Introduction
Gastric cancer (GC) is the most common malignant
tumor of the digestive system, and its incidence is ranked fourth
and its mortality is ranked third among malignant tumors (1). Most patients are at an advanced stage
when they are diagnosed with GC. Chemotherapy is the main treatment
for patients with advanced GC, however, although it can prolong the
overall survival, the adverse reactions are more prominent
(2). The liver is the main target
organ of GC metastasis. Shitara et al compiled 12,656
patients with advanced GC, of which the incidence of liver
metastasis was as high as 44% (3).
Patients who developed liver metastases from GC had limited
resection and the treatment was more difficult. GC patients with
liver metastases have a 5-year survival rate of only ~10% (4). With the in-depth study of the
molecular mechanism of the occurrence and development of GC,
molecular targeted therapy of GC has gradually emerged. Therefore,
it is urgent to understand the mechanisms involved in the
metastasis of GC, taking effective measures for early diagnosis and
targeted therapy for GC to improve their survival and life
quality.
SHC encodes three sub-subunits, including
p46Shc, p52Shc, and p66Shc, each of which has a carboxy-terminal
Src homology domain (SH2), a phospho-serine-binding domain (PTB)
with a free α-amino group, and a central proline-rich
collagen-homologous region (CH1), however, p66shc contains an amino
terminal region (CH2) (5,6). p46Shc and p52Shc are ubiquitous in
various cells, including cancer cells, such as breast and
endometrial cancer, however the amount of p66shc expression varies
depending on the cell type (7).
Tyrosine phosphorylation kinase receptors such as the growth factor
receptors EGFR, FGFR, erbB-2 and other tyrosine phosphorylation of
the intracellular domain recognize and bind to proteins in the
corresponding SH2 region of the cytoplasm, with the extracellular
signals passed down step by step (8).
SHC SH2 domain-binding protein 1 (SHCBP1) is an
important connexin on the SH2 domain of the SHC protein, and its
functional role has not been clearly established (9). The mRNA and protein of the
SHCBP1 gene are expressed in proliferating cells, such as
stem cells, lymphocytes and cancer cells, but are not expressed in
stable cells or permanent cells, such as skeletal muscle and
cardiomyocytes (10,11). SHCBP1 is an important intracellular
signaling pathway protein, which has been demonstrated to mediate
multiple signaling pathways such as RAS and PI3K/AKT and has a role
in regulating the cell cycle and promoting cell migration and
invasion (10,12). However, the exact role of SHCBP1 in
GC remains unclear.
In the present study, we attempted to reveal the
role of SHCBP1 in GC and its possible mechanism. SHCBP1 was
revealed to be overexpressed in GC tissues compared with adjacent
normal tissues from TCGA database. Downregulation of SHCBP1
inhibited proliferation and invasion and promoted apoptosis in
vitro. In addition, SHCBP1 knockdown decreased the expression
levels of cyclin D1 and CDK4. Hence, our study revealed that SHCBP1
may play a role in cell growth and metastasis and may be a
potential diagnosis biomarker and therapeutic target for GC.
Materials and methods
Materials and reagents
MGC-803 and SGC-7901 cell lines were purchased from
the American Type Culture Collection (ATCC; Manassas, VA, USA) and
cultured in Dulbecco's modified Eagle's medium (DMEM) containing
10% fetal bovine serum (FBS; both from Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Antibodies against cleaved
PARP (dilution 1:1,000; cat. no. 5625), Bax (dilution 1:1,000; cat.
no. 14796), Bcl-2 (dilution 1:1,000; cat. no. 3498), CDK4 (dilution
1:1,000; cat. no. 12790), cyclin D1 (dilution 1:1,000; cat. no.
2978) and cleaved-caspase-3 (dilution 1:500; cat. no. 9664) were
all purchased from Cell Signaling Technology, Inc. (Danvers, MA,
USA) and SHCBP1 (dilution 1:1,000; cat. no. ab184467) and GAPDH
(dilution 1:5,000; cat. no. ab181602) were purchased from Abcam
(Cambridge, MA, USA).
shRNA and RT-PCR
The shRNA sequence of SHCBP1 was:
CCGGCGAGGAAGTAAGGAAGGGAATCTCGAGATTCCCTTCCACTTCTCCGTTTTTG. Total RNA
was reversed to the first strand of cDNA with PrimeScript Reverse
Transcriptase (cat. no. RR047A; Takara Biotechnology Co., Ltd.,
Dalian, China). RT-PCR was performed using a SYBR Premix Ex Taq II
kit (RR420A; Takara Biotechnology Co., Ltd.) and detected by
StepOnePlus (Applied Biosystems; Thermo Fisher Scientific, Inc.)
using the following conditions: 95°C for 30 sec, followed by 40
cycles of 95°C for 5 sec and 60°C for 30 sec. The data were
analyzed using the ∆∆Cq method (13) with GAPDH as an internal control. The
SHCBP1 PCR primers were as follows: forward,
5′-GCTACCGTGATAAACCAGGTTC-3′ and reverse,
5′-AGGCTCTGAATCGCTCATAGA-3′.
Stable cell line construction
The target shRNA sequence to SHCBP1 and the negative
control RNA sequence were synthesized and inserted into the
lentivirus core vector expressing a GFP reporter and puromycin
resistance. Recombinant lentiviruses were provided by Shanghai
GeneChem Co., Ltd. (Shanghai, China). Cells were infected with the
corresponding lentivirus, and after 72 h, cells were selected with
puromycin (1 µg/ml) for 7 days. The expression level of SHCBP1 in
the selected cells was confirmed by qRT-PCR analysis and western
blot assays.
Western blotting
After cells expressing SHCBP1-shRNA (sh-SHCBP1) and
the control group were lysed by RIPA lysate (Beyotime Institute of
Biotechnology, Shanghai, China) and the protein concentration was
determined using BCA kit (Beyotime Institute of Biotechnology),
protein samples (40 µg/lane) were separated on SDS-PAGE gel (10%)
and transferred to polyvinylidene difluoride (PVDF) membranes.
After blocking with 5% milk for 1 h at room temperature, the
membranes were incubated with a primary antibody overnight at 4°C,
washed three times with TBST, and incubated with HRP-fused
secondary antibody (dilution 1:5,000; cat. no. ab6721; Abcam,
Cambridge, UK) for 1 h. The bands were displayed by ECL
illumination (EMD Millipore, Billerica, MA, USA). The experiment
was repeated three times.
Cell proliferation detection
Cell proliferation was assessed by Cell Counting
Kit-8 (CCK-8) kit (cat. no. CK04; Dojindo Molecular Technologies,
Inc., Kumamoto, Japan). Cells (104/100 µl)/well were
cultured in 96-well plates for 24–72 h. CCK-8 solution (10 µl) was
added to each well, and then the number of cells was assessed using
a 450-nm absorbance on a microplate reader. Three independent
experiments were performed.
Colony formation
Cells were trypsinized into single cell suspension
and seeded in 6-well plates at a density of 800 cells/well. After
14 days of culture, the cells were fixed with a 4% formaldehyde
solution for 30 min, stained with 0.1% crystal violet solution,
rinsed with tap water and dried in air and photographed under a
light microscope (Leica Microsystems, Wetzlar, Germany).
Quantitative analysis was performed by counting the number of
colonies stained in each well. Three independent experiments were
performed.
Cell cycle
The cell cycle assay kit (cat. no. C1052) was
purchased from Beyotime Institute of Biotechnology (Haimen, China).
The cells were fixed with 70% ethanol at 4°C for 24 h, and then
resuspended in cold phosphate-buffered saline (PBS). Then, the
cells were resuspended with a staining solution containing RNase A
and propidium iodide (PI), and the DNA content was assessed using a
flow cytometer. The number of cells in the different phases was
counted using ModFit version 3.2 (Verity Software House, Topsham,
ME, USA). Three independent experiments were performed.
Cell apoptosis
The apoptosis assay kit (cat. no. C1062) was
purchased from Beyotime Institute of Biotechnology. Cells were
double stained with Annexin V and PI according to the
manufacturer's instructions. The stained cells were analyzed by
flow cytometry and the data were analyzed using FlowJo version
10.5.3 (FlowJo LLC, Ashland, OR, USA) software. Three independent
experiments were performed.
Cell invasion and metastasis
The wound healing experiment was to inoculate
treated GC cells in a 6-well culture plate and grow to a density of
>90%. After 12 h, the wound was created by the tip and the image
of moved cells was captured after 48 h. In the cell invasion assay,
cells were placed in Transwell, 600 µl complete DMEM was added to
the lower chamber, and cells mixed with serum-free DMEM were added
to the upper chamber. After 24 h of culture, the cells that
migrated to the lower chamber were fixed with 4% paraformaldehyde
and stained with 0.1% crystal violet. Three independent experiments
were performed.
Differential expression analysis of
SHCBP1 using UALCAN
Based on The Cancer Genome Atlas (TCGA; http://cancergenome.nih.gov/), level 3 RNA-seq and
clinical data from stomach adenocarcinomas, the expression data of
SHCBP1 in GC and normal samples were retrieved and analyzed using
the online web portal UALCAN (http://ualcan.path.uab.edu).
Statistical analysis
Data analysis and statistics were performed by SPSS
17.0 software (SPSS, Inc., Chicago, IL, USA). T-tests and one-way
analysis of variance (ANOVA) followed by the Fishers' least
significant difference test (LSD) were used to determine
differences between groups. A P-value <0.05 was considered to
indicate a statistically significant result.
Results
High expression of SHCBP1 in GC
tissues and cell lines
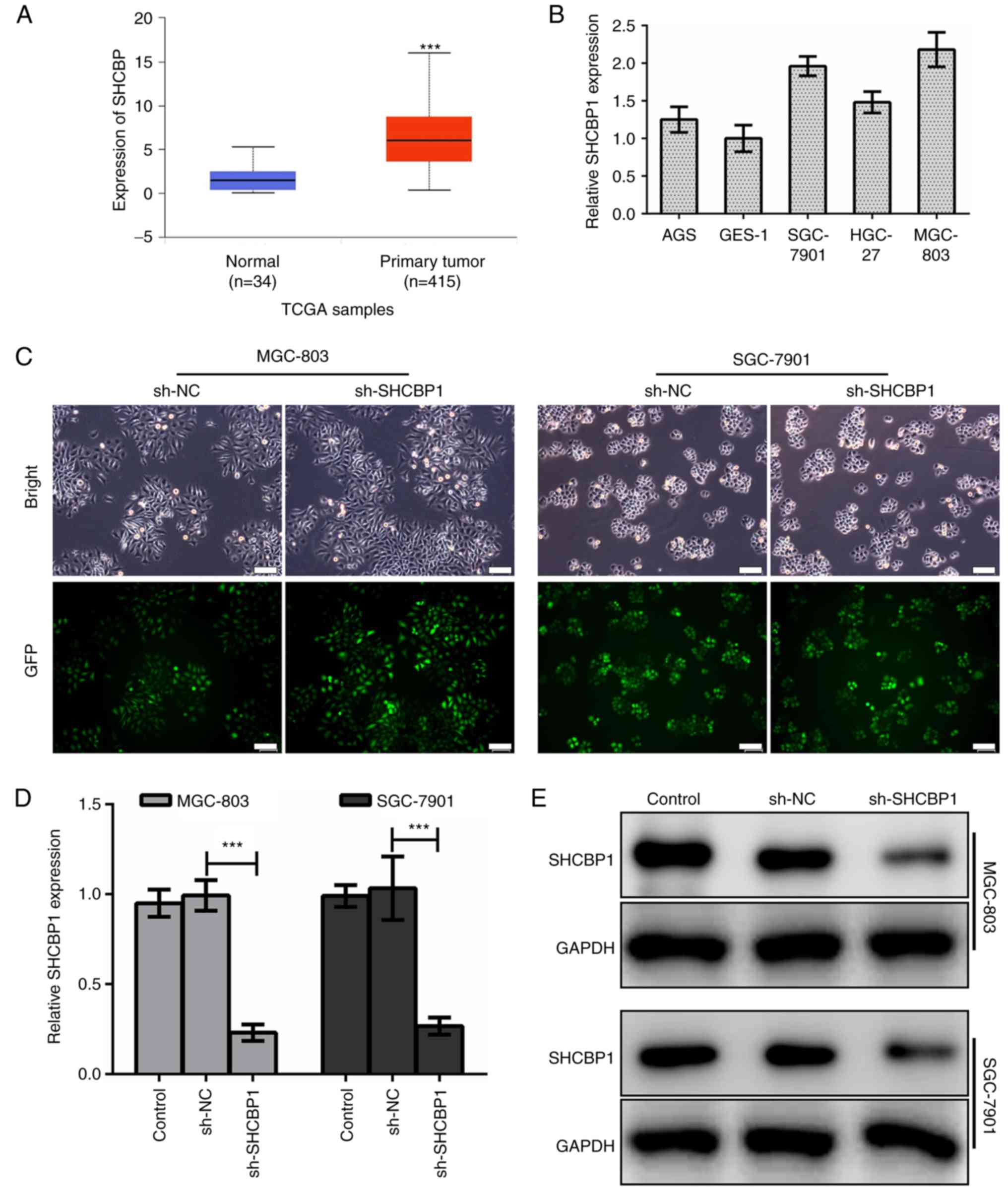
The results in TCGA database (https://cancergenome.nih.gov/) confirmed that the
expression of SHCBP1 in GC tissues was significantly higher than
that in normal tissues (Fig. 1A).
In various GC cell lines, we found that the expression of SHCBP1
was higher in MGC-803 and SGC-7901 cells (Fig. 1B), thus, these two cell lines were
used as research models. First, we established a human GC cell line
model with stable SHCBP1 knockdown. We constructed a green
fluorescent protein (GFP) fusion expression vector of negative
control-shRNA (sh-NC) and SHCBP1-shRNA (sh-SHCBP1), and introduced
shRNA into human GC cell lines MGC-803 and SGC-7901 by lentiviral
transfection (Fig. 1C). By RT-PCR,
we examined the knockdown efficiency of SHCBP1 mRNA in the MGC-803
and SGC-7901 cell lines expressing sh-SHCBP1 (Fig. 1D). Western blotting was used to
detect the expression of SHCBP1 protein in MGC-803 and SGC-7901
cells expressing sh-SHCBP1 (Fig.
1E). The results revealed that the expression of SHCBP1 was
successfully knocked down in the GC cell lines MGC-803 and
SGC-7901.
High expression of SHCBP1 promotes
proliferation of GC cells
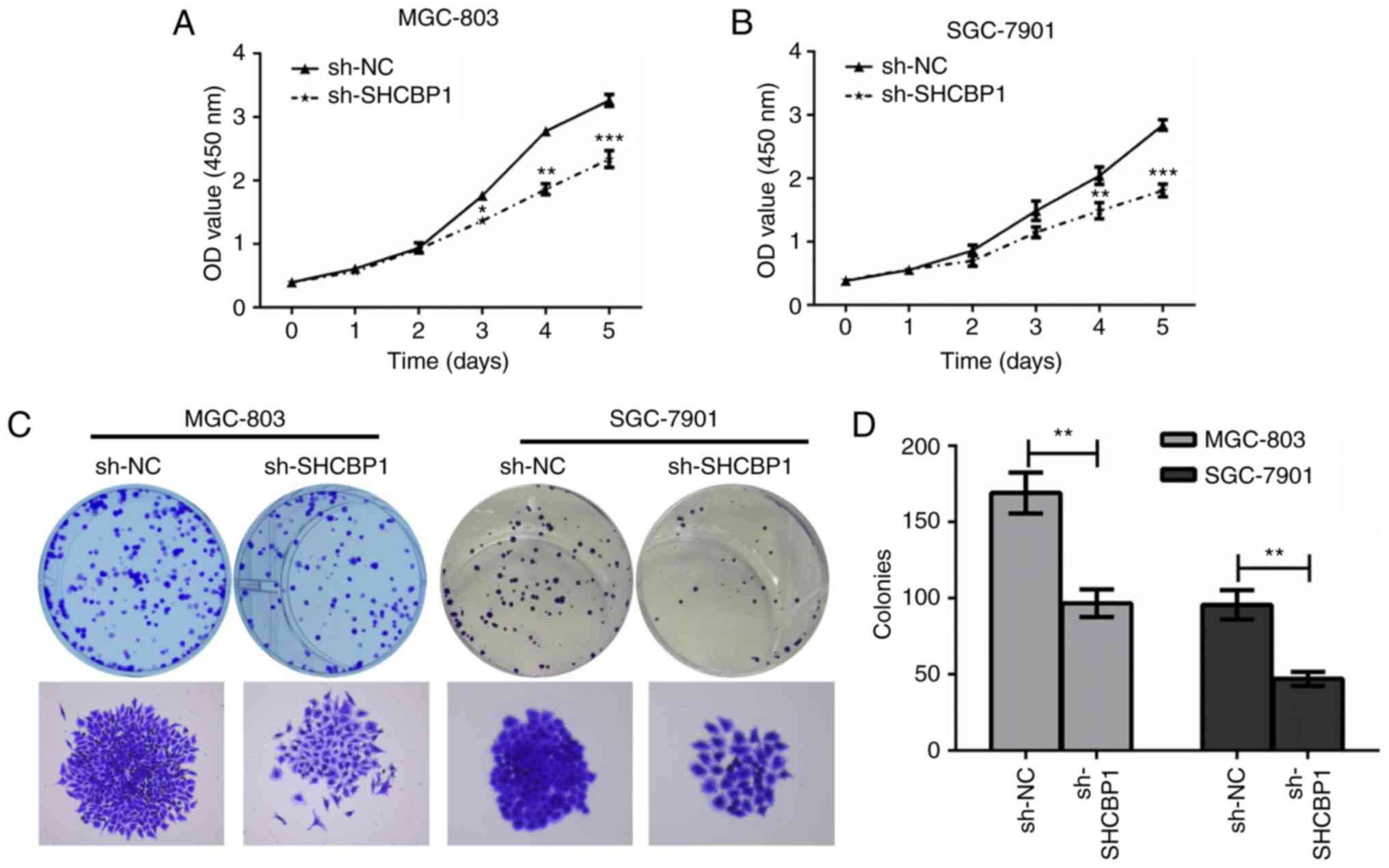
Next, the effect of SHCBP1 on the proliferation of
GC cells was examined. MGC-803 and SGC-7901 cells expressing sh-NC
and sh-SHCBP1 were cultured in 96-well plates, and cell
proliferation was assessed by CCK-8 assay. It was found that GC
cells expressing sh-SHCBP1 significantly inhibited cell
proliferation (Fig. 2A and B). Cell
colony assays of MGC-803 and SGC-7901 cells expressing control,
sh-NC and sh-SHCBP1 were determined (Fig. 2C), and the number of colonies formed
in each group was counted (Fig.
2D). The results revealed that the proliferation of MGC-803 and
SGC-7901 cells by shHCBP1 knockdown was significantly inhibited,
thus, SHCBP1 promoted the proliferation of GC cells.
SHCBP1 promotes cell cycle progression
of GC cells via CDK4-cyclin D1 cascade
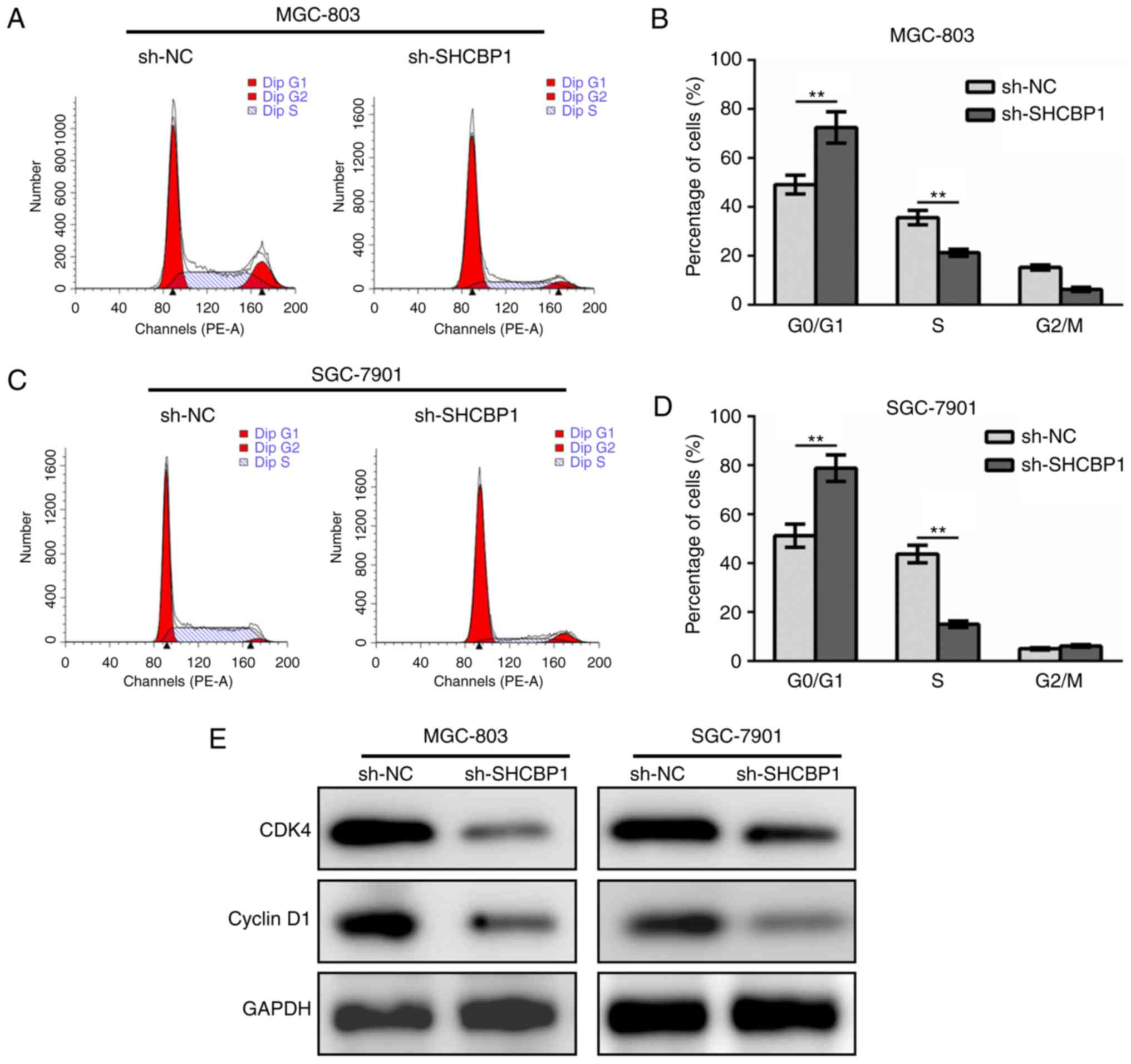
The GC cell lines MGC-803 (Fig. 3A and B) and SGC-7901 (Fig. 3C and D) expressing sh-NC and
sh-SHCBP1 were stained with PI, followed by cell cycle analysis by
flow cytometry. After SHCBP1 knockdown, the proportion of cells in
the G1 phase in the cell cycle increased while the cells in the S
phase decreased significantly, while the G2/M phase was not altered
(Fig. 3B and D). By detecting the
levels of key regulatory proteins in the cell cycle, it was found
that SHCBP1 knockdown resulted in downregulation of CDK4 and cyclin
D1 (Fig. 3E). These data indicated
that SHCBP1 promoted cell cycle progression in GC cells.
SHCBP1 alters the apoptosis pathway
and inhibits apoptosis of GC cells
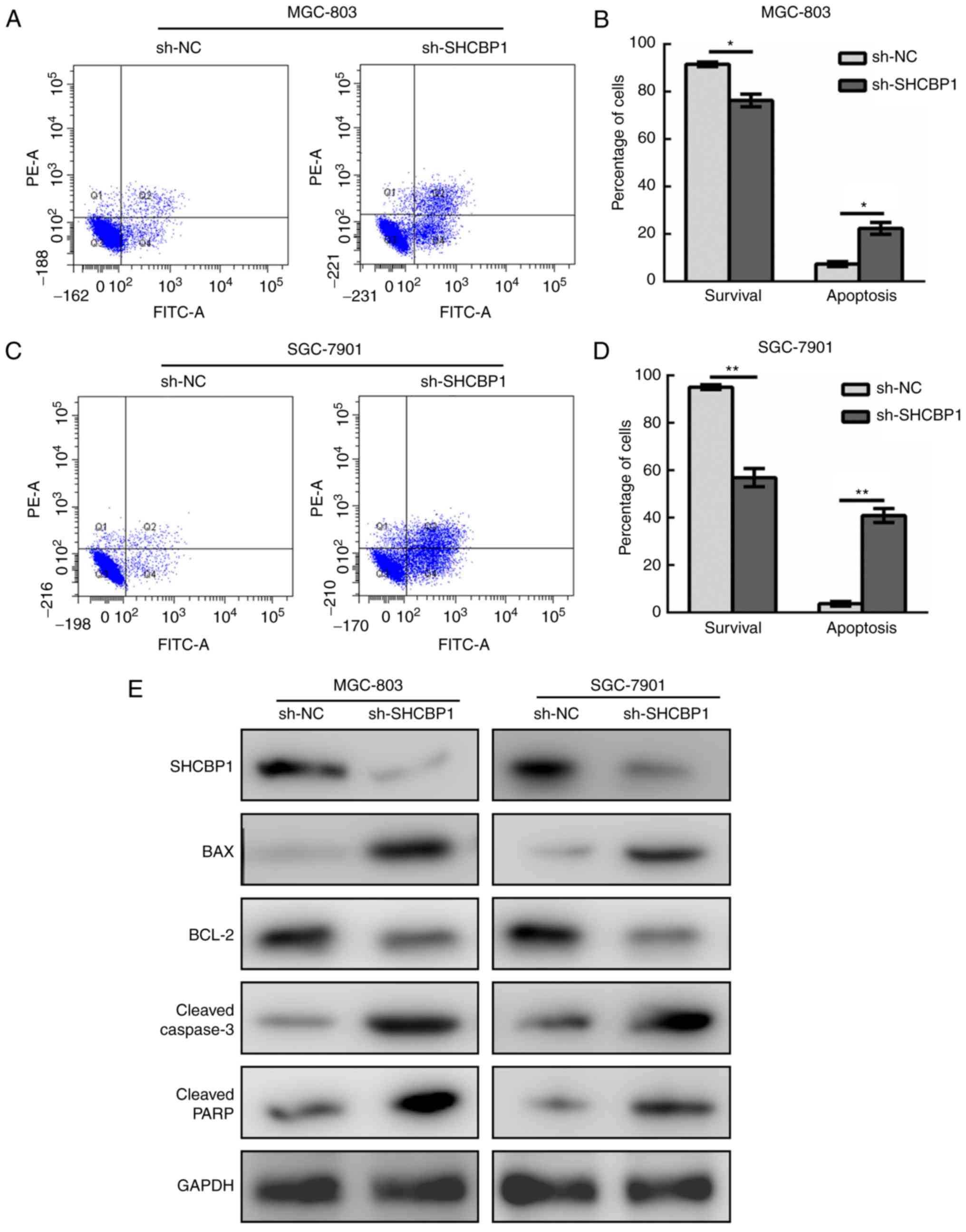
In a similar manner, GC cell lines MGC-803 (Fig. 4A and B) and SGC-7901 (Fig. 4C and D) expressing sh-NC and
sh-SHCBP1 were subjected to apoptosis analysis by flow cytometry.
It was found that the percentage of apoptotic GC cells increased
after SHCBP1 knockdown, and the proportion of surviving cells
decreased. The results of apoptosis-related markers revealed that
the levels of Bax, cleaved caspase-3 and cleaved PARP increased
with SHCBP1 knockdown (Fig. 4E).
Therefore, the role of SHCBP1 in GC cells was to inhibit
apoptosis.
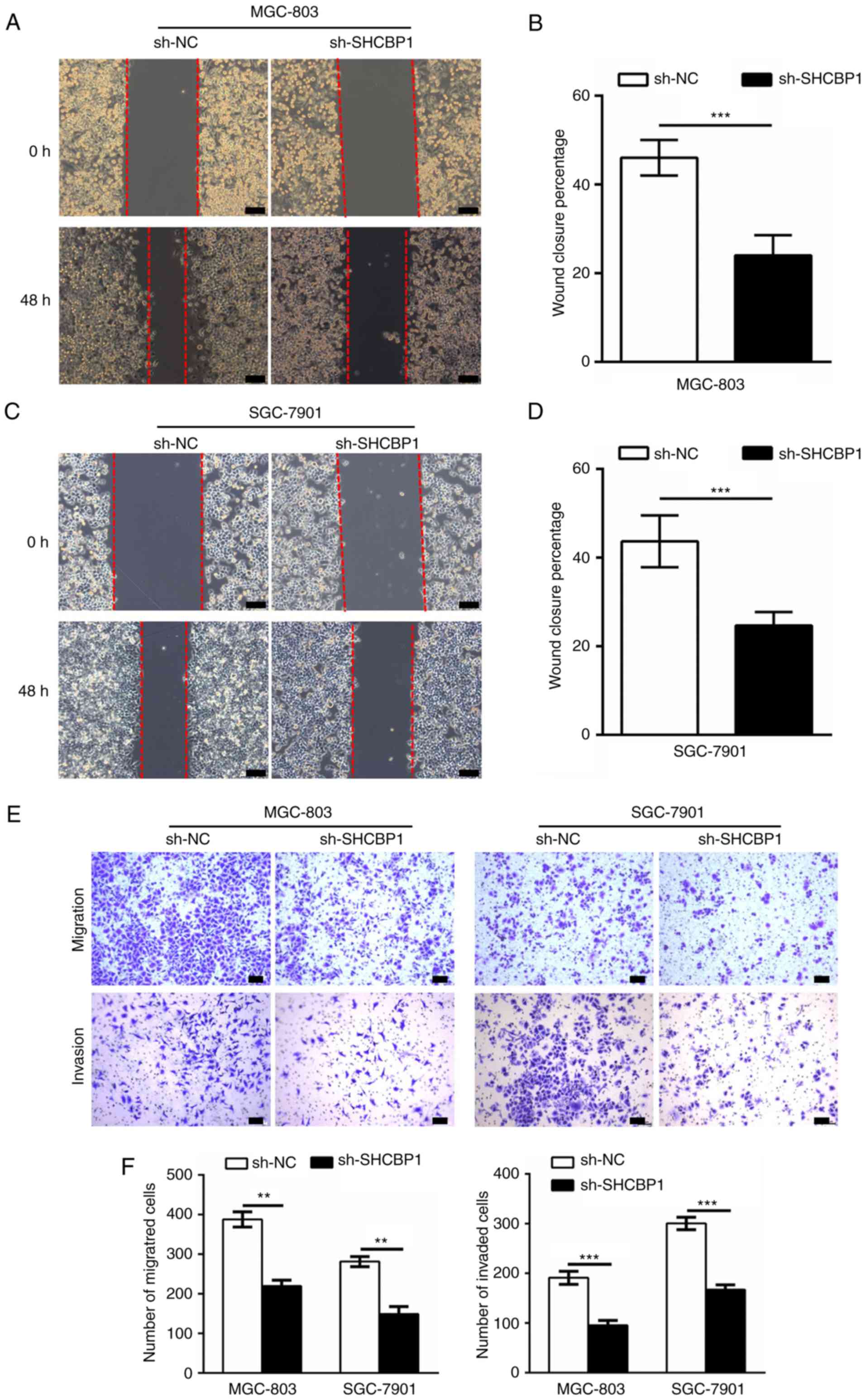
SHCBP1 promotes invasion and
metastasis of GC cells
The role of SHCBP1 in the invasion and metastasis of
GC cells was then investigated. The wound healing assays examined
the effect of knockdown of SHCBP1 on migration ability, and the
migration distance of sh-SHCBP1 MGC-803 (Fig. 5A and B) and SGC-7901 (Fig. 5C and D) cells was significantly
reduced compared to the control and sh-NC cells. In the invasion
and metastasis assays, we assessed the invasion and migration
abilities of treated MGC-803 and SGC-7901 cells by Transwell. The
number of invading and migrating cells in sh-SHCBP1 cells was
significantly reduced compared to control and sh-NC cells (Fig. 5E and F). These data indicated that
expression of SHCBP1 enhanced the ability of GC cells to invade and
metastasize.
Discussion
At present, the prognosis of GC still relies on
traditional pathological indicators such as tumor size and
histological grade (14,15). Therefore, it is imperative to study
the relevant molecular mechanisms in the development of GC. The
development of GC involves a series of oncogenes and tumor
suppressors. Screening for new oncogenes and studying their
mechanisms of action have important clinical diagnostic value for
the early diagnosis of biomarkers and potential molecular
therapeutic targets for GC. The metastasis of GC is a multi-step
pathological process involving a loss of epithelial properties,
such as reduced expression of E-cadherin, and a gain in mesenchymal
phenotypes, such as enhanced expression of N-cadherin and vimentin
(16). On one hand, tumor cells
regulate the changes of their own molecules, so that they can
develop in the direction of metastasis; on the other hand, they
interact with other cells to form a special metastatic
microenvironment and promote liver metastasis of GC (17). At present, there are still many
molecular mechanisms to be studied in the process of GC metastasis.
Although GC is easily transferred to the liver mainly due to the
function of the liver and intestine, numerous studies have shown
that tumor cells do not tend to metastasize to the nearest organ,
and different tumors have the specificity of organ metastasis
(18). The intrinsic determinants
of GC-specific metastasis are still unclear. Finding specific genes
important for GC liver metastasis is a priority for future
research. The development of targeted therapeutic drugs for these
specific molecules has important clinical application value. Each
process of GC liver metastasis is inseparable from the interaction
with the microenvironment, and the tumor microenvironment plays an
important role in promoting the metastatic process (19). Considering the invasion-promoting
ability of SHCBP1 in various cancers and in GC, it is of high
significance to further examine the relationship between SHCBP1
expression and liver metastasis. Additionally, enlarged human
tissues are required to assess the potential of SHCBP1 to serve as
an early diagnostic marker.
Research on the transfer microenvironment has
gradually become the main research direction in the field of
cancer, however research on the microenvironment of liver
metastasis of GC is still in its preliminary stage, and in-depth
research is required to have a clearer understanding of liver
metastasis of GC (20). In recent
years, tumor treatment strategies targeting the tumor
microenvironment have rapidly developed, and drugs targeting the
tumor extracellular matrix, endothelial cells or immune cells have
been increasingly developed and utilized (21). However, in GC, particularly liver
metastasis, there is still a lack of effective targeted therapies.
Since many patients with liver metastases have been unable to
undergo surgery, accurate targeted therapy and emerging
immunotherapy may be the main way to treat liver metastasis of GC
in the future.
To further clarify whether SHCBP1 can act on the
proliferation and invasion of GC cells, we first detected the
expression of SHCBP1 in different GC cells, and selected the cell
lines with higher relative expression. Then, shRNA interfered with
SHCBP1 expression, and subsequently an MTT assay, flow cytometry
and cell invasion assay were used to evaluate the effect of
interference with the expression of SHCBP1 on proliferation,
apoptosis and invasion of GC cells. At last, the effect of SHCBP1
on the downstream signal factors of proliferation was detected by
western blotting. The possible molecular mechanism of SHCBP1 in the
proliferation and invasion of GC cells was explored, which provided
a theoretical basis for the mechanism of SHCBP1 in the development
of GC.
The clinical treatment of tumors is gradually
shifting to individualization, and the discovery of new biomarkers
can be used for early diagnosis of tumors, prediction of the
probability of metastatic spread and recurrence and evaluation of
treatment effects. The SHCBP1 signaling pathway has been revealed
to be involved in the pathogenesis of a variety of malignant
tumors, promoting tumor proliferation, migration and invasion, and
inhibiting apoptosis and differentiation (11,22,23).
Therefore, the application of SHCBP1 and its signaling pathway in
the diagnosis and treatment of tumors may have a high significance.
Currently SHCBP1 is being used as follows: i) SHCBP1-specific
inhibitors are being used to antagonize binding to SHCBP1 and
inhibit tumor cell proliferation and migration; ii) siRNAs to
silence the SHCBP1 gene are being applied, thereby changing the
expression and function of SHCBP1, consequently preventing and
inhibiting the autocrine pathway and its mediated tumor cell
activity; iii) blocking the anti-apoptotic effect of the SHCBP1
downstream signaling pathway is being used; iv) intervention to
regulate the upstream signaling pathway of SHCBP1 is also being
utilized, thereby indirectly regulating the SHCBP1 signaling
pathway; v) in addition, radiolabels and drugs are being designed
to bind to SHCBP1 inhibitors, and transport radioactive markers or
drugs to tumor cells expressing SHCBP1 for imaging diagnosis and
targeted therapy of tumors. In summary, SHCBP1 plays an important
role in the development of GC and is expected to become an
important biomarker and therapeutic target for the early diagnosis
of tumors.
Acknowledgements
Not applicable.
Funding
The present study was supported in part by a grant
from the Health and Family Planning Commission of Henan Province
(no. 182102310205).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
DYL supervised and directed this study. YDD and YLY
performed most of the experiments. DYL, YDD and YLY contributed to
the project design. HBY, GJT and YDD performed the western blot
analysis and the Traswell assays. HBY and GJT contributed to the
RNA extraction. DYL, YDD and YLY analyzed the data and wrote the
manuscript. All authors read and approved the manuscript and agree
to be accountable for all aspects of the research in ensuring that
the accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors state that they have no competing
interests.
References
|
1
|
Whiting J, Sano T, Saka M, Fukagawa T,
Katai H and Sasako M: Follow-up of gastric cancer: A review.
Gastric Cancer. 9:74–81. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rosell R, Carcereny E, Gervais R,
Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R,
Pallares C, Sanchez JM, et al: Erlotinib versus standard
chemotherapy as first-line treatment for European patients with
advanced EGFR mutation-positive non-small-cell lung cancer
(EURTAC): A multicentre, open-label, randomised phase 3 trial.
Lancet Oncolog. 13:239–246. 2012. View Article : Google Scholar
|
|
3
|
Shitara K, Kondo C, Takahari D, Ura T,
Muro K and Matsuo K: Reporting patient characteristics and
stratification factors in randomized trials of systemic
chemotherapy for advanced gastric cancer. Gastric Cancer.
15:137–143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baek HU, Sang BK, Cho EH, Jin SH, Yu HJ,
Lee JI, Bang HY and Lim CS: Hepatic resection for hepatic
metastases from gastric adenocarcinoma. J Gastric Cancer. 13:86–92.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Heinrich JN, Kwak SP, Howland DS, Chen J,
Sturner S, Sullivan K, Lipinski K, Cheng KY, She Y, Lo F, et al:
Disruption of ShcA signaling halts cell
proliferation-Characterization of ShcC residues that influence
signaling pathways using yeast. Cell Signal. 18:795–806. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ferro M, Savino MT, Ortensi B, Finetti F,
Genovese L, Masi G, Ulivieri C, Benati D, Pelicci G and Baldari CT:
The shc family protein adaptor, rai, negatively regulates T cell
antigen receptor signaling by inhibiting ZAP-70 recruitment and
activation. PLoS One. 6:e298992011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shih HJ, Chen HH, Chen YA, Wu MH, Liou GG,
Chang WW, Chen L, Wang LH and Hsu HL: Targeting MCT-1 oncogene
inhibits Shc pathway and xenograft tumorigenicity. Oncotarget.
3:1401–1415. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Francia P, Cosentino F, Schiavoni M, Huang
Y, Perna E, Camici GG, Lüscher TF and Volpe M: p66Shc
protein, oxidative stress, and cardiovascular complications of
diabetes: The missing link. J Mol Med. 87:885–891. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Asano E, Hasegawa H, Hyodo T, Ito S, Maeda
M, Chen D, Takahashi M, Hamaguchi M and Senga T: SHCBP1 is required
for midbody organization and cytokinesis completion. Cell Cycle.
13:2744–2751. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tao HC, Wang HX, Dai M, Gu CY, Wang Q, Han
ZG and Cai B: Targeting SHCBP1 inhibits cell proliferation in human
hepatocellular carcinoma cells. Asian Pac J Cancer Prev.
14:5645–5650. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Asano E, Hasegawa H, Hyodo T, Ito S, Maeda
M, Takahashi M, Hamaguchi M and Senga T: The Aurora-B-mediated
phosphorylation of SHCBP1 regulates cytokinetic furrow ingression.
J Cell Sci. 126:3263–3270. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Peng C, Zhao H, Chen W, Song Y, Wang X, Li
J, Qiao Y, Wu D, Ma S, Wang X, et al: Identification of SHCBP1 as a
novel downstream target gene of SS18-SSX1 and its functional
analysis in progression of synovial sarcoma. Oncotarget.
7:66822–66834. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2ΔΔCT method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Van CE: The treatment of advanced gastric
cancer: New findings on the activity of the taxanes. Oncologist.
2:9–15. 2004.
|
|
15
|
Aoyama T, Maezawa Y and Sawazaki S:
Evaluation of clinic pathological characteristics and prognosis of
gastric cancer in elderly patients. Ann Cancer Res Ther. 26:31–32.
2018. View Article : Google Scholar
|
|
16
|
Deng X, Liu P, Zhao Y and Wang Q:
Expression profiling of CEACAM6 associated with the tumorigenesis
and progression in gastric adenocarcinoma. Genet Mol Res.
13:7686–7697. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ren G, Tian Q, An Y, Feng B, Lu Y, Liang
J, Li K, Shang Y, Nie Y, Wang X and Fan D: Coronin 3 promotes
gastric cancer metastasis via the up-regulation of MMP-9 and
cathepsin K. Mol Cancer. 11:672012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Obenauf AC and Massagué J: Surviving at a
distance: Organ specific metastasis. Trends Cancer. 1:76–91. 2015.
View Article : Google Scholar :
|
|
19
|
Romano F, Garancini M and Uggeri F,
Degrate L, Nespoli L, Gianotti L, Nespoli A and Uggeri F: Surgical
treatment of liver metastases of gastric cancer: State of the art.
World J Surg Oncol. 10:1572012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsuboi K, Kodera Y, Nakanishi H, Ito S,
Mochizuki Y, Nakayama G, Koike M, Fujiwara M, Yamamura Y and Nakao
A: Expression of CXCL12 and CXCR4 in pT3-stage gastric cancer does
not correlate with peritoneal metastasis. Oncol Rep. 20:1117–1123.
2008.PubMed/NCBI
|
|
21
|
Patra C, Boccaccini AR and Engel FB:
Vascularisation for cardiac tissue engineering: The extracellular
matrix. Thromb Haemost. 113:532–547. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peng C, Zhao H, Song Y, Chen W, Wang X,
Liu X, Zhang C, Zhao J, Li J, Cheng G, et al: SHCBP1 promotes
synovial sarcoma cell metastasis via targeting TGF-β1/Smad
signaling pathway and is associated with poor prognosis. J Exp
Clini Cancer Res. 36:1412017. View Article : Google Scholar
|
|
23
|
Feng W, Li HC, Xu K, Chen YF, Pan LY, Mei
Y, Cai H, Jiang YM, Chen T and Feng DX: SHCBP1 is over-expressed in
breast cancer and is important in the proliferation and apoptosis
of the human malignant breast cancer cell line. Gene. 587:91–97.
2016. View Article : Google Scholar : PubMed/NCBI
|