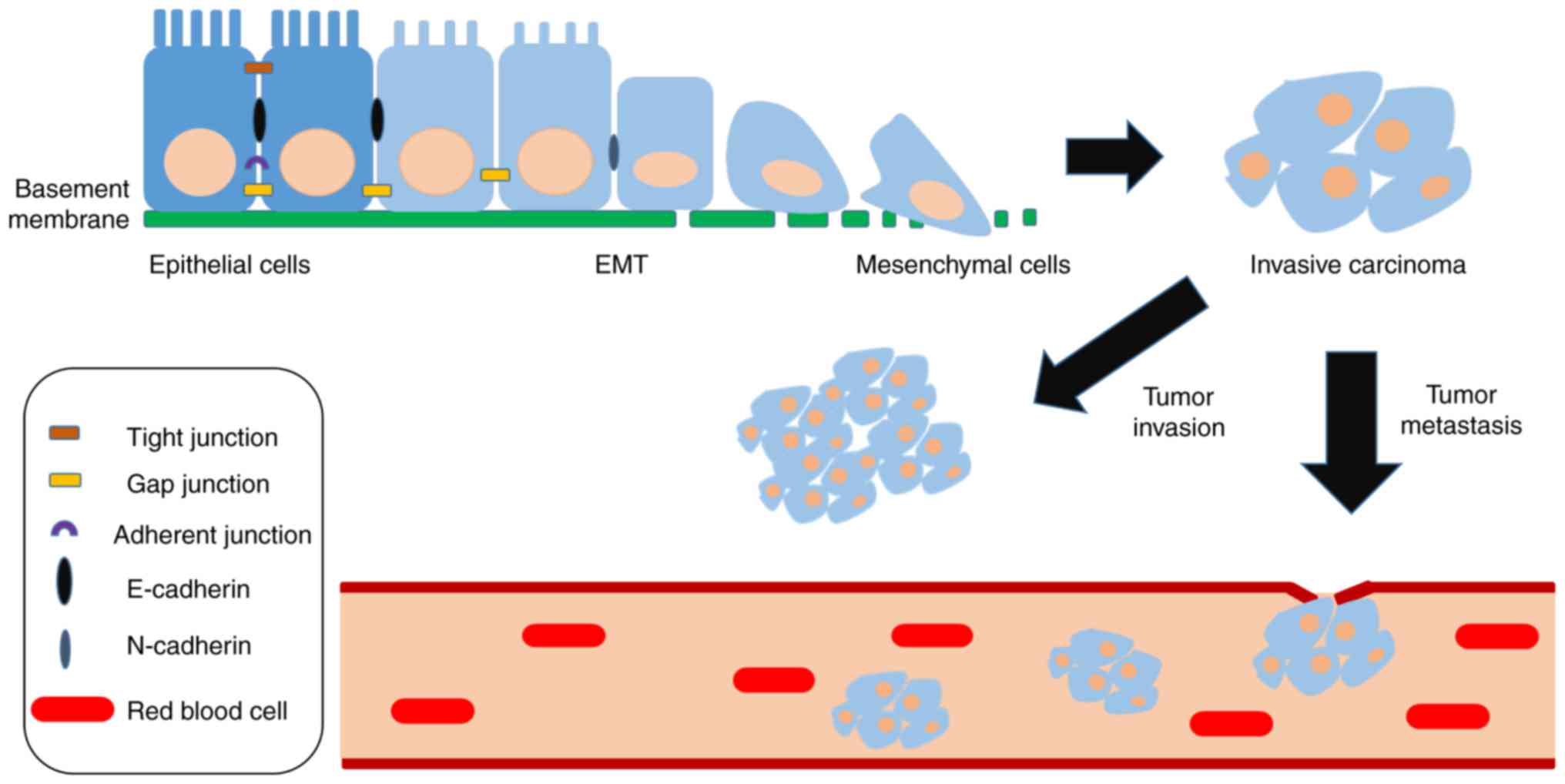
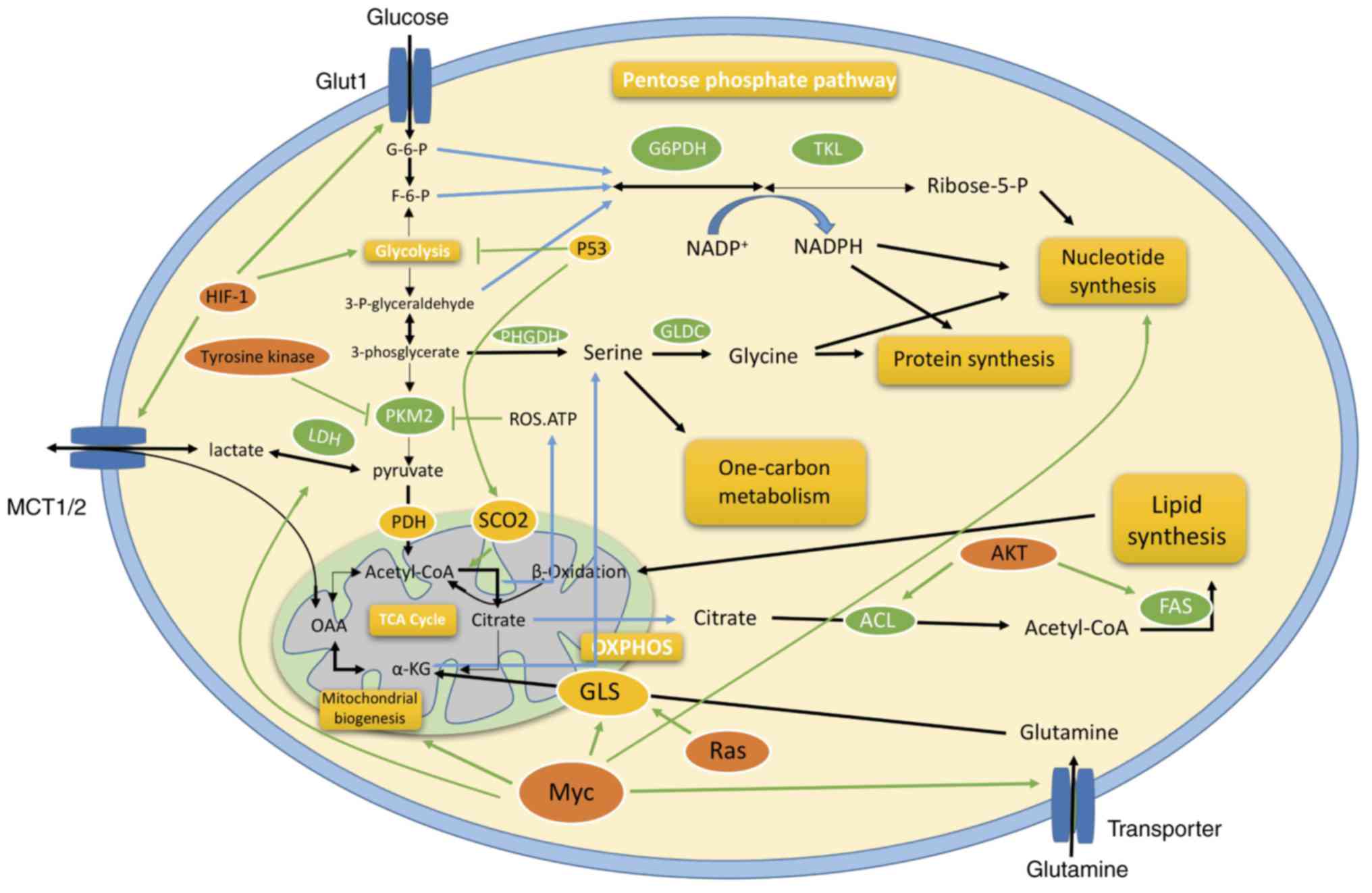
|
1
|
Kroemer G and Pouyssegur J: Tumor cell
metabolism: Cancer's Achilles' heel. Cancer Cell. 13:472–482. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mayevsky A: Mitochondrial function and
energy metabolism in cancer cells: Past overview and future
perspectives. Mitochondrion. 9:165–179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Walenta S and Mueller-Klieser WF: Lactate:
Mirror and motor of tumor malignancy. Semin Radiat Oncol.
14:267–274. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sakamoto S, Tsukada K, Sagara T, Kawachi
Y, Murakami S and Iwama T: Human colorectal malignancy and oral
UFT. Anticancer Res. 22:339–341. 2002.PubMed/NCBI
|
|
5
|
Santos CR and Schulze A: Lipid metabolism
in cancer. FEBS J. 279:2610–2623. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Furuta E, Okuda H, Kobayashi A and Watabe
K: Metabolic genes in cancer: Their roles in tumor progression and
clinical implications. Biochim Biophys Acta. 1805:141–152.
2010.PubMed/NCBI
|
|
7
|
Martin RM, Vatten L, Gunnell D, Romundstad
P and Nilsen TI: Components of the metabolic syndrome and risk of
prostate cancer: The HUNT 2 cohort, Norway. Cancer Causes Control.
20:1181–1192. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Siegel AB and Zhu AX: Metabolic syndrome
and hepatocellular carcinoma: Two growing epidemics with a
potential link. Cancer. 115:5651–5661. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stocks T, Lukanova A, Johansson M, Rinaldi
S, Palmqvist R, Hallmans G, Kaaks R and Stattin P: Components of
the metabolic syndrome and colorectal cancer risk; A prospective
study. Int J Obes. 32:304–314. 2008. View Article : Google Scholar
|
|
10
|
Kabat GC, Kim M, Chlebowski RT, Khandekar
J, Ko MG, McTiernan A, Neuhouser ML, Parker DR, Shikany JM,
Stefanick ML, et al: A longitudinal study of the metabolic syndrome
and risk of postmenopausal breast cancer. Cancer Epidemiol
Biomarkers Prev. 18:2046–2053. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hugo H, Ackland ML, Blick T, Lawrence MG,
Clements JA, Williams ED and Thompson EW: Epithelial-mesenchymal
and mesenchymal-epithelial transitions in carcinoma progression. J
Cell Physiol. 213:374–383. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Foroni C, Broggini M, Generali D and Damia
G: Epithelial-mesenchymal transition and breast cancer: Role,
molecular mechanisms and clinical impact. Cancer Treat Rev.
38:689–697. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thiery JP: Epithelial-mesenchymal
transition in development and pathologies. Curr Opin Cell Biol.
15:740–746. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yilmaz M and Christofori G: EMT, the
cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev.
28:15–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Christofori G and Semb H: The role of the
cell-adhesion molecule E-cadherin a tumour-suppresor gene. Trends
Biochemci Sci. 24:73–76. 1999. View Article : Google Scholar
|
|
16
|
Hiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Boyer B, Valls AM and Edme N: Induction
and regulation of epithelial-mesenchymal transitions. Biochem
Pharmacol. 60:1091–1099. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Saitoh M and Miyazawa K: Transcriptional
and post-transcriptional regulation in TGF-β-mediated
epithelial-mesenchymal transition. J Biochem. 151:563–71. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu J, Lamouille S and Derynck R:
TGF-beta-induced epithelial to mesenchymal transition. Cell Res.
19:156–172. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gradl D, Kuhl M and Wedlich D: The Wnt/Wg
signal transducer beta-catenin controls fibronectin expression. Mol
Cell Biol. 19:5576–5587. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gilles C, Polette M, Mestdagt M,
Nawrocki-Raby B, Ruggeri P, Birembaut P and Foidart JM:
Transactivation of vimentin by beta-catenin in human breast cancer
cells. Cancer Res. 63:2658–2664. 2003.PubMed/NCBI
|
|
22
|
Wang Z, Li Y, Kong D and Sarkar FH: The
role of Notch signaling pathway in epithelial-mesenchymal
transition (EMT) during development and tumor aggressiveness. Curr
Drug Targets. 11:745–751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kume T: The role of FoxC2 transcription
factor in tumor angiogenesis. J Oncol. 2012:2045932012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Harris TJ and Tepass U: Adherens
junctions: From molecules to morphogenesis. Nat Rev Mol Cell Biol.
11:502–514. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sullivan NJ, Sasser AK, Axel AE, Vesuna F,
Raman V, Ramirez N, Oberyszyn TM and Hall BM: Interleukin-6 induces
an epithelial-mesenchymal transition phenotype in human breast
cancer cells. Oncogene. 28:2940–2947. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cheng GZ, Zhang WZ, Sun M, Wang Q, Coppola
D, Mansour M, Xu LM, Costanzo C, Cheng JQ and Wang LH: Twist is
transcriptionally induced by activation of STAT3 and mediates STAT3
oncogenic function. J Biol Chem. 283:14665–14673. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cairns R, Harris IS and Mak TW: Regulation
of cancer cell metabolism. Nat Rev Cancer. 11:85–95. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hanahan D and Weinberg R: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Christiansen JJ and Rajasekaran AK:
Reassessing epithelial to mesenchymal transition as a prerequisite
for carcinoma invasion and metastasis. Cancer Res. 66:8319–8326.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kaartinen V, Voncken JW, Shuler C,
Warburton D, Bu D, Heisterkamp N and Groffen J: Abnormal lung
development and cleft palate in mice lacking TGF-beta-3 indicates
defects of epithelial-mesenchymal interaction. Nat Genet.
11:415–421. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Khoury H, Dankort DL, Sadekova S, Naujokas
MA, Muller WJ and Park M: Distinct tyrosine autophosphorylation
sites mediate induction of epithelial mesenchymal like transition
by an activated ErbB-2/Neu receptor. Oncogene. 20:788–799. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lester RD, Jo M, Montel V, Takimoto S and
Gonias SL: uPAR induces epithelial-mesenchymal transition in
hypoxic breast cancer cells. J Cell Biol. 178:425–436. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gheldof A and Berx G: Cadherins and
epithelial-to-mesenchymal transition. Prog Mol Biol Transl Sci.
116:317–336. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tiwari N, Gheldof A, Tatari M and
Christofori G: EMT as the ultimate survival mechanism of cancer
cells. Semin Cancer Biol. 22:194–207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Takai E, Tan X, Tamori Y, Hirota M, Egami
H and Ogawa M: Correlation of translocation of tight junction
protein Zonula occludens-1 and activation of epidermal growth
factor receptor in the regulation of invasion of pancreatic cancer
cells. Int J Oncol. 27:645–651. 2005.PubMed/NCBI
|
|
36
|
Ryeom SW, Paul D and Goodenough DA:
Truncation mutants of the tight junction protein ZO-1 disrupt
corneal epithelial cell morphology. Mol Biol Cell. 11:1687–1697.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Radisky DC, Levy DD, Littlepage LE, Liu H,
Nelson CM, Fata JE, Leake D, Godden EL, Albertson DG, Nieto MA, et
al: Rac1b and reactive oxygen species mediate MMP-3-induced EMT and
genomic instability. Nature. 436:123–127. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Miyoshi A, Kitajima Y, Sumi K, Sato K,
Hagiwara A, Koga Y and Miyazaki K: Snail and SIP1 increase cancer
invasion by upregulating MMP family in hepatocellular carcinoma
cells. Br J Cancer. 90:1265–1273. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jiang L, Sugiura H, Sugiura H, Huang X,
Ali A, Kuro-o M, Deberardinis RJ and Boothman DA: Metabolic
reprogramming during TGFβ1-induced epithelial-to-mesenchymal
transition. Oncogene. 34:3908–3916. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang H, Liu CY, Zha ZY, Zhao B, Yao J,
Zhao S, Xiong Y, Lei QY and Guan KL: TEAD transcription factors
mediate the function of TAZ in cell growth and
epithelial-mesenchymal transition. J Biol Chem. 284:13355–13362.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sahlgren C, Gustafsson MV, Jin S,
Poellinger L and Lendahl U: Notch signaling mediates
hypoxia-induced tumor cell migration and invasion. Proc Natl Acad
Sci USA. 105:6392–6397. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zheng H and Kang Y: Multilayer control of
the EMT master regulators. Oncogene. 33:1755–1763. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang Y, Shi J, Chai K, Ying X and Zhou BP:
The role of snail in EMT and tumorigenesis. Curr Cancer Drug
Targets. 13:963–972. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lin CY, Tsai PH, Kandaswami CC, Lee PP,
Huang CJ, Hwang JJ and Lee MT: Matrix metalloproteinase-9
cooperates with transcription factor Snail to induce
epithelial-mesenchymal transition. Cancer Sci. 102:815–827. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Haraguchi M, Sato M and Ozawa M:
CRISPR/Cas9n-mediated deletion of the snail 1gene (SNAI1)
reveals its role in regulating cell morphology, cell-cell
interactions, and gene expression in ovarian cancer (RMG-1) cells.
PLoS One. 10:e01322602015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kim NH, Cha YH, Lee J, Lee SH, Yang JH,
Yun JS, Cho ES, Zhang X, Nam M, et al: Snail reprograms glucose
metabolism by repressing phosphofructokinase PFKP allowing cancer
cell survival under metabolic stress. Nat Commun. 8:143742017.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yang F, Sun L, Li Q, Han X, Lei L, Zhang H
and Shang Y: SET8 promotes epithelial-mesenchymal transition and
confers TWIST dual transcriptional activities. EMBO J. 31:110–123.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fang X, Cai Y, Liu J, Wang Z, Wu Q, Zhang
Z, Yang CJ, Yuan L and Ouyang G: Twist2 contributes to breast
cancer progression by promoting an epithelial-mesenchymal
transition and cancer stem-like cell self-renewal. Oncogene.
30:4707–4720. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tania M, Khan MA and Fu J: Epithelial to
mesenchymal transition inducing transcription factors and
metastatic cancer. Tumour Biol. 35:7335–7342. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Gemmill RM, Roche J, Potiron VA, Nasarre
P, Mitas M, Coldren CD, Helfrich BA, Garrett-Mayer E, Bunn PA and
Drabkin HA: ZEB1-responsive genes in non-small cell lung cancer.
Cancer Lett. 300:66–78. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Halldorsson S, Rohatgi N, Magnusdottir M,
Choudhary KS, Gudjonsson T, Knutsen E, Barkovskaya A, Hilmarsdottir
B, Perander M, Mælandsmo GM, et al: Metabolic re-wiring of isogenic
breast epithelial cell lines following epithelial to mesenchymal
transition. Cancer Lett. 396:117–129. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Anastasiou D, Poulogiannis G, Asara JM,
Boxer MB, Jiang JK, Shen M, Bellinger G, Sasaki AT, Locasale JW,
Auld DS, et al: Inhibition of pyruvate kinase M2 by reactive oxygen
species contributes to cellular antioxidant responses. Science.
334:1278–1283. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Cairns RA, Harris IS and Mak TW:
Regulation of cancer cell metabolism. Nat Rev Cancer. 11:85–95.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Dong C, Yuan T, Wu Y, Wang Y, Fan TW,
Miriyala S, Lin Y, Yao J, Shi J, Kang T, et al: Loss of FBP1 by
Snail-mediated repression provides metabolic advantages in
Basal-like breast cancer. Cancer Cell. 23:316–331. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Warburg O, Posener K and Negelein E: Ueber
den stoffwechsel der tumoren. Biochemische Zeitschrif. 152:319–344.
1924.(In German).
|
|
57
|
Porporato PE, Payen VL, Perez-Escuredo J,
De Saedeleer CJ, Danhier P, Copetti T, Dhup S, Tardy M, Vazeille T,
Bouzin C, et al: A mitochondrial switch promotes tumor metastasis.
Cell Rep. 8:754–766. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wood T: Physiological functions of the
pentose phosphate pathway. Cell Biochem Funct. 4:241–247. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Schieber MS and Chandel NS: ROS links
glucose metabolism to breast cancer stem cell and EMT phenotype.
Cancer Cell. 23:265–267. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Liu HW, Zhu X, Zhang J, Zhang XB and Tan
W: A red emitting two-photon fluorescent probe for dynamic imaging
of redox balance meditated by a superoxide anion and GSH in living
cells and tissues. Analyst. 141:5893–5899. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie
MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M, et al: Association
of reactive oxygen species levels and radioresistance in cancer
stem cells. Nature. 458:780–783. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Locasale JW: Serine, glycine and
one-carbon units: Cancer metabolism in full circle. Nat Rev Cancer.
13:572–583. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Luka Z, Mudd SH and Wagner C: Glycine
N-methyltransferase and regulation of
S-adenosylmethionine levels. J Biol Chem. 284:22507–22511.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
McCabe MT, Ott HM, Ganji G, Korenchuk S,
Thompson C, Van Aller GS, Liu Y, Graves AP, Della Pietra A III,
Diaz E, et al: EZH2 inhibition as a therapeutic strategy for
lymphoma with EZH2-activating mutations. Nature. 492:108–112. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Cook CC, Kim A, Terao S, Gotoh A and
Higuchi M: Consumption of oxygen: A mitochondrial generated
progression signal of advanced cancer. Cell Death Dis. 3:e2582012.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Chiche J, Rouleau M, Gounon P,
Brahimi-Horn MC, Pouysségur J and Mazure NM: Hypoxic enlarged
mitochondria protect cancer cells from apoptotic stimuli. J Cell
Physiol. 222:648–657. 2010.PubMed/NCBI
|
|
67
|
Kamarajugadda S, Stemboroski L, Cai Q,
Simpson NE, Nayak S, Tan M and Lu J: Glucose oxidation modulates
anoikis and tumor metastasis. Mol Cell Biol. 32:1893–1907. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Andersen JL and Kornbluth S: Mcl-1 rescues
a glitch in the matrix. Nat Cell Biol. 14:563–565. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Morandi A, Taddei ML, Chiarugi P and
Giannoni E: Targeting the metabolic reprogramming that controls
epithelial-to-mesenchymal transition in aggressive tumors. Front
Oncol. 7:402017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Bonuccelli G, Tsirigos A, Whitaker-Menezes
D, Pavlides S, Pestell RG, Chiavarina B, Frank PG, Flomenberg N,
Howell A, Martinez-Outschoorn UE, et al: Ketones and lactate ‘fuel’
tumor growth and metastasis: Evidence that epithelial cancer cells
use oxidative mitochondrial metabolism. Cell Cycle. 9:3506–3514.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Doherty JR and Cleveland JL: Targeting
lactate metabolism for cancer therapeutics. J Clin Invest.
123:3685–3692. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Liu M, Quek LE, Sultani G and Turner N:
Epithelial-mesenchymal transition induction is associated with
augmented glucose uptake and lactate production in pancreatic
ductal adenocarcinoma. Cancer Metab. 4:192016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Goetze K, Walenta S, Ksiazkiewicz M,
Kunz-Schughart LA and Mueller-Klieser W: Lactate enhances motility
of tumor cells and inhibits monocyte migration and cytokine
release. Int J Oncol. 39:453–463. 2011.PubMed/NCBI
|
|
75
|
Sotnik JL, Lori JC, Rose BJ and Thamm DH:
Glycolysis inhibition by 2-deoxy-D-glucose reverts the metastatic
phenotype in vitro and in vivo. Clin Exp Metastasis. 28:865–875.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Christofk HR, Vander Heiden MG, Harris MH,
Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL and
Cantley LC: The M2 splice isoform of pyruvate kinase is important
for cancer metabolism and tumour growth. Nature. 452:230–233. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
David CJ, Chen M, Assanah M, Canoll P and
Manley JL: HnRNP proteins controlled by c-Myc deregulate pyruvate
kinase mRNA splicing in cancer. Nature. 463:364–368. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Christofk HR, Vander HM, Wu N, Asara JM
and Cantley LC: Pyruvate kinase M2 is a phosphotyrosine-binding
protein. Nature. 452:181–186. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Hittosugi T, Kang S, Vander Heiden MG,
Chung TW, Elf S, Lythgoe K, Dong S, Lonial S, Wang X, Chen GZ, et
al: Tyrosine phosphorylation inhibits PKM2 to promote the Warburg
effect and tumor growth. Sci Signal. 2:ra732009. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Luo W, Hu H, Chang R, Zhong J, Knabel M,
O'Meally R, Cole RN, Pandey A and Semenza GL: Pyruvate kinase M2 is
a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell.
145:232–744. 2011. View Article : Google Scholar
|
|
81
|
Elisa G, Maria LT, Andrea M, Comito G,
Calvani M, Bianchini F, Richichi B, Raugei G, Wong N, Tang D and
Chiarugi P: Targeting stromal-induced pyruvate kinase M2 nuclear
translocation impairs oxphos and prostate cancer metastatic spread.
Oncotarget. 6:24061–24074. 2015.PubMed/NCBI
|
|
82
|
Zhan C, Shi Y, Lu C and Wang Q: Pyruvate
kinase M2 is highly correlated with the differentiation and the
prognosis of esophageal squamous cell cancer. Dis Esophagus.
26:746–753. 2013.PubMed/NCBI
|
|
83
|
Li J, Yang Z, Zou Q, Yuan Y, Li J, Liang
L, Zeng G and Chen S: PKM2 and ACVR 1C are prognostic markers for
poor prognosis of gallbladder cancer. Clin Transl Oncol.
16:200–207. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Feng C, Gao Y, Wang C, Yu X, Zhang W, Guan
H, Shan Z and Teng W: Aberrant overexpression of pyruvate kinase M2
is associated with aggressive tumor features and the BRAF
mutation in papillary thyroid cancer. J Clin Endocrinol Metab.
98:E1524–E1533. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Goldberg MS and Sharp PA: Pyruvate kinase
M2-specific siRNA induces apoptosis and tumor regression. J Exp
Med. 209:217–224. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Yoo YG, Christensen J, Gu J and Huang LE:
HIF-1α mediates tumor hypoxia to confer a perpetual mesenchymal
phenotype for malignant progression. Sci Signal. 4:pt42011.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Düvel K, Yecies JL, Menon S, Raman P,
Lipovsky AI, Souza AL, Triantafellow E, Ma Q, Gorski R, Cleaver S,
et al: Activation of a metabolic gene regulatory network down
stream of m TOR complex 1. Mol Cell. 39:171–183. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Icard P, Kafara P, Steyaert JM, Schwartz L
and Lincet H: The metabolic cooperation between cells in solid
cancer tumors. Biochim Biophys Acta. 1846:216–225. 2014.PubMed/NCBI
|
|
89
|
Wu CY, Tsai YP, Wu MZ, Teng SC and Wu KJ:
Epigenetic reprogramming and post-transcriptional regulation during
the epithelial-mesenchymal transition. Trends Genet. 28:454–463.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Song IS, Wang AG, Yoon SY, Kim JM, Kim JH,
Lee DS and Kim NS: Regulation of glucose metabolism-related genes
and VEGF by HIF-1alpha and HIF-1beta, but not HIF-2alpha, in
gastric cancer. Exp Mol Med. 41:51–58. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Zhao T, Zhu Y, Morinibu A, Kobayashi M,
Shinomiya K, Itasaka S, Yoshimura M, Guo G, Hiraoka M and Harada H:
HIF-1-mediated metabolic reprogramming reduces ROS levels and
facilitates the metastatic colonization of cancers in lungs. Sci
Rep. 4:37932014. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Psaila B and Lyden D: The metastatic
niche: Adapting the foreign soil. Nat Rev Cancer. 9:285–293. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Erler JT, Bennewith KL, Cox TR, Lang G,
Bird D, Koong A, Le QT and Giaccia AJ: Hypoxia-induced lysyl
oxidase is a critical mediator of bone marrow cell recruitment to
form the premetastatic niche. Cancer Cell. 15:35–44. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Ippolito L, Marini A, Cavallini L, Morandi
A, Pietrovito L, Pintus G, Giannoni E, Schrader T, Puhr M, Chiarugi
P and Taddei ML: Metabolic shift toward oxidative phosphorylation
in docetaxel resistant prostate cancer cells. Oncotarget.
7:61890–61904. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Mashek DG and Coleman RA: Cellular fatty
acid uptake: The contribution of metabolism. Curr Opin Lipidol.
17:274–278. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Zechner R, Strauss JG, Haemmerle G, Lass A
and Zimmermann R: Lipolysis: Pathway under construction. Curr Opin
Lipidol. 16:333–340. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Nieman KM, Kenny HA, Penicka CV, Ladanyi
A, Buell-Gutbrod R, Zillhardt MR, Romero IL, Carey MS, Mills GB,
Hotamisligil GS, et al: Adipocytes promote ovarian cancer
metastasis and provide energy for rapid tumor growth. Nat Med.
17:1498–1503. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Menendez JA, Vellon L, Oza BP and Lupu R:
Does endogenous fatty acid metabolism allow cancer cells to sense
hypoxia and mediate hypoxic vasodilatation? Characterization of a
novel molecular connection between fatty acid synthase (FAS) and
hypoxia-inducible factor-1alpha (HIF-1alpha)-related expression of
vascular endothelial growth factor (VEGF) in cancer cells
overexpressing her-2/neu oncogene. J Cell Biochem. 94:857–863.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Swierczyński J and Sledziński T: Metabolic
and regulatory function of fatty acid synthase. Postepy Biochem.
58:175–185. 2012.(In Polish). PubMed/NCBI
|
|
100
|
Jeong NY, Lee JS, Yoo KS, Oh S, Choe E,
Lee HJ, Park BS, Choi YH and Yoo YH: Fatty acid synthase inhibitor
cerulenin inhibits topoisomerase I catalytic activity and augments
SN-38-induced apoptosis. Apoptosis. 18:226–237. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Fujita Y, Krause G, Scheffner M, Zechner
D, Leddy HE, Behrens J, Sommer T and Birchmeier W: Hakai, a
c-Cbl-like protein, ubiquitinates and induces endocytosis of the
E-cadherin complex. Nat Cell Biol. 4:222–231. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Li J, Dong L, Wei D, Wang X, Zhang S and
Li H: Fatty acid synthase mediates the epithelial-mesenchymal
transition of breast cancer cells. Int J Biol Sci. 10:171–180.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Rakheja D, Kapur P, Hoang MP, Roy LC and
Bennett MJ: Increased ratio of saturated to unsaturated C18 fatty
acids in colonic adenocarcinoma: Implications for cryotherapy and
lipid raft function. Med Hypotheses. 65:1120–1123. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Ikonnen E: Roles of lipid rafts in
membrane transport. Curr Oppin Cell Biol. 13:470–477. 2001.
View Article : Google Scholar
|
|
105
|
Russell DW: The enzymes, regulation, and
genetics of bile acid synthesis. Annu Rev Biochem. 72:137–174.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Pelton K, Freeman MR and Solomon KR:
Cholesterol and cancer. Curr Opin Pharmacol. 12:751–759. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Kitahara CM, Berrington de González A,
Freeman ND, Huxley R, Mok Y, Jee SH and Samet JM: Total cholesterol
and cancer risk in a large prospective study in Korea. J Clin
Oncol. 29:1592–1598. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Alikhani N, Ferguson RD, Novosyadlyy R,
Gallagher EJ, Scheinman EJ, Yakar S and LeRoith D: Mammary tumor
growth and pulmonary metastasis are enhanced in a hyperlipidemic
mouse model. Oncogene. 32:961–967. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Nielsen SF, Nordestgaard BG and Bojesen
SE: Statin use and reduced cancer-related mortality. N Engl J Med.
367:1792–1802. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Taras D, Blanc JF, Rullier A, Dugot-Senant
N, Laurendeau I, Vidaud M and Rosenbaum J: Pravastatin reduces lung
metastasis of rat hepatocellular carcinoma via a coordinated
decrease of MMP expression and activity. J Hepatol. 46:69–76. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Zhang J, Yang Z, Xie L, Xu L, Xu D and Liu
X: Statins, autophagy and cancer metastasis. Int J Biochem Cell
BIol. 45:745–752. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Roy M, Kung HJ and Ghosh PM: Statins and
prostate cancer: Role of cholesterol inhibition vs. prevention of
small GTP-binding proteins. Am J Cancer Res. 1:542–561.
2011.PubMed/NCBI
|
|
113
|
Simons K and Ikonen E: Functional rafts in
cell membranes. Nature. 387:569–572. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Freeman MR, Di Vizio D and Solomon KR: The
Rafts of the Medusa: Cholesterol targeting in cancer therapy.
Oncogene. 29:3745–3747. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Patra SK: Dissecting lipid raft
facilitated cell signaling pathways incancer. Biochim Biophys Acta.
1785:182–206. 2008.PubMed/NCBI
|
|
116
|
Mural T: The role of lipid rafts in cancer
cell adhesion and migration. Int J Cell Biol.
2012:7632832012.PubMed/NCBI
|
|
117
|
Rangaswami H, Bulbule A and Kundu GC:
Osteopontin: Role in cell signaling and cancer progression. Trends
Cell Biol. 16:79–87. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Murai T, Maruyama Y, Mio K, Nishiyama H,
Suga M and Sato C: Low cholesterol triggers membrane
microdomain-dependent CD44 shedding and suppresses tumor cell
migration. J Biol Chem. 286:1999–2007. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Nicholson RI, Gee JM and Harper ME: EGFR
and cancer prognosis. Eur J Cancer. 37 Suppl 4:S9–S15. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Irwin ME, Mueller KL, Bohin N, Ge Y and
Boerner JL: Lipid raft localization of EGFR alters the response of
cancer cells to the EGFR tyrosine kinase inhibitor gefitinib. J
Cell Physiol. 226:2316–2328. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Afshordel S, Kern B, Clasohm J, König H,
Priester M, Weissenberger J, Kögel D and Eckert GP: Lovastatin and
perillyl alcohol inhibit glioma cell invasion, migration, and
proliferation - impact of Ras-/Rho-prenylation. Pharmacol Res.
91:69–77. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Nelson ER, Wardell SE, Jasper JS, Park S,
Suchindran S, Howe MK, Carver NJ, Pillai RV, Sullivan PM, Sondhi V,
et al: 27-Hydroxycholesterol links hypercholesterolemia and breast
cancer pathophysiology. Science. 342:1094–1098. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Wu Q, Ishikawa T, Sirianni R, Tang H,
McDonald JG, Yuhanna IS, Thompson B, Girard L, Mineo C, Brekken RA,
et al: 27-Hydroxycholesterol promotes cell-autonomous, ER-positive
breast cancer growth. Cell Rep. 5:637–645. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Hensley CT, Wasti AT and Deberardinis RJ:
Glutamine and cancer: Cell biology, physiology and clinical
opportunities. J Clin Invest. 123:3678–3684. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Kim D, Fiske BP, Birsoy K, Freinkman E,
Kami K, Possemato RL, Chudnovsky Y, Pacold ME, Chen WW, Cantor JR,
et al: SHMT2 drives glioma cell survival in ischaemia but imposes a
dependence on glycine clearance. Nature. 520:363–367. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Roberts E and Frankel S: Free amino acid
in normal and neoplastic tissues of mice as studied by paper
chromatography. Cancer Res. 9645–648. (3 pl)1949.PubMed/NCBI
|
|
127
|
Roberts E and Borges PR: Patterns of free
amino acids in growing and regressing tumor. Cancer Res.
15:697–699. 1955.PubMed/NCBI
|
|
128
|
Yuneva M, Zamboni N, Oefner P,
Sachidanandam R and Lazebnik Y: Deficiency in glutamine but not
glucose induces MYC-dependent apoptosis in human cells. J Cell
Biol. 178:93–105. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Wise DR, Deberardinis RJ, Mancuso A, Sayed
N, Zhang XY, Pfeiffer HK, Nissim I, Daikhin E, Yudkoff M, McMahon
SB, et al: Myc regulates a transcriptional program that stimulates
mitochondrial glutaminolysis and leads to glutamine addiction. Proc
Natl Acad Sci USA. 105:18782–18787. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Gao P, Tchernyshyov I, Chang TC, Lee YS,
Kita K, Ochi T, Zeller KI, De Marzo AM, Van Eyk JE, Mendell JT and
Dang CV: c-Myc suppression of miR-23a/b enhances mitochondrial
glutaminase expression and glutamine metabolism. Nature.
458:762–765. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Wang JB, Erickson JW, Fuji R, Ramachandran
S, Gao P, Dinavahi R, Wilson KF, Ambrosio AL, Dias SM, Dang CV, et
al: Targeting mitochondrial glutaminase activity inhibits oncogenic
transformation. Cancer Cell. 18:207–219. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Jin L, LI D, Alesi GN, Fan J, Kang HB, Lu
Z, Boggon TJ, Jin P, Yi H, Wright ER, et al: Glutamate
dehydrogenase1 signals through antioxidant glutathione peroxidase1
to regulate redox homeostasis and tumor growth. Cancer Cell.
27:257–270. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Piskounova E, Agathocleous M, Murphy MM,
Hu Z, Huddlestun SE, Zhao Z, Leitch AM, Johnson TM, DeBerardinis RJ
and Morrison SJ: Oxidative stress inhibits distant metastasis by
human melanoma cells. Nature. 527:186–191. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Kung HN, Marks JR and Chi JT: Glutamine
synthetase is a genetic determinant of cell type-specific glutamine
independence in breast epithelia. PLoS Genet. 7:e10022292011.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Maher EA, Marin-Valencia I, Bachoo RM,
Mashimo T, Raisanen J, Hatanpaa KJ, Jindal A, Jeffrey FM, Choi C,
Madden C, et al: Metabolism of [U-13 C]glucose in human
brain tumors in vivo. NMR Biomed. 25:1234–1244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Guohua C, Stephanie L and Jian W: Glycine
decarboxylase is a target for transcriptional repressor Snail.
Cancer Metab. 2 Suppl 1:P832014. View Article : Google Scholar :
|
|
137
|
Lepage GA: In vitro incorporation of
glycine-2-C14 into purines and proteins. Cancer Res.
13:178–185. 1953.PubMed/NCBI
|
|
138
|
Zhang WC, Shyh-Chang N, Yang H, Rai A,
Umashankar S, Ma S, Soh BS, Sun LL, Tai BC, Nga ME, et al: Glycine
decarboxylase activity drives non-small cell lung cancer
tumor-initiating cells and tumorigenesis. Cell. 148:259–272. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Shen T: Biochemistry. 2nd. Beijing: Higher
Education Press; pp. 270–273. 1991, PubMed/NCBI
|
|
140
|
Rose ML, Madren J, Bunzendahl H and
Thurman RG: Dietary glycine inhibits the growth of B16 melanoma
tumors in mice. Carcinogenesis. 20:793–798. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Kalhan SC and Hanson RW: Resurgence of
serine: An often neglected but indispensable amino acid. J Biol
Chem. 287:19786–19791. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Possemato R, Marks KM, Shaul YD, Pacold
ME, Kim D, Birsoy K, Sethumadhavan S, Woo HK, Jang HG, Jha AK, et
al: Functional genomics reveal that the serine synthesis pathway is
essential in breast cancer. Nature. 476:346–350. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Pipkorn R, Wiessler M, Waldeck W, Hennrich
U, Nokihara K, Beining M and Braun K: Improved synthesis strategy
for peptide nucleic acids (PNA) appropriate for cell-specific
fluorescence imaging. Int J Med Sci. 9:1–10. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Lin J, Teo S, Lam DH, Jeyaseelan K and
Wang S: MicroRNA-10b pleiotropically regulates invasion,
angiogenicity and apoptosis of tumor cells resembling mesenchymal
subtype of glioblastoma multiforme. Cell Death Dis. 3:e3982012.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Gupta RA, Shah N, Wang KC, Kim J, Horlings
HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al: Long
non-coding RNA HOTAIR reprograms chromatin state to promote cancer
metastasis. Nature. 464:1071–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Zhang C, Zhang Z, Zhu Y and Qin S:
Glucose-6-phosphate dehydrogenase: A biomarker and potential
therapeutic target for cancer. Anticancer Agents Med Chem.
14:280–289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Wang R, Yu C, Zhao D, Wu M and Yang Z: The
mucin-type glycosylating enzyme polypeptide
N-acetylgalactosaminyltransferase 14 promotes the migration of
ovarian cancer by modifying mucin 13. Oncol Rep. 30:667–676. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Li Y, Liu H, Huang YY, Pu LJ, Zhang XD,
Jiang CC and Jiang ZW: Suppression of endoplasmic reticulum
stress-induced invasion and migration of breast cancer cells
through the downregulation of heparanase. Int J Mol Med.
31:1234–1242. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Lin RZ, Wang TP, Hung RJ, Chuang YJ, Chien
CC and Chang HY: Tumor-induced endothelial cell apoptosis: Roles of
NAD(P)H oxidase-derived reactive oxygen species. J Cell Physiol.
226:1750–1762. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Qian YR, Guo Y, Wan HY, Fan L, Feng Y, Ni
L, Xiang Y and Li QY: Angiotensin-converting enzyme 2 attenuates
the metastasis of non-small cell lung cancer through inhibition of
epithelial mesenchymal transition. Oncol Rep. 29:2408–2414. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Kim EK, Park JM, Lim S, Choi JW, Kim HS,
Seok H, Seo JK, Oh K, Lee DS, Kim KT, et al: Activation of
AMP-activated protein kinase is essential for lysophosphatidic
acid-induced cell migration in ovarian cancer cells. J Biol Chem.
286:24036–24045. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Qiu SL, Xiao ZC, Piao CM, Xian YL, Jia LX,
Qi YF, Han JH, Zhang YY and Du J: AMP-activated protein kinase α2
protects against liver injury from metastasized tumors via reduced
glucose deprivation-induced oxidative stress. J Biol Chem.
289:9449–9459. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Skubitz AP, Taras EP, Boylan KL, Waldron
NN, Oh S, Panoskaltsis-Mortari A and Vallera DA: Targeting CD133 in
an in vivo ovarian cancer model reduces ovarian cancer progression.
Gynecol Oncol. 130:579–587. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Chen J, Liu Q, Xiao J and Du J:
EpCAM-antibody-labeled noncytotoxic polymer vesicles for cancer
stem cells-targeted delivery of anticancer drug and siRNA.
Biomacromolecules. 16:1695–1705. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Lawson DA, Bhakta NR, Kessenbrock K,
Prummel KD, Yu Y, Takai K, Zhou A, Eyob H, Balakrishnan S, Wang CY,
et al: Single-cell analysis reveals a stem-cell program in human
metastatic breast cancer cells. Nature. 526:131–135. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Yang Y, Fan Y, Qi Y, Liu D, Wu K, Wen F
and Zhao S: Side population cells separated from A549 Lung cancer
cell line possess cancer stem cell-like properties and inhibition
of autophagy potentiates the cytotoxic. Oncol Rep. 34:929–935.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Iacopino F, Angelucci C, Piacentini R,
Biamonte F, Mangiola A, Maira G, Grassi C and Sica G: Isolation of
cancer stem cells from three human glioblastoma cell lines:
Characterization of two selected clones. PLoS One. 9:e1051662014.
View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Aguilar E, Marin de Mas I, Zodda E, Marin
S, Morrish F, Selivanov V, Meca-Cortés Ó, Delowar H, Pons M,
Izquierdo I, et al: Metabolic reprogramming and dependencies
associated with epithelial cancer stem cells independent of the
epithelial-mesenchymal transition program. Stem Cells.
34:1163–1176. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumors: Accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Oshimori N, Oristian D and Fuchs E: TGF-β
promotes heterogeneity and drug resistance in squamous cell
carcinoma. Cell. 160:963–976. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Gammon L, Biddle A, Heywood HK,
Johannessen AC and Mackenzie IC: Sub-sets of cancer stem cells
differ intrinsically in their patterns of oxygen metabolism. PLoS
One. 8:e624932013. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Biddle A, Liang X, Gammon L, Fazil B,
Harper LJ, Emich H, Costea DE and Mackenzie IC: Cancer stem cells
in squamous cell carcinoma switch between two distinct phenotypes
that are preferentially migratory or proliferative. Cancer Res.
71:5317–5326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Biddle A, Gammon L, Liang X, Costea DE and
Mackenzie IC: Phenotypic plasticity determines cancer stem cell
therapeutic resistance in oral squamous cell carcinoma.
EBioMedicine. 4:138–145. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Ying H, Kimmelman AC, Lyssiotis CA, Hua S,
Chu GC, Fletcher-Sananikone E, Locasale JW, Son J, Zhang H, Coloff
JL, et al: Oncogenic kras maintains pancreatic tumors through
regulation of anabolic glucose metabolism. Cell. 149:656–670. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Shi L, Jackstadt R, Siemens H, Li H,
Kirchner T and Hermeking H: p53-induced miR-15a/16-1 and AP4 form a
double-negative feedback loop to regulate epithelial-mesenchymal
transition and metastasis in colorectal cancer. Cancer Res.
74:532–542. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Khew-Goodall Y and Goodall GJ:
Myc-modulated miR-9 makes more metastases. Nat Cell Biol.
12:209–211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Colvin H, Nishida N, Konno M, Haraguchi N,
Takahashi H, Nishimura J, Hata T, Kawamoto K, Asai A, Tsunekuni K,
et al: Oncometabolite D-2-hydroxyglurate directly induces
epithelial-mesenchymal transition and is associated with distant
metastasis in colorectal cancer. Sci Rep. 8:362892016. View Article : Google Scholar
|
|
169
|
Kondoh H, Lleonart ME, Gil J, Wang J,
Degan P, Peters G, Martinez D, Carnero A and Beach D: Glycolytic
enzymes can modulate cellular life span. Cancer Res. 65:177–185.
2005.PubMed/NCBI
|
|
170
|
Hu J, Liu Z and Wang X: Does TP53 mutation
promote ovarian cancer metastasis to omentum by regulating lipid
metabolism? Med Hypotheses. 81:515–520. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Paschka P, Schlenk RF, Gaidzik VI, Habdank
M, Krönke J, Bullinger L, Späth D, Kayser S, Zucknick M, Götze K,
et al: IDH1IDH2 mutations are frequent genetic alterations
in acute myeloid leukemia and confer adverse prognosis in
cytogenetically normal acute myeloid leukemia with NPM1
mutation without FLT3 internal tandem duplication. J Clin
Oncol. 28:3636–3643. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Gaglio D, Metallo CM, Gameiro PA, Hiller
K, Danna LS, Balestrieri C, Alberghina L, Stephanopoulos G and
Chiaradonna F: Oncogenic K-Ras decouples glucose and glutamine
metabolism to support cancer cell growth. Mol Syst Biol. 7:5232011.
View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Weinberg F, Hamanaka R, Wheaton WW,
Weinberg S, Joseph J, Lopez M, Kalyanaraman B, Mutlu GM, Budinger
GR and Chandel NS: Mitochondrial metabolism and ROS generation are
essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci USA.
107:8788–8793. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Guo JY, Chen HY, Mathew R, Fan J,
Strohecker AM, Karsli-Uzunbas G, Kamphorst JJ, Chen G, Lemons JM,
Karantza V, et al: Activated Ras requires autophagy to maintain
oxidative metabolism and tumorigenesis. Genes Dev. 25:460–470.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Schwartzenberg-Bar-Yoseph F, Armoni M and
Karnieli E: The tumor suppressor p53 down-regulates glucose
transporters GLUT1GLUT4 gene expression. Cancer Res.
64:2627–2633. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Bensaad K, Tsuruta A, Selak MA, Vidal MN,
Nakano K, Bartrons R, Gottlieb E and Vousden KH: TIGAR, a
p53-inducible regulator of glycolysis and apoptosis. Cell.
126:107–120. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Matoba S, Kang JG, Patino WD, Wragg A,
Boehm M, Gavrilova O, Hurley PJ, Bunz F and Hwang PM: p53 regulates
mitochondrial respiration. Science. 312:1650–1653. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Li F, Wang Y, Zeller KI, Potter JJ, Wonsey
DR, O'Donnell KA, Kim JW, Yustein JT, Lee LA and Dang CV: Myc
stimulates nuclearly encoded mitochondrial genes and mitochondrial
biogenesis. Mol Cell Biol. 25:6225–6234. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Smith AP, Verrecchia A, Fagà G, Doni M,
Perna D, Martinato F, Guccione E and Amati B: A positive role for
Myc in TGFbeta-induced Snail transcription and
epithelial-to-mesenchymal transition. Oncogene. 28:422–430. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Lee SY, Jeon HM, Ju MK, Jeong EK, Kim CH,
Park HG, Han SI and Kang HS: Dlx-2 and glutaminase upregulate
epithelial-mesenchymal transition and glycolytic switch.
Oncotarget. 7:7925–7939. 2016.PubMed/NCBI
|
|
181
|
Liu W, Le A, Hancock C, Lane AN, Dang CV,
Fan TW and Phang JM: Reprogramming of proline and glutamine
metabolism contributes to the proliferative and metabolic responses
regulated by oncogenic transcription factor c-Myc. Proc Natl Acad
Sci USA. 109:8983–8988. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Kim JW, Gao P, Liu YC, Semenza GL and Dang
CV: Hypoxia-inducible factor 1 and dysregulated c-Myc cooperatively
induce vascular endothelial growth factor and metabolic switches
hexokinase 2 and pyruvate dehydrogenase kinase 1. Mol Cell Biol.
27:7381–7393. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Zhao H, Duan Q, Zhang Z, Li H, Wu H, Shen
Q, Wang C and Yin T: Up-regulation of glycolysis promotes the
stemness and EMT phenotypes in gemcitabine-resistant pancreatic
cancer cells. J Cell Mol Med. 21:2055–2067. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Guerra F, Guaragnella N, Arbini AA, Bucci
C, Giannattasio S and Moro L: Mitochondrial dysfunction: A novel
potential driver of epithelial-to-mesenchymal transition in cancer.
Front Oncol. 7:2952017. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Murphy TA, Dang CV and Young JD:
Isotopically nonstationary 13C flux analysis of
Myc-induced metabolic reprogramming in B-cells. Metab Eng.
15:206–217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Yan H, Parsons DW, Jin G, McLendon R,
Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ,
et al: IDH1IDH2 mutations in gliomas. N Engl J Med.
360:765–773. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Dang L, White DW, Gross S, Bennett BD,
Bittinger MA, Driggers EM, Fantin VR, Jang HG, Jin S, Keenan MC, et
al: Cancer-associated IDH1 mutations produce 2-hydroxyglutarate.
Nature. 462:739–744. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Ward PS, Patel J, Wise DR, Abdel-Wahab O,
Bennett BD, Coller HA, Cross JR, Fantin VR, Hedvat CV, Perl AE, et
al: The common feature of leukemia-associated IDH1 and IDH2
mutations is a neomorphic enzyme activity converting
alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell. 17:225–234.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Turcan S, Rohle D, Goenka A, Walsh LA,
Fang F, Yilmaz E, Campos C, Fabius AW, Lu C, Ward PS, et al: IDH1
mutation is sufficient to establish the glioma hypermethylator
phenotype. Nature. 483:479–483. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Figueroa ME, Abdel-Wahab O, Lu C, Ward PS,
Patel J, Shih A, Li Y, Bhagwat N, Vasanthakumar A, Fernandez HF, et
al: Leukemic IDH1 and IDH2 mutations result in a hypermethylation
phenotype, disrupt TET2 function, and impair hematopoietic
differentiation. Cancer Cell. 18:553–567. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Rohle D, Popovici-Muller J, Palaskas N,
Turcan S, Grommes C, Campos C, Tsoi J, Clark O, Oldrini B,
Komisopoulou E, et al: An inhibitor of mutant IDH1 delays growth
and promotes differentiation of glioma cells. Science. 340:626–630.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Grassian AR, Lin F, Barrett R, Liu Y,
Jiang W, Korpal M, Astley H, Gitterman D, Henley T, Howes R, et al:
Isocitrate dehydrogenase (IDH) mutations promote a reversible
ZEB1/microRNA (miR)-200-dependent epithelial-mesenchymal transition
(EMT). J Biol Chem. 287:42180–42194. 2012. View Article : Google Scholar : PubMed/NCBI
|