Introduction
Cytochalasin B (CB) is a mycogenic toxin extracted
from the fungus Phoma sp. CB permeates through the cell
membrane into the cytoplasm and binds to the ‘barbed’ end (‘plus’
end) of the filamentous actin (F-actin), while preventing the
superposition of actin monomer polymerization at this site.
Consequently, the polymerization of the actin cytoskeleton is
impeded and its conformation is altered (1,2),
ultimately affecting cell morphology and biological processes, such
as cell shrinkage, mitosis and apoptosis (3). Cytochalasins are extensively used to
investigate the role of the microfilament cytoskeleton in various
biological processes, including cell movement, differentiation and
mitosis. However, accumulating evidence indicates that
cytochalasins exert potent anticancer effects and induce apoptosis
in various malignant cell types (4,5).
Unlike the conventional microtubule-targeted agents (6), CB is a type of microfilament-directed
drug that can potentially increase the efficacy of chemotherapeutic
agents by acting synergistically with them (7,8). In
addition, malignant cells have a perturbed actin cytoskeleton,
which makes them susceptible to preferential damage by
cytochalasins. CB may induce apoptosis of various cancer cells
through intrinsic or extrinsic pathways (4,9).
However, there is currently no comprehensive information available
regarding the biomechanics and surface topography during early
apoptosis (10,11). In addition, although chemical
signals have been extensively investigated to characterize cell
apoptosis (12), only a limited
number of studies have systematically addressed the alterations in
biomechanics, cell surface topography and biological signals
related to the disruption of the microfilament cytoskeleton.
Ever since apoptosis was first described by Kerr
et al (13), numerous
studies have focused on the morphology, molecular biology and
underlying biological behaviors in an attempt to elucidate the
subtle molecular mechanisms involved in cell death (14,15).
Researchers have long believed that apoptosis occurs when key
proteins, such as initiators caspase-8 and −9, are cleaved and
activated (16,17), while overlooking the alterations in
biomechanics during early-stage apoptosis. Expanding knowledge and
advances in research methods have enabled researchers to examine
the changes in the cytoskeleton and cell elasticity. The decrease
in elastic modulus was usually measured 24 h after the cells were
treated (18,19). A number of studies have focused on
the decline in cellular elastic modulus following drug treatment.
Pelling et al (20) reported
that the cellular elastic modulus decreases during early-stage
apoptosis, and Schulze et al (21) observed that alterations in the actin
cytoskeleton led to changes in cellular morphology and elastic
modulus. These findings suggest that a certain correlation exists
among disruption of the F-actin cytoskeleton, mechanical
alterations and apoptosis.
F-actin is among the most important cytoskeletal
components involved in maintaining the shape and mechanical
properties of the cell. Alterations in F-actin organization are
inevitably accompanied by changes in cellular mechanical properties
(such as cell stiffness). Bio-type atomic force microscopy (AFM) is
a unique technique enabling direct measurement of the mechanical
properties of living cells and detection of nanostructures on the
cell surface (22). Researchers
have used AFM to investigate the nanoscale morphology and
mechanical properties of single living cells treated with
anticarcinogens (23), and the
results indicated that cell stiffness is altered when cells are
exposed to cytotoxic agents, such as those used for chemotherapy.
The alterations in the mechanical properties of individual cells
may be used as a biomarker for evaluating apoptosis (24,25).
These viewpoints indicate a subtle association among the
reorganization of the actin cytoskeleton, cellular mechanics and
apoptosis. However, these previous studies only focused on the
mechanical phenomena at 12 or even 24 h after cell treatment, and
overlooked the mechanical alterations during the early stages of
drug treatment. Therefore, the aim of the present study was to
investigate the early alterations in biomechanics, cellular
geometry, morphology and biological signals following cell exposure
to CB.
For this purpose, an apoptosis model was established
using HeLa cells induced by CB in order to explore the alterations
and possible correlations among biomechanical reconstruction,
morphological remodeling and apoptotic signal activation.
Materials and methods
Reagents and cell culture
All reagents, including CB and fluorescein
isothiocyanate (FITC)-phalloidin (cat. no. P5282), were purchased
from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany), unless
otherwise specified. Fetal bovine serum (FBS; SH30084) and BCA
protein assay kit (cat. no. 23227) were purchased from Thermo
Fisher Scientific, Inc. (Waltham, MA, USA). The primary anti-Fas
antibody (dilution 1:200, rabbit mAb; cat. no. 133619) and primary
anti-vinculin antibody (dilution 1:200, rabbit mAb; cat. no. 73412)
were obtained from Abcam (Cambridge, UK). An Annexin V-fluorescein
isothiocyanate (FITC)/propidium iodide (PI) kit (70-AP101-60) was
obtained from Hangzhou MultiSciences Biotech Co., Ltd. (Hangzhou,
China). Nitrocellulose membranes and cell membrane protein
extraction kit (P0033) were acquired from Beyotime Institute of
Biotechnology (Shanghai, China). CB (cat. no. C6762) was dissolved
in dimethyl sulfoxide (D2650). The secondary antibodies Texas red
fluorescent-conjugated goat anti-rabbit IgG (dilution 1:200; cat.
no. 6904-250), horseradish peroxidase-labeled goat anti-rabbit IgG
(dilution 1:200; cat. no. 6403-05), FITC fluorescent-conjugated IgG
(dilution 1:200; cat. no. 6401-05) were obtained from BioVision,
Inc. (San Francisco, CA, USA).
Human cervical cancer HeLa cells were obtained from
the Cell Bank of the Chinese Academy of Sciences (Shanghai, China)
and cultured in Dulbecco's modified Eagle's medium (DMEM; HyClone;
GE Healthcare, Chicago, IL, USA) supplemented with 10% FBS, 100
µg/ml streptomycin and 100 U/ml penicillin in a humidified
atmosphere of 5% CO2 at 37°C.
Fluorescence staining and F-actin
visualization
To visualize the F-actin organization, cells treated
with CB were stained with fluorochrome. The cell-climbing pieces
(sterile coverslips preplaced in a Petri dish) were treated with 5
µg/ml CB for different time periods, rinsed with phosphate-buffered
saline (PBS; pH 7.4), fixed with 4.0% cooled paraformaldehyde,
permeated with 0.2% Triton X-100 in PBS, blocked with 2% bovine
serum albumin (BSA; 9048-46-8; Beijing Solarbio Science &
Technology Co., Ltd., Beijing, China) in PBS, and incubated with
FITC fluorescent-conjugated phalloidin for 1 h at room temperature
in the dark. The nuclei were labeled with
4,6-diamidino-2-phenylindole (DAPI, D9542; Sigma-Aldrich; Merck
KGaA) at a concentration of 0.1 mg/ml. The coverslips were sealed
on the glass slides with Antifade Mounting Medium (Beijing Solarbio
Science & Technology Co., Ltd.). The specimens were observed
and imaged by an Olympus FV1000 laser scanning confocal microscope
(LSCM; Olympus Corp., Tokyo, Japan) in 1 week. The mean
fluorescence intensity of F-actin was analyzed with FV10-ASW 4.1
Viewer software (Olympus Corp.). No other filtering or adjustments
were applied.
Western blot analysis and death
receptor CD95/Fas expression
Vinculin, a ubiquitously expressed actin-binding
protein (ABP), can affect cell adhesion and signal transmission. To
investigate whether vinculin expression was inhibited by CB, its
expression was analyzed by western blotting. Samples were collected
by trypsinization (~1×107 cells). The cell membrane
protein extraction kit was used to extract the total proteins of
the cell membrane. The total protein contents of the lysates were
determined with the BCA protein assay kit. The proteins (30 g/well)
were separated by 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and then transferred onto nitrocellulose
membranes. Non-specific proteins were blocked with 5% skimmed milk
powder diluted in Tris-buffered saline containing 0.05% Tween-20 at
room temperature for 1 h. Then, primary anti-vinculin antibodies
(1:1,000 dilution) and anti-GAPDH antibodies (1:1,000 dilution;
cat. no. 22555; Abcam) were added and incubated at 4°C overnight.
The blots were incubated with horseradish peroxidase-labeled goat
anti-rabbit secondary antibodies at room temperature for 1 h. The
bands were exposed by a Tanon-5200 imaging system (Tanon Science
and Technology Co., Ltd., Shanghai, China), and the intensity was
quantified using the ImageJ 2× software [National Institutes of
Health (NIH) Bethesda, MD, USA].
After treatment, the cell-climbing pieces were
fixed, permeated and incubated with rabbit monoclonal primary
anti-Fas antibodies (1:200 dilution) at 4°C overnight, colored with
Texas Red fluorescent-conjugated secondary antibodies, then
incubated with rabbit polyclonal anti-vinculin antibodies (1:200
dilution) at 37°C for 2 h, and colored with FITC
fluorescent-conjugated secondary antibodies. The nuclei were
labeled with DAPI. All procedures were performed in the dark. The
specimens were observed and imaged using LSCM.
Annexin V-FITC/PI apoptotic
analysis
During apoptosis, phosphatidylserine (PS)
translocates to the membrane surface. Acting as an ‘eat me’ signal,
PS exposure prompts phagocytes to engulf apoptotic cells (26), which is a relatively late stage of
the apoptosis process. PS externalization is common in early
apoptosis (27). A recent study
demonstrated that PS exposure in response to apoptotic stimuli is
mediated by the phospholipid scramblase Xkr8, which is activated
directly by caspases. But the authors also pointed out that this
effect is only observed in the late stages of apoptosis (28). In addition, in early apoptosis, the
intracellular Ca2+ concentration increases, which
mediates PS exposure. Accordingly, the present study used PS
exposure to determine the occurrence of apoptosis, and then
designed subsequent experiments. Annexin V has a high affinity for
PS. Thus, PS translocation was analyzed by flow cytometry (FCM)
using an Annexin V-FITC/PI apoptosis detection kit, in accordance
with the manufacturer's instructions. The cells (5×105)
were loaded onto a 60-mm Petri dish, incubated overnight, treated
with 5 µg/ml CB, and collected by trypsinization. Then, the cell
pellets were resuspended in 100 µl binding buffer, followed by
staining with 5 µl Annexin V-FITC and 5 µl PI for 30 min at room
temperature in the dark. All the samples were analyzed with a
FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ,
USA) and the data were evaluated using the CellQuest software (BD
Biosciences).
AFM imaging and nanoindentation
mechanical analysis
A commercial NanoWizard III AFM (JPK Instruments,
Berlin, Germany) was utilized for cell imaging and probing the
force spectroscopy of living HeLa cells. This microscope was
combined with an inverted optical microscope (Carl Zeiss AG,
Oberkochen, Germany) to facilitate AFM and optical microscopy
imaging simultaneously. Silicon nitride cantilevers (PNP-DB;
NanoWorld AG, Neuchatel, Switzerland) with a nominal spring
constant of 0.03 N/m (fo: 17 kHz) were used in all
experiments. AFM images and force spectroscopy measurements were
collected under intermittent mode by using a square pyramidal
silicon nitride probe with a 4.0-nm tip diameter and a 25°C
half-opening angle. Prior to the AFM measurements, the sensitivity
and the spring constants of the cantilevers were calibrated using
JPK Instruments 4.2.61 software. A square pyramidal tip with a
spring constant of 0.029 N/m was used in the subsequent
experiments. The inverted optical microscope was used to select the
ideal cell and position of the AFM tip. All AFM images and
nanoindentation experiments were conducted in cell culture medium
at room temperature according to previously described operating
procedures (29,30). The AFM had a scanning range of 80×80
µm (x- and y-axes) and a vertical range (z-axis) of 15 µm. The
cells were imaged at 512×512 pixels. Then, 10–15 dots around the
nucleus (orange area) were selected to obtain the force-distance
curves from the indentation of the living cells. The interaction
between the tip and sample caused a cantilever deflection, which
was recorded as a function of the relative sample position, i.e., a
force-distance curve. The elastic modulus (cell stiffness) was
calculated by using JPK Instruments data processing 4.2.61 software
to analyze 130–160 approach curves of force spectroscopy.
Cell height, roughness and volume
measurement
The height of the cell, which was defined as the
distance between the top and bottom, was determined from the
height-measured images. The height, diameter and average roughness
of each cell were measured by cross-sectional analysis. Average
roughness (Ra) and root mean square (RMS) roughness (Rq) are key
surface roughness parameters for understanding the nanoscale
surface morphology of cells. The analysis of the surface roughness
provides novel quantitative data on cell nanomorphology. The
cellular volume is another important indicator for understanding
the state of the cell. If the cell is considered as the half of an
oblate ellipsoid, then the cell volume can be calculated as follows
(31):
V=23πr2h
where V is the volume, r is the radius
and h is the height of the cell.
Mechanical property measurement
AFM is an effective tool for assessing the
mechanical properties of single living cells via nanoindentation
tests under physiological conditions (24,25).
If the cell is considered as an elastomer of homogeneous structure,
the cell elastic modulus E is calculated according to the
Hertz model and the approach curves (32,33).
The indentation depth (5–10% of the height of the cell, ~200–500
nm) is fitted using JPK Data Processing software (JPK Instruments)
(34,35). The referential equation indicating
the relation between indentation force and depth is as follows:
F=δ22πE1-μ2tanθ
where F is the loading force, E is the
elastic modulus, δ is the indentation depth, µ is the
Poisson's ratio of the samples, and θ is the half-opening
angle of the pyramidal tip.
Cell surface visualization by scanning
electron microscopy
The spinous protrusions and surface morphological
changes at the nanoscale level were observed by CLSM and AFM after
the cells were treated with CB at different time-points. To further
elucidate these phenomena, we created specimens for scanning
electron microscopy (SEM) and observed the cell surface. The cells
were seeded (5×103 cells) onto a 24-well plate
(preplaced sterile coverslips), cultivated for 48 h, and treated
with 5 µg/ml CB for 0, 0.5 and 3 h. The samples were rinsed with
cold PBS, fixed with 3% pre-cooled glutaraldehyde solution at 4°C
overnight, and made into ultra-thin slices. The cell surface
morphology was observed and captured by using a scanning electron
microscope (JSM6380 LV; JEOL, Ltd., Tokyo, Japan).
Statistical analysis
Data are recorded as mean ± standard deviation and
analyzed using SPSS 22.0 (Statistical Product and Service
Solutions; IBM Corp. Armonk, NY, USA). Statistical comparisons were
performed using one-way analysis of variance ANOVA followed with
Dunnett's t-tests. P<0.05 or P<0.01 were considered to
indicate statistically significant differences. Single asterisks
(*) indicate a significant difference (P<0.05), and double
asterisks (**) denote an extremely significant difference
(P<0.01).
Results
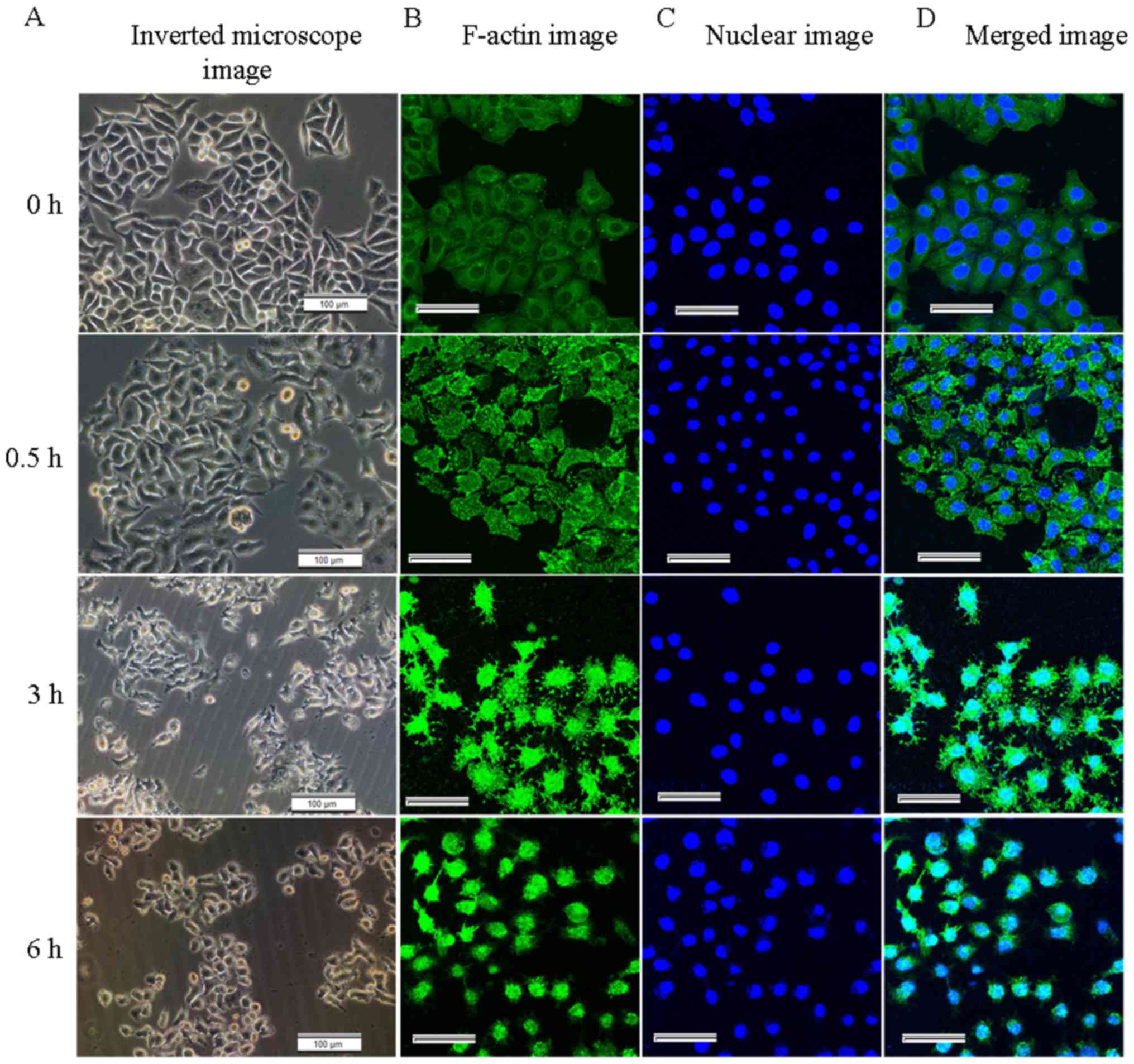
Morphological changes and actin
cytoskeletal depolymerization
The morphological changes of the HeLa cells treated
with CB were observed using an Olympus CKX31 inverted microscope
(Olympus Corp.). The untreated cells were well-spread and the cell
borders were clear. By contrast, the CB-treated cells gradually
shrank, rounded up, even became detached from the substrate and
floated (Fig. 1A). The
fluorescent-labelled F-actin and nucleus were observed by LSCM. In
the control groups, the F-actin was uniformly distributed beneath
the cell membrane around the nuclei. In the treated groups, the
F-actin was disrupted, with short or punctate actin fragments
(green fluorescence) occupied the background of the images.
Numerous spinous protrusions appeared at the periphery of the
cells, particularly at 3 h (Fig.
1B). These changes indicated that filamentous actin was
gradually depolymerized and disrupted by CB. As the disruption of
the actin cytoskeleton progressed, the adherent area of the cells
gradually decreased and the nucleus became irregular at 6 h
(Fig. 1C). The fluorescence
intensity of F-actin gradually increased, reaching its maximum at 2
h, and then gradually decreased (Fig.
2D), further reflecting the disruption of the actin
cytoskeleton, and the density of the intracellular actin fragments
increased.
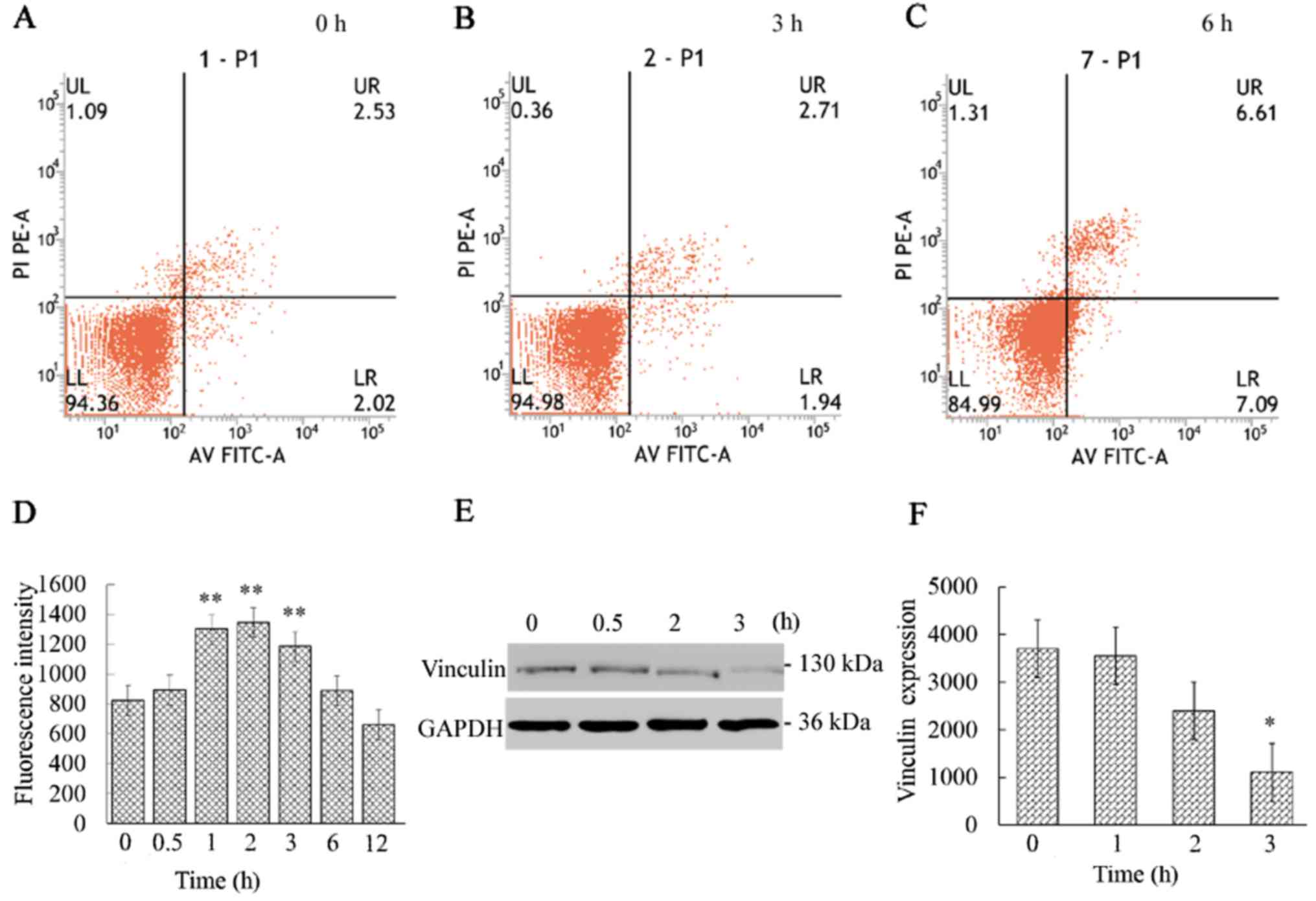
Effect of cytochalasin B on PS
exposure
Annexin V-FITC/PI apoptosis analysis revealed that
the apoptosis marker PS was almost undetectable during the initial
3 h (Fig. 2A-C). Then, the
apoptosis rate gradually increased, reaching 14.77% at 12 h (data
not shown).
The aforementioned results demonstrate that the
actin cytoskeleton was markedly disrupted after the cells were
treated with CB. The FCM analysis revealed that obvious apoptosis
was almost not detected during the initial 3 h and the disruption
of the F-actin cytoskeleton occurred prior to PS exposure on the
cell surface. This phenomenon indicates that CB mediates apoptosis
through targeting the actin cytoskeleton.
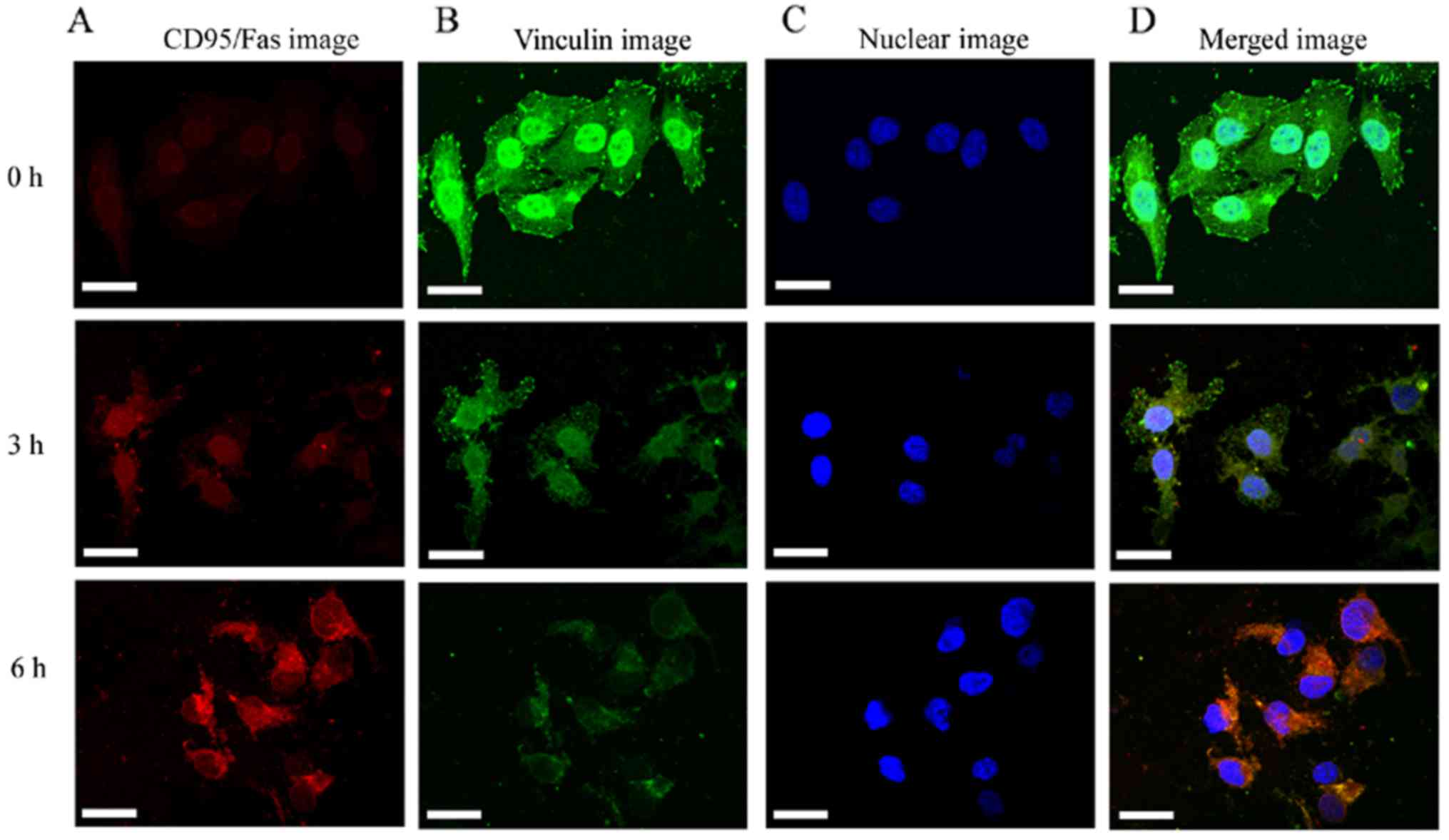
Downregulation of vinculin and
activation of CD95/Fas
The vinculin expression was continuously decreased
and was found to be significantly (P<0.05) reduced at 3 h
(Fig. 2E and F). The results
indicated that the destruction of the actin cytoskeleton resulted
in the reduction of vinculin expression, which destroyed the
signaling pathway that connects internal and external signal
transmission. The decrease in vinculin expression is likely the
cause of the activation of the cell membrane death receptor
CD95/Fas. Several studies have demonstrated that apoptosis was
accompanied by decreased vinculin expression and the activation of
CD95/Fas (4,36). These findings are consistent with
our results that the CD95/Fas (red fluorescence) was mildly
activated at 3 h and significantly activated at 6 h, whereas the
vinculin expression (green fluorescence) gradually decreased
(Fig. 3).
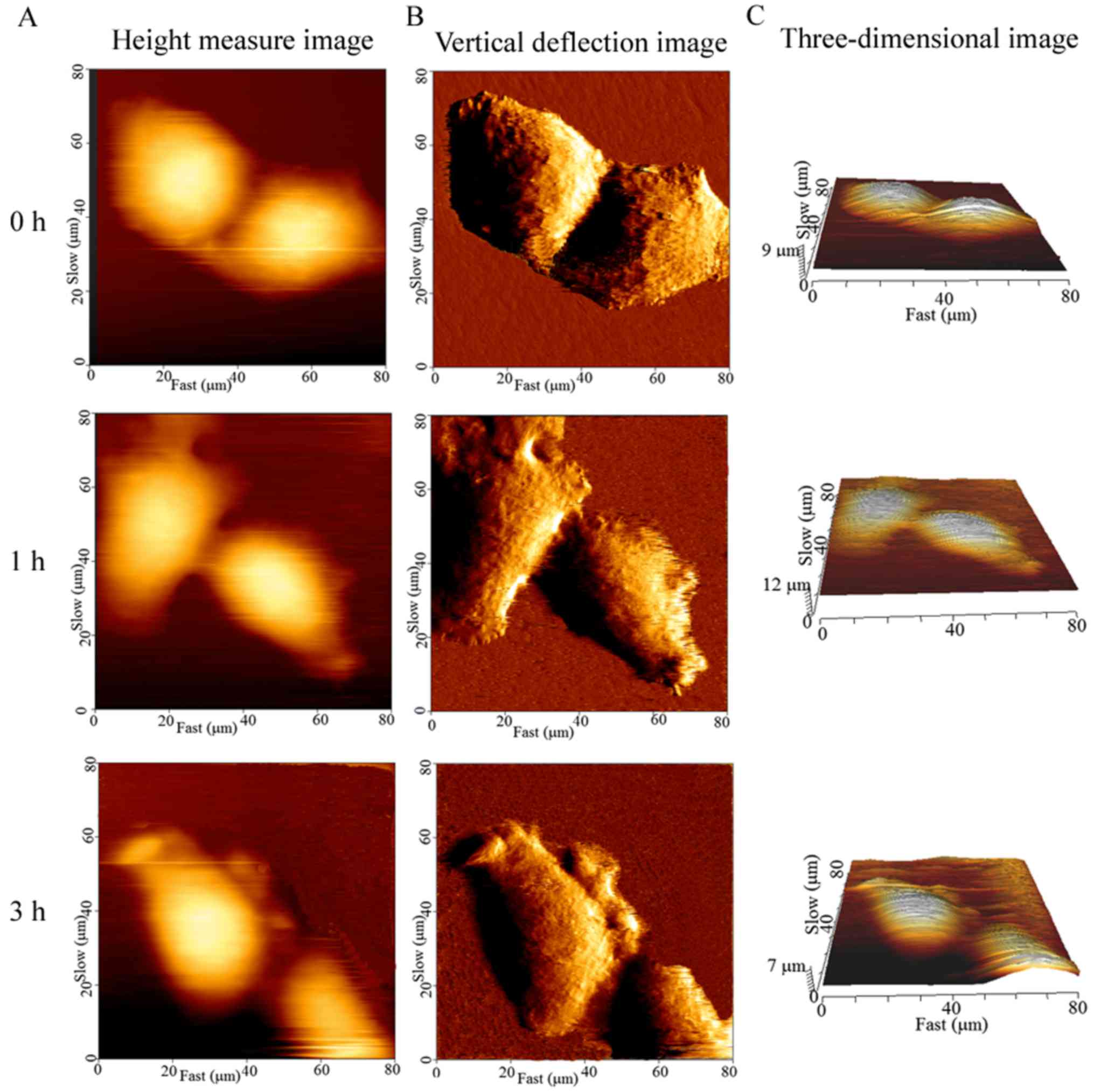
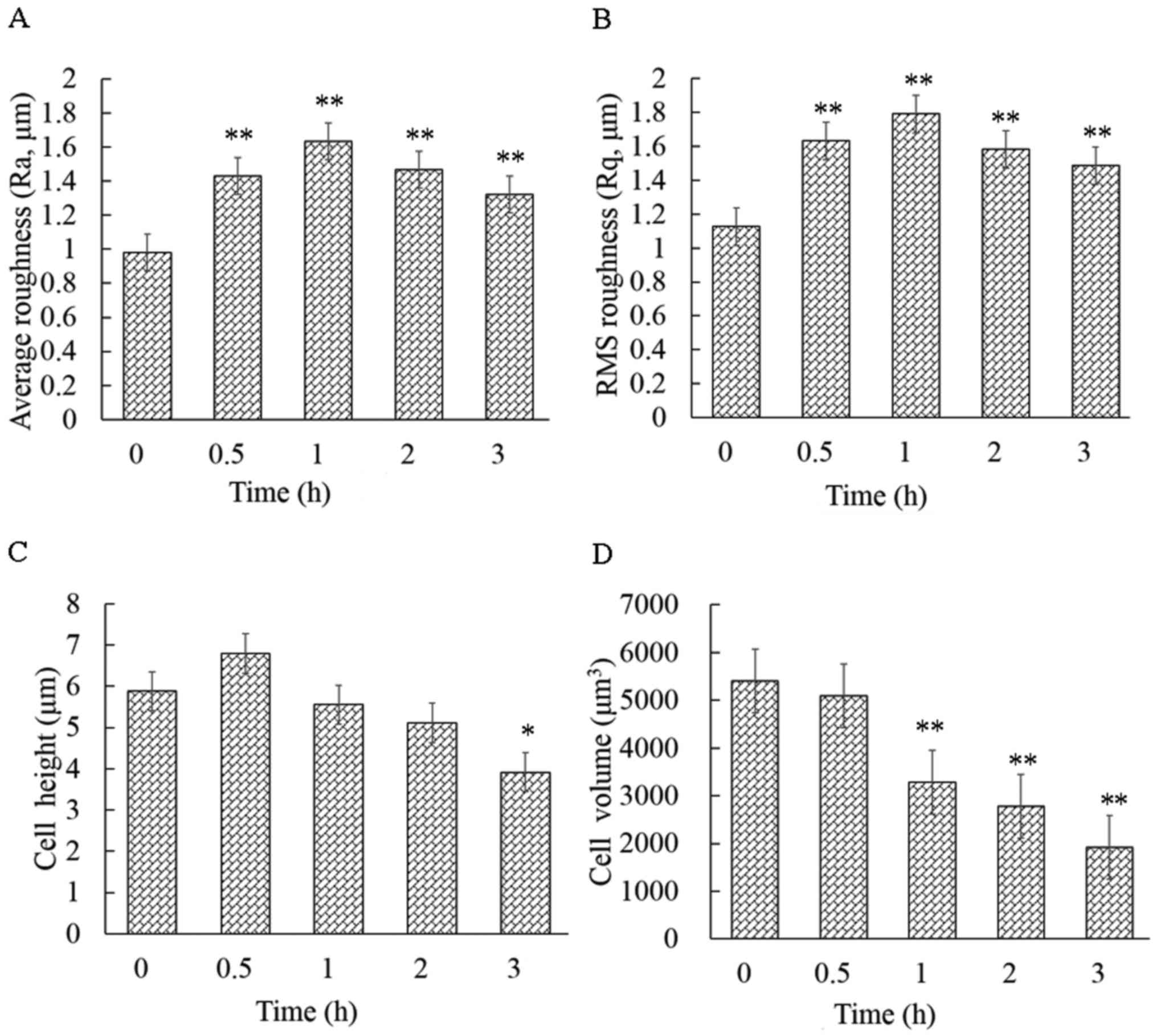
Geometric reconstruction and surface
roughness increase
The cellular height, diameter and roughness were
measured by the cross-sectional analysis of the height-measured
images (Fig. 4A-C). Ra and Rq are
key parameters for understanding the cellular surface topography at
the nanoscale level. The cell surface roughness differed
significantly before and after the cells were exposed to CB, and
this change occurred in a time-dependent manner. The control cells
displayed the smallest Ra (0.97±0.09 mm) and Rq (1.12±0.12 mm),
while the average Ra and Rq of the treated cells were significantly
higher (P<0.01) than that of the control (Fig. 5A and B). These findings were
consistent with the spinous protrusions at the periphery of cells,
as observed by LSCM. However, the cause of the increased cell
surface roughness remains unknown. Thus, the cell surface was then
observed by SEM.
In addition, the CB-induced apoptosis was
accompanied by a decrease in volume. The brightly colored area was
at the highest part of the cell on the height-measured images
(Fig. 4A). The mean cellular height
changed drastically, increasing at 30 min of exposure and
subsequently decreasing (Fig. 5C).
However, the cellular mean volume changed continuously, decreasing
from 5,404±752 to 1,919±506 µm3 in 3 h (Fig. 5D). In particular, the volume
significantly declined (P<0.01) with respect to the control
within 1 h of exposure.
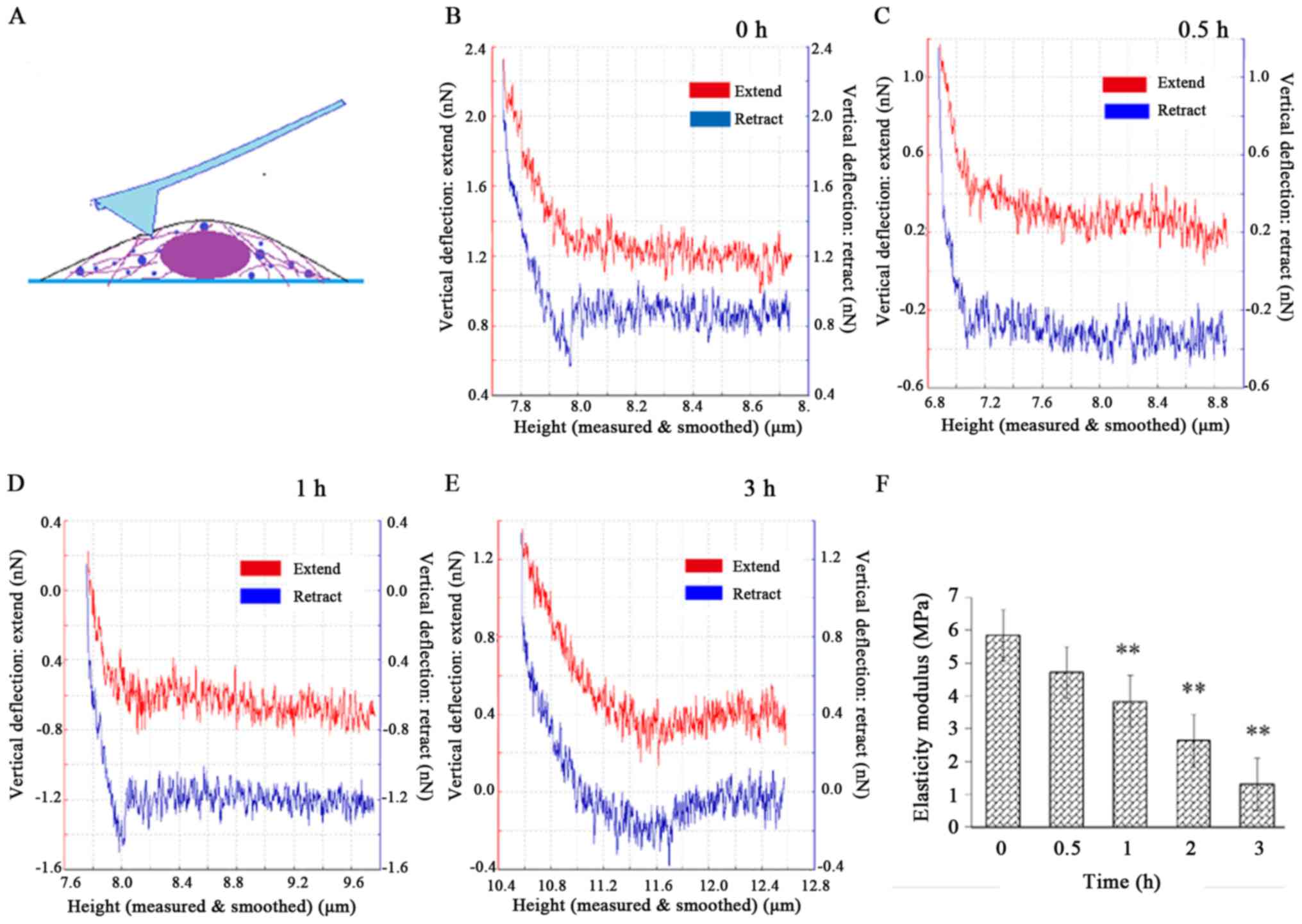
Decrease in the elastic modulus of
HeLa cells
After the cell height image was captured, 10–15 dots
were selected around the nucleus to perform the nanoindentation
experiment (Fig. 6A) and obtain the
force-distance curves (Fig. 6B-E).
The red and blue lines denote the extend curves and the retract
curves, respectively. The elastic modulus E was normally
distributed. Most of the E values were concentrated around
the mean E value of each group and the mean E was
5.84±0.88 MPa for the control. After the cells were exposed to CB,
E significantly decreased (P<0.01) to 1.30±0.33 MPa at 3
h (Fig. 6F), suggesting that the
cells became increasingly softer as F-actin was damaged during the
treatment.
The elastic modulus is mainly determined by the
F-actin component of the cytoskeleton. The mechanical properties
are also recognized as indicators of physiological processes, such
as malignant phenotype, differentiation and mitosis. In the present
experiment, the cellular elastic modulus significantly declined
within 3 h. However, PS, which is an early biological apoptosis
marker, remained undetectable at that time. Therefore, the elastic
modulus had obviously decreased before biological signals were
detected, indicating that the cellular mechanical reconstruction
preceded the biological alterations during CB-induced
apoptosis.
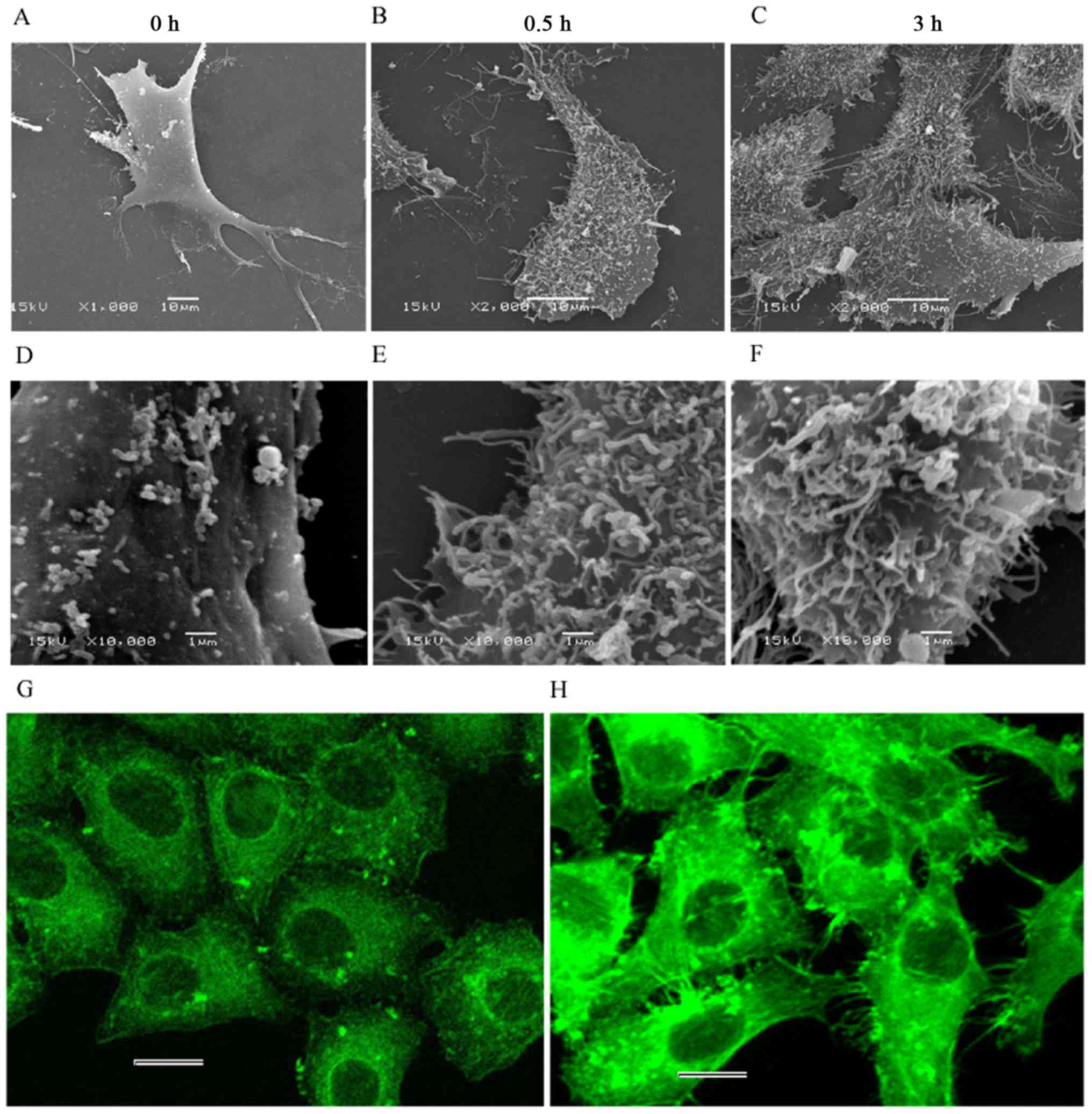
Ultrastructural changes in HeLa
cells
The surface of the control cells was smooth, with
only a few tiny points observed (Fig.
7A and D). Microvilli, lamellipodia and filopodia were
identifiable. By contrast, the surface of the treated cells became
considerably rough, with numerous filaments passing through the
membrane and located on cell surface (Fig. 7B and C, E and F). Actin fluorescence
staining also revealed a large number of filamentous structures at
the periphery of the cells (Fig.
7H) while these structures were not seen around the cells of
the control (Fig. 7G). This
phenomenon may be the reason for the rougher surface observed
during the treatment. However, only the filaments passing through
the membrane could be seen, as we were unable to determine whether
these filaments were the F-actin fragments disrupted by CB.
Discussion
In the present study, the alterations in
biomechanics, geometry and biological signals were systematically
investigated during cytochalasin B (CB)-triggered HeLa cell
apoptosis. The findings demonstrated that the destruction of
F-actin rapidly led to a decrease in the elastic modulus and volume
of the cells, and an increase in the cell surface roughness and
intracellular crowding, followed by gradual exposure of PS on the
plasma membrane and activation of the cell membrane death receptor
CD95/Fas. Previous studies by our research group have found that
there are coupling interplays between biological apoptotic signals
and biomechanical remodeling during apoptosis (28). Therefore, further studies on
alterations in biomechanics contribute to a better understanding of
the anticancer mechanism of action of actin-targeted drugs, such as
cytochalasins.
Apoptosis is a complex multi-step process involving
a series of biological signals. Its characterization includes
morphological changes (cell contraction, chromatin condensation,
nuclear fragmentation and cytoplasmic membrane blebbing) and
energy-dependent biochemical molecular event cascades (PS exposure,
caspase activation and protein cross-linking) (37). However, with the improvements in
research methods and the continuous elucidation of apoptosis, a
growing number of studies report that apoptosis is not only a
cascade of biochemical signals, but is also accompanied by cellular
mechanical remodeling. In the present study, it was found that the
biological apoptotic marker PS did not expose on the cell surface,
CD95/Fas clustering was not obvious within the first 3 h of CB
treatment. But the elastic modulus and volume of the cells were
significantly decreased, and the surface roughness was
significantly increased. This finding is consistent with the
results of a recent study (38),
supporting that the significant changes in mechanical activity were
measured while the alterations in biological activity were yet
undetected. These significant changes in cellular mechanics may be
measured due to the superiority of the means of mechanical
research. The AFM adopted in the present study does not require
complex cellular processing and directly detects the mechanical
behavior of cells at the nanometer level under physiological
conditions in a real-time, quantitative and free-labeled manner.
Therefore: i) compared with biological signals, mechanical and
geometrical reconstruction is more sensitive during apoptosis; and
ii) compared with biological technology, biomechanical technology
is more sensitive for the detection of apoptosis.
HeLa cells induced by CB were observed by SEM, and
it was found that the lamellipodia and filopodia contracted, and a
large number of filamentous structures appeared on the cell surface
at 30 min. These filamentous structures were denser at 3 h. Actin
fluorescence staining also revealed a large number of spiny
structures at the periphery of the cells (Fig. 7H). According to the apoptotic
morphological characteristics, cytoplasmic membrane blebbing
appears during apoptosis (39). The
morphological features of the cell surface upon cytochalasin B
treatment largely resemble the well-documented cytoplasmic membrane
blebbing during apoptosis, which is mediated by caspase-triggered
activation of the Rho effector ROCK I b promoting myosin light
chain protein phosphorylation (40). Therefore, it is possible that
mechanical and geometrical features induced by cytochalasin B could
be the results of biological signals of apoptotic cells, such as
activation of apoptosis-specific nucleases and proteases. However,
the ultrastructure of HeLa cells after treatment with Polyalthia
longifolia leaf extract (PLME) was observed by SEM, and
membrane blebbing appeared at ~12 h (41). This finding is roughly consistent
with the significant activation of caspase at ~16–24 h during
CB-mediated HeLa cell apoptosis (4,9).
However, the increased surface roughness was observed within 3 h
after the HeLa cells were treated with CB (its effect is equivalent
to that of PLME). Moreover, the filamentous structures were
completely different from the blebbing described in the literature
(41). CB induces apoptosis by
disruption of the actin cytoskeleton and the fragments of actin
aggravated intracellular crowding. As a matter of fact, eukaryotic
cells are surprisingly crowded (42). According to Monte Carlo simulations
(43), the increase in molecular
crowding leads to biophysical molecule redistribution. In
semi-permeable biofilm cavity, as the crowding increases, the
macromolecules are redistributed to the wall of the cavity,
allowing some molecules to protrude across the biofilm (44). The protrusions are the filamentous
structures observed by SEM and are also responsible for the
increased cell surface roughness. Taken together, these findings
suggest that the changes in cell surface roughness are caused by
the redistribution of protein molecules inside the cells.
In addition to the changes in mechanics, geometry
and surface roughness, cytoskeletal disruption was accompanied by
the downregulation of vinculin expression. Vinculin, a widely
expressed ABP, connects the actin cytoskeleton and the focal
adhesion proteins and is involved in the formation of the focal
adhesion-actin cytoskeleton signaling cascade (45). Vinculin can affect this cascade and
modulate cell survival and apoptosis through focal adhesion
proteins. For example, vinculin regulates apoptosis by disturbing
the interactions between focal adhesive kinase (FAK) and paxillin
(46). Interfering with FAK can
induce the activation of CD95/Fas-related death domains, leading to
apoptosis (47). The present study
only found that vinculin expression continuously decreased and the
CD95/Fas was gradually activated during apoptosis. The
downregulation of vinculin disrupted the focal
adhesion-cytoskeletal signaling cascade. However, there is not any
evidence to support a direct cause-effect relationship between the
vinculin expression and CD95/Fas activation. Vinculin expression
decrease was only correlated with the increase in CD95/Fas
expression.
The CB-triggered disruption of the actin
cytoskeleton rapidly caused the decrease in cellular elasticity and
volume, and the increase in cell surface roughness and
intracellular crowding, ultimately leading to apoptosis. Moreover,
these alterations occurred prior to the changes of biological
apoptosis signals, including PS and CD95/Fas.
In summary, the results of the present study
suggested that, compared with biological signals, biomechanical and
geometric reconstruction is more sensitive during apoptosis. The
CB-induced disruption of the actin cytoskeleton causes
redistribution of protein molecules inside the cells, and some
protein molecules protrude from the cell membrane, which is an
explanation for the observed increased cell surface roughness.
These findings may improve our understanding of the apoptosis
mechanisms of cancer cells mediated by cytochalasins.
Acknowledgements
The authors thank the Key Lab of Stomatology of
State Ethnic Affairs Commission (Northwest Minzu University) and
Ms. Fei Tang and Mr. Ming-zhong Chen for their technical assistance
with the flow cytometric analysis and imaging with CLSM,
respectively.
Funding
The present study was supported by grants from the
Fundamental Research Funds for the Central Universities (no.
31920150006, lzujbky-2017-ot11) and the National Nature Science
Foundation of China (nos. 11472119, 11421062 and 81660189).
Availability of data and materials
The datasets used during the present study are
available from the corresponding authors upon reasonable
request.
Authors' contributions
XS performed most of the experiments. JW and HK
designed the experiments and coordinated the project. GB, BZ and LZ
participated in the in vitro study. All authors participated
in the collection of the data. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of
interest.
References
|
1
|
Bonder EM and Mooseker MS: Cytochalasin B
slows but does not prevent monomer addition at the barbed end of
the actin filament. J Cell Biol. 102:282–288. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zigmond SH: Beginning and ending an actin
filament: Control at the barbed end. Curr Top Dev Biol. 63:145–188.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gourlay CW and Ayscough KR: The actin
cytoskeleton: A key regulator of apoptosis and ageing? Nat Rev Mol
Cell Biol. 6:583–589. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kulms D, Düssmann H, Pöppelmann B, Ständer
S, Schwarz A and Schwarz T: Apoptosis induced by disruption of the
actin cytoskeleton is mediated via activation of CD95 (Fas/APO-1).
Cell Death Differ. 9:598–608. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Trendowski M, Christen T, Acquafondata C
and Fondy T: Effects of cytochalasin congeners,
microtubule-directed agents, and doxorubicin alone or in
combination against human ovarian carcinoma cell lines in vitro.
BMC Cancer. 15:6322015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Walker SR, Chaudhury M, Nelson EA and
Frank DA: Microtubule-targeted chemotherapeutic agents inhibit
signal transducer and activator of transcription 3 (STAT3)
signaling. Mol Pharmacol. 78:903–908. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bezabeh T, Mowat MR, Jarolim L, Greenberg
AH and Smith IC: Detection of drug-induced apoptosis and necrosis
in human cervical carcinoma cells using 1H NMR spectroscopy. Cell
Death Differ. 8:219–224. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Raguz S and Yagüe E: Resistance to
chemotherapy: New treatments and novel insights into an old
problem. Br J Cancer. 99:387–391. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hwang J, Yi M, Zhang X, Xu Y, Jung JH and
Kim DK: Cytochalasin B induces apoptosis through the mitochondrial
apoptotic pathway in HeLa human cervical carcinoma cells. Oncol
Rep. 30:1929–1935. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lecuit T and Lenne PF: Cell surface
mechanics and the control of cell shape, tissue patterns and
morphogenesis. Nat Rev Mol Cell Biol. 8:633–644. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
DuFort CC, Paszek MJ and Weaver VM:
Balancing forces: Architectural control of mechanotransduction. Nat
Rev Mol Cell Biol. 12:308–319. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bakal C, Aach J, Church G and Perrimon N:
Quantitative morphological signatures define local signaling
networks regulating cell morphology. Science. 316:1753–1756. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kerr JF, Wyllie AH and Currie AR:
Apoptosis: A basic biological phenomenon with wide-ranging
implications in tissue kinetics. Br J Cancer. 26:239–257. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qiu M, Chen L, Tan G, Ke L, Zhang S, Chen
H and Liu J: A reactive oxygen species activation mechanism
contributes to JS-K-induced apoptosis in human bladder cancer
cells. Sci Rep. 5:151042015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fulda S: Molecular pathways: Targeting
inhibitor of apoptosis proteins in cancer-from molecular mechanism
to therapeutic application. Clin Cancer Res. 20:289–295. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li J and Yuan J: Caspases in apoptosis and
beyond. Oncogene. 27:6194–6206. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jeong JJ, Park N, Kwon YJ, Ye DJ, Moon A
and Chun YJ: Role of annexin A5 in cisplatin-induced toxicity in
renal cells: Molecular mechanism of apoptosis. J Biol Chem.
289:2469–2481. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim KS, Cho CH, Park EK, Jung MH, Yoon KS
and Park HK: AFM-detected apoptotic changes in morphology and
biophysical property caused by paclitaxel in Ishikawa and HeLa
cells. PLoS One. 7:e300662012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Susanto O, Stewart SE, Voskoboinik I,
Brasacchio D, Hagn M, Ellis S, Asquith S, Sedelies KA, Bird PI,
Waterhouse NJ, et al: Mouse granzyme A induces a novel death with
writhing morphology that is mechanistically distinct from granzyme
B-induced apoptosis. Cell Death Differ. 20:1183–1193. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pelling AE, Veraitch FS, Chu CP, Mason C
and Horton MA: Mechanical dynamics of single cells during early
apoptosis. Cell Motil Cytoskeleton. 66:409–422. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schulze C, Müller K, Käs JA and Gerdelmann
JC: Compaction of cell shape occurs before decrease of elasticity
in CHO-K1 cells treated with actin cytoskeleton disrupting drug
cytochalasin D. Cell Motil Cytoskeleton. 66:193–201. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Müller DJ and Dufrêne YF: Atomic force
microscopy as a multifunctional molecular toolbox in
nanobiotechnology. Nat Nanotechnol. 3:261–269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Samarakoon R and Higgins PJ: MEK/ERK
pathway mediates cell-shape-dependent plasminogen activator
inhibitor type I gene expression upon drug-induced disruption of
the microfilament and microtubule networks. J Cell Sci.
115:3093–3103. 2002.PubMed/NCBI
|
|
24
|
Galluzzi L, Maiuri MC, Vitale I, Zischka
H, Castedo M, Zitvogel L and Kroemer G: Cell death modalities:
Classification and pathophysiological implications. Cell Death
Differ. 14:1237–1243. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cross SE, Jin YS, Rao J and Gimzewski JK:
Nanomechanical analysis of cells from cancer patients. Nat
Nanotechnol. 2:780–783. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nagata S, Hanayama R and Kawane K:
Autoimmunity and the clearance of dead cells. Cell. 140:619–630.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Martin SJ, Reutelingsperger CP, McGahon
AJ, Rader JA, van Schie RC, LaFace DM and Green DR: Early
redistribution of plasma membrane phosphatidylserine is a general
feature of apoptosis regardless of the initiating stimulus:
Inhibition by overexpression of Bcl-2 and Abl. J Exp Med.
182:1545–1556. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Suzuki J, Denning DP, Imanishi E, Horvitz
HR and Nagata S: Xk-related protein 8 and CED-8 promote
phosphatidylserine exposure in apoptotic cells. Science.
341:403–406. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang B, Li L, Li Z, Liu Y, Zhang H and
Wang J: Carbon ion-irradiated hepatoma cells exhibit coupling
interplay between apoptotic signaling and morphological and
mechanical remodeling. Sci Rep. 6:351312016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang B, Liu B, Zhang H and Wang J:
Erythrocyte stiffness during morphological remodeling induced by
carbon ion radiation. PLoS One. 9:e1126242014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hessler JA, Budor A, Putchakayala K, Mecke
A, Rieger D, Banaszak Holl MM, Orr BG, Bielinska A, Beals J and
Baker J Jr: Atomic force microscopy study of early morphological
changes during apoptosis. Langmuir. 21:9280–9286. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gavara N: Combined strategies for optimal
detection of the contact point in AFM force-indentation curves
obtained on thin samples and adherent cells. Sci Rep. 6:212672016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sobiepanek A, Milner-Krawczyk M, Lekka M
and Kobiela T: AFM and QCM-D as tools for the distinction of
melanoma cells with a different metastatic potential. Biosens
Bioelectron. 93:274–281. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zuk A, Targosz-Korecka M and Szymonski M:
Effect of selected drugs used in asthma treatment on morphology and
elastic properties of red blood cells. Int J Nanomedicine.
6:249–257. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Geiger B, Spatz JP and Bershadsky AD:
Environmental sensing through focal adhesions. Nat Rev Mol Cell
Biol. 10:21–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Magro AM, Magro AD, Cunningham C and
Miller MR: Down-regulation of vinculin upon MK886-induced apoptosis
in LN18 glioblastoma cells. Neoplasma. 54:517–526. 2007.PubMed/NCBI
|
|
37
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wu S, Liu X, Zhou X, Liang XM, Gao D, Liu
H, Zhao G, Zhang Q and Wu X: Quantification of cell viability and
rapid screening anti-cancer drug utilizing nanomechanical
fluctuation. Biosens Bioelectron. 77:164–173. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Barros LF, Kanaseki T, Sabirov R,
Morishima S, Castro J, Bittner CX, Maeno E, Ando-Akatsuka Y and
Okada Y: Apoptotic and necrotic blebs in epithelial cells display
similar neck diameters but different kinase dependency. Cell Death
Differ. 10:687–697. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sebbagh M, Renvoizé C, Hamelin J, Riché N,
Bertoglio J and Bréard J: Caspase-3-mediated cleavage of ROCK I
induces MLC phosphorylation and apoptotic membrane blebbing. Nat
Cell Biol. 3:346–352. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Vijayarathna S, Chen Y, Kanwar JR and
Sasidharan S: Standardized Polyalthia longifolia leaf extract
(PLME) inhibits cell proliferation and promotes apoptosis: The
anti-cancer study with various microscopy methods. Biomed
Pharmacother. 91:366–377. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Doyle AD and Yamada KM: Cell biology:
Sensing tension. Nature. 466:192–193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lee B, Leduc PR and Schwartz R: Unified
regression model of binding equilibria in crowded environments. Sci
Rep. 1:972011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shew CY, Kondo K and Yoshikawa K: Rigidity
of a spherical capsule switches the localization of encapsulated
particles between inner and peripheral regions under crowding
condition: Simple model on cellular architecture. J Chem Phys.
140:0249072014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Humphries JD, Wang P, Streuli C, Geiger B,
Humphries MJ and Ballestrem C: Vinculin controls focal adhesion
formation by direct interactions with talin and actin. J Cell Biol.
179:1043–1057. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cui S, Wang J, Wu Q, Qian J, Yang C and Bo
P: Genistein inhibits the growth and regulates the migration and
invasion abilities of melanoma cells via the FAK/paxillin and MAPK
pathways. Oncotarget. 8:21674–21691. 2017.PubMed/NCBI
|
|
47
|
Xu LH, Yang X, Bradham CA, Brenner DA,
Baldwin AS Jr, Craven RJ and Cance WG: The focal adhesion kinase
suppresses transformation-associated, anchorage-independent
apoptosis in human breast cancer cells. Involvement of death
receptor-related signaling pathways. J Biol Chem. 275:30597–30604.
2000. View Article : Google Scholar : PubMed/NCBI
|