Introduction
Lung cancer is the leading cause of cancer mortality
worldwide and its incidence is constantly increasing (1). Non-small cell lung cancer (NSCLC) is
the main pathological type of lung cancer and its incidence is
>85% in patients with lung cancer. Platinum-based dual drug
regimens are the first-line treatment for NSCLC (1,2).
Cisplatin (DDP) is a classic platinum compound used in lung cancer
therapy, which binds to DNA strands and interferes with DNA
replication causing cell cycle arrest in the S phase (3). However, the increased ability of DNA
repair frequently affects the therapeutic efficacy of DDP. Thus, it
is indispensable to explore the mechanism of drug resistance and
provide alternative means to improve the antitumor efficacy of DDP.
Recent studies have proposed numerous mechanisms that could explain
DDP chemoresistance, including decreased drug accumulation in
cells, enhanced detoxification ability, enhanced DNA damage repair
function of the cells and inhibition or inactivation of the
apoptotic process (4,5). However, DDP resistance in the
aforementioned cases is associated with the increased ability of
DNA repair and nucleotide excision repair (NER) that are involved
in the removal of intra-strand DNA-cross-links caused by DDP
(6).
Xeroderma pigmentosum, complementation group C (XPC)
is a nuclear excision repair gene which mainly participates in the
identification of DNA damage (7).
Recent studies have identified that XPC is aberrantly expressed in
several human types of cancer, such as lung, breast, gastric and
ovarian cancer (8–11). The positive correlation of the
expression of XPC and the outcome of various types of cancer
indicated that XPC was closely associated with the incidence of
cancer and its progression. This in turn affected the response rate
and the overall survival rate of the affected patients. Emerging
evidence has suggested that XPC affects early stages of lung
carcinogenesis (12). It is
important to note that the expression deficiency or downregulation
of XPC affects DNA repair capacity and the sensitivity of the cells
to platinum-based drug chemotherapy (13). Thus, several studies have shown that
XPC expression deficiency can activate matrix metalloproteinase-1
(MMP1) and p53 transcription in order to promote induction of
apoptosis (14). The present study
revealed that XPC may play major roles in the progression of lung
cancer via its sensitivity to standard chemotherapy (14). However, the exact mechanism
underlying the upregulation of XPC expression levels and drug
resistance in lung cancer remains unclear.
In the present study, we aimed to investigate the
role of XPC on the resistance of A549/DDP lung adenocarcinoma cells
with regard to the efficacy of DDP. The expression levels of the
XPC protein and mRNA were determined in A549 and A549/DDP cells.
The proliferative, migratory and apoptotic activities of A549/DDP
cells were examined in comparison with XPC expression using gene
silencing approaches. The signaling pathways that mediated the
induction of XPC expression were investigated. The findings
revealed that XPC silencing significantly inhibited A549/DDP cell
proliferation and increased the induction of apoptosis. XPC further
regulated the major proteins involved in the PI3K/Akt/mTOR
signaling pathway and their downstream mediators in
vitro.
Materials and methods
Reagents and antibodies
DDP was obtained from Sigma- Aldrich; Merck
(Shanghai, China). XPC-siRNA (si-XPC) and the negative control
(siNC) were purchased from Shanghai GeneChem Co., Ltd. (Shanghai,
China). The FITC Annexin V Apoptosis Detection kit 1 (BD
Biosciences, Franklin Lakes, NJ, USA) was used for detecting cell
apoptosis by flow cytometry. The anti-Akt (dilution 1:1,000; cat.
no. 4691S), anti-p-Akt (dilution 1:1,000; cat. no. 4060S),
anti-caspase-3 (dilution 1:1,000; cat. no. 9662S),
anti-cleaved-caspase-3 (dilution 1:1,000; cat. no. 9661S) and
anti-caspase-9 (dilution 1:1,000; cat. no. 9502P) antibodies were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA);
and the anti-p-mTOR (dilution 1:1,000; cat. no. 13152-1) antibody
was purchased from Signalway Antibody LLC (College Park, MD, USA).
The anti-PI3K (dilution 1:1,000; cat. no. A0982) and anti-mTOR
(dilution 1:1,000; cat. no. A11928) antibodies were purchased from
ABclonal Technology (Wuhan, China), and the anti-XPC (dilution
1:2,000; cat. no. ab203693) antibody was purchased from Abcam
(Cambridge, UK). The anti-Bax (dilution 1:1,000; cat. no.
50599-1-AP) and anti-Bcl-2 (dilution 1:1,000; cat. no. 12789-1-AP)
antibodies were purchased from ProteinTech Group, Inc. (Rosemont,
IL, USA). The actin (dilution 1:1,000; cat. no. TA-09) and GAPDH
(dilution 1:1,000; cat. no. TA-08) antibodies were obtained from
OriGene Technologies, Inc. (Beijing, China). The secondary antibody
horseradish peroxidase conjugated goat anti-rabbit IgG (dilution
1:5,000; cat. no. ZB-2306), also was purchased from OriGene
Technologies, Inc.
Cell culture and transfection
A549 and A549/DDP human lung carcinoma cells were
obtained from the Chinese Academy of Sciences (Shanghai, China).
Both cell lines were cultured in RPMI-1640 medium (HyClone; GE
Healthcare Life Sciences, Logan, UT, USA) supplemented with 10%
fetal bovine serum (FBS; HyClone; GE Healthcare Life Sciences), 100
U/ml penicillin and 100 U/ml streptomycin (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) in a humidified atmosphere of
5% CO2 at 37°C. In addition, A549/DDP cells were
cultured in medium containing 2 mg/l DDP in order to retain the
drug resistant phenotype. The cells were divided into 3 groups as
follows: The blank group (without transfection), the group
transfected with the small interfering RNA targeting the XPC
(si-XPC) group and the negative control (si-NC) group. The target
sequence of si-XPC was: CTCTGACCTGTTACAAGTA. A total of
2×105 A549/DDP cells were added into a 6-well plate. The
cells were transfected at 70% confluence with si-XPC and/or si-NC
using Lipofectamine™ 2000 (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. The
transfection reagent was mixed with si-RNA or si-NC in a ratio of 2
µl:1 µg. We determined the transfection efficiency using western
blotting. Following 24 h of transfection, each group of cells that
were in the logarithmic growth phase was collected for the next
experiment.
Cell Counting Kit-8 assay
Cell Counting Kit-8 (CCK-8) assay (Dojindo Molecular
Technologies, Inc., Rockville, MA, USA) was used to detect cell
viability following different treatments. Briefly, A549 and
A549/DDP cells were transfected with si-XPC and si-NC and were
incubated in a 96-well plate (3,000 cells/well) overnight.
Following DDP treatment at the indicated doses (0, 0.5, 1, 2, 4, 8,
16 and 32 mg/l) for 24 h, 10 µl of CCK-8 solution was added to each
well and the plates were incubated for 1 h. The optical density
(OD) of each well was detected at 450 nm using a microplate reader
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) according to the
manufacturer's instructions. The half inhibitory concentration
(IC50) of the cells was determined by CCK-8 assay. The
half-maximal inhibitory concentration (IC50) of the
drugs was calculated on GraphPad Prism using the log(inhibitor) vs.
response-variable slope (4 parameters) equation under the
non-linear regression dialogue.
Wound healing assay
Following transfection of A549/DDP cells, the cells
were allowed to grow for 24 h. The monolayer was scratched with a
200-µl pipette tip. The attached cells were washed 3 times with
phosphate-buffered saline (PBS) in order to remove floating cells
and debris. Subsequently, serum-free medium was added. Moreover,
DDP was added in the petri dishes as described above. The cells
were continuously incubated for 48 h. The wounds were visualized
every 24 h, and the lines that were aligned with the wounds of each
group were photographed in each experiment under an inverted
microscope (TS100; Nikon Corp., Tokyo, Japan). In each well, at
least 8 regions of each condition was captured randomly at a
magnification of ×100. The images were analyzed using ImageJ
v2.1.4.7 software (National Institutes of Health, Bethesda, MD,
USA).
Flow cytometry
The rate of apoptosis was analyzed by flow cytometry
using an Annexin V-FITC/PI kit (BD Biosciences, Franklin Lakes, NJ,
USA). A549/DDP cells were seeded in a 6-well plate and incubated
overnight. Following transfection, the cells were incubated with
DDP in growth media for 24 h. The cells were collected and
resuspended in 500 µl of 1X binding buffer. Subsequently, staining
was conducted as described by the manufacturer's protocol.
Quantitative real-time PCR
Total RNA was extracted with TRIzol reagent (Axygen,
Inc.; Corning Inc., Corning, NY, USA). The cDNA was generated with
a cDNA Synthesis kit (Toyobo Life Science, Osaka, Japan). qRT-PCR
was performed using a SYBR-Green assay kit (Roche Diagnostics,
Basel, Switzerland) on an Applied Biosystems thermal cycler
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The qPCR
reactions were run on the ABI 7500 real-time PCR system using the
following conditions: 95°C for 30 sec, followed by 35 cycles at
62°C for 30 sec and 72°C for 30 sec, final extension at 72°C for 5
min. The relative mRNA expression levels of GAPDH were calculated
and quantified with the 2−∆∆Cq method (15). GAPDH was used as an internal
control. The primers for XPC and GAPDH were as follows:
5′-GACAAGCAGGAGAAGGCAAC-3′ and 5′-GGTTCGGAATCCTCATCAGA-3′ for the
XPC sense and reverse primers, respectively; and
5′-TGGACCTGACCTGCCGTCTA-3′ and 5′-AGGAGTGGGTGTCGCTGTTG-3′ for the
GAPDH sense and reverse primers, respectively.
Western blot assay
The expression levels of various proteins were
detected by western blot analysis. The total protein in the cells
was collected by RIPA lysis buffer (Beijing Solarbio Science &
Technology Co., Ltd., Beijing, China). The protein concentration
was determined using a BCA protein concentration assay kit
(Beyotime Institute of Biotechnology, Shanghai, China), and protein
products (8–12 µg/µl; 40–50 µg total) were separated by 10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
then transferred onto polyvinylidene fluoride (PVDF) membranes (EMD
Millipore, Bedford, MA, USA). Subsequently, the membranes were
blocked with BSA for 1 h at room temperature and incubated with
primary antibodies overnight at 4°C. Following washing with PBST
(Beijing Solarbio Science & Technology Co., Ltd.), the PVDF
membranes were subsequently incubated with the secondary antibodies
for 1 h. Following additional washing with PBST to remove the
secondary antibodies, the protein signals were detected by the
ChemiDoc XRS gel documentation system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) using ECL Western Blotting Substrate (Beijing
Solarbio Science and Technology Co., Ltd.). The results were
scanned and quantified using the ImageJ software v2.1.4.7.
Statistical analysis
Each experiment was conducted at least 3 times and
the results were presented as the mean values ± standard deviation
(SD). One-way analysis of variance (ANOVA) was carried out to
compare the differences among multiple groups, and the Bonferroni
test was performed followed ANOVA. In addition, the independent
samples t-test was performed to compare the differences between two
groups. Statistical analysis was performed using the GraphPad Prism
5 statistical software (GraphPad Software, Inc., San Diego, CA,
USA). A P-value of <0.05 was considered to indicate a
statistically significant difference.
Results
Upregulation of XPC in A549/DDP
cells
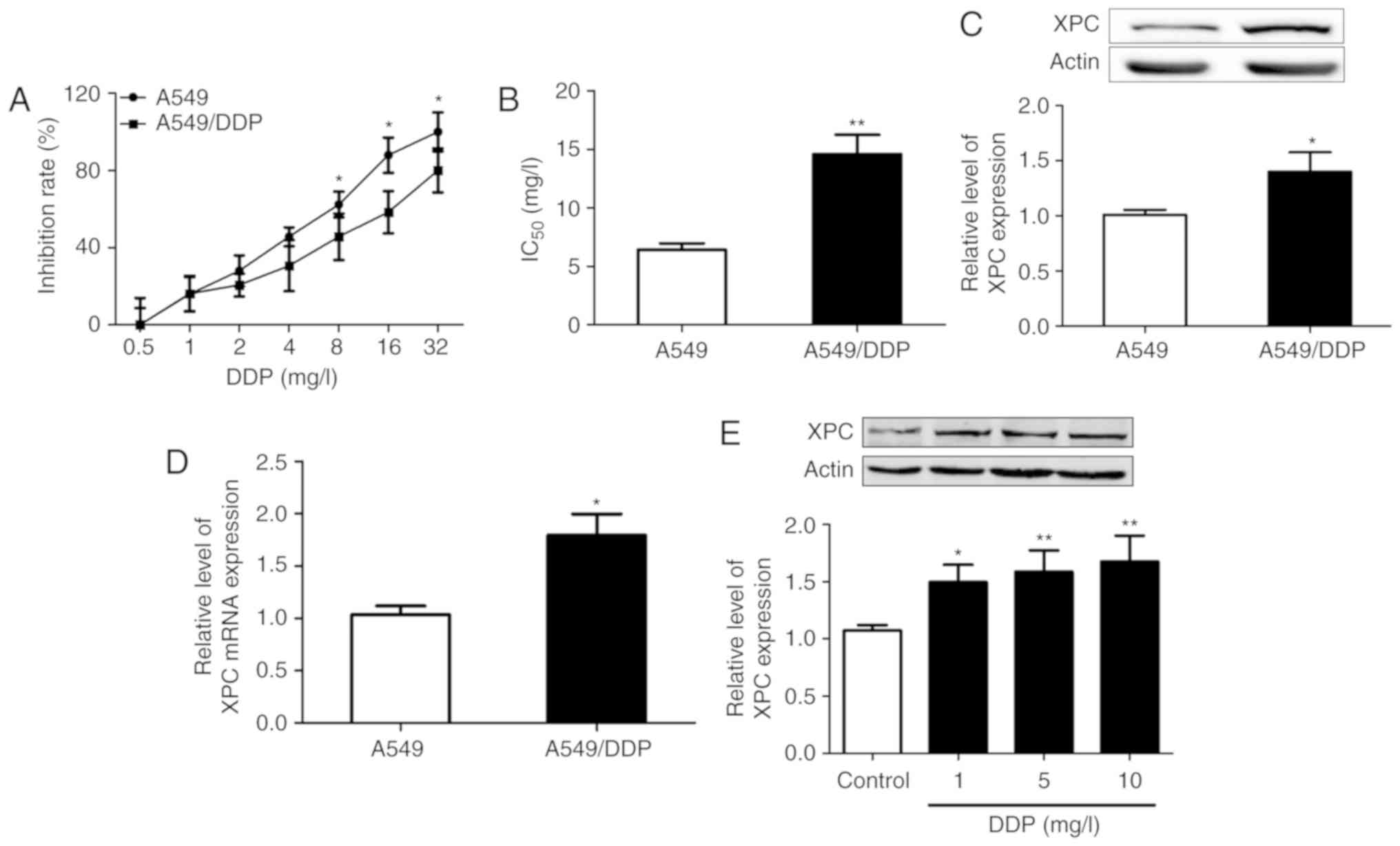
The cytotoxic effects of different concentrations of
DDP (0, 0.5, 1, 2, 4, 8, 16 and 32 mg/l) were examined in A549/DDP
and A549 cells. A CCK-8 assay was used to assess cell viability.
The results revealed that the growth inhibitory rate was increased
following the increase in the concentration of DDP, while the
inhibitory effect of DDP on A549 cells was significantly higher
than that noted in A549/DDP cells (Fig.
1A). Furthermore, we calculated the IC50 values of
A549 and A549/DDP cells using a CCK-8 assay, and the results
indicated that it was 6.43±0.89 mg/l in A549 cells and 14.6±2.87
mg/l in A549/DDP cells. A549/DDP cells were more resistant to DDP
treatment in vitro than A549 cells and 10 mg/l of DDP was
selected as the optimal concentration in the follow-up experiments
(Fig. 1B).
Western blot assays indicated that the expression
levels of XPC were significantly increased in A549/DDP cells
compared with those noted in A549 cells (P<0.05) (Fig. 1C). Moreover, to determine whether
upregulation of XPC expression was a result of increased
transcription, qRT-PCR was performed to analyze the mRNA levels of
XPC. The results demonstrated that the mRNA levels of XPC
were also upregulated (Fig. 1D).
DDP treatment induced an increase in XPC protein levels in A549/DDP
cells (Fig. 1E). These results
indicated that the expression levels of XPC were upregulated in
A549/DDP cells compared with those in A549 cells.
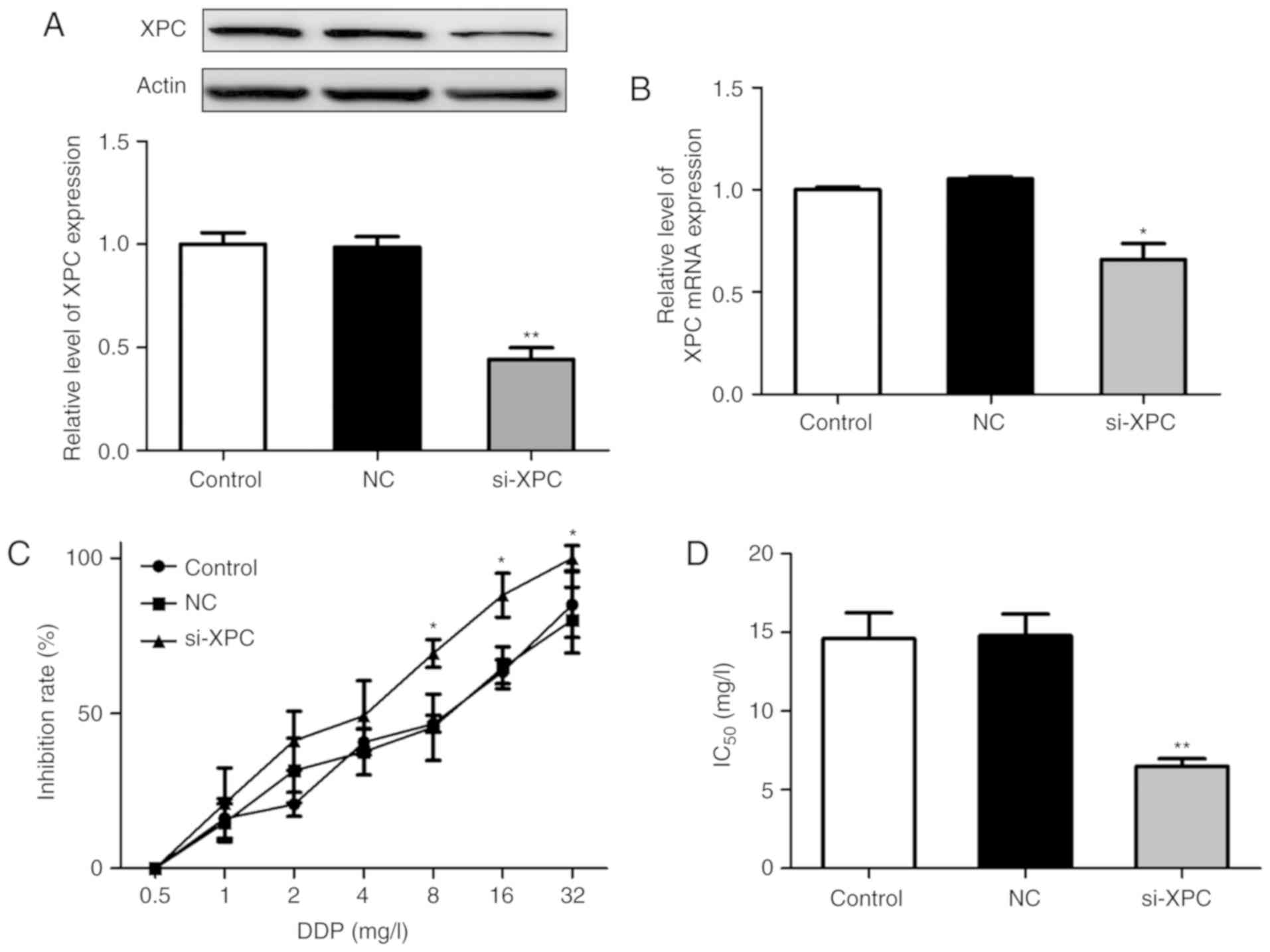
Efficient silencing of XPC expression
in A549/DDP cells using siRNA
To further explore the role of XPC in the resistance
of A549/DDP cells to chemotherapy, XPC knockdown in A549/DDP cells
was performed with siRNA. Based on previous studies, the most
effective siRNA (siRNA-178) was selected from the 3 knockdown
sequences (siRNA-177/178/179) for the following experiments.
A549/DDP cells were transfected with si-XPC or si-NC. Western
blotting detection demonstrated that the expression levels of XPC
were significantly downregulated compared with those noted in the
negative control group (Fig. 2A).
Furthermore, XPC mRNA levels were also inhibited following
si-XPC transfection (Fig. 2B). The
effects of XPC knockdown on the sensitivity of the resistant cells
to DDP were subsequently investigated. A CCK-8 assay was employed
to examine the cell viability of A549/DDP cells. The results
revealed that the inhibitory potencies of DDP were higher in the
si-XPC transfected group than those in the other 2 groups (Fig. 2C). In addition, the IC50
values of DDP in the 3 groups were calculated and as shown in
Fig. 2D, no significant differences
were noted in the IC50 values of DDP between the control
(14.6±2.87%) and the negative control groups (14.81±2.36%). In
contrast to these observations, the IC50 value of DDP
was significantly reduced in the si-XPC transfected group
(6.5±0.82) compared with that noted in the other 2 groups
(P<0.05). These results indicated that A549/DDP cells were more
sensitive to DDP treatment following efficient silencing of
XPC.
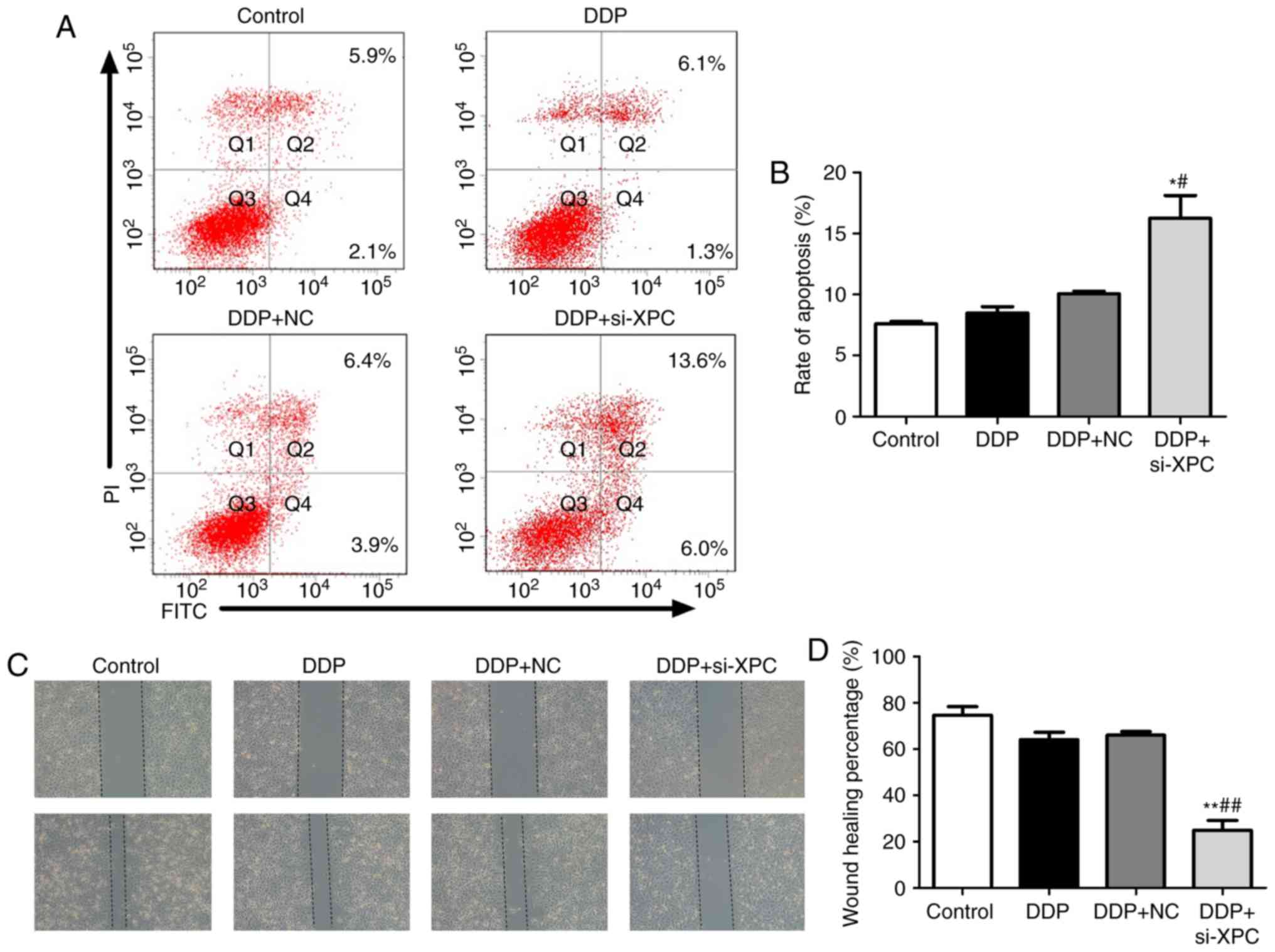
Silencing of XPC in A549/DDP cells by
si-XPC reduces proliferation and migration and promotes
apoptosis
The effect of XPC knockdown on the induction of
apoptosis using Annexin V-FITC/PI double staining was explored. As
revealed in Fig. 3A and B, the
apoptotic rate of the si-XPC group was increased to 19.6% in the
A549/DDP cells, indicating that XPC knockdown induced apoptosis in
A549/DDP cells. A CCK-8 assay was used to detect the proliferative
ability of A549/DDP cells. The results revealed that the
proliferation of A549/DDP cells was significantly reduced following
transfection with si-XPC (Fig. 2C).
Furthermore, a wound healing assay was used to detect the migratory
ability of A549/DDP cells. The results demonstrated that the
migratory distance noted in A549/DDP cells was significantly
reduced following transfection with si-XPC (Fig. 3C and D). Subsequently, the
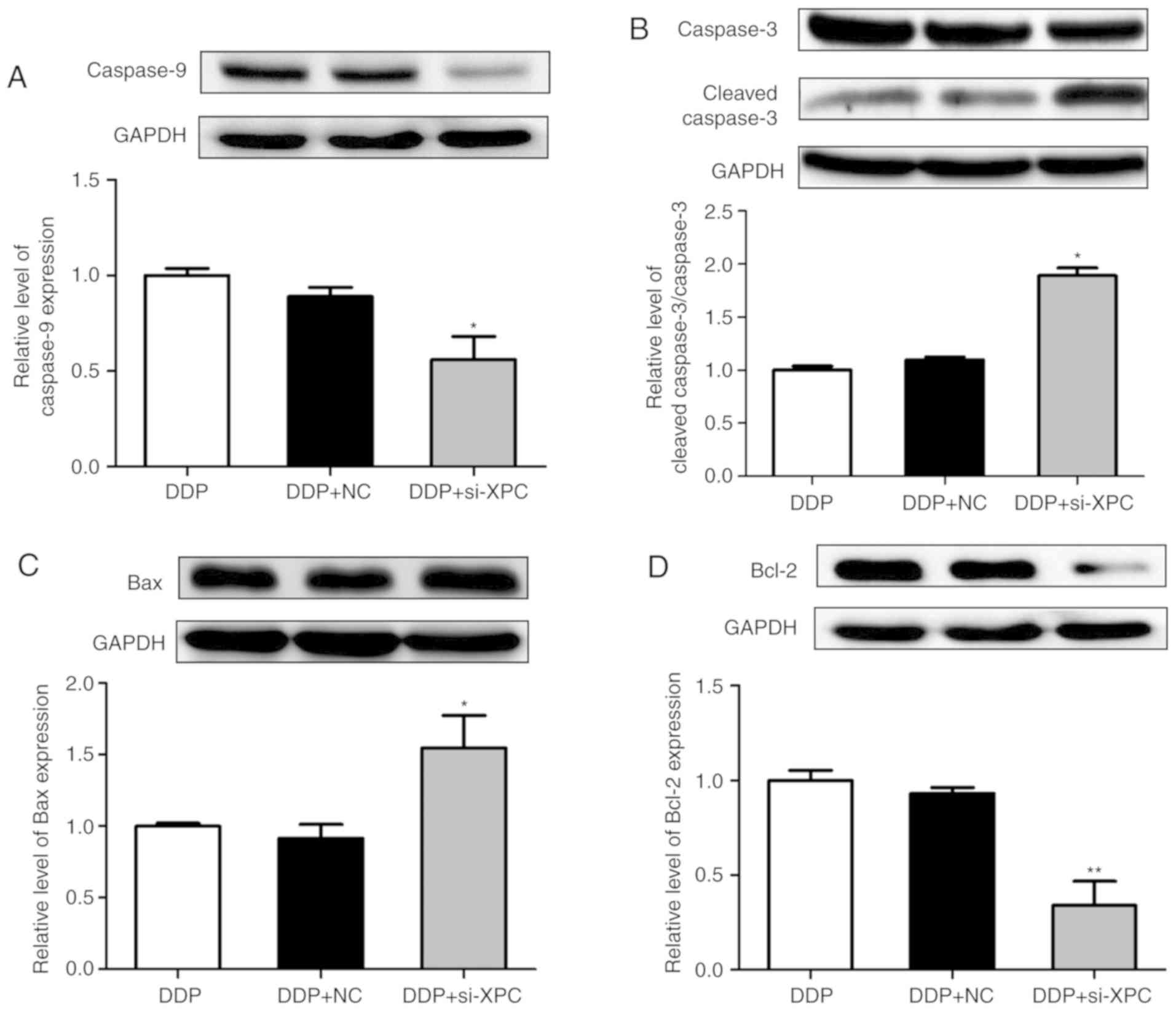
expression levels of the apoptosis-related proteins, including
caspase-3, caspase-9, Bax, Bcl-2 and active caspase-3 were also
determined by western blot assays. XPC knockdown increased the
expression levels of active caspase-3/caspase-3 and Bax/Bcl-2,
while it decreased the expression levels of caspase-9 in A549/DDP
cells that were treated with DDP for 24 h (Fig. 4). These results indicated that XPC
knockdown inhibited cell proliferation and survival.
Silencing of XPC in A549/DDP cells
activates the PI3K/Akt/mTOR pathway
In order to investigate whether the PI3K/Akt/mTOR
pathway is involved in the XPC-mediated cell apoptosis of A549/DDP
cells, the expression levels of apoptosis-related proteins were
detected. Initially, the expression levels of the phosphorylated
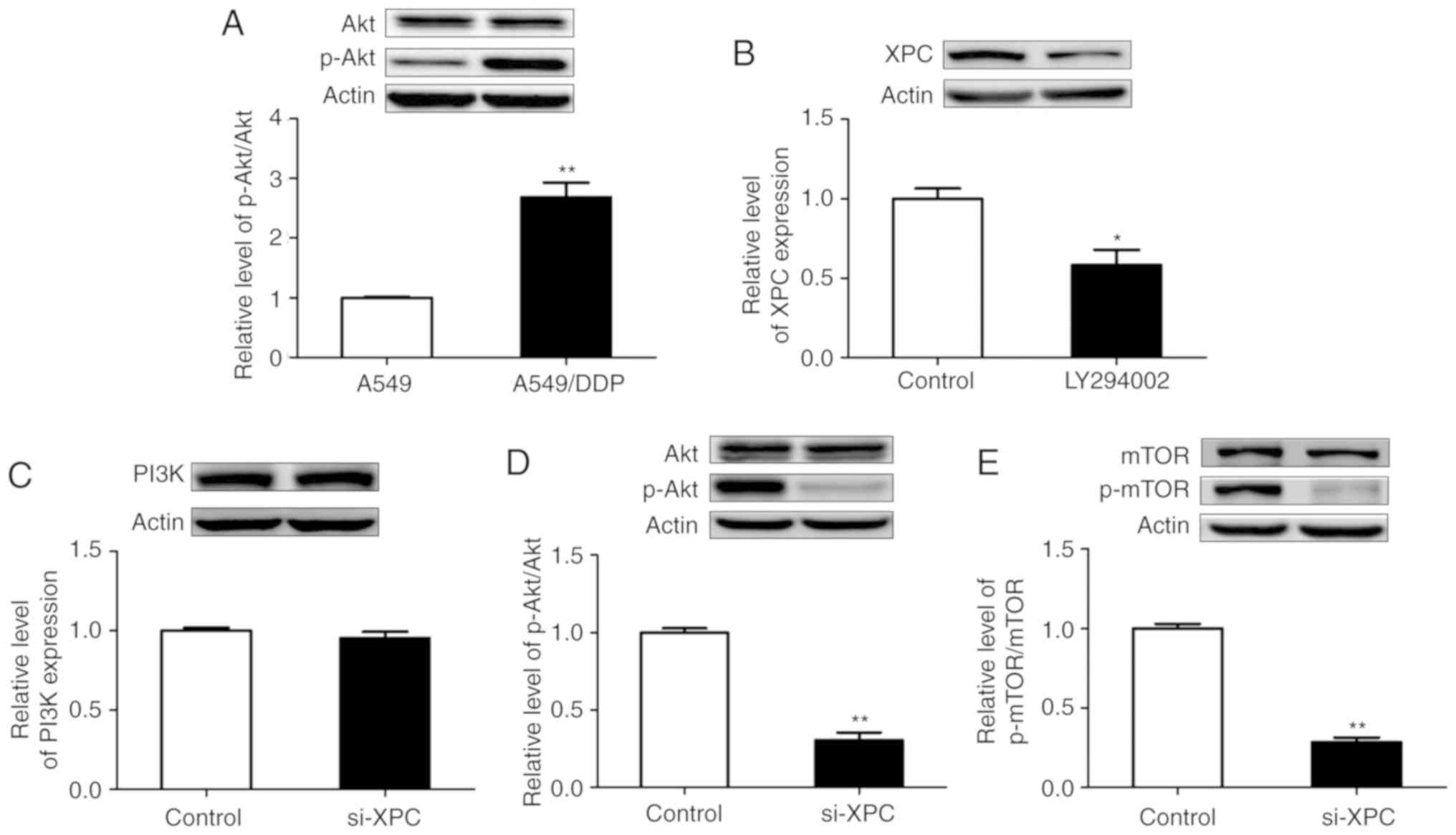
form of the Akt protein (Ser473) were significantly increased in
drug-resistant cells, while no significant change was noted in the
expression levels of the total Akt protein (Fig. 5A). Subsequently, DDP-resistant cells
were treated with PI3K inhibitors and western blotting was
performed in order to detect the expression levels of XPC. The
results revealed that XPC expression in the treatment group was
significantly lower than that in the control group (Fig. 5B). This indicated a potential
interaction between p-Akt and XPC.
XPC silencing using siRNA did not affect the protein
levels of PI3K (Fig. 5C). Silencing
of XPC caused a significant decrease in the expression levels of
p-Akt and p-mTOR proteins in A549/DDP cells (Fig. 5D and E). However, transfection of
si-XPC did not result in a significant change in the protein levels
of Akt and mTOR. This transfection caused a decrease in the levels
of the phosphorylated (activated) form of mTOR (Ser2448), a
downstream target of PI3K/Akt, which has been revealed to promote
cell growth. The results of the present study indicated the
potential impact of XPC on the Akt/mTOR signaling pathway.
Furthermore, silencing of XPC increased the levels of active
caspase-3 protein following DDP treatment (Fig. 4B). Collectively, the data indicated
the effects of XPC on the Akt/mTOR signaling pathway and confirmed
that XPC plays a vital role in the regulation of apoptosis in
A549/DDP cells.
Discussion
The resistance of cancer cells to conventional
cytotoxic drugs ultimately leads to chemotherapy failure and
increased patient mortality. DDP, a non-specific cell cycle agent,
has been used as a primary treatment against various malignancies,
notably those of lung, gastric, esophageal and ovarian origin. The
DNA repair capacity can maintain the genome integrity and
chromosomal stability. Therefore, the increased ability of DNA
repair can lead to DDP resistance. The NER is a key process
required for DNA repair (6).
Various studies have recently revealed that the defective
expression of NER-related genes is associated with tumor
progression and DDP resistance. The results indicated that the
reduced levels of excision repair cross-complementing 1 (ERCC1),
xeroderma pigmentosum, complementation group A (XPA) or xeroderma
pigmentosum, complementation group F (XPF) were associated with the
development and prognosis of lung carcinoma (16). Furthermore, in vitro studies
have linked high ERCC1 expression with platinum resistance in
several types of cancer, while the detection of ERCC1 has
been used as a biomarker for individualized treatment in lung
cancer patients (17–19). A previous study examined the
silencing of various NER genes in HeLa cells and demonstrated that
the suppression of XPC expression could lead to an increased
sensitivity for etoposide treatment combined with DDP (20). Furthermore, upregulation of XPC has
been revealed in lung adenocarcinoma tissue samples of DDP
insensitive patients (21). The
increased expression levels of XPC caused by the stimulation of the
cells with DDP indicate a potential biological mechanism of genomic
integrity leading to DDP resistance.
The serine/threonine kinase Akt is involved in the
progression of various types of cancer, whereas the Akt-mediated
signaling pathway plays an anti-apoptotic role by phosphorylating
target proteins in various downstream pathways (22,23).
The PI3K/Akt/mTOR is an important intracellular signaling pathway
that regulates the cell cycle during cell dormancy, proliferation,
cancer progression and necrosis. However, a previous study has
revealed that XPC expression levels were related to the
inactivation of the Akt pathway (24). Recently, several studies have also
revealed that Akt inhibition reduces cell survival and improves DDP
resistance (25,26). Cheng et al reported that the
levels of PI3K and pAkt in lung cancer cells were significantly
increased compared with those in normal cells and that the
application of PI3K inhibitors could promote lung cancer cell
apoptosis (27). Thus, we
hypothesized that XPC could alleviate the resistance of DDP through
the Akt signaling pathway in lung cancer cells.
In the present study, we propose a novel mechanism
that can reverse the effects of XPC on DDP resistance in lung
cancer cells. Initially, the results indicated that XPC mRNA and
XPC protein levels were markedly increased in the resistant cells
(A549/DDP cells) compared with those noted in the parent cells
(A549 cells) (P<0.05). In addition, the present study examined
the regulation of A549/DDP cell growth by detecting the induction
of apoptosis and the expression of the PI3K/Akt signaling pathway
proteins. The downregulation of XPC expression following
transfection with si-RNA increased the sensitivity to DDP and
reduced the expression of the p-Akt and p-mTOR proteins.
Concomitantly, the proliferative ability and the high
IC50 value were significantly reduced in A549/DDP cells
following XPC downregulation compared with those noted in the
negative control samples.
It has been confirmed that caspase-3 and Bax/Bcl-2
are involved in the regulation of apoptosis. The present study
demonstrated that the ratios of cleaved caspase-3/caspase-3 and
Bax/Bcl-2 were significantly increased following knockdown of XPC
expression in resistant cells (A549/DDP cells), while the
IC50 value and p-Akt expression levels of these cells
were reduced. Our results indicated that suppression of XPC
expression could significantly increase the induction of apoptosis
in chemotherapy-resistant cells. Therefore, we hypothesized that
XPC plays an essential role in the regulation of caspase-3, and in
determining the Bax/Bcl-2 ratio in lung cancer cells. These effects
can reverse the resistance of A549/DDP cells to DDP by accelerating
the induction of apoptosis and by inhibiting cancer cell
proliferation. The resistance of lung cancer cells to
chemotherapeutic drugs was associated with high XPC expression.
In conclusion, our results demonstrated that XPC
expression was linked with DDP resistance in lung cancer cells and
that the reversal of the resistance was mainly mediated by the
Akt/mTOR signaling pathway. The present study provides significant
findings in the potential clinical applications of XPC as a
prognostic marker for DDP resistance of NSCLC. Moreover, this
application can aid the efficacy of chemotherapy by providing
personalized treatment for each patient based on his
pharmacogenomic profile.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Heilongjiang
Provincial Health and Family Planning Commission Scientific
Research Subject (no. 2017-120), the Harbin Medical University
Cancer Hospital Haiyan Fund Youth funding project (no.
JJQN2014-04), the National Natural Science Foundation of China
(81700303) and the Fundamental Research Funds for the Provincial
Universities (2017LCZX17).
Availability of data and materials
All data used in the present study were included in
this manuscript.
Authors' contributions
YS and MD designed the study. XT and XFF wrote the
manuscript. XT, XFF, QL and SL performed the experiments including
cell culture, cell transfection and cell apoptosis assays. SL, DYW
and SYW participated in the western blot assays. DYW and SYW
participated in the real-time RT-PCR assays. YS, XT and QL
conducted the statistical analysis. YS and MD revised the
manuscript. All authors read and approved the manuscript and agreed
to be accountable for all aspects of the research in ensuring that
the accuracy or integrity of any part of the work were
appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NSCLC
|
non-small cell lung cancer
|
|
DDP
|
cisplatin
|
|
NER
|
nucleotide excision repair
|
|
XPC
|
xeroderma pigmentosum complementation
group C
|
|
MMP1
|
matrix metalloproteinase-1
|
|
CCK-8
|
Cell Counting Kit-8
|
|
ERCC1
|
excision repair cross-complementing
1
|
|
XPA
|
xeroderma pigmentosum, complementation
group A
|
|
XPF
|
xeroderma pigmentosum, complementation
group F
|
|
A549/DDP
|
cisplatin-resistant A549 cells
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
NSCLC Meta-Analyses Collaborative Group:
Chemotherapy in addition to supportive care improves survival in
advanced non-small-cell lung cancer: A systematic review and
meta-analysis of individual patient data from 16 randomized
controlled trials. J Clin Oncol. 26:4617–4625. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siddik ZH: Cisplatin: Mode of cytotoxic
action and molecular basis of resistance. Oncogene. 22:7265–7279.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Amable L: Cisplatin resistance and
opportunities for precision medicine. Pharmacol Res. 106:27–36.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Milane L, Ganesh S, Shah S, Duan ZF and
Amiji M: Multi-modal strategies for overcoming tumor drug
resistance: Hypoxia, the Warburg effect, stem cells, and
multifunctional nanotechnology. J Control Release. 155:237–247.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
De Silva IU, McHugh PJ, Clingen PH and
Hartley JA: Defining the roles of nucleotide excision repair and
recombination in the repair of DNA interstrand cross-links in
mammalian cells. Mol Cell Biol. 20:7980–7990. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shell SM, Hawkins EK, Tsai MS, Hlaing AS,
Rizzo CJ and Chazin WJ: Xeroderma pigmentosum complementation group
C protein (XPC) serves as a general sensor of damaged DNA. DNA
Repair (Amst). 12:947–953. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fautrel A, Andrieux L, Musso O, Boudjema
K, Guillouzo A and Langouët S: Overexpression of the two nucleotide
excision repair genes ERCC1 and XPC in human hepatocellular
carcinoma. J Hepatol. 43:288–293. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang LC, Hsiao YP, Lu CT, Huang CH, Chao
WR, Lin YT, Su HA, Chang SL and Chung JG: Xeroderma pigmentosum
complementation group C Protein (XPC) expression in basal cell
carcinoma. In Vivo. 29:35–38. 2015.PubMed/NCBI
|
|
10
|
Zhang Y, Yu JJ, Tian Y, Li ZZ, Zhang CY,
Zhang SF, Cao LQ, Zhang Y, Qian CY, Zhang W, et al: eIF3a improve
cisplatin sensitivity in ovarian cancer by regulating XPC and
p27Kip1 translation. Oncotarget. 6:25441–25451.
2015.PubMed/NCBI
|
|
11
|
Zhang Y, Cao J, Meng Y, Qu C, Shen F and
Xu L: Overexpression of xeroderma pigmentosum group C decreases the
chemotherapeutic sensitivity of colorectal carcinoma cells to
cisplatin. Oncol Lett. 15:6336–6344. 2018.PubMed/NCBI
|
|
12
|
Wang C, Nie H and Li Y, Liu G, Wang X,
Xing S, Zhang L, Chen X, Chen Y and Li Y: The study of the relation
of DNA repair pathway genes SNPs and the sensitivity to
radiotherapy and chemotherapy of NSCLC. Sci Rep. 6:265262016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hollander MC, Philburn RT, Patterson AD,
Velasco-Miguel S, Friedberg EC, Linnoila RI and Fornace AJ Jr:
Deletion of XPC leads to lung tumors in mice and is associated with
early events in human lung carcinogenesis. Proc Natl Acad Sci USA.
102:13200–13205. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu YH, Wu TC, Liao JW, Yeh KT, Chen CY and
Lee H: p53 dysfunction by xeroderma pigmentosum group C defects
enhance lung adenocarcinoma metastasis via increased MMP1
expression. Cancer Res. 70:10422–10432. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jordheim LP, Crosperrial E, Matera EL,
Bouledrak K and Dumontet C: Expression of domains for
protein-protein interaction of nucleotide excision repair proteins
modifies cancer cell sensitivity to platinum derivatives and
genomic stability. Clin Exp Pharmacol Physiol. 41:817–824. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Du P, Zhang X, Liu H and Chen L:
Lentivirus-mediated RNAi silencing targeting ERCC1 reverses
cisplatin resistance in cisplatin-resistant ovarian carcinoma cell
line. DNA Cell Biol. 34:497–502. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Z, Liang X, Cheng Z, Xu Y, Yin P, Zhu
H, Li Q, Qian X and Liu J: Induction of apoptosis and suppression
of ERCC1 expression by the potent amonafide analogue 8-c in human
colorectal carcinoma cells. Anticancer Drugs. 24:355–365. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang W, Zhang L, Liu L, Zheng Y, Zhang Y,
Yang S, Shi R and Wang S: Chemosensitizing effect of shRNA-mediated
ERCC1 silencing on a Xuanwei lung adenocarcinoma cell line and its
clinical significance. Oncol Rep. 37:1989–1997. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Despras E, Pfeiffer P, Salles B, Calsou P,
Kuhfittig-Kulle S, Angulo JF and Biard DS: Long-term XPC silencing
reduces DNA double-strand break repair. Cancer Res. 67:2526–2534.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lai TC, Chow KC, Fang HY, Cho HC, Chen CY,
Lin TY, Chiang IP and Ho SP: Expression of xeroderma pigmentosum
complementation group C protein predicts cisplatin resistance in
lung adenocarcinoma patients. Oncol Rep. 25:1243–1251.
2011.PubMed/NCBI
|
|
22
|
Zhang K, Wang X and Wang H: Effect and
mechanism of Src tyrosine kinase inhibitor sunitinib on the
drug-resistance reversal of human A549/DDP cisplatin-resistant lung
cancer cell line. Mol Med Rep. 10:2065–2072. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou D, Liu W, Liang S, Sun B, Liu A, Cui
Z, Han X and Yuan L: Apoptin-derived peptide reverses cisplatin
resistance in gastric cancer through the PI3K-AKT signaling
pathway. Cancer Med. 7:1369–1383. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rezvani HR, Kim AL, Rossignol R, Ali N,
Daly M, Mahfouf W, Bellance N, Taïeb A, de Verneuil H, Mazurier F
and Bickers DR: XPC silencing in normal human keratinocytes
triggers metabolic alterations that drive the formation of squamous
cell carcinomas. J Clin Invest. 121:195–211. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang W, Cheng Y, Zhao N, Li L, Shi Y,
Zong A and Wang F: Sulfated polysaccharide of Sepiella
Maindroni ink inhibits the migration, invasion and matrix
metalloproteinase-2 expression through suppressing EGFR-mediated
p38/MAPK and PI3K/Akt/mTOR signaling pathways in SKOV-3 cells. Int
J Biol Macromol. 107:349–362. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao M, Xu P, Liu Z, Zhen Y, Chen Y, Liu
Y, Fu Q, Deng X, Liang Z, Li Y, et al: Dual roles of miR-374a by
modulated c-Jun respectively targets CCND1-inducing PI3K/AKT signal
and PTEN-suppressing Wnt/β-catenin signaling in non-small-cell lung
cancer. Cell Death Dis. 9:782018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cheng H, Zou Y, Ross JS, Wang K, Liu X,
Halmos B, Ali SM, Liu H, Verma A, Montagna C, et al: RICTOR
amplification defines a novel subset of lung cancer patients who
may benefit from treatment with mTOR1/2 inhibitors. Cancer Discov.
5:1262–1270. 2015. View Article : Google Scholar : PubMed/NCBI
|