Introduction
Multiple myeloma (MM) is a common tumor of the
blood, which is characterized by plasma cell colonization and
prominent bone marrow damage. The majority of patients with MM
eventually exhibit relapse or multidrug resistance (MDR),
regardless of chemotherapy, targeted drug therapy or autologous
stem cell transplantation. The development of MDR to
chemotherapeutic agents remains a major obstacle in the effective
treatment of cancer (1). The
occurrence and recurrence of MM suggests the presence of cancer
stem cells (CSCs), which are responsible for tumor initiation,
self-renewal, drug resistance and metastasis (2). CSCs from acute myeloid leukemia,
breast cancer, glioma and numerous other types of cancer have been
successfully isolated using cancer-type specific surface markers
(3–5). However, CSCs have not been isolated
from MM, due to the lack of specific surface markers.
Despite the distinct surface markers of MM, CSCs
have not been definitively identified. The concept of side
population (SP) cells has been widely used as a unique source for
studying CSCs when specific markers are not available (6–12).
First described by Goodell et al (13), SP cells are a subset of enriched
progenitor cells that exhibit CSC-like phenotypes with distinct low
staining of Hoechst 33342 in several malignant tumors. Accumulating
evidence has indicated that CSCs are highly resistant to
conventional cancer therapies and contribute to MDR (14–18).
For example, SP cells sorted from glioma and primary esophageal
carcinoma have a lower sensitivity to chemotherapy drugs in
vitro (18,19). Although a few studies have
characterized SP cells compared with main population (MP) cells,
the stem-like properties and tumorigenicity of SP cells in MM
remains largely unknown.
Although MDR is a multifactorial phenomenon,
overexpression of ATP-binding cassette (ABC) drug transporter
proteins remains one of the most common mechanisms underlying MDR.
It is well known that CSCs often exhibit high ABC transporter
activity, particularly ABC subfamily G member 2 (ABCG2) activity.
ABCG2 is a surface molecule that contributes to drug resistance via
the efflux of intracellular drugs (20,21).
Phosphatidylinositol 3-kinases (PI3Ks) are a family of lipid
kinases that serve critical roles in regulating various cellular
processes. With subsequent activation of AKT serine-threonine
kinase (AKT) and other downstream effectors, such as mammalian
target of rapamycin (mTOR), the PI3K pathway is crucial in cancer
proliferation and also contributes to MDR in certain types of
cancer (22). However, the roles of
PI3K/AKT/mTOR signaling in maintaining MM stem cell properties have
not been extensively studied (23,24).
Therefore, the present study aimed to investigate whether ABCG2 may
be used as a surface marker for MM CSCs, and if a correlation
exists between ABCG2 expression and PI3K/AKT signaling in SP cells
in MM.
Materials and methods
MM cell lines and primary MM
cells
The U266 and NCI-H929 human MM cell lines were
originally obtained from American Type Culture Collection
(Manassas, VA, USA), and were further cultivated in our laboratory.
Cell lines were authenticated using a short-tandem repeat method
and were confirmed as mycoplasma contamination-free. Cells were
cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum
(TransGen Biotech Co., Ltd., Beijing, China) and 1%
penicillin/streptomycin (TransGen Biotech Co., Ltd.) at 37°C in a
humidified incubator containing 5% CO2.
A total of 30 patients diagnosed with MM, according
to the Updated Diagnostic Criteria and Staging System for MM
(25), were selected for the
present study. A total of 16 patients were men and 14 were women
(age, 22–82 years). With regards to the Durie-Salmon (DS) criteria,
two samples were DS stage I, five were DS stage II and 23 were DS
stage III; in addition, with regards to the International Staging
System (ISS) criteria, three samples were ISS stage I, 11 were ISS
stage II and 16 were ISS stage III. The control group consisted of
10 samples (three male patients and seven female patients; age,
31–52 years) from healthy individuals without hematological
diseases. Patients with MM and control individuals were recruited
from the Department of Hematology, China Medical University
(Shenyang, China) between January 1, 2015 and December 30, 2015.
Bone marrow mononuclear cells were obtained from total bone marrow
by density gradient centrifugation; briefly, lymphocyte separation
solution (GE Healthcare, Chicago, IL, USA) and bone marrow was
added to 15 ml centrifuge tubes and were centrifuged at 400 × g for
30 min at room temperature. The present study (AF-SOP-07-1.0–01)
was approved by the institutional review board of the First
Affiliated Hospital of China Medical University (Shenyang, China),
and written informed consent was obtained from all patients prior
to their participation, in accordance with the Declaration of
Helsinki.
Reagents and antibodies
Hoechst 33342 and Verapamil were obtained from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). Rabbit polyclonal
antibodies against phosphatase and tensin homolog (PTEN; cat. no.
9559), phosphorylated (p)-PI3K (cat. no. 4228), PI3K (cat. no.
4257), p-AKT (cat. no. 4060), AKT (cat. no. 9272), ABCG2 (cat. no.
42078) and β-actin (cat. no. 4970) were obtained from Cell
Signaling Technology, Inc. (Danvers, MA, USA). Arsenic trioxide
(As2O3), bortezomib (Bort), LY294002 and
rapamypin were purchased from Selleck Chemicals (Houston TX,
USA).
Lentiviral infection of ABCG2 in
NCI-H929 cells
Lentiviral particles were produced in 293T cells
(Shanghai GeneChem Co., Ltd., Shanghai, China) [5×106
cells/ml, cultured in Dulbecco's modified Eagle's medium (DMEM;
HyClone; GE Healthcare Life Sciences, Logan, UT, USA) containing
10% fetal bovine serum (HyClone; GE Healthcare Life Sciences] by
transiently co-transfecting control vector pGV358 (DMEM, 37°C, 24
h) (obtained from Dr Van Duyne, University of Pennsylvania,
Philadelphia, PA, USA) or pGV358-ABCG2 (ABCG2 cDNA was ontained
from Shanghai GeneChem Co., Ltd.; GenBank accession no.: NM-004827,
http://www.ncbi.nlm.nih.gov/genbank/)
with helper plasmids pMD2.G (Plasmid #12259; Addgene, Inc.,
Cambridge, MA, USA) and psPAX2 (Plasmid #12260; Addgene, Inc.)
using FuGENE® HD Transfection Reagent (Promega
Corporation, Madison, WI, USA). A total of 24 h post-infection of
NCI-H929 cells (5×105 cells/ml) with ABCG2 or
non-specific control (NC) lentiviruses. The lentiviral supernatants
were harvested post-transfection and were filtered (0.45 mM filter)
prior to infection of the cell lines at 37°C for 24 h at a
multiplicity of infection of 80, the cells were harvested and
resuspended in fresh medium. These cells were referred to as
NCI-H929/ABCG2+ or NCI-H929/NC cells.
Flow cytometry
NCI-H929, U266 and primary MM cells were suspended
at a concentration of 1×106 cells/ml and were stained
with Hoechst 33342 dye (5 µg/ml) in culture medium at 37°C
for 90 min. Samples were also treated with 50 µM Verapamil
for 90 min at 37°C as a negative control, which can inhibit the
efflux of Hoechst 33342. Propidium iodide (PI; 1 µg/ml) was
added to the cells at 4°C for 15 min prior to flow cytometric
analysis. PI-negative (live) cells were initially gated, and the
SP-gated cells were defined as the diminished area on the dot plot
(Fig. 1A) (26). SP and MP cells were sorted for
further experiments using fluorescence-activated cell sorting
(FACS) (FACSAria II, BD). NCI-H929 and NCI-H929/ABCG2+
cells treated with LY294002 (20 µM) or rapamycin (100 nM) at
37°C for 72 h were subjected to the same procedures for SP and MP
cell sorting. Cell cycle analysis was performed by fixing cells in
75% ethanol and staining them with PI, according to the protocol of
the cell cycle analysis kit (TransGen Biotech Co., Ltd.).
Determining drug sensitivity using a
cell proliferation assay
Drug sensitivity of sorted SP and MP cells was
determined using the Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc., Kumamato, Japan), according to the
manufacturer's protocol. Briefly, cells were suspended at
1×105 cells/ml and seeded in a 96-well plate. SP and MP
cells were treated with various doses of Bort (10, 25 and 50 nM) or
As2O3 (1.25, 2.5 and 5 µM) for 24 h at
37°C. Subsequently, CCK-8 reagent (10 µl) was added to each
well and absorbance was measured at 450 nm after 1 h. For NCI-H929
and NCI-H929/ABCG2+ cells, LY294002 (20 µM) or
rapamycin (100 nM) was added to the culture medium for 72 h prior
to the addition of As2O3 or Bort. The ratio
of dead cells was calculated using the following equation: Ratio =
[1 - (OD value of treatment - OD value of background)/(OD value of
control - OD value of background)] × 100%. OD refers to optical
density.
Soft agar colony formation assay
The soft agar colony formation assay was performed
as previously described (27).
Briefly, the soft agar used consisted of an upper layer (0.35%
agarose) and a lower layer (0.7% agarose). FACS-sorted SP or MP
cells (5×103/test) were inoculated and grown for 14 days
in the soft agar. Fresh medium was added to the cells every 3 days,
and the number of colonies was counted (colonies contained >50
cells) at day 14 under a BX51 microscope (Olympus Corporation,
Tokyo, Japan).
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
Bone marrow mononuclear cells were obtained from
total bone marrow by density gradient centrifugation. Total RNA was
extracted from cells using TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. RNA samples (500 ng each) were reverse transcribed into
cDNA using an M-MLV first strand cDNA synthesis kit (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. qPCR was performed using the 7500 real-time PCR system
and Power SYBR Green Master Mixture (both Applied Biosystems;
Thermo Fisher Scientific, Inc.). The thermocycling conditions ewre
as follows: 95°C for 5 min followed by 40 cycles at 95°C for 30
sec, 52°C for 30 sec, 72°C for 45 sec, and a final step at 62°C for
10 min. GAPDH was used as the internal control gene. Fold changes
in relative gene expression were calculated according to the ΔΔCq
method (28). In addition, the
amplified products were analyzed by DNA electrophoresis on a 2%
agarose gel and the gels were stained wih ethidium bromide
(Beyotime Institute of Biotechnology, Shanghai, China). Primer
sequences for specific gene amplification are listed in Table I.
 | Table I.List of primer sequences. |
Table I.
List of primer sequences.
| Gene name | Sequence (5′ to
3′) |
|---|
| ABCG2 | F:
ATTGAAGCCAAAGGCAGATG |
|
| R:
TGAGTCCTGGGCAGAAGTTT |
| ABCA3 | F:
AGAAATACGGTGCCGGCTATCACA |
|
| R:
CAATGCCCAGCTCTTTCTGCTTCT |
| ABCB1 | F:
GCTCCTGACTATGCCAAAGC |
|
| R:
TCTTCACCTCCAGGCTCAGT |
| ABCC1 | F:
CTGGGCTTATTTCGGATCAA |
|
| R:
TGAATGGGTCCAGGTTCATT |
| PTEN | F:
ATACCAGGACCAGAGGAAACC |
|
| R:
TTGTCATTATCCGCACGCT |
| GAPDH | F:
TCCTGCACCACCAACTGCTT |
|
| R:
GAGGGGCCATCCACAGTCTT |
Western blotting
Cells were lysed with Lysis Buffer (Beijing Solarbio
Science & Tecnology Co., Ltd., Beijing, China) and total
protein concentration was measured using the Pierce Bicinchoninic
Acid Protein Assay kit (Thermo Fisher Scientific, Inc.). Equal
amounts of protein (50 µg) were separated by 8% SDS-PAGE and
transferred to polyvinylidene fluoride membranes, which were
blocked with ice-cold transfer film buffer (0.29% Tris-base, 0.58%
glycine, 0.037% SDS and ddH2O) for 60–90 min. Primary
antibodies against PTEN (1:1,000), p-PI3K (1:1,000), PI3K
(1:1,000), p-AKT (1:1,000), AKT (1:1,000), ABCG2 (1:1,000) and
β-actin (1:1,000) were applied overnight at 4°C at a dilution of
1:1,000. Proteins were detected by enhanced chemiluminescence (EMD
Millipore, Billerica, MA, USA), following incubation with
horseradish peroxidase-conjugated secondary antibodies (1:10,000
dilution; cat. no. HAF008; R&D Systems China Co., Ltd.,
Shanghai, China) at room temperature for 1 h. Blots were
semi-quantified using ImageJ (V1.8.0; National Institutes of
Health, Bethesda, MD, USA).
Statistical analysis
SPSS software 13.0 (SPSS, Inc., Chicago, IL, USA)
and GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, ISA)
were used for statistical analysis. Data are presented as the means
± standard deviation. Differences between two groups were analyzed
using Student's t-test. Differences among multiple groups were
analyzed using one-way analysis of variance and the
Student-Newman-Keuls post hoc test. The correlations between PTEN
and ABCG2, PTEN expression and SP%, and ABCG2 expression and SP%
were assessed using Spearman correlation analysis. All experiments
were performed in triplicate with consistent results. *P<0.05
was considered to indicate a statistically signifcant difference,
**P<0.01 was considered to indicate a larger statistically
significant difference.
Results
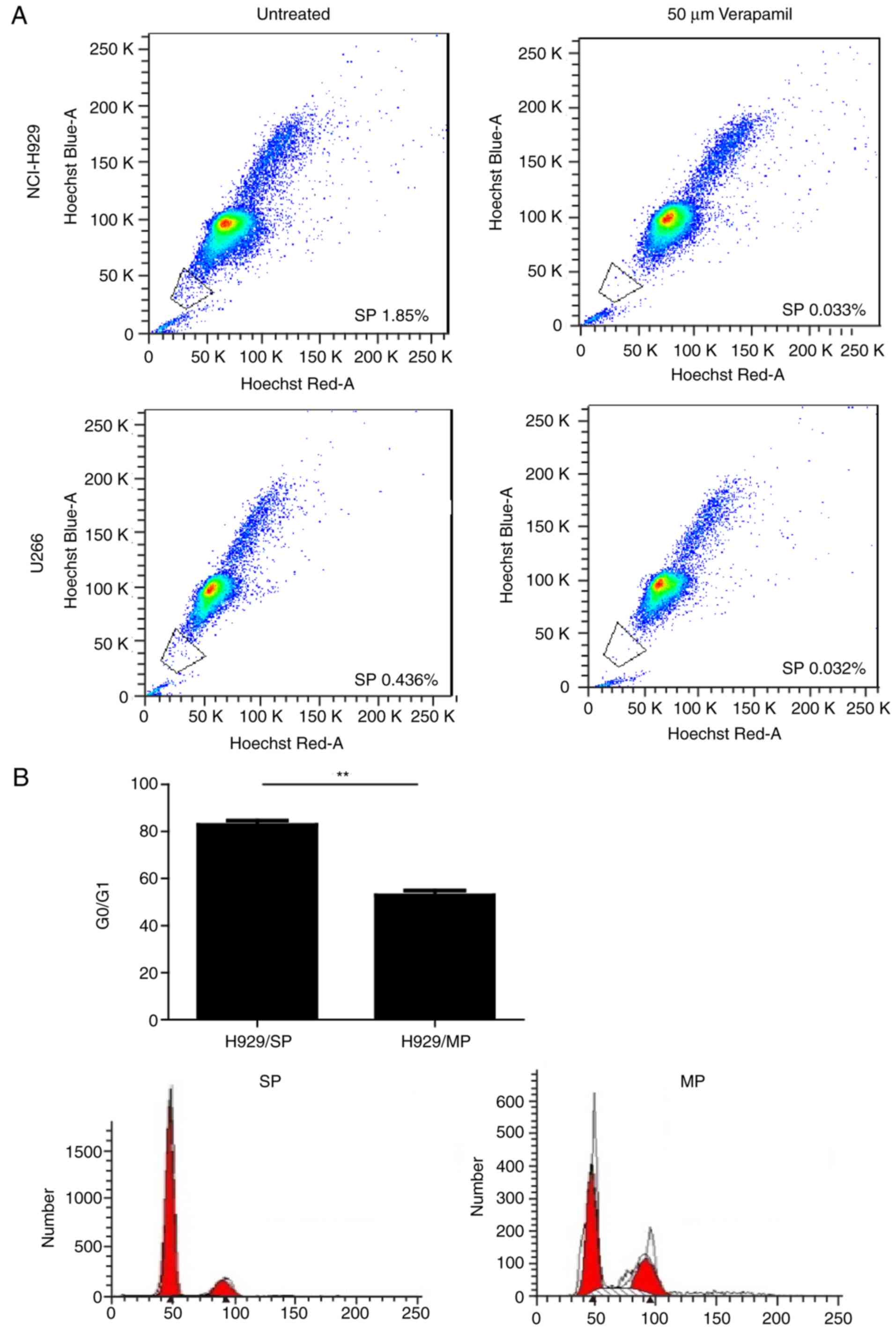
SP cells sorted from MM cell lines
exhibit CSC-like phenotypes and express high levels of ABCG2
The present study stained two human MM cell lines
(NCI-H929 and U266) with Hoechst 33342 and PI, in order to
determine whether SP cells can be identified in a similar manner as
in other tumors. Both MM cell lines contained SP cells, which
presented as a distinct ‘tail’ in the flow cytometric analysis. The
proportion of SP cells was 1.79±0.10% in the NCI-H929 cell line and
0.46±0.01% in the U266 cell line. Since NCI-H929 cells seemed to
have a higher proportion of SP cells, SP and MP cells isolated from
the NCI-H929 cell line were used for subsequent experiments. SP
cells have inherent properties that distinguish them from MP cells.
As shown in Fig. 1B, cell cycle
analysis indicated that the percentage of cells in
G0/G1 phase was significantly higher in SP
cells compared with in MP cells (P<0.01). The majority of SP
cells existed in an abnormal G0/G1 phase,
which is consistent with the characteristics of CSCs (29). Furthermore, FACS-sorted SP cells
exhibited increased proliferation compared with MP cells under the
same culture conditions, as demonstrated by CCK-8 assays (Fig. 1C). The colony forming ability of SP
and MP cells was determined using soft agar colony forming assays.
Both SP and MP cells generated colonies, whereas the number of
colonies generated by SP cells was significantly greater compared
with MP cells (P<0.01; Fig. 1D and
E); ~13.80% of SP cells formed visible colonies. These findings
suggested that SP cells may possess a prominent self-renewal
ability, which is a common characteristic of CSCs.
It is well known that CSCs often exhibit high ABC
transporter activity. The ABC protein family contains ABC subfamily
A member 3 (ABCA3), ABC subfamily B member 1 (ABCB1), ABC subfamily
C member 1 (ABCC1) and ABCG2. Therefore, the present study
performed qPCR to investigate the expression of these ABC family
members in SP and MP NCI-H929 cells. As expected, the mRNA
expression levels of ABCA3, ABCB1, ABCC1 and ABCG2 were higher in
SP cells compared with in MP cells (Fig. 2A). Notably, SP cells had ~10 times
more ABCG2 transcript compared with MP cells (Fig. 2B). Correspondingly, the protein
expression levels of ABCG2 were markedly increased in SP cells
compared with in MP cells (Fig.
2C). These data indicated that ABCG2 may be a potential primary
factor that maintains the stem cell-like characteristics of SP
NCI-H929 cells.
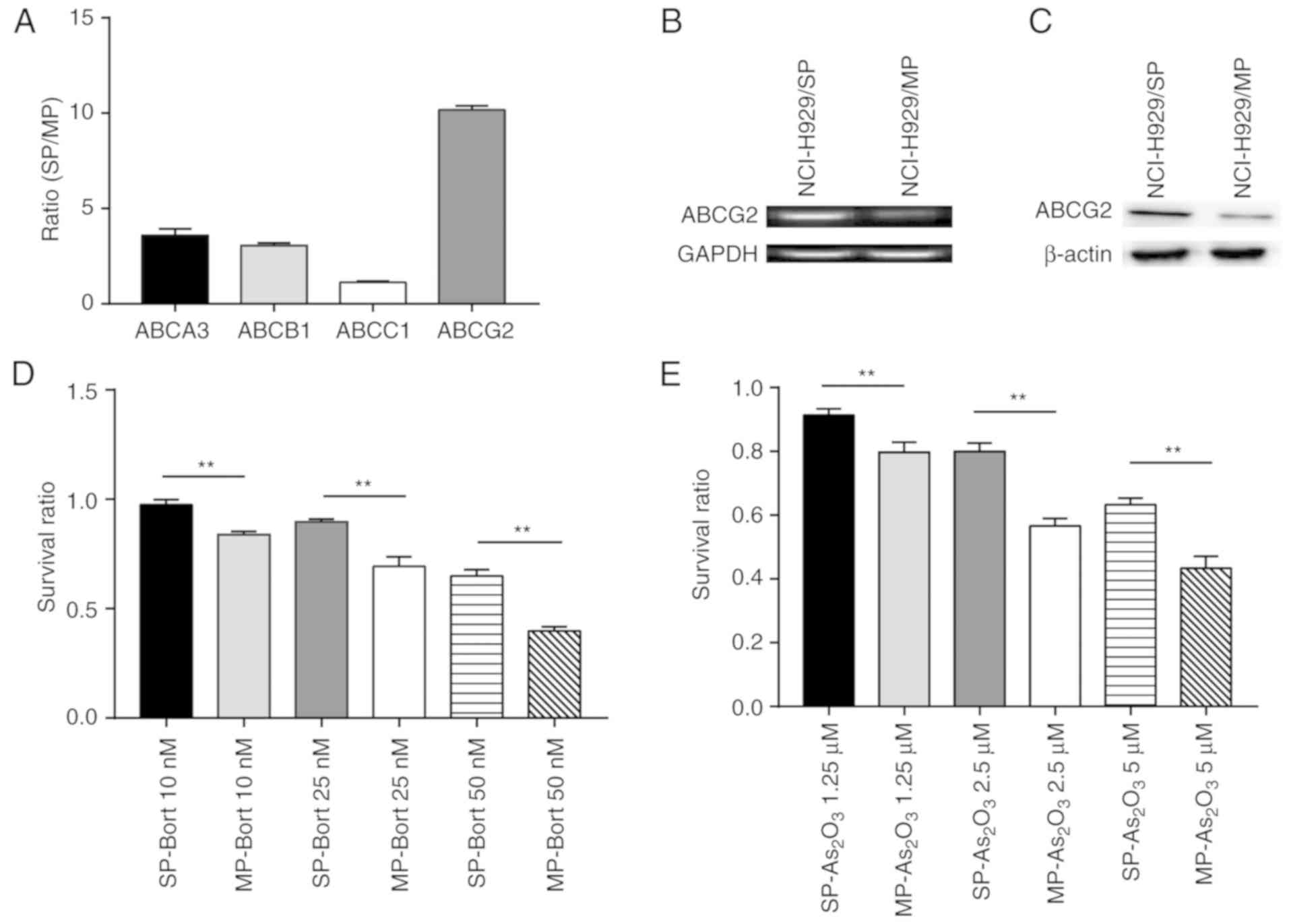 | Figure 2.SP cells express high levels of ABCG2
and display more potent chemoresistance than MP cells. (A) Ratios
of ABCA3, ABCB1, ABCC1 and ABCG2 transcripts in SP NCI-H929 cells
relative to MP NCI-H929 cells. (B) mRNA expression levels of ABCG2,
as determined using DNA agarose gel electrophoresis. (C) ABCG2
protein expression levels were increased in SP cells compared with
in MP cells. (D and E) Survival ratio of SP and MP cells treated
with various doses of (D) Bort or (E) As2O3.
**P<0.01. ABC, ATP binding cassette; ABCA3, ABC subfamily A
member 3; ABCB1, ABC subfamily B member 1; ABCC1, ABC subfamily C
member 1; ABCG2, ABC subfamily G member 2;
As2O3, arsenic trioxide; Bort, bortezomib;
MP, main population; SP, side population. |
The present study determined the sensitivity of SP
and MP cells to two common chemotherapy drugs, Bort and
As2O3. Regardless of the concentrations
tested, the survival rates were significantly higher in response to
treatment with either Bort (Fig.
2D) or As2O3 (Fig. 2E) in SP cells compared with in MP
cells. Taken together, SP cells from the NCI-H929 cell line
exhibited higher ABCG2 expression levels and displayed a more
potent chemoresistant phenotype compared with MP cells.
Association between ABCG2 expression,
SP cells and the PI3K/mTOR pathway in NCI-H929 cells
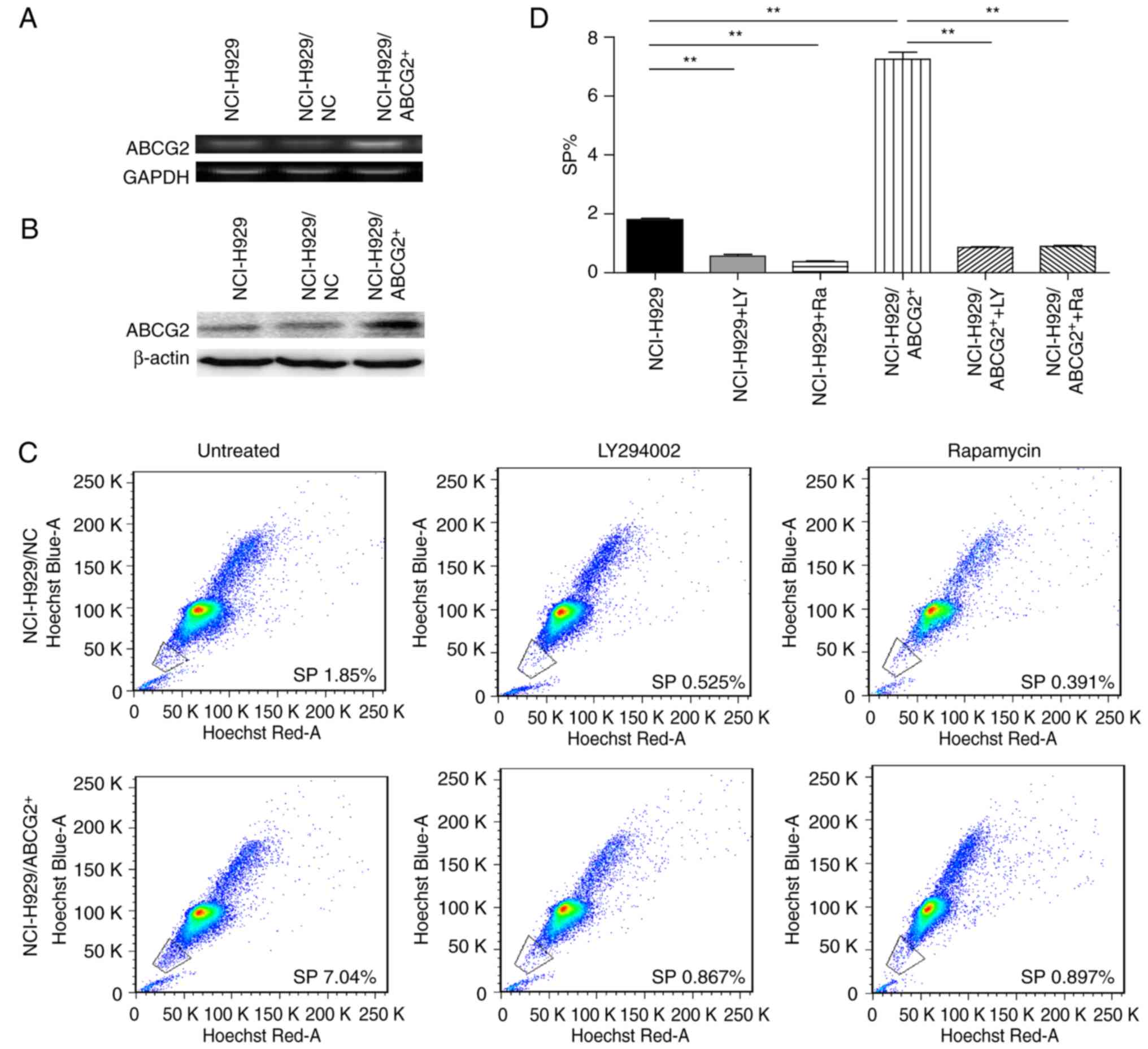
To further consolidate the role of ABCG2 in
maintaining the phenotype of CSCs, NCI-H929 cells were transfected
with either ABCG2-lentivirus (NCI-H929/ABCG2+) or
control lentivirus (NCI-H929/NC). Cells infected with green
fluorescence protein-tagged lentiviruses were sorted (Fig. 3), and the expression levels of
ABCG2, at both mRNA (Fig. 4A) and
protein (Fig. 4B) levels, were
detected. Considering the critical roles of the PI3K/mTOR pathway
in regulating MDR, the present study determined the effects of
LY294002 (an inhibitor of PI3K signaling) and rapamycin (an
inhibitor of mTOR signaling) on the frequency of SP cells in
NCI-H929 and NCI-H929/ABCG2+ cell lines. As shown in
Fig. 4C and D, the
NCI-H929/ABCG2+ cell line exhibited a higher percentage
of SP cells compared with the NCI-H929 cell line (7.0 vs. 1.8%;
P<0.01). In NCI-H929 and NCI-H929/ABCG2+ cell lines,
pretreatment with LY294002 or rapamycin significantly reduced the
abundance of SP cells. Notably, LY294002 and rapamycin decreased
the frequency of SP cells from 7.0 to ~1.5% in the
NCI-H929/ABCG2+ cell line. These data indicated that
overexpression of ABCG2 and activation of the PI3K/mTOR pathway may
contribute to SP cell maintenance in the NCI-H929 cell line.
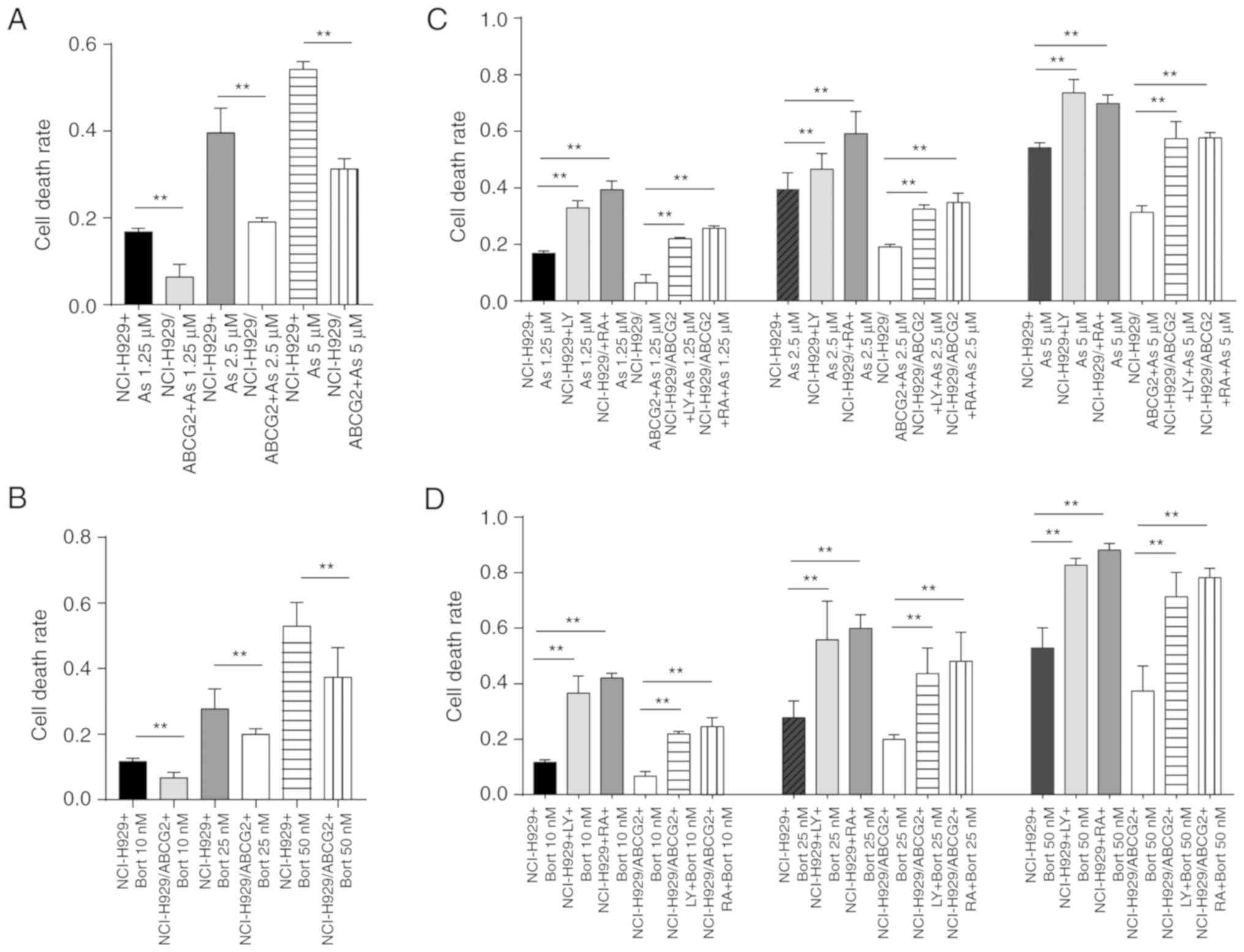
Protective effects of ABCG2 against
chemotherapeutic drugs is reversed by PI3K/mTOR pathway inhibition
in NCI-H929 cells
ABCG2 expression is associated with the
chemoresistance of SP cells in the NCI-H929 cell line. Consistent
with this finding, the cell death rate was significantly lower in
NCI-H929/ABCG2+ cells compared with NCI-H929 cells, in
response to various concentrations of As2O3
(Fig. 5A) or Bort (Fig. 5B). These data suggested that ABCG2
serves an important role in protecting tumor cells. Since ABCG2
expression and activation of the PI3K/mTOR pathway may be
correlated with the proportion of SP cells in the NCI-H929 cell
line, the present study detected the sensitivity of tumor cells to
chemotherapeutic drugs following inhibition of the PI3K/mTOR
pathway. Cell death rates were increased in a dose-dependent manner
in response to LY294002 or rapamycin following
As2O3 (Fig.
5C) or Bort (Fig. 5D) treatment
in NCI-H929 and NCI-H929/ABCG2+ cells. These findings
suggested that inhibition of the PI3K/mTOR pathway in MM may
enhance the sensitivity of tumor cells to chemotherapeutic drugs,
thus suggesting that inhibition of the PI3K/mTOR pathway
counteracts the protective effects of ABCG2.
ABCG2 expression is associated with
the activation of PI3K/mTOR signaling in NCI-H929 cells
Since inhibition of the PI3K/mTOR signaling pathway
attenuated the protective effects of ABCG2 overexpression on
NCI-H929/ABCG2+ cells, the present study aimed to
determine whether the PI3K/mTOR pathway regulates ABCG2 expression.
The results of RT-qPCR demonstrated that PTEN was downregulated in
NCI-H929/ABCG2+ cells; PTEN is an inhibitor molecule
upstream of PI3K/mTOR signaling (Fig.
6A and B). This finding suggested that ABCG2 overexpression may
be associated with activation of PI3K/mTOR signaling. Conversely,
inhibition of PI3K/mTOR signaling by LY294002 or rapamycin reduced
ABCG2 expression in NCI-H929 and NCI-H929/ABCG2+ cells
(Fig. 6C and D). Subsequently,
PI3K/mTOR activity was analyzed by measuring p-PI3K and its
downstream target p-AKT in NCI-H929 cells treated with LY294002 or
rapamycin. As shown in Fig. 6E, the
protein expression levels of p-PI3K and p-AKT were higher in
NCI-H929/ABCG2+ cells compared with in NCI-H929 cells.
In addition, PTEN expression was reduced in
NCI-H929/ABCG2+ cells. Notably, ABCG2 expression was
markedly decreased following LY294002 or rapamycin treatment of
NCI-H929 and NCI-H929/ABCG2+ cells. Therefore, it may be
hypothesized that the PI3K/AKT signaling pathway regulated ABCG2
protein expression, and ABCG2 regulated PTEN protein expression via
a possible negative feedback loop.
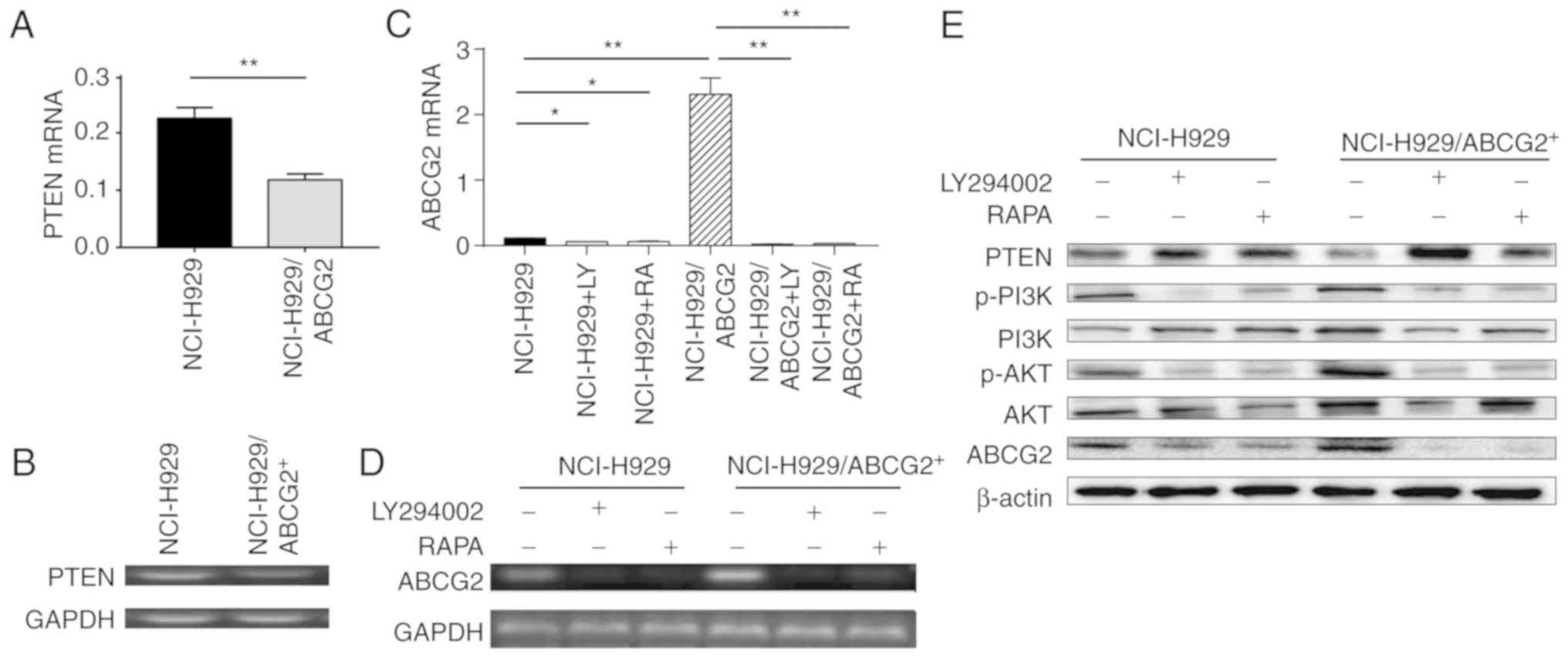 | Figure 6.The PI3K/AKT signaling pathway
regulates ABCG2 protein expression in NCI-H929 cells. (A and B)
PTEN mRNA expression was decreased in NCI-H929/ABCG2+
cells compared with in control NCI-H929 cells. (A) Summary data and
(B) representative images of DNA agarose gel electrophoresis are
shown. (C and D) ABCG2 mRNA expression was increased in
NCI-H929/ABCG2+ cells compared with in control NCI-H929
cells, whereas LY294002 or RAPA downregulated ABCG2 expression in
both cell types. (C) Summary data and (D) representative images of
DNA agarose gel electrophoresis are shown. n=3 tests/group. (E)
Association between PI3K/AKT signaling and ABCG2 protein expression
in NCI-H929 cells. Whereas PTEN protein levels were decreased,
ABCG2, p-PI3K and p-AKT protein levels were increased in
NCI-H929/ABCG2+ cells compared with in control NCI-H929
cells. LY294002 or RAPA treatment decreased p-PI3K, p-AKT and ABCG2
protein levels in NCI-H929 and NCI-H929/ABCG2+ cells.
*P<0.05, **P<0.01. ABCG2, ABC subfamily G member 2; AKT, AKT
serine/threonine kinase; p, phosphorylated; PI3K,
phosphatidylinositol 3-kinase; PTEN, phosphatase and tensin
homolog; RAPA, rapamycin. |
Proportion of SP cells, ABCG2
expression and activation of the PI3K/AKT pathway are associated
with disease progression in patients with MM
A total of 30 patients diagnosed with MM were
recruited to this study, in order to determine if the in
vitro findings in MM cell lines were applicable in clinical
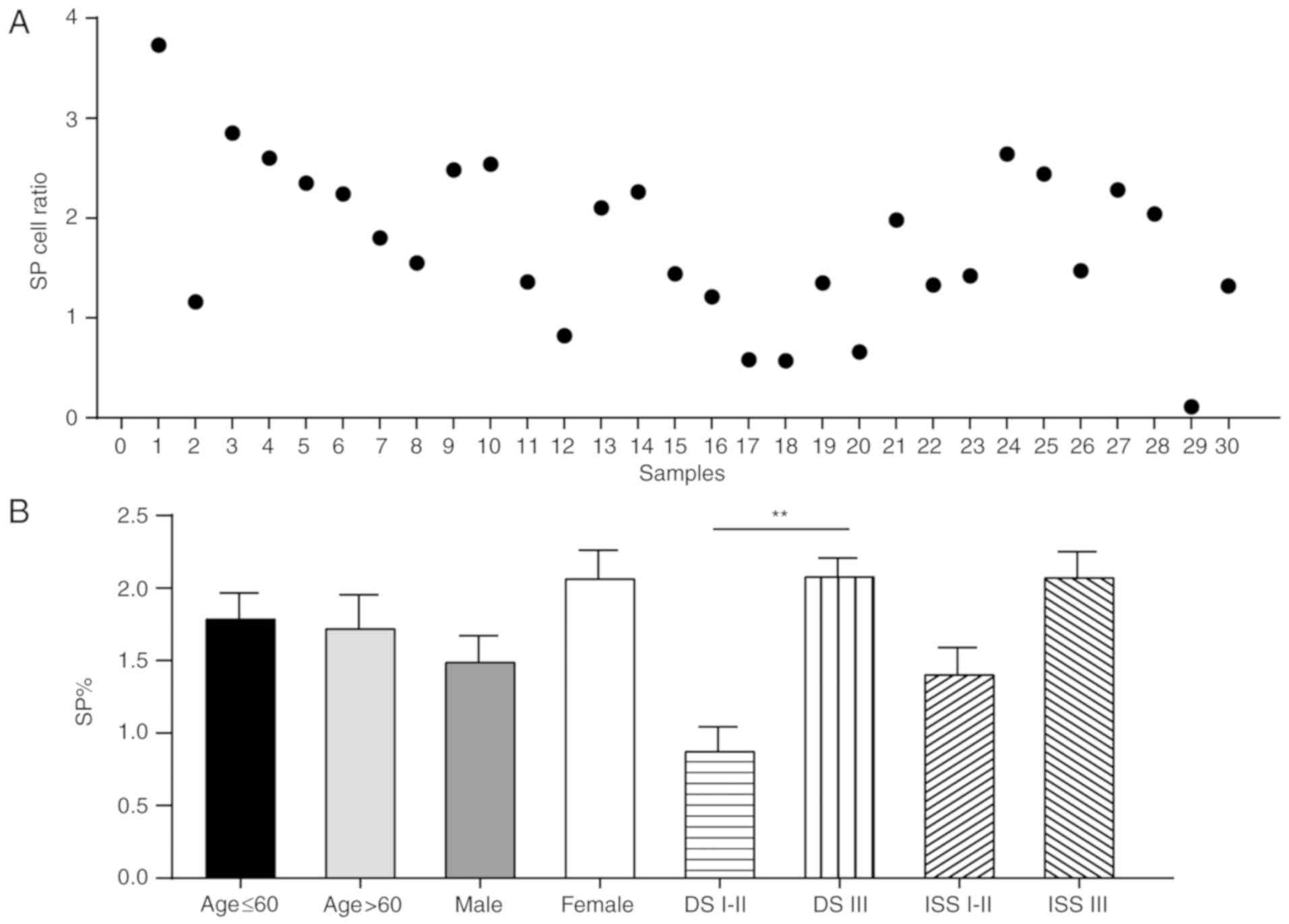
samples (Figs. 7–10). Flow cytometric analyses indicated
that the frequencies of SP cells in patient bone marrow samples
ranged between 0.11 and 3.73% (Fig.
7A). It was evident that patients with MM had a notably higher
proportions of SP cells compared with the control, and the
proportions were associated with DS stages, as patients with DS
stage III MM had significantly more SP cells compared with patients
with DS stage I–II MM (Fig. 8A).
However, the proportion of SP cells in patients with MM was not
associated with sex (male vs. female, P=0.321), age (<60 vs.
>60 years, P=0.309) or ISS stage (I–II vs. III, P=0.813)
(Fig. 7B).
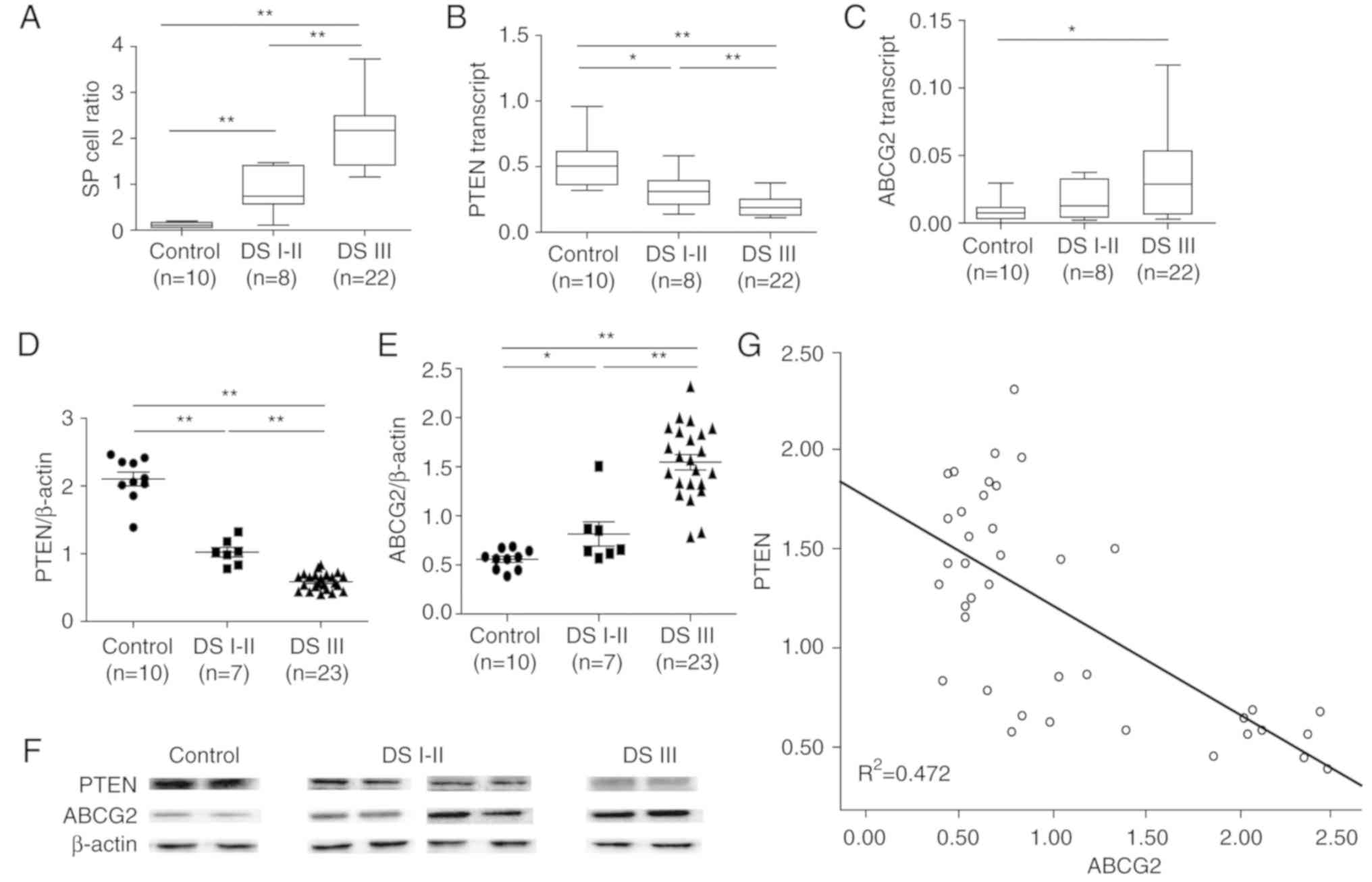
The patient samples were then divided into three
groups (Control, patients with DS I–II MM and patients with DS III
MM), and the mRNA and protein expression levels of ABCG2 and PTEN
were measured. Compared with in the control samples, the mRNA
expression levels of PTEN were significantly lower in patients with
MM, particularly in DS III patients (Fig. 8B). Conversely, the mRNA expression
levels of ABCG2 were significantly higher in patients with DS III
MM compared with in the control group. However, there was no
significant difference between DS I–II patients and the control
group; this may be due to the small sample size (Fig. 8C).
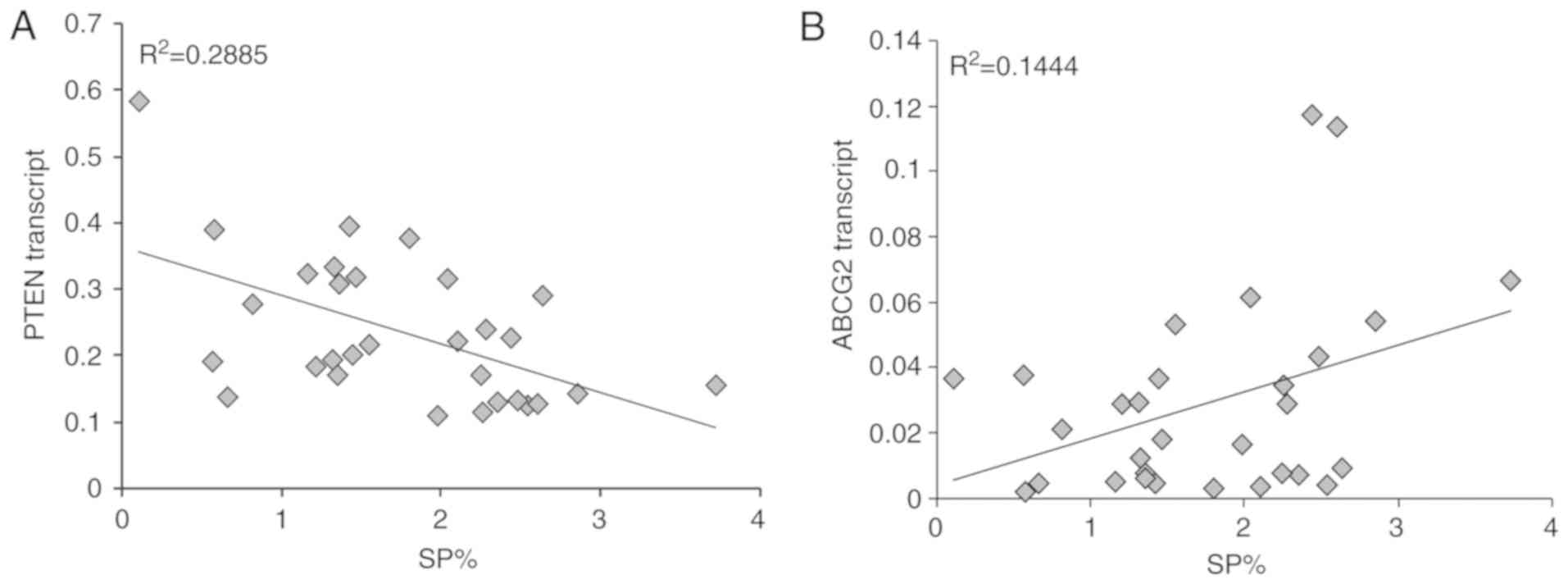
Notably, a significant negative correlation was
detected between PTEN mRNA expression and the proportion of SP
cells (R2=0.2885), whereas a significant positive
correlation was detected between ABCG2 mRNA expression and the
proportion of SP cells (R2=0.1444) in patients with MM
(Fig. 10). Accordingly, similar
protein expression patterns of PTEN and ABCG2 (Fig. 8D-F) were identified among the
control group, and DS I–II patient and DS III patient groups.
Specifically, PTEN protein levels were negatively correlated with
ABCG2 protein levels in all subjects (P<0.01,
R2=0.472 (Fig. 8G). In
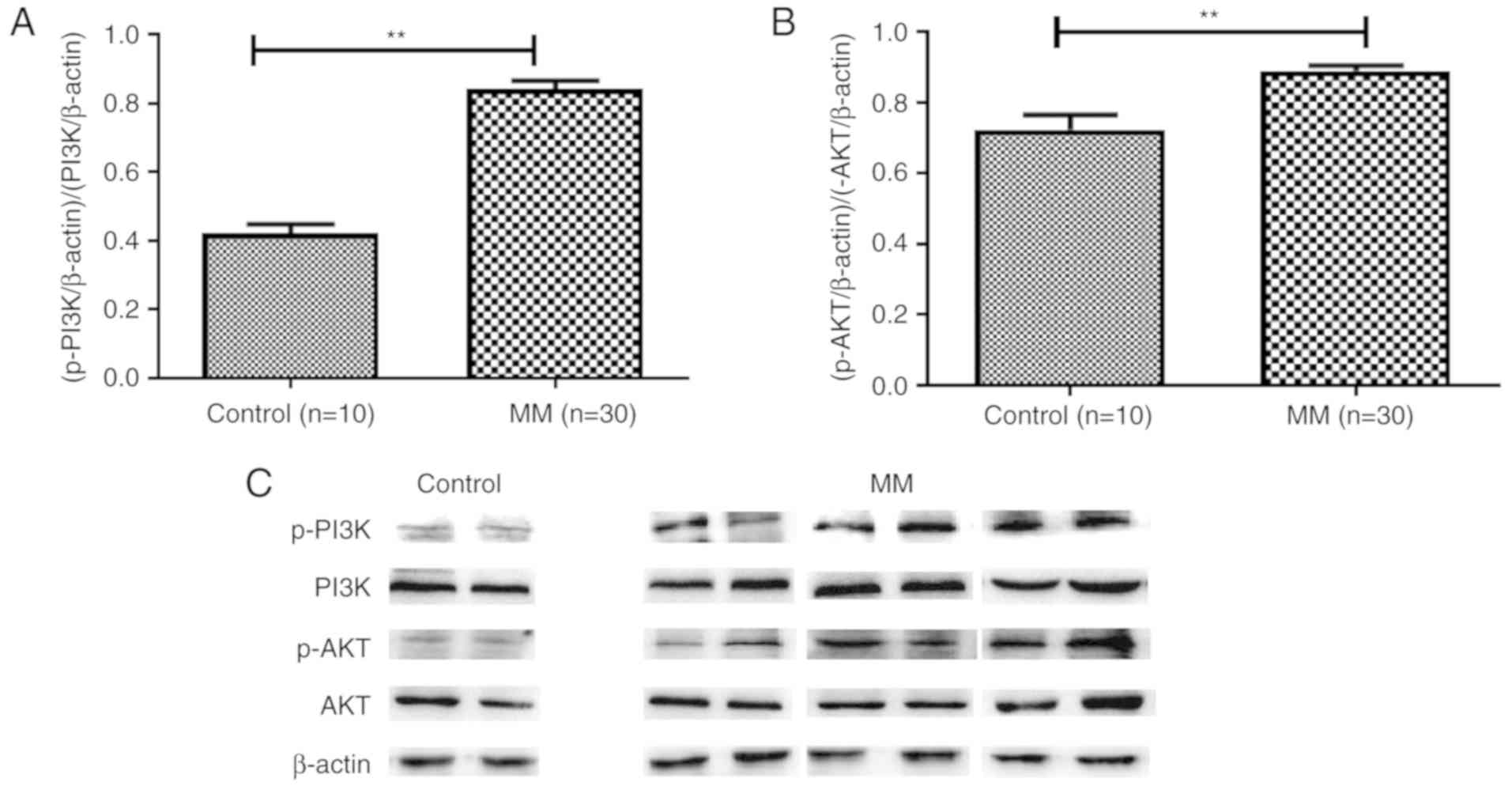
addition, increased levels of p-PI3K (Fig. 9A) and p-AKT (Fig. 9B and C) were detected in patients
with MM, thus indicating the importance of the PI3K/AKT signaling
pathway in MM pathogenesis.
Discussion
The global incidence of MM is increasing annually;
however, there is currently no optimal treatment for MM. Patients
receiving chemotherapy endure varying degrees of pain; therefore,
it is necessary to develop novel treatments to improve the
prognosis of MM. Generally, MM CSCs are associated with
chemoresistance, which is the primary cause for clinical failure of
the complete elimination of MM cells. In the present study, SP
cells were identified and MM CSC-like cells were isolated from MM
cell lines and patient samples. The present investigation suggested
that ABCG2 may be an indicator of MM disease progression, and the
reciprocal regulation identified between ABCG2 and the PI3K/AKT
pathway provided novel approaches to advance MM therapy in the
future.
The identification of SP cells from NCI-H929 and
U266 MM cell lines is consistent with the literature. Jakubikova
et al (30) reported that
0.5–2.5% of cells in MM cell lines are SP cells. As the present
study did not analyze MDR cell lines, the proportion of SP cells
was fewer than what has been reported in the literature. In
addition to increased cell cycle arrest at
G0/G1 phase and increased self-renewal, the
expression levels of ABCA3, ABCB1, ABCC1 and ABCG2 were
significantly increased in SP cells compared with in MP cells.
Notably, the expression levels of ABCG2 were ~10 times higher in SP
cells compared with in MP cells. These findings suggested that
ABCG2 may be one of the most important ABC family members for
maintaining the CSC-like biological characteristics of SP cells.
This observation is consistent with reports that the ABCG2
transporter is the most specific SP cell marker in solid tumours
(31). The survival rate of SP
cells was significantly higher than MP cells in response to
As2O3 or Bort. This finding indicated that SP
cells may possess lower sensitivity to chemotherapeutic agents
compared with MP cells. The present study revealed that SP cells
shared similar characteristics with CSCs; however, the surface
markers of MM CSCs were not clear (32). It may be hypothesized that targeting
SP cells is an effective strategy to eradicate malignant tumors of
the blood system, particularly if SP cells can be located and
targeted according to the expression pattern of ABCG2.
It has been reported that ABCG2 exists in both
normal and tumor tissues, including in gynecological cancer,
prostate cancer, respiratory system tumors, digestive system tumors
and blood system tumors (33–35).
Since ABCG2 is highly expressed in some CSCs, ABCG2 may be
considered a surface marker for CSCs. The present study aimed to
explore the biological function of ABCG2 in MM. The results
revealed that the death rate of NCI-H929/ABCG2+ cells
was significantly lower compared with NCI-H929 cells following
treatment with chemotherapeutic drugs. NCI-H929/ABCG2+
cells exhibited low sensitivity to chemotherapeutic agents,
indicating that ABCG2 overexpression may have a protective effect
on tumor cells. Conversely, the death rate of NCI-H929 and
NCI-H929/ABCG2+ cells was increased when the PI3K/AKT
pathway was inhibited, thus suggesting that suppressing the
PI3K/AKT signaling pathway may counteract the protective effects of
ABCG2 overexpression. It is necessary to further study whether the
occurrence, development and prognosis of MM can be altered through
regulating the biological behavior of ABCG2.
The PI3K/AKT signaling pathway serves an important
role in the occurrence and development of tumors. Activation of the
PI3K/AKT pathway has been associated with an increased incidence of
leukemia (26); however, the roles
of PI3K/AKT signaling in CSCs from MM remain largely unclear. In
the present study, NCI-H929/ABCG2+ cells exhibited
decreased PTEN and increased p-PI3K and p-AKT expression compared
with in control NCI-H929 cells; ABCG2 was also negatively
correlated with PTEN expression in MM. PTEN deficiencies are
closely associated with tumor incidence, including prostate cancer,
and significantly higher levels of p-PI3K and p-AKT in leukemia
(23). Notably, the present results
in NCI-H929 and NCI-H929/ABCG2+ cells suggested that the
PI3K/AKT signaling pathway may regulate the proportion of SP cells
and ABCG2 expression. Therefore, it may be concluded that PTEN
regulates SP cell frequency and ABCG2 expression via the PI3K/AKT
pathway in MM.
SP cells have been detected in numerous solid tumors
(36–38) and hematological malignancies
(39,40). In an early study on MM, SP cells
were detected in 18 out of 21 samples; the inability to detect SP
cells in the remaining three samples was likely due to biological
polymorphisms (41). In the present
study, the proportion of SP cells in patients with MM was
significantly higher compared with in the control group. Further
analysis indicated that the proportion of SP cells was positively
associated with DS stages; patients with DS III MM had higher
percentages of SP cells compared with patients with DS I–II MM.
Therefore, it may be suggested that the proportion of SP cell was
associated with MM disease progression.
Loss of PTEN increases the percentage of SP cells in
glioma (18), which is consistent
with the present finding that PTEN was significantly reduced in MM.
Specifically, PTEN in patients with DS III MM was significantly
lower compared with in patients with DS I–II, suggesting that PTEN
may be involved in MM pathogenesis and closely associated with
disease progression. Furthermore, a negative correlation was
identified between the proportion of SP cells and the expression
levels of PTEN. ABCG2 protein expression in patients with DS III MM
was significantly higher compared with in patients with DS I–II MM,
indicating that ABCG2 is an indicator of disease progression and
prognosis. Conversely, PTEN protein expression was significantly
negatively correlated with ABCG2 protein expression. The PI3K/AKT
pathway has been reported to serve an important role in the
maintenance of SP cells in primary esophageal carcinoma (19). Consistent with this finding, the
present study suggested that PTEN-mediated PI3K/AKT signaling may
contribute to the proportion of SP cells in MM, possibly via
regulating ABCG2 expression.
In conclusion, SP cells sorted from MM cell lines
exhibited high ABCG2 expression and shared similar biological
characteristics with CSCs. ABCG2 protected MM cells against the
cytotoxicity of chemotherapeutic drugs and was regulated by
PTEN-mediated PI3K/AKT signaling in NCI-H929 cells. Inhibition of
the PI3K/AKT pathway by LY294002 or rapamycin significantly
decreased the proportion of SP cells and enhanced the sensitivity
of NCI-H929 cells to chemotherapeutic drugs. The proportion of SP
cells, ABCG2 expression and activation of the PI3K/AKT pathway were
all positively associated with disease progression in patients with
MM. The present study offers insights into regulation of the
proportion of SP cells and provides a novel strategy to overcome
resistance of MM to existing therapies by targeting the ABCG2 and
PI3K/AKT signaling axis.
Acknowledgements
The authors would like to thank Dr Pingping Wang, Dr
Yazhu Wang and Dr Yue Wang (Department of Hematology, The First
Affiliated Hospital of China Medical University) for providing
technical assistance.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81600117).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LW, NL and YL conceived and designed the
experiments. LW performed the experiments, analyzed the data and
wrote the paper. YL contributed with reagents/materials/analytical
tools, critically read the manuscript and provided technical
assistance. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study (AF-SOP-07-1.0–01) was approved by
the institutional review board of the First Affiliated Hospital of
China Medical University, and written informed consent was obtained
from all patients prior to their participation, in accordance with
the Declaration of Helsinki.
Patient consent for publication
Patients provided consent for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nikesitch N, Lee JM, Ling S and Roberts
TL: Endoplasmic reticulum stress in the development of multiple
myeloma and drug resistance. Clin Transl Immunol. 7:e10072018.
View Article : Google Scholar
|
|
2
|
Issa ME, Cretton S and Cuendet M:
Targeting multiple myeloma cancer stem cells with natural
products-lessons from other hematological malignancies. Planta Med.
83:752–760. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Clarke RB, Anderson E, Howell A and Potten
CS: Regulation of human breast epithelial stem cells. Cell Prolif.
1:45–58. 2003. View Article : Google Scholar
|
|
6
|
Dai Y, Liu S, Zhang WQ, Yang YL, Hang P,
Wang H, Cheng L, Hsu PC, Wang YC, Xu Z, et al: YAP1 regulates ABCG2
and cancer cell side population in human lung cancer cells.
Oncotarget. 8:4096–4109. 2017.PubMed/NCBI
|
|
7
|
Prasanphanich AF, White DE, Gran MA and
Kemp ML: Kinetic modeling of ABCG2 transporter heterogeneity: A
quantitative, single-cell analysis of the side population assay.
PLoS Comput Biol. 12:e10051882016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wei Z, Liu Y, Wang Y, Zhang Y, Luo Q, Man
X, Wei F and Yu X: Downregulation of Foxo3 and TRIM31 by miR-551b
in side population promotes cell proliferation, invasion, and drug
resistance of ovarian cancer. Med Oncol. 33:1262016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang Y, Gao H, Liu M and Mao Q: Sorting
and biological characteristics analysis for side population cells
in human primary hepatocellular carcinoma. Am J Cancer Res.
6:1890–1905. 2016.PubMed/NCBI
|
|
10
|
Gu H, Wu XY, Fan RT, Wang X, Guo YZ and
Wang R: Side population cells from long-term passage non-small cell
lung cancer cells display loss of cancer stem cell-like properties
and chemoradioresistance. Oncol Lett. 12:2886–2893. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu H, Shi B, Yan Y, Niu L, Zhou Y, Cai H
and Ge G: CXCR4 promotes growth and sphere formation of hypoxic
breast cancer side 7 population cells via activation of c-Jun/ABCG2
pathway. Oncol Res. 2016. View Article : Google Scholar :
|
|
12
|
Wei Z, Lv S, Wang Y, Sun M, Chi G, Guo J,
Song P, Fu X, Zhang S and Li Y: Biological characteristics of side
population cells in a self-established human ovarian cancer cell
line. Oncol Lett. 12:41–48. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Goodell MA, Brose K, Paradis G, Conner AS
and Mulligan RC: Isolation and functional properties of murine
hematopoietic stem cells that are replicating in vivo. J Exp Med.
183:1797–1806. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Haraguchi N, Utsunomiya T, Inoue H, Tanaka
F, Mimori K, Barnard GF and Mori M: Characterization of a side
population of cancer cells from human gastrointestinal system. Stem
Cells. 24:506–513. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hirschmann-Jax C, Foster AE, Wulf GG,
Nuchtern JG, Jax TW, Gobel U, Goodell MA and Brenner MK: A distinct
‘side population’ of cells with high drug efflux capacity in human
tumor cells. Proc Natl Acad Sci USA. 101:14228–14233. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang J, Guo LP, Chen LZ, Zeng YX and Lu
SH: Identification of cancer stem cell-like side population cells
in human nasopharyngeal carcinoma cell line. Cancer Res.
67:3716–3724. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang D, Gao Q, Guo L, Zhang C, Jiang W,
Li H, Wang J, Han X, Shi Y and Lu SH: Isolation and identification
of cancer stem-like cells in esophageal carcinoma cell lines. Stem
Cells Dev. 18:465–473. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bleau AM, Hambardzumyan D, Ozawa T,
Fomchenko EI, Huse JT, Brennan CW and Holland EC: PTEN/PI3K/Akt
pathway regulates the side population phenotype and ABCG2 activity
in glioma tumor stem-like cells. Cell Stem Cell. 4:226–235. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li H, Gao Q, Guo L and Lu SH: The
PTEN/PI3K/Akt pathway regulates stem-like cells in primary
esophageal carcinoma cells. Cancer Biol Ther. 11:950–958. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chang FW, Fan HC, Liu JM, Fan TP, Jing J,
Yang CL and Hsu RJ: Estrogen enhances the expression of the
multidrug transporter gene ABCG2-Increasing drug resistance
of breast cancer cells through estrogen receptors. Int J Mol Sci.
18:E1632017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stacy AE, Jansson PJ and Richardson DR:
Molecular pharmacology of ABCG2 and its role in chemoresistance.
Mol Pharmacol. 84:655–669. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fischer B, Frei C, Moura U, Stahel R and
Felley-Bosco E: Inhibition of phosphoinositide-3 kinase pathway
down regulates ABCG2 function and sensitizes malignant pleural
mesothelioma to chemotherapy. Lung Cancer. 78:23–29. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gao T, Mei Y, Sun H, Nie Z, Liu X and Wang
S: The association of phosphatase and tensin homolog (PTEN)
deletion and prostate cancer risk: A meta-analysis. Biomed
Pharmacother. 83:114–121. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu H, Dai X and Wang E: Plumbagin inhibits
cell proliferation and promotes apoptosis in multiple myeloma cells
through inhibition of the PI3K/Akt-mTOR pathway. Oncol Lett.
12:3614–3618. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rajkumar SV: Updated diagnostic criteria
and staging system for multiple myeloma. Am Soc Clin Oncol Educ
Book. 35:e418–e423. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang FF, Wu DS, Zhang L, Yu YH, Yuan XY,
Li WJ, Chen XP, Zhao XL, Chen FP and Zeng H: Inactivation of PTEN
increases ABCG2 expression and the side population through the
PI3K/Akt pathway in adult acute leukemia. Cancer Lett. 336:96–105.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Borowicz S, Van Scoyk M, Avasarala S,
Karuppusamy Rathinam MK, Tauler J, Bikkavilli RK and Winn RA: The
soft agar colony formation assay. J Vis Exp. e519982014.PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2ΔΔCT method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sajadian S, Vatankhah M, Majdzadeh M,
Kouhsari SM, Ghahremani MH and Ostad SN: Cell cycle arrest and
apoptogenic properties of opium alkaloids noscapine and papaverine
on breast cancer stem cells. Toxicol Mech Methods. 25:388–395.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jakubikova J, Adamia S, Kost-Alimova M,
Klippel S, Cervi D, Daley JF, Cholujova D, Kong SY, Leiba M, Blotta
S, et al: Lenalidomide targets clonogenic side population in
multiple myeloma: Pathophysiologic and clinical implications.
Blood. 117:4409–4419. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhou S, Schuetz JD, Bunting KD, Colapietro
AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M,
Nakauchi H, et al: The ABC transporter Bcrp1/ABCG2 is expressed in
a wide variety of stem cells and is a molecular determinant of the
side-population phenotype. Nat Med. 7:1028–1034. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kellner J, Liu B, Kang Y and Li Z: Fact or
fiction-Identifying the elusive multiple myeloma stem cell. J
Hematol Oncol. 6:912013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guzel E, Karatas OF, Duz MB, Solak M,
Ittmann M and Ozen M: Differential expression of stem cell markers
and ABCG2 in recurrent prostate cancer. Prostate. 74:1498–1505.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sabnis NG, Miller A, Titus MA and Huss WJ:
The efflux transporter ABCG2 maintains prostate stem cells. Mol
Cancer Res. 15:128–140. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang H, Luo F, Zhu Z, Xu Z, Huang X, Ma R,
He H, Zhu Y, Shao K and Zhao J: ABCG2 is a potential prognostic
marker of overall survival in patients with clear cell renal cell
carcinoma. BMC Cancer. 17:2222017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Singh S, Bora-Singhal N, Kroeger J, Laklai
H and Chellappan SP: βArrestin-1 and Mcl-1 modulate self-renewal
growth of cancer stem-like side-population cells in non-small cell
lung cancer. PLoS One. 8:e559822013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhao R, Liu X, Wang Y, Jie X, Qin R, Qin
W, Zhang M, Tai H, Yang C, Li L, et al: Integrated glycomic
analysis of ovarian cancer side population cells. Clin Proteomics.
13:322016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Murota Y, Tabu K and Taga T: Requirement
of ABC transporter inhibition and Hoechst 33342 dye deprivation for
the assessment of side population-defined C6 glioma stem cell
metabolism using fluorescent probes. BMC Cancer. 16:8472016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yan W, Du J, Du Y, Pu H, Liu S, He J,
Zhang J and Hou J: Fenretinide targets the side population in
myeloma cell line NCI-H929 and potentiates the efficacy of
antimyeloma with bortezomib and dexamethasone regimen. Leuk Res.
51:32–40. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang Y, Yin C, Feng L, Ma L, Wei Y and
Sheng G: Sorting, identification and enrichment of side population
cells in THP-1 acute monocytic leukemia cells. Oncol Rep.
29:1923–1931. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Loh YS, Mo S, Brown RD, Yamagishi T, Yang
S, Joshua DE, Roufogalis BD and Sze DM: Presence of hoechst low
side populations in multiple myeloma. Leuk Lymphoma. 49:1813–1816.
2008. View Article : Google Scholar : PubMed/NCBI
|