Introduction
Biomarkers are important for guiding the diagnosis
and treatment of cancer. One such marker is the metastasis gene
osteopontin (OPN), the expression of which supports progression and
dissemination (1,2), and reduces the prospects for survival
(3,4). However, OPN is also secreted from
immune system cells and physiologically acts as a cytokine. The
gene product is subject to alternative splicing in transformed
cells, which generates three distinct forms (5). While in studies prior to 2006 total
OPN was measured, the splice variants (the expression of which
varies by cancer type) may be more sensitive markers than pan-OPN.
Further, any additional information that can put into context the
reliability of OPN splice variants in diagnosis, prognosis, and
prediction may be of value. In this regard, autoantibodies that
neutralize OPN function could be a modulating influence.
Being highly expressed in malignant tumors, the OPN
forms have the potential to act as tumor-associated antigens. This
possibility is particularly given for the splice variants OPN-b and
OPN-c, which are absent from healthy tissue. Therefore, the host
immune system is not tolerized to these potential autoantigens. In
various immune diseases, anti-OPN autoantibodies have been
described to arise (6,7–9), even
though they do not occur in healthy individuals (10). Cancer may also generate anti-OPN
autoimmunity (11,12). It is conceivable that such
autoantibodies (to OPN or its splice-variants) will neutralize OPN
function. Taken together, these elements raise the possibility that
antibodies to OPN forms, produced by some cancer patients, are
capable of inactivating their targets and thus having a propensity
for improving the prognosis. It is not known which splice forms may
be responsible for stimulating the generation of
autoantibodies.
Here we tested the prevalence of antibodies to the
three variant forms of OPN in the blood of cancer patients. We
hypothesized that the occurrence of OPN splice variants in these
patients may stimulate an autoimmune response, and that patients
who have autoantibodies to OPN splice variants experience a milder
course of the disease. We found autoantibodies in a variable
fraction of patients, averaging 21% across all cancers studied.
While the reactivity of the autoantibodies is consistent with the
differential appearance of splice variants in individual
malignancies, autoantibodies are much more common to full-length
osteopontin than to either osteopontin-b or osteopontin-c.
Materials and methods
Patients
This study contained serum samples from patients
enrolled at the Tumor Bank of the University of Cincinnati Cancer
Institute (UCCITB). Plasma samples from healthy donors were
obtained from CHTN Midwestern Division (Columbus, OH, USA). Serum
from thyroid tumors and controls was provided by Johns Hopkins
Medical Institute (JHMI). Of 175 patients, we tested serum. Of 29
patients we tested plasma, among whom 14 were non-cancer
controls.
Competitive ELISA
The antibodies used in this study were O-17 (cat.
no. 18625; IBL America, Minneapolis, MN, USA), anti-hOPNc IgY (cat.
no. AhOPNc; Gallus Immunotech, Cary, NC, USA; Exalpha Biologicals,
Shirley, MA, USA) and LF161 (Dr Larry Fisher, NIH). The polyclonal
rabbit antibody O-17 recognizes an epitope upstream of the splice
junctions and thus is common to all three forms of OPN
(anti-pan-OPN). The IgY antibody recognizes the OPN-c splice
junction. The polyclonal rabbit antibody LF161 has reactivity
selectively with exon 4 (present in OPN-a and -b).
We assessed the presence of anti-OPN autoantibodies
by competitive solid-phase enzyme-linked immunosorbent assay
(ELISA). For the detection of autoantibodies to OPN splice
variants, the plates were coated with GST-OPN (-a or -b or -c).
Specific autoantibodies in the serum can bind to the plated
antigens, but the binding is reduced if soluble antigen is added in
excess together with the serum to compete with the plated antigen
for the autoantibodies. The soluble Ag-Ab complex formed with the
soluble antigen is eliminated in the washes, and the absorbance
measured is reflective of the autoantibody bound to the coated
antigen. We avoided setting up a sandwich ELISA, because of
previously reported heterogeneity in the results with this format
(13).
GST fusion proteins of the human OPN splice variants
-a, -b, and -c were generated from pGEX-6P constructs and purified
over GSH-agarose columns. High-binding plates were coated with 40
ng/100 µl/well of recombinant osteopontin in phosphate buffer
saline (PBS) overnight at 4°C. On the following day, the plates
were blocked with 200 µl/well 5% bovine serum albumin (BSA) in PBS
for 2 h at room temperature with gentle shaking. Following three
washes with PBST (PBS containing 0.5% Tween-20), serum samples
(200-, 400-, or 800-fold final dilution) were added in PBST/1% BSA.
For competition wells, the same GST-OPN form as had been plated was
added at 2 µg/100 µl/well. Otherwise, PBST/1% BSA was added to a
volume of 100 µl/well. As reference standards, anti-osteopontin
antibodies (O-17, LF161, or anti-hOPNc IGY) were used (with and
without competing GST-OPN) in separate wells. The plates were
incubated for 2 h at room temperature with gentle shaking, before
washing five times. This was followed by addition of 100 µl/well of
HRP-conjugated goat anti-human IgG (1:2,000 diluted; cat. no.
5220-0279; KPL/SeraCare, Milford, MA, USA) for 1 h. The reference
wells received their appropriate secondary antibodies. After 3
washes, 100 µl/well of the detection reagent TMB
(3,3′,5,5′-tetramethylbenzidine; Surmodics BioFx, Eden Prairie, MN,
USA) was provided and the reaction was terminated with stopping
reagent (Surmodics TMB Stop Solution) at the appropriate time. The
optical density (OD) of each well was read at a wavelength of 450
nm.
A main challenge in using human serum samples in
ELISA is a high and variable background of non-specific binding. To
minimize this effect, we tested various blocking buffers, including
5% FBS (fetal bovine serum), ELISA Ultrablock (AbD Serotec), 5% BSA
(bovine serum albumin), and 1% BSA. Whereas ultra-block (which
contains predominantly fish protein) increased the non-specific
absorbance, the others had some efficacy in suppressing it. BSA
(5%) was determined to be the most suitable choice (Fig. 1).
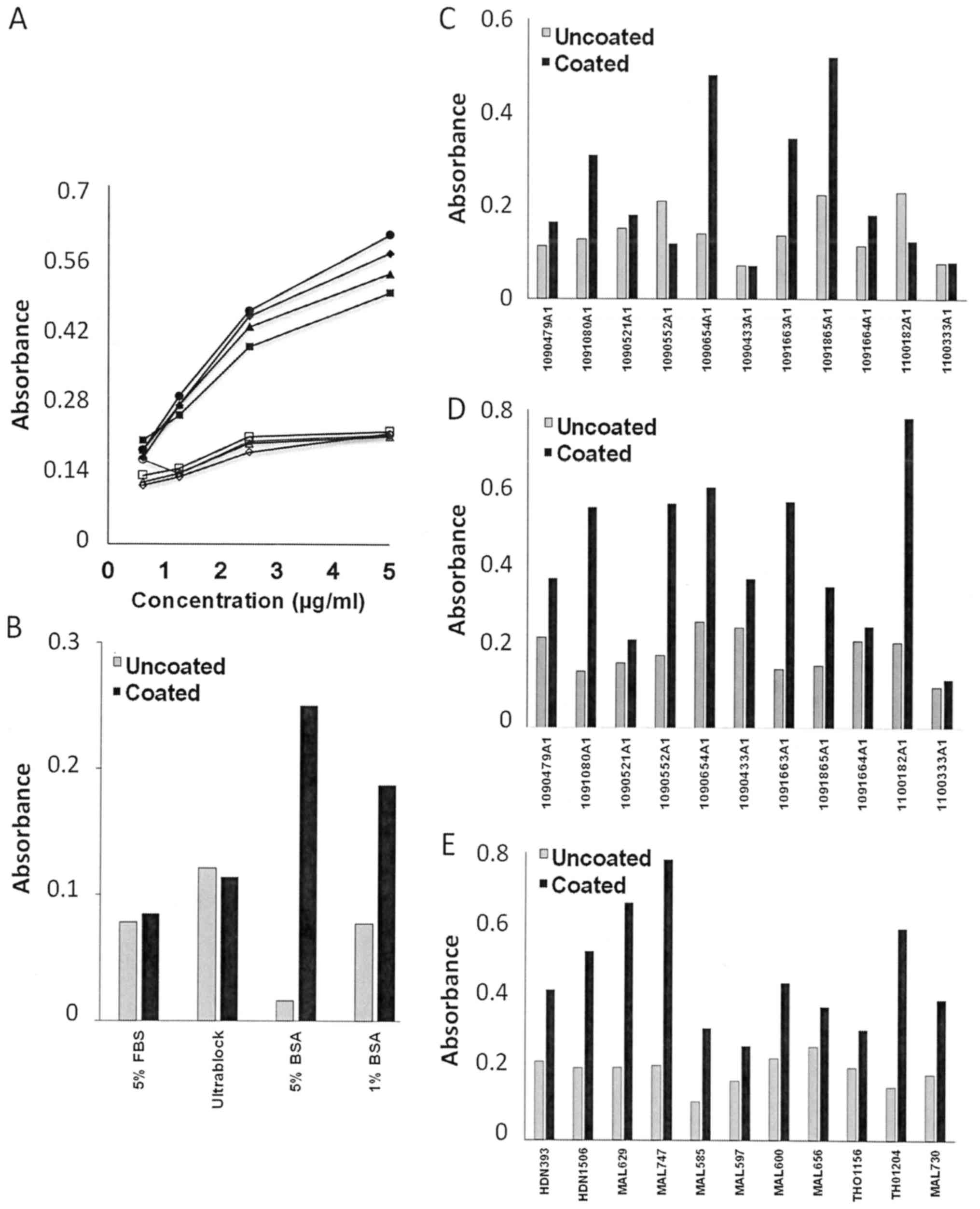 | Figure 1.Effects of various blocking modalities
in ELISA. (A) Purified Ig is inert to the modality of blocking. The
plate was coated with GST-OPN-c 40 ng/100 µl/well. The blocking
buffers compared included 5% FBS (circles), Ultrablock (diamonds),
5% BSA (triangles) and 1% BSA (squares). Probing commenced with
anti-hOPNc IgY (1:3,200, 1:6,400, 1:12,800 and 1:25,600), followed
by HRP-conjugated goat anti-human IgG (1:2,000), and specificity of
the signal was corroborated by comparing competition with soluble
GST-OPN-c 2 µg/100 µl/well (open symbols) vs. no competition
(filled symbols). (B) Various blocking buffers impact signal and
background absorbance of plasma samples. The plate was coated with
GST-OPN 40 ng/100 µl/well. On the next day, the plate was blocked
with 5% FBS, Ultrablock, 5% BSA or 1% BSA in distinct wells. A
representative plasma sample was added (1:400) to the
antigen-coated as well as the antigen-uncoated wells for detection
with HRP-anti-human Ig. (C and D) Testing 1% BSA (C) or 5% BSA (D)
with the same set of plasma samples. The plate was coated with or
without GST-OPN-a, then blocked with the indicated concentrations
of BSA. Plasma samples were added (1:400) to the antigen-coated
wells and antigen-uncoated wells. (E) BSA at 5% was also found to
be suitable for blocking of serum samples. Serum samples were added
(1:400) to the antigen-coated wells (OPN-a) and antigen-uncoated
wells. Each analysis was performed at least twice; one
representative experiment is shown. |
The format of a competitive ELISA may benefit from a
preincubation of the antibody with the competing antigen. However,
we found that preincubation of the serum with the competing GST-OPN
did not make a significant difference in relation to adding the
reagents directly to the coated wells. The absorbance values
between preincubated and directly added wells differed by only
±0.92% (data not shown). Two protocols of competition were
evaluated.
i) We applied competition with the GST-OPN form that
was identical to the plated form (Fig.
2A-E). This modality of competition assesses the specificity of
the absorbance signal, it does not identify the epitope or splice
variant specificity of auto-antibodies present.
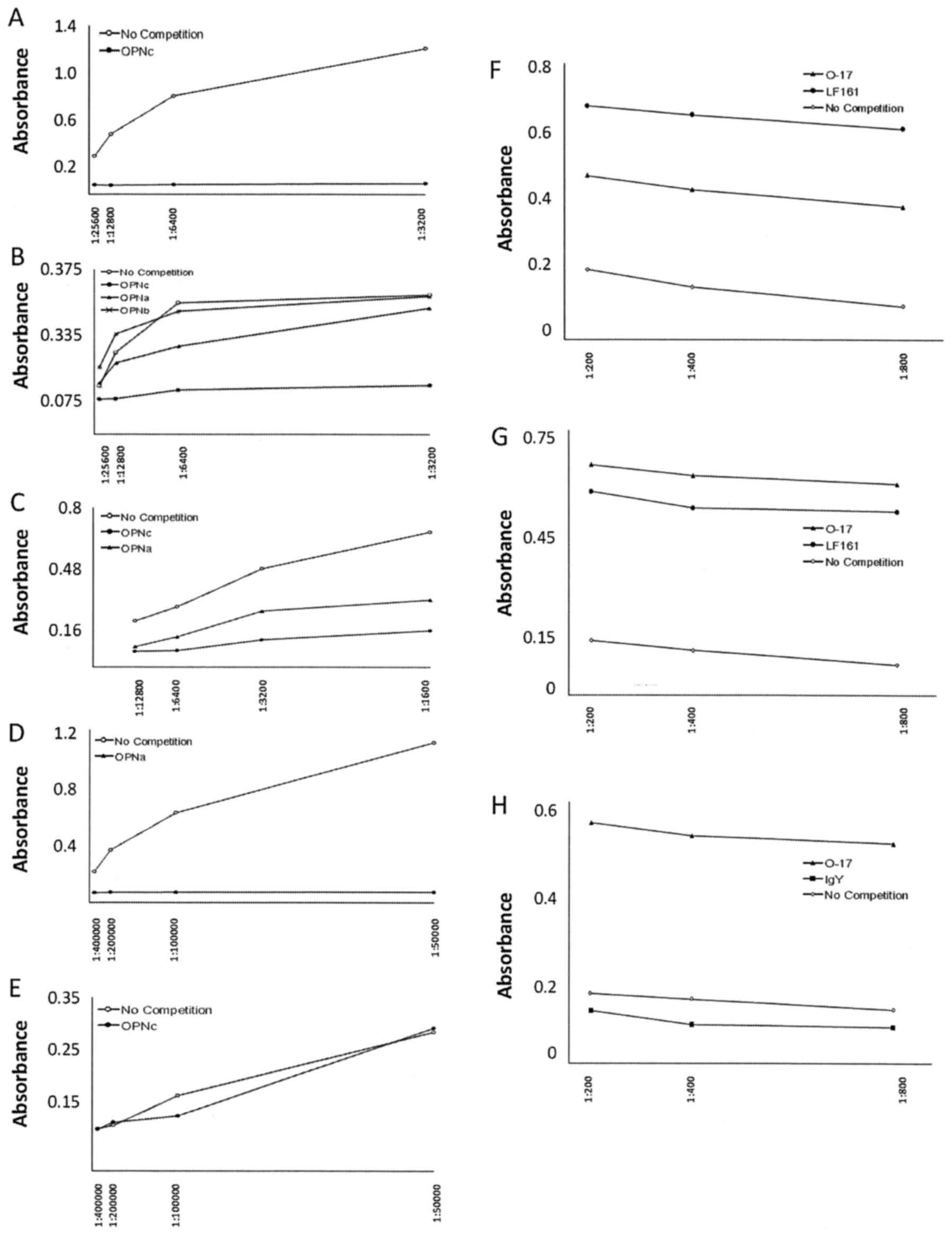 | Figure 2.ELISA validation. In all cases, the
plate was coated with the suitable form of GST-OPN overnight. The
next day, the plate was blocked with 5% BSA. Serial dilutions of
the primary antibody were added in the absence of competition or in
the presence of soluble GST-OPN. Detection was accomplished by
binding of secondary HRP-conjugated anti-Ig antibody and color
development with TMZ reagent. The graphs show one representative
experiment of many independent replicates. (A-E) Competition with
GST-OPN utilizing positive control antibodies. LF161 and IgY
recognize distinct forms of bound GST-OPN, while O-17 binds to a
common epitope. The absorbance signal generated in solid-phase
ELISA can be competed by the addition of a suitable form of soluble
GST-OPN at 2 µg/100 µl/well. (A) Testing the competition with
GST-OPN-c as an indicator for the binding specificity by
anti-osteopontin-c IgY, added in a dilution series (1:3,200,
1:6,400, 1:12,800, 1:25,600). (B) Testing the competition with
OPN-a and OPN-b (the plate was coated with GST-OPN-c.) vs.
anti-osteopontin-c IgY. No competitor or soluble GST-OPN-a,
GST-OPN-b or GST-OPN-c were added in separate wells. (C) Testing
the competition by GST-OPN-a or GST-OPN-c against the binding of
the anti-pan-OPN antibody O-17 (diluted at 1:1,600, 1:3,200,
1:6,400, 1:12,800). The plate was coated with GST-OPN-b. (D)
Testing the competition with GST-OPN-a as an indicator for the
binding specificity by anti-osteopontin-a/b LF161, added in a
dilution series (1:50,000, 1:100,000, 1:200,000, 1:400,000). The
plate was coated with GST-OPN-b. The curves reflect either no
competition of competition with soluble GST-OPN-a. (E) Testing the
competition with GST-OPN-c as an indicator for the binding
specificity by anti-osteopontin-a/b LF161. The plate was coated
with GST-OPN-a. (F-H) ELISA with antibody competition. Secondary
anti-human antibody crossreacts with rabbit Ig (O-17, LF161), but
not with chicken IgY (anti-hOPNc). (F) The plate was coated with
GST-OPN-a. On the following day, the wells were blocked with 5% BSA
for 2 h. A human serum sample was added for measurement at serial
dilution (1:100, 1:200, 1:400 and 1:800). For the competition wells
LF161, O-17 or no competition were added in separate wells.
Detection was achieved with HRP-conjugated goat anti-human IgG
(1:2,000), followed by the color reagents. (G) The plate was coated
with GST-OPN-b. A human serum sample was added for measurement at
serial dilution. For the competition wells LF161, O-17 or no
competition were added in separate wells. (H) The plate was coated
with GST-OPN-c. A human serum sample was added for measurement at
serial dilution. For the competition wells LF161, IgY or no
competition were added in separate wells. |
ii) The alternative competition with
anti-osteopontin antibodies O-17 (a polyclonal rabbit antibody
recognizes all forms of OPN), LF161 (a polyclonal rabbit antibody
that recognizes exon-4 in OPN-a and OPN-b) or IgY (anti-hOPN
antibody recognizes OPN-c) became problematic. The secondary
anti-hIg antibody cross-reacted with the rabbit polyclonal
antibodies O-17 and LF161. This left the competition with IgY as a
viable option for testing reactivity against osteopontin-c
(Fig. 2F-H).
Statistical analysis
To account for sample-to-sample variation in
background absorbance, the ratio of uncompeted to competed
absorbance (reflective of the specific signal) was calculated and
used in the biostatistical analysis. For each cancer specimen and
each of the OPN splice variants, the ratios from repeat experiments
were compared for significant differences to the mean values of
ratios from all healthy controls using a one-tailed t-test for
samples with unequal variance (accounting for
heteroscedasticity).
Results
Patient characteristics
Specimens from 204 patients and controls were
obtained from three sources: The University of Cincinnati Tumor
Bank, Collaborative Human Tissue Network (CHTN), and Johns Hopkins
Medical Institute. They covered thyroid cancers, skin cancers, lung
cancers, pancreatic cancers, and breast cancers, as well as
remission from breast cancers, benign breast tumors, and no-tumor
controls (Table I).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Cancer type | N | Sex
(male/female) | Average age
(range) | Race | Follow-up |
|---|
| Thyroid | 5 | 2/3 | 43.6 (19–65) | 3 W 2 B | 0 a; 1 b; 1 c |
| Thyroid | 50 | (unknown) | (unknown) | (unknown) | None |
| Non-melanoma
skin | 5 | 4/1 | 73.8 (70–78) | 5 W | 0 a; 1 b; 4 c |
| Lung | 52 | 27/25 | 62.1 (29–84) | 45 W 6 B 1 O | 0 a; 15 b; 20 c |
| Pancreas | 25 | 13/12 | 61.6 (28–83) | 12 W 4 B | 0 a; 5 b; 12 c |
| Breast | 49 | 0/49 | 58.9 (30–86) | 40 W 7 B 1 A 1 O | 7 a; 15 b; 10 c |
| Breast cancer
remission | 2 | 0/2 | 57.5 (49–66) | 2 W | None |
| Benign breast
tumor | 2 | 0/2 | 48.5 (39–58) | 2 W | None |
| Healthy controls | 14 | 4/7 (3 unknown) | 43.1 (18–65) | 13 W 1 B | None |
| Total | 204 |
|
|
|
|
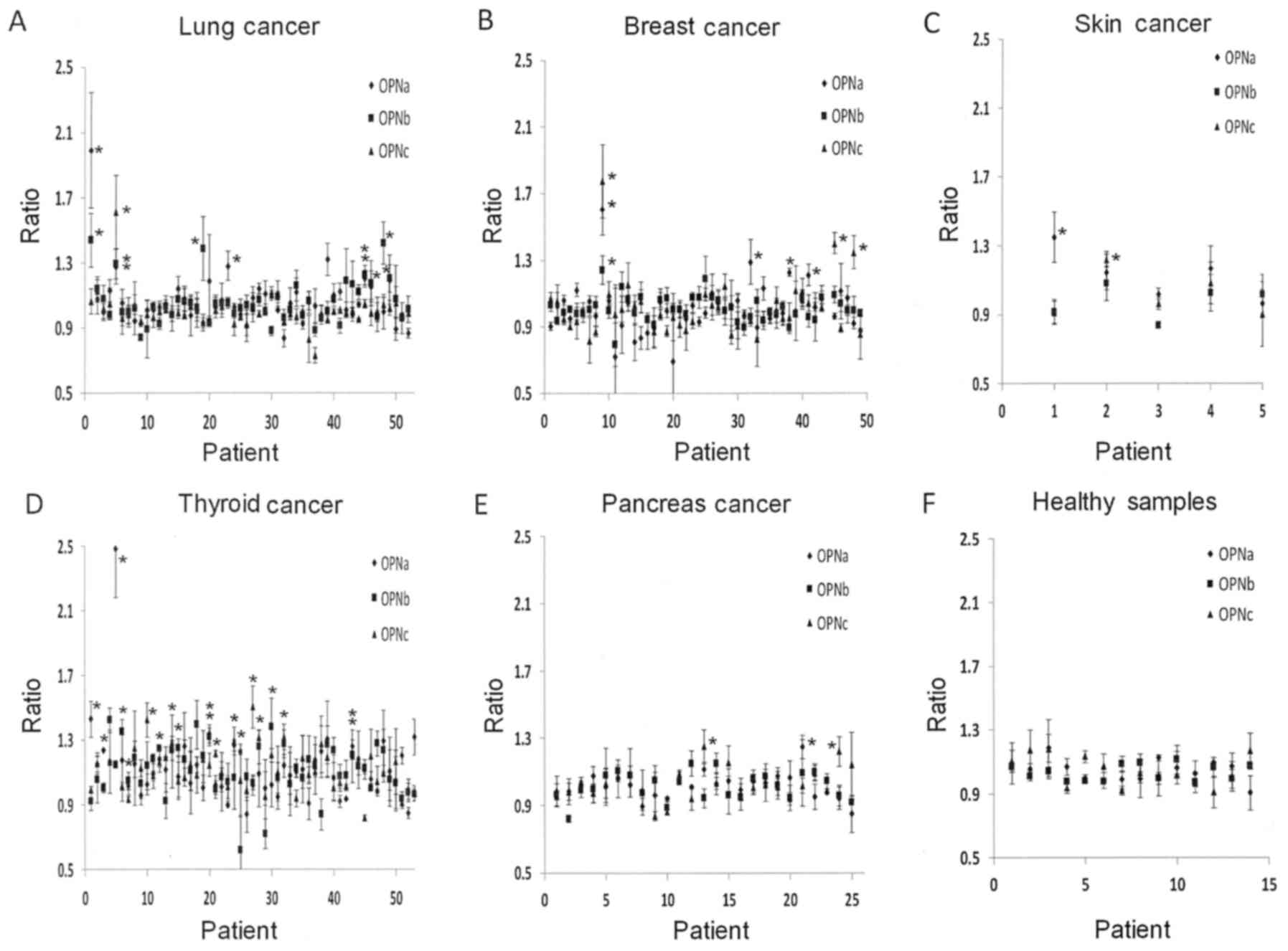
Presence of autoantibodies
Of the 186 cancer patients, 2 patients in remission
from breast cancer, and 2 patients with benign breast tumors (190
in total), 39 (21%) had autoantibodies to OPN. The largest fraction
of autoantibodies was found in thyroid cancers at 19 of 55 patients
(35%). Among all other tumor patients, 20 of 135 patients (15%) had
autoantibodies to OPN. Only 2 had pan-OPN reactivity, 4 were
specific for OPN-a/b, 1 was specific for OPN-a/c, 13 were
selectively specific for OPN-a, 7 for OPN-b, and 12 for OPN-c
(Fig. 3, summarized in Table II). Consistent with the
preferential expression of certain splice variants in individual
tumors (14,15), only autoantibodies were observed
that matched the occurrence of the cognate OPN form (no anti-OPNb
in breast cancer, from which OPN-b is absent; no anti-OPNc in lung
cancer, which does not produce this form).
 | Table II.Splice variant-specific osteopontin
autoantibodies in cancer. |
Table II.
Splice variant-specific osteopontin
autoantibodies in cancer.
| Cancer type | N | Anti-panOPN | Anti-OPNa/b | Anti-OPNa/c | Anti-OPNb/c | Anti-OPNa | Anti-OPNb | Anti-OPNc | Total | Percentage |
|---|
| Thyroid | 55 | – | 2 | 1 | – | 6 | 3 | 7 | 19 | 35 |
| Non-melanoma
skin | 5 | – | – | – | – | 1 | – | 1 | 2 | 40 |
| Lung | 52 | 1 | 2 | – | – | 2 | 4 | − | 9 | 17 |
| Pancreas | 25 | – | – | – | – | 1 | – | 2 | 3 | 12 |
| Breast | 49 | 1 | – | – | – | 3 | – | 2 | 6 | 12 |
| Breast cancer
remission | 2 | – | – | – | – | − | – | − | 0 | 0 |
| Benign breast
tumor | 2 | – | – | – | – | − | – | − | 0 | 0 |
| Total | 190 | 2 | 4 | 1 | 0 | 13 | 7 | 12 |
|
|
| Percentage |
| 1 | 2 | 1 | 0 | 7 | 4 | 6 |
|
|
| Non-thyroid
total | 135 | 2 | 2 | 0 | 0 | 7 | 4 | 5 |
|
|
| Non-thyroid
percentage |
| 1 | 1 | 0 | 0 | 5 | 3 | 4 |
|
|
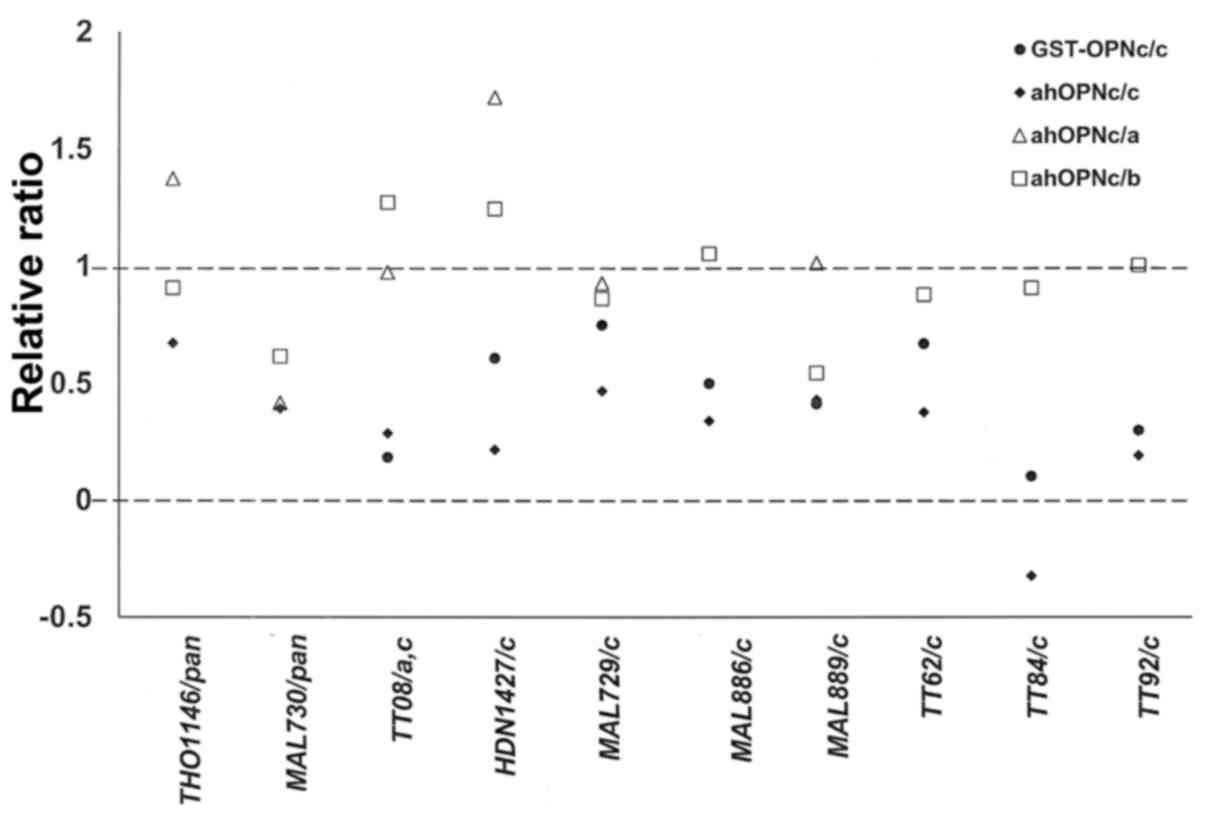
While competition with the same form of GST-OPN that
was plated indicated specificity of the auto-antibody signal, it
gave no indication of the affected epitope. Our anti-hOPNc chicken
antibody had been raised to the splice junction of OPN-c. We
therefore used it as a competing agent. Expectedly, the antibody
had only a minor effect on serum reactivity when GST-OPNa or
GST-OPNb was plated in the ELISA. Where GST-OPNc was plated, and
competition with GST-OPNc had previously indicated the presence of
OPN-c-specific autoantibodies, the competition with anti-hOPNc
confirmed that the autoantibodies were directed at the splice
junction. The effect was similar for a serum sample with reactivity
to OPN-a and OPN-c, however, against serum with anti-pan-OPN
autoantibodies, the competition with anti-hOPNc was not effective,
likely because the autoantigenic epitope was located away from the
spice junction (Fig. 4).
Autoantibodies and disease
severity
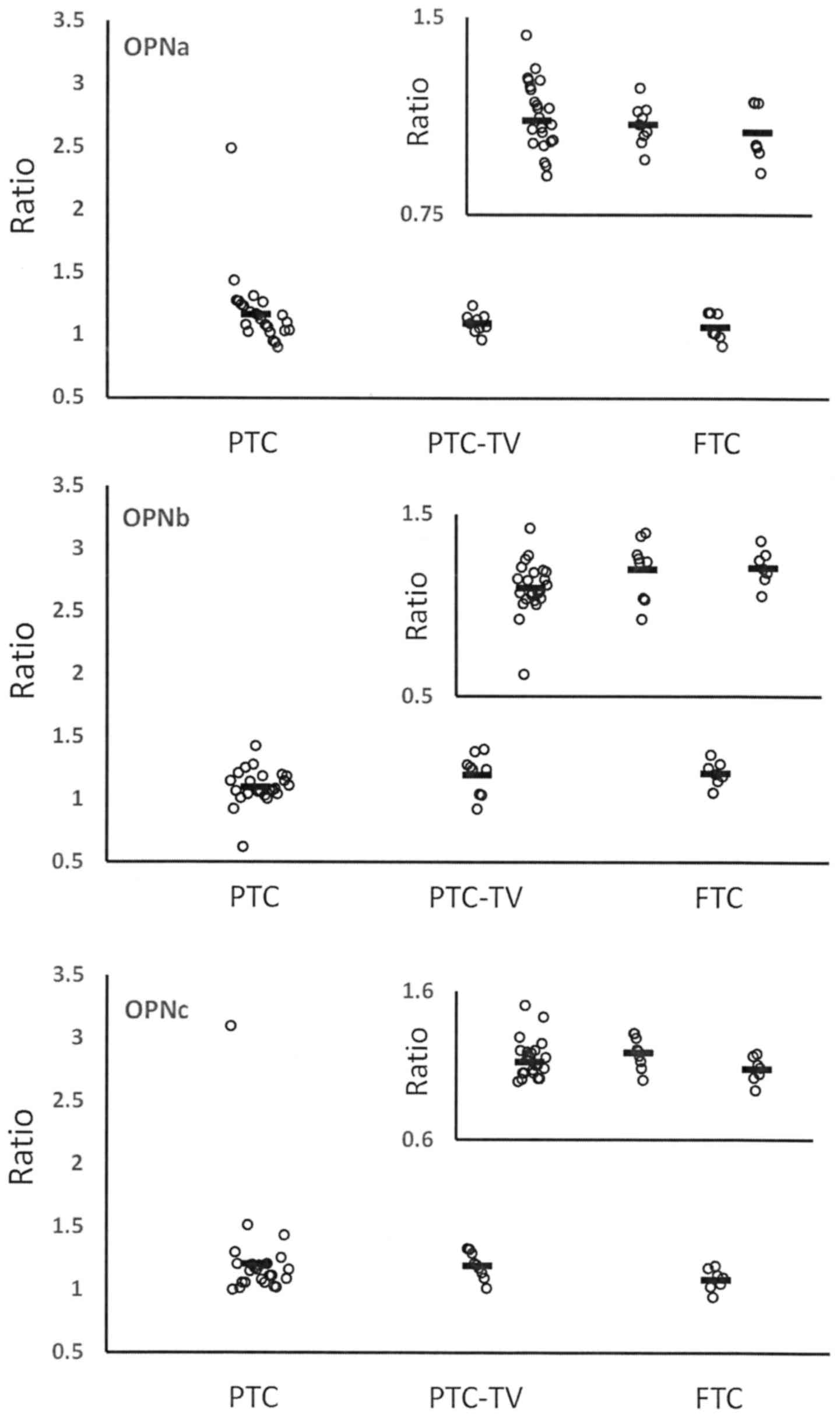
Papillary (PTC) and follicular thyroid carcinoma
(FTC) are distinct forms of differentiated thyroid carcinoma.
Within PTC, the follicular variant (PTC-FV) is common. While it
displays variations in clinical presentation, the long-term outcome
is similar to classical PTC (16).
Here, the two manifestations are grouped together. The tall cell
variant (PTC-TV) is more aggressive and has intermediate prognosis
between other forms of PTC and FTC (17). FTC is associated with poorer
survival than PTC (18). For the
thyroid cancers, the autoantibody reactivities to OPN-a and OPN-c
were higher in mild (PTC) vs. aggressive (FTC) disease (Fig. 5). We found no anti-OPNa or anti-OPNc
antibodies in the FTC specimens. This is consistent with the
possibility that neutralization of OPN-a and/or OPN-c may
counteract progression and ameliorate the course of the disease. By
contrast, there was no correlation of autoantibody reactivity with
the levels of TG (thyroglobulin), the presence of anti-TG
antibodies, TSH (thyroid-stimulating hormone), or RAI (radioactive
iodine) uptake (data not shown). This indicates that anti-OPN
auto-antibodies provide distinct information on the disease status
from the conventional molecular indicators.
Autoantibodies and prognosis
We hypothesized that the presence of autoantibodies
would generate a more favorable disease outcome. For the
non-thyroid cancers, no correlation was discernible with disease
stage or grade (data not shown). This could be due to a lack of
power in 20 patients with autoantibodies and 135 patients without
autoantibodies. The available follow-up information was very
limited (see Table I), with only 47
patients having been tracked for more than 12 months. Further
research will test the prognostic power of OPN variants in the
presence or absence of auto-antibodies in cancer.
Discussion
In the present study, we identified autoantibodies
to spliced forms of OPN. Approximately 21% of cancer patients were
found to harbor such antibodies. The proportion was particularly
high in thyroid cancers (35%) as compared to other tumor types
(15%). The reactivity of the autoantibodies was consistent with the
appearance of splice variants in individual cancers. Breast cancer
expresses OPN-a and -c, but not OPN-b (15,19,20).
Liver cancer has elevated levels of OPN-a and -b, but not -c
(14).
Without therapeutic intervention, the immunogenicity
of most cancers is low, and some even engage active mechanisms of
immune escape. Other cancers, by contrast, may express
tumor-associated antigens that can act in an immunostimulatory
fashion. This mechanism typically requires the presentation, via
MHC class I or class II, of epitopes to which the host is not
tolerized. It then leads to the production of tumor-reactive
effector T-cells or antibodies. Tumors considered to have strong
antigenicity include melanoma, lung cancer, renal carcinoma, and
colon cancer. By contrast, the paucity of breast antigenicity has
been a longstanding disappointment in the immunotherapy community.
For OPN variants, the ability to act as tumor-associated antigens
is uncertain. The unspliced form is present in the healthy body,
where it acts as an inducer of the cellular immune defense
(21) but does not stimulate
autoimmunity (7,10,11).
By contrast, the splice variants, which seem to have arisen very
recently in evolution (Table
III), are not synthesized without transformation and their
junctions represent tumor-associated neo-epitopes. Whereas the
uniqueness of their splice junction sequences renders the
acquisition of tolerance during immune maturation improbable, their
inherent immunogenicity is predicted to be low. The rare occurrence
of autoantibodies, specific to either OPN-b or OPN-c, suggests that
the breaking of tolerance to full-length OPN is more prevalent in
tumor antigenicity than a reaction to a poorly immunogenic
neo-epitope.
 | Table III.Prevalence of sequences that match
the splice junctions in osteopontin. |
Table III.
Prevalence of sequences that match
the splice junctions in osteopontin.
| A, pBlast search
was conducted for the splice junctions of OPN-b |
|---|
|
|---|
| Sequence | Description | Score | Query cover
(%) | E value | Ident. (%) | Accession no. | Uncon-firmed |
|---|
| AAV38944.1 | Secreted
phosphoprotein 1 (synthetic construct) | 47.3 | 100 | 5.00E-05 | 94 | AAV38944.1 | n/a |
| AAP36151.1 | Homo sapiens
secreted phosphoprotein 1 (synthetic construct) | 47.3 | 100 | 5.00E-05 | 94 | AAP36151.1 | n/a |
| XP_023053890.1 | Osteopontin isoform
X3 (Piliocolobus tephrosceles) | 47.3 | 100 | 5.00E-05 | 94 | XP_023053890.1 | * |
| NP_001126162.1 | Osteopontin
precursor (Pongo abelii) | 47.3 | 100 | 5.00E-05 | 94 | NP_001126162.1 | * |
| XP_021567722.1 | Osteopontin isoform
X2 (Carlito syrichta) | 47.3 | 100 | 5.00E-05 | 94 | XP_021567722.1 | * |
| BAA05950.1 | OPN-b (Homo
sapiens) | 47.3 | 100 | 5.00E-05 | 94 | BAA05950.1 |
|
| NP_000573.1 | Osteopontin isoform
OPN-b precursor (Homo sapiens) | 47.3 | 100 | 5.00E-05 | 94 | NP_000573.1 |
|
| ABM86681.1 | Secreted
phosphoprotein 1 (synthetic construct) | 47.3 | 100 | 5.00E-05 | 94 | ABM86681.1 | n/a |
| XP_004039151.2 | PREDICTED:
osteopontin isoform X2 (Gorilla gorilla gorilla) | 47.3 | 100 | 5.00E-05 | 94 | XP_004039151.2 |
|
| XP_003950415.1 | PREDICTED:
osteopontin isoform X2 (Pan troglodytes) | 47.3 | 100 | 5.00E-05 | 94 | XP_003950415.1 |
|
| XP_011807212.1 | PREDICTED:
osteopontin isoform X3 (Colobus angolensis palliatus) | 47.3 | 100 | 5.00E-05 | 94 | XP_011807212.1 |
|
| XP_011846123.1 | PREDICTED:
osteopontin isoform X3 (Mandrillus leucophaeus) | 47.3 | 100 | 5.00E-05 | 94 | XP_011846123.1 |
|
| XP_008961899.1 | PREDICTED:
osteopontin isoform X2 (Pan paniscus) | 47.3 | 100 | 5.00E-05 | 94 | XP_008961899.1 |
|
| XP_008141789.1 | PREDICTED:
osteopontin isoform X2 (Eptesicus fuscus) | 47.3 | 100 | 5.00E-05 | 94 | XP_008141789.1 |
|
| XP_007997396.1 | PREDICTED:
osteopontin isoform X2 (Chlorocebus sabaeus) | 47.3 | 100 | 5.00E-05 | 94 | XP_007997396.1 |
|
| XP_004661666.1 | PREDICTED:
osteopontin isoform X2 (Jaculus jaculus) | 43.9 | 100 | 8.00E-04 | 88 | XP_004661666.1 |
|
| XP_008828427.1 | PREDICTED:
osteopontin isoform X2 (Nannospalax galili) | 43.9 | 100 | 8.00E-04 | 88 | XP_008828427.1 |
|
| XP_006867814.1 | PREDICTED:
osteopontin isoform X2 (Chrysochloris asiatica) | 43.1 | 100 | 0% | 88 | XP_006867814.1 |
|
| XP_003790145.1 | PREDICTED:
osteopontin (Otolemur garnettii) | 43.1 | 100 | 0% | 88 | XP_003790145.1 |
|
| Search sequence:
SQKQNLLAPQETLPSKS |
|
| B, pBlast search
was conducted for the splice junctions of OPN-c |
|
|
Sequence |
Description | Score | Query cover
(%) | E value | Ident.
(%) |
|
|
|
| XP_023053891.1 | Osteopontin isoform
X4 (Piliocolobus tephrosceles) | 50.3 | 100 | 4.00E-06 | 100 |
|
|
| XP_021567723.1 | Osteopontin isoform
X3 (Carlito syrichta) | 50.3 | 100 | 4.00E-06 | 100 |
|
|
| NP_001035149.1 | Osteopontin isoform
OPN-c precursor (Homo sapiens) | 50.3 | 100 | 4.00E-06 | 100 |
|
|
| BAA05951.1 | OPN-c (Homo
sapiens) | 50.3 | 100 | 4.00E-06 | 100 |
|
|
| XP_005341953.1 | Osteopontin isoform
X2 (Ictidomys tridecemlineatus) | 44.3 | 93 | 5.00E-04 | 93 |
|
|
| XP_006931039.1 | Osteopontin isoform
X2 (Felis catus) | 44.3 | 100 | 5.00E-04 | 94 |
|
|
| XP_021066705.1 | Osteopontin (Mus
pahari) | 42.2 | 93 | 0.003 | 93 |
|
|
| XP_003434071.1 | Osteopontin isoform
X2 (Canis lupus familiaris) | 41.8 | 100 | 0.004 | 88 |
|
|
| ABA40758.1 | Osteopontin
(Canis lupus familiaris) | 41.8 | 100 | 0.004 | 88 |
|
|
| EPY87927.1 | Osteopontin isoform
OPN-c precursor (Camelus ferus) | 40.1 | 100 | 0.015 | 81 |
|
|
| AEA49027.1 | Osteopontin-C
(Homo sapiens) | 40.1 | 75 | 0.016 | 100 |
|
|
| XP_003950416.1 | PREDICTED:
osteopontin isoform X3 (Pan troglodytes) | 50.3 | 100 | 4.00E-06 | 100 |
|
|
| XP_014994236.1 | PREDICTED:
osteopontin isoform X1 (Macaca mulatta) | 50.3 | 100 | 4.00E-06 | 100 |
|
|
| XP_011807213.1 | PREDICTED:
osteopontin isoform X4 (Colobus angolensis palliatus) | 50.3 | 100 | 4.00E-06 | 100 |
|
|
| XP_011846124.1 | PREDICTED:
osteopontin isoform X4 (Mandrillus leucophaeus) | 50.3 | 100 | 4.00E-06 | 100 |
|
|
| XP_008961900.1 | PREDICTED:
osteopontin isoform X3 (Pan paniscus) | 50.3 | 100 | 4.00E-06 | 100 |
|
|
| XP_017652830.1 | PREDICTED:
osteopontin isoform X3 (Nannospalax galili) | 50.3 | 100 | 4.00E-06 | 100 |
|
|
| XP_012415080.1 | PREDICTED:
osteopontin isoform X3 (Trichechus manatus latirostris) | 50.3 | 100 | 4.00E-06 | 100 |
|
|
| XP_014334291.1 | PREDICTED:
osteopontin isoform X3 (Bos mutus) | 47.7 | 100 | 3.00E-05 | 94 |
|
|
| XP_004266185.1 | PREDICTED:
osteopontin isoform X3 (Orcinus orca) | 47.3 | 100 | 4.00E-05 | 94 |
|
|
| XP_007450745.1 | PREDICTED:
osteopontin isoform X3 (Lipotes vexillifer) | 47.3 | 100 | 4.00E-05 | 94 |
|
|
| XP_016016596.1 | PREDICTED:
osteopontin isoform X2 (Rousettus aegyptiacus) | 44.3 | 100 | 5.00E-04 | 88 |
|
|
| XP_008141790.1 | PREDICTED:
osteopontin isoform X3 (Eptesicus fuscus) | 44.3 | 100 | 5.00E-04 | 88 |
|
|
| XP_015354628.1 | PREDICTED:
osteopontin isoform X2 (Marmota marmota marmota) | 44.3 | 93 | 5.00E-04 | 93 |
|
|
| XP_019320322.1 | PREDICTED:
osteopontin isoform X2 (Panthera pardus) | 44.3 | 100 | 5.00E-04 | 94 |
|
|
| XP_014929118.1 | PREDICTED:
osteopontin isoform X2 (Acinonyx jubatus) | 44.3 | 100 | 5.00E-04 | 94 |
|
|
| XP_007074758.1 | PREDICTED:
osteopontin isoform X2 (Panthera tigris altaica) | 44.3 | 100 | 5.00E-04 | 94 |
|
|
| XP_006867815.1 | PREDICTED:
osteopontin isoform X3 (Chrysochloris asiatica) | 41.4 | 93 | 0.005 | 87 |
|
|
| Search sequence:
SGSSEEKQNAVSSEET |
The association of disease with deviations of the
immune system is common. At the fringes of the phenotypical
spectrum, this can manifest as immune suppression or as
autoimmunity. In cancer, the affliction of an organ such as the
thymus, which is involved in the development of the immune system,
predisposes to autoimmune reactions. Once the immune system has
turned against its host, epitope spreading may occur and lead to
the generation of autoantibodies against multiple antigens,
regardless of their roles in disease generation. Cytokines may be
the targets of autoantibodies in tumorous as well as in autoimmune
diseases.
Autoantibodies to multiple cytokines are ubiquitous
in healthy individuals, although their presence has been reported
to be variable. Focusing on the detection of cytokine/autoantibody
complexes in 15 healthy individuals, reactivity was detected to
IL-2, IL-8, TNF-α, VEGF and G-CSF, but not to OPN, IL-3 or M-CSF
(10). In patients with early-stage
osteoarthritis of the knee, the prevalence of autoantibodies to
cartilage OPN, intermediate layer protein, YKL-39, and cyclic
citrullinated peptide suggests that autoimmune processes are
involved in the disease that is widely considered to be caused by
wear and tear. Anti-OPN antibodies were detected in 0% (0/67) of
healthy controls and in 8.1% (11/136) of osteoarthritis patients,
specifically with grades II and III but not with grade IV (7). OPN is one of the autoantigens in
osteoarthritis and rheumatoid arthritis, with major epitopes being
conformational. Autoantibodies to native OPN were present in 9.5%
(11/105) of osteoarthritis serum samples and 15% (13/88) of
rheumatoid arthritis serum samples. The existence of anti-OPN
antibodies may be linked to disease severity in rheumatoid
arthritis as it correlates with high serum levels of rheumatoid
factor and C-reactive protein and with accelerated erythrocyte
sedimentation rate (8).
Insulin-dependent diabetes mellitus has an autoimmune origin, for
which autoantigens include insulin, glutamic acid decarboxylase,
and the tyrosine phosphatases IA-2ic, and IA-2b. About 7% of
Insulin-dependent diabetes mellitus sera may contain anti-OPN
antibodies. In the condition, OPN is an autoantigen, which is
located in the somatostatin cells of the islets (9). The blinding disease autoimmune uveitis
is caused by autoreactive T-cells attacking the inner eye, and is
accompanied by autoantibodies. In recurrent uveitis, autoantibodies
were identified to the three autoantigens OPN, extracellular matrix
protein 1, and metalloproteinase inhibitor 2. OPN reactivity was
present in 25.7% (19/74) of recurrent uveitis vitreous samples,
whereas all controls were negative (0/19) (6) (Table
IV).
 | Table IV.Literature data on the frequency of
anti-osteopontin autoantibodies. |
Table IV.
Literature data on the frequency of
anti-osteopontin autoantibodies.
| Healthy %
(n/total) | Osteoarthritis %
(n/total) | Rheumatoid
arthritis % (n/total) | Autoimmune uveitis
% (n/total) | Insulin-dependent
diabetes % (n/total) | Hashimoto
thyroiditis % (n/total) | Chronic hepatitis %
(n/total) | Liver cirrhosis %
(n/total) | Hepatocellular
carcinoma % (n/total) | Benign prostate
hyperplasia % (n/total) | Prostate cancer %
(n/total) | (Refs.) |
|---|
| 0% (0/15) |
|
|
|
|
|
|
|
|
|
| (10) |
| 0% (0/67) | 8.1% (11/136) |
|
|
|
|
|
|
|
|
| (7) |
| | 9.5% (11/105) | 15% (13/88) |
|
|
|
|
|
|
|
| (8) |
| 0% (0/19) |
|
| 25.7% |
|
|
|
|
|
|
| (6) |
|
|
|
| (19/74) |
|
|
|
|
|
|
|
|
| 3.9% (3/76) |
|
|
| 7.3% (5/68) | 100% (1/1) |
|
|
|
|
| (9) |
| 0% (0/75) |
|
|
|
|
| 3.1% (1/32) | 15.6% (5/32) | 12.8% (19/148) |
|
| (11) |
| 10% (3/30) |
|
|
|
|
|
|
|
| 33% (6/18) | 62% (18/29) | (12) |
Autoimmunity may be associated with cancer. The
frequency of anti-OPN autoantibodies in hepatocellular carcinoma
was found to be 12.8% (19/148), compared to liver cirrhosis at
15.6% (5/32), and chronic hepatitis at 3.1% (1/32). There was 0%
reactivity (0/75) in serum from healthy donors. The association of
autoantibodies with prognosis was not assessed (11). By immunoblotting, arguably a less
quantitative and less reliable technique, the frequency of anti-OPN
autoantibodies was described as 66% in prostate cancer, compared to
33% in benign prostate hyperplasia and 10% in healthy serum donors
(12).
Acknowledgements
We are grateful to Dr Neal Fedarko, Johns Hopkins
Medical Institute for generously sharing 50 thyroid cancer samples.
We also thank Dr Larry Fisher, NIH, for having generously provided
the antibody LF161 (anti-osteopontin-exon-4).
Funding
The present study was supported by the Marlene
Harris-Ride Cincinnati/Pilot Program to GFW.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author upon reasonable
request.
Authors' contributions
GFW designed the research. LKA mainly collected the
data. GFW and LKA performed the statistical analysis and analyzed
the data. Both authors read and approved the manuscript and agree
to be accountable for all aspects of the research in ensuring that
the accuracy or integrity of all parts of the study are
appropriately addressed.
Ethics approval and consent to
participate
The study was covered by University of
Cincinnati/IRB Protocol 04-01-29-01: Osteopontin splice variants in
human breast cancer, Exemption 4.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Senger DR, Perruzzi CA and Papadopoulos A:
Elevated expression of secreted phosphoprotein I (osteopontin, 2ar)
as a consequence of neoplastic transformation. Anticancer Res.
9:1291–1299. 1989.PubMed/NCBI
|
|
2
|
Craig AM, Smith JH and Denhardt DT:
Osteopontin, a transformation-associated cell adhesion
phosphoprotein, is induced by 12-O-tetradecanoylphorbol 13-acetate
in mouse epidermis. J Biol Chem. 264:9682–9689. 1989.PubMed/NCBI
|
|
3
|
Weber GF, Lett GS and Haubein NC:
Osteopontin is a marker for cancer aggressiveness and patient
survival. Br J Cancer. 103:861–869. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weber GF, Lett GS and Haubein NC:
Categorical meta-analysis of Osteopontin as a clinical cancer
marker. Oncol Rep. 25:433–441. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
He B, Mirza M and Weber GF: An osteopontin
splice variant induces anchorage independence in human breast
cancer cells. Oncogene. 25:2192–2202. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Merl J, Deeg CA, Swadzba ME, Ueffing M and
Hauck SM: Identification of autoantigens in body fluids by
combining pull-downs and organic precipitations of intact immune
complexes with quantitative label-free mass spectrometry. J
Proteome Res. 12:5656–5665. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Du H, Masuko-Hongo K, Nakamura H, Xiang Y,
Bao CD, Wang XD, Chen SL, Nishioka K and Kato T: The prevalence of
autoantibodies against cartilage intermediate layer protein,
YKL-39, osteopontin, and cyclic citrullinated peptide in patients
with early-stage knee osteoarthritis: Evidence of a variety of
autoimmune processes. Rheumatol Int. 26:35–41. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sakata M, Tsuruha JI, Masuko-Hongo K,
Nakamura H, Matsui T, Sudo A, Nishioka K and Kato T: Autoantibodies
to osteopontin in patients with osteoarthritis and rheumatoid
arthritis. J Rheumatol. 28:1492–1495. 2001.PubMed/NCBI
|
|
9
|
Fierabracci A, Biro PA, Yiangou Y, Mennuni
C, Luzzago A, Ludvigsson J, Cortese R and Bottazzo GF: Osteopontin
is an autoantigen of the somatostatin cells in human islets:
Identification by screening random peptide libraries with sera of
patients with insulin-dependent diabetes mellitus. Vaccine.
18:342–354. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Watanabe M, Uchida K, Nakagaki K, Kanazawa
H, Trapnell BC, Hoshino Y, Kagamu H, Yoshizawa H, Keicho N, Goto H
and Nakata K: Anti-cytokine autoantibodies are ubiquitous in
healthy individuals. FEBS Lett. 581:2017–2021. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ying X, Zhao Y, Wang JL, Zhou X, Zhao J,
He CC, Guo XJ, Jin GH, Wang LJ, Zhu Q and Han SX: Serum
anti-osteopontin autoantibody as a novel diagnostic and prognostic
biomarker in patients with hepatocellular carcinoma. Oncol Rep.
32:1550–1556. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tilli TM, Silva EA, Matos LC, Faget DV,
Dias BF, Vasconcelos JS, Yokosaki Y and Gimba ER: Osteopontin is a
tumor autoantigen in prostate cancer patients. Oncol Lett.
2:109–114. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Anborgh PH, Wilson SM, Tuck AB, Winquist
E, Schmidt N, Hart R, Kon S, Maeda M, Uede T, Stitt LW and Chambers
AF: New dual monoclonal ELISA for measuring plasma osteopontin as a
biomarker associated with survival in prostate cancer: Clinical
validation and comparison of multiple ELISAs. Clin Chem.
55:895–903. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hartung F and Weber GF: RNA Blood levels
of osteopontin splice variants are cancer markers. Springerplus.
2:1102013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mirza M, Shaughnessy E, Hurley JK,
Vanpatten KA, Pestano GA, He B and Weber GF: Osteopontin-c is a
selective marker for breast cancer. Int J Cancer. 122:889–897.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu XM, Schneider DF, Leverson G, Chen H
and Sippel RS: Follicular variant of papillary thyroid carcinoma is
a unique clinical entity: A population-based study of 10,740 cases.
Thyroid. 23:1263–1268. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ghossein R and Livolsi VA: Papillary
thyroid carcinoma tall cell variant. Thyroid. 18:1179–1181. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aboelnaga EM and Ahmed RA: Difference
between papillary and follicular thyroid carcinoma outcomes: An
experience from Egyptian institution. Cancer Biol Med. 12:53–59.
2015.PubMed/NCBI
|
|
19
|
Zduniak K, Agrawal A, Agrawal S, Hossain
MM, Ziolkowski P and Weber GF: Osteopontin splice variants are
differential predictors of breast cancer treatment responses. BMC
Cancer. 16:4412016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zduniak K, Ziolkowski P, Ahlin C, Agrawal
A, Agrawal S, Blomqvist C, Fjällskog ML and Weber GF: Nuclear
Osteopontin-c is a prognostic breast cancer marker. Br J Cancer.
112:729–738. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ashkar S, Weber GF, Panoutsakopoulou V,
Sanchirico ME, Janssen M, Zawaideh S, Rittling S, Denhardt DT,
Glimcher MJ and Cantor H: Eta-1 (Osteopontin): An early component
of type 1 (cell-mediated) immunity. Science. 287:860–864. 2000.
View Article : Google Scholar : PubMed/NCBI
|