Introduction
Gastric cancer (GC) is one of the most common
malignancies and a leading cause of cancer-related deaths worldwide
(1,2). Despite the great improvements achieved
in the diagnosis and treatment of gastric cancer, the long-term
prognosis of these patients remains poor due to the high rate of
invasion and metastasis (3).
However, the underlying mechanisms remain largely unknown (4,5).
Therefore, there is an urgent need to unveil the molecular
mechanisms underlying metastasis and identify novel therapeutic
markers and develop new efficient treatment strategies.
Tripartite motif-containing 14 (TRIM14), which is
located on chromosome 9q22, has been recognized as a cancer-related
protein in previous studies (6–8).
TRIM14 was found to be significantly upregulated in hepatocellular
carcinoma and its high expression was demonstrated to be associated
with poor prognosis of patients (9). Overexpression of TRIM14 was found to
promote tongue squamous cell carcinoma aggressiveness by activating
the NF-κB signaling pathway (10).
In breast cancer, TRIM14 promoted cell proliferation and inhibited
apoptosis via signal transducer and activator of transcription 3
(STAT3) signaling (11). These
results suggest that TRIM14 is an oncogene in tumors. However, in
non-small cell lung carcinoma (NSCLC), TRIM14 knockdown
significantly enhanced tumor growth in NSCLC xenograft mouse
models, while exogenous TRIM14 attenuated tumorigenesis. Moreover,
the TRIM14 mRNA levels are significantly associated with poorer
prognosis in early stage NSCLC patients (12). These results revealed that TRIM14 is
a tumor suppressor. Therefore, the role of TRIM14 in tumors is
tissue-specific. However, the expression levels and biological
functions of TRIM14 in the progression of GC remain largely
unknown.
Epithelial-to-mesenchymal transition (EMT) refers to
the conversion of epithelial cells into cells with mesenchymal
properties and appearance. EMT has been found to be a critical
process during development and carcinogenesis (13). EMT has been validated to promote
cancer cell dissemination and metastasis and maintain cancer stem
cell properties in a variety of cancers (14,15).
Recently, EMT was found to confer high migration and invasion
capacities, chemo-resistance, and strengthen the propensity for
cancer metastasis (16). However,
the regulatory mechanism of EMT in GC progression has not been
entirely understood.
In the present study, we demonstrated that TRIM14
expression was significantly increased in human clinical GC tissues
and cell lines. Its overexpression was associated with poor
clinical features and poor survival. Gain- and loss-of-function
experiments confirmed that TRIM14 regulated the migration and
invasion of GC cells by alteration of the expression of
EMT-associated factors. Moreover, we identified that the the
protein kinase B (AKT) signaling pathway mediated the biological
effects of TRIM14 in downstream and the upstream of TRIM expression
was regulated by miR-195-5p in GC cells. Taken together, our
findings suggest that TRIM14 plays a critical oncogenic role in GC
progression and highlight its potential as a therapeutic target for
GC treatment.
Materials and methods
Clinical samples and cell lines
A total of 117 GC tissues and matched adjacent
non-tumor tissues were collected at at The First Affiliated
Hospital of Xi'an Jiaotong University (Xi'an, China) from January
2007 to December 2009. Patients received no other curative strategy
with chemotherapy or radiotherapy before surgery. The Ethical
Committee of The First Affiliated Hospital of Xi'an Jiaotong
University approved the study protocol and written informed consent
was provided by all participating patients.
The human GC cell lines (MKN45, MGC803, BGC823,
SGC7901 and AGS) and normal gastric epithelial cell line GES-1
which were obtained from the Chinese Academy of Sciences (Shanghai,
China) were maintained in Invitrogen™ RPMI-1640 medium (Thermo
Fisher Scientific, Inc., Waltham, MA, USA) added with 10% HyClone™
fetal bovine serum (FBS; GE Healthcare Life Sciences, Logan, UT,
USA) and 1% penicillin/streptomycin (HyClone; GE Healthcare Life
Sciences). All cell lines were incubated in a humidified atmosphere
with 5% CO2 at 37°C.
Cell transfection
Lipofectamine 2000 reagent (Invitrogen Life
Technologies; Thermo Fisher Scientific, Inc.) was applied to
conduct cells transfection on the basis of the product
specification at a final concentration of 100 pmol. Mimic control
(miR-control; CmiR0001-MR04), miR-195-5p mimics (HmiR0270),
inhibitor control (anti-miR-NC; CmiR-AN0001-AM02) and miR-195-5p
inhibitors (HmiR-AN0282) were purchased from Genecopoeia Inc.
(Guangzhou, China). TRIM14 clones and TRIM14 shRNAs were obtained
from Shanghai Sangon Biotech Co., Ltd. (Shanghai, China). Cells
were transfected with the shRNAs and clones above using
Lipofectamine 2000 according to the manufacturer's instructions.
After 48 h of transfection, cells were used for the following
experiments.
RNA extraction and quantitative
real-time PCR (qRT-PCR)
The total RNA from GC cells and tissues was
extracted using Invitrogen™ TRIzol reagent (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. cDNA
was synthesized by TaqMan miRNA reverse transcription (Applied
Biosystems, Foster City, CA, USA) and a PrimeScript Reverse
Transcriptase kit (Takara Biotechnology Co., Ltd., Dalian, China).
The relative expression of miR-195-5p and TRIM14 mRNA were
quantified using miRNA-specific TaqMan miRNA Assay Kit (Applied
Biosystems) and the SYBR Premix Ex Taq™ Kit (Takara Bio Inc.,
Shiga, Japan) in the Applied Biosystems 7500 Sequence Detection
system. The cycling conditions were as follows: First 95°C for 10
min, then 40 cycles at 95°C for 15 sec and 60°C for 60 sec. Primers
for miR-195-5p and TRIM14 were obtained from Genecopoeia. The gene
expression levels were calculated using the delta-delta Cq method
with U6 or GAPDH as an internal control (17).
Immunofluorescence (IF)
We used 4% paraformaldehyde to fix transfected cells
and used 0.3% Triton X-100 to permeabilize. The primary antibody
E-cadherin, N-cadherin and vimentin (dilution 1:300; Cell Signaling
Technology, Danvers, MA, USA) was used. Then the Alexa
Fluor-conjugated secondary antibody was performed in the next
experiment. Lastly, the images were obtained by microscopy (Carl
Zeiss, Oberkochen, Germany).
Western blotting
The whole proteins were lysed in RIPA buffer
(Bio-Rad Laboratories, Hercules, CA, USA) supplemented with
protease and phosphatase inhibitors (Roche) and the concentrations
were quantified with BCA Protein Assay kit (Tiangen Biotech Co.,
Ltd., Beijing, China), and then 30 µg protein was separated by 10%
SDS-PAGE and transferred to PVDF membranes. Subsequently, the PVDF
membranes were probed with primary antibodies against TRIM14
(dilution 1:1,000; cat. no. ab85374; Abcam, Cambridge, MA, USA),
p-AKT (dilution 1:1,000; cat. no. 4060; Ser473; Cell Signaling
Technology, Beverly, MA, USA), AKT (dilution 1:1,000; cat. no.
4691; Cell Signaling Technology), E-cadherin (dilution 1:1,000;
cat. no. 14472; Cell Signaling Technology), vimentin (dilution
1:1,000; cat. no. 5741; Cell Signaling Technology), N-cadherin
(dilution 1:1,000; cat. no. 14215; Cell Signaling Technology) and
GAPDH (dilution 1:3,000; cat. no. sc-47724; Santa Cruz
Biotechnology, Santa Cruz, CA, USA), and then probed with
horseradish peroxidase (HRP)-conjugated secondary antibodies
(dilution 1:5,000; cat. nos. 7074 and 7076; Cell Signaling
Technology). The western blot was detected with enhanced
chemiluminescence regent (Thermo Scientific Inc., Waltham, MA, USA)
and analyzed using the Quantity One 1-D analysis software (Bio-Rad
Laboratories, Inc.).
Luciferase reporter assay
The sequence of TRIM14 3′-untranslated region (UTR)
containing the putative miR-195-5p binding region was amplified
from human genomic DNA. Then the sequence was cloned into pGL3
luciferase reporter vector (Promega, Madison, WI, USA). The
potential miR-195-5p binding sites were mutated by the Quick-change
site-directed mutagenesis kit (Agilent Technologies, Santa Clara,
CA, USA). The wild-type (wt) TRIM14 3′-UTR vector or mutant (mt)
TRIM14 3′-UTR vector and miR-195-5p mimics or miR-195-5p inhibitors
were co-transfected into MKN45 cells using Invitrogen™
Lipofectamine 2000 (Thermo Fisher Scientific, Inc.). The luciferase
activity was measured using Dual-Luciferase Reporter Assay system
(Promega) under a luminometer (Berthold Detection System,
Pforzheim, Germany) and luciferase activity was normalized to
Renilla activity.
Cell migration and invasion
assays
The indicated GC cells (1×104) were
seeded into the upper chamber coated with or without Matrigel (BD
Bioscience, San Jose, CA, USA) and RPMI-1640 without FBS was added.
Then, the chamber was placed into the cell culture plate containing
RPMI-1640 supplemented with 10% FBS and incubated at 37°C for 24 h.
Subsequently, the cells inside the upper chamber were carefully
removed with cotton swabs. Migrated and invaded cells were fixed
with 1% paraformaldehyde for 10 min and subsequently stained with
hematoxylin for 5 min. The migratory and invasive cells were
finally counted in 10 independent vision and counted under a Leica
TCS SP5 confocal microscope (Leica Microsystems, Wetzlar,
Germany).
In vivo metastasis assay
Four- to six-week-old male BALB/c nude mice (Centre
of Laboratory Animals, The Medical College of Xi'an Jiaotong
University, Xi'an, China) were randomized into two groups (n=5),
and either the LV-TRIM14 or TRIM14-shRNA transfected cells
(1×106) were injected into the tail veins for the
establishments of pulmonary metastatic model. The mice were
sacrificed 3 weeks post injection and examined microscopically by
H&E staining for the development of lung metastatic foci.
Animals were housed in cages under standard conditions. All in
vivo protocols were approved by the Institutional Animal Care
and Use Committee of Xi'an Jiaotong University.
Immunohistochemical (IHC)
staining
Paraformaldehyde-fixed paraffin GC tissue sections
were used for IHC staining. TRIM14 primary antibodies (dilution
1:300; cat. no. ab85374; Abcam, Cambridge, MA, USA) were diluted in
PBS to 1:100 and incubated at 4°C overnight. Sections were then
incubated with biotinylated secondary antibodies (dilution 1:1,000;
ZSGB-BIO, Beijing, China). Complexes were detected by
HRP-streptavidin conjugates (ZSGB-BIO) and visualized with DAB
(ZSGB-BIO).
Statistical analysis
All data are presented as mean ± standard deviation
(SD) and were analyzed using GraphPad Prism software version 5.0
(GraphPad Software, Inc., La Jolla, CA, USA). Statistical analysis
was calculated by a Chi-squared test, Student's t-test, ANOVA,
Pearson correlation analysis, Kaplan-Meier method and log-rank
test. P-value <0.05 was considered to indicate a statistically
significant result. Each experiment was repeated three times.
Results
TRIM14 is upregulated in GC tissues
and cell lines and is correlated with the survival of GC
patients
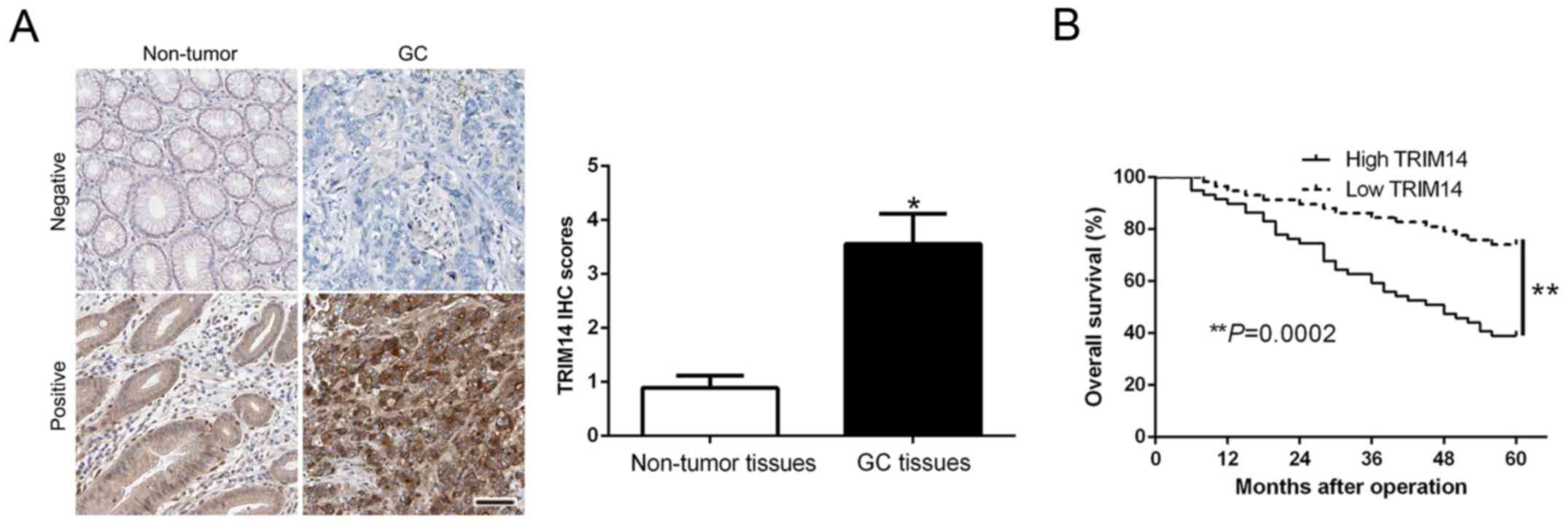
To determine the expression level of TRIM14 in GC,
we performed IHC staining to confirm TRIM14 expression and found
that the IHC scores of TRIM14 in GC tissues were obviously
increased compared to the scores noted in the normal tissues
(P<0.05, Fig. 1A). Kaplan-Meier
survival cure demonstrated that an increased TRIM14 in GC patients
was indicative of a shorter overall survival (OS) in GC patients
(P=0.0002, Fig. 1B). Subsequently,
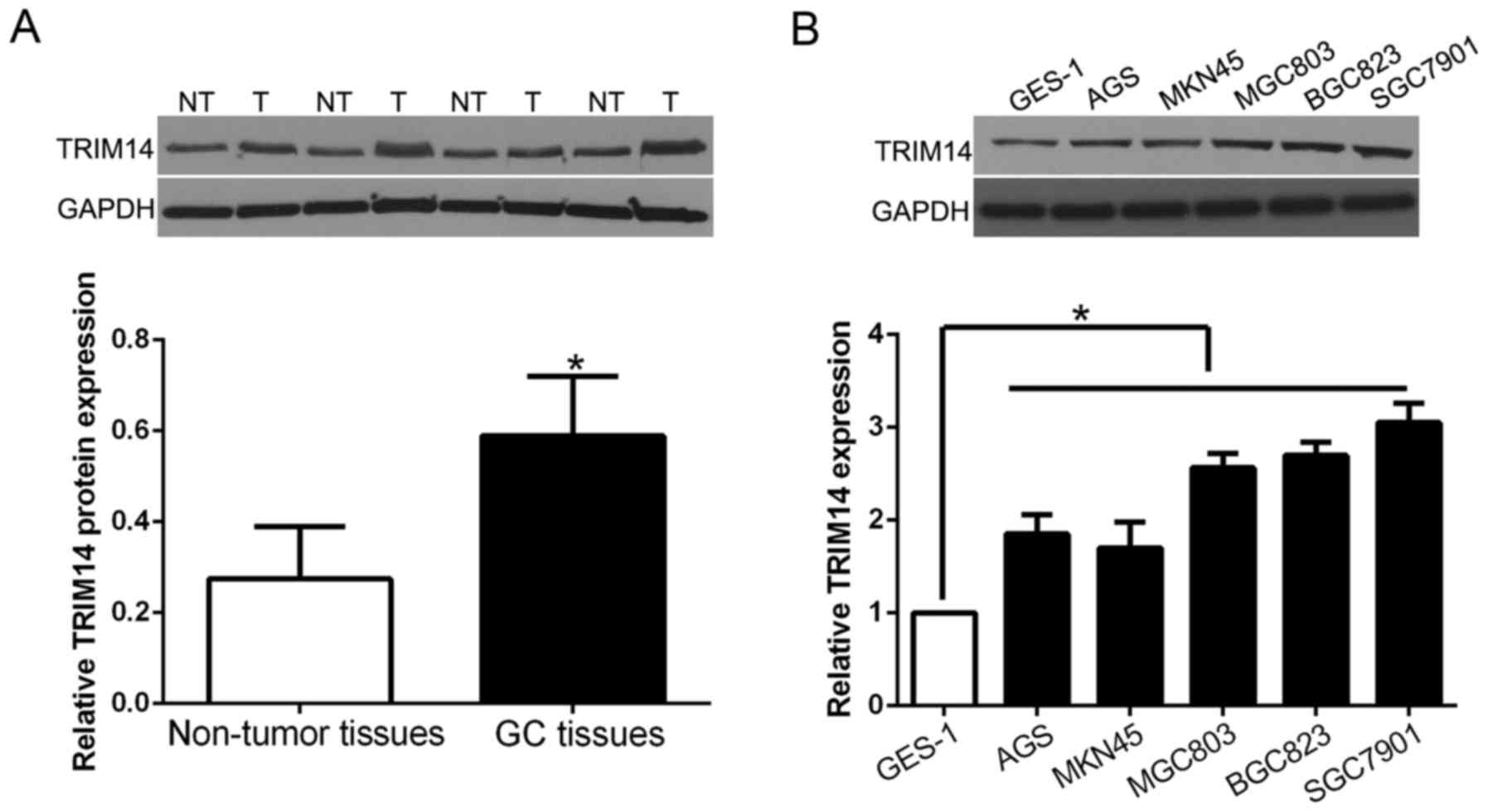
we randomly selected 40 GC tissues and paired adjacent normal
tissues to perform western blot analysis to validate these
findings. Our data showed that the expression of TRIM14 protein was
significantly higher in GC tissues than that in adjacent non-tumor
tissues (P<0.05; Fig. 2A).
Furthermore, we analyzed TRIM14 expression in GC cell lines and the
normal immortalized gastric epithelium cell line GES-1. The results
showed that TRIM14 was increased in GC cell lines when compared
with the level in GES-1 cells (P<0.05; Fig. 2B). These results suggest that TRIM14
is involved in GC progression.
Clinical significance of TRIM14 in GC
patients
To investigate the clinical role of TRIM14, we
analyzed the relevance between TRIM14 and the clinicopathological
features and prognosis of GC patients. We determine the mean value
of expression as the cut-off and high TRIM14 was significantly
associated with lymph node metastasis and advanced TNM stage
(P=0.007 and 0.02, respectively, Table
I). These results suggest that TRIM14 is involved in the
process of metastasis of GC.
 | Table I.Association between TRIM14 expression
and clinicopathological features of the GC patients (n=117). |
Table I.
Association between TRIM14 expression
and clinicopathological features of the GC patients (n=117).
|
|
| Expression level |
|
|---|
|
|
|
|
|
|---|
| Clinical
parameters | Cases (n) | TRIM14high
(n=59) | TRIM14low
(n=58) | P value |
|---|
| Age (years) |
|
|
| 0.629 |
|
<60 | 49 | 26 | 23 |
|
| ≥60 | 68 | 33 | 35 |
|
| Sex |
|
|
| 0.516 |
| Male | 76 | 40 | 36 |
|
|
Female | 41 | 19 | 22 |
|
| Tumor size (cm) |
|
|
| 0.227 |
| < | 94 | 50 | 44 |
|
| ≥5 | 23 | 9 | 14 |
|
| Histological
type |
|
|
| 0.961 |
|
Intestinal | 91 | 46 | 45 |
|
|
Diffuse | 26 | 13 | 13 |
|
| TNM stage |
|
|
| 0.020a |
| I+II | 33 | 11 | 22 |
|
| III+IV | 84 | 48 | 36 |
|
| Lymph node
metastasis |
|
|
| 0.007a |
| Present | 82 | 48 | 34 |
|
| Absent | 35 | 11 | 24 |
|
Knockdown of TRIM14 attenuates the
gastric cancer cell migration and invasion
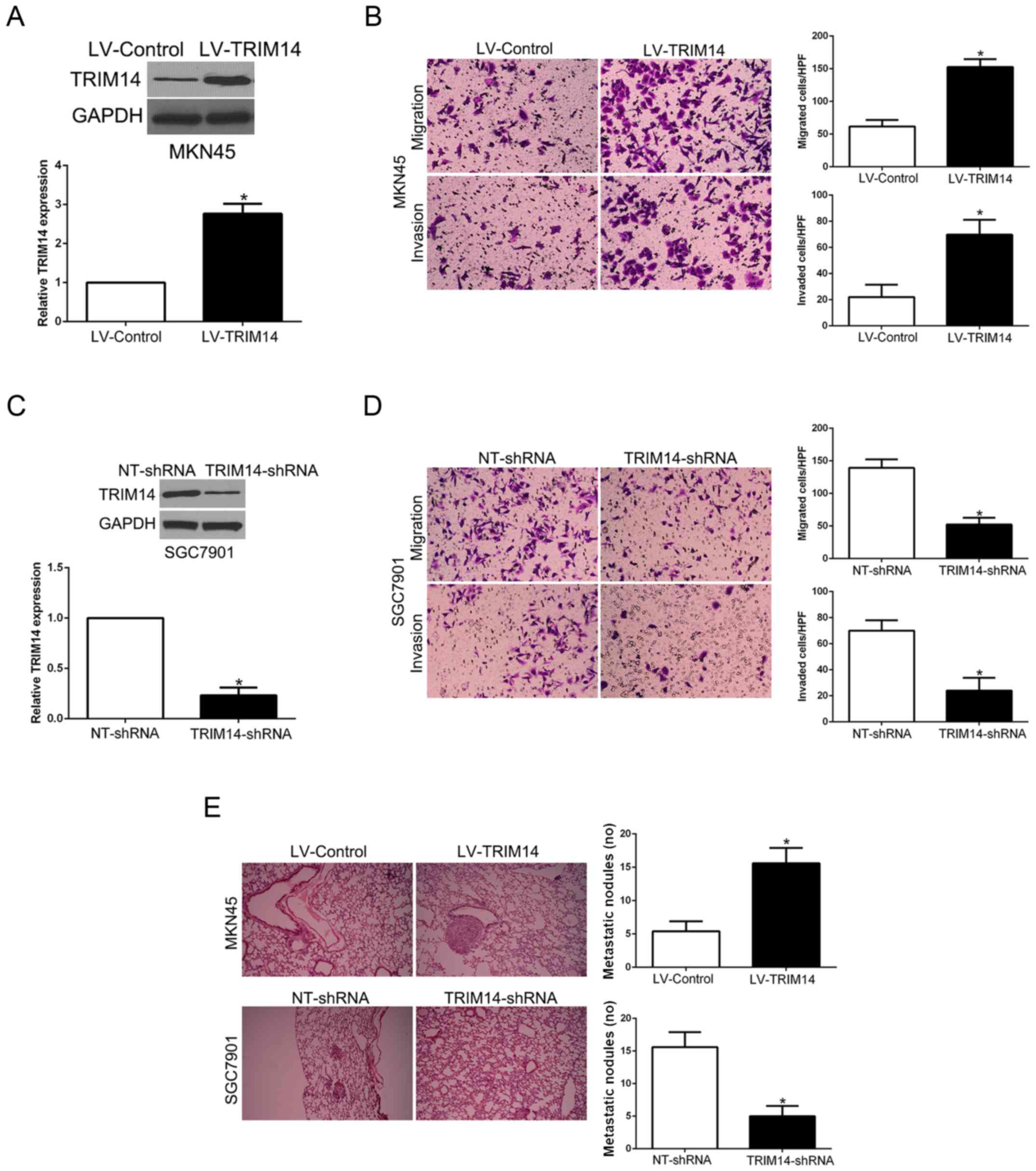
Since our clinical data revealed the correlation
between TRIM14 and metastatic features of GC, we performed Matrigel
uncoated and coated assays to determine the migration and invasion
capacity of GC. MKN45 cells with low TRIM14 level and SGC7901 cells
with high TRIM14 level were used for gain- and loss-of-function
experiments, respectively (P<0.05, Fig. 3A and C). Transwell assays showed
that TRIM14 overexpression significantly promoted the migration and
invasion of MKN45 cells (P<0.05, Fig. 3B), while TRIM14 knockdown
significantly inhibited migration and invasion of SGC7901 cells
(P<0.05, Fig. 3D). Furthermore,
to confirm the role of TRIM14 in vivo, we used tail vain
injection to construct a lung metastasis model. Our results showed
that TRIM14 overexpression significantly increased the number of
lung metastatic nodules derived from MKN45 cells whereas TRIM14
knockdown validly reduced the lung metastasis (P<0.05, Fig. 3E). Therefore, TRIM14 displays a
critical role in the migration and invasion of GC cells in
vitro and in vivo.
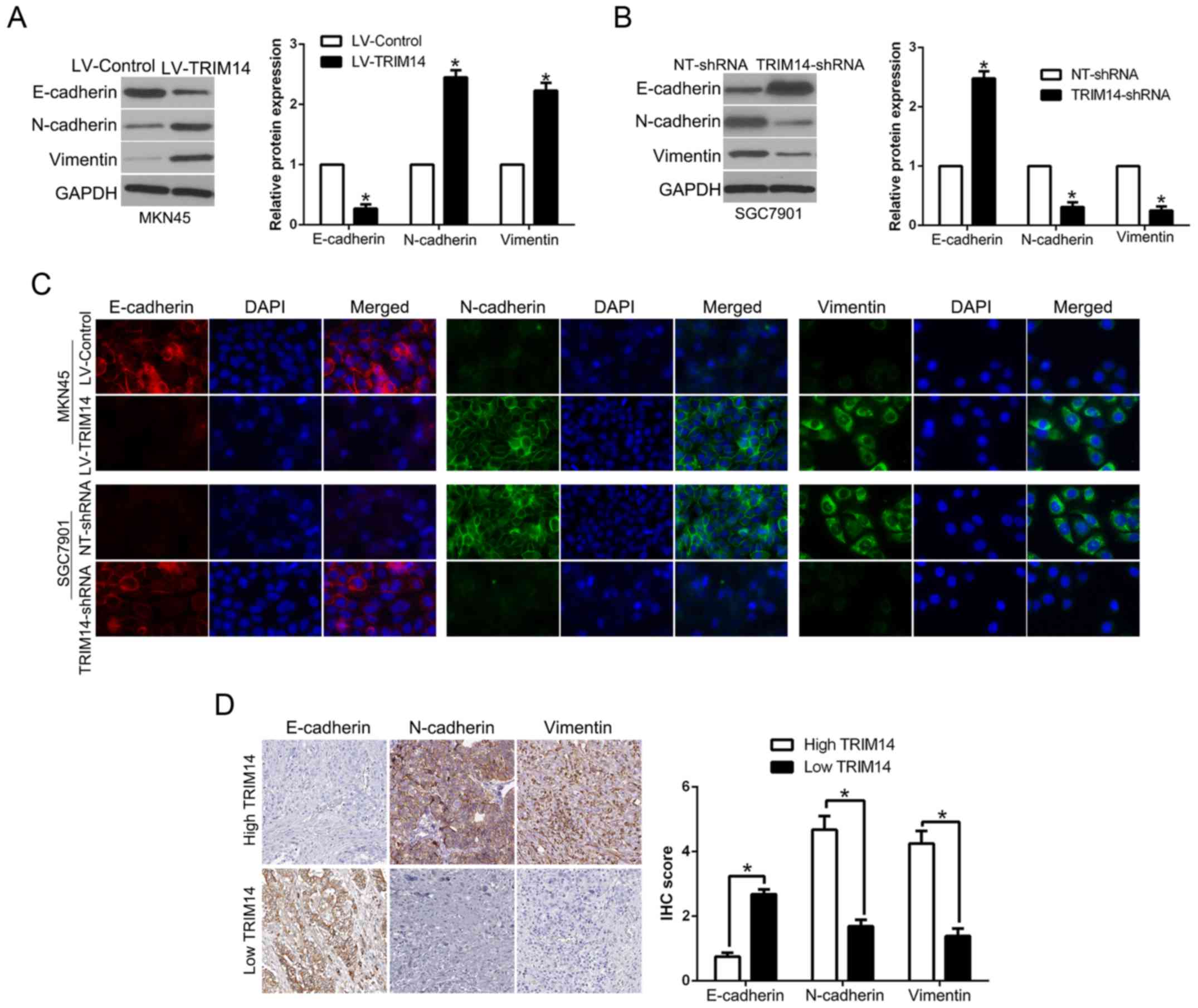
TRIM14 promotes EMT in GC
EMT plays a critical role in tumor invasion and
metastasis (18). To determine the
effect of TRIM14 on EMT, western blot analysis showed that TRIM14
overexpression significantly decreased the level of epithelial
marker E-cadherin, while it increased the levels of mesenchymal
markers N-cadherin and vimentin (P<0.05, Fig. 4A). In contrary, TRIM14 knockdown
showed the opposite effects (P<0.05, Fig. 4B). Moreover, immunofluorescence (IF)
confirmed the similar function of TRIM14 on the process of EMT
(Fig. 4C). In addition, we
demonstrated that E-cadherin was lower in the GC tissues with high
TRIM14 expression while vimentin was remarkably higher in GC
tissues with high TRIM14 than that in the low TRIM14 GC tissue
group (P<0.05, Fig. 4D). Taken
together, we verified that TRIM14 is an activator of the EMT
process in GC.
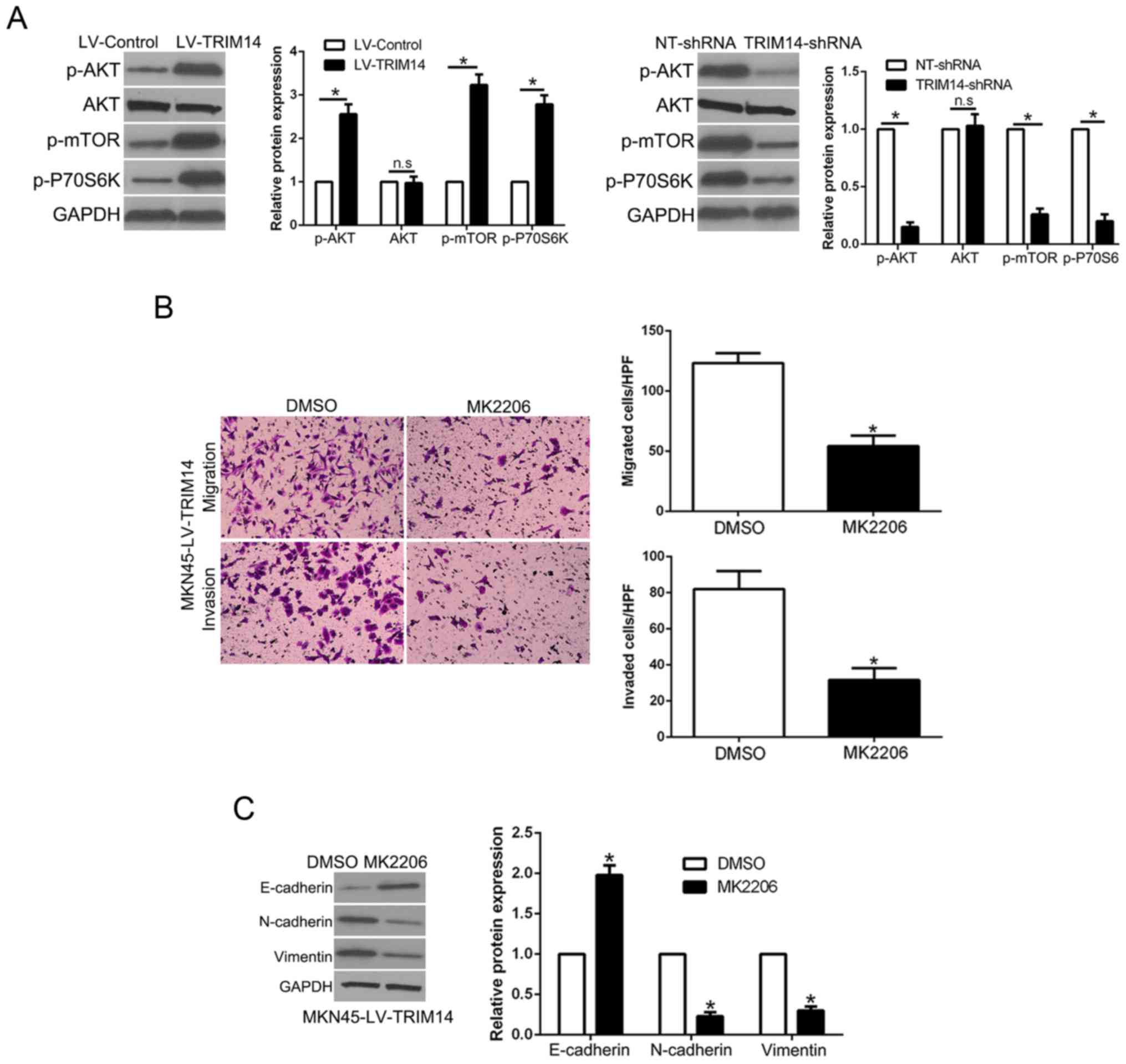
AKT phosphorylation mediates the
biological effects of TRIM14 in GC cells
Previous research confirmed that AKT signaling plays
an important role in GC metastasis (19). Moreover, among the TRIM family,
TRIM29 stimulates the AKT pathway in human cancers (20). Thus, we aimed to ascertain whether
TRIM14 regulates the AKT pathway in GC. Notably, TRIM14
overexpression promoted p-AKT, p-mTOR and p-P70S6K expression while
TRIM14 knockdown showed the opposite effects (P<0.05, Fig. 5A). To ascertain whether the AKT
pathway mediates the effects of TRIM14, we used AKT inhibitor
MK2206 to inhibit AKT phosphorylation in TRIM14-overexpressing GC
cells. The results showed that AKT inhibition reversed the
promotive effects of TRIM14 in regards to cell migration and
invasion (P<0.05, Fig. 5B).
Similarly, AKT inhibition abolished the effects of TRIM14 on EMT
progression (P<0.05, Fig. 5C).
Collectively, these data suggest that the AKT pathway at least
partially mediated the effects of TRIM14 on migration and invasion
of GC.
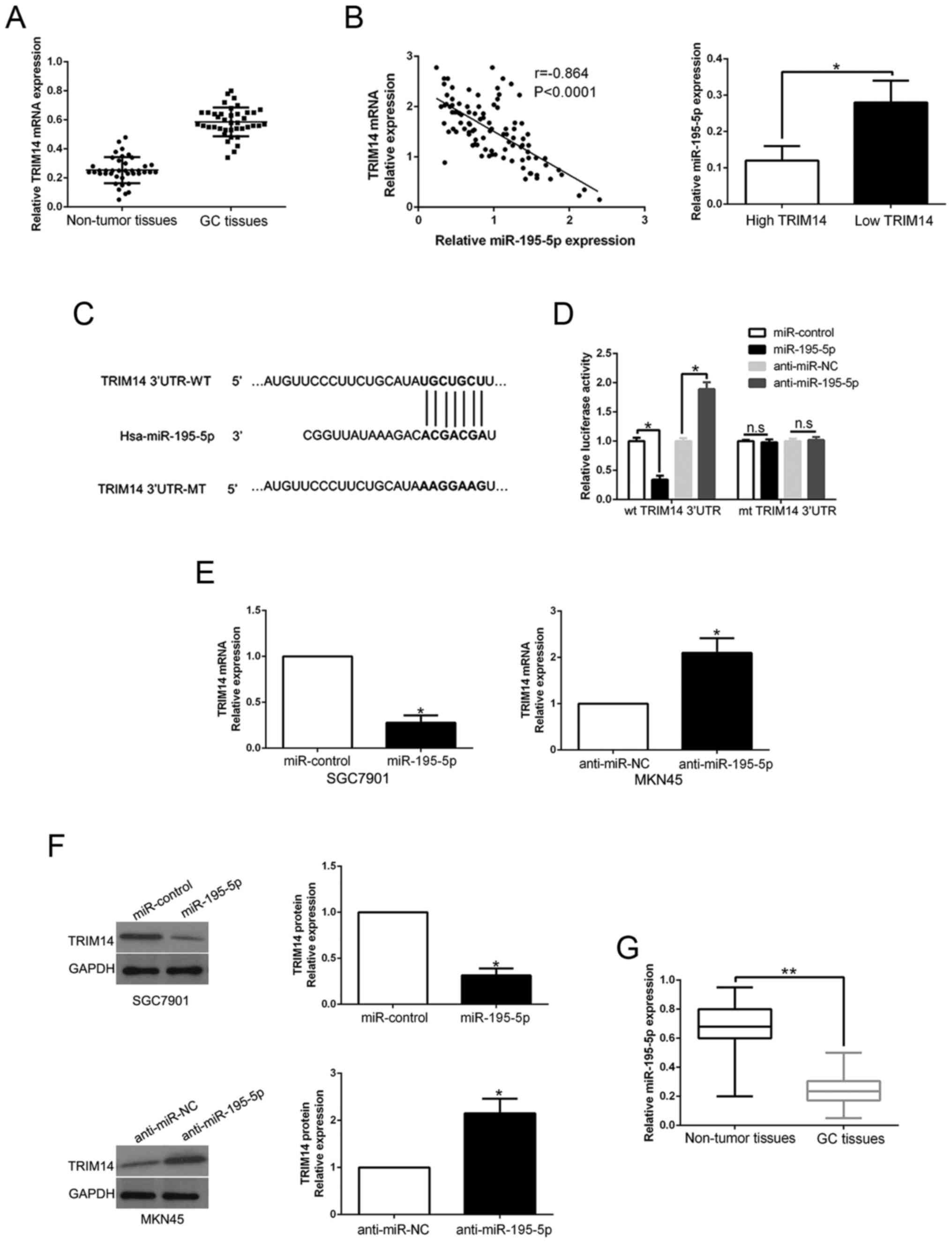
TRIM14 expression is regulated by
miR-195-5p
Previous research has confirmed that aberrant miRNA
expression contributes to GC progression and regulates protein
expression by binding to its 3′UTR (21). To elucidate the mechanisms involved
in the upregulation of TRIM14, we searched the TargetScan to
predict whether miR-195-5p could bind to the 3′UTR of TRIM14
(Fig. 6C). We performed qRT-PCR to
confirm that TRIM14 mRNA expression was higher in GC tissues than
those in adjacent non-tumor tissues (P<0.05, Fig. 6A). Notably, an inverse correlation
between TRIM14 mRNA and miR-195-5p was confirmed in GC tissues
(r=−0.864, P<0.05, Fig. 6B, left
graph). Furthermore, miR-195-5p expression in the high TRIM14 group
tissues was prominently lower than that in the GC tissues with low
TRIM14 expression (P<0.05, Fig.
6B, right histogram). Luciferase reporter assays confirmed that
miR-195-5p overexpression prominently reduced while miR-195-5p
knockdown increased the luciferase activity of cells with wt 3′UTR
of TRIM14 (P<0.05, Fig. 6D).
However, the activity had no change in mt 3′UTR of TRIM14.
Moreover, miR-195-5p overexpression significantly inhibited while
miR-195-5p knockdown promoted TRIM14 mRNA and protein in GC cells
(P<0.05, respectively, Fig. 6E and
F). In GC tissues, we demonstrated that miR-195-5p was
downregulated compared to that noted in the adjacent non-tumor
tissues (P<0.05, Fig. 6G). Taken
together, we first disclose that miR-195-5p regulates TRIM14
expression in GC tissues.
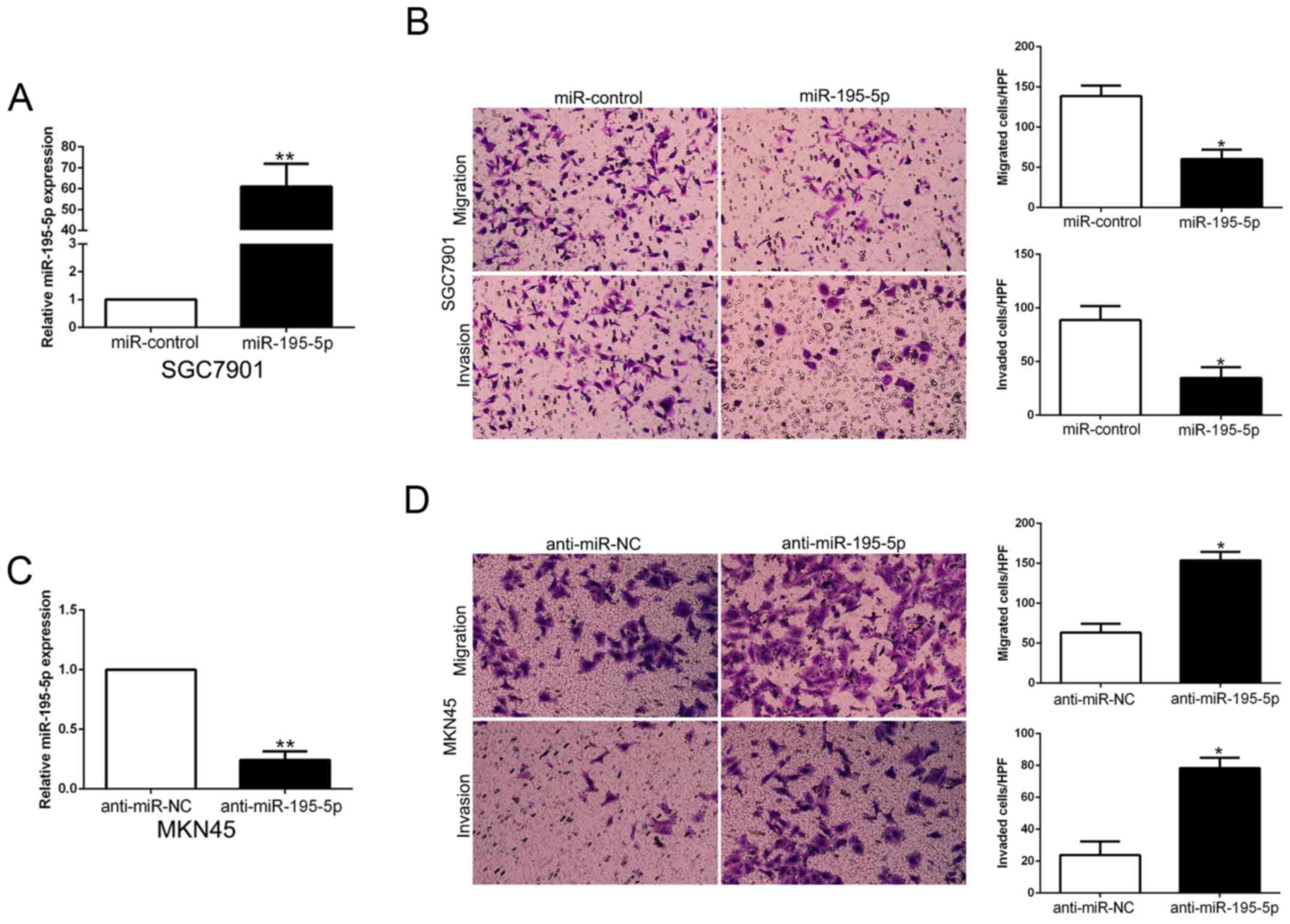
miR-195-5p inhibits the migration and
invasion of GC cells
To confirm the biological function of miR-195-5p in
GC tissues, we used overexpression or knockdown vectors to perform
gain- and loss-of-function experiment. The Transwell assays showed
that miR-195-5p overexpression significantly inhibited the
migration and invasion of SGC7901 cells (P<0.05, Fig. 7A and B). Furthermore, miR-195-5p
knockdown significantly facilitated the migration and invasion of
MKN45 cells (P<0.05, Fig. 7C and
D). Thus, miR-195-5p functions as a tumor suppressor in the
migration and invasion of GC cells.
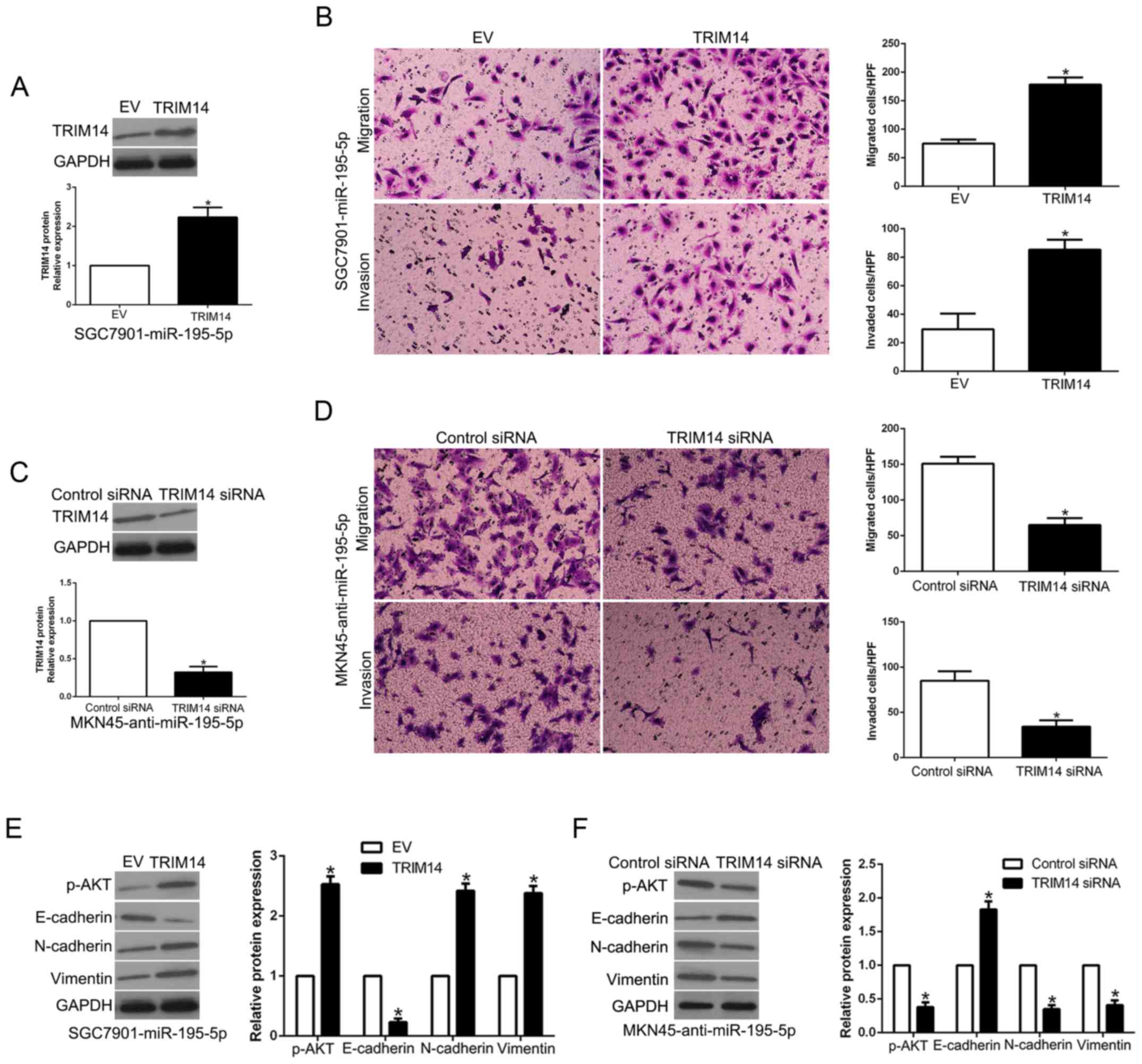
TRIM14 mediates the effects of
miR-195-5p in GC cells
To clarify whether TRIM14 is a biological mediator
of miR-195-5p, we perform a rescue experiment in which TRIM14 was
restored in miR-195-5p-overexpressing SGC7901 cells (P<0.05,
Fig. 8A). TRIM14 restoration
reversed the inhibitory effect of miR-195-5p on the migration and
invasion of SGC7901 cells (P<0.05, Fig. 8B). Moreover, TRIM14 restoration
increased the levels of p-AKT, N-cadherin and vimentin and reduced
E-cadherin expression in the miR-195-5p-overexpressing SGC7901
cells (P<0.05, Fig. 8E).
However, TRIM14 knockdown significantly inhibited the migration,
invasion and EMT progression of miR-195-5p-suppressed MKN45 cells
(P<0.05, Fig. 8C, D and F).
These results suggest that TRIM14 is a downstream mediator of
miR-195-5p in GC.
Discussion
The TRIM14 superfamily of proteins are important
regulators of cellular physiological and pathological processes
including cell proliferation, apoptosis, inflammation, immunity and
metastasis (8,22,23).
Recently, an increasing body of evidence has confirmed that TRIM14
is involved in cancer (24). TRIM14
was found to regulate cell proliferation and invasion in
osteosarcoma via promotion of AKT signaling (8). Moreover, a bispecific antibody against
TRIM14 suppressed osteosarcoma aggressiveness through regulation of
the NF-κB signaling pathway (25).
Here, we reported for the first time that TRIM14 mRNA and protein
were both increased in GC tissues and cell lines. Our clinical data
showed that high TRIM14 was significantly correlated with advanced
TNM stage and lymph node metastasis of GC patients. Moreover,
TRIM14 was found to be an important prognostic marker for 5-year OS
in GC patients. These results showed that TRIM14 play a critical
role in aggressive clinical characteristics and unfavorable
prognosis, and the development and progression of GC.
To confirm the biological function of TRIM14 in GC,
we performed gain- and loss-of-function experiment in vitro
and in vivo to confirm that TRIM14 promotes migration and
invasion of GC cells by regulating EMT phenotype progression. EMT
is critical for GC metastasis. We used western blot analysis and
immunofluorescence to confirm that TRIM14 regulates the EMT
process. Moreover, in GC tissues, TRIM14 was also significantly
associated with the EMT process. To explore the underlying
TRIM14-induced migration, invasion and EMT process in GC cells, we
demonstrated that AKT signaling was activated and inhibition of AKT
signaling reversed the TRIM14-induced migration, invasion and EMT
progression. This is consistent with previous results that TRIM14
activates the AKT signaling pathway-mediated cascade which is
involved in various processes.
Previous studies have reported that abnormal
expression of miRNAs has been verified to be vital regulators in
the initiation and progression of human cancers (26,27).
We searched bioinformation database and showed that miR-195-5p
could interact with TRIM14 3′UTR. Luciferase reporter assays
confirmed that miR-195-5p could directly bind to the 3′UTR of
TRIM14. Gain- and loss-of-function experiments confirmed that
miR-195-5p negatively regulated the expression of TRIM14 mRNA and
protein. In GC tissues, miR-195-5p showed an inverse correlation
with TRIM14 expression. Moreover, alteration of miR-195-5p
expression resulted in an inhibitory effect on migration and
invasion of GC cells. Through rescue experiments, we demonstrated
that alteration of TRIM14 abolished the effects of miR-195-5p on
migration, invasion and EMT progression in GC cells. These results
suggest that TRIM14 is a downstream target and mediator of
miR-195-5p in GC.
In conclusion, we demonstrated that TRIM14 is
increased in GC tissues and cell lines. Its high expression is
associated with malignant clinical features and unfavorable
prognosis. Gain- and loss-of-functional experiments confirmed that
TRIM14 promotes migration, invasion and EMT progression by
activating AKT signaling. Additionally, we determined that
miR-195-5p regulates TRIM14 expression and inhibits migration and
invasion of GC cells. Moreover, alteration of TRIM14 expression
abolished the effects of miR-195-5p on migration, invasion and the
EMT process in GC. These findings provide an improved understanding
of GC progression and support the potential role of TRIM14 as an
attractive therapeutic target for GC treatment.
Acknowledgements
Not applicable.
Funding
This study was supported by the Fundamental Research
Funds for the Central Universities (no. 1191329732).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
FW, JY, QZ and WW performed all the experiments and
drafted the manuscript. LR and FW participated in the study design,
data analysis and interpretation of results. All authors read and
approved the final manuscript and agree to be accountable for all
aspects of the research in ensuring that the accuracy or integrity
of any part of the work are appropriately investigated and
resolved.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The First Affiliated Hospital of Xi'an Jiaotong
University and written informed consent was obtained from all
enrolled patients. All in vivo protocols were approved by
the Institutional Animal Care and Use Committee of Xi'an Jiaotong
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zong L, Abe M, Seto Y and Ji J: The
challenge of screening for early gastric cancer in China. Lancet.
388:26062016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cervantes A, Roda D, Tarazona N, Roselló S
and Pérez-Fidalgo JA: Current questions for the treatment of
advanced gastric cancer. Cancer Treat Rev. 39:60–67. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Glockzin G and Piso P: Current status and
future directions in gastric cancer with peritoneal dissemination.
Surg Oncol Clin N Am. 21:625–633. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Meroni G and Diez-Roux G: TRIM/RBCC, a
novel class of ‘single protein RING finger’ E3 ubiquitin ligases.
Bioessays. 27:1147–1157. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang X, Guo H, Yao B and Helms J: miR-15b
inhibits cancer-initiating cell phenotypes and chemoresistance of
cisplatin by targeting TRIM14 in oral tongue squamous cell cancer.
Oncol Rep. 37:2720–2726. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu G, Guo Y, Xu D, Wang Y, Shen Y, Wang F,
Lv Y, Song F, Jiang D, Zhang Y, et al: TRIM14 regulates cell
proliferation and invasion in osteosarcoma via promotion of the AKT
signaling pathway. Sci Rep. 7:424112017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dong B and Zhang W: High levels of TRIM14
are associated with poor prognosis in hepatocellular carcinoma.
Oncol Res Treat. 41:129–134. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Su X, Wang J, Chen W, Li Z, Fu X and Yang
A: Overexpression of TRIM14 promotes tongue squamous cell carcinoma
aggressiveness by activating the NF-κB signaling pathway.
Oncotarget. 7:9939–9950. 2016.PubMed/NCBI
|
|
11
|
Hu G, Pen W and Wang M: TRIM14 promotes
breast cancer cell proliferation by inhibiting apoptosis. Oncol
Res. Mar 21–2018.(Epub ahead of print). View Article : Google Scholar
|
|
12
|
Hai J, Zhu CQ, Wang T, Organ SL, Shepherd
FA and Tsao MS: TRIM14 is a putative tumor suppressor and regulator
of innate immune response in non-small cell lung cancer. Sci Rep.
7:396922017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu Q, Xiang S, Ma J, Hui P, Wang T, Meng
W, Shi M and Wang Y: Long non-coding RNA CASC15 regulates gastric
cancer cell proliferation, migration and epithelial mesenchymal
transition by targeting CDKN1A and ZEB1. Mol Oncol. 12:799–813.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Z, Dou C, Yao B, Xu M, Ding L, Wang Y,
Jia Y, Li Q, Zhang H, Tu K, et al: Methylation-mediated repression
of microRNA-129-2 suppresses cell aggressiveness by inhibiting high
mobility group box 1 in human hepatocellular carcinoma. Oncotarget.
7:36909–36923. 2016.PubMed/NCBI
|
|
15
|
Chen Y, Liu J, Wang W, Xiang L, Wang J,
Liu S, Zhou H and Guo Z: High expression of hnRNPA1 promotes cell
invasion by inducing EMT in gastric cancer. Oncol Rep.
39:1693–1701. 2018.PubMed/NCBI
|
|
16
|
Zhao J, Geng L, Duan G, Xu W, Cheng Y,
Huang Z, Zhou Z and Gong S: REC8 inhibits EMT by downregulating
EGR1 in gastric cancer cells. Oncol Rep. 39:1583–1590.
2018.PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ceausu AR, Ciolofan A, Cimpean AM, Magheti
A, Mederle O and Raica M: The mesenchymal-epithelial and
epithelial-mesenchymal cellular plasticity of liver metastases with
digestive origin. Anticancer Res. 38:811–816. 2018.PubMed/NCBI
|
|
19
|
Chen D, Lin X, Zhang C, Liu Z, Chen Z, Li
Z, Wang J, Li B, Hu Y, Dong B, et al: Dual PI3K/mTOR inhibitor
BEZ235 as a promising therapeutic strategy against
paclitaxel-resistant gastric cancer via targeting PI3K/Akt/mTOR
pathway. Cell death Dis. 9:1232018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu J, Li Z, Su Q, Zhao J and Ma J: TRIM29
promotes progression of thyroid carcinoma via activating P13K/AKT
signaling pathway. Oncol Rep. 37:1555–1564. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xiao C, Hong H, Yu H, Yuan J, Guo C, Cao H
and Li W: MiR-340 affects gastric cancer cell proliferation, cycle,
and apoptosis through regulating SOCS3/JAK-STAT signaling pathway.
Immunopharmacol Immunotoxicol. 40:278–283. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tan P, He L, Cui J, Qian C, Cao X, Lin M,
Zhu Q, Li Y, Xing C, Yu X, et al: Assembly of the WHIP-TRIM14-PPP6C
mitochondrial complex promotes RIG-I-mediated antiviral signaling.
Mol Cell. 68:293–307.e5. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jia X, Zhou H, Wu C, Wu Q, Ma S, Wei C,
Cao Y, Song J, Zhong H, Zhou Z and Wang J: The ubiquitin ligase
RNF125 targets innate immune adaptor protein TRIM14 for
ubiquitination and degradation. J Immunol. 198:4652–4658. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang T, Ren Y, Liu R, Ma J, Shi Y, Zhang L
and Bu R: miR-195-5p suppresses the proliferation, migration, and
invasion of oral squamous cell carcinoma by targeting TRIM14.
Biomed Res Int. 2017:73781482017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu GH, Li AM, Li X, Yang Z and Peng H:
Bispecific antibody suppresses osteosarcoma aggressiveness through
regulation of NF-κB signaling pathway. Tumour Biol.
39:10104283177055722017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu Z, Wang Y, Dou C, Sun L, Li Q, Wang L,
Xu Q, Yang W, Liu Q and Tu K: MicroRNA-1468 promotes tumor
progression by activating PPAR-γ-mediated AKT signaling in human
hepatocellular carcinoma. J Exp Clin Cancer Res. 37:492018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu Z, Dou C, Yao B, Xu M, Ding L, Wang Y,
Jia Y, Li Q, Zhang H, Tu K, et al: Ftx non coding RNA-derived
miR-545 promotes cell proliferation by targeting RIG-I in
hepatocellular carcinoma. Oncotarget. 7:25350–25365.
2016.PubMed/NCBI
|