Introduction
Liver cancer is one of the most common causes of
cancer-associated mortality worldwide, particularly in China
(1). Hepatocellular carcinoma
accounts for 70–85% of all liver cancer cases (2). There are currently several treatment
options for liver cancer; however, none have been shown to be a
cure-all, with regards to recurrence, patient survival and
longevity (3). Patients with
early-stage liver cancer can be treated with surgery, which may
involve removal of part of the liver or transplantation; the 5-year
survival rate in these cases often exceeds 70%. However, the
majority of cases are not suitable for surgical treatment; the
overall 5-year survival rate in these cases is ~15% (4). Until 2017, there was no systemic
treatment option for patients with advanced liver cancer.
Therefore, it is important to investigate the molecular mechanisms
underlying liver carcinogenesis, and to identify novel approaches
that can effectively inhibit liver cancer cell growth and
metastasis.
Taurolidine (TRD) is a substance derived from the
amino sulfonic acid taurine, which was originally used to treat
peritonitis and catheter-associated infections (5). Previous studies have revealed that TRD
inhibits proliferation of cancer cells and induces the
differentiation of malignant cells, including glioblastoma
(6,7), melanoma (8,9),
mesothelioma (10,11), colon carcinoma (12,13),
squamous cell esophageal carcinoma (14) and sarcoma cell lines (15,16).
However, the effect and mechanism of TRD in liver cancer remain to
be elucidated.
Gene associated with retinoid-interferon-induced
mortality-19 (GRIM-19) is located on human chromosome 19p-13.1 and
encodes a 16-kD protein comprising 144 amino acids (17). GRIM-19 serves an important role in
the mitochondrial respiratory chain (18). Our previous study (19) revealed that GRIM-19 is an important
regulator of tumor cell survival. Notably, downregulation of
GRIM-19 and hyperactivation of phosphorylated (p)-signal transducer
and activator of transcription 3 (STAT3) expression in liver cancer
lesions are closely correlated with increased histological grading
in liver cancer (19,20). Favorable pharmacokinetic and safety
data have been reported for TRD following systemic application in
healthy volunteers (21), as well
as in patients with locally advanced gastric carcinoma and
glioblastoma (22–24). However, the role of TRD in the
induction of cell apoptosis remains to be fully elucidated. The
mitochondria-dependent pathway (7,15,25–27)
and the death receptor-associated pathway have been reported to be
associated with TRD-induced apoptosis (14,16,27,28).
However, it is not clear whether TRD can induce liver cancer cell
apoptosis; therefore, the potential mechanism requires further
exploration.
In the present study, the effects of TRD treatment
on GRIM-19, cyclin D1, STAT3, p-STAT3, B-cell lymphoma 2 (Bcl-2)
and Bcl-2-associated X protein (Bax) expression, and on the
apoptosis of liver cancer cells, were investigated in vitro.
The results indicated that treatment with TRD induced liver cancer
cell apoptosis in a dose-dependent manner, which was associated
with an increase in GRIM-19 expression and deactivating the STAT3
signaling pathway.
Materials and methods
Cell lines and transfection
HepG2 and SNU-423 human liver cancer cell lines were
obtained from the Shanghai Preservation Center Cell Bank (Shanghai,
China). HepG2 cells were cultured in Dulbecco's modified Eagle's
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
whereas SNU-423 cells were cultured in Roswell Park Memorial
Institute 1640 medium (Gibco; Thermo Fisher Scientific, Inc.), both
media were supplemented with 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml
streptomycin (Thermo Fisher Scientific, Inc.) at 37°C in an
atmosphere containing 5% CO2. Small interfering RNA
(siRNA) duplex oligoribonucleotides targeting the coding region of
GRIM-19 were obtained from Thermo Fisher Scientific, Inc. HepG2 and
SNU-423 cells (105 cells/well) were transfected with
control siRNA (5′-UUCUCCGAACGUGUCACGUTT-3′, 50 nM) or specific
GRIM-19 siRNA (5′-GCUUCAUGUGGUACACGUATT-3′, 50 nM) using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), according to the manufacturer's protocol. The
culture medium was replaced after 6 h of incubation. Subsequently,
48 h post-transfection, the cells were collected. GRIM-19 knockdown
cells were pretreated with niclosamide (p-STAT3 inhibitor, 10 µM;
50-65-7; Selleck Chemicals, Houston, TX, USA) for 30 min, after
which, 100 µM TRD (Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
was added for 24 h.
MTT assay
The effects of treatment with TRD on HepG2 and
SNU-423 cell viability were measured using the MTT assay. Cells
(103 cells/well) were cultured in 96-well plates
overnight and were treated with TRD at various concentrations (10,
50, 100 and 20 0 µM) for 0, 12, 24, 48 or 72 h at 37°C in an
atmosphere containing 5% CO2. During the last 4 h of
culture, the cells in each well were exposed to 20 µl MTT (5 mg/ml;
Merck KGaA, Darmstadt, Germany). The generated formazan in each
well was then dissolved in 150 µl dimethyl sulfoxide (DMSO) and the
absorbance was measured at 490 nm using a microplate reader, as
previously described (29).
Flow cytometry
HepG2 cells were treated with various concentrations
of TRD (50, 100 and 200 µM) and SNU-423 cells were treated with 200
µM TRD for 24 or 48 h at 37°C. In addition, GRIM-19 knockdown cells
were treated with 100 µM TRD for 24 h at 37°C. Cells
(3×105 cells/well) were then transferred to a tube in
which Annexin V (5 µl) and propidium iodide (5 µl) (APOAF;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) were added, and the
cells were incubated for 30 min at 37°C. Subsequently, the
percentages of apoptotic cells in the different groups were
determined by flow cytometry, using the BD FACSCelesta™ flow
cytometer (BD Biosciences, San Jose, CA, USA). Data were analysed
with FCS Express 4 (De Novo Software, Los Angeles, CA, USA). In
addition, some cells were stained with trypan blue (ab233465;
Abcam, Hong Kong, China) and surviving cells were counted under a
microscope (Olympus IX51; Leeds Precision Instruments, Inc.,
Minneapolis, MN, USA) in a blinded manner. Cell survival rate was
determined using the following equation: Survival rate (%) = number
of unstained cells/total number of cells observed × 100. Survival
rate of cells before TRD treatment exceeded 95% in all groups.
Immunofluorescence
HepG2 cells (105 cells/slide) were
cultured on glass cover slips in 24-well plates overnight, fixed
with 4% paraformaldehyde (Merck KGaA) in PBS and washed with PBS.
Following permeabilization, the glass cover slips were blocked with
1 mM CaCl2, 1 mM MgCl2, 5% normal goat serum
(SL038; Beijing Solarbio Science & Technology Co., Ltd.,
Beijing, China), 10% FBS and 2% bovine serum albumin (pH 7.6,
A8010; Beijing Solarbio Science & Technology Co., Ltd.) in PBS
and were incubated with mouse anti-human GRIM-19 antibody (1:1,000
dilution, ab110240; Abcam) at 4°C overnight. After washing with PBS
containing 0.1% Triton X-100, the glass cover slips were incubated
with tetramethyl rhodamine isothiocyanate-conjugated secondary
antibodies (1:100 dilution, BA1089; Boster Biological Technology,
Pleasanton, CA, USA) for 30 min at 37°C, followed by
counterstaining with DAPI and mounting with Gel Mount™ Aqueous
Mounting Medium (Merck KGaA). Images were acquired using a Leica™
fluorescence microscope equipped with a Leica™ camera
(LEICA-DFC320; Leica Microsystems, Inc., Buffalo Grove, IL,
USA).
Western blot analysis
Cells (3×105 cells/well) were cultured in
24-well plates overnight, and were harvested and lysed with
radioimmunoprecipitation assay buffer (R0020; Beijing Solarbio
Science & Technology Co., Ltd.). Protein concentrations were
determined using the Bicinchoninic Acid Reagent (Thermo Fisher
Scientific, Inc., after which, the cell lysates (30 µg/lane) were
separated by 8–15% SDS-PAGE and transferred onto nitrocellulose
membranes (GE Healthcare, Chicago, IL, USA). After blocking with 5%
non-fat dry milk (for GRIM-19, STAT3, Bcl-2, Bax and cyclin D1) or
5% bovine serum albumin (for p-STAT3) in 10 mM Tris-Cl (pH 8.0),
150 mM NaCl and 0.05% Tween 20 for 1 h, the membranes were
incubated with various antibodies overnight at 4°C. The bound
antibodies were detected using horseradish peroxidase-conjugated
immunoglobulin G secondary antibodies (1:5,000 dilutions, NA931 or
NA9340; GE Healthcare) for 1 h at room temperature and visualized
using enhanced chemiluminescence (GE Healthcare). The primary
antibodies included mouse polyclonal anti-human GRIM-19 (1:1,000
dilution, ab110240; Abcam), mouse monoclonal anti-human STAT3
(1:5,000 dilution, ab119352; Abcam), rabbit monoclonal anti-human
cyclin D1 (1:10,000 dilution, ab134175; Abcam), rabbit monoclonal
anti-human Bcl-2 (1:1,000 dilution, ab32124; Abcam), rabbit
monoclonal anti-human Bax (1:2,000 dilution, ab32503; Abcam), mouse
monoclonal anti-human p-STAT3 (Tyr705) (1:2,000 dilution, #4113;
Cell Signaling Technology, Inc., Danvers, MA, USA), and
anti-β-actin (1:1,000 dilution, sc-517582; Santa Cruz
Biotechnology, Inc.). The levels of target protein expression
relative to the control protein, β-actin, were analyzed by
densitometric analysis using a detection system (Amersham Imager
600; GE Healthcare).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from individual groups of
cells using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. RNA
was reverse transcribed into cDNA using the RT2 First
Strand kit (330404; Qiagen, Inc., Valencia, CA, USA), according to
the manufacturer's protocol. The expression levels of GRIM-19 mRNA
transcripts relative to the β-actin control were determined by
RT-qPCR using SYBR® Premix Ex Taq™ (Takara Biotechnology
Co., Ltd., Dalian, China) and specific primers on the LC480
Real-time PCR system (Roche Applied Science, Penzberg, Germany).
The thermocycling conditions were as follows: 95°C for 30 sec,
followed by 40 cycles at 95°C for 5 sec, 60°C for 30 sec and 72°C
for 30 sec, with a final extension step at 72°C for 5 min. The
primer sequences were as follows: GRIM-19 (116 bp), forward
5′-CATCGACTACAAACGGAACTTG-3′, reverse 5′-GCTCACGGTTCCACTTCATTA-3′;
and β-actin (196 bp), forward 5′-TGACGTGGACATCCGCAAAG-3′ and
reverse 5′-CTGGAAGGTGGACAGCGAGG-3′. The data were quantified using
the 2−ΔΔCq method (30,31).
Statistical analysis
All statistical analyses were performed using SPSS
10.0 software (SPSS, Inc., Chicago, IL, USA). All data are
expressed as the means ± standard deviation. Multiple comparisons
of means were determined using one- or two-way analysis of
variance, followed by the post hoc Newman-Keuls test. P<0.05 was
considered to indicate a statistically significant difference.
Results
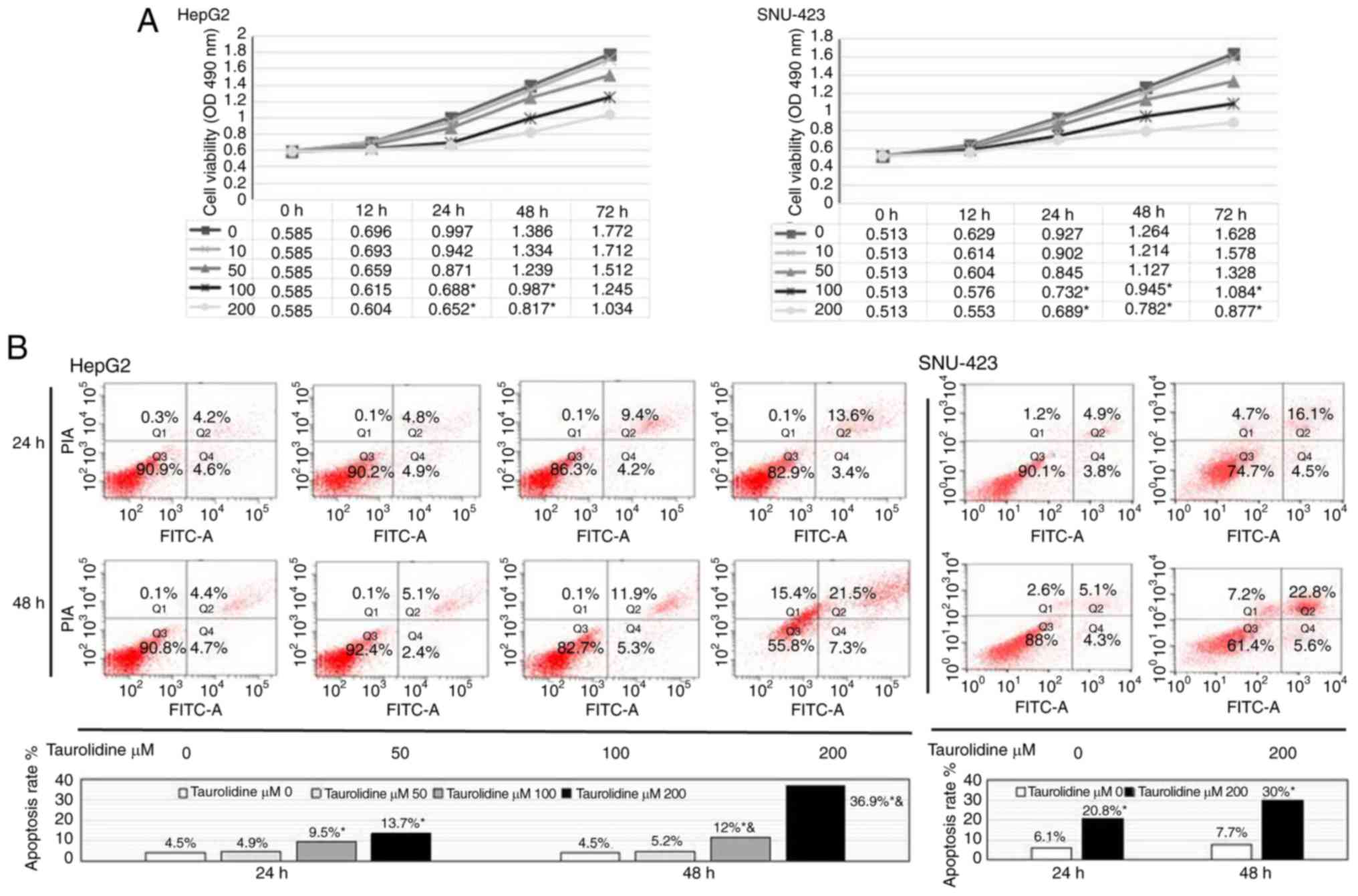
TRD significantly reduces HepG2 and
SNU-423 cell viability and induces apoptosis
To determine the effects of TRD on liver cancer cell
viability, HepG2 cells were treated with various concentrations of
TRD (10, 50, 100 and 200 µM) for 0, 12, 24, 48 or 72 h. Cell
viability was then analyzed using the MTT assay. As shown in
Fig. 1A, treatment with any
concentration of TRD treatment for 12 h, and with 10 and 50 µM TRD
at any time-point, did not significantly inhibit cell viability.
However, TRD (100 and 200 µM) significantly inhibited cell
viability in the 24-h treatment group (0.688±0.062 vs. 0.997±0.055,
P<0.01; 0.652±0.074 vs. 0.997±0.055, P<0.01), the 48-h
treatment group (0.987±0.037 vs. 1.386±0.063, P<0.01;
0.817±0.045 vs. 1.386±0.063, P<0.01) and the 72-h treatment
group (1.245±0.125 vs. 1.772±0.116, P<0.01; 1.034±0.108 vs.
1.772±0.116, P<0.01). These findings suggested that TRD
inhibited HepG2 cell viability in a dose-dependent manner. Notably,
the proliferation of HepG2 cells in the TRD group was significantly
reduced, but not completely stopped; similar results were observed
in the SNU-423 cells line.
Based on these results, HepG2 cells were then
treated with various concentrations of TRD (50, 100 and 200 µM) for
24 or 48 h, in order to observe cell apoptosis by flow cytometry.
The results revealed that TRD (100 and 200 µM) significantly
increased cell apoptosis in the 24- and 48-h groups (P<0.01). In
addition, TRD treatment (100 and 200 µM) for 48 h significantly
accelerated cell apoptosis compared with at 24 h (P<0.05;
P<0.01). TRD treatment (50 µM) for 24 or 48 h had no effect on
cell apoptosis (P=0.35; P=0.28). These findings indicated that TRD
induced apoptosis of HepG2 cells in a dose- and time-dependent
manner (Fig. 1B). Subsequently,
SNU-423 cells were treated with TRD (200 µM) for 24 and 48 h, and
cell apoptosis was detected by flow cytometric analysis. The
results further confirmed that TRD induced apoptosis of liver
cancer cells.
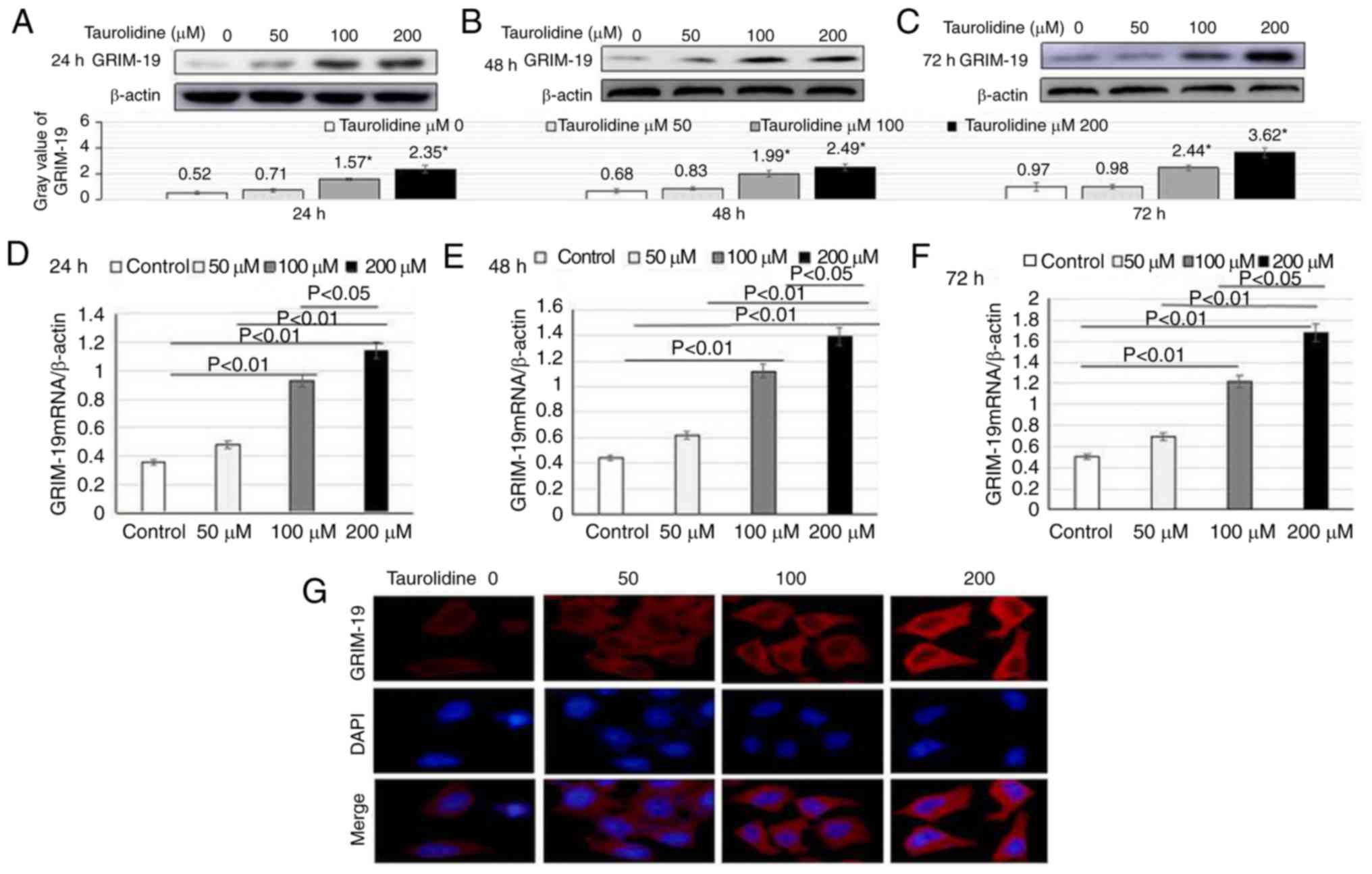
TRD upregulates GRIM-19 expression in
HepG2 cells
GRIM-19 is a regulator of cell death, which is
particularly associated with the stabilization and function of
mitochondrial complex 1 (32). To
understand the importance of GRIM-19 in TRD-induced apoptosis of
liver cancer cells, HepG2 cells were treated with TRD at various
concentrations (50, 100 and 200 µM) for 24, 48 or 72 h. The
expression levels of GRIM-19 were detected by western blotting. The
results revealed that treatment with TRD (100 and 200 µM)
significantly increased GRIM-19 protein expression in HepG2 cells
in the 24-h group (1.57±0.12 vs. 0.52±0.11, P<0.01; 2.35±0.31
vs. 0.52±0.11, P<0.01), in the 48-h group (1.99±0.22 vs.
0.68±0.17, P<0.01; 2.49±0.22 vs. 0.68±0.17, P<0.01) and in
the 72-h group (2.44±0.22 vs. 0.97±0.35, P<0.01; 3.62±0.37 vs.
0.97±0.35, P<0.01). The effects of TRD on the upregulation of
GRIM-19 protein expression appeared to be dose-dependent (Fig. 2A-C).
The mRNA expression levels of GRIM-19 mRNA in the
different groups were detected by RT-qPCR. TRD treatment (100 and
200 µM) significantly enhanced GRIM-19 mRNA expression in HepG2
cells in the 24-h group (0.93±0.07 vs. 0.36±0.05, P<0.01;
1.14±0.11 vs. 0.36±0.05, P<0.01), in the 48-h group (1.12±0.14
vs. 0.44±0.06, P<0.01; 1.39±0.21 vs. 0.44±0.06, P<0.01) and
in the 72-h group (1.22±0.22 vs. 0.5±0.09, P<0.01; 1.68±0.25 vs.
0.5±0.09, P<0.01) (Fig.
2D-F).
Our previous study revealed that GRIM-19 is
predominantly expressed in the cytoplasm of liver cancer tissue
slices, whereas its expression in nuclei is limited (19). To confirm this observation in HepG2
cells, the cellular localization of endogenous GRIM-19 was analyzed
by immunofluorescence. Consistent with the histological staining
results of our previous study, GRIM-19 expression was detected in
the cytoplasm, and its expression was markedly increased in the
presence of TRD (Fig. 2G).
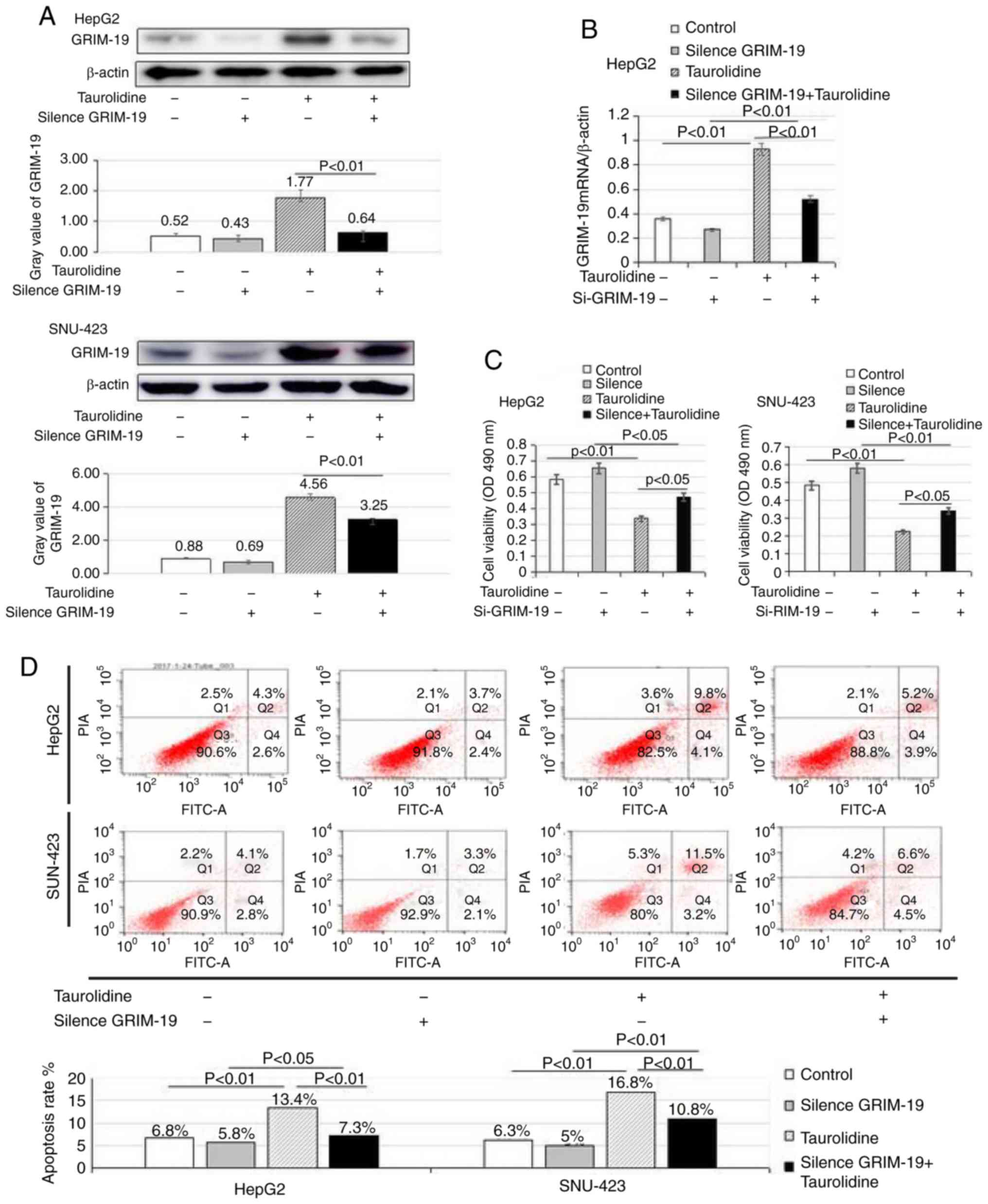
GRIM-19 serves a critical role in
TRD-induced inhibition of viability and promotion of apoptosis in
liver cancer cells
To determine the effects of GRIM-19 on the viability
and apoptosis of liver cancer cells, GRIM-19 expression was knocked
down by RNA interference in HepG2 and SNU-423 cells.
Post-transfection with GRIM-19 siRNA or control siRNA for 24 h, the
cells were treated with 100 µM TRD for 24 h. As shown in Fig. 3A and B, the protein and mRNA
expression levels of GRIM-19 were significantly decreased in the
TRD + GRIM-19 siRNA group compared with in the TRD treatment group
(protein: 0.64±0.06 vs. 1.77±0.25, P<0.01; mRNA: 0.52±0.04 vs.
0.93±0.17, P<0.01). However, there was no significant difference
between the GRIM-19 siRNA and control groups (protein: 0.43±0.11
vs. 0.52±0.09, P=0.23; mRNA: 0.27±0.08 vs. 0.36±0.03, P=0.25). This
may be related to the weak expression of GRIM-19 in the control
group. Furthermore, GRIM-19 protein and mRNA expression were
significantly increased in the TRD treatment group compared with in
the control group.
As shown in Fig. 3C,
TRD, which is an inducer of GRIM-19, significantly inhibited HepG2
cell viability compared with in the control group (0.339±0.09 vs.
0.585±0.13, P<0.01). Conversely, the effects of TRD on cell
viability were reversed following GRIM-19 knockdown (0.339±0.09 vs.
0.472±0.1, P<0.05); however, cell viability was not completely
recovered. Similar findings were observed in SNU-423 cells. There
may be other molecular mechanisms participating in the regulation
of TRD in liver cancer cell viability. Taken together, these
findings suggested that GRIM-19 was involved in the regulation of
cell viability.
As shown in Fig. 3D,
flow cytometry revealed that TRD treatment increased the percentage
of apoptotic cells compared with control siRNA transfection
(P<0.01). Cell apoptosis was significantly inhibited in the
GRIM-19 siRNA + TRD group compared with in the TRD group
(P<0.01) and the percentage of apoptotic cells was increased
compared with the GRIM-19 siRNA group (P<0.05). Cell apoptosis
in the TRD treatment group was the highest observed among the four
groups. In addition, there was no significant difference in cell
apoptosis between the GRIM-19 siRNA and control siRNA groups
(P=0.13).
GRIM-19 regulates HepG2 cell
proliferation and apoptosis via a STAT3-dependent pathway
To clarify the mechanism underlying the effects of
TRD and GRIM-19 on cell viability and apoptosis, the expression of
proliferation- and apoptosis-associated molecules, including STAT3,
p-STAT3, cyclin D1, Bcl-2 and Bax, was detected by western
blotting. As shown in Fig. 4A, the
expression levels of p-STAT3, cyclin D1 and Bcl-2 were increased in
the GRIM-19 siRNA group, whereas those of Bax were decreased. TRD
treatment was revealed to have the opposite effect, inhibiting the
expression of p-STAT3, cyclin D1 and Bcl-2, and increasing that of
Bax, compared with the control group. These results suggested that
GRIM-19 regulated HepG2 cell viability and apoptosis through the
STAT3 pathway.
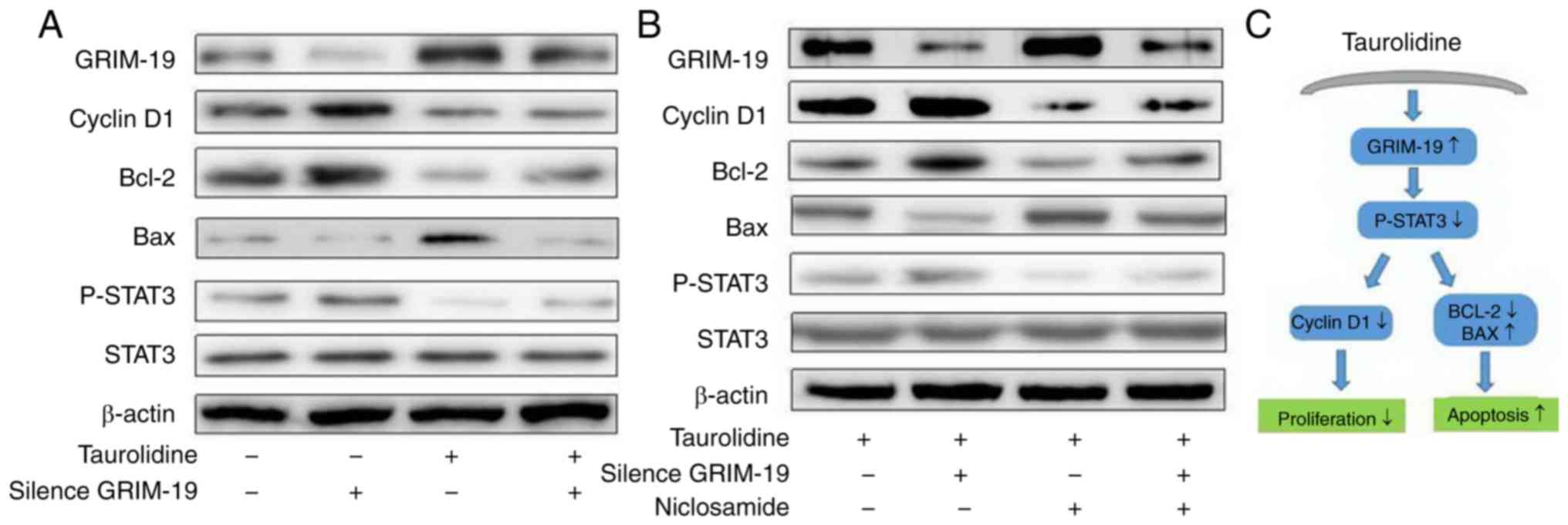 | Figure 4.GRIM-19 regulates HepG2 cell
proliferation and apoptosis via a STAT3-dependent pathway. The
expression levels of proliferation- and apoptosis-associated
molecules, STAT3, p-STAT3, cyclin D1, Bcl-2 and Bax, were detected
by western blotting. (A) Expression of p-STAT3, cyclin D1 and Bcl-2
was increased, and that of Bax was decreased, in the GRIM-19 siRNA
group, whereas TRD treatment had the opposite effect, inhibiting
the expression of p-STAT3, cyclin D1 and Bcl-2, and increasing that
of Bax, compared with the control group. (B) High expression levels
of GRIM-19 and Bax were detected in the TRD and TRD + niclosamide
groups, which were accompanied by low levels of cyclin D1, Bcl-2
and-STAT3. Conversely, low expression levels of GRIM-19 and Bax,
and high expression levels of cyclin D1, Bcl-2 and p-STAT3 were
detected in the TRD + GRIM-19 siRNA group. (C) Schematic diagram.
Data are representative images or expressed as the means ± standard
deviation of each group from three separate experiments. Bax,
Bcl-2-associated X protein; Bcl-2, B-cell lymphoma 2; GRIM-19,
retinoic-interferon-induced mortality 19; p-STAT3,
phosphorylated-STAT3; siRNA, small interfering RNA; STAT3, signal
transducer and activator of transcription 3; TRD, taurolidine. |
To further confirm the role of the STAT3 signaling
pathway in TRD treatment, GRIM-19 knockdown cells were pretreated
with a p-STAT3 inhibitor, niclosamide, for 30 min, and 100 µM TRD
was then added for 24 h. Western blot analysis detected high
GRIM-19 and Bax expression in the TRD and TRD + niclosamide groups,
accompanied by low expression levels of cyclin D1, Bcl-2 and
p-STAT3. Conversely, low expression levels of GRIM-19 and Bax, and
high expression levels of cyclin D1, Bcl-2 and p-STAT3 were
detected in the TRD + GRIM-19 siRNA group. Niclosamide reduced the
expression of p-STAT3, cyclin D1 and Bcl-2, and increased that of
Bax, in the TRD-treated HepG2 cells, irrespective of GRIM-19
knockdown. However, there was no effect on the expression levels of
GRIM-19, irrespective of the addition of niclosamide (Fig. 4B). This phenomenon indicated that
GRIM-19 was an upstream molecule of the STAT3 signaling pathway.
These findings suggested that TRD may enhance GRIM-19 expression
and induce apoptosis of human liver cancer cells via a
STAT3-dependent pathway.
Discussion
TRD is one of the most common types of human cancer,
and a leading cause of cancer-associated mortality worldwide.
Statistics have demonstrated that the majority of patients with
liver cancer are not suitable for surgical resection, thus carrying
a considerably poor prognosis. Particularly in patients with
advanced liver cancer, the benefits of conventional therapy are
limited; therefore, the need for more effective systemic treatments
is urgent. TRD is an antiseptic agent derived from the amino
sulfonic acid taurine, which has been used to treat peritonitis and
catheter-associated infections (5).
Recently, TRD has been used to treat malignant diseases (33–36),
including gastrointestinal carcinoma, glioblastoma, fibrosarcoma,
prostate cancer and melanoma (34,36–39).
However, its precise mechanism of action remains unclear. In the
present study, treatment with TRD significantly inhibited the
viability and triggered the apoptosis of liver cancer cells.
In our previous study (19), it was revealed that GRIM-19
expression is reduced in liver cancer compared with in adjacent
liver tissue. Furthermore, the decreased expression of GRIM-19 in
liver cancer lesions is closely associated with an increased
histological grading in liver cancer. In the present study,
treatment with TRD significantly increased GRIM-19 expression in
the cytoplasm of HepG2 cells. Simultaneously, cell viability was
inhibited, whereas apoptosis was induced in TRD-treated HepG2 and
SNU-423 cells, accompanied by an increase in GRIM-19 expression. In
addition, the viability and apoptosis of HepG2 and SNU-423 cells,
which had been affected by TRD treatment, was recovered when
GRIM-19 was knocked down. These findings indicated that TRD
inhibited cell viability and induced apoptosis via the GRIM-19
pathway in TRD. Wei et al (32) revealed that GRIM-19 is predominantly
expressed in the nuclei of HeLa cells. Haura et al (40) reported that GRIM-19 is predominantly
expressed in the inner mitochondrial membrane and is a component of
the mitochondrial complex I. In the present study, it was revealed
that GRIM-19 was predominantly expressed in the cytoplasm of liver
cancer cells, which is consistent with our previous results
(19).
The aim of the present study was to expand the
investigation of the in vitro effects of TRD treatment on
cell apoptosis. The apoptotic mechanism of cancer cells is composed
of both upstream regulators and downstream effector components. The
regulators can be divided into two major circuits. One receives and
processes extracellular death-inducing signals, and the other
senses and integrates various signals of intracellular origin.
Bcl-2 is an upstream effector molecule in the apoptotic pathway,
which has been identified as a potent suppressor of apoptosis.
Bcl-2 has been reported to form a heterodimer complex with the
proapoptotic Bax, thereby neutralizing its proapoptotic effects.
Therefore, the ratio of Bax/Bcl-2 is a decisive factor and serves
an important role in determining whether cells die or survive
(41–44). The expression levels of these
proteins were detected by western blot analysis in the present
study, in order to determine their involvement in the molecular
mechanisms underlying the role of TRD in liver cancer cell
apoptosis.
Our previous study detected a significantly higher
nuclear expression of p-STAT3 in liver cancer lesions. Notably, an
inverse correlation was identified between GRIM-19 and p-STAT3
expression in liver cancer tissues (19). To verify this mechanism in
vitro, a p-STAT3 inhibitor (niclosamide) was added and the
expression of proliferation- and apoptosis-associated molecules,
STAT3, p-STAT3, cyclin D1, Bcl-2 and Bax, was detected by western
blotting. The result demonstrated that treatment with TRD not only
enhanced the expression of GRIM-19, but also reduced the expression
of cyclin D1, Bcl-2 and p-STAT3 in HepG2 cells. This result further
supported the hypothesis that TRD may induce the apoptosis of human
liver cancer cells through GRIM-19-induced regulation of a
STAT3-dependent pathway. The present data demonstrated that
niclosamide reduced the expression levels of p-STAT3, cyclin D1 and
Bcl-2, and increased those of Bax in TRD-treated HepG2 cells,
irrespective of GRIM-19 knockdown. This phenomenon indicated that
GRIM-19 may be an upstream molecule of the STAT3 signaling
pathway.
In conclusion, the present study indicated that
GRIM-19 was predominantly expressed in the cytoplasm of liver
cancer cells, and the knockdown of GRIM-19 was accompanied by an
increase in the expression of cyclin D1, Bcl-2 and p-STAT3 in these
cells. As shown in Fig. 4C,
treatment with TRD significantly upregulated GRIM-19 expression,
but downregulated cyclin D1, Bcl-2 and p-STAT3 expression in HepG2
cells and SNU-423 cells. However, these expression levels were
recovered following GRIM-19 knockdown. In summary, TRD inhibited
liver cancer cell viability and induced apoptosis via
GRIM-19-induced regulation of a STAT3-dependent pathway; therefore,
TRD may serve as a novel effective treatment for liver cancer.
Acknowledgements
Not applicable.
Funding
This study was supported in part by grants from the
Shandong Province Medical and Health Science and Technology
Development Project (grant no. 2017WS465), the National Natural
Science Foundation of China (grant nos. 81600469 and 81472685), the
Science and Technology Development Projects of Shandong Province
(grant nos. 2017GSF218053 and 2016GSF201126), and the Major Special
Plan of Science and Technology of Shandong Province (grant no.
2015ZDXX0802A01).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FL contributed to experimentation and manuscript
writing. JQ was responsible for technical guidance and data
interpretation. CQ was responsible for data analysis and
statistical analysis. ZF and WR made substantial contributions to
conception and design. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
GRIM-19
|
gene associated with
retinoid-interferon-induced mortality-19
|
|
TRD
|
taurolidine
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
siRNA
|
small interfering RNA
|
|
STAT3
|
signal transducer and activator of
transcription 3
|
|
p-STAT3
|
phosphorylated-signal transducer and
activator of transcription protein 3
|
|
FBS
|
fetal bovine serum
|
|
DMSO
|
dimethyl sulfoxide
|
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mittal S and El-Serag HB: Epidemiology of
hepatocellular carcinoma: Consider the population. J Clin
Gastroenterol. 47 Suppl:S2–S6. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pinter M, Trauner M, Peck-Radosavljevic M
and Sieghart W: Cancer and liver cirrhosis: Implications on
prognosis and management. ESMO Open. 1:e0000422016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kanda T, Ogasawara S, Chiba T, Haga Y,
Omata M and Yokosuka O: Current management of patients with
hepatocellular carcinoma. World J Hepatol. 7:1913–1920. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chromik AM, Daigeler A, Bulut D, Flier A,
May C, Harati K, Roschinsky J, Sülberg D, Ritter PR, Mittelkötter
U, et al: Comparative analysis of cell death induction by
Taurolidine in different malignant human cancer cell lines. J Exp
Clin Cancer Res. 29:212010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stendel R, Stoltenburgdidinger G, Al Keikh
CL, Wattrodt M and Brock M: The effect of taurolidine on brain
tumor cells. Anticancer Res. 22:809–814. 2002.PubMed/NCBI
|
|
7
|
Stendel R, Biefer HR, Dékány GM, Kubota H,
Münz C, Wang S, Mohler H, Yonekawa Y and Frei K: The antibacterial
substance taurolidine exhibits anti-neoplastic action based on a
mixed type of programmed cell death. Autophagy. 5:194–210. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Braumann C, Jacobi CA, Rogalla S,
Menenakos C, Fuehrer K, Trefzer U and Hofmann M: The tumor
suppressive reagent taurolidine inhibits growth of malignant
melanoma-a mouse model. J Surg Res. 143:372–378. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun BS, Wang JH, Liu LL, Gong SL and
Redmond HP: Taurolidine induces apoptosis of murine melanoma cells
in vitro and in vivo by modulation of the Bcl-2 family proteins. J
Surg Oncol. 96:241–248. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Opitz I, Sigrist B, Hillinger S, Lardinois
D, Stahel R, Weder W and Hopkins-Donaldson S: Taurolidine and
povidone-iodine induce different types of cell death in malignant
pleural mesothelioma. Lung Cancer. 56:327–336. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aceto N, Bertino P, Barbone D, Tassi G,
Manzo L, Porta C, Mutti L and Gaudino G: Taurolidine and oxidative
stress: A rationale for local treatment of mesothelioma. Eur Respir
J. 34:1399–1407. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Braumann C, Schoenbeck M, Menenakos C,
Kilian M and Jacobi CA: Effects of increasing doses of a bolus
injection and an intravenous long-term therapy of taurolidine on
subcutaneous (metastatic) tumor growth in rats. Clin Exp
Metastasis. 22:77–83. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chromik AM, Daigeler A, Hilgert C, Bulut
D, Geisler A, Liu V, Otte JM, Uhl W and Mittelkötter U: Synergistic
effects in apoptosis induction by taurolidine and TRAIL in HCT-15
colon carcinoma cells. J Invest Surg. 20:339–348. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Daigeler A, Chromik AM, Geisler A, Bulut
D, Hilgert C, Krieg A, Klein-Hitpass L, Lehnhardt M, Uhl W and
Mittelkötter U: Synergistic apoptotic effects of taurolidine and
TRAIL on squamous carcinoma cells of the esophagus. Int J Oncol.
32:1205–1220. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Walters DK, Muff R, Langsam B, Gruber P,
Born W and Fuchs B: Taurolidine: A novel anti-neoplastic agent
induces apoptosis of osteosarcoma cell lines. Invest New Drugs.
25:305–312. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Daigeler A, Brenzel C, Bulut D, Geisler A,
Hilgert C, Lehnhardt M, Steinau HU, Flier A, Steinstraesser L,
Klein-Hitpass L, et al: TRAIL and Taurolidine induce apoptosis and
decrease proliferation in human fibrosarcoma. J Exp Clin Cancer
Res. 27:822008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chidambaram NV, Angell JE, Ling W, Hofmann
ER and Kalvakolanu DV: Chromosomal localization of human GRIM-19, a
novel IFN-beta and retinoic acid-activated regulator of cell death.
J Interferon Cytokine Res. 20:661–665. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen Y, Lu H, Liu Q, Huang G, Lim CP,
Zhang L, Hao A and Cao X: Function of GRIM-19, a mitochondrial
respiratory chain complex I protein, in innate immunity. J Biol
Chem. 287:27227–27235. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li F, Ren W, Zhao Y, Fu Z, Ji Y, Zhu Y and
Qin C: Downregulation of GRIM-19 is associated with hyperactivation
of p-STAT3 in hepatocellular carcinoma. Med Oncol. 29:3046–3054.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao J, Nie W, Li W, Zhou X, Sun H, Zhu J,
Chen J and Peng J: Interferon-β combined with all-trans retinoic
acid supresses proliferation and promote apoptosis by inhibiting
JAK2/STAT3 pathway in HepG2 human hepatocellular carcinoma cells.
Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 32:901–905. 2016.(In Chinese).
PubMed/NCBI
|
|
21
|
Gong L, Greenberg HE, Perhach JL, Waldman
SA and Kraft WK: The pharmacokinetics of taurolidine metabolites in
healthy volunteers. J Clin Pharmacol. 47:697–703. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Braumann C, Winkler G, Rogalla P,
Menenakos C and Jacobi CA: Prevention of disease progression in a
patient with a gastric cancer-re-recurrence. Outcome after
intravenous treatment with the novel antineoplastic agent
taurolidine. Report of a case. World J Surg Oncol. 4:342006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stendel R, Picht T, Schilling A,
Heidenreich J, Loddenkemper C, Jänisch W and Brock M: Treatment of
glioblastoma with intravenous taurolidine. First clinical
experience. Anticancer Res. 24:1143–1147. 2004.PubMed/NCBI
|
|
24
|
Stendel R, Scheurer L, Schlatterer K,
Stalder U, Pfirrmann RW, Fiss I, Möhler H and Bigler L:
Pharmacokinetics of taurolidine following repeated intravenous
infusions measured by HPLC-ESI-MS/MS of the derivatives taurultame
and taurinamide in glioblastoma patients. Clin Pharmacokinet.
46:513–524. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Darnowski JW, Goulette FA, Cousens LP,
Chatterjee D and Calabresi P: Mechanistic and antineoplastic
evaluation of taurolidine in the DU145 model of human prostate
cancer. Cancer Chemother Pharmacol. 54:249–258. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Han Z, Ribbizi I, Pantazis P, Wyche J,
Darnowski J and Calabresi P: The antibacterial drug taurolidine
induces apoptosis by a mitochondrial cytochrome c-dependent
mechanism. Anticancer Res. 22:1959–1964. 2002.PubMed/NCBI
|
|
27
|
Rodak R, Kubota H, Ishihara H, Eugster HP,
Könü D, Möhler H, Yonekawa Y and Frei K: Induction of reactive
oxygen intermediates-dependent programmed cell death in human
malignant ex vivo glioma cells and inhibition of the vascular
endothelial growth factor production by taurolidine. J Neurosurg.
102:1055–1068. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stendel R, Scheurer L,
Stoltenburg-Didinger G, Brock M and Möhler H: Enhancement of
Fas-ligand-mediated programmed cell death by taurolidine.
Anticancer Res. 23:2309–2314. 2003.PubMed/NCBI
|
|
29
|
Kolligs FT, Nieman MT, Winer I, Hu G, Van
Mater D, Feng Y, Smith IM, Wu R, Zhai Y, Cho KR, et al: ITF-2, a
downstream target of the Wnt/TCF pathway, is activated in human
cancers with beta-catenin defects and promotes neoplastic
transformation. Cancer Cell. 1:145–155. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ottaviano AJ, Sun L, Ananthanarayanan V
and Munshi HG: Extracellular matrix-mediated membrane-type 1 matrix
metalloproteinase expression in pancreatic ductal cells is
regulated by transforming growth factor-beta1. Cancer Res.
66:7032–7040. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wei D, Le X, Zheng L, Wang L, Frey JA, Gao
AC, Peng Z, Huang S, Xiong HQ, Abbruzzese JL and Xie K: Stat3
activation regulates the expression of vascular endothelial growth
factor and human pancreatic cancer angiogenesis and metastasis.
Oncogene. 22:319–329. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jacobi CA, Menenakos C and Braumann C:
Taurolidine-a new drug with anti-tumor and anti-angiogenic effects.
Anticancer Drugs. 16:917–921. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mccourt M, Wang JH, Sookhai S and Redmond
HP: Taurolidine inhibits tumor cell growth in vitro and in vivo.
Ann Surg Oncol. 7:685–691. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Petrovic L, Schlegel KA, Ries J, Park J,
Diebel E, Schultze-Mosgau S and Wiltfang J: In vitro effect of
taurolidine on squamous cell carcinoma in the oral cavity. Mund
Kiefer Gesichtschir. 7:102–107. 2003.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gallagher KA, Liu ZJ, Xiao M, Chen H,
Goldstein LJ, Buerk DG, Nedeau A, Thom SR and Velazquez OC:
Diabetic impairments in NO-mediated endothelial progenitor cell
mobilization and homing are reversed by hyperoxia and SDF-1. J Clin
Invest. 117:1249–1259. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Calabresi P, Goulette FA and Darnowski JW:
Taurolidine: Cytotoxic and mechanistic evaluation of a novel
antineoplastic agent. Cancer Res. 61:6816–6821. 2001.PubMed/NCBI
|
|
38
|
Braumann C, Henke W, Jacobi CA and Dubiel
W: The tumor-suppressive reagent taurolidine is an inhibitor of
protein biosynthesis. Int J Cancer. 112:225–230. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Neary PM, Hallihan P, Wang JH, Pfirrmann
RW, Bouchier-Hayes DJ and Redmond HP: The evolving role of
taurolidine in cancer therapy. Ann Surg Oncol. 17:1135–1143. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Haura EB, Turkson J and Jove R: Mechanisms
of disease: Insights into the emerging role of signal transducers
and activators of transcription in cancer. Nat Clin Pract Oncol.
2:315–324. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Oltvai ZN, Milliman CL and Korsmeyer SJ:
Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that
accelerates programed cell death. Cell. 74:609–619. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Korsmeyer SJ, Shutter JR, Veis DJ, Merry
DE and Oltvai ZN: Bcl-2/Bax: A rheostat that regulates an
anti-oxidant pathway and cell death. Semin Cancer Biol. 4:327–332.
1993.PubMed/NCBI
|
|
43
|
Tsukahara S, Yamamoto S, Tin-Tin-Win-Shwe,
Ahmed S, Kunugita N, Arashidani K and Fujimaki H: Inhalation of
low-level formaldehyde increases the Bcl-2/Bax expression ratio in
the hippocampus of immunologically sensitized mice.
Neuroimmunomodulation. 13:63–68. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Walensky LD: BCL-2 in the crosshairs:
Tipping the balance of life and death. Cell Death Differ.
13:1339–1350. 2006. View Article : Google Scholar : PubMed/NCBI
|