Introduction
Hepatocellular carcinoma (HCC) is the sixth most
prevalent type of cancer and ranks third among the most frequent
causes of solid tumor-related deaths worldwide (1). HCC is the most common primary
malignancy of the liver, and its incidence has increased in recent
decades (2). Patients with advanced
unresectable or metastatic HCC often have a poor prognosis, and
only a few chemotherapeutics have been proven to be effective
(3). Only early-stage HCC patients
can receive potentially curative therapies, such as surgical
resection and liver transplantation. Therefore, there is an urgent
need to identify and develop more effective treatments for HCC
(4). Sorafenib, the only systemic
therapy that improves the survival of patients with advanced HCC,
is a multi-kinase inhibitor (5).
The phosphoinositide 3 kinase (PI3K)/Akt/mammalian target of
rapamycin (mTOR) signaling axis, which plays a pivotal role in cell
proliferation, colonization and survival (6), is an emerging target in HCC that
contributes to disease progression and the development of
resistance to sorafenib. However, sensitivity to sorafenib after
the development of resistance may be partially restored by PI3K/Akt
inhibitors in vitro (7).
Therefore, targeting PI3K/Akt signaling may considerably improve
the management of HCC patients treated with sorafenib (8).
Capsaicin (8-methyl N-vanillyl-6 nonenamide) is a
natural plant extract and the major pungent component of hot
peppers of the genus Capsicum (9).
Capsaicin has potential antitumor properties (10) and produces apoptosis in various
types of malignancies, including breast cancer (11,12),
colon adenocarcinoma (13,14), nasopharyngeal carcinoma (15), esophageal epidermoid carcinoma
(16), HCC (17,18)
and prostate cancer (19).
Capsaicin has been reported to induce apoptosis and autophagy in
several types of human carcinoma cells via inhibition of the
PI3K/Akt/mTOR signaling pathway (15,18).
The activation of PI3K/Akt/mTOR signaling is associated with cancer
cell proliferation, colonization and survival. PI3K/Akt/mTOR
signaling may inhibit cell apoptosis (20) and autophagy (21), whereas upregulation of this
signaling pathway may promote angiogenesis (22), invasion and metastasis (23–25).
Therefore, this pathway holds promise as an effective target for
the treatment of HCC through the combined use of capsaicin and
sorafenib.
Epidermal growth factor receptor (EGFR) is a growth
factor receptor tyrosine kinase, and its isogenous ligands have
been found to be commonly affected in multiple cancer types and
appear to facilitate solid tumor growth (26). EGFR is located upstream of
PI3K/Akt/mTOR and is overexpressed in HCC cells (27). Therefore, the aim of the present
study was to investigate the antitumor activity of capsaicin and
sorafenib in in vitro and in vivo studies, alone as
well as in combination, in order to determine whether their
combination can induce HCC cell apoptosis and autophagy and inhibit
HCC cell proliferation, migration and invasion in a synergistic
manner.
Materials and methods
Chemicals and antibodies
Capsaicin and sorafenib were purchased from
Sigma-Aldrich; Merck KGaA (St. Louis, MO, USA) and Selleckchem
(Houston, TX, USA), respectively. Antibodies against GAPDH, Bax,
cleaved caspase-3 (Asp175), poly(ADP-ribose) polymerase (PARP),
beclin-1, LC3A/B, E-cadherin, vimentin, P-Akt (Ser473), Akt, P-mTOR
(Ser2448), mTOR, P-p70S6 kinase (P-p70S6K, Thr389), p70S6K and
Ki-67 were obtained from Cell Signaling Technology (Danvers, MA,
USA). The P62 antibody was obtained from Proteintech (Rosemont, IL,
USA). The antibodies against Bcl-2, N-cadherin, matrix
metalloproteinase (MMP)2, MMP9, P-EGFR, EGFR and PI3K p85α were
obtained from Abcam (Cambridge, MA, USA). The details on the
antibodies used in the present study are listed in Table I.
 | Table I.Details of the antibodies used in the
present study. |
Table I.
Details of the antibodies used in the
present study.
| Antibody | Dilution | Catalogue no. | Company
details |
|---|
| GAPDH | WB 1:1,000 | 5174 | Cell Signaling
Technologya |
| Bax | WB 1:1,000, IHC
1:200 | 5023 | Cell Signaling
Technologya |
| Cleaved caspase-3
(Asp175) | WB 1:1,000 | 9664 | Cell Signaling
Technologya |
| PARP antibody | WB 1:1,000 | 9542 | Cell Signaling
Technologya |
| Beclin-1 | WB 1:1,000 | 3495 | Cell Signaling
Technologya |
| LC3A/B
antibody | WB 1:1,000 | 4108 | Cell Signaling
Technologya |
| E-cadherin | WB 1:1,000 | 3195 | Cell Signaling
Technologya |
| Vimentin | WB 1:1,000 | 5741 | Cell Signaling
Technologya |
| Phospho-Akt
(Ser473) | WB 1:1,000, IHC
1:200 | 4060 | Cell Signaling
Technologya |
| Akt (pan) | WB 1:1,000 | 4691 | Cell Signaling
Technologya |
| Phospho-mTOR
(Ser2448) | WB 1:1,000 | 5536 | Cell Signaling
Technologya |
| mTOR | WB 1:1,000 | 2983 | Cell Signaling
Technologya |
| Phospho-p70 S6
kinase (Thr389) | WB 1:1,000 | 9234 | Cell Signaling
Technologya |
| p70 S6 kinase | WB 1:1,000 | 2708 | Cell Signaling
Technologya |
| Ki-67 | IHC 1:200 | 12202 | Cell Signaling
Technologya |
| P62/SQSTM1
antibody | WB 1:1,000, IHC
1:200 | 18420-1-AP |
Proteintechb |
| Anti-N cadherin
antibody | WB 1:1,000 | ab18203 | Abcamc |
| Anti-MMP2
antibody | WB 1:1,000, IHC
1:200 | ab37150 | Abcamc |
| Anti-PI 3 kinase
p85 alpha antibody | WB 1:1,000 | ab86714 | Abcamc |
| Anti-EGFR
antibody | WB 1:1,000, IHC
1:200 | ab52894 | Abcamc |
| Anti-MMP9
antibody | WB 1:1,000, IHC
1:200 | ab38898 | Abcamc |
| Anti-Bcl-2
antibody | WB 1:1,000 | ab32124 | Abcamc |
| Anti-EGFR (phospho
Y1068) antibody | WB 1:1,000, IHC
1:200 | ab40815 | Abcamc |
Cell lines and culture conditions
The LM3, Hep3B and HuH7 human HCC cell lines were
obtained from the Cell Bank of Type Culture Collection of Chinese
Academy of Sciences (Shanghai, China). The cells were maintained in
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific Inc.) at 37°C in
a humidified atmosphere (5% CO2, 95% air). The HCC LM3
cell line used in this study has been authenticated by STR
profiling.
Cell viability and colony formation
assays
To allow cells to attach completely, LM3, Hep3B and
HuH7 cells (5,000 cells/well) were seeded in a 96-well plate for 24
h; then, capsaicin and sorafenib were added to the culture media at
the indicated concentrations for another 48 h. Next, 10% Cell
Counting Kit (CCK)-8 solution was added to the culture media, and
the plates were incubated for 4 h. OD450 values were determined by
a spectrophotometer, and the results were analyzed to measure cell
growth.
For colony formation assays, adherent cells were
trypsinized, and 1,000 viable cells were re-seeded in 6-well plates
(in triplicate). After cell adherence, cells were allowed to form
colonies for 14 days with each of the treatments. To visualize the
colonies, the media were discarded, and the cells were submerged in
4% paraformaldehyde for 15 min and dyed with 0.1% crystal violet
staining solution.
Cell migration and invasion
assays
LM3 cells were seeded and cultured in a 6-well plate
for 24 h to adherence and confluence. The cell layers were
scratched with a 200-µl pipette tip to create a wound and
then washed three times with phosphate-buffered saline (PBS) to
remove floating cells. The medium was then replaced with serum-free
medium. The wound was photographed at 0, 48 and 72 h.
After trypsinization, 2×105 cells were
plated on Boyden chambers coated with 10 µg Matrigel (BD
Biosciences, Sparks, MD, USA) per well (for invasion assays), and
5×104 cells were plated on uncoated Boyden chambers (for
migration assays) in medium containing 1% FBS. Medium containing
10% FBS was added to the lower chamber as a chemoattractant.
Capsaicin, sorafenib or their combination was added to the upper
and lower chambers at the indicated concentrations. After 48 h, the
cells that had moved to the lower surface of the membrane were
fixed with methanol and stained with 0.1% crystal violet solution.
Photographs of three random fields of fixed cells were captured,
and the cells were counted. Each reported value was estimated from
three plates.
Immunofluorescence
Cells (2×105) were cultured on sterile
sheet glass and treated with capsaicin, sorafenib or their
combination for 48 h. The cells were submerged in 4%
paraformaldehyde solution at room temperature for 10 min after
treatment, then washed three times in PBS, permeabilized with 0.1%
Triton X-100/PBS for 5 min, and then blocked with 10% bovine serum
albumin for 1 h. The cells were incubated with primary antibodies
overnight at 4°C, washed three times in PBS for 15 min, and
incubated with secondary antibodies for 1 h at room temperature.
Unbound Ab was removed by washing with 1X TBST four times for 20
min each time; thereafter, the cell nuclei were stained with DAPI
(1:20). ProLong® Gold Antifade Mounting Agent (Thermo
Fisher Scientific, Inc.) was used for treating the cells, and
fluorescence images were captured by a fluorescence microscope.
Western blotting
Following treatment with capsaicin, sorafenib or
their combination, cells were lysed in radioimmunoprecipitation
assay buffer (Beyotime Institute of Biotechnology, Shanghai, China)
containing 1% phenylmethylsulfonyl fluoride (Beyotime Institute of
Biotechnology) and 10% phosphatase inhibitor (Roche Diagnostics
GmbH, Mannheim, Germany) for 30 min. Subsequently, the lysates were
centrifuged at 10,000 × g for 30 min at 4°C and the supernatant was
collected in a new tube. The protein concentrations were determined
using the BCA Protein Assay Kit (Beyotime Institute of
Biotechnology). After denaturation, protein from each group (50
µg) was fractionated with 8–12% Tris-glycine gel
electrophoresis. Subsequently, different proteins were transferred
to PVDF membranes and probed with the corresponding primary
antibodies for 12 h at 4°C, then incubated with same-species
secondary antibodies at 1:5,000 dilution for 1 h. Enhanced
chemiluminescence reagent (Thermo Fisher Scientific, Inc.) was used
to detect the proteins, and antibody binding was visualized by
Image Lab software (http://www.bio-rad.com/en-cn/product/image-lab-software?ID=KRE6P5E8Z).
Apoptosis analysis with flow cytometry
and TdT-mediated dUTP nick end labeling (TUNEL)
For the apoptosis analysis, LM3 cells were treated
with capsaicin, sorafenib or their combination for the indicated
times, trypsinized and collected in tubes, and incubated in binding
buffer with propidium iodide and FITC-conjugated Annexin V for 10
min in the dark at room temperature. Flow cytometry analysis was
used to estimate the cell apoptosis rate. The TUNEL method was
applied to visualize the 3′-OH ends of DNA fragments in apoptotic
cells according to the manufacturer's protocol (Roche Diagnostics
GmbH). LM3 cells were subjected to different treatments for 48 h
and fixed in 4% paraformaldehyde. The cells were then submerged in
methanol containing 0.3% H2O2 to inhibit
endogenous peroxidase activity. Following washing with PBS, cells
were covered with proteinase K solution for 10 min. Subsequently,
the cells were covered with the TUNEL reaction mixture and
incubated for 1 h at 37°C in the dark. After washing in PBS, the
cells were placed in Converter-POD (Roche Diagnostics GmbH) and
then incubated at 37°C for 30 min. After rinsing in PBS three times
for 5 min, the cells were dipped in DAB (Roche Diagnostics GmbH) at
room temperature for 10 min and observed under a microscope.
In vivo studies
Five-week-old BALB/C nude mice (SPF grade) were
supplied by SLAC Co., Ltd. (Shanghai, China). All the mice were
raised in the cabinet with laminar air flow under pathogen-free
conditions in a humidity- and temperature-controlled environment
with a 12 h light/dark schedule. The mice had ad libitum
access to food and water. Prior to the study initiation, the mice
were allowed to acclimatize for 1 week. Then, the mice received a
subcutaneous injection of 1×107 LM3 cells suspended in
100 µl sterile PBS into the right flank. Two weeks after the
inoculation, based on the initial tumor volume, the mice were
divided into four groups (n=6 per group) and received daily
treatments via i.p. injections: The control group received sterile
PBS with 1% dimethyl sulfoxide (DMSO), the capsaicin group was
treated with 5 mg/kg capsaicin containing 1% DMSO, the sorafenib
group received 50 mg/kg sorafenib containing 1% DMSO, and the
combination group was treated with 5 mg/kg capsaicin + 50 mg/kg
sorafenib containing 1% DMSO. Tumor volume and mouse weight were
measured every other day, and tumor volume was calculated according
to the formula V (mm3) = 1/2 (length ×
width2). The mice were treated with different compounds
for 28 days. All the mice were sacrificed by cervical dislocation
under pentobarbital sodium anesthesia administered through i.p.
injection, the livers and kidneys were harvested for
immunohistochemical examination and blood was collected for
biochemistry tests. All efforts were made to minimize animal
suffering. All animal procedures were conducted in accordance with
the guidelines of the National Institutes of Health and were
approved by the Ethical Committee of Wenzhou Medical University and
the Laboratory Animal Management Committee of Zhejiang Province
(Approval ID: wydw2017-0052).
Immunohistochemistry
The tumors were fixed with 4% paraformaldehyde
solution and embedded in paraffin, then cut into 4-µm
sections. The slides were incubated with antibodies against P-EGFR,
P-Akt, Ki67, Bax, P62, MMP2 and MMP9; then, the slides were washed,
stained with secondary antibody, and directly visualized by the
ChemMate EnVision Kit (ZSGB-BIO Beijing China). Images of the
stained sections were captured under a microscope at a
magnification of ×400. Histological analysis of liver and kidney
sections was conducted with hematoxylin and eosin (H&E)
staining. Details on the antibodies used may be found in Table I.
Statistical analysis
The data are presented as the mean ± standard error
of the mean for given samples, and were analyzed by one-way
analysis of variance (ANOVA) followed by Dunnett's multiple range
tests using SPSS v.22 statistical software (IBM Corp., Armonk, NY,
USA). The significance level was set at P<0.05.
Results
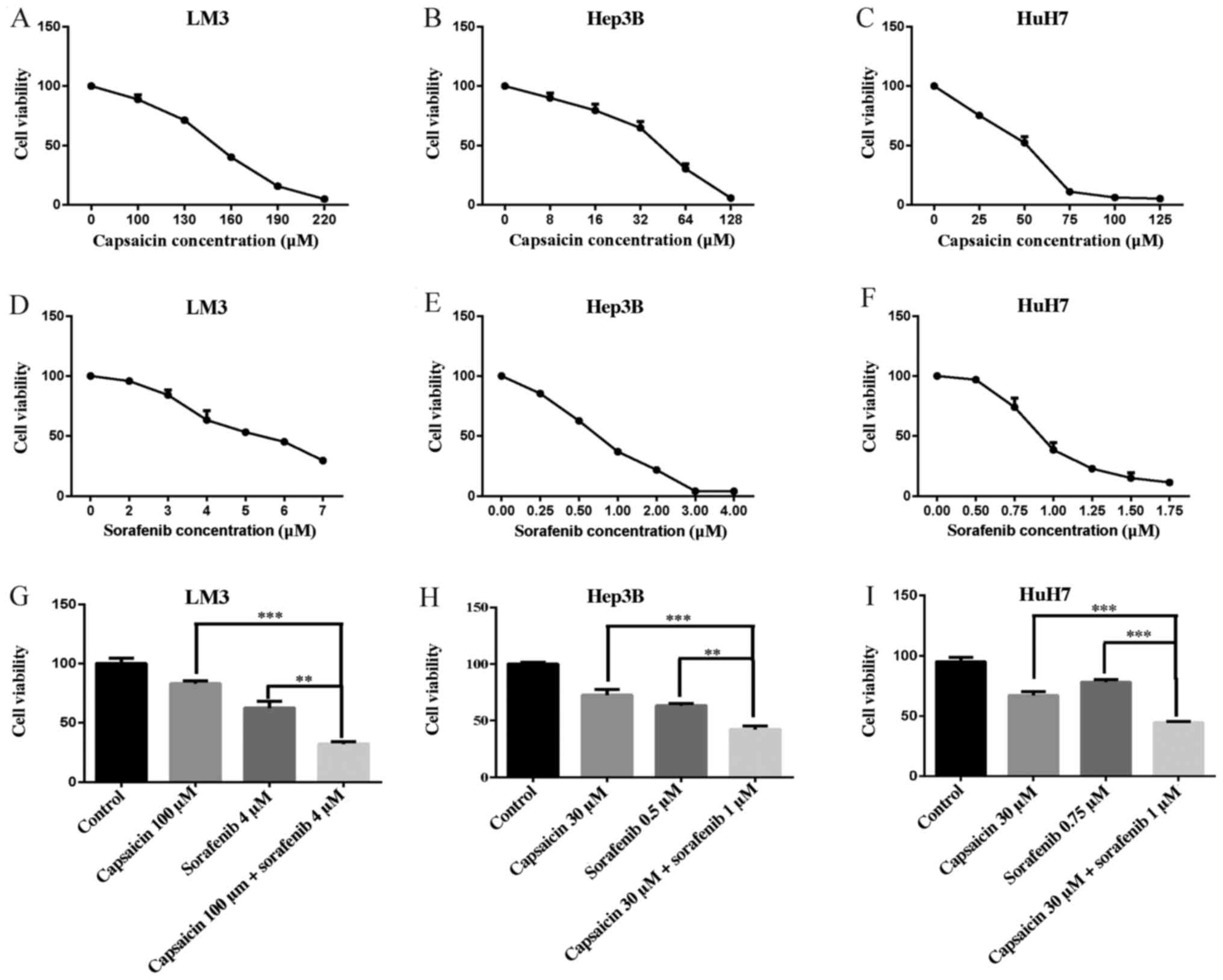
Capsaicin reduces cell viability and
potentiates the inhibitory effects of sorafenib in HCC lines
CCK-8 assays were performed to evaluate the effects
of capsaicin and sorafenib alone and in combination on three human
HCC cell lines, LM3, Hep3B and HuH7 (Fig. 1A-I). The survival and proliferation
of these four cell lines decreased with increasing concentrations
of capsaicin and sorafenib. The inhibition of cell survival and
proliferation was greatly enhanced by the combination of a low
concentration of capsaicin and a moderate concentration of
sorafenib.
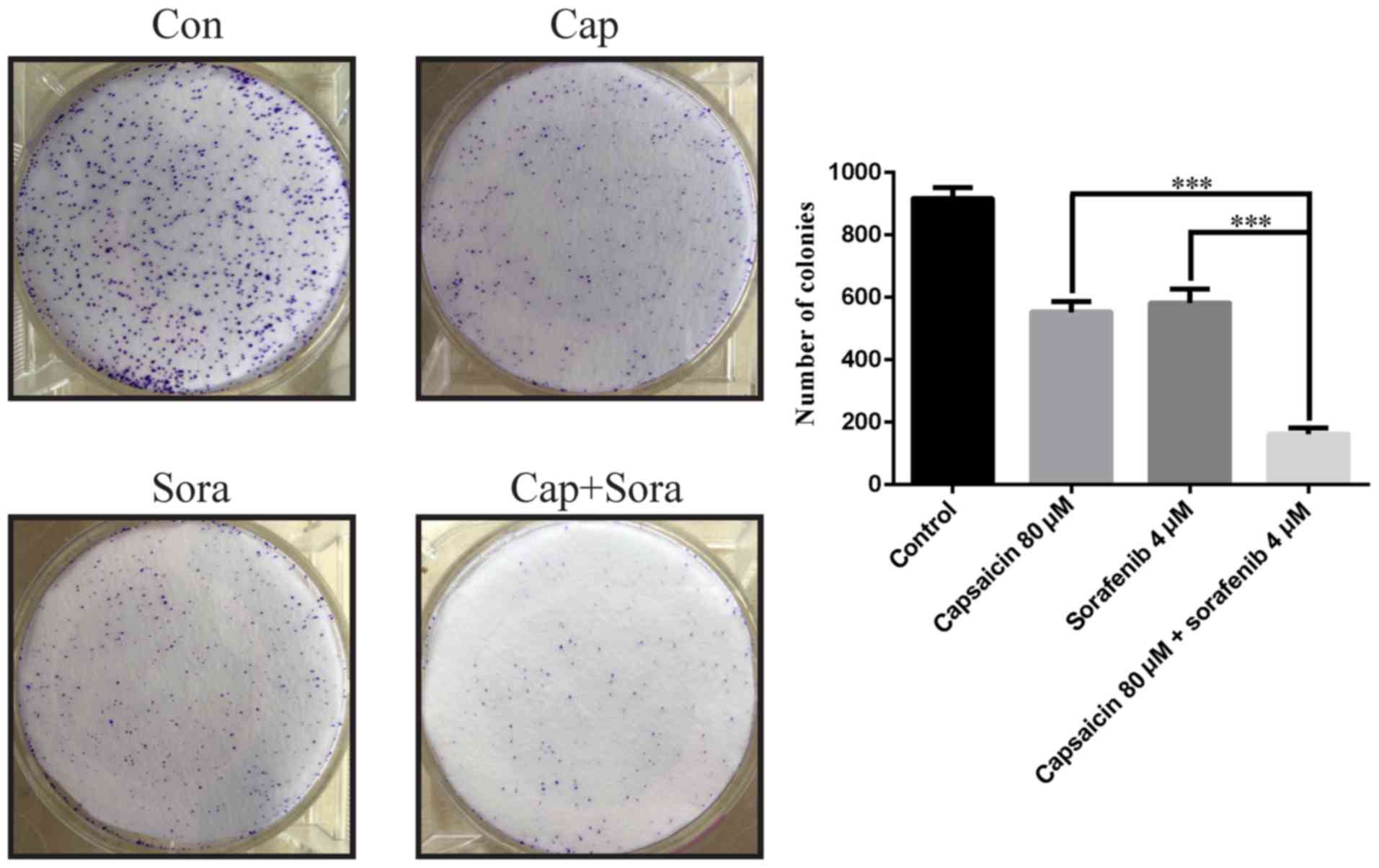
Different combinations of capsaicin
and sorafenib inhibit LM3 cell proliferation to various
extents
In colony formation assays, LM3 cells treated with
the combination of capsaicin and sorafenib exhibited decreased
colony formation ability compared with those treated with
monotherapy (Fig. 2). The effects
of capsaicin in combination with sorafenib on LM3 cells are further
detailed in Table II. In LM3
cells, the IC50 of sorafenib in combination with
capsaicin (80, 100 and 120 µM) was significantly decreased
from 3.987 µM to 2.989, 2.590 and 1.854 µM,
respectively, and significant synergy between sorafenib and
capsaicin was observed at 80, 100 and 120 µM capsaicin.
 | Table II.Cells were incubated with three
different concentrations of capsaicin, sorafenib and 9 types of
combination for 48 h prior to being subjected to Cell Counting
Kit-8 assay. |
Table II.
Cells were incubated with three
different concentrations of capsaicin, sorafenib and 9 types of
combination for 48 h prior to being subjected to Cell Counting
Kit-8 assay.
| Capsaicin
(µM) | Sorafenib
(µM) | OD | Inhibition ratio
(%) | CI |
IC50 |
|---|
| 0 | 0 | 1.259±0.217 |
|
|
|
| 80 | 0 | 1.169±0.408 | 8.49921 |
|
|
| 100 | 0 | 1.038±0.235 | 20.8752 |
|
|
| 120 | 0 | 0.8099±0.046 | 42.5308 |
|
|
| 0 | 2 | 1.116±0.119 | 13.4929 |
|
|
| 0 | 3 | 0.884±0.357 | 35.4692 |
| 3.987 |
| 0 | 4 | 0.755±0.146 | 47.7788 |
|
|
| 80 | 2 | 0.887±0.180 | 35.1351 | 0.388557 |
|
| 80 | 3 | 0.754±0.073 | 47.8033 | 0.367342 | 2.989 |
| 80 | 4 | 0.546±0.106 | 67.5735 | 0.283707 |
|
| 100 | 2 | 0.850±0.215 | 38.7417 | 0.394444 |
|
| 100 | 3 | 0.633±0.071 | 59.3175 | 0.329109 | 2.590 |
| 100 | 4 | 0.502±0.064 | 71.7346 | 0.279882 |
|
| 120 | 2 | 0.647±0.035 | 57.9597 | 0.335968 |
|
| 120 | 3 | 0.402±0.015 | 81.2204 | 0.237104 | 1.854 |
| 120 | 4 | 0.215±0.006 | 98.9005 | 0.137558 |
|
Capsaicin acts synergistically with
sorafenib to induce apoptosis in the LM3 cell line
Based on the results in Table II, the combination of 80 µM
capsaicin and 4 µM sorafenib was selected for the next
series of experiments. To ascertain the extent of apoptosis, cells
were examined by TUNEL staining. Following treatment with capsaicin
and sorafenib, microscopic examination revealed stained cells, and
the combination of capsaicin and sorafenib was associated with a
higher number of TUNEL-positive cells (Fig. 3A). To further validate apoptotic
cell death induced by capsaicin and sorafenib alone and in
combination, apoptosis was evaluated by flow cytometry analysis.
The combination treatment triggered apoptosis to a greater extent
than either monotherapy. As shown in Fig. 3B, the percentage of apoptotic cells
after 48 h of treatment with control, 80 µM capsaicin, 4
µM sorafenib and the combination of 80 µM capsaicin
and 4 µM sorafenib was 3.43±0.536, 16.58±0.629, 21.18±0.809
and 52.13±1.602%, respectively. To further elucidate the mechanism
underlying the increased apoptosis of LM3 cells, the protein levels
of two key apoptosis-related protein, Bcl2 and Bax, were
investigated. The pro-apoptotic protein Bax expression was
upregulated by the capsaicin and sorafenib combination, and the
expression of the anti-apoptotic protein Bcl2 was reduced (Fig. 3C). Caspase-3 was also examined by
western blot assays. As shown, exposure to 80 µM capsaicin
and 4 µM sorafenib for 24 h increased the cleavage of
caspase-3. The results mentioned above indicate that capsaicin
combined with sorafenib can modulate the activation of apoptotic
signaling pathways in LM3 cells.
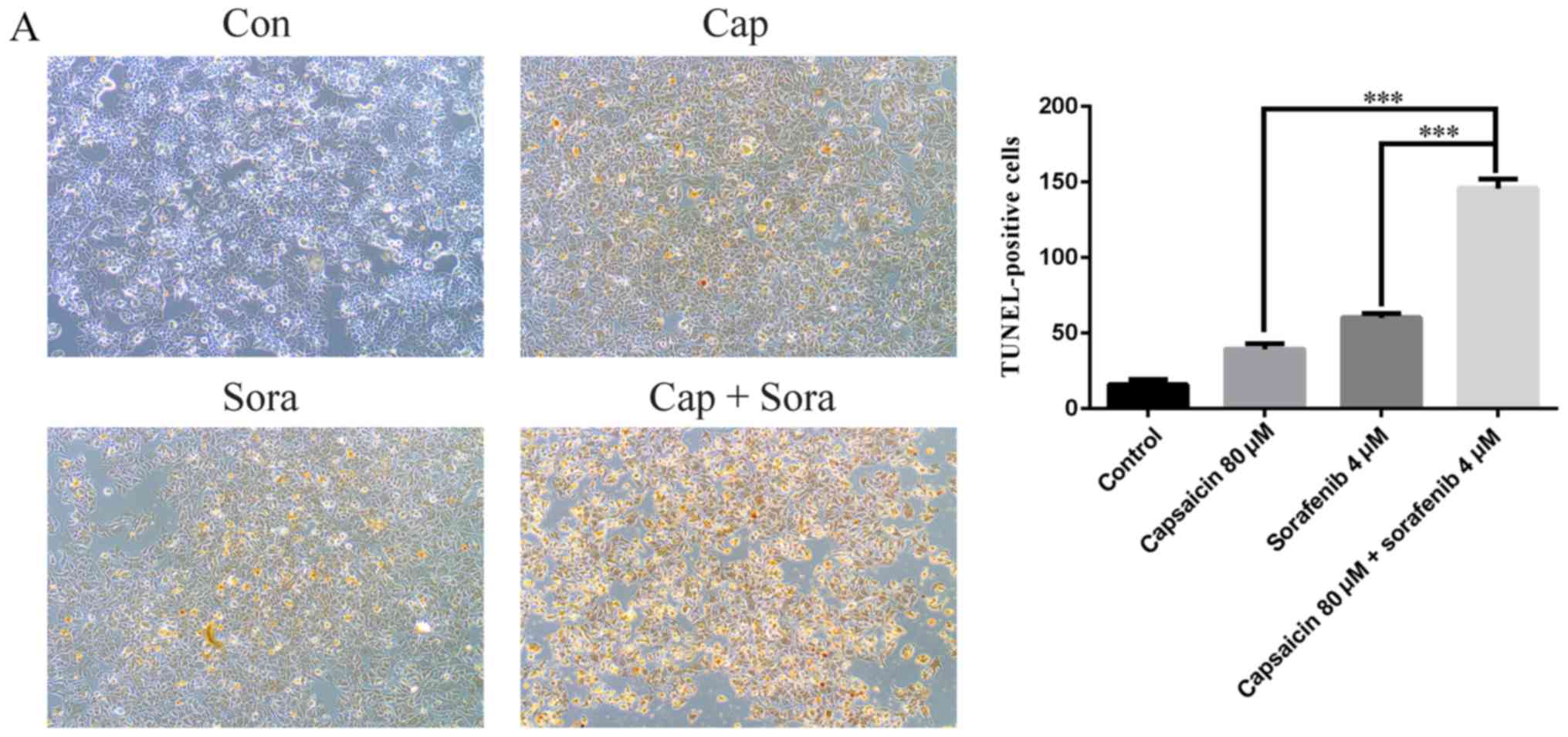 | Figure 3.Capsaicin and sorafenib induce
apoptosis in LM3 cells, and their combination exerts a synergetic
effect on cell apoptosis. (A) TUNEL staining was used to visualize
the 3′-OH ends of DNA fragments in apoptotic cells. Representative
photomicrographs of cells under control or drug treatments are
shown (magnification, ×100). (B) LM3 cells were treated with
capsaicin, sorafenib and their combination for 48 h and were then
stained with Annexin V and propidium iodide (PI). The early
apoptotic cells (Annexin V-positive, PI-negative) and the late
apoptotic cells (Annexin V-positive, PI-positive) are indicated as
the percentage of gated cells. The histogram represents the
apoptotic cells for each dose. Data are presented as the mean ±
standard error of the mean (SEM) of three experiments. (C) Western
blot analysis of Bax, Bcl-2, cleaved caspase-3 and cleaved PARP
after capsaicin, sorafenib and combination treatment for 12 h. The
results are representative of three separate experiments. GAPDH was
set as control. The data are presented as the mean ± SEM.
***P<0.005; #, Capsaicin vs. combination; +, sorafenib vs.
combination. ###/+++: P<0.005. Con, control; Cap, capsaicin 80
µM; Sora, sorafenib 4 µM; PI, propidium iodide; PARP,
poly(ADP-ribose) polymerase. |
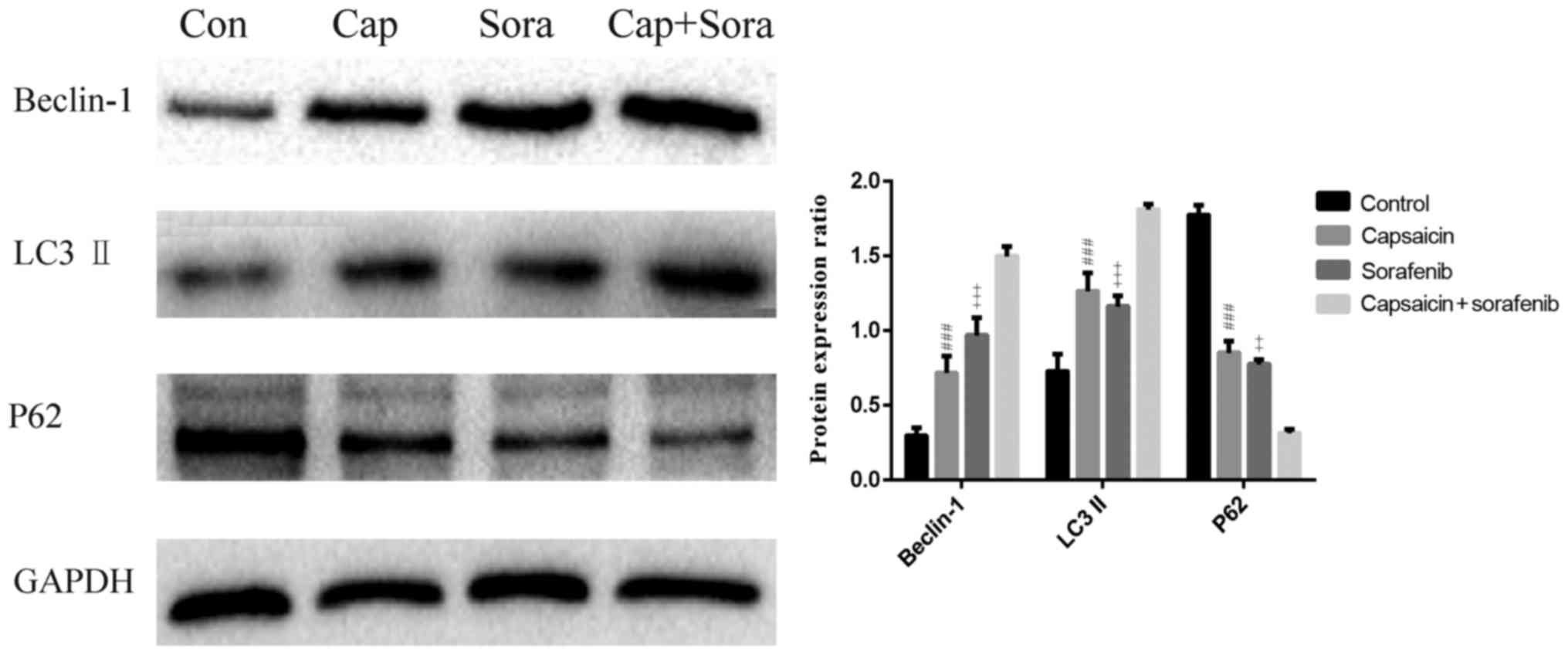
Capsaicin combined with sorafenib
enhances autophagy in the LM3 cell line
To determine the effect of the capsaicin and
sorafenib combination treatment on cell autophagy, the protein
expression of the autophagy-related genes beclin-1, P62 and
LC3A/B-II was examined by western blotting (Fig. 4). Beclin-1 and LC3A/B-II levels were
increased by the combination treatment compared with monotherapy.
Compared with the monotherapy groups, the autophagy-specific
substrate P62 was obviously reduced in the combination group.
The capsaicin and sorafenib
combination markedly inhibits LM3 cell invasion and migration
Cell scratch assays were used to determine whether
capsaicin and sorafenib inhibit HCC cell migration. As shown in
Fig. 5A, the LM3 cell-free area
after the combination treatment was wider compared with that after
monotherapy, and the wound was wider in the treatment groups
compared with that in the control group at 48 and 72 h. This result
demonstrated that capsaicin and sorafenib inhibit LM3 cell
migration in a synergistic manner. Transwell migration assays and
Matrigel invasion assays (Fig. 5B)
revealed that capsaicin and sorafenib can inhibit both the
migration and invasion of LM3 cells, and the combined treatment was
more effective than either monotherapy. Therefore, we next examined
the expression levels of E-cadherin, N-cadherin, vimentin, MMP2 and
MMP9 in the monotherapy and combination groups through western
blotting. The N-cadherin, vimentin, MMP2 and MMP9 levels were
markedly decreased in LM3 cells after treatment with the
combination of capsaicin and sorafenib, while the E-cadherin levels
were increased (Fig. 5C).
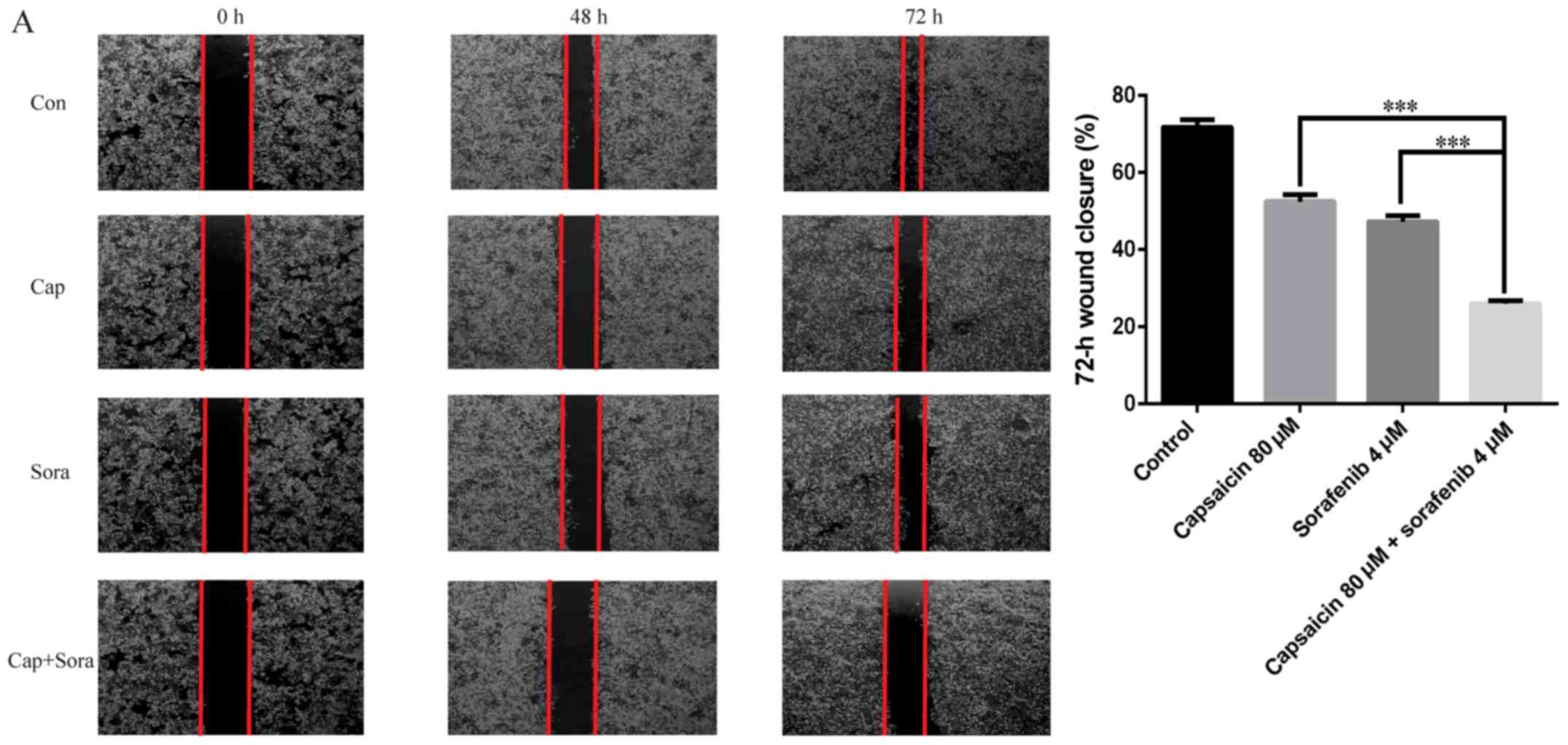 | Figure 5.Capsaicin and sorafenib inhibit the
invasion and migration of LM3 cells, and the combination treatment
can inhibit invasion and migration synergistically. (A) Capsaicin,
sorafenib and their combination inhibited migration of LM3 cells.
The cell migration was assessed by wound healing assay
(magnification, ×40). Con, control; Cap, capsaicin 80 µM;
Sora, sorafenib 4 µM. Capsaicin and sorafenib inhibit the
invasion and migration of LM3 cells, and the combination treatment
can inhibit invasion and migration synergistically. (B) Effects of
capsaicin on invasion and migration in LM3 cells. Cells were
pretreated with capsaicin, sorafenib and their combination for 48
h. After 48 h, cells on the lower side of the filter were counted
(magnification, ×200). (C) LM3 cells were treated with capsaicin,
sorafenib and their combination for 12 h, and the expression of
E-cadherin, N-cadherin, vimentin, matrix metalloproteinase (MMP)2
and MMP9 was examined by western blot analysis. GAPDH served as a
loading control. The results are representative of three separate
experiments. The data are presented as the mean ± standard error of
the mean. ***P<0.005. #, Capsaicin vs. combination; +, sorafenib
vs. combination. ##/++ and ###/+++: P<0.01 and 0.005,
respectively. Con, control; Cap, capsaicin 80 µM; Sora,
sorafenib 4 µM. |
Capsaicin combined with sorafenib
obviously inhibits the expression of EGFR and downstream effectors
of the EGFR/PI3K/Akt signaling pathway in LM3 cells
To elucidate the mechanism underlying the
synergistic effects of capsaicin and sorafenib, we investigated
PI3K/Akt/mTOR signal transduction, which is critically implicated
in the effects of capsaicin and sorafenib. Capsaicin and sorafenib
treatment decreased the levels of PI3K, P-Akt, P-mTOR and P-p70S6K,
and these levels were lower in LM3 cells after combination
treatment compared with after either monotherapy (Fig. 6). PI3K/Akt/mTOR signaling may be
activated by multiple stimuli. Growth factor receptor family
proteins are major upstream molecules of PI3K/Akt/mTOR signaling
(28). A number of solid tumors
display high or abnormal EGFR expression. EGFR is associated with
tumor cell proliferation, angiogenesis, invasion, metastasis and
inhibition of apoptosis (29).
Therefore, P-EGFR and EGFR levels were examined in LM3 cells
following treatment with capsaicin, sorafenib, or their
combination. The P-EGFR and EGFR levels decreased in LM3 cells
treated with capsaicin and sorafenib, and the combination treatment
synergistically downregulated P-EGFR and EGFR levels, which were
lower compared with those in the monotherapy groups. Therefore, it
was concluded that EGFR and PI3K/Akt/mTOR signaling was inhibited
synergistically by capsaicin and sorafenib.
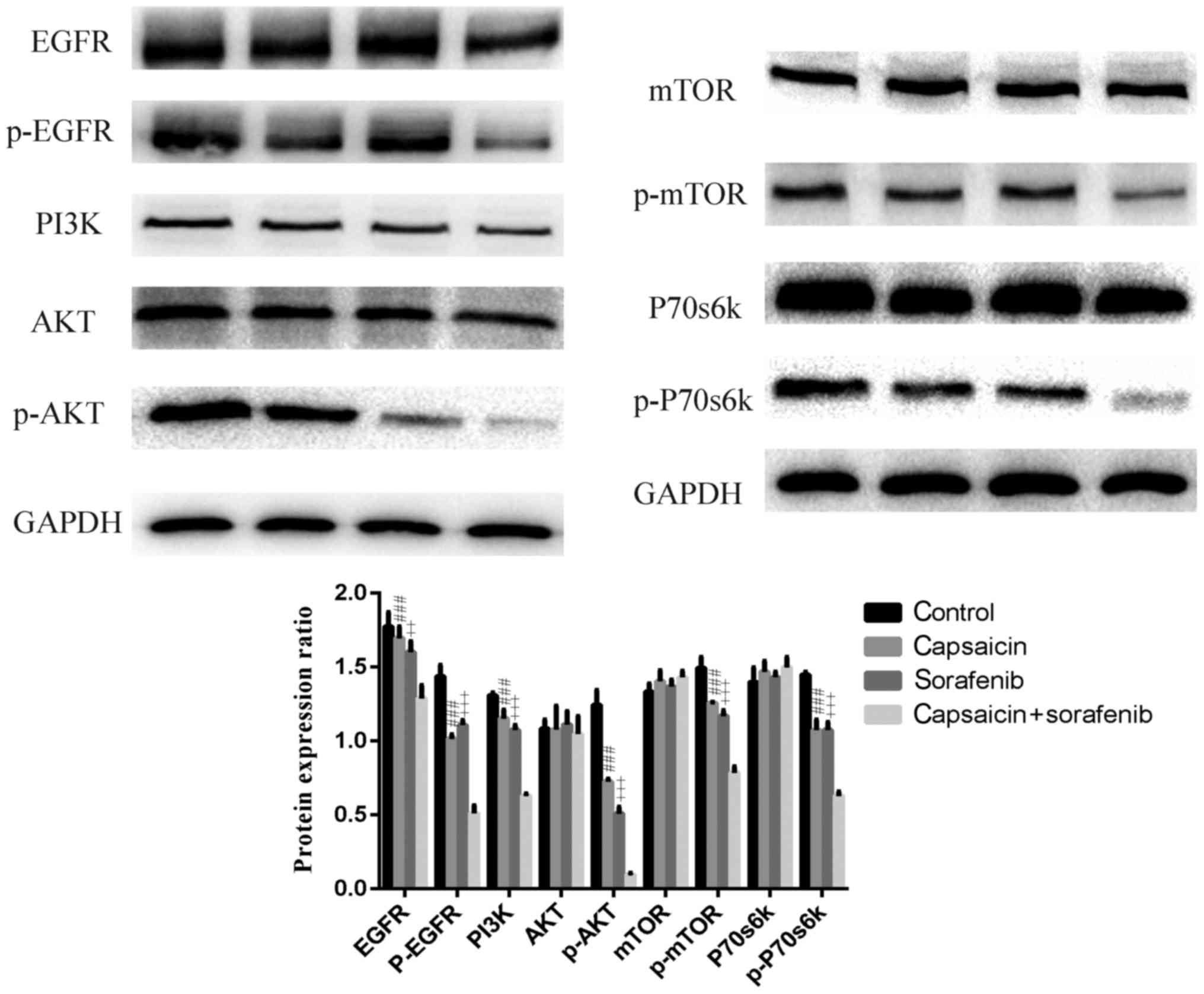 | Figure 6.Capsaicin and sorafenib reduce the
expression of EGFR, PI3K/Akt/mTOR pathway components. Capsaicin and
sorafenib downregulate EGFR, PI3K/Akt/mTOR signaling and the client
protein P-p70s6k in LM3 cells. The combination of capsaicin and
sorafenib exerted a synergistic effect on this pathway. Cells were
serum-starved for 24 h and then treated with capsaicin, sorafenib
and their combination for 12 h. Protein lysates were analyzed by
western blotting with the indicated antibodies. GAPDH served as a
loading control. Values represented the mean ± standard error of
the mean from three independent experiments. #, Capsaicin vs.
combination; +, sorafenib vs combination. ++ and ###/+++, P<0.01
and 0.005, respectively. EGFR, epidermal growth factor receptor;
PI3K, phosphoinositide-3 kinase; mTOR, mammalian target of
rapamycin. |
Capsaicin and sorafenib restrain the
growth of hepatocellular tumors synergistically in vivo
To further confirm capsaicin and sorafenib as
synergistic inhibitors of tumor growth in vivo,
1×107 LM3 cells were subcutaneously inoculated in nude
mice. Capsaicin and sorafenib treatment was administered i.p. for
28 days starting at the 7th day post-inoculation. It was observed
that the capsaicin and sorafenib combination treatment exerted an
obvious inhibitory effect on tumor volume (Fig. 7A and B). However, there were no
significant differences in body weight between the control and
treatment groups (Fig. 7A).
Furthermore, the expression levels of proteins related to
proliferation, apoptosis, autophagy, invasion and metastasis in
xenograft tumor tissues were evaluated by immunohistochemistry.
Proliferation, invasion and metastasis were inhibited in xenograft
tumors by capsaicin, sorafenib and their combination, while
apoptosis and autophagy were activated (Fig. 7C). Ki67 and Bax indicated the
presence of more apoptotic cells and obviously fewer proliferative
cells in tumors treated with the combination of capsaicin and
sorafenib. In addition, the highest autophagy level among the four
groups, as measured by P62, was observed in tumors of the
combination treatment group. Capsaicin and sorafenib decreased MMP2
and MMP9 expression in the tumors, and the combination treatment
enhanced this effect. Moreover, P-EGFR and P-Akt levels were
evaluated in xenograft tumors and it was observed that the
capsaicin and sorafenib combination decreased P-EGFR and P-Akt
levels to the greatest extent. The biochemical function of the
liver and kidney was monitored, and there were no significant
differences among the four groups (Fig.
7D). To further evaluate treatment-related toxicity, liver and
kidneys from the control and drug-treated groups were stained with
H&E. The histological structure of the liver and kidneys was
compared under the microscope, and no considerable histological
changes were observed following treatment with capsaicin,
sorafenib, or their combination (Fig.
7E). These results suggest that capsaicin and sorafenib
combination treatment can effectively inhibit the growth, invasion
and metastasis of xenograft hepatocellular tumors in vivo in
a synergistic manner, with well-tolerated toxicity.
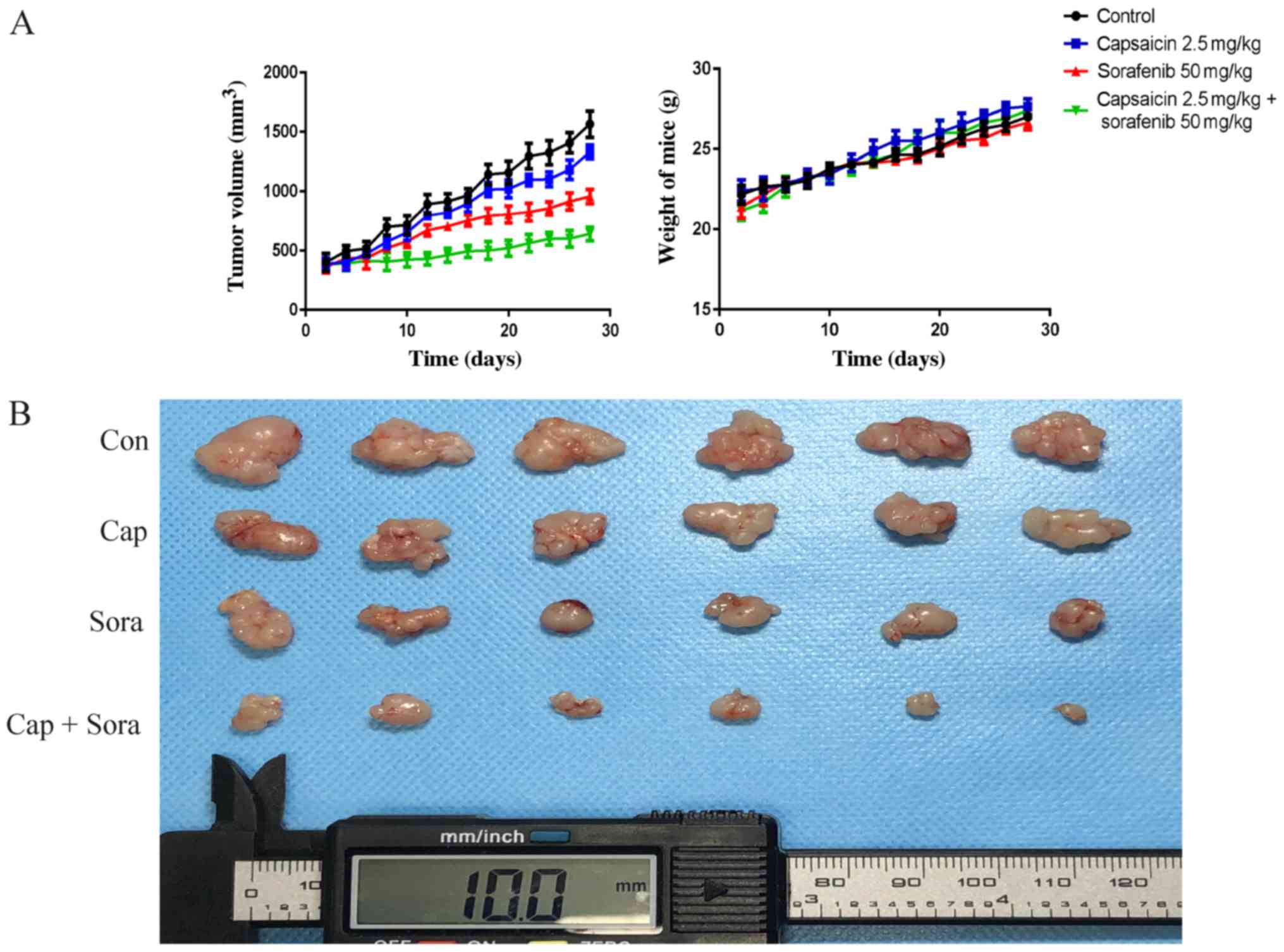 | Figure 7.Capsaicin and sorafenib inhibit HCC
cancer cell growth, invasion and metastasis synergistically in
vivo. (A) 1×107 LM3 cells were inoculated into
BALB/c-nude mice. The mice were randomized into 4 groups (n=6) and
treated with PBS (with DMSO), capsaicin (Cap), sorafenib (Sora) and
their combination daily for 28 days. Tumor volumes and body weight
were measured every 2 days. (B) The tumors were excised after the
last treatment. EGFR, epidermal growth factor receptor; PBS,
phosphate-buffered saline; DMSO, dimethyl sulfoxide. Capsaicin and
sorafenib inhibit HCC cancer cell growth, invasion and metastasis
synergistically in vivo. (C) The apoptosis, autophagy,
invasion, metastasis and phosphorylation of Ki67, Bax, P62, matrix
metalloproteinase (MMP)2, MMP9, P-Akt and P-EGFR in xenograft tumor
tissues were detected by immunohistochemistry (magnification,
×400). Con, control; Cap, capsaicin; Sora, sorafenib. Capsaicin and
sorafenib inhibit HCC cancer cell growth, invasion and metastasis
synergistically in vivo. (D) The liver and kidney
biochemical functions were evaluated. The AST, ALT, BUN, CR and
BUN/CR levels of were detected in mouse blood by ELISA. (E) The
liver and kidneys from the control and the three treatment groups
were stained with hematoxylin and eosin to evaluate the toxicity
after treatment. The histological structures of the liver and
kidney were observed and compared microscopically (magnification,
×200). AST, aspartate aminotransferase; ALT, alanine
aminotransferase; BUN, blood urea nitrogen; CR, creatinine; Con,
control; Cap, capsaicin; Sora, sorafenib. |
Discussion
The multi-kinase inhibitor sorafenib is the only
systemic therapy that improves the survival of patients with
advanced HCC, but its efficacy is not satisfactory. The aim of the
present study was to ascertain the synergistic effect of capsaicin
and sorafenib against HCC. It was observed that capsaicin and
sorafenib exerted synergistic antitumor effects on HCC cells in
vitro as well as in vivo. Capsaicin induces apoptosis
and autophagy through the PI3K/Akt/mTOR pathway (15) and suppresses EGF-induced invasion
and metastasis of tumor cells (30). Sorafenib inhibits HCC growth by
decreasing the expression of PI3K/Akt/mTOR pathway components
(31). In addition, inhibition of
the PI3K/Akt/mTOR pathway enhances sorafenib-induced autophagy in
HCC cells (32,33). EGFR is overexpressed in HCC cells
(27). Overexpression or mutation
of EGFR leads to activation of the PI3K/Akt/mTOR pathway. In
addition, activated Akt affects a variety of biological processes
via phosphorylation cascades involving numerous proteins; Akt
activation promotes tumor cell growth, proliferation, invasion and
metastasis, regulates tumor angiogenesis and inhibits apoptosis
(29).
In the present study, four HCC cell lines were
examined to observe the effects of capsaicin, sorafenib and their
combination on cell proliferation. Capsaicin inhibited the growth
of three HCC cell lines at 48 h, with IC50 values
between 44.7 µM (HuH7) and 95.7 µM (LM3). Sorafenib
inhibited HCC cell growth at 48 h, with IC50 values
between 0.99 µM (HuH7) and 4.02 µM (LM3). Then, HCC
cells were treated with a low concentration of capsaicin and a
moderate concentration of sorafenib, and synergistic inhibitory
effects were observed. The LM3 cell line was selected for
subsequent studies, as it has a strong capacity for growth and is
sensitive to both capsaicin and sorafenib. The 80 µM
capsaicin and 4 µM sorafenib combination was selected for
further studies after analyzing various combinations of capsaicin
and sorafenib in LM3 cells.
We investigated apoptosis, autophagy, invasion and
metastasis in LM3 cells treated with capsaicin and/or sorafenib,
and it was concluded that capsaicin and sorafenib inhibit
proliferation, invasion and metastasis and induce apoptosis and
autophagy in LM3 cells in a synergistic manner. Therefore, the
expression of EGFR/PI3K/Akt/mTOR signaling components was further
examined in LM3 cells. The results demonstrated that capsaicin and
sorafenib inhibited EGFR/PI3K/Akt/mTOR signaling and that their
combination exerted a synergistic inhibitory effect on
EGFR/PI3K/Akt/mTOR signaling in LM3 cells.
Compared with monotherapy, the capsaicin and
sorafenib combination treatment induced apoptosis and autophagy
synergistically, as evidenced by western blotting, TUNEL staining
and immunofluorescence staining. Apoptosis and autophagy play key
roles in cancer progression. Moreover, the same synergistic effect
on invasion and metastasis was observed. Cell scratch, Transwell
migration, Matrigel invasion and immunofluorescence assays all
demonstrated that capsaicin and sorafenib inhibited LM3 cell
invasion and metastasis in a synergistic manner. Activation of
PI3K/Akt/mTOR signaling increases tumor cell apoptosis, invasion
and metastasis; therefore, inhibiting PI3K/Akt/mTOR signaling can
inhibit tumor cell apoptosis, invasion and metastasis (34,35).
Additionally, it has been demonstrated by previous studies that
mTOR (particularly mTORC1) plays an important role in regulating
autophagy, with PI3K/Akt signaling as the key upstream effector
(36,37). In addition, it was reported that
autophagy in HCC cells can be induced by inhibiting the
PI3K/Akt/mTOR pathway (38,39). The present study demonstrated that
the capsaicin and sorafenib combination decreased the expression
levels of P-Akt, PI3K, P-mTOR and P-p70S6K and exerted a
synergistic inhibitory effect on PI3K/Akt/mTOR signaling in LM3
cells. It is likely that capsaicin and sorafenib combination
treatment inhibits LM3 cell proliferation, invasion and metastasis
and enhances apoptosis and autophagy synergistically through the
PI3K/Akt/mTOR pathway. The in vivo experiments yielded the
same results: The combination treatment exerted synergistic effects
on tumor proliferation, invasion and metastasis. P-EGFR and EGFR
levels were next investigated in LM3 cells and found that treatment
with capsaicin or sorafenib alone decreases p-EGFR and EGFR levels,
whereas the combination treatment exerts a synergistic effect. The
combination treatment also decreased P-EGFR and P-Akt levels in a
synergistic manner in mouse xenograft tumors.
In conclusion, a strong growth inhibitory effect of
capsaicin and sorafenib combination was observed in LM3 cells by
decreasing EGFR levels and PI3K/Akt/mTOR downstream signaling.
Capsaicin acted synergistically with sorafenib to inhibit LM3 cell
growth, invasion and metastasis and to enhance apoptosis and
autophagy in vitro as well as in vivo. The
combination treatment is associated with the inhibition of EGFR and
PI3K/Akt/mTOR signaling, with concomitant increases in cleaved
caspase-3, Bax, cleaved PARP, beclin-1, LC3A/B-II and E-cadherin,
and decreases in Bcl-2, P62, N-cadherin, MMP2, MMP9 and vimentin.
Therefore, this combination may be a promising approach to the
treatment of patients with advanced HCC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Funding of China (grant no. 81470874); the Zhejiang
Provincial Natural Science Foundation of China (grant no.
LY13H030009).
Availability of data and materials
All data generated/analyzed in the present study are
available from the corresponding author on reasonable request.
Authors' contributions
QH contributed to the conception of the study; PG
contributed significantly to the analysis and manuscript
preparation; ND and RY performed the data analyses and wrote the
manuscript; HC helped perform the analysis with constructive
discussions; QZ was involved in the conception of the study and
approved the final version of the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All animal procedures were conducted in accordance
with the guidelines of the National Institutes of Health and were
approved by the Ethical Committee of Wenzhou Medical University and
the Laboratory Animal Management Committee of Zhejiang Province
(Approval ID: wydw2017-0052).
Patient consent for publication
Not applicable.
Competing interests
All the authors declare that they have no competing
interests to disclose.
References
|
1
|
Laursen L: A preventable cancer. Nature.
516 Suppl:S2–S3. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gomaa AI, Khan SA, Toledano MB, Waked I
and Taylor-Robinson SD: Hepatocellular carcinoma: Epidemiology,
risk factors and pathogenesis. World J Gastroenterol. 14:4300–4308.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xie B, Wang DH and Spechler SJ: Sorafenib
for treatment of hepatocellular carcinoma: A systematic review. Dig
Dis Sci. 57:1122–1129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Colombo M and Sangiovanni A: Treatment of
hepatocellular carcinoma: Beyond international guidelines. Liver
Int. 35 Suppl 1:S129–S138. 2015. View Article : Google Scholar
|
|
5
|
Llovet JM and Hernandez-Gea V:
Hepatocellular carcinoma: Reasons for phase III failure and novel
perspectives on trial design. Clin Cancer Res. 20:2072–2079. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Osaki M, Oshimura M and Ito H: PI3K-Akt
pathway: Its functions and alterations in human cancer. Apoptosis.
9:667–676. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang H and Chen L: Tumor microenviroment
and hepatocellular carcinoma metastasis. J Gastroenterol Hepatol.
28 Suppl 1:S43–S48. 2013. View Article : Google Scholar
|
|
8
|
Gao JJ, Shi ZY, Xia JF, Inagaki Y and Tang
W: Sorafenib-based combined molecule targeting in treatment of
hepatocellular carcinoma. World J Gastroenterol. 21:12059–12070.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sricharoen P, Lamaiphan N, Patthawaro P,
Limchoowong N, Techawongstien S and Chanthai S: Phytochemicals in
Capsicum oleoresin from different varieties of hot chilli peppers
with their antidiabetic and antioxidant activities due to some
phenolic compounds. Ultrason Sonochem. 38:629–639. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Diaz-Laviada I and Rodriguez-Henche N: The
potential antitumor effects of capsaicin. Prog Drug Res
Fortschritte der Arzneimittelforschung Progres Des Recherches
Pharm. 68:181–208. 2014.
|
|
11
|
Nazıroğlu M, Çiğ B, Blum W, Vizler C,
Buhala A, Marton A, Katona R, Jósvay K, Schwaller B, Oláh Z and
Pecze L: Targeting breast cancer cells by MRS1477, a positive
allosteric modulator of TRPV1 channels. PLoS One. 12:e01799502017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee JY, Lee SY, Kim GG, Hur MG, Yang SD,
Park JH and Kim SW: Development of
68Ga-SCN-DOTA-Capsaicin as an imaging agent targeting
apoptosis and cell cycle arrest in breast cancer. Cancer Biother
Radiopharm. 32:169–175. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoshitani SI, Tanaka T, Kohno H and
Takashima S: Chemoprevention of azoxymethane-induced rat colon
carcinogenesis by dietary capsaicin and rotenone. Int J Oncol.
19:929–939. 2001.PubMed/NCBI
|
|
14
|
Kim YM, Hwang JT, Kwak DW, Lee YK and Park
OJ: Involvement of AMPK signaling cascade in capsaicin-induced
apoptosis of HT-29 colon cancer cells. Ann N Y Acad Sci.
1095:496–503. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin YT, Wang HC, Hsu YC, Cho CL, Yang MY
and Chien CY: Capsaicin induces autophagy and apoptosis in human
nasopharyngeal carcinoma cells by downregulating the PI3K/AKT/mTOR
pathway. Int J Mol Sci. 18:E13432017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu CC, Lin JP, Yang JS, Chou ST, Chen SC,
Lin YT, Lin HL and Chung JG: Capsaicin induced cell cycle arrest
and apoptosis in human esophagus epidermoid carcinoma CE 81T/VGH
cells through the elevation of intracellular reactive oxygen
species and Ca2+ productions and caspase-3 activation. Mutat Res.
601:71–82. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang SP, Chen JC, Wu CC, Chen CT, Tang
NY, Ho YT, Lo C, Lin JP, Chung JG and Lin JG: Capsaicin-induced
apoptosis in human hepatoma HepG2 cells. Anticancer Res.
29:165–174. 2009.PubMed/NCBI
|
|
18
|
Chen X, Tan M, Xie Z, Feng B, Zhao Z, Yang
K, Hu C, Liao N, Wang T, Chen D, et al: Inhibiting
ROS-STAT3-dependent autophagy enhanced capsaicin-induced apoptosis
in human hepatocellular carcinoma cells. Free Radic Res.
50:744–755. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee JH, Kim C, Baek SH, Ko JH, Lee SG,
Yang WM, Um JY, Sethi G and Ahn KS: Capsazepine inhibits JAK/STAT3
signaling, tumor growth, and cell survival in prostate cancer.
Oncotarget. 8:17700–17711. 2017.PubMed/NCBI
|
|
20
|
Xin M and Deng X: Nicotine inactivation of
the proapoptotic function of Bax through phosphorylation. J Biol
Chem. 280:10781–10789. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu X, Long YC and Shen HM: Differential
regulatory functions of three classes of phosphatidylinositol and
phosphoinositide 3-kinases in autophagy. Autophagy. 11:1711–1728.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Choudhari SK, Chaudhary M, Bagde S,
Gadbail AR and Joshi V: Nitric oxide and cancer: A review. World J
Surg Oncol. 11:1182013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qian Y, Corum L, Meng Q, Blenis J, Zheng
JZ, Shi X, Flynn DC and Jiang BH: PI3K induced actin filament
remodeling through Akt and p70S6K1: Implication of essential role
in cell migration. Am J Physiol Cell Physiol. 286:C153–C163. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim D, Kim S, Koh H, Yoon SO, Chung AS,
Cho KS and Chung J: Akt/PKB promotes cancer cell invasion via
increased motility and metalloproteinase production. FASEB J.
15:1953–1962. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Grille SJ, Bellacosa A, Upson J,
Klein-Szanto AJ, van Roy F, Lee-Kwon W, Donowitz M, Tsichlis PN and
Larue L: The protein kinase Akt induces epithelial mesenchymal
transition and promotes enhanced motility and invasiveness of
squamous cell carcinoma lines. Cancer Res. 63:2172–2178.
2003.PubMed/NCBI
|
|
26
|
Nicholson RI, Gee JM and Harper ME: EGFR
and cancer prognosis. Eur J Cancer. 37 Suppl 4:S9–S15. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kannangai R, Sahin F and Torbenson MS:
EGFR is phosphorylated at Ty845 in hepatocellular carcinoma. Mod
Pathol. 19:1456–1461. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
O'Reilly KE, Rojo F, She QB, Solit D,
Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL, et al:
mTOR inhibition induces upstream receptor tyrosine kinase signaling
and activates Akt. Cancer Res. 66:1500–1508. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cheng GZ, Park S, Shu S, He L, Kong W,
Zhang W, Yuan Z, Wang LH and Cheng JQ: Advances of AKT pathway in
human oncogenesis and as a target for anti-cancer drug discovery.
Curr Cancer Drug Targets. 8:2–6. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hwang YP, Yun HJ, Choi JH, Han EH, Kim HG,
Song GY, Kwon KI, Jeong TC and Jeong HG: Suppression of EGF-induced
tumor cell migration and matrix metalloproteinase-9 expression by
capsaicin via the inhibition of EGFR-mediated FAK/Akt, PKC/Raf/ERK,
p38 MAPK, and AP-1 signaling. Mol Nutr Food Res. 55:594–605. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang CZ, Wang XD, Wang HW, Cai Y and Chao
LQ: Sorafenib inhibits liver cancer growth by decreasing mTOR, AKT,
and PI3K expression. J BUON. 20:218–222. 2015.PubMed/NCBI
|
|
32
|
Cui SX, Shi WN, Song ZY, Wang SQ, Yu XF,
Gao ZH and Qu XJ: Des-gamma-carboxy prothrombin antagonizes the
effects of Sorafenib on human hepatocellular carcinoma through
activation of the Raf/MEK/ERK and PI3K/Akt/mTOR signaling pathways.
Oncotarget. 7:36767–36782. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jiang S, Wang Q, Feng M, Li J, Guan Z, An
D, Dong M, Peng Y, Kuerban K and Ye L: C2-ceramide enhances
sorafenib-induced caspase-dependent apoptosis via PI3K/AKT/mTOR and
Erk signaling pathways in HCC cells. Appl Microbiol Biotechnol.
101:1535–1546. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee DH, Szczepanski MJ and Lee YJ:
Magnolol induces apoptosis via inhibiting the EGFR/PI3K/Akt
signaling pathway in human prostate cancer cells. J Cell Biochem.
106:1113–1122. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang L, Wang F, Jiang Y, Xu S, Lu F, Wang
W and Sun X and Sun X: Migration of retinal pigment epithelial
cells is EGFR/PI3K/AKT dependent. Front Biosci (Schol Ed).
5:661–671. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
He C and Klionsky DJ: Regulation
mechanisms and signaling pathways of autophagy. Ann Rev Genet.
43:67–93. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jacinto E, Facchinetti V, Liu D, Soto N,
Wei S, Jung SY, Huang Q, Qin J and Su B: SIN1/MIP1 maintains
rictor-mTOR complex integrity and regulates Akt phosphorylation and
substrate specificity. Cell. 127:125–137. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li TT, Zhu D, Mou T, Guo Z, Pu JL, Chen
QS, Wei XF and Wu ZJ: IL-37 induces autophagy in hepatocellular
carcinoma cells by inhibiting the PI3K/AKT/mTOR pathway. Mol
Immunol. 87:132–140. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang SS, Chen YH, Chen N, Wang LJ, Chen
DX, Weng HL, Dooley S and Ding HG: Hydrogen sulfide promotes
autophagy of hepatocellular carcinoma cells through the
PI3K/Akt/mTOR signaling pathway. Cell Death Dis. 8:e26882017.
View Article : Google Scholar : PubMed/NCBI
|