Introduction
Lung cancer is considered the second most common
cancer and the leading cause of cancer-associated mortalities in
both males and females in the United States (1). Based on the differences in clinical
behavior and purposes of treatment, lung cancers can be divided
into two broad categories, including small cell lung cancer and
non-small cell lung cancer (NSCLC). NSCLC is the major type of lung
cancer which accounts for 85% of lung cancer cases (2,3).
Despite lung cancer patients can be successfully treated, at least
to some extent, by conventional therapeutic strategies including
surgery, radiotherapy and chemotherapy, however the majority of
patients do not respond well to chemo- and/or radio-therapeutic
regimens and the prognosis still remains poor, with a dismal 5-year
survival rate of <15% (4).
Therefore, it is of great importance to discover novel effective
therapeutic agents to treat lung cancer.
Unlike synthesized chemo-therapeutic agents which
have severe side-effects, traditional Chinese medicines (TCMs),
which are made from purely natural compounds, have attracted
increasing attention due to less adverse effects for the treatment
of cancer (5). A large number of
monomer compositions extracted from TCMs have been revealed to
exhibit anticancer effects through not only inhibiting cancer cell
proliferation, migration and invasion, but also promoting apoptosis
and programmed cell death (6). For
example, paclitaxel, one of the most successful TCMs extracted from
T. cuspidata trees, has been revealed to have significant
anticancer activity in clinical trials against many types of solid
tumors, particularly against metastatic breast cancer and
refractory ovarian cancer (7).
Notably, in recent years, the active molecular components extracted
from the roots of Salvia miltiorrhiza Bunge (also named
Danshen) have been widely used for treating numerous cardiovascular
and endocrine diseases such as coronary artery disease, angina
pectoris, hepatitis and menstrual disorders (8). Among them, cryptotanshinone (CPT) has
been demonstrated to have bioactivities including
anti-inflammatory, anti-oxidative stress and antiplatelet
aggregation properties (9).
Previously, increasing evidence indicates that CPT appears to exert
diverse anticancer properties against numerous human cancer cell
lines, such as prostate cancer (10), leukemia (11), gliomas (12), hepatic carcinomas (13), pancreatic (14), breast (15), colorectal (16) and melanoma cancer (17). However, only a few studies have
reported the anticancer effects of CPT on lung cancer, and more
importantly, the underlying mechanism is largely unknown.
The tyrosine kinase insulin-like growth factor 1
receptor (IGF-1R) plays a pivotal role in the development of cell
growth, differentiation and progression of various cancers
(18). It has been reported that
IGF-1R is overexpressed in many human carcinomas, including breast,
prostate and colon cancers (19,20).
Conversely, evidence shows that high circulating levels of IGF-1, a
ligand of IGF-1R, are associated with increased risk of multiple
types of cancers which mainly depends on activation of the
downstream signaling of the IGF-1/IGF-1R (21,22).
Following activation by IGF-1, the tyrosine kinase activity of
IGF-1R phosphorylates the downstream substrates, which in turn
results in activation of its downstream signaling pathways,
including the phosphatidylinositide 3-kinase/RAC-alpha
serine/threonine-protein kinase (PI3K/Akt) signaling cascade
(23). Over-activation of PI3K/Akt
signaling cascades are largely involved in tumorigenesis and
metastasis by extensively phosphorylating apoptotic effector
molecules, subsequently leading to cell proliferation and
anti-apoptosis (24). Thus,
interference with IGF-1R signaling is considered as a potentially
effective therapeutic strategy for cancer.
Given the inhibitory effects of CPT on
tumorigenesis, the present study aimed to investigate the
anticancer effects of CPT on human lung carcinoma A549 and H1299
cells, and explore the potential underlying mechanisms by which it
exerts these effects. The results demonstrated that CPT inhibited
IGF-1R/PI3K/Akt signaling, which resulted in the inhibition of cell
proliferation and migration of lung cancer.
Materials and methods
Materials
IGF-1 and LY294002 were purchased from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany). CPT was purchased from Tocris
Cookson, Inc. (Bristol, UK), Antibodies for western blot analysis,
including phospho-Tyr1135/1136 IGF-1R antibody, IGF-1R
antibody, phospho-Ser473Akt antibody, Akt antibody,
β-actin antibody, goat anti-rabbit horseradish peroxidase
(HRP)-conjugated were purchased from Cell Signaling Technology,
Inc. (Danvers, MA, USA). Reagents used for cell culture including
RPMI-1640 and fetal bovine serum (FBS) were purchased from Gibco
(Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Cell culture
Human lung adenocarcinoma cells including A549 and
H1299 were obtained from the Cell Bank of Chinese Academy of
Sciences (Shanghai, China) and cultured according to
specifications. The cells were grown in RPMI-1640 medium
supplemented with 10% fetal bovine serum (FBS) and were maintained
at 37°C in a humidified atmosphere containing 5%
CO2.
Cell proliferation assay
An MTT assay was used to analyze cell proliferation.
Briefly, cells in the logarithmic growth phase were seeded in
24-well culture plates at a density of 5×104 cells/well
and cultured overnight. Cells were treated with different
concentrations of CPT for indicated time points (12, 24, 36 and 48
h). Following incubation, 20 µl MTT (5 mg/ml, Beyotime Institute of
Biotechnology, Haimen, China) was added to each well according to
the manufacturer's protocol, and cells were incubated in complete
media at 37°C for 3 h. The supernatant was removed, then 500 µl
dimethyl sulfoxide was added to each well of the 24-well plate and
gently shaken for 10 min. The absorbance (optical density) at a
wavelength of 570 nm was measured using an enzyme-linked
immunosorbent assay reader and used to represent the viability of
cells. Results were calculated as percentage of absorbance in
control cultures.
Wound healing assay
A total of 2×105 cells were seeded into
6-well plates and cultured to reach 80–90% confluence. A yellow
pipette tip was used to scratch a wound on the midline of each
well. The migration of the cells was monitored photographically
under a light microscope (Olympus IX83; Olympus Corporation, Tokyo,
Japan) and the difference in the area of the wounds was measured
before or after application of CPT. The migration rate was
expressed as a percentage of the control and was calculated as the
proportion of the mean distance between both borderlines to the
distance that remained cell free after regrowth.
Colony formation assay
Cells were seeded in a 6-well plate (500
cells/plate) and then treated with CPT for another two weeks. The
medium containing CPT was replaced with fresh culture medium every
2–3 days. After two weeks, the supernatants were discarded and the
cells were carefully washed with phosphate-buffered saline (PBS).
Subsequently, cells were fixed with 4% paraformaldehyde for 15 min
(at room temperature) and then stained in crystal violet for 20 min
(at room temperature). Images of the colonies were captured by a
digital camera.
Transwell assay
Cell migration was assessed using 24-well Transwell
cell culture chambers (Corning Incorporated, Corning, NY, USA),
according to the manufacturer's protocol. Cells were treated with
different concentrations of CPT (5 and 20 µM) for 24 h. Cells were
digested with trypsin and resuspended in serum-free medium. Then
upper chambers were seeded with 200 µl cells (1×105
cells) in serum free medium, and 600 µl medium supplemented with
10% serum was added to the bottom chambers. The chamber was
incubated for 24 h at 37°C, and after washing twice with PBS, cells
were fixed with cold 4% paraformaldehyde at room temperature and
then stained with crystal violate for 15 min at room temperature.
Cells on the bottom surface of the membrane were imaged under an
inverted microscope and cells in five randomly selected fields were
counted and quantified.
Western blotting
Cells were lysed with SDS lysis buffer (Beyotime
Institute of Biotechnology, Haimen, China) supplemented with
protease inhibitor cocktail. After determination of protein
concentration using a modified BCA protein assay (Beyotime
Institute of Biotechnology, Haimen, China), equal amounts of
protein (20 µg) were separated by 12% (for pIGF-1R and IGF-1R, 10%)
SDS-PAGE and transferred to nitrocellulose membranes (EMD
Millipore, Billerica, MA, USA). Following blocking with
Tris-buffered saline containing 5% non-fat dry milk and 0.1%
Tween-20 for 1 h at room temperature, membranes were incubated
overnight at 4°C with anti-phospho-IGF-1R (cat. no. 3024; 1:2,000),
anti-IGF-1R (cat. no. 9750; 1:2,000), anti-phospho-Akt (cat. no.
9271; 1:2,000), anti-Akt (cat. no. 9272; 1:2,000), anti-β-actin
(cat. no. 4970; 1:4,000), followed by a 2 h incubation with
horseradish peroxidase (HRP)-linked secondary antibodies (cat. no.
7074; 1:20,000) at room temperature. Immunoreactive bands were
visualized by enhanced chemiluminescence (Pierce, Thermo Fisher
Scientific, Inc.), and semi-quantification was conducted using
ImageJ 1.47t software (National Institutes of Health, Bethesda, MD)
and normalization total protein.
Statistical analysis
Data are presented as the mean ± standard error of
the mean from at least three independent experiments and were
analyzed by GraphPad Software Prism 6.0 (GraphPad Software, Inc.,
La Jolla, CA, USA). Statistical analysis of data was performed
using one-way analysis of variance with Bonferroni's multiple
comparison tests, or Student's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
CPT inhibits the cell proliferation of
A549 and H1299 lung cancer cells
As lung cancer is caused by excessive cell growth in
the lung and then spreads to nearby tissue in metastasis, the
present study first investigated the potential role of CPT on cell
growth of lung cancer A549 cells. Cells were treated with various
concentrations of CPT (0, 1, 5, 10, 20, 30 and 50 µM) for 48 h. The
cell viability was determined by an MTT assay. As presented in
Fig. 1, CPT significantly inhibited
A549 cell viability in a dose-dependent manner (Fig. 1A and B). Compared with the control
group, concentrations of <5 µM had no significant effect,
whereas concentrations of >5 µM progressively inhibited the
growth of A549 cells (Fig. 1B).
Next, the effects of CPT for different treatment times on A549 cell
growth were explored, and cells were treated with 20 µM CPT for 12,
24, 36 or 48 h. As presented in Fig.
1C, CPT significantly inhibited A549 cell growth, except for at
12 h incubation, with the strongest effect at 48 h (Fig. 1C). To confirm whether CPT inhibited
cell proliferation of other lung cancer cells, the present study
detected the antitumor effect of CPT on H1299 cells, another human
non-small cell lung cancer cell line. As presented in Fig. 1D and E, similar results were
obtained in H1299 lung cancer cells.
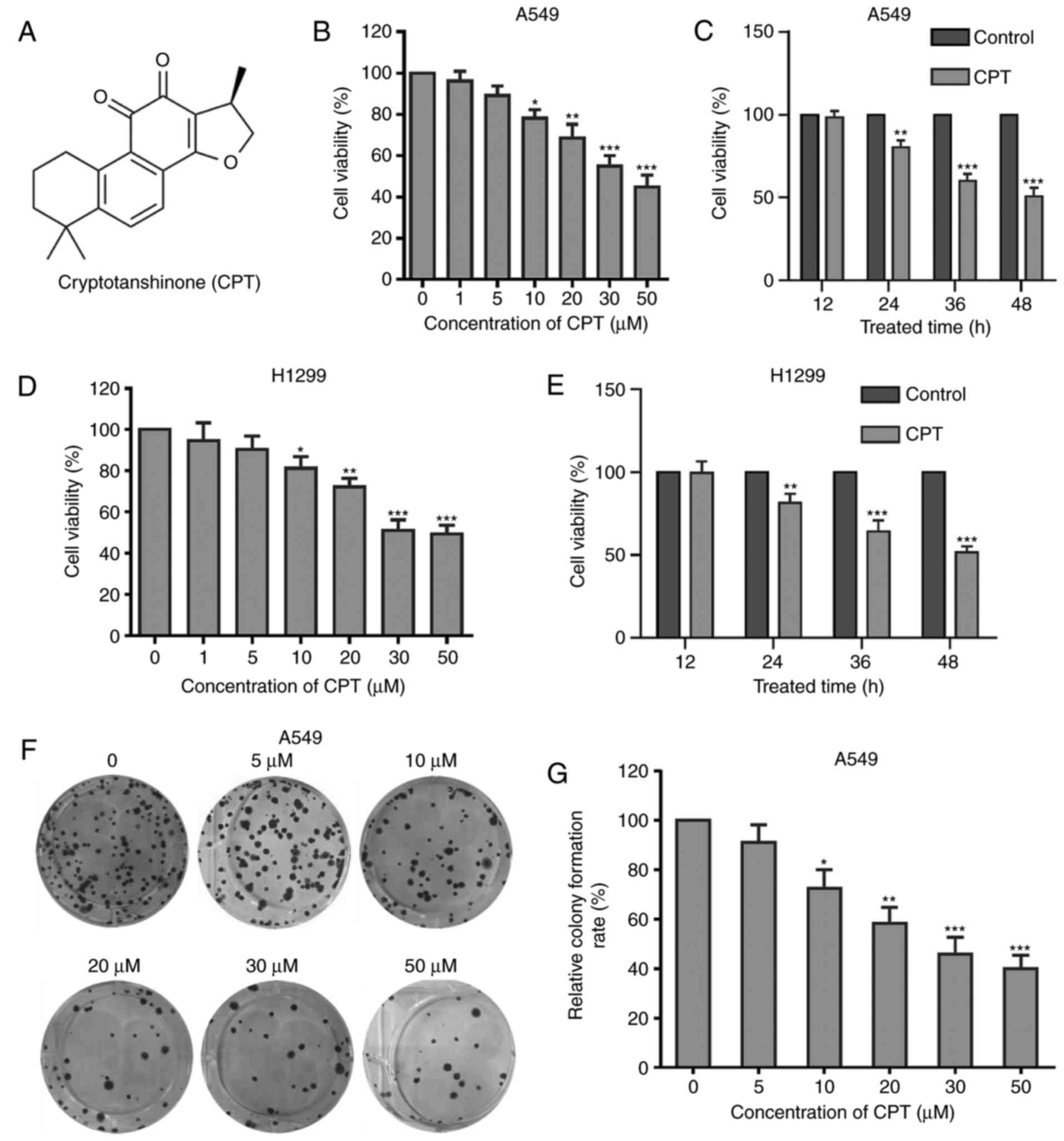 | Figure 1.CPT inhibits the cell proliferation
of A549 and H1299 cells. (A) The chemical structure of CPT. (B)
Effects of different concentrations of CPT on the cell viability of
A549 cells. Cells were treated with increasing concentrations of
CPT (1, 5, 10, 20, 30 or 50 µM) for 48 h. Cell viability was
measured by the MTT assay. (C) Effects of CPT (20 µM) on the
viability of A549 cells after 12, 24, 36 and 48 h treatment. (D)
Effects of different concentrations of CPT on the cell viability of
H1299 cells. (E) Effects of CPT (20 µM) on the viability of H1299
cells after 12, 24, 36 and 48 h treatment. Data are expressed as
the mean ± standard error of the mean SEM (three independent
experiments). (F) Cells were pretreated with CPT at the indicated
concentrations for two weeks and colonies were photographed. (G)
Quantitation of colony formation results. Data was expressed as
percentage of control. *P<0.05, **P<0.01, ***P<0.001 vs.
control group. CPT, crytotanshinone. |
To further explore the effects of CPT on A549 cell
growth, a colony formation assay was performed. Consistent with the
MTT assay, CPT significantly suppressed the colony formation of
A549 cells in a dose-dependent manner (Fig. 1F and G). Taken together, the
aforementioned results indicated that CPT exhibits a significant
inhibitory effect toward cell growth in lung cancer cells.
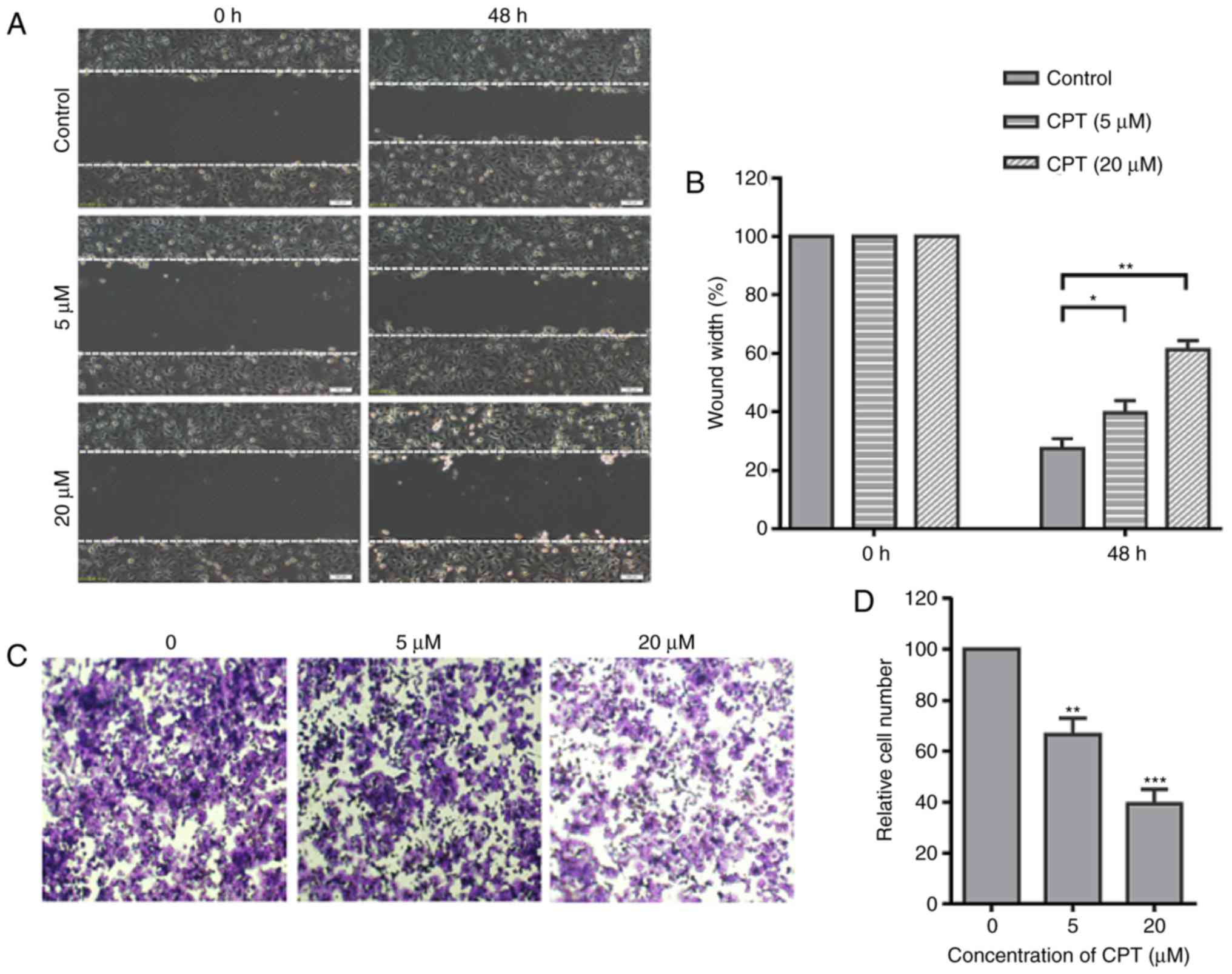
CPT significantly suppresses the
migration of A549 cells
Migration of cancer cells is a key factor
responsible for cancer metastasis, and the present study
investigated whether CPT could affect the migration of A549 cells.
To investigate the effects of CPT on the migration of A549 cells, a
typical scratch wound healing assay was performed to measure the
migration ratio. The confluent monolayer was scraped with a sterile
yellow micropipette tip to create a scratch wound. After incubation
with 5 or 20 µM CPT for 48 h, the cells migrated to the denuded
zone and then the width of gaps of the wound area were measured. As
presented in Fig. 2, CPT
significantly inhibited the migration of A549 cells at 48 h after
treatment (Fig. 2A and B). To
further confirm this result, a Transwell assay was performed to
quantify the migratory ability of A549 cells. The results revealed
that CPT induced a dose-dependent inhibition in migration with CPT
(Fig. 2C and D). These results
indicated that CPT significantly inhibits the migration of A549
cells, even at a low dose.
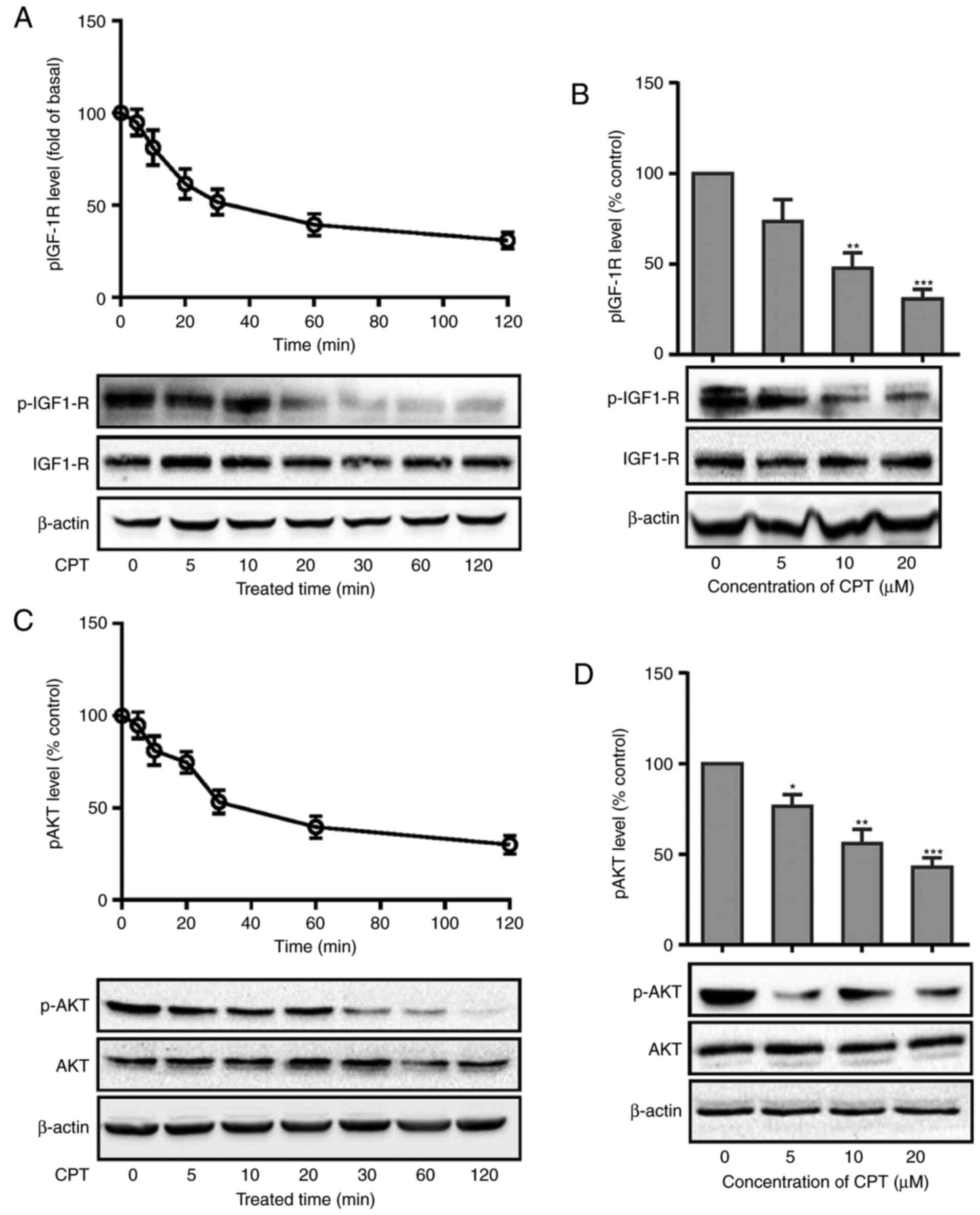
CPT inhibits IGF-1R and Akt
phosphorylation in A549 cells
The activation of receptor tyrosine kinases such as
IGF-1R signaling has been reported to be crucial for lung cancer
cell proliferation, survival, invasion and angiogenesis (25). Therefore, we explore whether IGF-1R
signaling is involved in the anti-proliferation effect of CPT.
Cells were treated with CPT (20 µM) for different time points as
indicated and IGF-1R phosphorylation was detected by western
blotting. As presented in Fig. 3A,
CPT caused a rapid decrease in the phosphorylation level of IGF-1R
(Fig. 3A). However, it had no
effect on the total IGF-1R expression levels. Following this, the
phosphorylation level of IGF-1R was detected after cells were
treated with different concentrations of CPT (5, 10 and 20 µM) for
30 min. As presented in Fig. 3B,
IGF-1R phosphorylation was also significantly decreased (Fig. 3B).
The PI3K/Akt signaling pathway, which is an
important downstream signaling cascade of IGF-1R, regulates various
cellular functions relevant to the growth and progression of lung
cancer (26). The present study
next measured the effects of CPT on the phosphorylation level of
Akt. As presented in Fig. 3C, CPT
significantly decreased the phosphorylation level of Akt in the
same manner as IGF-1R phosphorylation (Fig. 3C and D). The aforementioned results
suggest that CPT can significantly inhibit the IGF-1R/Akt signaling
pathway in A549 cells.
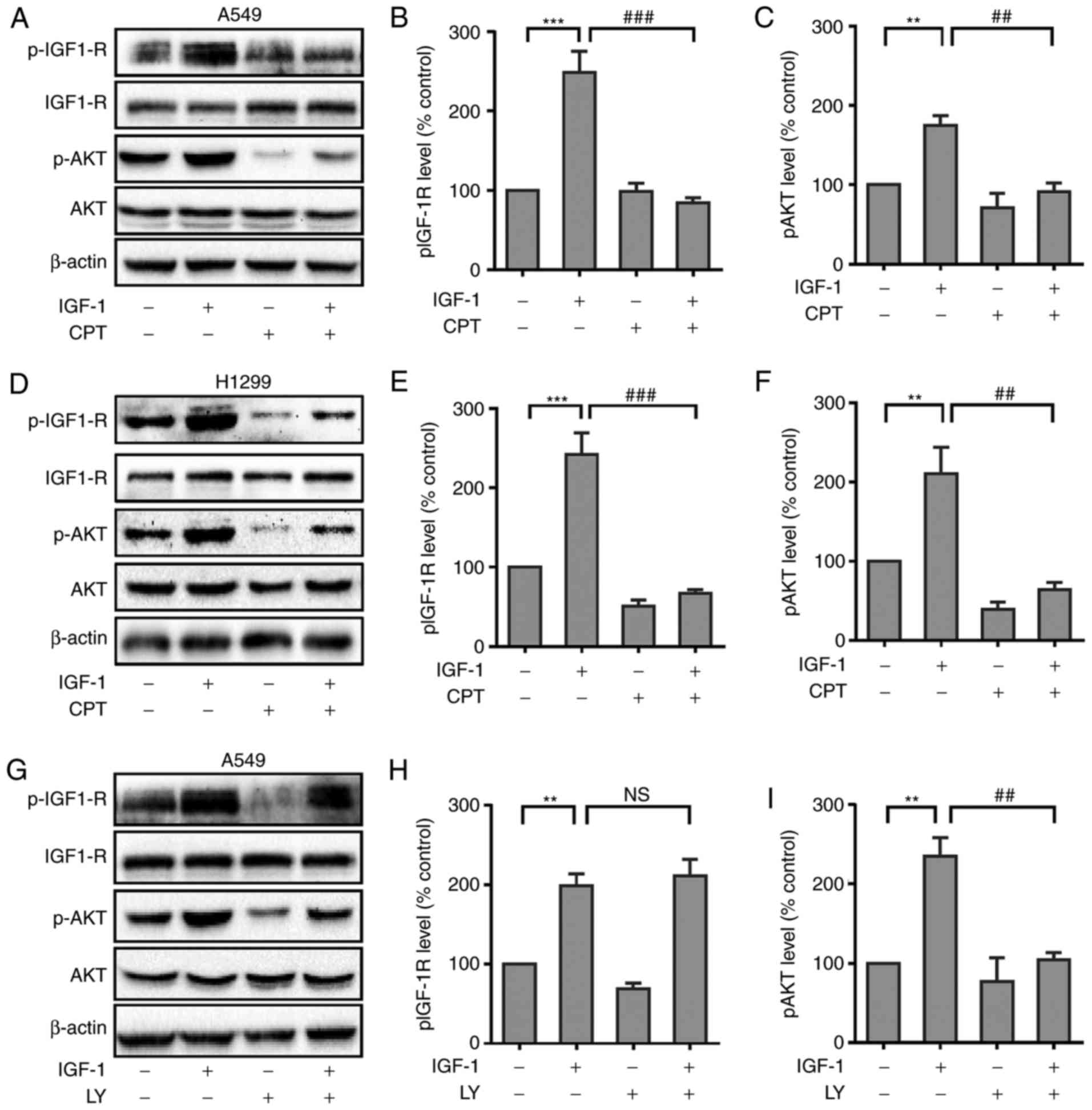
CPT inhibits IGF-1-induced Akt
phosphorylation in A549 and H1299 cells
It has been demonstrated that high circulating
levels of IGF-1 are associated with increased risk of multiple
types of cancers including lung cancer. It was then determined
whether CPT could inhibit IGF-1-induced activation of IGF-1R
signaling. As presented in Fig. 4A,
compared with control group, the phosphorylation level of IGF-1R
was greatly enhanced in A549 cells treated with IGF-1. As expected,
pretreatment of A549 cells with CPT significantly inhibited IGF-1
induced phosphorylation of IGF-1R (Fig.
4A and B). Furthermore, CPT also inhibited the IGF-1-induced
phosphorylation of Akt (Fig. 4A and
C). Additionally, similar results were observed in H1299 cells
(Fig. 4D-F), suggesting CPT can
significantly inhibit IGF-1R and Akt signaling cascades in lung
cancer cells.
To confirm whether Akt phosphorylation was
downstream of IGF-1R in lung cancer cells, the present study next
evaluated the effects of the PI3K selective inhibitor LY294002 on
IGF-1-induced Akt phosphorylation in A549 cells. Cells were
pretreated with LY294002 (20 µM) for 30 min and then stimulated
with IGF-1. It was demonstrated that LY294002 did not have a
significant effect on IGF-1R phosphorylation (Fig. 4G and H), however blocked
IGF-1-induced Akt phosphorylation (Fig.
4G and I), suggesting Akt acts as a downstream factor of PI3K
signaling in A549 cells. This suggested that PI3K acts as a
downstream signaling molecule of IGF-1R.
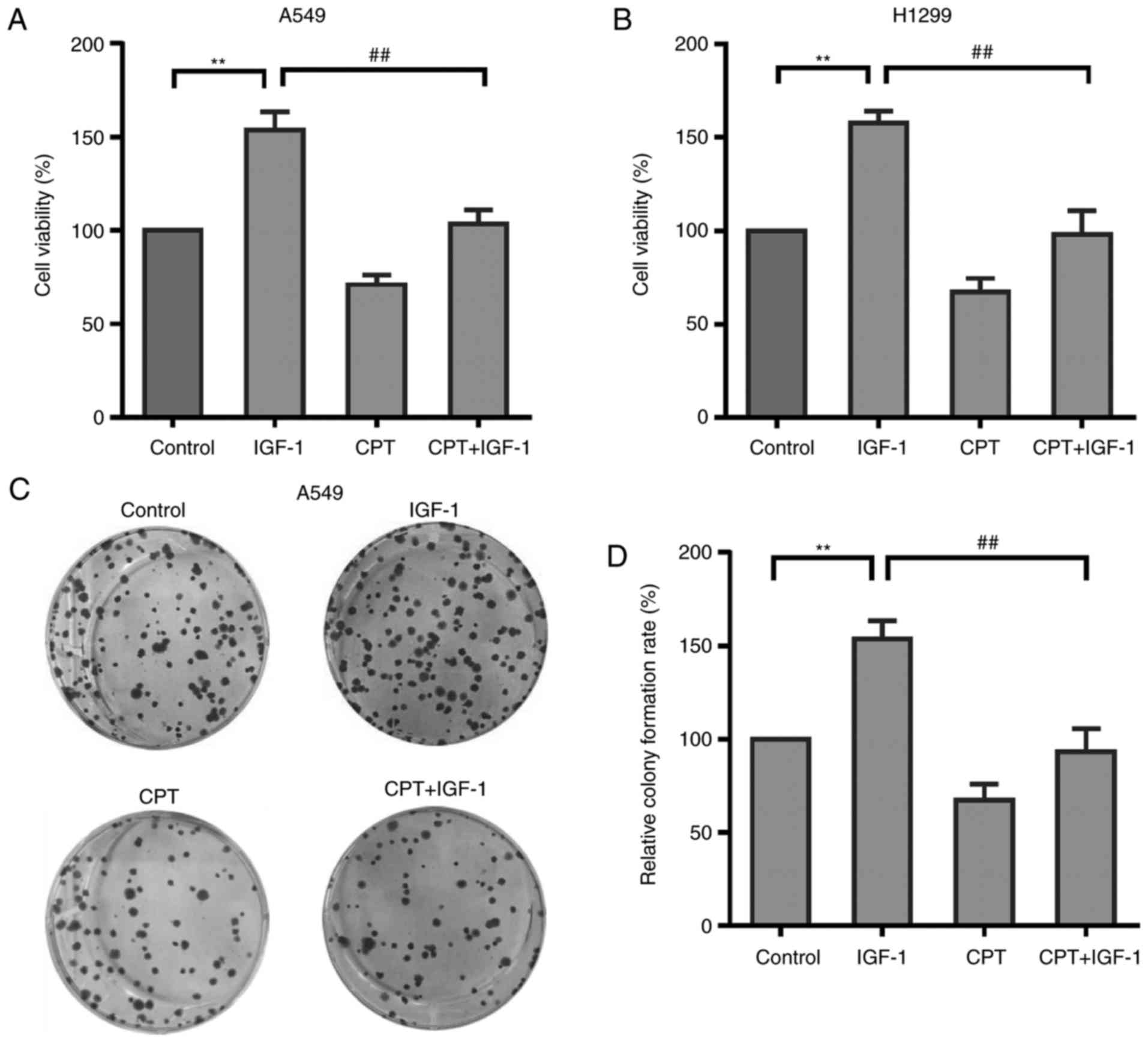
CPT inhibits IGF-1-induced A549 and
H1299 cell proliferation
Given the ability of CPT to significantly inhibit
proliferation and IGF-1R/PI3K/Akt phosphorylation in A549 cells, it
was hypothesized that the inhibitory effect of CPT on cell
proliferation was mediated by the IGF-1R/PI3K/Akt pathway. To
verify this hypothesis, the present study determined whether CPT
was able to inhibit IGF-1-induced cell proliferation of A549 cells
or not. The cells were treated with IGF-1 (20 ng/ml) alone or in
combination with CPT (20 µM) for 48 h and then cell viability was
measured by MTT assay. As presented in Fig. 5A, IGF-1 treatment alone
significantly increased cell proliferation of A549 cells, which is
associated with the activated IGF-1R/PI3K/Akt pathway. However,
this IGF-1-enhanced proliferation was significantly suppressed by
CPT pretreatment (Fig. 5A). CPT was
also able to inhibit IGF-1-induced cell proliferation in H1299
cells (Fig. 5B). The result was
verified by a colony formation assay. As expected, CPT
significantly inhibited IGF-1 induced colony formation capacity
(Fig. 5B and C). Taken together,
these results suggest that CPT may suppress A549 cell proliferation
via inhibiting IGF-1R/PI3K/Akt pathway.
Discussion
Lung cancer is a leading cause of cancer-associated
mortality worldwide. An increasing number of people, particularly
adult men, suffer fatalities from lung cancer than any other type
of cancers. Due to the high incidence and mortality of lung cancer,
development of novel therapeutic agents is urgently needed. The
present study investigated the anticancer effects of CPT on human
lung carcinoma A549 and H1299 cells and explored the potential cell
signaling mechanism. The results indicated that CPT not only
inhibited the proliferation and migration, but also suppressed the
IGF-1 induced proliferation of A549 and H1299 cells. Furthermore,
the anticancer effect of CPT may be attributable to inhibition of
the IGF-1R/PI3K/Akt signaling pathway.
The traditional Chinese medicine Salvia
miltiorrhiza Bunge has been used as an anticancer agent for
many years. To date, several effective compounds including
tanshinones I, IIA and IIB, dihydrotanshinone and CPT which are
extracted from the roots of Salvia miltiorrhiza Bunge have
been demonstrated to possess an anticancer effect in multiple
cancer types. However, most studies focused on the anti-oxidant,
anti-inflammatory and anticancer effects (7) of tanshinone I and IIA (8). In recent years, more and more studies
indicated that CPT also exerts anticancer effects, mainly by
inhibiting cancer cell proliferation and migration and inducing
cancer cell apoptosis in multiple cancer types. A recent study
showed that CPT inhibits the cell growth of lung cancer cells
(27). However, the underlying
molecular mechanisms of such an effect are not fully understood.
The present study investigated the potential anticancer effect of
CPT and revealed the underlying mechanisms: CPT suppressed lung
cancer cell proliferation and migration through inhibiting
IGF-1R/PI3K/Akt signaling cascades.
It has been reported that IGF-1R is aberrantly
expressed in many types of cancers. Both increased IGF-1R
expression levels and high circulating levels of its ligand IGF-1
are associated with higher risk of developing various malignant
tumors which mainly depends on activation of the downstream cascade
of IGF-1/IGF-1R signaling (21,28).
Notably, an increasing number of studies have demonstrated that
IGF-1R-mediated downstream signaling pathways are overactivated
during the early stages of carcinogenesis in various malignant
tumors including lung cancer, and a role for IGF-1R signaling has
been demonstrated not only in primary tumor formation but also in
progression to more aggressive lung adenocarcinoma (13). Based on the aforementioned findings,
IGF-1R is considered as an important target for the prevention of
lung cancer and various other types of cancer formation. The
present study demonstrated that CPT inhibited IGF-1R
phosphorylation in both A549 and H1299 lung cancer cells. CPT also
inhibited IGF-1-induced IGF-1R phosphorylation and cell
proliferation of these cells. These results suggested that CPT may
suppress A549 and H1299 cell proliferation through IGF-1R
signaling. However, the underlying mechanism by which CPT acts to
suppress IGF-1R phosphorylation requires further investigation in
the future. Since other growth factor receptors like epidermal
growth factor receptor (EGFR) are overexpressed in 40–80% of NSCLCs
(28), whether CPT can also inhibit
EGFR signaling remains elusive.
The PI3K/Akt pathway integrates signals from
external cellular stimuli such as growth factors and cytokines to
regulate essential cellular functions and is aberrantly activated
in various types of human cancers (24). Accumulating studies indicate that
the PI3K/Akt pathway plays a critical role in regulating various
cellular functions such as proliferation, survival, invasiveness
and metastasis of cancer cells (29,30).
Moreover, clinical studies also demonstrated that activation of the
PI3K/Akt pathway is highly correlated with tumor progression and
reduced patient survival (31).
Thus, the PI3K/Akt pathway is considered a promising target for
anticancer pharmacological agents (32). The present study demonstrated that
CPT not only blocked basal Akt phosphorylation but also inhibited
IGF-1 induced Akt activation. Together with the results that CPT
inhibited both basal level and IGF-1-induced A549 and H1299 cell
proliferation, the data suggest the involvement of the PI3K/Akt
pathway in CPT-induced inhibition of cell proliferation.
Deregulation of apoptosis is the hallmark of all
cancer cells, and the induction of apoptosis has been described as
a standard and the most efficient strategy in anticancer therapy
(33). The present study focused on
the effect of CPT on inhibiting proliferation and migration, and it
is worth noting that CPT also displays a pro-apoptosis effect in
several human cancers including melanoma cells, and colorectal
cancer cell lines (16,17). A previous study indicated that CPT
can promote apoptosis not only in human lung cancer cells, but also
in xeno-grafts and inhibit the growth of tumors in vivo
(27). However, the underlying
signaling mechanisms of CPT-mediated pro-apoptotic effects are not
fully understood. The present results indicated that CPT can reduce
IGF-1R phosphorylation and PI3K/Akt activation. It was hypothesized
that inhibition of IGF-1R and downstream signaling cascades may
also play important roles in the pro-apoptotic effect of CPT. IGF-1
is widely known as a mitogenic and anti-apoptotic factor, and
activation of IGF-1R results in both proliferative and
anti-apoptotic signals in cancer cells (34). Activation of PI3K/Akt signaling
cascades have been reported to induce a strong survival signal by
arresting cells in the G0/G1 phase of the cell cycle via modulation
of mammalian target of rapamycin and regulating cyclin D1 (35). Akt activation can extensively
phosphorylate apoptotic effector molecules, such as B cell
lymphoma-2, MCL-1, Bim, Bad and many others to promote cell
survival (24,36). However, whether CPT can induce
apoptosis in lung cancer cells remains unclear and requires further
investigation.
In conclusion, the results indicated that CPT not
only inhibited the proliferation and migration, but also suppressed
the IGF-1 induced proliferation of lung cancer cells. Furthermore,
CPT inhibited IGF-1 induced phosphorylation of IGF-1R and Akt,
suggesting the anticancer effect of CPT may be attributable to
inhibition of the IGF-1R/PI3K/Akt signaling pathway. The data
provides evidence that CPT could be developed as a potential
therapeutic agent for the treatment of lung cancer.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the Natural
Science Foundation of China (grant nos. 81460444, 81660391), the
Key Program of the Natural Science Foundation of Jiangxi Province
of China (grant no. S2016ZRZDB0105), the Natural Science Foundation
of Jiangxi Province of China (grant nos. 20132BBG20010 and
20151BAB205036).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
JZ and BY conceived and designed the study. JZ, GW
and LS performed most of the experiments. WY, RW and QZ performed
parts of the experiments. GZ performed and analyzed parts of the
western blotting data. JZ and BY wrote the manuscript. WY and GZ
reviewed and edited the manuscript. All authors read and approved
the manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CPT
|
cryptotanshinone
|
|
EGFR
|
epidermal growth factor receptor
|
|
IGF-1
|
insulin-like growth factor 1
|
|
IGF-1R
|
insulin-like growth factor 1
receptor
|
|
NSCLC
|
non-small cell lung cancer
|
|
PI3K
|
phosphoinositide 3-kinase
|
|
TCM
|
Traditional Chinese medicine
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sakashita S, Sakashita M and Tsao Sound M:
Genes and pathology of non-small cell lung carcinoma. Semin Oncol.
41:28–39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hu P, He J, Liu S, Wang M, Pan B and Zhang
W: beta2-adrenergic receptor activation promotes the proliferation
of A549 lung cancer cells via the ERK1/2/CREB pathway. Oncol Rep.
36:1757–1763. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Youlden DR, Cramb SM and Baade PD: The
international epidemiology of lung cancer: Geographical
distribution and secular trends. J Thorac Oncol. 3:819–831. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Efferth T, Li PC, Konkimalla VS and Kaina
B: From traditional Chinese medicine to rational cancer therapy.
Trends Mol Med. 13:353–361. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tan W, Lu J, Huang M, Li Y, Chen M, Wu G,
Gong J, Zhong Z, Xu Z, Dang Y, et al: Anticancer natural products
isolated from chinese medicinal herbs. Chin Med. 6:272011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Singla AK, Garg A and Aggarwal D:
Paclitaxel and its formulations. Int J Pharm. 235:179–192. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu CY, Cherng JY, Yang YH, Lin CL, Kuan
FC, Lin YY, Lin YS, Shu LH, Cheng YC, Liu HT, et al: Danshen
improves survival of patients with advanced lung cancer and
targeting the relationship between macrophages and lung cancer
cells. Oncotarget. 8:90925–90947. 2017.PubMed/NCBI
|
|
9
|
Chen X, Guo J, Bao J, Lu J and Wang Y: The
anticancer properties of Salvia miltiorrhiza Bunge
(Danshen): A systematic review. Med Res Rev. 34:768–794. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shin DS, Kim HN, Shin KD, Yoon YJ, Kim SJ,
Han DC and Kwon BM: Cryptotanshinone inhibits constitutive signal
transducer and activator of transcription 3 function through
blocking the dimerization in DU145 prostate cancer cells. Cancer
Res. 69:193–202. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim JH, Jeong SJ, Kwon TR, Yun SM, Jung
JH, Kim M, Lee HJ, Lee MH, Ko SG, Chen CY, et al: Cryptotanshinone
enhances TNF-alpha-induced apoptosis in chronic myeloid leukemia
KBM-5 cells. Apoptosis. 16:696–707. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu L, Li C, Li D, Wang Y, Zhou C, Shao W,
Peng J, You Y, Zhang X and Shen X: Cryptotanshinone inhibits human
glioma cell proliferation by suppressing STAT3 signaling. Mol Cell
Biochem. 381:273–282. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park IJ, Yang WK, Nam SH, Hong J, Yang KR,
Kim J, Kim SS, Choe W, Kang I and Ha J: Cryptotanshinone induces G1
cell cycle arrest and autophagic cell death by activating the
AMP-activated protein kinase signal pathway in HepG2 hepatoma.
Apoptosis. 19:615–628. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ge Y, Yang B, Chen Z and Cheng R:
Cryptotanshinone suppresses the proliferation and induces the
apoptosis of pancreatic cancer cells via the STAT3 signaling
pathway. Mol Med Rep. 12:7782–7788. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li S, Wang H, Hong L, Liu W, Huang F, Wang
J, Wang P, Zhang X and Zhou J: Cryptotanshinone inhibits breast
cancer cell growth by suppressing estrogen receptor signaling.
Cancer Biol Ther. 16:176–184. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li W, Saud SM, Young MR, Colburn NH and
Hua B: Cryptotanshinone, a Stat3 inhibitor, suppresses colorectal
cancer proliferation and growth in vitro. Mol Cell Biochem.
406:63–73. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ye T, Zhu S, Zhu Y, Feng Q, He B, Xiong Y,
Zhao L, Zhang Y, Yu L and Yang L: Cryptotanshinone induces melanoma
cancer cells apoptosis via ROS-mitochondrial apoptotic pathway and
impairs cell migration and invasion. Biomed Pharmacother.
82:319–326. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Werner H and Bruchim I: The insulin-like
growth factor-I receptor as an oncogene. Arch Physiol Biochem.
115:58–71. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Resnik JL, Reichart DB, Huey K, Webster NJ
and Seely BL: Elevated insulin-like growth factor I receptor
autophosphorylation and kinase activity in human breast cancer.
Cancer Res. 58:1159–1164. 1998.PubMed/NCBI
|
|
20
|
Weber MM, Fottner C, Liu SB, Jung MC,
Engelhardt D and Baretton GB: Overexpression of the insulin-like
growth factor I receptor in human colon carcinomas. Cancer.
95:2086–2095. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sharon C, Baranwal S, Patel NJ,
Rodriguez-Agudo D, Pandak WM, Majumdar AP, Krystal G and Patel BB:
Inhibition of insulin-like growth factor receptor/AKT/mammalian
target of rapamycin axis targets colorectal cancer stem cells by
attenuating mevalonate-isoprenoid pathway in vitro and in vivo.
Oncotarget. 6:15332–15347. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Teng JA, Wu SG, Chen JX, Li Q, Peng F, Zhu
Z, Qin J and He ZY: The activation of ERK1/2 and JNK MAPK signaling
by insulin/IGF-1 is responsible for the development of colon cancer
with type 2 diabetes mellitus. PLoS One. 11:e01498222016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou Y, Zeng C, Li X, Wu PL, Yin L, Yu XL,
Zhou YF and Xue Q: IGF-I stimulates ERbeta and aromatase expression
via IGF1R/PI3K/AKT-mediated transcriptional activation in
endometriosis. J Mol Med. 94:887–897. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hennessy BT, Smith DL, Ram PT, Lu Y and
Mills GB: Exploiting the PI3K/AKT pathway for cancer drug
discovery. Nat Rev Drug Discov. 4:988–1004. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim WY, Jin Q, Oh SH, Kim ES, Yang YJ, Lee
DH, Feng L, Behrens C, Prudkin L, Miller YE, et al: Elevated
epithelial insulin-like growth factor expression is a risk factor
for lung cancer development. Cancer Res. 69:7439–7448. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim WY, Prudkin L, Feng L, Kim ES,
Hennessy B, Lee JS, Lee JJ, Glisson B, Lippman SM, Wistuba II, et
al: Epidermal growth factor receptor and K-Ras mutations and
resistance of lung cancer to insulin-like growth factor 1 receptor
tyrosine kinase inhibitors. Cancer. 118:3993–4003. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen L, Wang HJ, Xie W, Yao Y, Zhang YS
and Wang H: Cryptotanshinone inhibits lung tumorigenesis and
induces apoptosis in cancer cells in vitro and in vivo. Mol Med
Rep. 9:2447–2452. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Peled N, Wynes MW, Ikeda N, Ohira T,
Yoshida K, Qian J, Ilouze M, Brenner R, Kato Y, Mascaux C, et al:
Insulin-like growth factor-1 receptor (IGF-1R) as a biomarker for
resistance to the tyrosine kinase inhibitor gefitinib in non-small
cell lung cancer. Cell Oncol. 36:277–288. 2013. View Article : Google Scholar
|
|
29
|
Engelman JA: Targeting PI3K signalling in
cancer: Opportunities, challenges and limitations. Nat Rev Cancer.
9:550–562. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Thorpe LM, Yuzugullu H and Zhao JJ: PI3K
in cancer: Divergent roles of isoforms, modes of activation and
therapeutic targeting. Nat Rev Cancer. 15:7–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun CH, Chang YH and Pan CC: Activation of
the PI3K/Akt/mTOR pathway correlates with tumour progression and
reduced survival in patients with urothelial carcinoma of the
urinary bladder. Histopathology. 58:1054–1063. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Luo J, Manning BD and Cantley LC:
Targeting the PI3K-Akt pathway in human cancer: Rationale and
promise. Cancer Cell. 4:257–262. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kelly PN and Strasser A: The role of Bcl-2
and its pro-survival relatives in tumourigenesis and cancer
therapy. Cell Death Differ. 18:1414–1424. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
LeRoith D and Roberts CT Jr: The
insulin-like growth factor system and cancer. Cancer Lett.
195:127–137. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
De Luca A, Maiello MR, D'Alessio A,
Pergameno M and Normanno N: The RAS/RAF/MEK/ERK and the PI3K/AKT
signalling pathways: Role in cancer pathogenesis and implications
for therapeutic approaches. Expert Opin Ther Targets. 16 Suppl
2:S17–S27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Datta SR, Dudek H, Tao X, Masters S, Fu H,
Gotoh Y and Greenberg ME: Akt phosphorylation of BAD couples
survival signals to the cell-intrinsic death machinery. Cell.
91:231–241. 1997. View Article : Google Scholar : PubMed/NCBI
|