Introduction
Glioma is the most common intracranial tumour. It is
assigned grades I–IV according to the WHO Classification of Tumours
of the Central Nervous System (1).
Among the different types of glioma, glioblastoma multiforme (GBM)
is the most common type of primary brain tumour in adults, and it
is associated with a poor prognosis (2). Patients with glioblastoma multiforme
usually survived less than 15 months following diagnosis and
treatment (3). There are no
adequate treatment methods for this disease. Currently, a
comprehensive approach, including surgery, radiation therapy and
chemotherapy, is the main glioma treatment strategy (4). Although tremendous progress has been
made in the genomic, transcriptomic and epigenetic fields regarding
glioma, the exact pathogenesis of glioma remains unknown (5). Most current research on high-grade
glioma has focused on DNA mismatch repair, a disorder of signalling
pathways, mutations of PI3K/AKT/PTEN, Ras and P53/RB1 pathway genes
and stem cell tumours (6,7).
The PI3K signalling pathway plays a critical role in
cell proliferation, differentiation, apoptosis, and glucose
transport. The Class I PI3Ks are composed of a regulatory and a
catalytic subunit and are further divided into class Ia and class
Ib. The regulatory subunits of class Ia PI3Ks included p110α and
p110β and are expressed by corresponding genes (PIK3CA and PIK3CB)
(8). Deregulation of the
PI3K/AKT/mTOR signalling pathway has been demonstrated to result
mainly from frequent inactivation of PTEN, which has been
identified in at least 60% of glioblastomas (9). Additionally, mutations in PIK3CA have
also been confirmed to exist in glioblastoma. These alterations of
genes are associated with tumourigenesis, and relevant studies have
revealed the importance of inhibitors targeting the PI3K/Akt/mTOR
pathway.
Although mutations in PIK3CA are generally
considered to be related to tumourigenesis (10–12),
recent research has shown that p110β plays an essential role in
specific PTEN-deficient cancer cells (13–16).
In order to investigate whether an inhibitor of p110β could be used
to treat glioma, AZD6282, a novel potential selective p110β
inhibitor, was selected to test its inhibitory properties in glioma
U87 and U118 cell lines.
Materials and methods
Analysis of TCGA and GDSC data
Mutation data from 273 samples were downloaded from
the cBioPortal online platform (www.cbioportal.org). The data visualization was
performed using the R package (‘complexheatmap’). The Genomics of
Drug Sensitivity in Cancer (GDSC) database (www.cancerrxgene.org) was used to search for compounds
with significant selectivity for PTEN mutations. Scatter plots were
generated via the GDSC online platform.
Cell culture
Human glioblastoma cell lines (U87 and U118) were
acquired from the State Key Laboratory of Molecular Biology,
Institute of Biochemistry and Cell Biology, Shanghai Institutes for
Biological Sciences, Chinese Academy of Sciences (Shanghai, China).
The U87 cell line has been found to be misidentified and originate
from an unknown glioblastoma. Additionally, the U118 cell line had
similar cytogenetics and derivative marker chromosomes with the
glioblastoma cell line U138MG. However, our U87 and U118 cell lines
were authenticated by STR profiling; thus, we used U87 and U118 as
our experimental cells. The cells were cultured in Dulbecco's
modified Eagle's medium (DMEM) supplemented with 10% foetal bovine
serum (FBS) (both from Gibco; Thermo Fisher Scientific, Inc,
Waltham, MA, USA), 100 µg/ml penicillin and 100 µg/ml streptomycin
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 37°C with 5%
CO2.
Chemicals and antibodies
AZD6482 (Fig. 2A)
was purchased from MedChemExpress (MCE, Shanghai, China) and
dissolved in dimethyl sulfoxide (DMSO), which was purchased from
Merck KGaA. Antibodies against phospho-GSK-3β (catalog number:
5558), GSK-3β (catalog number: 9315), Akt (catalog number: 4691),
phospho-Akt (catalog number: 4060), Bcl-2 (catalog number: 2872),
Bax (catalog number: 2774), cyclin D1 (catalog number: 2922),
β-actin (catalog number: 58169) and GAPDH (catalog number: 5174)
(all used at 1:1,000) were purchased from Cell Signaling Technology
(Danvers, MA, USA).
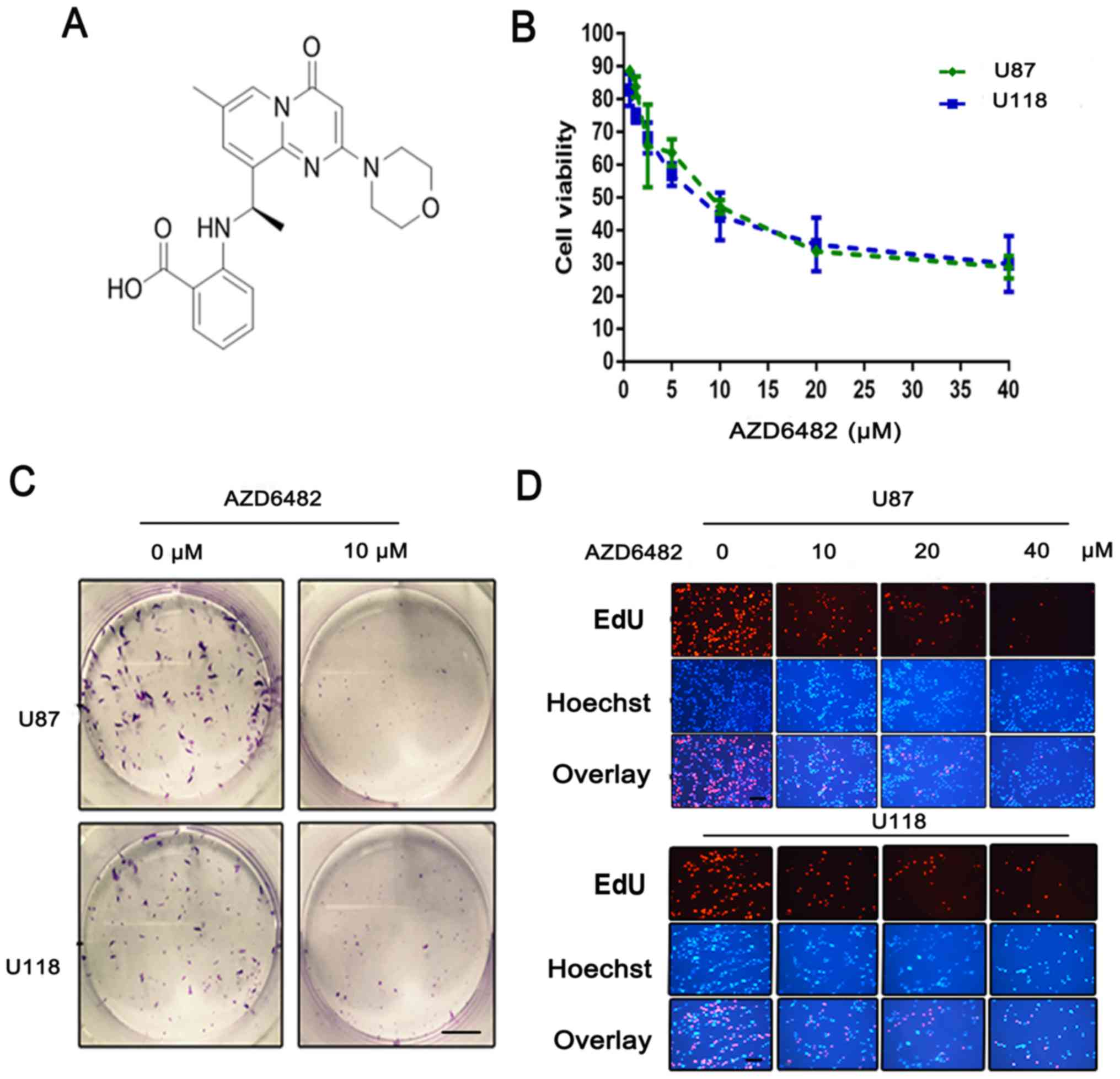 | Figure 2.Treatment with AZD6482 leads to an
anti-proliferative effect on glioblastoma cells. (A) The molecular
structure of AZD6482. (B) Cell viability was determined by CCK-8
assay after AZD6482 treatment at various concentrations (0, 0.625,
1.25, 2.5, 5, 10, 20, or 40 µM) for 48 h. Each cell line was
analysed in triplicate. (C) AZD6482 inhibited the colony formation
of U87 and U118 cells. Fewer colonies were formed in the treated
group compared with the control group. Scale bar, 50 µm. (D) DNA
replication activity was assessed by an EdU incorporation assay.
Nuclei were stained with Hoechst (blue), and the proliferative
cells were dyed red with EdU. Scale bar, 100 µm (same magnification
in all panels). |
Cell viability
Cell Counting Kit-8 (CCK-8) (Dojindo, Shanghai,
China) was used to determine the inhibitory effect of AZD6482 on
the proliferation of the U87 and U118 cells according to the
manufacturer's instructions. Cells in Dulbecco's modified Eagle's
medium (Gibco; Thermo Fisher Scientific, Inc, Waltham, MA, USA)
with 10% FBS were plated into 96-well plates (5×103
cells/100 µl/well) and cultured with 0.625, 1.25, 2.5, 5, 10, 20 or
40 µM of AZD6482 for 48 h. Then, 10 µl of CCK-8 was added, and the
cells were incubated for another 1 h. The absorbance value (OD) of
every well was measured with a spectrophotometric plate reader at
450 nm. Assays were performed in triplicate with three independent
experiments.
Colony formation assay
An appropriate number of cells were plated on a
6-well plate (500 cells/2 ml/well) and cultured in 2 ml of DMEM
with 10% FBS. Then, the cells were treated with 5 µM of AZD6482 for
three weeks until the cells in the plates had formed colonies that
were of approximately the correct size (50 cells per colony or
greater). The cells were fixed with 2 ml of 75% ethanol at room
temperature for 15 min and then stained with 0.5% crystal violet.
The counts of colonies were quantitatively evaluated using ImageJ
Software (National Institutes of Health, Bethesda, MD, USA).
5-Ethynyl-2′-deoxyuridine (EdU)
incorporation assay
The Cell-Light™ EdU Apollo® 643 In Vitro
Imaging kit (100T) was purchased from RiboBio Co., Ltd. (Guangzhou,
China). It was used according to the manufacturer's protocol. In
brief, 5×103 cells were seeded in a volume of 100 µl of
DMEM into each well of a 96-well plate and treated with 0, 10, 20
or 40 µM of AZD6482 for 48 h. Then, the cells were cultured in
medium with 50 µM EdU for 12 h and fixed with 4% paraformaldehyde
for 30 min. Cells were incubated in the Apollo reaction mixture for
30 min, and the DNA was stained with 5 µg/ml Hoechst 33342 for 30
min. The fluorescence images were visualized under a fluorescence
microscope (magnification, ×200; Olympus BX51; Olympus, Tokyo,
Japan).
Cell cycle distribution analysis
The effect of AZD6482 on the cell cycle distribution
was analysed by flow cytometry with propidium iodide (PI) staining.
Cells in 6-well plates were harvested and washed in PBS and then
treated with 0, 10, 20 or 40 µM AZD6482 for 48 h. Then, the cells
were fixed in cold 70% ethanol for 12 h at 4°C. Subsequently, the
cells were washed twice with PBS, treated with 50 µl of 100 µg/ml
RNase at 37°C and stained with 5 µl of PI from a 50 mg/ml stock
solution. The results were analysed by BD FACSAria (BD Biosciences,
Franklin Lakes, NJ, USA). The data were quantified using ModFit LT
4.0 (http://www.vsh.com/products/mflt/index.asp; Verity
Software House, Topsham, ME, USA).
Flow cytometric analysis of apoptosis
with PE/7-ADD staining
To analyse apoptosis, cells were treated with 0, 10,
20 or 40 µM AZD6482 for 48 h. The cells were suspended in 100 µl of
1X binding buffer (0.1 mM HEPES/NaOH, 1.4 M NaCl, 25 mM
CaCl2, pH 7.4) and stained with 5 µl of PE Annexin V and
5 µl of 7-amino-actinomycin (7-ADD) for 15 min at room temperature.
Then, 400 µl 1X binding buffer was added to each tube. Analysis of
the results was carried out with BD FACSAria (BD Biosciences,
Franklin Lakes, NJ, USA). Data were quantified with FlowJo software
(Tristar, San Carlos, CA, USA).
Wound-healing assay
The cells were seeded into 6-well plates at a
density (3×105 cells/well) and grown until they reached
70–80% confluence as a monolayer. Then, the cell monolayer was
gently and slowly scratched using a yellow pipette tip in each
well. Subsequently, the cells were gently washed with 1X PBS and
cultured in DMEM supplemented with 1% FBS. Simultaneously, 0 or 10
µM of AZD6482 was added to the medium for an additional 24 and 48
h. The images were visualized under a microscope (magnification,
×100; Olympus BX51; Olympus, Tokyo, Japan). The gap distance could
be quantitatively evaluated using ImageJ Software (National
Institutes of Health, Bethesda, MD, USA).
Cell invasion assays
Invasion assays were performed using a Transwell
chamber with an 8.0-µm pore polycarbonate membrane. First,
8×104 cells treated with 0, 5, or 10 µM of AZD6482 were
seeded into the top chambers and suspended in 100 µl of serum-free
DMEM. Then, 500 µl of 10% FBS DMEM was added into the lower
chamber. After 36 h of incubation, the cells on the lower side of
the insert membrane were fixed with 5% glutaraldehyde and stained
with 0.5% crystal violet. Finally, the results were visualized
under a microscope (magnification, ×200; Olympus BX51; Olympus,
Tokyo, Japan).
Western blot analysis
Western blotting was performed as described
previously (15). Briefly, the
cells were treated with 0, 5, 10, or 20 µM of AZD6482 for 48 h and
then lysed in RIPA buffer. Then, the same protein samples were
loaded onto a 10 or 12% SDS-PAGE and electrotransferred to a
polyvinylidene fluoride (PVDF) membrane for 60 or 90 min. After the
transfer, the membrane was blocked with 5% skim milk and then
incubated with anti-Akt, anti-p-Akt, anti-GSK-3B, anti-p-GSK-3B,
anti-Bcl-2, anti-Bax, anti-cyclin D1, anti-β-actin and anti-GAPDH
antibodies at 1:1000 overnight at 4°C. Subsequently, the membranes
were incubated with Alex Fluor 680/790-labelled goat anti-rabbit or
goat anti-mouse IgG secondary antibodies (catalog numbers:
926-68021/926-68020; Li-COR Biosciences, Lincoln, NE, USA) for 1 h.
The results were visualized using the LI-COR Odyssey Infrared
Imaging System (Li-COR Biosciences).
Statistical analysis
Statistical analyses were conducted using the SPSS
17.0 software package (SPSS Inc., Chicago, IL, USA) and GraphPad
Prism 6.0 software (GraphPad Software, Inc., La Jolla, CA, USA).
Data from the experiments are expressed as the means ± standard
deviation (SD). Statistical differences between groups were
analysed by one-way ANOVA and the Tukey test. All experiments were
repeated at least three times. Statistical significance is
indicated in the figures as follows: *P<0.05, **P<0.01 and
***P<0.001.
Results
Deregulation of the PI3K signalling
pathway resulting from frequent mutation of PTEN in glioblastoma
cells
Mutation data of glioblastoma were downloaded from
the cBioPortal online platform and visualized using the R package
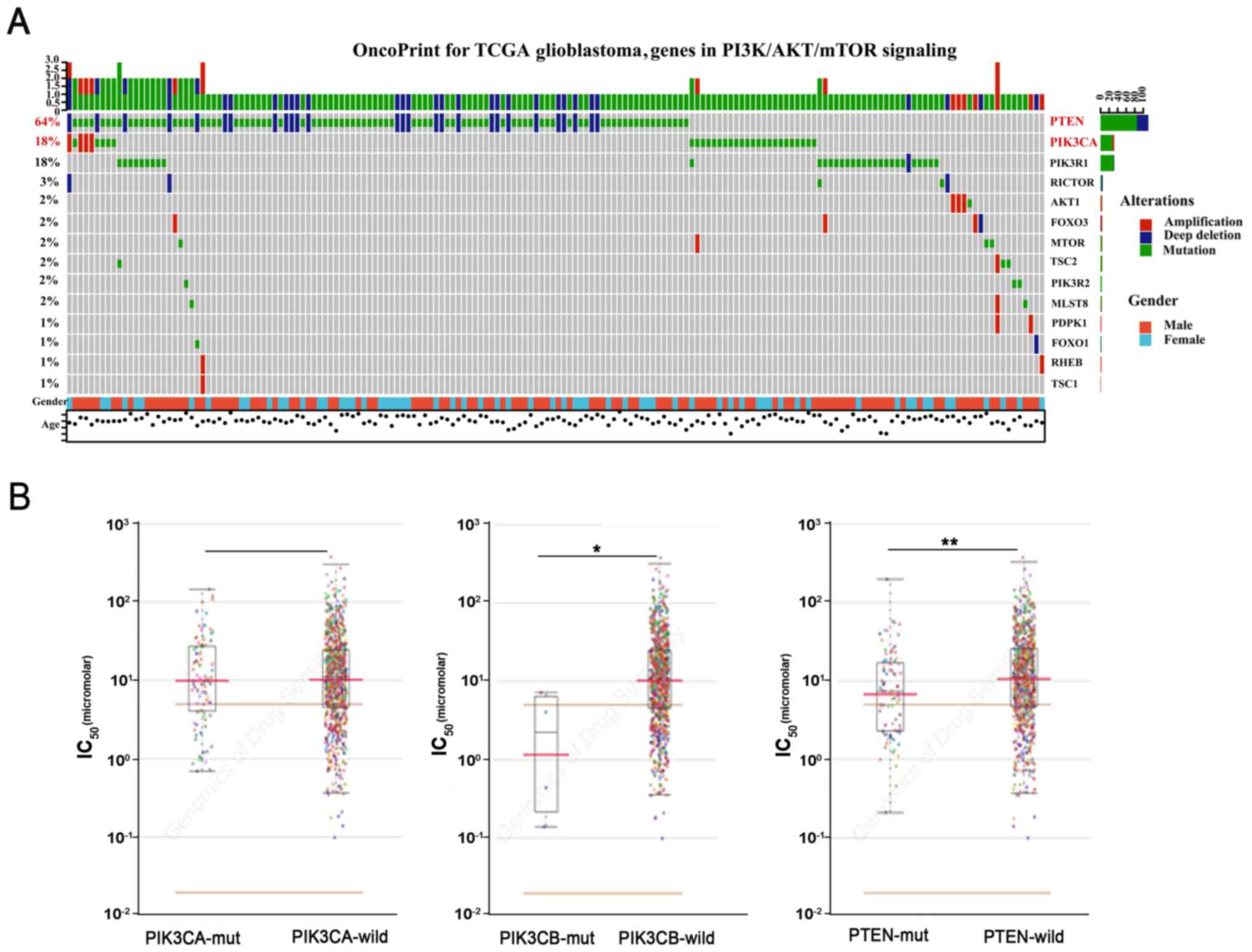
complexheatmap (17–19). Mutation of PTEN (64%) was found in
273 samples (Fig. 1A). Among them,
deep deletion of PTEN was the dominant mutation. Additionally,
PIK3CA was also frequently mutated in glioblastoma cells. However,
there were few mutations in PIK3CB. We further explored the GDSC
database. The datasets showed that PTEN-deficient cancer cells were
sensitive to AZD6482 (Fig. 1B)
(20).
AZD6482 suppresses the proliferation
of U87 and U118 cells
The antiproliferative effect of AZD6482 on U87 and
U118 cells was investigated using a Cell Counting Kit-8 (CCK-8).
The two cell lines were exposed to various concentrations of
AZD6482 (0.625–40 µM) for 48 h. The results showed that the
viability of the cell lines was significantly (P<0.0001)
suppressed in a dose-dependent manner (Fig. 2B). The results also showed that U118
cells were more sensitive than U87 cells, with IC50
values of 7.989 (95% CI, 6.5–9.7) and 9.061 (95% CI, 7.5–11),
respectively.
To further confirm the potential inhibitory action
of AZD6482, a colony formation assay was carried out to assess
proliferation. After three weeks of growth, the final counts of
colonies treated with AZD6482 (U87: 21 clones; U118: 37 clones)
were fewer than those of the non-treated cells (U87: 110 clones;
U118: 78 clones, P<0.01) (Fig.
2C). In addition, a 5-ethynyl-2′-deoxyuridine (EdU)
incorporation assay was performed with both U87 and U118 cells. The
fluorescence images showed that the percentage of EdU-positive
cells was decreased in a dose-dependent manner (Fig. 2D). This indicated that DNA
replication was inhibited by AZD6482. These results, along with the
viability data, confirmed the antiproliferative effect of AZD6482
on glioma cells.
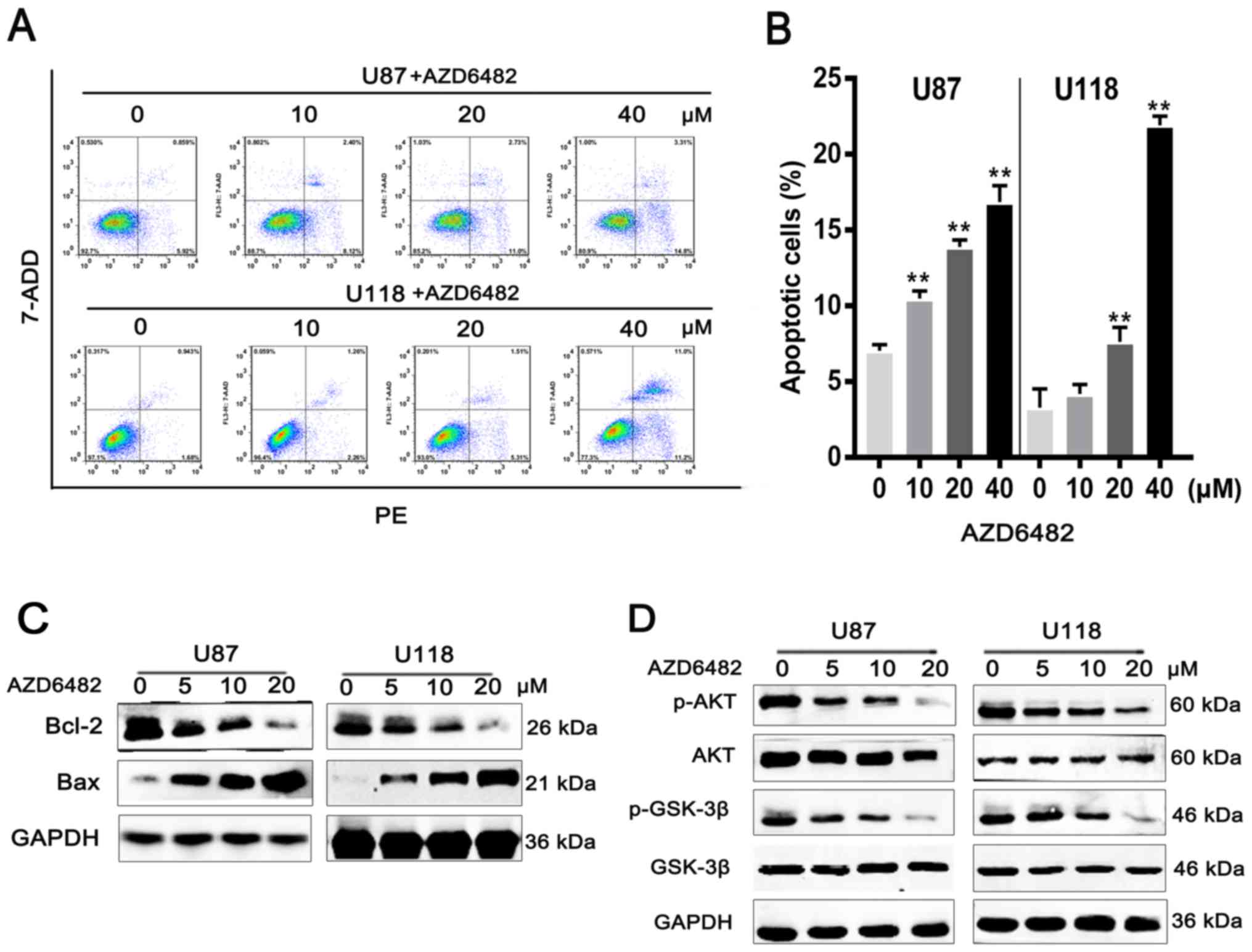
AZD6482 induces apoptosis in U87 and
U118 cells
To observe the influence of AZD6482 on cell
apoptosis, flow cytometric analysis with PE/7-ADD staining was
performed. After treatment with various concentrations of AZD6482,
the results showed that the early apoptotic cell population (U87:
13.5%; U118: 11.3%) was increased in a dose-dependent manner
(Fig. 3A and B). Moreover, western
blot results showed that the expression level of Bcl-2 was
downregulated with increasing drug concentration, and the
expression level of Bax was upregulated under the same conditions
(Fig. 3C). The ratio of Bcl-2/Bax
was also significantly decreased. It is well known that the
PI3K-AKT signalling pathway is involved in cell survival. Western
blotting was performed to confirm whether AZD6482 targeting of
PI3Kβ induced apoptosis by the PI3K-AKT signalling pathway. The
results showed that the levels of p-AKT and p-GSK-3β were decreased
in U87 and U118 cells (Fig. 3D)
with increasing concentrations of AZD6482. These results further
confirmed that AZD6482 induced apoptosis through the PI3K-AKT
signalling pathway in U87 and U118 cells.
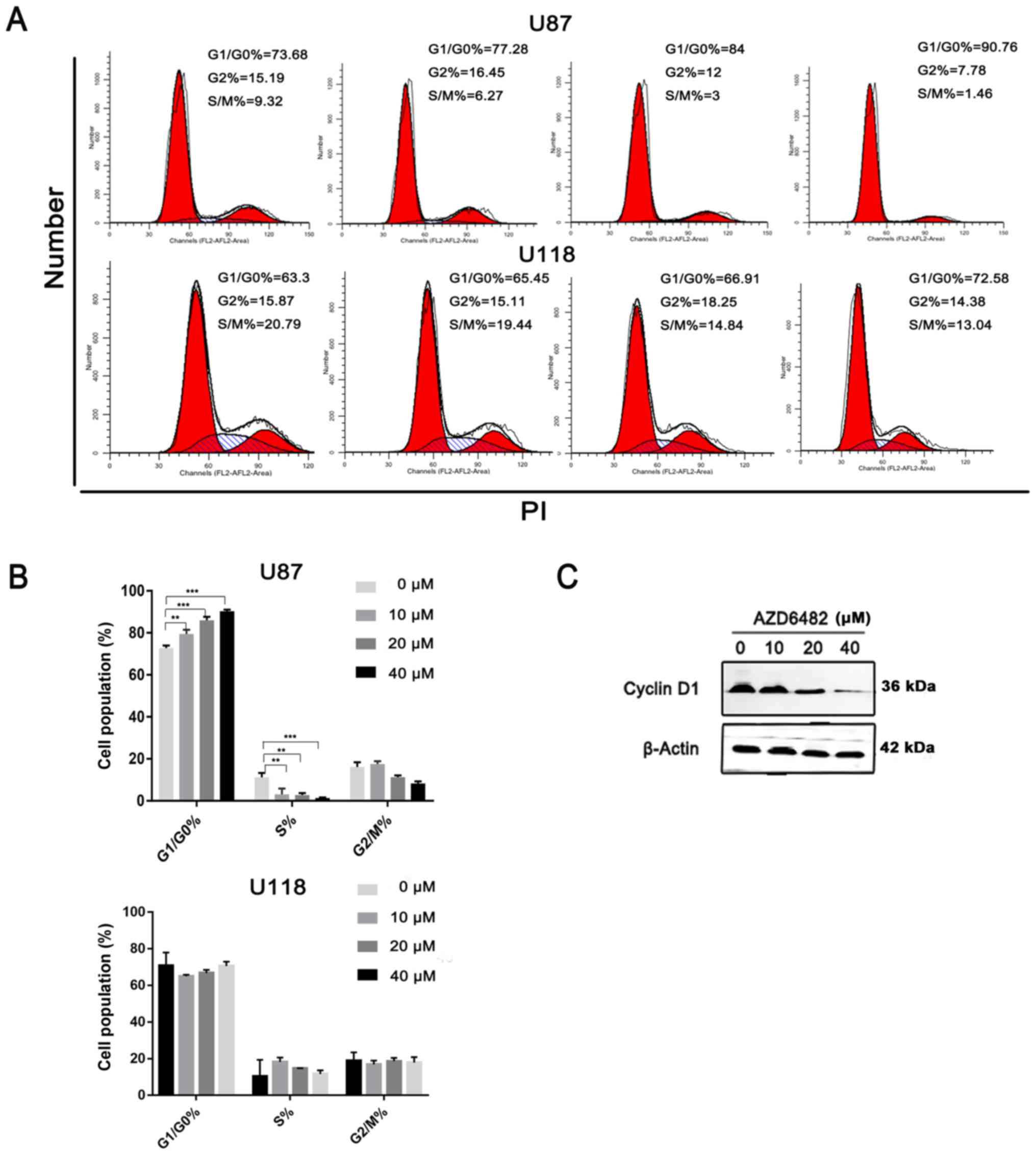
AZD6482 induces cell cycle arrest at
the G1 phase in U87 cells
The cell cycle is also related to the viability of
cells. The cells treated with various concentrations of AZD6282 for
48 h were stained with PI and analysed by flow cytometry. The
results showed that AZD6482 induced G1 arrest in the U87 cells
(Fig. 4A and B). However, no
significant accumulation of U118 cells in the G1 phase was found.
Meanwhile, previous studies reported that the expression of cyclin
D1 is mediated by GSK-3β (21–23),
and it was confirmed that the level of phosphorylation decreased
after AZD6482 treatment (Fig. 4C).
Therefore, AZD6482 induced G1 arrest in U87 cells via the PI3K-AKT
signalling pathway.
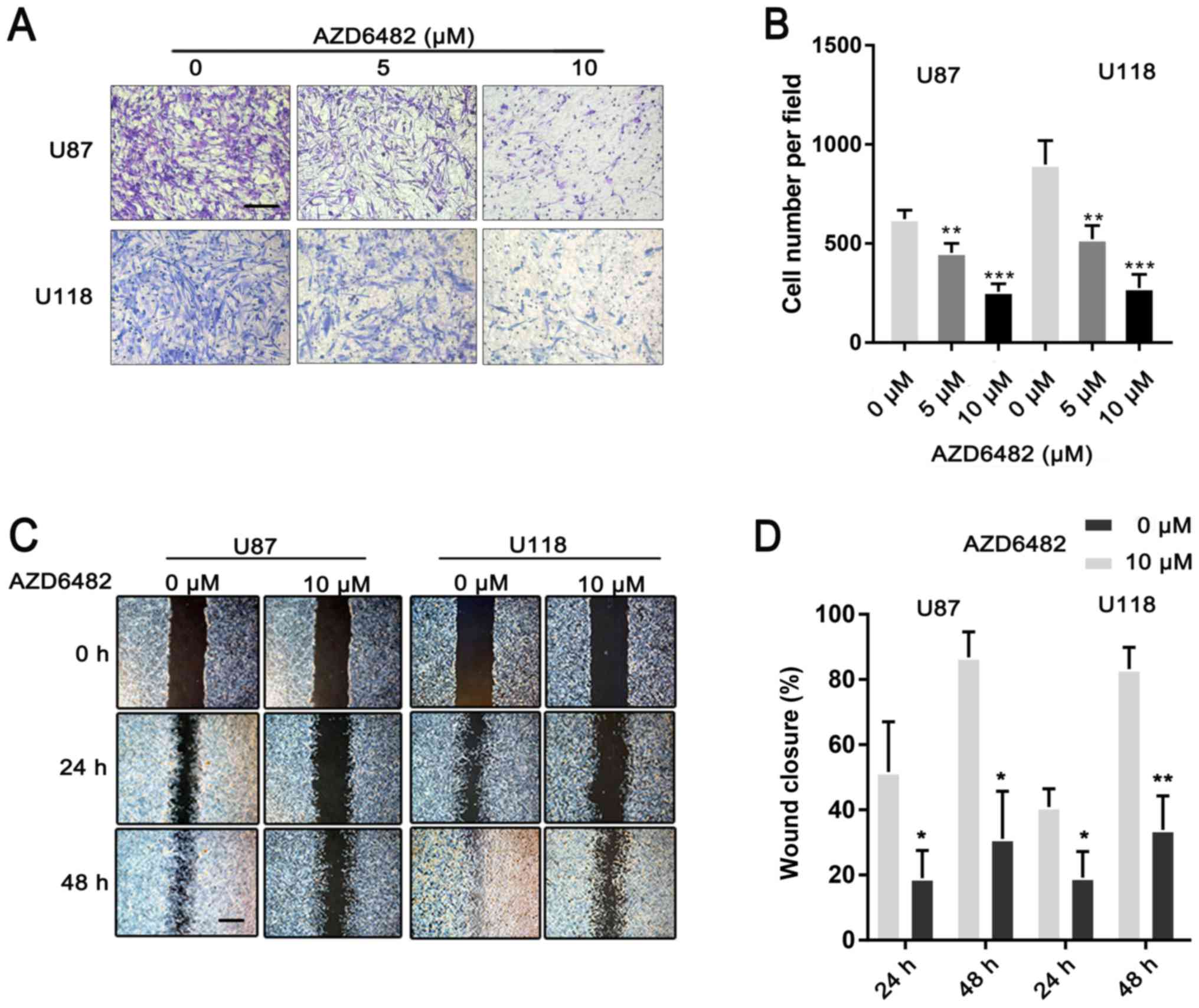
AZD6482 inhibits the migration and
invasion of U87 and U118 cells
To investigate the effect of AZD6482 on glioma
metastasis, wound-healing and invasion assays were performed.
Compared to the group treated with AZD6482, the untreated group
demonstrated a rapid increase in the gap distance up to 48 h of
growth (Fig. 5A and B). The cells
treated with AZD6482 also demonstrated a significant reduction in
invasive ability compared to the untreated cells (Fig. 5C and D). All of the results
demonstrated that AZD6482 inhibited the migration and invasion of
U87 and U118 cells.
Discussion
The PI3K signalling pathway is one of the most
critical signalling pathways in a variety of human cancers
(24,25). Mutations in PIK3CA or PTEN are the
most frequent genetic alterations in this pathway (9). Therefore, targeted PI3K inhibitors are
potential anticancer drugs. Currently, targeted p110a drugs are
developed faster than those targeting p110β. However, previous
studies have reported that p110β is thought to play a pivotal role
in PTEN loss-induced tumourigenesis (26,27).
Herein, we showed for the first time that a p110β-selective
inhibitor, AZD6482, could inhibit the induced cell proliferation of
glioma mediated by the loss of PTEN. Based on previous reports
(28), U87 and U118 cell lines
containing genomic mutations in PTEN were selected as the cell
lines for this research. Then, we confirmed that AZD6482 suppressed
the proliferation of U87 and U118 cells, with IC50
values of 9.061 and 7.989, respectively. AZD6482 not only promoted
G1 arrest and cell apoptosis but also had significant anticancer
activity by inhibiting cell migration and invasion.
The cell cycle is known to be regulated by the
cyclin-CDK complex and CDK inhibitor proteins. As a critical
component of the G1/S checkpoint, cyclin D1 forms a complex with
CDK4 and promotes cellular passage through the G1 phase (29,30).
Previous studies have indicated that GSK-3β increases the
expression of cyclin D1, which is a downstream molecule of AKT. As
shown in this study, treatment with AZD6482 led to a decrease in
p-GSK-3β and p-AKT expression. Therefore, the G1 arrest of U87
cells might be due to AZD6482 blockade of the PI3K signalling
pathway.
Flow cytometry with Annexin PE/7-ADD staining
suggested that AZD6482 induced apoptosis in U87 and U118 cells,
which affected cell proliferation. This result was also supported
by the expression levels of Bcl-2 and Bax. Finally, the
wound-healing and cell invasion assays confirmed that the migration
and invasion of human glioma cell lines was reduced by AZD6482.
Although there were notable discoveries in this
study, many limitations still exist. First, previous research
demonstrated that the effects of p110β inhibitors were transient
and caused the activation of PI3Kα and a rebound of downstream
signalling in PTEN-deficient tumours (13). In our study, this rebound was not
observed, perhaps because the drug treatment course was short.
Second, animal experiments have not yet been implemented. Although
some recent studies confirmed that the pharmacodynamics of AZD6482
are consistent in vivo and in vitro for some
non-nervous system tumours (16),
the consequence in glioma might be different due to the presence of
the blood-brain barrier. Third, our data demonstrated that AZD6482
only affected PI3K signalling in PTEN-deficient glioma cell lines.
Previous research confirmed that a few tumour cell lines with PTEN
mutations and wild-type PTEN were sensitive to AZD6482 (15). However, whether AZD6482 has the same
inhibition property in wild-type glioma cell lines needs to be
further verified. Despite its limitations, this study indicates
that AZD6482 is a promising drug candidate for glioma therapy.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Science Foundation of China (no. 81572489).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
PFX, JAY and QXC conceived and designed the study.
PFX conducted the experiments. JAY and JHL performed the
bioinformatics study and data interpretation. XY, JML, FEY and BHL
performed the statistical analysis. PFX and JAY wrote the
manuscript. JHL, XY, JML, FEY, BHL and QXC reviewed and edited the
manuscript. All authors read and approved the manuscript and agree
to be accountable for all aspects of the research in ensuring that
the accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Ethical approval was waived since we used only
publicly available data and materials in this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interest.
References
|
1
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 World Health Organization
Classification of Tumors of the Central Nervous System: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Omuro A and DeAngelis LM: Glioblastoma and
other malignant gliomas: A clinical review. JAMA. 310:1842–1850.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alifieris C and Trafalis DT: Glioblastoma
multiforme: Pathogenesis and treatment. Pharmacol Ther. 152:63–82.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Anjum K, Shagufta BI, Abbas SQ, Patel S,
Khan I, Shah SAA, Akhter N and Hassan SSU: Current status and
future therapeutic perspectives of glioblastoma multiforme (GBM)
therapy: A review. Biomed Pharmacother. 92:681–689. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aldape K, Zadeh G, Mansouri S,
Reifenberger G and von Deimling A: Glioblastoma: Pathology,
molecular mechanisms and markers. Acta Neuropathol. 129:829–848.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Network TC: Corrigendum: Comprehensive
genomic characterization defines human glioblastoma genes and core
pathways. Nature. 494:5062013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vanhaesebroeck B, Guillermet-Guibert J,
Graupera M and Bilanges B: The emerging mechanisms of
isoform-specific PI3K signalling. Nat Rev Mol Cell Biol.
11:329–341. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brennan CW, Verhaak RG, McKenna A, Campos
B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ,
Berman SH, et al: ; TCGA Research Network: The somatic genomic
landscape of glioblastoma. Cell. 155:462–477. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Avivar-Valderas A, McEwen R,
Taheri-Ghahfarokhi A, Carnevalli LS, Hardaker EL, Maresca M, Hudson
K, Harrington EA and Cruzalegui F: Functional significance of
co-ocπcurring mutations in PIK3CA and MAP3K1 in breast cancer.
Oncotarget. 9:21444–21458. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
García-Escudero R, Segrelles C, Dueñas M,
Pombo M, Ballestín C, Alonso-Riaño M, Nenclares P,
Álvarez-Rodríguez R, Sánchez-Aniceto G, Ruíz-Alonso A, et al:
Overexpression of PIK3CA in head and neck squamous cell carcinoma
is associated with poor outcome and activation of the YAP pathway.
Oral Oncol. 79:55–63. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Peyre M, Gaillard S, de Marcellus C, Giry
M, Bielle F, Villa C, Boch AL, Loiseau H, Baussart B, Cazabat L, et
al: Progestin-associated shift of meningioma mutational landscape.
Ann Oncol. 29:681–686. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schwartz S, Wongvipat J, Trigwell CB,
Hancox U, Carver BS, Rodrik-Outmezguine V, Will M, Yellen P, de
Stanchina E, Baselga J, et al: Feedback suppression of PI3Kα
signaling in PTEN-mutated tumors is relieved by selective
inhibition of PI3Kβ. Cancer Cell. 27:109–122. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yuzugullu H, Baitsch L, Von T, Steiner A,
Tong H, Ni J, Clayton LK, Bronson R, Roberts TM, Gritsman K, et al:
A PI3K p110β-Rac signalling loop mediates Pten-loss-induced
perturbation of haematopoiesis and leukaemogenesis. Nat Commun.
6:85012015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wee S, Wiederschain D, Maira SM, Loo A,
Miller C, deBeaumont R, Stegmeier F, Yao YM and Lengauer C:
PTEN-deficient cancers depend on PIK3CB. Proc Natl Acad Sci USA.
105:13057–13062. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ni J, Liu Q, Xie S, Carlson C, Von T,
Vogel K, Riddle S, Benes C, Eck M, Roberts T, et al: Functional
characterization of an isoform-selective inhibitor of PI3K-p110β as
a potential anticancer agent. Cancer Discov. 2:425–433. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao J, Aksoy BA, Dogrusoz U, Dresdner G,
Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al:
Integrative analysis of complex cancer genomics and clinical
profiles using the cBioPortal. Sci Signal. 6:pl12013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: An open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gu Z, Eils R and Schlesner M: Complex
heatmaps reveal patterns and correlations in multidimensional
genomic data. Bioinformatics. 32:2847–2849. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang W, Soares J, Greninger P, Edelman EJ,
Lightfoot H, Forbes S, Bindal N, Beare D, Smith JA, Thompson IR, et
al: Genomics of Drug Sensitivity in Cancer (GDSC): A resource for
therapeutic biomarker discovery in cancer cells. Nucleic Acids Res.
41:D955–D961. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Laco F, Woo TL, Zhong Q, Szmyd R, Ting S,
Khan FJ, Chai CLL, Reuveny S, Chen A and Oh S: Unraveling the
inconsistencies of cardiac differentiation efficiency induced by
the GSK3β inhibitor CHIR99021 in human pluripotent stem cells. Stem
Cell Reports. 10:pp. 1851–1866. 2018, View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu SL, Liu Z, Zhang LD, Zhu HQ, Guo JH,
Zhao M, Wu YL, Liu F and Gao FH: GSK3β-dependent cyclin D1 and
cyclin E1 degradation is indispensable for NVP-BEZ235 induced G0/G1
arrest in neuroblastoma cells. Cell Cycle. 16:2386–2395. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang J, Li XM, Bai Z, Chi BX, Wei Y and
Chen X: Curcumol induces cell cycle arrest in colon cancer cells
via reactive oxygen species and Akt/GSK3β/cyclin D1 pathway. J
Ethnopharmacol. 210:1–9. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lien EC, Dibble CC and Toker A: PI3K
signaling in cancer: Beyond AKT. Curr Opin Cell Biol. 45:62–71.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fruman DA, Chiu H, Hopkins BD, Bagrodia S,
Cantley LC and Abraham RT: The PI3K pathway in human disease. Cell.
170:605–635. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jia S, Liu Z, Zhang S, Liu P, Zhang L, Lee
SH, Zhang J, Signoretti S, Loda M, Roberts TM, et al: Corrigendum:
Essential roles of PI(3)K-p110β in cell growth, metabolism and
tumorigenesis. Nature. 533:2782016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen H, Mei L, Zhou L, Shen X, Guo C,
Zheng Y, Zhu H, Zhu Y and Huang L: PTEN restoration and PIK3CB
knockdown synergistically suppress glioblastoma growth in vitro and
in xenografts. J Neurooncol. 104:155–167. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mikheev AM, Mikheeva SA, Severs LJ, Funk
CC, Huang L, McFaline-Figueroa JL, Schwensen J, Trapnell C, Price
ND, Wong S, et al: Targeting TWIST1 through loss of function
inhibits tumorigenicity of human glioblastoma. Mol Oncol.
12:1188–1202. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Verreault M, Weppler SA, Stegeman A,
Warburton C, Strutt D, Masin D and Bally MB: Combined RNAi-mediated
suppression of Rictor and EGFR resulted in complete tumor
regression in an orthotopic glioblastoma tumor model. PLoS One.
8:e595972013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Malumbres M and Barbacid M: Cell cycle,
CDKs and cancer: A changing paradigm. Nat Rev Cancer. 9:153–166.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fedorov SN, Shubina LK, Bode AM, Stonik VA
and Dong Z: Dactylone inhibits epidermal growth factor-induced
transformation and phenotype expression of human cancer cells and
induces G1-S arrest and apoptosis. Cancer Res. 67:5914–5920. 2007.
View Article : Google Scholar : PubMed/NCBI
|