Introduction
Apoptosis and autophagy are the major processes of
the programmed cell death (PCD) and play important roles in
cellular homeostasis and diseases (1,2).
Induction of autophagic or apoptotic death in tumor cells is one of
the best strategies in chemotherapy (3,4).
Autophagy is a well-known double-edged sword, promoting survival
and/or inducing cell death (5,6).
Autophagy-mediated programmed cell death (autophagic PCD) is
elicited when the cells undergo stress such as cellular damage,
nutrient starvation, aging, and pathogen infection (5). Autophagy is characterized by an
increase in autophagosomes or autophagic vesicles (double-membrane
vesicles), which is mediated by phosphatidylinositol 3-kinase
(PI3K) class III, Beclin-1 and autophagy-related protein 14 (Atg14)
signals. In addition, autophagosome membrane engagement is complied
with the Atg16L1, Atg12, Atg7, Atg5, and LC3 ubiquitin-like
conjugation systems. Finally, the autophagosome fuses with the
lysosome thus forming an autophagolysosome and degradation of the
captured proteins or organelles is carried out by lysosomal enzymes
(7). In contrast, apoptosis is
characterized by DNA condensation and fragmentation, and the
blebbing of nuclear and apoptotic bodies (8,9). The
regulators of apoptotic death are Bcl-2 family molecules and
caspase cascade (8,10). Pro-apoptotic Bcl-2 family proteins
(such as Bax, Bak, Bim and Bid) and anti-apoptotic proteins (such
as Bcl-2, Bcl-xL and Mcl-1) can regulate the process of the
apoptotic pathway by the ratio of pro-apoptotic Bcl-2 family
proteins/anti-apoptotic proteins (10,11).
Polygonum cuspidatum Sieb. et Zucc. (Hu
Zhang) is a herbaceous perennial plant found in Taiwan, China,
Japan, and America and belongs to the family Polygonaceae
(12,13). P. cuspidatum has been
detected to have various phytochemicals, including essential oils,
quinones, stilbenes, flavonoids, coumarins and lignans (12–14).
P. cuspidatum has been used to remove jaundice and clear
heat-toxin, to promote blood circulation, dispel stasis, expel wind
and dampness, to dissipate phlegm, and to suppress coughing in
traditional Chinese medicine (TCM) treatments (12). Its clinical applications also
include anti-hepatitis, amenorrhea and leucorrhea therapy,
arthralgia therapy, and snake bite therapy (12–14).
In vitro and in vivo pharmacological studies have
demonstrated that the extract from P. cuspidatum has
anti-angiogenesis (15), anti-viral
(13,16), anti-microbial (17,18),
anti-inflammatory (19–21), and neuro-protective properties
(12,22). P. cuspidatum extract has
exhibited antiproliferative effects against various human cancer
cells of HL-60, A549, H1650, L-02, HepG2, SHZ-888 and MCF-7/ADM
cells (23–28). The methanol and ethyl acetate
extracts of P. cuspidatum have been revealed to trigger oral
cancer KB cell apoptosis through caspase-3 activation and the
regulation of specificity protein 1 (23). Although the various anticancer
effects of P. cuspidatum have been investigated, its
underlying mechanism of autophagy and apoptosis on drug-resistant
human oral cancer cells is still unclear. The present study aimed
to determine the mechanisms of autophagy and apoptosis induced by
the ethanol extract of P. cuspidatum (PCE) in
cisplatin-resistant human oral cancer CAR cells.
Materials and methods
Plant extraction procedures and
analytic method
Dried Polygonum cuspidatum Sieb. et Zucc.
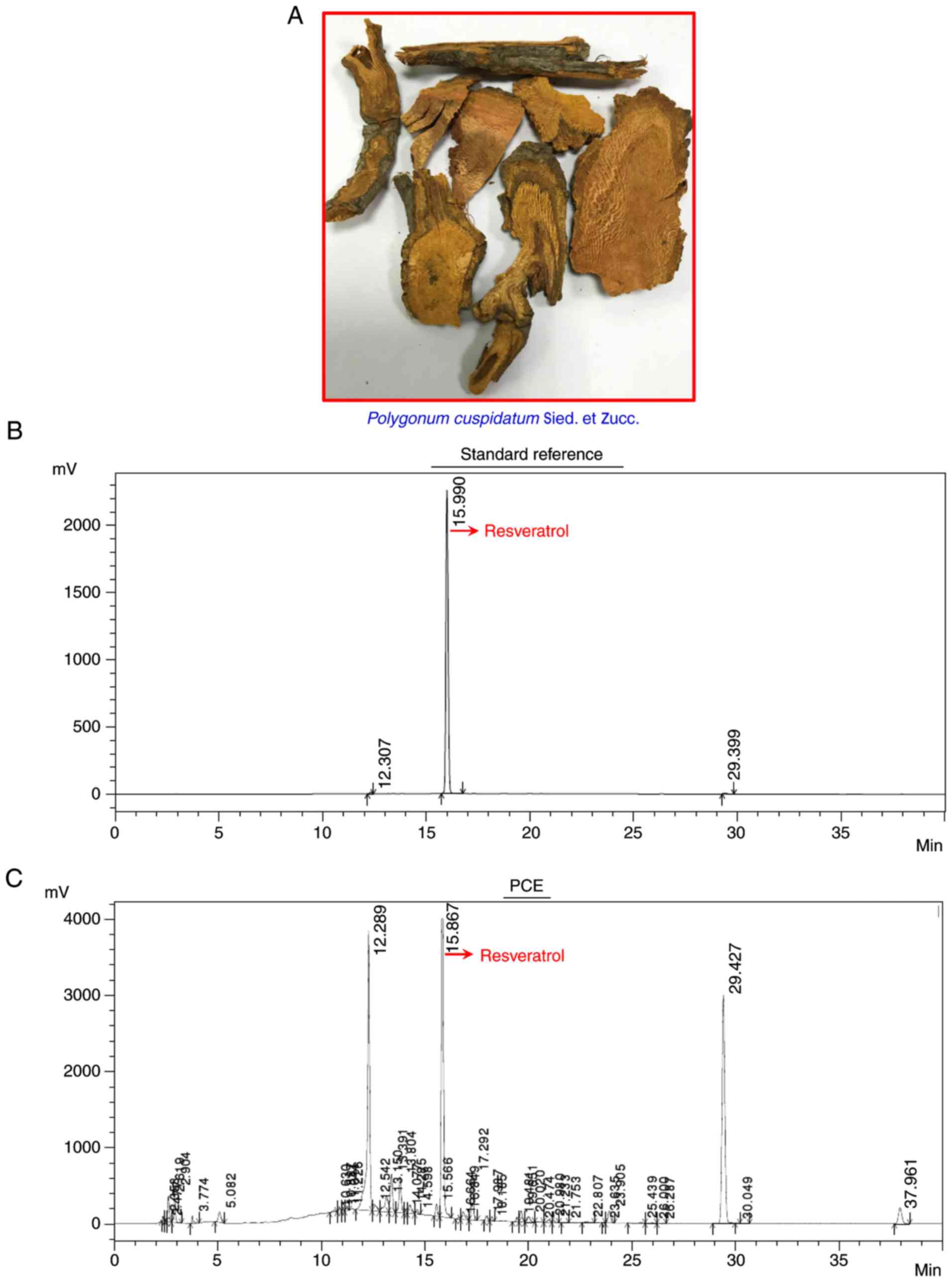
(Fig. 1A) was obtained from a
traditional Chinese medicine drugstore in Taichung, Taiwan. The
sample was powdered to a homogeneous size through a 60-mesh filter
in a mill and then sieved. The powder (100 g) was extracted twice
by 75% ethanol for 60 min under reflux at room temperature. The
ethanol extract of P. cuspidatum (PCE) was obtained by
combining the filtrates dried in a vacuum at 45°C, and then
collecting 40.32 g of brownish viscous residue, as previously
described (29). The analysis was
carried out via high-performance liquid chromatography (HPLC)
analysis, as previously described (30,31),
using a Shimadzu LC-20A system consisting of a CBM-20A HPLC pump, a
FRC-10A autosampler, UV and PDA detectors, and Merck Purospher STAR
RP-18 end-capped 250–4.6 mm (5 µm) column. Chromatograms were
monitored at 280 nm using a UV detector. H2O (containing
0.2% formic acid) was used as solvent A and acetonitrile was used
as solvent B. Following the injection of 10 µl of the sample, the
flow rate of the mobile phases was maintained at 1 ml/min. A linear
HPLC gradient was employed: i) 0.0–10.0 min linear gradient from 10
to 40% of solvent B; ii) 10.0–32.0 min linear gradient from 40 to
85% of solvent B; iii) 32.0–40.0 min isocratic at 85% of solvent B.
Furthermore, the PCE was re-suspended and dissolved in dimethyl
sulfoxide (DMSO) and used for further in vitro
experiments.
Chemicals and reagents
Dulbecco's modified Eagle's medium (DMEM), DMEM/F12
1:1 medium, fetal bovine serum (FBS), L-glutamine, and
penicillin/streptomycin were purchased from HyClone; GE Healthcare
Life Sciences (Logan, UT, USA). Cisplatin, DMSO,
monodansylcadaverine (MDC), thiazolyl blue tetrazolium bromide
(MTT), and resveratrol were obtained from Sigma-Aldrich; Merck KGaA
(Darmstadt, Germany). Acridine orange (AO),
4′,6-diamidino-2-phenylindole (DAPI), LysoTracker Red DND-99, and
trypsin-EDTA were obtained from Thermo Fisher Scientific, Inc.
(Waltham, MA, USA). The primary antibodies [anti-Atg5 (cat. no.
GTX113309), anti-Atg7 (cat. no. GTX113613), anti-Atg12 (cat. no.
GTX629815), anti-Beclin-1 (cat. no. GTX631396), anti-LC3 (cat. no.
GTX39752), anti-Bax (cat. no. GTX109683), anti-Bcl-2 (cat. no.
GTX100064), anti-caspase-3 (cat. no. GTX110543) (all 1:1,000
dilution) and anti-β-actin (cat. no. GTX109639) (1:5,000 dilution)]
and the anti-rabbit (cat. no. GTX213110-01) or anti-mouse (cat. no.
GTX213111-01) IgG-horseradish peroxidase (HRP) secondary antibodies
(1:10,000 dilution) were all purchased from GeneTex International
Corporation (Hsinchu, Taiwan).
Cell culture
The cisplatin-resistant CAR cells were established
as previous methods (3,32–39).
The parental human tongue squamous cell carcinoma CAL 27 cell line
was obtained from American Type Culture Collection (ATCC; Manassas,
VA, USA) after increasing exposure to 10–80 µM of cisplatin for 10
cycles and at least stably resistant to 80 µM cisplatin. CAR cells
were cultured in DMEM with 10% FBS, 100 U/ml penicillin, 100 µg/ml
streptomycin, 2 mM L-glutamine and 80 µM cisplatin. Normal human
primary gingival fibroblast (HGF) was purchased from CLS Cell Lines
Service GmbH (Eppelheim, Germany) and cultivated in DMEM/F12 1:1
medium with 10% FBS, 100 µg/ml streptomycin, 100 U/ml penicillin
and 2 mM L-glutamine. All cells were cultured in a 37°C humidified
incubator with 5% CO2.
Cell viability assay and morphological
changes
CAR or HGF cells (1×104 cells/well) were
plated on 96-well plates and exposed to 50, 100, 150 and 200 µg/ml
of PCE for 24 and 48 h. At the end of treatment, each medium
containing 500 µg/ml MTT solution was added for an additional 3 h
before the medium was discarded from each well. The blue formazan
product was dissolved by 100 µl DMSO, and the optical density was
spectrophotometrically detected at an absorbance of 570 nm, as
previously described (40,41). Cell morphological changes
(autophagic vacuoles and apoptotic characteristics) were visualized
and photographed via a phase-contrast microscope, as previously
described (40,42).
Autophagy assays
CAR cells (5×104 cells/ml) were plated on
sterile coverslips in tissue culture plates and then treated with
150 µg/ml PCE for 24 h. Cells were then individually stained with
either 1 µg/ml AO, 100 µM MDC, and 1 µg/ml LysoTracker Red DND-99
for 15 min, as previously described (35,36).
The autophagy marker LC3 was detected via the Premo Autophagy
Sensor LC3B-GFP (BacMam 2.0) Kit (Thermo Fisher Scientific, Inc.)
following the manufacturer's protocol. The fluorescent images were
immediately monitored and photographed by fluorescence microscopy
(Nikon Corp., Melville, NY, USA).
Apoptosis assay
CAR cells (2×105 cells/well) plated on
12-well plates were incubated with 150 µg/ml PCE. After exposure
for 24 h, TdT-mediated dUTP-X nick-end labeling (TUNEL) positive
cells were determined via the In Situ Cell Death Detection kit,
Fluorescein (Roche, Sigma-Aldrich; Merck KGaA) according to
manufacturer's instructions. The cell image was photographed using
a fluorescence microscope after being counterstained with 1 µg/ml
DAPI dye, as previously described (3).
Caspase-3 and −9 activity assays
CAR cells (5×106 cells/75T flask) were
treated with or without 150 µg/ml PCE for 12 and 24 h. Cell lysates
were collected, and the supernatant was incubated in the supplied
reaction buffer with dithiothreitol and the caspase-specific
substrates [Asp-Glu-Val-Asp (DEAD) for caspase-3; Leu-Glu-His-Asp
(LEHD) for caspase-9] labeled with p-nitroaniline (pNA) at 37°C for
2 h in the dark following the manufacturer's protocols (Caspase-3
and Caspase-9 Colorimetric Assay Kits; R&D Systems, Inc.,
Minneapolis, MN, USA).
Western blotting
CAR cells (5×106 cells/75T flask) were
exposed to 0, 150 and 200 µg/ml PCE for 24 h. Whole-cell lysates
were isolated with Trident RIPA Lysis Buffer (GeneTex International
Corp.), and the protein concentration was quantified using Pierce
BCA Protein Assay Kit (Thermo Fisher Scientific, Inc.). Equal
amounts (40 µg) of protein samples were separated using 10–12%
SDS-PAGE, as previously described (42). The separated protein was transferred
to an Immobilon-P Transfer Membrane (EMD Millipore, Billerica, MA,
USA) via use of electroblotting. The membranes were soaked in 5%
skim milk and individually incubated with the primary antibodies,
including Atg5, Atg7, Atg12, Beclin-1, LC3, Bax, Bcl-2, caspase-3
and β-actin overnight at 4°C, as well as the appropriate
horseradish peroxidase-conjugated secondary antibodies for 1 h at
room temperature to hybridize targeted protein using an Immobilon
Western Chemiluminescent HRP Substrate (EMD Millipore). All bands
were normalized to the level of β-actin for each lane, and their
densitometric quantification was carried out using NIH ImageJ 1.47
software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
All data were reported as the mean ± standard
deviation (SD) of triplicate samples. The significant differences
of data were subjected to one-way analysis of variance (ANOVA)
followed by Dunnett's test using SPSS software version 16.0 (SPSS,
Inc., Chicago, IL, USA). A P-value of <0.05 was considered to
indicate a statistically significant difference.
Results
Resveratrol is one of the major
compounds in PCE
The result from the HPLC analysis revealed that the
major peak of the standard reference (resveratrol) appeared at
15.990 min (Fig. 1B). In addition,
the data indicated that PCE had several peaks at various retention
time intervals, indicating that PCE possessed multiple components.
The retention time of the peak at 15.867 min was identified as
resveratrol (Fig. 1C). Therefore,
resveratrol may be one of the major compounds in PCE.
PCE inhibits cell viability in
cisplatin-resistant human oral cancer CAR cells
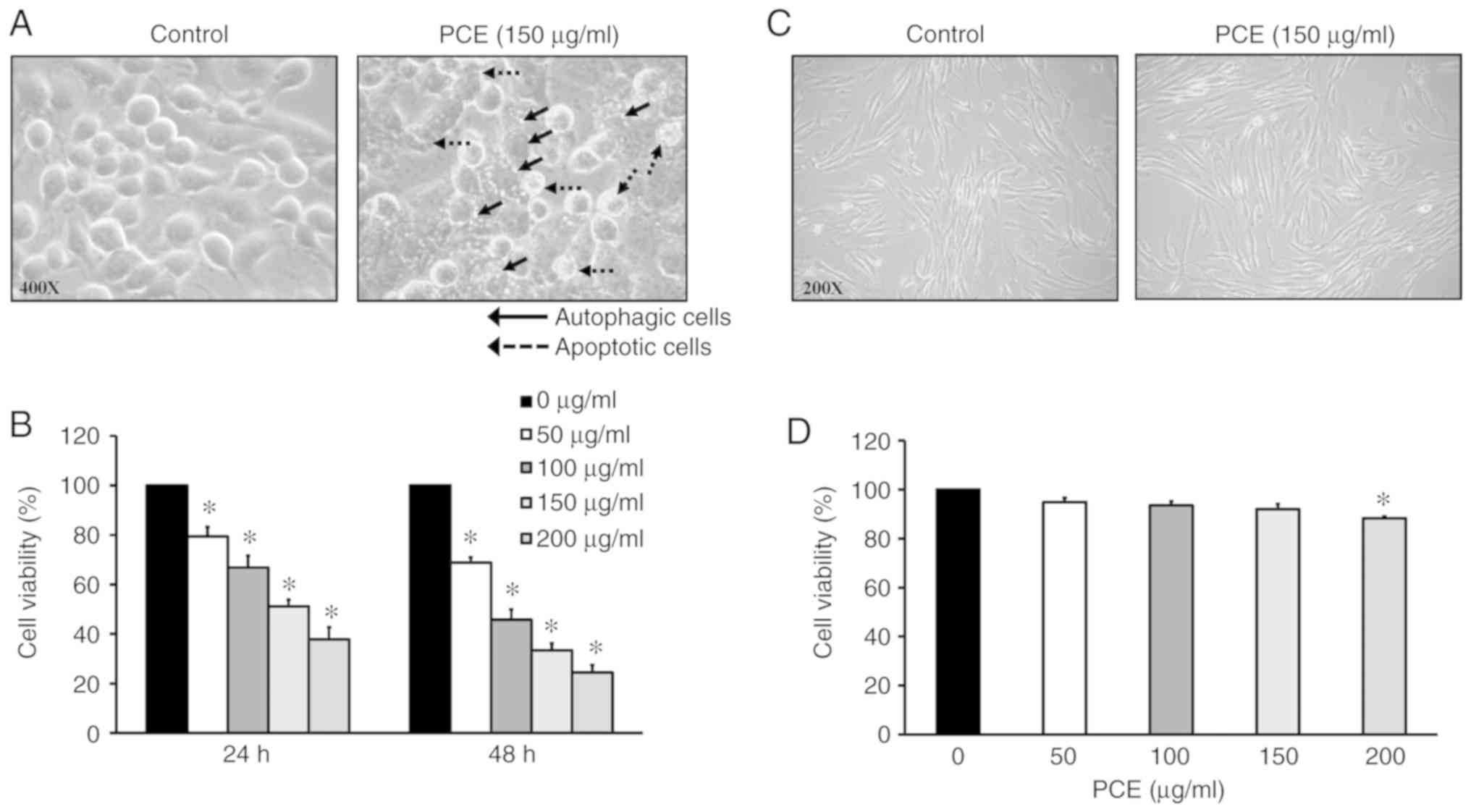
PCE promoted the formation of autophagic vacuoles
and apoptotic bodies after exposure to 150 µg/ml PCE for 24 h in
CAR cells (Fig. 2A). PCE also
reduced viable CAR cells in a time- and concentration-dependent
manner (Fig. 2B). These findings
indicated that autophagy and apoptotic mechanisms may be present in
PCE-treated CAR cells. Notably, no morphological changes (Fig. 2C) and cytotoxic effects (Fig. 2D) were found on normal HGF cells.
Thus, PCE exerted lower cytotoxicity in normal oral cells and
triggered autophagic and apoptotic mechanisms in CAR cells.
PCE induces autophagy in CAR
cells
To determine PCE-induced autophagy, CAR cells were
incubated with different concentrations of PCE for 24 h, and then
monitored for the formation of acidic vesicular organelles (AVOs)
and a punctate pattern of LC3. PCE increased the red fluorescence
intensity in the cytoplasm compared to the control cells via AO
staining, indicating that PCE led to AVO occurrence (Fig. 3A). PCE also caused the punctate
pattern of LC3-GFP in CAR cells (Fig.
3B). In addition, autophagic evidence was also demonstrated in
cells probed with MDC and LysoTracker Red, respectively. Our
results revealed that autophagic vacuoles and lysosome activity
were individually observed in PCE-treated CAR cells, and the
fluorescence intensity of MDC (green fluorescence) (Fig. 3C) and LysoTracker Red (red
fluorescence) (Fig. 3D) staining
was directly proportional to CAR cells at 150 µg/ml PCE exposure.
Therefore, PCE triggered an autophagic response in CAR cells.
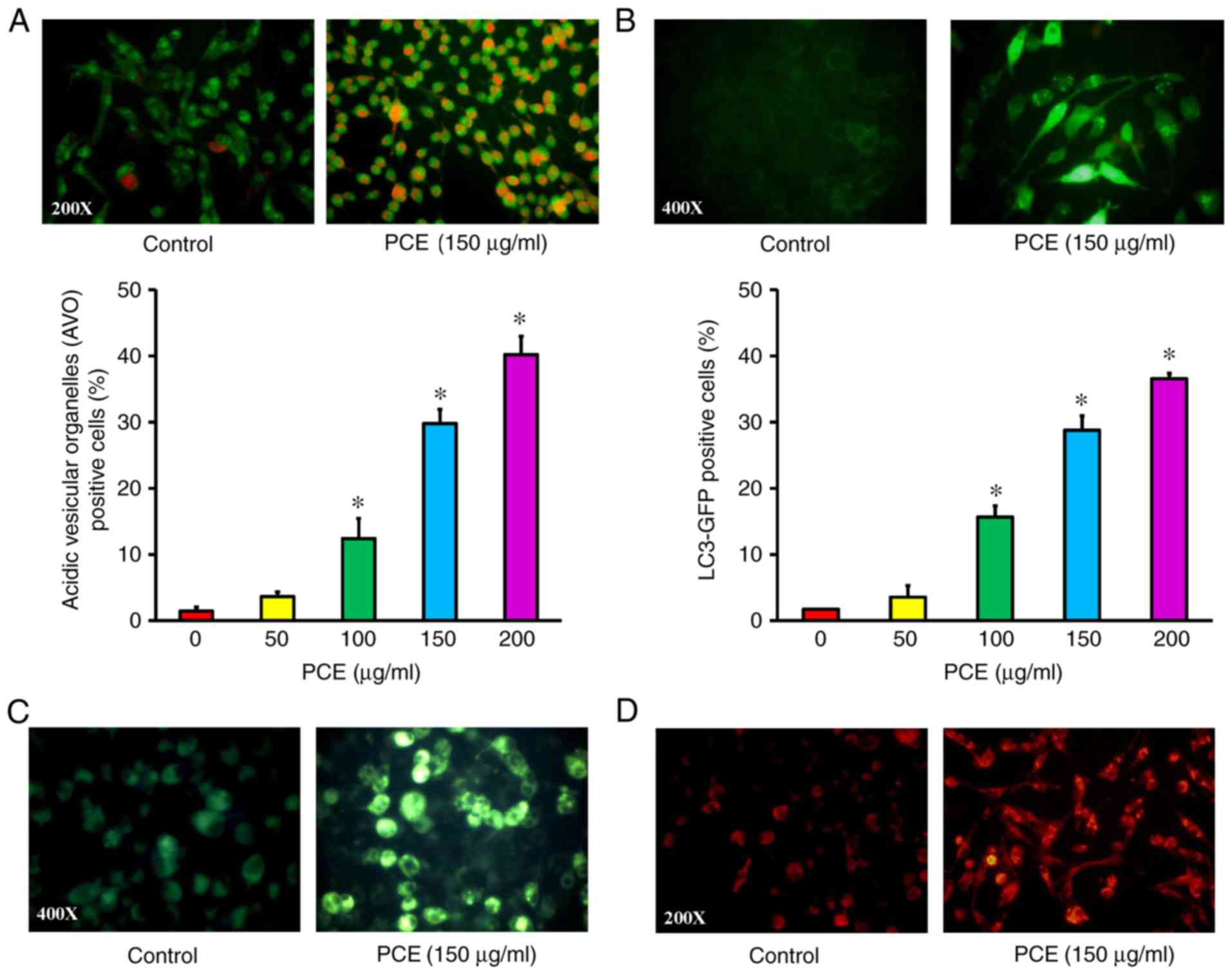 | Figure 3.Effect(s) of PCE on autophagy of CAR
cells. Cells were exposed to 0, 50, 100, 150 and 200 µg/ml of PCE
for 24 h and then were harvested. (A) AO staining was used to
detect AVOs (red fluorescence) (at ×200 magnifications), and the
data was quantified. (B) An LC3B-GFP kit was applied to monitor
LC3B expression (green fluorescence) (at ×400 magnifications), and
the data was quantified. The results are expressed as the mean ± SD
(n=3). *P<0.05 vs. untreated control. Cells were with or without
150 µg/ml PCE exposure for 24 h. Cells were individually stained
with (C) MDC dye (green fluorescence) (at ×400 magnifications) and
(D) LysoTracker Red DND-99 (red fluorescence) (at ×200
magnifications) to detect autophagic vacuoles and lysosomal enzyme
activity, respectively, as described in the Materials and methods.
PCE, P. cuspidatum extract; AO, acridine orange; AVOs, acidic
vesicular organelles; MDC, monodansylcadaverine. |
PCE induces the apoptotic process in
CAR cells
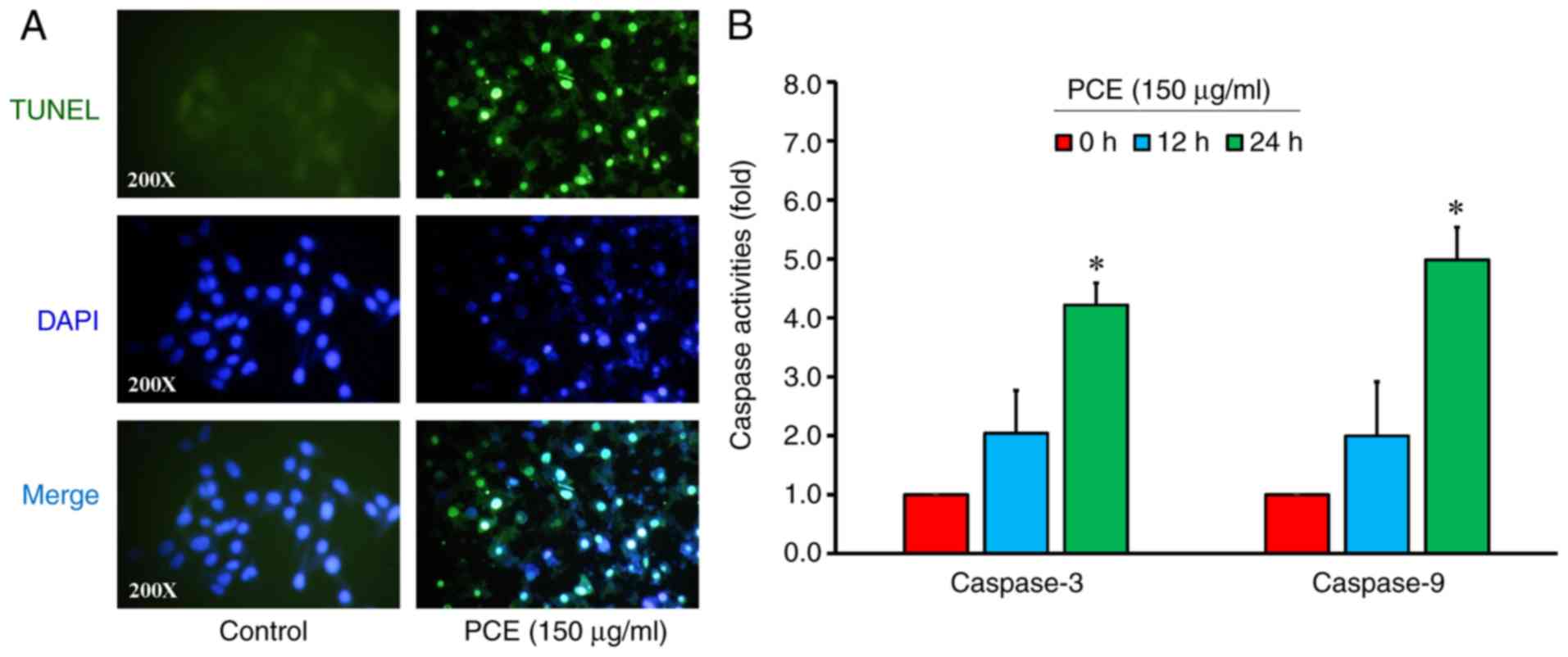
To assess CAR cell apoptosis induced by PCE, the
cells were treated with or without 150 µg/ml PCE for 24 h and
detected by TUNEL/DAPI staining and caspase-3/-9 activity assays.
PCE at 150 µg/ml markedly stimulated apoptotic DNA breaks (TUNEL
positive) and elicited the occurrence of DNA fragmentation and
condensation in CAR cells (Fig.
4A). To further confirm caspase-3 and caspase-9 signaling in
PCE-treated CAR cells, the activity of caspase-3 and caspase-9 was
detected. Our data revealed that PCE at 150 µg/ml for 24 h
increased the activities of caspase-3 and caspase-9 (Fig. 4B) in CAR cells. The results
indicated that PCE-triggered apoptosis resulted from the intrinsic
pathway (caspase-3/-9-dependent) in CAR cells.
PCE regulates autophagy- and
apoptosis-related protein signaling in CAR cells
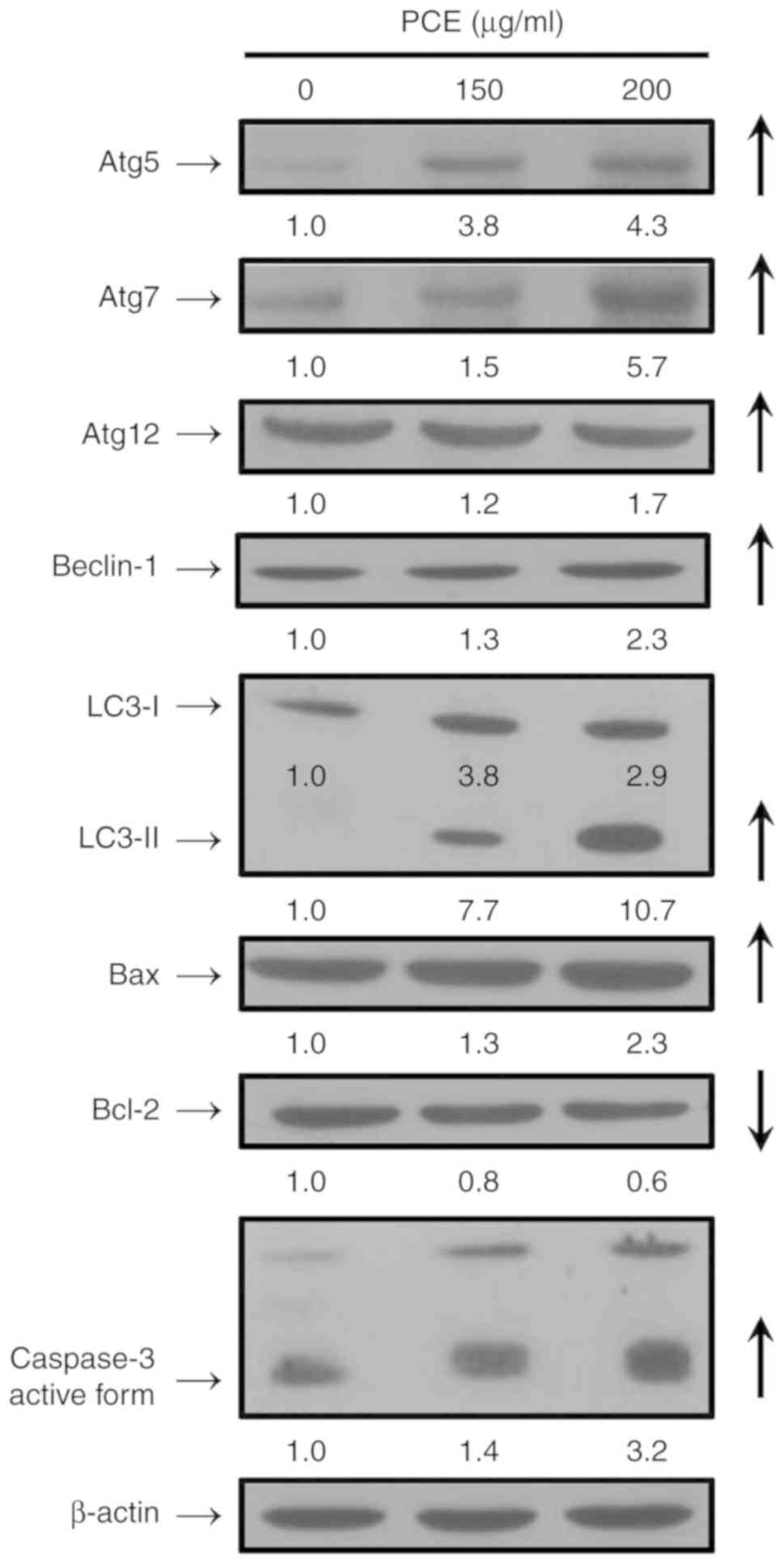
PCE at 150 and 200 µg/ml for 24 h increased the
protein levels of Atg5, Atg7, Atg12, Beclin-1, and LC3-II in CAR
cells (Fig. 5). Based on the key
activation of autophagy markers, it is suggested that PCE induced
autophagy in CAR cells. Moreover, after treatment with 150 and 200
µg/ml of PCE, the protein levels of Bax and active form of
caspase-3 were upregulated, while the level of Bcl-2 was
downregulated in CAR cells (Fig.
5). Our findings demonstrated that PCE induced apoptotic death
via a mitochondria-dependent pathway in CAR cells.
Discussion
Natural products and traditional Chinese medicine
(TCM) have exhibited anticancer activities and low toxicity, and
they have been investigated for anticancer activities for some time
now (32,43,44).
P. cuspidatum (Hu Zhang) is a TCM that has been studied for
the treatment of various types of cancers, inflammatory diseases,
hepatitis virus infection, HIV, and diarrhea (12–14).
It has been demonstrated that physcion, emodin, and resveratrol are
the main phytochemicals extracted from the P. cuspidatum
fraction (12–14,20,45).
P. cuspidatum is a major and abundant resource of
resveratrol since the average content of it is ~1–3 mg/g (13). Our data (Fig. 1B and C) were also consistent with
previous studies (13,30). Resveratrol has a wide spectrum of
pharmacological activities such as antioxidant, anti-inflammatory,
anti-atherosclerotic, and anticancer effects (46–50).
Our previous study reported that resveratrol triggered autophagy
and apoptosis in CAR cells (3).
Herein, the present study is the first to the best of our
knowledge, to report that PCE induced autophagy and apoptosis of
CAR cells. The cisplatin-resistant subline CAR cells were
originally from a human tongue cancer cell line CAL 27 and
established using the method of increasing the concentration of
cisplatin (to at least 80 µM) according to a previously described
method (3,32–37).
Furthermore, the differences from the parental CAL 27 cells and CAR
cells were investigated. Our findings revealed that CAR cells were
resistant to 80 µM cisplatin compared with the parental CAL 27
cells (3,32,34).
Notably, multidrug resistance protein 1 (MDR1) revealed a higher
expression in CAR cells than in parental CAL 27 cells. Thus far,
CAR cells have been a drug-resistant cell platform to test various
phytochemicals, traditional Chinese medicine, and novel compounds
(3,32–37,39).
In the present study, it was firstly demonstrated that PCE reduced
cell proliferation in CAR cells (Fig.
2). The half maximal inhibitory concentration (IC50)
for 24 and 48 h treatment of PCE in CAR cells were 162.89±6.28 and
110.34±8.21 µg/ml, respectively. PCE exhibited low toxicity to
normal HGF cells (IC50 >200 µg/ml) (Fig. 2). Additionally, our preliminary data
indicated that PCE reduced cell viability of the parental CAL 27
cells after 48-h treatment, and the IC50 value was
188.39±4.21 µg/ml (data not shown). As a result, PCE induced more
selective cytotoxicity in human cisplatin-resistant oral cancer CAR
cells while having a low toxicity to normal cells.
Autophagy and apoptosis are the major routes that
lead to cell death (2,5). Our study was undertaken to demonstrate
that PCE can induce the formation of autophagic vesicle and
apoptotic bodies (Fig. 2A). It was
suggested at the outset that PCE may induce CAR cell death through
autophagy and apoptotic pathways. The characteristic of autophagy
is to increase autophagosome accumulation in the cytoplasm of cells
(5,6,51). The
method used for monitoring autophagy in autophagosomes and
lysosomal enzyme activity was examining the uptake of fluorescent
dyes, such as acridine orange (AO), monodansylcadaverine (MDC), and
LysoTracker Red. Furthermore, autophagy-related proteins such as
ATGs and LC3 were also detected (4,49,51).
In the present study, the results from AO (Fig. 3A), LC3-GFP (Fig. 3B), and MDC staining (Fig. 3C) indicated that PCE induced the
formation of autophagic vesicles in a concentration-dependent
manner in CAR cells. The LysoTracker Red staining was also used to
assess lysosome activity following treatment with PCE (Fig. 3D). PCE also increased the protein
level of autophagic proteins, including Atg5, Atg7, Atg12,
Beclin-1, and LC3 (Fig. 5) in CAR
cells. Notably, 3-methyladenine (3-MA), a specific inhibitor of
PI3K kinase class III, inhibited the autophagic vesicle formation
induced by PCE (data not shown). Our results support the notion
that PCE-induced CAR cell death was mediated through the induction
of autophagic death.
In the present study, it was revealed that PCE
triggered apoptotic morphological changes in CAR cells (Fig. 2A). PCE induced DNA condensation and
fragmentation (Fig. 4A).
PCE-induced apoptosis was confirmed by the increase of the activity
(Fig. 4B) and protein levels
(Fig. 5) of caspase-3 in CAR cells.
When the cells undergo apoptotic cell death, the ratio of Bax/Bcl-2
is enhanced leading to cytochrome c release from the
mitochondria (52). Cytochrome
c activates the caspase-9/-3 cascade to induce cell
apoptosis (10,52). PCE increased the protein level of
Bax and decreased the Bcl-2 signal (Fig. 5), indicating that the involvement of
the mitochondrial apoptotic pathway contributed to CAR cell death
after PCE treatment.
It has been reported that autophagy precedes
apoptosis in cervical cancer HeLa cells (53,54)
and colorectal DLD1, HT-29 and COLO 201 cells induced by
resveratrol (55–57), suggesting that resveratrol triggered
apoptotic cell death following the autophagy. Recently, in a
separate study, it was demonstrated that resveratrol had an
extremely low toxicity in normal HGF cells (3). Resveratrol induced autophagic CAR cell
death as observed in AO, LC3-GFP and MDC staining (3). Moreover, resveratrol increased the
autophagy-related protein levels (Atg5, Atg7, Atg12, Atg14,
Atg16L1, Beclin-1, PI3K class III and LC3), but it decreased
rubicon protein expression (3).
Resveratrol also induced apoptotic DNA fragmentation, elicited the
caspase-3/-9 activities, and increased the protein levels of
caspase-3 and −9, and Bax, while it decreased the protein level of
Bcl-2 (3,51). The present results are in agreement
with previous studies (3,51) in reiterating that resveratrol may be
a major autophagic inducer in PCE.
In conclusion, our results demonstrated that PCE
treatment of CAR cells induced reduction rather than enhancement of
autophagic degradation that led to apoptotic cell death. Thus, the
present study provided new insight and motivated us to forge ahead
regarding the action(s) of PCE and its molecular mechanism(s). PCE
could be used for its potential therapeutic use against
drug-resistant oral cancer in the near future.
Acknowledgements
Not applicable.
Funding
The present study was supported by funds from the
Kaohsiung Armed Forces General Hospital (grant no. 105-15).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
YLW, CTH, CCL and FAC conceived and designed the
experiments; MTH, HCC, YSH, JSY, GKW and JHC performed the
experiments. MTH, JSY, JHC, HHC and CCL analyzed the data; YLW,
CTH, CCL and FAC wrote and modified the manuscript. All authors
read and approved the manuscript and agree to be accountable for
all aspects of the research in ensuring that the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wei Y, Yang P, Cao S and Zhao L: The
combination of curcumin and 5-fluorouracil in cancer therapy. Arch
Pharm Res. 41:1–13. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Galluzzi L, Vitale I, Aaronson SA, Abrams
JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews
DW and et al: Molecular mechanisms of cell death: Recommendations
of the nomenclature committee on cell death 2018. Cell Death
Differ. 25:486–541. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chang CH, Lee CY, Lu CC, Tsai FJ, Hsu YM,
Tsao JW, Juan YN, Chiu HY, Yang JS and Wang CC: Resveratrol-induced
autophagy and apoptosis in cisplatin-resistant human oral cancer
CAR cells: A key role of AMPK and Akt/mTOR signaling. Int J Oncol.
50:873–882. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marinkovic M, Šprung M, Buljubašić M and
Novak I: Autophagy modulation in cancer: Current knowledge on
action and therapy. Oxid Med Cell Longev. 2018:80238212018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang JS, Lu CC, Kuo SC, Hsu YM, Tsai SC,
Chen SY, Chen YT, Lin YJ, Huang YC, Chen CJ and et al: Autophagy
and its link to type II diabetes mellitus. Biomedicine. 7:82017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang SJ, Yang W, Wang C, He WS, Deng HY,
Yan YG, Zhang J, Xiang YX and Wang WJ: Autophagy: A double-edged
sword in intervertebral disk degeneration. Clin Chim Acta.
457:27–35. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li YJ, Lei YH, Yao N, Wang CR, Hu N, Ye
WC, Zhang DM and Chen ZS: Autophagy and multidrug resistance in
cancer. Chin J Cancer. 36:522017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han Y, Fan S, Qin T, Yang J, Sun Y, Lu Y,
Mao J and Li L: Role of autophagy in breast cancer and breast
cancer stem cells (Review). Int J Oncol. 52:1057–1070.
2018.PubMed/NCBI
|
|
9
|
Chowdhury RM, Singh G, Joshi A, Singh DK,
Bhatia S and Goswami S: Autophagy and oral cancers: A short review.
J Stomatol Oral Maxillofac Surg. 119:37–39. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Campbell KJ and Tait SWG: Targeting BCL-2
regulated apoptosis in cancer. Open Biol. 8(pii): 1800022018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Adams JM and Cory S: The BCL-2 arbiters of
apoptosis and their growing role as cancer targets. Cell Death
Differ. 25:27–36. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang H, Li C, Kwok ST, Zhang QW and Chan
SW: A Review of the pharmacological effects of the dried root of
Polygonum cuspidatum (Hu Zhang) and its constituents. Evid Based
Complement Alternat Med. 2013:2083492013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peng W, Qin R, Li X and Zhou H: Botany,
phytochemistry, pharmacology, and potential application of
Polygonum cuspidatum Sieb. et Zucc.: A review. J Ethnopharmacol.
148:729–745. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nonomura S, Kanagawa H and Makimoto A:
Chemical constituents of polygonaceous plants. I. Studies on the
components of Ko-J O-Kon. (Polygonum cuspidatum Sieb. Et Zucc.).
Yakugaku Zasshi. 83:988–990. 1963.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang S, Zheng Z, Weng Y, Yu Y, Zhang D,
Fan W, Dai R and Hu Z: Angiogenesis and anti-angiogenesis activity
of Chinese medicinal herbal extracts. Life Sci. 74:2467–2478. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin CJ, Lin HJ, Chen TH, Hsu YA, Liu CS,
Hwang GY and Wan L: Polygonum cuspidatum and its active components
inhibit replication of the influenza virus through toll-like
receptor 9-induced interferon beta expression. PLoS One.
10:e01176022015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiewei T, Lei W, Xiufeng L, Heming Z,
Xiaoguang L, Haiyan F and Yongqiang T: Microbial transformation of
resveratrol by endophyte Streptomyces sp. A12 isolated from
Polygonum cuspidatum. Nat Prod Res. 32:2343–2346. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun W, Qu D, Ma Y, Chen Y, Liu C and Zhou
J: Enhanced stability and antibacterial efficacy of a traditional
Chinese medicine-mediated silver nanoparticle delivery system. Int
J Nanomedicine. 9:5491–5502. 2014.PubMed/NCBI
|
|
19
|
Sohn E, Kim J, Kim CS, Lee YM and Kim JS:
Extract of Polygonum cuspidatum attenuates diabetic retinopathy by
inhibiting the high-mobility group box-1 (HMGB1) signaling pathway
in streptozotocin-induced diabetic rats. Nutrients. 8:1402016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fan P, Zhang T and Hostettmann K:
Anti-inflammatory activity of the invasive neophyte Polygonum
cuspidatum Sieb. and Zucc. (Polygonaceae) and the chemical
comparison of the invasive and native varieties with regard to
resveratrol. J Tradit Complement Med. 3:182–187. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Han JH, Koh W, Lee HJ, Lee HJ, Lee EO, Lee
SJ, Khil JH, Kim JT, Jeong SJ and Kim SH: Analgesic and
anti-inflammatory effects of ethyl acetate fraction of Polygonum
cuspidatum in experimental animals. Immunopharmacol Immunotoxicol.
34:191–195. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shen B, Truong J, Helliwell R,
Govindaraghavan S and Sucher NJ: An in vitro study of
neuroprotective properties of traditional Chinese herbal medicines
thought to promote healthy ageing and longevity. BMC Complement
Altern Med. 13:3732013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shin JA, Shim JH, Jeon JG, Choi KH, Choi
ES, Cho NP and Cho SD: Apoptotic effect of Polygonum Cuspidatum in
oral cancer cells through the regulation of specificity protein 1.
Oral Dis. 17:162–170. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chang CJ, Ashendel CL, Geahlen RL,
McLaughlin JL and Waters DJ: Oncogene signal transduction
inhibitors from medicinal plants. In Vivo. 10:185–190.
1996.PubMed/NCBI
|
|
25
|
Owen HC, Appiah S, Hasan N, Ghali L,
Elayat G and Bell C: Phytochemical modulation of apoptosis and
autophagy: Strategies to overcome chemoresistance in leukemic stem
cells in the bone marrow microenvironment. Int Rev Neurobiol.
135:249–278. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiao Y, Wu Y and Du D: Polydatin inhibits
cell proliferation, invasion and migration, and induces cell
apoptosis in hepatocellular carcinoma. Braz J Med Biol Res.
51:e68672018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee KH, Song JL, Park ES, Ju J, Kim HY and
Park KY: Anti-obesity effects of starter fermented kimchi on 3T3-L1
adipocytes. Prev Nutr Food Sci. 20:298–302. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu J, Zhang Q, Chen K, Liu J, Kuang S,
Chen W and Yu Q: Small-molecule STAT3 signaling pathway modulators
from Polygonum cuspidatum. Planta Med. 78:1568–1570. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chang JS, Liu HW, Wang KC, Chen MC, Chiang
LC, Hua YC and Lin CC: Ethanol extract of Polygonum cuspidatum
inhibits hepatitis B virus in a stable HBV-producing cell line.
Antiviral Res. 66:29–34. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun P, Qi Y, Mu Z and Wang K: Quantitative
determination of resveratrol in Polygonum cuspidatum and its
anti-proliferative effect on melanoma A375 cells. Biomed Res.
26:750–754. 2015.
|
|
31
|
Lu CC, Yang SH, Hsia SM, Wu CH and Yen GC:
Inhibitory effects of Phyllanthus emblica L. on hepatic steatosis
and liver fibrosis in vitro. J Funct Foods. 20:20–30. 2016.
View Article : Google Scholar
|
|
32
|
Chang HP, Lu CC, Chiang JH, Tsai FJ, Juan
YN, Tsao JW, Chiu HY and Yang JS: Pterostilbene modulates the
suppression of multidrug resistance protein 1 and triggers
autophagic and apoptotic mechanisms in cisplatin-resistant human
oral cancer CAR cells via AKT signaling. Int J Oncol. Mar
2–2018.(Epub ahead of print). doi: 10.3892/ijo.2018.4298.
View Article : Google Scholar
|
|
33
|
Chiu YJ, Yang JS, Hsu HS, Tsai CH and Ma
H: Adipose-derived stem cell conditioned medium attenuates
cisplatin-triggered apoptosis in tongue squamous cell carcinoma.
Oncol Rep. 39:651–658. 2018.PubMed/NCBI
|
|
34
|
Lee MR, Lin C, Lu CC, Kuo SC, Tsao JW,
Juan YN, Chiu HY, Lee FY, Yang JS and Tsai FJ: YC-1 induces G0/G1
phase arrest and mitochondria-dependent apoptosis in
cisplatin-resistant human oral cancer CAR cells. Biomedicine.
7:122017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yuan CH, Horng CT, Lee CF, Chiang NN, Tsai
FJ, Lu CC, Chiang JH, Hsu YM, Yang JS and Chen FA: Epigallocatechin
gallate sensitizes cisplatin-resistant oral cancer CAR cell
apoptosis and autophagy through stimulating AKT/STAT3 pathway and
suppressing multidrug resistance 1 signaling. Environ Toxicol.
32:845–855. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hsieh MT, Chen HP, Lu CC, Chiang JH, Wu
TS, Kuo DH, Huang LJ, Kuo SC and Yang JS: The novel pterostilbene
derivative ANK-199 induces autophagic cell death through regulating
PI3 kinase class III/beclin 1/Atg-related proteins in
cisplatin-resistant CAR human oral cancer cells. Int J Oncol.
45:782–794. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chang PY, Peng SF, Lee CY, Lu CC, Tsai SC,
Shieh TM, Wu TS, Tu MG, Chen MY and Yang JS: Curcumin-loaded
nanoparticles induce apoptotic cell death through regulation of the
function of MDR1 and reactive oxygen species in cisplatin-resistant
CAR human oral cancer cells. Int J Oncol. 43:1141–1150. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gosepath EM, Eckstein N, Hamacher A,
Servan K, von Jonquieres G, Lage H, Györffy B, Royer HD and Kassack
MU: Acquired cisplatin resistance in the head-neck cancer cell line
Cal27 is associated with decreased DKK1 expression and can
partially be reversed by overexpression of DKK1. Int J Cancer.
123:2013–2019. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen CF, Yang JS, Chen WK, Lu CC, Chiang
JH, Chiu HY, Tsai SC, Juan YN, Huang HJ and Way TD: Ursolic acid
elicits intrinsic apoptotic machinery by downregulating the
phosphorylation of AKT/BAD signaling in human cisplatin-resistant
oral cancer CAR cells. Oncol Rep. 40:1752–1760. 2018.PubMed/NCBI
|
|
40
|
Lu CC, Yang JS, Chiang JH, Hour MJ, Lin
KL, Lee TH and Chung JG: Cell death caused by quinazolinone HMJ-38
challenge in oral carcinoma CAL 27 cells: Dissections of
endoplasmic reticulum stress, mitochondrial dysfunction and tumor
xenografts. Biochim Biophys Acta. 1840:2310–2320. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lu CC, Huang BR, Liao PJ and Yen GC:
Ursolic acid triggers nonprogrammed death (necrosis) in human
glioblastoma multiforme DBTRG-05MG cells through MPT pore opening
and ATP decline. Mol Nutr Food Res. 58:2146–2156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chiang JH, Yang JS, Lu CC, Hour MJ, Chang
SJ, Lee TH and Chung JG: Newly synthesized quinazolinone HMJ-38
suppresses angiogenetic responses and triggers human umbilical vein
endothelial cell apoptosis through p53-modulated Fas/death receptor
signaling. Toxicol Appl Pharmacol. 269:150–162. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chang CH, Wu JB, Yang JS, Lai YJ, Su CH,
Lu CC and Hsu YM: The suppressive effects of geniposide and genipin
on helicobacter pylori infections in vitro and in vivo. J Food Sci.
82:3021–3028. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ho TJ, Jiang SJ, Lin GH, Li TS, Yiin LM,
Yang JS, Hsieh MC, Wu CC, Lin JG and Chen HP: The in vitro and in
vivo wound healing properties of the Chinese herbal medicine
‘Jinchuang Ointment’. Evid Based Complement Alternat Med.
2016:16540562016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang C, Yang S, Lu H, You H, Ni M, Shan W,
Lin T, Gao X, Chen H, Zhou Q, et al: A natural product from
Polygonum cuspidatum sieb. Et Zucc. Promotes Tat-dependent HIV
latency reversal through triggering P-TEFb's release from 7SK
snRNP. PLoS One. 10:e01427392015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lange KW and Li S: Resveratrol,
pterostilbene, and dementia. Biofactors. 44:83–90. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Rauf A, Imran M, Butt MS, Nadeem M, Peters
DG and Mubarak MS: Resveratrol as an anti-cancer agent: A review.
Crit Rev Food Sci Nutr. 58:1428–1447. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nunes S, Danesi F, Del Rio D and Silva P:
Resveratrol and inflammatory bowel disease: The evidence so far.
Nutr Res Rev. 31:85–97. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jardim FR, de Rossi FT, Nascimento MX, da
Silva Barros RG, Borges PA, Prescilio IC and de Oliveira MR:
Resveratrol and brain mitochondria: A review. Mol Neurobiol.
55:2085–2101. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Perez-Vizcaino F and Fraga CG: Research
trends in flavonoids and health. Arch Biochem Biophys. 646:107–112.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lin W and Xu G: Autophagy: A role in the
apoptosis, survival, inflammation, and development of the retina.
Ophthalmic Res. 1–8. 2018.(Epub ahead of print).
|
|
52
|
Peña-Blanco A and García-Sáez AJ: Bax, Bak
and beyond-mitochondrial performance in apoptosis. FEBS J.
285:416–431. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Garcia-Zepeda SP, Garcia-Villa E,
Díaz-Chávez J, Hernández- Pando R and Gariglio P: Resveratrol
induces cell death in cervical cancer cells through apoptosis and
autophagy. Eur J Cancer Prev. 22:577–584. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hsu KF, Wu CL, Huang SC, Wu CM, Hsiao JR,
Yo YT, Chen YH, Shiau AL and Chou CY: Cathepsin L mediates
resveratrol-induced autophagy and apoptotic cell death in cervical
cancer cells. Autophagy. 5:451–460. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hong EH, Heo EY, Song JH, Kwon BE, Lee JY,
Park Y, Kim J, Chang SY, Chin YW, Jeon SM, et al: Trans-scirpusin A
showed antitumor effects via autophagy activation and apoptosis
induction of colorectal cancer cells. Oncotarget. 8:41401–41411.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Talero E, Ávila-Roman J and Motilva V:
Chemoprevention with phytonutrients and microalgae products in
chronic inflammation and colon cancer. Curr Pharm Des.
18:3939–3965. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Trincheri NF, Follo C, Nicotra G,
Peracchio C, Castino R and Isidoro C: Resveratrol-induced apoptosis
depends on the lipid kinase activity of Vps34 and on the formation
of autophagolysosomes. Carcinogenesis. 29:381–389. 2008. View Article : Google Scholar : PubMed/NCBI
|