Introduction
Human cervical carcinoma is one of the most
preventable cancer types in women. Although an effective early
screening tool is available for cervical cancer, it remains the
third leading cause of female cancer-related deaths with a
mortality rate near 30% within 5 years of diagnosis (1). Therefore, it is critical to explore
and understand the mechanisms concerning the development and
metastasis of cervical cancer, which may help us impede cervical
cancer progression.
CTHRC1 is an extracellular matrix protein that is
associated with atherosclerosis (2). Recently, CTHRC1 was found to be
upregulated during the tumorigenesis and metastasis of certain
cancers (3) and promoted cancer
cell invasion and migration (4–6). A
recent study suggested that CTHRC1 enhances tumor cell invasion and
metastasis by promoting epithelial-mesenchymal transition (EMT),
invasion, migration and/or angiogenesis through several signaling
pathways (7). Recently, studies
have suggested that Cthrcl may activated the non-canonical planar
cell polarity (PCP) pathway, one of the 3 Wnt signaling
pathways.
Although Cthrcl is upregulated during tumorigenesis
and metastasis, the effects and actual mechanisms during those
processes have not been thoroughly elucidated to date.
HeLa cells were used in the present study to observe
the expression and changes of CTHRC1 in cervical carcinoma and to
demonstrate the function of CTHRC1 in the PCP pathway of
non-canonical Wnt signaling.
Materials and methods
Antibodies and reagents
Rabbit phospho-GSK-3β antibodies and monoclonal
phospho-Akt (Ser473) were purchased from the Cell Signaling
Technologies, Inc. (Beverly, MA, USA; cat. nos. 5558 and 4060,
respectively). Rabbit monoclonal CTHRC1, C-myc and Ror2 antibodies
(tyrosine-protein kinase transmembrane receptor Ror2) were
purchased from Abcam, Inc. (Cambridge, MA, USA; cat. nos. ab85739,
ab32072 and ab92379, respectively). Rabbit polyclonal c-Jun and
Wnt5a antibodies were obtained from WanleiBio (Shenyang, China;
cat. nos. wl0219a and wl0198, respectively). Goat anti-rabbit
HRP-conjugated secondary antibodies and goat monoclonal
HRP-conjugated antibody against GAPDH were obtained from Dingguo
Biological Technology (Beijing, China; cat. nos. IH-0011 and
SH-0031, respectively).
Patients and tissue specimens
Cervical carcinoma tissue specimens (n=37) were
collected from patients following radical surgery between January
2005 and December 2015 at Qianfoshan Hospital Affiliated to
Shandong University (Jinan, China) with informed consent obtained
concerning the use of surgically resected specimens for research
purposes. All human tissue and sample experiments were approved by
the Ethics Committee of the School of Stomatology, Shandong
University. No patient received any form of adjuvant therapy before
surgery.
Cell culture
HeLa cells were cultured in Dulbecco's modified
Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) and
1% penicillin-streptomycin. The cells were cultured in a humidified
atmosphere of 5% CO2 and 95% air at 37°C.
siRNA synthesis
Single siRNA1 strand was, GGTGGTGGACCTGTATAAT;
single siRNA2 strand was, GCTGTCAGCGTTGGTATTT and single siRNA3
strand was, GGAGATGCTTCTACTGGAT. All single siRNA strands were
synthesized at Invitrogen; Thermo Fisher Scientific, Inc. (Waltham,
MA, USA).
siRNA transfection
The plasmid pcDNA3.1-CTHRC1 was constructed by
Shinegene (Shanghai, China) and the sequence of human CTHRC1 was
obtained from PubMed (NM-138455.3). siRNA (1, 2 and 3) sequences
were designed by Guangzhou RiboBio Co., Ltd. (Guangzhou, China).
HeLa cells (2×105) were seeded in 6-well culture plates.
Plasmids (pcDNA3.1-CTHRC1 and pcDNA3.1) and siRNA were transfected
into cells using PolyJet reagent according to the manufacturer's
instructions (Invitrogen; Thermo Fisher Scientific, Inc.), when the
cultures reached ~80% confluence. For pcDNA3.1 or pcDNA3.1-CTHRC1
single plasmid transfection, 1 µg of DNA, 100 µl jetPRIME buffer
and 2 µl jetPRIME/well were used in a 6-well plate. Briefly, 1 µg
DNA was diluted into 100 µl jetPRIME® buffer and mixed
by vortexing. Then, 2 µl jetPRIME® was added, vortexed
and incubated for 10 min at room temperature (RT). Transfection mix
(100 µl)/well was added onto the cells, and distributed evenly by
gently rocking the plates back and forth. Medium was replaced after
6 h of transfection. For plasmid and siRNA co-transfection, 1 µg
DNA and 20 nM siRNA/well were used in a 6-well plate with 100 µl
jetPRIME buffer and 2 µl jetPRIME. After 48 h of incubation, the
cells were harvested for further analysis. Efficiency of the
transfection was assessed by western blotting.
Wound scratch assay
Using α-DMEM as complete cell culture medium,
3–4×106 HeLa cells were seeded at a high density in
6-well plates after serum-free incubation for 12 or 24 h and were
allowed to attach to 80% density. HeLa cell wounds were made by
scraping through the cell monolayer with 200-µl sterile pipette
tips. Cells of each well were completely exposed to serum-free
α-DMEM for up to 12 h with or without siRNA transfection. Images
were acquired at a magnification of ×100 under a phase-contrast
microscope at different time-points. The widths of the healing area
in the cell monolayer were quantified and calculated using
Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville,
MD, USA).
Transwell invasion assay
The Matrigel-coated filter system was used to
evaluate cell invasion. HeLa cells were disaggregated and
resuspended in α-MEM with 0.2% FBS, and 2×104 cells were
placed into the upper chamber of the Matrigel-coated Transwell
inserts (Vigorous Biotechnology, Beijing, China) (8-µm pore size)
precoated with Matrigel and exposed to serum-free medium with or
without siRNA. α-MEM supplemented with 10% FBS was placed in the
lower chamber, and cells were incubated for 24 h at 37°C with 95%
air and 5% CO2 for 24 h. Next, the invaded cells were
fixed with 100% cold methanol for 15 min and were stained with 0.5%
crystal violet in 0.01 ml of phosphate-buffered saline (PBS) for 10
min, and then the non-invasive cells remaining on the upper
membrane were removed with a cotton wool. The number of invaded
cells, which penetrated the lower side of the filter, was counted
(8 fields/filter), and images were captured under a light inverted
microscope at a magnification of ×200, as aforementioned.
Western blotting
HeLa cells were washed twice with ice-cold PBS,
harvested and then lysed in RIPA buffer (Beyotime Institute of
Biotechnology, Shanghai, China).
The protein concentration was detected using the BCA
protein assay kit. Equal amounts of total protein (10 µg) were
separated by 10% SDS-PAGE electrophoresis and were transferred onto
polyvinylidene difluoride (PVDF) membranes (EMD Millipore, Bedford,
MA, USA). After blocking in 5% non-fat dry milk in
Tween-20/Tris-buffered saline for 1 h, the membranes were probed
with various antibodies against Cthric1 (dilution 1:5,000; Abcam,
Inc.), Ror2 (dilution 1:2,000), Wnt5a (dilution 1:1,000), p-c-Jun
(dilution 1:500) and GAPDH (dilution 1:20,000) overnight at 4°C,
and were subsequently incubated with a species-specific
HRP-conjugated secondary antibody (dilution 1:20,000) for 1 h at
25°C. Finally, peroxidase activity on the PVDF membranes was
assessed on X-ray film using ECL Western Blotting Studio Software
(Beyotime Institute of Biotechnology).
Immunohistochemical staining
The expression of Cthric1 in cervical carcinoma
specimens was assessed in immunofluorescence studies.
Immunohistochemistry was performed using a two-step standard
protocol. In simple terms, human cervical carcinoma sample tissues
were prepared by 4% w/v paraformaldehyde. The tissues were
deparaffinized in xylene, hydrated through a graded alcohol series
and washed with PBS as aforementioned. After incubating overnight
with Cthric1 at 4°C (dilution 1:500) the slides were washed and
incubated with HRP-conjugated goat anti-rabbit secondary antibody
(dilution 1:1,000) for 30 min at room temperature. Sections treated
with PBS instead of the primary antibody were used as negative
controls. To observe Cthric1 expression, we used a method described
by Maruyama et al (8). The
absence of membrane expression and positive cytoplasmic and nuclear
expression were considered to indicate abnormal Cthric1 expression.
The tissues sections were imaged using a confocal fluorescence
microscope.
Immunofluorescence
HeLa cells were grown on glass coverslips in 6-well
plates with or without siRNA for 24 or 48 h. HeLa cells were fixed
with paraformaldehyde for 15 min, and then were washed with PBS at
room temperature. After blocking with normal goat serum, HeLa cells
were incubated with the associated antibody (β-catenin; dilution
1:200; cat. no. AC106) at 4°C overnight followed by incubation with
FITC-conjugated goat anti-rabbit IgG (cat. no. IH-0011) at room
temperature for 1 h. The slides were washed in PBS-Tween-20 (0.1%)
before being stained with DAPI, and the immunofluorescence signals
were visualized and recorded with a fluorescence microscope.
Statistical analysis
Statistical analyses were performed using SPSS 19.0
software (SPSS, Inc., Chicago, IL, USA). Comparison between groups
was performed using paired Student's t-test, and for multiple
comparisons significant levels were adjusted for the number of
tests. The experimental and the control group of cells using
independent sample t-test. All of the results were expressed as the
means ± standard deviation (SD). A value of P<0.05 was
considered to indicate a statistically significant result. The
survival analysis of patients was evaluated using Cox proportional
hazards regression analysis and the Kaplan Meier method, and
significant differences were examined using the log-rank test.
Results
CTHRC1 expression is increased in
cervical carcinoma patients
To reveal whether CTHRC1 is expressed in cervical
carcinoma and its effect, we collected 37 paraffin-embedded samples
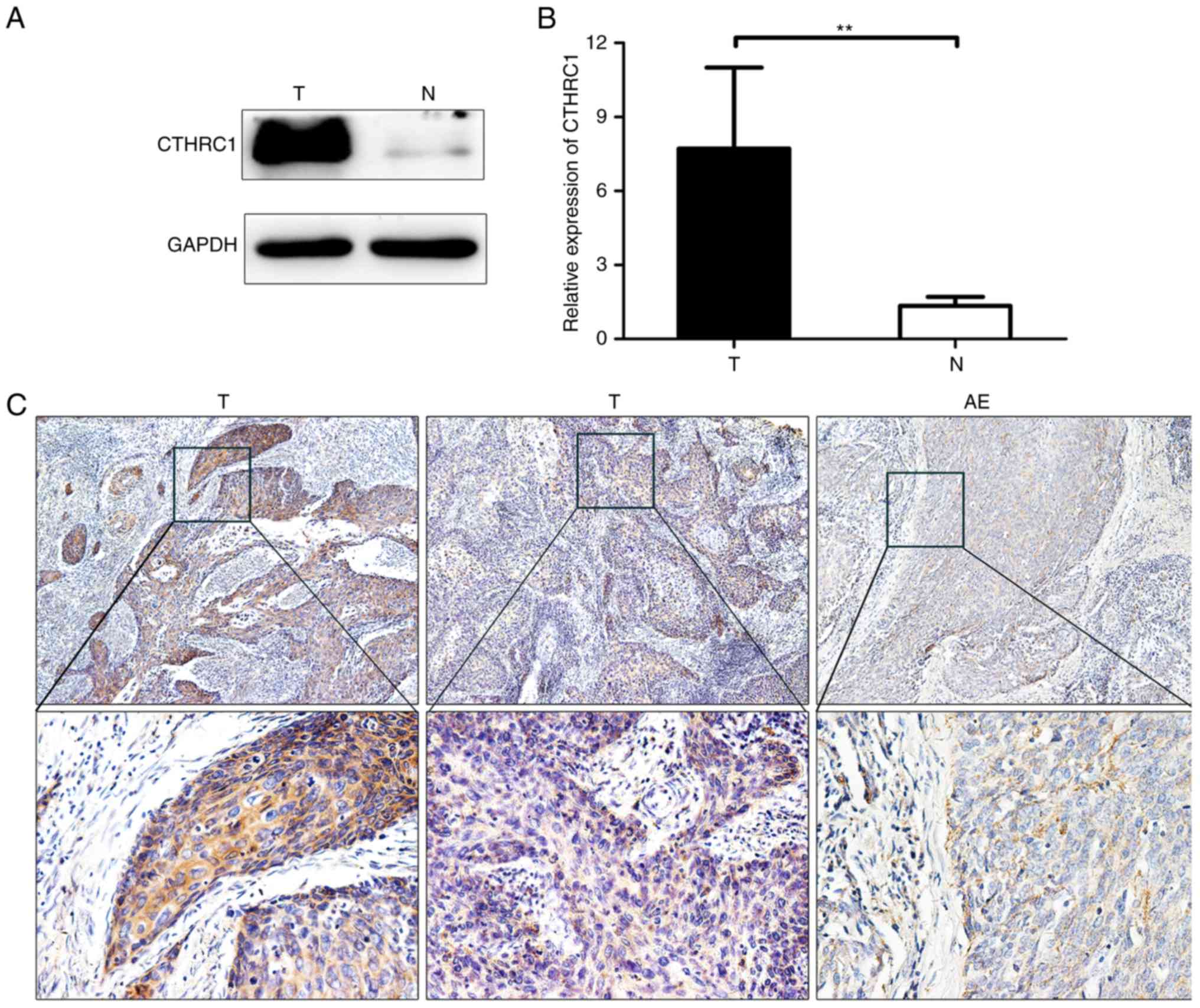
of cervical carcinoma. Western blotting revealed that CTHRC1
expression was significantly upregulated in tumor tissues compared
with that in adjacent non-cancerous tissues (Fig. 1A and B). CTHRC1 was further revealed
by immunohistochemical analysis of 37 tumor tissue specimens from
patients. CTHRC1 expression was found to be higher in cancer tissue
compared to adjacent non-cancerous epithelial tissues (Fig. 1C). Additionally, we observed by
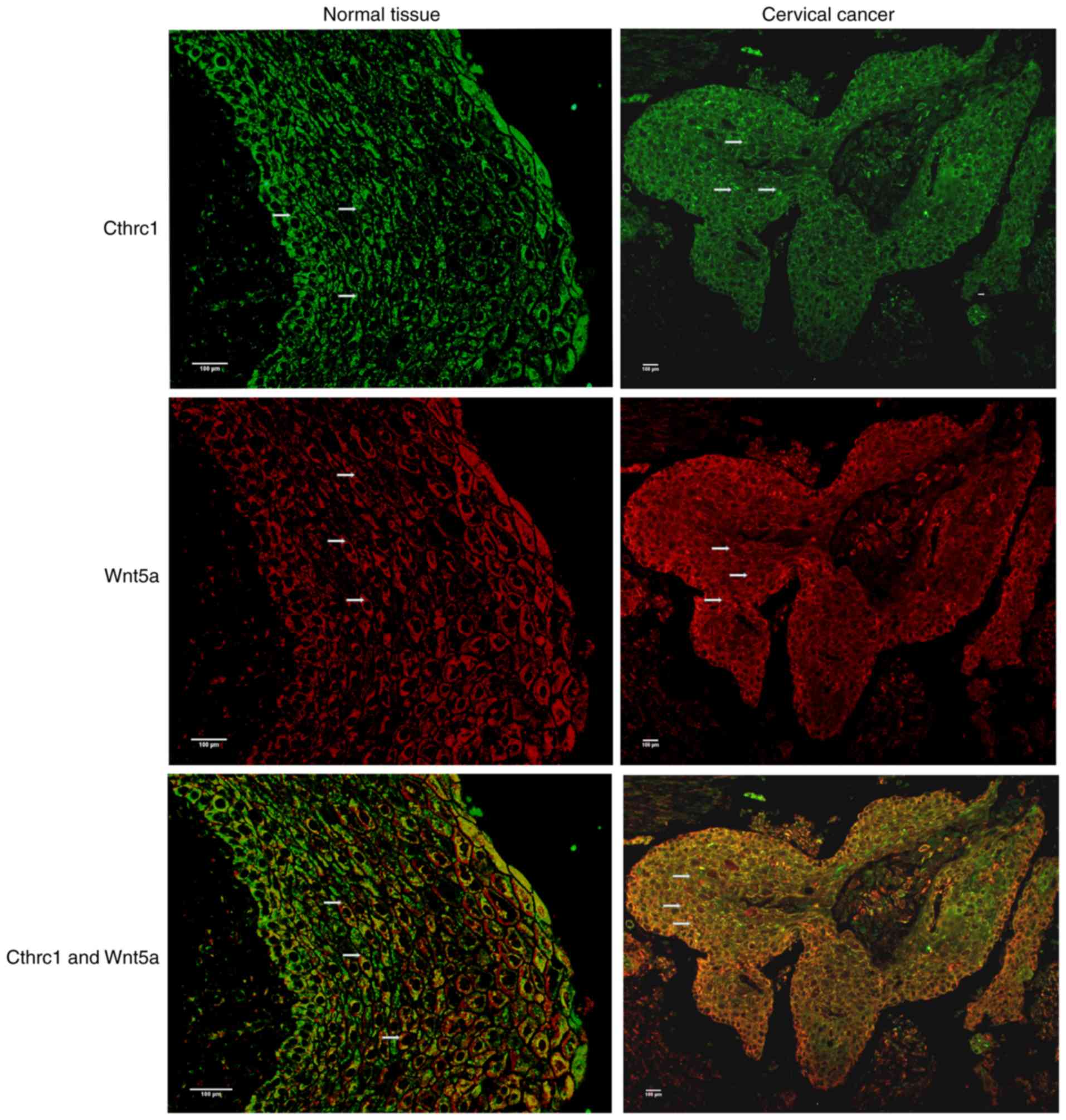
immunofluorescence that CTHRC1 was abundant in tumor tissues
(Fig. 2).
Relationship between survival in
cervical carcinoma patients and CTHRC1 expression
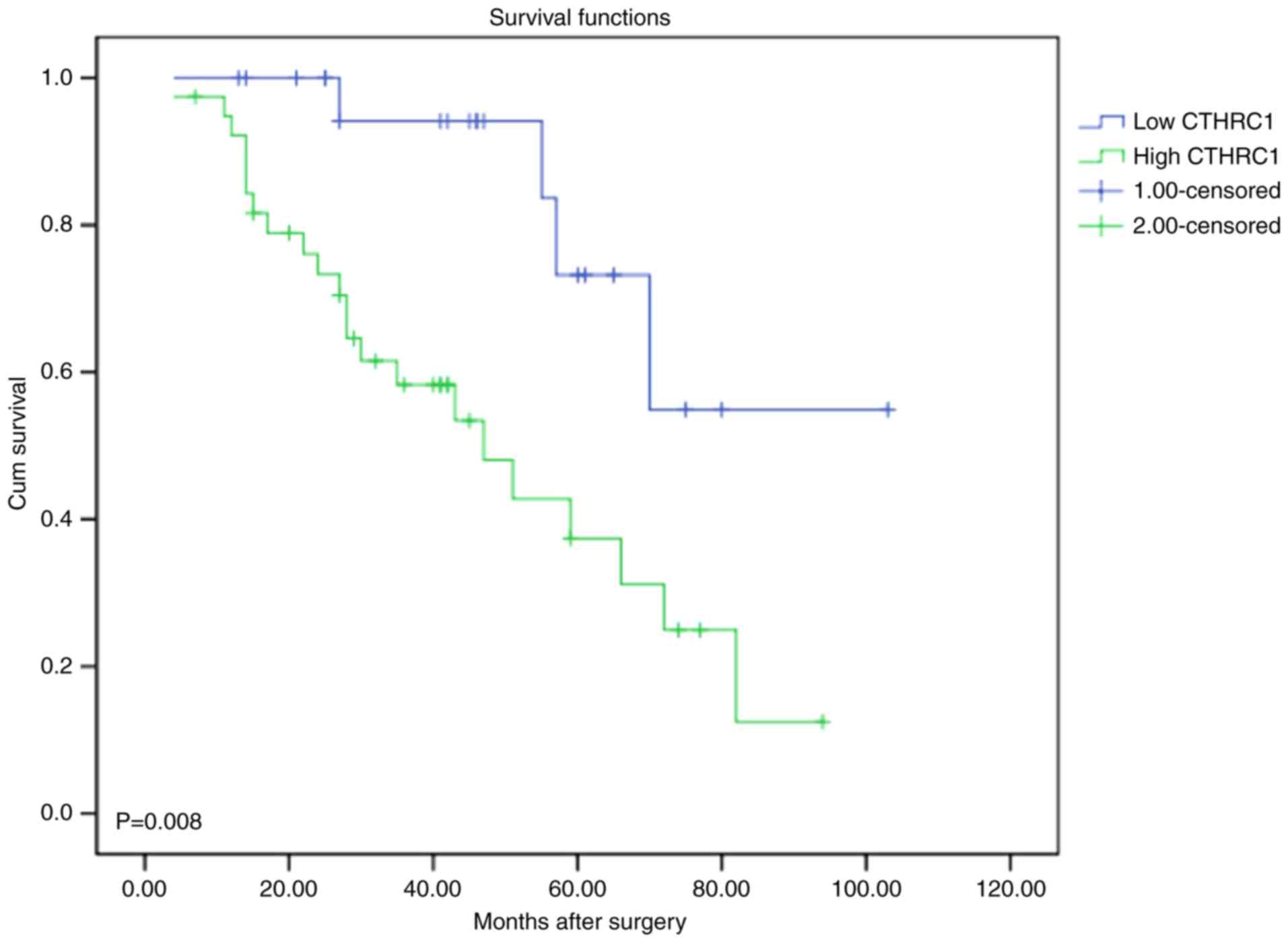
To detect the relationship between CTHRC1 expression
and cervical carcinoma patients, the correlation between CTHRC1
expression and the survival status of patients was analyzed. It
appears that the patients with low expression had better prognosis
and patients with high expression had worse prognosis (P=0.008;
Fig. 3).
To further evaluate whether CTHRC1 expression
represents a prognostic parameter in cervical carcinoma patients,
regression analysis using Cox proportional hazards model was
performed. In univariate analysis, variables such as low CTHRC1
expression and pN+ stage revealed a significant higher
hazard ratio (HR) for a significantly better prognosis.
Furthermore, multivariate analysis was performed using significant
variables surveyed in univariate analysis. The results indicated
that CTHRC1 expression was the only independent prognostic
predictor (P=0.031; Table I). These
results significantly indicated that downregulated CTHRC1
expression in cervical carcinoma patients was strongly associated
with a better prognosis.
 | Table I.Cox proportional hazards model
analysis of variables affecting survival in cervical carcinoma
patients. |
Table I.
Cox proportional hazards model
analysis of variables affecting survival in cervical carcinoma
patients.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Variables | Categories | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (years) | <55/≥55 | 1.384
(0.738–3.533) | 0.227 |
|
|
| Differentiation | Moderately +
poorly/well | 1.249
(0.623–2.655) | 0.458 |
|
|
| pT stage | T3-4/T1-2 | 1.635
(0.714–3.453) | 0.220 |
|
|
| pTNM stage | III–IV/I–II | 2.029
(0.961–4.719) | 0.063 |
|
|
| pN stage | N+/N° | 2.219
(1.055–5.095) | 0.034 | 1.581 (0.698-
3.623) | 0.272 |
| CTHRC1 | High/low | 3.937
(1.325–11.562) | 0.012 | 3.374
(1.201–9.294) | 0.031 |
Knockout of CTHRC1 reduces cervical
cancer cell invasion and migration in vitro
To explore the effect of CTHRC1 expression, the
correlation of CTHRC1 knockout treatment and the biological
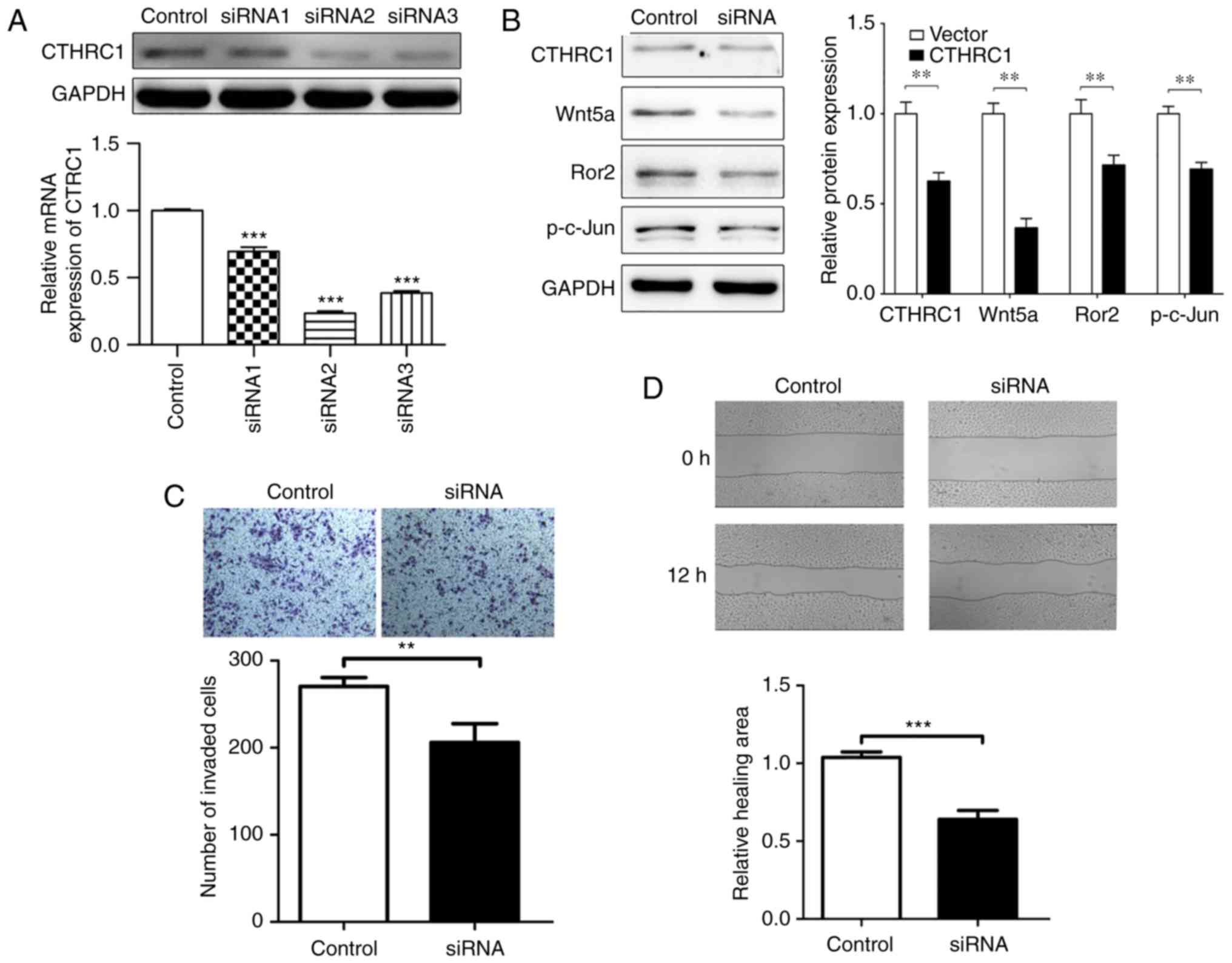
behaviors of HeLa cells was investigated. CTHRC1 siRNA2 (the most
effective siRNA compared to CTHRC1 siRNA1 and CTHRC1 siRNA3) was
chosen to knockout CTHRC1 protein (Fig.
4A). When CTHRC1 was knocked out, the expression of Wnt5a, Ror2
and p-c-Jun was downregulated as revealed by western blotting
(Fig. 4B). Compared with the
control group, the CTHRC1 group exhibited significant decreased
migration and invasion abilities as determined by Transwell
invasion and wound scratch assays (Fig.
4C and D). As shown in Fig. 4C,
the number of HeLa cells that invaded to the lower side of the
Transwell membrane in the knockdown CTHRC1 group was significantly
lower than that of the control group, indicating that CTHRC1 may
promote the invasion of HeLa cells. Fig. 4D revealed that in the wound scratch
assay, the control cells appear to show more healing ability
compared to the siRNA group at 12 h, indicating that CTHRC1
knockdown influenced the migration behavior of HeLa cells. The data
demonstrated that CTHRC1 could regulate the invasion and migration
of HeLa cells in vitro.
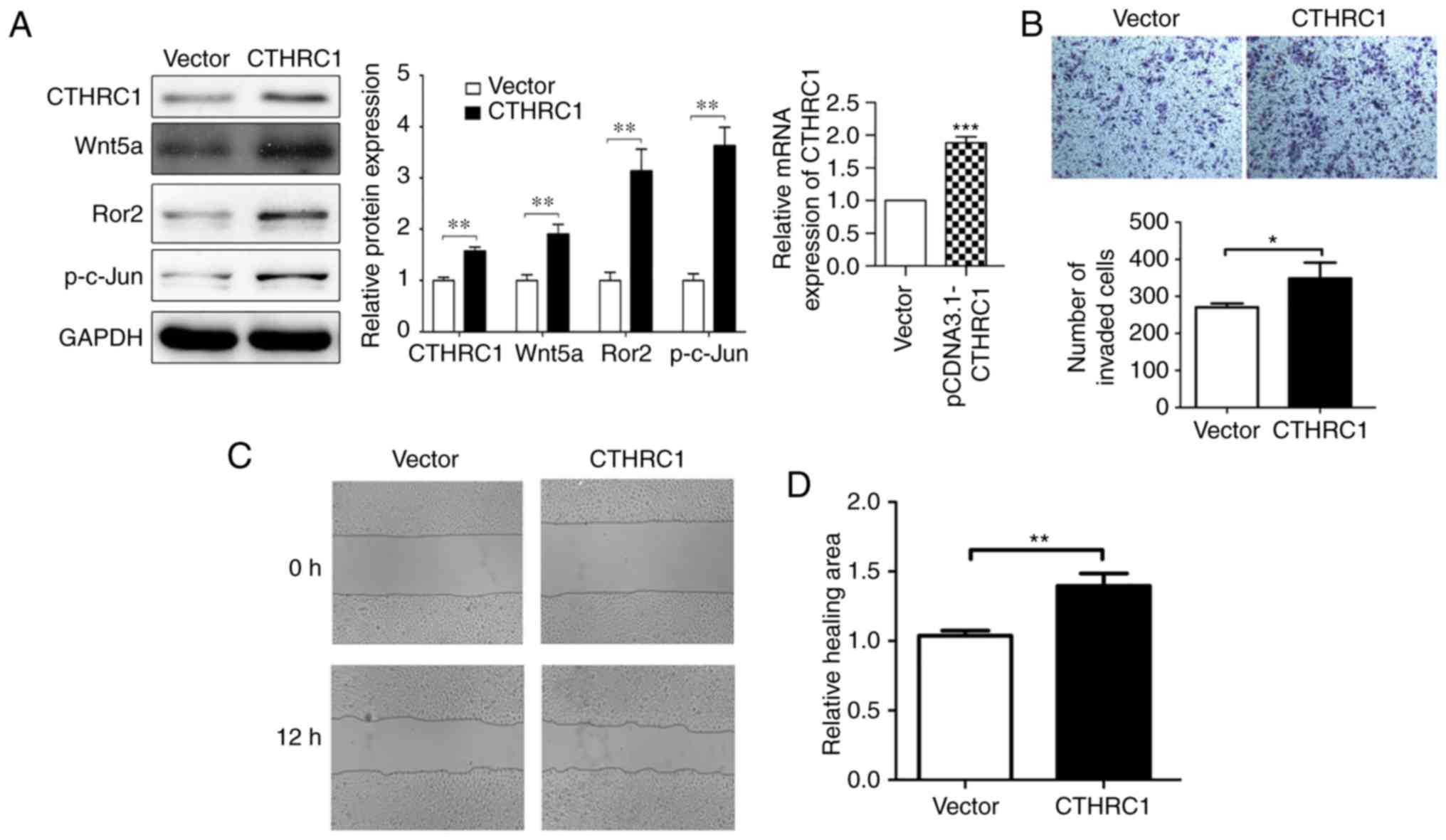
Overexpression of CTHRC1 promotes HeLa
cell migration and invasion in vitro
CTHRC1 expression was further assessed in HeLa cell
lines. Western blotting revealed that higher levels of CTHRC1
protein and mRNA expression were detected in HeLa cells when
compared to the vector (Fig. 5A),
and then further in vitro study was performed. The
relationship between the biological behaviors and CTHRC1
overexpression in HeLa cells was assessed. When CTHRC1 was
overexpressed, the expression of p-c-Jun, Ror2 and Wnt5a were
increased by western blotting (Fig.
5A). HeLa cell invasion and migration were markedly increased
when CTHRC1 was overexpressed using Transwell invasion and wound
scratch assays (Fig. 5B-D). As
revealed in Fig. 5C and D, in the
wound scratch assay, the CTHR1 overexpressed cells appear to show
more healing ability compared to the control group at 12 h,
indicating that CTHRC1 overexpression influenced the migration
behavior of HeLa cells. In Fig. 5B,
the number of HeLa cells that invaded to the lower side of the
Transwell membrane in the CTHRC1 overexpression group was
significantly lower than that in the control group, indicating that
CTHRC1 may promote the invasion of HeLa cells. These data
demonstrated that CTHRC1 overexpression could regulate and control
the invasion and migration of HeLa cells in vitro.
Discussion
Cervical cancer is one of the most prevalent cancers
among women of reproductive age in underdeveloped countries
(9). Moreover, the 5-year survival
rate of cervical cancer patients is relatively low using
traditional treatment. Recurrence and metastasis are the leading
causes of death in cervical cancer patients (10). Interactions between extracellular
matrix proteins and their receptors initiate downstream signaling
pathways leading to tumor invasion and metastasis (11).
CTHRC1 is a 28-kDa extracellular matrix glycoprotein
containing an NH2-terminal signaling peptide for
extracellular secretion, a short collagen triple-helix repeat of 36
amino acids, and a COOH-terminal globular domain (12). Recent studies have revealed that
CTHRC1 is expressed in human cancer and is upregulated in several
aggressive tumors, including gastrointestinal, colorectal and
breast cancer, and melanoma (13–16).
It has been suggested that CTHRC1 protein is undetectable in benign
nevi and in non-invasive melanoma tumors, but highly expressed in
invasive melanoma. Overexpression of CTHRC1 in melanoma cell lines
was revealed to enhance cell migration and adhesion, and protect
melanoma cells from serum deprivation-induced apoptosis (17). In breast cancer, the stromal
expression of CTHRC1 was enhanced in patients with bone metastasis
(18). These studies indicated that
CTHRC1 is a critical regulator of tumor development, metastasis and
invasion in the tumor microenvironment (19). At present, however, little is known
regarding the molecular mechanisms underlying CTHRC1 action in
cervical carcinoma. In the present study, we wondered whether
CTHRC1 played an important role in promoting migration and invasion
of HeLa cells in vitro. Thus, we revealed the expression of
CTHRC1 in cervical carcinoma and investigated the role of CTHRC1 in
the invasion and migration of this disease. In our experiment, it
was revealed that CTHRC1 was highly expressed in cervical carcinoma
and played a role in the invasion and migration of HeLa cells in
vitro. It was also inferred that CTHRC1 in HeLa cells markedly
promoted the ability of cell invasion and migration when CTHRC1 was
overexpressed in vitro as demonstrated by Transwell and
wound scratch assays. All of the results indicated that CTHRC1 may
be a tumor promoter in human cervical cancer.
The Wnt pathway plays an important role in the
microenvironment of cervical cancer carcinogenesis (20,21).
The most characterized Wnt canonical pathway determines cell fate
and regulation of growth, including the patterning of the
neuroectoderm, amplification of neural progenitors and formation of
the body axis (22). The
non-canonical Wnt/PCP pathway controls cell movement and tissue
polarity by activating c-jun N-terminal kinase (JNK), RHOA and
nemo-like kinase (NLK) signaling cascades (23). Research suggests that CTHRC1 can
interact with multiple extracellular components of Wnt signaling,
including the Wnt/PCP co-receptor Ror2. These components form a
CTHRC1/Wnt/Ror2 complex to selectively suppress the Wnt pathway and
selectively activate the canonical Wnt/PCP pathway (24) and CTHRC1, as a novel Wnt co-receptor
that acts to specifically cluster Wnts with Ror2 and Fzd, leading
to activation of the PCP pathway (25). Increasing evidence has suggested
that Wnt/Pcp plays an important role in regulating the metastasis
of cancer cells (26). In the
present study, it was revealed that CTHRC1 could concurrently
activate the Wnt/PCP signaling pathway through upregulation of
Wnt5a, Ror2 and p-c-Jun proteins to reverse cell migration and
invasion. In summary, the Wnt/PCP signal pathway partly explains
the mechanism of CTHRC1-induced migration and invasion in HeLa
cells in vitro. However, the specific mechanism of this
process remains unclear.
In conclusion, our findings demonstrated that CTHRC1
was overexpressed in cervical carcinoma tissues and HeLa cells and
played a critical role in cell invasion and migration, which was
likely mediated by activation of the Wnt/PCP pathway. Additional
experiments with cervical carcinoma are required to demonstrate the
specific underlying mechanism of CTHRC1 in cervical cancer
progression and metastasis.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (no. 81402298), and the Young
Scholars Program of Shandong University.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author on reasonable request.
Authors' contributions
MZ made substantial contributions to the conception
and design of the study; QZ made substantial contributions to the
analysis and interpretation of the data; XL was involved in the
conception of the study, drafted the manuscript and revised it
critically for important intellectual content, and was a major
contributor in writing the manuscript; CW performed the
histological examination of the cervical carcinoma tissue, and was
a major contributor in writing the manuscript; GL contributed to
the conception and design of the study and gave final approval of
the version to be published and agreement to be accountable for all
aspects of the work in ensuring that questions related to the
accuracy and integrity of any part of the work are appropriately
investigated and resolved. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All human tissue and sample experiments were
approved by the Ethics Committee of the School of Stomatology,
Shandong University. Informed consent concerning the use of
surgically resected specimens for research purposes was obtained
from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors state that they have no competing
interests.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pyagay P, Heroult M, Wang Q, Lehnert W,
Belden J, Liaw L, Friesel RE and Lindner V: Collagen triple helix
repeat containing 1, a novel secreted protein in injured and
diseased arteries, inhibits collagen expression and promotes cell
migration. Circ Res. 96:261–268. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang CY, Fong YC, Lee CY, Chen MY, Tsai
HC, Hsu HC and Tang CH: CCL5 increases lung cancer migration via
PI3K, Akt and NF-kappaB pathways. Biochem Pharmacol. 77:794–803.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim HC, Kim YS, Oh HW, Kim K, Oh SS, Kim
JT, Kim BY, Lee SJ, Choe YK, Kim DH, et al: Collagen triple helix
repeat containing 1 (CTHRC1) acts via ERK-dependent induction of
MMP9 to promote invasion of colorectal cancer cells. Oncotarget.
5:519–529. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ma MZ, Zhuang C, Yang XM, Zhang ZZ, Ma H,
Zhang WM, You H, Qin W, Gu J, Yang S, et al: CTHRC1 acts as a
prognostic factor and promotes invasiveness of gastrointestinal
stromal tumors by activating Wnt/PCP-Rho signaling. Neoplasia.
16:265–278, 278.e1-13. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eriksson J, Le Joncour V, Nummela P,
Jahkola T, Virolainen S, Laakkonen P, Saksela O and Hölttä E: Gene
expression analyses of primary melanomas reveal CTHRC1 as an
important player in melanoma progression. Oncotarget.
7:15065–15092. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Puliyappadamba VT, Cheriyan VT,
Thulasidasan AK, Bava SV, Vinod BS, Prabhu PR, Varghese R, Bevin A,
Venugopal S and Anto RJ: Nicotine-induced survival signaling in
lung cancer cells is dependent on their p53 status while its
down-regulation by curcumin is independent. Mol Cancer. 9:2202010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maruyama K, Ochiai A, Akimoto S, Nakamura
S, Baba S, Moriya Y and Hirohashi S: Cytoplasmic beta-catenin
accumulation as a predictor of hematogenous metastasis in human
colorectal cancer. Oncology. 59:302–309. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chidyaonga-Maseko F, Chirwa ML and Muula
AS: Underutilization of cervical cancer prevention services in low
and middle income countries: A review of contributing factors. Pan
Afr Med J. 21:2312015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Plummer M, Herrero R, Franceschi S, Meijer
CJ, Snijders P, Bosch FX, de Sanjosé S and Muñoz N;
IARCMulti-centre Cervical Cancer Study Group, : Smoking and
cervical cancer: Pooled analysis of the IARC multi-centric
case-control study. Cancer Causes Control. 14:805–814. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Multhaupt HA, Leitinger B, Gullberg D and
Couchman JR: Extracellular matrix component signaling in cancer.
Adv Drug Deliv Rev. 97:28–40. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hou M, Cheng Z, Shen H, He S, Li Y, Pan Y,
Feng C, Chen X, Zhang Y, Lin M, et al: High expression of CTHRC1
promotes EMT of epithelial ovarian cancer (EOC) and is associated
with poor prognosis. Oncotarget. 6:35813–35829. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Grando SA: Connections of nicotine to
cancer. Nat Rev Cancer. 14:419–429. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tan F, Liu F, Liu H, Hu Y, Liu D and and
Li G: CTHRC1 is associated with peritoneal carcinomatosis in
colorectal cancer: A new predictor for prognosis. Med Oncol.
30:4732013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang L, Dai DL, Su M, Martinka M, Li G and
Zhou Y: Aberrant expression of collagen triple helix repeat
containing 1 in human solid cancers. Clin Cancer Res. 12:3716–3722.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang P, Wang YC, Chen XY, Shen ZY, Cao H,
Zhang YJ, Yu J, Zhu JD, Lu YY and Fang JY: CTHRC1 is
upregulated by promoter demethylation and transforming growth
factor-β1 and may be associated with metastasis in human gastric
cancer. Cancer Sci. 103:1327–1333. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen YL, Wang TH, Hsu HC, Yuan RH and Jeng
YM: Overexpression of CTHRC1 in hepatocellular carcinoma promotes
tumor invasion and predicts poor prognosis. PLoS One. 8:e703242013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kharaishvili G, Cizkova M, Bouchalova K,
Mgebrishvili G, Kolar Z and Bouchal J: Collagen triple helix repeat
containing 1 protein, periostin and versican in primary and
metastatic breast cancer: An immunohistochemical study. J Clin
Pathol. 64:977–982. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lai YH, Chen J, Wang XP, Wu YQ, Peng HT,
Lin XH and Wang WJ: Collagen triple helix repeat containing-1
negatively regulated by microRNA-30c promotes cell proliferation
and metastasis and indicates poor prognosis in breast cancer. J Exp
Clin Cancer Res. 36:922017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Joyce JA and Pollard JW:
Microenvironmental regulation of metastasis. Nat Rev Cancer.
9:239–252. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Skeen VR, Paterson I, Paraskeva C and
Williams AC: TGF-β1 signalling, connecting aberrant inflammation
and colorectal tumorigenesis. Curr Pharm Des. 18:3874–3888. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Katoh M: WNT/PCP signaling pathway and
human cancer (Review). Oncol Rep. 14:1583–1588. 2005.PubMed/NCBI
|
|
23
|
Logan CY and Nusse R: The Wnt signaling
pathway in development and disease. Annu Rev Cell Dev Biol.
20:781–810. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamamoto S, Nishimura O, Misaki K, Nishita
M, Minami Y, Yonemura S, Tarui H and Sasaki H: Cthrc1 selectively
activates the planar cell polarity pathway of Wnt signaling by
stabilizing the Wnt-receptor complex. Dev Cell. 15:23–36. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kelley MW: Leading Wnt down a PCP path:
Cthrc1 acts as a coreceptor in the Wnt-PCP pathway. Dev Cell.
15:7–8. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsunoda K, Tsujino I, Koshi R, Sugano N,
Sato S and Asano M: Nicotine-mediated Ca2+-influx
induces IL-8 secretion in oral squamous cell carcinoma cell. J Cell
Biochem. 117:1009–1015. 2016. View Article : Google Scholar : PubMed/NCBI
|