Introduction
Inhibitors of apoptosis proteins (IAPs) are involved
in cell death, migration and cell cycle (1). They exhibit an important role in
maintaining the balance between cell death and cell growth
(2,3). IAPs may effectively suppress cell
apoptosis and promote cell growth (4). Additionally, IAP deregulation may
accelerate cell canceration and provide a new target for anticancer
therapy (5).
Following identification of the first human IAP
protein, neuronal apoptosis inhibitory protein (NAIP) (6), eight proteins of IAP family have been
identified: protein (NAIP/BIRC1/NLRB), cellular IAP1/human
IAP2/BIRC2, cellular IAP2/human IAP1/BIRC3, X-linked IAP
(XIAP)/BIRC4, survivin/BIRC5, baculoviral IAP repeat-containing
ubiquitin-conjugating enzyme/apollon/BIRC6,
Livin/melanoma-IAP/BIRC7/KIAP and inhibitory of apoptosis
proteins-like protein-2 (ILP-2)/testis-specific IAP
(Ts-IAP)/hILP-2/BIRC8 (5,7,8).
ILP-2 was initially detected in the human testis
(9,10) and in our previous study, we showed
that ILP-2 is a novel serological biomarker for breast cancer
(8). In this study, we identified
whether ILP-2 led to breast cancer progression. We analyzed the
expression levels of ILP-2 in breast cancer tissue samples by
immunohistochemistry, followed by detection of ILP-2 expression in
breast cancer cell lines through western blot analysis. We further
investigated the influence of ILP-2 in breast cancer growth via MTT
assay, scratch assay and acridine orange/ethidium bromide
(AO/EB)double-staining analysis in ascertaining the specific role
of ILP-2 in breast cancer. Furthermore, we knocked down ILP-2 by
siRNA to elucidate its role in breast cancer.
Materials and methods
Statement of Ethics
The present study was approved by Jishou University
Ethics Committee (Jishou, China). All participated patients
received and approved the written informed consent before joining
the study.
Patient samples
Fifty-nine pathological samples were provided by the
First Affiliated Hospital, College of Medical Science, Jishou
University (Jishou, China). These samples included 35 breast cancer
and 24 galactophore hyperplasia tissues. The average age of breast
cancer patients and galactophore hyperplasia patients was 47 and 32
years, respectively. These samples were collected from July 2013 to
October 2013. Breast cancer samples were collected from stage II
patients diagnosed according to cancer pathology standards, which
are scored on the formation of the gland tumor, the polymorphism of
nucleus and the fission count, and designated the infiltrating
ductal carcinoma for level I on the total score 3–5, level II on
6–7 and level III on 8–9. In addition, galactophore hyperplasia
samples were from breast fibroadenoma patients.
Immunohistochemistry
Tissue paraffin-embedded blocks were cut into 4
µm-thick slices and pasted on the glass slide. These blocks were
dewaxed by dimethylbenzene and dehydrated in graded series of
alcohol. Peroxidase was inactivated by incubation with 3% hydrogen
peroxide at 25°C for 10 min. Antigens were retrieved by heating a
citrate-buffered solution in the microwave for 10 min. Tissue
sections were incubated with 5% bovine serum albumin (BSA) for 13
min at room temperature and then incubated with ILP-2 (rabbit IgG;
dilution 1:500; cat. no. sc-130107; Santa Cruz Biotechnology, Inc.,
CA, USA) overnight at 4°C. Slices were visualized with
3,3′-diaminobenzidine kit (Beyotime Institute of Biotechnology,
Shanghai, China) for 5 min, and then stained by hematoxylin and
examined under light microscopy.
Western blot analysis
HCC-1937, MX-1, MCF-7 and MCF-10A cell lines
(5×106 cells/cell line) were separately collected and
western blot analysis was performed. Briefly, total proteins were
extracted from cells with RIPA buffer (20 mM Tris, pH 7.5, 150 mM
NaCl, 1% Triton X-100, 0.1% SDS and 1% deoxycholate) containing
protease inhibitors. The total protein concentration was determined
using a BCA Protein Assay kit (Applygen Technologies Inc., Beijing,
China). Thirty micrograms of total protein was separated on a 10%
SDS-polyacrylamide gel and then transferred onto polyvinylidene
fluoride (PVDF) membranes (Merck KGaA, Darmstadt, Germany). The
membranes were blocked with TBST (Tris-buffered saline and
Tween-20) and 5% skim milk powder for 2 h, then incubated with
primary antibodies against ILP-2 (rabbit IgG, 1:1,000; cat. no.
Ab9664; Abcam, Cambrige, UK) and tubulin (rabbit IgG, 1:1,000; cat.
no. 11224-1-AP; Proteintech Group, Wuhan, China) overnight at 4°C.
On the second day, the membranes were washed by TBST and incubated
with a goat anti-rabbit immunoglobulin G-horseradish peroxidase
(IgG-HRP) (anti-rabbit IgG, 1:5,000; cat. no. ZB-2301; ZSGB-BIO,
Beijing, China) for 1 h at room temperature. An enhanced
chemiluminescence detection system (Super ECL Plus; Applygen
Technologies) was used to visualize immunoreactive proteins. The
protein band was visualized by chemical chemiluminescence imaging
system (Beijing Sage Creation Science Co., Ltd., Beijing, China).
The images were analyzed with ImageJ (National Institutes of
Health, Bethesda, MD, USA). Tubulin was used as an internal
control.
RNA interference (RNAi)
According to the manufacturer's instructions,
Invitrogen™ Lipofectamine 2000 (Thermo Fisher Scientific, Inc., MA,
USA) and small interfering RNA [siRNA-5, siRNA-3, siRNA-1519,
siRNA-1643 and negative control (NC)] (Shanghai GenePharma Co.,
Ltd., Shanghai, China) were separately gently mixed at a volume
ratio of 1:1 and stored for 20 min at room temperature. Mixed
Lipofectamine 2000 and small interfering RNA were added to MCF-7
cells in the logarithmic growth phase and cultivated in 5%
CO2 for 24 h at 37°C, wherein the small interfering RNA
had a final concentration of 50 nM. Interference effects on ILP-2
expression were analyzed via western blot analysis.
Mixed Lipofectamine 2000 and small interfering RNA
(siRNA-5, siRNA-3 and NC) were correspondingly added to the
HCC-1937, MX-1 and MCF-7 cells in the logarithmic growth phase and
cultivated in 5% CO2 for 24, 48 and 72 h at 37°C. Total
proteins were separately extracted. After determining protein
concentration using the BCA kit (Beyotime Institute of
Biotechnology, Shanghai, China), proteins were analyzed by western
blot analysis.
MTT assays
HCC-1937, MX-1 and MCF-7 cells in the logarithmic
phase were simultaneously digested with trypsin, re-suspended in
complete culture medium and arranged in four groups: The siRNA-5
group, the siRNA-3 group, the NC group and HCC-1937 or MX-1 or
MCF-7 cell group. Four groups of cells were separately seeded on
2×103 cells/well in three 96-well plates, wherein every
cell group was arranged in 6-wells, incubated in 5% CO2
for 24 h at 37°C and mixed with the small interfering RNA.
Following cultivation of the four cell groups for 24, 48 and 72 h,
10 µl of MTT (5 mg/ml) (Beijing Dingguo Biotechnology, Beijing,
China) was separately added to each well. The medium was discarded
4 h later and 100 µl dimethyl sulfoxide (DMSO; Shanghai
Pharmaceutical Group Co., Ltd., Shanghai, China) was added to each
well to stop the reaction. After vortexing for 10 min at room
temperature, optical density was measured at 490 nm using a
microplate reader (Biotek ELx800; BioTek Instruments, Winooski, VT,
USA).
Scratch assays
Five parallel lines were scratched on the back of a
6-well plate per well. Four groups of HCC-1937, MX-1 and MCF-7
cells in the logarithmic phase, analogous to MTT assay procedure,
were simultaneously seeded on 1×104 cells/well in a
6-well plate, cultivated in 5% CO2 for 24 h at 37°C,
mixed with small interfering RNA and incubated for 24 h. Three
vertical scratch wounds were separately made on parallel lines by a
sterile pipette tip in the four cell groups. Cells were cultivated
in a 5% CO2 at 37°C. Three scratch wound areas were
measured by an Olympus CKX41 inverted microscope (Olympus Corp.,
Tokyo, Japan) for 0, 24, 48 and 72 h. Experiments were
triplicated.
AO/EB double staining analysis
HCC-1937, MX-1 and MCF-7 cells in the logarithmic
growth phase were adhered to coverslips in the well of a 6-well
plate (1×104 cells/well) and cultivated in 5%
CO2 for 24 h at 37°C. Mixed small interfering RNA
(siRNA-5, siRNA-3 and NC) and Lipofectamine 2000 were
simultaneously added to the HCC-1937, MX-1 and MCF-7 cells and
subsequently cultivated in 5% CO2 for 24, 48 and 72 h at
37°C. Culture medium was removed and cells were washed with
phosphate-buffered saline (PBS) twice. Dye Reagents 1 and 2 were
mixed at a volume ratio of 1:1 and diluted by PBS at a ratio of
1:25. Diluted staining agent was dropped into breast cancer cells
and cultivated in the darkroom for 5 min at room temperature.
Coverslips were examined under an Olympus BX51 inverted
fluorescence microscope (Olympus Corp.) at an excitation/emission
wavelength of 498/516 nm.
Statistical analysis
Histograms were constructed by GraphPad Prism 5
(GraphPad Software, Inc., La Jolla, CA, USA). Statistical analysis
was performed by conducting χ2 test, t-test, one-way
ANOVA, or post hoc Bonferroni tests by SPSS statistics 17.0 (SPSS,
Inc., Chicago, IL, USA). A level of P<0.05 was considered
statistically significant.
Results
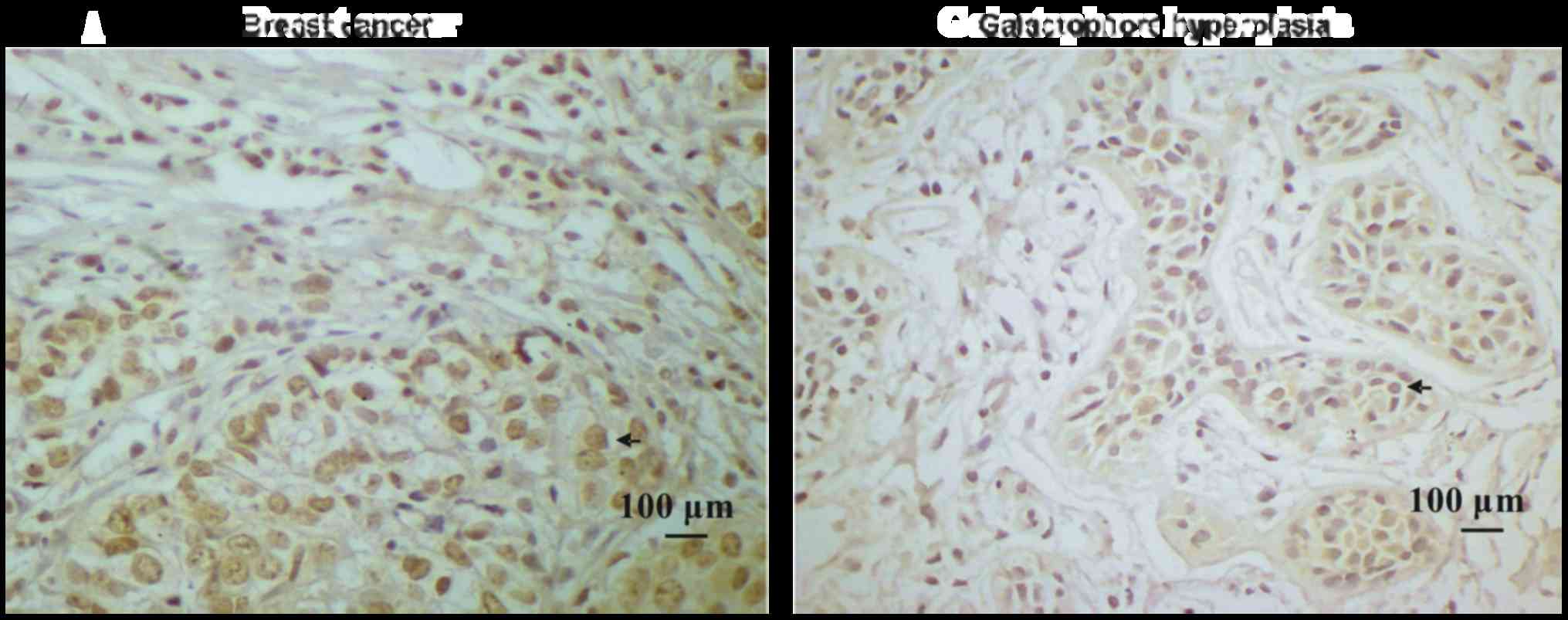
Breast cancer tissues have high ILP-2
expression
Paraffin-embedded blocks were analyzed using
immunohistochemical analysis to investigate ILP-2 protein
expression in the tissues. As shown in Fig. 1A and B, expression levels of ILP-2
in breast cancer tissues were significantly increased in comparison
to levels noted in the galactophore hyperplasia tissues
(P<0.05). In addition, positive rates of ILP-2 expression in
breast cancers were higher than that in the galactophore
hyperplasias (Table I)
(χ2=8.183, P<0.01). Our results indicate high ILP-2
expression levels in breast cancer tissues.
 | Table I.Immunohistochemical analysis of ILP-2
in breast tissues. |
Table I.
Immunohistochemical analysis of ILP-2
in breast tissues.
|
|
| Protein expression of
ILP-2 |
|
|---|
|
|
|
|
|
|---|
| Group | n | Positive | Negative | Positive rate
(%) |
|---|
| Breast cancer | 35 | 22 | 13 | 62.9a |
| Galactophore
hyperplasia | 24 | 6 | 18 | 25 |
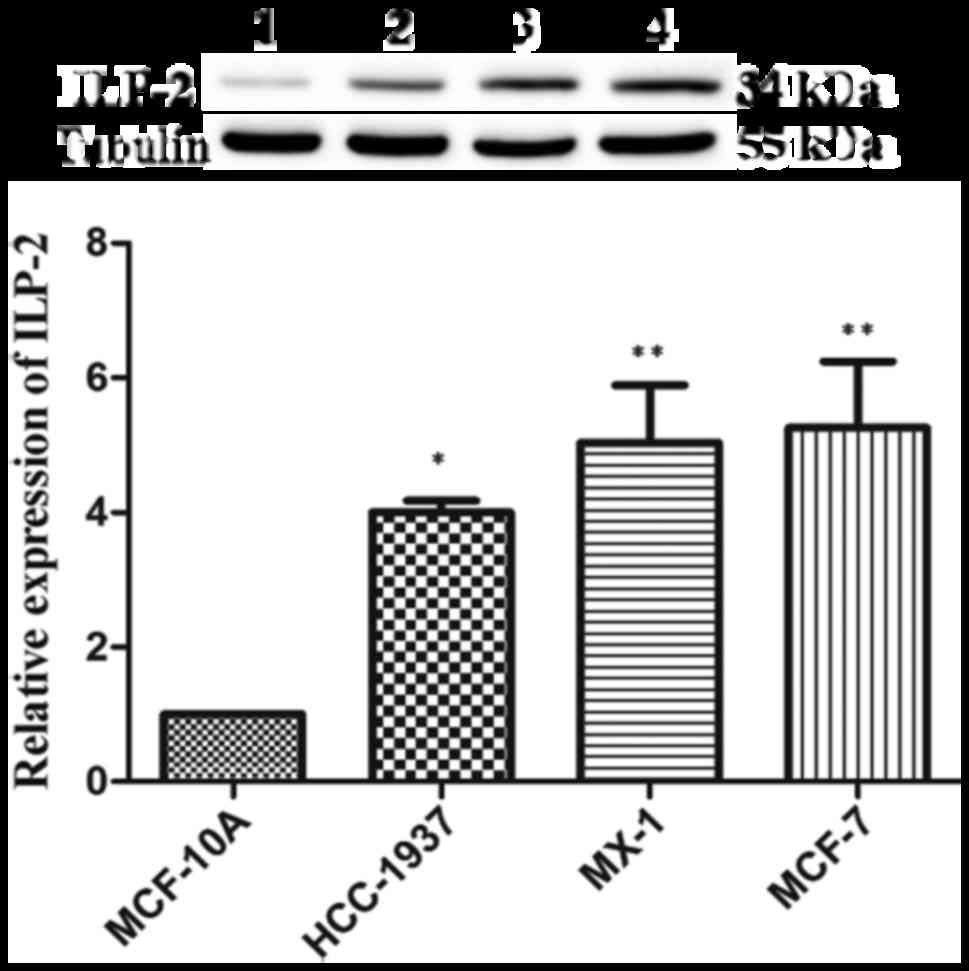
ILP-2 is highly expressed in breast
cancer cells
Western blot analysis was employed to analyze ILP-2
expression in the 4 breast cancer cell lines. As shown in Fig. 2, protein expression levels of ILP-2
in the breast cancer cell lines such as HCC-1937 (P<0.05), MX-1
(P<0.01) and MCF-7 (P<0.01) were significantly increased when
compared with that of the breast epithelial cell line MCF-10A.
These results corroborate the immunohistochemical results,
evidencing the highly expressed levels of ILP-2 breast cancer
cells.
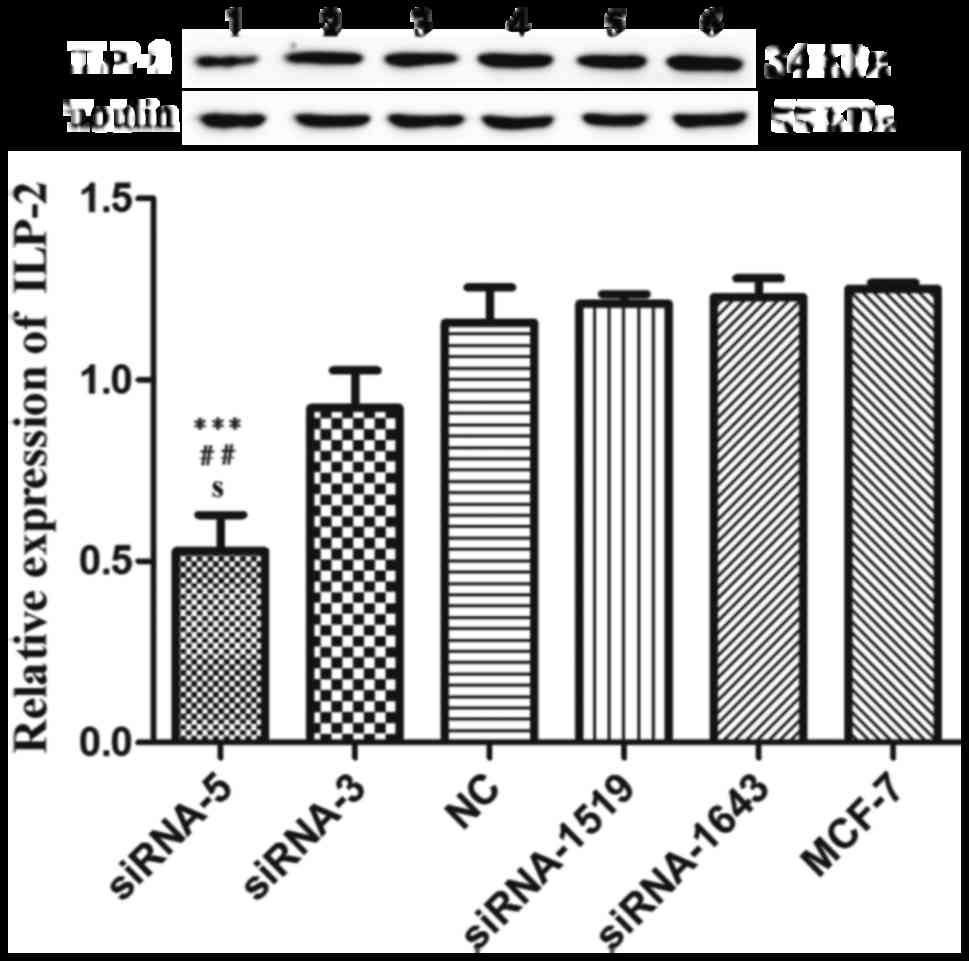
siRNA-5 sequence inhibits ILP-2
expression in breast cancer cell lines
We employed western blot analysis to analyze the
interference effect of five siRNAs on ILP-2 expression in the MCF-7
cell line. As shown in Fig. 3, when
ILP-2 was specifically knocked down by five siRNAs (siRNA-5,
siRNA-3, NC, siRNA-1519 and siRNA-1643), which follow the sequences
as depicted in Table II, ILP-2
expression was notably decreased in the siRNA-5 group (P<0.001)
in comparison to that of the MCF-7 group. These results indicate
that the siRNA-5 sequence show the highest interference efficiency
on ILP-2 expression.
 | Table II.siRNA sequences. |
Table II.
siRNA sequences.
| Name | Sense (5′-3′) | Antisense
(5′-3′) |
|---|
| siRNA-5 |
CUAUACGAAUGGGAUUUGATT |
UCAAAUCCCAUUCGUAUAGTT |
| siRNA-3 |
UGGUACAAACUACCAAGAATT |
UUCUUGGUAGUUUGUACCATT |
| siRNA-1519 |
GGUACAAACUACCAAGAAATT |
UUUCUUGGUAGUUUGUACCTT |
| siRNA-1643 |
GAGGAAAGAAUUCAAACAUTT |
AUGUUUGAAUUCUUUCCUCTT |
| Negative
control |
UUCUCCGAACGUGUCACGUTT |
ACGUGACACGUUCGGAGAATT |
As illustrated in Fig.
4, in the HCC-1937 cell line, the siRNA-5 group exhibited
significantly decreased ILP-2 expression similar to that of the
control HCC-1937 group (24 and 48 h, P<0.05; 72 h, P<0.01).
Likewise in the MX-1 cell line, the siRNA-5 group exhibited
significantly decreased ILP-2 expression at 48 h (P<0.001) and
72 h (P<0.05) when compared with that of the control MX-1 group.
In the MCF-7 cell line, ILP-2 expression in the siRNA-5 group was
significantly decreased at 48 h (P<0.05) and 72 h (P<0.01)
when compared with that of the MCF-7 group.
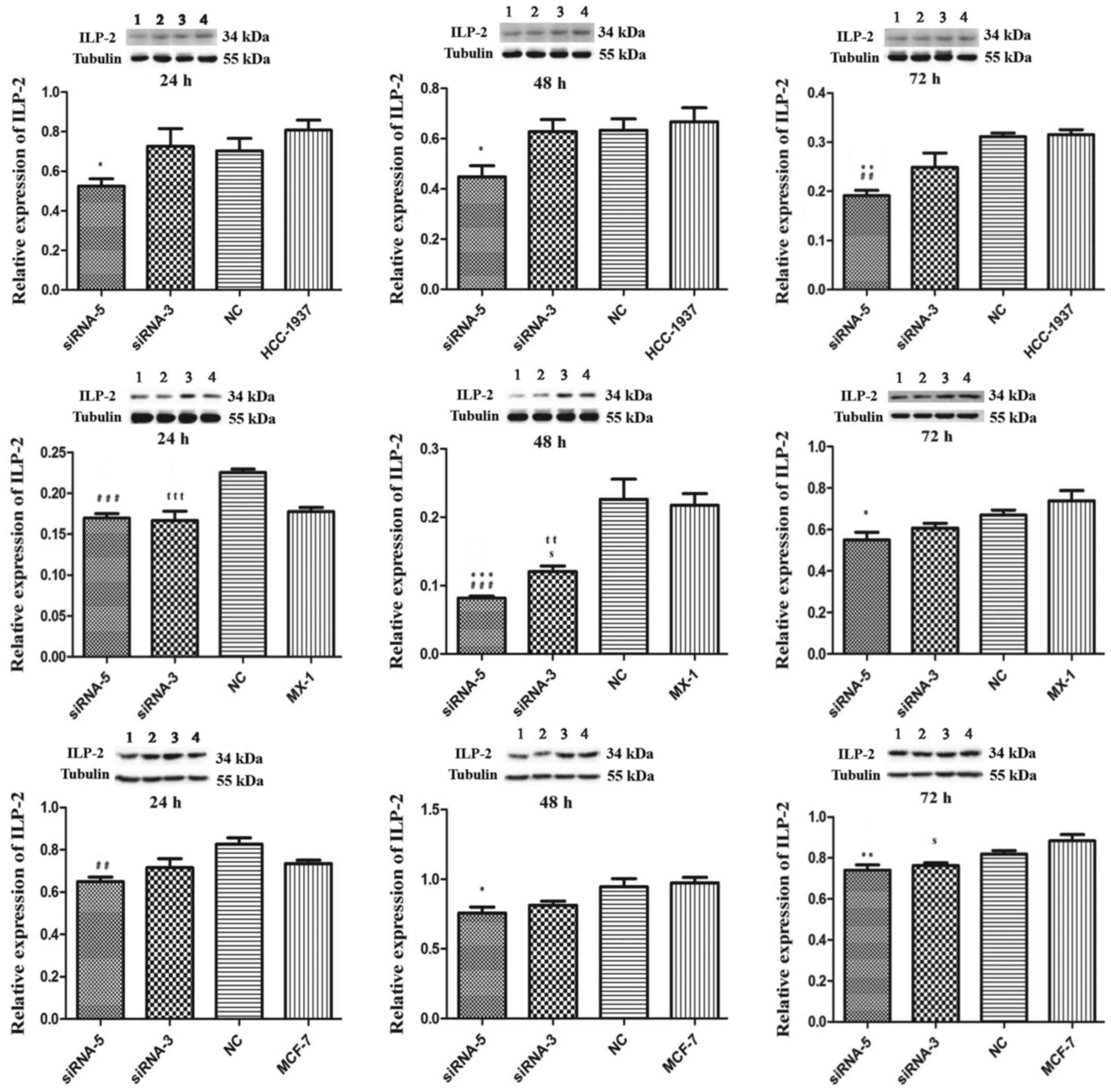 | Figure 4.Western blot analysis showing the
expression of ILP-2 in the HCC-1937, MX-1 and MCF-7 cell lines
following knockdown of ILP-2 for 24, 48 and 72 h. Lanes 1, 2, 3 and
4 separately indicate ILP-2 expressions in siRNA-5, siRNA-3, NC and
HCC-1937 or MX-1 or MCF-7 group. In regards to the HCC-1937 cell
line, expression of ILP-2 was significantly decreased in the
siRNA-5 group compared to the HCC-1937 group. *P<0.05,
**P<0.01 vs. the HCC-1937 group; ##P<0.01 vs. the
NC group. In regards to the MX-1 cell line, ILP–2 expression was
significantly decreased when compared to the MX-1 group following
the knockdown by the siRNA-5 sequence for 48 and 72 h. *P<0.05,
***P<0.001 vs. the MX-1 group; ###P<0.001 vs. the
NC group; ttP< 0.01, tttP< 0.001 vs.
the NC group; sP<0.05 vs. the MX-1group. In regards
to the MCF-7 cell line, ILP-2 expression was significantly
decreased when compared to MCF-7 group after knockdown by the
siRNA-5 sequence for 48 and 72 h. *P<0.05, **P<0.01 vs. the
MCF-7 group; ##P<0.01 vs. the NC group;
sP<0.05 vs. the MCF-7 group. Data are expressed as
mean ± SEM by ANOVA. Groups were compared by conducting Bonferroni
test; n=3. |
ILP-2 positively influences the cell
viability of breast cancer cell lines
MTT assays were performed to examine cell viability
when ILP-2 expression was knocked down by siRNA sequences for 24,
48 and 72 h in the HCC-1937, MX-1 and MCF-7 cell lines respectively
so as to determine the role of ILP-2 in breast cancer cell growth.
As shown in Fig. 5, cell viability
of the siRNA-5 groups was significantly reduced when compared to
that of the control group (Fig. 5A and
C, P<0.001; Fig. 5B, 24 and
48 h, P<0.001, 72 h, P<0.01), thus, indicating that ILP-2
positively promotes the growth of breast cancer cells.
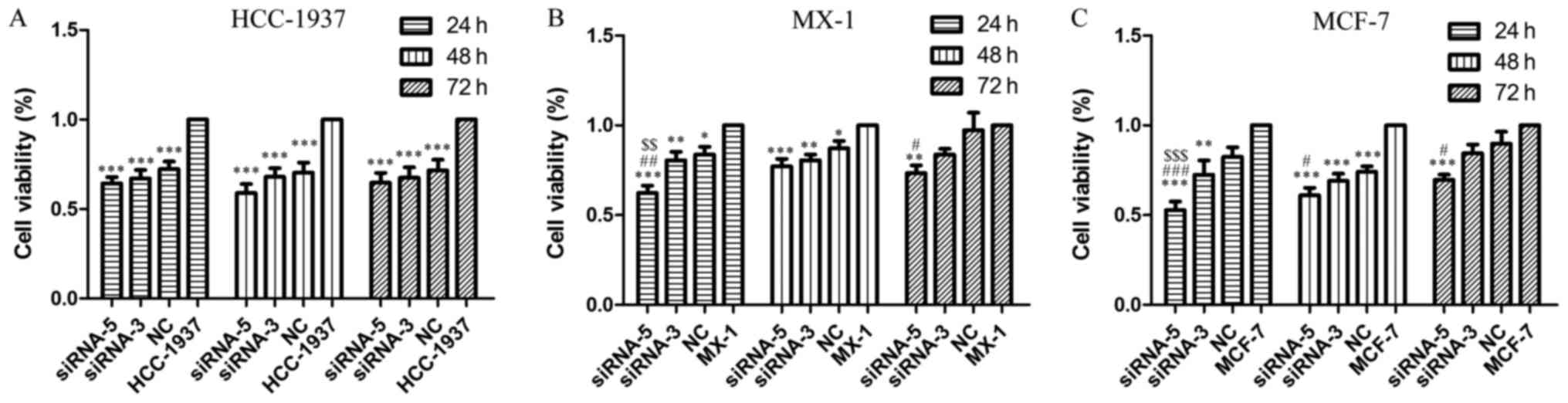 | Figure 5.MTT assay demonstrating the cell
viability of HCC-1937, MX-1 and MCF-7 cell lines when ILP-2 was
separately knocked down for 24, 48 and 72 h. In regards to the
HCC-1937 cell line, the cell viability of the siRNA-5 group was
notably decreased when compared to the HCC-1937 group.
***P<0.001 vs. the HCC-1937 group. In regards to the MX-1 cell
line, cell viability of the siRNA-5 group was significantly
decreased when compared to the MX-1 group. *P<0.05, **P<0.01,
***P<0.001 vs. the MX-1 group; #P<0.05,
##P<0.01 vs. the NC group; $$P<0.01 vs.
the siRNA-3 group. In regards to the MCF-7 cell line, cell
viability of the siRNA-5 group was significantly decreased when
compared to the MCF-7 group. **P<0.01, ***P<0.001 vs. the
MCF-7 group; #P<0.05, ###P<0.001 vs.
the NC group; $$$P<0.001 vs. the siRNA-3 group. Data
are expressed as mean ± SEM by ANOVA. Groups were compared by
conducting Bonferroni test; n=6. |
ILP-2 is actively involved in the cell
migration of breast cancer cell lines
Scratch assays were performed to study the rate of
cell migration in ILP-2 siRNA knockdown cell lines such as
HCC-1937, MX-1 and MCF-7 so as to ascertain whether ILP-2 is
involved in breast cancer cell migration. As shown in Fig. 6A-C, the rate of cell migration of
the siRNA-5 group was significantly decreased in comparison to that
of the control group (Fig. 6A, 24
h, P<0.001, 48 and 72 h, P<0.01; Fig. 6B, 24 h, P<0.01, 48 h, P<0.001,
72 h, P<0.05; Fig. 6C, 24 h,
P<0.01, 48 h, P<0.05, 72 h, P<0.001), with results
implying that ILP-2 positively accelerates the migration of breast
cancer cells.
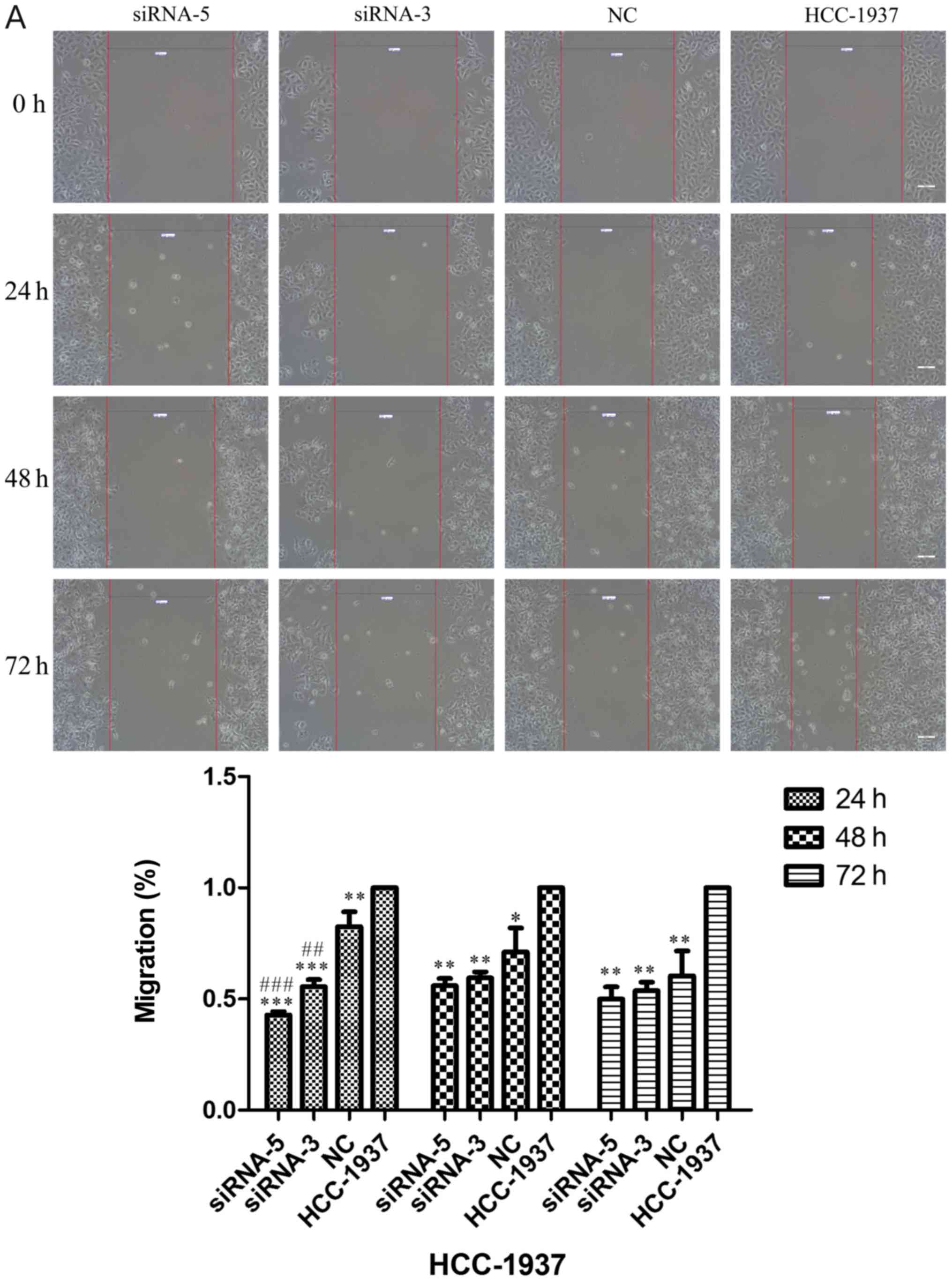 | Figure 6.Scratch analysis showing the rate of
cell migration of the HCC-1937, MX-1 and MCF-7 cell lines when
ILP-2 was separately knocked down for 24, 48 and 72 h. (A) In
regards to the HCC-1937 cell line, the rate of cell migration of
the siRNA-5 group was notably decreased compared to the HCC-1937
group. *P<0.05, **P<0.01, ***P<0.001 vs. the HCC-1937
group, ##P<0.01, ###P<0.001 vs. the NC
group. (B) In regards to the MX-1 cell line, the rate of cell
migration of the siRNA-5 group was notably decreased compared to
the MX-1 group. *P<0.05, **P<0.01, ***P<0.001 vs. the MX-1
group; ##P<0.01 vs. the NC group;
$P<0.05 vs. the siRNA-3 group. (C) In regards to the
MCF-7 cell line, the rate of cell migration of the siRNA-5 group
was notably decreased compared to the MCF-7 group. *P<0.05,
**P<0.01, ***P<0.001 vs. the MCF-7 group. Data are expressed
as mean ± SEM by ANOVA. Groups were compared by conducting
Bonferroni test. Scale bar, 200 µm; n=3. |
ILP-2 inhibits the apoptosis of breast
cancer cells
Considering the effect of ILP-2 on the apoptosis of
breast cancer cells, dual AO/EB staining analysis was separately
used to detect HCC-1937, MX-1 and MCF-7 cell apoptosis when ILP-2
was knocked down for 24, 48 and 72 h to further determine the role
of ILP-2 on breast cancer cell growth. As shown in Fig. 7A-C, the apoptosis rate of the
siRNA-5 group was significantly increased when compared with that
of the control group (P<0.001 at all times for all cell lines),
with results indicating that ILP-2 positively inhibits the
apoptosis of breast cancer cells and promotes the growth of breast
cancer cells.
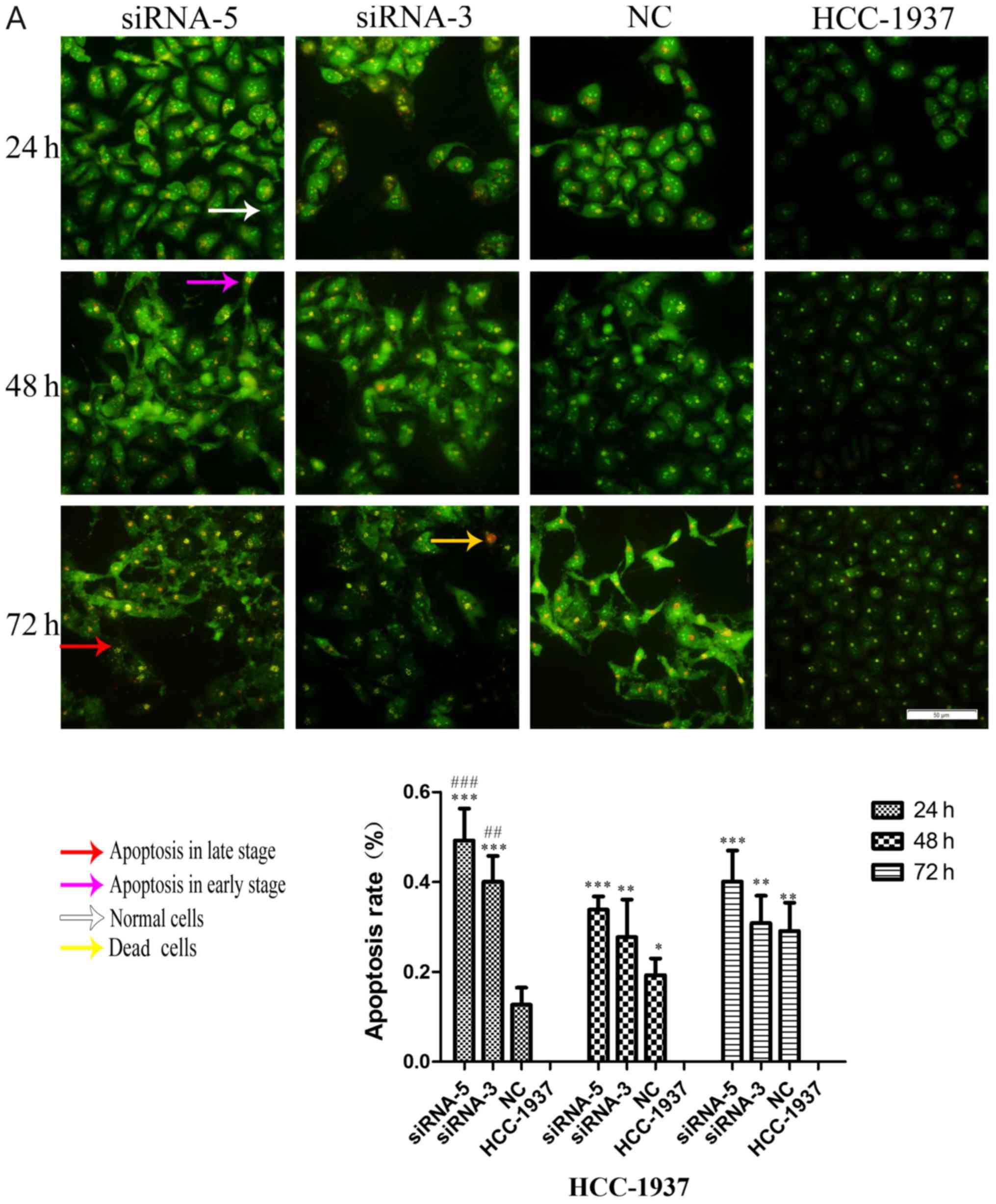 | Figure 7.AO-EB double staining analysis showing
the apoptotic rate of HCC-1937, MX-1 and MCF-7 cell lines when
ILP-2 was separately knocked down for 24, 48 and 72 h. (A) In
regards to the HCC-1937 cell line, the apoptosis rate of the
siRNA-5 group was notably increased when compared to the HCC-1937
group. *P<0.05, **P<0.01, ***P<0.001 vs. the HCC-1937
group; ##P<0.01, ###P<0.001 vs. the NC
group. (B) In regards to the MX-1 cell line, the apoptotic rate of
the siRNA-5group was notably increased when compared to the MX-1
group. *P<0.05, **P<0.01, ***P<0.001 vs. the MX-1 group;
#P<0.05, ##P<0.01,
###P<0.001 vs. the NC group; $P<0.05,
$$$P<0.001 vs. the siRNA-3 group. (C) In regards to
the MCF-7 cell line, the apoptotic rate of the siRNA-5 group was
notably increased when compared to the MCF-7 group. ***P<0.001
vs. the MCF-7 group; ##P<0.01,
###P<0.001 vs. the NC group; $$$P<0.001
vs. the siRNA-3 group. Data are expressed as mean ± SEM by ANOVA.
Groups were compared by conducting Bonferroni test. Scale bar, 100
µm; n=5. |
Discussion
ILP-2 is a novel apoptotic inhibitory protein
closely correlated to caspase-9 (9). ILP-2/BIRC-8 was found to inhibit HepG2
cell apoptosis and promote cell growth (11). Based on the results from the present
study, we hypothesize that ILP-2 accelerates the growth of breast
cancer cells.
Initially, ILP-2 was found to be solely expressed in
the testis of normal tissues (9,10). It
was later detected in lymphoblastoid cells (9). In our prior published study, we
demonstrated high ILP-2 expression in breast cancer patient sera
(8). In the present study, we found
high ILP-2 expression in the breast cancer tissues and breast
cancer cells. Livin protein expression is elevated in breast cancer
tissues and cell lines, which in turn promotes the progression and
metastasis of breast cancer (12).
Our present results indicate that ILP-2 plays an active role in
breast cancer cell growth.
XIAP (ILP-1) and Livin are members of the IAP
family, with a very similar domain to ILP-2 (4). RNAi technology can distinctively
inhibit ILP-1 and Livin expression (7,13–19).
We applied RNAi technology to knock down ILP-2 expression and
analyzed its role in breast cancer cell growth. Our results
demonstrated that siRNA-5 can inhibit the protein expression of
ILP-2 in breast cancer cells.
Livin participates in proliferation, migration and
invasion of breast cancer (20).
Overexpression of Livin promotes the migratory and invasive
abilities of MCF-7, with knockdown of Livin exhibiting contrasting
effect (12). After inhibiting
ILP-2 expression using siRNA-5 in breast cancer cells such as
HCC-1937, MX-1 and MCF-7 cell lines, cell viability and rate of
cell migration were respectively significantly decreased, the cell
apoptosis rate was separately significantly increased. These
results suggest that ILP-2 vigorously promotes breast cancer cell
growth.
In conclusion, our experiment confirms the
hypothesis that ILP-2 plays a significant role in the growth of
breast cancer cells and is a novel growth enhancer for breast
cancer. However, further mechanistic studies need to be carried out
to examine how ILP-2 promotes breast cancer cell growth.
Accordingly, we plan to further explore the underlying mechanism of
the growth-promoting activity of ILP-2 in breast cancer cells.
First, we will apply the co-immunoprecipitation technology to
search the protein which can interact with ILP-2 and verify it. In
addition, we will construct Lenti-ILP-2-shRNA which knocks down the
expression of ILP-2, transfect it into MCF-7 cells and filtrate the
stably transfected MCF-7 cells by addition of the puromycin to
MCF-7 cells. The stably transfected MCF-7 cells can be applied to
establish human breast cancer xenografts in nude mice. When the
solid tumors are successfully grown, we will excise them, draw
growth cures after measuring the solid tumor size and analyze the
expression of the ILP-2 downstream gene in its pathway by using the
solid tumors.
Acknowledgements
We thank Dr Benson O.A. Botchway and Dr Akhileshwar
Namani (Zhejiang University School of Medicine) for having
critically revised the manuscripts.
Funding
The present study was supported by the National
Natural Science Foundation of China (no. 81360397), the Scientific
Research Project in Jishou University (no. Jdy16024) and the
Scientific Research Project for Graduates in JiShou University (no.
JGY201772).
Availability of data and materials
All data generated or analyzed in this study are
included in this published article.
Authors' contributions
MX designed the manuscript and was also involved in
the conception of the study; LZ, WZ, XZ and SX wrote the
manuscript, collected clinical information and performed the
statistical analyses; MW, XP, YP, BL, PT, QC and DY assisted with
the immunohistochemistry and western blotting; ZC, ZS, SW and KY
assisted with MTT assays, scratch assays and AO/EB double staining
analysis. All authors read and approved the manuscript and agree to
be accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work is appropriately
investigated and resolved.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Jishou University (Jishou, China). All participated
patients received and approved the written informed consent before
joining the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lopez J and Meier P: To fight or
die-inhibitor of apoptosis proteins at the crossroad of innate
immunity and death. Curr Opin Cell Biol. 22:872–881. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Almagro MC and Vucic D: The inhibitor
of apoptosis (IAP) proteins are critical regulators of signaling
pathways and targets for anti-cancer therapy. Exp Oncol.
34:200–211. 2012.PubMed/NCBI
|
|
4
|
LaCasse EC, Mahoney DJ, Cheung HH,
Plenchette S, Baird S and Korneluk RG: IAP-targeted therapies for
cancer. Oncogene. 27:6252–6275. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Saleem M, Qadir MI, Perveen N and Ahmad B,
Saleem U, Irshad T and Ahmad B: Inhibitors of apoptotic proteins:
New targets for anticancer therapy. Chem Biol Drug Des. 82:243–251.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liston P, Roy N, Tamai K, Lefebvre C,
Baird S, Cherton-Horvat G, Farahani R, McLean M, Ikeda JE,
MacKenzie A, et al: Suppression of apoptosis in mammalian cells by
NAIP and a related family of IAP genes. Nature. 379:349–353. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hiscutt EL, Hill DS, Martin S, Kerr R,
Harbottle A, Birch-Machin M, Redfern CP, Fulda S, Armstrong JL and
Lovat PE: Targeting X-linked inhibitor of apoptosis protein to
increase the efficacy of endoplasmic reticulum stress-induced
apoptosis for melanoma therapy. J Invest Dermatol. 130:2250–2258.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xiang M, Zhou W, Gao D, Fang X and Liu Q:
Inhibitor of apoptosis protein-like protein-2 as a novel
serological biomarker for breast cancer. Int J Mol Sci.
13:16737–16750. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Richter BW, Mir SS, Eiben LJ, Lewis J,
Reffey SB, Frattini A, Tian L, Frank S, Youle RJ, Nelson DL, et al:
Molecular cloning of ILP-2, a novel member of the inhibitor of
apoptosis protein family. Mol Cell Biol. 21:4292–4301. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang L, Zhang Q, Liu B, Han M and Shan B:
Challenge and promise: roles for Livin in progression and therapy
of cancer. Mol Cancer Ther. 7:3661–3669. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chuturgoon AA, Phulukdaree A and Moodley
D: Fumonisin B1 inhibits apoptosis in HepG2 cells by
inducing Birc-8/ILP-2. Toxicol Lett. 235:67–74. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Han Y, Zhang L, Wang W, Li J and Song M:
Livin promotes the progression and metastasis of breast cancer
through the regulation of epithelial-mesenchymal transition via the
p38/GSK3β pathway. Oncol Rep. 38:3574–3582.
2017.PubMed/NCBI
|
|
13
|
Chen J, Xiao XQ, Deng CM, Su XS and Li GY:
Downregulation of xIAP expression by small interfering RNA inhibits
cellular viability and increases chemosensitivity to methotrexate
in human hepatoma cell line HepG2. J Chemother. 18:525–531. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Buneker CK, Yu R, Deedigan L, Mohr A and
Zwacka RM: IFN-γ combined with targeting of XIAP leads
to increased apoptosis-sensitisation of TRAIL resistant pancreatic
carcinoma cells. Cancer Lett. 316:168–177. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang S, Bai L, Lu J, Liu L, Yang CY and
Sun H: Targeting inhibitors of apoptosis proteins (IAPs) for new
breast cancer therapeutics. J Mammary Gland Biol Neoplasia.
17:217–228. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu H, Wang S, Sun H, Pan Z, Zhou W and Wu
M: Inhibition of tumorigenesis and invasion of hepatocellular
carcinoma by siRNA-mediated silencing of the livin gene. Mol Med
Rep. 3:903–907. 2010.PubMed/NCBI
|
|
17
|
Wang TS, Ding QQ, Guo RH, Shen H, Sun J,
Lu KH, You SH, Ge HM, Shu YQ and Liu P: Expression of livin in
gastric cancer and induction of apoptosis in SGC-7901 cells by
shRNA-mediated silencing of livin gene. Biomed Pharmacother.
64:333–338. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dasgupta A, Alvarado CS, Xu Z and Findley
HW: Expression and functional role of inhibitor-of-apoptosis
protein livin (BIRC7) in neuroblastoma. Biochem Biophys Res Commun.
400:53–59. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang D, Song X, Zhang J, Ye L, Wang S, Che
X, Wang J, Zhang Z and Wang L: Suppression of livin gene expression
by siRNA leads to growth inhibition and apoptosis induction in
human bladder cancer T24 cells. Biosci Biotechnol Biochem.
74:1039–1044. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li CJ, Cong Y, Liu XZ, Zhou X, Shi X, Wu
SJ, Zhou GX and Lu M: Research progress on the livin gene and
osteosarcomas. Asian Pac J Cancer Prev. 15:8577–8579. 2014.
View Article : Google Scholar : PubMed/NCBI
|