Introduction
Esophageal cancer (EC) is the eighth most commonly
diagnosed cancer and the sixth leading cause of cancer-associated
mortality worldwide (1). Radiation
therapy (RT) is a key part of multimodality EC therapy (2,3).
Radiotherapy resistance often leads to subsequent recurrence and
metastasis (4,5).
MicroRNAs (miRNAs/miRs) are a family of endogenous
non-coding RNAs comprised of 18–25 nucleotides in length that
function to regulate the stability of target genes by directly
binding to the 3′ untranslated region (3′ UTR) (6,7). An
increasing body of evidence has demonstrated that miRNAs can act as
tumor promoters or suppressors to regulate various basic cellular
functions (8–10). Furthermore, some studies have
revealed that miRNAs directly affect radioresistance by regulating
specific pathways (11), including
the repair of DNA double strand breaks (12), cell cycle checkpoint activation
(13), apoptosis (14) and autophagy (15). For example, Lin28-let7 regulates the
radiosensitivity of human cancers through activated KRAS signaling
(16).
miR-301a, located on chromosome 17q22-17q23, is an
important miRNA that has been studied in a variety of tumor types
(17). miR-301a expression is
upregulated in several types of cancer including pancreatic
(18), prostate cancer (19) and malignant melanoma (20); it also acts as a candidate oncogene
by promoting tumor proliferation and invasion. Recently, a previous
study demonstrated that miR-301a promoted radioresistance in
prostate cancer cells by downregulating N-myc downstream-regulated
gene 2 (NDRG2) (21). However, the
mechanism underlying the role of miR-301a in EC radioresistance
remains unclear.
In our previous study, we used a human miRNA
microarray to detect the differential expression of miRNAs,
comparing the human radioresistant esophageal squamous cell
carcinoma (ESCC) line KYSE-150R and the parental cell line KYSE-150
(22). Based on the predicted genes
and pathways of the miRNA target, the expression of miR-301a was
significantly downregulated in KYSE-150R cells and it was thought
to play an important role in regulating the radiosensitivity of
ESCC cells. In the present study, we confirmed the effect of
miR-301a on cell proliferation, epithelial-mesenchymal transition
(EMT) and radiosensitivity in ESCC cells. It was revealed that
miR-301a suppressed cell proliferation and migration, and enhanced
radiosensitivity in ESCC cells by directly targeting WNT1.
Materials and methods
Cell culture
The human ESCC cell line KYSE-150 was purchased from
the American Type Culture Collection (ATCC; Manassas, VA, USA). The
radioresistant cell line KYSE-150R was previously established at
our department by gradient dose irradiation treatment (22). KYSE-150 and KYSE-150R cells were
maintained in RPMI-1640 medium (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) with 100 U/ml of penicillin, 100 mg/ml of
streptomycin and 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.), in an incubator at 37°C containing 5%
CO2. The cell lines were sub-cultured every 2 to 3 days
following digestion at room temperature with 1 ml 0.25%
Trypsin-EDTA (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The
viability was reported as the percentage of the number of viable
cells to the total number of cells. The average viability over 95%
was determined by Trypan Blue staining, at 37°C in an incubator
containing 5% CO2.
Cell transfection
Cultured cells were transfected with miR-301a mimic
or inhibitor (Ambion; Thermo Fisher Scientific, Inc.) using
Lipofectamine 2000™ reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. Cells were
transfected with 0, 5, 10 and 20 nM miR-301a mimic or inhibitor or
negative control (NC) for 48 h, unless indicated otherwise.
Transwell assay
After transfection with miR-301a mimic or inhibitor,
5×104 cells were plated into the top chamber of the
insert (8 µm pore size; BD Biosciences, Franklin Lakes, NJ, USA) in
medium without serum. The lower chamber was loaded with medium
containing 10% FBS. The cells were incubated for 12 h and the cells
that had migrated to the bottom of the membrane were fixed with
methanol and stained with crystal violet. The number of stained
cells were counted at least five random microscopic fields by a
light microscope at a magnification of ×200 (Olympus Corp., Tokyo,
Japan).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT)
assay
Cells were seeded into a 96-well plate in triplicate
at a concentration of 4×103 cells/well. Cell growth was
measured by MTT bromide assay every 24 h from Day 1 to Day 6. Cells
were incubated with 5 mg/ml MTT for 4 h, and subsequently
solubilized in dimethyl sulfoxide (DMSO) (100 µl/well). The
absorbance was measured at 570 nm using an ELISA reader.
Clonogenic assay
Cells in the exponential phase of growth were plated
in triplicate onto 6-well plates with ~1×103 cells/well.
These logarithmic growth cells were irradiated with doses of 0, 2,
4, 6, 8, or 10 Gy using X-ray from a linear accelerator (Varian
2300C/D; Varian Medical Systems, Salt Lake, UT, USA) with an
average dose rate of 100 cGy/min. Immediately following
irradiation, the cells were incubated for 10 days at 37°C in 5%
CO2 to allow for colony formation and then fixed with
pure ethanol. Visible colonies consisting of at least 50 cells were
stained with 0.5% crystal violet (Sigma-Aldrich; Merck KGaA) and
counted. The surviving fraction was then estimated.
Dual-luciferase reporter assay
The wild-type (wt) or mutated (mut) 3′ UTR of WNT1,
containing three binding sites of miR-301a, were cloned into the
luciferase vector pMIR reporter. Cells were transfected with the
WNT1 luciferase reporter vector, Renilla luciferase vector
(Promega Corp., Madison, WI, USA) and the miRNA mimic or inhibitor
using Lipofectamine 2000™ reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). After 48 h, the cells were harvested, lysed and
luciferase activity was assessed using the Dual-Luciferase Reporter
Assay system (Promega Corp.) according to the manufacturer's
protocol. Renilla luciferase was used for normalization.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Total RNA (1 µg) was reverse transcribed
to cDNA using the SuperScript II reverse transcription kit (Thermo
Fisher Scientific, Inc.). RT-qPCR was performed using SYBR-Green
Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.) and
the ABI PRISM 7900 Sequence Detection System (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The RT-qPCR thermocycling
conditions were as follows: 95°C for 10 min, followed by 40 cycles
at 95°C for 15 sec and 60°C for 1 min. β-actin was used as an
internal control and the fold-changes of target genes were
calculated using the 2−ΔΔCq method (23). Primer sequences of Wnt pathway- and
EMT-associated genes are presented in Table I.
 | Table I.Primer sequences of Wnt pathway- and
EMT-associated genes. |
Table I.
Primer sequences of Wnt pathway- and
EMT-associated genes.
| Target gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| WNT1 |
TAAGCAGGTTCGTGGAGGAG |
GGTTTCTGCTACGCTGCTG |
| E-cadherin |
GACCGGTGCAATCTTCAAA |
TTGACGCCGAGAGCTACAC |
| Twist |
TCCATTTTCTCCTTCTCTGGAA |
CCTTCTCGGTCTGGAGGAT |
| Vimentin |
GCAAAGATTCCACTTTGCGT |
GAAATTGCAGGAGGAGATGC |
| Snail |
TCTGAGTGGGTCTGGAGGTG |
CTCTAGGCCCTGGCTGCTAC |
| TCF4 |
CATAGGGAGTCCCATCTCCA |
GGACCAACTTCTTTGGCAAG |
| Cyclin D1 |
GGCGGATTGGAAATGAACTT |
TCCTCTCCAAAATGCCAGAG |
| β-actin |
AAAGACCTGTACGCCAACAC |
GTCATACTCCTGCTTGCTGAT |
Western blot analysis
Proteins were extracted from cultured cells using
RIPA buffer (Thermo Fisher Scientific, Inc). Protein concentration
was determined using the BCA protein assay kit (Thermo Fisher
Scientific, Inc.). Lysates containing 100 µg of protein were mixed
with loading buffer containing 5% β-mercaptoethanol. Extracted
proteins were subjected to 10% SDS-PAGE and transferred to PVDF
membranes (0.45 µm). The membranes were then incubated with 5%
non-fat dry milk in phosphate-buffered saline (PBS) for 1 h at room
temperature to block non-specific binding sites. Then, the
membranes were incubated with primary antibodies overnight at 4°C,
followed by a secondary antibody (1:20,000; anti-rabbit or
anti-mouse IgG, HRP-linked antibody, cat. nos. 7071 and 7072,
respectively; Cell Signaling Technology, Inc.) incubation for 2 h
at room temperature. The protein bands were visualized with the
SuperSignal West Pico Chemiluminescent kit (Pierce; Thermo Fisher
Scientific, Inc.). The following primary antibodies were used:
Cyclin D1 (1:1,000; cat. no. 2978), E-cadherin (1:1,000; cat. no.
3195), β-catenin (1:1,000; cat. no. 8480) and Snail (1:1,000; cat.
no. 3879; all from CST Biological Reagents Co., Ltd., Shanghai,
China), WNT1 (1:1,000; cat. no. ab15251; Abcam, Cambridge, MA, USA)
and vimentin (1:1,000; cat. no. 5741; CST Biological Reagents Co.,
Ltd.) and Tubulin (1:4,000; cat. no. T5168; Sigma-Aldrich; Merck
KGaA).
Immunofluorescence staining
Cells were fixed with 4% formaldehyde, permeabilized
with 0.5% Triton, and then incubated with an E-cadherin (1:500) or
vimentin (1:500) antibody overnight. After washing with PBS, the
cells were incubated with Alexa Fluor 488-labeled secondary
antibody (1,000-fold dilution; cat. no. 34201; Invitrogen; Thermo
Fisher Scientific, Inc.) for 45 min. Cells were counterstained with
DAPI to label the nuclei. Images were captured with a Nikon
fluorescent microscope.
Statistical analysis
Data were expressed as the mean ± standard deviation
from at least three independent experiments. Student's t-test was
used to compare the differences between two groups. All statistical
analyses were performed using SPSS 15.0 software (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-301a inhibits cell proliferation
and migration, and increases radioresistant cell sensitivity to
irradiation in ESCC
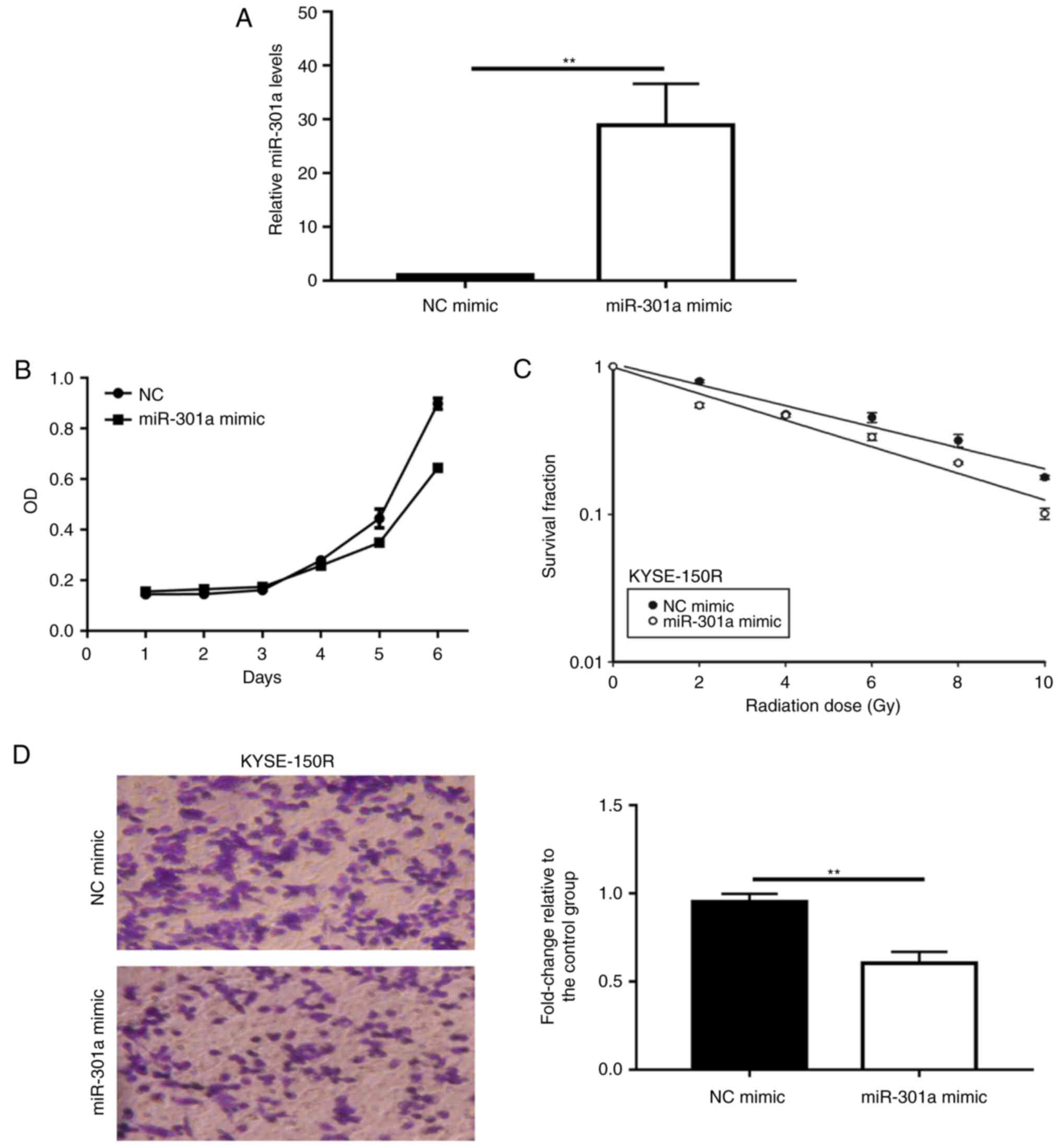
The present study initially transfected KYSE-150R
cells with the miR-301a mimic in order to upregulate miR-301a in
vitro (P<0.01; Fig. 1A). The
overexpression of miR-301a in KYSE-150R cells decreased cell
viability when compared with cells transfected with NC mimic cells
(P<0.05; Fig. 1B). The results
of the colony formation assays revealed that overexpressing
miR-301a significantly enhanced the effects of radiotherapy in
KYSE-150R cells (P<0.05; Fig.
1C). Upregulation of miR-301a in KYSE-150R cells led to a
30–40% reduction in the levels of migration when compared with the
NC-mimic cells (P<0.01; Fig.
1D).
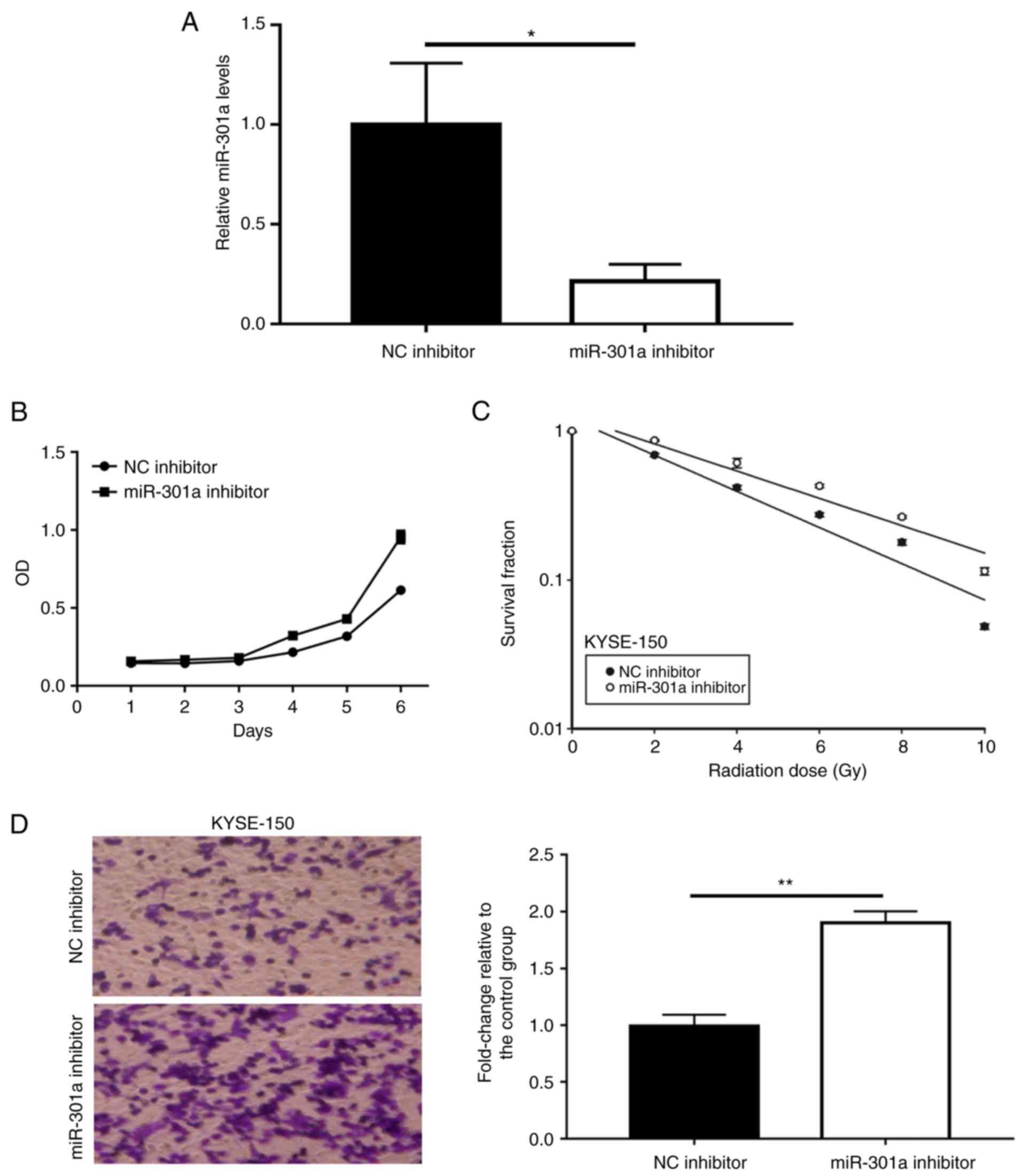
Next, the present study suppressed the expression of
miR-301a in KYSE-150 cells via transfection with a miR-301a
inhibitor (P<0.05; Fig. 2A). The
miR-301a inhibitor groups exhibited enhanced cell proliferation
when compared with the NC inhibitor group (P<0.05; Fig. 2B). The KYSE-150 cells with reduced
miR-301a levels had a significantly greater survival fraction when
compared with the NC cells (P<0.05; Fig. 2C), indicating that these cells had
decreased radiosensitivity. Anti-miR301a cells displayed
significantly higher migration potential, compared to the NC group
(P<0.01; Fig. 2D).
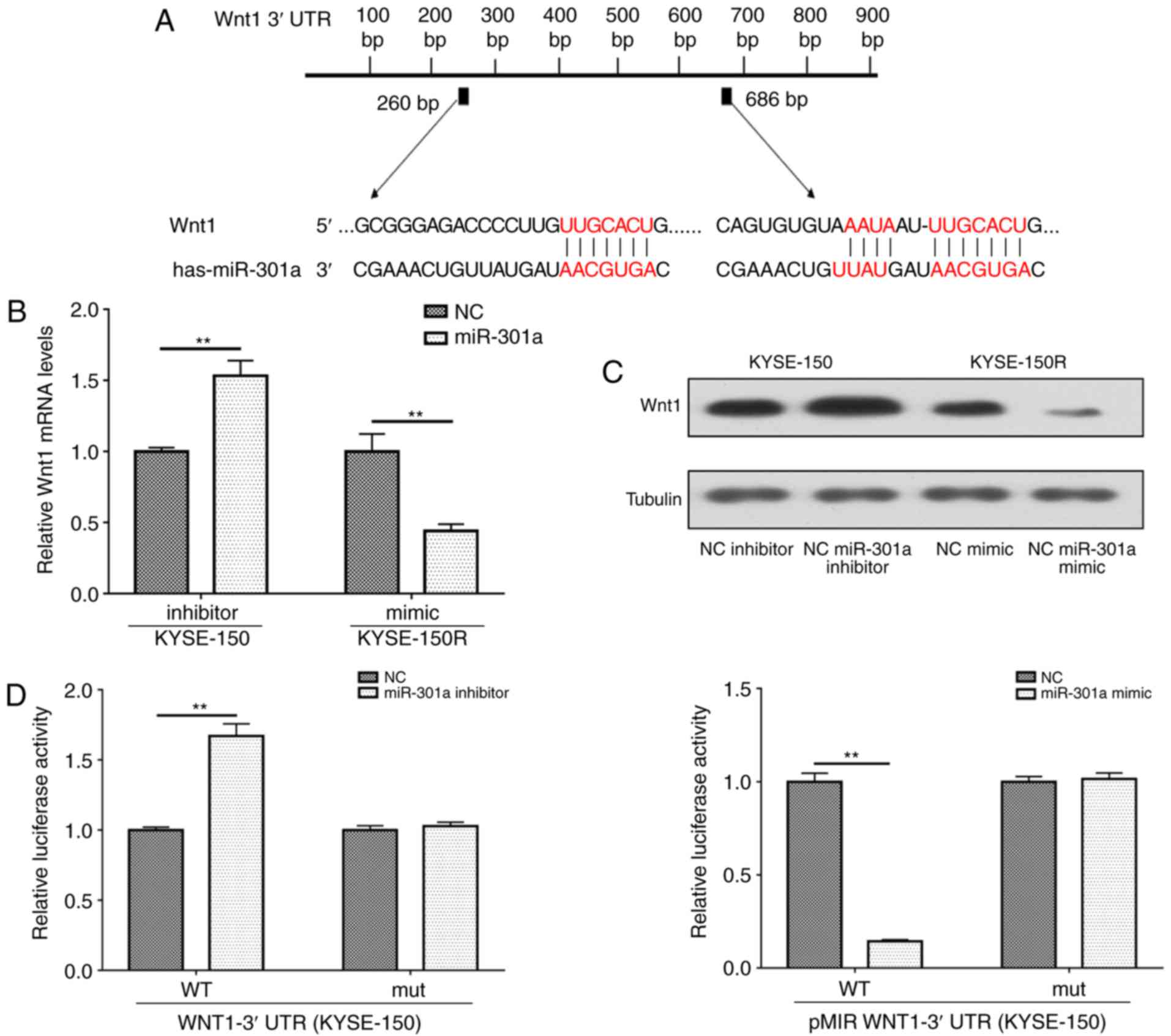
miR-301a directly targets the coding
region of WNT1
Next the present study evaluated the potential
targets of miR-301a. Bioinformatics analysis was performed to
identify potential candidate targets of miR-301a using publicly
available algorithms. As revealed in Fig. 3A, the 3′ UTR WNT1 contained the
target sequence of miR-301a. Subsequently, RT-qPCR and western
blotting were employed to determine the ability of miR-301a to
regulate the expression of WNT1. Overexpression of miR-301a
markedly downregulated the mRNA and protein levels of WNT1 in
KYSE-150R cells. Conversely, inhibition of miR-301a markedly
increased the expression of WNT1 at the mRNA and protein levels in
the KYSE-150 group (Fig. 3B and
C).
To further explore whether miR-301a could interact
with the 3′ UTR of WNT1, the present study performed luciferase
reporter assays. As indicated in Fig.
3D, the miR-301a mimic significantly inhibited luciferase
activity in cells expressing pMIR-WNT1-wt 3′ UTR. However,
co-transfection with the pMIR-WNT1-mut plasmid and the miR-301a
mimic did not significantly alter the luciferase activity when
compared with the NC group (P>0.05). Collectively, these results
demonstrated that WNT1 is directly targeted by miR-301a.
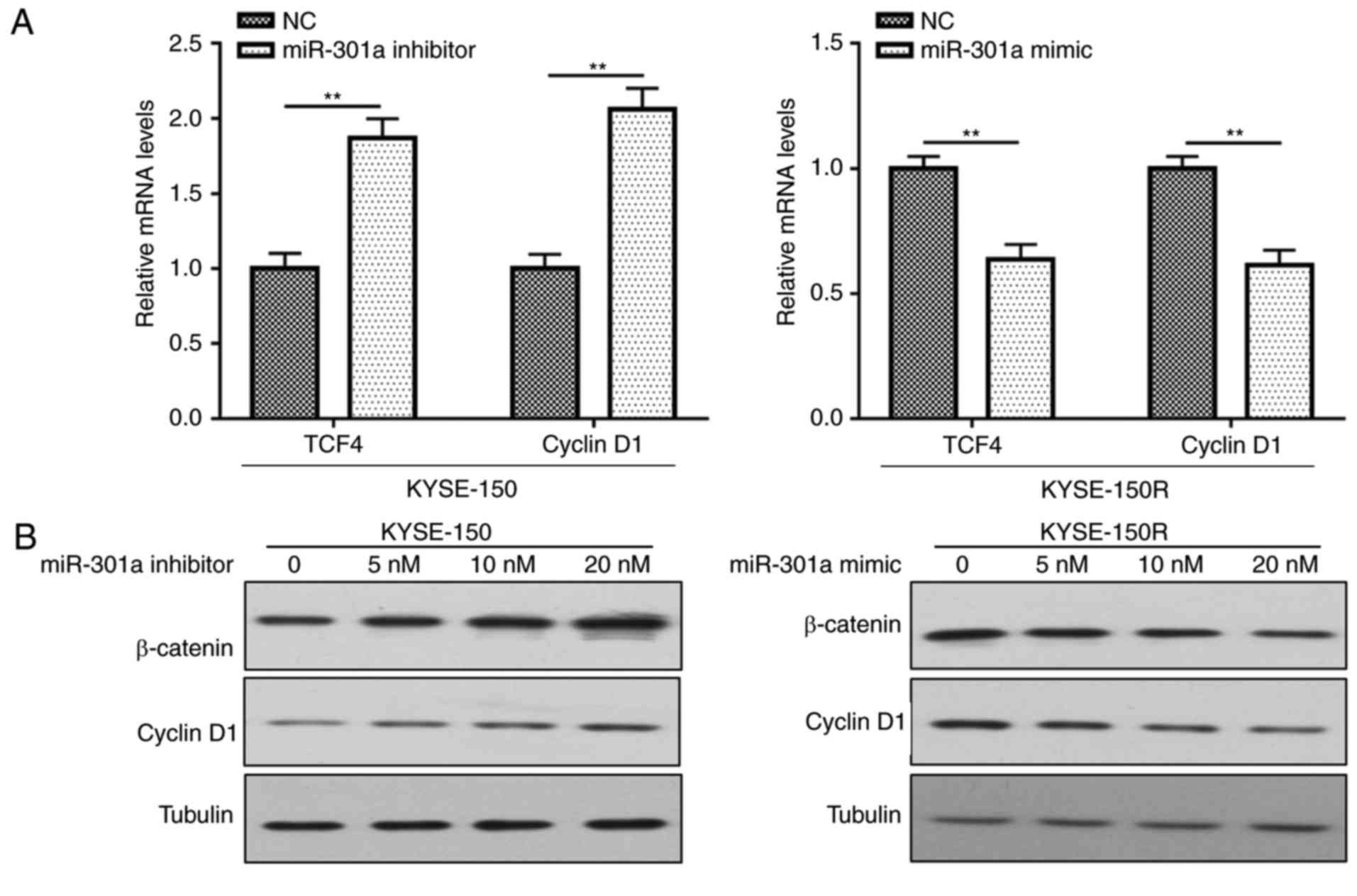
Comparisons between the expression of
Wnt/β-catenin signaling pathway-related genes and proteins among
the different groups
RT-qPCR and western blot analyses were conducted to
investigate whether miR-301a expression could markedly affect the
expression of Wnt/β-catenin-related proteins. As revealed in
Fig. 4A, miR-301a mimic-transfected
cells (at the greatest concentration of 20 nM) had significantly
lower mRNA expression of transcription factor 4 (TCF4) and cyclin
D1 when compared to the KYSE-150R control groups. In miR-301a
inhibitor-transfected cells, TCF4 and cyclin D1 were significantly
increased. Furthermore, the protein expression of β-catenin and
cyclin D1 was reduced in the miR-301a mimic-transfected group.
However, the miR-301a inhibitor-transfected group exhibited the
opposite results (P<0.05; Fig.
4B).
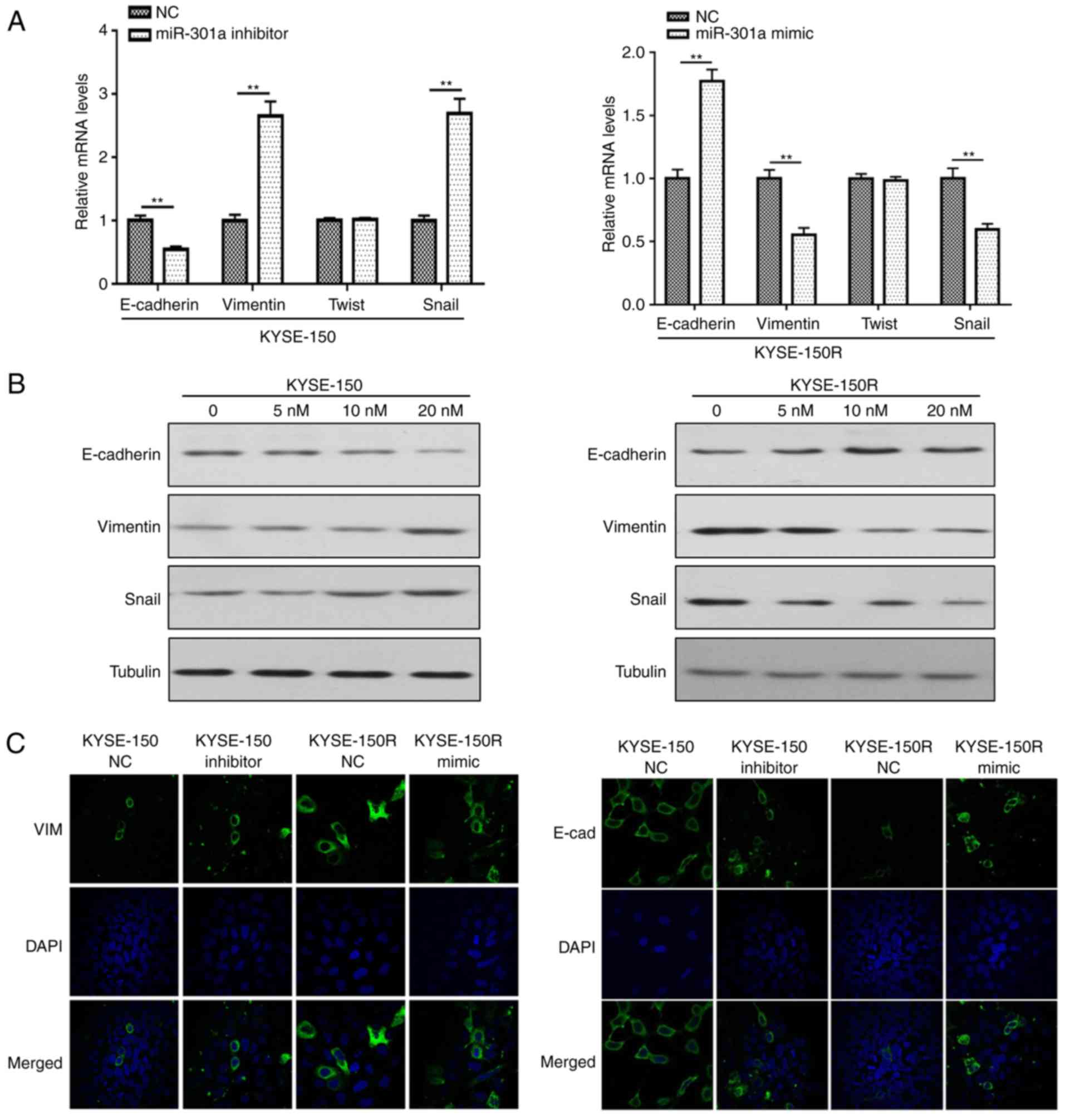
miR-301a affects EMT-related protein
expression
Our previous study demonstrated that EMT mediates
radioresistance in human ESCC cells (24). Therefore, in the present study
classic EMT biomarkers and transcription factors were detected in
the different cell groups. The results of the RT-qPCR assay and
western blotting revealed that the gene and protein expression
levels of E-cadherin in KYSE-150 cells transfected with miR-301a
inhibitors were decreased than those observed in the NC groups,
while E-cadherin expression in the miR-301a-mimic groups was
significantly increased when compared with the NC groups (Fig. 5A and B). Furthermore, expression of
vimentin and Snail in the miR-301a-inhibitor group was upregulated,
whereas the expression of vimentin and Snail was downregulated in
the miR-301a-mimic group. However, the regulation of miR-301a did
not significantly affect Twist expression (P≥0.05; Fig. 5A and B).
Immunofluorescence analysis was conducted to confirm
the localization of vimentin and E-cadherin in the miR-301a
overexpression and knockout groups. Intense staining in the cell
membrane indicated that E-cadherin was shuttled to the cell
membrane in miR-301a-overexpressing cells, while less staining in
knockout miR-301a cells indicated that E-cadherin was downregulated
(Fig. 5C). Conversely the
expression of vimentin in the cell membrane of miR-301a
mimic-transfected cells was reduced. These results indicated that
miR-301a may be an upstream regulator of E-cadherin and vimentin
expression, and an inducer of EMT phenotypes.
Discussion
Acquired radioresistance is considered to be an
important cause of treatment failure in EC patients. Our previous
study revealed that miR-301a was a candidate in the aberrant
profile of radiosensitive miRNAs and was associated with ESCC
radiosensitivity (22). However,
there is limited research on its function. In the present study,
miR-301a was revealed to regulate the radiosensitivity of ESCC by
targeting WNT1. The results indicated that miR-301a may be a new
type of radiosensitivity-related miRNA for future EC radioresistant
therapeutic strategies.
miR-301a has been identified as a candidate oncogene
in different types of cancers. In colorectal cancer, ectopic
miR-301a expression was observed to promote migration and invasion
by downregulating transforming growth factor β receptor (17). Nam et al (25) demonstrated that miR-301a played an
oncogenic role in prostate cancer by directly targeting the p63
tumor suppressor. Kawano et al (26) demonstrated that restoration of
miR-301a downregulation decreased ESC proliferation and
tumorigenesis, and that the oncogenic activity may involve the
negative regulation of the target gene, phosphatase and tensin
homolog. In the present study, upregulation of miR-301a in
KYSE-150R cells was revealed to inhibit cell proliferation and
migration, and increase cell sensitivity to irradiation.
Furthermore, inhibition of miR-301a in KYSE-150 cells reduced cell
sensitivity to irradiation. However, miR-301a function in the
present study was markedly different to that reported in previous
studies. This may be due to the fact that the different functions
of miRNAs depend on the particular type of cancer cell line used
because of its particular genetic background.
miRNAs exert their biological functions by binding
with the 3′ UTR of their target genes. Wang et al (21) observed that higher expression levels
of miR-301a and miR-301b resulted in elevated autophagy and
increased radioresistance in LNCaP cells by targeting NDRG2. The
present study confirmed that WNT1 was a target of miR-301a based on
the results of the luciferase reporter assays. In agreement with
this, the present study also revealed that miR-301a overexpression
significantly inhibited WNT1 expression at both the protein and
mRNA levels, indicating that miR-301a suppressed ESCC cell
activities by targeting WNT1.
Numerous previous studies have focused on WNT1, a
major member of Wnt/β-catenin signaling, and have often reported
that it is altered in EC. The Wnt/β-catenin signaling pathway plays
a crucial role in EC biological processes, including cell
proliferation, apoptosis, and motility. Recently, previous research
has reported that aberrant activation of canonical Wnt/β-catenin
signaling after long-term exposure to fractionated irradiation was
associated with the development of radioresistance in many types of
human cancers. In our pervious study, significantly upregulated
expression levels of WNT1, catenin β1 and cyclin D1 were observed
in the radioresistant ESCC KYSE-150R cell line, indicating that the
Wnt/β-catenin signaling pathway was activated (24). As crucial biological regulators,
miRNAs inhibit targets of signaling pathways and can promote or
inhibit cancer progression in a context- or target-dependent
manner. (27). miR-185-3p regulated
nasopharyngeal carcinoma radioresistance by targeting WNT2B
(28). The present study revealed
that β-catenin and cyclin D1 may be key molecules in the
Wnt/β-catenin signaling pathway, as their expression was altered
when miR-301a was upregulated. These studies provide the basis for
the future exploration of the potential mechanisms underlying
miR-301a functions and how WNT1 is involved in EC radiation
resistance.
Notably, the present study demonstrated that
miR-301a not only enhanced radiosensitivity but also affected EMT
by changing vimentin and E-cadherin expression. EMT is a vitally
important biological process during which epithelial cells lose
their polarity and change to a mesenchymal phenotype (29). Numerous studies have demonstrated
that EMT plays an important role in cancer malignant behaviors,
including radiation resistance (30), drug resistance (31) and cancer stem cells (32). The epithelial marker E-cadherin and
the mesenchymal marker vimentin are often used as markers of EMT
during metastatic progression. Our previous study reported that the
ESCC cell line KYSE-150R presented with typical morphological
changes of the EMT phenotype with significantly decreased
E-cadherin and increased vimentin expression. The results of the
present study revealed that miR-301a and WNT1 affected the
expression of EMT-related biomolecules, indicating that miR-301a
and WNT1 may induce EC radioresistance through EMT.
However, there are some limitations in our study.
For example, we only used one radioresistant cell line to conduct
the study. In vivo models will be set up to define the roles
of miR-301a in EC radioresistance in further research. In
conclusion, upregulation of miR-301a inactivated the Wnt/β-catenin
signaling pathway by targeting WNT1, leading to increased
radiosensitivity and reduced migration of cancer cells. In
addition, targeting this novel miR-301a/Wnt/β-catenin axis may be a
promising strategy for the future treatment of EC
radioresistance.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of Zhejiang Province (grant nos. LQ15H160013,
LY17H160051 and LY15H280013) and the Natural Science Foundation of
China (grant no. 81602658).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
MC designed the experiments. HS, YW, LS, and ZF
performed the biological experiments. MC, YW, LS and HS provided
administrative support and funded the experiments. HS, YF, ZF and
CX analyzed the data. HS, YF, CX and MC drafted the manuscript. All
authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Napier KJ, Scheerer M and Misra S:
Esophageal cancer: A Review of epidemiology, pathogenesis, staging
workup and treatment modalities. World J Gastrointest Oncol.
6:112–120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fokas E, Weiss C and Rödel C: The role of
radiotherapy in the multimodal management of esophageal cancer. Dig
Dis. 31:30–37. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sjoquist KM, Burmeister BH, Smithers BM,
Zalcberg JR, Simes RJ, Barbour A and Gebski V: Australasian
Gastro-Intestinal Trials Group: Survival after neoadjuvant
chemotherapy or chemoradiotherapy for resectable oesophageal
carcinoma: An updated meta-analysis. Lancet Oncol. 2:681–692. 2011.
View Article : Google Scholar
|
|
4
|
Barker HE, Paget JT, Khan AA and
Harrington KJ: The tumour microenvironment after radiotherapy:
Mechanisms of resistance and recurrence. Nat Rev Cancer.
15:409–425. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maier P, Hartmann L, Wenz F and Herskind
C: Cellular pathways in response to ionizing radiation and their
targetability for tumor radiosensitization. Int J Mol Sci. 17(pii):
E1022016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lim LP, Lau NC, Garrett-Engele P, Grimson
A, Schelter JM, Castle J, Bartel DP, Linsley PS and Johnson JM:
Microarray analysis shows that some microRNAs downregulate large
numbers of target mRNAs. Nature. 433:769–773. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Garzon R, Marcucci G and Croce CM:
Targeting microRNAs in cancer: Rationale, strategies and
challenges. Nat Rev Drug Discov. 9:775–778. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hummel R, Hussey DJ and Haier J:
MicroRNAs: Predictors and modifiers of chemo- and radiotherapy in
different tumour types. Eur J Cancer. 46:298–311. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Farazi TA, Spitzer JI, Morozov P and
Tuschl T: miRNAs in human cancer. J Pathol. 223:102–115. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao L, Lu X and Cao Y: MicroRNA and
signal transduction pathways in tumor radiation response. Cell
Signal. 25:1625–1634. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu B, Wang X, Hu S, Ying X, Wang P, Zhang
X, Wang J, Wang H and Wang Y: miR-21-mediated radioresistance
occurs via promoting repair of DNA double strand breaks. J Biol
Chem. 292:3531–3540. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bhattacharya A, Schmitz U, Wolkenhauer O,
Schönherr M, Raatz Y and Kunz M: Regulation of cell cycle
checkpoint kinase WEE1 by miR-195 in malignant melanoma. Oncogene.
32:3175–3183. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang S, Pan Y, Zhang R, Xu T, Wu W, Zhang
R, Wang C, Huang H, Calin CA, Yang H and Claret FX: Hsa-miR-24-3p
increases nasopharyngeal carcinoma radiosensitivity by targeting
both the 3′UTR and 5′UTR of Jab1/CSN5. Oncogene. 35:6096–6108.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang P, Zhang J, Zhang L, Zhu Z, Fan J,
Chen L, Zhuang L, Luo J, Chen H, Liu L, et al: MicroRNA 23b
regulates autophagy associated with radioresistance of pancreatic
cancer cells. Gastroenterology. 145:1133–1143. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oh JS, Kim JJ, Byun JY and Kim IA:
Lin28-let7 modulates radiosensitivity of human cancer cells with
activation of K-Ras. Int J Radiat Oncol Biol Phys. 76:5–8. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang W, Zhang T, Jin R, Zhao H, Hu J,
Feng B, Zang L, Zheng M and Wang M: MicroRNA-301a promotes
migration and invasion by targeting TGFBR2 in human colorectal
cancer. J Exp Clin Cancer Res. 33:1132014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xia X, Zhang K, Luo G, Cen G, Cao J, Huang
K and Qiu Z: Downregulation of miR-301a-3p sensitizes pancreatic
cancer cells to gemcitabine treatment via PTEN. Am J Transl Res.
9:1886–1895. 2017.PubMed/NCBI
|
|
19
|
Damodaran C, Das TP, Papu John AM, Suman
S, Kolluru V, Morris TJ, Faber EN, Rai SN, Messer JC, Alatassi H,
et al: miR-301a expression: A prognostic marker for prostate
cancer. Urol Oncol. 34:e13–e20. 2016. View Article : Google Scholar
|
|
20
|
Cui L, Li Y, Lv X, Li J, Wang X, Lei Z and
Li X: Expression of microRNA-301a and its functional roles in
malignant melanoma. Cell Physiol Biochem. 40:230–244. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang W, Liu M, Guan Y and Wu Q:
Hypoxia-responsive miR-301a and miR-301b promote radioresistance of
prostate cancer cells via downregulating NDRG2. Med Sci Monit.
22:2126–2132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Su H, Jin X, Zhang X, Xue S, Deng X, Shen
L, Fang Y and Xie C: Identification of microRNAs involved in the
radioresistance of esophageal cancer cells. Cell Biol Int.
38:318–325. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Su H, Jin X, Zhang X, Zhao L, Lin B, Li L,
Fei Z, Shen L, Fang Y, Pan H, et al: FH535 increases the
radiosensitivity and reverses epithelial-to-mesenchymal transition
of radioresistant esophageal cancer cell line KYSE-150R. J Transl
Med. 13:1042015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nam RK, Benatar T, Wallis CJ, Amemiya Y,
Yang W, Garbens A, Naeim M, Sherman C, Sugar L and Seth A: MiR-301a
regulates E-cadherin expression and is predictive of prostate
cancer recurrence. Prostate. 76:869–884. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kawano M, Tanaka K, Itonaga I, Iwasaki T
and Tsumura H: MicroRNA-301a promotes cell proliferation via PTEN
targeting in Ewing's sarcoma cells. Int J Oncol. 48:1531–1540.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Song JL, Nigam P, Tektas SS and Selva E:
microRNA regulation of Wnt signaling pathways in development and
disease. Cell Signal. 27:1380–1391. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li G, Wang Y, Liu Y, Su Z, Liu C, Ren S,
Deng T, Huang D, Tian Y and Qiu Y: miR-185-3p regulates
nasopharyngeal carcinoma radioresistance by targeting WNT2B in
vitro. Cancer Sci. 105:1560–1568. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Marie-Egyptienne DT, Lohse I and Hill RP:
Cancer stem cells, the epithelial to mesenchymal transition (EMT)
and radioresistance: Potential role of hypoxia. Cancer Lett.
341:63–72. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shibue T and Weinberg RA: E MTCS Cs, and
drug resistance: The mechanistic link and clinical implications.
Nat Rev Clin Oncol. 14:611–629. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Martin-Belmonte F and Perez-Moreno M:
Epithelial cell polarity, stem cells and cancer. Nat Rev Cancer.
12:23–38. 2011. View
Article : Google Scholar : PubMed/NCBI
|