Introduction
Head and neck cancer remains as one of the most
debilitating human neoplasms, and is the sixth most commonly
diagnosed cancer worldwide. Histologically, 90% of head and neck
cancers are head and neck squamous cell carcinoma (HNSCC), and
tongue squamous cell carcinoma (TSCC) contributes to a large
proportion of HNSCC (1). In recent
decades, advancements have been achieved in the conventional
treatment of these cancers, including surgery, radiotherapy and
chemotherapy. However, the prognosis of TSCC remains poor (2). Thus, a greater understanding of the
pathogenesis of TSCC is needed in order to identify effective
antitumor drugs that can be used to clinically control TSCC.
To this end, the present study focuses on a natural
flavonoid that possesses anticancer effects. The potential
chemotherapeutic effects of many flavonoids have been tested in
different clinical trials (3).
Morin is a naturally occurring bioflavonoid originally isolated
from members of the Moraceae family of flowering plants. Morin can
be isolated from amygdala (P. guajava L.) as a yellow
pigment (4,5). Morin exerts anticancer activity
through the modulation of various gene-signaling pathways that are
involved in cell proliferation and differentiation (6–10).
Previous studies have also demonstrated that morin is able to
induce apoptosis in prostate cancer, human leukemia HL-60 and
multiple myeloma cells. Recently, studies have demonstrated that
treatment with morin induces tumor cell apoptosis by activating the
mitochondrial and caspase-3 pathway (11,12).
The Hippo pathway is an important tumor suppressor
pathway in various human cancers (13–18).
The Hippo kinase cascade involves mammalian sterile 20-like 1/2
(MST1/2, also known as STK4/3), salvador (SAV1), large tumor
suppressor 1/2 (LATS1/2), MOB kinase activator 1A/B (MOB1a/b) and
Yes-associated protein (YAP) (15,18,19).
Activation of the Hippo kinase cascade leads to the phosphorylation
of YAP and restriction of YAP translocsation into the cytoplasm for
the induction of YAP degradation (16,17).
However, while the Hippo signaling cascade is inactive, YAP is
translocated into the nucleus where it promotes the expression of
various genes involved in cell proliferation and survival (15). In addition, MOB1 is an adapter
protein and it can bind to upstream MST1; thus, it can regulate
MST1 phosphorylation or bind to the downstream LATS1 protein to
enable the trans-phosphorylation of YAP (14). The activity of the Hippo pathway
regulates organ size during embryonic development in animals
through the regulation of cell proliferation and apoptosis, and
alteration of this pathway occurs in various types of human cancer
(13,20). Aberrant expression of the Hippo
pathway proteins promotes the translocation of YAP into the nucleus
and drives transcription of YAP-targeting genes and, therefore,
enhances cancer cell proliferation and inhibits apoptosis (20–22).
YAP can also stimulate epithelial-to-mesenchymal transition of
tumor cells and drive the tumor-initiation capacity of cancer stem
cells (23). To date, elevated YAP
expression has been observed in many types of human cancers,
including lung, liver, ovary, breast and colon cancers (24–28).
Previous studies have demonstrated that morin was able to impede
YAP nuclear translocation through the activation of Hippo signaling
and foster apoptosis in human liver cancer HepG2 cells (29). Additionally, morin has demonstrated
antitumor activity in human TSCC cells (6). In the present study, we further
assessed whether morin treatment could regulate MST1 and MOB1
expression to constitutively activate the Hippo signaling pathway
and inactivate YAP in TSCC cells in vitro. We determined the
effect of morin treatment on the modulation of TSCC cell
proliferation, apoptosis and migration and on the regulation of the
phosphorylation of the protein components of the Hippo pathway and
YAP target genes. We aimed to provide insightful information
regarding the effect of morin on TSCC cells and to identify morin
as a potential treatment strategy or preventative agent for
TSCC.
Materials and methods
Cell line culture and drug
treatment
The TSCC cell line, CAL27, was obtained from
Shanghai Ninth People's Hospital (Shanghai, China) and cultured in
a high-glucose Dulbecco's modified Eagle's medium (DMEM; HyClone
Laboratories; GE Healthcare Life Sciences, Logan, UT, USA)
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) with 5% CO2 at 37°C.
For morin treatment, tumor cells were cultured in a complete cell
culture medium supplemented with different doses of morin (0, 50,
100 and 150 µM; Yuanye Chemicals Co., Ltd., Shanghai, China), while
dimethyl sulfoxide (DMSO; Beijing Solarbio Science & Technology
Co., Ltd., Beijing, China), the solvent, was used as the control
treatment for up to 14 days.
Cell viability assay
Tumor cell viability was assayed following morin
treatment using a cell viability Cell Counting Kit-8 (CCK-8;
Dojindo Laboratories, Kumamoto, Japan). Briefly, Cal27 cells were
seeded into 96-well plates at a density of 3,000 cells per well and
grown overnight in a complete cell culture medium (see above) and
then refreshed with morin-containing medium for 24 to 48 h. At the
end of each experiment, cell growth medium was added to 10 µl of
CCK-8 working solution and cells were further cultured for 90 min
at 37°C. The optical density value (OD) of the plates was then
measured using a microplate reader at 450 nm. The experiment was
performed in triplicate and repeated at least three times.
Colony formation assay
Cal27 cells were plated into 6-well plates (1,000
cells/well) and grown overnight. Following this incubation, the
cell culture medium was refreshed with morin-containing medium (0,
50, 100 and 150 µM) every 2 days for 14 days. At the end of the
experiments, cells were washed with ice-cold phosphate-buffered
saline (PBS), fixed using freshly made 4% polyformaldehyde/PBS
solution and stained with 0.1% crystal violet. Cell colonies with
more than 50 cells were counted under an inverted microscope
(Olympus Corp., Tokyo, Japan). The experiment was performed in
triplicate and repeated at least three times.
5-Ethynyl-2′-deoxyuridine (EdU)
incorporation assay
We performed the EdU incorporation assay using an
EdU kit from Guangzhou RiboBio Co., Ltd. (Guangzhou, China)
according to the manufacturer's instructions. Specifically, Cal27
cells were plated into 24-well plates at a density of 50,000
cells/well and grown overnight. The cells were then treated with
different concentrations of morin (0, 50, 100 and 150 µM) for 24 h.
Following this, cell culture was added to 50 µl of the EdU working
solution and further cultured for 2 h. At the end of the
experiment, cells were washed with ice-cold PBS and fixed in 4%
paraformaldehyde for 15 min. The cells were then treated with 2
mg/ml glycine at room temperature for 5 min and stained with
Apollo® 567 and Hoechst working solution, in the dark,
for 30 min. Finally, the cell images were obtained using
fluorescence microscopy (Olympus Corp.).
Wound-healing assay
Cal27 cells were grown and treated with various
concentrations of morin (0, 50, 100 and 150 µM) for 24 h. For the
tumor cell wound-healing assay, the cells were tripsinized and
reseeded into 6-well plates at a density of 1×106
cells/well and grown overnight to reach ~90% confluency. The cell
monolayer was then scratched with a sterile 200-µl pipette tip
across the plates, washed with DMEM twice and cultured for up to 24
h in DMEM/0.1% FBS as well as different the concentrations of
morin. At each time-point (0, 6 or 24 h), the cell monolayers were
photographed using an inverted microscope (Olympus Corp.). The
wound healing areas were measured using ImageJ software 14.8 for
Windows (National Institutes of Health, Bethesda, MD, USA). The
experiment was performed in triplicate and repeated at least three
times.
Flow cytometric Annexin V/PI apoptosis
assay
Cal27 cells were first plated into 6-well plates
(1×106 cells/well) and incubated overnight at 37°C.
Cells were then treated with different doses of morin (0, 50, 100
and 150 µM) for up to 48 h. At the end of each experiment, both
detached and attached cells were collected and incubated with the
Annexin V-FITC/propidium iodide (PI) apoptosis detection kit
(eBioscience, Vienna, Austria) according to the manufacturer's
protocol. Specifically, ~20,000 cells were suspended in the binding
buffer and 5 µl of Annexin V-FITC was added. Cells were incubated
for 10 min, followed by a further 5-min incubation after the
addition of 5 µl of the PI staining solution at room temperature in
the dark. Finally, the apoptosis level was measured using flow
cytometry (FACSCalibur; BD Biosciences, San Jose, CA, USA).
Flow cytometric cell cycle assay
Cal27 cells were plated into 6-well plates
(1×106 cells/well) and incubated overnight. Cells were
then treated with different doses of morin (0, 50, 100 and 150 µM)
for 24 h. The cells were then harvested and washed with ice-cold
PBS once and fixed in 70% ethanol at 4°C for 12 h. On the next day,
the cells were washed again with PBS and then stained with PI
working solution at room temperature for 30 min in the dark. The
cell cycle distribution of the cells was analyzed using flow
cytometry (FACSCalibur; BD Biosciences).
Protein extraction and western blot
analysis
Morin-treated Cal27 cells were lysed in
radioimmunoprecipitation assay buffer (RIPA buffer; Beyotime
Institute of Biotechnology, Shanghai, China) containing 1%
phenylmethylsulfonyl fluoride (PMSF; Beyotime Institute of
Biotechnology) for 30 min on ice. Both cytoplasmic and nuclear
extracts were then extracted using a nuclear fractionation
protocol, following the manufacturer instructions (Beijing Solarbio
Science & Technology Co., Ltd.). The concentration of each
protein sample was assayed using the bicinchoninic acid assay kit
(Beijing Solarbio Science & Technology) and equal amounts (20
µg of each) of protein samples were separated using 10% sodium
dodecyl sulfate-polyacrylamide gel (Beyotime Institute of
Biotechnology) electrophoresis and electrically transferred onto
polyvinylidene fluoride (PVDF) membranes (Invitrogen; Thermo Fisher
Scientific). For western blotting, the membranes were first
incubated in 5% non-fat milk at room temperature for 1 h and then
incubated with a rabbit monoclonal anti-human YAP antibody (cat.
no. 14074), a rabbit monoclonal anti-human phospho-YAP antibody
(cat. no. 13619), a rabbit polyclonal anti-human MST1 antibody
(cat. no. 3682), a rabbit polyclonal anti-human phospho-MST1
antibody (cat. no. 3681), a rabbit monoclonal anti-human MOB1
antibody (cat. no. 13730), and a rabbit monoclonal anti-human
phospho-MOB1 antibody (cat. no. 8699) (all from Cell Signaling
Technology, Inc., Danvers, MA, USA) at 4°C overnight. The next day,
the membranes were washed with Tris-based saline-Tween-20 (TBS-T;
20 mmol/l Tris-HCl, 150 mmol/l NaCl and 0.05% Tween-20) three times
for 10 min each time and then incubated in 1:5,000 HRP-conjugated
goat anti-rabbit IgG (cat. no. 7074S; Cell Signaling Technology,
Inc.). The protein bands were visualized using the Chemiluminescent
HRP Substrate kit (EMD Millipore, Billerica, MA, USA). The level of
protein expression was then normalized to that of GAPDH
(cytoplasmic protein) and Histone H3 (nuclear protein).
Quantitative RT-PCR (qRT-PCR)
The total cellular RNA was isolated from Cal27 cells
that had been treated with morin for 24 h using a
TRIzol® reagent (Takara Bio, Inc., Otsu, Japan). The RNA
was then reversely transcribed into cDNA using a Reverse
Transcriptase kit (Takara Bio) according to the manufacturer's
protocols. These cDNA samples were then amplified with qPCR in 20
µl of the reaction system, which contained the SYBR®
Primix Ex Taq™ (Takara Bio), cDNA, each primer and RNase-free
H2O, following the manufacturer's protocol.
Amplification conditions were set to an initial step of 95°C for 30
sec and then 45 cycles of 95°C for 5 sec, 60°C for 35 sec, and 72°C
for 60 sec and a final step at 40°C for 30 sec. The level of GAPDH
mRNA was used as a control and the relative expression level of
each RNA sample was calculated using the 2−ΔΔCq method
(30). All experiments were
performed in triplicate and repeated at least once. The primer
sequences were GAPDH, 5′-GCACCGTCAAGGCTGAGAAC-3′ and
5′-TGGTGAAGACGCCAGTGGA-3′; CTGF, 5′-TCTCCAACCTCTCCTACTAC-3′ and
5′-GCACGTAGTTTCGATCACT3′; CYR61, 5′-CCTTGTGGACAGCCAGTGTA-3′ and
5′-ACTTGGGCCGGTATTTCTTC-3′; and ANKRD, 5′-AGTAGAGGAACTGGTCACTGG-3′
and 5′-TGGGCTAGAAGTGTCTTCAGAT-3′.
Statistical analysis
All data are summarized as the mean ± standard error
of the mean (SEM) of at least three replicates of each experiment
and statistically analyzed using GraphPad Prism 5 (GraphPad
Software Inc., La Jolla, CA, USA) with the two-tailed Student's
t-test for two groups of data or the one-way analysis of variance
(ANOVA) plus Tukey's post hoc test for multiple groups of data.
P<0.05 indicates statistical significance.
Results
Morin reduces TSCC cell
proliferation
To evaluate the effects of morin on the regulation
of TSCC cell proliferation, we performed cell viability (CCK-8),
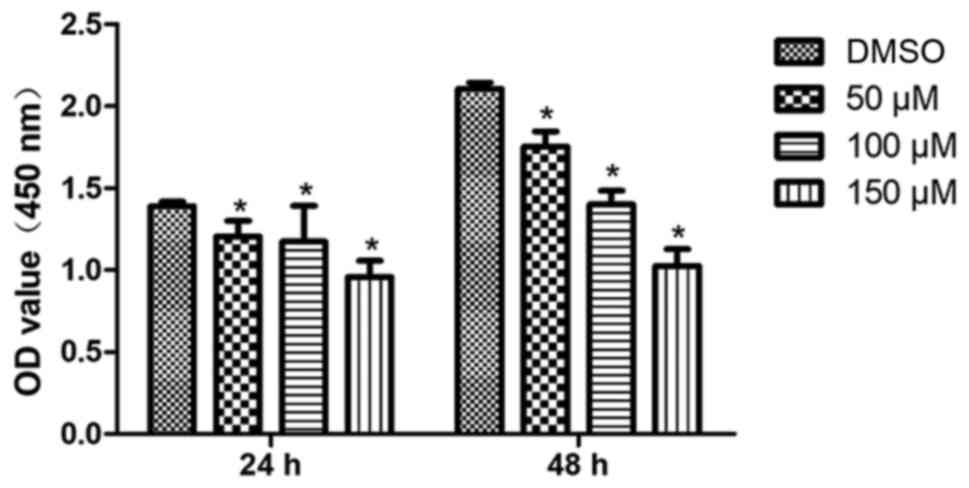
EdU incorporation and colony formation assays. Our CCK-8 assay data
revealed that morin treatment significantly reduced tumor cell
viability in a dose-dependent manner both at 24 and 48 h (Fig. 1). The results from the tumor cell
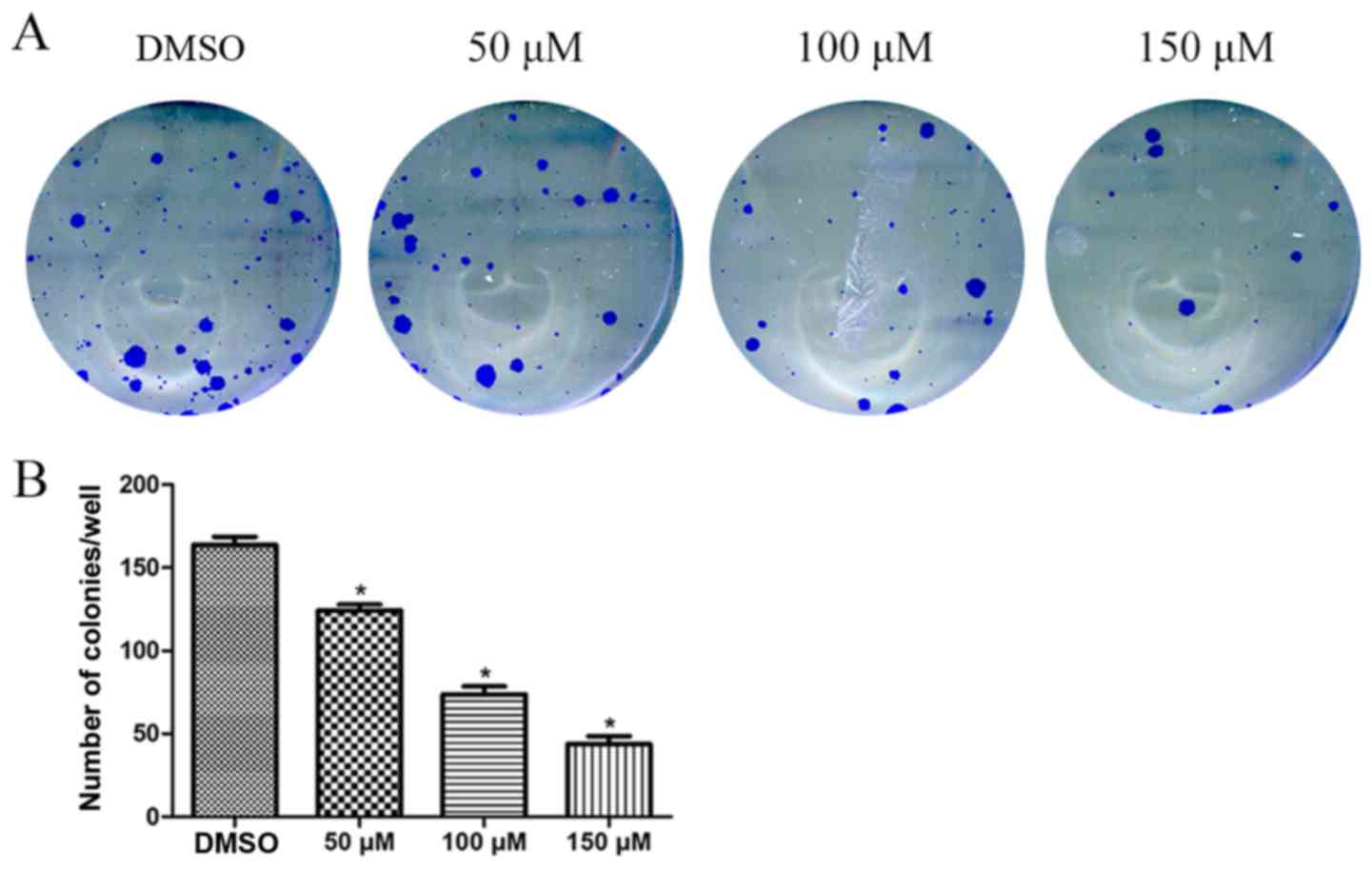
colony formation assay also revealed that morin treatment
significantly reduced the number of tumor cell colonies compared
with the number of tumor cell colonies observed in the untreated
control cells (Fig. 2). Moreover,
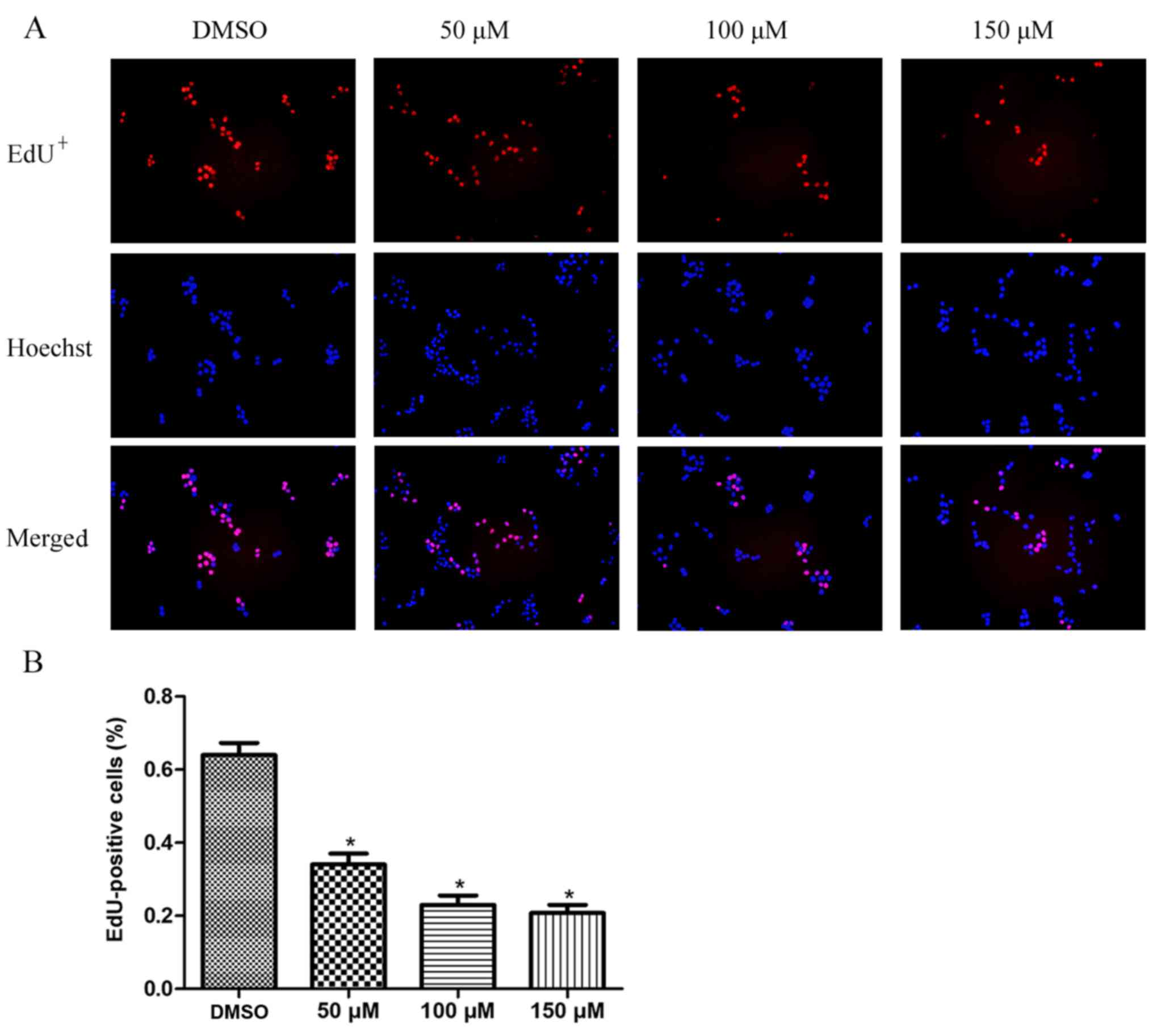
morin treatment for 24 h led to a significantly lower percentage of
EdU-positive cells than that observed in the control cells, in a
dose-dependent manner (Fig. 3).
These data indicated that morin inhibited TSCC cell
proliferation.
Morin induces tumor cell cycle arrest
and apoptosis
We further investigated how morin treatment inhibits
tumor cell proliferation using flow cytometric apoptosis and cell
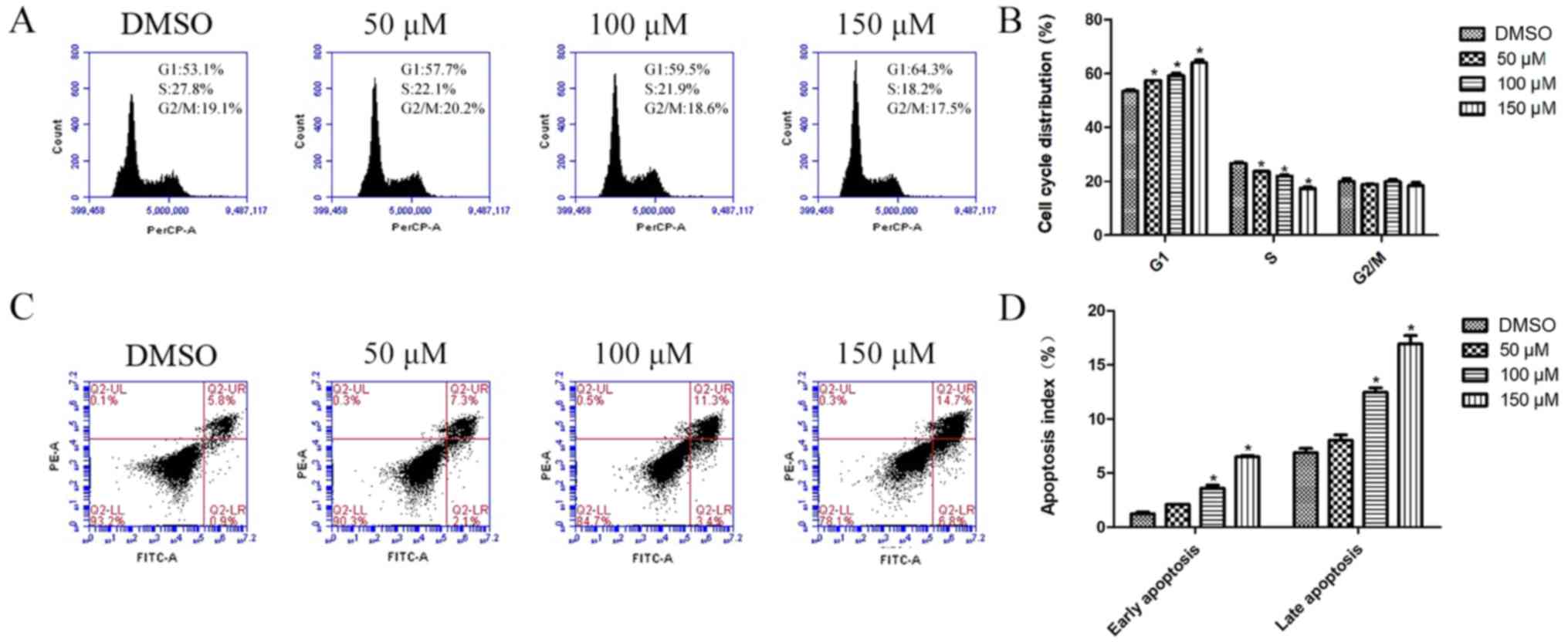
cycle assays. Our data showed that morin treatment, for 24 h,
arrested tumor cells at the G1 phase of the cell cycle, and showed
a significantly smaller percentage of S phase cells than this
percentage in the control group (Fig.
4A and B). Moreover, morin-treated tumor cells underwent
apoptosis. Both the percentages of early and late apoptotic cells
were significantly higher at 48 h of morin treatment at 100 and 150
µM than in the control cells (Fig. 4C
and D).
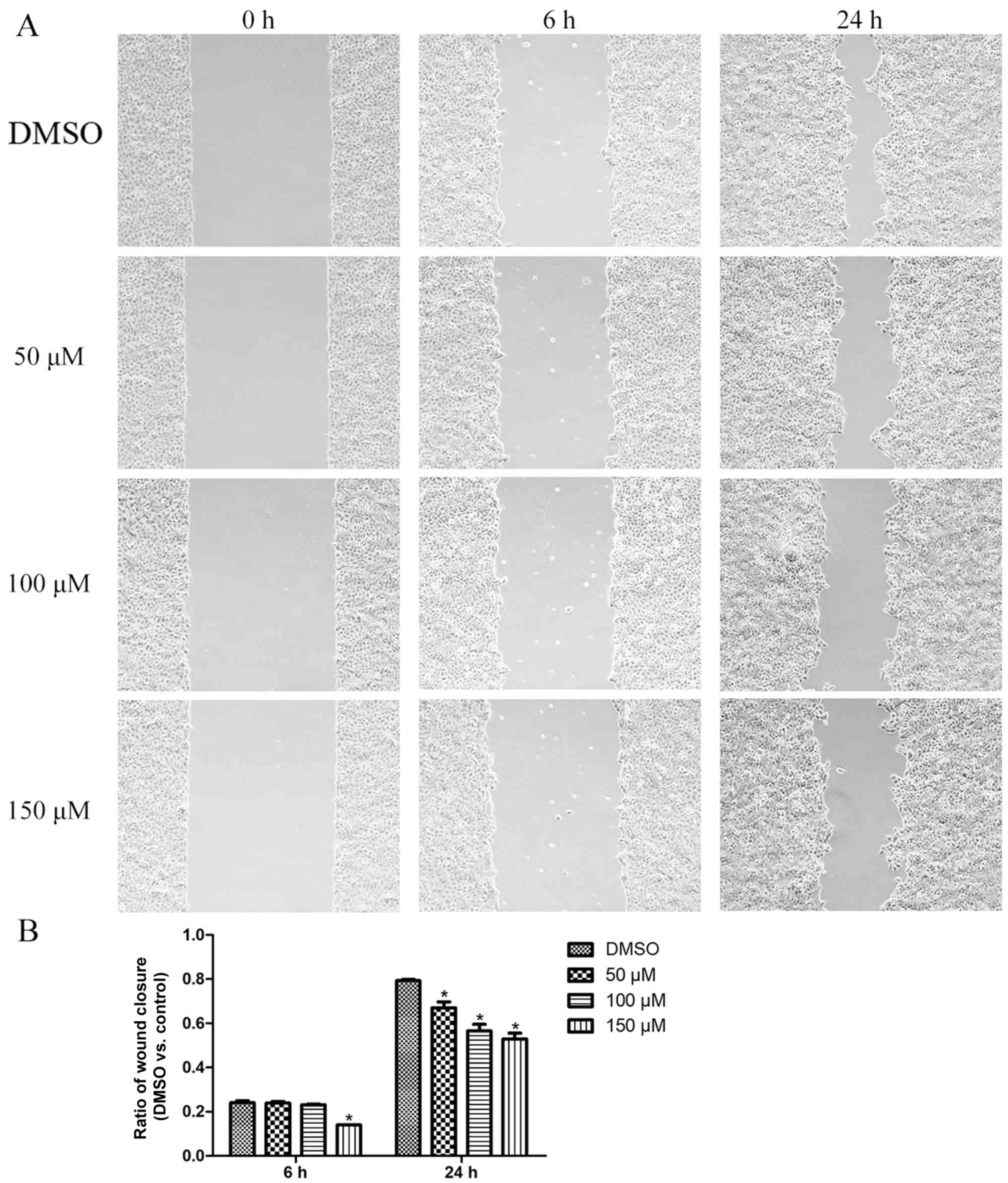
Morin reduces TSCC cell migration
We then performed the tumor cell wound-healing assay
to investigate the effect of morin on the migratory capacity of
Cal27 cells. We found that, following morin treatment, cells showed
a lower tumor cell wound healing capacity than the control cells,
which showed quick closure of the wound (Fig. 5).
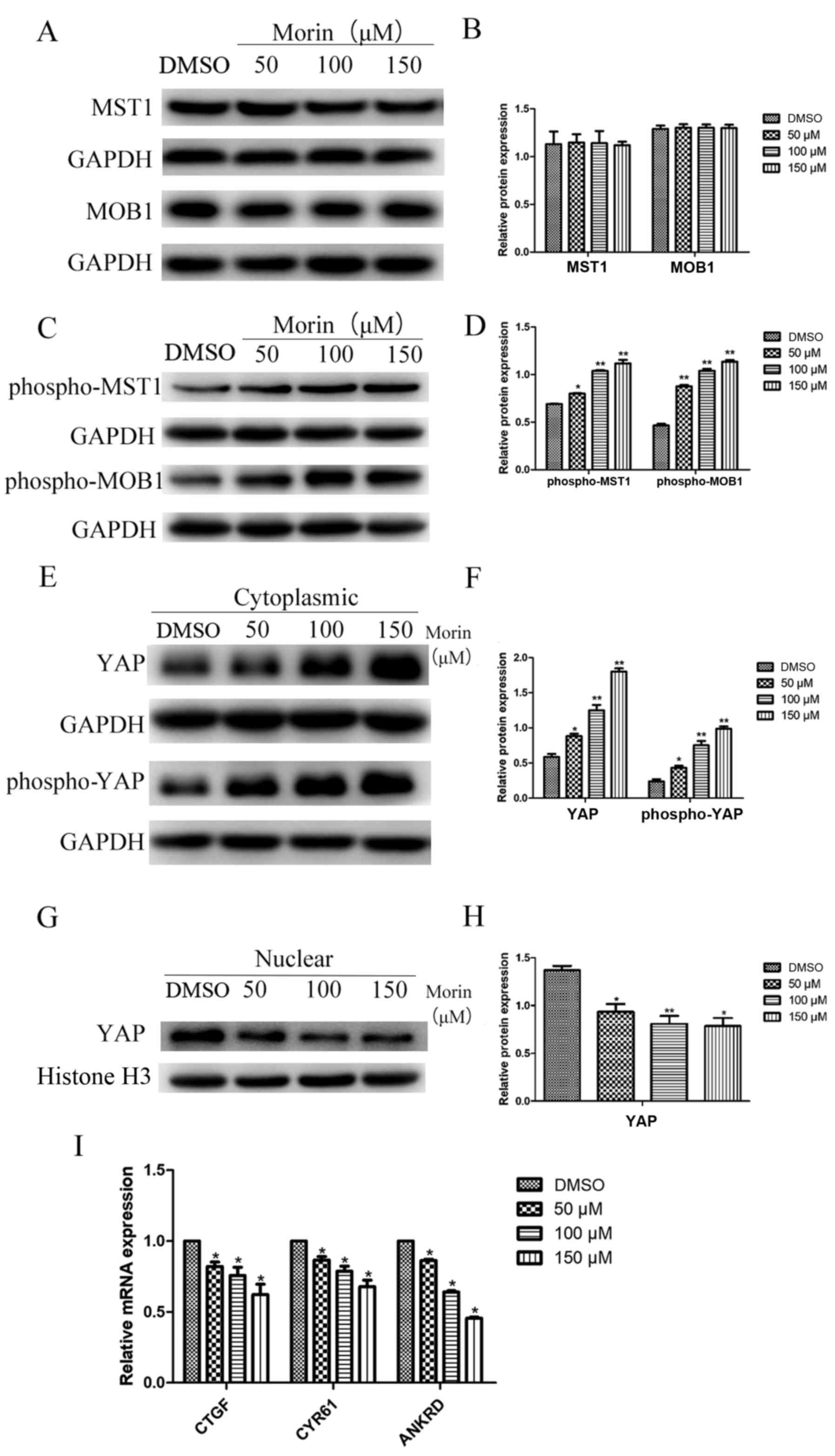
Morin activates Hippo pathway proteins
and inhibits YAP nuclear translocation
Thus far, we demonstrated the antitumor activity of
morin in TSCC cells in vitro. To further this study, we
aimed to assess the underlying molecular pathways responsible for
the action of morin on TSCC cells. Our western blot analysis
revealed that the total levels of MST1 and MOB1 proteins were not
significantly altered after morin treatment of TSCC cells (there
was no significant difference between the results of the treated
cells and the control cells; Fig. 6A
and B). In contrast, the expression levels of phospho-MST1 and
phospho-MOB1 proteins were significantly higher in morin-treated
TSCC cells than these levels in the control cells (Fig. 6C and D). Furthermore, the
cytoplasmic level of YAP protein was significantly upregulated in
morin-treated TSCC cells and the ratio of phosphorylated YAP was
also greater in the morin-treated tumor cells (Fig. 6E and F). In contrast, TSCC cells
treated with high concentrations of morin exhibited a lower level
of nuclear YAP protein than that of the control cells (Fig. 6G and H). We further investigated
whether the changes in YAP protein localization that were observed
following morin treatment correlated with an alteration in
YAP-targeting proteins, such as CTGF, CYR61 and ANKRD. Our data
showed that the expression of CTGF, CYR61 and ANKRD genes was
significantly lower in the morin-treated Cal27 cells than that in
the control cells (Fig. 6I). Taken
together, our current data demonstrated that morin treatment
upregulated the activity of the Hippo pathway but suppressed YAP
nuclear translocation and YAP-related transcriptional activity in
Cal27 cells.
Discussion
Morin displays wide-ranging pharmacological
activities and low cytotoxicity against different types of human
cancer cells (31). For example,
previous studies have shown that morin possesses anti-liver cancer
activity in the promotion stage of an in vivo liver
carcinogenesis model (10).
Additionally, morin has been shown to suppress breast cancer
malignant behaviors through the inhibition of tumor cell
epithelial-mesenchymal transition and Akt activation (32). Morin has also been suggested to
induce apoptosis of human histiocytic lymphoma U937 cells through
the upregulation of the Bcl-2-associated death promoter (BAD)
protein levels (33). In tongue
squamous cell carcinoma (TSCC), a recent study demonstrated that
human TSCC cells were sensitive to morin-induced tumor cell growth
inhibition (6). However, the
underlying mechanism of morin in TSCC cells remains to be defined.
Thus, the present study assessed the antitumor effect of morin in
TSCC Cal27 cells and explored the underlying mechanisms. We found
that morin treatment effectively decreased cell proliferation,
colony formation, and migration of Cal27 cells in a dose-dependent
manner. In the wound healing assay, cells were cultured for up to
24 h. During this period of time cells do not proliferate to a
great extent, thus this enabled us to avoid the effect of morin on
cell proliferation in the wound healing assay. Our data showed that
the wound healing capacity was inhibited to a greater extent in the
morin-treated tumor cells than that observed in the control group,
over a period of 24 h. Additionally, the cell viability assay
showed a significant reduction in tumor cell survival in the
morin-treated group after a 24-h incubation period. Taken together,
we have reason to believe that the wound assay will show a more
significant difference between the drug treatment groups and the
control group after 24 h. Furthermore, our cell cycle analysis
showed that morin treatment led to cell cycle arrest at the G1
phase, with a significantly lower percentage of cells in the S
phase than that noted in the controls. Moreover, the data
concerning the apoptosis analysis showed that morin treatment
significantly increased the percentage of Cal27 cells that were
undergoing early and late apoptosis. The morin-induced inhibition
of TSCC cell growth and migration was demonstrated in our in
vitro results.
Indeed, previous studies have demonstrated that
different phytochemicals, including morin, possess antitumor
activity in various types of human cancers (reviewed in ref.
4); morin has shown different
pharmacological activities with very low cytotoxicity in humans
(29); thus, in the present study,
we treated TSCC Cal27 cells with up to 150 µM morin, while a
previous study of nude mouse melanoma cell xenografts treated the
mice with 50 mg/kg of morin intraperitoneally (34). These doses could clinically be
achievable (35). Previous studies
have demonstrated that a low dose of morin reduced the
cisplatin-induced toxicity of 293 cells and mouse kidney cells
(36) and showed neuroprotective
effect of morin in lead acetate-induced apoptosis in the rat brain
(37). The induction of tumor cell
apoptosis in response to morin at higher doses has been further
confirmed in our current data.
Upon further exploration of the underlying mechanism
of the morin antitumor activity in TSCC, we found that, at the gene
level, morin upregulated the phosphorylation of the Hippo pathway
proteins and inhibited YAP nuclear translocation. It is known that
the Hippo pathway regulates cell growth, proliferation, apoptosis
and organ size during embryonic development through alterations of
the subcellular localization of YAP (38). For example, Song et al
demonstrated that Hippo pathway-inactivated mice exhibited induced
YAP phosphorylation with nuclear translocation, which led to the
development of hepatocellular carcinoma (HCC) in mice (39). In contrast, re-activation of the
Hippo pathway, in the HCC-derived cell line, promoted YAP
phosphorylation and the suppression of HCC (39). Immunohistochemical studies of
non-small cell lung cancer revealed that an elevated ratio of
nuclear localization of YAP protein is associated with advanced
tumor features and poor patient outcome (40–42).
Furthermore, the level of YAP protein has been found to be
upregulated in precancerous lesions in a rat model of liver cancer
and upregulation of the nuclear level of YAP protein is frequently
found in fully developed HCC (43).
Elevated YAP expression and nuclear localization also occurs in
mouse models of pancreatic cancer, and in tumors derived from human
pancreatic adenocarcinoma cell lines (44,45).
The pancreas-specific YAP knockout inhibits tumor progression in a
mouse pancreatic cancer model (46). In the present study, we also
demonstrated that morin treatment was able to activate Hippo
signaling through the upregulation of phospho-MST1 and phospho-MOB1
proteins. Additionally, YAP phosphorylation was induced by morin,
and the level of YAP nuclear translocation and the expression of
YAP-targeting CTGF, CYR61 and ANKRD genes were inhibited.
This study demonstrated that morin possesses
antitumor activity in Cal27 cells through the activation of the
Hippo pathway and suppression of YAP nuclear translocation in
vitro. However, the use of only one cell line is a limitation
of this study. Future study with more cell lines is needed to
confirm our current data. In addition, we do know that morin, as a
photochemical, should be able to target multiple gene or gene
pathways, as is seen with other phytochemicals (33,34).
Thus, future studies will further explore the gene targets of
morin.
In conclusion, the present proof-of-principle study
demonstrated that morin treatment inhibited TSCC Cal27 cell
proliferation and migration, but induced TSCC cell apoptosis and
arrested tumor cells in the G1 phase of the cell cycle. At the gene
level, morin treatment activated the Hippo pathway and inhibited
YAP activity, indicating that morin possesses antitumor activity in
TSCC in vitro.
Acknowledgements
The authors would like to thank the Shandong
Provincial Key Laboratory of Oral Tissue Regeneration for their
support of the present study.
Funding
The present study was supported in part by grants
from the Shandong Provincial Natural Science Foundation (no.
ZR2017MH031) and the Construction Engineering Special Fund of
Taishan Scholars (no. ts201511106).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
XX and XQ designed the study and the experiments. YJ
and LJ conducted the experiments. YZ, YX, DZ, XW and BZ analyzed
the data and YJ wrote the manuscript. All authors read and approved
the manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang SS, Cen X, Liang XH and Tang YL:
Macrophage migration inhibitory factor: A potential driver and
biomarker for head and neck squamous cell carcinoma. Oncotarget.
8:10650–10661. 2017.PubMed/NCBI
|
|
2
|
Kang H, Kiess A and Chung CH: Emerging
biomarkers in head and neck cancer in the era of genomics. Nat Rev
Clin Oncol. 12:11–26. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Birt DF and Bresnick E: Chemoprevention by
nonnutrient components of vegetables and fruits. Springer US;
1991
|
|
4
|
Aggarwal BB and Shishodia S: Molecular
targets of dietary agents for prevention and therapy of cancer.
Biochem Pharmacol. 71:1397–1421. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wijeratne SS, Abou-Zaid MM and Shahidi F:
Antioxidant polyphenols in almond and its coproducts. J Agric Food
Chem. 54:312–318. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brown J, O'Prey J and Harrison PR:
Enhanced sensitivity of human oral tumours to the flavonol, morin,
during cancer progression: Involvement of the Akt and stress kinase
pathways. Carcinogenesis. 24:171–177. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sivaramakrishnan V and Niranjali DS: Morin
regulates the expression of NF-kappaB-p65, COX-2 and matrix
metalloproteinases in diethylnitrosamine induced rat hepatocellular
carcinoma. Chem Biol Interact. 180:353–359. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sivaramakrishnan V and Devaraj SN: Morin
fosters apoptosis in experimental hepatocellular carcinogenesis
model. Chem Biol Interact. 183:284–292. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Madankumar P, Naveenkumar P, Manikandan S,
Devaraj H and Niranjalidevaraj S: Morin ameliorates chemically
induced liver fibrosis in vivo and inhibits stellate cell
proliferation in vitro by suppressing Wnt/β-catenin signaling.
Toxicol Appl Pharmacol. 277:210–220. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Madankumar P, Naveenkumar P, Devaraj H and
Niranjalidevaraj S: Morin, a dietary flavonoid, exhibits
anti-fibrotic effect and induces apoptosis of activated hepatic
stellate cells by suppressing canonical NF-κB signaling. Biochimie.
110:107–118. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kuo HM, Chang LS, Lin YL, Lu HF, Yang JS,
Lee JH and Chung JG: Morin inhibits the growth of human leukemia
HL-60 cells via cell cycle arrest and induction of apoptosis
through mitochondria dependent pathway. Anticancer Res. 27:395–405.
2007.PubMed/NCBI
|
|
12
|
Gupta SC, Phromnoi K and Aggarwal BB:
Morin inhibits STAT3 tyrosine 705 phosphorylation in tumor cells
through activation of protein tyrosine phosphatase SHP1. Biochem
Pharmacol. 85:898–912. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saucedo LJ and Edgar BA: Filling out the
Hippo pathway. Nat Rev Mol Cell Biol. 8:613–621. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bichsel SJ, Tamaskovic R, Stegert MR and
Hemmings BA: Mechanism of activation of NDR (nuclear Dbf2-related)
protein kinase by the hMOB1 protein. J Biol Chem. 279:35228–35235.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pan D: The hippo signaling pathway in
development and cancer. Dev Cell. 19:491–505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oka T, Mazack V and Sudol M: Mst2 and Lats
kinases regulate apoptotic function of yes kinase-associated
protein (YAP). J Biol Chem. 283:27534–27546. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang J, Wu S, Barrera J, Matthews K and
Pan D: The hippo signaling pathway coordinately regulates cell
proliferation and apoptosis by inactivating Yorkie, the
Drosophila homolog of YAP. Cell. 122:421–434. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu FX and Guan KL: The Hippo pathway:
Regulators and regulations. Genes Dev. 27:355–371. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Halder G and Johnson RL: Hippo signaling:
Growth control and beyond. Development. 138:9–22. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao B, Tumaneng K and Guan KL: The Hippo
pathway in organ size control, tissue regeneration and stem cell
self-renewal. Nat Cell Biol. 13:877–883. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zeng Q and Hong W: The emerging role of
the hippo pathway in cell contact inhibition, organ size control,
and cancer development in mammals. Cancer Cell. 13:188–192. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu FX, Zhao B and Guan KL: Hippo pathway
in organ size control, tissue homeostasis, and cancer. Cell.
163:811–828. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cordenonsi M, Zanconato F, Azzolin L,
Forcato M, Rosato A, Frasson C, Inui M, Montagner M, Parenti AR,
Poletti A, et al: The Hippo transducer TAZ confers cancer stem
cell-related traits on breast cancer cells. Cell. 147:759–772.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Overholtzer M, Zhang J, Smolen GA, Muir B,
Li W, Sgroi DC, Deng CX, Brugge JS and Haber DA: Transforming
properties of YAP, a candidate oncogene on the chromosome 11q22
amplicon. Proc Natl Acad Sci USA. 103:12405–12410. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Steinhardt AA, Gayyed MF, Klein AP, Dong
J, Maitra A, Pan D, Montgomery EA and Anders RA: Expression of
Yes-associated protein in common solid tumors. Hum Pathol.
39:1582–1589. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fernandez-L A, Northcott PA, Dalton J,
Fraga C, Ellison D, Angers S, Taylor MD and Kenney AM: YAP1 is
amplified and up-regulated in hedgehog-associated medulloblastomas
and mediates Sonic hedgehog-driven neural precursor proliferation.
Genes Dev. 23:2729–2741. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Song Y, Li L, Ou Y, Gao Z, Li E, Li X,
Zhang W, Wang J, Xu L, Zhou Y, et al: Identification of genomic
alterations in oesophageal squamous cell cancer. Nature. 509:91–95.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mohseni M, Sun J, Lau A, Curtis S,
Goldsmith J, Fox VL, Wei C, Frazier M, Samson O, Wong KK, et al: A
genetic screen identifies an LKB1-MARK signalling axis controlling
the Hippo-YAP pathway. Nat Cell Biol. 16:108–117. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Perumal N, Perumal M, Kannan A, Subramani
K, Halagowder D and Sivasithamparam N: Morin impedes Yap nuclear
translocation and fosters apoptosis through suppression of
Wnt/β-catenin and NF-κB signaling in Mst1 overexpressed HepG2
cells. Exp Cell Res. 15:124–141. 2017. View Article : Google Scholar
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gutiérrez RM, Mitchell S and Solis RV:
Psidium guajava: A review of its traditional uses,
phytochemistry and pharmacology. J Ethnopharmacol. 117:1–27. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jin H, Lee WS, Eun SY, Jung JH, Park HS,
Kim G, Choi YH, Ryu CH, Jung JM, Hong SC, et al: Morin, a flavonoid
from Moraceae, suppresses growth and invasion of the highly
metastatic breast cancer cell line MDA-MB-231 partly through
suppression of the Akt pathway. Int J Oncol. 45:1629–1637. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Park C, Lee WS, Go SI, Nagappan A, Han MH,
Hong SH, Kim GS, Kim GY, Kwon TK, Ryu CH, et al: Morin, a flavonoid
from moraceae, induces apoptosis by induction of BAD protein in
human leukemic cells. Int J Mol Sci. 16:645–659. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hu J, Guo X and Yang L: Morin inhibits
proliferation and self-renewal of CD133+ melanoma cells
by upregulating miR-216a. J Pharmacol Sci. 136:114–120. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hoque A and Xu XC: Basic and translational
research on dietary phytochemicals and cancer prevention. Targeting
Cellular Signaling for Cancer Prevention and Therapy by
Phytochemicals. 127–156. 2013. View Article : Google Scholar
|
|
36
|
Singh MP, Chauhan AK and Sun CK: Morin
hydrate ameliorates cisplatin-induced ER stress, inflammation and
autophagy in HEK-293 cells and mice kidney via PARP-1 regulation.
Int Immunopharmacol. 56:156–167. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Thangarajan S, Vedagiri A, Somasundaram S,
Sakthimanogaran R and Murugesan M: Neuroprotective effect of morin
on lead acetate-induced apoptosis by preventing cytochrome c
translocation via regulation of Bax/Bcl-2 ratio. Neurotoxicol
Teratol. 66:35–45. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ling P, Lu TJ, Yuan CJ and Lai MD:
Biosignaling of mammalian Ste20-related kinases. Cell Signal.
20:1237–1247. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Song H, Mak KK, Topol L, Yun K, Hu J,
Garrett L, Chen Y, Park O, Chang J, Simpson RM, et al: Mammalian
Mst1 and Mst2 kinases play essential roles in organ size control
and tumor suppression. Proc Natl Acad Sci USA. 107:1431–1436. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xie M, Zhang L, He CS, Hou JH, Lin SX, Hu
ZH, Xu F and Zhao HY: Prognostic significance of TAZ expression in
resected non-small cell lung cancer. J Thorac Oncol. 7:799–807.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Su LL, Ma WX, Yuan JF, Shao Y, Xiao W and
Jiang SJ: Expression of Yes-associated protein in non-small cell
lung cancer and its relationship with clinical pathological
factors. Chin Med J. 125:4003–4008. 2012.PubMed/NCBI
|
|
42
|
Noguchi S, Saito A, Horie M, Mikami Y,
Suzuki HI, Morishita Y, Ohshima M, Abiko Y, Mattsson JS, König H,
et al: An integrative analysis of the tumorigenic role of TAZ in
human non-small cell lung cancer. Clin Cancer Res. 20:4660–4672.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Perra A, Kowalik MA, Ghiso E,
Ledda-Columbano GM, Di Tommaso L, Angioni MM, Raschioni C, Testore
E, Roncalli M, Giordano S, et al: YAP activation is an early event
and a potential therapeutic target in liver cancer development. J
Hepatol. 61:1088–1096. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Morvaridi S, Dhall D, Greene MI, Pandol SJ
and Wang Q: Role of YAP and TAZ in pancreatic ductal adenocarcinoma
and in stellate cells associated with cancer and chronic
pancreatitis. Sci Rep. 5:167592015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Diep CH, Zucker KM, Hostetter G, Watanabe
A, Hu C, Munoz RM, Von Hoff DD and Han H: Down-regulation of yes
associated protein 1 expression reduces cell proliferation and
clonogenicity of pancreatic cancer cells. PLoS One. 7:e327832012.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang W, Nandakumar N, Shi Y, Manzano M,
Smith A, Graham G, Gupta S, Vietsch EE, Laughlin SZ, Wadhwa M, et
al: Downstream of mutant KRAS, the transcription regulator YAP is
essential for neoplastic progression to pancreatic ductal
adenocarcinoma. Sci Signal. 7:ra422014. View Article : Google Scholar : PubMed/NCBI
|