In current cancer therapy, the limitations of
traditional treatments cannot be ignored. Thus, we must explore new
treatment methods. Cancer immunotherapy, which has been ignored for
many years, has again become a focus of researchers and become a
pillar of cancer therapy. For its outstanding efficacy, cancer
immunotherapy was rated by Science as the Breakthrough of
the Year in 2013 (1), and
Nature considered Emily Whitehead, the first child in the
world whose leukemia was treated with CAR-T therapy, to be one of
10 people who mattered in 2017 (2).
As a pillar of cancer immunotherapy, immune checkpoint inhibitors
have been applied in cancer therapy and have led to promising
clinical responses. In this review, we discuss immune checkpoint
inhibitors that prevent tumor immune escape and recent clinical
studies of immune checkpoint therapy. The efficacy of combination
immunotherapies and the link between the gut microbiome and immune
system are also described in this review.
With the development of cancer research, tumors have
gradually come to be regarded as complete organs rather than masses
of transformed cells (3). These
organs mainly include tumor cells, cancer-associated fibroblasts
(CAFs), endothelial cells and immune cells. These non-cancerous
cells affect the development of tumors (4). In the initial stage of tumor
formation, only a small fraction of the microenvironment contains
infiltrated immune cells, but eventually, various immune cells are
recruited to the microenvironment and T cells may be the most
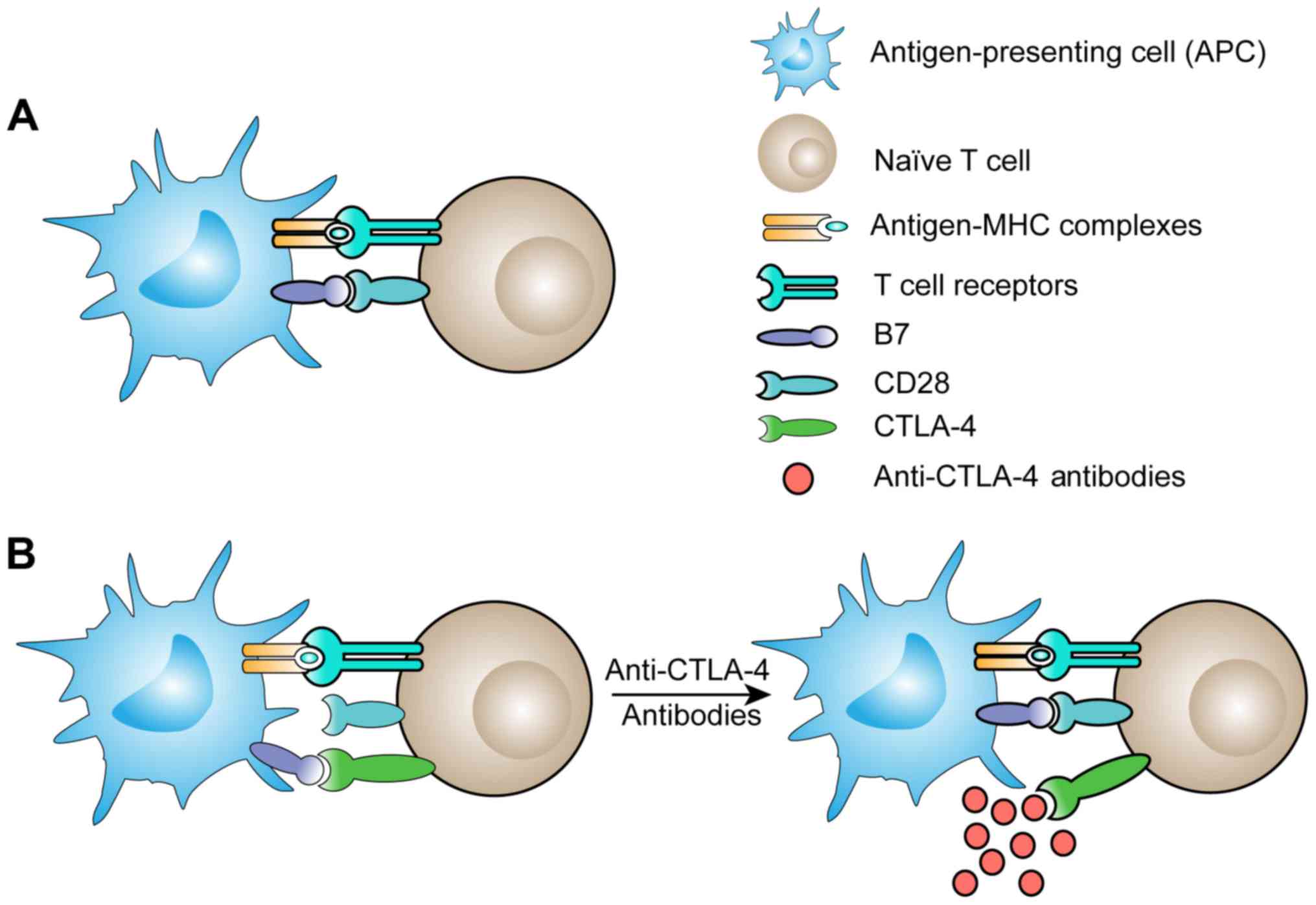
important type of immune cell. Tumor antigens can be displayed by
antigen-presenting cells (APCs), which express major
histocompatibility complex (MHC) molecules on their surfaces. T
cells become activated through the engagement of antigen-MHC
complexes with their T cell receptors (TCRs), molecules that are
mainly expressed on CD4+ T cells and CD8+ T
cells, after which the T cells proliferate and differentiate
(5). Additional costimulatory
signals are also required to activate naïve T cells. CD28 is one of
the proteins expressed on T cells that acts as a B7 receptor and is
constitutively expressed on naïve T cells. When CD28 binds to CD80
and CD86, two B7 molecules, costimulatory signals are provided
(6). B7 molecules are only
expressed by cells that can efficiently present antigens. However,
tumor cells are easily overlooked by the immune system. The
inflammatory response is a component of the immune system that
permits antigen-presenting cells to take up tumor antigens and then
present antigen-MHC complexes and permits B7 molecules to activate
T cells (Fig. 1A).
Inefficient antigen presentation is an obstacle that
must be overcome to allow activated T cells to infiltrate the tumor
microenvironment. When tumor-specific T cells encounter tumor
antigens in tumor-draining lymph nodes, an effective immune
response that attacks tumor cells develops (7). Another hurdle that acts to lower the
responsiveness of antitumor immunity also needs to be overcome.
This hurdle is comprised of tumor cells, regulatory T cells,
myeloid-derived suppressor cells, and other inhibitory factors in
the tumor microenvironment. The accumulation of a sufficient number
of obstacles that the antitumor immune response cannot develop into
an effective reaction underlies this hurdle. How to eliminate the
immunosuppressive state of tumors and mobilize the immune system
may be the next direction of tumor immune studies.
How can the suppression of the antitumor immune
response be weakened? Understanding the fundamental mechanisms of
immune regulation in the immune system, especially in T cells, can
help us solve this problem. We know that the activation of T cells
is complex, requiring not only proliferation and functional
differentiation but also attenuation and termination. In the early
stages of T cell activation, a gene called CTLA-4, which has
high homology to CD28, is expressed. CTLA-4 downregulates the T
cell response by displacing CD28 costimulation (8). Researchers have proposed that the
antitumor functions of T cells can be restored by inhibiting the
binding of CTLA-4 to B7 molecules (Fig.
1B). Further animal experiments confirmed that treatment with
anti-CTLA-4 antibodies in mouse tumor models enhanced the antitumor
response of T cells (9,10). Based on the data generated by
experiments in mice, ipilimumab, a human anti-CTLA-4 antibody, was
developed. A clinical trial indicated that patients with metastatic
melanoma who were treated with ipilimumab achieved a significant
improvement in overall survival (11).
The successful implementation of anti-CTLA-4
antibodies has led to a growing interest in immune checkpoint
therapy (12). As research
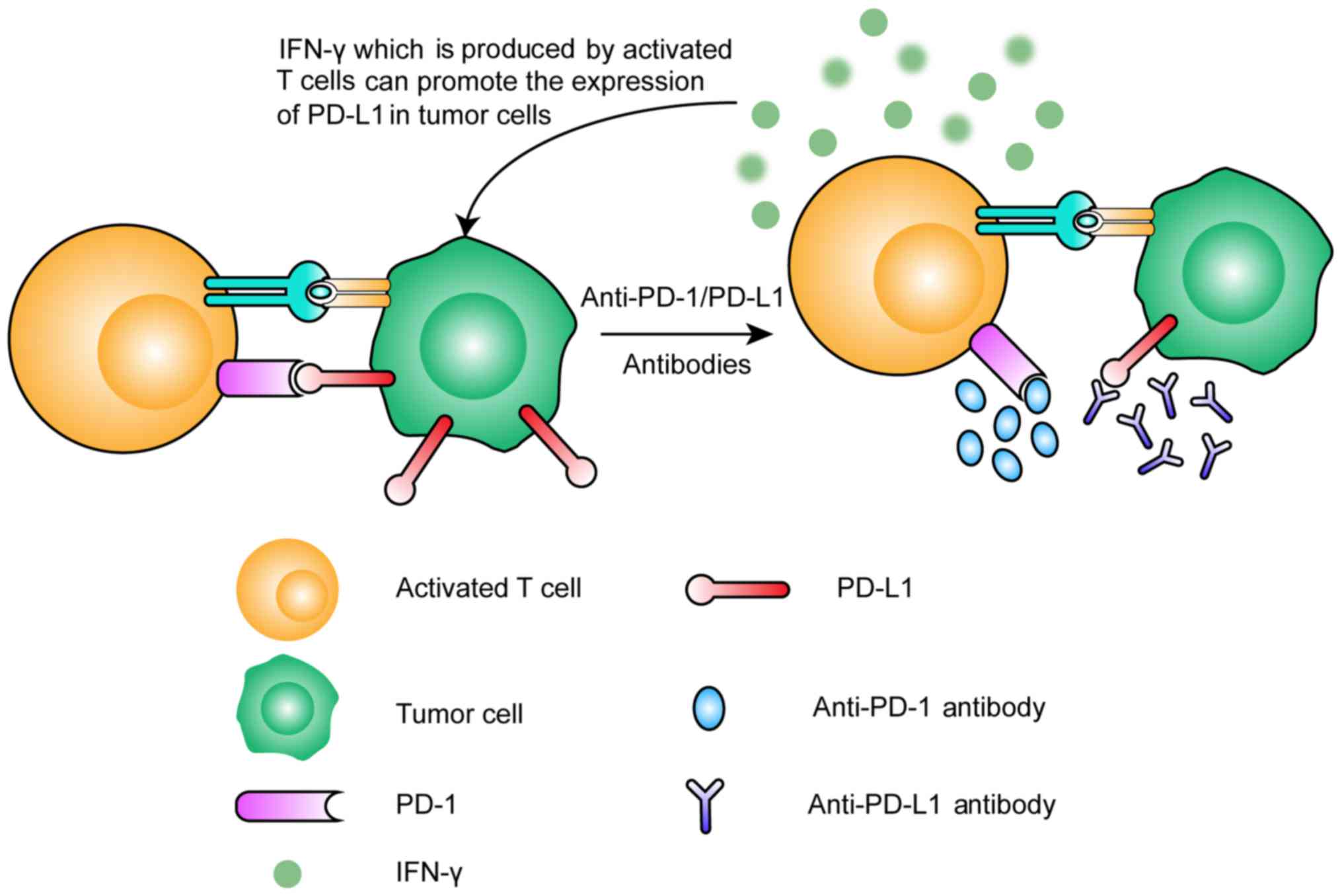
progresses, more immune checkpoints have been found. Programmed
cell death-1 (PD-1), an important immune checkpoint, was discovered
in 2000. PD-1 is expressed only in T cells that have been activated
by antigen (13) and contributes to
the inhibitory response that interferes with the function of the
T-cell receptor by binding with its ligands PD-L1 and PD-L2
(14). The PD-1 expression level
decreases when the activating antigen is cleared during acute
responses, but its level remains high if the antigen is present for
a long time (15–17). Neoantigens are one type of antigen
that can cause high and sustained PD-1 expression, which is one
reason cancer cells can be ignored by T cells (18,19).
PD-1 ligands (PD-L1 and PD-L2) are expressed by many cell types
(such as APCs, dendritic cells, macrophages and vascular
endothelial cells), but PD-L1 is the ligand that is usually
expressed on cancer cells (20,21).
When the PD-L1 molecules on cancer cells bind with PD-1 on T cells,
immunosuppression occurs (Fig. 2).
Activated T cells will produce the cytokine interferon-γ (IFN-γ),
which can promote the expression of PD-L1 in tumor cells (22), subsequently promoting
immunosuppression. This process in normal tissues acts as a
negative feedback mechanism to protect normal cells from attack by
T cells. However, in tumor tissues, this mechanism leads to the
tumor cells becoming invisible to the immune system. Once we have
prevented PD-1 binding with its ligands, we can weaken the
suppression of antitumor immunity. That is the mechanism that
anti-PD-1/PD-L1 therapy is based on.
Anti-CTLA-4 antibodies, such as ipilimumab, are the
pioneers of immune checkpoint blockade therapy (23). Clinical trials revealed that
metastatic melanoma patients treated with ipilimumab exhibited
considerable improvement in their overall survival. Based on the
outstanding efficacy, ipilimumab earned FDA approval for the
treatment of metastatic and non-resectable melanomas in March 2011.
Four years later, ipilimumab was approved as an adjuvant therapy
for stage III melanoma patients.
A multinational phase III trial was conducted with
951 patients after complete resection of stage III cutaneous
melanoma. This trial demonstrated that recurrence-free survival for
these patients was significantly improved by long-term treatment
with a low dose of ipilimumab, but the incidence of adverse events
was higher than that observed in advanced melanoma patients treated
with ipilimumab (24). Secondary
outcomes from this trial also revealed that health-related quality
of life (HRQoL) was slightly impaired (25). The NCT00094653 trial revealed the
efficacy of ipilimumab in metastatic melanoma patients. This phase
III trial compared the median overall survival (OS) between
ipilimumab and other treatments in metastatic melanoma patients
with previous medical therapy. The results revealed that ipilimumab
can improve OS in metastatic melanoma patients (ipilimumab alone
vs. gp100 monotherapy: hazard ratio (HR): 0.66, P=0.003;
combination therapy vs. gp100 alone: HR: 0.68, P<0.001)
(11). Ipilimumab has demonstrated
an antitumor effect in patients who were previously treated for
melanoma and was also tested in patients without previous therapy.
Another phase III trial involving 502 metastatic melanoma patients
without previous therapy has been reported. This trial revealed
that ipilimumab also improved OS in metastatic melanoma patients
without previous therapy, and patients without previous therapy
suffered no new types of adverse events compared with those in
prior studies of ipilimumab (26).
Aside from melanoma, ipilimumab has been studied in other diseases,
such as renal cell carcinoma (27),
prostate (28) and pancreatic
cancer (29), but its efficacy in
these solid tumors is limited (Table
I). The intrinsic resistance of solid tumors, caused by
features such as low tumor immunogenicity and potently
immunosuppressive tumor microenvironments, can explain these
results (30).
Ipilimumab has exhibited a clear beneficial response
in the clinic, but immune-related adverse events (irAEs) have
become an obstacle limiting who can benefit from it. Negative
regulators of immunity are important for the body to maintain
immunologic homeostasis, and when they are blocked by ipilimumab,
irAEs occur. The most common immune-related toxic effects that have
been established include rashes, colitis, hepatitis,
endocrinopathies and pneumonitis (31,32).
Do irAEs have an adverse impact on quality of life? A phase III
trial appears to provide evidence regarding this matter (25). The results revealed that although
some investigators reported grades 3–4 adverse events (11,27),
HRQoL showed little impairment. Nearly half of the patients
withdrew from this trial due to drug-related toxic effects, which
led to a significant reduction in the credibility of the trial.
It is undeniable that ipilimumab has a significant
survival benefit in patients with malignant neoplasms, especially
melanoma. However, adverse events and limited efficacy have ceased
it from benefiting more patients. What can we do in the future to
improve anti-CTLA-4 therapy? A more suitable dose can balance the
efficacy and adverse events, despite 3 mg/kg being the FDA approved
dose for advanced-stage disease patients. In addition, establishing
a set of ipilimumab adverse reaction risk assessment systems and
interventions for patients at higher risk may reduce the incidence
of adverse effects. Of course, determining the resistance
mechanisms to anti-CTLA-4 antibodies is also a crucial step in
solving the efficacy problem.
Anti-PD-1 therapy is a feasible way to relieve
immunosuppression. To date, the success of this therapeutic method
has been demonstrated in clinical trials. The phase Ib study
NCT01953692 (33) enrolled 31
patients with classical Hodgkin lymphoma who did not respond to
brentuximab vedotin therapy to receive pembrolizumab, an anti-PD-1
antibody, at a dose of 10 mg/kg every 2 weeks. The results revealed
that 5 of the 20 patients who exhibited an objective response
achieved complete remission, and 15 patients achieved partial
remission, for an overall response rate (ORR) of 65% (90% CI,
48–79%). In addition to hematological tumor therapy, anti-PD-1
antibodies also play an important role in solid tumor therapy. A
total of 493 patients with advanced gastric or gastro-esophageal
junction cancer were enrolled in a phase III trial (NCT02267343)
(34). With 8.59–8.87 months of
median follow-up, the median overall survival in the nivolumab
group was 1.12 months longer than the median overall survival in
the placebo group (5.26 months vs. 4.14 months; HR 0.63, 95% CI,
0.51–0.78; P<0.0001). The 12-month overall survival rate in the
nivolumab group was 26.2% (95% CI, 20.7–32.0), and that in the
placebo group was 10.9% (95% CI, 6.2–17.0).
Between anti-CTLA-4 antibodies and anti-PD-1
antibodies, which approach will achieve better results in patients?
Interim analyses of the phase III NCT01866319 trial (35) revealed that pembrolizumab provides
superior progression-free survival and overall survival vs.
ipilimumab (the overall survival data remain immature; Table II). Regarding nivolumab, the
NCT02388906 study demonstrated that it provided significantly
longer recurrence-free survival than ipilimumab (36). In addition, both of these studies
revealed that anti-PD-1 antibodies had a lower rate of serious
adverse events than ipilimumab (35,36).
Anti-PD-L1 therapy is another cancer treatment based
on the PD-1 inhibitory pathway. In the phase II NCT02108652 trial
(37), 119 patients with
cisplatin-ineligible urothelial carcinoma received the anti-PD-L1
antibody atezolizumab as their first-line treatment. After a median
follow-up period of 17.2 months, the ORR was 23%, the complete
response rate was 9%, and the median overall survival was 15.9
months in all patients. In IC2/3 patients (those with PD-L1
expression on ≥5% of tumor-infiltrating immune cells), the ORR
increased to 28%. This result demonstrated the encouragingly
durable activity of atezolizumab in untreated metastatic urothelial
cancer patients, especially in patients with high PD-L1 expression
levels. The treatment-related adverse events (TRAEs) in this trial
were acceptable. Unfortunately, the results of a phase III
randomized controlled study (NCT02302807) were not as ideal as
those of the phase II trial (38,39).
The phase III study revealed that overall survival in the IC2/3
population did not differ significantly between the treatment
groups (HR 0.87, 95% CI 0.63–1.21, P=0.41), which precluded further
formal statistical comparisons. The objective response rates were
similar (atezolizumab vs. chemotherapy: 23 vs. 22%), but the
duration of response in the atezolizumab group was longer than that
in the chemotherapy group (Table
II). The reasons for these results warrant further analysis.
Notably, the exploratory biomarker analysis in this study revealed
that the median overall survival for patients treated with
atezolizumab was numerically longer than the median overall
survival for patients treated with chemotherapy in the high tumor
mutation burden population (11.3 months vs. 8.3 months; HR 0.68,
95% CI, 0.51–0.90), whereas survival was similar between the groups
in the low tumor mutation burden population. Furthermore, in IC2/3
patients with a high tumor mutation burden, the median survival for
the atezolizumab group was longer than that for the chemotherapy
group (17.8 months vs. 10.6 months; HR 0.50, 95% CI, 0.29–0.86).
These results indicated that IC2/3 patients with a high tumor
mutation burden will experience a significantly greater benefit
from anti-PD-L1 therapy and that the level of tumor mutation burden
and the PD-L1 expression level of tumor-infiltrating immune cells
can be used to select a population suitable for anti-PD-L1
therapy.
Data on atezolizumab in the treatment of NSCLC have
also been reported. A phase II study (40) of patients with previously treated
NSCLC demonstrated the effectiveness of atezolizumab. The overall
survival in the treatment group was 12.6 months, and the overall
survival in the control group was 9.7 months (HR 0.73, 95% CI,
0.53–0.99; P=0.04). However, having so few patients limited the
ability to draw further conclusions. Then, the phase III
NCT02008227 study (41) enrolled
1,225 patients with previously treated NSCLC. As was observed in
the phase II study (40), the
overall survival in the treatment group was significantly
prolonged. Furthermore, this study revealed that atezolizumab
treatment resulted in an improvement in overall survival compared
with docetaxel, regardless of PD-L1 expression levels (Table II). Overall survival improvement
for patients with squamous or non-squamous histology was also
analyzed, and the conclusion was similar for these 2 histology
types.
Another anti-PD-L1 immune checkpoint inhibitor named
avelumab has also been tested in various tumor types. The first
type avelumab was tested in was metastatic Merkel cell carcinoma,
which was approved by the FDA for treatment with avelumab in March
2017. The phase II trial revealed the promising efficacy of
avelumab in metastatic Merkel cell carcinoma. In a cohort of 88
patients with this carcinoma, treatment with avelumab achieved
objective responses in 28 (31.8%) patients, and 8 (28.6%) of the 28
patients achieved complete responses. In addition, responses are
still ongoing in most patients who achieved an objective response,
and TRAEs in this study were acceptable (42). In solid tumors, avelumab also
revealed marked efficacy. A phase Ia dose-escalation trial reported
that a dose of 20 mg/kg was acceptable and had no negative effect
on the counts of immune cell subsets (43). In a phase Ib dose-expansion trial
(44), 184 patients with NSCLC were
enrolled, and 22 patients (12%) achieved confirmed objective
responses, and 92 patients (50%) achieved disease control. The
reported median OS was 8.4 months (95% CI, 7.3–10.6) in this trial.
Additionally, 23 (13%) of the 184 patients had grade 3 or worse
TRAEs. These results revealed the promising efficacy and acceptable
safety of avelumab in NSCLC. However, nearly 33.2% of the enrolled
patients received 2 or more previous treatments, which prevented
accurate conclusions about the efficacy of avelumab. We look
forward to findings from ongoing phase III trials, which will
provide us with much more powerful evidence. Trials with other
anti-PD-L1 antibodies, such as durvalumab, have shown promising
results in advanced urothelial bladder cancer and NSCLC (45–47).
Anti-PD-1/PD-L1 therapy provides a reason for us to
believe that immunotherapy will achieve greater success in the
future. However, in different patients, there is a great difference
in the efficacy of anti-PD-1/PD-L1 therapy (48). A study performed whole exome
sequencing of 35 patients with metastatic clear cell renal cell
carcinoma and found that patients whose PBRM1 gene had
loss-of-function mutations had significant clinical benefit from
anti-PD-1/PD-L1 therapy compared to patients without
loss-of-function mutations (49).
This study provided a basis for selecting patients for
anti-PD-1/PD-L1 therapy. Another severe problem that needs to be
solved is that large numbers of patients do not show long-lasting
remission. A team from the University of Pennsylvania revealed a
hint to this answer. They found that using a PD-1 inhibitor in mice
could restart failing T cells and lead to a low level of memory T
cell development, but the restarted T cells failed later due to
transient changes in their epigenetics (50).
Immune checkpoint blockers (ICBs), at the forefront
in the field of immunotherapy, have shown enormous success in
cancer therapy. Unfortunately, a large number of patients must
cease their treatment. The appearance of TRAEs is an important
reason for stopping treatment. However, attention should also be
paid to the heterogeneity of responses to ICBs. Reported clinical
data have indicated that only 10–15% of patients respond to
ipilimumab therapy (11,26). What has led to this phenomenon?
Tumors render immune checkpoint therapy ineffective
by intrinsic and extrinsic factors. The intrinsic factors include
genetic and epigenetic alterations, and presentation disrupting the
action of cytotoxic T cells. Non-cancerous stromal or immune cells,
an inhospitable tumor microenvironment for antitumor T cells, and
other systemic influences (such as the gut microbiome) are
considered extrinsic factors (51–53).
As the mechanisms of resistance to ICBs are further
elucidated, several trials are underway to relieve the resistance.
Combination therapies are in various stages of development
(54), and personalized cancer
vaccines hat enhance immune memory show promise (55); furthermore, biomarkers associated
with resistance will be identified in additional research (53). Ultimately, clinical activity will be
optimized, and the rate of clinical response will increase in the
future.
Immune checkpoint inhibitors have achieved exciting
results in some types of cancers (56–59).
However, only a subset of patients benefit from immune checkpoint
inhibition (60). Short-lasting
remission (50) and toxic effects
(61–63) are the major problems we cannot
ignore. Combination anticancer therapies are useful methods to
resolve these issues.
The answer may be yes. On T lymphocytes, both CTLA-4
and PD-1 are expressed, but their mechanisms for inhibiting the
function of immune cells are different (14,69).
This difference provides the rationale for combination therapy. The
phase III NCT01844505 study compared nivolumab alone, ipilimumab
alone, and the combination of the two in advanced melanoma
(70). This study found that the
clinical activity of the combination therapy was better than that
of either monotherapy. Health-related quality of life results from
this study revealed no clinically meaningful deterioration
(71). The phase I NCT01454102
trial in advanced non-small cell lung cancer revealed results
analogous with NCT01844505 (72).
An open-label phase Ib clinical trial observed a similar objective
response rate in its pembrolizumab plus ipilimumab therapy group
compared with the objective response rate in the nivolumab plus
ipilimumab therapy group in the NCT01844505 study (61 vs. 58%)
(70,73).
In addition, according to previous studies,
combination with radiotherapy or targeted therapy enhanced the
anticancer effect of immune checkpoint inhibition (74,75).
Another powerful combination strategy that combines oncolytic
viruses with immune checkpoint inhibitors has been studied in phase
Ib and phase II trials (76,77).
Both trials indicated that this combination strategy had greater
antitumor activity vs. monotherapy. In addition, the incidence of
TRAEs was acceptable. Further clinical trials using combination
anticancer therapies are underway, and the current results show
promise that these therapies will reveal more in the future.
The gut microbiome, which includes bacteria,
microbial eukaryotes, and viruses, lives in the human intestine
(78). Its composition and status
are closely related to the health of the host (79) and to the outcome of cancer
immunotherapy (80).
The ability of intestinal microbiota to modulate the
response to immune checkpoint inhibitors was revealed by
preclinical studies. A study in mice revealed that commensal
Bifidobacterium in the intestine promoted antitumor immunity
and anti-PD-L1 efficacy (81).
Melanoma growth in mice harboring distinct commensal microbiota was
compared in this study, and it was found that spontaneous antitumor
immunity varied. In addition, inoculated Bifidobacterium in
intestines facilitated the curative effect of anti-PD-L1 therapy,
enhancing the number of CD8+ T cells. Another study in
mice demonstrated that gut microbiota directly affected anti-CTLA-4
antibody efficacy (82).
Preclinical studies indicated that immune checkpoint
inhibitors rely on the gut microbiome in mice. Does this also occur
in humans? Clinical data from patients with epithelial tumors were
analyzed (83). The results
revealed a shorter median overall survival in patients who used
antibiotics before or at the beginning of anti-PD-1 therapy, which
meant that intestinal flora disorder inhibited the clinical benefit
of immune checkpoint inhibitors. This inhibitory effect is due to
the lack of Akkermansia muciniphila in this study, and this
type of bacteria can increase the recruitment of CD4+ T
cells into the tumor microenvironment by producing interleukin-12,
which promotes antitumor activities (83). Two additional studies focused on
melanoma. These studies demonstrated that greater diversity in the
gut microbiomes of melanoma patients led to better clinical
effects. Furthermore, responding patients had a higher abundance of
bacterial species, including Ruminococcaceae, Bifidobacterium
longum, Collinsella aerofaciens and Enterococcus faecium
(84,85).
In addition to CTLA-4 and PD-1/PD-L1, several new
immune checkpoints have emerged. The tumor glyco-code as a novel
immune checkpoint should not be overlooked (87). Several studies have shown that the
tumor glyco-code modifies immunity by affecting lectin receptors
expressed by immune cells. For example, Tn antigen (one of the most
common tumor-associated glycans) drives an immune suppressive
program in macrophages by enriching for molecules (such as MUC1,
CD43 and CD45) that interact with macrophage galactose-specific
lectin (MGL) on macrophages. This program is characterized by the
induction of effector T cell apoptosis and upregulation of IL-10
(88,89). Altered glycosylation of malignant
cells often occurs in the early stages of malignant transformation,
and different types of cancer have been marked by certain
tumor-associated glycans, making the early diagnosis of cancer
possible (90–92). Monoclonal antibodies can be used to
characterize changes in the tumor glyco-code that serve as cancer
biomarkers (93,94), and the expression statuses of glycan
and lectin synthesis genes could serve as novel diagnostic tools or
prognostic predictors (95,96). Notably, the tumor glyco-code can be
harnessed not only for cancer diagnosis but also for cancer
therapy. Several therapies based on the tumor glyco-code have been
developed, such as anti-glycan vaccines, inhibitors capable of
blocking glycan-lectin interactions, new tumor glyco-specific CAR-T
cells and dendritic cell targeting (97–100).
We can predict that the development of research on the tumor
glyco-code will benefit patients who fail to respond to current
immunotherapy regimens.
Another novel immune checkpoint that should be
focused on is T-cell immunoglobulin mucin 3 (TIM-3)/galectin-9.
TIM-3 and its ligand galectin-9 play important roles in
tumor-associated immune suppression (101,102). Initial evidence has revealed that
interactions between these proteins induce negative regulation of
the Th1 and Th17 responses (103,104). Further studies revealed that
CD8+ T cells, which are the most suppressed population,
are marked by TIM-3 in preclinical cancer models. The TIM-3 pathway
together with the PD-1 pathway may promote a more severe
dysfunctional phenotype than the TIM-3 pathway by itself in
CD8+ T cells of malignant tumors (105). In addition, other studies have
reported that TIM-3 plays a crucial role in the biology of Tregs in
tumors. In preclinical models, compared with TIM-3−
Tregs, TIM-3+ Tregs have been revealed to be more
immunosuppressive in the tumor tissue, which is likely due to
increased levels of IL-10 and other key effector molecules,
including perforin and granzymes (106). Based on these studies, TIM3 can be
regarded as a target for immunotherapy. Preclinical studies have
revealed very promising results for TIM-3 blockade in colon
carcinoma, Wilms tumor and prostate cancer (107). Notably, better effectiveness has
been demonstrated when TIM-3 blockade is combined with PD-1
blockade, but the molecular mechanisms of these effects have not
been elucidated (104,108). The ligand galectin-9 also plays a
role in preventing cancer progression and immune escape. In breast
cancer cells, galectin-9, which exists in the cytoplasm, induces
cancer cell aggregation and prevents tumor metastasis (109). In addition, galectin-9 induced
apoptosis and inhibited the growth of hepatocellular carcinoma
cells in an in vivo study (110). Synthetic galectin-9 has been
demonstrated to be effective without significant side effects in
mice (111), but it remains to be
investigated in humans. Galectin-9 may be a potential antitumor
agent but requires thorough investigation in the future (112).
An immune checkpoint is a protective factor that
prevents healthy cells from damage caused by excessive T cell
activity. Tumor cells use this mechanism to suppress immune cells
and escape from the body's immune system. Immune checkpoint
inhibitors can relieve this inhibitory effect and reactivate T
cells to destroy cancer cells.
Anti-CTLA-4 antibodies and anti-PD-1/PD-L1
antibodies have revealed encouraging results in a broad range of
tumors, but these therapies do not achieve objective responses in
every patient. Furthermore, some responding patients have to
discontinue these treatments due to serious treatment-related
adverse effects. In addition, some patients are resistant to ICBs
and fail to achieve long-lasting remission. All of these problems
are urging researchers to determine a certain standard with which
to select more suitable patients for immune checkpoint inhibition.
Recent studies have indicated that patients with a higher tumor
mutation burden and higher PD-L1 expression exhibit better clinical
effects. Combination anticancer therapy is a new approach that
combines the benefits of different cancer treatments. Choosing the
right combination of agents at the right dose is essential for
benefiting more patients. Consideration of the gut microbiome with
immune checkpoint inhibitors is another therapeutic method for
anticancer therapy. A high abundance of bacterial species in the
intestine leads to better clinical effects. Inoculation of specific
microbial species can improve the effect of immunotherapy in mice.
The excellent results in animal experiments have made us excited
about the effect of this treatment in humans. Several new immune
checkpoints have been found. Research targeting these checkpoints
in antitumor therapies is underway. We believe that inhibitors of
these checkpoints will be anticancer agents in the future following
a thorough investigation. As immune checkpoint therapies are
developed, more patients will experience benefits.
YF especially wishes to thank Xiaoming Fan (Hengdian
Thermal Electricity Co., Ltd, Jinhua, China) and Jinyun Lu (Dongxin
Wushu School, Jinhua, China) who have given him powerful spiritual
support over the past decades.
This study was supported by the National Nature
Science Foundation of China (grant no. 81702861).
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
YF, CZ, SJ and DB conceived and designed the study.
YF, AW, JC, DL and QW researched the literature. YF and CZ
performed the analysis of data and drafted the manuscript. YF, ZG,
XS and JC jointly designed the figures. YF, CZ, SJ, ZG, JC, AW, DL,
QW, XS and DB critically revised the article for important
intellectual content. All authors read and approved the manuscript
and agree to be accountable for all aspects of the research in
ensuring that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
McNutt M: Cancer immunotherapy. Science.
342:14172013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ledford H: Engineered cell therapy for
cancer gets thumbs up from FDA advisers. Nature. 547:2702017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Junttila MR and de Sauvage FJ: Influence
of tumour micro-environment heterogeneity on therapeutic response.
Nature. 501:346–354. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nagarsheth N, Wicha MS and Zou W:
Chemokines in the cancer microenvironment and their relevance in
cancer immunotherapy. Nat Rev Immunol. 17:559–572. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Church SE and Galon J: Tumor
microenvironment and immunotherapy: The whole picture is better
than a glimpse. Immunity. 43:631–633. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Greenwald RJ, Freeman GJ and Sharpe AH:
The B7 family revisited. Annu Rev Immunol. 23:515–548. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Swartz MA and Lund AW: Lymphatic and
interstitial flow in the tumour microenvironment: Linking
mechanobiology with immunity. Nat Rev Cancer. 12:210–219. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Linsley PS, Brady W, Urnes M, Grosmaire
LS, Damle NK and Ledbetter JA: CTLA-4 is a second receptor for the
B cell activation antigen B7. J Exp Med. 174:561–569. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Leach DR, Krummel MF and Allison JP:
Enhancement of antitumor immunity by CTLA-4 blockade. Science.
271:1734–1736. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zamarin D, Holmgaard RB, Subudhi SK, Park
JS, Mansour M, Palese P, Merghoub T, Wolchok JD and Allison JP:
Localized oncolytic virotherapy overcomes systemic tumor resistance
to immune checkpoint blockade immunotherapy. Sci Transl Med.
6:226ra322014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hodi FS, O'Day SJ, McDermott DF, Weber RW,
Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel
JC, et al: Improved survival with ipilimumab in patients with
metastatic melanoma. N Engl J Med. 363:711–723. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Agata Y, Kawasaki A, Nishimura H, Ishida
Y, Tsubata T, Yagita H and Honjo T: Expression of the PD-1 antigen
on the surface of stimulated mouse T and B lymphocytes. Int
Immunol. 8:765–772. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wei SC, Levine JH, Cogdill AP, Zhao Y,
Anang NAS, Andrews MC, Sharma P, Wang J, Wargo JA, Pe'er D, et al:
Distinct cellular mechanisms underlie anti-CTLA-4 and anti-PD-1
checkpoint blockade. Cell. 170:1120–1133.e17. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pauken KE and Wherry EJ: Overcoming T cell
exhaustion in infection and cancer. Trends Immunol. 36:265–276.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Barber DL, Wherry EJ, Masopust D, Zhu B,
Allison JP, Sharpe AH, Freeman GJ and Ahmed R: Restoring function
in exhausted CD8 T cells during chronic viral infection. Nature.
439:682–687. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Crawford A, Angelosanto JM, Kao C, Doering
TA, Odorizzi PM, Barnett BE and Wherry EJ: Molecular and
transcriptional basis of CD4+ T cell dysfunction during
chronic infection. Immunity. 40:289–302. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Restifo NP, Smyth MJ and Snyder A:
Acquired resistance to immunotherapy and future challenges. Nat Rev
Cancer. 16:121–126. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen DS and Mellman I: Elements of cancer
immunity and the cancer-immune set point. Nature. 541:321–330.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sharpe AH and Pauken KE: The diverse
functions of the PD1 inhibitory pathway. Nat Rev Immunol.
18:153–167. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ribas A and Hu-Lieskovan S: What does
PD-L1 positive or negative mean? J Exp Med. 213:2835–2840. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dong H, Strome SE, Salomao DR, Tamura H,
Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al:
Tumor-associated B7-H1 promotes T-cell apoptosis: A potential
mechanism of immune evasion. Nat Med. 8:793–800. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sharma P, Hu-Lieskovan S, Wargo JA and
Ribas A: Primary, adaptive, and acquired resistance to cancer
immunotherapy. Cell. 168:707–723. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eggermont AM, Chiarion-Sileni V, Grob JJ,
Dummer R, Wolchok JD, Schmidt H, Hamid O, Robert C, Ascierto PA,
Richards JM, et al: Adjuvant ipilimumab versus placebo after
complete resection of high-risk stage III melanoma (EORTC 18071): A
randomised, double-blind, phase 3 trial. Lancet Oncol. 16:522–530.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Coens C, Suciu S, Chiarion-Sileni V, Grob
JJ, Dummer R, Wolchok JD, Schmidt H, Hamid O, Robert C, Ascierto
PA, et al: Health-related quality of life with adjuvant ipilimumab
versus placebo after complete resection of high-risk stage III
melanoma (EORTC 18071): Secondary outcomes of a multinational,
randomised, double-blind, phase 3 trial. Lancet Oncol. 18:393–403.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Robert C, Thomas L, Bondarenko I, O'Day S,
Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, et al:
Ipilimumab plus dacarbazine for previously untreated metastatic
melanoma. N Engl J Med. 364:2517–2526. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hammers HJ, Plimack ER, Infante JR, Rini
BI, McDermott DF, Lewis LD, Voss MH, Sharma P, Pal SK, Razak ARA,
et al: Safety and efficacy of nivolumab in combination with
ipilimumab in metastatic renal cell carcinoma: The CheckMate 016
Study. J Clin Oncol. 35:3851–3858. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kwon ED, Drake CG, Scher HI, Fizazi K,
Bossi A, Van den Eertwegh AJ, Krainer M, Houede N, Santos R,
Mahammedi H, et al: ; CA184-043 Investigators: Ipilimumab versus
placebo after radiotherapy in patients with metastatic
castration-resistant prostate cancer that had progressed after
docetaxel chemotherapy (CA184-043): A multicentre, randomised,
double-blind, phase 3 trial. Lancet Oncol. 15:700–712. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Le DT, Lutz E, Uram JN, Sugar EA, Onners
B, Solt S, Zheng L, Diaz LA Jr, Donehower RC, Jaffee EM, et al:
Evaluation of ipilimumab in combination with allogeneic pancreatic
tumor cells transfected with a GM-CSF gene in previously treated
pancreatic cancer. J Immunother. 36:382–389. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao X and Subramanian S: Intrinsic
resistance of solid tumors to immune checkpoint blockade therapy.
Cancer Res. 77:817–822. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Friedman CF, Proverbs-Singh TA and Postow
MA: Treatment of the immune-related adverse effects of immune
checkpoint inhibitors: A review. JAMA Oncol. 2:1346–1353. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cousin S, Seneschal J and Italiano A:
Toxicity profiles of immunotherapy. Pharmacol Ther. 181:91–100.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Armand P, Shipp MA, Ribrag V, Michot JM,
Zinzani PL, Kuruvilla J, Snyder ES, Ricart AD, Balakumaran A, Rose
S, et al: Programmed death-1 blockade with pembrolizumab in
patients with classical Hodgkin lymphoma after brentuximab vedotin
failure. J Clin Oncol. 34:3733–3739. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kang YK, Boku N, Satoh T, Ryu MH, Chao Y,
Kato K, Chung HC, Chen JS, Muro K, Kang WK, et al: Nivolumab in
patients with advanced gastric or gastro-oesophageal junction
cancer refractory to, or intolerant of, at least two previous
chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet.
390:2461–2471. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schachter J, Ribas A, Long GV, Arance A,
Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, et al:
Pembrolizumab versus ipilimumab for advanced melanoma: Final
overall survival results of a multicentre, randomised, open-label
phase 3 study (KEYNOTE-006). Lancet. 390:1853–1862. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Weber J, Mandala M, Del Vecchio M, Gogas
HJ, Arance AM, Cowey CL, Dalle S, Schenker M, Chiarion-Sileni V,
Marquez-Rodas I, et al: ; CheckMate 238 collaborators: Adjuvant
nivolumab versus ipilimumab in resected stage III or IV melanoma. N
Engl J Med. 377:1824–1835. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Balar AV, Galsky MD, Rosenberg JE, Powles
T, Petrylak DP, Bellmunt J, Loriot Y, Necchi A, Hoffman-Censits J,
Perez-Gracia JL, et al: IMvigor210 Study Group: Atezolizumab as
first-line treatment in cisplatin-ineligible patients with locally
advanced and metastatic urothelial carcinoma: A single-arm,
multicentre, phase 2 trial. Lancet. 389:67–76. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Powles T, Durán I, van der Heijden MS,
Loriot Y, Vogelzang NJ, De Giorgi U, Oudard S, Retz MM, Castellano
D, Bamias A, et al: Atezolizumab versus chemotherapy in patients
with platinum-treated locally advanced or metastatic urothelial
carcinoma (IMvigor211): A multicentre, open-label, phase 3
randomised controlled trial. Lancet. 391:748–757. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rosenberg JE, Hoffman-Censits J, Powles T,
van der Heijden MS, Balar AV, Necchi A, Dawson N, O'Donnell PH,
Balmanoukian A, Loriot Y, et al: Atezolizumab in patients with
locally advanced and metastatic urothelial carcinoma who have
progressed following treatment with platinum-based chemotherapy: A
single-arm, multicentre, phase 2 trial. Lancet. 387:1909–1920.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fehrenbacher L, Spira A, Ballinger M,
Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D,
Artal-Cortes A, Lewanski C, et al: ; POPLAR study group:
Atezolizumab versus docetaxel for patients with previously treated
non-small-cell lung cancer (POPLAR): A multicentre, open-label,
phase 2 randomised controlled trial. Lancet. 387:1837–1846. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rittmeyer A, Barlesi F, Waterkamp D, Park
K, Ciardiello F, Von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols
MC, et al: ; OAK study group: Atezolizumab versus docetaxel in
patients with previously treated non-small-cell lung cancer (OAK):
A phase 3, open-label, multicentre randomised controlled trial.
Lancet. 389:255–265. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kaufman HL, Russell J, Hamid O, Bhatia S,
Terheyden P, D'Angelo SP, Shih KC, Lebbé C, Linette GP, Milella M,
et al: Avelumab in patients with chemotherapy-refractory metastatic
Merkel cell carcinoma: A multicentre, single-group, open-label,
phase 2 trial. Lancet Oncol. 17:1374–1385. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Heery CR, O'Sullivan-Coyne G, Madan RA,
Cordes L, Rajan A, Rauckhorst M, Lamping E, Oyelakin I, Marté JL,
Lepone LM, et al: Avelumab for metastatic or locally advanced
previously treated solid tumours (JAVELIN Solid Tumor): A phase 1a,
multicohort, dose-escalation trial. Lancet Oncol. 18:587–598. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gulley JL, Rajan A, Spigel DR, Iannotti N,
Chandler J, Wong DJL, Leach J, Edenfield WJ, Wang D, Grote HJ, et
al: Avelumab for patients with previously treated metastatic or
recurrent non-small-cell lung cancer (JAVELIN Solid Tumor):
Dose-expansion cohort of a multicentre, open-label, phase 1b trial.
Lancet Oncol. 18:599–610. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Antonia SJ, Villegas A, Daniel D, Vicente
D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, De Wit M, et
al: ; PACIFIC Investigators: Durvalumab after chemoradiotherapy in
stage III non-small-cell lung cancer. N Engl J Med. 377:1919–1929.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Massard C, Gordon MS, Sharma S, Rafii S,
Wainberg ZA, Luke J, Curiel TJ, Colon-Otero G, Hamid O, Sanborn RE,
et al: Safety and efficacy of durvalumab (MEDI4736), an
anti-programmed cell death ligand-1 immune checkpoint inhibitor, in
patients with advanced urothelial bladder cancer. J Clin Oncol.
34:3119–3125. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Garassino MC, Cho BC, Kim JH, Mazières J,
Vansteenkiste J, Lena H, Corral Jaime J, Gray JE, Powderly J,
Chouaid C, et al: ; ATLANTIC investigators: Durvalumab as
third-line or later treatment for advanced non-small-cell lung
cancer (ATLANTIC): An open-label, single-arm, phase 2 study. Lancet
Oncol. 19:521–536. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Luke JJ, Flaherty KT, Ribas A and Long GV:
Targeted agents and immunotherapies: Optimizing outcomes in
melanoma. Nat Rev Clin Oncol. 14:463–482. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Miao D, Margolis CA, Gao W, Voss MH, Li W,
Martini DJ, Norton C, Bossé D, Wankowicz SM, Cullen D, et al:
Genomic correlates of response to immune checkpoint therapies in
clear cell renal cell carcinoma. Science. 359:801–806. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Pauken KE, Sammons MA, Odorizzi PM, Manne
S, Godec J, Khan O, Drake AM, Chen Z, Sen DR, Kurachi M, et al:
Epigenetic stability of exhausted T cells limits durability of
reinvigoration by PD-1 blockade. Science. 354:1160–1165. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Pitt JM, Vétizou M, Daillère R, Roberti
MP, Yamazaki T, Routy B, Lepage P, Boneca IG, Chamaillard M,
Kroemer G, et al: Resistance mechanisms to immune-checkpoint
blockade in cancer: Tumor-intrinsic and -extrinsic factors.
Immunity. 44:1255–1269. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Joyce JA and Fearon DT: T cell exclusion,
immune privilege, and the tumor microenvironment. Science.
348:74–80. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jenkins RW, Barbie DA and Flaherty KT:
Mechanisms of resistance to immune checkpoint inhibitors. Br J
Cancer. 118:9–16. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Smyth MJ, Ngiow SF, Ribas A and Teng MW:
Combination cancer immunotherapies tailored to the tumour
microenvironment. Nat Rev Clin Oncol. 13:143–158. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J,
Bozym DJ, Zhang W, Luoma A, Giobbie-Hurder A, Peter L, et al: An
immunogenic personal neoantigen vaccine for patients with melanoma.
Nature. 547:217–221. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Barata PC and Rini BI: Treatment of renal
cell carcinoma: Current status and future directions. CA Cancer J
Clin. 67:507–524. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Mathew M, Enzler T, Shu CA and Rizvi NA:
Combining chemotherapy with PD-1 blockade in NSCLC. Pharmacol Ther.
186:130–137. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Bourgeois-Daigneault MC, Roy DG, Aitken
AS, El Sayes N, Martin NT, Varette O, Falls T, St-Germain LE, Pelin
A, Lichty BD, et al: Neoadjuvant oncolytic virotherapy before
surgery sensitizes triple-negative breast cancer to immune
checkpoint therapy. Sci Transl Med. 10(pii): eaao16412018.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Overman MJ, McDermott R, Leach JL, Lonardi
S, Lenz HJ, Morse MA, Desai J, Hill A, Axelson M, Moss RA, et al:
Nivolumab in patients with metastatic DNA mismatch repair-deficient
or microsatellite instability-high colorectal cancer (CheckMate
142): An open-label, multicentre, phase 2 study. Lancet Oncol.
18:1182–1191. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chen DS and Mellman I: Oncology meets
immunology: The cancer-immunity cycle. Immunity. 39:1–10. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yshii LM, Hohlfeld R and Liblau RS:
Inflammatory CNS disease caused by immune checkpoint inhibitors:
Status and perspectives. Nat Rev Neurol. 13:755–763. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Varricchi G, Galdiero MR and Tocchetti CG:
Cardiac toxicity of immune checkpoint inhibitors: Cardio-oncology
meets immunology. Circulation. 136:1989–1992. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Shoushtari AN, Friedman CF,
Navid-Azarbaijani P, Postow MA, Callahan MK, Momtaz P, Panageas KS,
Wolchok JD and Chapman PB: Measuring toxic effects and time to
treatment failure for nivolumab plus ipilimumab in melanoma. JAMA
Oncol. 4:98–101. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Powles T, Eder JP, Fine GD, Braiteh FS,
Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL, et
al: MPDL3280A (anti-PD-L1) treatment leads to clinical activity in
metastatic bladder cancer. Nature. 515:558–562. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Mahoney KM, Rennert PD and Freeman GJ:
Combination cancer immunotherapy and new immunomodulatory targets.
Nat Rev Drug Discov. 14:561–584. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Govindan R, Szczesna A, Ahn MJ, Schneider
CP, Gonzalez Mella PF, Barlesi F, Han B, Ganea DE, Von Pawel J,
Vladimirov V, et al: Phase III trial of ipilimumab combined with
paclitaxel and carboplatin in advanced squamous non-small-cell lung
cancer. J Clin Oncol. 35:3449–3457. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Reck M, Luft A, Szczesna A, Havel L, Kim
SW, Akerley W, Pietanza MC, Wu YL, Zielinski C, Thomas M, et al:
Phase III randomized trial of ipilimumab plus etoposide and
platinum versus placebo plus etoposide and platinum in
extensive-stage small-cell lung cancer. J Clin Oncol. 34:3740–3748.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Lynch TJ, Bondarenko I, Luft A,
Serwatowski P, Barlesi F, Chacko R, Sebastian M, Neal J, Lu H,
Cuillerot JM, et al: Ipilimumab in combination with paclitaxel and
carboplatin as first-line treatment in stage IIIB/IV non-small-cell
lung cancer: Results from a randomized, double-blind, multicenter
phase II study. J Clin Oncol. 30:2046–2054. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Gubin MM, Zhang X, Schuster H, Caron E,
Ward JP, Noguchi T, Ivanova Y, Hundal J, Arthur CD, Krebber WJ, et
al: Checkpoint blockade cancer immunotherapy targets
tumour-specific mutant antigens. Nature. 515:577–581. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wolchok JD, Chiarion-Sileni V, Gonzalez R,
Rutkowski P, Grob JJ, Cowey CL, Lao CD, Wagstaff J, Schadendorf D,
Ferrucci PF, et al: Overall survival with combined nivolumab and
ipilimumab in advanced melanoma. N Engl J Med. 377:1345–1356. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Schadendorf D, Larkin J, Wolchok J, Hodi
FS, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL,
Lao C, et al: Health-related quality of life results from the phase
III CheckMate 067 study. Eur J Cancer. 82:80–91. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hellmann MD, Rizvi NA, Goldman JW,
Gettinger SN, Borghaei H, Brahmer JR, Ready NE, Gerber DE, Chow LQ,
Juergens RA, et al: Nivolumab plus ipilimumab as first-line
treatment for advanced non-small-cell lung cancer (CheckMate 012):
Results of an open-label, phase 1, multicohort study. Lancet Oncol.
18:31–41. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Long GV, Atkinson V, Cebon JS, Jameson MB,
Fitzharris BM, McNeil CM, Hill AG, Ribas A, Atkins MB, Thompson JA,
et al: Standard-dose pembrolizumab in combination with reduced-dose
ipilimumab for patients with advanced melanoma (KEYNOTE-029): An
open-label, phase 1b trial. Lancet Oncol. 18:1202–1210. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Herrera FG, Bourhis J and Coukos G:
Radiotherapy combination opportunities leveraging immunity for the
next oncology practice. CA Cancer J Clin. 67:65–85. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Lee JM, Cimino-Mathews A, Peer CJ, Zimmer
A, Lipkowitz S, Annunziata CM, Cao L, Harrell MI, Swisher EM,
Houston N, et al: Safety and clinical activity of the programmed
death-ligand 1 inhibitor durvalumab in combination with poly
(ADP-Ribose) polymerase inhibitor olaparib or vascular endothelial
growth factor receptor 1–3 inhibitor cediranib in women's cancers:
A dose-escalation, phase I study. J Clin Oncol. 35:2193–2202. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Chesney J, Puzanov I, Collichio F, Singh
P, Milhem MM, Glaspy J, Hamid O, Ross M, Friedlander P, Garbe C, et
al: Randomized, open-label phase II study evaluating the efficacy
and safety of talimogene laherparepvec in combination with
ipilimumab versus ipilimumab alone in patients with advanced,
unresectable melanoma. J Clin Oncol. 36:1658–1667. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ribas A, Dummer R, Puzanov I, VanderWalde
A, Andtbacka RHI, Michielin O, Olszanski AJ, Malvehy J, Cebon J,
Fernandez E, et al: Oncolytic virotherapy promotes intratumoral T
cell infiltration and improves anti-PD-1 immunotherapy. Cell.
170:1109–1119. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Sender R, Fuchs S and Milo R: Revised
estimates for the number of human and bacteria cells in the body.
PLoS Biol. 14:e10025332016. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Hall AB, Tolonen AC and Xavier RJ: Human
genetic variation and the gut microbiome in disease. Nat Rev Genet.
18:690–699. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
York A: Microbiome: Gut microbiota sways
response to cancer immunotherapy. Nat Rev Microbiol. 16:1212018.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Sivan A, Corrales L, Hubert N, Williams
JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B,
Alegre ML, et al: Commensal Bifidobacterium promotes antitumor
immunity and facilitates anti-PD-L1 efficacy. Science.
350:1084–1089. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Vétizou M, Pitt JM, Daillère R, Lepage P,
Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong
CP, et al: Anticancer immunotherapy by CTLA-4 blockade relies on
the gut microbiota. Science. 350:1079–1084. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Routy B, Le Chatelier E, Derosa L, Duong
CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C,
Roberti MP, et al: Gut microbiome influences efficacy of PD-1-based
immunotherapy against epithelial tumors. Science. 359:91–97. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Matson V, Fessler J, Bao R, Chongsuwat T,
Zha Y, Alegre ML, Luke JJ and Gajewski TF: The commensal microbiome
is associated with anti-PD-1 efficacy in metastatic melanoma
patients. Science. 359:104–108. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Gopalakrishnan V, Spencer CN, Nezi L,
Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman
K, Wei SC, et al: Gut microbiome modulates response to anti-PD-1
immunotherapy in melanoma patients. Science. 359:97–103. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Jobin C: Precision medicine using
microbiota. Science. 359:32–34. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
RodrÍguez E, Schetters STT and Van Kooyk
Y: The tumour glyco-code as a novel immune checkpoint for
immunotherapy. Nat Rev Immunol. 18:204–211. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Van Vliet SJ, Bay S, Vuist IM, Kalay H,
García-Vallejo JJ, Leclerc C and Van Kooyk Y: MGL signaling
augments TLR2-mediated responses for enhanced IL-10 and TNF-α
secretion. J Leukoc Biol. 94:315–323. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Van Vliet SJ, Gringhuis SI, Geijtenbeek TB
and Van Kooyk Y: Regulation of effector T cells by
antigen-presenting cells via interaction of the C-type lectin MGL
with CD45. Nat Immunol. 7:1200–1208. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Drake RR: Glycosylation and cancer: Moving
glycomics to the forefront. Adv Cancer Res. 126:1–10. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Pinho SS and Reis CA: Glycosylation in
cancer: Mechanisms and clinical implications. Nat Rev Cancer.
15:540–555. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Kaur S, Kumar S, Momi N, Sasson AR and
Batra SK: Mucins in pancreatic cancer and its microenvironment. Nat
Rev Gastroenterol Hepatol. 10:607–620. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Kim J, Bamlet WR, Oberg AL, Chaffee KG,
Donahue G, Cao XJ, Chari S, Garcia BA, Petersen GM and Zaret KS:
Detection of early pancreatic ductal adenocarcinoma with
thrombospondin-2 and CA19-9 blood markers. Sci Transl Med. 9(pii):
eaah55832017. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Yang AP, Liu J, Lei HY, Zhang QW, Zhao L
and Yang GH: CA72-4 combined with CEA, CA125 and CAl9-9 improves
the sensitivity for the early diagnosis of gastric cancer. Clin
Chim Acta. 437:183–186. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Ashkani J and Naidoo KJ:
Glycosyltransferase gene expression profiles classify cancer types
and propose prognostic subtypes. Sci Rep. 6:264512016. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Syed P, Gidwani K, Kekki H, Leivo J,
Pettersson K and Lamminmäki U: Role of lectin microarrays in cancer
diagnosis. Proteomics. 16:1257–1265. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Miles D and Papazisis K: Rationale for the
clinical development of STn-KLH (Theratope®) and
anti-MUC-1 vaccines in breast cancer. Clin Breast Cancer.
3:S134–S138. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Cagnoni AJ, Pérez Sáez JM, Rabinovich GA
and Mariño KV: Turning-off signaling by siglecs, selectins, and
galectins: Chemical inhibition of glycan-dependent interactions in
cancer. Front Oncol. 6:1092016. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Posey AD Jr, Schwab RD, Boesteanu AC,
Steentoft C, Mandel U, Engels B, Stone JD, Madsen TD, Schreiber K,
Haines KM, et al: Engineered CAR T cells targeting the
cancer-associated Tn-glycoform of the membrane mucin MUC1 control
adenocarcinoma. Immunity. 44:1444–1454. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Unger WW, Mayer CT, Engels S, Hesse C,
Perdicchio M, Puttur F, Streng-Ouwehand I, Litjens M, Kalay H,
Berod L, et al: Antigen targeting to dendritic cells combined with
transient regulatory T cell inhibition results in long-term tumor
regression. Oncoimmunology. 4:e9704622014. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Chou FC, Chen HY, Kuo CC and Sytwu HK:
Role of galectins in tumors and in clinical immunotherapy. Int J
Mol Sci. 1(pii): E4302018. View Article : Google Scholar
|
|
102
|
Alderton GK: Tumour immunology: TIM3
suppresses antitumour DCs. Nat Rev Immunol. 12:620–621. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Sundar R, Soong R, Cho BC, Brahmer JR and
Soo RA: Immunotherapy in the treatment of non-small cell lung
cancer. Lung Cancer. 85:101–109. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Anderson AC: Tim-3: An emerging target in
the cancer immunotherapy landscape. Cancer Immunol Res. 2:393–398.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Liu J, Zhang S, Hu Y, Yang Z, Li J, Liu X,
Deng L, Wang Y, Zhang X, Jiang T, et al: Targeting PD-1 and Tim-3
pathways to reverse CD8 T-cell exhaustion and enhance ex vivo
T-cell responses to autologous dendritic/tumor vaccines. J
Immunother. 39:171–180. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Sakuishi K, Ngiow SF, Sullivan JM, Teng
MW, Kuchroo VK, Smyth MJ and Anderson AC:
TIM3+FOXP3+ regulatory T cells are
tissue-specific promoters of T-cell dysfunction in cancer.
OncoImmunology. 2:e238492013. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Ngiow SF, von Scheidt B, Akiba H, Yagita
H, Teng MW and Smyth MJ: Anti-TIM3 antibody promotes T cell
IFN-γ-mediated antitumor immunity and suppresses established
tumors. Cancer Res. 71:3540–3551. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Zhou Q, Munger ME, Veenstra RG, Weigel BJ,
Hirashima M, Munn DH, Murphy WJ, Azuma M, Anderson AC, Kuchroo VK,
et al: Coexpression of Tim-3 and PD-1 identifies a CD8+
T-cell exhaustion phenotype in mice with disseminated acute
myelogenous leukemia. Blood. 117:4501–4510. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Irie A, Yamauchi A, Kontani K, Kihara M,
Liu D, Shirato Y, Seki M, Nishi N, Nakamura T, Yokomise H, et al:
Galectin-9 as a prognostic factor with antimetastatic potential in
breast cancer. Clin Cancer Res. 11:2962–2968. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Fujita K, Iwama H, Sakamoto T, Okura R,
Kobayashi K, Takano J, Katsura A, Tatsuta M, Maeda E, Mimura S, et
al: Galectin-9 suppresses the growth of hepatocellular carcinoma
via apoptosis in vitro and in vivo. Int J Oncol.
46:2419–2430. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Mengshol JA, Golden-Mason L, Arikawa T,
Smith M, Niki T, McWilliams R, Randall JA, McMahan R, Zimmerman MA,
Rangachari M, et al: A crucial role for Kupffer cell-derived
galectin-9 in regulation of T cell immunity in hepatitis C
infection. PLoS One. 5:e95042010. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Fujita K, Iwama H, Oura K, Tadokoro T,
Samukawa E, Sakamoto T, Nomura T, Tani J, Yoneyama H, Morishita A,
et al: Cancer therapy due to apoptosis: Galectin-9. Int J Mol Sci.
18(pii): E742017. View Article : Google Scholar : PubMed/NCBI
|