Introduction
Solid tumors are often characterized by hypoxic
areas with a pO2 of less than 5 mmHg (equivalent to 0.7%
O2) due to poor vascularization and uncontrolled growth
(1). One of the most universal
characteristics of solid tumors is the so called ‘Warburg effect’,
i.e. adaption of hypoxic tumor cells' metabolism from oxidative
phosphorylation to glycolysis to generate energy in the absence of
oxygen (2). This adaptation
increases the production of lactic acid and the generation of
CO2 by neutralizing protons (H+) in the
cytoplasm, a process catalyzed by carbonic anhydrases (CA).
Consequently, the extracellular environment is acidified because of
the export of lactic acid (monocarboxylate transporter, MCT) and
CO2 release from the tumor cells (3,4). Tumor
cells are able to adapt to the resulting acidic environment,
whereas it is toxic to normal cells (4). In various tumor entities, one of the
main mediators of the hypoxic response is the transcriptional
factor hypoxia-inducible factor 1 (HIF-1). HIF-1 governs the
cellular adaption to oxygen deficiency by regulating tumor-relevant
genes involved in important processes such as glucose transport
(GLUT-1), angiogenesis (VEGF), proliferation
(IGF-2) and pH regulation (CA9) (5). Furthermore, the expression of
membrane-bound CAIX is highly upregulated by HIF-1α and catalyzes
the hydration of carbon dioxide (CO2) to bicarbonate
ions (HCO3−) and protons (H+)
(6). CAIX thus contributes to the
acidification of the extracellular pH (pHe) under hypoxic
conditions. Additionally, bicarbonate is actively transported back
into cells by a sodium-dependent co-transporter, where it
neutralizes H+ to maintain a favorable intracellular pH
(pHi) (7). Moreover, a low pH of
the extracellular environment has been proven to influence
CAIX-mediated tumor growth, invasion and metastasis (8–10).
In vivo CAIX is overexpressed in many tumor entities and is
associated with a poor survival of cancer patients (11). Especially in patients with invasive
breast cancer, immunohistochemical CAIX expression also correlates
with worse relapse-free and overall survival (10,12,13).
This poor prognosis may be due to hypoxia-mediated resistance to
drug therapy or radiotherapy (3).
Hypoxia-induced CAIX expression may also be directly linked to
radioresistance. A study of breast cancer patients who received
radiotherapy revealed that high CAIX expression in tumor tissue
correlates with poor recurrence-free survival (14). This finding was supported by another
recent clinical study showing that CAIX overexpression was
significantly associated with poor disease-free and overall
survival in a cohort of patients with triple-negative
(ER−, PR− and Her2−) breast cancer
treated with radiotherapy, which suggests a correlation between
CAIX expression and response to radiotherapy (15). Initial studies in a colorectal
carcinoma xenograft model revealed that inhibiting CAIX with
different CAIX inhibitors (CAI: acetazolamide, DH348 or 11c) or
knockdown of CA9 with shRNA delayed tumor growth and
radiosensitized tumor cells (16,17).
Furthermore, it has been supposed that the prognostic significance
of CAIX expression differs depending on the breast cancer subtype
of patients (18).
In the present study, we investigated the cellular
and radiobiological effects of CAIX inhibition in two human breast
cancer cell lines of different subtypes: The highly invasive
metastatic breast cancer cell line MDA-MB-231 (basal,
triple-negative) and the less invasive non-metastatic breast cancer
cell line MCF-7 (luminal, ER+, PR+ and
Her2−). In the present study, inhibition of CAIX was
performed by two alternative strategies, namely treatment with
CA9 siRNA or exposure to the CAIX/CAXII selective inhibitor
U104, which is currently being tested in a phase I clinical trial
in patients with advanced solid tumors (19). Our previous study indicated that
betulin 3,28-disulfamate, which is described as a CAI by Winum
et al (20), radiosensitized
MDA-MB-231 breast cancer cells (21). Recently, CAI FC9403A but not CAI S4
showed synergistic effects with irradiation in MDA-MB-231 breast
cancer spheroids (22). However, to
the best of our knowledge, there are no studies regarding the
cellular and radiobiological effects of a selective CA9
knockdown with RNA interference or a CAIX/CAXII specific inhibition
in human breast cancer cells. We hypothesized that selective
CA9/CAIX knockdown/inhibition will decrease the
proliferation, migration and clonogenic survival of both breast
cancer cell lines and that the ureidosulfonamide U104 (or
SLC-0111)-induced inhibition of CAIX and CAXII will cause stronger
effects on radiosensitivity in breast cancer cells than RNA
interference.
Materials and methods
Cell culture conditions and treatments
of breast cancer cells
The breast cancer cell lines MDA-MB-231 and MCF-7
were cultured with RPMI-1640 containing 25 mM HEPES and L-glutamine
(Lonza, Walkersville, MD, USA) and supplemented with 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA), 1% pyruvate, 185 U/ml penicillin and 185 µg/ml
streptomycin (Invitrogen; Thermo Fisher Scientific, Inc.) in a
humidified atmosphere of 3% CO2 at 37°C. All experiments
were conducted with cells in the logarithmic growth phase.
Twenty-four hours before treatment, the cells were
seeded in cell culture flasks (Greiner Bio-One, Kremsmünster,
Austria). Breast cancer cells were transfected with 100 nM
CA9 targeting siRNA (Table
I) using INTERFERin™ reagent as recommended by the manufacturer
(Polyplus Transfection, Illkirch, France). To inhibit CAIX
activity, breast cancer cells were incubated with the CAIX
inhibitor U104 (R&D Systems, Minneapolis, MN, USA) diluted in
dimethyl sulfoxide (DMSO) (Sigma-Aldrich Chemie; Merck KGaA,
Darmstadt, Germany). Following treatment, the cells were exposed to
hypoxia for 24 to 72 h. To achieve hypoxic conditions (0.1%
O2) we used a gas generator system (Anaerocult P; Merck
Millipore, Darmstadt, Germany) as previously described (23).
 | Table I.siRNAs and primers. |
Table I.
siRNAs and primers.
| Gene | Sequence 5′→3′ | Localization | Source |
|---|
| siRNAs | CA9 | Sense |
5′-CAGTGCCTATGAGCAGTTG-3′ | 900-918 | Eurofins Genomics,
Ebersberg, Germany |
|
|
| Antisense |
5′-CAACTGCTCATAGGCACTG-3′ |
|
|
|
| Nonsense | Sense |
5′-CGTACGCGGAATACTTCGA-3′ |
|
|
|
|
| Antisense |
5′-TCGAAGTATTCCGCGTACG-3′ |
|
|
| Primers | CA9 | Forward |
5′-GAAAACAGTGCCTATGAGCAGTTG-3′ | 895-918 | Sigma-Aldrich;
Merck KGaA, |
|
|
| Reverse |
5′-TGCTTAGCACTCAGCATCAC-3′ | 1,106–1,087 | Darmstadt,
Germany |
|
| HPRT | Forward |
5′-TTGCTGACCTGCTGGATTAC-3′ | 391-410 |
|
|
|
| Reverse |
5′-CTTGCGACCTTGACCATCTT-3′ | 652-633 |
|
|
| POLR2A | Forward |
5′-CTTGCCCCGTGCCATGCAGA-3′ | 1,358–1,377 |
|
|
|
| Reverse |
5′-CTCGCACCCGGCCTTCCTTG-3′ | 1,440–1,421 |
|
Irradiation was performed under hypoxic conditions
(0.1% O2) 24 h after treatment with U104 and 72 h after
siRNA transfection. The cells were irradiated with 2, 6 and 10 Gy
at a dose rate of 2 Gy/min accomplished with 6 MV photons and
adequate bolus material on an Elekta Synergy linear accelerator
(Elekta AB, Stockholm, Sweden).
Quantitative real-time PCR (qPCR) and
western blot hybridization
RNA isolation, cDNA synthesis and qPCR were
performed as previously described (24). The primers used are cited in
Table I. A no-template reaction was
used as a negative control. HPRT (hypoxanthine-guanine
phosphoribosyltransferase) and POLR2A (DNA-directed RNA
polymerase II subunit A) served as housekeeping genes and were used
for normalization. We used plasmid DNA standards for each gene
(107−103 copies/µl) to calculated the copy
number of gene of interest or the housekeeping gene.
To isolate protein, breast cancer cells were lysed
in cell lysis buffer supplemented with protease inhibitors (Cell
Signaling Technology, Inc., Danvers, MA, USA) and homogenized by
ultrasound. The protein concentration was determined using Bradford
method. The proteins were separated by gel electrophoresis [4–12%
Bis-Tris mini gels (Invitrogen; Thermo Fisher Scientific, Inc.] and
transferred to a polyvinylidene difluoride (PVDF) membrane (Merck
Millipore, Darmstadt, Germany). The membranes were blocked with 10%
non-fat milk/TBST (50 mM NaCl, 30 mM Tris-HCl pH 8.0, 0.1%
Tween-20) and incubated with monoclonal mouse anti-CAIX antibody
(dilution 1:2,000; clone no. M75; Bioscience Slovakia, Bratislava,
Slovak Republic), rabbit anti-human cleaved PARP (Asp214) antibody
(dilution 1:2,000; cat. no. 9541; Cell Signaling Technology, Inc.)
or monoclonal mouse anti-β-actin antibody (dilution 1:10,000; cat.
no. A5441; Sigma-Aldrich; Merck KGaA), followed by incubation with
HRP-conjugated secondary antibodies (goat anti-rabbit, cat. no.
P0448; and rabbit anti-mouse, cat. no. P0260; both diluted at
1:5,000; Dako Deutschland GmbH, Hamburg, Germany). Further washing
steps were followed by the visualization of immune complexes with
an ECL detection system (GE Healthcare, Chicago, IL, USA).
Quantification of western blot signals was performed by the use of
Image Studio Lite 5.2 software (LI-COR Biosciences, Lincoln, NE,
USA).
Measurement of CAIX activity and
intracellular pH (pHi)
Breast cancer cells were cultured under normoxic or
hypoxic conditions, respectively, for 24 h. Afterwards, hypoxic
cells were treated with 50 µM U104 for 3 h. To measure CAIX
activity, breast cancer cells were washed with cold isotonic buffer
(130 mM NaCl, 5 mM KCl, 20 mM Hepes; pH adjusted to pH 8.2 at 4°C),
scraped down and resuspended in 2 ml of isotonic buffer. The pHe
was measured with a microelectrode (WTW, Weilheim, Germany) for 1
min before adding 1 ml of CO2-saturated water while
monitoring the pH every 5 sec for 10 min. The CAIX activity was
calculated according to the Wilbur-Anderson-method
(WAU/mg=2*(T0-T)/T*mg protein). The duration (T) to lower the pH of
the isotonic buffer from 8.0 to 6.6 at 4°C was determined [T0,
unanalyzed reaction (isotonic buffer); T, catalyzed reaction (e.g.,
normoxia, hypoxia)].
The pHi was measured with BCECF
(2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein,
acetoxymethyl ester) (Thermo Fisher Scientific, Inc.) as previously
described (25). The cells were
seeded on poly-lysine-treated coverslips and cultured under hypoxic
conditions for 24 h. Subsequently, the cells were incubated with pH
6.6 ringer solution with/without 50 µM U104 for 3 h before
measuring the pHi.
Sulforhodamine B assay
The SRB assay was performed as previously described
(21). MDA-MB-231 and MCF-7 cells
were seeded in 96-well plates (TPP Techno Plastic Products AG,
Trasadingen, Switzerland) and treated with CA9 siRNA or CAI
U104 (10, 100 and 250 µM) under hypoxic conditions for 72 h. The
cells were then fixed, washed and dyed with 0.4% sulforhodamine,
and absorbance was measured at 540 nm using a GENios microplate
reader (Tecan Group AG, Männedorf, Switzerland).
Clonogenic survival assay and
radiosensitivity
The colony-forming assay was performed 24 h after
incubation with the CAIX inhibitor U104 or 72 h after siRNA
transfection under hypoxic conditions. For determination of
radiosensitivity, the cells were irradiated 24 h (U104) and 72 h
(siRNA) after treatment and the clonogenic survival assay was
performed 1 h after irradiation, as previously described (26). The survival fraction is the ratio of
the plating efficiencies of treated cells (e.g., siRNA treatment,
irradiation) to that of control cells (e.g., nonsense siRNA,
non-irradiated). Additionally, the DMF10 (dose-modifying factor
10%), i.e., the ratio of radiation doses with or without treatment
resulting in 10% survival, and the EF10Gy (enhancement
factor), i.e., the ratio of survival fraction of treated and
control cells, were determined. Data were fitted to a linear
quadratic model (-lnS=αD + βD2) using OriginPro 8G
(OriginLab Corp., Northampton, MA, USA).
Migration assay and cell cycle
analysis
To analyze migration, we performed a wound scratch
assay as previously described (26). In brief, cells were seeded in
24-well plates (Greiner Bio-One) and cultured until they reached
100% confluency. In detail, 24 h after U104 treatment and 48 h
after siRNA transfection, respectively, the cells were wounded by
creating a cell-free area with a 200-µl pipette tip and washed
twice to remove detached cells. Images were captured immediately (0
h) and 16 h after scratching to quantify the extent of the wounded
area using the software Photoshop (Adobe Systems Inc., San Jose,
CA, USA).
The cell cycle was analyzed 72 h after siRNA
transfection or incubation with U104 as previously described
(24). Briefly, propidium iodide
(PI) was used to label DNA, and the DNA content was measured by
flow cytometry on a FACScan instrument (BD Biosciences, Franklin
Lakes, NJ, USA). The cell cycle phase distribution was then
analyzed using the software ModFit (Verity Software House, Topsham,
ME, USA).
Statistical analysis
Data represent at least three independent
experiments. All data represent the mean value and standard
deviation (+ SD). The significance of differences was assessed
using one-way ANOVA followed by Tukey's post hoc test or Dunnett's
post hoc test, or unpaired two-sided Student's t-test. A P-value
<0.05 was considered to indicate a significant difference in
reference to the population of negative control cells (nonsense
siRNA or DMSO), if not otherwise indicated.
Results
CAIX expression under different cell
culture conditions
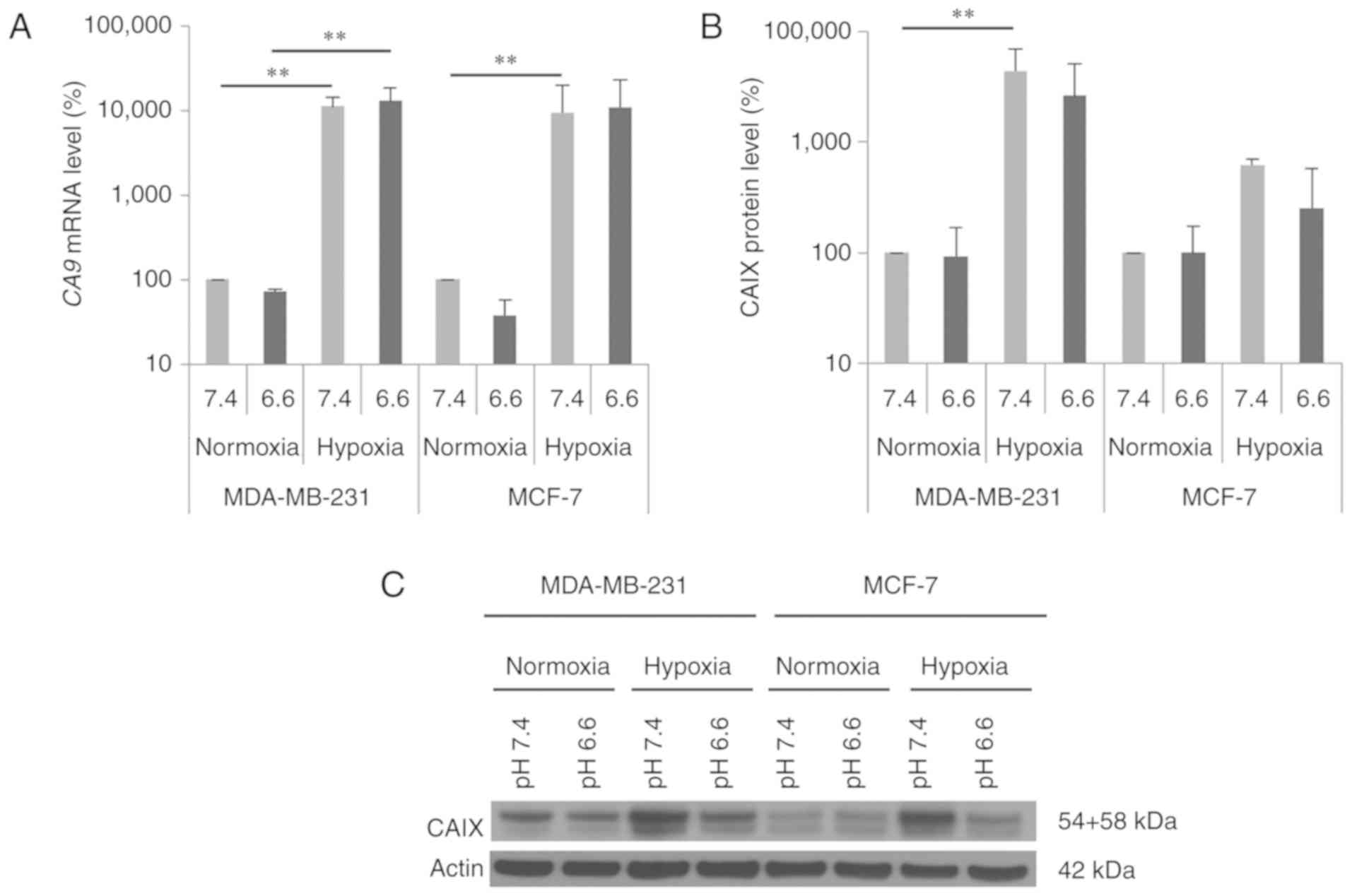
We investigated CA9 mRNA and CAIX protein
expression levels under different oxygen conditions (21 vs. 0.1%
O2) and different pH values (pH 6.6 vs. 7.4) in the
breast cancer cell lines MDA-MB-231 and MCF-7. As expected, the
expression of CA9 mRNA and CAIX protein increased in both cell
lines under hypoxic conditions compared to levels observed under
normoxic conditions (Fig. 1). The
CA9 mRNA level was significantly elevated 100-fold under
hypoxia in both breast cancer cell lines (Fig. 1A, pH 7.4: MDA-MB-231: P=0.002;
MCF-7: P=0.009), whereas the increase in CAIX protein level was
higher in the MDA-MB-231 cells (pH 7.4: 40-fold; P=0.002) than that
noted in the MCF-7 cells (pH 7.4: 6-fold; P=0.5). In addition,
hypoxia-induced CAIX expression was independent of the
extracellular pH (pHe 6.6 and 7.4) (Fig. 1B and C). Under normoxic and hypoxic
conditions, CA9 mRNA levels were not significantly altered
by acidic conditions (pHe 6.6) in both breast cancer cell lines
(Fig. 1A). The CAIX protein level
was also not influenced by the acidic medium under normoxic
conditions. However, hypoxic conditions produced a trend towards
reduced CAIX protein levels in both cell lines cultured in acidic
medium (Fig. 1B and C).
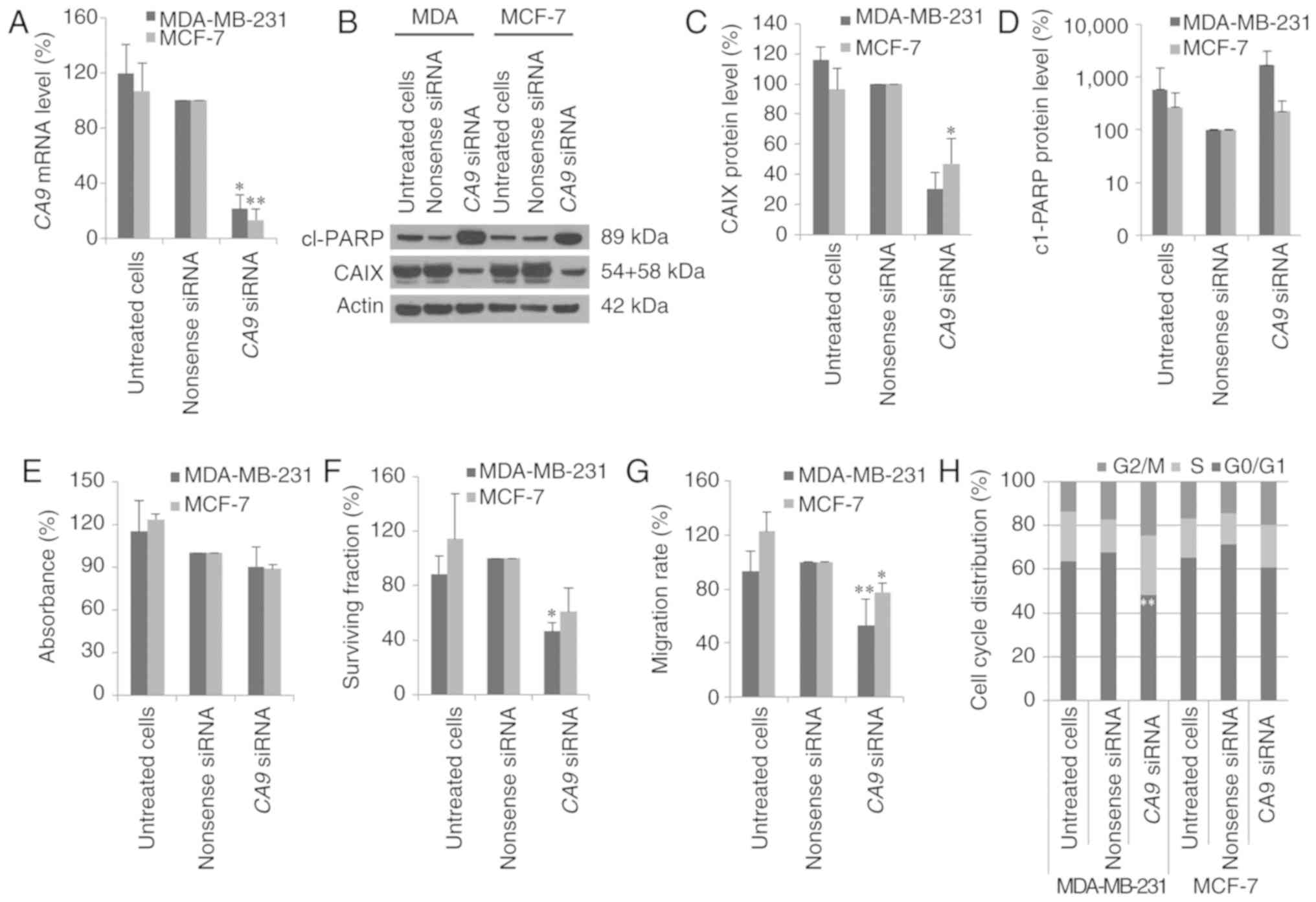
Effects of CA9 siRNAs in breast cancer
cells
RNA interference (RNAi) technique was used to
transfect chemically synthesized siRNAs against CA9 into
breast cancer cells and the expression of CA9 mRNA and CAIX
protein expression levels were examined by real-time PCR and via
western blot analyses. In MDA-MB-231 and MCF-7 cells, the
CA9 mRNA expression levels were reduced by 79 to 87%
(P<0.01) 48 h after transfection with CA9 siRNA (Fig. 2A). Moreover, CA9 siRNA
continued to suppress CA9 mRNA expression 72 h after siRNA
transfection (data not shown). Due to the long half-life of CAIX
protein, we observed a strong reduction in CAIX protein level in
both cell lines 72 h after transfection with CA9 siRNA
(Fig. 2B and C). Additionally, the
sulforhodamine B (SRB) assay showed that CA9 knockdown did
not affect the proliferation of breast cancer cells (Fig. 2E). However, poly(ADP-ribose)
polymerase (PARP) cleavage, a marker of apoptosis, increased after
treatment with CA9 siRNA in both cell lines (Fig. 2D). In MDA-MB-231 cells, CA9
siRNA reduced clonogenic survival by 54% (P=0.05), and in MCF-7
cells, treatment with CA9 siRNA resulted in reduced
clonogenic survival of ~40% (P=0.3) (Fig. 3F).
In a wound scratch assay, the poorly invasive MCF-7
cells migrated to a lower extent compared to highly invasive
MDA-MB-231 cells (data not shown). The migration of MDA-MB-231
cells was significantly inhibited by 50% (P=0.008) after CA9
knockdown (Fig. 2G). In contrast,
migration of MCF-7 cells was reduced by 20% after transfection with
CA9 siRNA (P=0.02). A subsequent flow cytometric analysis
revealed that CAIX inhibition decreased the number of MDA-MB-231
cells in the G0/G1 phase (P=0.005) and
increased the number of cells in the G2/M phase (P=0.2) (Fig. 2H). In contrast, inhibition of CAIX
expression in MCF-7 cells did not affect cell cycle distribution
(Fig. 2H).
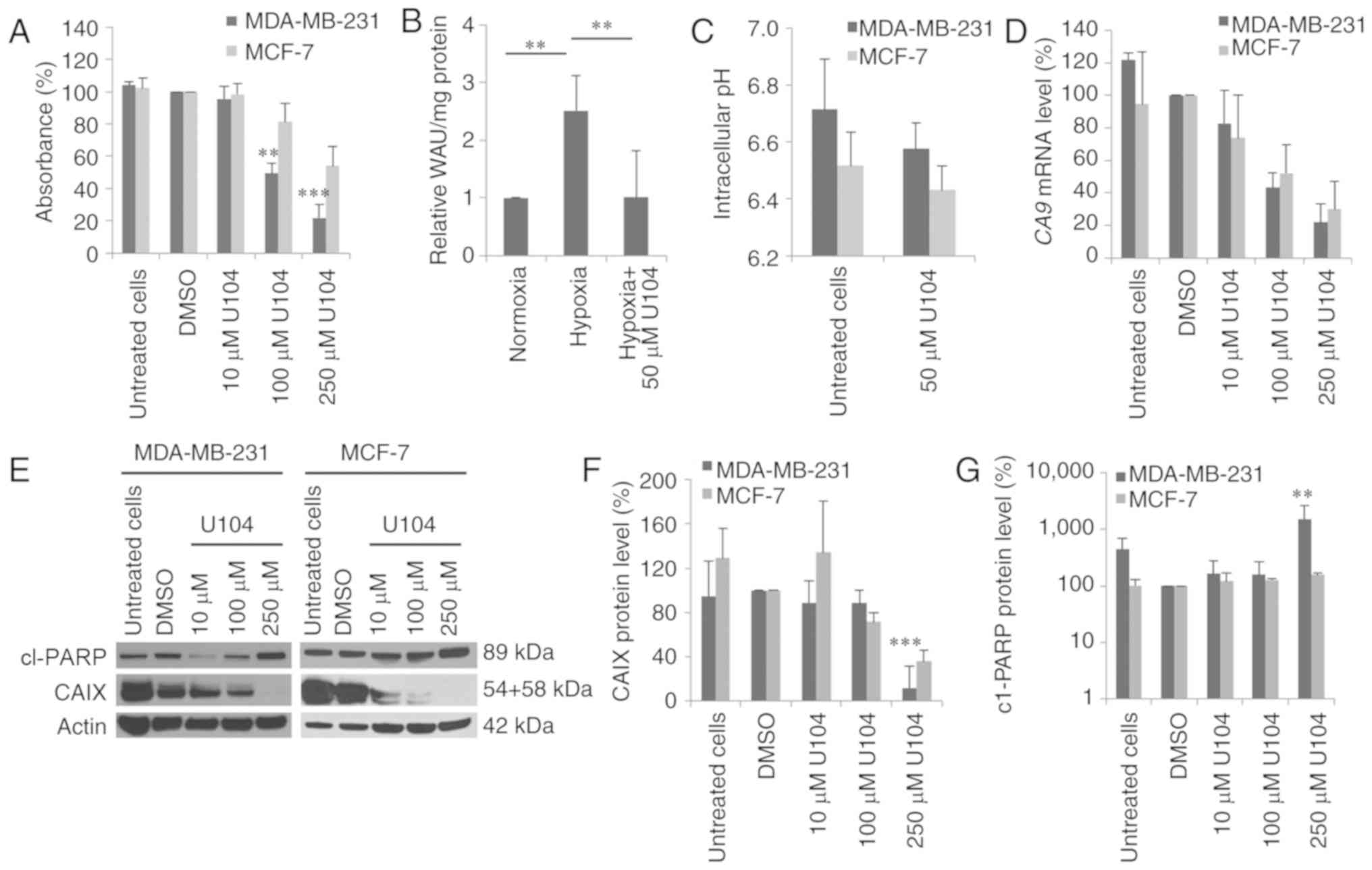
CAIX inhibition with U104
Inhibition of CAIX was also performed by an
alternative strategy, namely treatment with the CAIX inhibitor
U104, which specifically inhibits CAIX activity. Cytotoxicity
assays revealed that U104 was more cytotoxic in MDA-MB-231 cells
than in MCF-7 cells (Fig. 3A). The
concentration that reduced survival by half (IC50) was
determined using a dose-response curve fitting. Specifically, the
IC50 of U104 was 112.6±21.8 µM in MDA-MB-231 cells,
whereas in MCF-7 cells the IC50 was much higher
(306.9±37.9 µM; P<0.001). The measurement of the CAIX activity
in MDA-MB-231 cells revealed a 2.5-fold increase in the CAIX
activity under hypoxic conditions compared to normoxic conditions
(P=0.002). Treatment with 50 µM U104 reduced CAIX activity to basal
normoxic CAIX activity level (P=0.002) (Fig. 3B). In MCF-7 cells, no change in the
CAIX activity was detected after exposure to hypoxia (data not
shown). Additionally, we investigated the pHi after U104 treatment.
The extracellular pH (pHe) was adjusted to pH 6.6, and untreated
MDA-MB-231 cells had a pHi of 6.71 (Fig. 3C). Treatment with 50 µM U104 reduced
the pHi by 0.13 pH units to 6.58 (P=0.2). Furthermore, in MCF-7
cells a lower effect of treatment with CAI U104 on pHi was observed
compared to MDA-MB-231 cells. The untreated MCF-7 cells had a pHi
of 6.52, which was reduced by 0.09 pH units (P=0.4) after
incubation with U104 (Fig. 3C).
Moreover, inhibition of CAIX with U104 reduced the
CA9 mRNA expression level (Fig.
3D). In particular, the CA9 mRNA level in MDA-MB-231
cells was reduced by 60 and 80% 72 h after treatment with 100 and
250 µM U104, respectively. In MCF-7 cells, the CA9 mRNA
level was reduced to a lesser extent by 50 and 70% 72 h after
treatment with 100 and 250 µM, respectively (Fig. 3D). Additionally, the CAIX protein
level was decreased in both cell lines 72 h after incubation with
10 and 100 µM U104, and CAIX protein could no longer be detected
after incubation with 250 µM U104 (MDA-MB-231: P=0.0009) (Fig. 3E and F). PARP cleavage was
significantly increased after treatment with U104 in MDA-MB-231
cells (P=0.001) (Fig. 3E and
G).
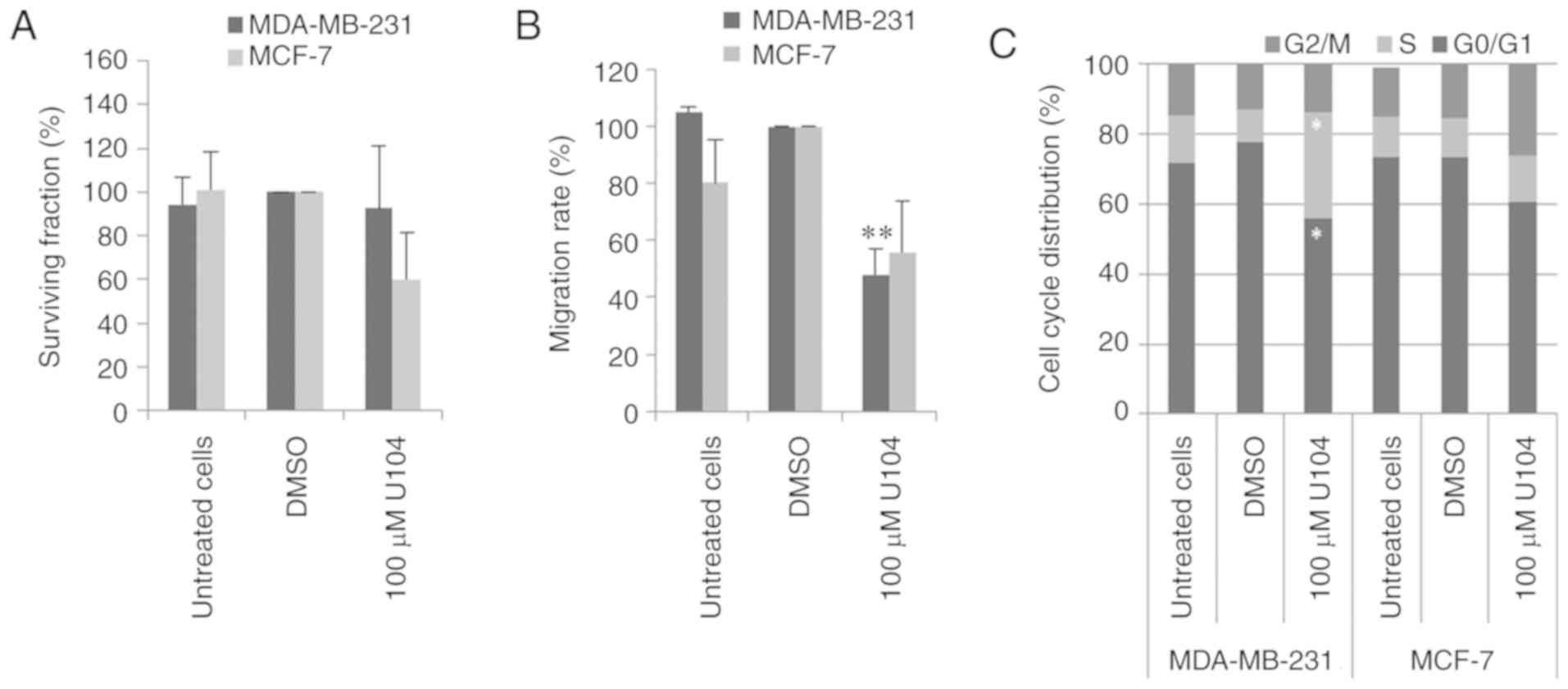
CAIX inhibition by U104 did not affect the
clonogenic survival of MDA-MB-231 cells but reduced the clonogenic
survival of MCF-7 cells by 55% (P=0.2) (Fig. 4A). Incubation with U104 reduced the
migration rate of MDA-MB-231 and MCF-7 cells by 52% (P=0.008) and
44% (P=0.8), respectively (Fig.
4B). Investigation of the cell cycle distribution of MDA-MB-231
cells revealed an increased number of cells in the S phase and a
statistically significantly reduced number of cells in
G0/G1 (P=0.02 and P=0.01, respectively) after
treatment with CAI U104 (Fig. 4C).
However, CAIX inhibition with U104 increased the number of MCF-7
cells in G2/M and reduced the number of MCF-7 cells in
G0/G1 (P=0.1 and P=0.1, respectively)
(Fig. 4C).
Radiosensitivity after CAIX
inhibition
Combination of CAIX inhibition by siRNA and
irradiation had little or no effects on the radiosensitivity of
MDA-MB-231 and MCF-7 cells, respectively (Fig. 5A and C). Specifically, a DMF10 and
EF10Gy of 1.10±0.17 (P=0.9) and 1.79±0.36 (P=0.4) were
calculated for MDA-MB-231 cells and a DMF10 and EF10Gy
of 0.97±0.36 (P=0.9) and 0.82±0.7 (P=0.9) for MCF-7 cells.
Inhibition of CAIX with CAI U104 revealed stronger effects on the
radiosensitivity of MDA-MB-231 and MCF-7 cells (Fig. 5B and D). The DMF10 and
EF10Gy were 1.57±0.16 (P=0.05) and 2.62±0.09 (P=0.02) in
MDA-MB-231 cells. In MCF-7 cells a DMF10 of 1.77±0.65 (P=0.2) and
an EF10Gy of 4.27±2.80 (P=0.1) was calculated.
Discussion
Hypoxia-regulated protein carbonic anhydrase IX
(CAIX) is associated with tumor-relevant processes, such as
migration, proliferation and invasion and its expression correlates
with the prognosis of cancer patients (27,28).
Although CAIX is primarily responsible for the acidification of the
tumor environment, we did not identify an altered CA9 mRNA
expression but a slightly reduced CAIX protein level in response to
acidic (pH 6.6) and hypoxic (0.1% O2) culture conditions
in both breast cancer cell lines (Fig.
1). Recently, we detected similar effects in AT1 prostate
cancer cells (29). In contrast to
our investigations, Tang et al observed a decrease in
CA9 mRNA level in MCF-7 cells after incubation with lactic
acid under hypoxic conditions (30). On the other hand, we previously
showed that the response of CAIX expression to acidic conditions
(upregulated or downregulated) depends on the cell line (31). For example, culturing glioblastoma
and osteosarcoma cells under acidic conditions (pH 6.4) increased
CA9 gene transcription and CAIX protein level under normoxic
and hypoxic conditions (32,33).
Therefore, acidic conditions-in contrast to hypoxia-have different,
cell-type specific consequences in human tumor cells.
With RNA interference and CAI U104, two different
strategies were used to target CAIX under hypoxic conditions in two
breast cancer cell lines. Both methods resulted in decreased
CA9 mRNA and CAIX protein levels (Figs. 2 and 3). These effects are in line with previous
reports that also demonstrated reductions in CA9 mRNA and
CAIX protein level in tumor cells in response to treatment with RNA
interference or CAI U104 (10,21,22,34–36).
CAI-induced changes were attributed to the possible degradation or
internalization of CAIX (21,36).
In addition, treatment with CAI U104 decreased the pHi and reduced
CAIX activity in MDA-MB-231 breast cancer cells (Fig. 3B and C). Accordingly, the pHi of
CAIX-expressing fibroblasts was previously shown to be more
alkaline than the pHi of fibroblasts that do not express CAIX
(8). Contrary to MDA-MB-231 cells,
a hypoxia-induced increase in CAIX activity was lacking in MCF-7
breast cancer cells. It is conceivable that the CAIX activity assay
is insufficiently sensitive for measurement of this slight increase
of CAIX protein level in MCF-7 cells after hypoxic exposure. In
agreement with this, Meehan et al detected only partial
activation of HIF signaling in acutely hypoxic MCF-7 cells
(22).
In the present study, RNA interference or CAI U104
affected migration, clonogenic survival, cell cycle distribution
and apoptosis in both breast cancer cell lines (Figs. 2–4).
Other studies investigating various ureido-sulfamate CAIX
inhibitors also revealed a reduction in the migration, invasion or
metastasis of breast cancer cells in vitro or in xenograft
models (10,37). In soft tissue sarcoma cells
(HT-1080), the depletion of CAIX expression decreased migration,
invasion and expansive growth (9).
CAIX influences the expression of genes that regulate several
processes, such as cell motility, cell-cell contact, focal adhesion
formation and epithelial-mesenchymal transition (38). In cervical carcinoma cells, CAIX
increased migration and invasion via the CA9-dependent
inactivation of Rho-GTPase (39).
However, CA9 knockdown did not affect the proliferation of
breast cancer cells after 3 days (Fig.
2E). Initial studies of breast cancer cell lines revealed that
silencing of CA9 with siRNAs inhibited long-term
proliferation (after 6 days) and clonogenic survival under hypoxic
conditions (34). In confirmation
with our results concerning ureidosulfonamide U104, Dubois et
al observed a decrease in proliferation and induction of
apoptosis after inhibiting CAIX in colorectal cancer (16). The decreased pHi may be responsible
for the decreased proliferation and increased apoptosis rate
(16,28). This study of rat prostate cancer
cells confirmed the intracellular acidification, anti-proliferative
and pro-apoptotic effects of CAIX inhibition with CAI U104
(29). In addition, recently, our
previous results confirmed CAI-induced apoptosis for betulinyl
sulfamates in MDA-MB-231 and MCF7 cells using different methods
(21,40). A further study detected CAI-induced
apoptosis in different cancer cell lines (41). However, the molecular mechanism
underlying this effect remains to be elucidated.
The effects of RNA interference or CAI U104 were
even stronger in the basal triple-negative MDA-MB-231 breast cancer
cells compared to luminal MCF-7 cells (Figs. 2–4).
In agreement with our results, silencing of CA9 with shRNA
revealed a stronger inhibition of invasion and increased
doxorubicin-mediated reduction of spheroid-forming ability in
triple-negative MDA-MB-231 cells compared to breast cancer cell
lines with other subtypes (18). In
addition, the prognostic significance of the CA9 mRNA level
differed depending on the subtype of breast cancer (18). For generalization of these effects,
further breast cancer cell lines with each intrinsic breast cancer
subtype must be analyzed.
We determined additive/synergistic effects after
CA9 knockdown or U104-induced CAIX inhibition in combination
with irradiation in MDA-MB-231 and MCF-7 breast cancer cells under
hypoxic conditions (Fig. 5).
Initial studies of a colorectal carcinoma xenograft model revealed
that CAIX inhibition with different CAIX inhibitors (CAI:
Acetazolamide, DH348 or 11c) or CA9 knockdown by shRNA
delayed tumor growth and radiosensitized tumor cells (16,17).
These findings corroborate other studies showing that inhibition of
carbonic anhydrases with different CAIs radiosensitized different
tumor cell lines (21,35,42).
This effect may be due to the reduced acidification of the
extracellular environment (16,17) or
the intracellular acidification (Fig.
3C) caused by CAIX inhibition. Fibroblasts lacking CAIX were
strongly radiosensitized when cultured under acidic conditions (pH
7.0) compared to normal conditions (pH 7.5) because they cannot
maintain their intracellular pH (42). However, this effect could be
abrogated by stably transfecting cells with CAIX. In further
studies we plan investigations with stable knockdown of the CAIX
gene in mammary carcinoma cell lines. It should be noted that the
effects of CAI U104 on radiosensitization were stronger than the
effects caused by RNAi, especially in MCF-7 cells. The isoenzyme
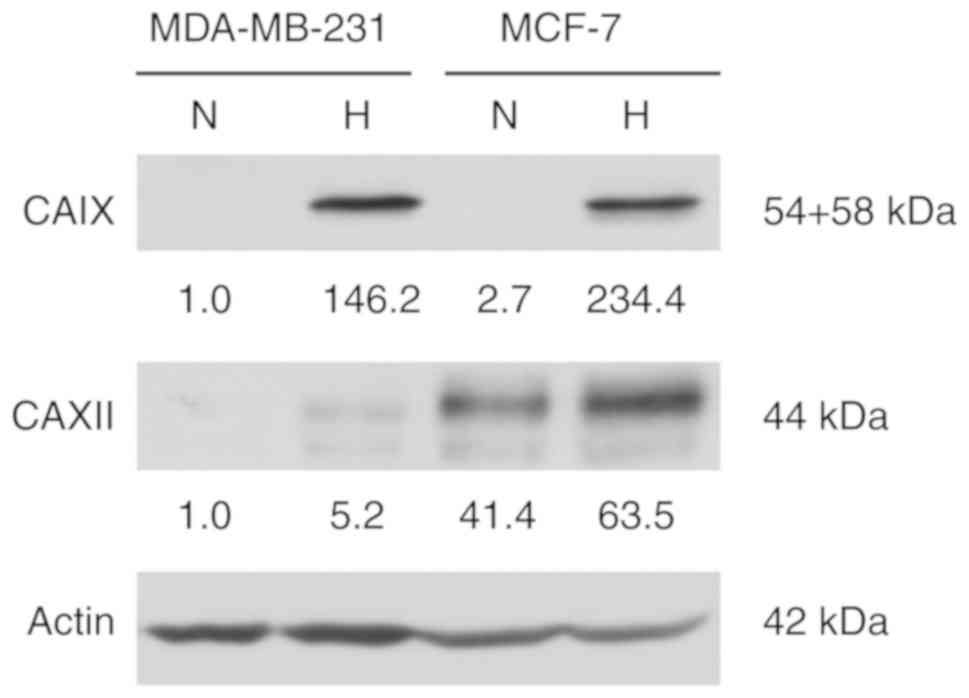
CAXII is expressed at higher levels in MCF-7 cells compared to
MDA-MB-231 cells under normoxic and hypoxic conditions (Fig. 6). The effects of CAIX inhibition by
siRNA may be compensated by CAXII, since siRNA selectively
inhibited CA9, not CA12 mRNA expression. However, CAI
U104 inhibited activity of both isoenzymes CAIX and CAXII and
therefore caused stronger effects on radiosensitivity (10). In accordance with that, combined
knockdown of CA9 and CA12 gene expression revealed
stronger radiosensitization of colon carcinoma cells than single
knockdown of CA9 or CA12 gene expression (42). Due to possible compensatory effects
of CAXII, investigation of CAXII function in tumor relevant
processes is warranted.
In summary, it was demonstrated that specifically
targeting CA9 or inhibiting CAIX/CAXII activity influences
intracellular and extracellular pH that is important for clonogenic
survival, apoptosis, migration and radiosensitivity of both breast
cancer cell lines. CAIX alone and in combination with CAXII are
significant targets for novel combination strategies with
radiotherapy in breast cancer.
Acknowledgements
We would like to thank our colleagues from the
Department of Radiotherapy and the Julius Bernstein Institute of
Physiology for their contribution to this study and their
continuous support. We would also like to thank Gabriele Thomas,
Kathrin Theile and Sarah Reime for their excellent technical
assistance.
Funding
The present study was supported by the Wilhelm
Sander Stiftung (grant no. FKZ: 2013.090.1).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
AG designed the study, performed the experimental
procedures, analyzed the data and drafted the manuscript. KT
performed the experimental procedures and analyzed the data. AR, HW
and JK substantially contributed to the data acquisition and
interpretation and reviewed the manuscript. OT, MB and DV designed
the study, substantially contributed to the acquisition and
interpretation of the data and reviewed the manuscript. All authors
read and approved the manuscript and agree to be accountable for
all aspects of the research in ensuring that the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors state that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CAIX
|
carbonic anhydrase IX
|
|
CAI
|
carbonic anhydrase inhibitor
|
|
pHe
|
extracellular pH
|
|
pHi
|
intracellular pH
|
|
PARP
|
poly(ADP-ribose)-polymerase
|
|
HPRT
|
hypoxanthine-guanine
phosphoribosyltransferase
|
|
POLR2A
|
DNA-directed RNA polymerase II
subunit A
|
|
BCECF
|
2′,7′-bis-
(2-carboxyethyl)-5-(and-6)-carboxyfluorescein, acetoxymethyl
ester
|
|
SRB
|
sulforhodamine B
|
|
DMF10
|
dose-modifying factor 10%
|
|
EF
|
enhancement factor
|
References
|
1
|
Brown JM and Wilson WR: Exploiting tumour
hypoxia in cancer treatment. Nat Rev Cancer. 4:437–447. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gatenby RA and Gillies RJ: Why do cancers
have high aerobic glycolysis? Nat Rev Cancer. 4:891–899. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brown JM and Giaccia AJ: The unique
physiology of solid tumors: Opportunities (and problems) for cancer
therapy. Cancer Res. 58:1408–1416. 1998.PubMed/NCBI
|
|
5
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wykoff CC, Beasley NJ, Watson PH, Turner
KJ, Pastorek J, Sibtain A, Wilson GD, Turley H, Talks KL, Maxwell
PH, et al: Hypoxia-inducible expression of tumor- associated
carbonic anhydrases. Cancer Res. 60:7075–7083. 2000.PubMed/NCBI
|
|
7
|
Swietach P, Vaughan-Jones RD and Harris
AL: Regulation of tumor pH and the role of carbonic anhydrase 9.
Cancer Metastasis Rev. 26:299–310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lou Y, McDonald PC, Oloumi A, Chia S,
Ostlund C, Ahmadi A, Kyle A, Auf dem Keller U, Leung S, Huntsman D,
et al: Targeting tumor hypoxia: Suppression of breast tumor growth
and metastasis by novel carbonic anhydrase IX inhibitors. Cancer
Res. 71:3364–3376. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Radvak P, Repic M, Svastova E, Takacova M,
Csaderova L, Strnad H, Pastorek J, Pastorekova S and Kopacek J:
Suppression of carbonic anhydrase IX leads to aberrant focal
adhesion and decreased invasion of tumor cells. Oncol Rep.
29:1147–1153. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chiche J, Ilc K, Laferrière J, Trottier E,
Dayan F, Mazure NM, Brahimi-Horn MC and Pouysségur J:
Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell
growth by counteracting acidosis through the regulation of the
intracellular pH. Cancer Res. 69:358–368. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
van Kuijk SJA, Yaromina A, Houben R,
Niemans R, Lambin P and Dubois LJ: Prognostic significance of
carbonic anhydrase IX expression in cancer patients: A
meta-analysis. Front Oncol. 6:692016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hussain SA, Ganesan R, Reynolds G, Gross
L, Stevens A, Pastorek J, Murray PG, Perunovic B, Anwar MS,
Billingham L, et al: Hypoxia-regulated carbonic anhydrase IX
expression is associated with poor survival in patients with
invasive breast cancer. Br J Cancer. 96:104–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chia SK, Wykoff CC, Watson PH, Han C, Leek
RD, Pastorek J, Gatter KC, Ratcliffe P and Harris AL: Prognostic
significance of a novel hypoxia-regulated marker, carbonic
anhydrase IX, in invasive breast carcinoma. J Clin Oncol.
19:3660–3668. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brennan DJ, Jirstrom K, Kronblad A,
Millikan RC, Landberg G, Duffy MJ, Rydén L, Gallagher WM and
O'Brien SL: CA IX is an independent prognostic marker in
premenopausal breast cancer patients with one to three positive
lymph nodes and a putative marker of radiation resistance. Clin
Cancer Res. 12:6421–6431. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jin MS, Lee H, Park IA, Chung YR, Im SA,
Lee KH, Moon HG, Han W, Kim K, Kim TY, et al: Overexpression of
HIF1α and CAXI predicts poor outcome in early-stage triple negative
breast cancer. Virchows Arch. 469:183–190. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dubois L, Peeters SG, van Kuijk SJ,
Yaromina A, Lieuwes NG, Saraya R, Biemans R, Rami M, Parvathaneni
NK, Vullo D, et al: Targeting carbonic anhydrase IX by
nitroimidazole based sulfamides enhances the therapeutic effect of
tumor irradiation: A new concept of dual targeting drugs. Radiother
Oncol. 108:523–528. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dubois L, Peeters S, Lieuwes NG, Geusens
N, Thiry A, Wigfield S, Carta F, McIntyre A, Scozzafava A, Dogné
JM, et al: Specific inhibition of carbonic anhydrase IX activity
enhances the in vivo therapeutic effect of tumor irradiation.
Radiother Oncol. 99:424–431. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ivanova L, Zandberga E, Siliņa K, Kalniņa
Z, Ābols A, Endzeliņš E, Vendina I, Romanchikova N, Hegmane A,
Trapencieris P, et al: Prognostic relevance of carbonic anhydrase
IX expression is distinct in various subtypes of breast cancer and
its silencing suppresses self-renewal capacity of breast cancer
cells. Cancer Chemother Pharmacol. 75:235–246. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McDonald PC, Chafe SC and Dedhar S:
Overcoming hypoxia-mediated tumor progression: Combinatorial
approaches targeting pH regulation, angiogenesis and immune
dysfunction. Front Cell Dev Biol. 4:272016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Winum JY, Pastorekova S, Jakubickova L,
Montero JL, Scozzafava A, Pastorek J, Vullo D, Innocenti A and
Supuran CT: Carbonic anhydrase inhibitors: Synthesis and inhibition
of cytosolic/tumor-associated carbonic anhydrase isozymes I, II,
and IX with bis-sulfamates. Bioorg Med Chem Lett. 15:579–584. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bache M, Münch C, Güttler A, Wichmann H,
Theuerkorn K, Emmerich D, Paschke R and Vordermark D: Betulinyl
sulfamates as anticancer agents and radiosensitizers in human
breast cancer cells. Int J Mol Sci. 16:26249–26262. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Meehan J, Ward C, Turnbull A,
Bukowski-Wills J, Finch AJ, Jarman EJ, Xintaropoulou C,
Martinez-Perez C, Gray M, Pearson M, et al: Inhibition of pH
regulation as a therapeutic strategy in hypoxic human breast cancer
cells. Oncotarget. 8:42857–42875. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kessler J, Hahnel A, Wichmann H, Rot S,
Kappler M, Bache M and Vordermark D: HIF-1α inhibition by siRNA or
chetomin in human malignant glioma cells: Effects on hypoxic
radioresistance and monitoring via CA9 expression. BMC Cancer.
10:6052010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Güttler A, Giebler M, Cuno P, Wichmann H,
Keßler J, Ostheimer C, Söling A, Strauss C, Illert J, Kappler M, et
al: Osteopontin and splice variant expression level in human
malignant glioma: Radiobiologic effects and prognosis after
radiotherapy. Radiother Oncol. 108:535–540. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Riemann A, Ihling A, Thomas J, Schneider
B, Thews O and Gekle M: Acidic environment activates inflammatory
programs in fibroblasts via a cAMP-MAPK pathway. Biochim Biophys
Acta. 1853:299–307. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hahnel A, Wichmann H, Kappler M, Kotzsch
M, Vordermark D, Taubert H and Bache M: Effects of osteopontin
inhibition on radiosensitivity of MDA-MB-231 breast cancer cells.
Radiat Oncol. 5:822010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McDonald PC, Winum JY, Supuran CT and
Dedhar S: Recent developments in targeting carbonic anhydrase IX
for cancer therapeutics. Oncotarget. 3:84–97. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pastorek J and Pastorekova S:
Hypoxia-induced carbonic anhydrase IX as a target for cancer
therapy: From biology to clinical use. Semin Cancer Biol. 31:52–64.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Riemann A, Güttler A, Haupt V, Wichmann H,
Reime S, Bache M, Vordermark D and Thews O: Inhibition of carbonic
anhydrase IX by ureidosulfonamide inhibitor U104 reduces prostate
cancer cell growth, but does not modulate daunorubicin or cisplatin
cytotoxicity. Oncol Res. 26:191–200. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tang X, Lucas JE, Chen JL, LaMonte G, Wu
J, Wang MC, Koumenis C and Chi JT: Functional interaction between
responses to lactic acidosis and hypoxia regulates genomic
transcriptional outputs. Cancer Res. 72:491–502. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vordermark D, Kaffer A, Riedl S, Katzer A
and Flentje M: Characterization of carbonic anhydrase IX (CA IX) as
an endogenous marker of chronic hypoxia in live human tumor cells.
Int J Radiat Oncol Biol Phys. 61:1197–1207. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Matsubara T, Diresta GR, Kakunaga S, Li D
and Healey JH: Additive influence of extracellular pH, oxygen
tension, and pressure on invasiveness and survival of human
osteosarcoma cells. Front Oncol. 3:1992013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ihnatko R, Kubes M, Takacova M, Sedlakova
O, Sedlak J, Pastorek J, Kopacek J and Pastorekova S: Extracellular
acidosis elevates carbonic anhydrase IX in human glioblastoma cells
via transcriptional modulation that does not depend on hypoxia. Int
J Oncol. 29:1025–1033. 2006.PubMed/NCBI
|
|
34
|
Said HM, Hagemann C, Carta F, Katzer A,
Polat B, Staab A, Scozzafava A, Anacker J, Vince GH, Flentje M and
Supuran CT: Hypoxia induced CA9 inhibitory targeting by two
different sulfonamide derivatives including acetazolamide in human
glioblastoma. Bioorg Med Chem. 21:3949–3957. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Duivenvoorden WCM, Hopmans SN, Gallino D,
Farrell T, Gerdes C, Glennie D, Lukka H and Pinthus JH: Inhibition
of carbonic anhydrase IX (CA9) sensitizes renal cell carcinoma to
ionizing radiation. Oncol Rep. 34:1968–1976. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Robertson N, Potter C and Harris AL: Role
of carbonic anhydrase IX in human tumor cell growth, survival, and
invasion. Cancer Res. 64:6160–6165. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ward C, Meehan J, Mullen P, Supuran C,
Dixon JM, Thomas JS, Winum J-Y, Lambin P, Dubois L, Pavathaneni NK,
et al: Evaluation of carbonic anhydrase IX as a therapeutic target
for inhibition of breast cancer invasion and metastasis using a
series of in vitro breast cancer models. Oncotarget. 6:24856–24870.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sedlakova O, Svastova E, Takacova M,
Kopacek J, Pastorek J and Pastorekova S: Carbonic anhydrase IX, a
hypoxia-induced catalytic component of the pH regulating machinery
in tumors. Front Physiol. 4:4002014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shin HJ, Rho SB, Jung DC, Han IO, Oh ES
and Kim JY: Carbonic anhydrase IX (CA9) modulates tumor-associated
cell migration and invasion. J Cell Sci. 124:1077–1087. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Vanchanagiri K, Emmerich D, Bruschke M,
Bache M, Seifert F, Csuk R, Vordermark D and Paschke R: Synthesis
and biological investigation of new carbonic anhydrase IX (CAIX)
inhibitors. Chem Biol Interact. 284:12–23. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cianchi F, Vinci MC, Supuran CT, Peruzzi
B, De Giuli P, Fasolis G, Perigli G, Pastorekova S, Papucci L, Pini
A, et al: Selective inhibition of carbonic anhydrase IX decreases
cell proliferation and induces ceramide-mediated apoptosis in human
cancer cells. J Pharmacol Exp Ther. 334:710–719. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Doyen J, Parks SK, Marcié S, Pouysségur J
and Chiche J: Knock-down of hypoxia-induced carbonic anhydrases IX
and XII radiosensitizes tumor cells by increasing intracellular
acidosis. Front Oncol. 2:1992013. View Article : Google Scholar : PubMed/NCBI
|