Introduction
Lung cancer, which is frequently diagnosed in both
males and females, is the leading cause of cancer-related deaths
worldwide (1,2). In 2008, there were more than 1.6
million lung cancer cases diagnosed, comprising ~12.7% of all new
cancer cases (3). In general, small
cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC) are
the two major types of lung cancer. Evidence suggests that tobacco
smoking is the main cause of lung cancer, accounting for probably
90% of all lung cancer diagnoses (4,5). The
risk of lung cancer among active smokers is 10-fold higher than
that among non-smokers (6). Despite
improved survival intervals, the survival rate for lung cancer is
still discouragingly low (7).
Migration and invasion are widely recognized as two
major hallmarks of malignancy and are closely related to the
biological behavior of cancer cells. Actin, a main component of the
cytoskeleton, plays an important role in cell movement (8,9). The
Ras homologue GTPase activation protein 6 (ARHGAP6) is a novel Rho
GTPase-activating protein (RhoGAP) gene that plays a crucial role
in regulating actin polymerization in several cellular processes,
including tumor growth and metastasis (10,11).
It was reported that ARHGAP6 inhibited the proliferation and
metastasis of cervical carcinoma, caused cell cycle arrest, and
induced cell apoptosis (12).
ARHGAP6 isoform 1 variant, an Hb3 antigen, may be a novel biomarker
of the progression of colorectal cancer (CRC); 8.3% of patients
with CRC develop pulmonary metastases despite undergoing curative
surgery (13). However, the role of
ARHGAP6 expression in lung cancer remains unclear. Matrix
metalloproteinase-9 (MMP9) and vascular endothelial growth factor
(VEGF) are commonly highly expressed in lung cancer and play an
important role in lung cancer progression (14–16).
Studies have associated MMP9 expression to tumor growth, metastasis
and angiogenesis (17,18). VEGF is a major growth factor
involved in angiogenesis, and VEGF-mediated angiogenesis is a
crucial process for tumor growth and metastasis (19,20).
In addition, signal transducer and activator of transcription 3
(STAT3), a transcription factor, has been found to be activated in
lung cancer (21). Indirectly or
directly inhibiting STAT3 activity affects the transcription of
several genes, including VEGF, somewhat inhibits angiogenesis and
affects the formation of tumors (22,23). A
previous study reported that interleukin 6 (IL-6) acted as a strong
activator of STAT3 signaling in lung cancer (24). IL-6 is a key cytokine frequently
upregulated in cancer and considered a mediator of malnutrition in
lung cancer patients (25,26).
In this study, significantly reduced ARHGAP6 levels
were observed in tumor tissues from patients with lung cancer,
accompanied by high levels of MMP9 and VEGF, which indicated that
ARHGAP6 was involved in lung cancer progression. In vitro
upregulation of ARHGAP6 significantly inhibited the growth and
metastasis of A549 and H1299 cells, accompanied by decreased
protein levels of MMP9, VEGF and p-STAT3. Moreover, IL-6-induced
migration, invasion and expression of MMP9, VEGF and p-STAT3 were
suppressed by ARHGAP6 upregulation. These results indicated that
ARHGAP6 upregulation may benefit lung cancer patients through the
suppression of MMP9, VEGF and STAT3 signaling.
Materials and methods
Lung cancer tissue and adjacent normal
tissue
After written informed consent was obtained,
twenty-five pairs of tumor and adjacent normal tissue from lung
cancer patients (13 men and 12 women among the age of 50–80 years)
treated at Liaoning Cancer Hospital and Institute (Liaoning, China)
were collected from May to December 2016 and immediately frozen in
liquid nitrogen. After pretreatment, the expression of ARHGAP6,
MMP9 and VEGF in these samples was detected by real-time PCR. All
experiments in this study were approved by the Ethics Committee of
Liaoning Cancer Hospital and Institute.
Cell culture
The A549 and H1299 human lung cancer cell lines and
the 16HBE pulmonary epithelial cell line were purchased from the
Cell Bank of the Chinese Academy of Sciences (Shanghai, China).
These cells were cultured in RPMI-1640 medium (product no.
SH30809.01B; HyClone Laboratories; GE Healthcare Life Sciences,
Logan, UT, USA) containing 10% fetal bovine serum (cat. no.
16000-044; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
and 1% antibiotics (100X, a mixture of penicillin and streptomycin;
product code: P1400-100; Beijing Solarbio Science & Technology
Co., Ltd., Beijing, China) in a 5% CO2 incubator (Thermo
Forma 3111; Thermo Fisher Scientific, Inc.) at 37°C. The medium was
replaced according to the growth demands of the cells during
incubation.
Construction of the lentivirus
The mRNA sequence of the target gene was queried in
NCBI, and primers and restriction sites were then designed for the
coding sequence (CDS) and the selected vector. The 2925-bp
full-length CDS of ARHGAP6 (NM_013427.2) synthesized by Genewiz
Company (Shanghai, China) was inserted into the
EcoRI/BamHI restriction sites of the pLVX-Puro
plasmid, and the resulting plasmid was confirmed by DNA sequencing
(Majorbio Bio-Pharm Technology Co., Ltd., Shanghai, China). The
core plasmid pLVX-Puro-ARHGAP6 and the viral packaging plasmids
psPAX2 and pMD2G (Addgene, Inc., Cambridge, MA, USA) were
co-transfected into 293T cells with Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.). After 48 h of transfection, the
virus particles in the medium were collected (15).
Experimental groups
To upregulate the expression of ARHGAP6 in the A549
and H1299 cell lines, lentivirus-mediated RNA overexpression was
used. A549 and H1299 cells were divided into three groups: Infected
with medium (control), infected with negative control lentivirus
(vector) and infected with ARHGAP6 recombinant lentivirus
(oe-ARHGAP6). After 48 h of infection, real-time PCR, western blot
analysis and Transwell assays were carried out. In addition,
proliferation assays were performed at 0, 24, 48 and 72 h.
To further investigate the effects of ARHGAP6 on
lung cancer cells, A549 and H1299 cells were divided into three
groups and then treated with medium (control), negative control
lentivirus and 50 ng/ml IL-6 (Vector + IL-6), or ARHGAP6
recombinant lentivirus and 50 ng/ml IL-6 (oe-ARHGAP6 + IL-6). After
48 h of treatment, western blot analysis and Transwell assays were
carried out.
Proliferation assay
A549 and H1299 cells in the logarithmic growth phase
were digested with 0.25% trypsin (Beijing Solarbio Science &
Technology Co., Ltd.), inoculated in triplicate in 96-well culture
plates at a density of 3×103 cells/well, and cultured in
a humidified incubator overnight at 37°C with 5% CO2.
The following day, the cells were infected with medium, negative
control lentivirus, or ARHGAP6 recombinant lentivirus, and after 0,
24, 48 and 72 h of infection, 100 µl of 10% Cell Counting Kit-8
(CCK-8; SAB, College Park, MD, USA) solution in serum-free medium
was added to each well, and the plates were then placed in an
incubator for 1 h. The absorbance at 450 nm was assessed by a
microplate reader (Perlong Medical Equipment Co., Ltd., Beijing,
China).
Real-time polymerase chain reaction
(RT-PCR) assay
Total RNA was isolated from lentivirus-infected A549
and H1299 cells by TRIzol (Invitrogen; Thermo Fisher Scientific,
Inc.), and RNA quality was confirmed by 1% agarose gel
electrophoresis after quantification. A reverse transcriptase kit
(Fermentas; Thermo Fisher Scientific, Inc.) was used to reverse
transcribe RNA into cDNA. RT-PCR with cDNA as a template was
conducted on an ABI PRISM 7300 instrument (Applied Biosystems;
Thermo Fisher Scientific, Inc.) using a SYBR-Green PCR kit (Thermo
Fisher Scientific, Inc.). The data were normalized to GAPDH, and
the mRNA level of ARHGAP6 was calculated by the 2−ΔΔCq
method (27). The primers were as
follows: ARHGAP6, 5′-GAATTTGACCGTGGGATTG-3′ and
5′-CAGGGAGGTAGAAGGTATATG-3′; GAPDH, 5′-AATCCCATCACCATCTTC-3′ and
5′-AGGCTGTTGTCATACTTC-3′. The RT-PCR procedure was as follows: 95°C
for 10 min, followed by 40 cycles at 95°C for 15 sec and 60°C for
45 sec; one cycle at 95°C for 15 sec and 60°C for 1 min; and one
cycle at 95°C for 15 sec and 60°C for 15 sec (28).
Western blot analysis
Treated A549 and H1299 cells were lysed in RIPA
buffer (Beijing Solarbio Science & Technology Co., Ltd.)
containing protease and phosphatase inhibitors and incubated for
~30 min on ice. The proteins in the supernatant of the cell lysates
were collected after centrifugation for 10 min at 12,000 × g at 4°C
and quantified by a BCA quantification kit (Thermo Fisher
Scientific, Inc.). After being separated by SDS-PAGE (JRDUN
Biotechnology Co., Ltd, Shanghai, China), the proteins on the gel
were transferred onto polyvinylidene fluoride (PVDF) membranes (EMD
Millipore, Billerica, MA, USA) by semidry electroblotting. The PVDF
membranes were blocked in 5% skimmed milk (BD Biosciences, Franklin
Lakes, NJ, USA) for 1 h at room temperature, followed by incubation
with primary antibodies against ARHGAP6 (1:1,000; cat. no.
NBPI-80837; Biocompare, San Francisco, CA, USA), MMP9 (1:1,000;
cat. no. ab38898), VEGF (1:2,000; cat. no. ab69479), STAT3 (1:100;
cat. no. ab50761), p-STAT3 (1:20,0000; cat. no. ab76315; all from
Abcam, Cambridge, MA, USA) or GAPDH (1:2,000; cat. no. 5174; Cell
Signaling Technology, Inc., Danvers, MA, USA) at 4°C overnight with
gentle shaking. After 5–6 washes with TBST, the membranes were
incubated with secondary antibodies (1:1,000; Beyotime Institute of
Biotechnology, Haimen, China) of goat anti-rabbit (cat. no. A0208)
and goat anti-mouse (cat. no. A0216) for 1 h at 37°C. Finally, the
target protein bands were visualized on an ECL imaging system
(Tanon-5200; Tanon Science and Technology Co., Ltd., Shanghai,
China) after a 5-min incubation with chemiluminescent detection
reagent (EMD Millipore) and the protein levels were calculated by
ImageJ software 1.47v (National Institutes of Health, Bethesda, MD,
USA).
Cell migration and invasion
assays
The migration and invasion activities of A549 and
H1299 cells were measured by Transwell assays using a modified
Boyden chamber (Transwell Costar; cat. no. 342; Corning Inc.,
Corning, NY, USA) as previously described (29). The 24-well plates and Transwell
chambers were soaked in 1X PBS for 5 min before inoculation (one
more step for the invasion assay: 80 µl of Matrigel was placed in
small chambers and clotted in a 37°C incubator for 30 min). After
24 h of serum starvation, infected and treated cells were digested
by trypsin, washed, resuspended in serum-free medium, and
inoculated in the upper chamber at a density of 5×104
cells/well. Then, 0.7 ml of RPMI-1640 medium containing 10% FBS as
a chemoattractant was added to the lower chamber. After 24 h of
incubation in a 37°C incubator, the migrating and invading cells on
the lower side of the membrane were fixed with 1 ml of 4%
formaldehyde for 10 min. After removing the fixative and washing
once with 1X PBS, the cells were incubated in 1 ml of 0.5% crystal
violet for 30 min, washed once with 1X PBS and dried. The number of
cells that migrated and invaded from the upper chamber to the lower
surface was counted with a microscope at ×200.
Statistical analysis
GraphPad Prism 7.0 (GraphPad Software, Inc., La
Jolla, CA, USA) was used to perform statistical analyses.
Quantitative data comparisons between two groups were analyzed by
paired Student's t-test (for parametric data), while differences
among multiple groups were analyzed by one-way analysis of variance
(ANOVA) followed by Tukey's multiple comparison. Pearson analysis
was used to determine correlations between two groups. The data are
presented as the mean ± SD of three independent experiments, and a
P-value of <0.05 was considered to indicate a statistically
significant difference.
Results
ARHGAP6 levels are significantly
decreased in tumor tissues from lung cancer patients
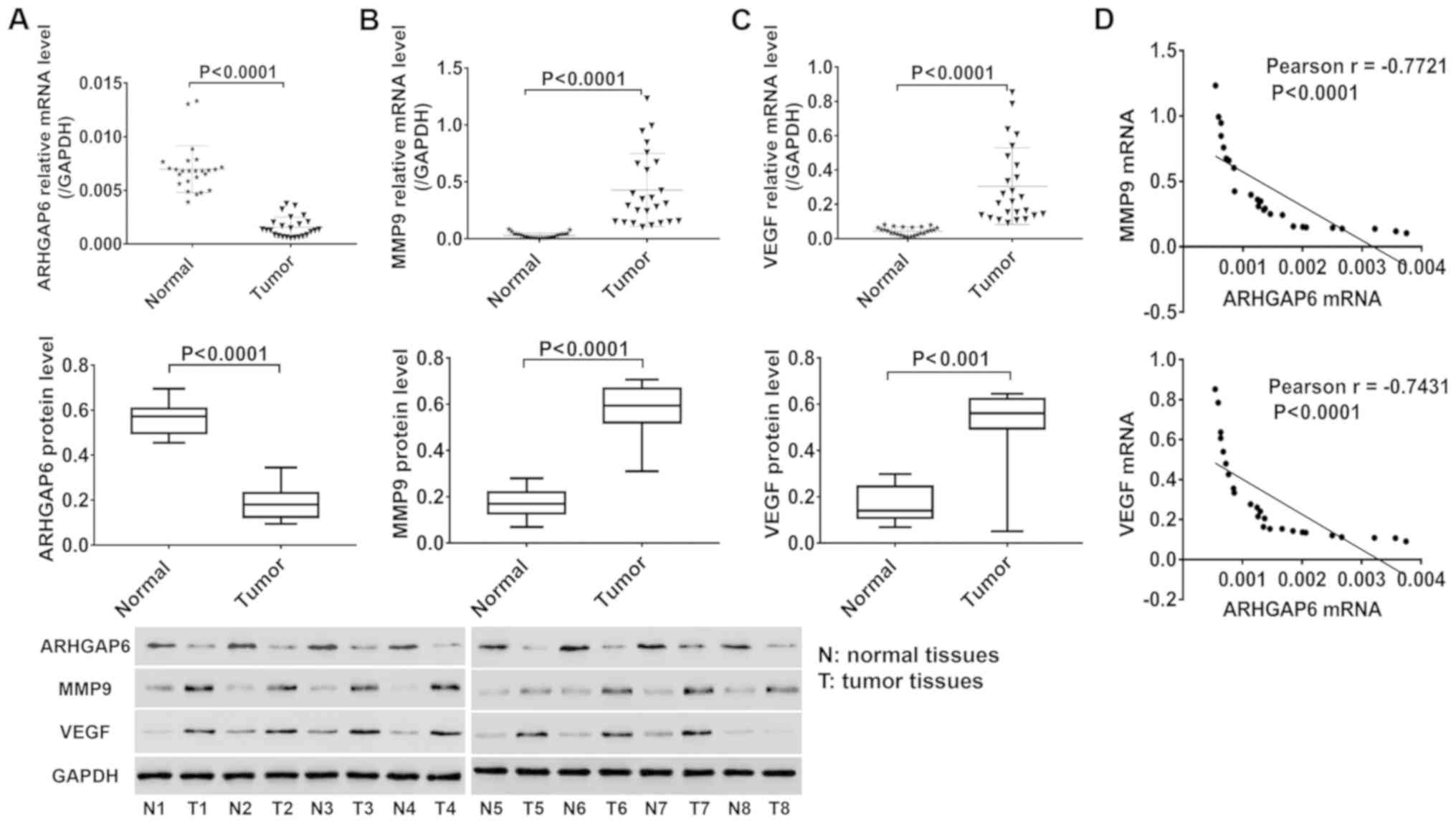
Twenty-five pairs of tumor and adjacent normal
tissues from lung cancer patients were collected, and the
expression of ARHGAP6, MMP9 and VEGF in these tissues was
quantified by RT-PCR and western blotting. As shown in Fig. 1, ARHGAP6 mRNA (Fig. 1A, upper image) and protein (Fig. 1A, lower image) levels were
significantly reduced in tumor tissues from lung cancer patients
compared to adjacent normal tissues, accompanied by significantly
increased levels of MMP9 (Fig. 1B)
and VEGF (Fig. 1C). Pearson
analysis revealed negative correlations between ARHGAP6 and MMP9
(Fig. 1D, upper image) and between
ARHGAP6 and VEGF (Fig. 1D, lower
image). These data indicated that ARHGAP6 may be implicated in the
progression of lung cancer.
The expression of ARHGAP6 in the
16HBE, A549 and H1299 cell lines
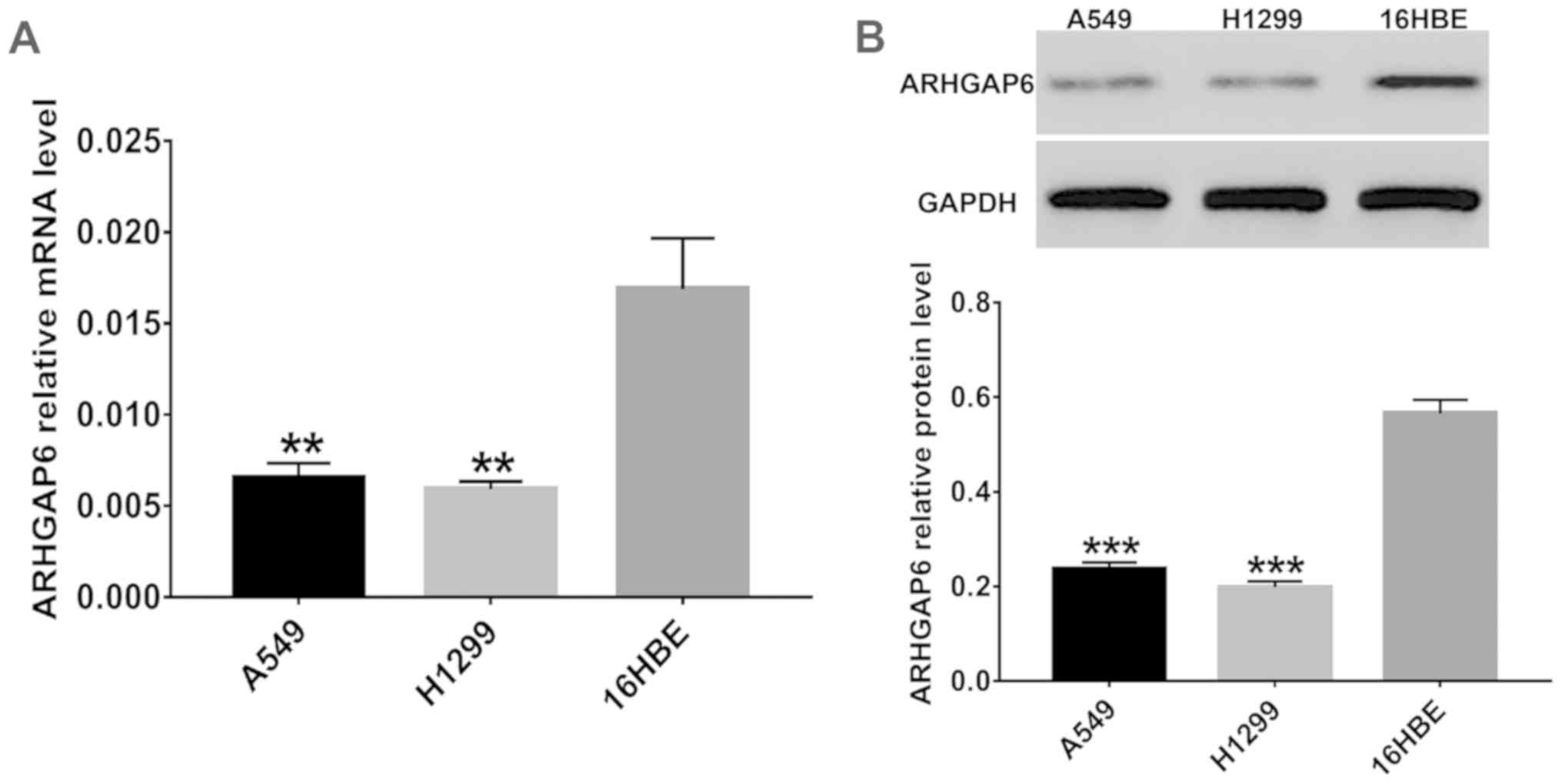
The expression of ARHGAP6 in three cell lines
(16HBE, A549 and H1299) was detected in vitro. As shown in
Fig. 2, the levels of ARHGAP6 mRNA
(Fig. 2A) and protein (Fig. 2B) in A549 and H1299 cells were much
lower than those in the 16HBE cells, which indicated that ARHGAP6
expression was closely associated with lung cancer. The A549 and
H1299 cell lines were used for further study.
Upregulation of ARHGAP6 in the A549
and H1299 cell lines
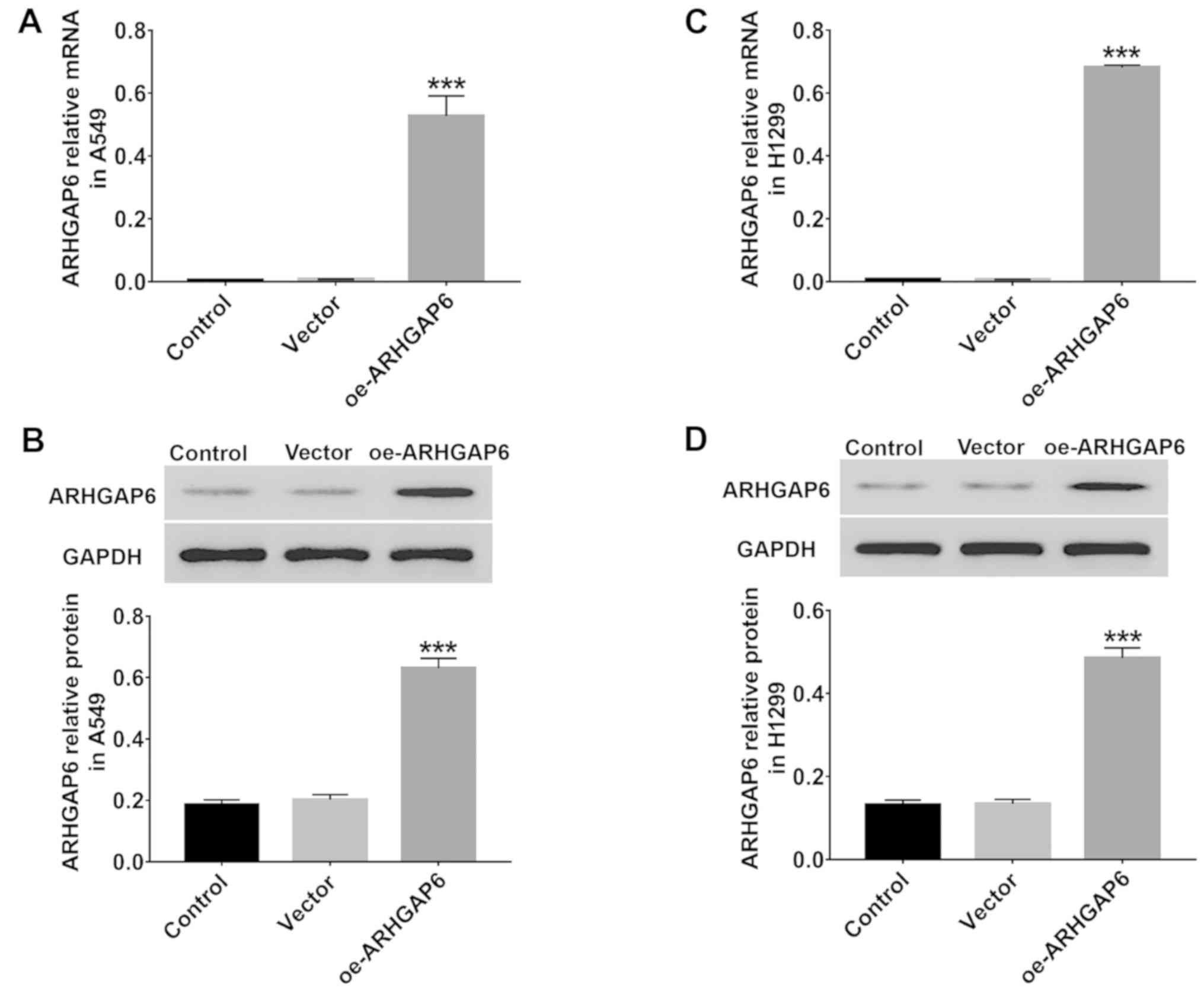
For further study, ARHGAP6 recombinant lentivirus
was used to upregulate the expression of ARHGAP6 in A549 and H1299
cells. After 48 h of infection, the levels of ARHGAP6 mRNA and
protein were quantified by RT-PCR and western blotting,
respectively. The results revealed in Fig. 3 indicated that in both A549
(Fig. 3A and B) and H1299 (Fig. 3C and D) cells, the expression of
ARHGAP6 was markedly upregulated by infection with ARHGAP6
recombinant lentivirus. Therefore, the ARHGAP6 lentivirus was used
in the following experiments due to its effective upregulation of
ARHGAP6 expression.
Effect of ARHGAP6 upregulation on the
proliferation, migration and invasion of A549 and H1299 cells
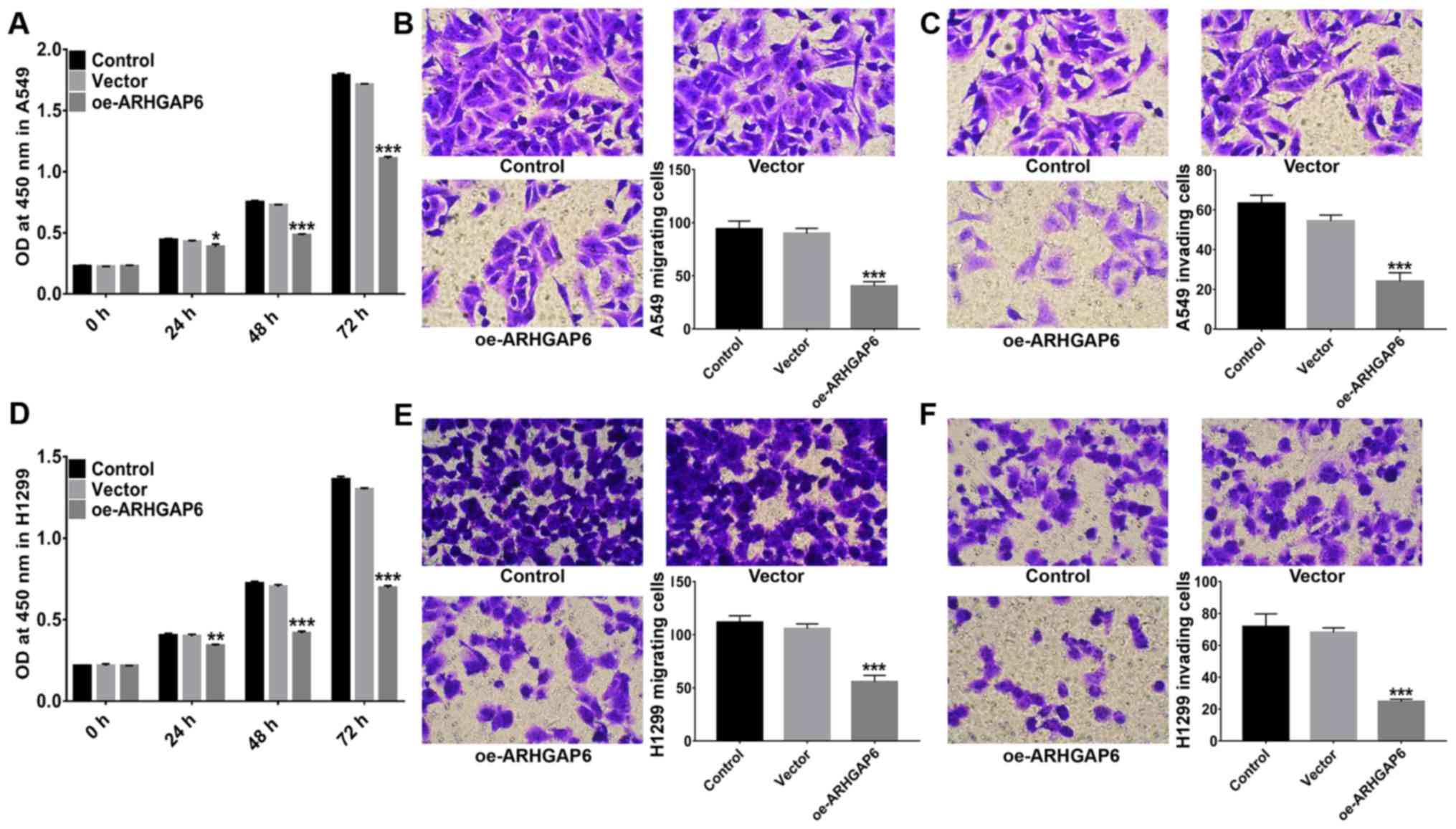
Following the upregulation of ARHGAP6 expression,
the proliferation of A549 and H1299 cells was evaluated by CCK-8
assays. As revealed in Fig. 4, in
both A549 (Fig. 4A) and H1299 cells
(Fig. 4D), proliferation was
significantly inhibited when ARHGAP6 was upregulated. In addition,
the migration activities of A549 (Fig.
4B) and H1299 cells (Fig. 4E)
were significantly suppressed after ARHGAP6 upregulation.
Similarly, ARHGAP6-overexpressing A549 (Fig. 4C) and H1299 (Fig. 4F) cells were less invasive. These
findings demonstrated that ARHGAP6 upregulation had an inhibitory
effect on the growth and metastasis of lung cancer cells. Targeting
ARHGAP6 may be a potential strategy for the treatment and
prevention of lung cancer.
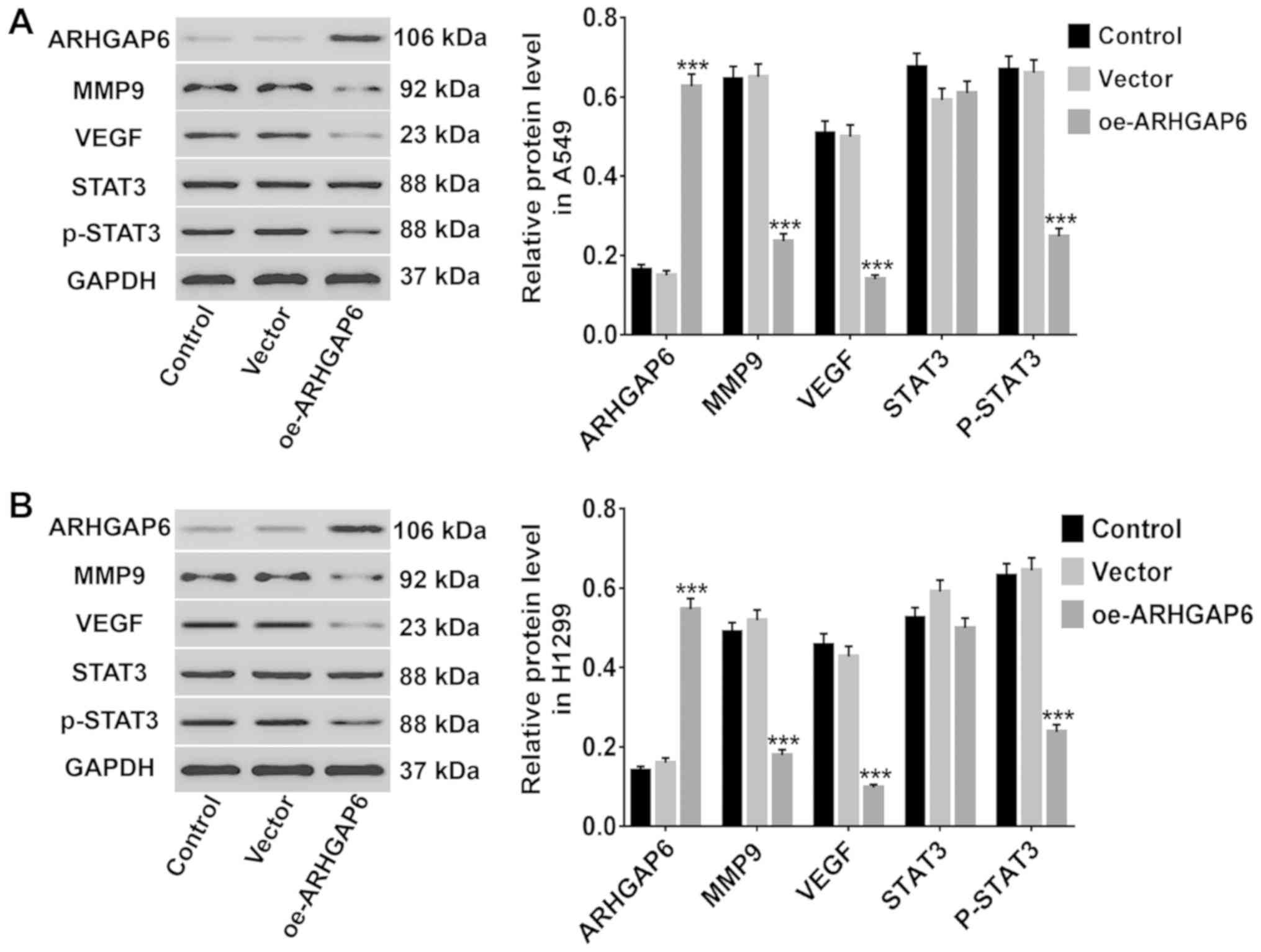
The altered expression of several
associated genes after ARHGAP6 upregulation
Several cancer-associated genes (MMP9, VEGF and
STAT3/p-STAT3) were analyzed to further study the effects of
ARHGAP6 upregulation on lung cancer cells. After ARHGAP6 was
upregulated in A549 and H1299 cells, the levels of these genes were
analyzed by western blotting. As revealed in Fig. 5, in both A549 (Fig. 5A) and H1299 (Fig. 5B) cells, the protein levels of MMP9,
VEGF, and p-STAT3 were markedly reduced after ARHGAP6 upregulation,
while the levels of STAT3 were unchanged. Activation of MMP9 and
VEGF transcription has been revealed to enhance tumor migration and
the invasion of lung cancer (30,31).
STAT3, which mediates the transcription of several genes, including
VEGF, is critically important for the progression of human lung
cancer (32). Thus, we concluded
that ARHGAP6 upregulation may inhibit the growth and metastasis of
lung cancer cells by inhibiting MMP9, VEGF, and STAT3
signaling.
ARHGAP6 upregulation significantly
suppresses IL-6-induced migration, invasion, and MMP9, VEGF and
p-STAT3 expression
It has been reported that IL-6 is an important
multifunctional cytokine that regulates the growth of a variety of
tumors and plays a crucial role in carcinogenesis (16,33,34).
Here, after lentivirus infection and treatment with 50 ng/ml IL-6,
the migration and invasion activities of H1299 cells and the
protein levels of MMP9, VEGF, STAT3 and p-STAT3 were detected. As
shown in Fig. 6, IL-6 induced the
migration (Fig. 6A) and invasion
(Fig. 6B) of H1299 cells, and
ARHGAP6 upregulation significantly inhibited these changes induced
by IL-6. Moreover, the IL-6-induced expression of MMP9, VEGF and
p-STAT3 was significantly reduced by ARHGAP6 upregulation, while
the level of STAT3 was unchanged (Fig.
6C). All the data demonstrated that the upregulation of ARHGAP6
inhibited lung cancer metastasis through the suppression of MMP9,
VEGF, and STAT3 signaling.
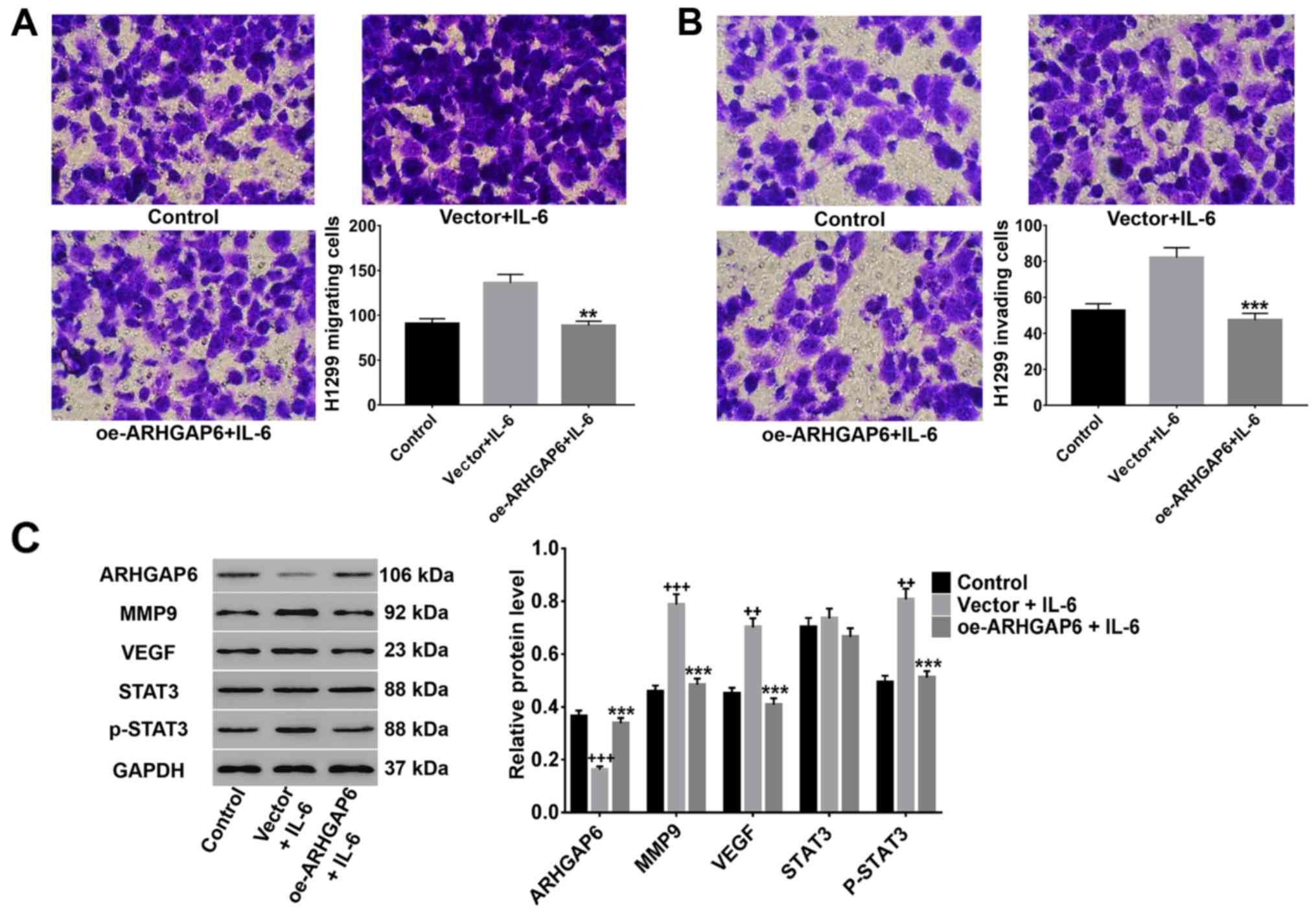 | Figure 6.ARHGAP6 upregulation significantly
suppresses IL-6-induced migration and invasion, as well as MMP9,
VEGF and p-STAT3 expression. After treatment with ARHGAP6
lentivirus and 50 ng/ml IL-6, the (A) migrated and (B) invaded A549
and H1299 cells were determined by Transwell assays. (C) In
addition, the expression of MMP9, VEGF, STAT3 and p-STAT3 was
detected by western blot analysis. All data are presented as the
mean ± SD of three independent experiments (++P<0.01,
+++P<0.001 compared to the control, ***P<0.001
compared to the vector + IL-6 group). ARHGAP6, Ras homologue GTPase
activation protein 6; MMP9, matrix metalloproteinase-9; VEGF,
vascular endothelial growth factor. |
Discussion
Lung cancer, due to its typical diagnosis at later
stages and poor long-term prognosis (35), causes more deaths than other types
of cancer, such as breast and colorectal cancer (6). Thus, new potential treatments or
prevention methods to reduce the incidence and mortality of lung
cancer are urgently needed. Several members of the RhoGAP family
have been discovered to function in lung cancer. For example, a
study has reported that ARHGAP10 acts as a tumor suppressor in lung
cancer (36), and the metastasis of
lung cancer cells was effectively suppressed by downregulation of
the protumorigenic protein ARHGAP5 (37). In this study, we found that ARHGAP6
upregulation was beneficial for lung cancer treatment in that it
suppressed cell proliferation, migration and invasion via
inhibition of STAT3 signaling activation and the expression of MMP9
and VEGF.
Multiple studies have observed a significant
increase in MMP9 in lung cancer, and MMP9 performs important roles
in tumor growth and metastasis (14,15).
In addition to lung cancer, other cancers such as breast cancer
exhibit high expression of MMP9 (38–40).
MMP9 is associated with pathological type, and positive
immunostaining for MMP9 has prognostic value for the distant
metastasis or local recurrence of lung cancer (17,41,42).
VEGF has been reported to induce tumor blood vessel proliferation
and possibly contributes to the extravasation of cancer cells and
the formation of metastasis (43).
These previous findings are consistent with our discovery that
ARHGAP6 was decreased and that MMP9 and VEGF were elevated in
tumors from lung cancer patients. Similar to ARHGAP10 and ARHGAP5,
we concluded that ARHGAP6 is likely a potentially attractive target
for lung cancer diagnosis and treatment. ARHGAP6 upregulation had
inhibitory effects on the growth and metastasis of A549 and H1299
cells; the high levels of MMP9, VEGF, and p-STAT3 were markedly
reduced, while STAT3 was unchanged. These data indicated that
ARHGAP6 was critically important for the growth and metastasis of
lung cancer cells, and MMP9 and VEGF, which play a previously
described crucial role in lung cancer (18,43),
were negatively regulated by ARHGAP6 expression. STAT3 signaling,
which is activated in NSCLC cells, was reported to regulate VEGF
transcription, and disruption of its activity inhibits tumor
angiogenesis (22,23). Therefore, we speculated that the
inhibitory effects of ARHGAP6 upregulation on the growth and
metastasis of lung cancer cells possibly occurs through the
suppression of MMP9, VEGF, and STAT3 signaling. This hypothesis was
further supported by the finding that ARHGAP6 upregulation
significantly suppressed the effects of IL-6 treatment on lung
cancer cells. There are still some limitations in our research such
as the small sample size. Although a small sample size may lead to
false positives in statistical analysis, our results are still of
clinical value. In the future, we will expand the sample size to
more fully ascertain our findings.
In conclusion, we found that upregulation of ARHGAP6
had an inhibitory effect on the cell growth and metastasis of lung
cancer, possibly through the suppression of MMP9, VEGF, and STAT3
signaling. Targeting ARHGAP6 may be beneficial for the treatment
and prevention for lung cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YW and YM conceived and designed the study. YW, MX,
RH and KX performed the experiments. YW and YM wrote the
manuscript. All authors read and approved the manuscript and agree
to be accountable for all aspects of the research in ensuring that
the accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
All experiments in this study were approved by the
Ethics Committee of Liaoning Cancer Hospital and Institute. Written
informed consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Jemal A, Grey N, Ferlay J and
Forman D: Global cancer transitions according to the Human
Development Index (2008–2030): A population-based study. Lancet
Oncol. 13:790–801. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Shin HR, Bray F, Mathers C and
Parkin DM: GLOBOCAN2008, cancer incidence and mortality worldwide:
IARC CancerBase no. 10. Int J cancer. 136:E359–E386. 2012.
View Article : Google Scholar
|
|
3
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brawley OW, Glynn TJ, Khuri FR, Wender RC
and Seffrin JR: The first Surgeon General's report on smoking and
health: The 50th anniversary. CA Cancer J Clin. 64:5–8. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Prevention NCfCD, Smoking HPOo Health, .
The Health Consequences of Smoking-50 Years of Progress: A Report
of the Surgeon General. Public Health Service. Office of the
Surgeon General. (United States). 2014.
|
|
6
|
Nana-Sinkam SP and Powell CA: Molecular
biology of lung cancer: Diagnosis and management of lung cancer,
3rd edition: American College of Chest Physicians evidence-based
clinical practice guidelines. Chest. 143:e30S–e39S. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Egile C, Rouiller I, Xu XP, Volkmann N, Li
R and Hanein D: Mechanism of filament nucleation and branch
stability revealed by the structure of the Arp2/3 complex at actin
branch junctions. PLoS Biol. 3:e3832005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ridley AJ: Rho-related proteins: Actin
cytoskeleton and cell cycle. Curr Opin Genet Dev. 5:24–30. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schaefer L, Prakash S and Zoghbi HY:
Cloning and characterization of a novelrho-type
GTPase-activating protein gene (ARHGAP6) from the critical
region for microphthalmia with linear skin defects. Genomics.
46:268–277. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tribioli C, Droetto S, Bione S, Cesareni
G, Torrisi MR, Lotti LV, Lanfrancone L, Toniolo D and Pelicci P: An
X chromosome-linked gene encoding a protein with characteristics of
a rhoGAP predominantly expressed in hematopoietic cells. Proc Natl
Acad Sci USA. 93:695–699. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li J, Yang L and Yin Y: Inhibitory effects
of Arhgap6 on cervical carcinoma cells. Tumour Biol. 37:1411–1425.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo F, Liu Y, Huang J, Li Y, Zhou G, Wang
D, Li Y, Wang J and Xie P: Identification of Rho GTPase activating
protein 6 isoform 1 variant as a new molecular marker in human
colorectal tumors. Pathol Oncol Res. 16:319–326. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Faraji SN, Mojtahedi Z, Ghalamfarsa G and
Takhshid MA: N-myc downstream regulated gene 2 overexpression
reduces matrix metalloproteinase-2 and −9 activities and cell
invasion of A549 lung cancer cell line in vitro. Iran J Basic Med
Sci. 18:773–779. 2015.PubMed/NCBI
|
|
15
|
Xu X, Cao L, Zhang Y, Yin Y, Hu X and Cui
Y: Network analysis of DEGs and verification experiments reveal the
notable roles of PTTG1 and MMP9 in lung cancer. Oncol Lett.
15:257–263. 2018.PubMed/NCBI
|
|
16
|
Wójcik E, Jakubowicz J, Skotnicki P,
Saskorczyńska B and Kulpa JK: IL-6 and VEGF in small cell lung
cancer patients. Anticancer Res. 30:1773–1778. 2010.PubMed/NCBI
|
|
17
|
Cai J, Li R, Xu X, Zhang L, Wu S, Yang T,
Fang L, Wu J, Zhu X, Li M and Huang Y: URGCP promotes non-small
cell lung cancer invasiveness by activating the NF-κB-MMP-9
pathway. Oncotarget. 6:36489–36504. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zuo J, Wen M, Li S, Lv X, Wang L, Ai X and
Lei M: Overexpression of CXCR4 promotes invasion and migration of
non-small cell lung cancer via EGFR and MMP-9. Oncol Lett.
14:7513–7521. 2017.PubMed/NCBI
|
|
19
|
Goudar RK and Vlahovic G: Hypoxia,
angiogenesis, and lung cancer. Curr Oncol Rep. 10:277–282. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lucchi M, Mussi A, Fontanini G, Faviana P,
Ribechini A and Angeletti CA: Small cell lung carcinoma (SCLC): The
angiogenic phenomenon. Eur J Cardiothorac Surg. 21:11052002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu H and Jove R: The STATs of cancer-new
molecular targets come of age. Nat Rev Cancer. 4:94–105. 2004.
View Article : Google Scholar
|
|
22
|
Leong H, Mathur PS and Greene GL: Green
tea catechins inhibit angiogenesis through suppression of STAT3
activation. Breast Cancer Res Treat. 117:505–515. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song L, Rawal B, Nemeth JA and Haura EB:
JAK1 activates STAT3 activity in non-small-cell lung cancer cells
and IL-6 neutralizing antibodies can suppress JAK1-STAT3 signaling.
Mol Cancer Ther. 10:481–494. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yeh HH, Lai WW, Chen HH, Liu HS and Su WC:
Autocrine IL-6-induced Stat3 activation contributes to the
pathogenesis of lung adenocarcinoma and malignant pleural effusion.
Oncogene. 25:4300–4309. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Martín F, Santolaria F, Batista N, Milena
A, González-Reimers E, Brito MJ and Oramas J: Cytokine levels (IL-6
and IFN-gamma), acute phase response and nutritional status as
prognostic factors in lung cancer. Cytokine. 11:80–86. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yanagawa H, Sone S, Takahashi Y, Haku T,
Yano S, Shinohara T and Ogura T: Serum levels of interleukin 6 in
patients with lung cancer. Br J Cancer. 71:1095–1098. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hong J, Kang B, Kim A, Hwang S, Ahn J, Lee
S, Kim J, Park JH and Cheon DS: Development of a highly sensitive
real-time one step RT-PCR combined complementary locked primer
technology and conjugated minor groove binder probe. Virol J.
8:3302011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kobayashi T, Hino S, Oue N, Asahara T,
Zollo M, Yasui W and Kikuchi A: Glycogen synthase kinase 3 and
h-prune regulate cell migration by modulating focal adhesions. Mol
Cell Biol. 26:898–911. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cheng X, Yang Y, Fan Z, Yu L, Bai H, Zhou
B, Wu X, Xu H, Fang M, Shen A, et al: MKL1 potentiates lung cancer
cell migration and invasion by epigenetically activating MMP9
transcription. Oncogene. 34:5570–5581. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Takeda A, Stoeltzing O, Ahmad SA, Reinmuth
N, Liu W, Parikh A, Fan F, Akagi M and Ellis LM: Role of
angiogenesis in the development and growth of liver metastasis. Ann
Surg Oncol. 9:610–616. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang ZH, Bi-Dan HU, Min YU and Zhang YB:
Expressions of leptin, STAT3, p-STAT3 and bcl-2 in lung cancer and
their clinical significance. Tumor. 30:529–534. 2010.
|
|
33
|
Heinrich PC, Behrmann I, Haan S, Hermanns
HM, Müller-Newen G and Schaper F: Principles of interleukin
(IL)-6-type cytokine signaling and its regulation. Biochem J.
374:1–20. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schafer ZT and Brugge JS: IL-6 involvement
in epithelial cancers. J Clin Invest. 117:3660–3663. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang T, Nelson RA, Bogardus A and Grannis
FW Jr: Five-year lung cancer survival: Which advanced stage
nonsmall cell lung cancer patients attain long-term survival?
Cancer. 116:1518–1525. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Teng JP, Yang ZY, Zhu YM, Ni D, Zhu ZJ and
Li XQ: The roles of ARHGAP10 in the proliferation, migration and
invasion of lung cancer cells. Oncol Lett. 14:4613–4618. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang J, Tian X, Han R, Zhang X, Wang X,
Shen H, Xue L, Liu Y, Yan X, Shen J, et al: Downregulation of
miR-486-5p contributes to tumor progression and metastasis
by targeting protumorigenic ARHGAP5 in lung cancer.
Oncogene. 33:1181–1189. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zuo J, Ishikawa T, Boutros S, Xiao Z,
Humtsoe JO and Kramer RH: Bcl-2 overexpression induces a partial
epithelial to mesenchymal transition and promotes squamous
carcinoma cell invasion and metastasis. Mol Cancer Re. 8:170–182.
2010. View Article : Google Scholar
|
|
39
|
Gao J, Liu X, Yang F, Liu T, Yan Q and
Yang X: By inhibiting Ras/Raf/ERK and MMP-9, knockdown of EpCAM
inhibits breast cancer cell growth and metastasis. Oncotarget.
6:27187–27198. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shon SK, Kim A, Kim JY, Kim KI, Yang Y and
Lim JS: Bone morphogenetic protein-4 induced by NDRG2 expression
inhibits MMP-9 activity in breast cancer cells. Biochem Biophys Res
Commun. 385:198–203. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Elbadrawy MK, Yousef AM, Shaalan D and
Elsamanoudy AZ: Matrix metalloproteinase-9 expression in lung
cancer patients and its relation to serum mmp-9 activity,
pathologic type, and prognosis. J Bronchology Interv Pulmonol.
21:327–334. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu J, Ping W, Zu Y and Sun W:
Correlations of lysyl oxidase with MMP2/MMP9 expression and its
prognostic value in non-small cell lung cancer. Int J Clin Exp
Pathol. 7:6040–6047. 2014.PubMed/NCBI
|
|
43
|
Matsuyama W, Hashiguchi T, Mizoguchi A,
Iwami F, Kawabata M, Arimura K and Osame M: Serum levels of
vascular endothelial growth factor dependent on the stage
progression of lung cancer. Chest. 118:948–951. 2000. View Article : Google Scholar : PubMed/NCBI
|