Introduction
Gastric cancer (GC) is the third major cause of
cancer-associated fatality in Japan (1). The prognosis has improved because of
early diagnosis and surgical treatment, followed by chemotherapy
and molecular targeting therapy using anti-human epidermal growth
factor receptor 2 antibody and anti-programmed death-ligand 1
antibody (2). However, the
prognosis of GC at an advanced stage is still unfavorable (3). New strategies for the diagnosis and
treatment of GC are therefore required.
Protein disulfide isomerase A3 (PDIA3), which is
also known as GRP58/ERp57, is a chaperone protein that supports the
folding and processing of protein synthesized in the endoplasmic
reticulum (4). PDIA3 is involved in
multiple biological functions, including stabilization of
receptors, antigen processing and presentation, and degradation of
proteins (5). It has also been
indicated that PDIA3 is involved in the proliferation and cell
death in human carcinomas (6). High
expression levels of PDIA3 are associated with poor prognosis of
laryngeal cancer, hepatocellular carcinoma and diffuse glioma
(7–9). However, high expression levels of
PDIA3 are also associated with a favorable prognosis of uterine
cervical cancer (10). Regarding
GC, it has been reported that high expression of PDIA3 is
associated with favorable prognosis in GC at early stage; however,
an association has not been identified in GC at the advanced stage
(11). The association of PDIA3
with prognosis of GC in Japanese cases is not known. Furthermore,
the mechanism of favorable prognosis in GC with high expression of
PDIA3 remains undetermined.
In the present study, the association of PDIA3
expression with clinicopathological features, including prognosis,
was investigated in Japanese cases of GC. A possible mechanism of
how PDIA3 affects the prognosis of GC was also suggested.
Materials and methods
Cases of GC
Cases of GC were retrieved from the archives of the
pathology records of Nippon Medical School Hospital (Tokyo, Japan)
between January 2006 and December 2008. A total of 52 cases were
used for the study. The cases were randomly selected from the cases
of GC. The patients did not receive chemotherapy or radiation prior
to the surgery. Clinical information and the time of death
following surgery were retrieved from the clinical records. The
study was conducted according to the declaration of Helsinki and
the Japanese Society of Pathology. The study was approved by the
Ethics Committee of the Nippon Medical School Hospital (Tokyo,
Japan; no. 29-06-764). Informed consent was obtained from all
patients.
Histological examination of GC
The histology of the pathological specimens of GC
was reviewed by three individuals. Resected stomachs were fixed in
10% formalin at room temperature for 24 h and processed to be
embedded in paraffin. Three µm-thick sections of the entire area of
the carcinoma were stained with hematoxylin and eosin (stained for
5 min with hematoxylin and 3 min with eosin at room temperature)
and observed using microscope (ECLIPSE 50i; Nikon Corp., Tokyo,
Japan). The histological subtype was determined as the predominant
histology of the carcinoma tissue according to the classification
indicated by Laurén (12). The
depth of the invasion and lymph node metastasis were also evaluated
(13). The pathological stage was
classified according to the classification of the Union for
International Cancer Control (UICC) (14). Helicobacter pylori infection
state was evaluated with 3 µm-thick sections stained with
hematoxylin and eosin and viewed using a microscope (ECLIPSE 50i;
Nikon Corp.) at high magnification (×1,000). The infection state
was also considered positive if the serum was positive for
anti-Helicobacter pylori antibody.
Immunohistochemistry and
semi-quantitative evaluation
Immunohistochemistry was conducted using a
polymer-based two-step method. Briefly, 3-µm-thick paraffin
sections were deparaffinized and hydrated in phosphate-buffered
saline (PBS). Sections were pretreated in Tris-HCl (pH 9.0) for
PDIA3 and citrate buffer (pH 6.0) for Ki-67 at 121°C for 15 min.
Endogenous peroxidase was blocked in 3% H2O2
in methanol at room temperature for 30 min. The sections were
subsequently incubated with mouse anti-PDIA3 antibody (cat. no.
ab13506; dilution, 1:2,000; Abcam K.K., Tokyo, Japan) and mouse
anti-Ki-67 antibody (cat. no. MIB1; dilution, 1:100; Agilent
Technologies Japan, Ltd., Tokyo, Japan) overnight at 4°C. Following
this, the sections were incubated with Histofine Simple Stain
MAX-PO (M) (Nichirei Bioscience Inc., Tokyo, Japan) at room
temperature for 30 min. The peroxidase activity was visualized
using diaminobenzidine at room temperature for 2 min.
The intensity and proportion of stained carcinoma
cells were scored using a semi-quantitative method (15). The immunostained sections were
observed using a microscope (ECLIPSE 50i; Nikon Corp.) at a
magnification of ×400. Cytoplasmic staining was considered to
indicate a positive reaction. The intensity score was divided into
four grades as follows: no staining, score of 0; moderate staining
similar to that of the cells in the glands with intestinal
metaplasia (IM), score of 2; clear but weaker staining than that of
the metaplastic cells, score of 1; and stronger staining than that
of these cells, score of 3. Furthermore, the proportion of positive
cells of each intensity score was evaluated as a percentage in 10%
increments. The total score was calculated using the following
formula: 1 × (proportion of score 1 cells) + 2 × (proportion of
score 2 cells) + 3 × (proportion of score 3 cells). Two individuals
evaluated the intensity score and proportion of the positive cells
in a blind manner. When the values were discrepant, the two
individuals discussed the results and the most appropriate value
was determined.
The Ki-67 labeling index was calculated as the
percentage of Ki-67-positive cells in ~1,000 tumor cells in the
areas of the highest nuclear labeling under ×400 magnification
using the eCount image analysis software version 4.7 (e-Path Co.,
Ltd., Fujisawa, Kanagawa, Japan).
Terminal deoxynucleotidyl transferase
dUTP nick-end labeling (TUNEL) assay
Apoptotic cell death was determined by TUNEL assay
using the Apoptag Peroxidase In Situ Apoptosis Detection Kit
(EMD Millipore, Temecula, CA, USA). Briefly, deparaffinized
3-µm-thick paraffin section was digested with 20 µg/ml proteinase K
(cat. no. S3004; Agilent Technologies Japan, Ltd.) at 37°C for 30
min. The labeling was performed in the mixture of terminal
deoxyribonucleotide transferase and digoxygenin-dUTP at 37°C for 1
h. Incorporated digoxygenin-dUTP was detected by peroxidase-labeled
anti-digoxygenin antibody, which was included in the kit. The
peroxidase activity was visualized using diaminobenzidine at room
temperature for 2 min. Nuclear staining was considered positive.
The TUNEL index was calculated as the percentage of TUNEL-positive
cells in 1,000 counted carcinoma cells in the areas of highest
nuclear labeling under ×400 magnification using the eCount image
analysis software version 4.7 (e-Path Co., Ltd.).
Extraction of total RNA and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from paraffin sections of
GCs. The sections were deparaffinized and hydrated. Following this,
carcinoma tissues were dissected and collected into 1.5-ml tubes.
For the standardization, normal mucosa with IM were used. Total RNA
was extracted using the RNeasy FFPE Kit (Qiagen, K.K., Tokyo,
Japan) following the protocol recommended by the manufacturer, and
the concentration of total RNA was quantified. cDNA was synthesized
by random primer method using SuperScript VILO cDNA Synthesis Kit
(Thermo Fisher Scientific, K.K., Tokyo, Japan). The qPCR was
performed with a 20-µl mixture of 1X TaqMan Master Mix (Thermo
Fisher Scientific, K.K.), the primers and probes of PDIA3
(Hs04194196) or 18S rRNA (Hs03928990) (both from Thermo Fisher
Scientific, K.K.) and cDNA synthesized from 20 ng of total RNA. The
sequences of primers and probes used for the TaqMan assay are not
published by the company. The reaction program was initiated at
95°C for 20 sec, followed by 40 cycles of 95°C for 1 sec and 60°C
for 20 sec. The changes in fluorescence were monitored using a
StepOne Plus Real-Time PCR System (Thermo Fisher Scientific, K.K.).
Quantification cycles (Cq) of PDIA3 and 18S rRNA were determined as
the cycle where the linear increase in fluorescence reached the
threshold level. For standardization, ΔCq was calculated by
subtracting Cq18S rRNA from CqPDIA3. Then,
ΔΔCq was calculated by the subtraction of ΔCq of IM from ΔCq of GC.
The relative expression levels were calculated using the
2−ΔΔCq method (16). The
expression levels of PDIA3 mRNA in GC were calculated as fold
expression relative to IM.
Cell culture
The experiment was performed with four cell lines of
GC: NS-8, MKN-7, NUGC-4 and KATO-III (Cell Resource Center for
Biomedical Research Institute of Development, Aging and Cancer,
Tohoku University, Sendai, Japan). The cells were cultured in
Dulbecco's Modified Eagle's medium (DMEM; Thermo Fisher Scientific,
K.K.) supplemented with 10% fetal bovine serum (Nichirei Bioscience
Inc.). The cells were cultured in the medium with 100 ng/ml
interferon γ (IFNγ; cat. no. 80385; Cell Signaling Technology
Japan, K.K., Tokyo, Japan) for 48 h. Following a wash step with
PBS, the cells were lysed in a 0.5% SDS/50 mM Tris-HCl (pH 7.6)
buffer and sonicated for 10 min. The protein concentration was
quantified using a Pierce 660 nm Protein Assay Reagent (Thermo
Fisher Scientific, K.K.) and used for western blot analysis.
Co-immunoprecipitation analysis
KATO-III cells were cultured in DMEM supplemented
with 100 ng/ml IFNγ for 48 h, and the cells were collected into
15-ml tubes with a cell scraper. The cells were washed with PBS and
centrifuged at 1,500 × g at 4°C for 5 min. Then, the cells were
lysed with immunoprecipitation (IP) lysis/wash buffer (Thermo
Fisher Scientific, K.K.) with protease inhibitor cocktail (cat. no.
P8340; dilution, 1:100; Sigma-Aldrich Japan K.K., Tokyo, Japan),
and incubated on ice for 10 min. The solution was centrifuged at
12,000 × g at 4°C for 5 min. The supernatant was transferred to a
new tube, and the protein concentration was quantified using a
Pierce 660 nm Protein Assay Reagent (Thermo Fisher Scientific,
K.K.). Protein from each cell line (1 mg) was incubated in 800 µl
IP lysis/wash buffer mixed with Protein A/G PLUS-Agarose (Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and 2 µg of
anti-PDIA3 antibody (cat. no. ab13506; dilution, 1:400; Abcam
K.K.), anti-HLA class I-A, B, C antibody (cat. no. AB-46-H;
dilution, 1:400; Hokudo System Co. Ltd., Sapporo, Japan) and
isotype normal mouse IgG (cat. no. SC2025; dilution, 1:320; Santa
Cruz Biotechnology, Inc.) at 4°C overnight. The mixture was
subsequently applied to Sigma Prep Spin Columns with Break-Away
Tips (Sigma-Aldrich Japan K.K.), and the column was washed with a
buffer of 0.01 M Tris-HCl (pH 7.6)/150 mM NaCl/0.05% Tween-20
(TBS-T) three times. The proteins were eluted with 30 µl of Laemmli
Sample Buffer (Bio-Rad Laboratories, Inc., Tokyo, Japan) with
3-mercaptethanol and used for western blot analysis.
Western blot analysis
The protein samples were electrophoresed in a 5–20%
gradient gel (e-PAGEL; cat. no. E-T520L; ATTO Corporation, Tokyo,
Japan). For the analysis of protein expression in cultured cells,
10 µg of cell lysates were mixed with Laemmli Sample Buffer
(Bio-Rad Laboratories, Inc.) and loaded to the wells. For the
analysis of immunoprecipitated proteins, 20 µl of eluted solutions
were loaded to the wells. The electrophoresed proteins were blotted
onto a polyvinylidene difluoride membrane. Following the blocking
of the membrane with 5% skim milk in TBS-T at room temperature for
1 h, the membrane was incubated with monoclonal antibodies at 4°C
overnight. The antibodies used in the analysis were anti-PDIA3
(cat. no. ab13506; dilution 1:2,000; Abcam K.K.), anti-HLA class
I-A, B, C antibody (cat. no. AB-46-H; dilution, 1:500; Hokudo
System Co. Ltd.) and anti-β-actin antibody (cat. no. A5316;
dilution, 1:10,000; Sigma-Aldrich Japan K.K.). The membrane was
subsequently incubated with horse radish peroxidase-labeled
anti-mouse IgG antibody (TrueBlot; cat. no. 18-8817-30; dilution,
1:10,000; Rockland Immunochemicals Inc., Pottstown, PA, USA) at
room temperature for 1 h. The peroxidase activity was detected as
chemiluminescence using SuperSignal West Dura Extended Duration
Substrate (Thermo Fisher Scientific, K.K.). The positive bands were
quantified using Quantity One Software version 4.6.2 (Bio-Rad
Laboratories, Inc.).
Statistical analysis
Data were indicated as the mean ± standard
deviation. The data of two groups were compared using the
Mann-Whitney U test. Clinicopathological parameters were analyzed
using the χ2 and Fisher's exact tests. The Cox
proportional hazards model was used to identify independent
factors, which had a significant influence on the survival. Overall
survival was analyzed by the Kaplan-Meier's method and the log rank
test. P<0.05 was considered to indicate a statistically
significant difference. All statistical analyses were performed
using SPSS (version 23; IBM Corp., Armonk, NY, USA).
Results
Cases of GC
A total of 52 cases of GC were retrieved from the
archives of pathology records. The ages of the cases used in the
present study ranged between 45 and 81 years old. Thirty-five men
and 17 women represented the cases. The pathological stages ranged
from I to IV. The follow-up duration ranged between 6 and 92 months
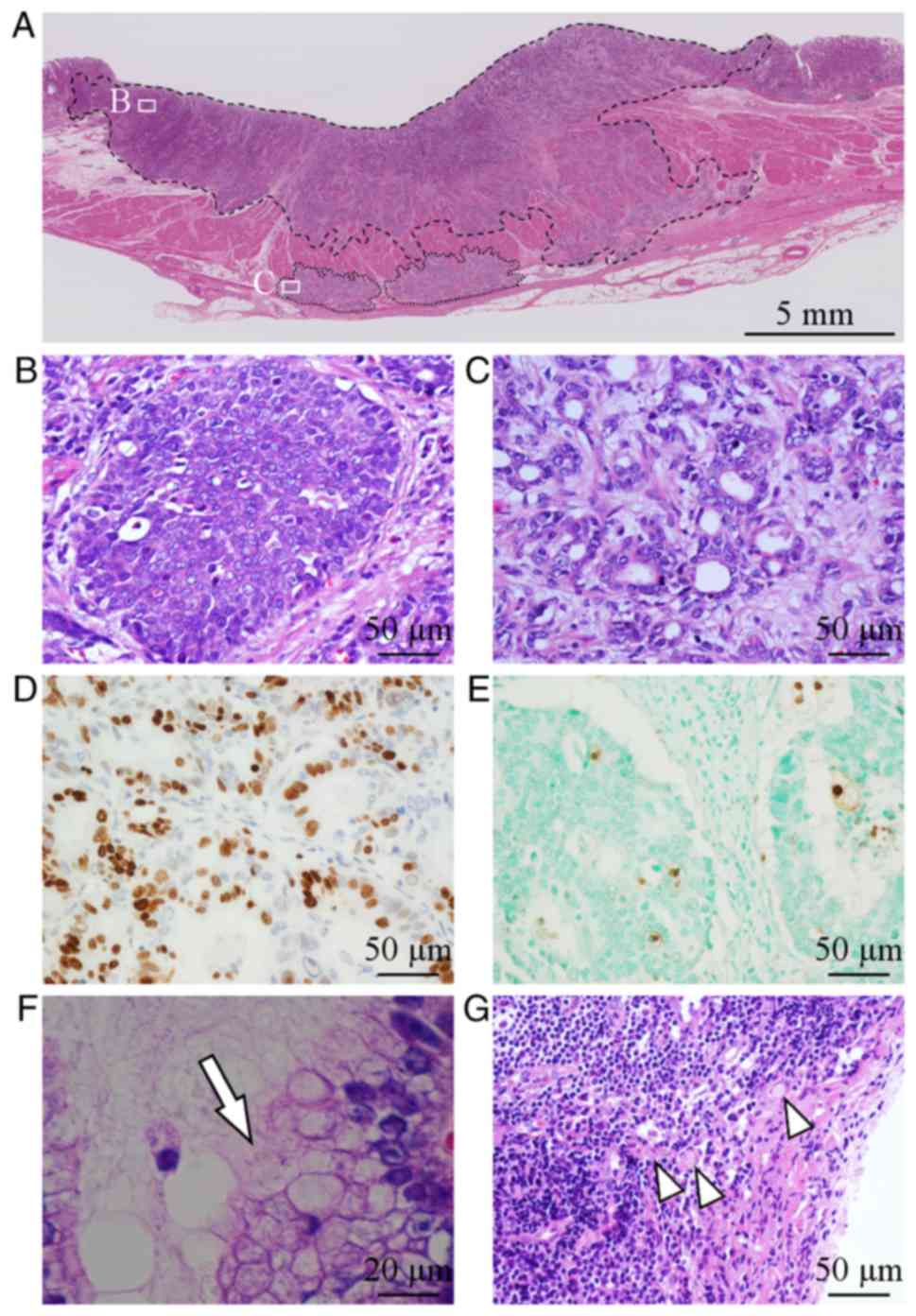
(mean, 59 months). Representative histology images of diffuse and
intestinal type GC were indicated (Fig.
1A-C). Twenty-three cases were classified into the intestinal
type and 29 cases were considered the diffuse type. Ki-67 labeling
index ranged from 2.1 to 97.3% (Fig.
1D). Apoptotic cell death, which was evaluated by TUNEL, ranged
from 1.3 to 37.1% (Fig. 1E).
Infection of Helicobacter pylori was noted in 45 (88%) cases
(Fig. 1F). Lymph node metastasis
(Fig. 1G) and distant metastasis
were identified in 29/52 (56%) cases and 10/52 (19%) cases,
respectively.
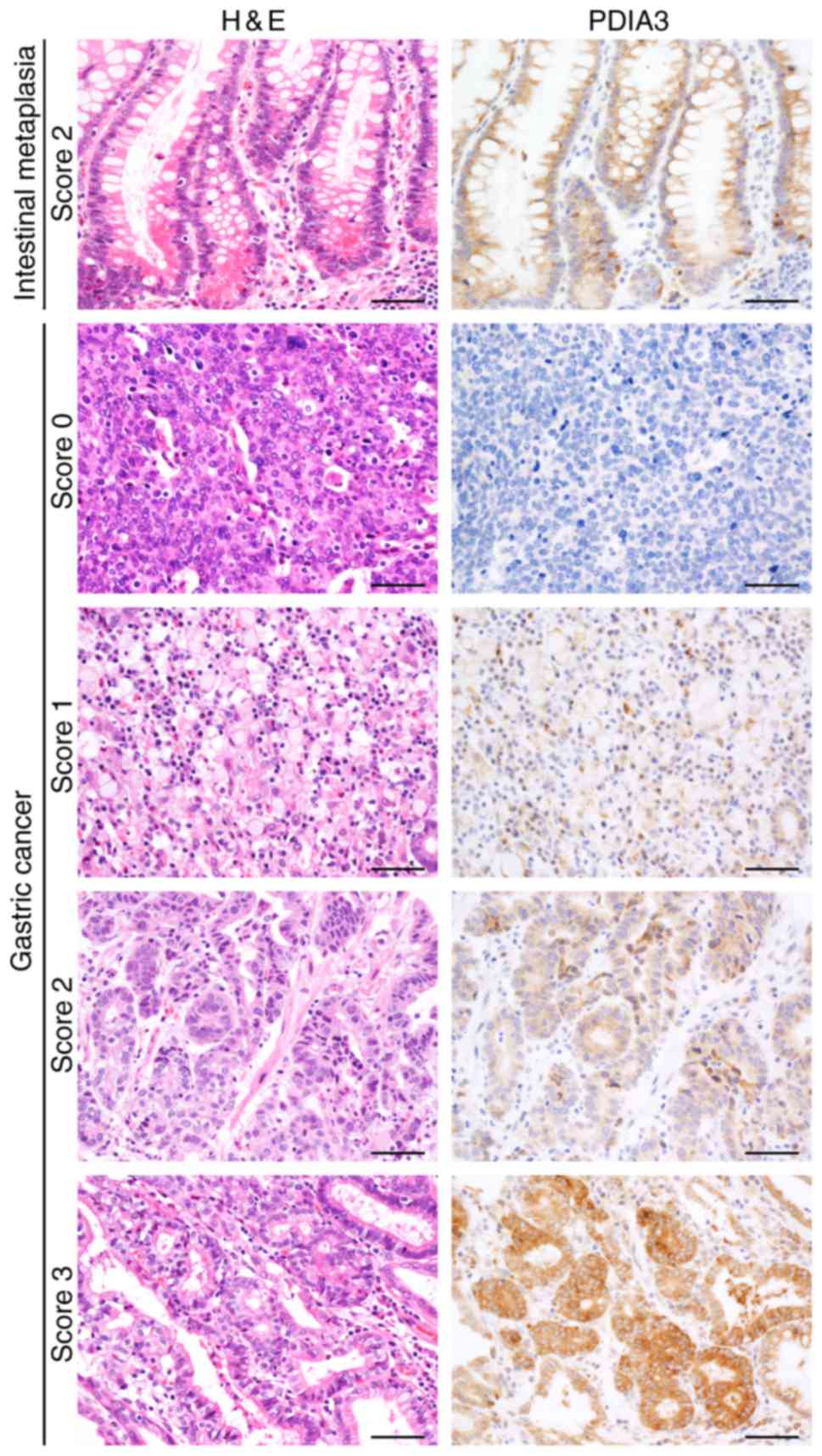
PDIA3 expression in GC samples
Glandular cells of the IM exhibited a positive
reaction for PDIA3 (Fig. 2). The
positive staining was observed in the cytoplasm of the carcinoma
cells; however, their staining intensity varied largely (Fig. 2). Well-differentiated carcinomas
tended to exhibit an intense positive reaction, whereas poorly
differentiated adenocarcinomas and signet ring cells exhibited only
a weak or faint positive reaction. The score of PDIA3 expression
ranged between 0 and 300, and the median was 95. Cases with the
total score ≥95 were classified as PDIA3-High and those with a
total score <95 were classified as PDIA3-Low.
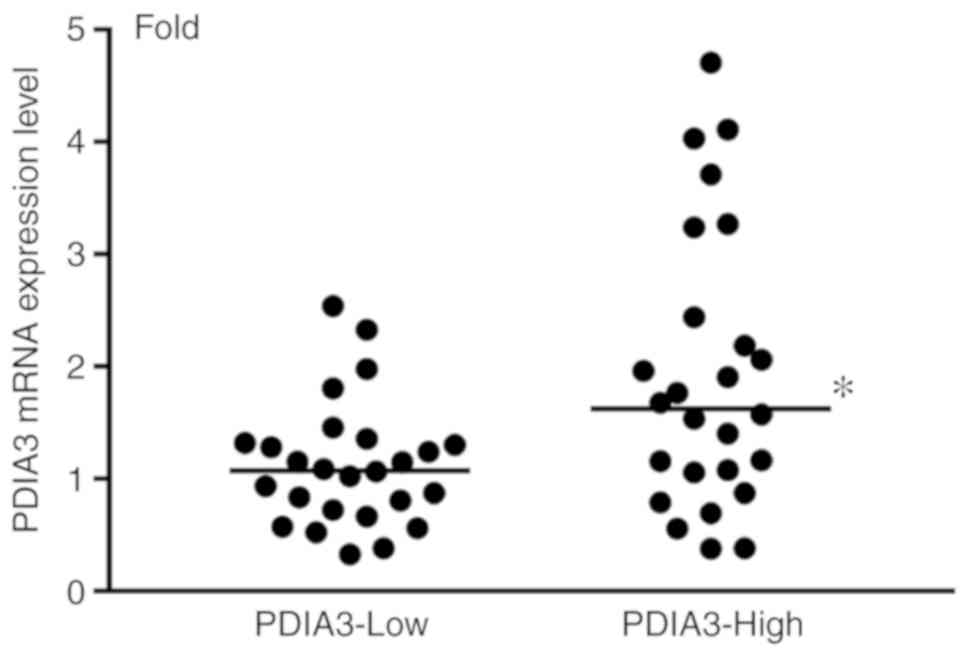
Expression of PDIA3 mRNA in GC
samples
The expression levels of PDIA3 were further
verified by RT-qPCR. The expression levels of mRNA in PDIA3-High GC
samples were significantly elevated compared with PDIA3-Low GC
samples (Fig. 3).
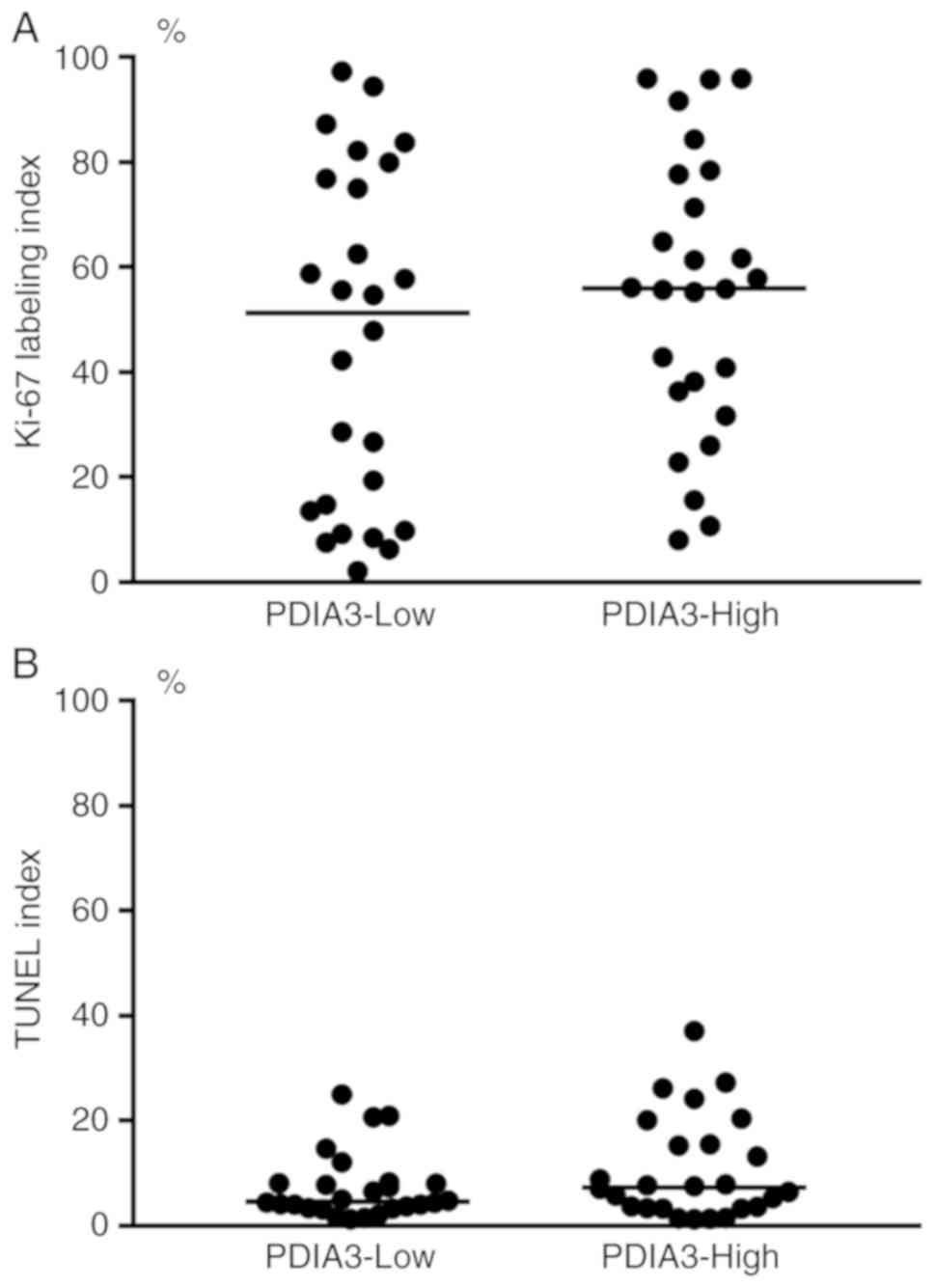
Clinicopathological features of
GC
The clinicopathological features are summarized in
Table I. The intestinal type was
the predominant histopathological subtype in PDIA3-High GC, whereas
the diffuse type was predominant in PDIA3-Low GC. There was no
significant difference in the frequency of Ki-67 labeling and TUNEL
staining between PDIA3-High and PDIA3-Low GC (Fig. 4). There was also no significant
difference in other assessed clinicopathological features between
PDIA3-High and PDIA3-Low GC.
 | Table I.Association of PDIA3 with
clinicopathological features of 52 cases of gastric cancer. |
Table I.
Association of PDIA3 with
clinicopathological features of 52 cases of gastric cancer.
|
|
| PDIA3
expression |
|
|---|
|
|
|
|
|
|---|
| Factors | Cases | High | Low | P-value |
|---|
| Age, years |
|
≥65 | 29 | 15 | 14 | 0.780 |
|
<65 | 23 | 11 | 12 |
|
| Sex |
|
Male | 35 | 18 | 17 | 0.768 |
|
Female | 17 | 8 | 9 |
|
| Location |
|
Upper | 9 | 4 | 5 | 0.697 |
|
Middle | 22 | 10 | 12 |
|
|
Lower | 21 | 12 | 9 |
|
| Histological
subtype |
|
Intestinal type | 23 | 17 | 6 | 0.002 |
| Diffuse
type | 29 | 9 | 20 |
|
| Ki-67 labeling
index |
|
≥55.6% | 26 | 15 | 11 | 0.267 |
|
<55.6% | 26 | 11 | 15 |
|
| TUNEL index |
|
≥6.1% | 26 | 15 | 11 | 0.267 |
|
<6.1% | 26 | 11 | 15 |
|
| Helicobacter
pylori infection |
|
Positive | 45 | 22 | 23 | 0.500 |
|
Negative | 7 | 4 | 3 |
|
| Depth of tumor
invasion |
|
pT1 | 21 | 11 | 10 | 0.748 |
|
pT2 | 3 | 1 | 2 |
|
|
pT3 | 8 | 5 | 3 |
|
|
pT4 | 20 | 9 | 11 |
|
| Lymph node
metastasis |
|
Positive | 29 | 16 | 13 | 0.402 |
|
Negative | 23 | 10 | 13 |
|
| Distant
metastasis |
|
Positive | 10 | 3 | 7 | 0.159 |
|
Negative | 42 | 23 | 19 |
|
| UICC stage |
| I | 21 | 10 | 11 | 0.402 |
| II | 7 | 4 | 3 |
|
|
III | 14 | 9 | 5 |
|
| IV | 10 | 3 | 7 |
|
Survival analysis of GC
The overall survival of PDIA3-High and PDIA3-Low GC
cases was analyzed using univariate and multivariate analyses. With
univariate analysis, the hazard ratio of the histological subtype,
PDIA3 expression and UICC stage were significantly increased
(Table II). In multivariate
analysis, PDIA3 expression and UICC stage were determined as
independent factors (Table
III).
 | Table II.Univariate survival analysis of
overall survival in 52 cases of gastric cancer. |
Table II.
Univariate survival analysis of
overall survival in 52 cases of gastric cancer.
| Factors | Hazard ratio | 95% CI | P-value |
|---|
| Age, years (<65
vs. ≥65) | 0.58 | 0.23–1.47 | 0.248 |
| Sex (male vs.
female) | 0.80 | 0.29–2.24 | 0.672 |
| Location (upper vs.
middle/lower) | 0.73 | 0.24–2.22 | 0.578 |
| Histological
subtype (intestinal vs. diffuse) | 3.20 | 1.05–9.79 | 0.042 |
| Ki-67 labeling
index (<55.6 vs. ≥55.6%) | 1.66 | 0.64–4.29 | 0.295 |
| TUNEL index
(<6.1 vs. ≥6.1%) | 0.63 | 0.24–1.63 | 0.340 |
| PDIA3 (low vs.
high) | 4.51 | 1.47–13.78 | 0.008 |
| Helicobacter
pylori infection (negative vs. positive) | 0.62 | 0.18–2.15 | 0.454 |
| UICC stage (I vs.
II/III/IV) | 8.01 | 1.83–35.07 | 0.006 |
 | Table III.Multivariate survival analysis of
overall survival in 52 cases of gastric cancer. |
Table III.
Multivariate survival analysis of
overall survival in 52 cases of gastric cancer.
| Factors | Hazard ratio | 95% CI | P-value |
|---|
| Histological
subtype (intestinal vs. diffuse) |
1.24 | 0.38–4.09 | 0.721 |
| PDIA3 (low vs.
high) |
5.56 | 1.67–18.46 | 0.005 |
| Stage (I vs.
II/III/IV) | 10.13 | 2.26–45.36 | 0.002 |
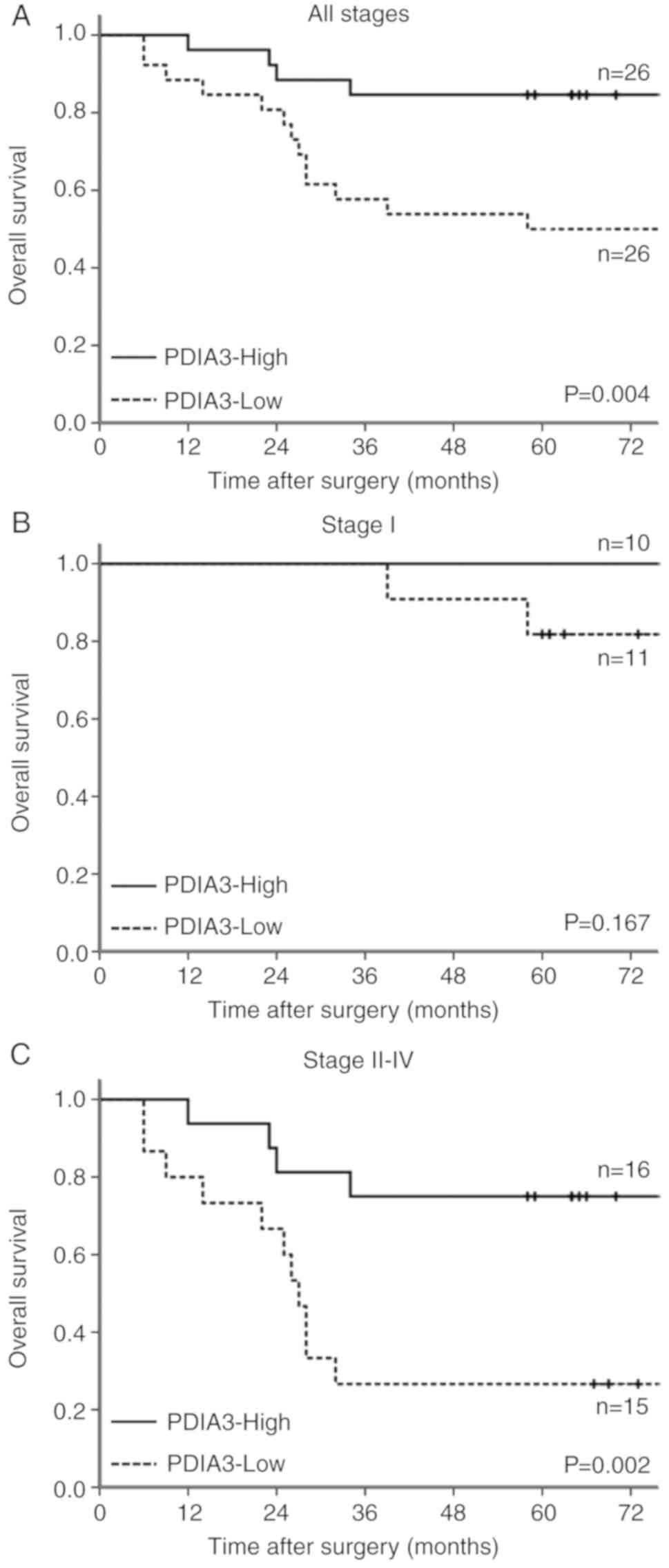
The overall survival of PDIA3-High GC cases was
significantly favorable compared with that in PDIA3-Low GC cases
(Fig. 5A). Survival was analyzed in
the early and advanced stages. In the early stage, survival tended
to be worse in PDIA3-Low GC cases (Fig.
5B). In the advanced stage, the survival was significantly
favorable in PDIA3-High GC cases. The 5-year survival was 75%
(Fig. 5C). Survival was
significantly worse in PDIA3-Low GC cases, and 5-year survival of
this subset was 27%.
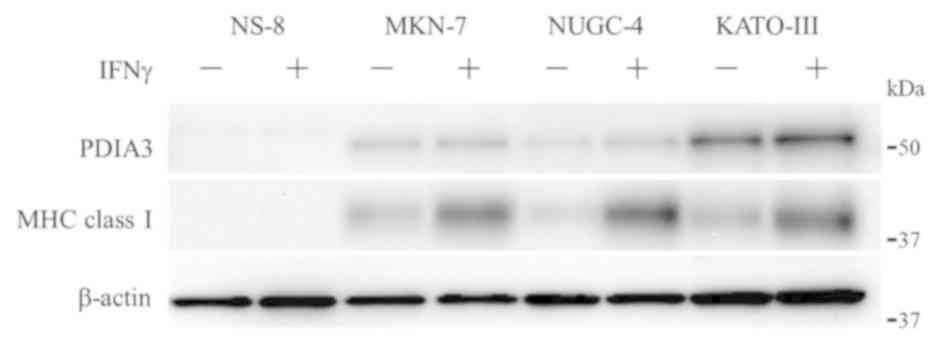
Expression of PDIA3 and MHC class I in
GC culture cells
The expression levels of PDIA3 and MHC class I were
examined in four cell lines, GC, NS-8, MKN-7, NUGC-4 and KATO-III
(Fig. 6). The expression levels of
these proteins were also examined in cells under the stimulation of
IFNγ, which is known to induce the expression of MHC class I
(17). PDIA3 was not expressed in
NS-8. However, PDIA3 was expressed in the other three cell lines,
and the expression level appeared to be lower in MKN-7 and NUGC-4
compared with KATO-III. The expression of MHC class I was
identified in all cell lines except NS-8. Under the stimulation of
IFNγ, the expression level of PDIA3 appeared to be increased in
KATO-III; however, the upregulation was not evident in IFNγ-treated
MKN-7 and NUGC-4. Notably, the expression of MHC class I was
increased by 4.5-, 18.3-, and 3.5-fold in IFNγ-treated MKN-7,
NUGC-4 and KATO-III, respectively (8.8±8.3-fold on average).
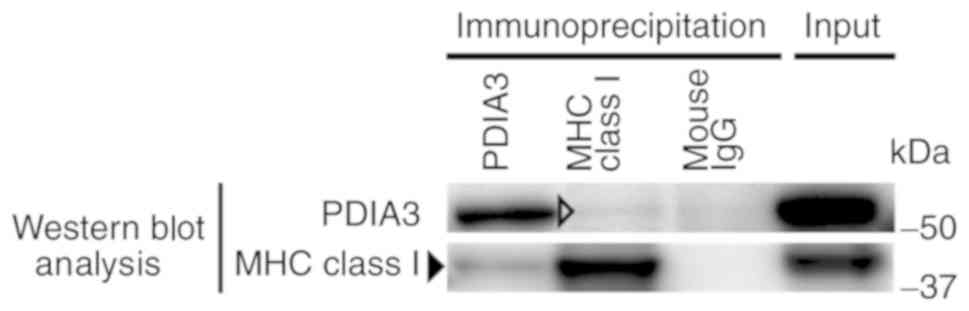
Co-immunoprecipitation analysis
The formation of a PDIA3 and MHC class I complex was
examined in the KATO-III cell line stimulated with IFNγ (Fig. 7). Western blot analysis with
anti-PDIA3 antibody revealed a clear positive signal in the sample
immunoprecipitated with anti-PDIA3 antibody and a faint band in the
sample immunoprecipitated with anti-MHC class I antibody. Western
blot analysis with an anti-MHC class I antibody indicated a clear
band in the sample immunoprecipitated with anti-MHC class I
antibody and a faint band in the sample immunoprecipitated with
anti-PDIA3 antibody. These results suggested that PDIA3 and MHC
class I formed a complex in KATO-III cells.
Discussion
The present study demonstrated the expression of
PDIA3 in GC and a favorable prognosis of PDIA3-High GC. The results
are in line with a previous report that also presented a favorable
prognosis of GC highly expressing PDIA3 (11). However, there is a slight difference
in prognosis between the previous and present study. In the
previous study, the prognosis was favorable in the cases at early
stage but not in the cases at advanced stage. The present study
demonstrated a significantly favorable prognosis in PDIA3-High GC
of all stages. In the cases of stage I, the prognosis appeared to
be improved in PDIA3-High GC, although there was no statistical
significance. In the cases of stage II to IV, the prognosis of
PDIA3-High GC was significantly favorable compared with that of
PDIA3-Low GC.
There are several differences in the study design
between the present study and the previous study (11). The previous study included cases
from several different ethnic groups. Furthermore, the histological
and immunohistochemical evaluations were conducted using tissue
microarray. However, the present study included only Japanese cases
of GC. In addition, histological examinations were performed with
tissue sections, which contain the whole area of carcinoma. As GC
is heterogenous in histology and molecular alterations (18), it is necessary to evaluate the whole
area of carcinoma. These factors may have led to the slight
difference reported in the prognosis of GC. To the best of our
knowledge, this is the first report on a favorable prognosis in
Japanese cases of GC with highly expressed PDIA3.
There have been several reports on the association
of PDIA3 expression with prognosis in types of cancer other than
GC. High PDIA3 expression has been associated with a worse
prognosis in laryngeal and hepatocellular carcinoma, and diffuse
glioma (7–9). However, high expression has also been
associated with a favorable prognosis in uterine cervical cancer
(10) and GC (11). The present study also suggested the
association of PDIA3 expression with favorable prognosis in GC. The
biological significance of PDIA3 on the prognosis of carcinomas
varies largely among carcinomas of various sites. This may reflect
the difference in carcinogenesis and histology of the carcinomas.
As PDIA3 exerts various cellular functions (4), the prognosis is influenced by the
cellular function, in which PDIA3 serves a critical role in
carcinoma cells. Cell proliferation and tumor immunity are possible
cellular functions with which PDIA3 is involved.
PDIA3 is involved in cell proliferation and
stabilizing receptors on the cell membrane (19,20)
and intracellular signaling molecules, including signal transducer
and activator of transcription 3 (21) and mechanistic target of rapamycin
(22). In hepatocellular carcinoma,
high expression of PDIA3 was associated with high proliferative
activity and low apoptotic cell death, whereas low expression was
associated low proliferative activity and high apoptotic cell death
(8,9). The unfavorable prognosis in
hepatocellular carcinoma with elevated expression of PDIA3 may be,
in part, accounted for by increased cell proliferation, which is
supported by PDIA3 expression. However, in GC, there was no
significant difference in the proliferation and apoptotic cell
death between PDIA3-High and PDIA3-Low GC in the present study. It
was thus considered that the role of PDIA3 on the proliferation or
apoptotic cell death was only limited in GC. This may also reflect
the difference in carcinogenic machinery between hepatocellular
carcinoma (23) and GC (24). The findings suggest that the
favorable prognosis in PDIA3-High GC is influenced by a factor
other than cell proliferation.
PDIA3 is involved in the processing and transport of
antigens to the cell membrane during an immune response (25,26).
Thus, the association of PDIA3 with MHC class I was examined in
culture cell lines of GC in the present study. The expression of
MHC class I was indicated in three of four assessed cell lines, and
the expression of MHC class I was upregulated under the stimulation
of IFNγ (17). The expression
levels of PDIA3 varied largely among the cell lines, and it
appeared to be comparable with the expression level in human cases
of GC. Upregulation of PDIA3 was evident in one cell line,
KATO-III, which had the most abundant expression of PDIA3 among the
cell lines. Co-immunoprecipitation experiments demonstrated that
MHC class I formed a complex with PDIA3. It is therefore
conceivable that PDIA3 serves a critical role in tumor immunity,
forming a complex with MHC class I in GC.
The expression of MHC class I in carcinoma cells may
induce cytotoxic effects of lymphocytes and other immune cells
(27,28). Carcinoma cells that do not express
MHC class I may evade from the cytotoxic effects of immune cells.
Notably, the expression and upregulation of MHC class I was
indicated in the cell line, KATO-III, that revealed high expression
of PDIA3 in the present study. The expression of PDIA3 in carcinoma
cells may suggest a sufficient capacity to form a complex with MHC
class I and to transport antigens to the cell membrane. It may thus
be speculated that a favorable prognosis of PDIA3-High GC is
attributed, in part, to a sufficient immune response mediated by
PDIA3. The present findings suggest that PDIA3 may serve an
important role in the pathobiology of GC. The expression of PDIA3
may be a useful biomarker for the prediction of prognosis of
GC.
Acknowledgements
The authors would like to acknowledge the excellent
assistance of Ms. Kiyoko Kawahara, Mr. Takenori Fujii, Mr. Kiyoshi
Teduka, Ms. Yoko Kawamoto and Ms. Taeko Kitamura of the Department
of Integrated Diagnostic Pathology, Nippon Medical School (Tokyo,
Japan).
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
TS, RW and ZN designed the study and wrote the
manuscript draft. TS, RW, SK, RO performed histological
examinations. TS, KI, MK conducted biochemical examinations, data
analyses and statistical analyses. TS prepared the figures/tables.
IF, EU and HY provided clinical data of the patients and assisted
with revising the manuscript. ZN supervised the experimental design
and manuscript writing, revised the manuscript, and gave the final
approval of the version to be published. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Nippon Medical School Hospital (Tokyo, Japan; no. 29-06-764).
Informed consent was obtained from all patients.
Patient consent for publication
Informed consent was obtained from all patients.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
GC
|
gastric cancer
|
|
HLA
|
human leukocyte antigen
|
|
IFN γ
|
interferon γ
|
|
IHC
|
immunohistochemistry
|
|
IM
|
intestinal metaplasia
|
|
MHC
|
major histocompatibility complex
|
|
PBS
|
phosphate-buffered saline
|
|
PDIA3
|
protein disulfide isomerase A3
|
|
TBS-T
|
Tris-buffered saline/Tween-20
|
References
|
1
|
Hori M, Matsuda T, Shibata A, Katanoda K,
Sobue T and Nishimoto H; Japan Cancer Surveillance Research Group,
: Cancer incidence and incidence rates in Japan in 2009: A study of
32 population-based cancer registries for the Monitoring of Cancer
Incidence in Japan (MCIJ) project. Jpn J Clin Oncol. 45:884–891.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim HJ and Oh SC: Novel systemic therapies
for advanced gastric cancer. J Gastric Cancer. 18:1–19. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Song Z, Wu Y, Yang J, Yang D and Fang X:
Progress in the treatment of advanced gastric cancer. Tumour Biol.
39:10104283177146262017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Turano C, Gaucci E, Grillo C and
Chichiarelli S: ERp57/GRP58: A protein with multiple functions.
Cell Mol Biol Lett. 16:539–563. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ni M and Lee AS: ER chaperones in
mammalian development and human diseases. FEBS Lett. 581:3641–3651.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hettinghouse A, Liu R and Liu CJ:
Multifunctional molecule ERp57: From cancer to neurodegenerative
diseases. Pharmacol Ther. 181:34–48. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Choe MH, Min JW, Jeon HB, Cho DH, Oh JS,
Lee HG, Hwang SG, An S, Han YH and Kim JS: ERp57 modulates STAT3
activity in radioresistant laryngeal cancer cells and serves as a
prognostic marker for laryngeal cancer. Oncotarget. 6:2654–2666.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takata H, Kudo M, Yamamoto T, Ueda J,
Ishino K, Peng WX, Wada R, Taniai N, Yoshida H, Uchida E, et al:
Increased expression of PDIA3 and its association with cancer cell
proliferation and poor prognosis in hepatocellular carcinoma. Oncol
Lett. 12:4896–4904. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zou H, Wen C, Peng Z, Shao YY, Hu L, Li S,
Li C and Zhou HH: P4HB and PDIA3 are associated with tumor
progression and therapeutic outcome of diffuse gliomas. Oncol Rep.
39:501–510. 2018.PubMed/NCBI
|
|
10
|
Chung H, Cho H, Perry C, Song J, Ylaya K,
Lee H and Kim JH: Downregulation of ERp57 expression is associated
with poor prognosis in early-stage cervical cancer. Biomarkers.
18:573–579. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Leys CM, Nomura S, LaFleur BJ, Ferrone S,
Kaminishi M, Montgomery E and Goldenring JR: Expression and
prognostic significance of prothymosin-alpha and ERp57 in human
gastric cancer. Surgery. 141:41–50. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lauren P: The two histological main types
of gastric carcinoma: Diffuse and so-called intestinal-type
carcinoma. An attempt at a histo-clinical classification. Acta
Pathol Microbiol Scand. 64:31–49. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Washington K: 7th edition of the AJCC
cancer staging manual: Stomach. Ann Surg Oncol. 17:3077–3079. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sano T, Coit DG, Kim HH, Roviello F,
Kassab P, Wittekind C, Yamamoto Y and Ohashi Y: Proposal of a new
stage grouping of gastric cancer for TNM classification:
International gastric cancer association staging project. Gastric
Cancer. 20:217–225. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pirker R, Pereira JR, von Pawel J,
Krzakowski M, Ramlau R, Park K, de Marinis F, Eberhardt WE,
Paz-Ares L, Störkel S, et al: EGFR expression as a predictor of
survival for first-line chemotherapy plus cetuximab in patients
with advanced non-small-cell lung cancer: Analysis of data from the
phase 3 FLEX study. Lancet Oncol. 13:33–42. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou F: Molecular mechanisms of IFN-gamma
to up-regulate MHC class I antigen processing and presentation. Int
Rev Immunol. 28:239–260. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wong SS, Kim KM, Ting JC, Yu K, Fu J, Liu
S, Cristescu R, Nebozhyn M, Gong L, Yue YG, et al: Genomic
landscape and genetic heterogeneity in gastric adenocarcinoma
revealed by whole-genome sequencing. Nat Commun. 5:54772014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Doroudi M, Olivares-Navarrete R, Boyan BD
and Schwartz Z: A review of 1α,25(OH)2D3
dependent Pdia3 receptor complex components in Wnt5a non-canonical
pathway signaling. J Steroid Biochem Mol Biol. 152:84–88. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gaucci E, Altieri F, Turano C and
Chichiarelli S: The protein ERp57 contributes to EGF receptor
signaling and internalization in MDA-MB-468 breast cancer cells. J
Cell Biochem. 114:2461–2470. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Coe H, Jung J, Groenendyk J, Prins D and
Michalak M: ERp57 modulates STAT3 signaling from the lumen of the
endoplasmic reticulum. J Biol Chem. 285:6725–6738. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ramirez-Rangel I, Bracho-Valdés I,
Vázquez-Macías A, Carretero-Ortega J, Reyes-Cruz G and
Vázquez-Prado J: Regulation of mTORC1 complex assembly and
signaling by GRp58/ERp57. Mol Cell Biol. 31:1657–1671. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li CW, Chang PY and Chen BS: Investigating
the mechanism of hepatocellular carcinoma progression by
constructing genetic and epigenetic networks using NGS data
identification and big database mining method. Oncotarget.
7:79453–79473. 2016.PubMed/NCBI
|
|
24
|
Katona BW and Rustgi AK: Gastric cancer
genomics: Advances and future directions. Cell Mol Gastroenterol
Hepatol. 3:211–217. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Blees A, Januliene D, Hofmann T, Koller N,
Schmidt C, Trowitzsch S, Moeller A and Tampé R: Structure of the
human MHC-I peptide-loading complex. Nature. 551:525–528.
2017.PubMed/NCBI
|
|
26
|
Garbi N, Hämmerling G and Tanaka S:
Interaction of ERp57 and tapasin in the generation of MHC class
I-peptide complexes. Curr Opin Immunol. 19:99–105. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen Y, Lin J, Guo ZQ, Lin WS, Zhou ZF,
Huang CZ, Chen Q and Ye YB: MHC I-related chain a expression in
gastric carcinoma and the efficacy of immunotherapy with
cytokine-induced killer cells. Am J Cancer Res. 5:3221–3230.
2015.PubMed/NCBI
|
|
28
|
Ribeiro CH, Kramm K, Gálvez-Jirón F, Pola
V, Bustamante M, Contreras HR, Sabag A, Garrido-Tapia M, Hernández
CJ, Zúñiga R, et al: Clinical significance of tumor expression of
major histocompatibility complex class I-related chains A and B
(MICA/B) in gastric cancer patients. Oncol Rep. 35:1309–1317. 2016.
View Article : Google Scholar : PubMed/NCBI
|