Introduction
Gastric cancer (GC) and colorectal cancer (CRC) are
worldwide public health problems; GC is the fourth most common type
of carcinoma and the second most common cause of
carcinoma-associated mortality, and CRC is the second most common
cause of carcinoma-associated mortality with an annual incidence of
1,000,000 cases and an annual mortality of >500,000 cases
(1). In China, the incidence of GC
ranks third among all malignant tumors and the mortality rate was
26.3/100,000 in 2005 (2); the
incidence of CRC ranked fourth of all cancer types and the
estimated mortality rate was ranked the fifth leading cause of
cancer-associated mortality in all cancer types in 2011 (3). However, the pathogenesis of these
diseases remains to be fully elucidated. Previous studies have
focused on the induced oncogenes and inhibited tumor suppressor
genes, as well as the dysfunction of mismatch base repair in
nuclear DNA, which does not fully explain the pathogenesis and
development of these diseases. Mitochondria can generate adenosine
triphosphate via oxidative phosphorylation and in turn control
essential cellular activities. The displacement loop region
(D-loop) is the main noncoding area of mitochondrial DNA (mtDNA).
mtDNA mutations in the D-loop region and somatic mtDNA mutations
have been described to be common in various types of primary human
cancer types including hepatocellular carcinoma, and bladder,
breast and lung cancers (4,5); however, their role in the pathogenesis
of gastrointestinal cancer (GIC) is controversial. In the present
study, whether somatic mtDNA and D-loop mutations occurred in
Chinese patients with GIC and their association with disease
progression were investigated.
Materials and methods
Patients and tissues
Tumor and para-tumor tissues were obtained from GC
and CRC patients who underwent surgical tumor resection at the
Fourth General Surgery Division, Shandong Cancer Hospital (Jinan,
China) between February 2012 and August 2012. Tumor tissues were
pathologically diagnosed as GC or CRC, and para-tumor tissues were
confirmed to be non-cancerous by experienced pathologists. The
inclusion criteria included: i) Preoperative pathological biopsy
and postoperative histopathology confirmed the diagnosis of GIC;
ii) patients without other diagnosed tumors or diseases; and iii)
patients or their families all provided signed informed consent.
The exclusion criteria included: ii) Patients with incomplete
clinical data available; ii) patients diagnosed with other types of
tumors and diseases; and iii) patients and their families who
refused to provide informed consent. Patient demographics and
clinical characteristics are listed in Table I. Among the participants, there were
18 patients with GC, 21 patients with colon cancers (CC) and 30
patients with rectal cancer (RC); their average ages were 55.1, 54
and 57.4 years, respectively. Men appeared to be over-represented,
accounting for 13/18 GC patients, 13/21 CC patients and 20/30 RC
patients. The majority of these patients were diagnosed with tumor
node metastasis (TNM) stages II–III and grades I–III. The present
study was approved by the Ethics Committees of Shandong Cancer
Hospital. Written informed consent was obtained from all study
participants.
 | Table I.Demographics and clinical
characteristics of the patients recruited to the present study. |
Table I.
Demographics and clinical
characteristics of the patients recruited to the present study.
|
| Carcinoma type |
|---|
|
|
|
|---|
|
Characteristics | Gastric cancer
(n=18) | Colon cancer
(n=21) | Rectum cancer
(n=30) |
|---|
| Sex (male) | 13 | 13 | 20 |
| Age (years) |
|
Mean | 55.1 | 54.0 | 57.4 |
|
Range | 40–68 | 30–71 | 34–75 |
| TNM stage (n) |
| I | 0 | 0 | 4 |
| II | 2 | 10 | 9 |
|
III | 14 | 5 | 14 |
| IV | 2 | 6 | 3 |
| Grade (n) |
| I | 2 | 7 | 5 |
| II | 6 | 12 | 20 |
|
III | 10 | 1 | 5 |
| IV | 0 | 1 | 0 |
| Type of surgery
(n) |
| Local
resection | 10 | 17 | 16 |
| Organ
resection | 8 | 4 | 14 |
|
Multiorgan resection | 0 | 0 | 0 |
| CEA (n ≥5
µg/l) | 5 | 11 | 10 |
| CA19-9 (n ≥37
U/ml) | 6 | 10 | 3 |
| CA72-4 (n ≥6
U/ml) | 6 | N/A | N/A |
| Risk factors |
| Tobacco use
(n) |
|
Current | 7 | 7 | 3 |
|
Former | 3 | 2 | 3 |
|
Never | 8 | 12 | 24 |
| Alcohol use
(n) |
|
Current | 8 | 4 | 3 |
|
Former | 1 | 3 | 1 |
|
Never | 9 | 14 | 26 |
| Family
history (n) | 1 | 3 | 1 |
| Polyps
(n) | 1 | 3 | 1 |
|
Inflammatory disease
(n)a | 3 | 2 | 0 |
|
Unhealthy diet
(n)b | 1 | 0 | 0 |
Immunohistochemistry staining
GI tissues were fixed with 10% formalin at 4°C for
24 h and then paraffin-embedded; sections 4-µm-thick were cut and
immunohistochemical staining was performed. Tissue sections were
first deparaffinized and rehydrated, followed by heat-induced
epitope retrieval at 95–100°C, washing with 100% Ethanol 2 for 5
min, 90% Ethanol 1 for 5 min, 70% Ethanol 1 for 5 min and
ddH2O 1 for 5 min, and then treated with a 10 mmol/l
citrate buffer (pH 6.0). Next, 3% H2O2 was
used to block endogenous peroxidase and sections were blocked with
PBS containing 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) at room temperature for 1 h.
Then, sections were incubated with anti-8-hydroxyguanine (oxo-G;
cat. no. 4354-MC-050; Trevigen, Inc., Gaithersburg, MD, USA) and
anti-8-oxo-20-deoxyguanosine glycosylase 1 (OGG1; cat. no.
NBP2-52724; Novus Biologicals, LLC, Littleton, CO, USA) antibodies,
diluted with blocking reagent (1:1,000), overnight at 4°C, followed
by incubation with a biotin-free horseradish peroxidase-conjugated
secondary antibody (cat. no. pv9005; Beijing Zhongshan Golden
Bridge Biotechnology Co., Ltd., Beijing, China); diluted with
blocking reagent (1:1,000) for 1 h at room temperature.
Visualization was performed with 3,3′-diaminobenzidine. The slides
were viewed and photographed under a fluorescent inverted
microscope (Olympus IX71; Olympus Corporation, Tokyo, Japan), and
positively stained cells were counted using Image Pro Plus 6.0
software (Media Cybernetics, Inc., Rockville, MD, USA).
DNA isolation, and cloning and
sequencing of the mtDNA D-loop
Total DNA from the tumor and para-tumor tissues was
isolated using a DNA extraction kit (Qiagen China Co., Ltd.,
Shanghai, China) following the manufacturer's protocol, and
polymerase chain reaction (PCR) was performed to amplify the mtDNA
D-loop region using high-fidelity Platinum Taq polymerase
(Invitrogen; Thermo Fisher Scientific, Inc.). The primer pairs and
PCR procedure for the D-loop in the present study have been
described in our previous study (6). The pGEM-18T vector (Takara
Biotechnology Co., Ltd., Dalian, China) was used to clone the PCR
products, and 10–12 randomly selected clones/samples were sequenced
on an ABI 3730 genetic analyzer (Thermo Fisher Scientific, Inc.).
The D-loop nucleotide sequences from each clone were analyzed and
manually adjusted using NCBI BLAST(blast.ncbi.nlm.nih.gov/Blast.cgi) and free BioEdit
software (version 7.1.3; Ibis Therapeutics, Carlsbad, CA, USA). All
sequences have been submitted to GenBank (accession nos.
KY402468-KY403500).
Reverse transcription-quantitative PCR
(RT-qPCR)
The RT-qPCR assay for mtDNA deletion quantification
was performed using the SYBR Green (Beijing Biomed Biotechnology
Co., Ltd., Beijing, China)method based on the relative nicotinamide
adenine dinucleotide hydride dehydrogenase subunit 1
(ND1)/ND4-quantification method as well as 2−ΔΔCq as
previously reported (6,8–11), and
was performed using the TaqMan 7900HT system (Thermo Fisher
Scientific, Inc.). The primers used in the present study were as
follows: Reference gene, Homo sapiens mitochondrion complete
genome (Gen-Bank NC 012920); ND1 forward,
5′-CCCTAAAACCCGCCACATCT-3′ and reverse,
5′-TGGAATCGAGAGTGGTAGCGAG-3′; ND4 forward,
5′-CCATTCTCCTCCTATCCCTCAAC-3′ and reverse,
5′-TTTATATCAAATTGGTTTTGTAGTCTAACAC-3′ (synthesized by Invitrogen;
Thermo Fisher Scientific, Inc.). The procedures were similar to
those described in our previous report (7,12).
Briefly, 250 nM probe and 300 nM primer were used in the PCR
reaction mix. The qPCR thermocycling conditions were as follows: 5
min at 95°C, followed by 50 cycles of 15 sec at 95°C and 1 min at
60°C. qPCR reactions were performed in triplicate for each sample.
Double-distilled water was used as a control reaction and was
subjected to the same conditions as the test reactions.
Sequence analysis
The sequences were analyzed as previously described
(13–15). Briefly, the obtained nucleotide
sequences from each clone were assembled and error checked using
the Vector NTI suite 7.0 ContigExpress software package (Thermo
Fisher Scientific, Inc.). The sequences were then aligned to the
reference sequence (Gen-Bank NC 012920) using the Clustal W
multiple sequence alignment program (www.ebi.ac.uk/Tools/msa/clustalw2/), then the
nucleotide mutations were identified and calculations were
performed. Shannon entropy (www.hiv.lanl.gov/content/sequence/ENTROPY/entropytwo.html),
as a measure of variation in mtDNA D-loop sequence alignments, was
used to determine whether there were more highly variable areas in
tumors when compared with the para-tumors. The sequences were then
aligned to the reference sequence using the MITOMASTER web tool in
the Mitomap database (www.mitomap.org/) to check for mtDNA mutations.
Microsatellite instability (MSI), a simple repetitive sequence
change caused by a mismatch repair gene mutation, was also examined
in 69 Chinese patients via alignment of sequences to the reference
sequence.
Statistical analysis
The results are presented as the mean ± standard
error of the mean. Statistical significance was determined by
one-way analysis of variance with post hoc correction using the
Tukey's multiple comparison test. Nonparametric Mann-Whitney,
Chi-square, or Fisher's exact tests were used to compare
nonparametric data. All statistical analyses were performed using
SPSS software (version 16.0; SPSS Inc., Chicago, IL, USA), and
P<0.05 was considered to indicate a statistically significant
difference.
Results
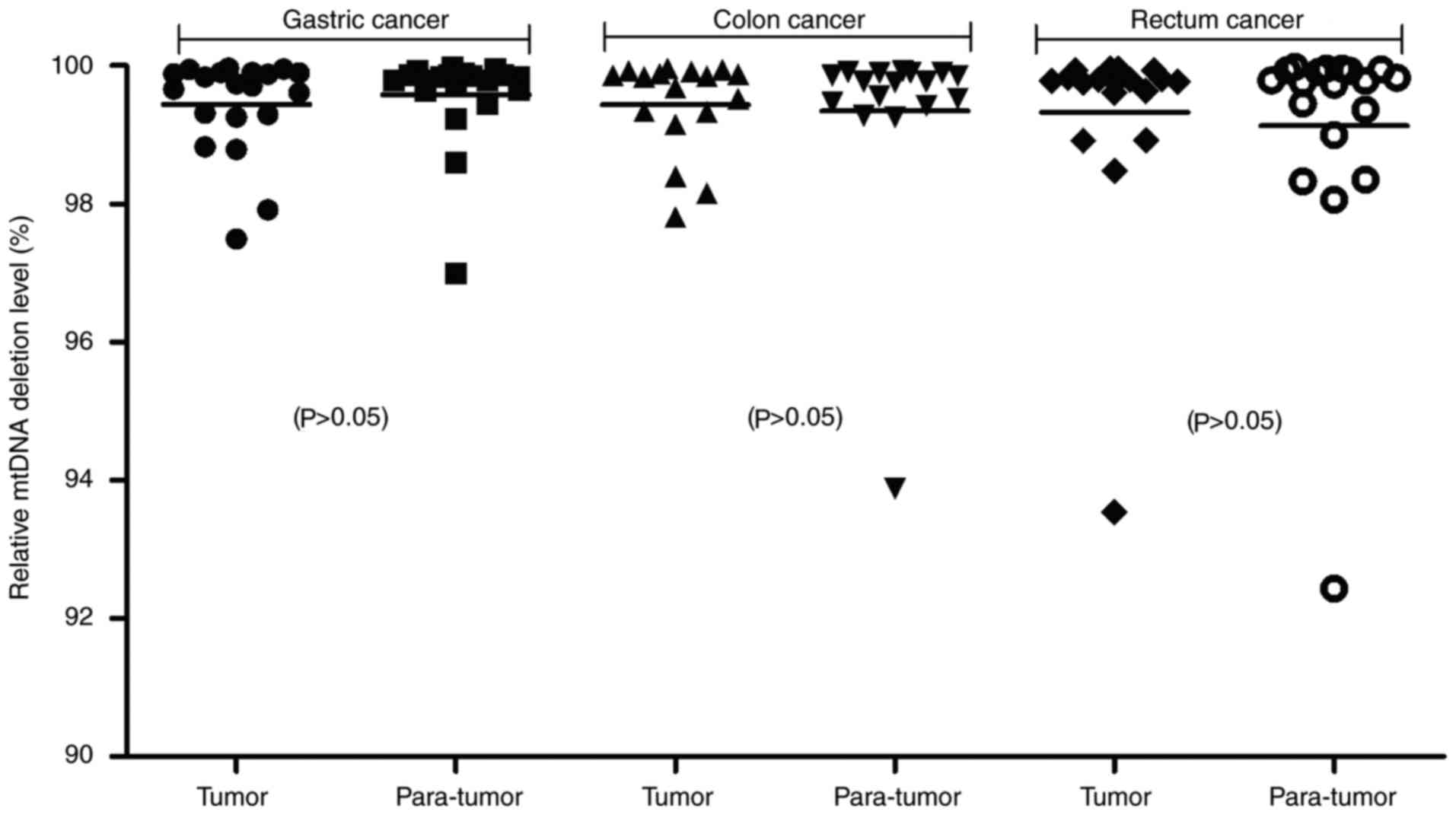
No increased mtDNA loss was observed
in GIC
In the present study, whether mtDNA deletions
occurred in GIC were assessed via qPCR. The ND1 gene is located in
the minor arc of mtDNA and is rarely deleted; however, the ND4 gene
is located at the major arc of mtDNA and is frequently deleted.
ND1/ND4 relative qPCR was used to detect mtDNA deletions via the
2−ΔΔCq method in the present study. The results
demonstrated that the relative amount of mtDNA copies were 99.44%
in GC tumors and 99.58% in GC para-tumors, 99.44% in CC tumors and
99.35% in CC para-tumors, and 99.32% in RC tumors and 99.13% in RC
para-tumors (Fig. 1). No
significant mtDNA deletions were noted in the GC, CC or RC tumor
tissues when compared with para-tumor tissues. Furthermore, the
relative amount of mtDNA copies between the different clinical
carcinoma stages were compared; however, no mtDNA deletions in
stage III–IV GC, CC or RC were identified (data not shown).
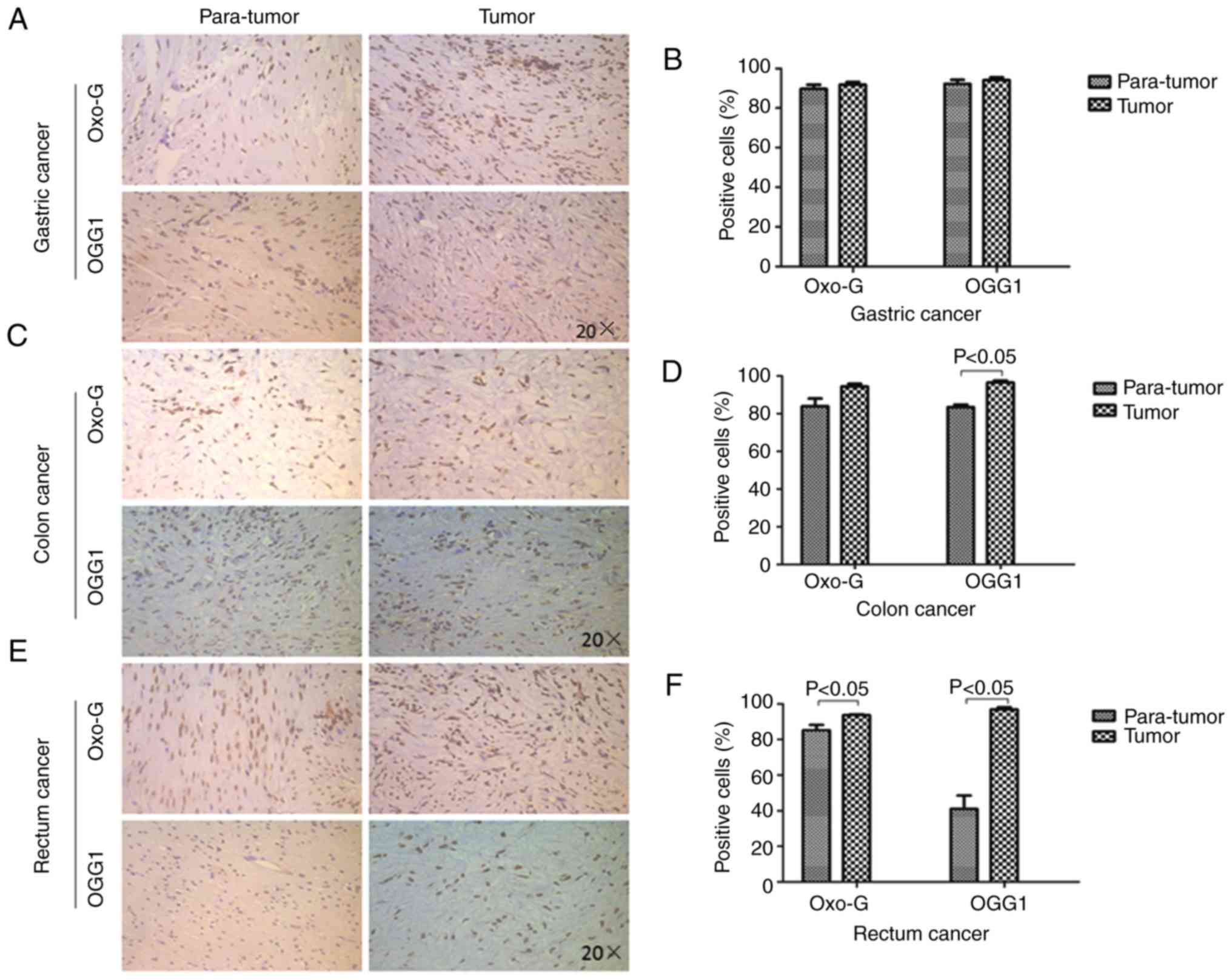
DNA oxidative damage and
over-activated DNA repair in GIC
Oxidative damage has been reported in the mtDNA of
tumor cells (16). DNA oxidative
damage commonly produces oxo-G, and OGG1initiates base excision
repair, which removes oxo-G damaged DNA (17). In the present study, the expression
of oxo-G and OGG1 was determined in GIC tumor and para-tumor
tissues using immunohistochemistry staining, as described in our
previous studies (7,18). No significant increases were
observed in oxo-G (92±1 vs. 90±2%) or OGG1 (94±1 vs. 92±2%)
expression in GC tissues when compared with para-GC tissues
(Fig. 2A and B). In addition, no
significant difference in oxo-G expression was observed between CC
tissues (94±1%) and para-CC tissues (84±4%), but OGG1 expression
was higher in CC tissues (97±1%) when compared with para-CC tissues
(83±1%; Fig. 2C and D). However,
oxo-G (94±4 vs. 83±1%) and OGG1 (97±1 vs. 41±7%) expression levels
were increased in RC tissues when compared with para-RC tissues
(Fig. 2E and F). These results
indicated that oxidative damage and over-activated DNA repair
functioning occurred in GIC.
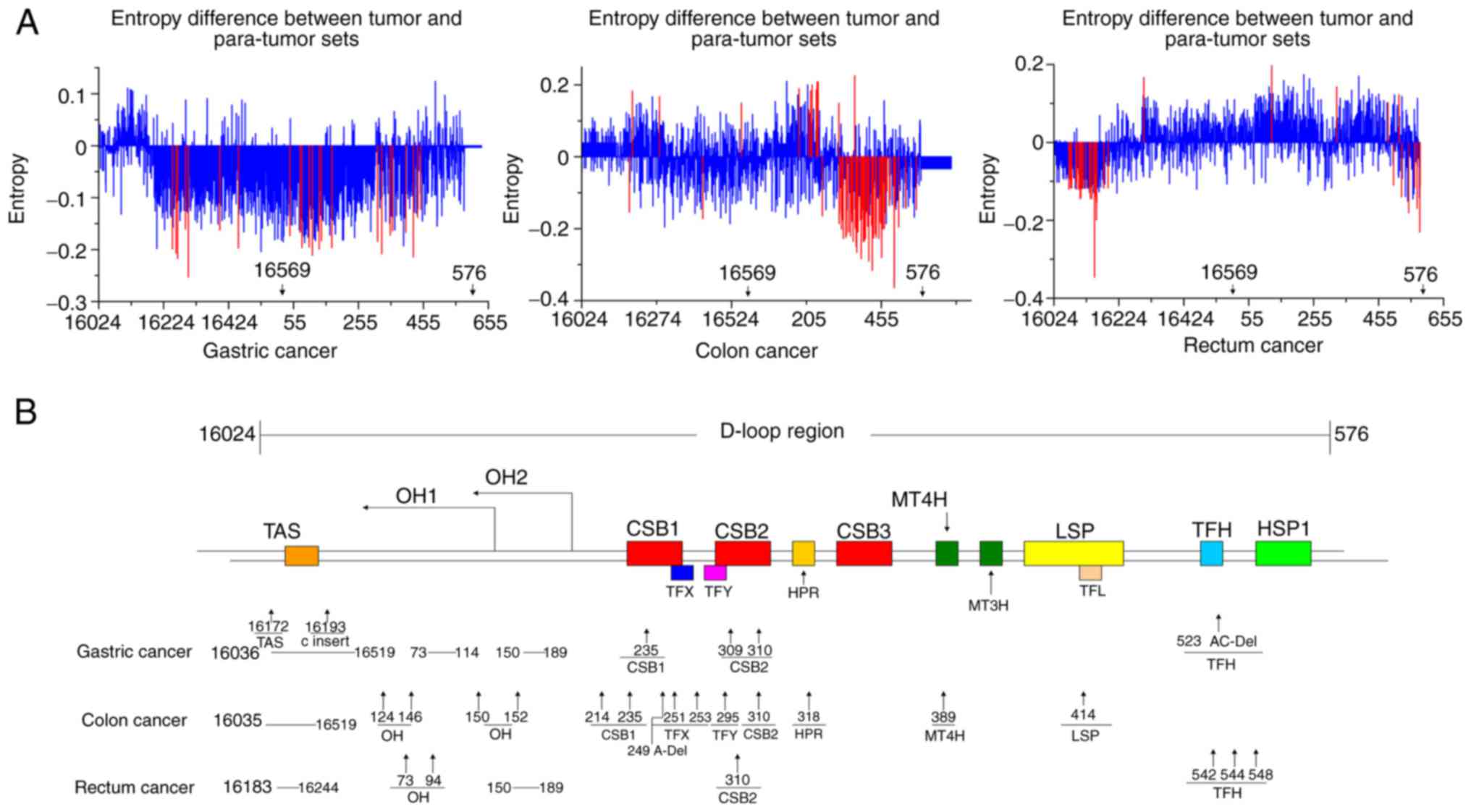
Somatic mtDNA D-loop mutations in
GIC
To identify somatic mutations in the D-loop of mtDNA
in GIC, Shannon entropy was used to identify highly variant regions
in the D-loop of mtDNA. The results revealed that GC had two highly
variant regions located at nucleotide position (np) 75–173 and np
314–447; CC had a highly variant region located at np 307–476,
which was similar to the second highly variant region of GC; and RC
had two highly variant regions located at np 16069–16177 and np
517–576, which were significantly different from the locations in
GC and CC (Fig. 3A). Then, the
sequences in the mtDNA D-loop region from tumor and para-tumor
tissues from 18 GC, 21 CC and 30 RC patients were directly
analyzed. The results demonstrated a total of 221 mutations in 196
sequences from the tumor tissues of the 18 patients with GC; this
number was significantly higher than that observed in the paired
para-tumor tissues, which contained 141 mutations in 179 sequences
(P<0.05). Similarly, a high frequency of mutation(s) was
identified in the 21 patients with CC (153 mutations in 155
sequences from the tumor tissues vs. 107 mutations in 154 sequences
from the para-tumor tissues) and the 30 patients with RC (166
mutations in 183 sequences from the tumor tissues vs. 104 mutations
in 168 sequences from the para-tumor tissues; both P<0.05;
Table II). These results indicated
that all three types of GIC contained more somatic mutations than
the normal para-tumor tissues. Based on the delineation of the
functional regions of the mtDNA D-loop in previous reports
(19,20), the mutation sites were analyzed, and
it was revealed that these mutations clustered in the replication
origin of the H-strand (P<0.05) and conserved sequence block 2.
Furthermore, GC and RC also possessed somatic mutation(s) in an
unknown functional region (P<0.05; Fig. 3B; Table
II).
 | Table II.Distribution of mtDNA D-loop
mutations in gastrointestinal cancer. |
Table II.
Distribution of mtDNA D-loop
mutations in gastrointestinal cancer.
|
| Gastric cancer | Colon cancer | Rectum cancer |
|---|
|
|
|
|
|
|---|
| Mitomap | Tumor
(na=196) | Para-tumor
(n=179) | P-value | Tumor (n=153) | Para-tumor
(n=154) | P-value | Tumor (n=183) | Para-tumor
(n=168) | P-value |
|---|
| OH1 | 135 | 87 | <0.05 | 68 | 40 | <0.05 | 85 | 48 | <0.05 |
| OH2 | – | – | – | 16 | 12 | >0.05 | 18 | 12 | >0.05 |
| CSB1 | 1 | 1 | – | 6 | 4 | – | – | – | – |
| TFX | – | – | – | 3 | 0 | – | – | – | – |
| TFY | – | – | – | 1 | 0 | – | – | – | – |
| CSB2 | 17 | 8 | >0.05 | 17 | 16 | >0.05 | 16 | 11 | >0.05 |
| HPR | – | – | – | 1 | 1 | – | – | – | – |
| CSB3 | – | – | – | – | – | – | – | – | – |
| MT4H | – | – | – | – | – | – | – | – | – |
| MT3H | 1 | 0 | – | 15 | 11 | >0.05 | – | – | – |
| LSP | – | – | – | 1 | 0 | – | – | – | – |
| TFL | – | – | – | – | – | – | – | – | – |
| TFH | 9 | 7 | – | – | – | – | 3 | 0 | – |
| HSP1 | – | – | – | – | – | – | – | – | – |
| TAS | 7 | 6 | – | 1 | 1 | – | – | – | – |
| UNKNOW | 51 | 32 | <0.05 | 25 | 22 | >0.05 | 44 | 33 | <0.05 |
| Sum | 221 | 141 | <0.05 | 154 | 107 | <0.05 | 166 | 104 | <0.05 |
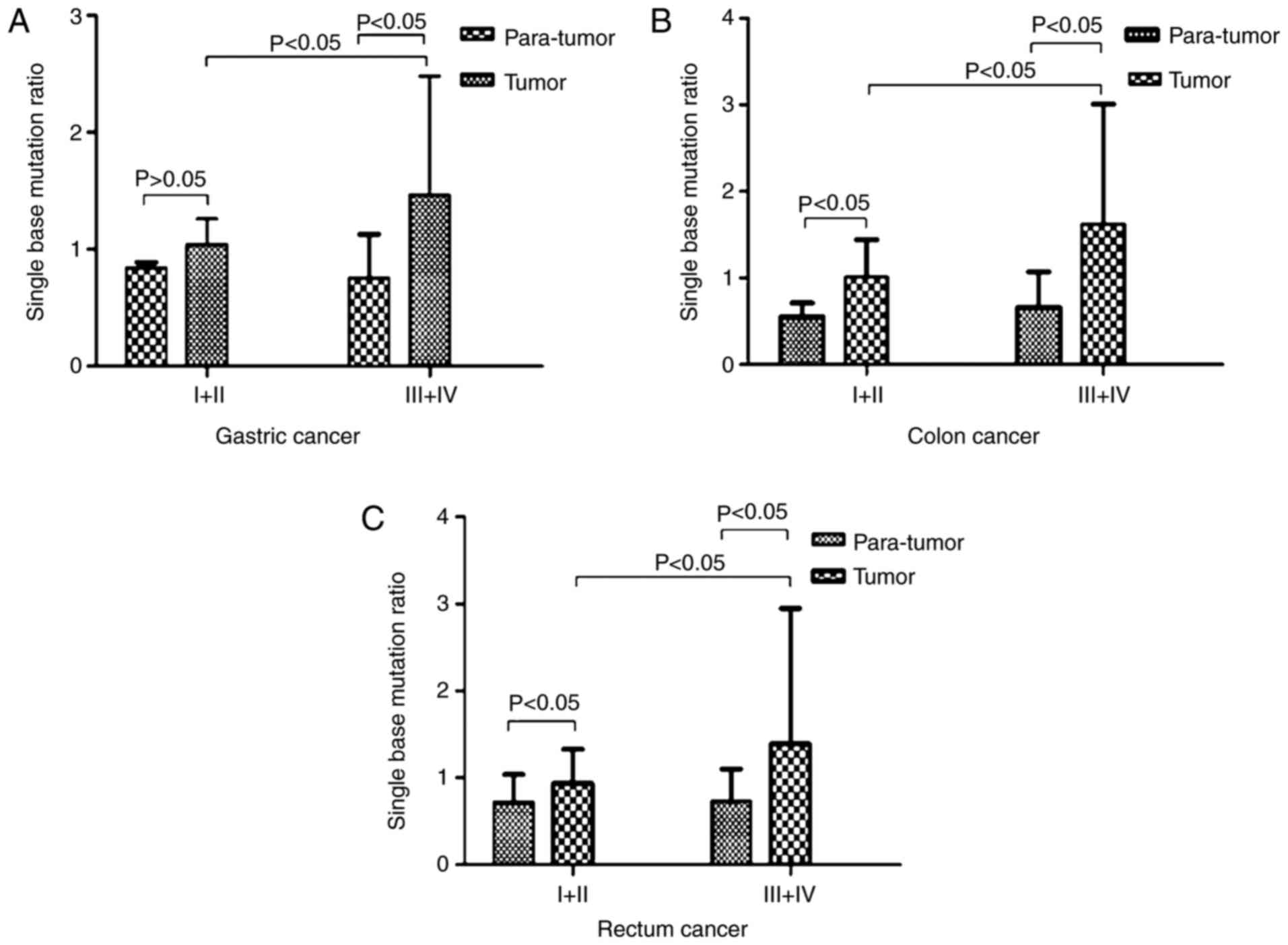
More severe mtDNA D-loop mutations are
observed in advanced stages of GIC
It is well known that tumor staging is an important
prognostic factor for malignant neoplasms (21). In the present study, the mutation
rate of the mtDNA D-loop region between tumors of different stages
and para-tumor tissues were compared. The results demonstrated that
the mean mutation rate of the mtDNA D-loop region was significantly
higher in stage III–IV GC tissues when compared with in para-tumor
tissues (1.46±1.02 vs. 0.75±0.38; P<0.05); however, the increase
in the D-loop mutation rate was not significant in stage I–II GC
(1.04±0.22 vs. 0.84±0.05; P>0.05; Fig. 4A). Similar analysis revealed that
early and advanced stages of CC and RC had a significantly higher
D-loop mutation rate when compared with para-tumors: 1.01±0.43 vs.
0.55±0.16 in stage I–II CC; 1.62±1.39 vs. 0.66±0.41 in stage III–IV
CC; 0.94±0.39 vs. 0.72±0.32 stage I–II RC; and 1.39±1.56 vs.
0.73±0.37 stage III–IV RC (all P<0.05). The mutation rates of
stage III–IV tumors were greater when compared with those of stage
I–II tumors (Fig. 4B and C). These
results indicated that the later the GC stage, the more severe the
mtDNA D-loop mutations.
Homoplasmic mutations of the mtDNA
D-loop in GIC
Certain studies have indicated that mtDNA mutations
in the coding regions of CRC are primarily transitions; A-T and G-C
are common in the D-loop region (22). In the present study, the mutation
types in the mtDNA D-loop in GIC were also analyzed. The results
revealed that of the single-base mutations, transitions accounted
for 83.86% in GC, 96.74% in CC and 93.9% in RC. The T-C base
substitution was the most common (44.11% in GC, 52.29% in CC and
45.12% in RC), followed by C-T (20.10% in GC, 22.88% in CC and
18.29% in RC); and the G-A transition was relatively rare (2.94% in
GC, 5.23% in CC and 2.44% in RC; Table III). These results suggested that
the mutations of the mtDNA D-loop were primarily homoplasmic in GIC
and were often transitions at pyrimidine sites.
 | Table III.Subtypes of single base mutation in
gastrointestinal cancer. |
Table III.
Subtypes of single base mutation in
gastrointestinal cancer.
|
| Gastric cancer | Colon cancer | Rectum cancer |
|---|
|
|
|
|
|
|---|
| Mutation
subtype | Sites | Tumor | PCT (%) | Sites | Tumor | PCT (%) | Sites | Tumor | PCT (%) |
|---|
| Transition |
|
A-G | 12 | 34 | 16.67 | 6 | 25 | 16.34 | 6 | 46 | 28.05 |
|
G-A | 4 | 6 | 2.94 | 6 | 8 | 5.23 | 3 | 4 | 2.44 |
|
C-T | 14 | 41 | 20.10 | 10 | 35 | 22.88 | 8 | 30 | 18.29 |
|
T-C | 18 | 90 | 44.11 | 18 | 80 | 52.29 | 13 | 74 | 45.12 |
|
Sum |
|
| 83.86 |
|
| 96.74 |
|
| 93.9 |
| Transversion |
|
A-C | 2 | 11 | 5.39 | 1 | 4 | 2.61 | 1 | 8 | 4.88 |
|
A-T | 1 | 1 | 0.49 | 0 | 0 | 0 | 0 | 0 | N/A |
|
C-A | 0 | 0 | 0 | 1 | 1 | 0.65 | 1 | 1 | 0.61 |
|
C-G | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0.61 |
|
G-C | 1 | 1 | 0.49 | 0 | 0 | 0 | 0 | 0 | N/A |
|
T-G | 1 | 20 | 9.80 | 0 | 0 | 0 | 0 | 0 | N/A |
|
Sum |
|
| 16.14 |
|
| 3.26 |
|
| 6.1 |
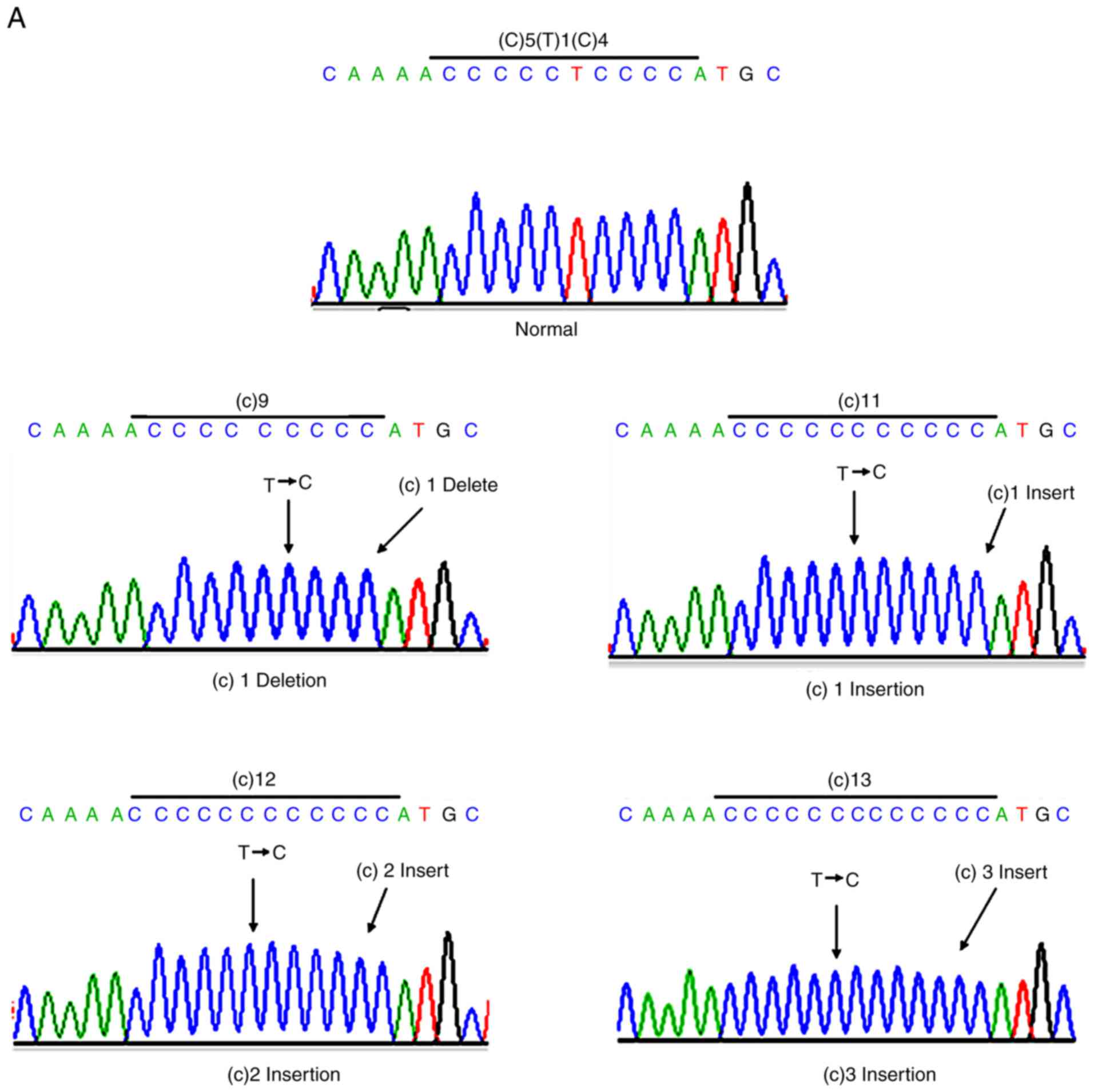
Mitochondrial MSI (mtMSI) in GIC
mtMSI has been reported to frequently occur in GC
and CRC (23,24). In the present study, the mtMSI in 69
Chinese patients with GIC were analyzed. It was revealed that
polynucleotide stretches were common and resulted from a 1–3
cytosine insertion or cytosine deletion. Among them, the percentage
of sequences containing a continuous 10-cytosine stretch was 11.7%
in GC, 13.8% in CC 11.7 and 20.9% in RC. The percentage of
sequences containing a continuous 7-cytosine stretch was 40.3% in
GC, 68.6% in CC 11.7 and 47% in RC (Table IV). Furthermore, repeated AC
stretches were observed in GC, which were caused by a 1–2 CA
insertion or CA deletion, and the total percentage of this sequence
was 37.2% (Table IV). The
formation of the polynucleotide stretch is presented in Fig. 5. These results suggested that mtMSI
occurs in Chinese patients with GIC.
 | Table IV.Mitochondrial microsatellite
instability in gastrointestinal cancer. |
Table IV.
Mitochondrial microsatellite
instability in gastrointestinal cancer.
|
| CCCCCTCCCC
(16184–16193a,
T→C,(C)10b) (%) |
| CCCCCCC (303–309,
(C)7) (%) |
| CA (515–524 (CA) 5)
(%) |
|---|
|
|
|
|
|
|
|
|---|
| Type | Gastric cancer | Colon cancer | Rectum cancer | Type | Gastric cancer | Colon cancer | Rectum cancer | Type | Gastric cancer |
|---|
| C1 insert | 7.1 | 7.2 | 7.7 | C1 insert | 34.7 | 45.1 | 35.0 | CA1 insert | 4.1 |
| C2 insert | 3.6 | 3.9 | 4.4 | C2 insert | 5.6 | 15.0 | 10.9 | CA2 insert | 1.5 |
| C3 insert | 1.0 | 2.0 | 2.2 | C3 insert | 0 | 5.9 | 1.1 | CA1 deletion | 31.6 |
| C1 deletion | 0 | 0.7 | 6.6 | C3 deletion | 0 | 2.6 | 0 | – | – |
| Sum | 11.7 | 13.8 | 20.9 | Sum | 40.3 | 68.6 | 47 | Sum | 37.2 |
Discussion
It is thought that the inactivation of mitochondrial
energy metabolism does not occur in cancer cells with mutations in
mitochondrial genes; however, the mitochondrial bioenergetic and
biosynthetic state may be altered through a series of modulations
of signal transduction pathways between the nucleus and
mitochondria (25,26). Although certain studies do not
support the idea of D-loop alterations of the mtDNA genome and
their carcinogenic role in CRC (27,28),
increased mtDNA mutations, deletions and even mitochondrial
dysfunction have been identified in GIC (29–33),
and even in certain precancerous lesions, including ulcerative
colitis lesions and adenomatous polyps (34). Mitochondrial dysfunction is
associated with tumor development and progression (35). Various mtDNA mutations have been
observed to modify tumor progression depending on the level of
respiratory complex I (36), and
defective mitochondrial respiration may be restored and
tumor-forming ability regained via mitochondrial acquisition
(37). In GC, the mtDNA repair
system does not appear to be disrupted (38). The present study supports the
occurrence of mtDNA D-loop mutations, but does not provide evidence
of mtDNA deletions in Chinese patients with GIC; more severe mtDNA
D-loop mutations may be identified in the advanced stages of
GIC.
A previous study focused on the location of the
tumorigenic mtDNA D-loop mutations, and identified
carcinogenesis-specific nucleoside sites and poly-C variations
(39). In addition, one study
indicated that the np 16189 T-C transition of the mtDNA D-loop may
contribute to polyC instability in GC (19). Furthermore, another previous study
reported that the minor haplotype of nucleotide 16290T and the
frequent haplotype of nucleotide 16298T in the hypervariable
segment 1 region were associated with a high survival rate of CRC,
and the nucleotide site of 16290 was an independent predictor of
CRC (40). However, a controversial
opinion is that site-specific nucleotide mutations may result from
mtDNA heterogeneity, and may not contribute to carcinogenesis
and/or tumor progression (41).
Thus, single nucleotide polymorphisms in the D-loop of mtDNA have
been reported to be associated with an increased risk of GC and CC,
including the frequent alleles of 73G/A, 146T/C, 195T/C, 324C/G,
16261C/T and 16304T/C; additionally, the majority of mtDNA
mutations are transitions (42–44).
In the present study, the T-C transition was the most common and
clustered in specific areas in GIC; poly-C variations were evident
in GIC.
It has been reported that mtMSI is a frequent
occurrence in CRC (45), and mtDNA
D-loop mutations and mtMSI appear to be associated with reactive
oxygen species, apoptosis and proliferation in GC (46). To the best of our knowledge, no
association has been identified between mtDNA mutations and mtMSI
status, and no mtMSI-positive GC cases have exhibited large
deletions in mtDNA (44).
Furthermore, mtMSI appears to be particularly frequent at the D310
locus; however, the high prevalence of mtMSI was not associated
with the prognosis of patients with CRC (23,47).
Notably, a previous study reported that stromal mtMSI may have
possibly served an independent role in the pathogenesis of CC
(48). In the present study, in
addition to the poly-C stretch, a CA repeat sequence that was
involved in GC-associated mtMSI may have been identified in GC.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81571178,
81371399 and 81470098), the National Key R&D Program of China
(grant no. 2017YFC1201100), the Project of Construction of
Innovative Teams and Teacher Career Development for Universities
and Colleges Under Beijing Municipality (grant no. IDHT20150502),
and the Project of Shandong Medical and Health Technology
Development Plan (grant no. 2017WS835).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BW collected the clinical data, analyzed the
sequencing results and was a major contributor in writing the
manuscript. LQ and JZ performed RT-qPCR. YW and DC conducted
immunohistochemistry staining. HG and YZ assisted with the
experiments and with writing the article. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committees of Shandong Cancer Hospital (Shandong, China). Written
informed consent was obtained from all study participants.
Patient consent for publication
All patients agreed to the publication of their
results and signed informed consents.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Herszenyi L and Tulassay Z: Epidemiology
of gastrointestinal and liver tumors. Eur Rev Med Pharmacol Sci.
14:249–258. 2010.PubMed/NCBI
|
|
2
|
Zhu X and Li J: Gastric carcinoma in
china: Current status and future perspectives (Review). Oncol Lett.
1:407–412. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu S, Zheng R, Zhang M, Zhang S, Sun X
and Chen W: Incidence and mortality of colorectal cancer in China,
2011. Chin J Cancer Res. 27:22–28. 2015.PubMed/NCBI
|
|
4
|
Chatterjee A, Dasgupta S and Sidransky D:
Mitochondrial subversion in cancer. Cancer Prev Res. 4:638–654.
2011. View Article : Google Scholar
|
|
5
|
Damas J, Samuels DC, Carneiro J, Amorim A
and Pereira F: Mitochondrial DNA rearrangements in health and
disease-a comprehensive study. Hum Mutat. 35:1–14. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang Y, Yang X, Zhai F, et al: Dietary
guidelines for chinese (2016). J Acad Nutr Dietetics. 116:A37.
2016. View Article : Google Scholar
|
|
7
|
Zhang Y, Wang M, Li H, Zhang H, Shi Y, Wei
F, Liu D, Liu K and Chen D: Accumulation of nuclear and
mitochondrial DNA damage in the frontal cortex cells of patients
with HIV-associated neurocognitive disorders. Brain Res. 1458:1–11.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
He L, Chinnery PF, Durham SE, Blakely EL,
Wardell TM, Borthwick GM, Taylor RW and Turnbull DM: Detection and
quantification of mitochondrial DNA deletions in individual cells
by real-time PCR. Nucleic Acids Res. 30:e682002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Krishnan KJ, Bender A, Taylor RW and
Turnbull DM: A multiplex real-time PCR method to detect and
quantify mitochondrial DNA deletions in individual cells. Anal
Biochem. 370:127–129. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Perier C, Bender A, Garcia-Arumi E, Melià
MJ, Bové J, Laub C, Klopstock T, Elstner M, Mounsey RB, Teismann P,
et al: Accumulation of mitochondrial DNA deletions within
dopaminergic neurons triggers neuroprotective mechanisms. Brain.
136:2369–2378. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Y, Song F, Gao Z, Ding W, Qiao L,
Yang S, Chen X, Jin R and Chen D: Long-term exposure of mice to
nucleoside analogues disrupts mitochondrial DNA maintenance in
cortical neurons. PLoS One. 9:e856372014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shi Y, Wang J, Wang Y, Wang A, Guo H, Wei
F, Mehta SR, Espitia S, Smith DM, Liu L, et al: A novel mutant
10ala/arg together with mutant 144Ser/arg of hepatitis B virus X
protein involved in hepatitis B virus-related hepatocarcinogenesis
in HepG2 cell lines. Cancer Lett. 371:285–291. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu F, Yu DM, Huang SY, Yu JL, Zhang DH,
Gong QM and Zhang XX: Clinical implications of evolutionary
patterns of homologous, full-length hepatitis B virus quasispecies
in different hosts after perinatal infection. J Clin Microbiol.
52:1556–1565. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ouyang Y, Liu L, Zhang Y, Yuan L, Liu Z,
Yang S, Wei F, Qiao L and Chen D: Discordant patterns of
tissue-specific genetic characteristics in the HIV-1 env gene from
HIV-associated neurocognitive disorder (HAND) and non-HAND
patients. J Neurovirol. 20:332–340. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oberley TD: Oxidative damage and cancer.
Am J Pathol. 160:403–408. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Boiteux S and Radicella JP: Base excision
repair of 8-hydroxyguanine protects DNA from endogenous oxidative
stress. Biochimie. 81:59–67. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liang Q, Zeng J, Wu J, Qiao L, Chen Q,
Chen D and Zhang Y: Nucleoside reverse transcriptase inhibitors
induced hepatocellular mitochondrial DNA lesions and compensatory
enhancement of mitochondrial function and DNA repair. Int J
Antimicrob Agents. 51:385–392. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Han CB, Li F, Zhao YJ, Ma JM, Wu DY, Zhang
YK and Xin Y: Variations of mitochondrial D-loop region plus
downstream gene 1 2S rRNA-tRNAphe and gastric
carcinomas. World J Gastroenterol. 9:1925–1929. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee HC, Li SH, Lin JC, Wu CC, Yeh DC and
Wei YH: Somatic mutations in the D-loop and decrease in the copy
number of mitochondrial DNA in human hepatocellular carcinoma.
Mutat Res. 547:71–78. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Levy M, Visokai V, Lipska L and Topolcan
O: Tumor markers in staging and prognosis of colorectal carcinoma.
Neoplasma. 55:138–142. 2008.PubMed/NCBI
|
|
22
|
Chatterjee A, Mambo E and Sidransky D:
Mitochondrial DNA mutations in human cancer. Oncogene.
25:4663–4674. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Venderbosch S, van Vliet S, Craenmehr MH,
Simmer F, de Haan AF, Punt CJ, Koopman M and Nagtegaal ID:
Mitochondrial microsatellite instability in patients with
metastatic colorectal cancer. Virchows Archiv. 466:495–502. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jeong CW, Lee JH, Sohn SS, Ryu SW and Kim
DK: Mitochondrial microsatellite instability in gastric cancer and
gastric epithelial dysplasia as a precancerous lesion. Cancer
Epidemiol. 34:323–327. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wallace DC: Mitochondria and cancer. Nat
Rev Cancer. 12:685–698. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Boland ML, Chourasia AH and Macleod KF:
Mitochondrial dysfunction in cancer. Front Oncol. 3:2922013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Webb E, Broderick P, Chandler I, Lubbe S,
Penegar S, Tomlinson IP and Houlston RS: Comprehensive analysis of
common mitochondrial DNA variants and colorectal cancer risk. Br J
Cancer. 99:2088–2093. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Heerdt BG, Chen J, Stewart LR and
Augenlicht LH: Polymorphisms, but lack of mutations or instability,
in the promotor region of the mitochondrial genome in human colonic
tumors. Cancer Res. 54:3912–3915. 1994.PubMed/NCBI
|
|
29
|
Hibi K, Nakayama H, Yamazaki T, Takase T,
Taguchi M, Kasai Y, Ito K, Akiyama S and Nakao A: Detection of
mitochondrial DNA alterations in primary tumors and corresponding
serum of colorectal cancer patients. Int J Cancer. 94:429–431.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu CW, Yin PH, Hung WY, Li AF, Li SH, Chi
CW, Wei YH and Lee HC: Mitochondrial DNA mutations and
mitochondrial DNA depletion in gastric cancer. Genes Chromosomes
Cancer. 44:19–28. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hung WY, Wu CW, Yin PH, Chang CJ, Li AF,
Chi CW, Wei YH and Lee HC: Somatic mutations in mitochondrial
genome and their potential roles in the progression of human
gastric cancer. Biochim Biophys Acta. 1800:264–270. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Burgart LJ, Zheng J, Shu Q, Strickler JG
and Shibata D: Somatic mitochondrial mutation in gastric cancer. Am
J Pathol. 147:1105–1111. 1995.PubMed/NCBI
|
|
33
|
Lievre A, Chapusot C, Bouvier AM,
Zinzindohoué F, Piard F, Roignot P, Arnould L, Beaune P, Faivre J
and Laurent-Puig P: Clinical value of mitochondrial mutations in
colorectal cancer. J Clin Oncol. 23:3517–3525. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kassem AM, El-Guendy N, Tantawy M,
Abdelhady H, El-Ghor A and Wahab Abdel AH: Mutational hotspots in
the mitochondrial D-loop region of cancerous and precancerous
colorectal lesions in egyptian patients. DNA Cell Biol. 30:899–906.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ferreira A, Serafim TL, Sardao VA and
Cunha-Oliveira T: Role of mtDNA-related mitoepigenetic phenomena in
cancer. Eur J Clin Invest. 1 Suppl 45:S44–S49. 2015. View Article : Google Scholar
|
|
36
|
Iommarini L, Kurelac I, Capristo M,
Calvaruso MA, Giorgio V, Bergamini C, Ghelli A, Nanni P, De
Giovanni C, Carelli V, et al: Different mtDNA mutations modify
tumor progression in dependence of the degree of respiratory
complex I impairment. Hum Mol Genet. 23:1453–1466. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Berridge MV, Dong L and Neuzil J:
Mitochondrial DNA in tumor initiation, progression, and metastasis:
Role of horizontal mtDNA transfer. Cancer Res. 75:3203–3208. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tamura G, Nishizuka S, Maesawa C, Suzuki
Y, Iwaya T, Sakata K, Endoh Y and Motoyama T: Mutations in
mitochondrial control region DNA in gastric tumours of Japanese
patients. Eur J Cancer. 35:316–319. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hung WY, Lin JC, Lee LM, Wu CW, Tseng LM,
Yin PH, Chi CW and Lee HC: Tandem duplication/triplication
correlated with poly-cytosine stretch variation in human
mitochondrial DNA D-loop region. Mutagenesis. 23:137–142. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang C, Zhao S, Du Y and Guo Z: Single
nucleotide polymorphisms in the D-loop region of mitochondrial DNA
is associated with colorectal cancer outcome. Mitochondrial DNA A
DNA Mapp Seq Anal. 27:4361–4363. 2016.PubMed/NCBI
|
|
41
|
Yoneyama H, Hara T, Kato Y, Yamori T,
Matsuura ET and Koike K: Nucleotide sequence variation is frequent
in the mitochondrial DNA displacement loop region of individual
human tumor cells. Mol Cancer Res. 3:14–20. 2005.PubMed/NCBI
|
|
42
|
Guo Z, Zhao S, Fan H, Du Y, Zhao Y and
Wang G: Identification of sequence polymorphisms in the D-Loop
region of mitochondrial DNA as a risk factor for colon cancer.
Mitochondrial DNA A DNA Mapp Seq Anal. 27:4244–4245.
2016.PubMed/NCBI
|
|
43
|
Akouchekian M, Houshmand M, Hemati S,
Ansaripour M and Shafa M: High rate of mutation in mitochondrial
DNA displacement loop region in human colorectal cancer. Dis Colon
Rectum. 52:526–530. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Maximo V, Soares P, Seruca R, Rocha AS,
Castro P and Sobrinho-Simoes M: Microsatellite instability,
mitochondrial DNA large deletions, and mitochondrial DNA mutations
in gastric carcinoma. Genes Chromosomes Cancer. 32:136–143. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Habano W, Nakamura S and Sugai T:
Microsatellite instability in the mitochondrial DNA of colorectal
carcinomas: Evidence for mismatch repair systems in mitochondrial
genome. Oncogene. 17:1931–1937. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhao YB, Yang HY, Zhang XW and Chen GY:
Mutation in D-loop region of mitochondrial DNA in gastric cancer
and its significance. World J Gastroenterol. 11:3304–3306. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chang SC, Lin PC, Yang SH, Wang HS, Liang
WY and Lin JK: Mitochondrial D-loop mutation is a common event in
colorectal cancers with p53 mutations. Int J Colorectal Dis.
24:623–628. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kim HS, Lim HS, Lee SH, Lee JW, Nam SW,
Park WS, Lee YS, Lee JY and Yoo NJ: Mitochondrial microsatellite
instability of colorectal cancer stroma. Int J Cancer.
119:2607–2611. 2006. View Article : Google Scholar : PubMed/NCBI
|