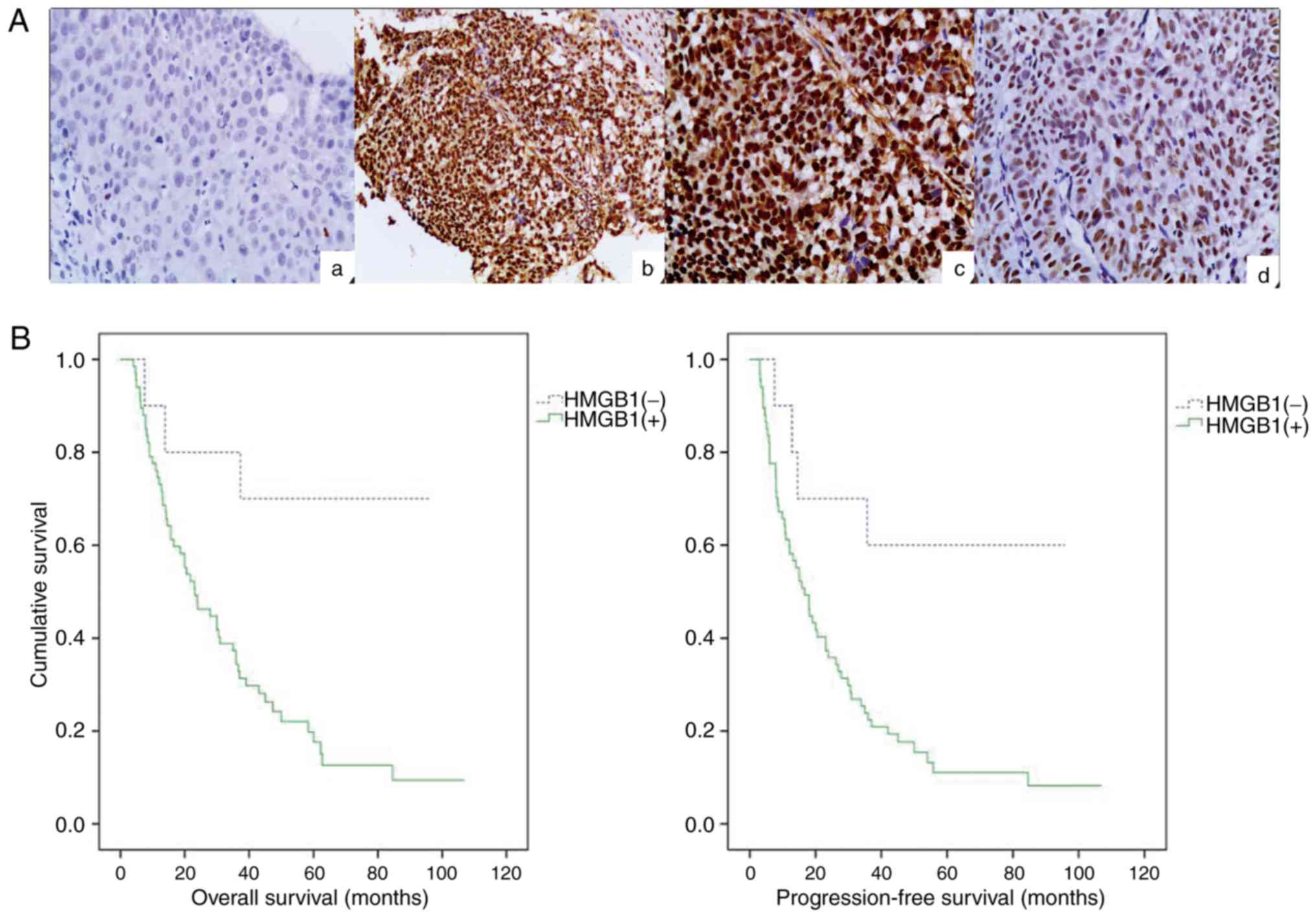
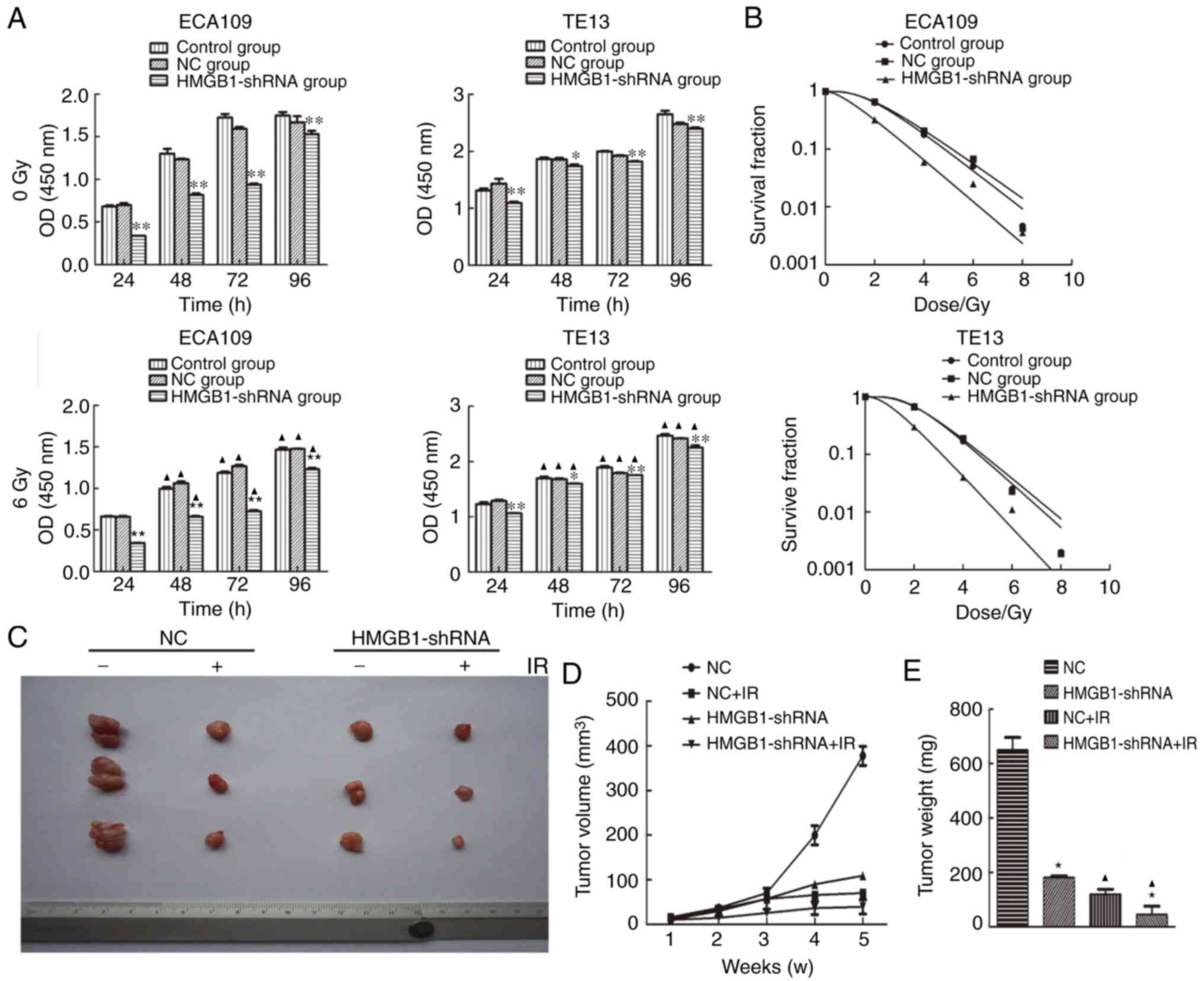
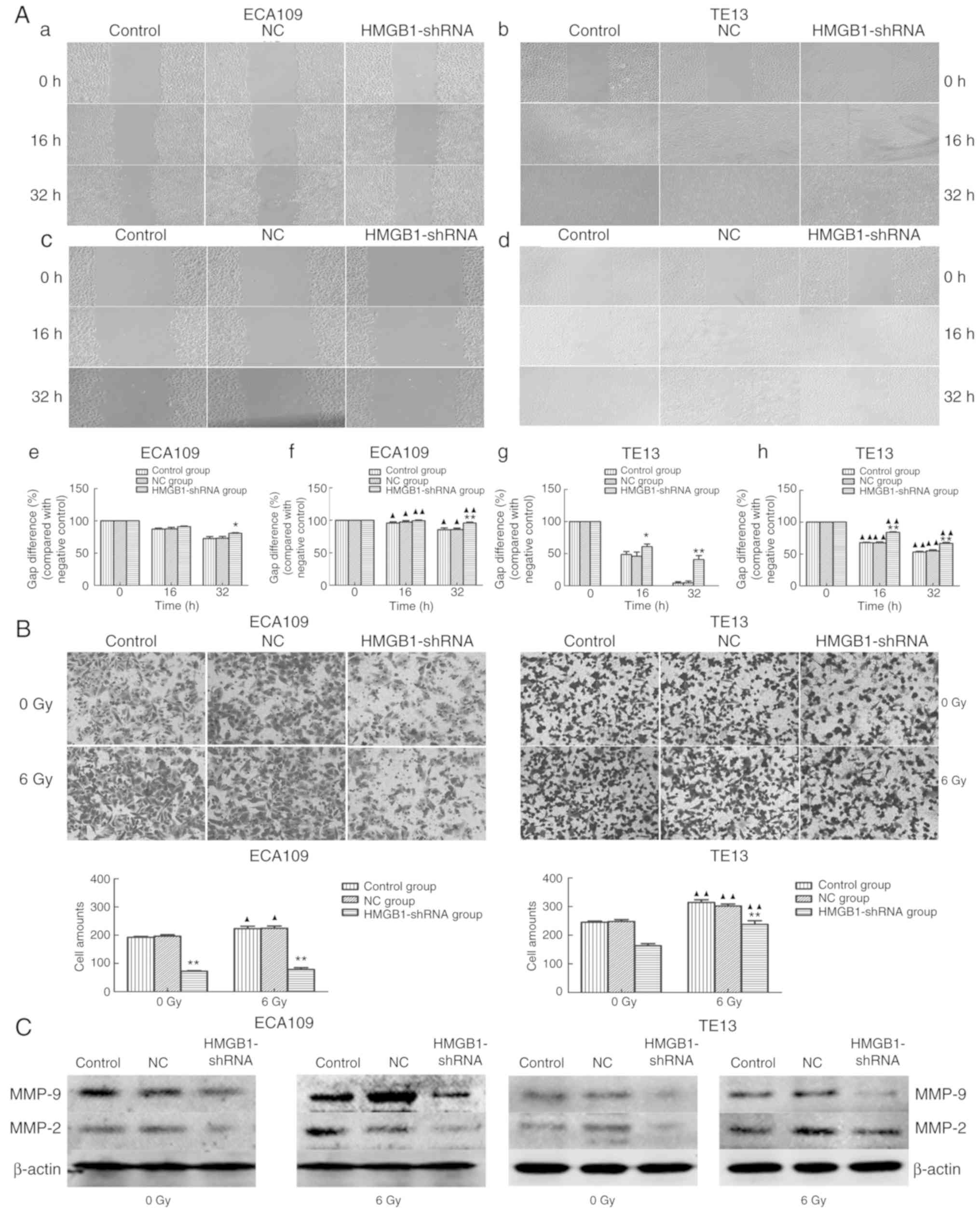
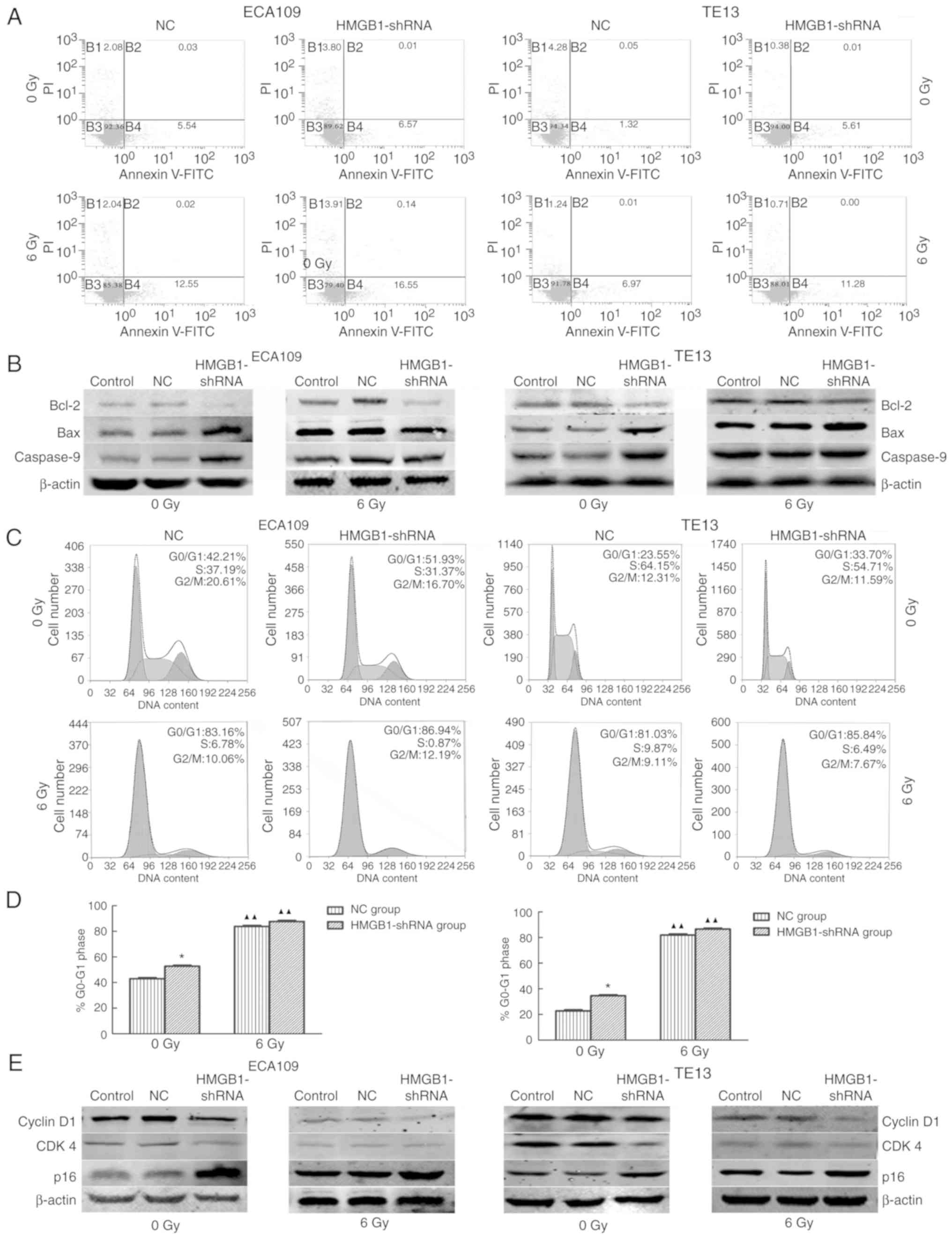
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pennathur A, Gibson MK, Jobe BA and
Luketich JD: Oesophageal carcinoma. Lancet. 381:400–412. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ishihara R, Yamamoto S, Iishi H, Takeuchi
Y, Sugimoto N, Higashino K, Uedo N, Tatsuta M, Yano M, Imai A, et
al: Factors predictive of tumor recurrence and survival after
initial complete response of esophageal squamous cell carcinoma to
definitive chemoradiotherapy. Int J Radiat Oncol Biol Phys.
76:123–129. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tang D, Kang R, Zeh HJ III and Lotze MT:
High-mobility group box 1 and cancer. Biochim Biophys Acta 1799.
131–140. 2010.
|
|
5
|
Kang R, Zhang Q, Zeh HJ III, Lotze MT and
Tang D: HMGB1 in cancer: Good, bad, or both? Clin Cancer Res.
19:4046–4057. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yuan F, Gu L, Guo S, Wang C and Li GM:
Evidence for involvement of HMGB1 protein in human DNA mismatch
repair. J Biol Chem. 279:20935–20940. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lange SS, Mitchell DL and Vasquez KM: High
mobility group protein B1 enhances DNA repair and chromatin
modification after DNA damage. Proc Natl Acad Sci USA.
105:10320–10325. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shrivastava S, Mansure JJ, Almajed W, Cury
F, Ferbeyre G, Popovic M, Seuntjens J and Kassouf W: The role of
HMGB1 in radioresistance of bladder cancer. Mol Cancer Ther.
15:471–479. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin HJ, Liu HH, Lin CD, Kao MC, Chen YA,
Chiang-Ni C, Jiang ZP, Huang MZ, Lin CJ, Lo UG, et al: Cytolethal
distending toxin enhances radiosensitivity in prostate cancer cells
by regulating autophagy. Front Cell Infect Microbiol. 7:2232017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ke S, Zhou F, Yang H, Wei Y, Gong J, Mei
Z, Wu L, Yu H and Zhou Y: Downregulation of high mobility group box
1 modulates telomere homeostasis and increases the radiosensitivity
of human breast cancer cells. Int J Oncol. 46:1051–1058. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hongo K, Kazama S, Tsuno NH, Ishihara S,
Sunami E, Kitayama J and Watanabe T: Immunohistochemical detection
of high-mobility group box 1 correlates with resistance of
preoperative chemoradiotherapy for lower rectal cancer: A
retrospective study. World J Surg Oncol. 13:72015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Soumaoro LT, Uetake H, Higuchi T, Takagi
Y, Enomoto M and Sugihara K: Cyclooxygenase-2 expression: A
significant prognostic indicator for patients with colorectal
cancer. Clin Cancer Res. 10:8465–8471. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu D, Ding Y, Wang S, Zhang Q and Liu L:
Increased expression of high mobility group box 1 (HMGB1) is
associated with progression and poor prognosis in human
nasopharyngeal carcinoma. J Pathol. 216:167–175. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boonstra JJ, van der Velden AW, Beerens
EC, van Marion R, Morita-Fujimura Y, Matsui Y, Nishihira T,
Tselepis C, Hainaut P, Lowe AW, et al: Mistaken identity of widely
used esophageal adenocarcinoma cell line TE-7. Cancer Res.
67:7996–8001. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2ΔΔCT method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang XX, Ma M, Sang MX, Wang XX, Song H,
Liu ZK and Zhu SC: Radiosensitization of esophageal carcinoma cells
by knockdown of RNF2 expression. Int J Oncol. 48:1985–1996. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Y: Epidemiology of esophageal
cancer. World J Gastroenterol. 19:5598–5606. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sohun M and Shen H: The implication and
potential applications of high-mobility group box 1 protein in
breast cancer. Ann Transl Med. 4:2172016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang H, Hu X, Zhang H and Li W:
Down-regulation of LncRNA TUG1 enhances radiosensitivity in bladder
cancer via suppressing HMGB1 expression. Radiat Oncol. 12:652017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shi Z, Huang Q, Chen J, Yu P, Wang X, Qiu
H, Chen Y and Dong Y: Correlation of HMGB1 expression to
progression and poor prognosis of adenocarcinoma and squamous
cell/adenosquamous carcinoma of gallbladder. Am J Transl Res.
7:2015–2025. 2015.PubMed/NCBI
|
|
21
|
Tabata C, Shibata E, Tabata R, Kanemura S,
Mikami K, Nogi Y, Masachika E, Nishizaki T and Nakano T: Serum
HMGB1 as a prognostic marker for malignant pleural mesothelioma.
BMC Cancer. 13:2052013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peng T, Hu M, Wu T, Chen Z, Zhang C, Huang
S and Zhou X: Effects of high-mobility group box 1 knockdown on
proliferation, migration and invasion of the HONE-1 human
nasopharyngeal carcinoma cell line. Mol Med Rep. 12:7531–7537.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Suzuki Y, Mimura K, Yoshimoto Y, Watanabe
M, Ohkubo Y, Izawa S, Murata K, Fujii H, Nakano T and Kono K:
Immunogenic tumor cell death induced by chemoradiotherapy in
patients with esophageal squamous cell carcinoma. Cancer Res.
72:3967–3976. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gebhardt C, Riehl A, Durchdewald M, Németh
J, Fürstenberger G, Müller-Decker K, Enk A, Arnold B, Bierhaus A,
Nawroth PP, et al: RAGE signaling sustains inflammation and
promotes tumor development. J Exp Med. 205:275–285. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu Z, Falo LD Jr and You Z: Knockdown of
HMGB1 in tumor cells attenuates their ability to induce regulatory
T cells and uncovers naturally acquired CD8 T cell-dependent
antitumor immunity. J Immunol. 187:118–125. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
van Beijnum JR, Nowak-Sliwinska P, van den
Boezem E, Hautvast P, Buurman WA and Griffioen AW: Tumor
angiogenesis is enforced by autocrine regulation of high-mobility
group box 1. Oncogene. 32:363–374. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Giavara S, Kosmidou E, Hande MP, Bianchi
ME, Morgan A, d'Adda di Fagagna F and Jackson SP: Yeast Nhp6A/B and
mammalian Hmgb1 facilitate the maintenance of genome stability.
Curr Biol. 15:68–72. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tang D, Kang R, Livesey KM, Kroemer G,
Billiar TR, Van Houten B, Zeh HJ III and Lotze MT: High-mobility
group box 1 is essential for mitochondrial quality control. Cell
Metab. 13:701–711. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Taguchi A, Blood DC, del Toro G, Canet A,
Lee DC, Qu W, Tanji N, Lu Y, Lalla E, Fu C, et al: Blockade of
RAGE-amphoterin signalling suppresses tumour growth and metastases.
Nature. 405:354–360. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livesey KM, Kang R, Vernon P, Buchser W,
Loughran P, Watkins SC, Zhang L, Manfredi JJ, Zeh HJ III, Li L, et
al: p53/HMGB1 complexes regulate autophagy and apoptosis. Cancer
Res. 72:1996–2005. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Krynetskaia NF, Phadke MS, Jadhav SH and
Krynetskiy EY: Chromatin-associated proteins HMGB1/2 and PDIA3
trigger cellular response to chemotherapy-induced DNA damage. Mol
Cancer Ther. 8:864–872. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Soutoglou E and Misteli T: Activation of
the cellular DNA damage response in the absence of DNA lesions.
Science. 320:1507–1510. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Krynetskaia N, Xie H, Vucetic S, Obradovic
Z and Krynetskiy E: High mobility group protein B1 is an activator
of apoptotic response to antimetabolite drugs. Mol Pharmacol.
73:260–269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kessenbrock K, Wang CY and Werb Z: Matrix
metalloproteinases in stem cell regulation and cancer. Matrix Biol.
44-46:184–190. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee CC, Wang CN, Lee YL, Tsai YR and Liu
JJ: High mobility group box 1 induced human lung myofibroblasts
differentiation and enhanced migration by activation of MMP-9. PLoS
One. 10:e01163932015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Agami R and Bernards R: Distinct
initiation and maintenance mechanisms cooperate to induce G1 cell
cycle arrest in response to DNA damage. Cell. 102:55–66. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cheng P, Dai W, Wang F, Lu J, Shen M, Chen
K, Li J, Zhang Y, Wang C, Yang J, et al: Ethyl pyruvate inhibits
proliferation and induces apoptosis of hepatocellular carcinoma via
regulation of the HMGB1-RAGE and AKT pathways. Biochem Biophys Res
Commun. 443:1162–1168. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Allan LA and Clarke PR: Apoptosis and
autophagy: Regulation of caspase-9 by phosphorylation. FEBS J.
276:6063–6073. 2009. View Article : Google Scholar : PubMed/NCBI
|