Introduction
Medulloblastomas comprise 15 to 20% of all the
central nervous system (CNS) primary pediatric malignant tumors,
and have recently been classified into four molecular subgroups:
Wingless (WNT), Sonic hedgehog (SHH) and the numerical groups 3 and
4, which display differences in disease course and clinical outcome
(1,2). Current advances in medulloblastoma
diagnosis, in combination with therapeutic regimens with surgery,
craniospinal radiotherapy or chemotherapy, have contributed to an
increase in the 5-year survival rate of up to 80% (2). However, drug resistance remains as one
of the most difficult barriers to overcome, and is frequently
associated with therapeutic failure, elevated morbidity rates,
relapses and long-term sequelae in neurocognitive development
(3,4).
Most solid tumors exhibit zones characterized by
gradients of low oxygen tension, termed hypoxia, a condition
considered to be a key factor in the promotion of resistance to
diverse chemotherapeutic agents in CNS tumors, including
medulloblastoma (3,5). Moderate to severe hypoxia (2.5–0.1%
O2, respectively), has for example been documented in
astrocytomas, oligodendrogliomas and glioblastoma multiforme
(6). Cellular responses to oxygen
variations are regulated by HIF-1 (hypoxia-inducible factor 1), a
heterodimeric transcription factor constituted by the HIF-1α
subunit, whose expression, protein stability and sub-cellular
localization depend on O2 levels, and HIF-1β, a nuclear
subunit constitutively expressed (7). HIF-1α overexpression has been
associated with the resistance of several types of tumors to a
variety of drugs (8–12), supporting the hypothesis that
hypoxia and the HIF-1 pathway contribute to modulate the
effectiveness and toxicity of anticancer chemotherapeutic drugs
(13).
The standard treatment of medulloblastoma includes
antineoplastic pro-drugs such as cyclophosphamide (CPA) and
ifosfamide (IFA), whose antitumoral activity requires chemical
transformation to produce alkylating cytotoxic intermediary
metabolites, a mechanism that depends on redox reactions catalyzed
predominantly by the cytochrome P450 (CYP) isoforms 2B6, 3A4 and
3A5 (14). These heme-thiolated
enzymes display differential expression in several solid tumors,
including osteosarcoma, breast, prostate and lung cancers (15–18),
and is widely accepted that alterations in the tumor expression of
CYP enzymes contribute to changes in the metabolic deactivation of
anticancer agents or the activation of pro-drugs (19,20).
However, to date, little is known concerning CYP expression in
medulloblastomas.
Recent studies demonstrated that moderate hypoxia
(1% O2) downregulates CYP3A4 expression in the human
hepatocarcinoma HepG2 cell line, resulting in decreased metabolic
activation of antineoplastic acridine compounds (21,22).
Furthermore, CYP2B6 selectively mediates the activation of the
pro-drug AQN4 to the cytotoxin AQ4 in hypoxic cells (23), evidencing that limited oxygen
availability in tumors may alter drug metabolism and therapeutic
effectiveness, mainly by interfering with the regulation of CYP
expression, with consequences for chemoresistance (21,24–26).
Despite intense research focusing on elucidating the
relationship between hypoxia and tumor drug resistance, the
underlying mechanisms in medulloblastomas have not been clearly
delineated. In this study, we showed that the in vitro
exposure of DAOY medulloblastoma cells to moderate (1%
O2) or severe (0.1% O2) hypoxia, produced
resistance to CPA or IFA, characterized by increased half-maximal
inhibitory concentration values (IC50), alongside
decreased protein levels of CYP2B6, CYP3A4 and CYP3A5, inhibition
of cell proliferation and arrest in the G1 phase of the cell cycle.
These responses depended on the activation of the HIF-1 pathway, as
evidenced by the fact that the chemical inhibition of its
transcriptional activity with 2-methoxyestradiol (2-ME) acted in an
additive manner with CPA or IFA to exert cytotoxic activity and
increase apoptosis.
Materials and methods
Reagents
Cyclophosphamide monohydrate, ifosfamide, MTT [3-(4,
5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide],
propidium iodide (PI) and RNase A were purchased from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). 2-Methoxyestradiol
(2-ME) was obtained from Tocris Bioscience (Bristol, UK). The
antibodies used and their sources were: Rabbit polyclonal
anti-HIF-1α (cat. no. NB100-449), from Novus Biologicals
(Littleton, CO, USA); mouse monoclonal anti-CA-IX (cat. no.
sc365900), mouse monoclonal anti-PCNA (proliferating cell nuclear
antigen) (cat. no. sc-25280), goat anti-rabbit IgG (cat. no.
sc-2004) and rabbit anti-goat IgG (cat. no. sc-2768), from Santa
Cruz Biotechnology (Santa Cruz, CA, USA); rabbit monoclonal
anti-CYP2B6 (cat. no. ab140609), rabbit polyclonal anti-CYP3A4
(cat. no. ab135813), rabbit polyclonal anti-CYP3A5 (cat. no.
ab89811) and mouse monoclonal anti-CDKN1B (cyclin-dependent kinase
inhibitor 1B) (cat. no. ab54563) from Abcam (Cambridge, MA, USA);
mouse monoclonal anti-α-actin (cat. no. GTX80809) and goat
anti-mouse IgG (cat. no. GTX213111-01), were purchased from GeneTex
(Irvine, CA, USA).
Cell culture and hypoxic
conditions
Monolayer cultures of DAOY cells (human desmoplastic
cerebellar medulloblastoma cell line; HTB-186; ATCC, Manassas, VA,
USA) were routinely maintained in Eagle's minimal essential medium
(EMEM) supplemented with 10% fetal bovine serum (FBS; Biowest,
Riverside, CA, USA), 100 U/ml penicillin and 100 µg/ml streptomycin
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), at 37°C
in a humidified atmosphere of 95% air and 5% CO2.
Normoxia was considered as 16.2% O2 (ppO2 588
mm Hg) as México City is located 2,240 m above sea level. Hypoxia
was generated by a pre-equilibrated Bactrox hypoxic chamber (Shel
Lab; Sheldon Manufacturing, Inc., Cornelius, OR, USA) and oxygen
was balanced with N2 and CO2. Once 90%
confluence was reached, medulloblastoma cells were incubated for 24
h under moderate (1% O2) or severe (0.1% O2)
hypoxia.
Immunoblot analysis/western
blotting
Total, cytosolic and nuclear protein extracts were
processed according to Jewell et al (27). Briefly, cells were grown in EMEM
supplemented with 10% FBS and antibiotics in T-75 culture flasks
(106 cells) until 90% confluence was reached. The medium
was replaced by fresh medium and cells were then incubated in
normoxia or hypoxia for 24 h. Adherent cells were scraped in 200 µl
cell lysis buffer (10 mM Tris-HCl pH 8.0, 1 mM EDTA and 150 mM
NaCl), containing 0.5% IGEPAL (Sigma-Aldrich; Merck KGaA), 1 mM
Mini-Complete protease inhibitor cocktail (Roche; Mannheim,
Germany) and 1 mM PMSF (Sigma-Aldrich; Merck KGaA). Samples were
incubated for 10 min on ice before centrifugation at 20,000 × g for
5 min at 4°C. Cytosolic proteins were removed and pellets were
re-suspended in nuclear extraction buffer (20 mM HEPES, pH 7.9, 400
mM NaCl, 1 mM EDTA and 1 mM DTT), incubated on ice for 15 min, and
then vortexed and centrifuged at 20,000 × g for 5 min at 4°C. Total
cell lysates were prepared using 100 µl RIPA buffer (Sigma-Aldrich;
Merck KGaA). After centrifugation (4,000 × g, 20 min, 4°C), protein
levels were determined by the Bradford colorimetric assay, using
bovine serum albumin (BSA) as standard reference.
Nuclear (30 µg protein), cytosolic (30 µg) and total
(30–50 µg) lysates were electrophoretically separated using 8–12%
SDS-PAGE and proteins were then transferred to polyvinylidene
difluoride membranes (Immobilon-P; Millipore, Temecula, CA, USA).
Membranes were blocked with 5% non-fat dry milk in Tris-buffered
saline solution (TBS; 50 mM Tris-HCl, pH 7.5, 150 mM NaCl)
containing 0.1% Tween-20 (Sigma-Aldrich; Merck KGaA) at room
temperature for 1 h, followed by overnight incubation with primary
antibodies: HIF-1α (1:2,000 dilution); CA-IX, (1:200); PCNA
(1:1,000); CYP2B6 (1:2,000); CYP3A4 (1:3,000); CYP3A5 (1:2,000);
CDKN1B (1:200) and α-actin (1:10,000). After extensive washing with
TBS, membranes were incubated for 1 h with horseradish
peroxidase-conjugated secondary antibodies: goat anti-mouse IgG
(1:3,000), goat anti-rabbit IgG (1:3,000) or rabbit anti-goat IgG
(1:5,000). Protein signals were detected by chemiluminescence using
the Immobilon™ Western HRP substrate (Millipore; Merck
KGaA), and images were captured with Bio-Rad Fluor S Max
MultiImager and analyzed with Quantity One 1-D Analysis Software
version 4.4.1 (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Protein levels were expressed as relative optical density,
normalized with respect to the levels of an internal control
(α-actin), with the exception of nuclear HIF-1α, normalized to PCNA
levels.
Immunocytochemistry
DAOY cells (104 cells/well) were grown in
EMEM supplemented with 10% FBS and antibiotics in Lab-Tek-8 well
chambers. After 24 h, the medium was replaced by fresh medium, and
cells were incubated in normoxic or hypoxic conditions for 24 h.
Cells were then fixed for 20 min in 4% formalin in water (w/v), and
rinsed twice with ice-cold phosphate-buffered saline solution (PBS;
137 mM NaCl, 2.7 mM KCl, 10 mM NaHPO4, 2 mM
KH2PO4, pH 7.4). Samples were incubated in
antigen retrieval buffer (10 mM sodium citrate, pH 6.0) for 15 min
at 95°C and then rinsed three times with ice-cold PBS. Endogenous
peroxidases were blocked with H2O2 (3%, v/v,
in methanol) for 15 min and cells were then rinsed with distilled
H2O and PBS for 5 min. Non-specific binding was blocked
with normal swine serum (2%, v/v, in PBS) at room temperature for 1
h. Cells were then incubated overnight at room temperature in
blocking solution containing an anti-HIF-1α antibody (1:1,000
dilution) in a humidified chamber. The Biotinylated Link Universal
Secondary Antibody (Dako; Agilent Technologies, Inc., Santa Clara,
CA, USA) and streptavidin conjugated with horseradish peroxidase
(cat. no. K069011) were used as secondary antibodies. Color was
generated by adding the substrate 3′3-diaminobenzidine (1:1,000
dilution) for 1 min, and counterstaining was performed with
hematoxylin. Finally, the cells were subsequently dehydrated in 70,
90 and 100% ethanol, ethanol-xylene (1:1) and xylene (100%), and
then covered with resin. Images were obtained with Image-Pro Plus
(Aperio; Leica Biosystems, Inc., Buffalo Grove, IL, USA) with an
Olympus BX-40 Microscope (Olympus Corp., Center Valley, PA,
USA).
Determination of cellular density and
viability by the trypan-blue exclusion assay
DAOY cells were grown in EMEM supplemented with 10%
FBS and antibiotics in 6-well plates (2×105 cells/well)
and cultured for 24 h. The medium was replaced by fresh medium
before incubation under normoxic and hypoxic conditions for 24 h.
The medium was then removed, cells were rinsed twice with 5 ml
ice-cold PBS and then incubated with 400 µl 0.25% trypsin-EDTA for
5 min. The trypsin action was terminated with 2 ml supplemented
EMEM medium. Cells (1:5 dilution) were exposed to 0.4% trypan-blue
solution in 300 µl PBS for 10 min and the number of cells was
determined by using a Neubauer chamber. CPA and IFA were dissolved
in EMEM at a final concentration of 100 mM. 2-ME was dissolved in
DMSO (5 mM stock), and stored at −20°C until required. All
dilutions were freshly prepared before addition to the cells. The
DMSO final concentration was 0.1% to avoid cellular damage.
MMT assay
DAOY cells were grown in EMEM supplemented with 10%
FBS and antibiotics in 96-well plates (5×103 cells/well)
and incubated for 24 h at 37°C and 5% CO2. Thereafter,
the cells were exposed to CPA or IFA (0.1–100 mM) under normoxic or
hypoxic conditions for 24 h. In parallel determinations, cells were
incubated under the described conditions in the presence of 2-ME
(5–40 µM). Control experimentation was performed in cells treated
with the corresponding vehicles. After 48 h of total incubation,
cells were analyzed with the MTT assay. Briefly, 10 µl of an MTT
solution (5 mg/ml sterile water) was added to the cells for 3 h.
The medium was removed and 100 µl MTT solvent (4 mM HCl and 0.1%
IGEPAL in isopropyl alcohol) was added and the plate was placed on
an orbital shaker for 15 min at 25°C. Spectrophotometrically
determinations at 560 nm were then performed (Modulus™
II Microplate; Turner BioSystems, Inc.; Thermo Fisher Scientific,
Inc.).
Cell cycle distribution
Cell cycle distribution was analyzed using the
modified assay described by Box and Demetrick (28). Cells (2×105 cells/well)
were grown in EMEM supplemented with 10% FBS and antibiotics in
6-well plates. After 24 h the medium was replaced by fresh medium
and cells were incubated under normoxia or hypoxia for 24 h. The
cells were then washed and trypsinized with 400 µl 0.25%
trypsin-EDTA for 5 min. The trypsin action was terminated with 2 ml
supplemented EMEM medium, the cell suspension was centrifuged (200
× g, 5 min, 4°C) and the pellet was re-suspended in 1 ml PBS
containing 1 mM EDTA. Absolute ethanol (2.3 ml, −20°C) was added
and the suspension was stored overnight at −20°C. The cell
suspension was centrifuged at 200 × g for 5 min, re-suspended in
500 µl DNA extraction buffer (0.2% Triton X-100 in PBS) and kept
for 10 min at room temperature. Cells were centrifuged again at 200
× g for 5 min at 4°C and the pellet was re-suspended in 200 µl
RNAase A solution (1 mg/ml) and kept for 10 min at room
temperature, centrifuged at 200 × g for 5 min, re-suspended in 500
µl PBS; 15 µl propidium iodide solution (1 mg/ml) were added and
the mixture was incubated for 50 min at room temperature in the
dark. After centrifugation at 200 × g (5 min, 4°C), cells were
re-suspended in 400 µl PBS. Flow cytometry was performed with BD
LSRFortessa™ equipment (BD Biosciences, San Jose, CA, USA) and the
cell cycle was analyzed using ModFit LT software (Verity Software
House, Topsham, ME, USA).
Apoptosis assay
DAOY cells (2×105 cells/well) were grown
in EMEM supplemented with 10% FBS and antibiotics in 6-well plates
for 24 h and then exposed to CPA and IFA (IC50 values
determined previously, see Results) in the absence or presence of
2-ME (5 µM). The medium was then removed and the cells were rinsed
twice with 5 ml ice-cold PBS and trypsinized with 400 µl 0.25%
trypsin-EDTA for 5 min. The trypsin action was terminated with 2 ml
supplemented EMEM medium. After centrifugation at 200 × g (5 min,
4°C), cells were re-suspended in 1 ml PBS (1 mM EDTA). The
apoptotic cell fraction was revealed with Annexin V/PI according to
the protocol of the Annexin V Apoptosis Detection Kit FITC
(eBiosciences; Thermo Fisher Scientific, Inc.). Flow cytometry was
performed with BD LSRFortessa™ and apoptosis was analyzed with
Cyflogic software (CyFlo Ltd., Turku, Finland).
Effect of drug combinations
The cytotoxic effects of CPA or IFA plus 2-ME were
analyzed by determining the resistance index (RI), with the
following equation: RI = Se (1×2)/So (1+2), where experimental
survival (Se) is the product of the survival observed with drug 1
and the survival observed with drug 2, and survival observed (So)
is the survival observed with the drug combination of 1 and 2. RI
values >1 indicate additive effects, whereas RI >2 indicates
synergistic effects (29).
Statistical analysis
Data are expressed as means ± standard error (SEM).
The statistical analysis was performed with Prism 5.0 (GraphPad
Software, La Jolla, CA, USA). Data were compared with one-way ANOVA
followed by post hoc Tukey's or Dunnett's tests, or with two-way
ANOVA followed by Bonferroni's test. Probability values (P)
<0.05 were considered statistically significant.
Results
Hypoxia activates the HIF-1α pathway
and upregulates CA-IX expression in medulloblastoma cells
Cell hypoxia limits the post-translational chemical
modifications of HIF-1α, leading to increased protein stabilization
and subsequent translocation to the nucleus, triggering thus the
transcriptional regulation of diverse genes (7). Considering that in solid tumors
gradients of low oxygen levels coexist, and in order to examine the
possible differences in protein stabilization of HIF-1α in response
to oxygen variations, in a first approach DAOY medulloblastoma
cells were exposed to 1 or 0.1% O2 for 24 h, and the
expression levels of HIF-1α were analyzed by western blot analysis
and immunocytochemistry.
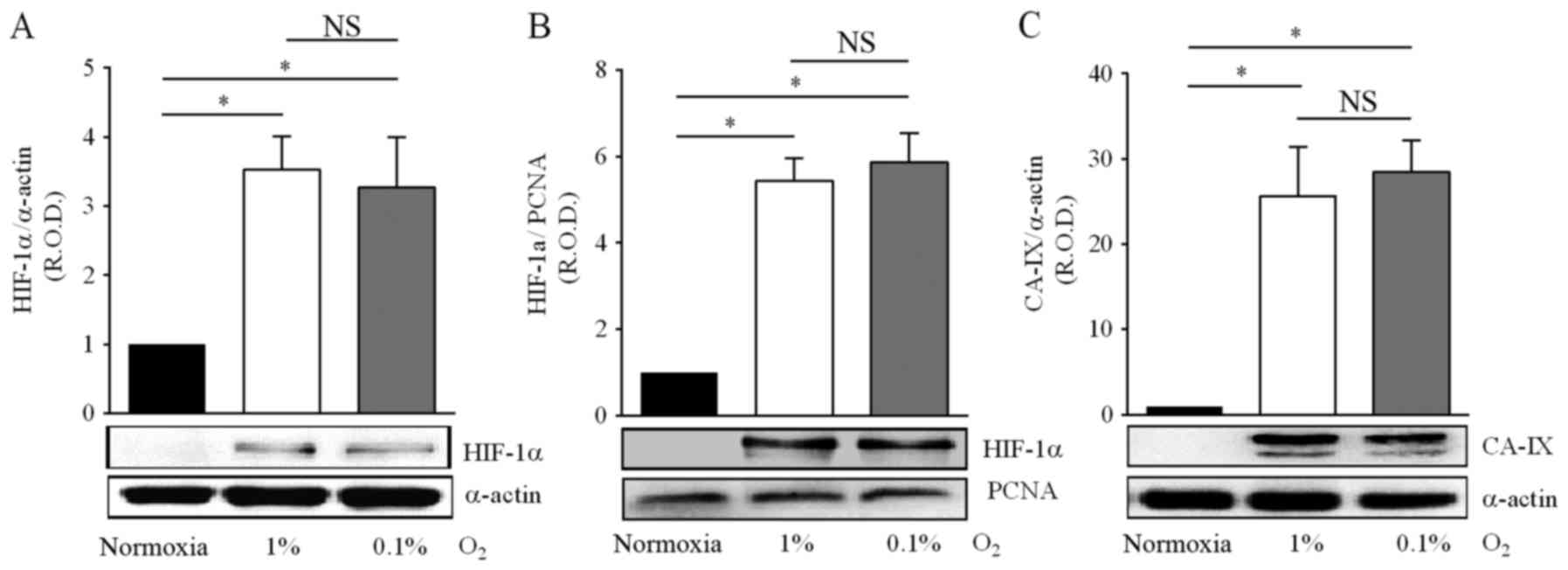
As expected, hypoxia increased the nuclear and
cytosolic protein levels of HIF-1α in medulloblastoma cells, by 3-
to 5-fold in 1% O2, and by 3- to 6-fold in 0.1%
O2 (Fig. 1A and B). Of
note, there was no significant difference in the magnitude of the
HIF-1α induction between both hypoxic conditions. Activation of the
HIF-1α pathway by hypoxia leads to the transcriptional regulation
of numerous target genes, including the gene codifying for CA-IX,
which plays a significant role in the modulation of the
extracellular pH, is therefore considered as a biomarker of
hypoxia, and is clinically associated to poor survival outcome in
CNS tumors (30). In the present
study, optical density analysis of the immunoblots for CA-IX was
performed on the bands corresponding to the transmembrane protein
(Fig. 1C), and showed increased
expression in cells incubated in either 1% O2 (19-fold)
or 0.1% O2 (22-fold). Likewise for HIF-1α levels,
changes in CA-IX protein levels in response to hypoxia did not
depend on the hypoxia levels, 1 or 0.1% O2 (Fig. 1A-C). These results indicate that in
DAOY cells moderate or severe hypoxia activates the HIF-1α
signaling pathway to a similar extent.
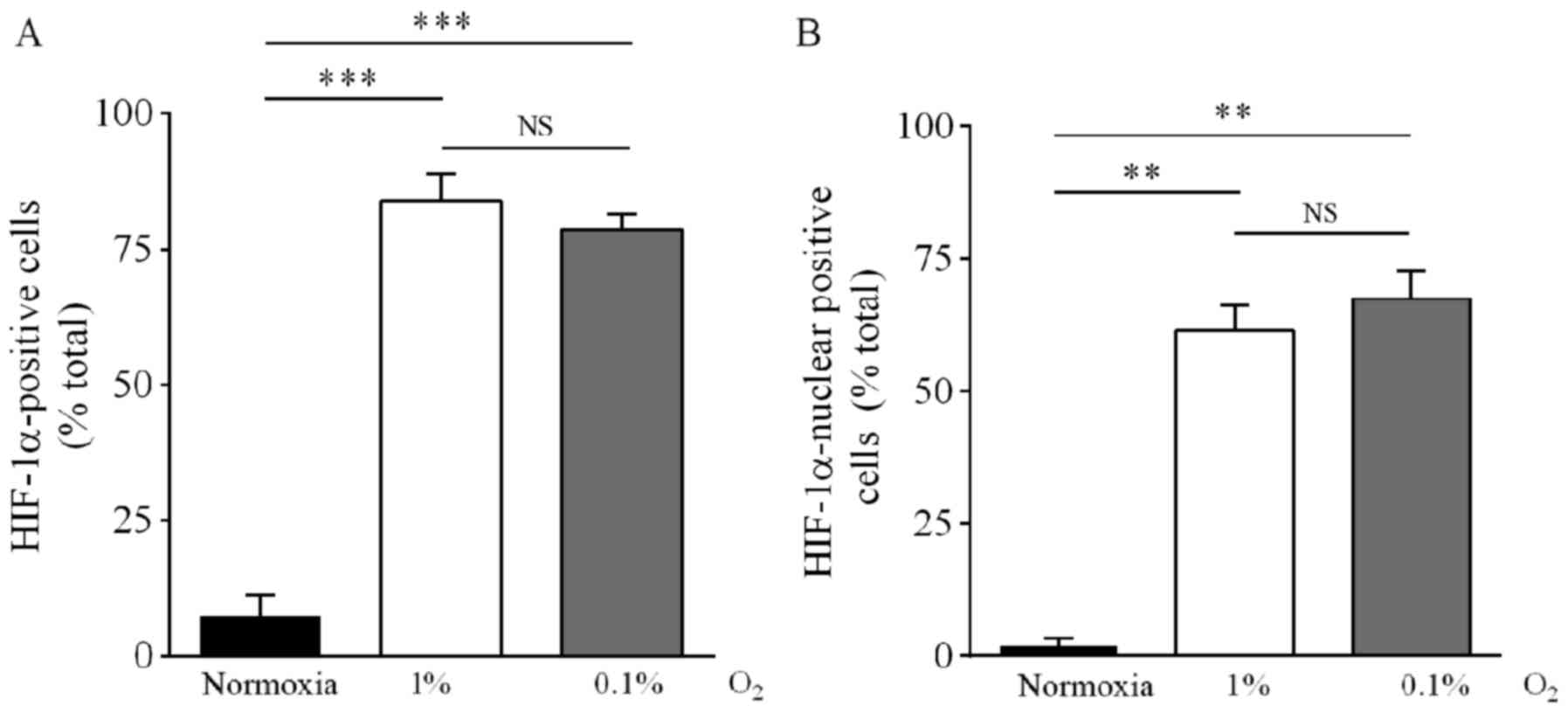
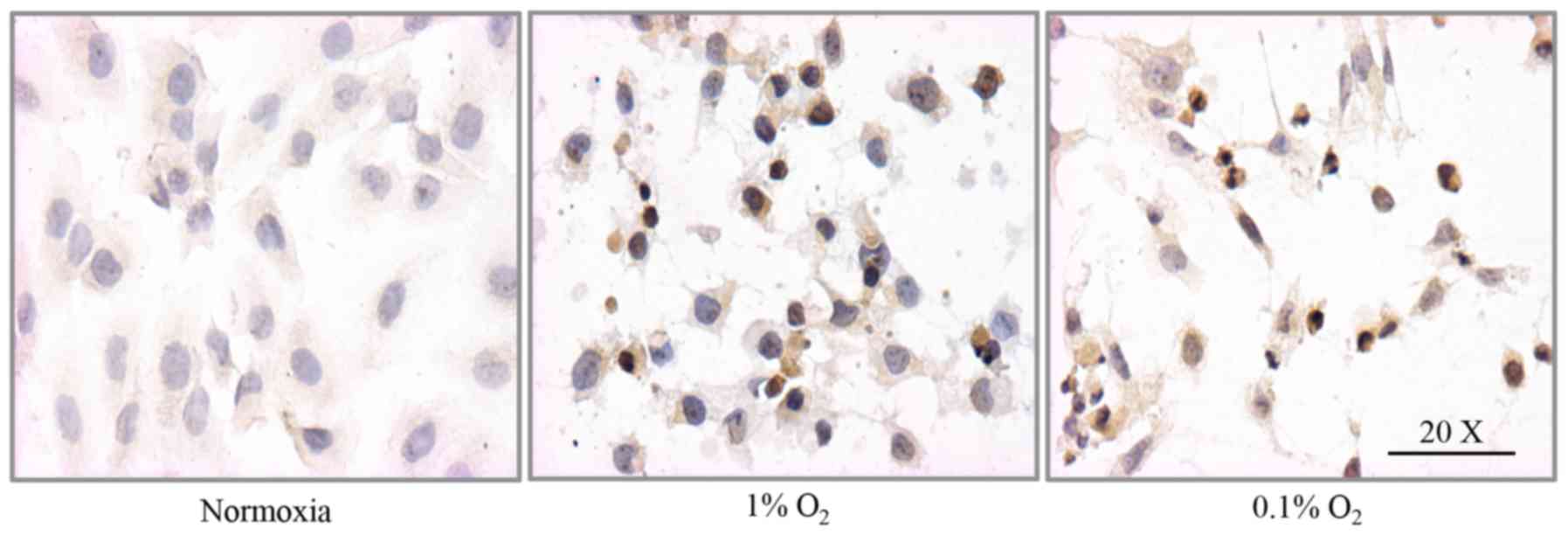
HIF-1α nuclear and cytosolic distribution was
corroborated by immunocytochemical analysis. Under 1%
O2, the fraction of cells positive to cytosolic HIF-1α
increased from 7.5% (normoxia) to 84%, whereas the fraction
positive for nuclear expression increased from 2.0% (normoxia) to
62%. Under 0.1% O2, the fraction of cells with cytosolic
HIF-1α immunoreactivity increased from 7.5 to 78% and the fraction
with nuclear immunoreactivity increased from 2.0 to 67% (Figs. 2A and B, and 3). There was no statistical difference
between cells incubated in 1 or 0.1% O2.
Hypoxia increases the resistance to
the cytotoxic effect of the pro-drugs CPA and IFA
HIF-1α overexpression has been associated with the
resistance of diverse solid tumors to a variety of drugs (8–12).
Current chemotherapeutic regimens for medulloblastoma include the
use of the pro-drugs CPA and IFA that undergo complex metabolic
activation to produce intermediary metabolites and finally the
phosphoramide mustard compounds that act as DNA alkylating agents
(31). Resistance to the CPA or IFA
cytotoxic effect promoted by hypoxia was analyzed on the basis of
changes in the concentrations required to halve the viability of
medulloblastoma cells (IC50). IC50 values
calculated for CPA and IFA under normoxia were 19 and 15 mM,
respectively (Table I). Under 1%
O2 the IC50 values for CPA and IFA increased
by 2- and 1.5-fold, respectively, whereas under 0.1% O2
the IC50 values increased by 2- and 3-fold,
respectively, indicating a reduction in the cytotoxic activity for
both hypoxic conditions (Table I).
Although these chemotherapeutic agents share metabolic activation
pathways and antineoplastic mechanisms, slight differences were
observed in the effect of hypoxia; for example, for CPA there was
no statistical difference in the IC50 values between 1
and 0.1% O2, but for IFA the IC50 value
obtained under 0.1% O2 was significantly different from
the value obtained under 1% O2 (Table I).
 | Table I.Effect of hypoxia on the cytotoxic
action of CPA or IFA in DAOY cells. |
Table I.
Effect of hypoxia on the cytotoxic
action of CPA or IFA in DAOY cells.
|
| CPA | IFA |
|---|
|
|
|
|
|---|
| O2
(%) |
pIC50 | IC50
(mM) | n |
pIC50 | IC50
(mM) | n |
|---|
| 16.2 | 1.73±0.05 | 18.6 | 7 | 1.83±0.06 | 14.75 | 5 |
| 1 |
1.44±0.06a | 36.1 | 7 |
1.65±0.04a | 22.4 | 5 |
| 0.1 |
1.43±0.05a | 36.8 | 5 |
1.32±0.08a,b | 47.5 | 5 |
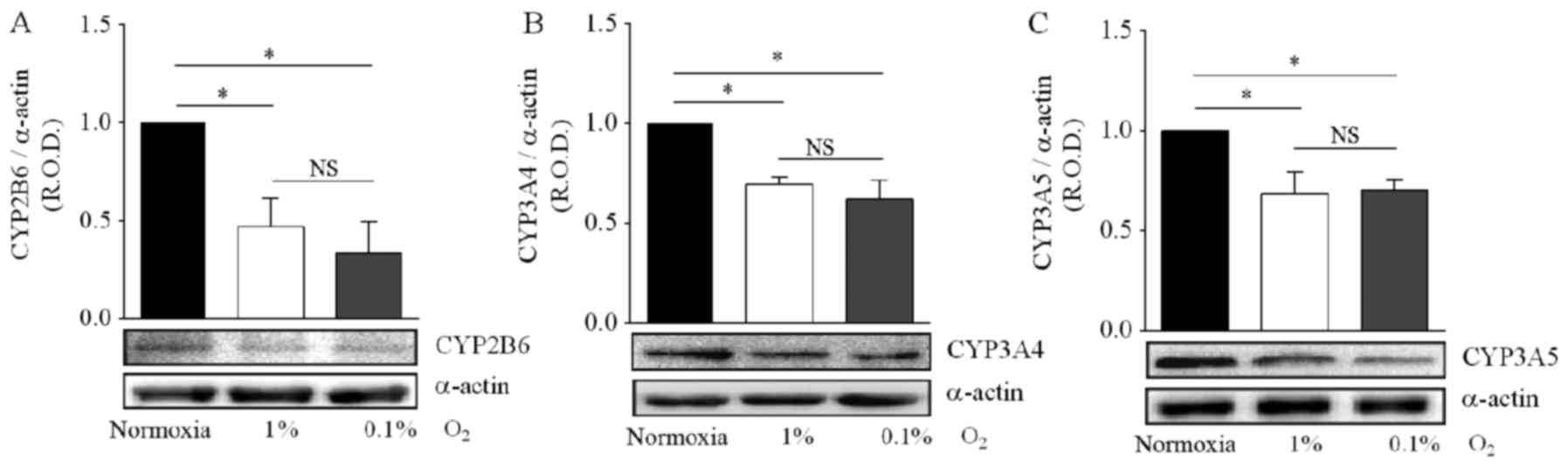
Hypoxia inhibits the expression of the
CYP2B6, CYP3A4 and CYP3A5 enzymes
The expression of various functionally active CYP
enzymes has been documented in diverse tumors, supporting a role in
the oxidative metabolism of a range of anticancer drugs (19). Specifically, the CYP2B6, CYP3A4 and
CYP3A5 isoforms are important for the biotransformation of CPA and
IFA to produce intermediary hydroxylated metabolites and exert
their antitumor activity (14,32).
However, to the best of our knowledge there is no information on
CYP2B6, CYP3A4 and CYP3A5 expression in regards to
medulloblastomas. For this reason, we initially evaluated the
protein expression of CYP2B6, CYP3A4 and CYP3A5 by DAOY cells.
Detectable protein levels of these CYP enzymes were visualized by
western blotting, and were compared with positive controls of
expression (HepG2 cell line), to corroborate the similar
electrophoretic mobility in SDS-PAGE (data not shown). When the
hypoxia effect on CYP isoform expression was analyzed, incubation
in 1 and 0.1% O2 produced a significant decrease in
CYP2B6 protein levels (−50 and −70%, respectively), in comparison
with DAOY cells maintened under normoxic conditions (Fig. 4A).
Similar results were obtained for the CYP3A4 and
CYP3A5 enzymes, with incubation in 1 or 0.1% O2 reducing
by 30% the protein levels of both proteins (Fig. 4B and C). For all three CYP isoforms,
the decrease in protein levels was not significantly different
between the two hypoxic conditions (Fig. 4A-C). Together, these results
indicate that hypoxia reduces the expression of CYP isoforms,
leading to a possible reduction in the metabolism of CPA and IFA,
and thus to drug resistance.
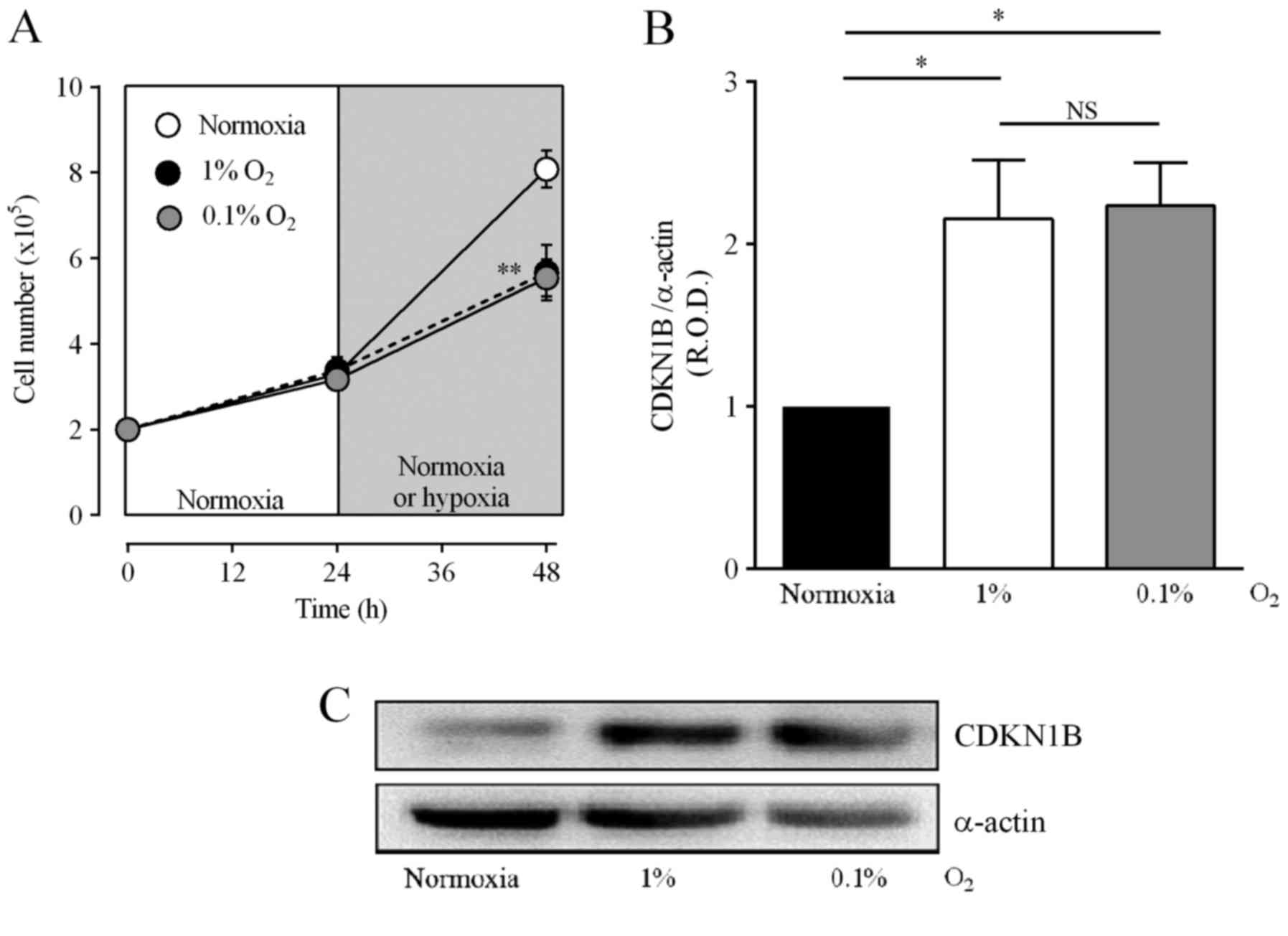
Hypoxia arrests medulloblastoma cells
in the G1 phase, increases the expression of CDKN1B and inhibits
cell proliferation
The alkylating intermediary metabolites derived from
CPA and IFA by the action of CYP enzymes, react to form chemical
cross-links within DNA strands, leading to cell apoptosis, an
action affected by the distribution of the cell cycle (33,34).
Incubation in 1 or 0.1% O2 increased the fraction of
DAOY cells in the G1 phase and decreased the cell population in the
S phase (Table II). Fig. 5A shows that hypoxia also decreased
the number of cells to 70.4±8.7 and 68.6±4.7% of the values for
normoxia in 1 or 0.1% O2, respectively. The arrest of
the cell cycle is controlled by several cyclins, and an increase in
CDKN1B is important for arrest in the G1 phase (35). Fig. 5B
and C show that incubation of DAOY cells under hypoxic
conditions increased CDKN1B levels to 216±36 and 223±26% of
normoxia values for 1 and 0.1% O2, respectively. These
results suggest that hypoxia induces cell arrest, which in turn may
reduce the alkylating effect of CPA and IFA.
 | Table II.Effect of hypoxia on the cell cycle
distribution of DAOY cells. |
Table II.
Effect of hypoxia on the cell cycle
distribution of DAOY cells.
|
| Cell cycle phase
(%) |
|---|
|
|
|
|---|
| O2
(%) | G1 | S | G2-M |
|---|
| 16.2 | 56.9±0.4 | 33.5±0.4 | 9.7±0.1 |
| 1 |
77.0±0.3a |
15.6±1.0a |
7.4±1.1NS |
| 0.1 |
73.1±0.5a |
19.2±0.4a |
7.7±0.1NS |
Effect of the chemical inhibition of
HIF-1α on hypoxia-induced changes in cell viability and in the
expression of CYP2B6, CYP3A4, CYP3A5 and CDKN1B
Our results suggested that in DAOY medulloblastoma
cells hypoxia induced resistance to CPA or IFA via activation of
the HIF-1α signaling pathway, which was correlated with decreased
expression of the CYP2B6, CYP3A4 and CYP3A5 enzymes, inhibition of
cell proliferation and arrest of the cell cycle in the G1 phase. In
order to evaluate whether these mechanisms are dependent on HIF-1α,
we next investigated the effects of the inhibition
pharmacologically, with 2-methoxyestradiol (2-ME) of the
transcriptional activity of HIF-1α (36).
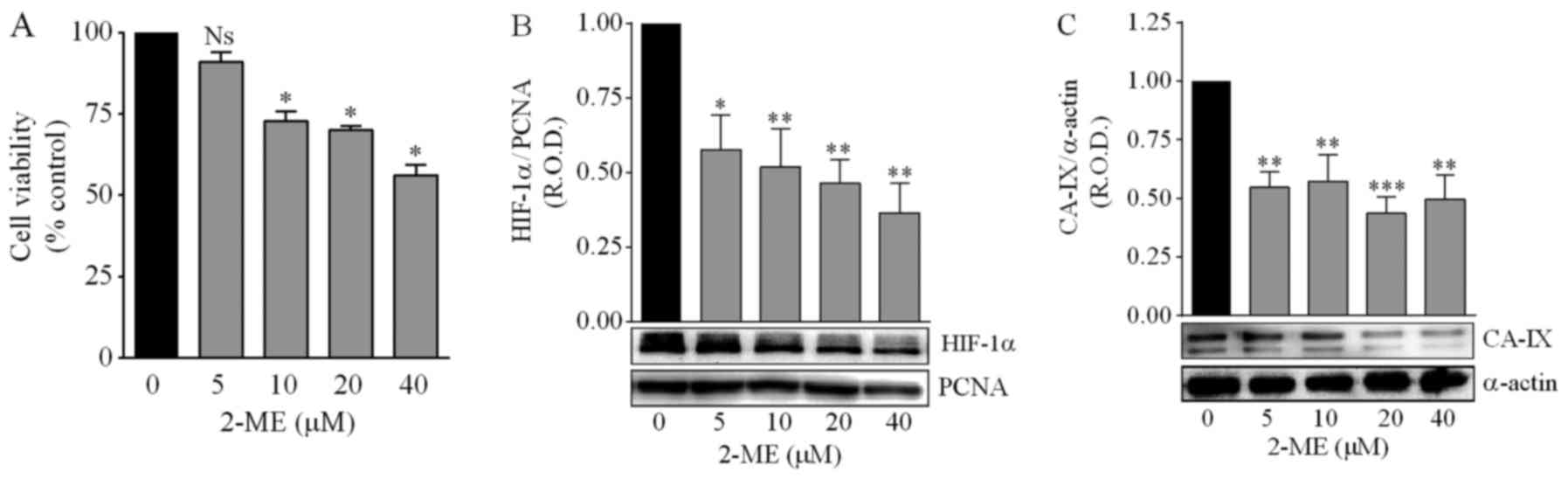
Concentrations of 2-ME >5 µM significantly
decreased the viability of DAOY cells (Fig. 6A). Treatment with 2-ME (5–40 µM)
significantly reduced the nuclear expression of HIF-1α in a
concentration-dependent manner (Fig.
6B), as well as the protein levels of the hypoxia biomarker
CA-IX, although in this case the effect did not depend on the 2-ME
concentration (Fig. 6C).
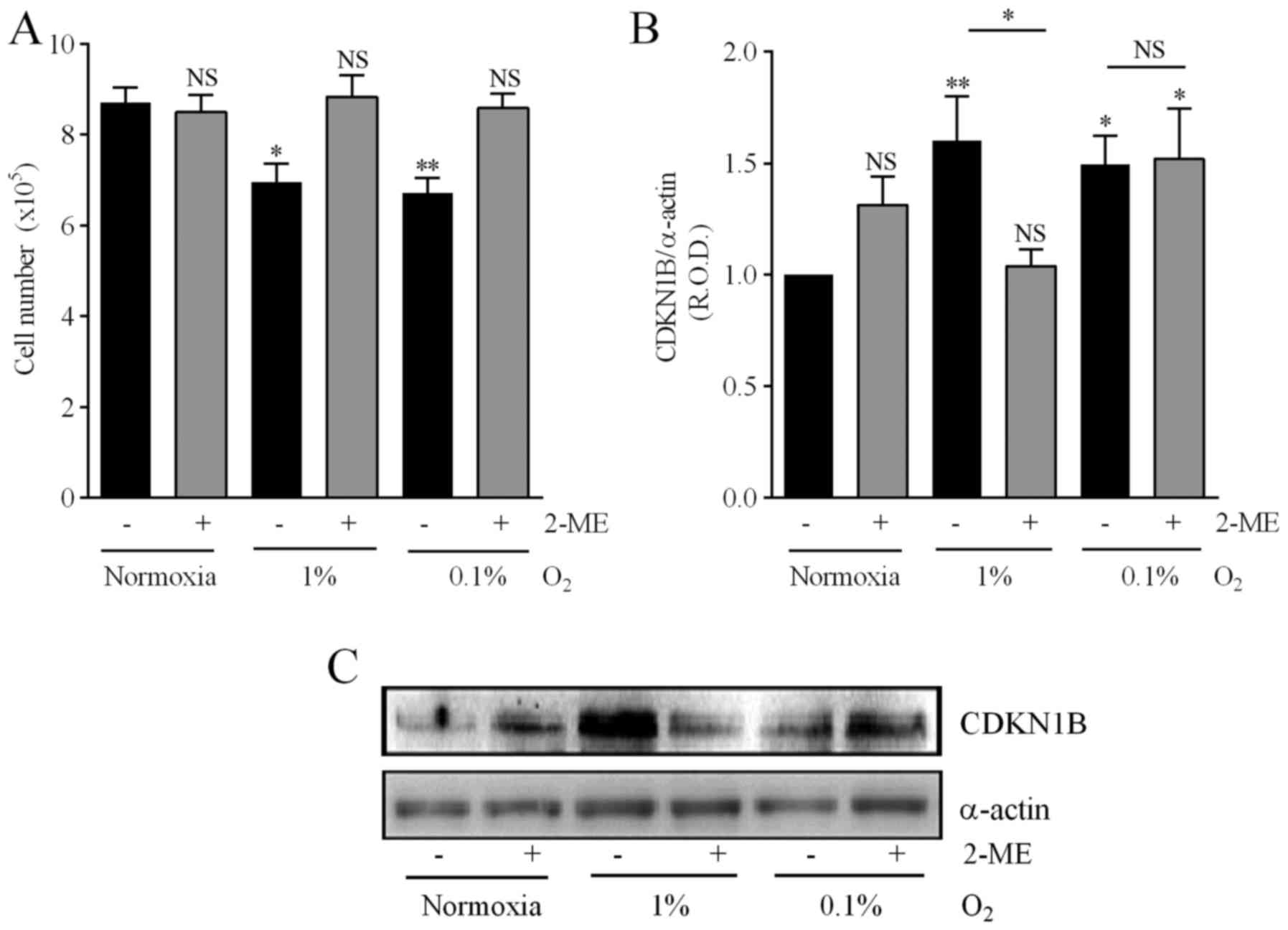
Considering these results, and in order to analyze the consequences
of inhibiting the HIF-1α pathway on the mechanisms associated with
drug resistance described in the present study, 5 µM was chosen as
the 2-ME optimal concentration. This concentration prevented the
reduction in cell number provoked by either 1 or 0.1% O2
(Fig. 7A). Furthermore, both 0.1
and 1% O2 increased the expression of CDKN1B by
medulloblastoma cells, although treatment with 2-ME prevented this
effect only in cells incubated in 1% O2 (Fig. 7B and C).
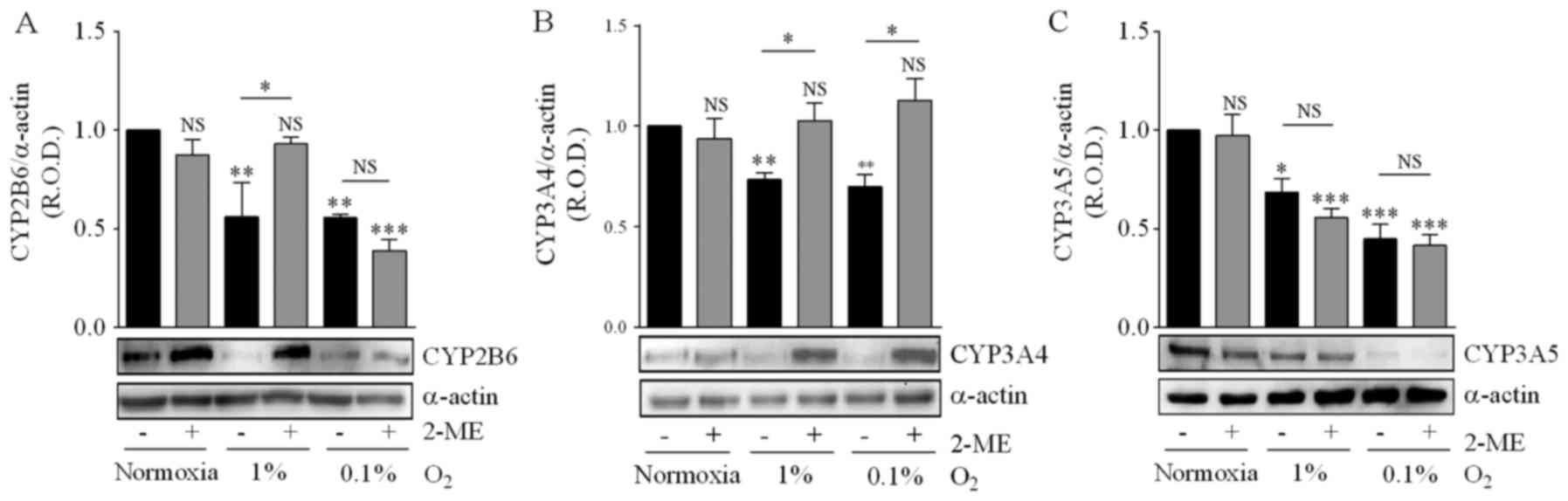
Hypoxia inhibited CYP2B6 expression, and incubation
of medulloblastoma cells with 5 µM 2-ME prevented the effect of 1%
O2 but not that of 0.1% O2 (Fig. 8A). CYP3A4 expression was also
reduced by incubation in either 1 or 0.1% O2, and 2-ME
prevented the effect of both hypoxic conditions (Fig. 8B). CYP3A5 expression was reduced by
incubation in 1 and 0.1% O2, but in this case 2-ME did
not prevent this effect (Fig. 8C).
These results indicate that HIF-1α inhibition differentially
affects the action of hypoxia on the expression of these CYP
isoforms.
2-ME sensitizes medulloblastoma cells
to CPA and IFA cytotoxicity
Treatment with CPA or IFA alone or in combination
with 2-ME (5 µM) was tested under normoxic and hypoxic conditions,
using the IC50 values for CPA or IFA as previously
determined (Table I). Table III shows that under normoxia the
reduction in cell viability induced by CPA (−43.2±1.7%) or IFA
(−46.3±2.2%) was not significantly modified by 2-ME (−45.8±3.3 and
−51.4±2.0% for CPA and IFA, respectively). In contrast, in cells
incubated under 1% O2, the reduction in cell viability
induced by CPA (−45.7±1.9%) or IFA (−50.3±2.5%) was significantly
enhanced by the presence of 2-ME, to −72.7±2.1 and −76.4±2.3%,
respectively. Likewise, under 0.1% O2, 2-ME increased
the cytotoxic effect of both CPA and IFA from −44.5±1.5 and
−51.2±2.9% to −69.8±2.1 and −73.5±1.9%, respectively.
 | Table III.Cytotoxicity induced by CPA or IFA
alone or in combination with 2-ME. |
Table III.
Cytotoxicity induced by CPA or IFA
alone or in combination with 2-ME.
| O2
(%) | 2-ME | CPA | CPA+2-ME | R index | Effect |
|---|
| 16.2 | 94.35±7.3 |
56.8±1.7a | 54.2±3.3 | 0.99±0.06 | No effect |
| 1 | 95.6±7.0 |
54.3±1.9a |
27.3±2.1b |
1.92±0.13c | Additivity |
| 0.1 | 93.2±3.0 |
55.5±1.5a |
30.2±2.1b |
1.74±0.11c | Additivity |
|
|
| 2-ME | IFA |
IFA+2-ME | R index | Effect |
|
| 16.2 | 93.9±1.0 |
53.7±2.2a | 48.6±2.0 | 1.04±0.02 | No effect |
| 1 | 94.4±1.2 |
49.7±2.5a |
23.6±2.3b |
2.03±0.12c | Synergy |
| 0.1 | 95.4±1.6 |
48.8±2.9a |
26.5±1.9b |
1.77±0.10c | Additivity |
The resistance index (RI) for cells incubated in
normoxia was not significantly greater than that observed for CPA
or IFA in combination with 2-ME. In contrast, the analysis showed
that CPA and 2-ME additively decreased cell viability in 1%
O2 (RI 1.9±0.1) and 0.1% O2 (RI 1.7±0.1). IFA
and 2-ME synergistically decreased cell viability in 1%
O2 (RI 2.0±0.1) and additively in 0.1% O2 (RI
1.8±0.1). These results suggest that the combination of CPA or IFA
with 2-ME enhances the effectiveness of the treatment in cells
exposed to hypoxic conditions.
Inhibition of HIF-1α by 2-ME increases
the apoptotic effect of CPA and IFA
Table IV shows that
the fraction of apoptotic cells (Annexin
V+/PI+) significantly increased in DAOY cells
incubated in 1% O2 and treated with CPA in combination
with 2-ME (78.8±2.4%) as compared with cells treated with CPA alone
(62.9±1.4%). Likewise, treatment with IFA in combination with 2-ME
significantly increased the fraction of apoptotic cells (86.6±2.7
vs. 67.8±1.1% for IFA alone). Similar results were obtained for
DAOY cells incubated in 0.1% O2, for which the fraction
of apoptotic cells increased significantly in cells treated with
CPA and 2-ME (68.9±6.6%) compared with CPA alone (38.2±4.7%), and
in cells treated with IFA plus 2-ME (75.9±2.6%) compared with IFA
alone (42.0±1.7%). These results indicate that HIF-1α inhibition
enhances the apoptotic effect of CPA and IFA under hypoxic
conditions.
 | Table IV.Apoptosis induced by CPA or IFA alone
or in combination with 2-ME. |
Table IV.
Apoptosis induced by CPA or IFA alone
or in combination with 2-ME.
|
| Annexin
V+/IP+ cells (%) |
|---|
|
|
|
|---|
|
| 16.2%
O2 | 1%
O2 | 0.1%
O2 |
|---|
| Control | 5.5±1.1 | 11.0±2.1 | 6.1±2.5 |
| 2-ME |
9.7±3.8a |
19.4±5.7a |
13.9±2.1a |
| CPA | 22.5±2.8 | 62.9±1.4 | 38.2±4.7 |
| CPA+2-ME |
27.6±4.7b |
78.8±2.4b |
68.9±6.6d |
| IFA | 24.0±4.4 | 67.8±1.1 | 42.0±1.7 |
| IFA+2-ME | 22.9±4.5 |
86.6±2.7c |
75.9±2.6e |
Discussion
In CNS tumors the presence of hypoxia has been
correlated with a more aggressive behavior (6), and in several other cancer types the
activation of the HIF-1α pathway in response to variations in
oxygen concentration was found to increase tumor resistance to
diverse chemotherapeutic agents (7–11).
Oxygen deprivation leads to the immediate cytosolic stabilization
of HIF-1α and its subsequent nuclear translocation to dimerize with
HIF-1β and modulate transcriptionally the expression of diverse
genes (7).
Herein we showed that in human medulloblastoma DAOY
cells, hypoxia increased the cytosolic and nuclear protein levels
of HIF-1α, and that this effect was correlated with a high
expression of CA-IX, a transmembrane protein with an extracellular
domain that catalyzes the reversible conversion of CO2
to bicarbonate and is involved in the regulation of the
extracellular pH (37). CA-IX
overexpression is consistently associated with poor survival in
diverse malignancies, including medulloblastoma (38), and in this neoplasia it modulates
E-cadherin-mediated cell adhesion via its interaction with
β-catenin, leading to increased tumor progression (39). Furthermore, CA-IX has been reported
to be an endogenous sensor of HIF-1 activity due to the presence of
a hypoxia response element (HRE) sequence located immediately
upstream of the transcription start (40), and the concomitant high expression
of HIF-1α and CA-IX was found to be correlated with chemoresistance
in lung cancer (41), similar to
that observed in the present study for CPA and IFA in
medulloblastoma cells.
Chemical transformation of CPA and IFA is performed
by diverse hepatic CYP enzymes, including CYP2B6, CYP3A4 and CYP3A5
(14), and it is widely recognized
that the expression of these enzymes in tumor cells may contribute
in situ to changes in the metabolic activation of these
pro-drugs (19). In relation to
medulloblastoma, Liu et al documented a link between the
expression of CYP1A1 and CYP1B1 and its enzymatic functionality in
the metabolic activation of resveratrol in the medulloblastoma
UW228-3 cell line (42). In the
present study, the CYP2B6, CYP3A4 and CYP3A5 isoforms were
detectable at the protein level in DAOY cells, and interestingly
their levels were significantly decreased in both hypoxic
conditions, 1 and 0.1% O2, coinciding with resistance to
CPA and IFA. In the liver, CPA is 4-hydroxylated by CYP2B6 (44.8%),
CYP3A4 (24.7%) and CYP3A5 (~11.0%), whereas IFA is also
4-hydroxylated by these isoforms although to a different extent:
CYP3A4, 64.8%; CYP3A5, 8.0%; CYP2B6, 6.5% (14,32).
In addition, hypoxia downregulates CYP1A1, CYP1A2, CYP2B6, CYP2C9
and CYP2C19, and decreases drug biotransformation (24). The reduction in CYP isoform
expression would thus favor resistance to both CPA and IFA in DAOY
cells.
Alterations in the expression of CYP enzymes in some
neoplasias such as breast cancer, lymphomas and epithelial ovarian
cancer, have been linked to resistance to ifosfamide, docetaxel,
doxorubicin, etoposide and paclitaxel (43–46).
Hypoxia and HIF-related pathways are linked to central cancer
hallmarks by promoting metabolic reprogramming in tumor cells
(47), that in turn alters the
function of CYP enzymes by compromising their mono-oxygenase
activity and redox reactions (48).
However, through its binding to HRE, HIF-1α has been shown to
downregulate diverse genes, including the tissue factor pathway
inhibitor (TFPI), peroxisome proliferator-activated receptors
(PPARα and PPARγ), estrogen receptor α (ER-α), peroxiredoxin 3
(Prx3), the vasodilator-stimulated phosphoprotein (VASP) and BID
(49,50–52).
In regard to CYP expression, in vivo and ex vivo
models show that low oxygen levels also downregulate the expression
of some CYP isoforms in different human or animal tissues, and cell
lines (21,22,53),
therefore interfering with the action of a wide variety of
anticancer drugs (22,48,54).
For example, moderate hypoxia was found to downregulate CYP3A4
expression in the HepG2 cell line, resulting in decreased metabolic
activation of antineoplastic compounds (21,22).
Inhibition of CYP expression promoted by hypoxia may thus be
considered as an adaptive response (48). Furthermore, overexpression in mice
of an orthologous isoform of the human CYP46A1 appeared to be
controlled by HIF-1α via HRE (55),
and hypoxia was found to downregulate CYP3A4 expression in the
human hepatic progenitor HepaRG cell line, an effect attributed to
the presence of an HRE sequence in the 5′-flanking region
(−674/-661) of the CYP3A4 gene, although functional analyses
failed to demonstrate the involvement of the HRE sequence (21).
We performed a preliminary in silico analysis
on the sequences of the 57 CYP human genes in the non-coding
regions (−3,500 to +1 pb) by alignment with CLUSTAL2W and JASPAR
databases, and we identified HRE sequences (5′-RCGTG-3′) with a
high probability of functional activity (relative score ≥0.95) in
CYP2B6, CYP3A4 and CYP3A5. The location of the identified HRE
sequences differed among these CYP isoforms, this location being
closer to the region of transcription for CYP2B6 (−972 to −965 pb)
than for CYP3A4 (−3215 to −3208 and −3496 to −3489 pb) or CYP3A5
(−3293 to −3288 pb). This differential location may contribute to
explain the pattern of regulation by HIF-1α found in this study
(CYP2B6 > CYP3A4 = CYP3A5). However, functional assays are
required to establish the biological activity of the putative HRE
sequences.
Once metabolized into their active metabolites, CPA
and IFA exert their cytotoxic effect on proliferating cells and it
is thus noteworthy that hypoxia diminished the proliferation of
DAOY cells by ~30%, in accord with the effect reported for prostate
cancer PC-3 cells (56), colorectal
cancer HT-29 and SW480 cells (57)
and glioblastoma D566, U87 and U251 cells (58). This effect could be explained by the
arrest in the G1 phase of the cell cycle (28–35%) observed in this
study, which could in turn be responsible, at least in part, for
the diminished cytotoxic action of CPA and IFA, as reported for
cervical cancer HeLa and breast cancer HTB-30 cells (28). Arrest in the G1 phase is controlled
by several cyclins, including CDKN1B, and in DAOY cells both
hypoxic conditions increased CDKN1B expression by 2.2 fold.
In order to corroborate whether the increased
resistance to CPA and IFA promoted by hypoxia, alongside the
inhibition of the expression of CYP2B6, CYP3A4 and CYP3A5,
inhibition of cell proliferation and arrest of the cell cycle in G1
phase, were all effects dependent on the HIF-1α pathway, the
pharmacological inhibition by 2-ME of HIF-1α activation was tested.
2-ME is a natural nonestrogenic metabolite derivative of
17β-estradiol with anti-angiogenic and pro-apoptotic activities,
and has been tested in combination with canonical antineoplastic
drugs in clinical phase II studies for the treatment of several
types of cancer (59,60). The mechanisms of action of 2-ME are
complex and still unclear, but it has been proposed that the drug
inhibits the polymerization of tubulin, thus disrupting the normal
microtubule function necessary for HIF-1α translocation to the
nucleus (36).
Incubation with 2-ME produced additive or
synergistic cytotoxic actions in combination with CPA or IFA, as
reported also for paclitaxel in head and neck squamous cells, such
as UM-SCC-1, −6, −11A, −11B and −46 cells (61), and sorafenib in hepatocellular
carcinoma (HCC) cells (62). Our
data support thus an important role for HIF-1α in the drug
resistance of medulloblastoma and confirm that its inhibition
enhances treatment effectiveness.
HIF-1α inhibition with 2-ME prevented the effect on
CYP2B6 expression in the 1% O2 condition but not in 0.1%
O2, as well as the effect of both hypoxic conditions on
CYP3A4 expression, but failed to modify the downregulation of
CYP3A5 expression. We do not have an explanation for the
differential rescuing action of 2-ME on the expression of the three
CYP isoforms studied. We propose that hypoxia induces
HIF-1α-dependent transcriptional repression of CYP2B6, CYP3A4 and
CYP3A5; however, our results do not discard additional
HIF-1α-independent regulatory mechanisms, including the activation
of transcriptional repressors such as the small heterodimer partner
(SHP), which has been implicated in the inhibition of the CYP7A1
isoform under hypoxic conditions, and the downregulation of nuclear
receptors implicated in the positive regulation of CYP2B6, CYP3A4
and CYP3A5, namely PPARα, PPARγ or ER-α, as well as the
constitutive androstane and pregnane X receptors, whose mRNA was
found to be reduced by hypoxia (1% O2, 24 h) in hepatic
HepaRG stem cells (21,51,52,63–65) In
addition, post-transcriptional regulation of CYP expression
implicates the action of diverse mRNA stabilizing molecules,
microRNAs and long noncoding RNAs (66). Finally, 2-ME itself could act as a
chemical modulator of CYP expression, because CYP substrates or
their metabolites are capable of inducing CYP expression (19). Therefore, the role of hypoxia and
HIF-1α in CYP transcriptional regulation appears to be complex and
probably does not only depend on HIF-1α alone, with alternative
mechanisms requiring further investigation.
Hypoxia (0.1 and 1% O2) increased CDKN1B
expression in DAOY cells, and in human medulloblastoma tissues
CDKN1B expression was detected in 57% of samples, with four
positive out of five samples from desmoplastic tumors correlating
with low apoptosis levels (67).
Interestingly, 2-ME induces CDKN1B expression in leukemia and
sarcoma cells; however, the mechanism underlying the effect of
hypoxia is poorly understood (68,69).
In medulloblastoma cells, 2-ME treatment decreased CDKN1B
expression in 1% O2 but not in 0.1% O2 and
alternative regulatory mechanisms may therefore be involved in the
lack of effect of 2-ME on the upregulation of CDKN1B expression in
0.1% O2. For example, the p27 promoter contains binding
sites for several transcription factors including Sp1,
cAMP-response element (CRE), Myb, NF-κB, and acute lymphocytic
leukaemia-1 fused gene from chromosome X (70). Since CRE, NF-κB and Sp1 are
regulated by hypoxia, HIF-1α-indepent mechanisms, not prevented by
2-ME, could have contributed to the action of 0.1% O2,
explaining thus the lack of effect of 2-ME on the upregulation of
CDKN1B.
In conclusion, in previous studies 2-ME has been
proposed as a potent anticancer agent and as an alternative to the
existing therapies for the treatment of diverse tumors, including
medulloblastoma (71–73). This research and a few others
provide experimental evidence that supports that 2-ME potentiates
the chemotherapeutic effectiveness of certain antineoplastic
agents, even under hypoxic conditions. Our results indicate that by
stimulating HIF-1α activity, hypoxia downregulates the expression
of CYP2B6, CYP3A4 and CYP3A5, that in turn leads to decreased
conversion of CPA and IFA into their active metabolites and thus to
diminished cytotoxicity. Accordingly, the pharmacological
inhibition of HIF-1α enhanced CPA and IFA cytotoxic actions. These
results support that the use of HIF-1α inhibitors in combination
with canonical antineoplastic agents provides a potential
therapeutic alternative against medulloblastoma.
Acknowledgements
We thank Juan Escamilla-Sánchez and Raúl
González-Pantoja for excellent technical assistance. Jesús
Valencia-Cervantes holds a Conacyt graduate scholarship
(21983).
Funding
The present study was partially funded by the
Instituto Nacional de Pediatría, SSA (Programa E022 Recursos
Federales Destinados a la Investigación, grant INP 39/2010),
Conacyt (grants CB-2010/152919 to VMD-B and CB-2013/220448 to
J-AA-M), and Cinvestav.
Availability of data and materials
Most data generated or analyzed during this study
are included in this published article. Datasets that were not
included (specified in the manuscript) are available from the
corresponding authors on a reasonable request.
Authors' contributions
JVC, JAAM and VMDB conceived and designed the study;
JVC, SHY and GAJ performed the experiments; JVC, SRE, DMF, JAAM and
VMDB performed the analysis and interpretation of the data; JVC,
SHY, GAJ, SRE, DMF, JAAM and VMDB were involved in drafting the
manuscript and revising it critically. All authors revised and
approved the manuscript and agree to be accountable for all aspects
of the work in ensuring that questions related to the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 World Health Organization
classification of tumors of the central nervous system: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Massimino M, Biassoni V, Gandola L, Garrè
ML, Gatta G, Giangaspero F, Poggi G and Rutkowski S: Childhood
medulloblastoma. Crit Rev Oncol Hematol. 105:35–51. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Warnke PC, Kopitzki K, Timmer J and
Ostertag CB: Capillary physiology of human medulloblastoma: Impact
on chemotherapy. Cancer. 107:2223–2227. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Palmer SL: Neurodevelopmental impacts on
children treatment for medulloblastoma: A review and proposed
conceptual model. Dev Dis Res Rev. 14:203–210. 2008. View Article : Google Scholar
|
|
5
|
Huang GH, Xu QF, Cui YH, Li N, Bian XW and
Lv SQ: Medulloblastoma stem cells: Promising targets in
medulloblastoma therapy. Cancer Sci. 107:583–589. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Evans SM, Judy KD, Dunphy I, Jenkins WT,
Hwang WT, Nelson PT, Lustig RA, Jenkins K, Magarelli DP, Hahn SM,
et al: Hypoxia is important in the biology and aggression of human
glial brain tumors. Clin Cancer Res. 10:8177–8184. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Michel G, Minet E, Ernest I, Roland I,
Durant F, Remacle J and Michiels C: A model for the complex between
the hypoxia-inducible factor-1 (HIF-1) and its consensus DNA
sequence. J Biomol Struct Dyn. 18:169–179. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hussein D, Estlin EJ, Dive C and Makin GW:
Chronic hypoxia promotes hypoxia-inducible factor-1alpha-dependent
resistance to etoposide and vincristine in neuroblastoma cells. Mol
Cancer Ther. 5:2241–2250. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu L, Ning X, Sun L, Zhang H, Shi Y, Guo
C, Han S, Liu J, Sun S, Han Z, et al: Hypoxia-inducible factor-1
alpha contributes to hypoxia-induced chemoresistance in gastric
cancer. Cancer Sci. 99:121–128. 2008.PubMed/NCBI
|
|
10
|
Rho JK, Choi YJ, Lee JK, Ryoo BY, Na II,
Yang SH, Kim CH, Yoo YD and Lee JC: Gefitinib circumvents
hypoxia-induced drug resistance by the modulation of HIF-1alpha.
Oncol Rep. 21:801–807. 2009.PubMed/NCBI
|
|
11
|
Doublier S, Belisario D, Polimeni M,
Annaratone L, Riganti C, Allia E, Ghigo D, Bosia A and Sapino A:
HIF-1 activation induces doxorubicin resistance in MCF7 3-D
spheroids via P-glycoprotein expression: A potential model of the
chemoresistance of invasive micropapillary carcinoma of the breast.
BMC Cancer. 12:42012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roncuzzi L, Pancotti F and Baldini N:
Involvement of HIF-1α activation in the doxorubicin resistance of
human osteosarcoma cells. Oncol Rep. 32:389–394. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Phillips RM: Targeting the hypoxic
fraction of tumours using hypoxia-activated prodrugs. Cancer Chemo
Pharmacol. 77:441–457. 2016. View Article : Google Scholar
|
|
14
|
Roy P, Yu L, Crespi C and Waxman D:
Development of a substrate-activity based approach to identify the
major human liver P-450 catalysts of cyclophosphamide and
ifosfamide activation based on cDNA-expressed activities and liver
microsomal P-450 profiles. Drug Metab Dispos. 27:655–666.
1999.PubMed/NCBI
|
|
15
|
Dhaini HR, Thomas DG, Giordano TJ, Johnson
TD, Biermann JS, Leu K, Hollenberg PF and Baker LH: Cytochrome P450
CYP3A4/5 expression as a biomarker of outcome in osteosarcoma. J
Clin Oncol. 21:2481–2485. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Murray GI, Patimalla S, Stewart KN, Miller
ID and Heys SD: Profiling the expression of cytochrome P450 in
breast cancer. Histopathology. 57:202–211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen TC, Sakaki T, Yamamoto K and Kittaka
A: The roles of cytochrome P450 enzymes in prostate cancer
development and treatment. Anticancer Res. 32:291–298.
2012.PubMed/NCBI
|
|
18
|
Qixing M, Juqing X, Yajing W, Gaochao D,
Wenjie X, Run S, Anpeng W, Lin X, Feng J and Jun W: The expression
levels of CYP3A4 and CYP3A5 serve as potential prognostic
biomarkers in lung adenocarcinoma. Tumour Biol.
39:10104283176983402017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McFadyen MC, Melvin WT and Murray GI:
Cytochrome P450 enzymes: Novel options for cancer therapeutics. Mol
Cancer Ther. 3:363–371. 2004.PubMed/NCBI
|
|
20
|
Rodriguez-Antona C and Ingelman-Sundberg
M: Cytochrome P450 pharmacogenetics and cancer. Oncogene.
25:1679–1691. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Legendre C, Hori T, Loyer P, Aninat C,
Ishida S, Glaise D, Lucas-Clerc C, Boudjema K, Guguen-Guillouzo C,
Corlu A and Morel F: Drug-metabolising enzymes are down-regulated
by hypoxia in differentiated human hepatoma HepaRG cells:
HIF-1alpha involvement in CYP3A4 repression. Eur J Cancer.
45:2882–2892. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Niemira M, Dastych J and Mazerska Z:
Pregnane X receptor dependent up-regulation of CYP2C9 and CYP3A4 in
tumor cells by antitumor acridine agents, C-1748 and C-1305,
selectively diminished under hipoxia. Biochem Pharmacol.
86:231–241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
McErlane V, Yakkundi A, McCarthy HO,
Hughes CM, Patterson LH, Hirst DG, Robson T and McKeown SR: A
cytochrome P450 2B6 meditated gene therapy strategy to enhance the
effects of radiation or cyclophosphamide when combined with the
bioreductive drug AQ4N. J Gene Med. 7:851–859. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fradette C and Souich P: Effect of hypoxia
on cytochrome P450 activity and expression. Curr Drug Metab.
5:257–271. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Michaelis UR, Fisslthaler B,
Barbosa-Sicard E, Falck JR, Fleming I and Busse R: Cytochrome P450
epoxygenases 2C8 and 2C9 are implicated in hypoxia-induced
endothelial cell migration and angiogenesis. J Cell Sci.
118:5489–5498. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fradette C, Batonga J, Teng S,
Piquette-Miller M and du Souich P: Animal models of acute moderate
hypoxia are associated with a down-regulation of CYP1A1, 1A2, 2B4,
2C5, and 2C16 and up-regulation of CYP3A6 and P-glycoprotein in
liver. Drug Metab Dispos. 35:765–771. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jewell UR, Kvietikova I, Scheid A, Bauer
C, Wenger RH and Gassmann M: Induction of HIF-1alpha in response to
hypoxia is instantaneous. FASEB J. 15:1312–1314. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Box AH and Demetrick DJ: Cell cycle kinase
inhibitor expression and hypoxia-induced cell cycle arrest in human
cancer cell lines. Carcinogenesis. 25:2325–2335. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Longley DB, Allen WL, McDermott U, Wilson
TR, Latif T, Boyer J, Lynch M and Johnston PG: The roles of
thymidylate synthase and p53 in regulating Fas-mediated apoptosis
in response to antimetabolites. Clin Cancer Res. 10:3562–3571.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chiche J, Brahimi-Horn MC and Pouysségur
J: Tumour hypoxia induces a metabolic shift causing acidosis: A
common feature in cancer. J Cell Mol Med. 14:771–794. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Swift LH and Golsteyn RM: Genotoxic
anti-cancer agents and their relationship to DNA damage, mitosis,
and checkpoint adaptation in proliferating cancer cells. Int J Mol
Sci. 15:3403–3431. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang Z, Roy P and Waxman DJ: Role of
human liver microsomal CYP3A4 and CYP2B6 in catalyzing
N-dechloroethylation of cyclophosphamide and ifosfamide. Biochem
Pharmacol. 59:961–972. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
O'Connor PM, Wassermann K, Sarang M,
Magrath I, Bohr VA and Kohn KW: Relationship between DNA
cross-links, cell cycle, and apoptosis in Burkitt's lymphoma cell
lines differing in sensitivity to nitrogen mustard. Cancer Res.
51:6550–6557. 1991.PubMed/NCBI
|
|
34
|
Shah MA and Schwartz GK: Cell
cycle-mediated drug resistance: An emerging concept in cancer
therapy. Clin Cancer Res. 7:2168–2181. 2001.PubMed/NCBI
|
|
35
|
Sun C, Wang G, Wrighton KH, Lin H,
Songyang Z, Feng XH and Lin X: Regulation of p27kip1
phosphorylation and G1 cell cycle progression by protein
phosphatase PPM1G. Am J Cancer Res. 6:2207–2220. 2016.PubMed/NCBI
|
|
36
|
Mabjeesh NJ, Escuin D, LaVallee TM,
Pribluda VS, Swartz GM, Johnson MS, Willard MT, Zhong H, Simons JW
and Giannakakou P: 2ME2 inhibits tumor growth and angiogenesis by
disrupting microtubules and dysregulating HIF. Cancer Cell.
3:363–75. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kaluz S, Kaluzová M, Liao SY, Lerman M and
Stanbridge EJ: Transcriptional control of the tumor- and
hypoxia-marker carbonic anhydrase 9: A one transcription factor
(HIF-1) show? Biochim Biophys Acta. 1795:162–172. 2009.PubMed/NCBI
|
|
38
|
Nordfors K, Haapasalo J, Korja M, Niemelä
A, Laine J, Parkkila AK, Pastorekova S, Pastorek J, Waheed A, Sly
WS, et al: The tumour-associated carbonic anhydrases CA II, CA IX
and CA XII in a group of medulloblastomas and supratentorial
primitive neuroectodermal tumours: An association of CA IX with
poor prognosis. BMC Cancer. 10:1482010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Svastová E, Zilka N, Zat'ovicová M,
Gibadulinová A, Ciampor F, Pastorek J and Pastoreková S: Carbonic
anhydrase IX reduces E-cadherin-mediated adhesion of MDCK cells via
interaction with beta-catenin. Exp Cell Res. 290:332–345. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wykoff CC, Beasley NJ, Watson PH, Turner
KJ, Pastorek J, Sibtain A, Wilson GD, Turley H, Talks KL, Maxwell
PH, et al: Hypoxia-inducible expression of tumor-associated
carbonic anhydrases. Cancer Res. 60:7075–7083. 2000.PubMed/NCBI
|
|
41
|
Sowa T, Menju T, Chen-Yoshikawa TF,
Takahashi K, Nishikawa S, Nakanishi T, Shikuma K, Motoyama H,
Hijiya K, Aoyama A, et al: Hypoxia-inducible factor 1 promotes
chemoresistance of lung cancer by inducing carbonic anhydrase IX
expression. Cancer Med. 6:288–297. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu J, Wang Q, Wu DC, Wang XW, Sun Y, Chen
XY, Zhang KL and Li H: Differential regulation of CYP1A1 and CYP1B1
expression in resveratrol-treated human medulloblastoma cells.
Neurosci Lett. 363:257–261. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Schmidt R, Baumann F, Knüpfer H,
Brauckhoff M, Horn LC, Schönfelder M, Köhler U and Preiss R: CY
P3A4 CY P2C9 and CYP2B6 expression and ifosfamide turnover in
breast cancer tissue microsomes. Br J Cancer. 90:911–916. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Miyoshi Y, Taguchi T, Kim SJ, Tamaki Y and
Noguchi S: Prediction of response to docetaxel by
immunohistochemical analysis of CYP3A4 expression in human breast
cancers. Breast Cancer. 12:11–15. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rodríguez-Antona C, Leskelä S, Zajac M,
Cuadros M, Alvés J, Moneo MV, Martín C, Cigudosa JC, Carnero A,
Robledo M, et al: Expression of CYP3A4 as a predictor of response
to chemotherapy in peripheral T-cell lymphomas. Blood.
110:3345–3351. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhu Z, Mu Y, Qi C, Wang J, Xi G, Guo J, Mi
R and Zhao F: CYP1B1 enhances the resistance of epithelial ovarian
cancer cells to paclitaxel in vivo and in vitro. Int
J Mol Med. 35:340–348. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yoshida GJ: Metabolic reprogramming: The
emerging concept and associated therapeutic strategies. J Exp Clin
Cancer Res. 34:1112015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Donovan L, Welford SM, Haaga J, LaManna J
and Strohl KP: Hypoxia-implications for pharmaceutical
developments. Sleep Breath. 14:291–298. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Erler JT, Cawthorne CJ, Williams KJ,
Koritzinsky M, Wouters BG, Wilson C, Miller C, Demonacos C,
Stratford IJ and Dive C: Hypoxia-mediated down-regulation of Bid
and Bax in tumors occurs via hypoxia-inducible factor 1-dependent
and -independent mechanisms and contributes to drug resistance. Mol
Cell Biol. 24:2875–2889. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cui XY, Skretting G, Tinholt M, Stavik B,
Dahm AEA, Sahlberg KK, Kanse S, Iversen N and Sandset PM: A novel
hypoxia response element regulates oxygen-related repression of
tissue factor pathway inhibitor in the breast cancer cell line
MCF-7. Thromb Res. 157:111–116. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li D, Du Y, Yuan X, Han X, Dong Z, Chen X,
Wu H, Zhang J, Xu L, Han C, et al: Hepatic hypoxia-inducible
factors inhibit PPARα expression to exacerbate acetaminophen
induced oxidative stress and hepatotoxicity. Free Radic Biol Med.
110:102–116. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Padró M, Louie RJ, Lananna BV, Krieg AJ,
Timmerman LA and Chan DA: Genome-independent hypoxic repression of
estrogen receptor alpha in breast cancer cells. BMC Cancer.
17:2032017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Fradette C, Bleau AM, Pichette V, Chauret
N and Du Souich P: Hypoxia-induced down-regulation of CYP1A1/1A2
and up-regulation of CYP3A6 involves serum mediators. Br J
Pharmacol. 137:881–891. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Nishida CR, Lee M and de Montellano PR:
Efficient hypoxic activation of the anticancer agent AQ4N by CYP2S1
and CYP2W1. Mol Pharmacol. 78:497–502. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Soncini M, Corna G, Moresco M, Coltella N,
Restuccia U, Maggioni D, Raccosta L, Lin CY, Invernizzi F,
Crocchiolo R, et al: 24-Hydroxycholesterol participates in
pancreatic neuroendocrine tumor development. Proc Natl Acad Sci
USA. 113:E6219–E6227. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Anderson KM, Guinan P and Rubenstein M:
The effect of normoxia and hypoxia on a prostate (PC-3) CD44/CD41
cell side fraction. Anticancer Res. 31:487–494. 2011.PubMed/NCBI
|
|
57
|
Murono K, Tsuno NH, Kawai K, Sasaki K,
Hongo K, Kaneko M, Hiyoshi M, Tada N, Nirei T, Sunami E, et al:
SN-38 overcomes chemoresistance of colorectal cancer cells induced
by hypoxia, through HIF1alpha. Anticancer Res. 32:865–872.
2012.PubMed/NCBI
|
|
58
|
Richards R, Jenkinson MD, Haylock BJ and
See V: Cell cycle progression in glioblastoma cells is unaffected
by pathophysiological levels of hypoxia. Peer J. 4:e17552016.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Harrison MR, Hahn NM, Pili R, Oh WK,
Hammers H, Sweeney C, Kim K, Perlman S, Arnott J, Sidor C, et al: A
phase II study of 2-methoxyestradiol (2ME2) NanoCrystal®
dispersion (NCD) in patients with taxane-refractory, metastatic
castrate-resistant prostate cancer (CRPC). Invest New Drugs.
29:1465–1474. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kulke MH, Chan JA, Meyerhardt JA, Zhu AX,
Abrams TA, Blaszkowsky LS, Regan E, Sidor C and Fuchs CS: A
prospective phase II study of 2-methoxyestradiol administered in
combination with bevacizumab in patients with metastatic carcinoid
tumors. Cancer Chemother Pharmacol. 68:293–300. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ricker JL, Chen Z, Yang XP, Pribluda VS,
Swartz GM and Van Waes C: 2-methoxyestradiol inhibits
hypoxia-inducible factor 1alpha, tumor growth, and angiogenesis and
augments paclitaxel efficacy in head and neck squamous cell
carcinoma. Clin Cancer Res. 10:8665–8673. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ma L, Li G, Zhu H, Dong X, Zhao D, Jiang
X, Li J, Qiao H, Ni S and Sun X: 2-methoxyestradiol synergizes with
sorafenib to suppress hepatocellular carcinoma by simultaneously
dysregulating hypoxia-inducible factor-1 and −2. Cancer Lett.
355:96–105. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Moon JY and Gwak HS: Role of the nuclear
pregnane X receptor in drug metabolism and the clinical response.
Receptors Clin Investig. 2:e9962015.
|
|
64
|
Handschin C and Meyer UA: Induction of
drug metabolism: The role of nuclear receptors. Pharmacol Rev.
55:649–673. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Plant N: The human cytochrome P450
sub-family: Transcriptional regulation, inter-individual variation
and interaction networks. Biochim Biophys Acta. 1770:478–488. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Yu AM, Tian Y, Tu MJ, Ho PY and Jilek JL:
MicroRNA pharmacoepigenetics: Posttranscriptional regulation
mechanisms behind variable drug disposition and strategy to develop
more effective therapy. Drug Metab Dispos. 44:308–319. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Adesina AM, Dunn ST, Moore WE and
Nalbantoglu J: Expression of p27kip1 and p53 in medulloblastoma:
Relationship with cell proliferation and survival. Pathol Res
Pract. 196:243–250. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Batsi C, Markopoulou S, Kontargiris E,
Charalambous C, Thomas C, Christoforidis S, Kanavaros P,
Constantinou AI, Marcu KB and Kolettas E: Bcl-2 blocks
2-methoxyestradiol induced leukemia cell apoptosis by a
p27Kip1-dependent G1/S cell cycle arrest in conjunction
with NF-κB activation. Biochem Pharmacol. 78:33–44. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Maran A, Shogren KL, Benedikt M, Sarkar G,
Turner RT and Yaszemski MJ: 2-methoxyestradiol-induced cell death
in osteosarcoma cells is preceded by cell cycle arrest. J Cell
Biochem. 104:1937–1945. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Alkarain A and Slingerland J: Deregulation
of p27 by oncogenic signaling and its prognostic significance in
breast cancer. Breast Cancer Res. 6:13–21. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Kumar AP, Garcia GE, Orsborn J, Levin VA
and Slaga TJ: 2-methoxyestradiol interferes with NF kappa B
transcriptional activity in primitive neuroectodermal brain tumors:
Implications for management. Carcinogenesis. 24:209–216. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Bruce JY, Eickhoff J, Pili R, Logan T,
Carducci M, Arnott J, Treston A, Wilding G and Liu G: A phase II
study of 2-methoxyestradiol nanocrystal colloidal dispersion alone
and in combination with sunitinib malate in patients with
metastatic renal cell carcinoma progressing on sunitinib malate.
Invest New Drugs. 30:794–802. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Gorska M, Kuban-Jankowska A, Zmijewski MA,
Gorzynik M, Szkatula M and Wozniak M: Neuronal nitric oxide
synthase induction in the antitumorigenic and neurotoxic effects of
2-methoxyestradiol. Molecules. 19:13267–13281. 2014. View Article : Google Scholar : PubMed/NCBI
|