Introduction
The liver has a key role in the metabolism and
detoxification of drugs. This role induces the exposure of the
liver to metabolites and toxins that, in turn, can cause chronic
inflammation (1) and in some cases
the development and pathogenesis of acute and chronic hepatic
diseases (2–4). Chronic inflammation of the liver
causes cellular damage and the deposit of a high amount of lipids
in the hepatocytes which is associated with a higher risk of
steatohepatitis, fibrosis and cancer (5–9). In
detail, liver tissue consists of several cell components involved
in inflammatory processes. These cell components include leukocytes
and Kupffer cells, that can be stimulated by toxins or other
inflammatory reactions to produce cytokines such as transforming
growth factor (TGF)-β, tumor necrosis factor (TNF)-α and
interleukin (IL)-6 (10). Among
these, TNF-α activates several intracellular pathways regulating
inflammation, cell death and proliferation (11,12).
TNF-α is mainly produced in response to lipopolysaccharides (LPS)
and other bacterial products. During hepatocyte injury, Kupffer
cells are able to produce TNF-α which increases the secretion of
IL-6 in an autocrine manner with subsequent induction of hepatocyte
proliferation. IL-6 is crucial for hepatocyte homeostasis and
mitogenic activity; in fact, it is involved in liver regeneration
but extended activation of the IL-6/IL-6-R signaling pathway is
crucial in the initiation and progression of hepatocellular
carcinoma (HCC) (13,14). In patients affected by HCC, a high
amount of macrophages infiltrating in the tumor is correlated with
the negative prognosis of the disease. This finding can be due to
IL-6 hypersecretion that, in turn, increases the generation of HCC
progenitor cells (HcPCs). Moreover, TGF-β is a growth factor with
pro-fibrogenic and immunosuppressive activity (15,16).
Therefore, its expression induces hepatocyte death and metabolic
modulation of cells involved in the wound-healing response such as
hepatic stellate cells and liver fibroblasts (17,18).
Overexpression of TGF-β is involved in several liver diseases
(19) and could be directly
involved in the tumorigenic process (20).
During the last few years, great emphasis has been
given to the ‘gut-liver axis’, and on the role of intestinal
bacterial flora in inducing positive or negative effects on liver
health protecting tissue from inflammatory injuries (21). For example, saccharolytic
fermentation (carried out mainly by Lactobacillus spp) of
unabsorbed and indigestible carbohydrates of soluble dietary
fibres, is an essential mechanism leading to the production of
short-chain fatty acids (SCFAs) (i.e. acetate, propionate and
butyrate) that display a well-known anti-inflammatory action in
liver tissues (22–24). Among the SCFAs, intestinal
epithelial cells derive the most useful energy for processes such
as proliferation and differentiation, secretion of mucus, decrease
in inflammation in different organs, from butyric acid (BA).
Specifically, BA decreases the production of cytokines such as
IL-6, IL-8, TNF-α and TGF-β, that have a central function in liver
inflammation and are involved in triggering fibrosis as well as
cancer (25,26). However, oral administration of BA is
clinically not easy due to both its unpleasant taste and
inefficient intestinal absorption (27,28).
Nevertheless, in the last few years some randomized controlled
trials have demonstrated the beneficial effects of BA after oral
administration even though an important limitation in these
clinical trials was its low bio-availability due to the
interference of gut microbiota that can change its concentration
and absorption (29). The recent
use of nanotechnology in medicine has brought important advances in
the delivery of drugs in inflamed and cancer tissues. In light of
this, liposomes are lipidic nanocarriers able to protect a drug
from the external environment, enzymatic attack and immune
recognition with consequently increased bioavailability, controlled
drug delivery, biodistribution in targets such as cancer tissues
and reduced toxicity (30–32). Liposomes and other nanocarriers are
directly involved in altering the biodistribution of certain
anticancer agents that cannot be efficiently delivered, in their
free formulation, in cancer tissues or cannot be appropriately
adsorbed by the gut (33).
Liposomes loaded with bioactive molecules, such as polyphenol or
SCFAs, and orally administered can represent important tools with
which to enhance bio-drug plasma concentration and specific
delivery to the liver (34,35). For the oral administration of
liposomes, surface liposome coating with natural polymers could be
an efficient strategy to increase gut absorption and, among these,
chitosan is one of the most promising. Chitosan is a natural
polysaccharide derived from chitin and due to its properties, such
as hydrophilicity, bioadhesivity, biocompatibility,
biodegradability and low toxicity can be considered as a novel drug
delivery system that improves the oral bioavailability of drugs by
prolonging the residence time at the site of intestinal absorption
(36). Moreover, chitosan induces a
redistribution of cytoskeletal F-actin and tight junction protein
ZO-1 via interaction between its positive charges and the
enterocyte surface negative charges, which results in increased
paracellular permeability for hydrophilic macromolecules. In the
present study, we investigated the in vitro anticancer
activity and anti-inflammatory properties of chitosan-coated and
-uncoated liposomes loaded with BA in hepatoblastoma (HB) HepG2
cells (37).
Materials and methods
Materials
Butyric acid, cholesterol, sodium
phosphatidylcholines, fluoresceineamine (FA), Spectra/Por Biotech
cellulose ester membrane (cut-off 5 kDa) and ethanol were purchased
from Sigma-Aldrich/Merck KGaA (Milan, Italy). To carry out
synthesis and characterization, we used purified and distillated
water by reverse osmosis (Milli-Q Plus; Thermo Fisher Scientific,
Milan, Italy).
Methods
Synthesis and chemical
characterization of liposomes and fluorescent liposomes
Thin-film hydration tecnique was used to prepare
liposomes either loaded or not with BA and chitosan-coated or
uncoated, as previously described (38) but with some modifications (36). Specifically, as an example for the
synthesis of uncoated liposomes loaded with BA, we used chloroform
to dissolve sodium phosphatidylcholines (SPC)/cholesterol/butyric
acid (20/5/4, w/w) and rotary evaporation at 37°C to dry and form a
thin film. The organic solvent was completely removed by drying
under vacuum. To hydrate the thin film we used 20 ml of 50 mM
citric acid and this solution was shaken and mixed. Finally, the pH
of the suspension was adjusted to 6.8 with 50 mM
Na2CO3 and, in order to obtain small and
homogeneous liposomes, the obtained nanocarriers were sonicated for
10 min (1 mHz) by using a sonicator (Sonics VCX 500 Vibra Cell™;
Sonics & Materials, Inc., Newton, CT, USA). An aliquot of
liposomes loaded with BA was added with the same volume of chitosan
(0.1%) in PBS (phosphate-buffered saline; pH 6.8) and then
incubated at 4°C for 1 h to prepare the chitosan-coated liposomes
loaded with BA.
For the biological studies of cellular
internalization and imaging by confocal laser scanning microscopy
(CLSM) fluorescently, chitosan-coated and -uncoated liposomes were
prepared with a solution of 0.1 mg/ml of fluoresceineamine (FA) in
PBS to the lipid solution before preparing liposomes as described
before. The particle sizes and zeta (ζ) potentials of the final
products were measured with a Zetasizer ZS nano series ZEN 3600
(Malvern Instruments Ltd., Malvern, UK) and 50 runs were carried
out for each measurement for ζ potential analysis, whereas the
default refractive index ratio (1.52) and 5 runs for each
measurement (1 run lasting 100 sec) were used in the calculations
of the particle size distribution.
Cell viability
To evaluate the cytotoxicity of all substances on
human hepatoblastoma (HB) HepG2 cells (HB-8065™; ATCC®,
American Type Culture Collection, Manassas, VA, USA), through their
mitochondrial dehydrogenase activity, a modified MTT test
[3-(4,5-dimethyldiazol-2-yl)-2,5- diphenyltetrazolium bromide] was
carried out according to the manufacturer's instructions (Dojindo
Molecular Technologies Inc., Rockville, MD, USA). Specifically, the
culture medium used to grow the HepG2 cell line (HB-8065 ATCC) was
Dulbecco's modified Eagle's medium (DMEM; Sigma Aldrich S.L.R.,
Milan, Italy) supplemented with 10% FBS and 1% Pen-Strep and cells
was seeded in 96-well plates at a density of 10,000 cells per well
at 37°C in a humidified 5% CO2 atmosphere. After 24 h of
growth, the following formulations were added to the cells in full
medium: empty liposomes (Lip), empty chitosan-coated liposomes
(Chit-Lip), uncoated liposomes loaded with BA (Lip-BA),
chitosan-coated liposomes loaded with BA (Chit-Lip-BA), free BA
(BA); for both loaded nanocarriers and free BA the fatty acid was
tested always at the corresponding concentrations ranging from 0.05
to 10 mM. Cells were then incubated from 24 to 72 h under standard
conditions and subsequently the cells were washed three times with
PBS at pH 7.4 and incubated with 100 µl with a MTT solution (0.5
mg/ml in cell culture medium) for 4 h at 37°C. Tecan Infinite M200
plate-reader (Tecan Group, Ltd., Mannedorf, Switzerland) with
I-control software (Tecan Infinite i-control 1.8.50.0) was used to
acquire the absorbance at 450 nm. The relative cell viability (%)
was calculated by the formula
[A]test/[A]control × 100, where
‘[A]test’ is the absorbance of the test sample, and
‘[A]control’ is the absorbance of the control cells
incubated solely with culture medium. After this evaluation was
carried out, the Micro BCA protein assay kit (Pierce) was utilized
to quantify total protein content. This method provides washing of
the cells with ice-cold PBS, and the incubation of these for 15 min
in 150 µl of lysis buffer (0.5% v/v Triton X-100 in PBS), and 150
µl of Micro BCA protein assay kit reagent (prepared following the
instructions of the manufacturer) and finally the absorbance was
measured at 562 nm. The cytotoxicity measurements were then
normalized by the amount of total protein content in each well.
Cellular uptake studies
Imaging by confocal laser scanning microscope. The
culture medium used to grow the HepG2 cells (HB-8065 ATCC) was DMEM
supplemented with 10% FBS and 1% Pen-Strep and cells were seeded in
24-well plates at a density of 5×103 cells/well at 37°C
in a humidified 5% CO2 atmosphere for 24 h. For the
imaging studies, we followed the same procedure described in the
literature (39,40) where the medium was replaced with 0.3
mg/ml of Chit-Lip-FA solution in culture medium and incubated for
0.5, 4, 8 and 24 h; after incubation time with fluorescent
liposomes, the cells were washed three times with PBS and fixed
with 2.5% glutaraldehyde in PBS for 20 min. Concanavalin A
tetramethylrhodamine conjugate (Invitrogen, Life Technology; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) was used to stain the
membrane at a final concentration of 100 µg/ml. After washing in
PBS, human cells were blocked with 1% BSA in PBS for 20 min and
washed three times with PBS. For data acquisition, we used a
confocal microscope (C1 Nikon; Nikon Cor., Tokyo, Japan) with EZ-C1
software (Nikon Corp.) and 60× or 100× oil immersion objective,
imaging of the fluorescent liposomes was evaluated by
excitation/emission at 492/518 nm, and the cell membrane with
excitation/emission at 555/580 nm.
Quantification of uptake
The culture medium used to grow HepG2 cells (ATCC
HB-8065) was supplemented with DMEM with 10% FBS and 1% Pen-Strep
and cells were seeded in 24-well plates at a density of
5×103 per well at 37°C in a humidified 5% CO2
atmosphere for 24 h. For uptake quantification, we followed the
same protocol used in our previous work (32). Substantially, each well with the
proper cell concentration was washed and 0.1 ml of 1 mg/ml solution
of either fluorescent uncoated (Lip-FA) or chitosan-coated
(Chit-Lip-FA) liposomes was added to the culture medium and cells
were incubated for a time ranging from 0.5 to 24 h. After this
time, the supernatant was removed and cells were washed three times
with 10 mM PBS and, then, the lysate with 0.1 ml of 0.5% Triton
X-100 in 0.2 N NaOH. The fluorescence of the cell lysate
(λexc = 485 nm, λem = 535 nm) allowed the
evaluation and quantification of the membrane-bound and
internalized fluorescent liposomes, using a calibration curve
ranged from 0.001 up to 0.6 mg/ml of fluorescent liposomes
dispersed in a cell lysate solution (106 untreated cells
dissolved in 1 ml of the Triton X-100/0.2 N NaOH solution). For
both calibration curve and Lip-FA/Chit-Lip-FA cellular uptake
determination, the fluorescence was measured at the proper
wavelengths by using a spectrofluorometer (xMark Microplate
spectrofluorometer; Bio-Rad Laboratories, Milan, Italy).
Mechanistic studies
In order to understand the mechanisms of liposome
internalization in HepG2 cells, we studied the effects of specific
pharmacological treatments, such as bafilomycin A1 [selective
reversible inhibitor of vacuolar H+-ATPases (V-ATPases)
which inhibits autophagy by preventing vacuolar acidification
necessary for autophagosome maturation] (41,42),
filipin (inhibitor of caveolae-mediated endocytosis), nocodazol
(rapidly reversible inhibitor of microtubule polymerization),
cytochalasin D (a well-known selective inhibitor of actin
polymerization), hypertonic sucrose and potassium-free buffer
(which inhibit the clathrin-mediated uptake with lower or higher
selectivity, respectively) and sodium azide (a metabolic inhibitor
of cell respiration,
Cell uptake experiments were performed at 4 h of
incubation with fluorescent uncoated (Lip-FA) and chitosan-coated
(Chit-Lip-FA) liposomes in the presence of these inhibitors,
specifically: 0.45 M sucrose, 0.1 mg/ml of cytochalasin D, 1 mg/ml
of nocodazole, 0.1 mg/ml of filipin and 2×10−7 M of
bafilomycin A1. To quantify the energy dependence of the process,
we performed other experiments in which cancer cells were incubated
with 10−2 M of sodium azide for 30 min prior to liposome
uptake. HepG2 cells were pre-incubated at 4°C for 30 min with
inhibitors and then at 37°C for 4 h without them, as previously
reported (43). In order to study
the effect of intracellular potassium depletion, HepG2 cells were
rinsed twice and incubated with a potassium-free buffer solution
with the following substances: 0.14 M NaCl, 0.02 M of MES buffer,
10−3 M of CaCl2 and 1 mg/ml of glucose pH 7.4
for 30 min before uptake experiments were performed in the same
medium, as previously reported (44).
Analysis of cytokine expression
With the ELISA test, it was possible to assess the
expression of IL-6, IL-8, TGF-β and TNF-α in human HB cells,
following the same procedure previously published by our group
(45). Briefly, HepG2 cells
(1.2×105 cells/well) were seeded in 12-well plates in
DMEM supplemented with 10% FBS and 1% Pen-Strep at 37°C in a
humidified 5% CO2 atmosphere. Thereafter, the cells were
incubated for 24 h in serum-free medium for 2.5 h. Subsequently,
the cells were incubated with or without 0.1 ml of unformulated
butyric acid or chitosan-coated or uncoated liposomes both loaded
with BA (in all cases, fatty acid was tested at 2.5, 5 and 10 mM)
for 5 h before exposure to LPS (40 ng/ml) for 12 h. LPS was used to
stimulate inflammation. Thereafter, using a VEGF ELISA kit (Sigma
Aldrich; Merck KGaA, Milan, Italy) according to the manufacturer's
instructions, the quantification of IL-6 and IL-8 was performed.
This assay can detect cytokines in a 10–32.000 pg/ml range with a
sensitivity less than 10 (pg/ml).
Statistical analysis
The analysis of variance (ANOVA) and Tukey's
multiple comparison test in SigmaPlot Software (Systat Software,
Inc., San Jose, CA, USA) was used to analyze the statistical
difference between experimental groups. The lowest acceptable
significant threshold, for statistical analysis of all data, was
P<0.05.
Results
Synthesis and chemical
characterization of the liposomes and fluorescent liposomes
As reported in Table
I, the obtained liposomes showed a specific distribution size
linked to the presence or absence of the coating on the surface. In
fact, uncoated liposomes either empty or loaded with drug or
fluorophore had a mean hydrodynamic size of ~88.5 nm (88.6±4.3,
92.1±4.1, 84.5±3.6 nm, respectively) with a polydispersity index
(PDI) always <0.3 indicating a good and homogeneous dispersion
of the liposome sizes. On the other hand, the coating of the
liposomes with chitosan, as expected, induced an increase in the
hydrodynamic size with a mean value of 126 nm (Table I) and with an acceptable PDI.
Regarding ζ potential, as clearly shown in Table I, coating of the nanocarriers caused
the formation of a net positive surface charge of the liposomes
indicating in an indirect manner the presence of chitosan on their
surface.
 | Table I.Physical-chemical characteristics of
the liposomes used: hydrodynamic size, polydispersity index (PDI)
and ζ potential. |
Table I.
Physical-chemical characteristics of
the liposomes used: hydrodynamic size, polydispersity index (PDI)
and ζ potential.
| Liposomes | Hydrodynamic size
(SD) | PDI (SD) | ζ potential
(SD) |
|---|
| Empty liposomes
(Lip) | 84.5 nm
(3.6) | 0.26 (0.03) | −10.2 mV (1.2) |
| Empty
chitosan-coated liposomes (Chit-Lip) | 126.3 nm (6.4) | 0.18 (0.01) | 12.2 mV (1.1) |
| Uncoated liposomes
loaded with BA (Lip-BA) | 92.1 nm
(4.1) | 0.18 (0.05) | - 9.3 mV (1.5) |
| Chitosan-coated
liposomes loaded with BA (Chit-Lip-BA) | 132.2 nm (2.3) | 0.22 (0.03) | 15.3 mV (2.1) |
| Fluorescent
uncoated liposomes (Lip-FA) | 88.6 nm
(4.3) | 0.24 (0.02) | −12.3 mV (2.2) |
| Fluorescent
chitosan-coated liposomes (Chit-Lip-FA) | 119.5 nm (3.3) | 0.19 (0.04) | 13.5 mV (1.6) |
Cell viability
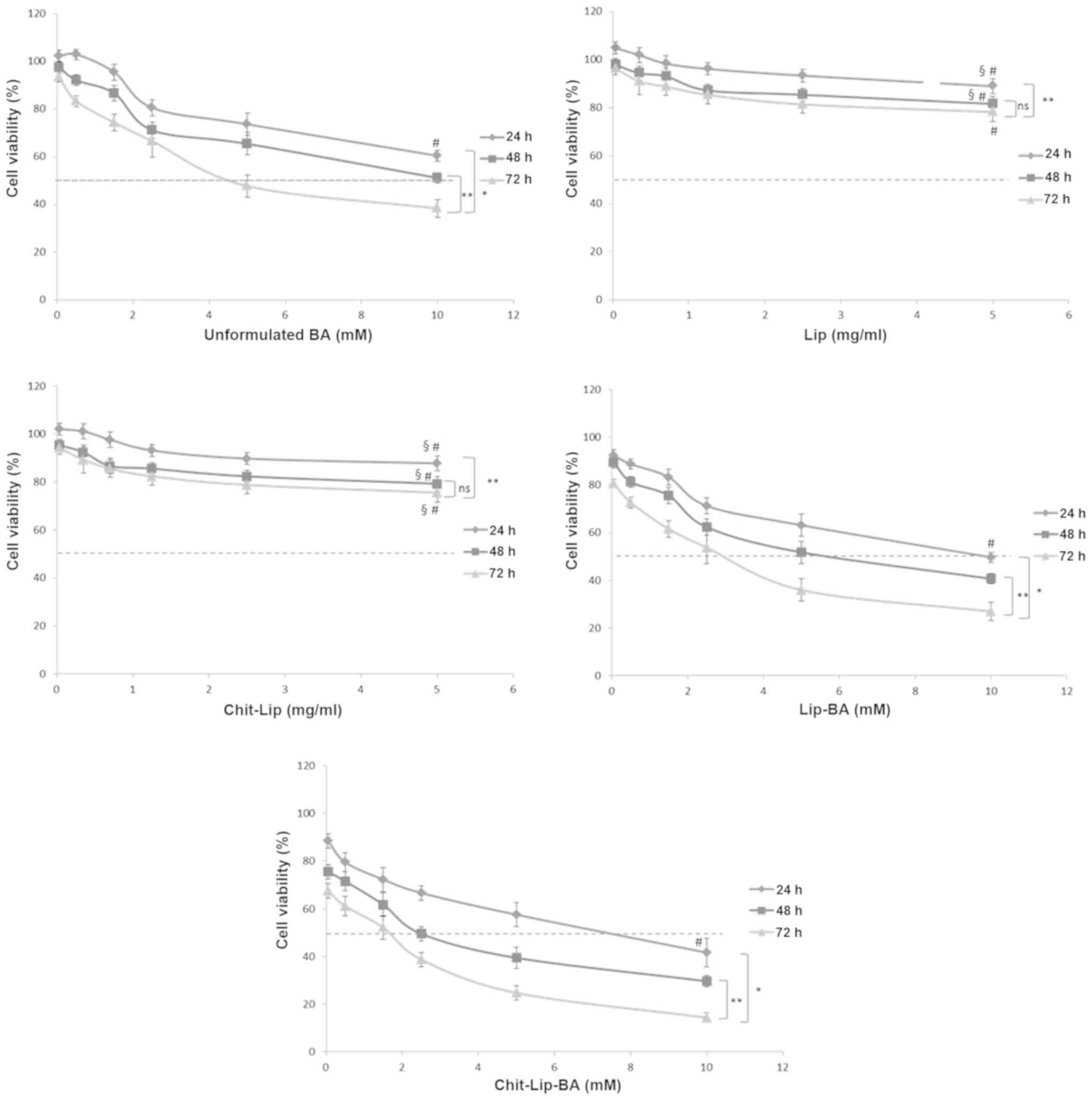
As shown in Fig. 1,
empty liposomes showed a very slight toxicity against HepG2 cells
at all the times and the same concentrations used. Specifically, at
a very high concentration of uncoated and coated liposomes,
corresponding to 5 mg/ml, after 72 h of incubation only
approximately 15–20% of the cells were not viable (P<0.05,
compared to the control). This biological behavior is already well
known as these lipidic nanocarriers are highly biocompatible and
easily metabolized by cells (46).
Interestingly, the chitosan coating did not increase cell
cytotoxicity against the HB cells, compared to the uncoated ones,
in all assessed concentrations indicating a good biocompatibility
of the polymer. As reported in the literature (47), free BA showed a marked antitumor
effect against human cells without reaching an IC50
(concentration inhibiting the 50% of cells) value after 24 h of
incubation at all assessed concentrations; only after 48 and 72 h
of incubation the IC50 value was obtained in cells
treated with free BA (10 and 4.5 mM, respectively) indicating a
time-dependent cytotoxicity of the fatty acid against HepG2 cells.
On the other hand, formulations of BA encapsulated in both coated
and uncoated liposomes induced a significantly improved
antiproliferative activity in the HepG2 cells. In detail, uncoated
liposomes loaded with BA (Lip-BA) induced an approximately 50% of
cell growth inhibition at 10, 5.5 and 2.7 mM after 24, 48 and 72 h
of incubation, respectively, indicating an increase in anticancer
activity of about 45 and 40% after 48 and 72 h of incubation,
respectively, when compared to free BA (Fig. 1). The best results were obtained
with the chitosan-coated liposomes (Chit-Lip-BA) that caused an
approximately 50% cell growth inhibition at 7.5, 2.5 and 1.6 mM
after 24, 48 and 72 h of incubation, respectively, with a 25, 55
and 41% (P<0.05) increased cytotoxic activity when compared to
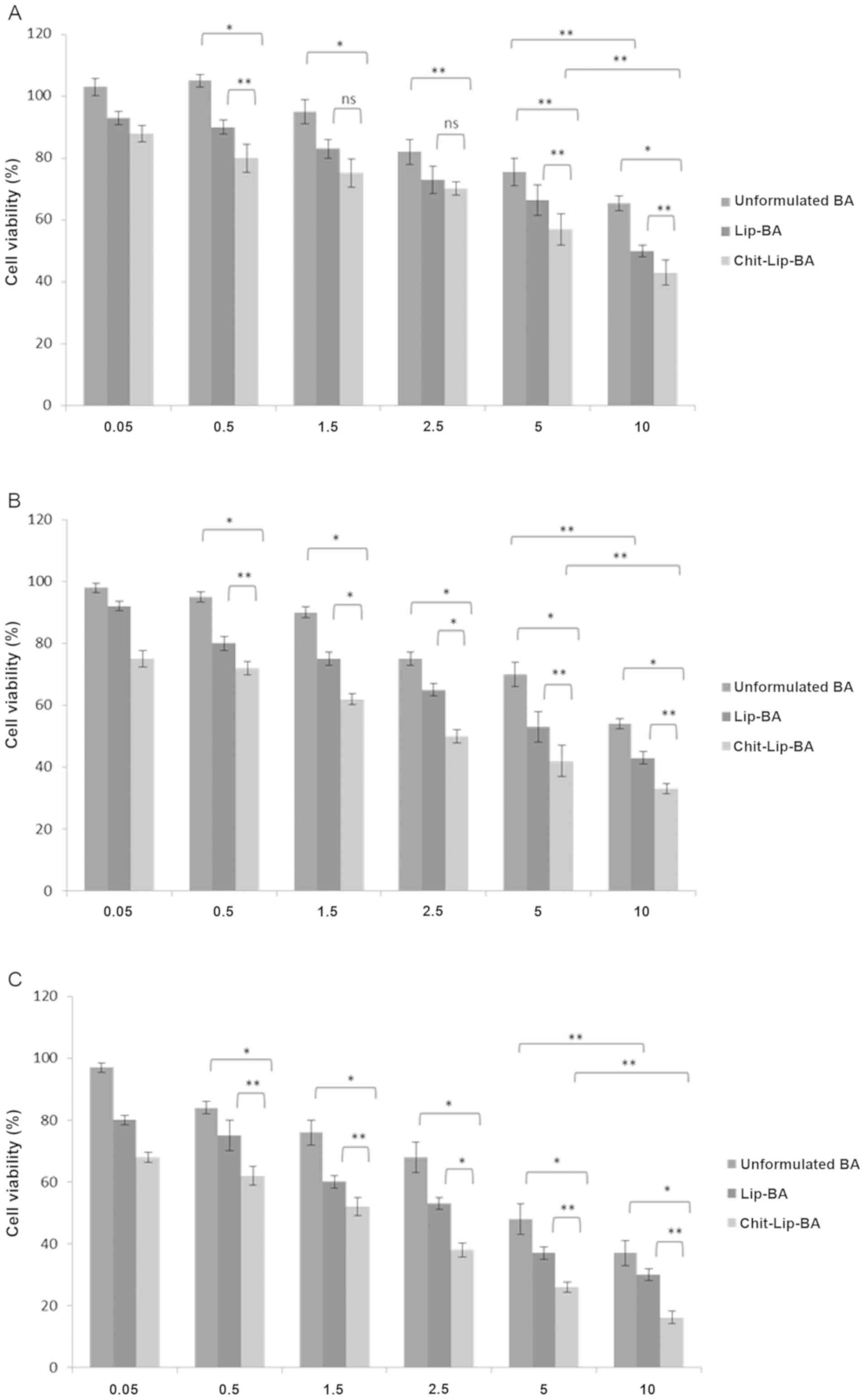
the uncoated liposomes, respectively. Comparing unformulated BA
with chitosan uncoated and coated liposomes loaded with BA
(Fig. 2), it was obvious that, at
48 (Fig. 2B) and 72 h (Fig. 2C) of incubation, there was a
statistically significant difference between Lip-BA and
Chit-Lip-BA. These differences, in the same cases, were not
significant at 24 h of incubation (at 1.5 and 2.5 mM of BA)
(Fig. 2A). However, these findings
suggest that BA cytotoxicity against human HB cells was greatly
improved by its encapsulation in liposomes coated with chitosan
with an IC50 value at 48 and 72 h of incubation that was
decreased at approximately 75 and 65.5% (P<0.01 for both) when
compared to free BA.
Cellular uptake studies
Imaging by confocal laser scanning
microscope
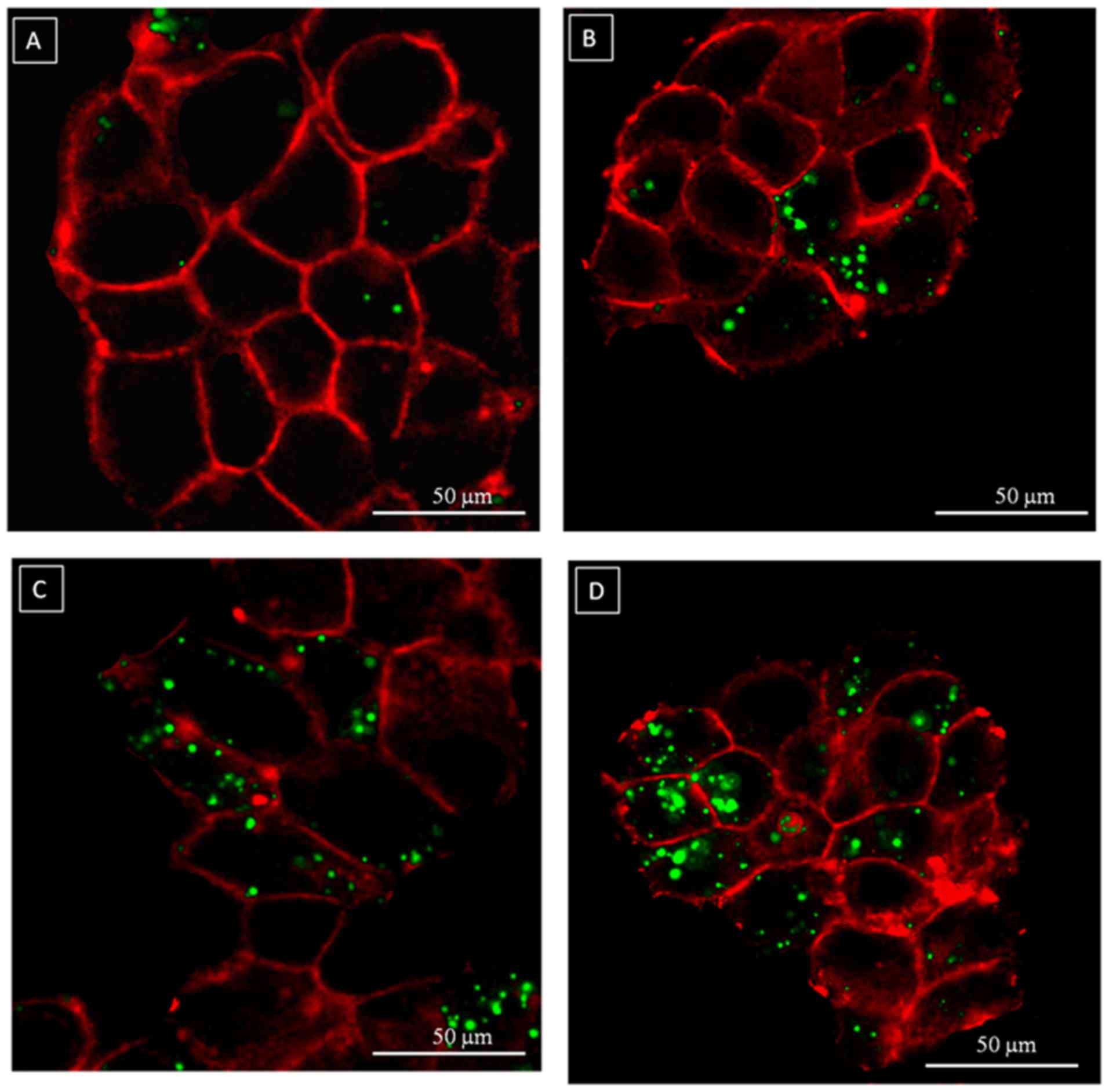
The images obtained with confocal laser scanning
microscope (Fig. 3) showed a
time-dependent uptake of fluorescent chitosan-coated liposomes with
a perimembrane localization after 0.5 and 4 h and a perinuclear
localization after 24 h (Fig. 3D).
Chitosan-coated liposomes appeared to be located especially on the
cell membrane and in the juxtamembrane region (Fig. 3A and B) at a short time of
incubation (0.5 and 4 h). Notably, the images showed the overall
spheric shape of the fluorescent nanocarrier with a characteristic
point-like dispersion in the intracellular microenvironment.
Uptake quantification
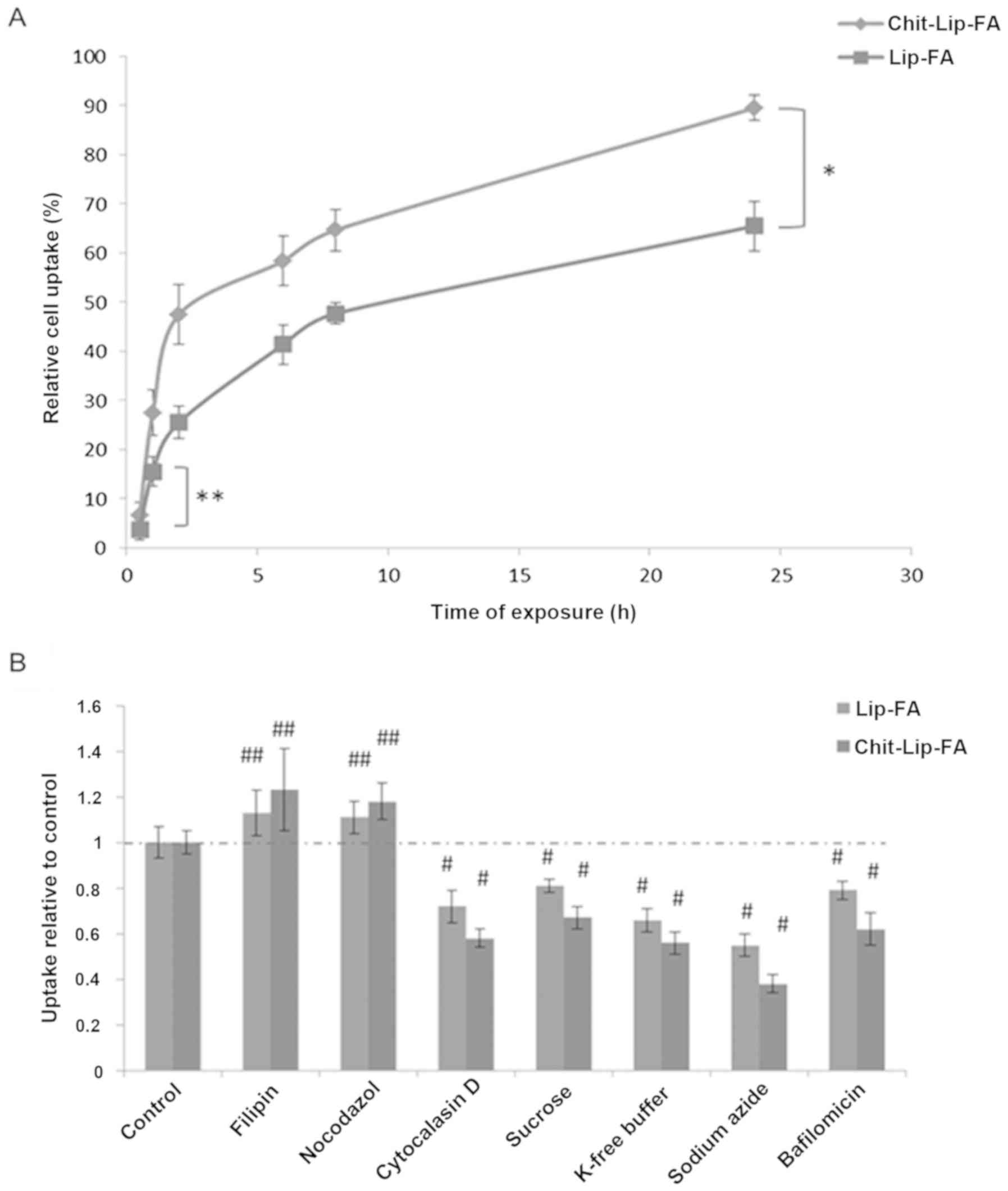
As shown in Fig. 4A,
the uptake of fluorescent-coated and -uncoated liposomes was
time-dependent in the HepG2 cells with a statistically significant
difference between the two different nanocarriers. Importantly,
just after 2 h of incubation, approximately 25.5±3.3 and 47.5±6% of
the uncoated and coated liposomes, respectively, were internalized
in the HepG2 cells while the maximal internalization was observed
at 24 h of incubation. In fact, 65.5±5 and 89.5±2.6% of the
uncoated and coated nanocarriers were found to be incorporated in
the cells (Fig. 4A) corresponding
to a theoretical overall molar concentration of BA of 1.31 and 1.79
mM, respectively.
Mechanistic studies
As it was established that liposomes can be
internalized in human HepG2 cells in a time-dependent manner, by
the use of a small library of inhibitors of general active
transport processes it can be possible to analyze cell uptake,
endosomal acidification, caveolae-mediated endocytosis, membrane
ruffling and vescicular transport on microtubuli or actin fibers.
These inhibitors had differential effects in HepG2 cells on the
internalization of fluorescent-coated and -uncoated liposomes. As
shown in Fig. 4B, both nanocarriers
had the same internalization mechanism in HepG2 cells. In detail,
the inhibition of the endocytosis of chitosan-coated liposomes by
sodium azide (62% of inhibition) and by cytochalasin D (42% of
inhibition) and the absence of any significant effect induced by
nocodazole suggested an energy-dependent mechanism of Chit-Lip-FA
internalization with the involvement of stress fibers, but not of
microtubules; filipin did not inhibit their internalization
excluding also a caveolae-mediated endocytic mechanism. On the
other hand, bafilomycin A1 caused an approximately 38% reduction in
endocytosis when compared to the control. Clathrin-selective
potassium-free buffer (42% of inhibition) exhibited the same
inhibitory action as the well-known hypertonic sucrose, that
inhibits also macropinocytic and caveolar uptake. These findings
demonstrate that a clathrin-dependent endocytosis could be the
preferential internalization mechanism in human HB cells (Fig. 4B).
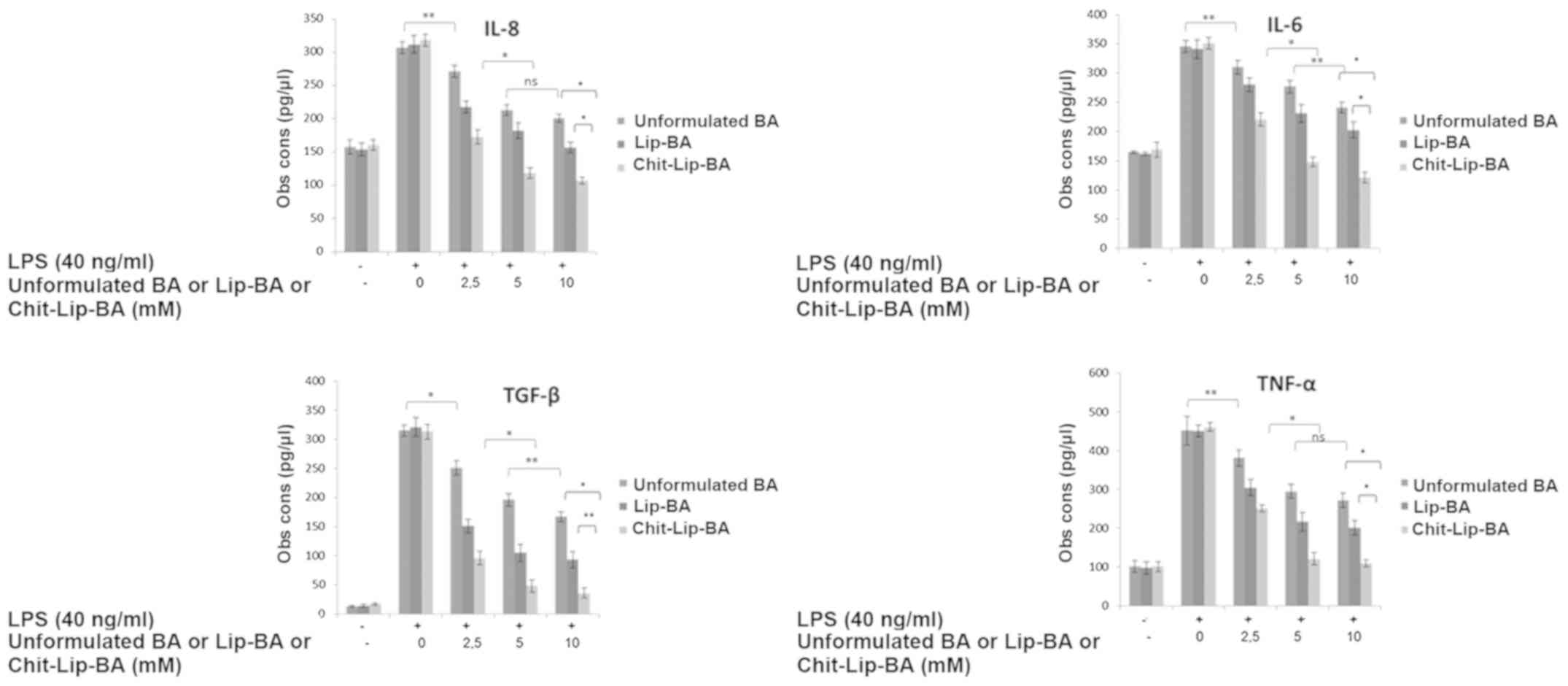
Evaluation of cytokine expression
Regarding the anti-inflammatory properties of free
or liposome-encapsulated BA, we observed that pre-treatment with
all the assessed substances at the corresponding concentrations of
2.5, 5 and 10 mM consistently induced a significant decrease in
IL-8, IL-6, TGF-β and TNF-α expression (Fig. 5) when compared to the untreated
cells. However, some interesting differences could be recorded. In
detail, unformulated BA administration at a concentration of 2.5 mM
reduced the increase in the cell amounts of IL-8, IL-6, TGF-β and
TNF-α of approximately 11.8, 10.4, 20.4 and 16.7%, respectively,
when compared to the LPS-treated HepG2 cells (P<0.001) (Fig. 5). The anti-inflammatory effects were
concentration-dependent since following the doubling of the BA
concentration, the increase in the cell amounts of IL-8, IL-6,
TGF-β and TNF-α were reduced by approximately 30.6, 20.0, 37.8 and
34.8%, respectively, compared to the untreated and LPS-treated HB
cells (P<0.001) (Fig. 5).
Interestingly, uncoated liposomes loaded with BA consistently
induced a higher and statistically significant anti-inflammatory
effects than free BA at all assessed concentrations; in fact, they
reduced IL-8, IL-6, TGF-β and TNF-α production by 30.0, 18.0, 53.0
and 32.4% at 2.5 mM and 42.0, 33.0, 68.0 and 52.0% at 5 mM of BA
concentration, respectively (all values compared to untreated
cells, P<0.001). On the other hand, chitosan-coated liposomes
loaded with BA caused higher anti-inflammatory effects when
compared to both free BA and uncoated liposomes loaded with BA at
all the assessed concentrations. In detail, they decreased IL-8,
IL-6, TGF-β and TNF-α production of 46.0, 37.0, 70.0 and 45.6% at
2.5 mM and 64.0, 58.0, 85.0 and 73.8% at 5 mM BA concentration,
respectively (all values compared to untreated cells, P<0.001).
Comparing the two different liposomal formulations, at 5 mM BA
concentration, chitosan-coated liposomes encapsulating BA reduced
IL-8, IL-6, TGF-β and TNF-α production by 22.0, 25.0, 17.0 and
22.0%, respectively, more than the uncoated liposomes encapsulating
BA. These results are in agree with the internalization and cell
viability experiments and support the hypothesis that
chitosan-coated liposomes improved significantly the
anti-inflammatory and anticancer functions of BA in liver cancer
cells.
Discussion
Butyric acid (BA) is an important fatty acid with
possible implications in cancer prevention and therapy, as
previously shown (48). Despite its
anti-inflammatory, immune-modulating, epigenetic and anticancer
properties, its main crucial limits are based on the poor
accumulation in target organs such as the liver where its
metabolism could have a key function in the treatment of cancer
conditions and altered lipidogenesis (49). This short-chain fatty acid (SCFA) is
known to modulate the tissue microenvironment by inhibiting
interleukin and cytokine secretion by fibroblasts and macrophages
but also by cancer cells that can release growth factors that act
in an autocrine or paracrine manner in order to stimulate their
survival, metastasis and chemo-radioresistance. Interestingly, BA
has shown anticancer properties against HCC cells principally based
on its histone deacetylase (HDAC) inhibitory activity (50). Another study demonstrated the
effects of BA on E-cadherin, vimentin, N-cadherin markers and on
TGF-β1 in HCC cells (51).
In the present study, we produced and characterized
biodegradable and biocompatible liposomes encapsulating BA, coated
or uncoated with chitosan on their surface in order to increase
both human cancer HepG2 cell uptake and BA internalization.
Liposomes are among the most versatile and biodegradable
nanocarriers used to increase the pharmacokinetic profile,
stability and targeting of anticancer agents for both diagnosis and
treatment (52). Chitosan is a
positively charged polysaccharide at physiological pH with
significant clinical applications as carriers of drugs after
systemic or oral administration in mice and humans; in fact,
chitosan was selected for its ability to resist degradation in the
gastro-intestinal tract and for its muco-adhesive capacity in the
intestinal lumen with interesting application for the oral delivery
of drugs (53). Following the
synthesis methods described above, the liposome distribution size
was always in agreement with the presence or absence of surface
coating with a mean value of approximately 100 nm that is well
studied in the literature as an efficient nanocarrier model for
delivery of anti-inflammatory and anticancer agents. Chitosan
coating increased the overall liposome diameter of approximately
30–40 nm and changed their surface charge from negative to positive
(Table I), an indirect
demonstration of the efficient coating process. The presence of
chitosan on the liposome surface always achieved the best
biological effects in all the experimental conditions; in fact,
their cell internalization efficiency was significantly greater
than the uncoated liposomes at all incubation times in the HepG2
cells; internalization properties of this type of liposomes were
supported by imaging with confocal microscopy. Moreover, on the
basis of the results obtained with specific uptake inhibitors, we
can hypothesize that both liposomes used the same internalization
mechanism in the HepG2 cells characterized by an endocytic manner
which use for their uptake the clathrin-coated pits and actin
filaments. However, chitosan-coated nanocarriers appeared to be
more dependent on these mechanisms when compared to the uncoated
liposomes.
Comparing the cytotoxicity of free BA with both
nanocarrier formulations, similar results were observed as
suggested by greater anticancer effects induced by chitosan-coated
liposomes with a reduction in the IC50 values at 72 h of
more than 65% when compared to free BA. As evident by previously
reported data, BA induces strong anti-inflammatory effects in the
liver microenvironment and IL-8, IL-6, TNF-α and TGF-β, produced
during inflammation, have a key function in liver cancer biology as
in non-cancer diseases such as steatosis. The effects of BA on
liver production of these interleukins have a translational value
in oncology and hepatology. In fact, the use of nonsteroidal
anti-inflammatory drugs decrease the incidence and/or recurrence of
HCC (54). Inhibition of the
inflammation status in the liver enhances various pharmacological
therapies; for example, the combination therapy of zoledronic acid
and sorafenib to treat advanced HCC is being evaluated in phase II
studies (NCT01259193) (55).
Numerous therapeutics have been designed to block cytokine
receptors and downstream signaling pathways such as receptor
kinases and STAT3 to inhibit inflammation-driven protumoral
signals. IL-8 and IL-6 are both related to poor chemotherapeutics,
specifically doxorubicin response and tumorigenicity in HCC
(56). An interesting clinical
trial on HCC cancer patients, demonstrated that serum IL-8 levels
were a significant prognostic factor in terms of disease-free and
overall survival. Moreover, patients with a serum IL-8 level of
>17.6 pg/ml had a poorer disease-free survival than those with a
level of <17.6 pg/ml (median disease-free survival 4.7 vs. 19.2
months) (57). In addition, other
small molecules such as TGF-β and TNF-α play a crucial role in
several liver disorders including cancer and steatosis (58) and are implicated in drug resistance
processes, as example in sorafenib and doxorubicin treatments, as
they upregulate the expression of multiple receptor tyrosine
kinases (RTKs), including IGF1R, EGFR, PDGFβR, and FGFR1 in HCC
cells (59).
Based on these considerations, many researchers have
discussed the use of single inhibitors of IL-8, IL-6 or TGF-β, also
in association with anticancer drugs. But considering the multiple
pathways involved in drug resistance processes as well as in cancer
cell progression and survival, an integrated approach able to
inhibit in a multiple manner more interleukins and growth factors
in the liver, could be an innovative and useful integrative tool in
HCC management. Here, we focused our attention on the role of BA as
a modulator of the liver microenvironment exploiting its abilities
in decreasing inflammation.
On the basis of these aims, we investigated the
abilities of two BA-loaded nanoformulations in hepatoblastoma (HB)
cell uptake and cell viability status. The obtained results
consistently showed that the chitosan-coated nanocarriers have the
best internalization capabilities and biological activities
compared to the uncoated one. These effects are in agreement with
other previously publiched research of our group (by loading
curcumin) (36). The
anti-inflammatory activity obtained with the coated liposomes
warrants the possible management of the liver cancer
microenvironment through the use of these formulations. Overall,
these results prompt us to perform additional experiments in
animals to get definitive data concerning the anti-inflammatory
properties of our formulations. In details, the expectation in the
future is to take benefit of the pharmacological function of
liposomes for the oral delivery of BA considering the ability of
chitosan-coated nanocarriers, such as nanoemulsions, to accumulate
in lthe iver after oral administration, as recently demonstrated
(35). However, one limitation of
our study is based on the absence of more biological studies such
as gene expression and proteomic studies and our future
investigations, planned by our research group, are based on the
evaluation of different biological effects of BA in human cancer
and non-cancer (such as fatty hepatocytes) liver cells considering
the well known anti-steatotic effects of BA.
Acknowledgements
We thank the University Data Managers of the
University Vanvitelli and University of Medicine of Salerno for
assistance in the data analysis.
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
VQ and MM coordinated and wrote the study. VQ and EA
performed the experiments in cells and synthesis of liposomes. MB
and AG coordinated the writing of the manuscript and the data
analysis. MC, MP and AB coordinated scientifically the work and
decided the choice of treatments performed. All authors read and
approved the manuscript and agree to be accountable for all aspects
of the research in ensuring that the accuracy or integrity of any
part of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
No potential competing interests are disclosed.
References
|
1
|
Marra M, Sordelli IM, Lombardi A, Lamberti
M, Tarantino L, Giudice A, Stiuso P, Abbruzzese A, Sperlongano R,
Accardo M, et al: Molecular targets and oxidative stress biomarkers
in hepatocellular carcinoma: An overview. J Transl Med. 9:1712011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Czaja AJ: Hepatic inflammation and
progressive liver fibrosis in chronic liver disease. World J
Gastroenterol. 20:2515–2532. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kershenobich Stalnikowitz D and Weissbrod
AB: Liver fibrosis and inflammation. A review. Ann Hepatol.
2:159–163. 2003.PubMed/NCBI
|
|
5
|
Li S, Tan H-Y, Wang N, Zhang ZJ, Lao L,
Wong CW and Feng Y: The role of oxidative stress and antioxidants
in liver diseases. Int J Mol Sci. 16:26087–26124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Seki E and Schwabe RF: Hepatic
inflammation and fibrosis: Functional links and key pathways.
Hepatology. 61:1066–1079. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang L, Song HY and Ji G: The joint
pathway of metabolism and inflammation in nonalcoholic fatty liver
disease. Zhonghua Gan Zang Bing Za Zhi. 19:395–397. 2011.(In
Chinese). PubMed/NCBI
|
|
8
|
Shivappa N, Hébert JR, Polesel J,
Zucchetto A, Crispo A, Montella M, Franceschi S, Rossi M, La
Vecchia C and Serraino D: Inflammatory potential of diet and risk
for hepatocellular cancer in a case-control study from Italy. Br J
Nutr. 115:324–331. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alzahrani B, Isel TJ and Hebbard LW:
Non-viral causes of liver cancer: Does obesity led inflammation
play a role? Cancer Lett. 345:223–229. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang LJ and Wang XZ: Interleukin-10 and
chronic liver disease. World J Gastroenterol. 12:1681–1685. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Budhu A and Wang XW: The role of cytokines
in hepatocellular carcinoma. J Leukoc Biol. 80:1197–1213. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wan S, Zhao E, Kryczek I, Vatan L,
Sadovskaya A, Ludema G, Simeone DM, Zou W and Welling TH:
Tumor-associated macrophages produce interleukin 6 and signal via
STAT3 to promote expansion of human hepatocellular carcinoma stem
cells. Gastroenterology. 147:1393–1404. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hatting M, Spannbauer M, Peng J, Al
Masaoudi M, Sellge G, Nevzorova YA, Gassler N, Liedtke C, Cubero FJ
and Trautwein C: Lack of gp130 expression in hepatocytes attenuates
tumor progression in the DEN model. Cell Death Dis. 6:e16672015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamazaki K, Masugi Y and Sakamoto M:
Molecular pathogenesis of hepatocellular carcinoma: Altering
transforming growth factor-β signaling in hepatocarcinogenesis. Dig
Dis. 29:284–288. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mamiya T, Yamazaki K, Masugi Y, Mori T,
Effendi K, Du W, Hibi T, Tanabe M, Ueda M, Takayama T, et al:
Reduced transforming growth factor-beta receptor II expression in
hepatocellular carcinoma correlates with intrahepatic metastasis.
Lab Invest. 90:1339–1345. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Korkaya H, Kim GI, Davis A, Malik F, Henry
NL, Ithimakin S, Quraishi AA, Tawakkol N, D'Angelo R, Paulson AK,
et al: Activation of an IL6 inflammatory loop mediates trastuzumab
resistance in HER2+ breast cancer by expanding the
cancer stem cell population. Mol Cell. 47:570–584. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Milagre CS, Gopinathan G, Everitt G,
Thompson RG, Kulbe H, Zhong H, Hollingsworth RE, Grose R, Bowtell
DD, Hochhauser D, et al: Adaptive upregulation of EGFR limits
attenuation of tumor growth by neutralizing IL6 antibodies, with
implications for combined therapy in ovarian cancer. Cancer Res.
75:1255–1264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Freudlsperger C, Bian Y, Contag Wise S,
Burnett J, Coupar J, Yang X, Chen Z and Van Waes C: TGF-β and NF-κB
signal pathway cross-talk is mediated through TAK1 and SMAD7 in a
subset of head and neck cancers. Oncogene. 32:1549–1559. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin L, Amin R, Gallicano GI, Glasgow E,
Jogunoori W, Jessup JM, Zasloff M, Marshall JL, Shetty K, Johnson
L, et al: The STAT3 inhibitor NSC 74859 is effective in
hepatocellular cancers with disrupted TGF-beta signaling. Oncogene.
28:961–972. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bajaj JS, Hylemon PB and Younossi Z: The
intestinal microbiota and liver disease. Am J Gastroenterol Suppl.
1:9–14. 2012. View Article : Google Scholar
|
|
22
|
Juárez-Hernández E, Chávez-Tapia NC, Uribe
M and Barbero-Becerra VJ: Role of bioactive fatty acids in
nonalcoholic fatty liver disease. Nutr J. 15:722016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sharma V, Garg S and Aggarwal S:
Probiotics and liver disease. Perm J. 17:62–67. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Endo H, Niioka M, Kobayashi N, Tanaka M
and Watanabe T: Butyrate-producing probiotics reduce nonalcoholic
fatty liver disease progression in rats: New insight into the
probiotics for the gut-liver axis. PLoS One. 8:e633882013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mattace Raso G, Simeoli R, Russo R, Iacono
A, Santoro A, Paciello O, Ferrante MC, Canani RB, Calignano A and
Meli R: Effects of sodium butyrate and its synthetic amide
derivative on liver inflammation and glucose tolerance in an animal
model of steatosis induced by high fat diet. PLoS One.
8:e686262013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Meijer K, de Vos P and Priebe MG: Butyrate
and other short-chain fatty acids as modulators of immunity: What
relevance for health? Curr Opin Clin Nutr Metab Care. 13:715–721.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Clemente JC, Ursell LK, Parfrey LW and
Knight R: The impact of the gut microbiota on human health: An
integrative view. Cell. 148:1258–1270. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Saito H, Morizane T, Watanabe T, Kagawa T,
Miyaguchi S, Kumagai N and Tsuchiya M: Differentiating effect of
sodium butyrate on human hepatoma cell lines PLC/PRF/5, HCC-M and
HCC-T. Int J Cancer. 48:291–296. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Banasiewicz T, Krokowicz Ł, Stojcev Z,
Kaczmarek BF, Kaczmarek E, Maik J, Marciniak R, Krokowicz P,
Walkowiak J and Drews M: Microencapsulated sodium butyrate reduces
the frequency of abdominal pain in patients with irritable bowel
syndrome. Colorectal Dis. 15:204–209. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu J and Zern MA: Modification of
liposomes for liver targeting. J Hepatol. 24:757–763. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rosso F, Quagliariello V, Tortora C, Di
Lazzaro A, Barbarisi A and Iaffaioli RV: Cross-linked hyaluronic
acid sub-micron particles: In vitro and in vivo biodistribution
study in cancer xenograft model. J Mater Sci Mater Med.
24:1473–1481. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vecchione R, Iaccarino G, Bianchini P,
Marotta R, D'autilia F, Quagliariello V, Diaspro A and Netti PA:
Ultrastable liquid-liquid interface as viable route for controlled
deposition of biodegradable polymer nanocapsules. Small.
12:3005–3013. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Caraglia M, Marra M, Misso G, Lamberti M,
Salzano G, De Rosa G and Abbruzzese A: Tumour-specific uptake of
anti-cancer drugs: The future is here. Curr Drug Metab. 13:4–21.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Camilleri JP, Williams AS, Amos N,
Douglas-Jones AG, Love WG and Williams BD: The effect of free and
liposome-encapsulated clodronate on the hepatic mononuclear
phagocyte system in the rat. Clin Exp Immunol. 99:269–275. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Van Rooijen N and Sanders A: Kupffer cell
depletion by liposome-delivered drugs: Comparative activity of
intracellular clodronate, propamidine, and
ethylenediaminetetraacetic acid. Hepatology. 23:1239–1243. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vecchione R, Quagliariello V, Calabria D,
Calcagno V, De Luca E, Iaffaioli RV and Netti PA: Curcumin
bioavailability from oil in water nano-emulsions: In vitro and in
vivo study on the dimensional, compositional and interactional
dependence. J Control Release. 233:88–100. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sasaki A, Murahashi N, Yamada H and
Morikawa A: Syntheses of novel galactosyl ligands for liposomes and
their accumulation in the rat liver. Biol Pharm Bull. 17:680–685.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Watanabe M, Pesando JM and Hakomori S:
Effect of liposomes containing sodium butyrate conjugated with
anti-CD19 monoclonal antibody on in vitro and in vivo growth of
malignant lymphoma. Cancer Res. 50:3245–3248. 1990.PubMed/NCBI
|
|
39
|
Altieri F, Di Stadio CS, Severino V,
Sandomenico A, Minopoli G, Miselli G, Di Maro A, Ruvo M, Chambery
A, Quagliariello V, et al: Anti-amyloidogenic property of human
gastrokine 1. Biochimie. 106:91–100. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Di Stadio CS, Altieri F, Miselli G, Elce
A, Severino V, Chambery A, Quagliariello V, Villano V, de Dominicis
G, Rippa E, et al: AMP18 interacts with the anion exchanger SLC26A3
and enhances its expression in gastric cancer cells. Biochimie.
121:151–160. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Baravalle G, Schober D, Huber M, Bayer N,
Murphy RF and Fuchs R: Transferrin recycling and dextran transport
to lysosomes is differentially affected by bafilomycin, nocodazole,
and low temperature. Cell Tissue Res. 320:99–113. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Barbarisi M, Iaffaioli RV, Armenia E,
Schiavo L, De Sena G, Tafuto S, Barbarisi A and Quagliariello V:
Novel nanohydrogel of hyaluronic acid loaded with quercetin alone
and in combination with temozolomide as new therapeutic tool, CD44
targeted based, of glioblastoma multiforme. J Cell Physiol.
233:6550–6564. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Qaddoumi MG, Gukasyan HJ, Davda J,
Labhasetwar V, Kim KJ and Lee VH: Clathrin and caveolin-1
expression in primary pigmented rabbit conjunctival epithelial
cells: Role in PLGA nanoparticle endocytosis. Mol Vis. 9:559–568.
2003.PubMed/NCBI
|
|
44
|
Huang M, Ma Z, Khor E and Lim LY: Uptake
of FITC-chitosan nanoparticles by A549 cells. Pharm Res.
19:1488–1494. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Quagliariello V, Iaffaioli RV, Armenia E,
Clemente O, Barbarisi M, Nasti G, Berretta M, Ottaiano A and
Barbarisi A: Hyaluronic acid nanohydrogel loaded with quercetin
alone or in combination to a macrolide derivative of rapamycin
RAD001 (Everolimus) as a new treatment for hormone-responsive human
breast cancer. J Cell Physiol. 232:2063–2074. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Nagasaki T, Hara M, Nakanishi H, Takahashi
H, Sato M and Takeyama H: Interleukin-6 released by colon
cancer-associated fibroblasts is critical for tumour angiogenesis:
anti-interleukin-6 receptor antibody suppressed angiogenesis and
inhibited tumour-stroma interaction. Br J Cancer. 110:469–478.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Meng J, Guo F, Xu H, Liang W, Wang C and
Yang XD: Combination therapy using co-encapsulated resveratrol and
paclitaxel in liposomes for drug resistance reversal in breast
cancer cells in vivo. Sci Rep. 6:223902016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gillet R, Jeannesson P, Sefraoui H,
Arnould-Guérin ML, Kirkiacharian S, Jardillier JC and Pieri F:
Piperazine derivatives of butyric acid as differentiating agents in
human leukemic cells. Cancer Chemother Pharmacol. 41:252–255. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Siregar C, Wasito EB and Sudiana IK:
Effect of butyric acid on p53 expression and apoptosis in colon
epithelial cells in mice after treated with
9,10-dimethyl-1,2-benz(a)anthracene. Procedia Chem. 18:141–146.
2016. View Article : Google Scholar
|
|
50
|
Coradini D and Speranza A: Histone
deacetylase inhibitors for treatment of hepatocellular carcinoma.
Acta Pharmacol Sin. 26:1025–1033. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang HG, Huang XD, Shen P, Li LR, Xue HT
and Ji GZ: Anticancer effects of sodium butyrate on hepatocellular
carcinoma cells in vitro. Int J Mol Med. 31:967–974. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Caraglia M, De Rosa G, Salzano G, Santini
D, Lamberti M, Sperlongano P, Lombardi A, Abbruzzese A and Addeo R:
Nanotech revolution for the anti-cancer drug delivery through
blood-brain barrier. Curr Cancer Drug Targets. 12:186–196. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Dash M, Chiellini F, Ottenbrite RM and
Chiellini E: Chitosan - A versatile semi-synthetic polymer in
biomedical applications. Prog Polym Sci. 36:981–1014. 2011.
View Article : Google Scholar
|
|
54
|
Wu CY, Chen YJ, Ho HJ, Hsu YC, Kuo KN, Wu
MS and Lin JT: Association between nucleoside analogues and risk of
hepatitis B virus–related hepatocellular carcinoma recurrence
following liver resection. JAMA. 308:1906–1914. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S,
Kim JS, Luo R, Feng J, Ye S, Yang TS, et al: Efficacy and safety of
sorafenib in patients in the Asia-Pacific region with advanced
hepatocellular carcinoma: A phase III randomised, double-blind,
placebo-controlled trial. Lancet Oncol. 10:25–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Park SY, Han J, Kim JB, Yang MG, Kim YJ,
Lim HJ, An SY and Kim JH: Interleukin-8 is related to poor
chemotherapeutic response and tumourigenicity in hepatocellular
carcinoma. Eur J Cancer. 50:341–350. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ren Y, Poon RT, Tsui HT, Chen WH, Li Z,
Lau C, Yu WC and Fan ST: Interleukin-8 serum levels in patients
with hepatocellular carcinoma: Correlations with
clinicopathological features and prognosis. Clin Cancer Res.
9:5996–6001. 2003.PubMed/NCBI
|
|
58
|
Theron AJ, Anderson R, Rossouw TM and
Steel HC: The role of transforming growth factor beta-1 in the
progression of HIV/AIDS and development of non-AIDS-defining
fibrotic disorders. Front Immunol. 8:14612017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ungerleider N, Han C, Zhang J, Yao L and
Wu T: TGFβ signaling confers sorafenib resistance via induction of
multiple RTKs in hepatocellular carcinoma cells. Mol Carcinog.
56:1302–1311. 2017. View Article : Google Scholar : PubMed/NCBI
|