Introduction
Lung cancer is the primary type of malignant tumor
that threatens human health; it accounts for the majority of cancer
mortalities worldwide and 80% of cases have non-small cell lung
cancer (NSCLC) pathology (1).
Although improvements to diagnostic and treatment methods have
greatly improved the rate of early diagnosis for NSCLC and provided
guidance for personalized treatment, ~66% of patients are diagnosed
when the disease has reached an advanced stage (2). Late diagnosis limits the effectiveness
of first-line treatment to chemotherapy and the expected survival
time for these patients is ~8 months. Research into the molecular
phenotypes of lung cancer contribute to more accurate early
diagnoses, advanced molecular classification and improved prognosis
estimates (3). The molecular
network underlying the development of lung cancer has been
partially identified at the protein and gene level. Over the past
10 years, gene therapy based on the underlying molecular network of
lung cancer development has advanced, however, the overall 5-year
mortality rate for patients with lung cancer has not significantly
improved (4).
Platinum-based chemotherapy regimens are the
first-line treatment against NSCLC; however, the body may rapidly
develop resistance to platinum drugs, which is a key obstacle in
the clinical treatment of NSCLC (5). Platinum resistance is generally
considered to be caused by multiple factors, including
resistance-associated genetic changes, reduced drug accumulation,
increased drug detoxication, enhanced apoptosis inhibition and DNA
repair capacity, which involve multiple signaling pathways and
numerous key factors (6). Recently,
microRNAs (miRNAs) have been identified as a novel, alternative
pathway for research into tumor drug resistance (7). Previous research has indicated that
miRNAs are closely associated with the pathogenesis and drug
resistance of tumors (7). The
upregulation or downregulation of miRNA expression may directly
cause the abnormal protein expression of target genes, which
ultimately alter drugs for tumor cells through the cellular
signaling pathways (8).
Research on the epidermal growth factor receptor
(EGFR) signaling pathway tyrosine kinase inhibitor is the most
thorough (9). At present, over 90%
of all identified NSCLC EGFR gene mutations are located in the
exons of chromosomes 19–21 (9).
EGFR is expressed in epithelial, mesenchymal and neurogenic
tissues, which serve a vital role in regulating the proliferation,
differentiation and growth of healthy cells. In addition, EGFR is
closely associated with tumor cell growth, angiogenesis, tumor
metastasis and cell apoptosis inhibition (10). A ligand binds with the N-terminal
extracellular domain of EGFR to form a homodimer or heterodimer,
which phosphorylates the tyrosine residue in the cell, thereby
activating the downstream signaling pathways. These signaling
pathways include the RAS/RAF/extracellular signal-regulated
kinase/mitogen-activated protein kinase pathway, the
phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) pathway and
the signal transducer and activator of transcription 3/5 signal
transduction pathway (11). Ligand
binding ultimately results in a series of abnormal biological
behaviors in tumor cells, including excessive proliferation and
invasion, metastasis and angiogenesis (12).
Mutations within the EGFR are an important indicator
of NSCLC progression within the Asian population (13). EGFR-tyrosine kinase inhibitors,
including gefitinib and erlotinib, have a response rate of 60–80%
when administered to NSCLC patients with EGFR mutation (14). Gefitinib has recently been
demonstrated to be clinically effective in patients with NSCLC and
brain metastasis, which indicates that EGFR mutations may lead to
brain metastases. Therefore, it is important to investigate the
association between EGFR mutations and NSCLC brain metastases
(13).
The recent identification of a novel class of
non-protein-coding miRNA, with no open reading frame, has confirmed
that the genomic complexity of cancer far exceeds original
expectations (15). miRNAs are a
type of small endogenous non-coding RNA with no open reading frame,
which are 20–25 nucleotides in length. They may regulate hundreds
of target genes at a post-transcriptional level and therefore
participate in numerous biological functions, including regulation
of cell differentiation, proliferation and apoptosis (16). As miRNAs are regulatory factors of
endogenous gene expression, they are also involved in the
pathophysiological processes of a number of diseases through the
regulation of multiple genes and their genetic networks (16). Previous studies have revealed that
miRNAs may participate in tumor development or regression as either
an oncogene or tumor-suppressor gene, respectively (8). Zhang et al revealed that
miRNA-145 (miR-145), miR-20a, miR-21 and miR-223 in the plasma, are
novel biomarkers for screening of early-stage NSCLC. However, the
mechanism of miR-145-regulated cell death in NSCLC remains unknown.
The aim of the present study was to evaluate the therapeutic
effects and the underlying molecular mechanisms of miR-145 in NSCLC
by chemotherapy.
Materials and methods
Cell lines and clinical specimens
The A549 human NSCLC cell line was purchased from
the Cancer Research Institute of Hebei Medical University and
cultured in Dulbecco's modified Eagle's medium (DMEM; Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% (m/v) fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C in a
5% CO2.
The NSCLC samples (n=6) and normal healthy
volunteers (n=6) samples were derived from patients undergoing
surgical procedures at the Fourth Hospital of Hebei Medical
University (Table I). Peripheral
blood (10 ml) of NSCLC samples and normal healthy volunteers
samples were centrifuged at 2,000 × g for 10 min at 4°C and serum
was saved at −80°C. All human studies were approved by the Ethics
Committee of Fourth Hospital of Hebei Medical University. All
patients signed written informed consent forms prior to the
study.
 | Table I.Basic knowledge of patients with
NSCLC. |
Table I.
Basic knowledge of patients with
NSCLC.
| Variables | Volunteers
(n=6) | NSCLC (n=6) |
|---|
| Age (years) |
|
|
|
≤55 | 3 | 3 |
|
>55 | 3 | 3 |
| Sex |
|
|
|
Female | 0 | 0 |
|
Male | 6 | 6 |
| Tumor size
(cm) |
|
|
|
≤3.0 | 0 | 2 |
|
>3.0 | 0 | 4 |
| Edmondson
grade |
|
|
| I | 0 | 0 |
| II | 0 | 2 |
|
III | 0 | 4 |
RNA extraction and quantitative
RT-PCR
Total RNAs were isolated using TRIzol reagent
(Thermo Fisher Scientific, Inc.). RT reactions were performed using
10 ng of total RNA samples and GeneChip WT (Takara Biomedical
Technology Co., Ltd., Beijing, China). Quantitative RT-PCR was
performed using the standard TaqMan MicroRNA assay protocol on a
7500 Real-Time PCR System (Applied Biosystems; Thermo Fisher
Scientific, Inc.) at 95°C for 15 min, followed by 45 cycles at 94°C
for 15 sec, 55°C for 30 sec, and 70°C for 30 sec. The primer
sequences were as follows: miR-145 forward,
5′-ATCGTCCAGTTTTCCCAGG-3′ and reverse, 5′-CGCCTCCACACACTCACC-3′; U6
forward, 5′-ATTGGAACGATACAGAGAAGATT-3′ and reverse,
5′-GGAACGCTTCACGAATTTG-3′. The fold change of miRNA-145 was
calculated using the 2−ΔΔCq equation (17).
Cell transfection
The cells (1×105 cells/well) were plated
in 6-well plates and transfected with either miR-145 mimics (100
nm) or miR-145 inhibitor (100 nm; both from Sangon Biotech, Co.,
Ltd., Shanghai, China) using Lipofectamine 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. After 48 h of transfection, the cells were collected
for the following experiments. In experiments where the EGFR
inhibitor or PI3K inhibitor was used, after 4 h of transfection,
the cells were treated with EGFR inhibitor (0.5 nM; cat. no.
AEE788; MedChemExpress, Monmouth Junction, NJ, USA) or PI3K
inhibitor (1,3-dicaffeoylquinic acid; 10 µM; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) for 48 h.
MTT assay and lactic dehydrogenase
(LDH) activity
The cells (5×103 cells/well) were plated
in 96-well plates and MTT (5 mg/ml) was added to the cells for 4 h
at 37°C. Subsequently, the MTT solution was removed and DMSO
solution was added into the cells for 20 min at 37°C. The
absorbance was measured using a multi-well plate reader (Tecan
Schweiz AG, Maennedorf, Switzerland) at 492 nm.
LDH activity was assessed using an LDH activity kit
(Beyotime Institute of Biotechnology, Shanghai, China). The
absorbance was measured using a multi-well plate reader (Tecan
Schweiz AG) at 450 nm.
Transwell assays
For the cell migration Transwell assays, 24-well
plates with 8.0-µm pore size polycarbonate membranes
(5×104, 6.5 mm diameter; Corning Inc., Corning, NY, USA)
were used. Serum-free medium (100 µl) was seeded into the upper
chamber and 600 µl medium containing 10% FBS was added to the
bottom chamber. Cell were cultured for 48 h and fixed with 4%
paraformaldehyde and stained with 0.1% crystal violet. The cells
were observed using an optical microscope (Optotech Co., Ltd.,
Changchun, China).
Flow cytometry (FCM)
The cells (1×106 cells/well) were plated
in 6-well plates, collected and washed twice with PBS before being
suspended with 500 µl of binding buffer (BD Biosciences, Franklin
Lakes, NJ, USA). The cells were stained with 5 µl of Annexin
V-fluorescein isothiocyanate and 5 µl of propidium iodide (PI) for
15 min at room temperature. A BD FACSCalibur flow cytometer (BD
Biosciences) was used to detect cell apoptosis.
Western blot analysis and caspase-3/-9
activity levels
Cell were washed and splitted using RIPA lysis
buffer (Beyotime Institute of Biotechnology) after centrifugation
at 10,000 × g for 10 min at 4°C. The protein concentration was
calculated with a Pierce BCA protein assay kit (Thermo Fisher
Scientific, Inc., Rockford, IL, USA). Protein (50 µg) was separated
on 8–10% SDS-PAGE gels and transferred to polyvinylidene difluoride
(PVDF) membranes (EMD Millipore, Bedford, MA, USA). The membranes
were blocked with 5% non-fat milk at room temperature for 1 h and
immunostained with primary antibodies: Bax (1:500; cat. no.
sc-6236), EGFR (1:500; sc-71034), PI3K (1:500; cat. no. sc-7174),
p-AKT (Ser473; 1:500; cat. no. sc-7985-R) and GAPDH (1:2,000; cat.
no. sc-25778; all from Santa Cruz Biotechnology, Inc., Dallas, TX,
USA) at 4°C overnight. The membranes were then washed three times
in TBST, and then incubated with anti-rabbit secondary antibody
(1:2,000; cat. no. sc-2030; Santa Cruz Biotechnology, Inc.) at room
temperature for 1 h. Subsequently, the membranes were detected with
ECL Plus (GE Healthcare, Piscataway, NJ, USA) and quantitatively
determined via densitometry with ImageJ software 2.1.4.7
(imagej.nih.gov/).
Protein (10 µg) was used to assess caspase-3 and
caspase-9 activity levels using caspase-3/-9 activity kits
(Beyotime Institute of Biotechnology). The absorbance was measured
using a multi-well plate reader (Tecan Schweiz AG) at 405 nm.
Statistical analysis
The results were expressed as the mean ± standard
deviation (n=3). Statistical significance of the results between
each group was evaluated using one-way ANOVA followed by Tukey's
post test. P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-145 expression
Initially gene chip technology was used to measure
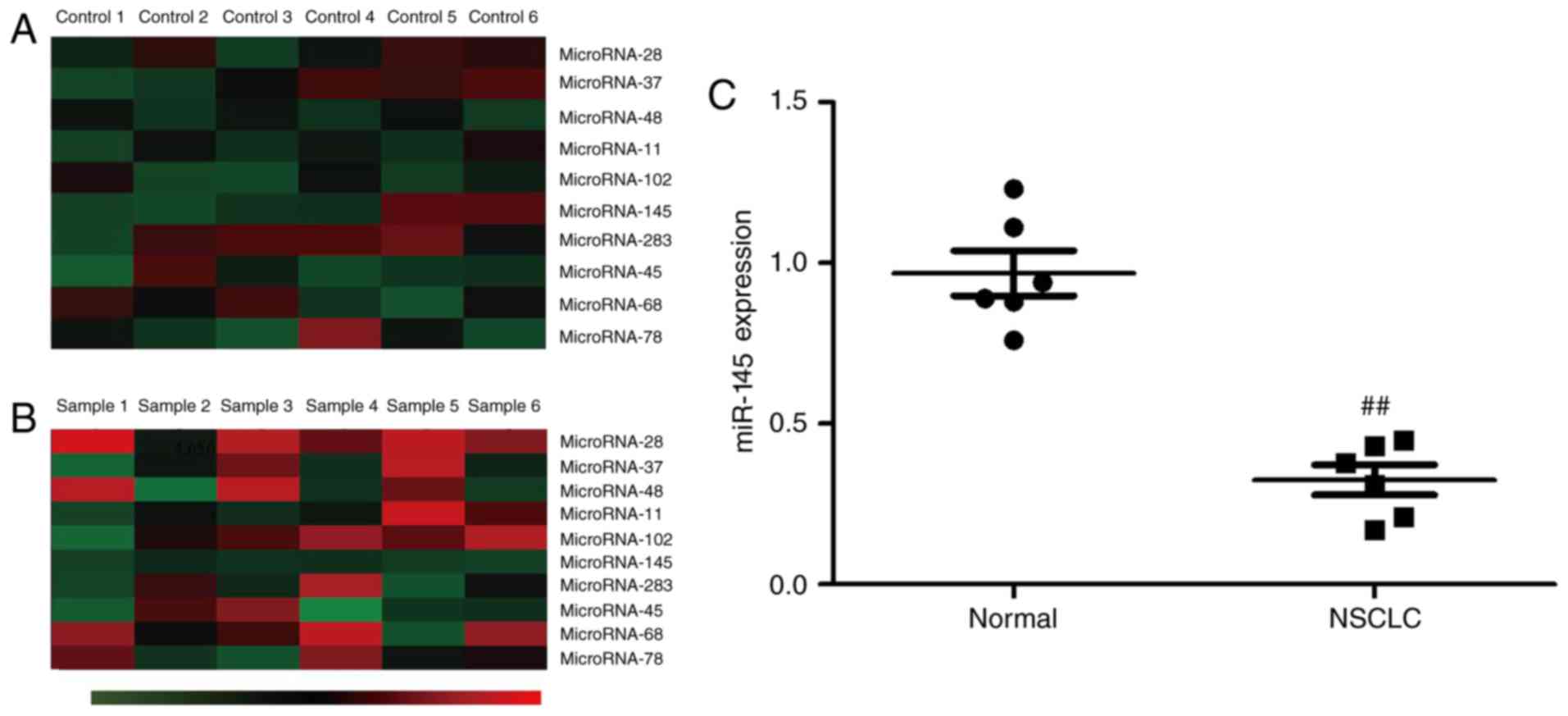
the expression of miRNAs. It was observed that miR-145 expression
was downregulated in the serum of patients with NSCLC compared with
normal healthy volunteers (Fig. 1A and
B). In addition, miR-145 expression was assessed using RT-PCR.
The results revealed that miR-145 expression was downregulated in
NSCLC patients compared to normal healthy volunteers (Fig. 1C).
Effect of miR-145 downregulation on
cell growth in A549 cells
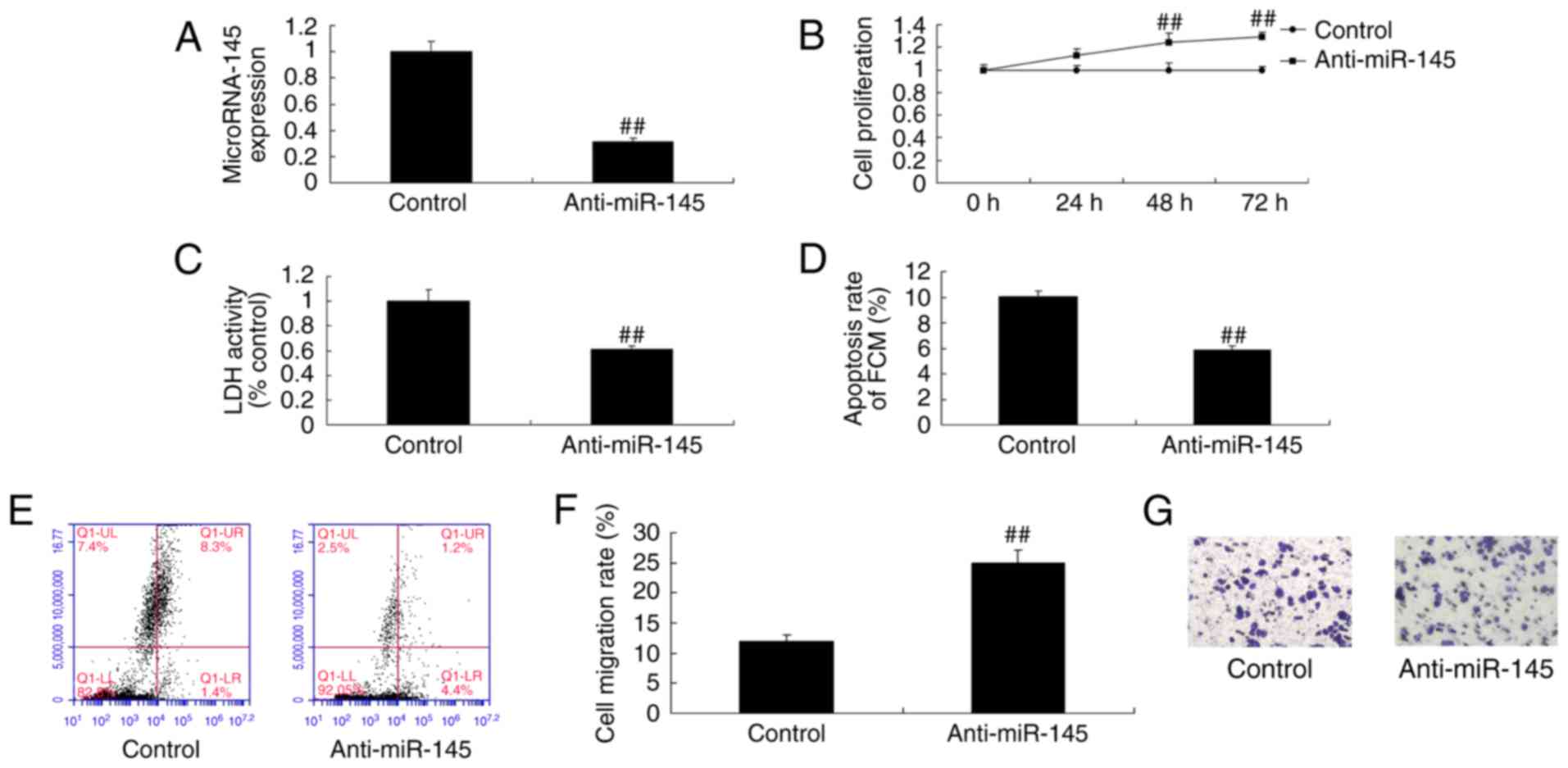
To confirm the potential role of miR-145 in patients
with NSCLC, a miR-145 inhibitor was used to inhibit the expression
of miR-145 in A549 cells (Fig. 2A).
The miR-145 inhibitor significantly increased cell proliferation
(Fig. 2B) and migration (Fig. 2F and G), and reduced LDH activity
(Fig. 2C) and apoptosis (Fig. 2D and E) in A549 cells compared with
the control group.
Effect of miR-145 downregulation on
caspase-3/-9 activity levels and Bax protein expression in A549
cells
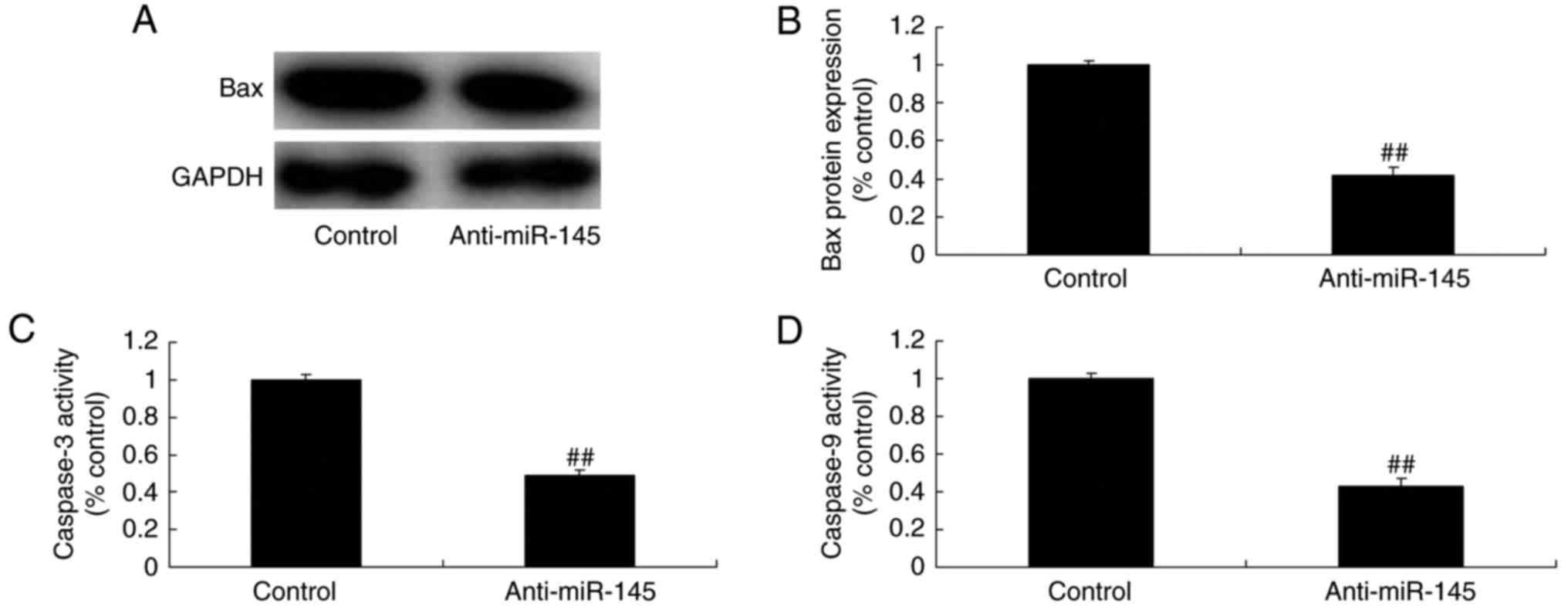
miR-145 downregulation resulted in significant
inhibition of caspase-3/-9 activity levels and Bax protein
expression in A549 cells compared with the control group (Fig. 3).
Effect of miR-145 upregulation on cell
growth in A549 cells
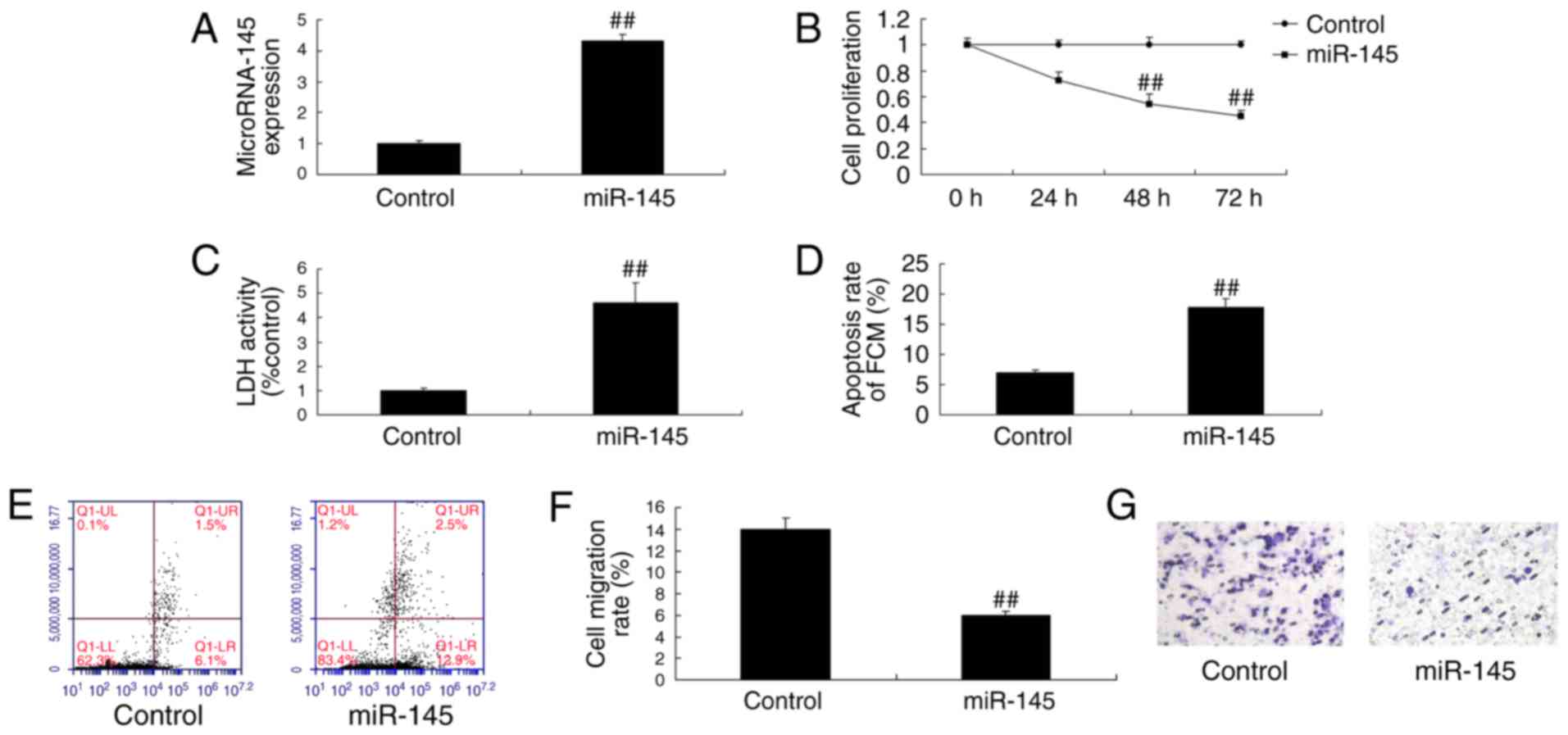
miR-145 mimics were used to verify the effect of
miR-145 upregulation on cell growth in A549 cells. The miR-145
mimics increased the expression of miR-145 in A549 cells (Fig. 4A). The miR-145 mimics also
significantly inhibited cell proliferation (Fig. 4B) and migration (Fig. 4F and G) and induced LDH activity
(Fig. 4C) and apoptosis (Fig. 4D and E) in A549 cells.
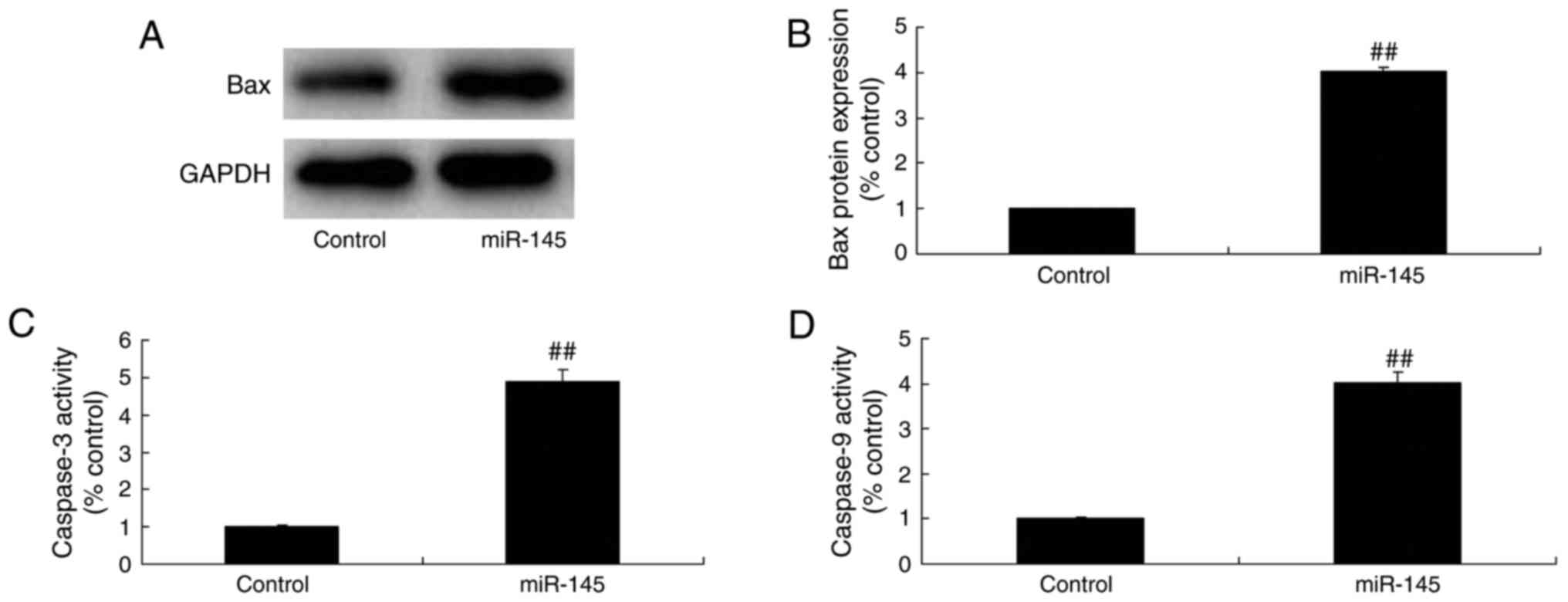
Effect of miR-145 upregulation on
caspase-3/-9 activity levels and Bax protein expression in A549
cells
Caspase-3/-9 levels and Bax protein expression are
common indicators of the apoptosis signaling pathway. It was
analyzed whether miR-145 upregulation altered caspase-3/-9 activity
levels and Bax protein expression in A549 cells. miR-145
upregulation caused a significant promotion in caspase-3/-9
activity levels and Bax protein expression in A549 cells compared
with the control group (Fig.
5).
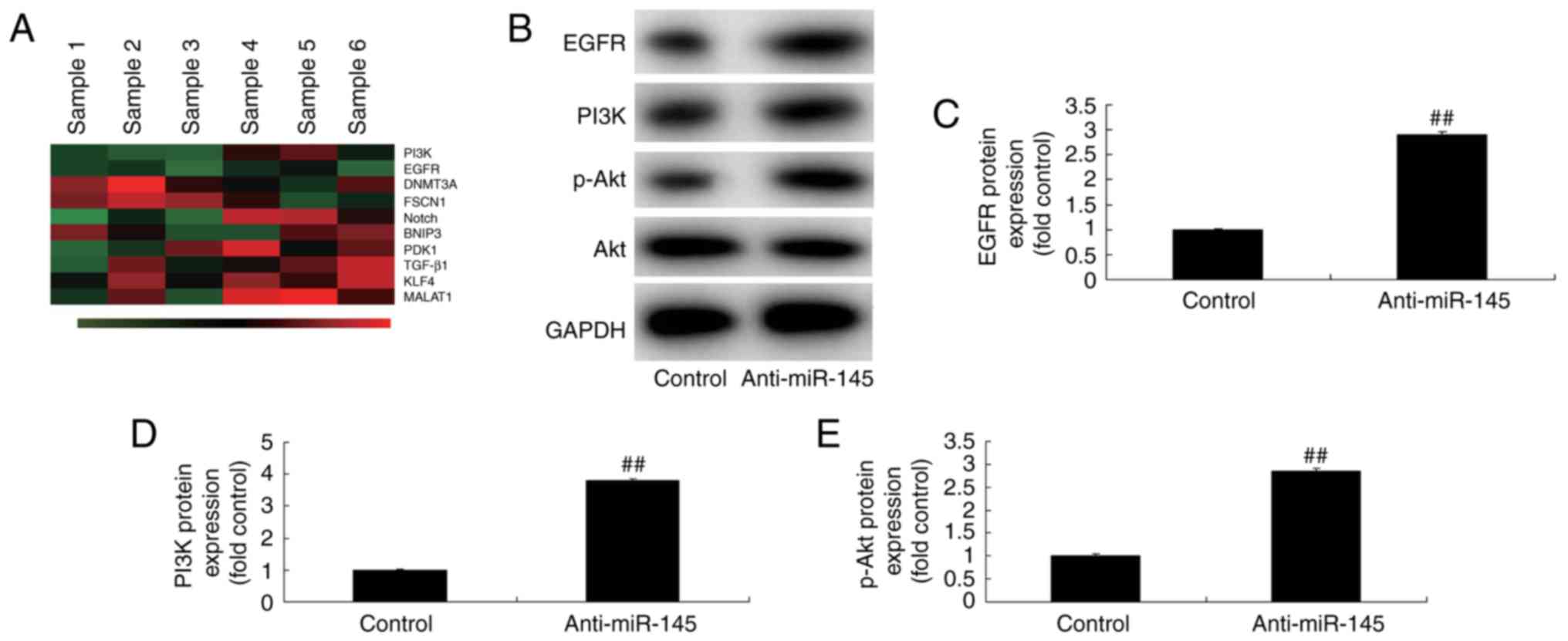
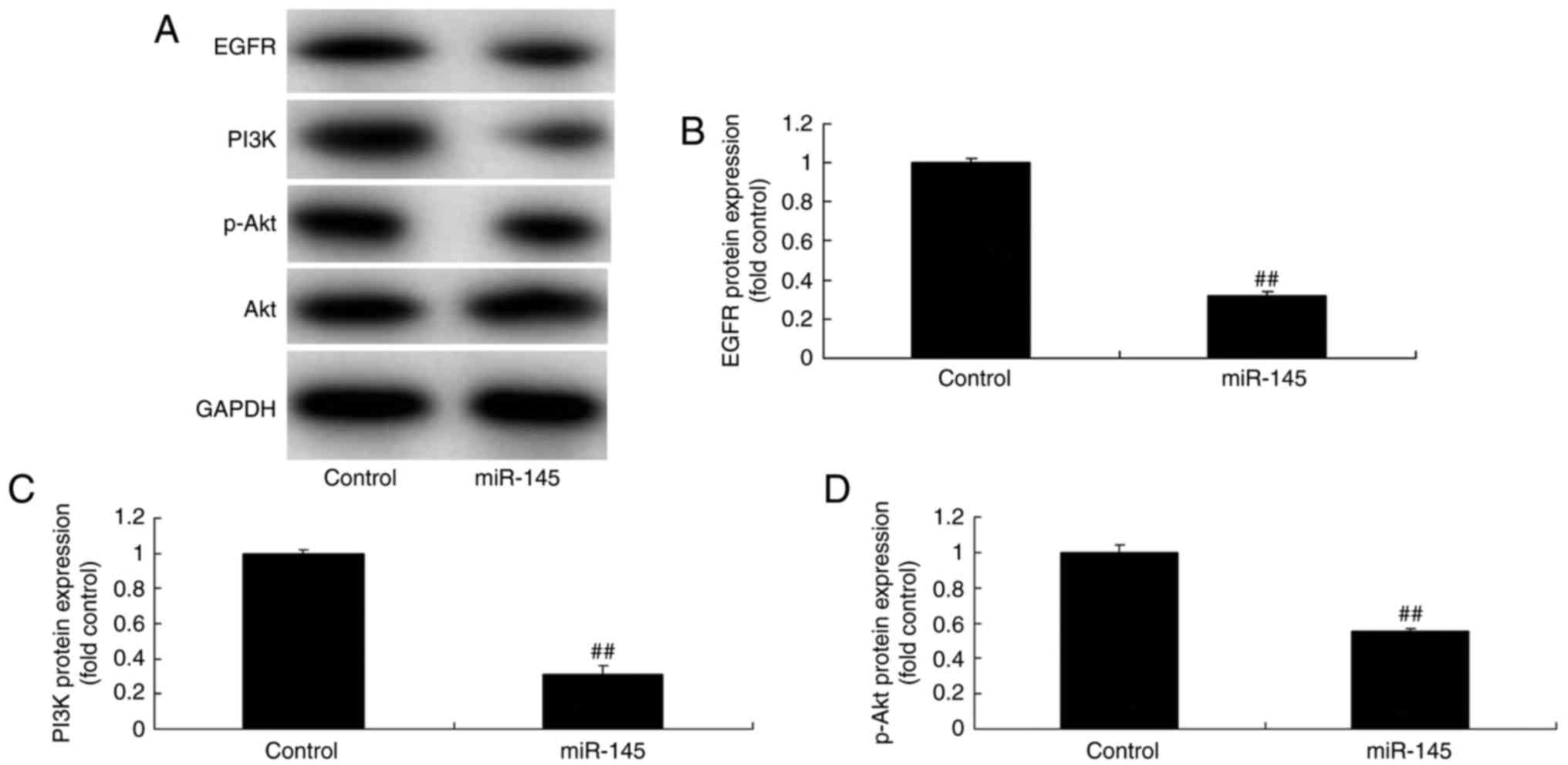
Effects of miR-145 on the
EGFR/PI3K/AKT signaling pathway in A549 cells
The underlying mechanism of miR-145 on apoptosis was
explored in A549 cells. Gene chip technology revealed that EGFR and
PI3K expression were downregulated in A549 cells compared with the
control group when miR-145 was overexpressed (Fig. 6A). In addition, miR-145
downregulation significantly induced the EGFR/PI3K/AKT signaling
pathway in A549 cells compared with the control group (Fig. 6B-E). The EGFR/PI3K/AKT signaling
pathway was significantly suppressed in A459 cells when miR-145 was
upregulated, compared with the control group (Fig. 7). These results further demonstrated
that miR-145 may serve an important role in A549 cells in
vitro.
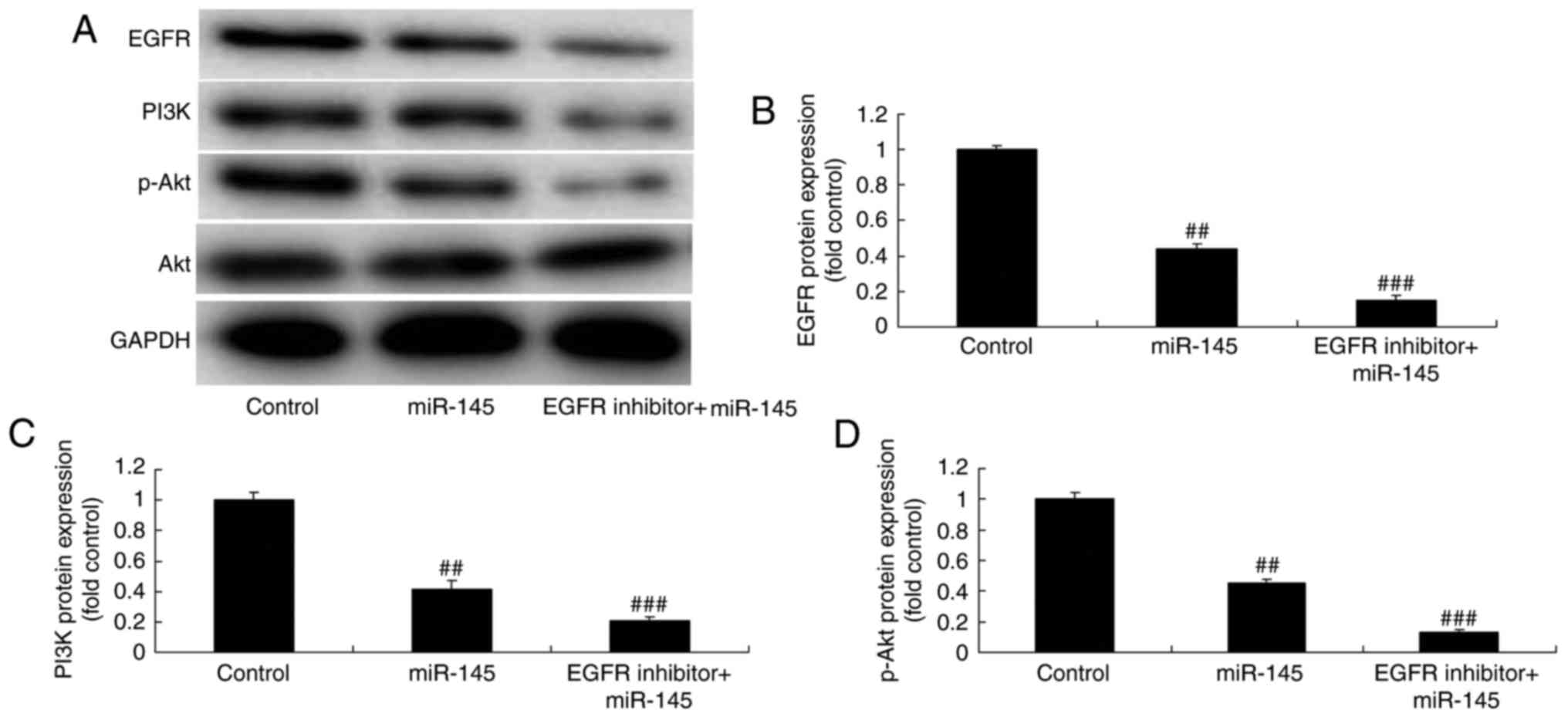
EGFR inhibitor suppresses the
EGFR/PI3K/AKT signaling pathway in A549 cells following miR-145
upregulation
The effect of EGFR on the EGFR/PI3K/AKT signaling
pathway was investigated in A549 cells following miR-145
upregulation. The EGFR/PI3K/AKT signaling pathway was significantly
suppressed in A549 cells treated with EGFR inhibitor (0.5 nM, 48 h;
cat. no. AEE788) following miR-145 upregulation, compared with the
miR-145 upregulation group (Fig.
8).
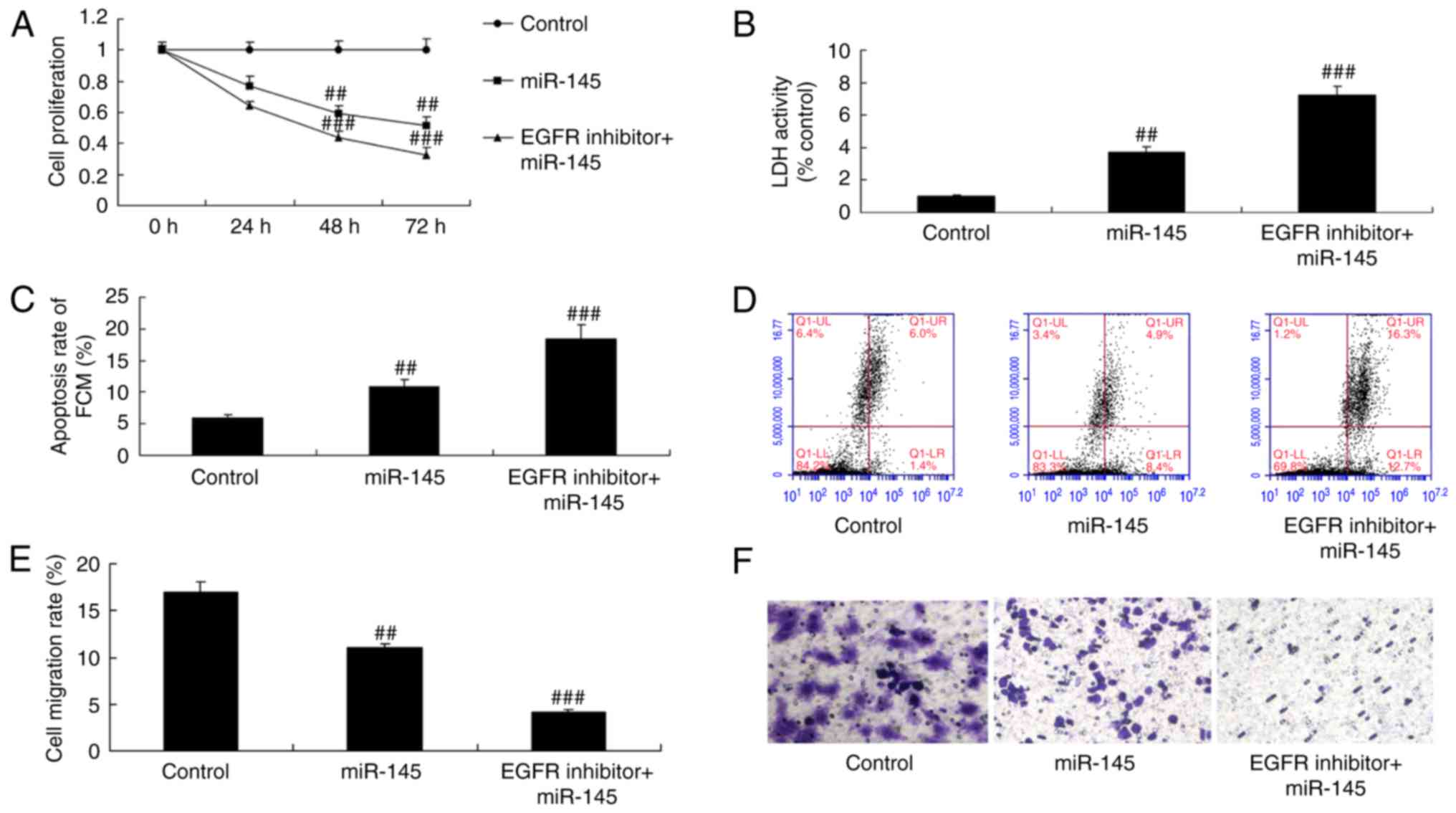
EGFR inhibitor suppresses cell growth
in A549 cells following miR-145 upregulation
Significant inhibition of cell proliferation and
migration was observed, as well as increases in LDH activity and
apoptosis in A549 cells following treatment with miR-145 and EGFR
inhibitor, compared with the miR-145 upregulation group (Fig. 9).
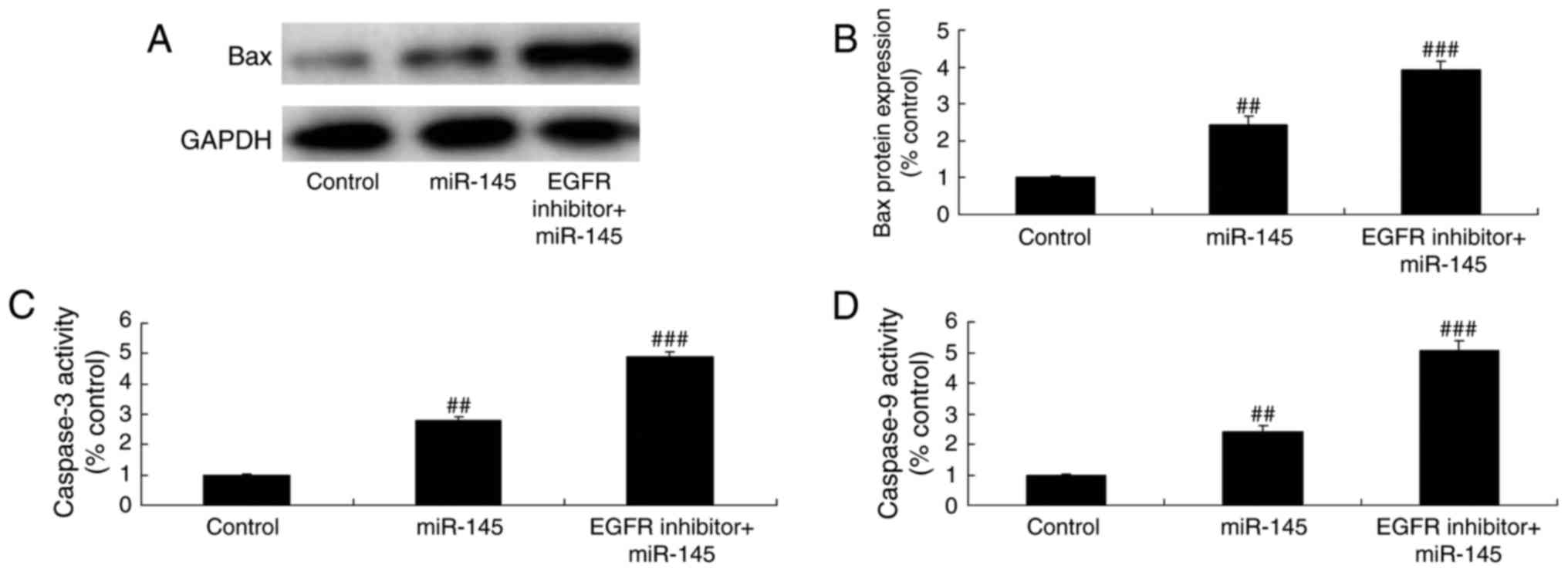
EGFR inhibitor suppresses caspase-3/-9
activity levels and Bax protein expression in A549 cells following
miR-145 upregulation
A significant promotion in caspase-3/-9 activity
levels and Bax protein expression was observed in A549 cells
following treatment with miR-145 and the EGFR inhibitor, compared
with the miR-145 upregulation group (Fig. 10). These results indicated that
EGFR is an important factor, which may enhance the effects of
miR-145 on NSCLC chemotherapy.
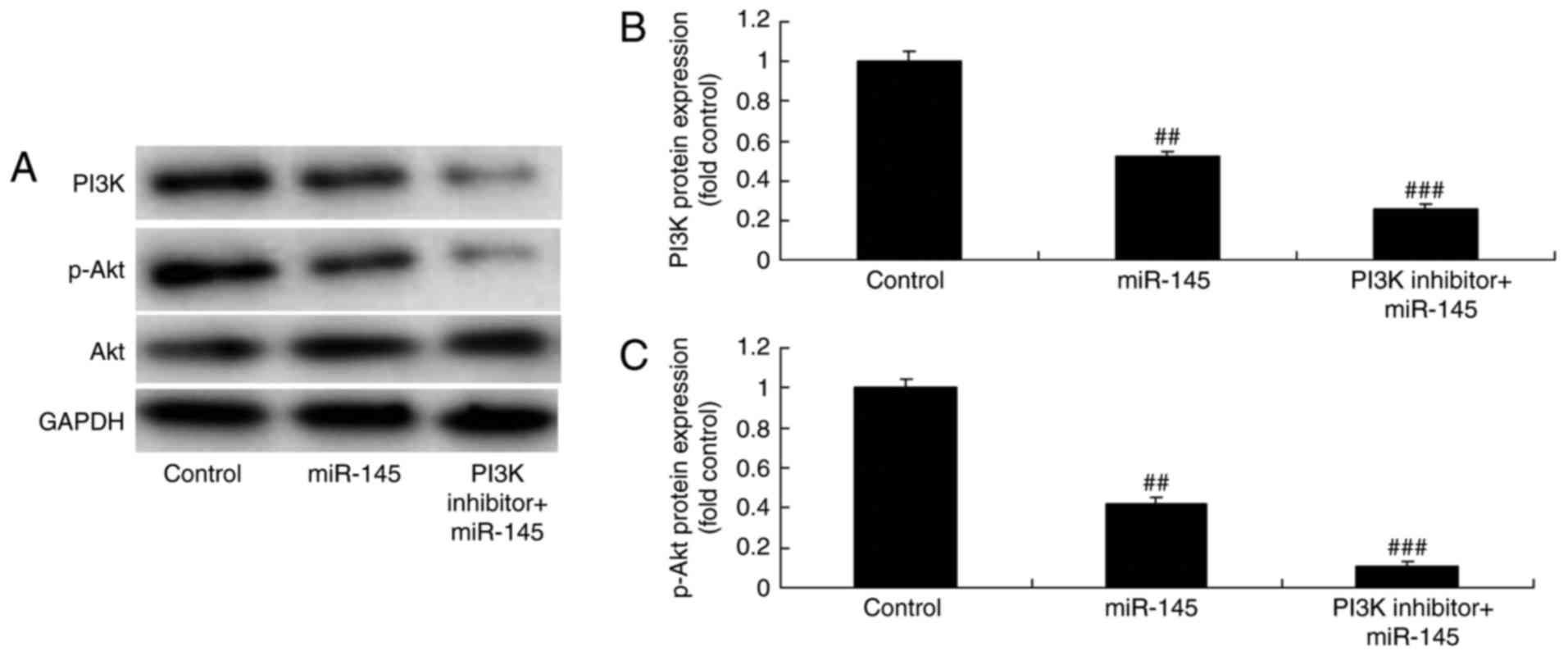
PI3K inhibitor suppresses the PI3K/AKT
signaling pathway in A549 cells following miR-145 upregulation
The function of PI3K and the effect of miR-145 on
the EGFR/PI3K/AKT signaling pathway was investigated in A549 cells.
The PI3K inhibitor (1,3-dicaffeoylquinic acid; 10 µM, 48 h)
significantly suppressed the PI3K/AKT signaling pathway in A549
cells following miR-145 upregulation, compared with the miR-145
upregulation group (Fig. 11).
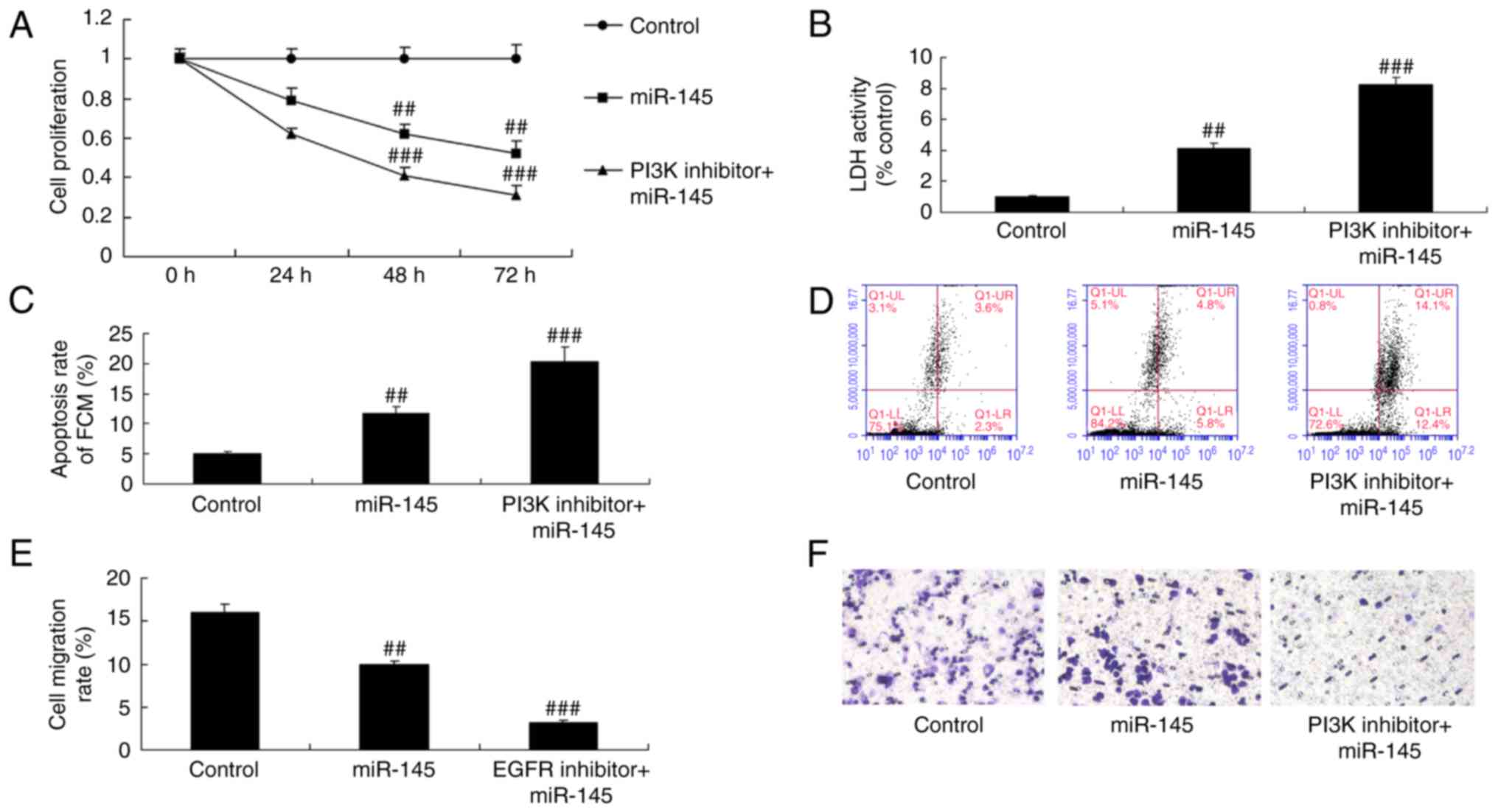
PI3K inhibitor suppresses cell growth
in A549 cells following miR-145 upregulation
The inhibition of PI3K significantly inhibited cell
proliferation and migration, while it increased LDH activity and
apoptosis in A549 cells following miR-145 upregulation, compared
with the miR-145 upregulation group (Fig. 12).
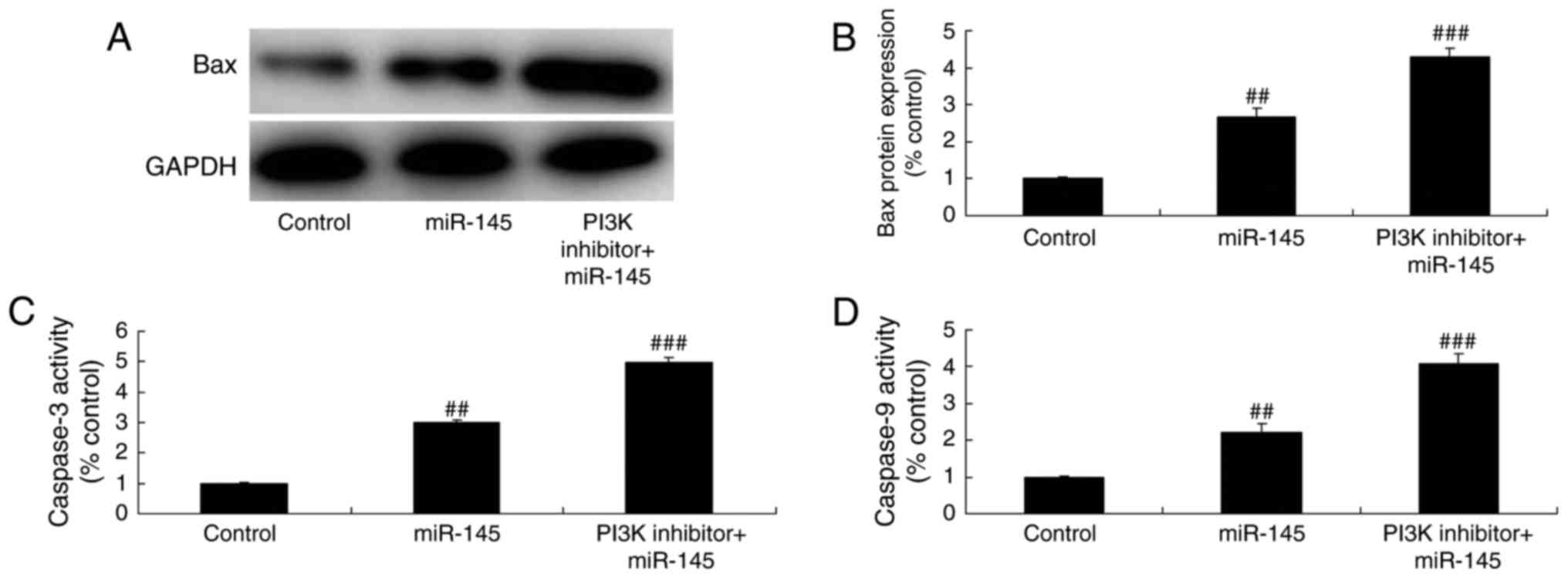
PI3K inhibitor suppresses caspase-3/-9
activity levels and Bax protein expression in A549 cells following
miR-145 upregulation
The inhibition of PI3K significantly increased the
effects of miR-145 on caspase-3/-9 activity levels and Bax protein
expression in A549 cells, compared with the miR-145 upregulation
group (Fig. 13). The
aforementioned results indicated that the effects of miR-145 occur
through the inhibition of the PI3K/AKT signaling pathway in
NSCLC.
Discussion
At present, the principal methods of lung cancer
classification and staging used within a clinical setting are
primarily based on imaging diagnoses, and morphological changes in
tissues and cells observed under a microscope (18). However, these diagnostic methods
provide limited information and are insufficient for an accurate
and in depth understanding of the molecular changes taking place
during tumor genesis and development (1). Therefore, research into the molecular
classification of lung cancer may contribute to more targeted
treatments and accurate prognosis (4). The present study revealed that miR-145
expression was downregulated in patients with NSCLC compared with
normal healthy volunteers, however there were only a total of six
individuals in each group, which is a small sample size and
represents a limitation of the study. Future studies should analyze
data from larger groups to increase the validity of the results.
The results of the present study demonstrated that the
downregulation of miR-145 in A549 cells reduced LDH and apoptosis,
increased cell proliferation, and inhibited caspase-3/-9 levels and
Bax protein expression. Zhu et al (19) revealed that miR-122, miR-145 and
let-7b were underexpressed in castration-resistant prostate cancer.
However, the present study only used A549 cells, which represents
another limitation and in future studies it is recommended that
additional cell lines are used.
In the majority of advanced NSCLC cases, the tumor
pathology and EGFR status are determined using the primary tumor in
the lung as opposed to the brain metastasis, as it is far easier to
access and obtain (20). However,
certain patients receive craniocerebral surgical resection or
biopsy following the initial symptom of brain metastasis, which is
able to positively confirm the diagnosis of NSCLC (21). When this occurs the brain metastasis
sample may be utilized for EGFR detection. If there is consistency
in the EGFR expression between the primary lesion and the brain
metastasis, as determined by either diagnostic method, targeted
therapies may be applied based on the results (22,23).
Therefore, the authors consider that miR-145 upregulation
suppressed the EGFR/PI3K/AKT signaling pathway in A549 cells. Cheng
et al demonstrated that miRNA-145 downregulates mucin 5AC to
alleviate airway remodeling through EGFR expression (24). p-EGFR may also participate in the
effects of miR-145 in A549 cells, however, only EGFR protein
expression was analyzed in present study. Further studies should
analyze the function of p-EGFR protein expression and the effect of
miR-145 on its expression in A549 cells.
The PI3K/AKT signaling pathway has been known for
over 10 years. When PI3K is phosphorylated it triggers the
production of the secondary messenger phosphatidyl inositol
triphosphate (PIP3) on the plasma membrane (12). PIP3 then binds with a domain in the
N-terminal of AKT, while AKT translocates from the cytoplasm to the
cell membrane. Activated AKT either activates or inhibits
downstream target proteins through their phosphorylation and
thereby regulates cell proliferation, differentiation, apoptosis
and migration (25). The results of
the present study demonstrated that the EGFR inhibitor suppressed
the EGFR/PI3K/AKT signaling pathway and increased the anticancer
effects of miR-145 upregulation in A549 cells. Zhang et al
(26) reported that synthetic
miR-145 expression inhibits multiple myeloma cell growth through
the PI3K/AKT signaling pathway. The present study only investigated
the effect of the EGFR inhibitor on miR-145 upregulation in A549
cells, therefore future studies should also explore the effect of
miR-145 inhibitors combined with EGFR inhibitors in A549 cells.
All members of the PI3K family are oncogenes, which
are important kinases of inositol and phosphatidylinositol
(27). PI3K consists of a
regulatory subunit p85 and a catalytic subunit p110, which promotes
the phosphorylation of the 3′hydroxyl on the inositol ring
(28). AKT is a serine/threonine
protein kinase with a molecular weight of 57 kDa; it is a homologue
of the viral AKT oncogene in mammals (27). Activated AKT influences the active
state of multiple downstream effector molecules, however these
effects only occur after the PI3K/AKT signaling pathway is
activated (27). AKT may inhibit
cell apoptosis and activate effector molecules, including Bad,
caspase-9, FKHR1 and nuclear factor-κB. AKT also participates in
cell cycle regulation (it is clarified at present that AKT
upregulates c-myc expression by increasing its transcription),
promotes tumor angiogenesis (AKT may activate nitric oxide synthase
and thereby stimulates the growth and proliferation of endothelial
cells, increases vascular permeability and promotes angiogenesis
following angiectasis, which provides sufficient nutrition for
tumor cells) and enhances cell invasion and metastasis (11,29).
In addition, it was revealed that the PI3K inhibitor suppresses the
PI3K/AKT signaling pathway and reversed the anticancer effects of
miR-145 upregulation in A549 cells. Boufraqech et al suggest
that miR-145 suppresses thyroid cancer growth and metastasis
through AKT3 expression (30). The
PI3K/AKT signaling pathway regulates a number of anticancer
signaling pathways, including mammalian target of rapamycin,
glycogen synthase kinase-3 and Bad (31). Future studies should investigate the
wide variety of anticancer signaling pathways that may be affected
by miR-145 in NSCLC.
In summary, the present study revealed the potential
role of miR-145 in patients with NSCLC. It was also demonstrated
that the EGFR/PI3K/AKT signaling pathway was inhibited by miR-145,
which ultimately inhibited tumor development. miR-145 has been
revealed as a novel potential therapy for the targeted treatment of
NSCLC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
BL designed the experiment; CMD, YXL, JCP, NG and
WWQ performed the experiment; BL and CMD analyzed the data; BL
wrote the manuscript. All authors read and approved the manuscript
and agree to be accountable for all aspects of the research in
ensuring that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
All human studies were approved by the Ethics
Committee of Fourth Hospital of Hebei Medical University.
Patient consent for publication
All patients signed written informed consent forms
prior to the study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhu Z, Liu W, Gillin M, Gomez DR, Komaki
R, Cox JD, Mohan R and Chang JY: Assessing the robustness of
passive scattering proton therapy with regard to local recurrence
in stage III non-small cell lung cancer: A secondary analysis of a
phase II trial. Radiat Oncol. 9:1082014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ahn MJ, Kim SW, Cho BC, Ahn JS, Lee DH,
Sun JM, Massey D, Kim M, Shi Y and Park K: Phase II study of
Afatinib as third-line treatment for patients in Korea with stage
IIIB/IV non-small cell lung cancer harboring wild-type EGFR.
Oncologist. 19:702–703. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou ZY, Xu L, Li HG, Tian JH, Jiao LJ,
You SF, Han ZF, Jiang Y, Guo HR and Liu H: Chemotherapy in
conjunction with traditional Chinese medicine for survival of
elderly patients with advanced non-small-cell lung cancer: Protocol
for a randomized double-blind controlled trial. J Integr Med.
12:175–181. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yamasaki M, Murakami I, Nakano K, Doi M,
Kitaguchi S, Kondo T, Sakurai J, Hattori N and Arita KI:
Carboplatin plus Weekly paclitaxel combined with bevacizumab as
first-line treatment for non-small cell lung cancer. Anticancer
Res. 37:923–928. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reck M, Rodriguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Pembrolizumab versus Chemotherapy for PD-L1-positive
non-small-cell lung cancer. N Engl J Med. 375:1823–1833. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hida T, Nakagawa K, Seto T, Satouchi M,
Nishio M, Hotta K, Takahashi T, Ohe Y, Takeda K, Tatsuno M, et al:
Pharmacologic study (JP28927) of alectinib in Japanese patients
with ALK+ non-small-cell lung cancer with or without
prior crizotinib therapy. Cancer Sci. 107:1642–1646. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Voortman J, Goto A, Mendiboure J, Sohn JJ,
Schetter AJ, Saito M, Dunant A, Pham TC, Petrini I, Lee A, et al:
MicroRNA expression and clinical outcomes in patients treated with
adjuvant chemotherapy after complete resection of non-small cell
lung carcinoma. Cancer Res. 70:8288–8298. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhan X, Wu W, Han B, Gao G, Qiao R, Lv J,
Zhang S, Zhang W, Fan W, Chen H, et al: Hsa-miR-196a2 functional
SNP is associated with severe toxicity after platinum-based
chemotherapy of advanced nonsmall cell lung cancer patients in a
Chinese population. J Clin Lab Anal. 26:441–446. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Neal JW, Dahlberg SE, Wakelee HA, Aisner
SC, Bowden M, Huang Y, Carbone DP, Gerstner GJ, Lerner RE, Rubin
JL, et al: Erlotinib, cabozantinib, or erlotinib plus cabozantinib
as second-line or third-line treatment of patients with EGFR
wild-type advanced non-small-cell lung cancer (ECOG-ACRIN 1512): A
randomised, controlled, open-label, multicentre, phase 2 trial.
Lancet Oncol. 17:1661–1671. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Planken S, Behenna DC, Nair SK, Johnson
TO, Nagata A, Almaden C, Bailey S, Ballard TE, Bernier L, Cheng H,
et al: Discovery of
N-((3R,4R)-4-fluoro-1-(6-((3-methoxy-1-methyl-1H-pyrazol-4-yl)amino)-9-methyl-9H-purin-2-yl)pyrrolidine-3-yl)acrylamide
(PF-06747775) through structure-based drug design: A high affinity
irreversible inhibitor targeting oncogenic EGFR mutants with
selectivity over wild-type EGFR. J Med Chem. 60:3002–3019. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Q, Zhu H, Xu X, Li L, Tan H and Cai
X: Inactivated Sendai virus induces apoptosis and autophagy via the
PI3K/Akt/mTOR/p70S6K pathway in human non-small cell lung cancer
cells. Biochem Biophys Res Commun. 465:64–70. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin CH, Lin HH, Kuo CY and Kao SH:
Aeroallergen Der p 2 promotes motility of human non-small cell lung
cancer cells via toll-like receptor-mediated up-regulation of
urokinase-type plasminogen activator and integrin/focal adhesion
kinase signaling. Oncotarget. 8:11316–11328. 2017.PubMed/NCBI
|
|
13
|
Qiao L, Wang J, Long G and Jiang Y:
Sequential treatment of tyrosine kinase inhibitor and
platinum-based doublet chemotherapy on EGFR mutant non-small cell
lung cancer: A meta-analysis of randomized controlled clinical
trials. Onco Targets Ther. 10:1279–1284. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Uchibori K, Inase N, Araki M, Kamada M,
Sato S, Okuno Y, Fujita N and Katayama R: Brigatinib combined with
anti-EGFR antibody overcomes osimertinib resistance in EGFR-mutated
non-small-cell lung cancer. Nat Commun. 8:147682017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo Y, Sun W, Gong T, Chai Y, Wang J, Hui
B, Li Y, Song L and Gao Y: miR-30a radiosensitizes non-small cell
lung cancer by targeting ATF1 that is involved in the
phosphorylation of ATM. Oncol Rep. 37:1980–1988. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fang C, Chen YX, Wu NY, Yin JY, Li XP,
Huang HS, Zhang W, Zhou HH and Liu ZQ: MiR-488 inhibits
proliferation and cisplatin sensibility in non-small-cell lung
cancer (NSCLC) cells by activating the eIF3a-mediated NER signaling
pathway. Sci Rep. 7:403842017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu X, Long L, Liu J, Zhang J, Wu T, Chen
X, Zhou B and Lv TZ: Gambogic acid suppresses inflammation in
rheumatoid arthritis rats via PI3K/Akt/mTOR signaling pathway. Mol
Med Rep. 16:7112–7118. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mulvenna P, Nankivell M, Barton R,
Faivre-Finn C, Wilson P, McColl E, Moore B, Brisbane I, Ardron D,
Holt T, et al: Dexamethasone and supportive care with or without
whole brain radiotherapy in treating patients with non-small cell
lung cancer with brain metastases unsuitable for resection or
stereotactic radiotherapy (QUARTZ): Results from a phase 3,
non-inferiority, randomised trial. Lancet. 388:2004–2014. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu J, Wang S, Zhang W, Qiu J, Shan Y,
Yang D and Shen B: Screening key microRNAs for castration-resistant
prostate cancer based on miRNA/mRNA functional synergistic network.
Oncotarget. 6:43819–43830. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Marquez-Medina D and Popat S: Eventual
role of EGFR-tyrosine kinase inhibitors in early-stage
non-small-cell lung cancer. Future Oncol. 12:815–825. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang W, Jiang X, Song Z and Zhang Y:
Patients harboring EGFR mutation after primary resistance to
crizotinib and response to EGFR-tyrosine kinase inhibitor. Onco
Targets Ther. 9:211–215. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nand M, Maiti P, Pant R, Kumari M, Chandra
S and Pande V: Virtual screening of natural compounds as inhibitors
of EGFR 696–1022 T790M associated with non-small cell lung cancer.
Bioinformation. 12:311–317. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sheng Z and Zhang Y: EGFR-TKIs combined
with chemotherapy versus EGFR-TKIs single agent as first-line
treatment for molecularly selected patients with non-small cell
lung cancer. Med Oncol. 32:4202015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cheng Z, Dai LL, Wang X, Jia LQ, Jing XG,
Li PF, Liu M, Wang H and An L: MicroRNA-145 down-regulates mucin
5AC to alleviate airway remodeling and targets EGFR to inhibit
cytokine expression. Oncotarget. 8:46312–46325. 2017.PubMed/NCBI
|
|
25
|
Kim N, Jeong S, Jing K, Shin S, Kim S, Heo
JY, Kweon GR, Park SK, Wu T, Park JI, et al: Docosahexaenoic acid
induces cell death in human non-small cell lung cancer cells by
repressing mTOR via AMPK activation and PI3K/Akt inhibition. Biomed
Res Int. 2015:2397642015.PubMed/NCBI
|
|
26
|
Zhang Q, Yan W, Bai Y, Xu H, Fu C, Zheng
W, Zhu Y and Ma J: Synthetic miR-145 mimic inhibits multiple
myeloma cell growth in vitro and in vivo. Oncol Rep.
33:448–456. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vansteenkiste JF, Canon JL, De Braud F,
Grossi F, De Pas T, Gray JE, Su WC, Felip E, Yoshioka H, Gridelli
C, et al: Safety and efficacy of Buparlisib (BKM120) in patients
with PI3K pathway-activated non-small cell lung cancer: Results
from the phase II BASALT-1 study. J Thorac Oncol. 10:1319–1327.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Z, Shen Z, Li Z, Duan J, Fu S, Liu Z,
Bai H, Zhang Z, Zhao J, Wang X, et al: Activation of the BMP-BMPR
pathway conferred resistance to EGFR-TKIs in lung squamous cell
carcinoma patients with EGFR mutations. Proc Natl Acad Sci USA.
112:9990–9995. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang XY, Kuang JL, Yan CS, Tu XY, Zhao
JH, Cheng XS and Ye XQ: NRSN2 promotes non-small cell lung cancer
cell growth through PI3K/Akt/mTOR pathway. Int J Clin Exp Pathol.
8:2574–2581. 2015.PubMed/NCBI
|
|
30
|
Boufraqech M, Zhang L, Jain M, Patel D,
Ellis R, Xiong Y, He M, Nilubol N, Merino MJ and Kebebew E: miR-145
suppresses thyroid cancer growth and metastasis and targets AKT3.
Endocr Relat Cancer. 21:517–531. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pérez-Ramirez C, Cañadas-Garre M, Molina
MA, Faus-Dáder MJ and Calleja-Hernández MA: PTEN and PI3K/AKT in
non-small-cell lung cancer. Pharmacogenomics. 16:1843–1862. 2015.
View Article : Google Scholar : PubMed/NCBI
|