Introduction
Odontogenic keratocysts (OKCs), previously defined
as keratocystic odontogenic tumors, are common jaw cysts with the
potential to be aggressive and the tendency to recur (1). An extensive volume of research has
demonstrated that the epithelium of OKCs has a unique growth
potential compared with that of other cysts (2,3). In
particular, there is ample evidence that a large number of OKCs
(sporadic and syndromic) harbor patched1 gene mutations, and the
aggressive growth potential of these developmental cysts have been
attributed to this (2,3). Although OKC belongs to a group of
developmental jaw cysts (4), it is
frequently exposed to microorganisms and the majority of OKCs often
occur along with focal inflammatory infiltrates (5). Radicular cysts (RCs) are the most
common jaw cysts, accounting for 52% of jaw cystic lesions
(6). RCs are a result of the
inflammatory process in the periapical tissues associated with
necrotic and infected pulps, and usually occur along with diffused
inflammatory cell infiltrations (6). It is widely accepted that the
cytokines from neutrophils, monocytes and other immune system cells
could facilitate the growth and enlargement of RC (7). Dentigerous cysts (DCs) are associated
with the crown of an unerupted tooth, with inflammation ranging
between little to relatively extensive (8). Cysts of the jaw lesions are known as
common osseous-destructive lesions. The inflammatory cells, upon
stimulation, are able to release cytokines and other undetected
functional protein carriers into the cyst cavity. It is possible
that variations in the inflammatory infiltrate could result in the
different osteolytic activity.
Cell-derived microparticles (MPs) are small
(100–1,000 nm in diameter) membrane-enclosed vesicles secreted from
cells by direct budding from the plasma membrane (9). Nearly all eukaryocytes can release
MPs. Among them, leukocyte-derived MPs (LMPs) originate from
neutrophils, monocytes/macrophages and lymphocytes (10). LMPs express markers from their
parental cells, including cluster of differentiation (CD)4, CD14
and CD15. Given that they arise from the rearrangement of the
plasma, MPs also present with phosphatidylserine (PS) on their
surface. Therefore, a previous study used the mother cell antigens
and Annexin V detection in flow cytometry to positively define MPs
(11). The invasion of bacteria or
bacterial toxins into the jaw cystic cavity involves initial
inflammatory reactions and leads to the accumulation of leukocytes
(5,12). Since odontogenic cysts are directly
immersed in the milieu of cyst fluids, leukocytes may release MPs
into the cavity. However, whether LMPs are present in the cyst
fluid of odontogenic lesions and their biological functions remain
unknown.
To the best of our knowledge, the present study was
the first to demonstrate increased percentages of LMPs in inflamed
OKCs compared with those in DCs. It was also demonstrated that MPs
derived from leukocytes express IL-15 and other cytokines that
potently induce the expression of RANKL and MMP-9 in OKC
fibroblasts, which promotes osteoclastogenesis. The present study
revealed a novel mechanism for the lymphocyte-induced bone
resorption of OKCs.
Materials and methods
Study population
All procedures were performed according to the
National Institutes of Health guidelines regarding the use of human
tissues, and the present study was approved by the Review Board of
the Medical Ethics Committee of the Hospital of Stomatology, Wuhan
University (Wuhan, Hubei, China) and was conducted between April
2016 and August 2017. All patients provided written informed
consent to participate in the study and for its publication. The
present study included a total of 20 patients with focal inflamed
OKC, 3 patients with uninflamed OKC, 15 patients with RC and 12
patients with focal inflamed DCs, diagnosed according to the fourth
edition of the World Health Organization Classification of Head and
Neck Tumors (13). Since diffused
inflamed or severe inflamed OKCs or DCs are difficult to diagnose,
the present study only included focal inflamed OKCs or DCs.
Patients with nevoid basal cell carcinoma syndrome-related OKCs
were excluded from the study. Hematoxylin and eosin (HE) staining
of odontogenic cysts was performed for diagnostic purposes.
Cyst fluid collection and isolation of
cyst fluid MPs
Samples of cystic fluids were obtained from the cyst
cavities by aspiration using a syringe attached to an 18G
sterilized needle prior to marsupialization or enucleation. Cyst
fluids were immediately centrifuged at 3,000 × g for 20 min at 4°C
(centrifuge 5810R; Eppendorf, Hamburg, Germany). Supernatants were
collected and centrifuged at 3,000 × g for another 20 min to obtain
cell-free cyst fluids. Subsequently, the supernatants were diluted
with an equal volume of Ca2+/Mg2+-free highly
purified phosphate-buffered saline (PBS; Beyotime Institute of
Biotechnology, Haimen, China) and were then centrifuged at 10,000 ×
g for 40 min at 4°C. This step aimed to remove apoptotic bodies and
larger vesicles. Next, the supernatants were centrifuged at 50,000
× g for 1 h at 4°C (Avanti J-26 XP; Beckman Coulter, Inc., Brea,
CA, USA) to pellet cystic fluid MPs (CFMPs) as previously described
(14). CFMP pellets were
resuspended in 150 µl PBS. Subsequently, 50 µl of each CFMP sample
was prepared for flow cytometry and the other 100 µl was
immediately stored at −80°C for further study.
Characterization of CFMPs by
transmission electron microscopy (TEM)
TEM was performed at the Wuhan Institute of Virology
(Wuhan, China). Freshly-isolated CFMPs and Jurkat cell-derived MPs
were placed on a copper grid for observation. The grids were
stained with 1% v/v uranyl acetate and the samples were examined on
a Hitachi HT7700 transmission electron microscope (Hitachi, Ltd.,
Tokyo, Japan).
Characterization of CFMPs by
carboxyfluorescein succinimidyl ester (CFSE) staining
The freshly isolated CFMPs were incubated with 10 µM
CFSE (Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C for
30 min in the dark. Subsequently, the samples were analyzed using a
fluorescence microscope (Leica Microsystems GmbH, Wetzlar, Germany)
or a FACSAria II flow cytometer (BD Biosciences, Franklin Lakes,
NJ, USA).
Characterization of CFMPs by dynamic
light scattering
The hydrodynamic diameters of CFMPs were determined
by a Nano-ZS ZEN 3600 (Malvern Instruments, Inc., Westborough, MA,
USA), which was equipped with a He-Ne laser (633 nm), as previously
described (15).
Detection and analysis of CFMPs by
flow cytometry
Flow cytometric analysis was performed using a BD
LSRFortessa flow cytometer (BD Biosciences). The size of CFMPs was
identified using Nile Red particles with a diameter of 0.7–0.9 µm
(Spherotech, Lake Forest, IL, USA). To quantify the CFMPs, a known
number of calibrator flow-count fluorosphere beads (10-µm diameter;
Beckman Coulter, Inc.) was added to determine the number of CFMPs.
As flow-count fluorospheres have a definite concentration, when
identical volumes of a sample and flow-count fluorospheres were
added and tested, the concentration of CFMPs could be calculated
using the following formula: CFMPs concentration = (total number of
events for the sample/total number of events for flow-count
fluorospheres) × flow-count fluorosphere assayed concentration.
Next, the concentrations of CFMPs were calculated using the initial
volumes of cyst fluids listed in Tables
I–IV. The subpopulations of
CFMPs were detected according to membrane-specific antigens.
Subsets of the CFMPs were identified as follows: Monocyte-derived
MPs (CD14+/Annexin V+), neutrophil-derived
MPs (CD15+/Annexin V+) and lymphocyte-derived
MPs (CD4+/Annexin V+). Antibodies were added
to 10 µl CFMPs as follows: 2 µl anti-CD14-APC (cat. no. 555399; BD
Pharmingen; BD Biosciences, Inc.), anti-CD4-APC-Cy7 (cat. no.
557871; BD Pharmingen; BD Biosciences, Inc.), anti-CD15-PE-Cy7
(cat. no. 560827; BD Pharmingen; BD Biosciences, Inc.) and 2 µl
Annexin V-PerCP-Cy5.5 (cat. no. 640936; BioLegend, Inc., San Diego,
CA, USA). Additionally, Annexin V binding buffer containing calcium
(BD Biosciences, Inc.) was used for Annexin V detection. These
antibodies and binding buffers were mixed with samples for 30 min
at 4°C in the dark. A total of 380 µl PBS was added to the CFMPs
and then analyzed by flow cytometry. The results were calculated
using FlowJo 9.3.2 software (FlowJo LLC, Ashland, Oregon, USA).
 | Table I.Summary of clinical features of
patients with focal inflamed dentigerous cysts. |
Table I.
Summary of clinical features of
patients with focal inflamed dentigerous cysts.
| Patient no. | Sex | Age, years | Location | Cyst fluid drained,
ml |
|---|
| 1 | F | 30 | Left maxilla | 5 |
| 2 | F | 18 | Right mandible | 2 |
| 3 | M | 18 | Maxilla | 3 |
| 4 | F | 33 | Right maxilla | 2 |
| 5 | M | 51 | Right mandible | 9 |
| 6 | M | 42 | Left maxilla | 2 |
| 7 | F | 50 | Left mandible | 11 |
| 8 | M | 34 | Right mandible | 3.5 |
| 9 | M | 34 | Right mandible | 2 |
| 10 | M | 56 | Right mandible | 4 |
| 11 | F | 28 | Left maxilla | 5 |
| 12 | M | 36 | Right maxilla | 4 |
 | Table IV.Summary of clinical features of
patients with focal inflamed odontogenic keratocysts. |
Table IV.
Summary of clinical features of
patients with focal inflamed odontogenic keratocysts.
| Patient no. | Sex | Age, years | Location | Cyst fluid drained,
ml |
|---|
| 1 | F | 24 | Left mandible | 2.5 |
| 2 | F | 24 | Right mandible | 2 |
| 3 | M | 32 | Left mandible | 4.5 |
| 4 | M | 52 | Left mandible | 1 |
| 5 | M | 79 | Right mandible | 5 |
| 6 | M | 24 | Right mandible | 2 |
| 7 | M | 11 | Right mandible | 5 |
| 8 | F | 52 | Right maxilla | 2 |
| 9 | F | 45 | Right mandible | 3 |
| 10 | M | 34 | Left mandible | 0.8 |
| 11 | F | 28 | Right mandible | 3 |
| 12 | M | 37 | Left maxilla | 7 |
| 13 | M | 26 | Right maxilla | 4 |
| 14 | M | 24 | Left mandible | 1 |
| 15 | F | 32 | Left mandible | 1 |
| 16 | M | 16 | Right mandible | 2 |
| 17 | F | 30 | Left maxilla | 2 |
| 18 | M | 38 | Right mandible | 1 |
| 19 | F | 27 | Maxilla | 1 |
| 20 | M | 27 | Right maxilla | 1 |
Cell culture and Jurkat cell-derived
MP collection
Jurkat Clone E6-1 human T cells (an immortal
lymphoma T-cell line) were grown in RPMI-1640 medium supplemented
with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc.) at 37°C in 5% CO2. For the induction of apoptosis,
1 µM staurosporine (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
or 1 µg/ml camptothecin (Sigma-Aldrich; Merck KGaA) was added to
the Jurkat cells. Following these treatments, cells were cultured
for an additional 48 h without FBS culture medium and then the
supernatant was centrifuged at 400 × g for 5 min to remove cells
(centrifuge 5810R; Eppendorf) at 4°C. Supernatant was transferred
to a fresh tube and recentrifuged again at 2,000 × g for 20 min at
4°C (centrifuge 5810R; Eppendorf). The supernatant was centrifuged
for 1 h at 50,000 × gat 4°C via the Avanti J-26 XP (Beckman
Coulter, Inc.). The pellet was resuspended in PBS (Beyotime
Institute of Biotechnology) and stored at −80°C.
Bicinchoninic acid assay (BCA)
determination of Jurkat cell-derived MPs
Jurkat cell-derived MPs were lysed in
radioimmunoprecipitation (RIPA) assay buffer (Thermo Fisher
Scientific, Inc.) and exposed to sonication, and then the proteins
were quantified using the BCA assay (cat. no., 23225; Pierce;
Thermo Fisher Scientific, Inc.). In brief, 5 µl albumin standard
with concentrations of 2,000, 1,000, 500, 250, 125, 62.5, 0 ng/µl
and 5 µl Jurkat cell-derived MPs (concentrations to be determined)
were added to the working solution and incubated for 30 min at 37°C
in 96-well plates. Next, the plate was read at 570 nm and the
protein concentrations of Jurkat cell-derived MPs were calculated
with the albumin standard proteins.
Cytokine antibody arrays
Jurkat cell-derived MPs and the Jurkat cell
supernatants were analyzed on a Bio-Plex Pro Human Cytokine GrpI
Panel 27-plex (#M500KCAF0Y), according to the manufacturer's
protocols (Bio-Plex MAGPIX System; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Specifically, 50 µg Jurkat cell-derived MPs and
the Jurkat cell supernatants were loaded and tested. The cytokine
concentrations were calculated using Bio-Plex Manager software 6.1
(Bio-Rad Laboratories, Inc.).
Fibroblast isolation and culture
Fibroblast cultures were established from fresh
samples of OKCs from 3 patients and the procedure was approved by
the review board of the Ethics Committee of Hospital of
Stomatology, Wuhan University. The detailed information of the
patients is listed in Table V. As
previously described, but with modification (16), the OKC tissues were washed and cut
into 5×5-mm2 cubes and plated into a T25 flask, which
was incubated at 37°C for 2 h. Next, 3 ml Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.)
containing 1% antibiotics (100 U/ml penicillin and 100 µg/ml
streptomycin; Sigma-Aldrich; Merck KGaA) and 10% FBS was gently
added to the flask, and the medium was replaced every 3 days. The
fibroblasts were observed at 5–7 days post-culture. Fibroblasts
were isolated and routinely cultured in DMEM, containing 1%
antibiotics and 10% FBS. Fibroblasts from passages 4–6 were used
for subsequent experiments.
 | Table V.Summary of clinical features of
patients with odontogenic keratocysts for fibroblasts
isolation. |
Table V.
Summary of clinical features of
patients with odontogenic keratocysts for fibroblasts
isolation.
| Patient no. | Sex | Age, years | Location | Diameter, cm |
|---|
| 1 | F | 35 | Left mandible | 2 |
| 2 | M | 26 | Right maxilla | 3 |
| 3 | F | 39 | Mandible | 4.5 |
Immunofluorescence
Fibroblasts were seeded onto glass slides (Nest,
Beijing, China) and grown to 80% confluence in 12-well plates.
Following 3 washes with PBS, the cells were fixed in 4%
paraformaldehyde in PBS for 20 min at 37°C, washed 3 times with PBS
for 5 min and permeabilized with 0.2% Triton X-100 for 10 min at
room temperature. The cells were blocked with 0.5% bovine serum
albumin for 1 h at 37°C, prior to being incubated with monoclonal
mouse antibodies against α-smooth muscle actin (α-SMA; 1:100; cat.
no. ZM-0003; OriGene Technologies, Inc., Beijing, China) and
fibroblast activation protein (FAP; 1:100; cat. no. sc-65398; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) overnight at 4°C. The
slides were washed again twice with PBS, prior to being incubated
with fluorescence-labeled secondary antibodies (FITC-labeled goat
anti-mouse IgG; 1:200; cat. no. A0568; Beyotime Institute of
Biotechnology) in a dark chamber for 45 min at 37°C. Cell nuclei
were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) at
room temperature for 15 min. The glass coverslips were examined
under a fluorescence microscope with appropriate filters.
Fibroblast treatments
OKC fibroblasts were plated and cultured in a T25
flask at a cell density of 2×105/ml. When the OKC
fibroblasts reached 50% confluence, they were co-cultured with
staurosporine-treated Jurkat cell-derived MPs (5 or 10 µg/ml) or
with 100 ng/ml anti-human IL-15Rα antibody (goat; cat. no. AF247;
R&D Systems, Inc., Minneapolis, MN, USA) for 72 h at 37°C. For
co-culture with Jurkat cells, OKC fibroblasts were plated and
cultured in a T25 flask at a cell density of 2×105/ml,
together with the Jurkat cell suspensions at a cell density of
2×105/ml or 6×105/ml, for a period of 72 h at
37°C.
Cellular uptake assay
To investigate the biological functions of LMPs,
cellular uptake assays were performed as described in our previous
study (17). The OKC primary
fibroblasts were labeled with CellMask (1:2,000; Thermo Fisher
Scientific, Inc.) at 37°C for 30 min in the dark and cultured on
cover slips in 12-well plates. LMPs (5 µg) from Jurkat cells were
labeled with CFSE at 37°C for 30 min, prior to being added to the
CellMask-labeled OKC fibroblasts, which were co-cultured for a
period of 2 h at 37°C. Subsequently, OKC fibroblasts were fixed in
4% paraformaldehyde for 10 min at room temperature, stained with
DAPI for 15 min at 37°C and observed under a confocal microscope
(Revolution XD; Andor, Belfast, UK) equipped with an Olympus IX 81
microscope (Olympus Corporation, Tokyo, Japan) and an EMCCD (iXon
DU897U single photon detector; Andor).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Isolation of total RNA, synthesis of cDNA and
RT-qPCR were performed as previously described (18). Briefly, total RNA was extracted from
OKC fibroblasts using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). Next, 2 µg RNA was reverse transcribed to 20 µl
cDNA with random primer with RevertAid™ First Strand cDNA Synthesis
kit (cat. no. K1622; Thermo Fisher Scientific, Inc.). Subsequently,
one-fifth of the cDNA was used for PCR using FastStart Universal
SYBR-Green Master mix (Roche Diagnostics, Basel, Switzerland) in a
7900HT Real-Time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). GAPDH was selected as an internal control. The
primer nucleotide sequences for PCR were designed as follows: RANKL
forward, 5′-TGATTCATGTAGGAGAATTAAACAGG-3′ and reverse,
5′-GATGTGCTGTGATCCAACGA-3′; MMP-9 forward,
5′-TGTACCGCTATGGTTACACTCG-3′ and reverse,
5′-GGCAGGGACAGTTGCTTCT-3′; and GAPDH forward,
5′-CGTCATGGGTGTGAACCATTGAGAAG-3′ and reverse,
5′-GCATGGACTGTGGTCATGAGTCCTT-3′. The thermal cycling conditions
comprised 94°C for 30 sec, 60°C for 1 min, 72°C for 1 min and a
final elongation at 72°C for 10 min, amplifying for 36 cycles. The
2−ΔΔCq method was used for the analysis of the data
(19).
Enzyme-linked immunosorbent assay
(ELISA)
Human RANKL levels were determined in the
supernatant of fibroblasts using a commercial ELISA kit (cat. no.
CSB-E05125 h; Cusabio Technology LLC, Wuhan, China) following the
manufacturer's protocols. Specifically, 5×105 OKC
fibroblasts were placed in 6-well plates with serum-free DMEM. For
the experiments, fibroblasts were treated with LMPs (5 or 10 µg/ml)
for 24 or 48 h. Next, 200 µl of the supernatant of OKC fibroblasts
were collected for ELISA assay.
Osteoclast formation assay
Raw264.7 cells obtained from the China Center for
Type Culture Collection (Wuhan, China), were seeded onto slides at
a density of 1,000 cells/well in 12-well plates and cultured with
DMEM (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10%
FBS containing 1% antibiotics (100 U/ml penicillin and 100 µg/ml
streptomycin; Sigma-Aldrich; Merck KGaA) at 37°C with a supply of
5% CO2 for 3 days. Next, the Raw264.7 cells were
cultured with OKC fibroblast supernatant or Jurkat MP-treated
fibroblasts supernatant (10 µg/ml; 48 h) with or without anti-human
sRANKL (1,000 ng/ml; R&D Systems, Inc.). The medium was
replaced every 2 days for 8 days. Cells were stained for
tartrate-resistant acid phosphatase (TRAP; kit cat. no. 387A-1;
Sigma-Aldrich; Merck KGaA), according to the manufacturer's
protocol. TRAP-positive cells containing no less than three nuclei
were counted as osteoclast-like cells. Raw264.7 cells routinely
cultured in DMEM were used as a negative control.
Statistical analysis
Data were analyzed using GraphPad 6.0 software
(GraphPad Software, Inc., La Jolla, CA, USA). Concentrations and
proportions of LMPs, gene expression in fibroblasts, sRANKL
concentrations and TRAP+ multinucleated cells (MNCs)
were compared using one-way analysis of variance, followed by
Tukey's post hoc test. Data are presented as the mean ± standard
deviation. P<0.05 was considered to indicate a statistically
significant difference.
Results
Clinical characteristics of patients
with cysts
A total of 12 patients with inflamed DCs (7 male and
5 female), 15 patients with RCs (10 male and 5 female), 3 patients
with uninflamed OKCs (all female) and 20 patients with inflamed
OKCs (12 male and 8 female) were enrolled in the present study
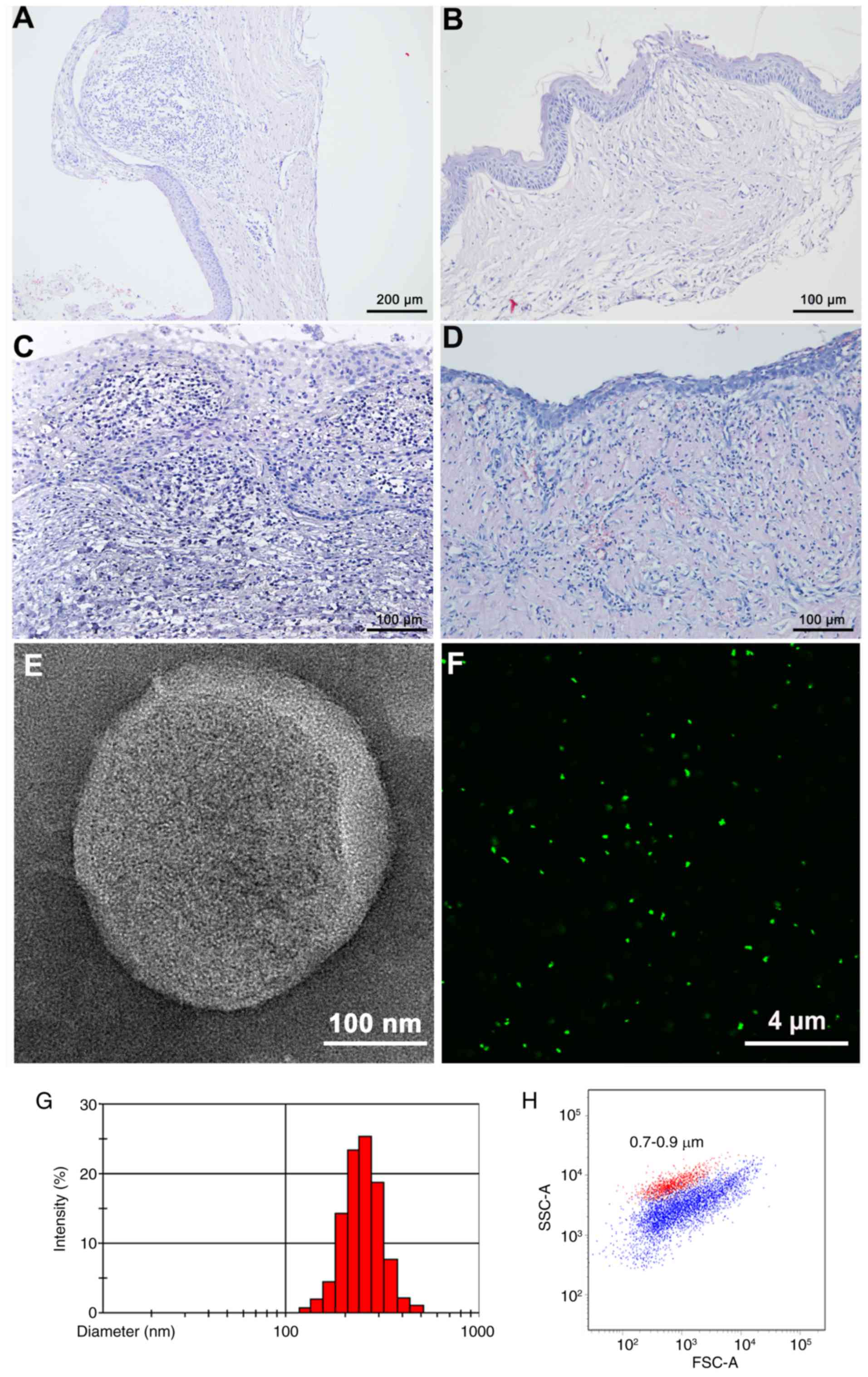
(Tables I–IV). H&E staining revealed
inflammatory cell distributions among the inflamed DCs, RCs, and
uninflamed and inflamed OKCs (Fig.
1A-D). The mean ages of the patients with inflamed DCs, RCs,
and uninflamed and inflamed OKCs were 36±12, 48±14, 33±9 and 33±15
years, respectively. No significant differences in age or sex
distribution were identified in the present study.
CFMP identification and analysis
CFMPs from patients with odontogenic cysts were
purified by differential centrifugation. To directly visualize the
CFMPs, TEM and CFSE fluorescence labeling were performed. CFMPs
were membrane-bound vesicles with a round or ellipse structure by
TEM (Fig. 1E). CFMPs could also be
successfully stained by CFSE, indicating their envelope structure
(Fig. 1F). Dynamic light scattering
demonstrated that the purified CFMPs had a size distribution
ranging between 100 and 1,000 nm (Fig.
1G). Using the Nile Red fluorescent particles with known
diameters (0.7–0.9 µm), the diameters of the CFMPs were determined,
ranging between 100 and 1,000 nm (Fig.
1H).
Increased percentages of
neutrophil-derived MPs in patients with inflamed OKC
For comparison of the subtypes of LMPs, three types
of leukocyte cell (neutrophils, monocytes and lymphocytes) were
analyzed by flow cytometry using anti-CD15, -CD14 and -CD4 staining
combined with Annexin V staining (Fig.
2A-F). The results demonstrated that the percentages of
CD4+lymphocyte-derived CFMPs were significantly higher
in patients with inflamed OKCs compared with those in patients with
inflamed DCs (Fig. 2G). The
percentages of monocyte-derived CFMPs showed no significant
difference in patients with inflamed DCs, RCs, and inflamed or
uninflamed OKCs (Fig. 2H). There
was also an increase in the percentage of neutrophil-derived CFMPs
in inflamed OKCs compared with that in RCs (Fig. 2I). Although the majority of the LMP
concentrations of inflamed OKCs were higher, only the concentration
of neutrophil-derived CFMPs was significantly higher than that of
the inflamed DCs (Fig. 2J-L).
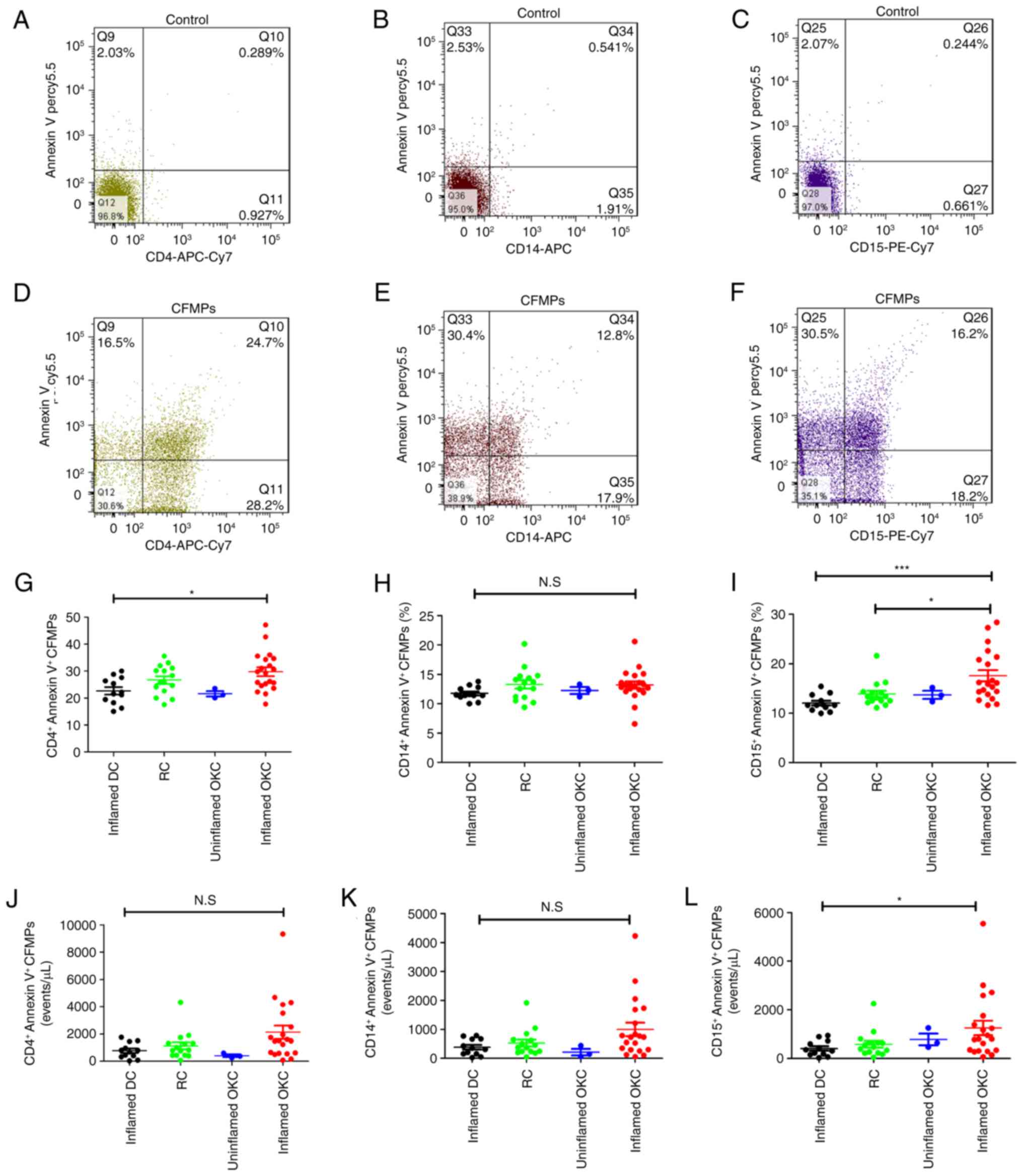 | Figure 2.Quantification of the concentration
and proportion of leukocyte-derived MPs within odontogenic lesions.
Representative flow cytometric images for (A) unstained
CD4+ lymphocyte-derived CFMPs, (B) unstained
monocyte-derived CFMPs and (C) unstained neutrophil-derived CFMPs.
Representative flow cytometric images for (D) stained
CD4+ lymphocyte-derived CFMPs, (E) stained monocyte
-derived CFMPs and (F) stained neutrophil-derived CFMPs.
Quantitative analysis for the percentages of (G)
CD4+/Annexin V+ CFMPs, (H)
CD14+/Annexin V+ CFMPs and (I)
CD15+/Annexin V+ CFMPs in uninflamed DCs,
RCs, and uninflamed and inflamed OKCs. Quantitative analysis for
the concentrations of (J) CD4+/Annexin V+
CFMPs, (K) CD14+/Annexin V+ CFMPs and (L)
CD15+/Annexin V+ CFMPs in inflamed DCs, RCs,
and uninflamed and inflamed OKCs. Data are presented as the mean ±
standard deviation. *P<0.05 and ***P<0.001. N.S, not
significant; CFMPs, cyst fluid microparticles; OKCs, odontogenic
keratocysts; DCs, dentigerous cysts; RCs, radicular cysts; CD,
cluster of differentiation, PE, phycoerythrin; APC,
allophycocyanin; Cy7, cyanine 7. |
Increased release of MPs from Jurkat
cells following staurosporine treatment
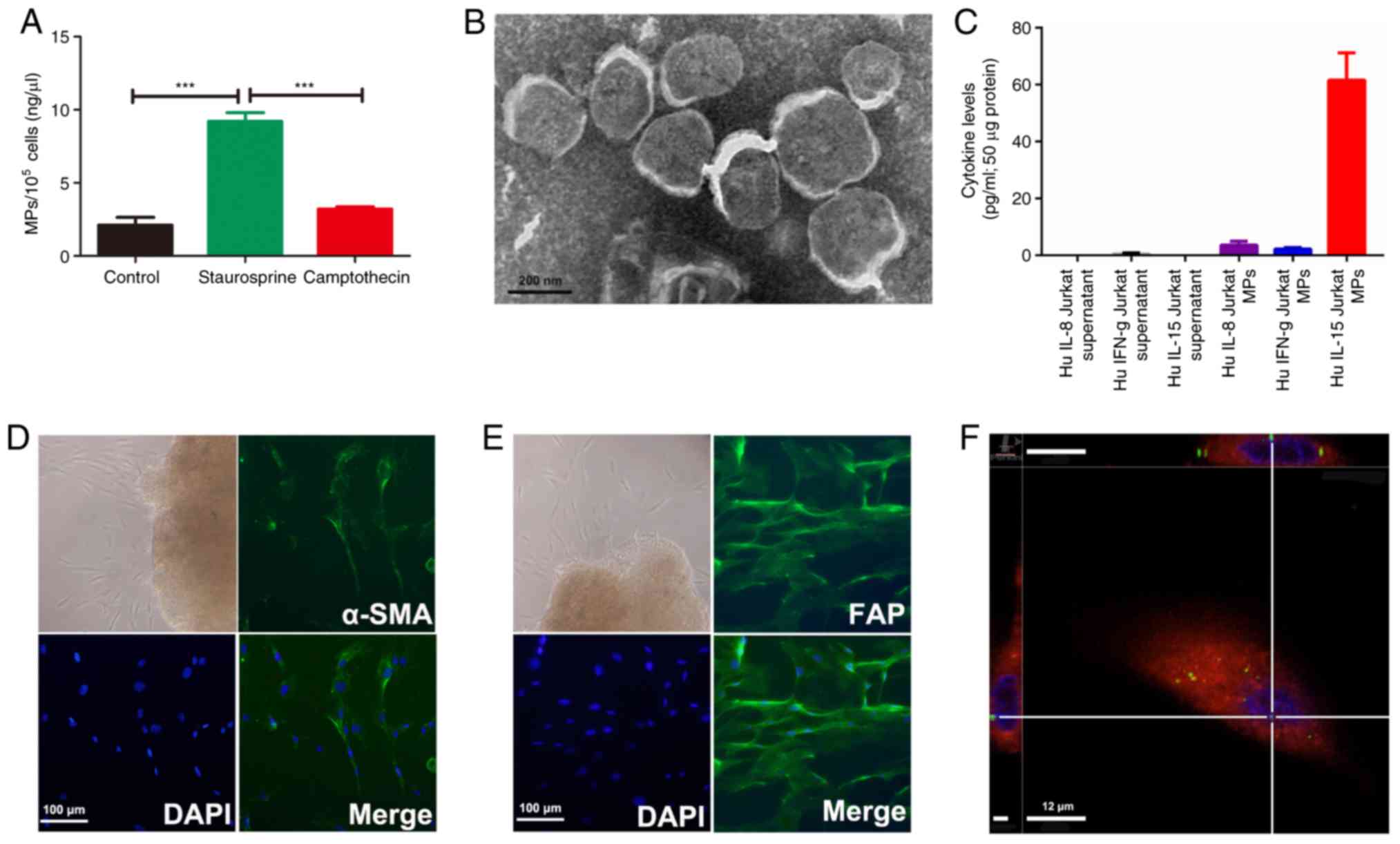
The release of MPs was first examined in Jurkat
cells at the basal state and following treatments to induce
apoptosis. A BCA assay demonstrated that 1×105 Jurkat
cells released 2 ng/µl MPs constitutively. The concentration of MPs
released from Jurkat cells was significantly increased up to 9
ng/µl following stimulation with staurosporine (Fig. 3A). Furthermore, TEM revealed a high
density of bound membrane vesicles derived from
staurosporine-treated Jurkat cells (Fig. 3B). However, there was no significant
change in MPs concentration in the camptothecin-treated Jurkat
cells compared with the basal state of the Jurkat cells.
Significant increases ininflammatory
cytokines in Jurkat cell-derived MPs compared with that in Jurkat
cell supernatants
To determine the difference between the Jurkat cell
derived-MPs and the Jurkat cell supernatant, an equal weight of the
Jurkat cell MPs and supernatants were determined by cytokine
antibody arrays. The results demonstrated that certain cytokines,
including IL-15, IL-8, interferon-γ and macrophage inflammatory
protein-1β were detected in Jurkat cell-derived MPs. Among them,
the highest concentration was in IL-15 (Fig. 3C).
Jurkat cell-derived MPs carrying IL-15
significantly promote MMP-9 and RANKL expression in OKC
fibroblasts, and promote osteoclastogenesis
After 5–7 days of culturing from the cyst wall of
OKCs, explants grew spindle-shaped fibroblast-like cells that were
positive for α-SMA and FAP staining, thereby confirming their
mesenchymal origin (Fig. 3D and E).
To determine the biological effects of Jurkat cell-derived MPs on
primary OKC fibroblasts, a cellular uptake assay was performed. The
results demonstrated that Jurkat cell-derived MPs (green dots) were
detected in the cytoplasm of OKC fibroblasts within 2 h (Fig. 3F). The bone resorption-related mRNAs
were investigated, and the results indicated that the mRNA
expression levels of MMP-9 and RANKL were increased in Jurkat
cell-derived MPs-treated (5 or 10 µg/ml) and Jurkat cell
co-cultured OKC fibroblasts compared with that in the control group
(Fig. 4A and B). Additionally, the
mRNA levels of MMP-9 and RANKL were decreased by the use of
anti-human IL-15Rα antibody in the Jurkat cell-derived MP-treated
group. Therefore, the Jurkat cell-derived MPs containing IL-15
could lead to higher levels of RANKL and MMP-9 mRNAs in OKC
fibroblasts. Moreover, the Jurkat cell-derived MPs could
significantly upregulate the RANKL levels within the supernatant of
OKC fibroblasts. Specifically, the 10 µg/ml Jurkat MPs-treated OKC
fibroblasts (48 h) showed approximately two-fold higher sRANKL
within the supernatant of OKC fibroblasts than the that in the
control group (Fig. 4C). In
addition, Raw264.7 cells were co-cultured with normal OKC
fibroblast supernatant and Jurkat MPs-treated fibroblast
supernatant (10 µg/ml, 48 h). TRAP+ MNCs were detected
in all co-culture groups. Specifically, the supernatant of the
Jurkat cell MP-treated fibroblast group had more abundant
TRAP+ MNCs (14±5 cells) than the OKC fibroblast
supernatant group (7±1 cells) for each well (Fig. 4D). The addition of anti-sRANKL to
Jurkat cell MPs-treated fibroblast supernatant decreased the trend
of TRAP+MNCs (3±3) (Fig.
4D).
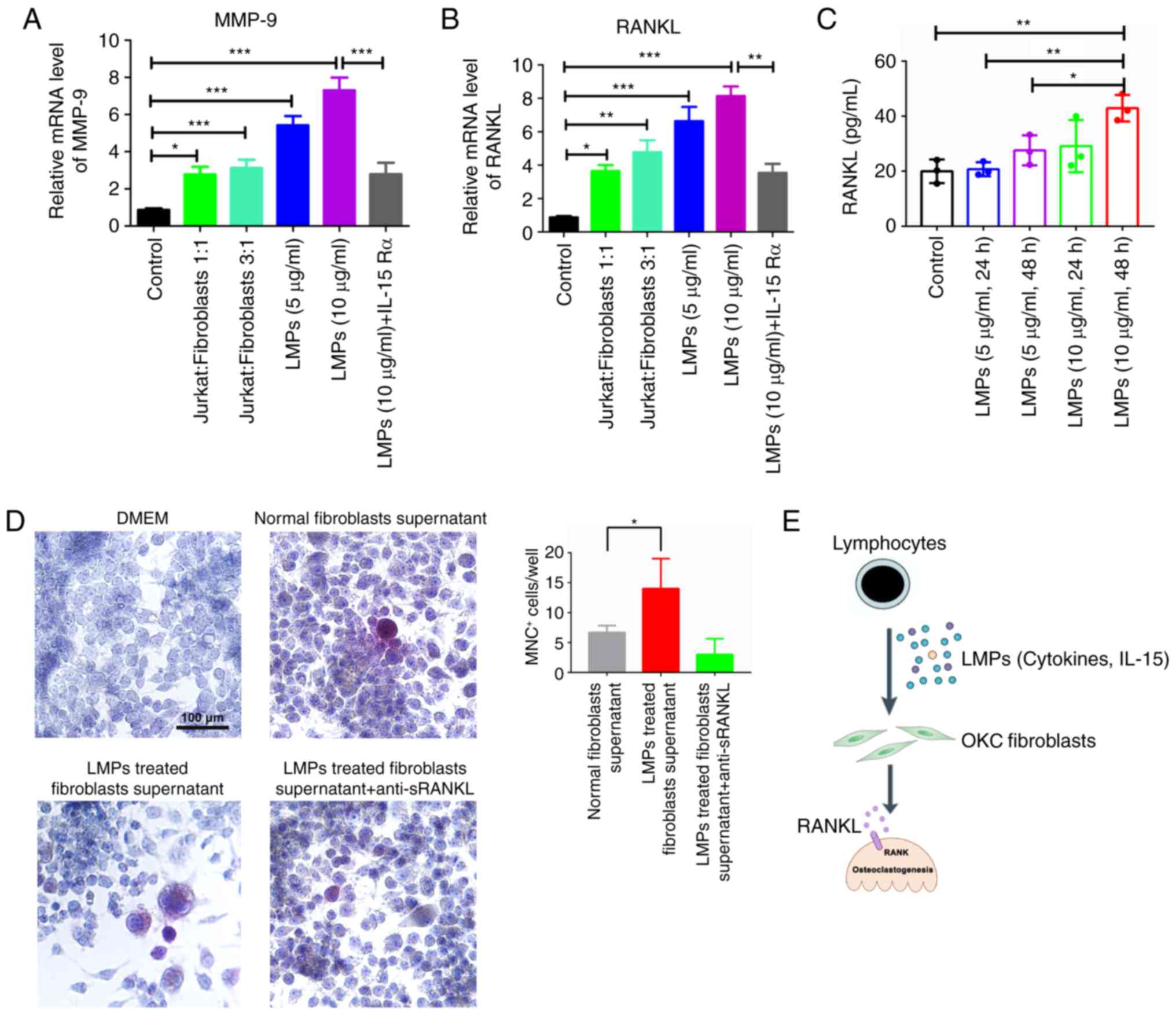 | Figure 4.Apoptotic Jurkat MPs could boost
RANKL expression of OKC fibroblasts and stimulate
osteoclastogenesis. The OKC fibroblasts were treated with Jurkat
cell-derived MPs and Jurkat cell suspension for 72 h, and the (A)
MMP-9 and (B) RANKL mRNAs were analyzed by reverse
transcription-quantitative polymerase chain reaction. The results
are presented as the relative ratio to the control group. Data are
presented as the mean ± standard deviation. *P<0.05, **P<0.01
and ***P<0.001 vs. control group. (C) The level of sRANKL was
determined in the supernatant from the Jurkat MP-treated
fibroblasts by ELISA assay. (D) The MNC+ cells were
determined by TRAP staining in fibroblasts supernatant and Raw264.7
cell co-culture systems. (E) Lymphocyte-derived MPs as
ostoclastogenesis players in the pathogenesis of odontogenic
keratocysts. MPs are released from infiltrating leucocytes upon
activation or apoptosis, and accumulate in the cyst fluid of
inflamed OKCs. LMPs carry IL-15 and other cytokines activate
fibroblasts of OKC, and potently stimulate MMP-9 and RANKL
expression. Aberrant activation of fibroblasts by LMPs may
therefore directly contribute toward bone destruction in OKC.
sRANKL, soluble receptor activator of nuclear factor-κB ligand;
MMP-9, matrix metallopeptidase 9; LMP, leukocyte-derived
microparticle; DMEM, Dulbecco's modified Eagle's medium; MNC,
multinucleated cell; TRAP, tartrate-resistant acid phosphatase;
OKC, odontogenic keratocyst. |
Discussion
MPs are released by cell membrane budding, which
could occur either spontaneously or in response to various stimuli
(9). Various body fluids, including
saliva, blood, pleural fluid, urine, synovial fluid and ascites
contain MPs (20). In the present
study, the distribution of MPs shed from neutrophils, monocytes and
lymphocytes in human cyst fluids was analyzed. The study
demonstrated an accumulation of LMPs in patients with inflamed OKC.
In addition, Jurkat cell-derived MPs carrying IL-15 and other
cytokines could stimulate the expression of RANKL and MMP-9 in OKC
fibroblasts, which accelerates osteoclastogenesis (Fig. 4E).
LMPs are generated from the neutrophils,
monocytes/macrophages and lymphocytes, which are associated with
the development of various diseases. It has previously been
confirmed that LMPs could stimulate the expression of
proinflammatory genes in endothelial cells, leading to the
production of cytokines in vitro (21). In inflammatory diseases, including
Ebola fever and sepsis, the number of tissue factor-positive
monocyte-derived MPs (CD14+) is increased and this is
associated with disease progression (22,23).
Additionally, there have been a few studies on the effect of LMPs
on fibroblasts. In rheumatoid arthritis, MPs from the Jurkat cells
and U937 monocytes could significantly induce MMP-9 mRNA expression
and the expression of cytokines, including IL-6, IL-8 and MCP,
which are released by synovial fibroblasts (24). Also, the presence of sonic hedgehog
(Shh) in MPs generated from activated/apoptotic human T lymphocytes
(CEM T-cell line) had been reported (25,26).
However, the present study employed the Jurkat cell line, and
without phytohemagglutinin and phorbol-12-myristate-13-acetate
activation treatment, the results showed no expressions of Shh in
Jurkat cell-derived MPs by western blot assay.
During the release of MPs, the asymmetric
distribution of phospholipids of the plasma membrane is lost,
leading to PS exposure (27).
Typically, MPs expose the anionic phospholipid PS on their membrane
surface, enabling their detection by Annexin V staining (28). The cyst fluid of OKCs usually
presents as a semi-solid form, and is characterized by a large
number of keratins (29). Due to
the similar size distributions, it is difficult to distinguish MPs
from protein fragments within the body fluids, which otherwise
results in a significant amount of background noise for the
quantification of MPs (30).
Therefore, the present study defined CFMPs using positive staining
for Annexin V in order to distinguish MPs from cell debris or
precipitates, as previously described (30). In addition, as MPs carry surface
membrane antigens from their donor cells, the present study tested
the various cellular origins of MPs using Annexin V and specific
markers, including CD15 (neutrophil marker), CD14 (monocyte marker)
and CD4 (lymphocyte marker).
It has been demonstrated that LMPs serve a role in
the pathogenesis of bone-related diseases (24). Previous studies have confirmed that
activated T cells could activate osteoclast precursors to
facilitate bone resorption (31,32).
Therefore, it is likely that the enhanced activation of leucocytes
observed in patients with odontogenic cysts results in an increased
release of MPs from these cells. In agreement with this hypothesis,
the present study detected higher proportions of LMPs in inflamed
OKCs. However, it is unclear why the number of LMPs in RCs was not
as high as that in the focal inflamed OKCs, since diffused
inflammatory cell infiltration has always been detected in RCs.
Theoretically, more inflammatory cells are present in the tissue of
RCs, which are located more diffusely than focal inflamed OKCs. It
may be that the different microenvironments in the RCs and OKCs
influence the release of LMPs. Additionally, inflamed OKCs
exhibited a higher percentage and concentration of LMPs than
uninflamed OKCs. However, due to the small group of patients with
uninflamed OKC, this difference was not statistically
significant.
To mimic the leukocyte-derived MPs, the present
study collected the MPs in untreated and staurosporine- and
camptothecin-treated Jurkat cells, an immortalized human T
lymphocyte cell line. In line with the results of a previous study
(33), the staurosporine-treated
Jurkat cells exhibited a significantly higher concentration of MPs
than the normal and camptothecin-treated groups. However, the
contents of the Jurkat MPs, particularly the cytokine profiles of
Jurkat cell-derived MPs, remain unknown. By using cytokine antibody
arrays, the present study demonstrated that a panel of inflammatory
cytokines was significantly upregulated compared with the Jurkat
cell supernatant. Among them, IL-15, with the highest
concentration, was detected in the Jurkat MPs. IL-15 has
chemoattractant and pro-inflammatory properties, and may promote
bone destruction (34). In line
with this view, IL-15 was previously shown to stimulate the
formation of mature osteoclasts in rat bone marrow cultures, and
blocking IL-15 relieved the destruction of cartilage and bone
(35,36). IL-15 could also induce the RANKL
expression of synovial fibroblasts, which ultimately facilitates
the process of osteoclastogenesis (37). In the present study, the Jurkat
cell-derived MPs-treated fibroblasts exhibited elevated expression
of MMP-9 and RANKL mRNAs compared with the Jurkat cell co-cultured
OKC fibroblasts, indicating that Jurkat-derived MPs are important
message carriers in the crosstalk between OKC fibroblasts and T
lymphocyte cells. Following the IL-15 blocking assay, the mRNA
levels of MMP-9 and RANKL were significantly decreased, indicating
that the Jurkat cell-derived MPs could carry IL-15 and induce the
expression of bone resorption-related mRNAs. Also, significant
elevation of sRANKL was detected in the Jurkat MPs-treated OKC
fibroblasts. In addition, the co-culture experiment revealed that
Jurkat MPs-treated fibroblasts had greater potential to promote
osteoclastogenesis via RANKL. Therefore, MPs serve as novel
communication tools that contribute toward the pathogenesis of OKCs
by inducing RANKL and MMP-9 expression of OKC fibroblasts, which
promote bone resorption. The present study reveals a novel
mechanism between the inflammation and bone resorption of OKCs.
Acknowledgements
The authors would like to thank technician Juan Min
from Wuhan Institute of Virology (Wuhan, China) for supporting the
flow cytometric analysis.
Funding
This research was supported by the National Natural
Science Foundation of China (grant no. 81570994).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
QWM and LZZ were responsible for the conception and
design of the study, manuscript preparation and submission. YZ,
YYZ, JYL, YFZ and BL were responsible for the data interpretation.
YFZ and BL revised the manuscript and all authors agreed to submit
for peer review.
Ethics approval and consent to
participate
The use of human samples was approved by the Review
Board of the Medical Ethics Committee of the Hospital of
Stomatology, Wuhan University (Wuhan, China). All patients agreed
to participate in the study and signed informed consent forms.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shear M: The aggressive nature of the
odontogenic keratocyst: Is it a benign cystic neoplasm? Part 1.
Clinical and early experimental evidence of aggressive behaviour.
Oral Oncol. 38:219–226. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mendes R, Carvalho J and van der Waal I:
Characterization and management of the keratocystic odontogenic
tumor in relation to its histopathological and biological features.
Oral Oncol. 46:219–225. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Oliveira MG, Ida Lauxen S, Chaves AC,
Rados PV and Filho Sant'Ana M: Odontogenic epithelium:
immunolabeling of Ki-67, EGFR and survivin in pericoronal
follicles, dentigerous cysts and keratocystic odontogenic tumors.
Head Neck Pathol. 5:1–7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kramer IR, Pindborg JJ and Shear M: The
WHO histological typing of odontogenic tumours. A commentary on the
second edition. Cancer. 70:2988–2994. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Scalas D, Roana J, Boffano P, Mandras N,
Gallesio C, Amasio M, Banche G, Allizond V and Cuffini AM:
Bacteriological findings in radicular cyst and keratocystic
odontogenic tumour fluids from asymptomatic patients. Arch Oral
Biol. 58:1578–1583. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Avelar RL, Antunes AA, Carvalho RW,
Bezerra PG, Neto Oliveira PJ and Andrade ES: Odontogenic cysts: A
clinicopathological study of 507 cases. J Oral Sci. 51:581–586.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jurisic V, Terzic T, Colic S and Jurisic
M: The concentration of TNF-alpha correlate with number of
inflammatory cells and degree of vascularization in radicular
cysts. Oral Dis. 14:600–605. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Martins CA, Rivero ER, Dufloth RM,
Figueiredo CP and Vieira DS: Immunohistochemical detection of
factors related to cellular proliferation and apoptosis in
radicular and dentigerous cysts. J Endod. 37:36–39. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Colombo M, Raposo G and Théry C:
Biogenesis, secretion, and intercellular interactions of exosomes
and other extracellular vesicles. Annu Rev Cell Dev Biol.
30:255–289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Angelillo-Scherrer A: Leukocyte-derived
microparticles in vascular homeostasis. Circ Res. 110:356–369.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ardoin SP, Shanahan JC and Pisetsky DS:
The role of microparticles in inflammation and thrombosis. Scand J
Immunol. 66:159–165. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iatrou IA, Legakis N, Ioannidou E and
Patrikiou A: Anaerobic bacteria in jaw cysts. Br J Oral Maxillofac
Surg. 26:62–69. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wright JM and Vered M: Update from the 4th
edition of the world health organization classification of head and
neck tumours: Odontogenic and maxillofacial bone tumors. Head Neck
Pathol. 11:68–77. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ren JG, Man QW, Zhang W, Li C, Xiong XP,
Zhu JY, Wang WM, Sun ZJ, Jia J, Zhang WF, et al: Elevated level of
circulating platelet-derived microparticles in oral cancer. J Dent
Res. 95:87–93. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen G, Zhu JY, Zhang ZL, Zhang W, Ren JG,
Wu M, Hong ZY, Lv C, Pang DW and Zhao YF: Transformation of
cell-derived microparticles into quantum-dot-labeled nanovectors
for antitumor siRNA delivery. Angew Chem Int Ed Engl. 54:1036–1040.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang HC and Li TJ: The growth and
osteoclastogenic effects of fibroblasts isolated from keratocystic
odontogenic tumor. Oral Dis. 19:162–168. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang W, Yu ZL, Wu M, Ren JG, Xia HF, Sa
GL, Zhu JY, Pang DW, Zhao YF and Chen G: Magnetic and folate
functionalization enables rapid isolation and enhanced
tumor-targeting of cell-derived microvesicles. ACS Nano.
11:277–290. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu JY, Ren JG, Zhang W, Wang FQ, Cai Y,
Zhao JH, Chen G and Zhao YF: Characterization of microparticles in
patients with venous malformations of the head and neck. Oral Dis.
23:110–119. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expressiondata using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gong J, Jaiswal R, Dalla P, Luk F and
Bebawy M: Microparticles in cancer: A review of recent developments
and the potential for clinical application. Semin Cell Dev Biol.
40:35–40. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mesri M and Altieri DC: Leukocyte
microparticles stimulate endothelial cell cytokine release and
tissue factor induction in a JNK1 signaling pathway. J Biol Chem.
274:23111–23118. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Geisbert TW, Young HA, Jahrling PB, Davis
KJ, Kagan E and Hensley LE: Mechanisms underlying coagulation
abnormalities in ebola hemorrhagic fever: Overexpression of tissue
factor in primate monocytes/macrophages is a key event. J Infect
Dis. 188:1618–1629. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nieuwland R, Berckmans RJ, McGregor S,
Böing AN, Romijn FP, Westendorp RG, Hack CE and Sturk A: Cellular
origin and procoagulant properties of microparticles in
meningococcal sepsis. Blood. 95:930–935. 2000.PubMed/NCBI
|
|
24
|
Distler JH, Jüngel A, Huber LC, Seemayer
CA, Reich CF III, Gay RE, Michel BA, Fontana A, Gay S, Pisetsky DS,
et al: The induction of matrix metalloproteinase and cytokine
expression in synovial fibroblasts stimulated with immune cell
microparticles. Proc Natl Acad Sci USA. 102:2892–2897. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Agouni A, Mostefai HA, Porro C, Carusio N,
Favre J, Richard V, Henrion D, Martinez MC and Andriantsitohaina R:
Sonic hedgehog carried by microparticles corrects endothelial
injury through nitric oxide release. FASEB J. 21:2735–2741. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Martinez MC, Larbret F, Zobairi F,
Coulombe J, Debili N, Vainchenker W, Ruat M and Freyssinet JM:
Transfer of differentiation signal by membrane microvesicles
harboring hedgehog morphogens. Blood. 108:3012–3020. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Piccin A, Murphy WG and Smith OP:
Circulating microparticles: Pathophysiology and clinical
implications. Blood Rev. 21:157–171. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mallat Z, Hugel B, Ohan J, Leseche G,
Freyssinet JM and Tedgui A: Shed membrane microparticles with
procoagulant potential in human atherosclerotic plaques: A role for
apoptosis in plaque thrombogenicity. Circulation. 99:348–353. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Aragaki T, Michi Y, Katsube K, Uzawa N,
Okada N, Akashi T, Amagasa T, Yamaguchi A and Sakamoto K:
Comprehensive keratin profiling reveals different histopathogenesis
of keratocystic odontogenic tumor and orthokeratinized odontogenic
cyst. Hum Pathol. 41:1718–1725. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dey-Hazra E, Hertel B, Kirsch T, Woywodt
A, Lovric S, Haller H, Haubitz M and Erdbruegger U: Detection of
circulating microparticles by flow cytometry: Influence of
centrifugation, filtration of buffer, and freezing. Vasc Health
Risk Manag. 6:1125–1133. 2010.PubMed/NCBI
|
|
31
|
Park JC, Kim BK, Jung IH, Choi E and Kim
CS: Alveolar bone resorption induced by CD4+CD45RB
high-density T-cell transfer in immunocompromised mice. J
Periodontol. 85:e339–e347. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wisitrasameewong W, Kajiya M, Movila A,
Rittling S, Ishii T, Suzuki M, Matsuda S, Mazda Y, Torruella MR,
Azuma MM, et al: DC-STAMP is an osteoclast fusogen engaged in
periodontal bone resorption. J Dent Res. 96:685–693. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Reich CF III and Pisetsky DS: The content
of DNA and RNA in microparticles released by Jurkat and HL-60 cells
undergoing in vitro apoptosis. Exp Cell Res. 315:760–768. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Okabe I, Kikuchi T, Mogi M, Takeda H, Aino
M, Kamiya Y, Fujimura T, Goto H, Okada K, Hasegawa Y, et al: IL-15
and RANKL play a synergistically important role in
osteoclastogenesis. J Cell Biochem. 118:739–747. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ferrari-Lacraz S, Zanelli E, Neuberg M,
Donskoy E, Kim YS, Zheng XX, Hancock WW, Maslinski W, Li XC, Strom
TB, et al: Targeting IL-15 receptor-bearing cells with an
antagonist mutant IL-15/Fc protein prevents disease development and
progression in murine collagen-induced arthritis. J Immunol.
173:5818–5826. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ogata Y, Kukita A, Kukita T, Komine M,
Miyahara A, Miyazaki S and Kohashi O: A novel role of IL-15 in the
development of osteoclasts: Inability to replace its activity with
IL-2. J Immunol. 162:2754–2760. 1999.PubMed/NCBI
|
|
37
|
Park MK, Her YM, Cho ML, Oh HJ, Park EM,
Kwok SK, Ju JH, Park KS, Min DS, Kim HY, et al: IL-15 promotes
osteoclastogenesis via the PLD pathway in rheumatoid arthritis.
Immunol Lett. 139:42–51. 2011. View Article : Google Scholar : PubMed/NCBI
|