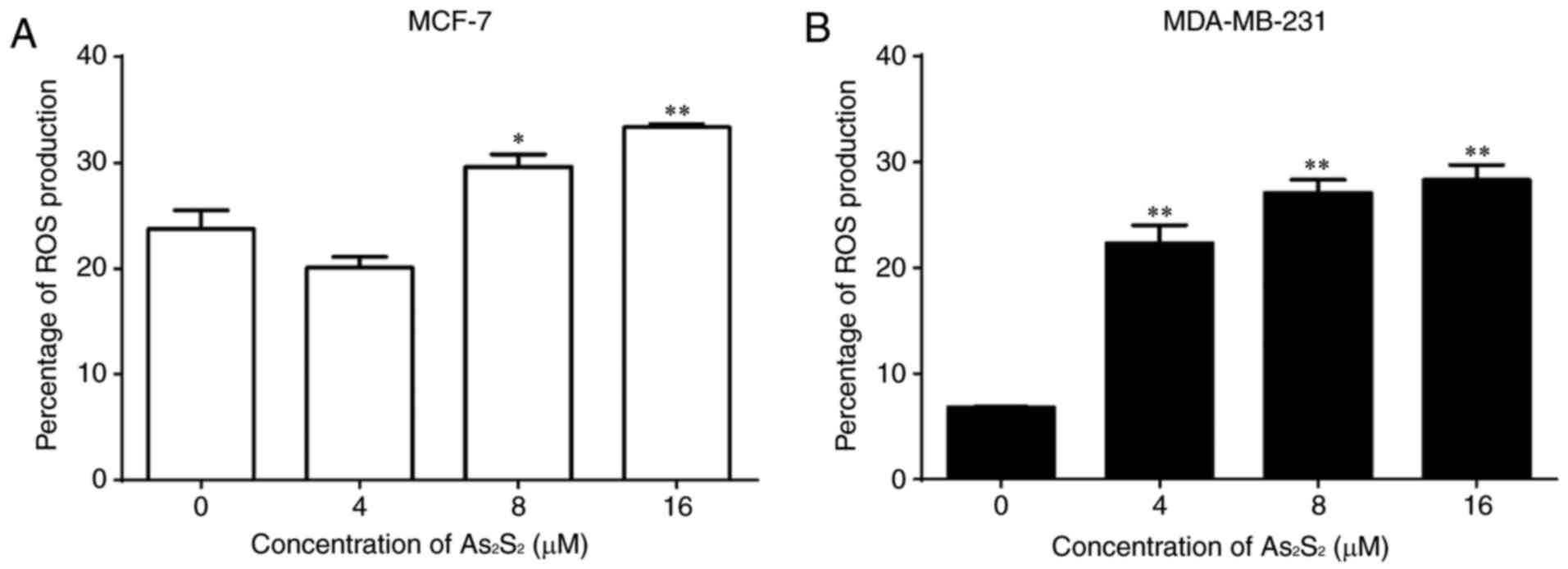
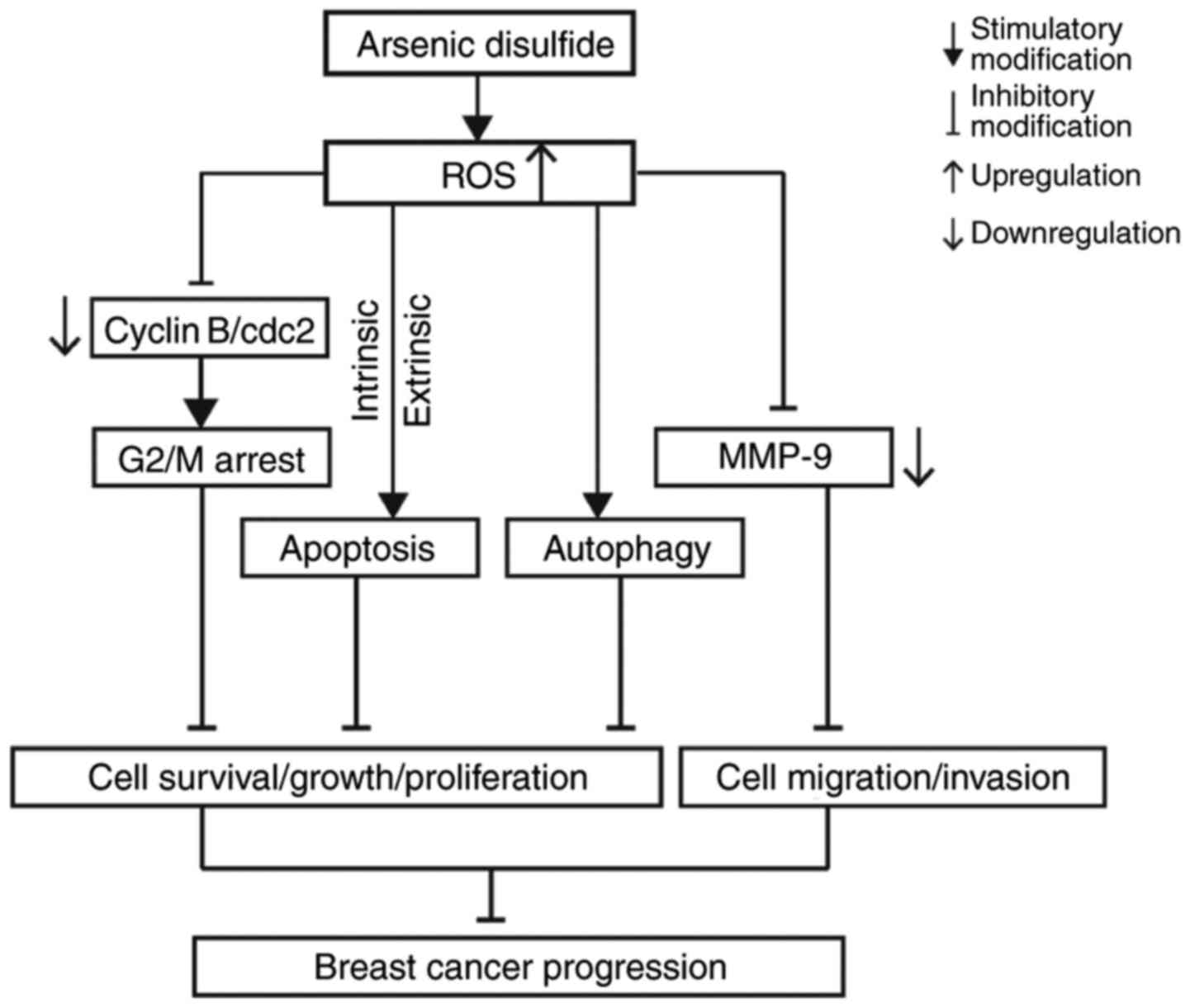
|
1
|
Wang H, Liu Z, Gou Y, Qin Y, Xu Y, Liu J
and Wu JZ: Apoptosis and necrosis induced by novel realgar quantum
dots in human endometrial cancer cells via endoplasmic reticulum
stress signaling pathway. Int J Nanomedicine. 10:5505–5512. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang L, Zhou GB, Liu P, Song JH, Liang Y,
Yan XJ, Xu F, Wang BS, Mao JH, Shen ZX, et al: Dissection of
mechanisms of Chinese medicinal formula Realgar-Indigo naturalis as
an effective treatment for promyelocytic leukemia. Proc Natl Acad
Sci USA. 105:4826–4831. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang QY, Mao JH, Liu P, Huang QH, Lu J,
Xie YY, Weng L, Zhang Y, Chen Q, Chen SJ, et al: A systems biology
understanding of the synergistic effects of arsenic sulfide and
Imatinib in BCR/ABL-associated leukemia. Proc Natl Acad Sci USA.
106:3378–3383. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu Y, He P, Cheng X and Zhang M:
Long-term outcome of 31 cases of refractory acute promyelocytic
leukemia treated with compound realgar natural indigo tablets
administered alternately with chemotherapy. Oncol Lett.
10:1184–1190. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang L, Kim S, Ding W, Tong Y, Zhang X,
Pan M and Chen S: Arsenic sulfide inhibits cell migration and
invasion of gastric cancer in vitro and in vivo. Drug Des Devel
Ther. 9:5579–5590. 2015.PubMed/NCBI
|
|
6
|
Wang G, Zhang T, Sun W, Wang H, Yin F,
Wang Z, Zuo D, Sun M, Zhou Z, Lin B, et al: Arsenic sulfide induces
apoptosis and autophagy through the activation of ROS/JNK and
suppression of Akt/mTOR signaling pathways in osteosarcoma. Free
Radic Biol Med. 106:24–37. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Song P, Chen P, Wang D, Wu Z, Gao Q, Wang
A, Zhu R, Wang Y, Wang X, Zhao L, et al: Realgar transforming
solution displays anticancer potential against human hepatocellular
carcinoma HepG2 cells by inducing ROS. Int J Oncol. 50:660–670.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qin YU, Wang H, Liu ZY, Liu J and Wu JZ:
Realgar quantum dots induce apoptosis and necrosis in HepG2 cells
through endoplasmic reticulum stress. Biomed Rep. 3:657–662. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu JZ and Ho PC: Evaluation of the in
vitro activity and in vivo bioavailability of realgar nanoparticles
prepared by cryo-grinding. Eur J Pharm Sci. 29:35–44. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ding W, Zhang L, Kim S, Tian W, Tong Y,
Liu J, Ma Y and Chen S: Arsenic sulfide as a potential anti-cancer
drug. Mol Med Rep. 11:968–974. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tse WP, Cheng CH, Che CT and Lin ZX:
Arsenic trioxide, arsenic pentoxide, and arsenic iodide inhibit
human keratinocyte proliferation through the induction of
apoptosis. J Pharmacol Exp Ther. 326:388–394. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao Y, Onda K, Yuan B, Tanaka S, Kiyomi
A, Sugiyama K, Sugiura M, Takagi N and Hirano T: Arsenic
disulfide-induced apoptosis and its potential mechanism in two- and
three-dimensionally cultured human breast cancer MCF-7 cells. Int J
Oncol. 52:1959–1971. 2018.PubMed/NCBI
|
|
13
|
Zhao Y, Yuan B, Onda K, Sugiyama K, Tanaka
S, Takagi N and Hirano T: Anticancer efficacies of arsenic
disulfide through apoptosis induction, cell cycle arrest, and
pro-survival signal inhibition in human breast cancer cells. Am J
Cancer Res. 8:366–386. 2018.PubMed/NCBI
|
|
14
|
Uematsu N, Zhao Y, Kiyomi A, Yuan BO, Onda
K, Tanaka S, Sugiyama K, Sugiura M, Takagi N, Hayakawa A and Hirano
T: Chemo-sensitivity of two-dimensional monolayer and
three-dimensional spheroid of breast cancer MCF-7 cells to
daunorubicin, docetaxel, and arsenic disulfide. Anticancer Res.
38:2101–2108. 2018.PubMed/NCBI
|
|
15
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: Defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Michailidou K, Hall P, Gonzalez-Neira A,
Ghoussaini M, Dennis J, Milne RL, Schmidt MK, Chang-Claude J,
Bojesen SE, Bolla MK, et al: Large-scale genotyping identifies 41
new loci associated with breast cancer risk. Nat Genet. 45:353–361,
361e1-2. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Recht A, Come SE, Henderson IC, Gelman RS,
Silver B, Hayes DF, Shulman LN and Harris JR: The sequencing of
chemotherapy and radiation therapy after conservative surgery for
early-stage breast cancer. N Engl J Med. 334:1356–1361. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cicconi L and Lo-Coco F: Current
management of newly diagnosed acute promyelocytic leukemia. Ann
Oncol. 27:1474–1481. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chow SK, Chan JY and Fung KP: Suppression
of cell proliferation and regulation of estrogen receptor alpha
signaling pathway by arsenic trioxide on human breast cancer MCF-7
cells. J Endocrinol. 182:325–337. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang X, Zhang X, Xu Z, Wang Z, Yue X and
Li H: Reversal effect of arsenic sensitivity in human leukemia cell
line K562 and K562/ADM using realgar transforming solution. Biol
Pharm Bull. 36:641–648. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie QJ, Cao XL, Bai L, Wu ZR, Ma YP and Li
HY: Anti-tumor effects and apoptosis induction by Realgar
bioleaching solution in Sarcoma-180 cells in vitro and transplanted
tumors in mice in vivo. Asian Pac J Cancer Prev. 15:2883–2888.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Engelberg-Kulka H, Amitai S, Kolodkin-Gal
I and Hazan R: Bacterial programmed cell death and multicellular
behavior in bacteria. PLoS Genet. 2:e1352006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fuchs Y and Steller H: Programmed cell
death in animal development and disease. Cell. 147:742–758. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maiuri MC, Zalckvar E, Kimchi A and
Kroemer G: Self-eating and self-killing: Crosstalk between
autophagy and apoptosis. Nat Rev Mol Cell Biol. 8:741–752. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Booth LA, Tavallai S, Hamed HA,
Cruickshanks N and Dent P: The role of cell signalling in the
crosstalk between autophagy and apoptosis. Cell Signal. 26:549–555.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zimmermann KC, Bonzon C and Green DR: The
machinery of programmed cell death. Pharmacol Ther. 92:57–70. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Levine B and Deretic V: Unveiling the
roles of autophagy in innate and adaptive immunity. Nat Rev
Immunol. 7:767–777. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Eisenberg-Lerner A, Bialik S, Simon HU and
Kimchi A: Life and death partners: Apoptosis, autophagy and the
cross-talk between them. Cell Death Differ. 16:966–975. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zambrano J and Yeh ES: Autophagy and
apoptotic crosstalk: Mechanism of therapeutic resistance in
HER2-positive breast cancer. Breast Cancer. 10:13–23.
2016.PubMed/NCBI
|
|
30
|
Shi D, Liu Y, Xi R, Zou W, Wu L, Zhang Z,
Liu Z, Qu C, Xu B and Wang X: Caveolin-1 contributes to realgar
nanoparticle therapy in human chronic myelogenous leukemia K562
cells. Int J Nanomedicine. 11:5823–5835. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pastorek M, Gronesova P, Cholujova D,
Hunakova L, Bujnakova Z, Balaz P, Duraj J, Lee TC and Sedlak J:
Realgar (As4S4) nanoparticles and arsenic trioxide (As2O3) induced
autophagy and apoptosis in human melanoma cells in vitro.
Neoplasma. 61:700–709. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shi X, Zhang Y, Zheng J and Pan J:
Reactive oxygen species in cancer stem cells. Antioxid Redox
Signal. 16:1215–1228. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gupta SC, Hevia D, Patchva S, Park B, Koh
W and Aggarwal BB: Upsides and downsides of reactive oxygen species
for cancer: The roles of reactive oxygen species in tumorigenesis,
prevention, and therapy. Antioxid Redox Signal. 16:1295–1322. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Trachootham D, Alexandre J and Huang P:
Targeting cancer cells by ROS-mediated mechanisms: A radical
therapeutic approach? Nat Rev Drug Discov. 8:579–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jing Y, Dai J, Chalmers-Redman RM, Tatton
WG and Waxman S: Arsenic trioxide selectively induces acute
promyelocytic leukemia cell apoptosis via a hydrogen
peroxide-dependent pathway. Blood. 94:2102–2111. 1999.PubMed/NCBI
|
|
36
|
Chen YC, Lin-Shiau SY and Lin JK:
Involvement of reactive oxygen species and caspase 3 activation in
arsenite-induced apoptosis. J Cell Physiol. 177:324–333. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Maeda H, Hori S, Nishitoh H, Ichijo H,
Ogawa O, Kakehi Y and Kakizuka A: Tumor growth inhibition by
arsenic trioxide (As2O3) in the orthotopic
metastasis model of androgen-independent prostate cancer. Cancer
Res. 61:5432–5440. 2001.PubMed/NCBI
|
|
38
|
Jayakumar AR, Bak LK, Rama Rao KV,
Waagepetersen HS, Schousboe A and Norenberg MD: Neuronal cell death
induced by mechanical percussion trauma in cultured neurons is not
preceded by alterations in glucose, lactate and glutamine
metabolism. Neurochem Res. 41:307–315. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT,
Liu B and Bao JK: Programmed cell death pathways in cancer: A
review of apoptosis, autophagy and programmed necrosis. Cell
Prolif. 45:487–498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li Y, Chang Y, Ye N, Dai D, Chen Y, Zhang
N, Sun G and Sun Y: Advanced glycation end products inhibit the
proliferation of human umbilical vein endothelial cells by
inhibiting cathepsin D. Int J Mol Sci. 18:E4362017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Aparicio IM, Martin Muñoz P, Salido GM,
Peña FJ and Tapia JA: The autophagy-related protein LC3 is
processed in stallion spermatozoa during short- and long-term
storage and the related stressful conditions. Animal. 10:1182–1191.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Singh BN, Singh HB, Singh A, Naqvi AH and
Singh BR: Dietary phytochemicals alter epigenetic events and
signaling pathways for inhibition of metastasis cascade:
Phytoblockers of metastasis cascade. Cancer Metastasis Rev.
33:41–85. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhou J, Zhu YF, Chen XY, Han B, Li F, Chen
JY, Peng XL, Luo LP, Chen W and Yu XP: Black rice-derived
anthocyanins inhibit HER-2-positive breast cancer
epithelial-mesenchymal transition-mediated metastasis in vitro by
suppressing FAK signaling. Int J Mol Med. 40:1649–1656.
2017.PubMed/NCBI
|
|
44
|
Si L, Yan X, Hao W, Ma X, Ren H, Ren B, Li
D, Dong Z and Zheng Q: Licochalcone D induces apoptosis and
inhibits migration and invasion in human melanoma A375 cells. Oncol
Rep. 39:2160–2170. 2018.PubMed/NCBI
|
|
45
|
Jacob A and Prekeris R: The regulation of
MMP targeting to invadopodia during cancer metastasis. Front Cell
Dev Biol. 3:42015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Diaz-Moralli S, Tarrado-Castellarnau M,
Miranda A and Cascante M: Targeting cell cycle regulation in cancer
therapy. Pharmacol Ther. 138:255–271. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Stewart ZA, Westfall MD and Pietenpol JA:
Cell-cycle dysregulation and anticancer therapy. Trends Pharmacol
Sci. 24:139–145. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Marconett CN, Morgenstern TJ, San Roman
AK, Sundar SN, Singhal AK and Firestone GL: BZ L101, a
phytochemical extract from the Scutellaria barbata plant, disrupts
proliferation of human breast and prostate cancer cells through
distinct mechanisms dependent on the cancer cell phenotype. Cancer
Biol Ther. 10:397–405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mariño G, Niso-Santano M, Baehrecke EH and
Kroemer G: Self-consumption: The interplay of autophagy and
apoptosis. Nat Rev Mol Cell Biol. 15:81–94. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ricci MS and Zong WX: Chemotherapeutic
approaches for targeting cell death pathways. Oncologist.
11:342–357. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Earnshaw WC, Martins LM and Kaufmann SH:
Mammalian caspases: Structure, activation, substrates, and
functions during apoptosis. Annu Rev Biochem. 68:383–424. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kadam CY and Abhang SA: Apoptosis markers
in breast cancer therapy. Adv Clin Chem. 74:143–193. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Suen DF, Norris KL and Youle RJ:
Mitochondrial dynamics and apoptosis. Genes Dev. 22:1577–1590.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Dickens LS, Boyd RS, Jukes-Jones R, Hughes
MA, Robinson GL, Fairall L, Schwabe JW, Cain K and Macfarlane M: A
death effector domain chain DISC model reveals a crucial role for
caspase-8 chain assembly in mediating apoptotic cell death. Mol
Cell. 47:291–305. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Martinou JC and Youle RJ: Mitochondria in
apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev
Cell. 21:92–101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Pettersson F, Dalgleish AG, Bissonnette RP
and Colston KW: Retinoids cause apoptosis in pancreatic cancer
cells via activation of RAR-gamma and altered expression of
Bcl-2/Bax. Br J Cancer. 87:555–561. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Liu F, Gao S, Yang Y, Zhao X, Fan Y, Ma W,
Yang D, Yang A and Yu Y: Curcumin induced autophagy anticancer
effects on human lung adenocarcinoma cell line A549. Oncol Lett.
14:2775–2782. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kim KY, Park KI, Kim SH, Yu SN, Park SG,
Kim YW, Seo YK, Ma JY and Ahn SC: Inhibition of autophagy promotes
salinomycin-induced apoptosis via reactive oxygen species-mediated
PI3K/AKT/mTOR and ERK/p38 MAPK-dependent signaling in human
prostate cancer cells. Int J Mol Sci. 18:E10882017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Tang F, Wang B, Li N, Wu Y, Jia J, Suo T,
Chen Q, Liu YJ and Tang J: RN F185, a novel mitochondrial ubiquitin
E3 ligase, regulates autophagy through interaction with BNIP1. PLoS
One. 6:e243672011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Li Y, Cui Y, Wang W, Ma M, Li M and Chen
S: Effect of the serum inhibited gene (Si1) on autophagy and
apoptosis in MCF-7 breast cancer cells. Cell Physiol Biochem.
41:2268–2278. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zeng Y, Li S, Wu J, Chen W, Sun H, Peng W,
Yu X and Yang X: Autophagy inhibitors promoted aristolochic acid I
induced renal tubular epithelial cell apoptosis via mitochondrial
pathway but alleviated nonapoptotic cell death in mouse acute
aritolochic acid nephropathy model. Apoptosis. 19:1215–1224. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Subramani R, Gonzalez E, Arumugam A, Nandy
S, Gonzalez V, Medel J, Camacho F, Ortega A, Bonkoungou S, Narayan
M, et al: Nimbolide inhibits pancreatic cancer growth and
metastasis through ROS-mediated apoptosis and inhibition of
epithelial-to-mesenchymal transition. Sci Rep. 6:198192016.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wang L, Li P, Hu W, Xia Y, Hu C, Liu L and
Jiang X: CD44+CD24+ subset of PANC-1 cells
exhibits radiation resistance via decreased levels of reactive
oxygen species. Oncol Lett. 14:1341–1346. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Banerjee A, Banerjee V, Czinn S and
Blanchard T: Increased reactive oxygen species levels cause ER
stress and cytotoxicity in andrographolide treated colon cancer
cells. Oncotarget. 8:26142–26153. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Li B, Zhao S, Geng R, Huo Z and Zhang H:
The sineoculis homeobox Homolog 1 (SIX1) gene regulates paclitaxel
resistance by affecting reactive oxygen species and autophagy in
human hepatocellular carcinoma cell line HepG2. Med Sci Monit.
24:2271–2279. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Apel K and Hirt H: Reactive oxygen
species: Metabolism, oxidative stress, and signal transduction.
Annu Rev Plant Biol. 55:373–399. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Li L, Cao W, Zheng W, Fan C and Chen T:
Ruthenium complexes containing 2,6-bis(benzimidazolyl)pyridine
derivatives induce cancer cell apoptosis by triggering DNA
damage-mediated p53 phosphorylation. Dalton Trans. 41:12766–12772.
2012. View Article : Google Scholar : PubMed/NCBI
|