistical analyses were performed with SPSS
version 20.0 (IBM Corporation, Armonk, NY, USA) and R 3.3.0
(https://www.r-project.org/).
Results
Study population
There were 444 cases identified in the miRNA-seq
dataset, and the corresponding survival profiles were downloaded
from the TCGA data portal (10).
Patients lacking survival data and those with a survival time of
zero were excluded from the study. A total of 425 COAD patients
were included in the study and further analyzed. Information on
age, sex and tumor stage was obtained from the TCGA portal. Tumor
stage was significantly associated with COAD OS, and advanced
stages were significantly correlated with an increased risk of
death [stages I and II vs. stages III and IV: log-rank P<0.0001;
hazard ratio (HR), 3.204; 95% confidence interval (CI),
2.069–4.963; Table I]. Therefore,
tumor stage was included in the multivariate Cox proportional
hazards regression model for adjustment.
 | Table I.Correlation between OS and
clinicopathological features of COAD patients. |
Table I.
Correlation between OS and
clinicopathological features of COAD patients.
| Variables | Events/total
(n=425) | MST (days) | Crude HR (95%
CI) | Log-rank
P-value |
|---|
| Age
(years)a |
|
|
|
0.109 |
|
≤65 | 29/165 | NA | 1 |
|
|
>65 | 67/258 | 2,475 | 1.425
(0.922–2.204) |
|
| Sex |
|
|
|
0.497 |
|
Female | 44/200 | NA | 1 |
|
|
Male | 53/225 | 2,475 | 1.149
(0.769–1.716) |
|
| Tumor
stageb |
|
|
| <0.001 |
| I | 4/71 | NA | 1 |
|
| II | 26/159 | 2,821 | 2.133
(0.742–6.133) |
|
|
III | 31/123 | NA | 4.067
(1.434–11.538) |
|
| IV | 31/61 | 858 | 11.032
(3.889–31.292) |
|
| Tumor
stageb |
|
|
| <0.001 |
|
I+II | 30/230 | NA | 1 |
|
|
III+IV | 62/184 | 332 | 3.204
(2.069–4.963) |
|
Screening of prognosis-related
miRNAs
After normalization, a total of 578 miRNAs were
included in the screening for prognosis-related miRNAs.
Multivariate Cox proportional hazards regression analysis was
performed with the survival package in the R platform after
adjusting for tumor stage and grouping by the median value of each
miRNA. The analysis identified 30 miRNAs that were significantly
associated with COAD OS (Table
II). Among these 30 miRNAs, those with expression values of
zero in more than half of the samples were excluded. Finally, 27
prognostic miRNAs were included in the evaluation of the prognostic
signature combination using the ‘step’ function.
 | Table II.Multivariate survival analysis
results of the miRNAs. |
Table II.
Multivariate survival analysis
results of the miRNAs.
| ID 95% CI |
P-valuea | HR | Low 95% CI | High |
|---|
| hsa-mir-1248 | 0.001 | 2.022 | 1.312 | 3.114 |
| hsa-mir-940 | 0.004 | 0.539 | 0.354 | 0.820 |
| hsa-mir-6783 | 0.004 | 0.538 | 0.353 | 0.821 |
| hsa-mir-141 | 0.005 | 1.848 | 1.210 | 2.824 |
| hsa-mir-550a-3 | 0.009 | 1.742 | 1.149 | 2.641 |
| hsa-mir-210 | 0.011 | 1.730 | 1.134 | 2.638 |
| hsa-mir-200a | 0.013 | 0.581 | 0.379 | 0.891 |
| hsa-mir-151b | 0.013 | 1.707 | 1.119 | 2.605 |
| hsa-mir-3613 | 0.015 | 0.596 | 0.393 | 0.905 |
| hsa-mir-891a | 0.015 | 1.680 | 1.105 | 2.555 |
| hsa-mir-147b | 0.018 | 1.654 | 1.090 | 2.512 |
| hsa-mir-197 | 0.018 | 1.657 | 1.089 | 2.522 |
| hsa-mir-200b | 0.019 | 0.607 | 0.400 | 0.921 |
| hsa-mir-216a | 0.019 | 1.651 | 1.086 | 2.511 |
| hsa-mir-641 | 0.019 | 1.644 | 1.084 | 2.496 |
| hsa-mir-500a | 0.026 | 0.618 | 0.405 | 0.943 |
| hsa-mir-1271 | 0.026 | 1.613 | 1.059 | 2.455 |
| hsa-mir-328 | 0.029 | 1.592 | 1.049 | 2.414 |
| hsa-mir-887 | 0.030 | 1.596 | 1.047 | 2.432 |
| hsa-mir-378d-1 | 0.031 | 1.577 | 1.044 | 2.382 |
| hsa-mir-3187 | 0.031 | 1.580 | 1.043 | 2.394 |
| hsa-mir-92a-1 | 0.032 | 0.633 | 0.417 | 0.962 |
| hsa-mir-92a-2 | 0.033 | 0.636 | 0.419 | 0.965 |
| hsa-mir-518c | 0.034 | 1.598 | 1.035 | 2.466 |
| hsa-mir-6854 | 0.041 | 0.647 | 0.426 | 0.982 |
| hsa-mir-1249 | 0.041 | 0.645 | 0.424 | 0.982 |
| hsa-mir-4709 | 0.041 | 1.544 | 1.017 | 2.343 |
| hsa-mir-126 | 0.042 | 1.539 | 1.016 | 2.332 |
| hsa-mir-33b | 0.043 | 1.538 | 1.013 | 2.335 |
| hsa-mir-526b | 0.049 | 1.512 | 1.001 | 2.284 |
Prognostic signature construction
After evaluation using the ‘step’ function
for these 27 prognostic miRNAs, the most effective combinations
based on the expression of candidate prognostic miRNAs were
selected. The following 10 prognostic miRNAs were used for
construction of the prognostic signature: hsa-mir-891a,
hsa-mir-6854, hsa-mir-216a, hsa-mir-378d-1, hsa-mir-92a-1,
hsa-mir-4709, hsa-mir-92a-2, hsa-mir-210, hsa-mir-940 and
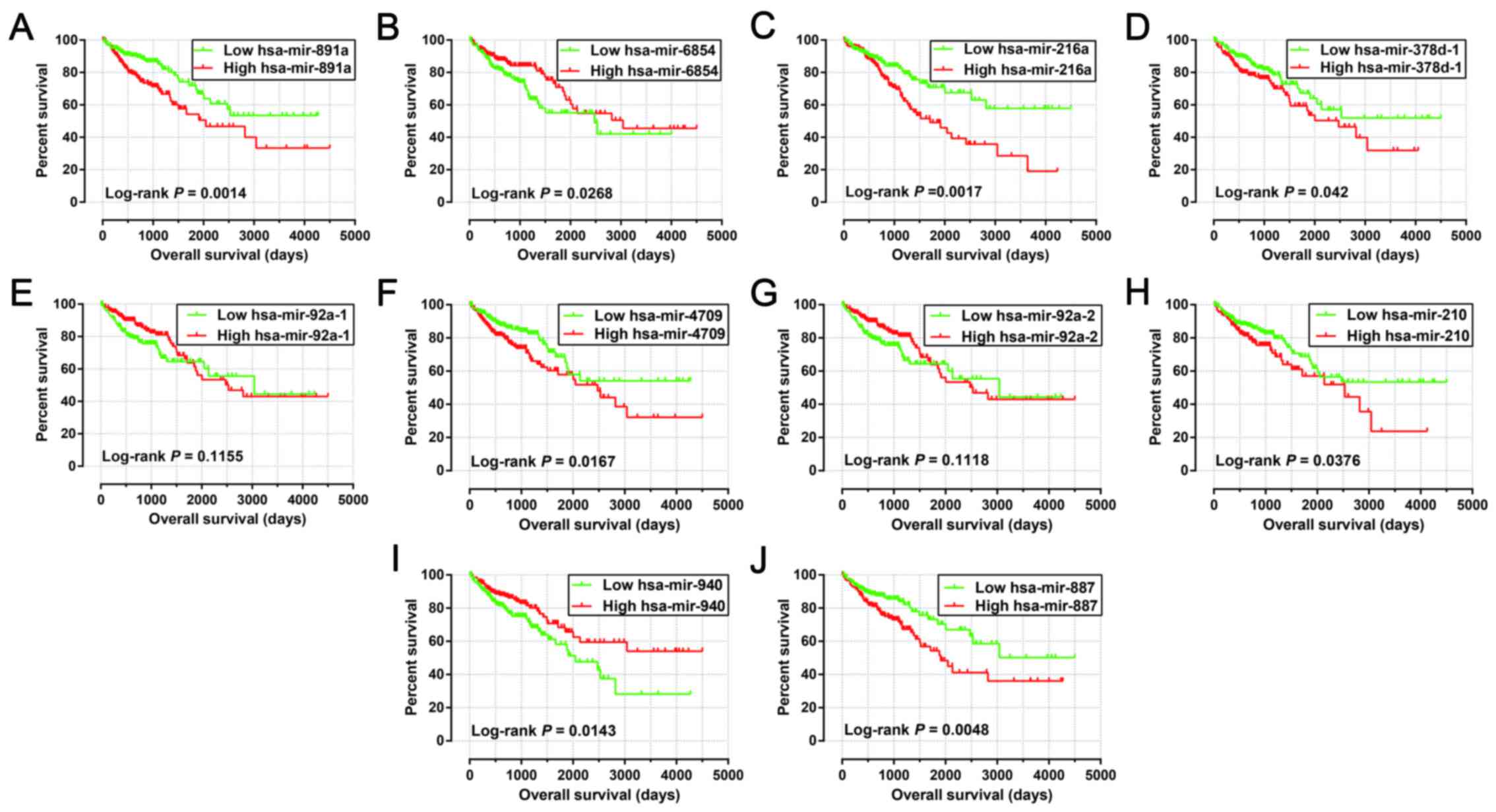
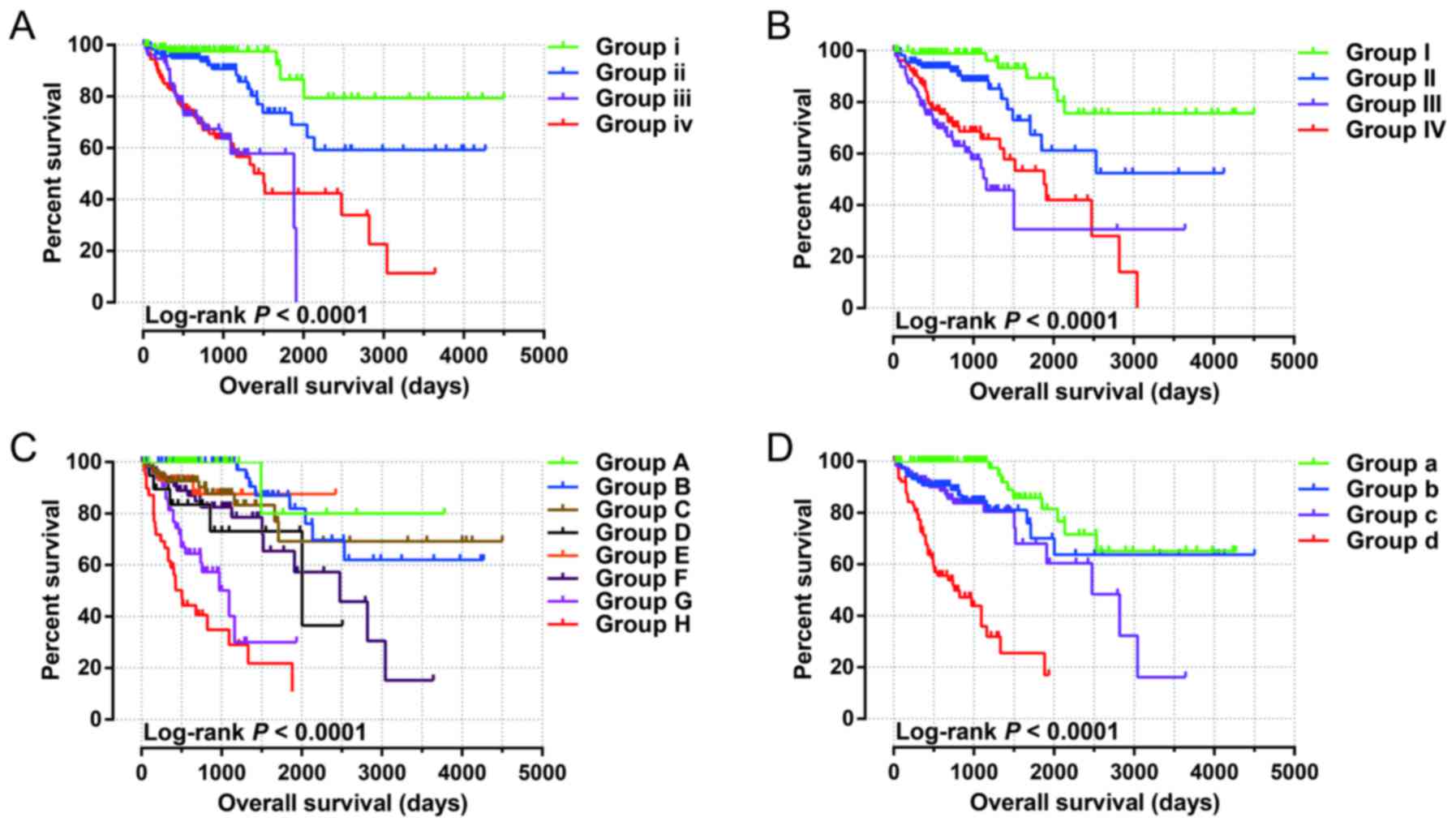
hsa-mir-887. The results of the Kaplan-Meier analysis of these
prognosis-related miRNAs are shown in Fig. 1A-J. The relative contribution of
these prognostic miRNAs was assessed using the multivariate Cox
proportional hazard regression model, with the multivariate Cox
regression coefficient (β) as the weight. The risk score formula
was as follows: risk score = hsa-mir-891a × (0.185) + hsa-mir-6854
× (−0.215) + hsa-mir-216a × (0.430) + hsa-mir-378d-1 × (0.471) +
hsa-mir-92a-1 × (−4.915) + hsa-mir-4709 × (0.233) + hsa-mir-92a-2 ×
(5.104) + hsa-mir-210 × (0.271) + hsa-mir-940 × (−0.247) +
hsa-mir-887 × (0.446). Patients were divided into low- and
high-risk groups according to the median risk scores, and survival
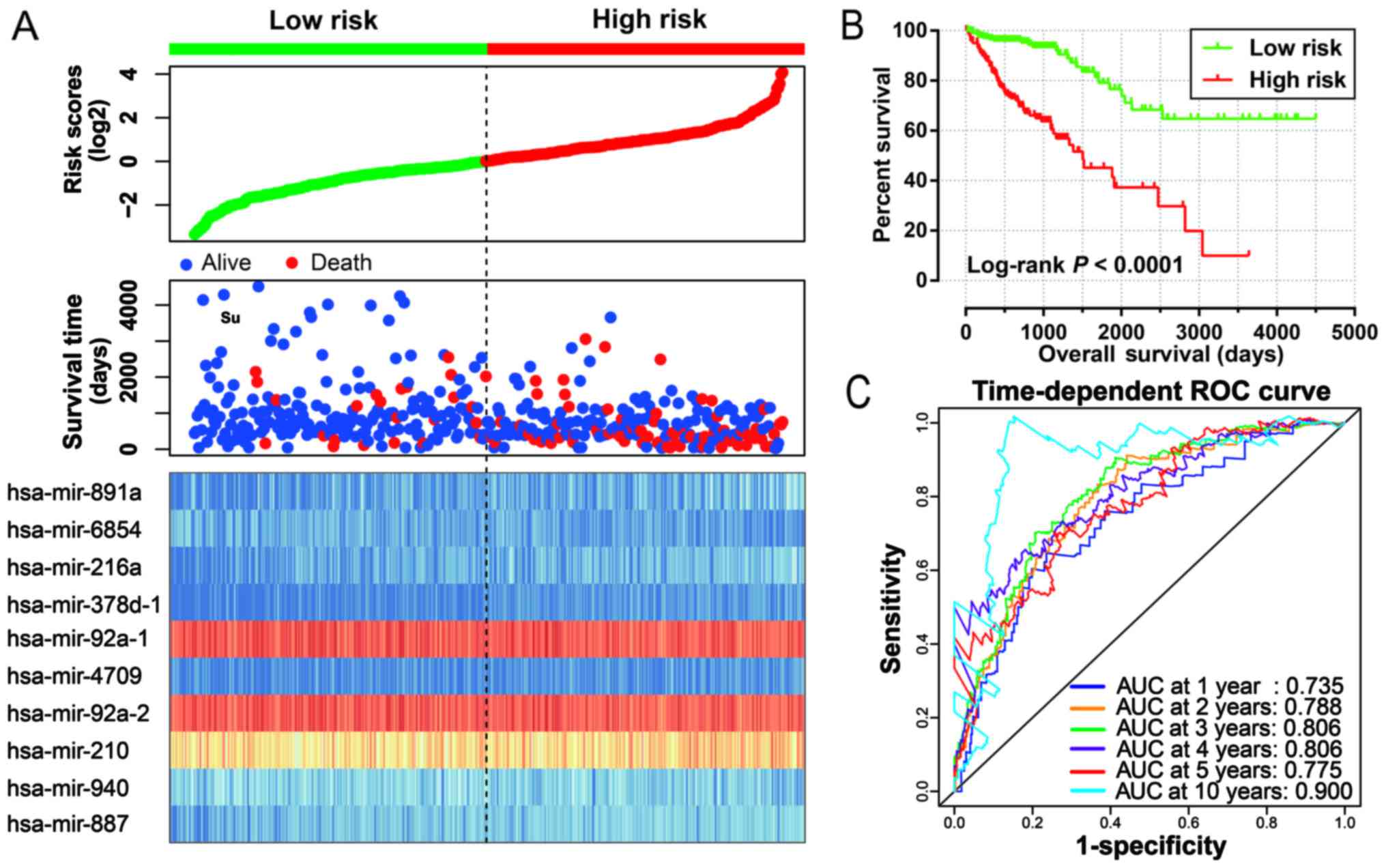
analysis indicated that patients with high risk scores were
significantly associated with a poor clinical outcome and increased
risk of death (adjusted P<0.0001; adjusted HR, 4.580; 95% CI,
2.783–7.538; Fig. 2A and B).
Time-dependent ROC curve analysis was used to evaluate the
predictive accuracy of this prognostic signature, and the results
suggested that the prognostic signature identified in the current
study performed well regarding 1-, 2-, 3-, 4-, 5- and 10-year
survival predictions. The area under the curve (AUC) for 1-, 2-,
3-, 4-, 5- and the 10-year predictions were 0.735, 0.788, 0.806,
0.806, 0.775 and 0.900, respectively (Fig. 2C). The distribution of the
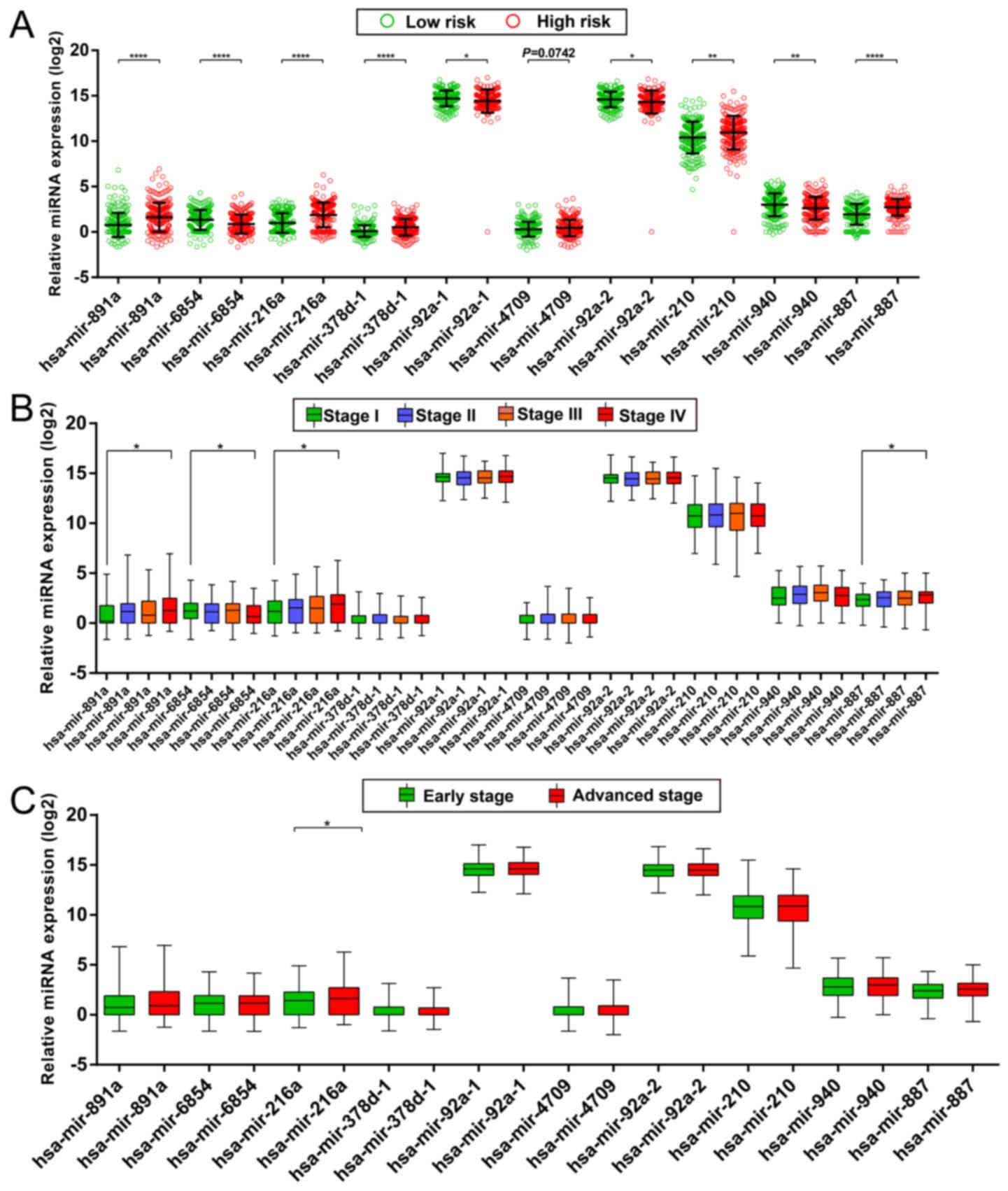
expression of miRNAs in the high- and low-risk groups is shown in
Fig. 3A, and the distribution of
miRNA expression according to tumor stage is shown in Fig. 3B and C. Comparison of the expression
levels of the identified miRNAs between different tumor stages
showed that hsa-mir-216a expression was considerably higher in
tumor stage IV than in tumor stage I and significantly increased in
advanced tumor stages. These results indicated that hsa-mir-216a
may play a role in COAD progression.
Stratified and joint effects
analysis
The relation between the prognostic signature and
the clinical characteristics associated with COAD OS was further
investigated by performing a comprehensive analysis of the
nomogram, stratified and joint effects survival analysis.
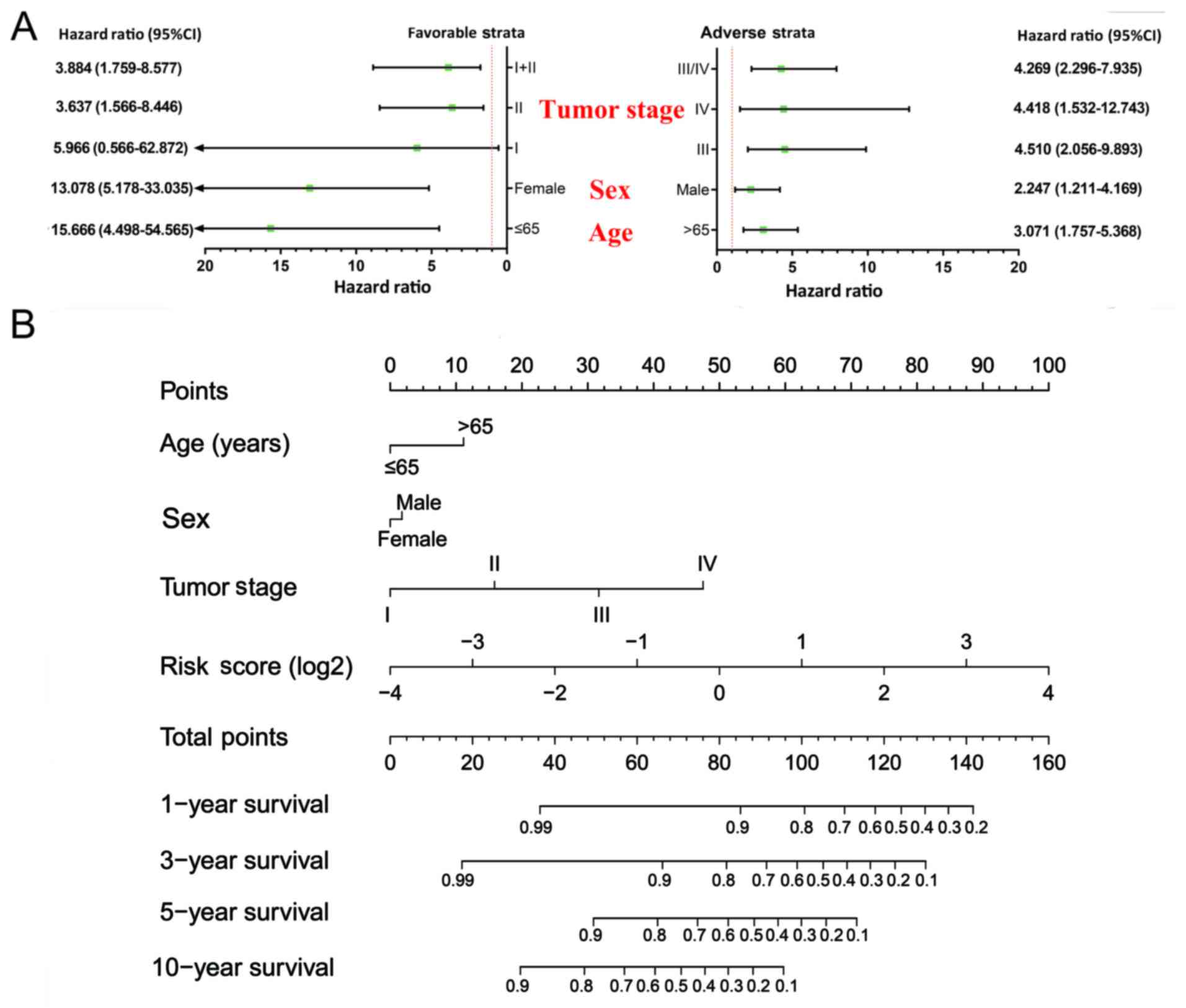
Stratified analysis indicated that patients with a high-risk score
showed a significantly increased risk of death in all favorable
strata and all adverse strata except in patients with stage I
(Fig. 4A). A nomogram was
visualized by rms and its auxiliary packages based on the
clinical characteristics of COAD and risk scores; results
demonstrated that the 10-miRNA prognostic signature contributed the
most risk points (ranged 0–100), whereas the other clinical
characteristics contributed much less (Fig. 4B).
Joint effects survival analysis of the 10-miRNA
prognostic signature and clinical parameters suggested that this
prognostic signature performed well in COAD OS predictions, and its
combination with clinical parameters significantly associated with
COAD OS considerably increased its predictive value for COAD OS
(Fig. 5A-D and Table III).
 | Table III.Joint effects survival analysis of
clinical factors and the risk score with OS in the COAD
patients. |
Table III.
Joint effects survival analysis of
clinical factors and the risk score with OS in the COAD
patients.
| Group | Risk score | Variables | Events/total
(n=425) | MST (days) | Crude HR (95%
CI) | Crude P-value | Adjusted HR (95%
CI) | Adjusted
P-valuea |
|---|
|
|
| Age
(years)b |
|
|
|
|
|
|
| i | Low risk | ≤65 | 5/85 | NA | 1 |
| 1 |
|
| ii | Low risk | >65 | 18/126 | NA | 2.622
(0.973–7.067) | 0.057 | 3.791
(1.394–10.309) | 0.009 |
| iii | Low risk | ≤65 | 24/80 | 1,881 | 9.806
(3.693–26.037) | <0.001 | 9.646
(3.521–26.431) | <0.001 |
| iv | High risk | >65 | 49/132 | 1,503 | 9.314
(3.698–23.459) | <0.001 | 12.037
(4.667–31.049) | <0.001 |
|
|
| Sex |
|
|
|
|
|
|
| I | Low risk | Female | 7/99 | NA | 1 |
| 1 |
|
| II | Low risk | Male | 17/114 | NA | 2.894
(1.195–7.011) | 0.019 | 3.053
(1.262–7.387) | 0.013 |
| III | High risk | Female | 37/101 | 1,162 | 11.092
(4.855–25.344) | <0.001 | 11.862
(5.100–27.588) | <0.001 |
| IV | High risk | Male | 36/111 | 1,881 | 7.822
(3.450–17.733) | <0.001 | 7.094
(3.061–16.442) | <0.001 |
|
|
| Tumor
stagec |
|
|
|
|
|
|
| A | Low risk | I | 1/42 | NA | 1 |
| 1 |
|
| B | Low risk | II | 8/77 | NA | 2.595
(0.324–20.810) | 0.369 | 2.595
(0.324–20.810) | 0.369 |
| C | Low risk | III | 10/69 | NA | 4.334
(0.553–33.949) | 0.163 | 4.334
(0.553–33.949) | 0.163 |
| D | Low risk | IV | 5/20 | 2,003 | 10.075
(1.175–86.413) | 0.035 | 10.075
(1.175–86.413) | 0.035 |
| E | High risk | I | 3/29 | NA | 5.416
(0.562–52.192) | 0.144 | 5.416
(0.562–52.192) | 0.144 |
| F | High risk | II | 18/82 | 2,475 | 8.553
(1.141–64.090) | 0.037 | 8.553
(1.141–64.090) | 0.037 |
| G | High risk | III | 21/54 | 1,094 | 25.841
(3.459–193.045) | 0.002 |
25.841(3.459–193.045) | 0.002 |
| H | High risk | IV | 26/41 | 504 | 44.775
(6.052–331.249) | <0.001 | 44.775
(6.052–331.249) | <0.001 |
|
|
| Tumor
stagec |
|
|
|
|
|
|
| a | Low risk | I+II | 9/119 | NA | 1 |
| 1 |
|
| b | Low risk | III+IV | 15/89 | NA | 2.436
(1.066–5.571) | 0.035 | 6.346
(1.772–22.721) | 0.005 |
| c | High risk | I+II | 21/111 | 2,475 | 3.606
(1.645–7.906) | 0.001 | 3.510
(1.600–7.702) | 0.002 |
| d | High risk | III+IV | 47/95 | 758 | 15.518
(7.392–32.577) | <0.001 | 33.853
(10.485–109.305) | <0.001 |
Target prediction and enrichment
analysis
The target genes of the 10 miRNAs were analyzed
using three independent miRNA target gene prediction websites:
TargetScan, miRDB and miRTarBase. Target genes overlapping in the
three websites were regarded as miRNA-target genes. Among the 10
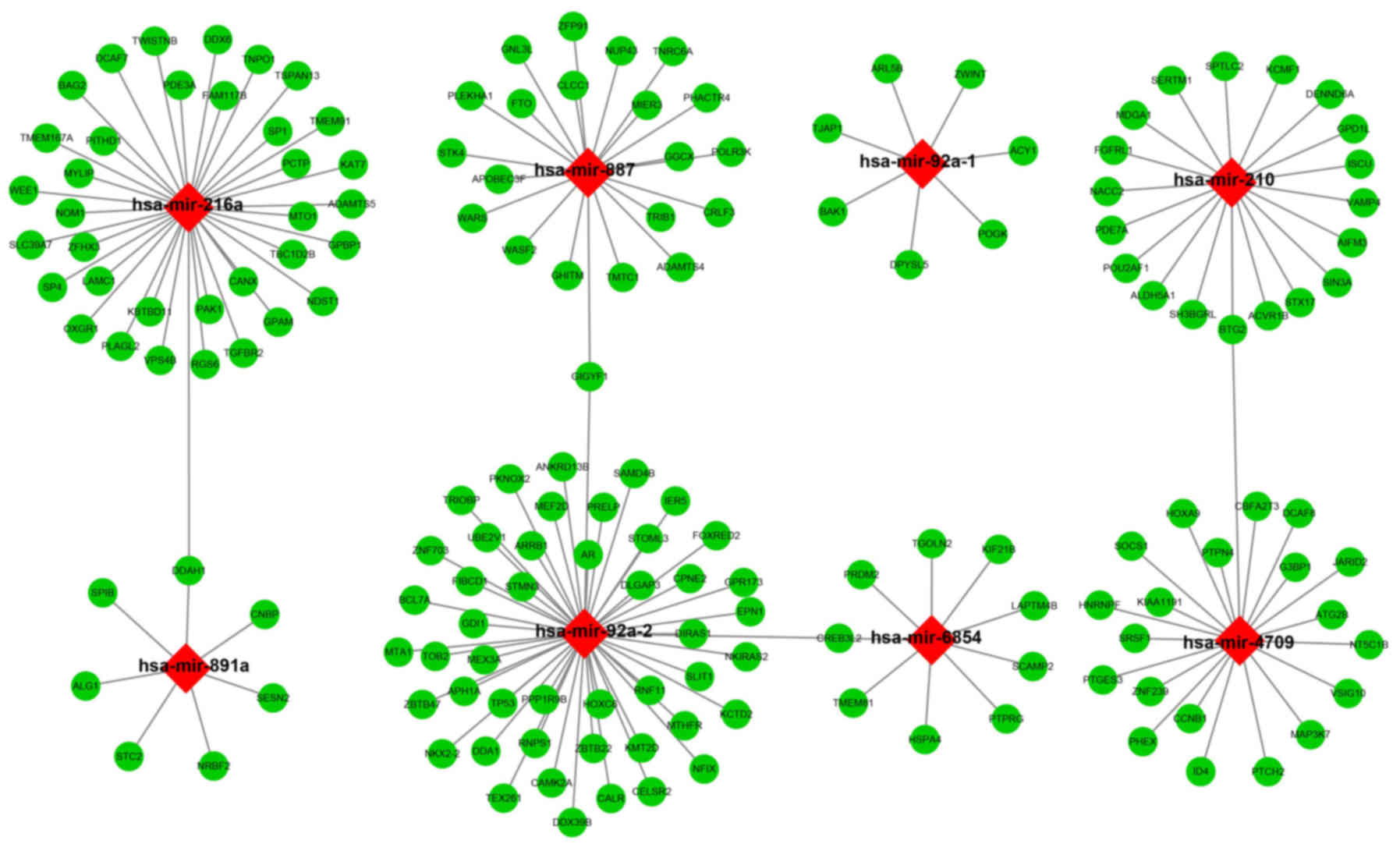
miRNAs, hsa-mir-216a, hsa-mir-887, hsa-mir-92a-1, hsa-mir-210,
hsa-mir-891a, hsa-mir-92a-2, hsa-mir-6854, and hsa-mir-4709 had
overlapping target genes in the three websites (Fig. 6). Enrichment analysis of these
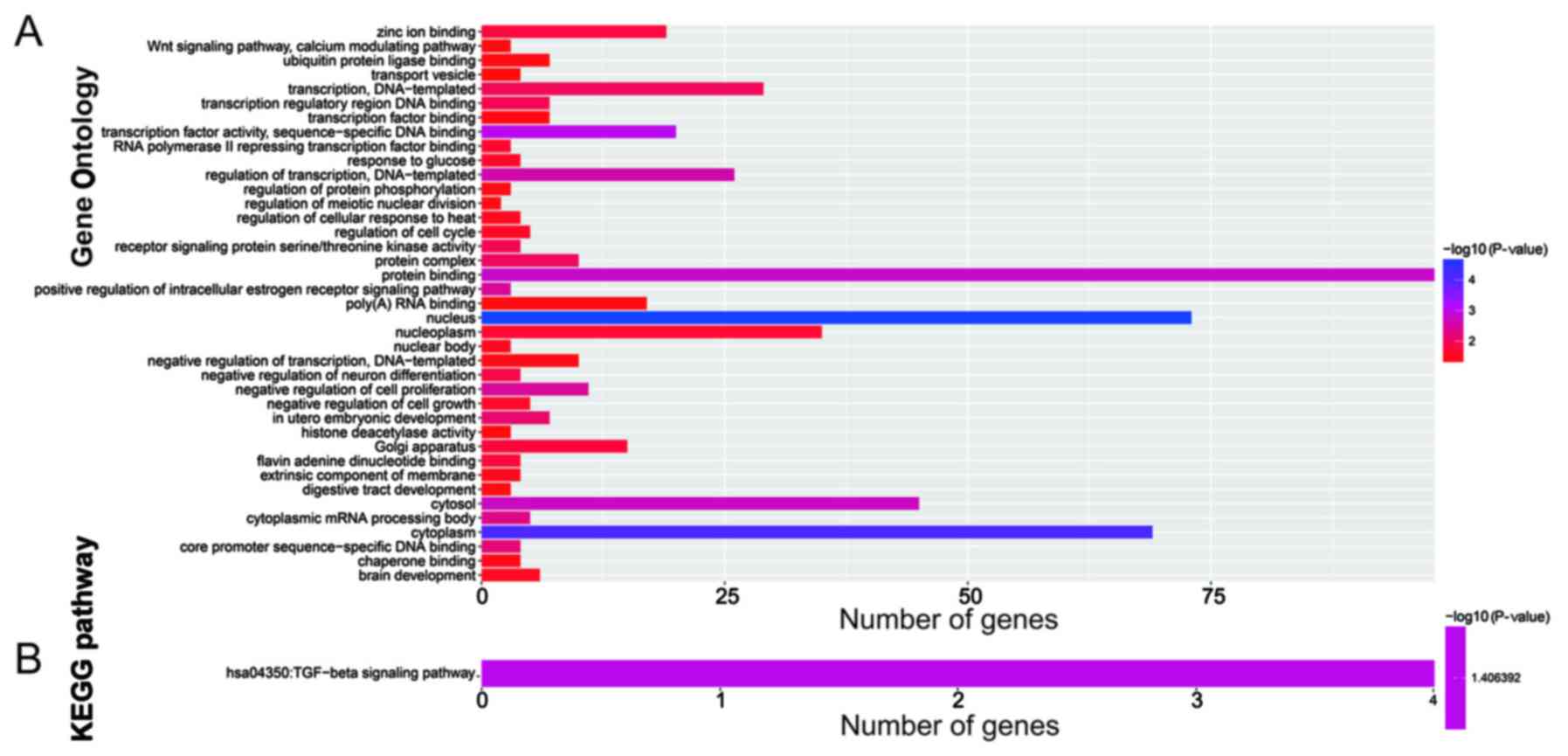
target genes was performed using DAVID v6.8. Gene Ontology (GO)
term enrichment results suggested that the target genes were
significantly enriched in the Wnt signaling pathway, calcium
modulating pathway, regulation of protein phosphorylation,
regulation of cell cycle, negative regulation of cell growth,
negative regulation of cell proliferation, regulation of
transcription, and DNA-templated biological processes (Fig. 7A). Kyoto Encyclopedia of Genes and
Genomes (KEGG) enrichment analysis indicated that these target
genes were significantly correlated with the transforming growth
factor-β (TGF-β) signaling pathway (Fig. 7B).
Among these 425 patients in the miRNA-seq cohorts,
423 patient tumor samples received RNA sequencing, and the RNA-seq
dataset was also normalized using the DESeq package in the R
platform (12). To further
investigate the role of the identified target genes in COAD OS,
survival analysis was performed using the survival package.
Among 164 target genes identified, 11 were significantly correlated
with COAD OS in the multivariate Cox proportional hazards
regression model after adjusting for tumor stage and grouping
according to the median expression value of each miRNA (Table IV). The Kaplan-Meier curves of
these 11 target genes are shown in Fig.
8A-K.
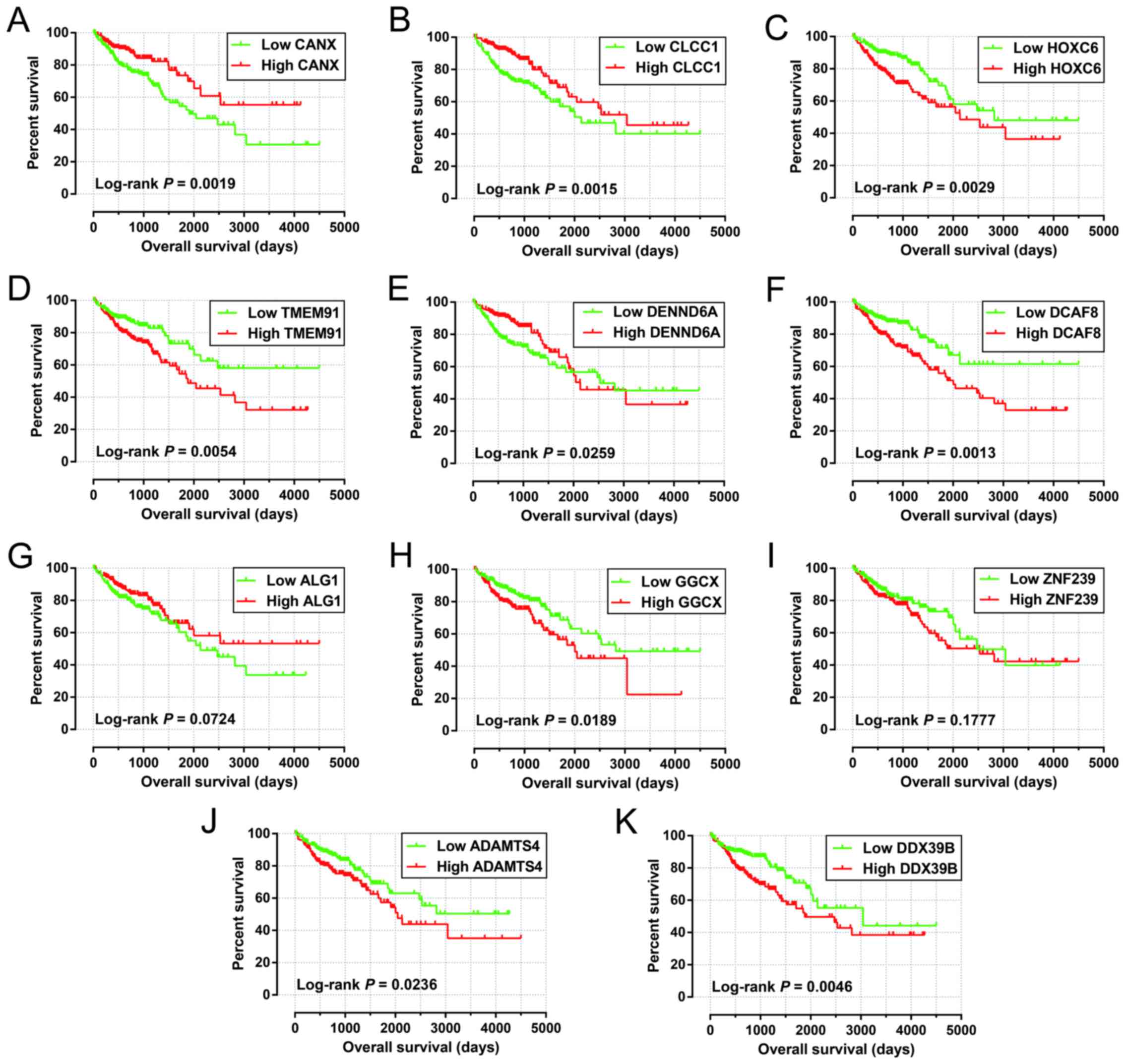 | Figure 8.Survival analysis of the target genes
significantly associated with COAD OS. The order of Kaplan-Meier
curves of the top five significant target genes are as follows:
CANX (A), CLCC1 (B), HOXC6 (C), TMEM91
(D), DENND6A (E), DCAF8 (F), ALG1 (G),
GGCX (H), ZNF239 (I), ADAMTS4 (J), and
DDX39B (K). OS, overall survival; COAD, colon
adenocarcinoma. |
 | Table IV.Multivariate survival analysis
results of the prognostic-related miRNA target genes. |
Table IV.
Multivariate survival analysis
results of the prognostic-related miRNA target genes.
| ID |
P-valuea | HR | Low 95% CI | High 95% CI |
|---|
| CANX | 0.002 | 0.508 | 0.331 | 0.779 |
| CLCC1 | 0.004 | 0.529 | 0.345 | 0.811 |
| HOXC6 | 0.008 | 1.765 | 1.158 | 2.690 |
| TMEM91 | 0.010 | 1.754 | 1.144 | 2.689 |
| DENND6A | 0.013 | 0.586 | 0.384 | 0.895 |
| DCAF8 | 0.017 | 1.710 | 1.102 | 2.651 |
| ALG1 | 0.019 | 0.605 | 0.397 | 0.921 |
| GGCX | 0.025 | 1.609 | 1.061 | 2.441 |
| ZNF239 | 0.027 | 1.606 | 1.055 | 2.446 |
| ADAMTS4 | 0.030 | 1.587 | 1.045 | 2.409 |
| DDX39B | 0.033 | 1.584 | 1.038 | 2.418 |
Discussion
TCGA uses a genome-wide approach to reveal the
genetic characteristics of cancers, and these datasets are open
access (10,25). Numerous previous studies have used
the TCGA dataset to screen for diagnostic and prognostic biomarkers
for several cancers including COAD (26–28).
Wang et al identified eight differentially expressed miRNAs
as potential diagnostic biomarkers for COAD by comparing tumor and
adjacent non-tumor tissues from TCGA using a genome-wide screening
approach (26). Yang et al
identified and validated several miRNAs (miR-15b, miR-215, miR-145,
miR-192, and let-7g) that are significantly correlated with
progression-free survival and/or OS in patients with COAD (27). Jacob et al identified a
16-miRNA signature as an independent biomarker of recurrence in
patients with stage II and III COAD using a LASSO regression
analysis (28). However, these
studies did not fully examine the COAD miRNA dataset of TCGA. The
present study, on the other hand, used the survival package
to perform a multivariate survival analysis of each miRNA
associated with COAD, and then constructed a prognostic signature,
using the step function to screen for the optimum
combination of these independent prognostic miRNAs. In addition, we
used the prognostic signature to construct a nomogram, and explored
its efficacy for determining individualized prognostic scores.
In the present study, we identified a 10-miRNA
prognostic signature for COAD prognosis prediction. Among the
miRNAs identified, seven (hsa-mir-891a, hsa-mir-216a,
hsa-mir-92a-1, hsa-mir-92a-2, hsa-mir-210, hsa-mir-940, and
hsa-mir-887) were previously reported to have crucial roles in
cancer. Of these seven miRNAs, hsa-mir-891a, hsa-mir-92a-1,
hsa-mir-92a-2, and hsa-mir-887 were analyzed in studies for their
potential role in cancer. Ye et al reported that
hsa-mir-891a is overexpressed in the exosomes of human
nasopharyngeal carcinoma (NPC) sera or cells, and its involvement
in the mitogen-activated protein kinase signaling pathway affects
cell proliferation and differentiation (29). Previous studies have shown that
hsa-mir-92a-1 is upregulated in prostate cancer and esophageal
cancer by analyzing the miRNA-seq dataset from TCGA, and may have
potential clinical applications in cancer diagnosis (30,31). A
six miRNA expression-based prognostic signature constructed by Xiao
et al, including hsa-mir-92a-1, performed well in prostate
cancer prognosis prediction (30).
Another miRNA belonging to the hsa-mir-92a cluster, hsa-mir-92a-2,
was found to be markedly upregulated in the tumor tissues of small
cell lung cancer (SCLC) patients with chemoresistance, compared
with patients without chemoresistance. These authors also observed
that higher tumor miR-92a-2 levels are significantly associated
with chemoresistance and prognosis in patients with SCLC (32). miR-887-5p expression was found to be
markedly higher in the sera of endometrial cancer patients than in
those of healthy subjects, and may serve as a potential diagnostic
biomarker for endometrial cancer (33).
The involvement of miR-216a in tumorigenesis was
reported previously; however, miR-216a has different functions in
various types of cancer and can act either as a tumor suppressor or
as an oncogenic miRNA (34–41). The tumor suppressor role of miR-216a
was observed in multiple types of cancer including CRC, non-small
cell lung cancer (NSCLC), oral squamous cell carcinoma, and
pancreatic cancer (PC), and it is downregulated in these cancer
tissues. Overexpression of miR-216a reduced the migration and
invasion of CRC cells in vitro, and inhibited xenograft
tumor metastasis in vivo (35). In addition, low expression of
miR-216a in NSCLC tumor tissues was found to be significantly
associated with poor OS (36).
However, a study by Xia et al demonstrated an opposite role
of miR-216a in hepatocellular carcinoma (HCC), showing that
miR-216a is upregulated in HCC tumor tissue samples and its
upregulation is associated with tumor recurrence (34). miR-216a was identified as a
prognostic biomarker for HCC recurrence, and high expression of
miR-216a in HCC tumor tissues was demonstrated to be significantly
correlated with poor disease-free survival (34). The present findings were consistent
with those of previous studies, as we showed that high expression
of miR-216a in CRC tumor tissues was significant associated with
poor OS. Therefore, the specific role of miR-216a in different
cancers needs further confirmation.
Several previous studies reported that miR-210 is a
marker of hypoxia and it is upregulated in cells with low oxygen
(42). Hypoxia induces the
dysregulation of several miRNAs, which in turn increase the
adaptive response to low oxygen in tumors (42,43).
miR-210 expression is increased in CRC tumor tissues (44,45)
and in hypoxic CRC cells (45–47).
Hypoxia-induced upregulation of miR-210 was found to promote the
self-renewal capacity of colon tumor-initiating cells by repressing
iron-sulfur cluster assembly enzymes and by inducing lactate
production (45), and autophagy was
demonstrated to contribute to the reduction in radiosensitivity in
the hypoxic environment mediated by the hypoxia-inducible factor
1α/miR-210/B-cell lymphoma 2 pathway in CRC (47). Chen et al reported that the
upregulation of miR-210 in CRC after surgery and chemotherapy may
indicate local recurrence, distant metastasis and poor prognosis
(44). The results of the present
study support previous reports by showing that high expression of
miR-210 was significantly associated with poor OS in COAD.
Furthermore, previous studies also suggested miR-210 as a
prognostic biomarker in multiple cancers, and overexpression of
miR-210 is significantly associated with poor clinical outcomes
(48), including in HCC (49), breast cancer (50–53),
glioma (54,55), and pediatric osteosarcoma (56). However, the potential roles of
miR-210 in cancer are complex, and overexpression of miR-210
predicts a better prognosis in lung cancer (57) and renal cancer (58). In addition, dysregulation of miR-210
shows a potential diagnostic value in cancers, as miR-210 is
upregulated in HCC (49), glioma
(54,55), renal cancer (58), and pediatric osteosarcoma (56) tumor tissues.
Another miRNA, hsa-mir-940, was identified
previously for its involvement in cancer. It acts as a tumor
suppressor miRNA in multiple cancers including NPC (59), HCC (60,61),
ovarian cancer (OC) (62,63), prostate cancer (64), triple-negative breast cancer (TNBC)
(65), and pancreatic ductal
adenocarcinoma (PDAC) (66).
However, hsa-mir-940 also plays an oncogenic role in gastric cancer
(GC) (67) and pancreatic carcinoma
(JF305 and SW1990 cell lines) (68). Expression of hsa-mir-940 is markedly
downregulated in HCC (60,61), prostate cancer (64), TNBC (65), and PDAC (66) tumor tissues, as well as in GC serum
(69). However, hsa-mir-940
upregulation was also reported in GC tumor tissues (67) and PC salivary samples (70). Furthermore, hsa-mir-940 showed a
good performance as a prognostic marker in HCC (60,61),
OC (63), PDAC (66), and GC (67). High expression of hsa-mir-940 is
significantly associated with better clinical outcomes in HCC
(60,61), OC (63), and PDAC (66), whereas it predicts a poor OS and
recurrence-free survival in GC (67).
The present study had several limitations. First,
the clinical parameters downloaded from TCGA database were not
comprehensive, and we were unable to perform a comprehensive
evaluation of the risk scores model. Second, there was no
additional validation cohort in this study; therefore, an extra
validation cohort is needed to confirm our results.
Despite these limitations, the present study
constructed a 10-miRNA expression-based prognostic signature that
may serve as an independent indicator of COAD OS, and it performed
better than other traditional clinicopathological parameters. We
also assessed the potential functions of these miRNAs using GO and
KEGG enrichment analysis and identified the potential roles of
their target genes in COAD prognosis. These results may improve our
understanding of the role of miRNAs in COAD prognosis, and may have
potential clinical application value in COAD prognosis monitoring
and for guiding treatment strategy selection.
In conclusion, in the present study, we screened the
genome-wide miRNA-seq data of COAD from TCGA and identified a
10-miRNA expression-based signature that may serve as an
independent indicator of COAD prognosis. However, the present
findings require further verification in future studies.
Acknowledgements
The authors also thank the contributors of the TCGA
(https://portal.gdc.cancer.gov/) for
sharing their data on open access. In addition, we also would like
to acknowledge the helpful comments on this paper received from our
reviewers.
Funding
The present study was supported in part by the
International Communication of Guangxi Medical University Graduate
Education, the Self-Raised Scientific Research Fund of the Health
and Family Planning Commission of the Guangxi Zhuang Autonomous
Region (grant no. Z2015198) and the Nanning Scientific Research and
Technology Development Project (Key Research and Development Plan;
grant no. 20173018-3).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable request.
All raw miRNA-seq and RNA-seq data of COAD, which include into
current study, can be downloaded from TCGA (https://portal.gdc.cancer.gov/).
Authors' contributions
HTW and ENG designed this manuscript; HTW, ENG, XWL,
LSC, JlW, MN and CL conducted and further performed the study,
processed and analyzed the data. HTW wrote this manuscript. All
authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Since all datasets of COAD included in the present
study were downloaded from TCGA, additional approval by an Ethics
Committee was not needed.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
Professor Hao-Tang Wei: ORCID ID: https://orcid.org/0000-0003-2977-9866.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA:
Cancer J Clin. 65:87–108. 2015.PubMed/NCBI
|
|
2
|
McGuire S: World cancer report 2014.
Geneva, Switzerland: World Health Organization, International
Agency for Research on Cancer, WHO press, 2015. Adv Nutr.
7:418–419. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in china,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zeng H, Zheng R, Guo Y, Zhang S, Zou X,
Wang N, Zhang L, Tang J, Chen J, Wei K, et al: Cancer survival in
China, 2003–2005: A population-based study. Int J Cancer.
136:1921–1930. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Towler BP, Jones CI and Newbury SF:
Mechanisms of regulation of mature miRNAs. Biochem Soc Trans.
43:1208–1214. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin S and Gregory RI: MicroRNA biogenesis
pathways in cancer. Nat Rev Cancer. 15:321–333. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Strubberg AM and Madison BB: MicroRNAs in
the etiology of colorectal cancer: Pathways and clinical
implications. Dis Model Mech. 10:197–214. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tomczak K, Czerwinska P and Wiznerowicz M:
The Cancer Genome Atlas (TCGA): An immeasurable source of
knowledge. Contemp Oncol. 19:A68–A77. 2015.
|
|
9
|
Cancer Genome Atlas Research Network, .
Weinstein JN, Collisson EA, Mills GB, Shaw KR, Ozenberger BA,
Ellrott K, Shmulevich I, Sander C and Stuart JM: The cancer genome
atlas pan-cancer analysis project. Nat Genet. 45:1113–1120. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cancer Genome Atlas Network: Comprehensive
molecular characterization of human colon and rectal cancer.
Nature. 487:330–337. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Anders S and Huber W: Differential
expression analysis for sequence count data. Genome Biol.
11:R1062010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liao X, Huang K, Huang R, Liu X, Han C, Yu
L, Yu T, Yang C, Wang X and Peng T: Genome-scale analysis to
identify prognostic markers in patients with early-stage pancreatic
ductal adenocarcinoma after pancreaticoduodenectomy. OncoTargets
Ther. 10:4493–4506. 2017. View Article : Google Scholar
|
|
13
|
Zhou M, Zhao H, Wang Z, Cheng L, Yang L,
Shi H, Yang H and Sun J: Identification and validation of potential
prognostic lncRNA biomarkers for predicting survival in patients
with multiple myeloma. J Exp Clin Cancer Res. 34:1022015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang R, Liao X and Li Q: Identification
and validation of potential prognostic gene biomarkers for
predicting survival in patients with acute myeloid leukemia.
OncoTargets Ther. 10:5243–5254. 2017. View Article : Google Scholar
|
|
15
|
Lossos IS, Czerwinski DK, Alizadeh AA,
Wechser MA, Tibshirani R, Botstein D and Levy R: Prediction of
survival in diffuse large-B-cell lymphoma based on the expression
of six genes. N Engl J Med. 350:1828–1837. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Heagerty PJ and Zheng Y: Survival model
predictive accuracy and ROC curves. Biometrics. 61:92–105. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 4:doi: 10.7554/eLife.05005. 2015. View Article : Google Scholar
|
|
18
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang X: Improving microRNA target
prediction by modeling with unambiguously identified
microRNA-target pairs from CLIP-ligation studies. Bioinformatics.
32:1316–1322. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wong N and Wang X: miRDB: An online
resource for microRNA target prediction and functional annotations.
Nucleic Acids Res. 43:D146–D152. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chou CH, Shrestha S, Yang CD, Chang NW,
Lin YL, Liao KW, Huang WC, Sun TH, Tu SJ, Lee WH, et al: miRTarBase
update 2018: A resource for experimentally validated
microRNA-target interactions. Nucleic Acids Res. 46:D296–D302.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hsu SD, Lin FM, Wu WY, Liang C, Huang WC,
Chan WL, Tsai WT, Chen GZ, Lee CJ, Chiu CM, et al: miRTarBase: A
database curates experimentally validated microRNA-target
interactions. Nucleic Acids Res. 39:D163–D169. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
da Huang W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
da Huang W, Sherman BT and Lempicki RA:
Bioinformatics enrichment tools: Paths toward the comprehensive
functional analysis of large gene lists. Nucleic Acids Res.
37:1–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cancer Genome Atlas Research Network.
Electronic address simplewheeler@bcm.edu; Cancer
Genome Atlas Research Network: Comprehensive and integrative
genomic characterization of hepatocellular carcinoma. Cell.
169(1327–1341): e13232017.
|
|
26
|
Wang JY, Wang CL, Wang XM and Liu FJ:
Comprehensive analysis of microRNA/mRNA signature in colon
adenocarcinoma. Eur Rev Med Pharmacol Sci. 21:2114–2129.
2017.PubMed/NCBI
|
|
27
|
Yang J, Ma D, Fesler A, Zhai H,
Leamniramit A, Li W, Wu S and Ju J: Expression analysis of microRNA
as prognostic biomarkers in colorectal cancer. Oncotarget.
8:52403–52412. 2016.PubMed/NCBI
|
|
28
|
Jacob H, Stanisavljevic L, Storli KE,
Hestetun KE, Dahl O and Myklebust MP: Identification of a
sixteen-microRNA signature as prognostic biomarker for stage II and
III colon cancer. Oncotarget. 8:87837–87847. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ye SB, Li ZL, Luo DH, Huang BJ, Chen YS,
Zhang XS, Cui J, Zeng YX and Li J: Tumor-derived exosomes promote
tumor progression and T-cell dysfunction through the regulation of
enriched exosomal microRNAs in human nasopharyngeal carcinoma.
Oncotarget. 5:5439–5452. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xiaoli Z, Yawei W, Lianna L, Haifeng L and
Hui Z: Screening of target genes and regulatory function of mirnas
as prognostic indicators for prostate cancer. Med Sci Monit.
21:3748–3759. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao JY, Wang F, Li Y, Zhang XB, Yang L,
Wang W, Xu H, Liu DZ and Zhang LY: Five mirnas considered as
molecular targets for predicting esophageal cancer. Med Sci Monit.
21:3222–3230. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ranade AR, Cherba D, Sridhar S, Richardson
P, Webb C, Paripati A, Bowles B and Weiss GJ: MicroRNA 92a-2*: A
biomarker predictive for chemoresistance and prognostic for
survival in patients with small cell lung cancer. J Thorac Oncol.
5:1273–1278. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jiang Y, Wang N, Yin D, Li YK, Guo L, Shi
LP and Huang X: Changes in the expression of serum MiR-887-5p in
patients with endometrial cancer. Int J Gynecolo Cancer.
26:1143–1147. 2016. View Article : Google Scholar
|
|
34
|
Xia H, Ooi LL and Hui KM:
MicroRNA-216a/217-induced epithelial-mesenchymal transition targets
PTEN and SMAD7 to promote drug resistance and recurrence of liver
cancer. Hepatology. 58:629–641. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang D, Zhao L, Shen Q, Lv Q, Jin M, Ma
H, Nie X, Zheng X, Huang S, Zhou P, et al: Down-regulation of
KIAA1199/CEMIP by miR-216a suppresses tumor invasion and metastasis
in colorectal cancer. Int J Cancer. 140:2298–2309. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang RT, Xu M, Xu CX, Song ZG and Jin H:
Decreased expression of miR216a contributes to non-small-cell lung
cancer progression. Clin Cancer Res. 20:4705–4716. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li L and Ma HQ: MicroRNA-216a inhibits the
growth and metastasis of oral squamous cell carcinoma by targeting
eukaryotic translation initiation factor 4B. Mol Med Rep.
12:3156–3162. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lu J, Li X, Wang F, Guo Y, Huang Y, Zhu H,
Wang Y, Lu Y and Wang Z: YB-1 expression promotes pancreatic cancer
metastasis that is inhibited by microRNA-216a. Exp Cell Res.
359:319–326. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang Y, Tang X, Shi M, Wen C and Shen B:
MiR-216a decreases MALAT1 expression, induces G2/M arrest and
apoptosis in pancreatic cancer cells. Biochem Biophys Res Commun.
483:816–822. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hou BH, Jian ZX, Cui P, Li SJ, Tian RQ and
Ou JR: miR-216a may inhibit pancreatic tumor growth by targeting
JAK2. FEBS Lett. 589:2224–2232. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang S, Chen X and Tang M: MicroRNA-216a
inhibits pancreatic cancer by directly targeting Janus kinase 2.
Oncol Rep. 32:2824–2830. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bavelloni A, Ramazzotti G, Poli A, Piazzi
M, Focaccia E, Blalock W and Faenza I: MiRNA-210: A current
overview. Anticancer Res. 37:6511–6521. 2017.PubMed/NCBI
|
|
43
|
Huang X and Zuo J: Emerging roles of
miR-210 and other non-coding RNAs in the hypoxic response. Acta
Biochim Biophys Sin. 46:220–232. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen J, Wang W, Zhang Y, Chen Y and Hu T:
Predicting distant metastasis and chemoresistance using plasma
miRNAs. Med Oncol. 31:7992014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ullmann P, Qureshi-Baig K, Rodriguez F,
Ginolhac A, Nonnenmacher Y, Ternes D, Weiler J, Gäbler K, Bahlawane
C, Hiller K, et al: Hypoxia-responsive miR-210 promotes
self-renewal capacity of colon tumor-initiating cells by repressing
ISCU and by inducing lactate production. Oncotarget. 7:65454–65470.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Nijhuis A, Thompson H, Adam J, Parker A,
Gammon L, Lewis A, Bundy JG, Soga T, Jalaly A, Propper D, et al:
Remodelling of microRNAs in colorectal cancer by hypoxia alters
metabolism profiles and 5-fluorouracil resistance. Hum Mol Genet.
26:1552–1564. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sun Y, Xing X, Liu Q, Wang Z, Xin Y, Zhang
P, Hu C and Liu Y: Hypoxia-induced autophagy reduces
radiosensitivity by the HIF-1alpha/miR-210/Bcl-2 pathway in colon
cancer cells. Int J Oncol. 46:750–756. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang J, Zhao J, Shi M, Ding Y, Sun H, Yuan
F and Zou Z: Elevated expression of miR-210 predicts poor survival
of cancer patients: A systematic review and meta-analysis. PLoS
One. 9:e892232014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhan M, Li Y, Hu B, He X, Huang J, Zhao Y,
Fu S and Lu L: Serum microRNA-210 as a predictive biomarker for
treatment response and prognosis in patients with hepatocellular
carcinoma undergoing transarterial chemoembolization. J Vasc Interv
Radiol. 25(1279–1287): e12712014.
|
|
50
|
Li Y, Ma X, Zhao J, Zhang B, Jing Z and
Liu L: microRNA-210 as a prognostic factor in patients with breast
cancer: Meta-analysis. Cancer Biomark. 13:471–481. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hong L, Yang J, Han Y, Lu Q, Cao J and
Syed L: High expression of miR-210 predicts poor survival in
patients with breast cancer: A meta-analysis. Gene. 507:135–138.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Camps C, Buffa FM, Colella S, Moore J,
Sotiriou C, Sheldon H, Harris AL, Gleadle JM and Ragoussis J:
hsa-miR-210 is induced by hypoxia and is an independent prognostic
factor in breast cancer. Clin Cancer Res. 14:1340–1348. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Toyama T, Kondo N, Endo Y, Sugiura H,
Yoshimoto N, Iwasa M, Takahashi S, Fujii Y and Yamashita H: High
expression of microRNA-210 is an independent factor indicating a
poor prognosis in Japanese triple-negative breast cancer patients.
Jpn J Clin Oncol. 42:256–263. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lai NS, Dong QS, Ding H, Miao ZL and Lin
YC: MicroRNA-210 overexpression predicts poorer prognosis in glioma
patients. J Clin Neurosci. 21:755–760. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lai NS, Wu DG, Fang XG, Lin YC, Chen SS,
Li ZB and Xu SS: Serum microRNA-210 as a potential noninvasive
biomarker for the diagnosis and prognosis of glioma. Br J Cancer.
112:1241–1246. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Cai H, Lin L, Cai H, Tang M and Wang Z:
Prognostic evaluation of microRNA-210 expression in pediatric
osteosarcoma. Med Oncol. 30:4992013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Eilertsen M, Andersen S, Al-Saad S,
Richardsen E, Stenvold H, Hald SM, Al-Shibli K, Donnem T, Busund LT
and Bremnes RM: Positive prognostic impact of miR-210 in non-small
cell lung cancer. Lung Cancer. 83:272–278. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
McCormick RI, Blick C, Ragoussis J,
Schoedel J, Mole DR, Young AC, Selby PJ, Banks RE and Harris AL:
miR-210 is a target of hypoxia-inducible factors 1 and 2 in renal
cancer, regulates ISCU and correlates with good prognosis. Br J
Cancer. 108:1133–1142. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ma J, Sun F, Li C, Zhang Y, Xiao W, Li Z,
Pan Q, Zeng H, Xiao G, Yao K, et al: Depletion of intermediate
filament protein Nestin, a target of microRNA-940, suppresses
tumorigenesis by inducing spontaneous DNA damage accumulation in
human nasopharyngeal carcinoma. Cell Death Dis. 5:e13772014.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ding D, Zhang Y, Yang R, Wang X, Ji G, Huo
L, Shao Z and Li X: miR-940 Suppresses tumor cell invasion and
migration via regulation of CXCR2 in hepatocellular carcinoma.
Biomed Res Int. 2016:76183422016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yuan B, Liang Y, Wang D and Luo F: MiR-940
inhibits hepatocellular carcinoma growth and correlates with
prognosis of hepatocellular carcinoma patients. Cancer Sci.
106:819–824. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wang F, Wang Z, Gu X and Cui J: miR-940
Upregulation suppresses cell proliferation and induces apoptosis by
targeting PKC-δ in ovarian cancer OVCAR3 cells. Oncol Res.
25:107–114. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Rashed MH, Kanlikilicer P,
Rodriguez-Aguayo C, Pichler M, Bayraktar R, Bayraktar E, Ivan C,
Filant J, Silva A, Aslan B, et al: Exosomal miR-940 maintains
SRC-mediated oncogenic activity in cancer cells: A possible role
for exosomal disposal of tumor suppressor miRNAs. Oncotarget.
8:20145–20164. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Rajendiran S, Parwani AV, Hare RJ,
Dasgupta S, Roby RK and Vishwanatha JK: MicroRNA-940 suppresses
prostate cancer migration and invasion by regulating MIEN1. Mol
Cancer. 13:2502014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Hou L, Chen M, Yang H, Xing T, Li J, Li G,
Zhang L, Deng S, Hu J, Zhao X, et al: MiR-940 inhibited cell growth
and migration in triple-negative breast cancer. Med Sci Monit.
22:3666–3672. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Song B, Zhang C, Li G, Jin G and Liu C:
MiR-940 inhibited pancreatic ductal adenocarcinoma growth by
targeting MyD88. Cell Physiol Biochem. 35:1167–1177. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Liu X, Ge X, Zhang Z, Zhang X, Chang J, Wu
Z, Tang W, Gan L, Sun M and Li J: MicroRNA-940 promotes tumor cell
invasion and metastasis by downregulating ZNF24 in gastric cancer.
Oncotarget. 6:25418–25428. 2015.PubMed/NCBI
|
|
68
|
Yang HW, Liu GH, Liu YQ, Zhao HC, Yang Z,
Zhao CL, Zhang XF and Ye H: Over-expression of microRNA-940
promotes cell proliferation by targeting GSK3beta and sFRP1 in
human pancreatic carcinoma. Biomed Pharmacother. 83:593–601. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Liu X, Kwong A, Sihoe A and Chu KM: Plasma
miR-940 may serve as a novel biomarker for gastric cancer. Tumour
Biol. 37:3589–3597. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Xie Z, Yin X, Gong B, Nie W, Wu B, Zhang
X, Huang J, Zhang P, Zhou Z and Li Z: Salivary microRNAs show
potential as a noninvasive biomarker for detecting resectable
pancreatic cancer. Cancer Prev Res. 8:165–173. 2015. View Article : Google Scholar
|