Introduction
Cancer invasion and the ability of malignant tumor
cells to metastasize are closely related to cancer progression and
prognosis (1). Invasive cancer is
characterized by spreading beyond the layers of the developed
tissue and growing into the surrounding healthy tissue. As
demonstrated by numerous studies, highly invasive cancer cells have
a systemic mechanism for increasing invasive activity (2). Cell-cell adhesion is lost and turned
into a spindle shape while pericellular proteolysis occurs by the
release of proteolytic enzyme in the direction of cancer migration
followed by cancer invasion (3).
Invadopodium is a protruding membrane that is actively formed in
invasive cancer cells (4). It is
rich in actin cytoskeleton and matrix metalloprotease (MMP). It
increases the invasiveness of cancer. When infiltration is needed,
cancer cells induce actin polymerization based on the Arp2/3
complex and N-WASP to stabilize the protruding structure by joining
many molecules such as Tsk5, cortactin and integrin. When protease
is secreted intensively around the protruding structure, the
extracellular matrix is deeply degraded, acting as a mechanism to
increase cancer cell invasion (2).
The regulation of invadopodium formation by various
growth factors has been reported (2). Epidermal growth factor (EGF),
transforming growth factor β (TGF- β), platelet-derived growth
factor (PDGF), hepatocyte growth factor (HGF) and heparin-binding
EGF (HB-EGF) are known as typical inducers, although their
activities differ depending on the type of cancer cells. Since the
cancer microenvironment is closely involved in the regulation of
invadopodium formation, understanding the cancer microenvironment
is important for controlling cancer invasion and progression.
During Transwell invasion experiments, we observed that Matrigel
increased fascin expression. These expression changes were not
observed in Transwell invasion experiments without Matrigel. Fascin
is an actin bundling protein that stabilizes actin in invadopodia
and increases the invasiveness of cancer (5). Matrigel® (Corning Costar,
Inc., Corning, NY, USA) is composed of extracellular matrix
proteins such as laminin, collagen IV and entactin. It is known to
contain several growth factors. These results suggest that
increased fascin expression during the infiltration process is
controlled by the tumor microenvironment. The aim of the present
study was to investigate stromal factors that could regulate fascin
expression in cancer cells and to understand the cell signaling
process involved.
Materials and methods
Cell lines and culture conditions
YD-10B human OSCC cells, which were derived from
tongue cancer patient tissues, were obtained from the Department of
Oral Pathology, College of Dentistry, Yonsei University (Seoul,
Korea). Cells were grown in DMEM/F12 (3:1 ratio) medium
supplemented with 10% fetal bovine serum (FBS), 1×10−10
M cholera toxin, 0.4 mg/ml hydrocortisone, 5 µg/ml insulin, 5 µg/ml
apo-transferrin and 2×10−11 M triiodothronine (T3) in a
humidified atmosphere of 5% CO2 at 37°C.
Normal gingival fibroblasts (NFs) were obtained from a patient who
had wisdom teeth extracted and who did not have oral mucosal
disease. Cancer-associated fibroblasts (CAFs) were obtained from a
surgical specimen of a patient with oral squamous cell carcinoma
(OSCC) and were selected from explanted cancer tissues in Versene
solution [200 mg EDTA and 1 mg glucose in 1 liter
phosphate-buffered saline (PBS) buffer] (6). Briefly, the tissue was washed with
betadine, cut into small pieces with scissors and washed three
times with PBS. Fibroblasts were selected in Versene solution and
characterized by immunohistochemical staining with anti-vimentin
(dilution 1:100; cat. no. M0725; Dako, Carpinteria, CA, USA) and
anti-α-smooth muscle actin (α-SMA) (dilution 1:100; cat. no.
ab5694; Abcam, Cambridge, MA, USA) antibodies. Early passages
(<9) of the fibroblasts were subjected to analysis. Informed
consent was provided by the patients whose CAFs and NFs were used
in this study and approval was granted by the Institutional Review
Board of Yonsei University College of Dentistry. The isolated
fibroblasts were maintained in DMEM/F12 (3:1 ratio) complete medium
containing 1% penicillin/streptomycin. Dimethyl sulfoxide (DMSO)
[0.1% (v/v)] was used in cell culture for control analysis.
Reagents
Dulbecco's modified Eagle's medium (DMEM), Ham's
F-12 nutrient mixture, FBS, antibiotic-antimycotic (100X), PBS and
0.25% trypsin-EDTA (1X) were purchased from Gibco-BRL (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Cholera toxin,
hydrocortisone, insulin, apo-transferrin, T3 and DMSO were obtained
from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Lysophosphatidic acid (LPA) was purchased from Enzo Life Sciences
(Plymouth Meeting, PA, USA). Recombinant human transforming growth
factor (TGF-β1), epidermal growth factor (EGF), and interleukin
(IL)-1β were obtained from EMD Millipore (Billerica, MA, USA).
SB431542, AG1478 and IL-1R antagonist were purchased from
Calbiochem (Merck KGaA). The following antibodies were purchased
from their respective sources: fascin (cat. no. M3567) and
E-cadherin (dilution 1:1,000; cat. no. M0725) (Dako); total (cat.
no. 9242)/phosphor (cat. no. 9246) forms of IκBα (dilution 1:1,000;
both from Cell Signaling Technology, Danvers, MA, USA); p50 (cat.
no. sc-166588) and p65 (cat. no. sc-71675) subunit of NF-κB
(dilution 1:1,000; both from Santa Cruz Biotechnology, Santa Cruz,
CA, USA); β-actin (cat. no. A5441) and GAPDH (cat. no. G9545)
(dilution 1:1,000; both from Sigma-Aldrich; Merck KGaA). Alexa
Fluor 568 phalloidin and Oregon Green 488 gelatin were purchased
from Molecular Probes (Thermo Fisher Scientific, Inc.).
Fascin depletion
Fascin 1-specific shRNA (h) lentiviral particles
were transduced in cultured cells with 5 µg/ml Polybrene according
to the manufacturer's protocol (Santa Cruz Biotechnology).
Continual selection was followed with 1 mg/ml puromycin to
establish the fascin-depleted stable cell line. Control shRNA (h)
lentiviral particles-A (Santa Cruz Biotechnology) were also used as
a negative control. The extent of fascin depletion was evaluated by
western blot analysis.
Western blotting
Protein (50 µg) was separated on 10%
SDS-polyacrylamide gel and were transferred to a polyvinylidene
difluoride (PVDF) membrane (EMD Millipore). The membrane was
blocked with 10% skim milk in PBS containing 0.1% Tween-20 (PBS-T)
and subsequently incubated overnight with a 1:1,000 dilution of the
primary antibody against its specific protein at 4°C.
The blots were then incubated with a 1:3,000 dilution of their
respective horseradish peroxidase-conjugated secondary antibodies
for 2 h at room temperature and were washed with PBS-T. The
targeted proteins were visualized using an enhanced
chemiluminescence detection kit (Amersham Life Science, Arlington
Heights, IL, USA) according to the manufacturer's instructions.
Nuclear extracts were prepared using a Nonidet P-40 lysis method.
Band intensities were measured with the ImageJ 1.52a software
program (National Institutes of Health, Bethesda, MD, USA)
Conditioned media (CM)
NFs and CAFs (5×104 cells) in a 100-mm
Petri dish were cultured in 6 ml of 1% FBS media for 48 h. The
media were centrifuged at 10,000 × g for 5 min and the supernatant
was used as CM.
Gelatin zymography
The conditioned medium was collected, and the
protein concentration was determined by the Bradford method
(Bio-Rad Laboratories, Hercules, CA, USA). Equal amounts of protein
(20 µg) were used for analysis on 8% sodium dodecyl sulfate
(SDS)-polyacrylamide gel containing 0.1% (w/v) gelatin. The gel was
washed with zymogram renaturation buffer (Bio-Rad Laboratories) for
1 h and incubated with zymogram development buffer (Bio-Rad
Laboratories) for 16 h at 37°C in a shaking incubator. The gel was
stained with 0.2% Coomassie Brilliant Blue and gelatinolytic
activity of MMP-9 and MMP-2 were detected as clear bands in a dark
blue background (7).
Invasion assay
Polycarbonate nucleopore filter inserts (8-µm
pore-sized) in a 24-well Transwell chamber (Corning Costar,
Cambridge, MA, USA) were coated with Matrigel (30 µg/well; BD
Biosciences, Franklin Lakes, NJ, USA). Cells (5×104
cells) were added into the upper chamber, and complete medium was
added to the bottom chamber and maintained for 48 h in a 37°C
incubator. Invaded cells on the lower surface of the membrane was
fixed with ethanol and non-invasive cells were removed with a
cotton swab (8). Then, cells were
stained with hematoxylin. Invaded cells from five fields were
counted under a fluorescence microscope (EVOS™ XL Cell Imaging
System; Life Technologies; Thermo Fisher Scientific, Inc.). CM was
diluted 1:1 with complete media and added to the bottom of the
Transwell chamber. Specific inhibitors were also added in the
bottom of Transwell chamber for inhibitor studies. For fascin
expression, cells in the upper and lower chamber were collected at
appropriate assay times and were analyzed.
ECM degradation assay
FITC-conjugated gelatin-coated coverslips were
prepared as previously described (9). Cells (3×103 cells) were
plated on Oregon Green 488 gelatin-coated coverslips in 12-well
plates and cultured for 16 h. Cells were fixed with 4%
paraformaldehyde followed by permeabilization with 0.5% Triton
X-100/PBS and stained for actin with Alexa Fluor 568 phalloidin.
Areas of matrix degradation was identified by a loss of
fluorescence using an EVOS FL monochrome microscope (Thermo Fisher
Scientific Inc.). To quantify invadopodium-mediated ECM
degradation, black and white images of gelatin degradation were
analyzed using ImageJ 1.52a software (National Institutes of
Health).
3D culture
The cancer cells were cultured on a dermal
equivalent that was generated with a Type I-A collagen mixture
(Nitta Gelatin Inc., Osaka, Japan) with eight volumes of ice-cold
collagen solution, one volume of 10X reconstitution solution (0.022
g/ml NaHCO3, 0.0477 g/ml HEPES and 0.05 N NaOH) and one
volume 10X DMEM. Gingiva fibroblast (1.5×105 cells)
suspensions in culture medium were then added. This mixture was
poured onto polycarbonate filter inserts (3-µm pore size, 12-mm
diameter; EMD Millipore) and placed in 6-well plates (Corning
Costar). After a 24-h incubation at 37°C, 3 ml of medium was added
to the 6-well plates. Cancer cells (1×106 cells) from
each cell line were seeded onto the dermal equivalent. After 48 h,
the media in the 6-well plates were changed, and culture media were
added to the epidermal equivalent. After 48 h, the cultures were
exposed to air by removing the medium from the epithelial layer to
generate an air-liquid interface microenvironment. The culture
medium was then changed every 2–3 days for 2 weeks. Each culture
was performed independently three times and then formalin-fixed,
paraffin-embedded and histologically examined. To measure invasive
areas and depth, the culture tissue was stained with hematoxylin
and eosin (H&E) (6).
Luciferase reporter assay
Transcriptional activity of NF-κB was measured by
luciferase reporter assay using the pNF-κB-Luc reporter plasmid
(Clontech Laboratories, Palo Alto, CA, USA). Mock and the
fascin-depleted cell line (fascindep) at 70–80%
confluence in 6-well plate were co-transfected with 1 µg of NF-κB
reporter constructs and 0.5 µg pSV-β-galactosidase for 8 h in
serum- and antibiotic-free Opti-MEM (Gibco-BRL; Themo Fisher
Scientific, Inc.) with Lipofectamine 2000 reagent (Invitrogen;
Themo Fisher Scientific, Inc.). Luciferase and β-galactosidase
activities were assayed according to the manufacturer's protocol
(Promega, Madison, WI, USA), using a microplate spectrofluorometer
(Molecular Devices, Palo Alto, CA, USA). Luciferase activity was
normalized by β-galactosidase activity in cell lysate and expressed
as an average of three independent experiments. pTAL construct was
used as negative control.
Animal study
All animal studies were performed in accordance with
the experimental protocols that were approved by the Animal Ethics
Committee of Eulji University. Male Balb/C athymic nude mice (5
weeks of age, 10 g of body weight; provided by the Central Animal
Laboratory, Seoul, Korea) were maintained at 20–22°C on a 12-h
light/dark cycle. Mock (n=8) and fascin-depleted cells
(fascindep) (n=8) (5×104 cells/0.1 ml PBS)
were submucosally injected into the tongues of mice under
anesthesia using a 0.5 ml insulin syringe (n=5/group). Growth of
the tumor xenografts in mice was observed for 5 weeks. Tissues were
collected and fixed in formalin and processed for paraffin
embedding for histopathological studies. The tumor areas in each
tongue were acquired with a fluorescence microscope (EVOS™ XL Cell
Imaging system; Life Technologies; Thermo Fisher Scientific, Inc.)
with a ×40 magnification and digitally quantified using ImageJ
1.52a software (National Institutes of Health).
Statistical analysis
The analysis for statistical purposes was conducted
using InStat GraphPad Prism version 5.01 statistical software
(GraphPad Software, Inc., San Diego, CA, USA). One-way ANOVA and
Tukey's honestly significant difference (HSD) post-hoc test were
applied. The non-parametric Mann-Whitney test was used to evaluate
the western blot analysis. Results are expressed as mean ± standard
deviation (SD). A P-value <0.05 was considered to indicate a
statistically significant result.
Results
Fascin depletion affects cancer
invasion
In a previous study, we performed array-comparative
genomic hybridization (CGH) analysis by separating the surgical
tissue of oral squamous cell carcinoma (OSCC) patients into
carcinoma, dysplasia (marginal tissue) and adjacent normal
epithelial cells (10). In the
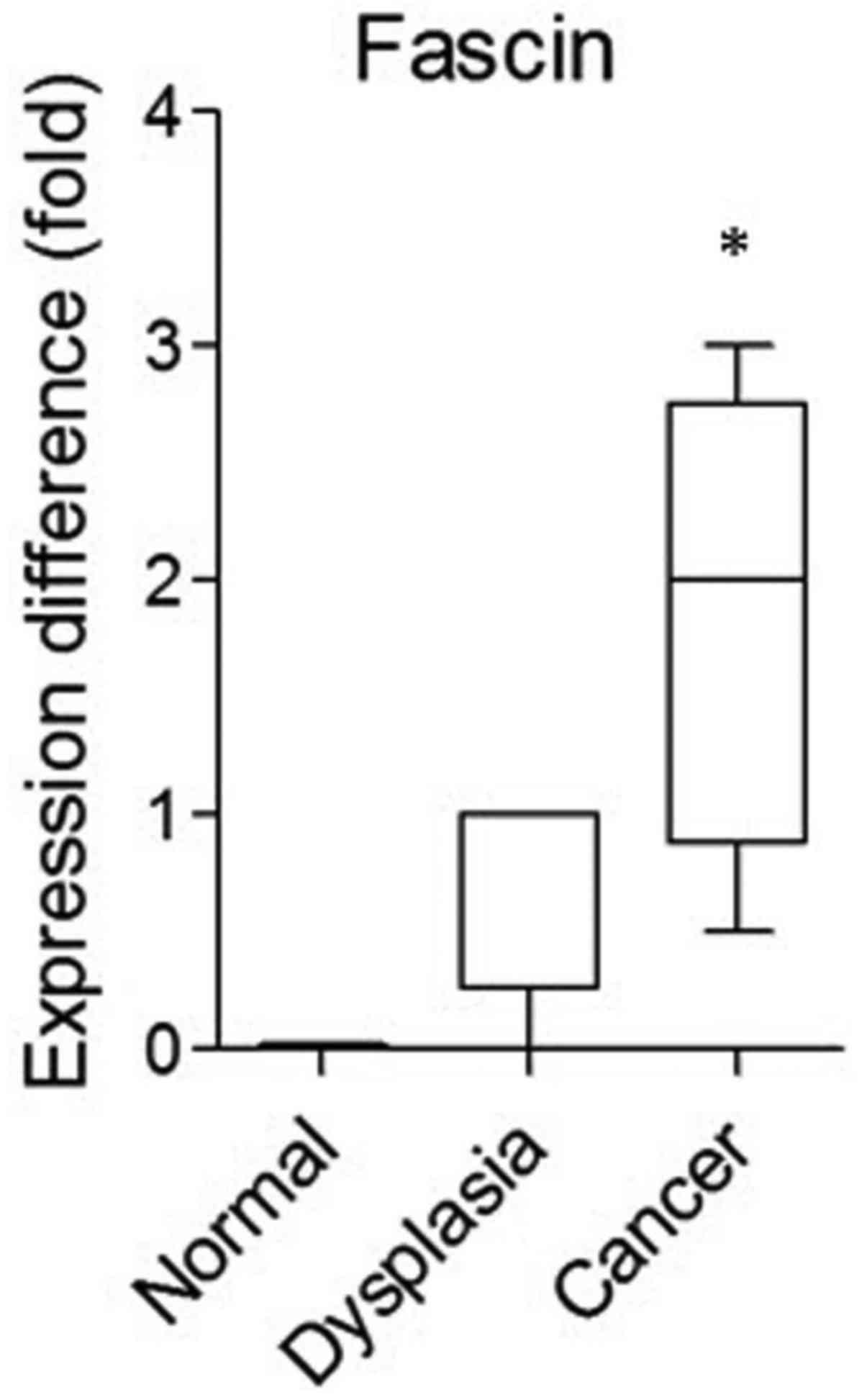
array-CGH data, fascin showed a higher expression level in cancer
specimens than that in normal specimens and dysplasia (Fig. 1). To confirm the role of fascin in
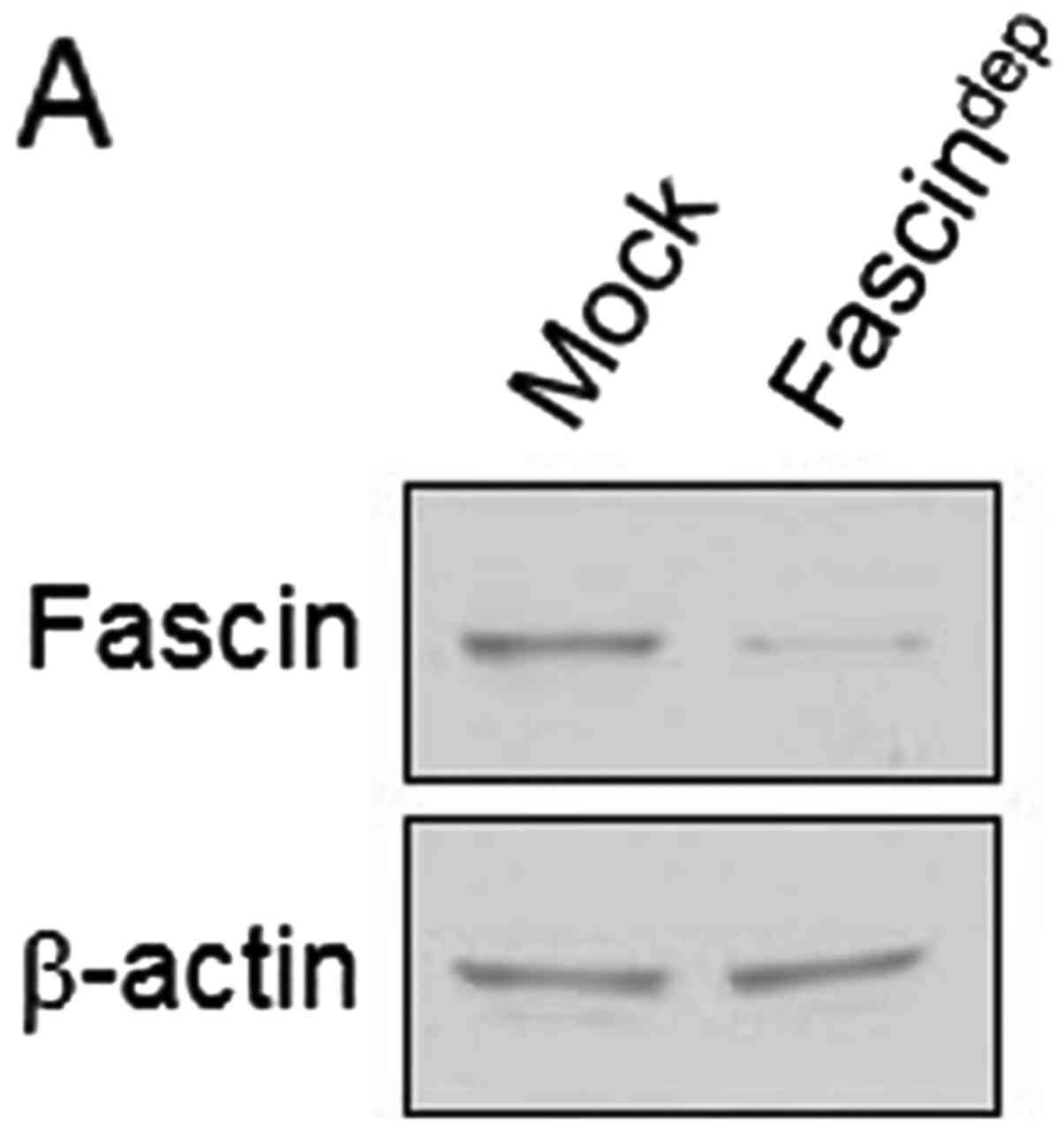
OSCC, fascin depletion was performed with lentiviral short hairpin
RNA (shRNA) against fascin mRNA and a stable cell line
(fascindep) was established (Fig. 2A). The fascindep cell
line had reduced invadopodia spot formation and FITC-gelatin
degradation activity (Fig. 2B).
This result indicates that fascin is involved in invadopodium
formation and extracelluar matrix degradation activity. In the
Matrigel-coated Transwell system, fascindep cells showed
reduced invasion activity compared to wild-type Mock cells
(Fig. 2C), respectively. Invasive
areas into the dermal equivalents were also determined using
three-dimensional (3D) cultures. Fascindep cells
possessed low invasion activity compared to the Mock (Fig. 2D). In the zymography assay,
gelatinolytic activities of pro-MMP-9 and active-MMP-2 in
conditioned media (CM) from fascindep cells were
significantly lower than those in the Mock (Fig. 2E). No statistically significant
changes were observed in the active-MMP-9 and pro-MMP-2
activity.
Fascin depletion affects tumor growth
in mouse xenografts
The effect of fascin on tumor growth was tested in
nude mice with orthotopic tongue tumors. Submucosally inoculated
Mock and fascindep cells were subjected to growth for 5
weeks. As shown in Fig. 3A, tumor
growth was significantly increased in mice inoculated with Mock
cells. However, the tumor area (mm2) was significantly
lower in the mice inoculation with the fascindep cells.
The difference in tumor area between the two groups was
statistically significant (Fig.
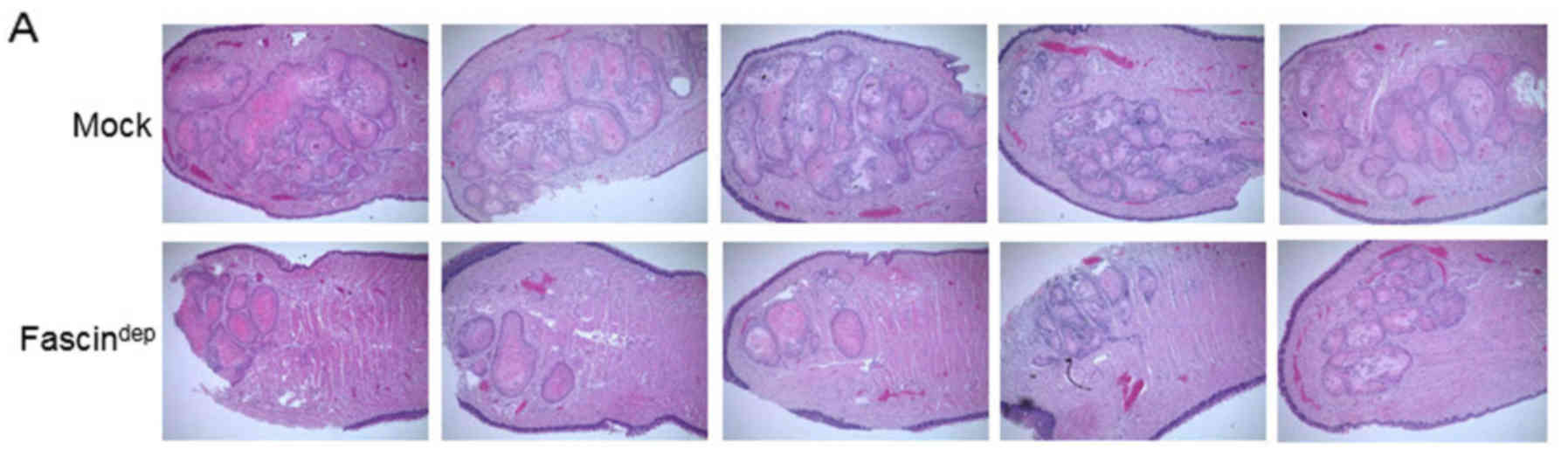
3B). Fascin expression was strongly observed in the invasive
tumor front in the tissue of the Mock group. In the
fascindep group, fascin expression was relatively low
and encapsulated tumor was observed (Fig. 3C).
Fascin expression is regulated by
stromal factors
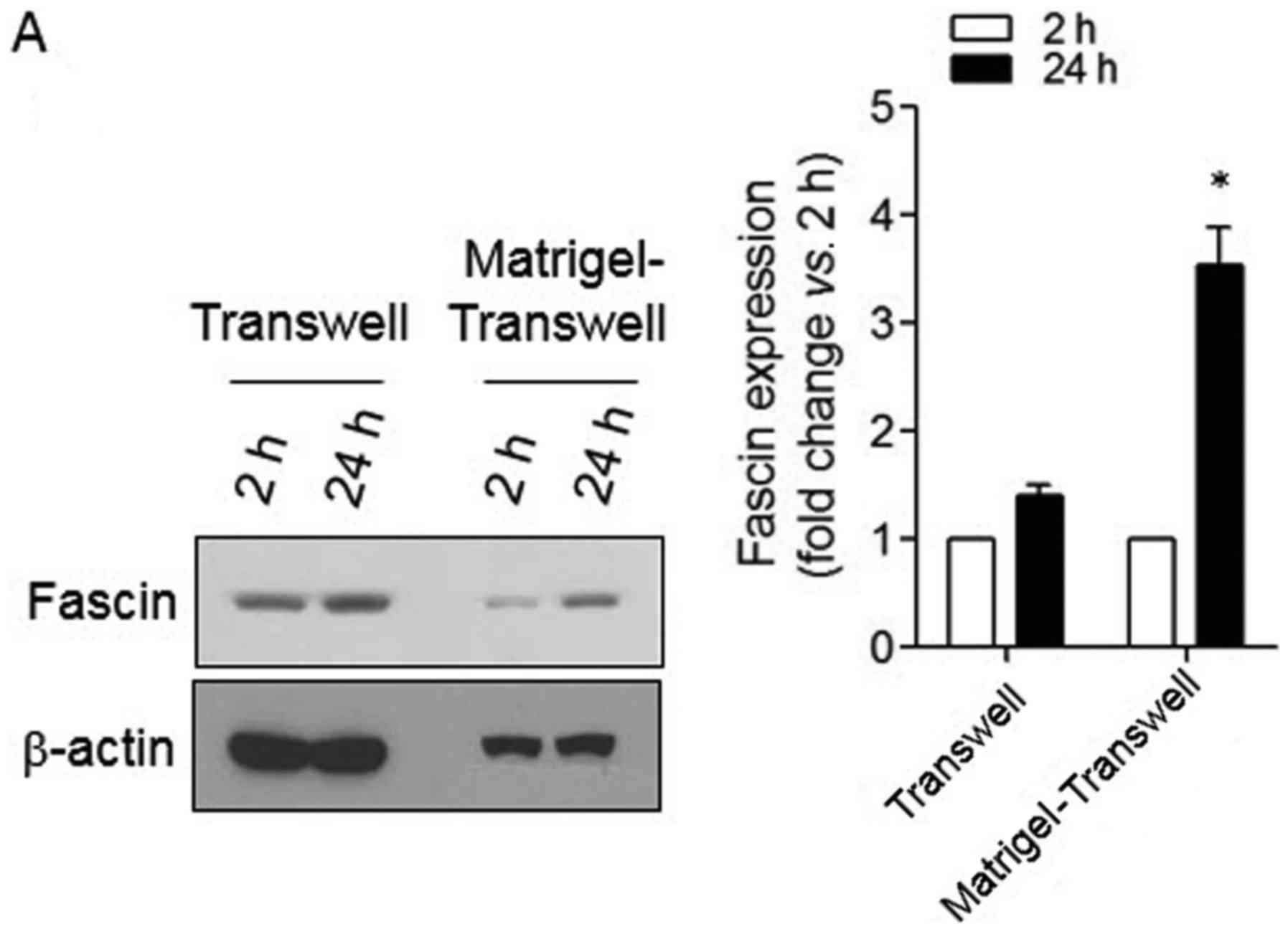
To verify changes in fascin expression during the
infiltration process, a Matrigel-coated Transwell invasion assay
was performed. In Transwell infiltration without Matrigel, there
was no significant change in fascin expression. However, expression
of fascin was increased during Matrigel-coated Transwell
infiltration (Fig. 4A). In
addition, unlike the reaction induced by conditioned media (CM)
from normal fibroblasts (NFs), the expression of fascin was
significantly increased by CM from cancer-derived fibroblasts
(CAFs) (Fig. 4B). These results
indicate that the expression of fascin which occurs during invasion
is regulated by the microenvironment surrounding the cancer. In
order to investigate the mechanism involved in fascin expression,
various molecules derived from the tumor microenvironment were used
to treat cancer cells and fascin expression was examined (Fig. 4C). Lysophosphatidic acid (LPA), a
phospholipid derivative that acts as a signaling molecule, was not
involved in fascin expression. However, stimulation by TGF-β1, EGF,
or IL-1 β significantly increased fascin expression. Selective
inhibitor SB431542 (TGF- β RI kinase inhibitor), AG1478 (EGF
receptor tyrosine kinase inhibitor), or IL-1 receptor (IL-1R)
antagonist inhibited Matrigel-induced-fascin expression (Fig. 4D). CAF stimulated-fascin expression
was also diminished by treatment with SB431542, AG1478, or IL-1
receptor antagonist (Fig. 4E).
RhoA and NF-κB signaling are involved
in fascin expression
The RhoA-NF-κB signaling axis plays a pivotal role
in cancer invasion and motility (11). RhoA, a small GTPase protein in the
Rho family, affects the invasive behavior of tumor cells by
stimulating actin polymerization at the leading edge (12). Constitutively active RhoA activates
NF-κB activity during invasion (13). The YD-10B OSCC cell line used in
this study is a highly invasive cancer cell line with originally
very low E-cadherin levels (6). In
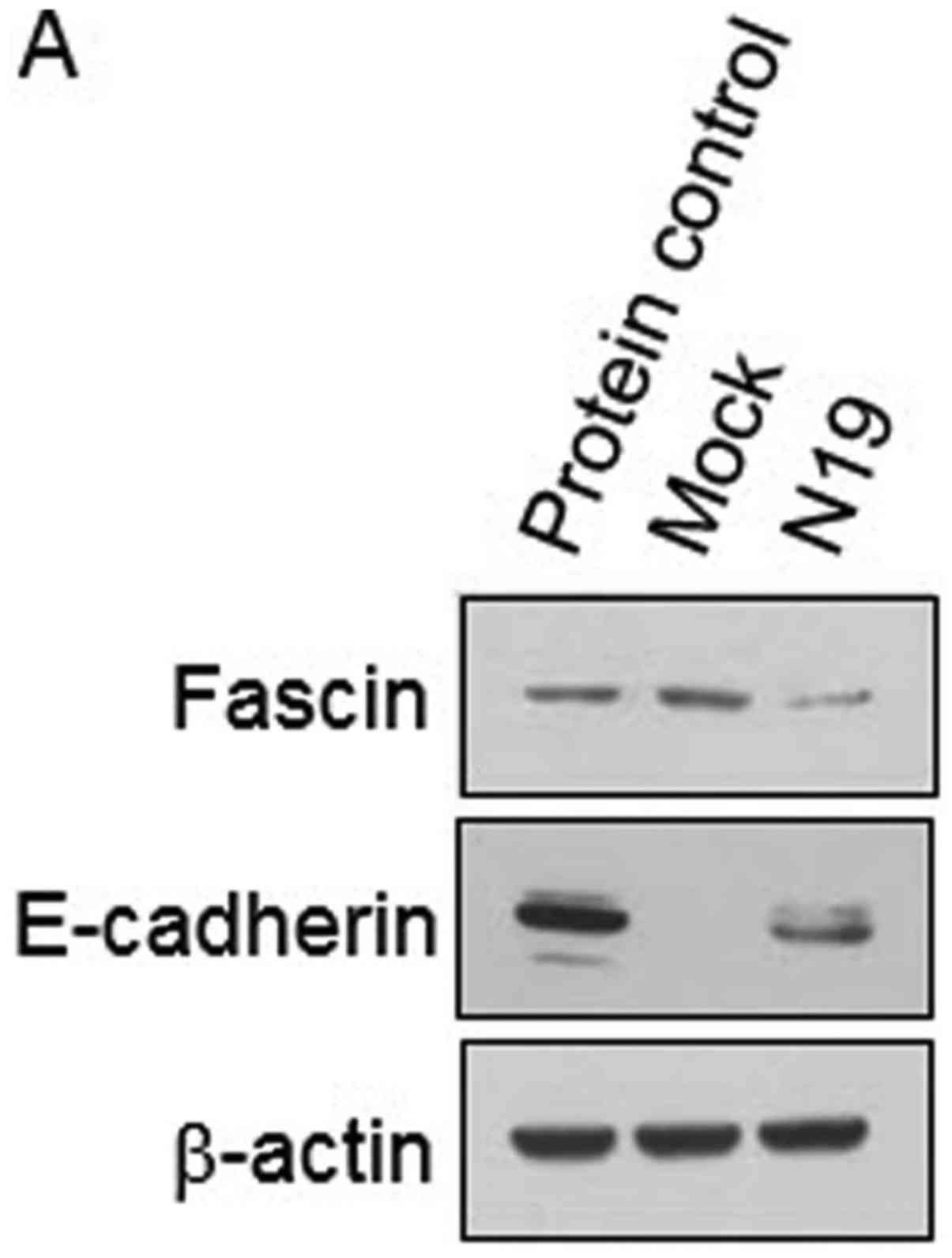
a stable cell line with dominant-negative RhoA N19, fascin
expression was relatively suppressed compared to that in the Mock
while the expression of epithelial phenotype marker E-cadherin was
increased (Fig. 5A). In addition,
IκBα an inhibitory molecule of NF-κB showed low phosphorylation
levels in the fascindep cells (Fig. 5B). Consistent with these results,
nuclear translocation levels of the p50 and p65 NF-κB subunit were
decreased in the fascindep cell line compared to those
in the Mock (Fig. 5C). Relatively
low activity of NF-κB was found in the fascindep cells
using a NF-κB luciferase reporter (Fig.
5D). These results indicate that RhoA and IκBα/NF-κB signaling
are involved in fascin expression in OSCC cells.
Discussion
Stroma is a part of tissues or organs with a
structural and connective role. It is composed of basement
membrane, fibroblasts, extracellular matrix, immune cells and
vascular system. Normal stromal tissue maintains homeostasis
through its action to inhibit inflammation and cancer formation.
However, it promotes cancer growth and malignancy in the tumor
environment (13). Cancer cells
produce several factors such as transforming growth factor (TGF)-β
that can induce transdifferentiation of normal fibroblasts to
carcinoma-associated fibroblasts (CAFs), thereby serving as pivotal
inducers of tumor growth, invasion and metastasis (14,15).
CAFs also secrete various chemokines and cytokines that can affect
tumor and other stromal cells, leading to cancer growth and
invasion. In addition, these factors can stimulate angiogenesis
around the tumor and draw bone marrow-derived cells or immune cells
around the tumor (16,17). MMPs as extracellular matrix (ECM)
degrading enzymes are also partly derived from CAFs (18). Therefore, the reciprocal cross-talk
between stromal tissue and the tumor plays a pivotal role in cancer
growth and progression.
Array-comparative genomic hybridization (CGH) can be
used to screen repeated changes of gene fragments in the genome or
to observe quantitative changes in the number of repetitions.
Unlike normal cells, cancer cells have structural abnormalities or
chromosomal micro-deletions, thus array-CGH analysis is used to
identify chromosomal abnormalities in cancer cells (19). In previous array-CGH studies, fascin
showed a higher expression level in cancer specimens than that in
normal specimens and dysplasia (10). Fascin has been reported to be
involved in cancer invasion and has been proposed as a diagnostic
marker for several tumors including breast, prostate and esophageal
squamous cell carcinoma (20–25).
Nevertheless, understanding of mechanisms underlying the
significance of fascin expression in cancer cells is unknown, and
further studies are needed. In the present study, we observed the
role of fascin in cancer cell invasion using a fascin-depleted cell
line and confirmed the expression mechanism of fascin by tumor
stromal factors, thereby demonstrating increased cancer invasion
through stroma-cancer crosstalk. YD-10 OSCC cells showed high
invasive activity in a Matrigel-coated Transwell chamber system. In
this experiment, infiltrated cells through the Transwell membrane
were harvested and electrophoresed and increased fascin expression
was observed compared to that in cells before they were placed in
the Transwell chamber. According to the manufacturer's product
information, Matrigel contains heparan sulfate proteoglycan
(perlecan), TGF-β, epidermal growth factor (EGF), insulin-like
growth factor (IGF), fibroblast growth factor (FGF), tissue
plasminogen activator (tPA), and other growth factors as well as
extracellular matrix proteins, such as laminin, collagen IV and
entactin (https://www.corning.com/media/worldwide/cls/documents/CLS-DL-CC-026%20DL.pdf).
The increased expression of fascin induced by Matrigel during
invasion indicates that fascin expression might be regulated by
stimulation of stromal factors during the invasion process. To
verify this possibility, normal gingival fibroblasts (NFs) and
carcinoma-associated fibroblasts (CAFs) were isolated and fascin
expression induced by the conditioned media from NFs and CAFs was
determined. The experimental results showed that fascin expression
in cancer cells was significantly increased in the culture with
CAFs compared to that in the culture with NFs.
Treatment of cancer cells with growth factors
resulted in increased expression of fascin in this study. It was
observed that the fascin expression level was significantly
increased by TGF-β1, EGF, or IL-1β. The increased expression of
fascin was inhibited by specific inhibitors of these growth
factors. Increased expression of fascin by TGF-β1 has also been
reported in gastric cancer as a Smad3 phosphorylation-dependent
response (26). In addition, fascin
expression by TGF-β1 Smad4 signal was abrogated through inhibition
of DNA binding of Smad4 by transcription factor GATA3 in breast
cancer cells (27). We observed
that fascin had a low expression level in a dominant-negative RhoA
(N19) cell line. In addition, the fascin-depleted cell line
(fascindep) showed a low phosphorylation level of IκBα
and translocation of NF-κB subunit into the nucleus.
Phosphorylation of inhibitory subunit (IκBα) leads to translocation
of NF-κB to the nucleus (11).
Al-Tweigeri et al reported that fascin is a key regulator of
breast cancer invasion and NF-κB can enhance its activity (28). Fascin-deleted cells had low NF-κB
luciferase reporter activity after TNF-α stimulation whereas fascin
overexpressed cells showed significantly increased NF-κB luciferase
activity after TNF-α stimulation. Moreover, in fascin-positive
colon carcinoma cells, cAMP response element-binding protein (CREB)
and aryl hydrocarbon receptor (AhR) were associated with the FSCN1
promoter region (−219/+114) and involved in the regulation of
fascin transcription (29). These
results indicate that fascin expression can be controlled through
multiple stimuli and intracellular signaling pathways during the
invasion process. RhoA and IκBα/NF-κB signal are also involved as
intracellular signals in fascin expression.
In order to understand cellular communication in
vivo, it is important to mimic an in vivo cellular
environment in vitro. Three-dimensional (3D) collagen matrix
method is widely used to reproduce the biological environment in
vitro (30). Various cellular
changes such as cell invasion, differentiation, survival, and
growth can be examined with this method. In the persent study, we
also observed the influence of fascin depletion on invasion
activity of cancer cells using 3D culture. Unlike the wild-type
Mock in which the cell mass deeply invaded into the matrix, the
fascindep cells showed low invasion activity and limited
infiltration with only a few single cells. We also found that
fascin was important for tumor growth through a xenograft model in
mice using the fascindep cell line.
In conclusion, through stimulation with CAFs and
growth factors, we demonstrated that fascin expression was
regulated by stromal factors of the microenvironment surrounding
the tumor. Gene depletion studies showed that fascin is a critical
factor for cancer growth and invasion. Therefore, more research is
needed to understand the reciprocal crosstalk of cancer-stroma as
the cancer microenvironment is an important target for
understanding cancer biology.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education, Science and Technology
(nos. 2015R1D1A1A01056946 and 2018R1D1A1B07042035).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
YSH, XZ and IHC designed the research, analyzed the
data, wrote and revised the manuscript. YSH, XZ, IHC, JHP and MKL
performed the experiments. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
All studies were performed in accordance with
experimental protocols that were approved by the Animal Ethics
Committee of Eulji University and by the Institutional Review Board
of Yonsei University College of Dentistry. The samples were used
according to ethical standards.
Patient consent for publication
Not applicable.
Competing interests
The authors state that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
OSCC
|
oral squamous cell carcinoma
|
|
CM
|
conditioned media
|
|
NF
|
normal fibroblast
|
|
CAF
|
cancer-associated fibroblast
|
|
MMP
|
matrix metalloprotease
|
|
Fascindep
|
fascin-depleted cells
|
References
|
1
|
Guan X: Cancer metastases: Challenges and
opportunities. Acta Pharm Sin B. 5:402–418. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eddy RJ, Weidmann MD, Sharma VP and
Condeelis JS: Tumor cell invadopodia: Invasive protrusions that
orchestrate metastasis. Trends Cell Biol. 27:595–607. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brabletz T, Kalluri R, Nieto MA and
Weinberg RA: EMT in cancer. Nat Rev Cancer. 18:128–134. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gould CM and Courtneidge SA: Regulation of
invadopodia by the tumor microenvironment. Cell Adh Migr.
8:226–235. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li A, Dawson JC, Forero-Vargas M, Spence
HJ, Yu X, König I, Anderson K and Machesky LM: The actin-bundling
protein fascin stabilizes actin in invadopodia and potentiates
protrusive invasion. Curr Biol. 20:339–345. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim EJ, Che ZM, Park YJ, Hwang YS, Kim KY,
Jung DW, Jeon NK, Choi YW, Lee EJ and Kim J: Morphogenesis and
biological significance of spindle cell transformation in a spindle
cell carcinoma. Cancer Lett. 275:61–71. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Toth M, Sohail A and Fridman R: Assessment
of gelatinases (MMP-2 and MMP-9) by gelatin zymography. Methods Mol
Biol. 878:121–135. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hwang YS, Park KK and Chung WY: Stromal
transforming growth factor-beta 1 is crucial for reinforcing the
invasive potential of low invasive cancer. Arch Oral Biol.
59:687–694. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bowden ET, Coopman PJ and Mueller SC:
Invadopodia: Unique methods for measurement of extracellular matrix
degradation in vitro. Methods Cell Biol. 63:613–627. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hwang YS, Park KK, Cha IH, Kim J and Chung
WY: Role of insulin-like growth factor-II mRNA-binding protein-3 in
invadopodia formation and the growth of oral squamous cell
carcinoma in athymic nude mice. Head Neck. 34:1329–1339. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hodge JC, Bub J, Kaul S, Kajdacsy-Balla A
and Lindholm PF: Requirement of RhoA activity for increased nuclear
factor kappaB activity and PC-3 human prostate cancer cell
invasion. Cancer Res. 63:1359–1364. 2003.PubMed/NCBI
|
|
12
|
Struckhoff AP, Rana MK and Worthylake RA:
RhoA can lead the way in tumor cell invasion and metastasis. Front
Biosci (Landmark Ed). 16:1915–1926. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bremnes RM, Dønnem T, Al-Saad S, Al-Shibli
K, Andersen S, Sirera R, Camps C, Marinez I and Busund LT: The role
of tumor stroma in cancer progression and prognosis: Emphasis on
carcinoma-associated fibroblasts and non-small cell lung cancer. J
Thorac Oncol. 6:209–217. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shiga K, Hara M, Nagasaki T, Sato T,
Takahashi H and Takeyama H: Cancer-associated fibroblasts: Their
characteristics and their roles in tumor growth. Cancers.
7:2443–2458. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
De Wever O and Mareel M: Role of tissue
stroma in cancer cell invasion. J Pathol. 200:429–447. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li J, Jia Z, Kong J, Zhang F, Fang S, Li
X, Li W, Yang X, Luo Y, Lin B, et al: Carcinoma-associated
fibroblasts lead the invasion of salivary gland adenoid cystic
carcinoma cells by creating an invasive track. PLoS One.
11:e01502472016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wen S, Niu Y, Yeh S and Chang C: BM-MSCs
promote prostate cancer progression via the conversion of normal
fibroblasts to cancer-associated fibroblasts. Int J Oncol.
47:719–727. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Taniwaki K, Fukamachi H, Komori K, Ohtake
Y, Nonaka T, Sakamoto T, Shiomi T, Okada Y, Itoh T, Itohara S, et
al: Stroma-derived matrix metalloproteinase (MMP)-2 promotes
membrane type 1-MMP-dependent tumor growth in mice. Cancer Res.
67:4311–4319. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
van Beers EH and Nederlof PM: Array-CGH
and breast cancer. Breast Cancer Res. 8:2102006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Al-Alwan M, Olabi S, Ghebeh H, Barhoush E,
Tulbah A, Al-Tweigeri T, Ajarim D and Adra C: Fascin is a key
regulator of breast cancer invasion that acts via the modification
of metastasis-associated molecules. PLoS One. 6:e273392011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang FK, Han S, Xing B, Huang J, Liu B,
Bordeleau F, Reinhart-King CA, Zhang JJ and Huang XY: Targeted
inhibition of fascin function blocks tumour invasion and metastatic
colonization. Nat Commun. 6:74652015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Min KW, Chae SW, Kim DH, Do SI, Kim K, Lee
HJ, Sohn JH, Pyo JS, Kim DH, Oh SJ, et al: Fascin expression
predicts an aggressive clinical course in patients with advanced
breast cancer. Oncol Lett. 10:121–130. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Darnel AD, Behmoaram E, Vollmer RT, Corcos
J, Bijian K, Sircar K, Su J, Jiao J, Alaoui-Jamali MA and Bismar
TA: Fascin regulates prostate cancer cell invasion and is
associated with metastasis and biochemical failure in prostate
cancer. Clin Cancer Res. 15:1376–1383. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang CQ, Tang CH, Chang HT, Li XN, Zhao
YM, Su CM, Hu GN, Zhang T, Sun XX, Zeng Y, et al: Fascin-1 as a
novel diagnostic marker of triple-negative breast cancer. Cancer
Med. 5:1983–1988. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Takikita M, Hu N, Shou JZ, Giffen C, Wang
QH, Wang C, Hewitt SM and Taylor PR: Fascin and CK4 as biomarkers
for esophageal squamous cell carcinoma. Anticancer Res. 31:945–952.
2011.PubMed/NCBI
|
|
26
|
Li L, Cao F, Liu B, Luo X, Ma X and Hu Z:
TGF-β induces fascin expression in gastric cancer via
phosphorylation of smad3 linker area. Am J Cancer Res. 5:1890–1896.
2015.PubMed/NCBI
|
|
27
|
Sun J, He H, Pillai S, Xiong Y, Challa S,
Xu L, Chellappan S and Yang S: GATA3 transcription factor abrogates
Smad4 transcription factor-mediated fascin overexpression,
invadopodium formation, and breast cancer cell invasion. J Biol
Chem. 288:36971–36982. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ghebeh H, Al-Khaldi S, Olabi S, Al-Dhfyan
A, Al-Mohanna F, Barnawi R, Tulbah A, Al-Tweigeri T, Ajarim D and
Al-Alwan M: Fascin is involved in the chemotherapeutic resistance
of breast cancer cells predominantly via the PI3K/Akt pathway. Br J
Cancer. 111:1552–1561. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hashimoto Y, Loftis DW and Adams JC:
Fascin-1 promoter activity is regulated by CREB and the aryl
hydrocarbon receptor in human carcinoma cells. PLoS One.
4:e51302009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Langhans SA: Three-dimensional in vitro
cell culture models in drug discovery and drug repositioning. Front
Pharmacol. 9:62018. View Article : Google Scholar : PubMed/NCBI
|