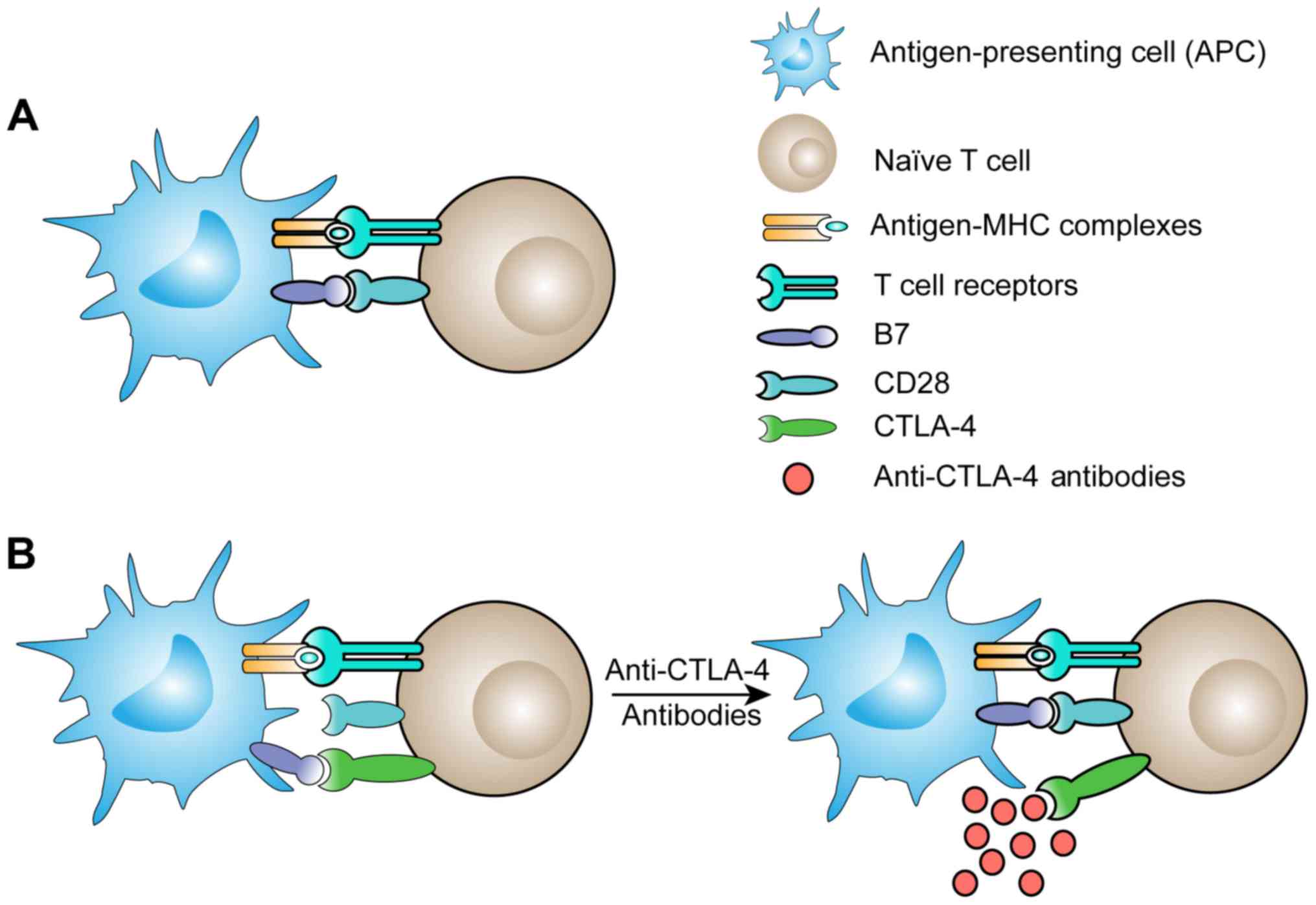
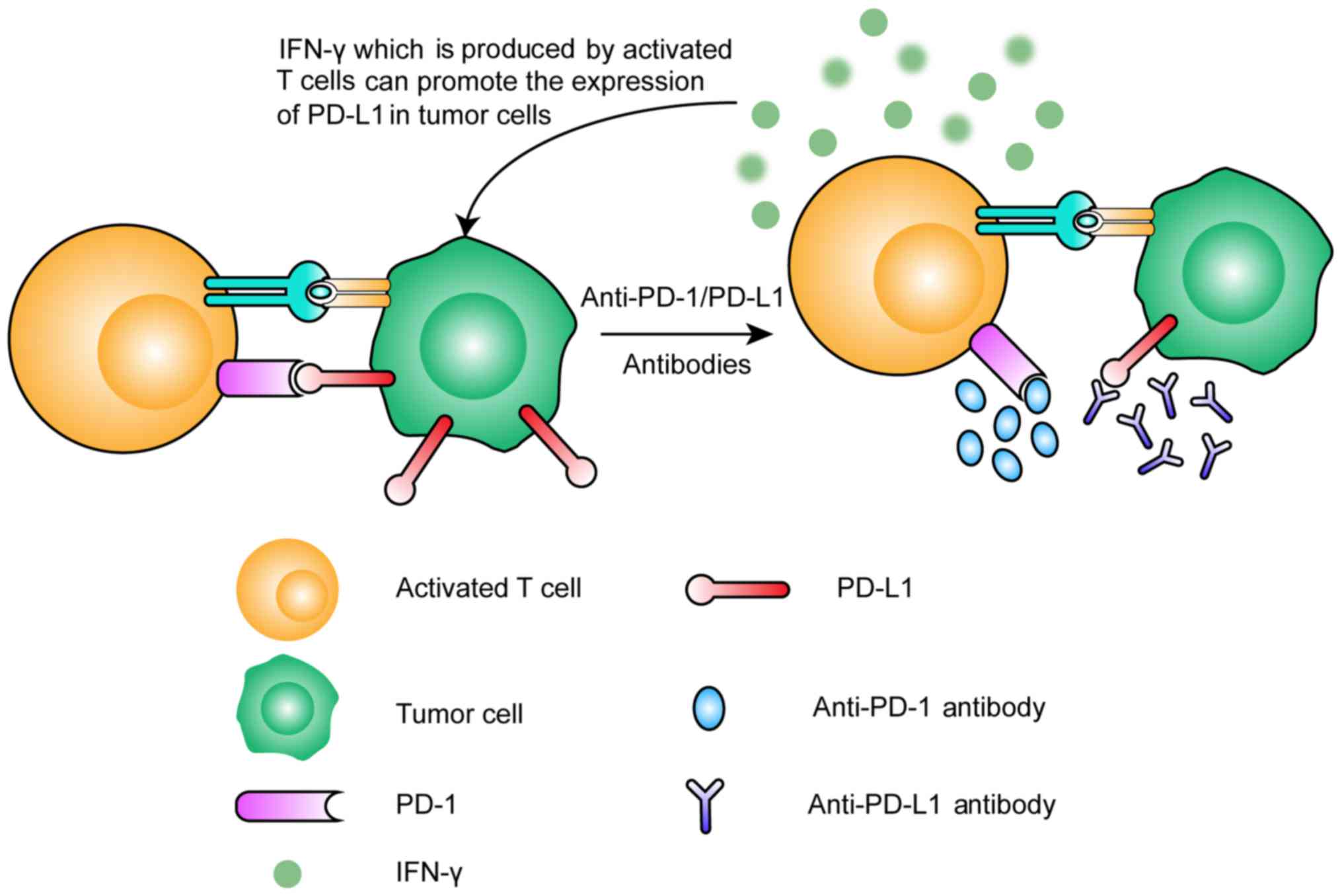
|
1
|
McNutt M: Cancer immunotherapy. Science.
342:14172013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ledford H: Engineered cell therapy for
cancer gets thumbs up from FDA advisers. Nature. 547:2702017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Junttila MR and de Sauvage FJ: Influence
of tumour micro-environment heterogeneity on therapeutic response.
Nature. 501:346–354. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nagarsheth N, Wicha MS and Zou W:
Chemokines in the cancer microenvironment and their relevance in
cancer immunotherapy. Nat Rev Immunol. 17:559–572. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Church SE and Galon J: Tumor
microenvironment and immunotherapy: The whole picture is better
than a glimpse. Immunity. 43:631–633. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Greenwald RJ, Freeman GJ and Sharpe AH:
The B7 family revisited. Annu Rev Immunol. 23:515–548. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Swartz MA and Lund AW: Lymphatic and
interstitial flow in the tumour microenvironment: Linking
mechanobiology with immunity. Nat Rev Cancer. 12:210–219. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Linsley PS, Brady W, Urnes M, Grosmaire
LS, Damle NK and Ledbetter JA: CTLA-4 is a second receptor for the
B cell activation antigen B7. J Exp Med. 174:561–569. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Leach DR, Krummel MF and Allison JP:
Enhancement of antitumor immunity by CTLA-4 blockade. Science.
271:1734–1736. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zamarin D, Holmgaard RB, Subudhi SK, Park
JS, Mansour M, Palese P, Merghoub T, Wolchok JD and Allison JP:
Localized oncolytic virotherapy overcomes systemic tumor resistance
to immune checkpoint blockade immunotherapy. Sci Transl Med.
6:226ra322014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hodi FS, O'Day SJ, McDermott DF, Weber RW,
Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel
JC, et al: Improved survival with ipilimumab in patients with
metastatic melanoma. N Engl J Med. 363:711–723. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Agata Y, Kawasaki A, Nishimura H, Ishida
Y, Tsubata T, Yagita H and Honjo T: Expression of the PD-1 antigen
on the surface of stimulated mouse T and B lymphocytes. Int
Immunol. 8:765–772. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wei SC, Levine JH, Cogdill AP, Zhao Y,
Anang NAS, Andrews MC, Sharma P, Wang J, Wargo JA, Pe'er D, et al:
Distinct cellular mechanisms underlie anti-CTLA-4 and anti-PD-1
checkpoint blockade. Cell. 170:1120–1133.e17. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pauken KE and Wherry EJ: Overcoming T cell
exhaustion in infection and cancer. Trends Immunol. 36:265–276.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Barber DL, Wherry EJ, Masopust D, Zhu B,
Allison JP, Sharpe AH, Freeman GJ and Ahmed R: Restoring function
in exhausted CD8 T cells during chronic viral infection. Nature.
439:682–687. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Crawford A, Angelosanto JM, Kao C, Doering
TA, Odorizzi PM, Barnett BE and Wherry EJ: Molecular and
transcriptional basis of CD4+ T cell dysfunction during
chronic infection. Immunity. 40:289–302. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Restifo NP, Smyth MJ and Snyder A:
Acquired resistance to immunotherapy and future challenges. Nat Rev
Cancer. 16:121–126. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen DS and Mellman I: Elements of cancer
immunity and the cancer-immune set point. Nature. 541:321–330.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sharpe AH and Pauken KE: The diverse
functions of the PD1 inhibitory pathway. Nat Rev Immunol.
18:153–167. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ribas A and Hu-Lieskovan S: What does
PD-L1 positive or negative mean? J Exp Med. 213:2835–2840. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dong H, Strome SE, Salomao DR, Tamura H,
Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al:
Tumor-associated B7-H1 promotes T-cell apoptosis: A potential
mechanism of immune evasion. Nat Med. 8:793–800. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sharma P, Hu-Lieskovan S, Wargo JA and
Ribas A: Primary, adaptive, and acquired resistance to cancer
immunotherapy. Cell. 168:707–723. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eggermont AM, Chiarion-Sileni V, Grob JJ,
Dummer R, Wolchok JD, Schmidt H, Hamid O, Robert C, Ascierto PA,
Richards JM, et al: Adjuvant ipilimumab versus placebo after
complete resection of high-risk stage III melanoma (EORTC 18071): A
randomised, double-blind, phase 3 trial. Lancet Oncol. 16:522–530.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Coens C, Suciu S, Chiarion-Sileni V, Grob
JJ, Dummer R, Wolchok JD, Schmidt H, Hamid O, Robert C, Ascierto
PA, et al: Health-related quality of life with adjuvant ipilimumab
versus placebo after complete resection of high-risk stage III
melanoma (EORTC 18071): Secondary outcomes of a multinational,
randomised, double-blind, phase 3 trial. Lancet Oncol. 18:393–403.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Robert C, Thomas L, Bondarenko I, O'Day S,
Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, et al:
Ipilimumab plus dacarbazine for previously untreated metastatic
melanoma. N Engl J Med. 364:2517–2526. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hammers HJ, Plimack ER, Infante JR, Rini
BI, McDermott DF, Lewis LD, Voss MH, Sharma P, Pal SK, Razak ARA,
et al: Safety and efficacy of nivolumab in combination with
ipilimumab in metastatic renal cell carcinoma: The CheckMate 016
Study. J Clin Oncol. 35:3851–3858. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kwon ED, Drake CG, Scher HI, Fizazi K,
Bossi A, Van den Eertwegh AJ, Krainer M, Houede N, Santos R,
Mahammedi H, et al: ; CA184-043 Investigators: Ipilimumab versus
placebo after radiotherapy in patients with metastatic
castration-resistant prostate cancer that had progressed after
docetaxel chemotherapy (CA184-043): A multicentre, randomised,
double-blind, phase 3 trial. Lancet Oncol. 15:700–712. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Le DT, Lutz E, Uram JN, Sugar EA, Onners
B, Solt S, Zheng L, Diaz LA Jr, Donehower RC, Jaffee EM, et al:
Evaluation of ipilimumab in combination with allogeneic pancreatic
tumor cells transfected with a GM-CSF gene in previously treated
pancreatic cancer. J Immunother. 36:382–389. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao X and Subramanian S: Intrinsic
resistance of solid tumors to immune checkpoint blockade therapy.
Cancer Res. 77:817–822. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Friedman CF, Proverbs-Singh TA and Postow
MA: Treatment of the immune-related adverse effects of immune
checkpoint inhibitors: A review. JAMA Oncol. 2:1346–1353. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cousin S, Seneschal J and Italiano A:
Toxicity profiles of immunotherapy. Pharmacol Ther. 181:91–100.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Armand P, Shipp MA, Ribrag V, Michot JM,
Zinzani PL, Kuruvilla J, Snyder ES, Ricart AD, Balakumaran A, Rose
S, et al: Programmed death-1 blockade with pembrolizumab in
patients with classical Hodgkin lymphoma after brentuximab vedotin
failure. J Clin Oncol. 34:3733–3739. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kang YK, Boku N, Satoh T, Ryu MH, Chao Y,
Kato K, Chung HC, Chen JS, Muro K, Kang WK, et al: Nivolumab in
patients with advanced gastric or gastro-oesophageal junction
cancer refractory to, or intolerant of, at least two previous
chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet.
390:2461–2471. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schachter J, Ribas A, Long GV, Arance A,
Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, et al:
Pembrolizumab versus ipilimumab for advanced melanoma: Final
overall survival results of a multicentre, randomised, open-label
phase 3 study (KEYNOTE-006). Lancet. 390:1853–1862. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Weber J, Mandala M, Del Vecchio M, Gogas
HJ, Arance AM, Cowey CL, Dalle S, Schenker M, Chiarion-Sileni V,
Marquez-Rodas I, et al: ; CheckMate 238 collaborators: Adjuvant
nivolumab versus ipilimumab in resected stage III or IV melanoma. N
Engl J Med. 377:1824–1835. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Balar AV, Galsky MD, Rosenberg JE, Powles
T, Petrylak DP, Bellmunt J, Loriot Y, Necchi A, Hoffman-Censits J,
Perez-Gracia JL, et al: IMvigor210 Study Group: Atezolizumab as
first-line treatment in cisplatin-ineligible patients with locally
advanced and metastatic urothelial carcinoma: A single-arm,
multicentre, phase 2 trial. Lancet. 389:67–76. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Powles T, Durán I, van der Heijden MS,
Loriot Y, Vogelzang NJ, De Giorgi U, Oudard S, Retz MM, Castellano
D, Bamias A, et al: Atezolizumab versus chemotherapy in patients
with platinum-treated locally advanced or metastatic urothelial
carcinoma (IMvigor211): A multicentre, open-label, phase 3
randomised controlled trial. Lancet. 391:748–757. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rosenberg JE, Hoffman-Censits J, Powles T,
van der Heijden MS, Balar AV, Necchi A, Dawson N, O'Donnell PH,
Balmanoukian A, Loriot Y, et al: Atezolizumab in patients with
locally advanced and metastatic urothelial carcinoma who have
progressed following treatment with platinum-based chemotherapy: A
single-arm, multicentre, phase 2 trial. Lancet. 387:1909–1920.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fehrenbacher L, Spira A, Ballinger M,
Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D,
Artal-Cortes A, Lewanski C, et al: ; POPLAR study group:
Atezolizumab versus docetaxel for patients with previously treated
non-small-cell lung cancer (POPLAR): A multicentre, open-label,
phase 2 randomised controlled trial. Lancet. 387:1837–1846. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rittmeyer A, Barlesi F, Waterkamp D, Park
K, Ciardiello F, Von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols
MC, et al: ; OAK study group: Atezolizumab versus docetaxel in
patients with previously treated non-small-cell lung cancer (OAK):
A phase 3, open-label, multicentre randomised controlled trial.
Lancet. 389:255–265. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kaufman HL, Russell J, Hamid O, Bhatia S,
Terheyden P, D'Angelo SP, Shih KC, Lebbé C, Linette GP, Milella M,
et al: Avelumab in patients with chemotherapy-refractory metastatic
Merkel cell carcinoma: A multicentre, single-group, open-label,
phase 2 trial. Lancet Oncol. 17:1374–1385. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Heery CR, O'Sullivan-Coyne G, Madan RA,
Cordes L, Rajan A, Rauckhorst M, Lamping E, Oyelakin I, Marté JL,
Lepone LM, et al: Avelumab for metastatic or locally advanced
previously treated solid tumours (JAVELIN Solid Tumor): A phase 1a,
multicohort, dose-escalation trial. Lancet Oncol. 18:587–598. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gulley JL, Rajan A, Spigel DR, Iannotti N,
Chandler J, Wong DJL, Leach J, Edenfield WJ, Wang D, Grote HJ, et
al: Avelumab for patients with previously treated metastatic or
recurrent non-small-cell lung cancer (JAVELIN Solid Tumor):
Dose-expansion cohort of a multicentre, open-label, phase 1b trial.
Lancet Oncol. 18:599–610. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Antonia SJ, Villegas A, Daniel D, Vicente
D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, De Wit M, et
al: ; PACIFIC Investigators: Durvalumab after chemoradiotherapy in
stage III non-small-cell lung cancer. N Engl J Med. 377:1919–1929.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Massard C, Gordon MS, Sharma S, Rafii S,
Wainberg ZA, Luke J, Curiel TJ, Colon-Otero G, Hamid O, Sanborn RE,
et al: Safety and efficacy of durvalumab (MEDI4736), an
anti-programmed cell death ligand-1 immune checkpoint inhibitor, in
patients with advanced urothelial bladder cancer. J Clin Oncol.
34:3119–3125. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Garassino MC, Cho BC, Kim JH, Mazières J,
Vansteenkiste J, Lena H, Corral Jaime J, Gray JE, Powderly J,
Chouaid C, et al: ; ATLANTIC investigators: Durvalumab as
third-line or later treatment for advanced non-small-cell lung
cancer (ATLANTIC): An open-label, single-arm, phase 2 study. Lancet
Oncol. 19:521–536. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Luke JJ, Flaherty KT, Ribas A and Long GV:
Targeted agents and immunotherapies: Optimizing outcomes in
melanoma. Nat Rev Clin Oncol. 14:463–482. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Miao D, Margolis CA, Gao W, Voss MH, Li W,
Martini DJ, Norton C, Bossé D, Wankowicz SM, Cullen D, et al:
Genomic correlates of response to immune checkpoint therapies in
clear cell renal cell carcinoma. Science. 359:801–806. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Pauken KE, Sammons MA, Odorizzi PM, Manne
S, Godec J, Khan O, Drake AM, Chen Z, Sen DR, Kurachi M, et al:
Epigenetic stability of exhausted T cells limits durability of
reinvigoration by PD-1 blockade. Science. 354:1160–1165. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Pitt JM, Vétizou M, Daillère R, Roberti
MP, Yamazaki T, Routy B, Lepage P, Boneca IG, Chamaillard M,
Kroemer G, et al: Resistance mechanisms to immune-checkpoint
blockade in cancer: Tumor-intrinsic and -extrinsic factors.
Immunity. 44:1255–1269. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Joyce JA and Fearon DT: T cell exclusion,
immune privilege, and the tumor microenvironment. Science.
348:74–80. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jenkins RW, Barbie DA and Flaherty KT:
Mechanisms of resistance to immune checkpoint inhibitors. Br J
Cancer. 118:9–16. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Smyth MJ, Ngiow SF, Ribas A and Teng MW:
Combination cancer immunotherapies tailored to the tumour
microenvironment. Nat Rev Clin Oncol. 13:143–158. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J,
Bozym DJ, Zhang W, Luoma A, Giobbie-Hurder A, Peter L, et al: An
immunogenic personal neoantigen vaccine for patients with melanoma.
Nature. 547:217–221. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Barata PC and Rini BI: Treatment of renal
cell carcinoma: Current status and future directions. CA Cancer J
Clin. 67:507–524. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Mathew M, Enzler T, Shu CA and Rizvi NA:
Combining chemotherapy with PD-1 blockade in NSCLC. Pharmacol Ther.
186:130–137. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Bourgeois-Daigneault MC, Roy DG, Aitken
AS, El Sayes N, Martin NT, Varette O, Falls T, St-Germain LE, Pelin
A, Lichty BD, et al: Neoadjuvant oncolytic virotherapy before
surgery sensitizes triple-negative breast cancer to immune
checkpoint therapy. Sci Transl Med. 10(pii): eaao16412018.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Overman MJ, McDermott R, Leach JL, Lonardi
S, Lenz HJ, Morse MA, Desai J, Hill A, Axelson M, Moss RA, et al:
Nivolumab in patients with metastatic DNA mismatch repair-deficient
or microsatellite instability-high colorectal cancer (CheckMate
142): An open-label, multicentre, phase 2 study. Lancet Oncol.
18:1182–1191. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chen DS and Mellman I: Oncology meets
immunology: The cancer-immunity cycle. Immunity. 39:1–10. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yshii LM, Hohlfeld R and Liblau RS:
Inflammatory CNS disease caused by immune checkpoint inhibitors:
Status and perspectives. Nat Rev Neurol. 13:755–763. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Varricchi G, Galdiero MR and Tocchetti CG:
Cardiac toxicity of immune checkpoint inhibitors: Cardio-oncology
meets immunology. Circulation. 136:1989–1992. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Shoushtari AN, Friedman CF,
Navid-Azarbaijani P, Postow MA, Callahan MK, Momtaz P, Panageas KS,
Wolchok JD and Chapman PB: Measuring toxic effects and time to
treatment failure for nivolumab plus ipilimumab in melanoma. JAMA
Oncol. 4:98–101. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Powles T, Eder JP, Fine GD, Braiteh FS,
Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL, et
al: MPDL3280A (anti-PD-L1) treatment leads to clinical activity in
metastatic bladder cancer. Nature. 515:558–562. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Mahoney KM, Rennert PD and Freeman GJ:
Combination cancer immunotherapy and new immunomodulatory targets.
Nat Rev Drug Discov. 14:561–584. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Govindan R, Szczesna A, Ahn MJ, Schneider
CP, Gonzalez Mella PF, Barlesi F, Han B, Ganea DE, Von Pawel J,
Vladimirov V, et al: Phase III trial of ipilimumab combined with
paclitaxel and carboplatin in advanced squamous non-small-cell lung
cancer. J Clin Oncol. 35:3449–3457. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Reck M, Luft A, Szczesna A, Havel L, Kim
SW, Akerley W, Pietanza MC, Wu YL, Zielinski C, Thomas M, et al:
Phase III randomized trial of ipilimumab plus etoposide and
platinum versus placebo plus etoposide and platinum in
extensive-stage small-cell lung cancer. J Clin Oncol. 34:3740–3748.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Lynch TJ, Bondarenko I, Luft A,
Serwatowski P, Barlesi F, Chacko R, Sebastian M, Neal J, Lu H,
Cuillerot JM, et al: Ipilimumab in combination with paclitaxel and
carboplatin as first-line treatment in stage IIIB/IV non-small-cell
lung cancer: Results from a randomized, double-blind, multicenter
phase II study. J Clin Oncol. 30:2046–2054. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Gubin MM, Zhang X, Schuster H, Caron E,
Ward JP, Noguchi T, Ivanova Y, Hundal J, Arthur CD, Krebber WJ, et
al: Checkpoint blockade cancer immunotherapy targets
tumour-specific mutant antigens. Nature. 515:577–581. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wolchok JD, Chiarion-Sileni V, Gonzalez R,
Rutkowski P, Grob JJ, Cowey CL, Lao CD, Wagstaff J, Schadendorf D,
Ferrucci PF, et al: Overall survival with combined nivolumab and
ipilimumab in advanced melanoma. N Engl J Med. 377:1345–1356. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Schadendorf D, Larkin J, Wolchok J, Hodi
FS, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL,
Lao C, et al: Health-related quality of life results from the phase
III CheckMate 067 study. Eur J Cancer. 82:80–91. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hellmann MD, Rizvi NA, Goldman JW,
Gettinger SN, Borghaei H, Brahmer JR, Ready NE, Gerber DE, Chow LQ,
Juergens RA, et al: Nivolumab plus ipilimumab as first-line
treatment for advanced non-small-cell lung cancer (CheckMate 012):
Results of an open-label, phase 1, multicohort study. Lancet Oncol.
18:31–41. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Long GV, Atkinson V, Cebon JS, Jameson MB,
Fitzharris BM, McNeil CM, Hill AG, Ribas A, Atkins MB, Thompson JA,
et al: Standard-dose pembrolizumab in combination with reduced-dose
ipilimumab for patients with advanced melanoma (KEYNOTE-029): An
open-label, phase 1b trial. Lancet Oncol. 18:1202–1210. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Herrera FG, Bourhis J and Coukos G:
Radiotherapy combination opportunities leveraging immunity for the
next oncology practice. CA Cancer J Clin. 67:65–85. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Lee JM, Cimino-Mathews A, Peer CJ, Zimmer
A, Lipkowitz S, Annunziata CM, Cao L, Harrell MI, Swisher EM,
Houston N, et al: Safety and clinical activity of the programmed
death-ligand 1 inhibitor durvalumab in combination with poly
(ADP-Ribose) polymerase inhibitor olaparib or vascular endothelial
growth factor receptor 1–3 inhibitor cediranib in women's cancers:
A dose-escalation, phase I study. J Clin Oncol. 35:2193–2202. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Chesney J, Puzanov I, Collichio F, Singh
P, Milhem MM, Glaspy J, Hamid O, Ross M, Friedlander P, Garbe C, et
al: Randomized, open-label phase II study evaluating the efficacy
and safety of talimogene laherparepvec in combination with
ipilimumab versus ipilimumab alone in patients with advanced,
unresectable melanoma. J Clin Oncol. 36:1658–1667. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ribas A, Dummer R, Puzanov I, VanderWalde
A, Andtbacka RHI, Michielin O, Olszanski AJ, Malvehy J, Cebon J,
Fernandez E, et al: Oncolytic virotherapy promotes intratumoral T
cell infiltration and improves anti-PD-1 immunotherapy. Cell.
170:1109–1119. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Sender R, Fuchs S and Milo R: Revised
estimates for the number of human and bacteria cells in the body.
PLoS Biol. 14:e10025332016. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Hall AB, Tolonen AC and Xavier RJ: Human
genetic variation and the gut microbiome in disease. Nat Rev Genet.
18:690–699. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
York A: Microbiome: Gut microbiota sways
response to cancer immunotherapy. Nat Rev Microbiol. 16:1212018.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Sivan A, Corrales L, Hubert N, Williams
JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B,
Alegre ML, et al: Commensal Bifidobacterium promotes antitumor
immunity and facilitates anti-PD-L1 efficacy. Science.
350:1084–1089. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Vétizou M, Pitt JM, Daillère R, Lepage P,
Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong
CP, et al: Anticancer immunotherapy by CTLA-4 blockade relies on
the gut microbiota. Science. 350:1079–1084. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Routy B, Le Chatelier E, Derosa L, Duong
CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C,
Roberti MP, et al: Gut microbiome influences efficacy of PD-1-based
immunotherapy against epithelial tumors. Science. 359:91–97. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Matson V, Fessler J, Bao R, Chongsuwat T,
Zha Y, Alegre ML, Luke JJ and Gajewski TF: The commensal microbiome
is associated with anti-PD-1 efficacy in metastatic melanoma
patients. Science. 359:104–108. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Gopalakrishnan V, Spencer CN, Nezi L,
Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman
K, Wei SC, et al: Gut microbiome modulates response to anti-PD-1
immunotherapy in melanoma patients. Science. 359:97–103. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Jobin C: Precision medicine using
microbiota. Science. 359:32–34. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
RodrÍguez E, Schetters STT and Van Kooyk
Y: The tumour glyco-code as a novel immune checkpoint for
immunotherapy. Nat Rev Immunol. 18:204–211. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Van Vliet SJ, Bay S, Vuist IM, Kalay H,
García-Vallejo JJ, Leclerc C and Van Kooyk Y: MGL signaling
augments TLR2-mediated responses for enhanced IL-10 and TNF-α
secretion. J Leukoc Biol. 94:315–323. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Van Vliet SJ, Gringhuis SI, Geijtenbeek TB
and Van Kooyk Y: Regulation of effector T cells by
antigen-presenting cells via interaction of the C-type lectin MGL
with CD45. Nat Immunol. 7:1200–1208. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Drake RR: Glycosylation and cancer: Moving
glycomics to the forefront. Adv Cancer Res. 126:1–10. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Pinho SS and Reis CA: Glycosylation in
cancer: Mechanisms and clinical implications. Nat Rev Cancer.
15:540–555. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Kaur S, Kumar S, Momi N, Sasson AR and
Batra SK: Mucins in pancreatic cancer and its microenvironment. Nat
Rev Gastroenterol Hepatol. 10:607–620. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Kim J, Bamlet WR, Oberg AL, Chaffee KG,
Donahue G, Cao XJ, Chari S, Garcia BA, Petersen GM and Zaret KS:
Detection of early pancreatic ductal adenocarcinoma with
thrombospondin-2 and CA19-9 blood markers. Sci Transl Med. 9(pii):
eaah55832017. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Yang AP, Liu J, Lei HY, Zhang QW, Zhao L
and Yang GH: CA72-4 combined with CEA, CA125 and CAl9-9 improves
the sensitivity for the early diagnosis of gastric cancer. Clin
Chim Acta. 437:183–186. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Ashkani J and Naidoo KJ:
Glycosyltransferase gene expression profiles classify cancer types
and propose prognostic subtypes. Sci Rep. 6:264512016. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Syed P, Gidwani K, Kekki H, Leivo J,
Pettersson K and Lamminmäki U: Role of lectin microarrays in cancer
diagnosis. Proteomics. 16:1257–1265. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Miles D and Papazisis K: Rationale for the
clinical development of STn-KLH (Theratope®) and
anti-MUC-1 vaccines in breast cancer. Clin Breast Cancer.
3:S134–S138. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Cagnoni AJ, Pérez Sáez JM, Rabinovich GA
and Mariño KV: Turning-off signaling by siglecs, selectins, and
galectins: Chemical inhibition of glycan-dependent interactions in
cancer. Front Oncol. 6:1092016. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Posey AD Jr, Schwab RD, Boesteanu AC,
Steentoft C, Mandel U, Engels B, Stone JD, Madsen TD, Schreiber K,
Haines KM, et al: Engineered CAR T cells targeting the
cancer-associated Tn-glycoform of the membrane mucin MUC1 control
adenocarcinoma. Immunity. 44:1444–1454. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Unger WW, Mayer CT, Engels S, Hesse C,
Perdicchio M, Puttur F, Streng-Ouwehand I, Litjens M, Kalay H,
Berod L, et al: Antigen targeting to dendritic cells combined with
transient regulatory T cell inhibition results in long-term tumor
regression. Oncoimmunology. 4:e9704622014. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Chou FC, Chen HY, Kuo CC and Sytwu HK:
Role of galectins in tumors and in clinical immunotherapy. Int J
Mol Sci. 1(pii): E4302018. View Article : Google Scholar
|
|
102
|
Alderton GK: Tumour immunology: TIM3
suppresses antitumour DCs. Nat Rev Immunol. 12:620–621. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Sundar R, Soong R, Cho BC, Brahmer JR and
Soo RA: Immunotherapy in the treatment of non-small cell lung
cancer. Lung Cancer. 85:101–109. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Anderson AC: Tim-3: An emerging target in
the cancer immunotherapy landscape. Cancer Immunol Res. 2:393–398.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Liu J, Zhang S, Hu Y, Yang Z, Li J, Liu X,
Deng L, Wang Y, Zhang X, Jiang T, et al: Targeting PD-1 and Tim-3
pathways to reverse CD8 T-cell exhaustion and enhance ex vivo
T-cell responses to autologous dendritic/tumor vaccines. J
Immunother. 39:171–180. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Sakuishi K, Ngiow SF, Sullivan JM, Teng
MW, Kuchroo VK, Smyth MJ and Anderson AC:
TIM3+FOXP3+ regulatory T cells are
tissue-specific promoters of T-cell dysfunction in cancer.
OncoImmunology. 2:e238492013. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Ngiow SF, von Scheidt B, Akiba H, Yagita
H, Teng MW and Smyth MJ: Anti-TIM3 antibody promotes T cell
IFN-γ-mediated antitumor immunity and suppresses established
tumors. Cancer Res. 71:3540–3551. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Zhou Q, Munger ME, Veenstra RG, Weigel BJ,
Hirashima M, Munn DH, Murphy WJ, Azuma M, Anderson AC, Kuchroo VK,
et al: Coexpression of Tim-3 and PD-1 identifies a CD8+
T-cell exhaustion phenotype in mice with disseminated acute
myelogenous leukemia. Blood. 117:4501–4510. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Irie A, Yamauchi A, Kontani K, Kihara M,
Liu D, Shirato Y, Seki M, Nishi N, Nakamura T, Yokomise H, et al:
Galectin-9 as a prognostic factor with antimetastatic potential in
breast cancer. Clin Cancer Res. 11:2962–2968. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Fujita K, Iwama H, Sakamoto T, Okura R,
Kobayashi K, Takano J, Katsura A, Tatsuta M, Maeda E, Mimura S, et
al: Galectin-9 suppresses the growth of hepatocellular carcinoma
via apoptosis in vitro and in vivo. Int J Oncol.
46:2419–2430. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Mengshol JA, Golden-Mason L, Arikawa T,
Smith M, Niki T, McWilliams R, Randall JA, McMahan R, Zimmerman MA,
Rangachari M, et al: A crucial role for Kupffer cell-derived
galectin-9 in regulation of T cell immunity in hepatitis C
infection. PLoS One. 5:e95042010. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Fujita K, Iwama H, Oura K, Tadokoro T,
Samukawa E, Sakamoto T, Nomura T, Tani J, Yoneyama H, Morishita A,
et al: Cancer therapy due to apoptosis: Galectin-9. Int J Mol Sci.
18(pii): E742017. View Article : Google Scholar : PubMed/NCBI
|