Introduction
Breast cancer is the second most common cause of
cancer-associated mortality in women following lung cancer; it is
estimated that, 266,120 women will be diagnosed with breast cancer
and 40,920 women will succumb to mortality from breast cancer in
the United States in 2018 (1).
In order to reduce the side effects and enhance the
efficacy of treatment, traditional Chinese medicine (TCM)
prescriptions of synergistic combinations of different herbs have
emerged as an alternative strategy for cancer therapy. The
combination of cucurbitacin B (Cu B) with another appropriate drug,
for example curcumin, can decrease the dosage of Cu B, resulting in
reduced toxicity and fewer side effects (2). Our previous study found that Cu B
synergistically eliminated the resistance of Burkitt's lymphoma
Ramos cells to apoptosis via arsenic trioxide through inhibiting
the phosphorylation of signal transducer and activator of
transcription 3 (3).
Trichosanthes kirilowii Maxim and Radix
Aconiti Lateralis Preparata are included in the 18 incompatible
medications that have been long established in TCM. The use of
these two herbs together has also been recorded in ancient
formulas, including Qian Jin Yi Fang (4) compiled by Simiao Sun in the Tang
Dynasty, Sheng Ji Zong Lu (5) from
the Song Dynasty, and Jin Gui Yao Lue (6), compiled by Zhongjing Zhang in the
Eastern Han Dynasty. Cu B, a tetracyclic triterpene compound
extracted from T. kirilowii Maxim, is reported to have
antiproliferative activity in various types of cancer cell,
including lung (7), liver (8), colorectal (9), pancreatic cancer (10) and lymphoma (3). In the toxic herb Radix Aconiti
Lateralis Preparata, several components are toxic, including
aconitine, which is the major toxic substance. However, higenamine
can be used as a food supplement (11), as a potential β2-adrenergic receptor
agonist (12), and as a dietary
supplement without adverse drug reactions (13), which confirms its toxicity is lower
than that of aconitine. Therefore, higenamine was selected in the
present study and Cu B, extracted from T. kirilowii Maxim,
was combined with higenamine, the active ingredient of Radix
Aconiti Lateralis Preparata, for the treatment of breast cancer.
The aim of the present study was to clarify whether higenamine
enhances the anticancer effects of Cu B in breast cancer cell
lines.
The combinations within TCM form a complex system
with multiple components and targets; however, network pharmacology
can bridge the gap between TCM and modern medicine, link drugs and
targets, and can be analyzed to reveal the possible mechanisms
(14). Therefore, in the present
study, to provide an explanation for the possible mechanisms,
network pharmacology methods were used.
The present study aimed to clarify whether
incompatible drugs can be combined together at a specific ratio to
exert synergistic effects. In TCM, there are 18 incompatible herbs
which include T. kirilowii Maxim and Radix Aconiti Lateralis
Preparata. The incompatible herbs indicate that when both herbs are
used together, severe toxicity or side effects occur. The present
study suggested the 18 incompatible herbs are not strictly
forbidden.
Materials and methods
Cell culture and compounds
The SKBr3 and T47D cell lines were purchased from
the Chinese Academy of Medicine Science (Beijing, China) and
cultured in RPMI-1640, supplemented with 10% fetal bovine serum
(FBS; Hyclone Laboratories; GE Healthcare Life Sciences, Logan, UT,
USA) at 37°C in a humidified atmosphere with 5% CO2. Cu
B was purchased from Tianjin Pharmacopoeia Standard Instrument
Pharmaceutical Development Company (Tainjin, China) and dissolved
in dimethylsulfoxide (DMSO) to produce a stock solution of 70
µmol/ml. Higenamine was purchased from Shanghai Yuanye Biological
Technology Co., Ltd. (Shanghai, China), and dissolved in DMSO at 10
µmol/ml. MK-2206 was purchased from Selleck Chemicals (Houston, TX,
USA).
Cell proliferation assay
The cells (5×104 cells/ml) were seeded in
96-well plates, with three wells seeded per treatment group, and
were treated with Cu B at different concentrations at 37°C. The
T47D cells were exposed to serial concentrations of 5, 10 or 20 µM
of Cu B and/or 0.5, 1 or 2 µM of higenamine for 24, 48 or 72 h. The
SKBr3 cells were exposed to 15, 30 or 60 nM of Cu B and/or 1.5, 3
or 6 nM of higenamine for 24, 48 or 72 h. Cell Counting Kit-8
(CCK-8) (10 µl; Dojindo Molecular Technologies, Inc., Kumamoto,
Japan) was added to each well containing 100 µl culture medium and
the absorbance at 450 nm of each well was measured using an
ultramicroplate reader. The results shown are the mean values of
three independent experiments.
Apoptosis analysis
The Annexin V-FITC Apoptosis Detection kit (BD
Pharmingen; BD Biosciences, San Jose, CA, USA) was used to measure
apoptosis. For the assay, the SKBr3 or T47D cells
(2×104/ml) in 2 ml were seeded in 6-well plates and
treated with the indicated concentrations of Cu B and/or higenamine
for 24 h. The SKBr3 cells were treated with 30 nM Cu B and/or 3 nM
higenamine for 24 h. The T47D cells were treated with 5 µM Cu B
and/or 500 nM higenamine for 24 h.
Nuclear staining with
4,6-diamidino-2-phenylindole (DAPI)
For staining, 2×104 cells (SKBr3 and
T47D) were plated on sterilized coverslips in 12-well plates and
treated with Cu B and/or higenamine. The SKBr3 cells were treated
with 30 nM Cu B and/or 3 nM higenamine for 24 h. The T47D cells
were treated with 5 µM Cu B and/or 500 nM higenamine for 24 h.
Following incubation, the cells were fixed in 4% paraformaldehyde
for 20 min at room temperature and permeabilized with 0.2% Triton
X-100 for 5 min. Finally, the cells were stained with DAPI solution
and examined using a fluorescence microscope (Leica TCS SP5; Leica
Microsystems, Inc., Buffalo Grove, IL, USA).
Cell cycle analysis
The T47D and SKBr3 cells (2×104/ml) were
seeded in 6-well plates and treated with Cu B and higenamine. The
SKBr3 cells were treated with 30 nM Cu B and/or 3 nM higenamine for
24 h. The T47D cells were treated with 5 µM Cu B and/or 500 nM
higenamine for 24 h. The cells were then trypsinized, washed with
ice-cold phosphate-buffered saline (PBS), fixed in 70% ethanol
overnight at −20°C, and then stained with PI (50 µg/ml) and RNase
(100 µg/ml) for 30 min at 37°C. The DNA distribution was analyzed
by flow cytometry using ModFit 3.1 software (Verity Software House,
Groton, MA, USA).
Predicted mechanism of action of the
combination of Cu B and higenamine in breast cancer treatment
The predicted targets of Cu B and higenamine were
obtained from ChemMapper (http://lilab.ecust.edu.cn/chemmapper/) (15), which predicts the
polypharmacological effects and modes of action for small molecules
based on 3D similarity. The simplified molecular-input line-entry
system (SMILES) notations for Cu B and higenamine were obtained
from PubChem (https://pubchem.ncbi.nlm.nih.gov/) (16). For Cu B, the SMILES notation was
CC(=O)OC(C)(C)/C=C/C(=O)[C@@](C)([C@H]1[C@@H](C[C@@]2([C@@]1(CC(=O)[C@@]3([C@H]2CC=C4[C@H]3C[C@@H](C(=O)C4(C)C)O)C)C)C)O)O
and for higenamine, the SMILES notation was
C1CNC(C2=CC(=C(C=C21)O)O)CC3=CC=C(C=C3)O. As Cu B has clear, direct
anti-breast cancer effects, the targets of Cu B were compared with
breast cancer treatment targets, which were elucidated in our
previous study (17). The
interactions of the matched targets and the targets of higenamine
were obtained from the Search Tool for the Retrieval of Interacting
Genes (STRING) database (version 10.5) (https://string-db.org/) (18) and remapped using Cytoscape software
(19) (version 3.4.0). Pathway
enrichment analysis was performed online using the Comparative
Toxicogenomics Database (http://ctdbase.org/tools/analyzer.go) (20) and FunRich software (version 3.1.3)
(21) using the targets of
higenamine that have direct interactions with the matched targets
of Cu B.
In vivo SKBr3 ×enograft model
The present study was approved by the Committee on
the Ethics of Animal Experiments of Tianjin Medical University
Cancer Institute and Hospital (Tianjin, China; permit no. 2017083).
A total of 29 female BALB/c nude mice (3–4 weeks old) weighing
15–18 g were obtained from Beijing Vital River Laboratory Animal
Technology Co., Ltd. (Beijing, China). The mice were housed in a
specific pathogen-free grade laboratory in a 12-h light/black cycle
environment at 22±2°C with ad libitum access to water and
food. SKBr3 cells (0.1 ml, 0.2×107/ml) were
subcutaneously implanted in the right flank of one mouse. When the
tumor diameter reached ~1.0 cm, the mice were sacrificed and the
tumors were excised. The tumors were cut into sections of
approximate volume of 8 mm3 and transplanted
subcutaneously into the other 28 female nude mice. The mice were
then randomized into the following treatment groups: control
(saline, n=7); Cu B (n=7); higenamine (n=7); and Cu B + higenamine
(n=7). The mice were intraperitoneally injected with vehicle, Cu B
(500 µg/kg), and/or higenamine (25 µg/kg) every other day for 13
days. The tumor volume and body weight were monitored once every 2
days. The tumor volume was calculated as L × W2/2, where
L and W are the length and width of the tumor, respectively. After
13 days, the mice were sacrificed with pentobaribital sodium at a
dose of 100 mg/kg, and the tumors were excised. The major organs,
including the heart, liver, spleen, lung and kidney, were excised
and weighed.
Western blot analysis
The T47D or SKBr3 cells (2×104/ml) were
plated in 6-well plates. The SKBr3 cells were treated with 30 nM Cu
B and/or 3 nM higenamine for 24 h. The T47D cells were treated with
5 µM Cu B and/or 500 nM higenamine for 24 h. For network
pharmacology evaluation, the SKBr3 cell line was first exposed to
the AKT inhibitor, MK-2206, for 6 h, and treated with Cu B for 24
h. Following treatment, the cells were collected, washed twice with
ice-cold PBS, and lysed with RIPA buffer supplemented with 1%
phosphatase inhibitor, 1% protease inhibitor and 1 mM PMSF. The
protein concentration was determined by BCA method. Equal amounts
of samples were loaded and separated by 10% SDS-PAGE, transferred
electrophoretically onto polyvinylidene difluoride membranes (EMD
Millipore, Billerica, MA, USA). Membranes were blocked with
Tris-buffered saline and Tween-20 (TBST) containing 5% skim milk
and blotted with antibodies against AKT produced in rabbit
(1:1,000; cat. no. YT6111; ImmunoWay Biotechnology Company, Plano,
TX, USA), phosphorylated (p-)AKT produced in rabbit (Ser473,
1:1,000; cat. no. 4060S; Cell Signaling Technology, Inc., Danvers,
MA, USA), B-cell lymphoma 2 produced in rabbit (Bcl-2; 1:1,000;
cat. no. ab32124; Abcam, Cambridge, MA, USA), Bcl-2-associated X
protein produced in rabbit (BAX; 1:1,000, cat. no. ab32503, Abcam),
cyclin A2 produced in mouse (CCNA2; 1:2,000; cat. no. 4656S; Cell
Signaling Technology, Inc., Danvers, MA, USA), cyclin-dependent
kinase (CDK)2 produced in rabbit (1:1,000; cat. no. ab32147;
Abcam), p-CDK2 produced in rabbit (Thr39, 1:300; cat. no. ab79379;
Abcam), p-histone H3 produced in rabbit (Ser 10, 1:200; cat. no.
ab47297; Abcam), β-actin produced in mouse (1:2,000; cat. no.
A1978; Sigma Chemical Co., St. Louis, MO, USA) overnight at 4°C.
After washing with TBST, the membranes were incubated with
horseradish peroxidase-conjugated goat anti-rabbit (1:500; cat. no.
SH-0031; Ding Guo Chang Sheng, Beijing, China) or anti-mouse IgG
(1:500; cat. no. SH-0021; Ding Guo Chang Sheng) at 4°C for 1 h. And
the protein levels were detected using enhanced chemiluminescence
reagent. The protein levels were detected using ImageJ 1.47v
(National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
The results of the CCK-8 experiments and in
vivo experiments are presented as the mean ± standard
deviation. The statistical significance was analyzed by one-way
analysis of variance computed using SPSS 21.0 software (IBM Corp.,
Armonk, NY, USA). Student's t-test was used to analyze the
significance of the two single-agent groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Higenamine enhances the inhibitory
effects of Cu B on breast cancer cells in vitro
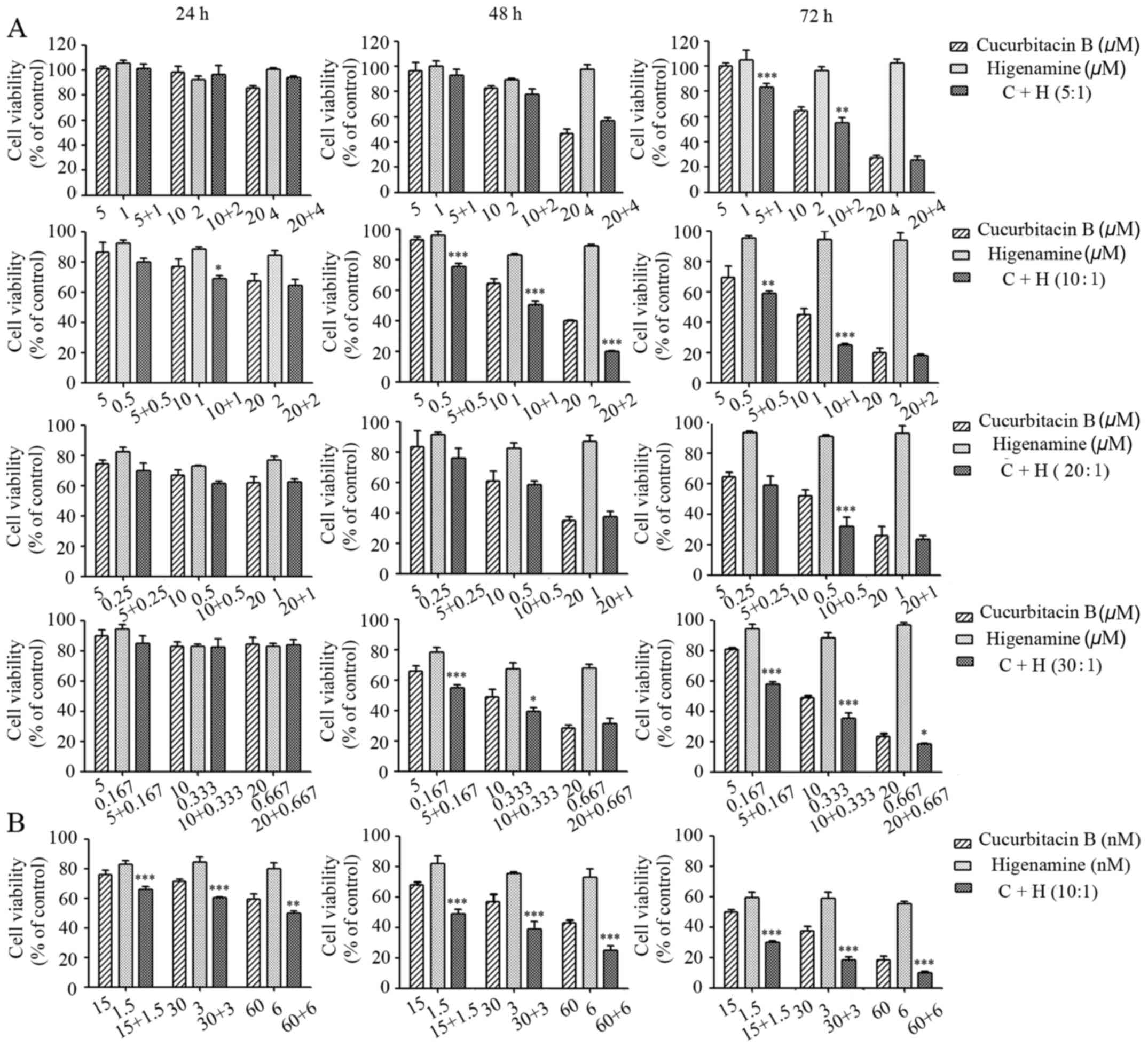
The viability of T47D breast cancer cells treated
individually with Cu B or higenamine, or their combination, was
analyzed following treatment for 24, 48 and 72 h using the CCK-8
assay. As shown in Fig. 1A, the
growth of T47D cells was significantly inhibited by Cu B in a
concentration- and time-dependent manner. By contrast, higenamine
only marginally affected cell viability and no dose-dependency was
observed. The ratios at 1:1, 1:2 and 2:1 (Cu B:higenamine) were
examined, and it was found that higenamine did not enhance the
inhibitory effect of Cu B at the ratios of 1:1 or 1:2. At the ratio
of 2:1, higenamine enhanced the inhibitory effect of Cu B with no
dose-independent effect (data not shown). Therefore, the proportion
of Cu B was increased in the combination with higenamine and the
effects at the ratios of 5:1, 10:1, 20:1, and 30:1 were compared.
Following combination treatment of Cu B and higenamine at the
ratios of 5:1, 10:1, 20:1 and 30:1, the combination of the agents
significantly enhanced the antiproliferative effect compared with
administration of the drugs separately. Among the ratios assessed,
the 10:1 ratio resulted in the most marked inhibition of cell
growth. The combined antitumor effects of Cu B and higenamine were
assessed in another breast cancer cell line, SKBr3, at the 10:1
ratio. The SKBr3 cells were exposed to 15, 30 or 60 nM Cu B, and
1.5, 3 or 6 nM of higenamine, respectively, for 24, 48 and 72 h. As
shown in Fig. 1B, the individual
administration of Cu B also inhibited the growth of the SKBr3
cells; furthermore, the inhibitory effect was enhanced by
higenamine.
Combination of Cu B and higenamine in
the induction of apoptosis
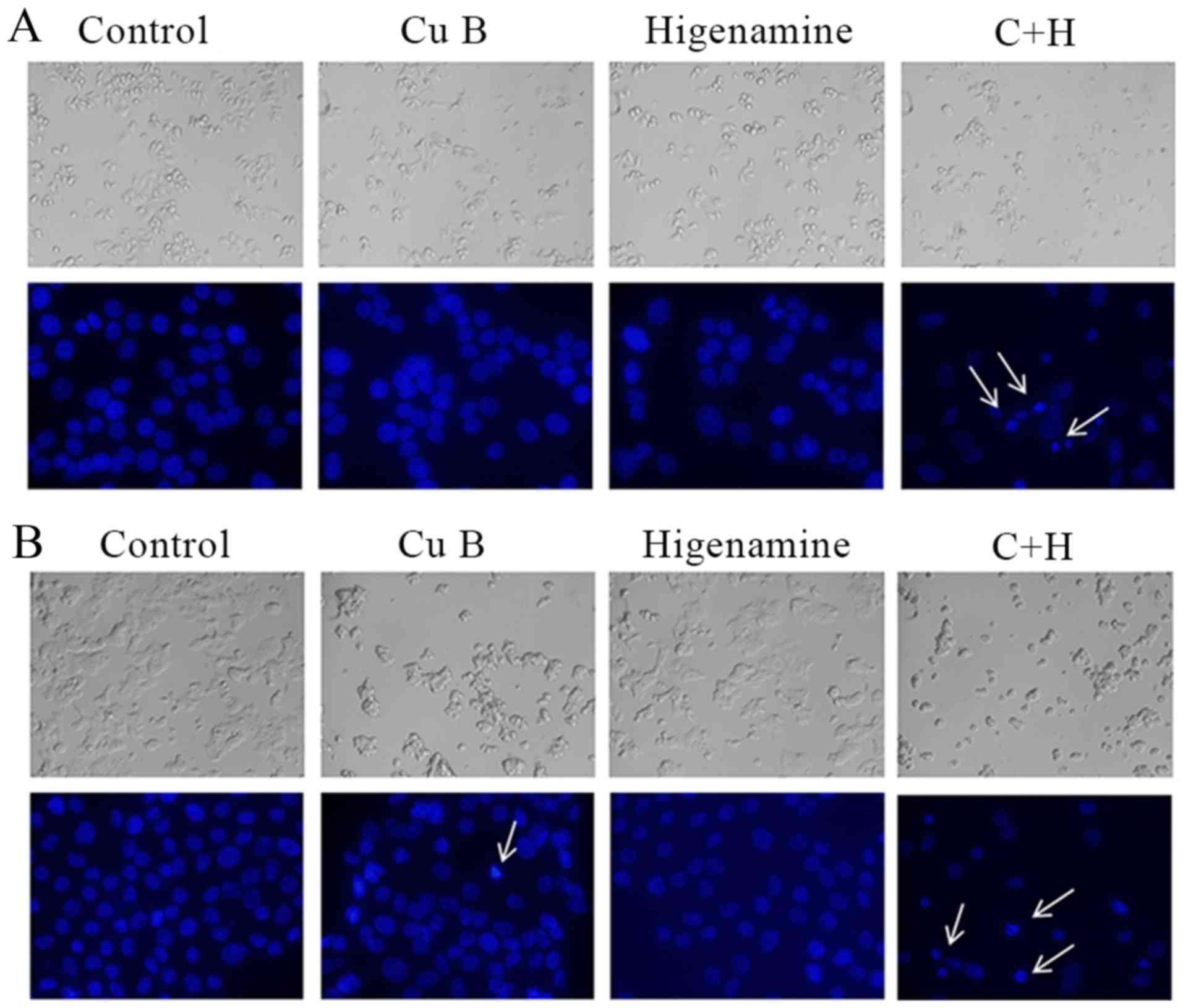
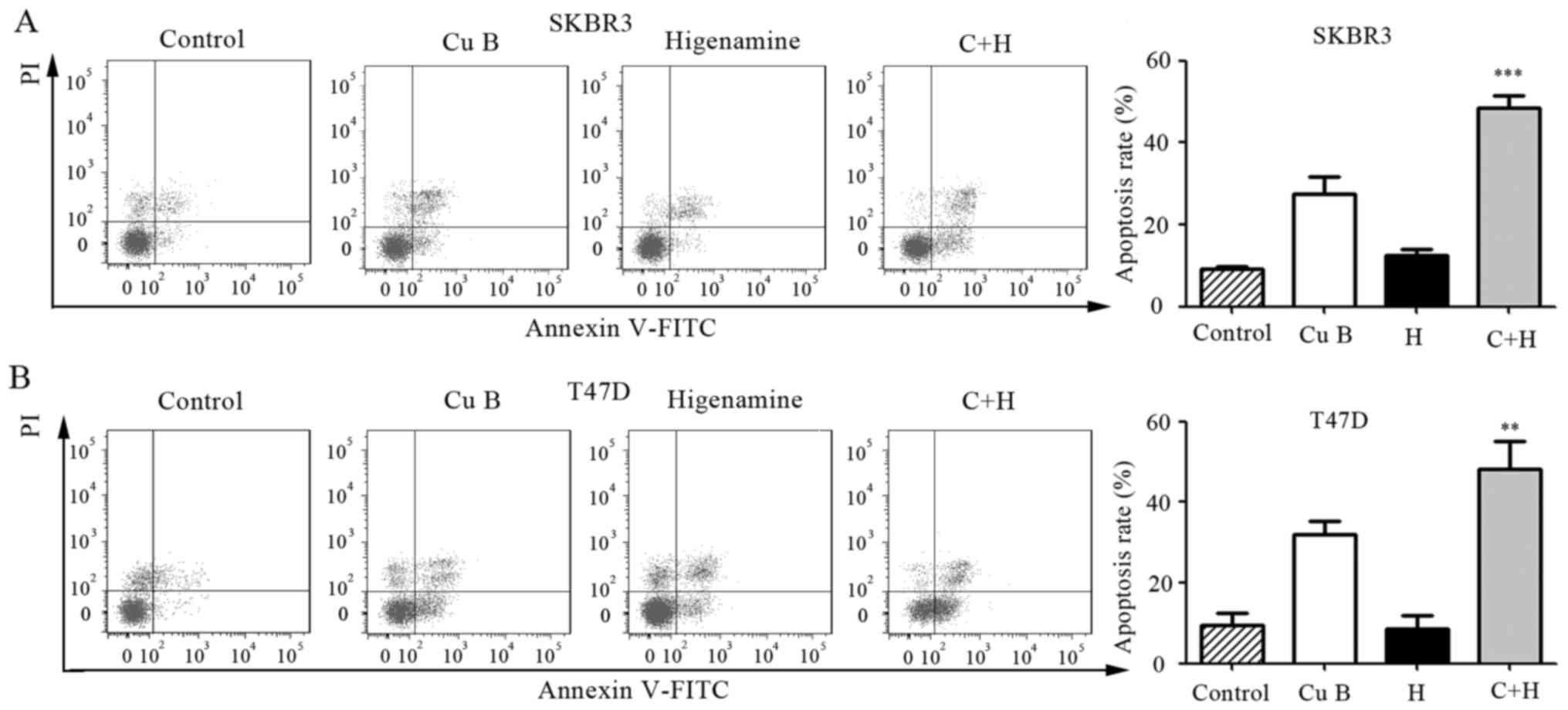
The induction of apoptosis by the combination
treatment of Cu B and higenamine in breast cancer cells was
assessed using DAPI staining and Annexin V/PI double staining. The
number of the breast cancer cells markedly decreased (Fig. 2A and B) and the cell condition
gradually deteriorated following treatment with Cu B and the
combination, as determined using microscopic observation. The DAPI
staining showed that the combination of Cu B and higenamine induced
nuclear chromatin condensation and granular apoptotic bodies. The
proportion of apoptotic cells was higher following the combination
treatment of Cu B and higenamine compared with the administration
of either agent individually in the SKBr3 and T47D cell lines, as
shown by flow cytometric evaluation of the Annexin V/PI
double-stained cells (Fig. 3A and
B).
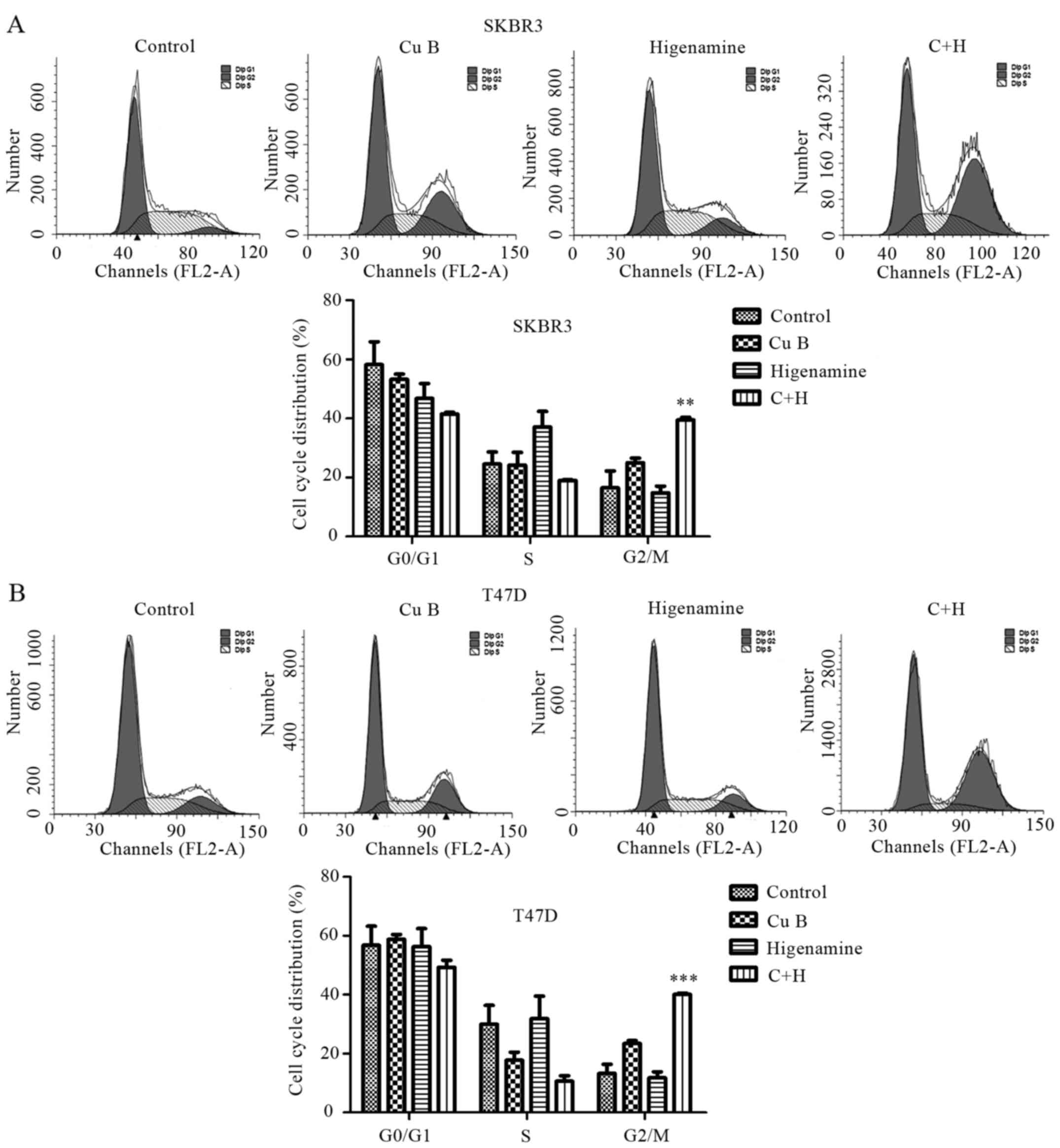
Combination of Cu B and higenamine in
the induction of G2/M cell cycle arrest in breast cancer cells
To examine whether Cu B and/or higenamine inhibited
cell proliferation via cell cycle arrest, flow cytometric analysis
was performed to investigate the cell cycle distribution following
Cu B and/or higenamine treatment using flow cytometry. Cu B induced
G2/M phase arrest and the combination treatment resulted in a
marked accumulation of cells in the G2/M phase compared with the
treatment of Cu B alone in the SKBr3 (Fig. 4A) and T47D (Fig. 4B) cell lines.
Predicted mechanisms of Cu B and
higenamine combination in breast cancer treatment
Using ChemMapper (data not shown), 119 predicted
targets of Cu B and 42 predicted targets of higenamine were
obtained. The cell viability results showed that Cu B exerted
direct anti-breast cancer effects, suggesting that the predicted
targets may be closely associated with the development of breast
cancer. Therefore, these predicted targets were matched with the
breast cancer treatment targets determined in our previous study.
AKT1, estrogen receptor (ER), farnesyltransferase, CAAX box, α
(FNTA), platelet-derived growth factor receptor α (PDGFRA),
peroxisome proliferator-activated receptor (PPAR), RET
proto-oncogene (RET), and vascular endothelial growth factor A
(VEGFA) were found to be directly associated with the anti-breast
cancer effects. Therefore, AKT1, ER, FNTA, PDGFRA, PPAR, RET and
VEGFA were considered to be the major targets of Cu B in breast
cancer.
As the direct anti-breast cancer effects of
higenamine remained to be elucidated, it was suggested that
higenamine may enhance the curative effects of Cu B in breast
cancer through target interactions. The protein interactions of the
major targets of Cu B and the whole predicted targets of higenamine
were determined using the STRING database and the validated
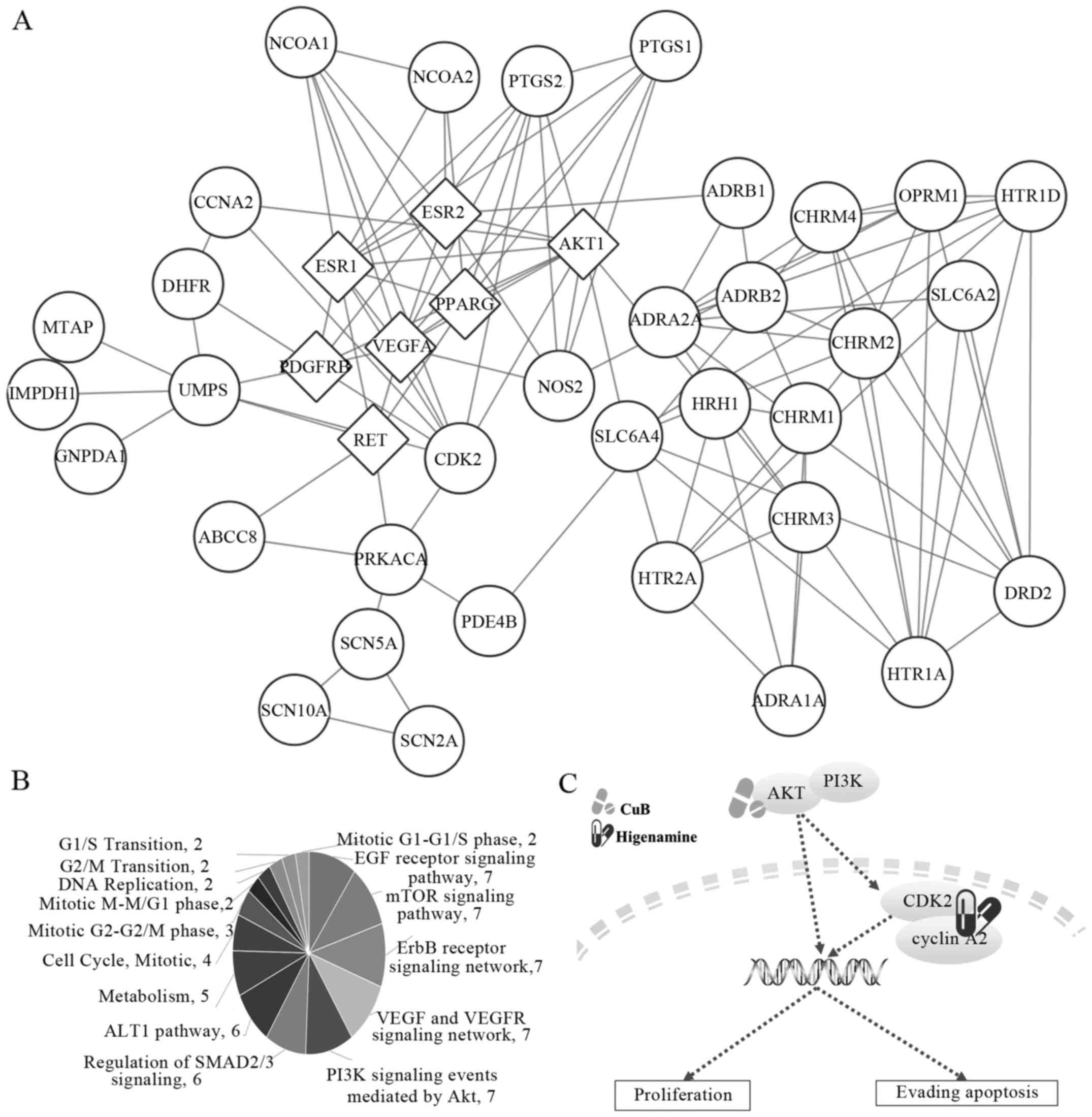
interactions were remapped through Cytoscape (Fig. 5A). Certain targets of higenamine
interacted directly with the major targets of Cu B, including
CCNA1, CDK2, dihydrofolate reductase (DHFR), uridine monophosphate
synthetase (UMPS), ATP binding cassette subfamily C member 8
(ABCC8), protein kinase CAMP-activated catalytic subunit α
(PRKACA), histamine receptor H1 (HRH1), solute carrier family 6
member 4 (SLC6A4), nitric oxide synthase 2 (NOS2),
prostaglandin-endoperoxide synthase 2 (PTGS2), adrenoceptor β1
(ADRB1), prostaglandin-endoperoxide synthase 1 (PTGS1), nuclear
receptor coactivator (NCOA)1 and NCOA2. Pathway enrichment analysis
was performed on these targets using the Comparative Toxicogenomics
Database (Table I) and FunRich,
respectively (Fig. 5B).
Predominantly, these targets were enriched in metabolism-, cell
cycle- and cancer-related signaling pathways. The metabolic
functions of higenamine have been reported in cardiovascular
disease treatment (10,19–21).
Therefore, the present study focused on the effects of higenamine
on the cell cycle, particularly on targets CCNA2, CDK2 and PRKACA,
according to the pathway analysis (Table I), which was accordance with our
experiment results. PRKACA can interact with RET, the target of Cu
B. However, AKT was the downstream effector target of ER, VEGFR and
RET, which were all targets of Cu B, and can directly interact with
CDK2 and CCNA2. Therefore, the present study focused on AKT, CDK2
and CCNA2. The associated pathways were summarized by KEGG pathway
analysis (Fig. 5C) and it was found
that AKT may be the key target of Cu B, and CDK2 and CCNA2 may be
the key targets of higenamine, respectively.
 | Table I.Pathways associated with the targets
of higenamine, which has direct interactions with the major targets
of Cu B. |
Table I.
Pathways associated with the targets
of higenamine, which has direct interactions with the major targets
of Cu B.
| Pathway | n | Annotated
genes |
|---|
| Metabolism | 8 | ABCC8, DHFR, NCOA1,
NCOA2, PRKACA, PTGS1, PTGS2, UMPS |
| Calcium signaling
pathway | 4 | ADRB1, HRH1, NOS2,
PRKACA |
| Cell Cycle,
mitotic | 4 | CCNA2, CDK2, DHFR,
PRKACA |
| G1/S
transition | 3 | CCNA2, CDK2,
DHFR |
| Mitotic G1-G1/S
phases | 3 | CCNA2, CDK2,
DHFR |
| G2/M
transition | 3 | CCNA2, CDK2,
PRKACA |
| Mitotic G2-G2/M
phases | 3 | CCNA2, CDK2,
PRKACA |
Western blot analysis of predicted key
targets
To clarify the anti-breast cancer mechanism of Cu B
and the mechanism through which higenamine enhanced the antitumor
activities of Cu B, the activity of AKT and CDK2 and the expression
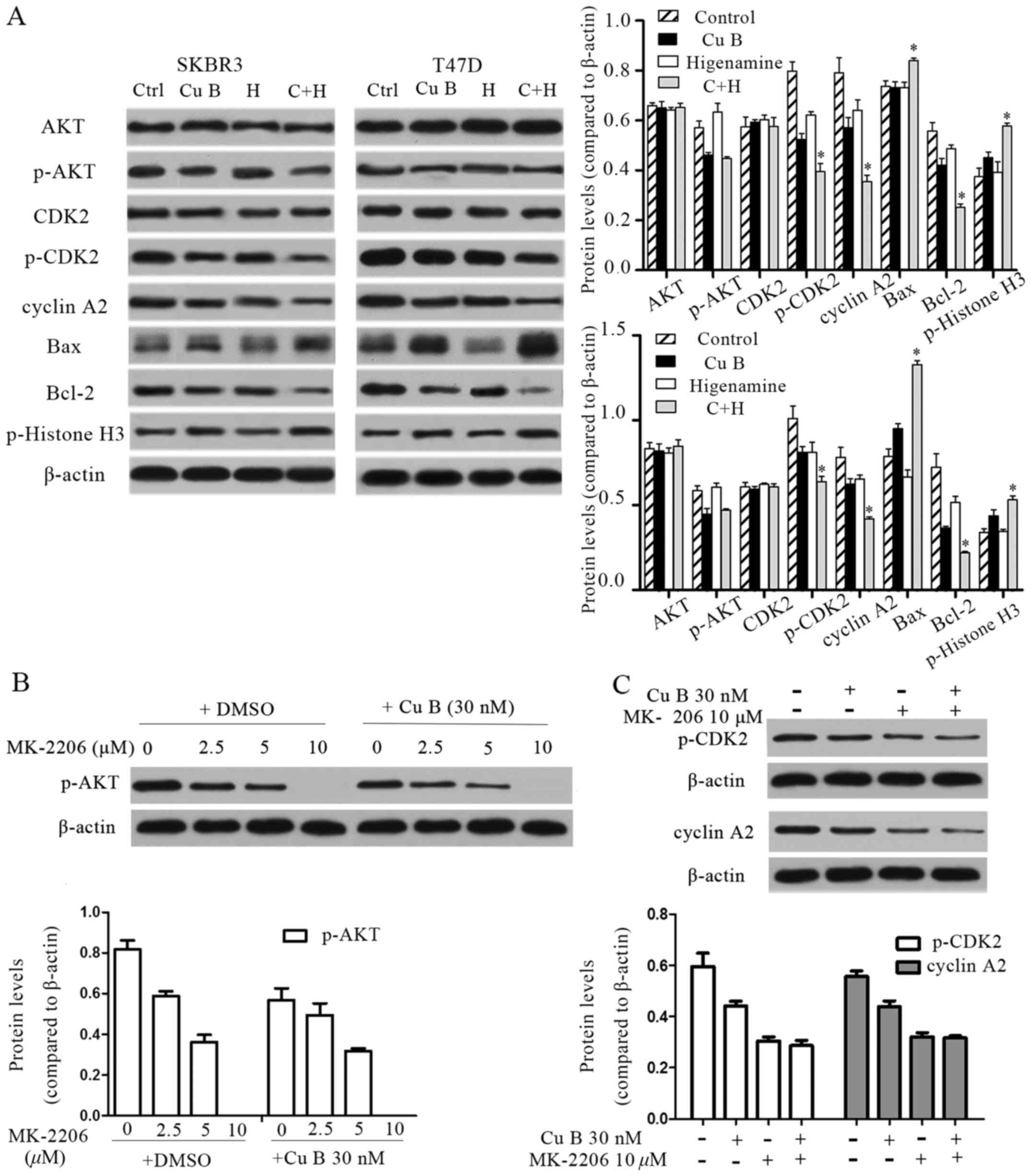
of CCNA2 were evaluated using western blot analysis. As shown in
Fig. 6A, the levels of total AKT
and CDK2 were not altered, however, the phosphorylation of AKT at
Serine 473 and of CDK2 at Threonine 39 were reduced following Cu B
treatment. Only marginal reductions in the activity of CDK2 and the
expression of CCNA2 were observed following treatment with
higenamine. The activity of CDK2 and the expression of CCNA2 were
significantly reduced following the combination treatment. The
expression of p-histone H3 was increased following the combination
treatment compared with the level following treatment with Cu B
(Fig. 6A), which was indicative of
the enhanced G2/M arrest described above. Treatment with the AKT
inhibitor, MK-2206, alone and together with Cu B further supported
the conclusion that Cu B can downregulate the phosphorylation of
AKT. Treatment with 10 µM of MK-2206 inhibited the phosphorylation
of AKT (Fig. 6B). Furthermore, 10
µM of MK-2206 reduced the phosphorylation of CDK2 and the
expression of CCNA2, which supported the close associations of AKT,
CDK2 and CCNA2. Compared with 10 µM of MK-2206, p-CDK2 and CCNA2
were not affected by 30 nM of Cu B combined with 10 µM of MK-2206,
which also suggested that CDK2 and CCNA2 were the downstream
effectors of AKT (Fig. 6C). These
results suggested that Cu B exerted anti-breast cancer effects
through inhibiting the activity of the AKT pathway, and that the
synergetic inhibition induced by the combination of Cu B and
higenamine was most likely a result of cell cycle-related pathways
triggered by CDK2 and CCNA2.
Effects of Cu B and higenamine in
vivo
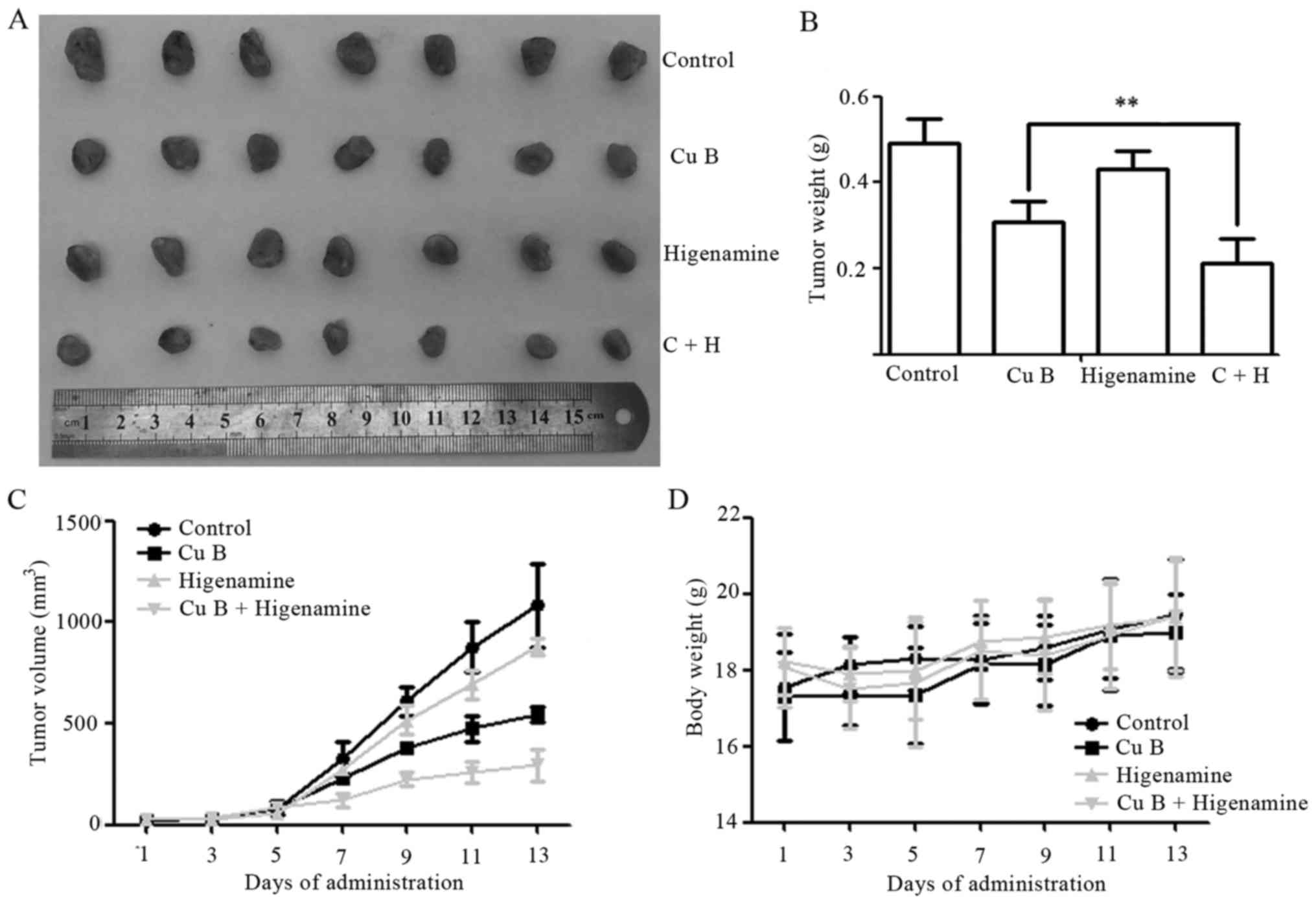
To further evaluate the antitumor efficacy of Cu B
and higenamine in vivo, BALB/c nude mice with SKBr3
×enografts were treated with Cu B and/or higenamine. The surviving
implanted mice were randomly divided into four groups. There were
no statistical differences among the sizes of the groups (all
P>0.05). Representative images of the excised tumors are shown
in Fig. 7A. After 13 days, the
combination treatment markedly and effectively suppressed tumor
growth, with a reduction in the tumor weights of 31.45 and 51.07%
compared with those in the single treatment groups with Cu B
(P<0.01) and higenamine (P<0.001), respectively (Fig. 7B and C). Compared with the control,
no significant difference in body weight gain was observed
following treatment with Cu B, higenamine, or the two in
combination (Fig. 7D). In addition,
no differences were observed in the organ indices for the heart,
liver, spleen, lung or kidney between the control and other groups
(all P>0.05; data not shown).
Discussion
T. kirilowii Maxim. has been used for
approximate nine hundred years in TCM and its antitumor effects
have been the subject of previous studies (25,26).
In the present study, a component of this herb, Cu B, was combined
with higenamine to determine whether the antitumor effect of Cu B
was enhanced in breast cancer cells. Aconitine, which is a
component in Radix Aconiti Lateralis Preparata, was also assessed,
but resulted in an unsatisfactory result (data not shown). The data
obtained suggested that higenamine enhanced the inhibitory effect
of Cu B on breast cancer, as demonstrated through the analysis of
growth inhibition, cell cycle arrest, and apoptosis. Cu B inhibited
breast cancer in a dose- and time-dependent manner. However, a 10:1
(Cu B:higenamine) ratio was optimal for the combination treatment
of breast cancer. In accordance with compatibility of herb-partners
in TCM, different proportions are used to treat different
diseases.
Network pharmacology methods were used to predict
the possible mechanism through which higenamine potentiated the
treatment effect of Cu B. It was found that AKT1, ER, FNTA, PDGFRA,
PPAR, RET and VEGFA were the major targets of Cu B in breast
cancer. As the direct anti-breast cancer effects of higenamine were
unclear, the protein-protein interactions with the major targets of
Cu B and all the predicted targets of higenamine were analyzed to
identify the possible targets through which the synergistic action
of higenamine was mediated. CCNA1, CDK2, DHFR, UMPS, ABCC8, PRKACA,
HRH1, SLC6A4, NOS2, PTGS2, ADRB1, PTGS1, NCOA1 and NCOA2 were found
to have direct interactions with the major targets of Cu B. These
targets were mainly enriched in metabolism and cell cycle. The
metabolic functions of higenamine are well established in
cardiovascular disease treatment (13,22–24).
Therefore, the present study focused on the effects of higenamine
on the cell cycle, particularly on the targets of CCNA2, CDK2, DHFR
and PRKACA. To better understand the predicted mechanism of Cu B
combined with higenamine in breast cancer treatment, the associated
pathways were summarized by KEGG pathway analysis (Fig. 5C) and it was found that AKT may be
the key target of Cu B, and CDK2 and CCNA2 may be the key targets
of higenamine, respectively. This was supported by the cell cycle
results, which showed that higenamine increased the accumulation of
G2/M arrest compared with Cu B treatment alone. The potentiated
apoptotic effect may result from inhibition of the activity of CDK2
(27). Apoptosis was verified by
the increased expression of Bax and the decreased expression of
Bcl-2. Several studies have reported that inhibiting the activity
of AKT and CDK2 suppresses breast cancer cell growth (28–31);
the results of the cell viability assay in the present study
correlated with this.
The activity of AKT and CDK2, and the expression of
CCNA2, were further evaluated by western blot analysis. The
inhibitory effect of Cu B treatment was clear. Higenamine treatment
alone exerted a modest effect on the activity of CDK2 and the
expression of CCNA2, whereas the combination treatment resulted in
marked inhibition of the activity of CDK2 and expression of CCNA2.
This suggested that the anti-breast cancer effects of Cu B were
mediated through the inhibition of AKT pathway activity, and that
the enhanced inhibitory effect induced by Cu B and higenamine
combination treatment was likely to be a result of cell
cycle-related pathways triggered by CDK2 and CCNA2.
Despite the above findings, the cell cycle arrest
and cell viability inhibition induced by higenamine only had a
modest effect on the activity of CDK2 and the expression of CCNA2.
It was reported previously that human colon cancer cell lines
proliferated normally following the depletion of CDK2 (32,33)
and the viability of CDK2-knockout mice was confirmed (34), which indicated that CDK2 was not
required for cell cycle progression in immortalized human cell
lines; therefore, the inhibition of CDK2 only does not offer a
promising strategy for cancer therapy. AKT regulates cell cycle
progression in the G1/S and G2/M phases through either the direct
phosphorylation of or the indirect regulation of the expression of
various substrates, including, but not limited to, CDK2, p21waf1,
p27kip1, c-Myc, glycogen synthase kinase 3β, CCND1 and forkhead box
O3 (35). The AKT-mediated
phosphorylation of CDK2 and the altered subcellular localizations
are important for cell cycle progression from the S to the G2/M
phase (27). Therefore, the
combination of higenamine and Cu B resulted in the marked
inhibition of CDK2 activity and arrest of the cell cycle.
Due to the limited funding, the present study did
not examine the effects of Cu B on normal cells. Therefore,
associated experiments are required in the future.
In conclusion, the present study demonstrated that
growth inhibition by the combination of Cu B and higenamine in
breast cancer cells resulted from cell cycle arrest and the
induction of apoptosis. Cu B exerted anti-breast cancer effects
through inhibition of the activity of the AKT pathway, and the
synergistic inhibition induced by the combination treatment of Cu B
and higenamine was likely a result of CDK2 and CCNA2.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81503383 and
81400321).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
XZW and DC conceived and designed the study. ZQJ,
JH, XY, JL, JWY, YM, DL and RC performed the experiments. ZQJ and
JH wrote the manuscript. DC, JHH and XL contributed to the analysis
and interpretation of data. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The present study was approved by the Committee on
the Ethics of Animal Experiments of Tianjin Medical University
Cancer Institute and Hospital (permit no. 2017083).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
Cu B
|
cucurbitacin B
|
|
TCM
|
traditional Chinese medicine
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun Y, Zhang J, Zhou J, Huang Z, Hu H,
Qiao M, Zhao X and Chen D: Synergistic effect of cucurbitacin B in
combination with curcumin via enhancing apoptosis induction and
reversing multidrug resistance in human hepatoma cells. Eur J
Pharmacol. 768:28–40. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ding X, Chi J, Yang X, Hao J, Liu C, Zhu
C, Wang X, Liu X, Niu Y, Ji W, et al: Cucurbitacin B
synergistically enhances the apoptosis-inducing effect of arsenic
trioxide by inhibiting STAT3 phosphorylation in lymphoma Ramos
cells. Leuk Lymphoma. 58:2439–2451. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun SM; Qian Jin, Yi Fang, Jiao Shi, Li
JR, Su L, Ren JL, Jiao ZL and Li PZ: People's Medical Publishing
House. Beijing: pp. 3241998
|
|
5
|
Zhao JC; Sheng Ji, Zong Lu, Zhuan Yao, Yu
YA, Wang LX, Li JR and Wu JH: Anhui: Science & Technology
Publishing House. Anhui: pp. 63–64. 1987
|
|
6
|
Zhang ZJ; Jin Gui, Yao Lue and Fan YS:
Chinese Press of Traditional Chinese Medicine. Beijing: pp.
2232003
|
|
7
|
Kausar H, Munagala R, Bansal SS, Aqil F,
Vadhanam MV and Gupta RC: Cucurbitacin B potently suppresses
non-small-cell lung cancer growth: Identification of intracellular
thiols as critical targets. Cancer Lett. 332:35–45. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou X, Yang J, Wang Y, Li W, Li-Ling J,
Deng Y and Zhang M: Cucurbitacin B inhibits
12-O-tetradecanoylphorbol 13-acetate-induced invasion and migration
of human hepatoma cells through inactivating mitogen-activated
protein kinase and PI3K/Akt signal transduction pathways. Hepatol
Res. 42:401–411. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saglam Yar AS, Alp E, Elmazoglu Z and
Menevse S: Treatment with cucurbitacin B alone and in combination
with gefitinib induces cell cycle inhibition and apoptosis via EGFR
and JAK/STAT pathway in human colorectal cancer cell lines. Hum Exp
Toxicol. 35:526–543. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang M, Sun C, Shan X, Yang X, Li-Ling J
and Deng Y: Inhibition of pancreatic cancer cell growth by
cucurbitacin B through modulation of signal transducer and
activator of transcription 3 signaling. Pancreas. 39:923–929. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang N, Lian Z, Peng X, Li Z and Zhu H:
Applications of Higenamine in pharmacology and medicine. J
Ethnopharmacol. 196:242–252. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bai G, Yang Y, Shi Q, Liu Z, Zhang Q and
Zhu YY: Identification of higenamine in Radix Aconiti Lateralis
Preparata as a beta2-adrenergic receptor agonist1. Acta Pharmacol
Sin. 29:1187–1194. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee SR, Schriefer JM, Gunnels TA, Harvey
IC and Bloomer RJ: Acute oral intake of a higenamine-based dietary
supplement increases circulating free fatty acids and energy
expenditure in human subjects. Lipids Health Dis. 12:1482013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yuan H, Ma Q, Cui H, Liu G, Zhao X, Li W
and Piao G: How can synergism of traditional medicines benefit from
network pharmacology? Molecules. 22:11352017. View Article : Google Scholar
|
|
15
|
Gong J, Cai C, Liu X, Ku X, Jiang H, Gao D
and Li H: ChemMapper: A versatile web server for exploring
pharmacology and chemical structure association based on molecular
3D similarity method. Bioinformatics. 29:1827–1829. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bolton EE, Wang Y, Thiessen PA and Bryant
SH: PubChem: Integrated platform of small molecules and biological
activities. Annu Rep Comput Chem. 4:217–241. 2008. View Article : Google Scholar
|
|
17
|
Mao Y, Hao J, Jin ZQ, Niu YY, Yang X, Liu
D, Cao R and Wu XZ: Network pharmacology-based and clinically
relevant prediction of the active ingredients and potential targets
of Chinese herbs in metastatic breast cancer patients. Oncotarget.
8:27007–27021. 2017.PubMed/NCBI
|
|
18
|
von Mering C, Jensen LJ, Snel B, Hooper
SD, Krupp M, Foglierini M, Jouffre N, Huynen MA and Bork P: STRING:
Known and predicted protein-protein associations, integrated and
transferred across organisms. Nucleic Acids Res. 33:D433–D437.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Davis AP, Grondin CJ, Lennon-Hopkins K,
Saraceni-Richards C, Sciaky D, King BL, Wiegers TC and Mattingly
CJ: The comparative toxicogenomics database's 10th year
anniversary: Update 2015. Nucleic Acids Res. 43(D1): D914–D920.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pathan M, Keerthikumar S, Ang CS, Gangoda
L, Quek CY, Williamson NA, Mouradov D, Sieber OM, Simpson RJ, Salim
A, et al: FunRich: An open access standalone functional enrichment
and interaction network analysis tool. Proteomics. 15:2597–2601.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu XJ, Wagner HN Jr and Tao S:
Measurement of effects of the Chinese herbal medicine higenamine on
left ventricular function using a cardiac probe. Eur J Nucl Med.
8:233–236. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Feng S, Jiang J, Hu P, Zhang JY, Liu T,
Zhao Q and Li BL: A phase I study on pharmacokinetics and
pharmacodynamics of higenamine in healthy Chinese subjects. Acta
Pharmacol Sin. 33:1353–1358. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kang YJ, Lee YS, Lee GW, Lee DH, Ryu JC,
Yun-Choi HS and Chang KC: Inhibition of activation of nuclear
factor kappaB is responsible for inhibition of inducible nitric
oxide synthase expression by higenamine, an active component of
aconite root. J Pharmacol Exp Ther. 291:314–320. 1999.PubMed/NCBI
|
|
25
|
Ni L, Zhu X, Gong C, Luo Y, Wang L, Zhou
W, Zhu S and Li Y: Trichosanthes kirilowii fruits inhibit
non-small cell lung cancer cell growth through mitotic cell-cycle
arrest. Am J Chin Med. 43:349–364. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Oh H, Mun YJ, Im SJ, Lee SY, Song HJ, Lee
HS and Woo WH: Cucurbitacins from Trichosanthes kirilowii as
the inhibitory components on tyrosinase activity and melanin
synthesis of B16/F10 melanoma cells. Planta Med. 68:832–833. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Maddika S, Ande SR, Wiechec E, Hansen LL,
Wesselborg S and Los M: Akt-mediated phosphorylation of CDK2
regulates its dual role in cell cycle progression and apoptosis. J
Cell Sci. 121:979–988. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sliva D, Jedinak A, Kawasaki J, Harvey K
and Slivova V: Phellinus linteus suppresses growth, angiogenesis
and invasive behaviour of breast cancer cells through the
inhibition of AKT signalling. Br J Cancer. 98:1348–1356. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zakikhani M, Blouin MJ, Piura E and Pollak
MN: Metformin and rapamycin have distinct effects on the AKT
pathway and proliferation in breast cancer cells. Breast Cancer Res
Treat. 123:271–279. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Caffarel MM, Andradas C, Mira E,
Pérez-Gómez E, Cerutti C, Moreno-Bueno G, Flores JM, García-Real I,
Palacios J, Mañes S, et al: Cannabinoids reduce ErbB2-driven breast
cancer progression through Akt inhibition. Mol Cancer. 9:1962010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Teixeira C and Pratt MA: CDK2 is a target
for retinoic acid-mediated growth inhibition in MCF-7 human breast
cancer cells. Mol Endocrinol. 11:1191–1202. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tetsu O and McCormick F: Proliferation of
cancer cells despite CDK2 inhibition. Cancer Cell. 3:233–245. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ortega S, Prieto I, Odajima J, Martín A,
Dubus P, Sotillo R, Barbero JL, Malumbres M and Barbacid M:
Cyclin-dependent kinase 2 is essential for meiosis but not for
mitotic cell division in mice. Nat Genet. 35:25–31. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Berthet C, Aleem E, Coppola V, Tessarollo
L and Kaldis P: Cdk2 knockout mice are viable. Curr Biol.
13:1775–1785. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liang J and Slingerland JM: Multiple roles
of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell
Cycle. 2:339–345. 2003. View Article : Google Scholar : PubMed/NCBI
|